Tratamentos para o priapismo em crianças e adultos com doença das células falciformes
Referencias
References to studies included in this review
Ir a:
Additional references
Ir a:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind, placebo‐controlled parallel group trial followed by an open‐label extension for 8 weeks. Conducted prospectively from June 2008 to November 2012. | |
| Participants | 13 participants recruited from regional haematology and urology clinics randomised to sildenafil or placebo. Inclusion criteria were occurrences of at least two self‐reported priapism episodes per week and ability to provide written informed consent. Exclusion criteria were estimated GFR < 50 mL/min, clinical cirrhosis, pulmonary hypertension based on echocardiography, alcohol use exceeding 2 standard drinks daily, formal contraindications for using phosphodiesterase type 5 inhibitor therapy. Participant age: sildenafil (n = 6) mean (SD) age: 21.7 (5.3) years; placebo (n = 7) mean (SD) age: 23.0 (8.7) years. Disease status: SCD (confirmed SS or SC haemoglobinopathy) and recurrent ischaemic priapism. Hypertension no (%) placebo 1 (14.3%), sildenafil 2 (33.3%). Stroke no (%) placebo 3 (42.9%), sildenafil 1 (16.7%). Avascular necrosis no (%) placebo 1 (14.3%), sildenafil 0 (0). Acute chest syndrome no (%) placebo 1 (14.3%), sildenafil 2 (33.3%). Asthma no (%) placebo 2 (28.6%), sildenafil 2 (33.3%). Smoker no (%) placebo 1 (14.3%), sildenafil 2 (33.3%). Alcohol use no (%) placebo 3 (42.9%), sildenafil 3 (50%). | |
| Interventions | Intervention: sildenafil 50 mg. Placebo: sugar pill. Either placebo or sildenafil was taken daily for 8 weeks. Participants were instructed to take the medication in the morning a few hours after awakening and without sexual stimulation. An 8‐week open‐label phase followed. | |
| Outcomes | Primary efficacy outcome was 50% reduction in frequency in priapism episodes bi‐weekly from baseline at the end of the 8‐week double‐blind phase. Secondary outcomes included subjective improvements in episode frequency/duration and decrease in the number of of bi‐weekly episodes of priapism using a scoring system: 0 = no episodes; 1 = 1 to 2 episodes; 2 = 3 to 4 episodes; 3 = 5 to 8 episodes; 4 = 9 to 16 episodes; 5 = > 16 episodes. Adverse effects were also recorded for the whole trial period including the open label phase. Outcomes were measured via twice‐weekly nurse co‐ordinator phone calls to record progress, medication changes and adverse events. In‐clinic evaluations were carried out at baseline, week four and week eight which included repeat administration of trial instruments. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participats were randomised in a 1:1 allocation. Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind trial where the participant, caregiver and investigator were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind trial where the participant, caregiver and investigator were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis. Both ITT and per protocol analyses were carried out. No data were shown for decreased median weekly change in priapism episode score, just that there were no significant differences between groups. Adverse effects were not listed in the methods section but were reported in the results. Adverse events were reported for the whole trial period including the open label phase (confirmed by author team). |
| Selective reporting (reporting bias) | Unclear risk | The publication states that there were no significant differences between groups for decreased median weekly change in priapism episode score but no data were shown. The same paper reported adverse effects in the published paper but did not include this as an outcome in either the methods section or in the trial registration document. |
| Other bias | Low risk | None identified. |
| Methods | Double‐blind, placebo controlled parallel group trial with four arms. The first phase of the trial was an observational phase lasting a minimum of three weeks and a maximum of 13 weeks to give a baseline before participants were randomised to intervention or placebo. The intervention phase ran for 26 weeks. | |
| Participants | 131 participants were enrolled onto the trial. 86 participants completed the observational phase of the trial. 78 participants were randomised to 1 of the 4 treatment arms. Inclusion criteria: males aged 12 or over with confirmed sickle cell anaemia or sickle cell beta‐thalassaemia on haemoglobin electrophoresis and stuttering priapism. Participants had to be in active attendance at a designated care centre (1 visit in last 6 months). Participant characteristics were only reported for the complete group of 86 not specifically for the 46 randomised participants. Age (participants who completed phase A n = 86): mean age was 23.6 (range 14.5 ‐ 46.1). Median weekly number of attacks in phase A ‐ 1.5 (range 0 – 7). Median weekly duration of attacks in phase A – 2.0h (range 0 – 42 h). Number (%) of weekly attacks > 4h duration in phase A – 22 (26%). Average attack pain score (mean (range)) in phase A – 3.4 (0 – 8.8). Participants were excluded if there was a history of an attack lasting longer than 4 hours or who needed acute hospital. admission and intervention or history of cerebrovascular accident or hypertension. | |
| Interventions | Group 1: participants received 15 mg ephedrine per day. Group 2: participants received 30 mg ephedrine per day. Group 3: participants received 50 mg etilefrine per day. Group 4: participants received a placebo. All treatments were taken once a day at bedtime for 6 months. | |
| Outcomes | Weekly total number of attacks expressed as a ratio of phase B to phase A. Mean difference in pain score from phase A to phase B. Number of participants who had an attack lasting more than four hours. Adverse events (palpitations, tachycardia, lack of sleep, hand shaking, anxiety, dry mouth) experienced during the trial period. Participants were reviewed on a 6‐weekly basis when diaries were monitored and BP was taken. | |
| Notes | None of the participants were on a chronic hypertransfusion program. One participant had been on hydroxyurea for 18 months prior to the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of the trial drug was carried out by Bilcare Ltd clinical trial supplies although the authors do not state who was blinded and what methods were used. It is a double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of the trial drug was carried out by Bilcare Ltd clinical trial supplies although the authors do not state who was blinded and what methods were used. It is a double‐blind trial. |
| Incomplete outcome data (attrition bias) | High risk | 86 participants completed the observational phase and of these, 59% entered the intervention phase. 78 participants were randomised to the intervention phase but data was only available for 46. The remainder were lost to follow‐up. 46 participants entered the intervention phase and 25 participants (54%) completed 26 weeks. |
| Selective reporting (reporting bias) | Low risk | No protocol available therefore outcomes reported in the results section against the methods section of the paper; no discrepancies found. |
| Other bias | Low risk | None identified. |
| Methods | Double‐blind, placebo‐controlled cross‐over trial, with two 14‐day treatment periods. Allocation concealment and method of randomisation was unclear. | |
| Participants | 11 males with stuttering priapism and homozygous sickle cell (SS) disease were randomised. 9 completed the trial, 1 participant defaulted after baseline and 1 participant had a painful crisis which aborted the priapism before any tablets were taken. Participants came from the sickle cell clinic of the University Hospital of the West Indies, Kingston, Jamaica. | |
| Interventions | Stilboestrol 5 mg daily versus placebo. | |
| Outcomes | Reduction in frequency of stuttering priapism. Return of priapism. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 participant defaulted, and in two participants the attacks of stuttering priapism ceased spontaneously during the baseline observation period. There was a fourth participant who initially did not take the placebo because a painful crisis aborted the stuttering priapism prior to taking the assigned tablet. In this participant (identified as patient 9) attacks of priapism recurred 2 weeks later and data on a second baseline period were collected. The paper did not discuss an ITT analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol available therefore outcomes reported in the results section against the methods section of the paper; no discrepancies found. |
| Other bias | Low risk | None identified. |
BP: blood pressure
ITT: intention‐to‐treat
SCD: sickle cell disease
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Silboesterol versus placebo (stuttering priapism), Outcome 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol). | ||||
| 1.1 No attacks into the second week of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil). | ||||
| 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2 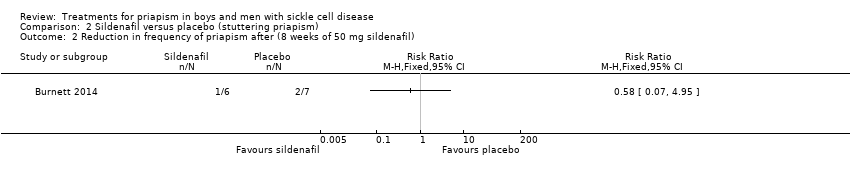 Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate side effects (palpitations) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 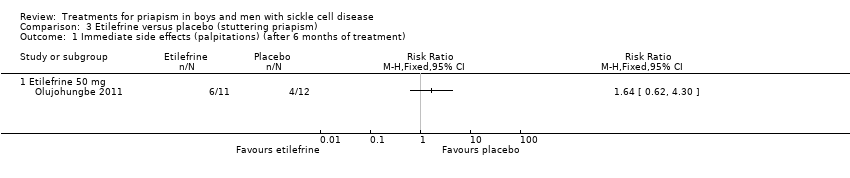 Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment). | ||||
| 1.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment). | ||||
| 2.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep). | ||||
| 3.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking). | ||||
| 4.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment). | ||||
| 5.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.6 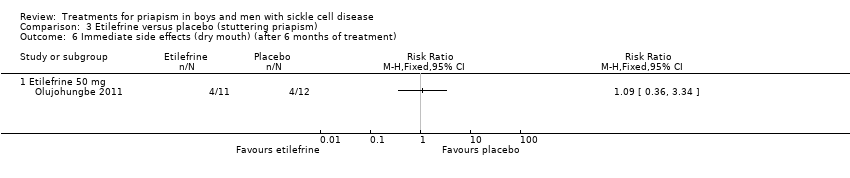 Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment). | ||||
| 6.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate side effects (palpitations) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment). | ||||
| 1.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment). | ||||
| 2.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3 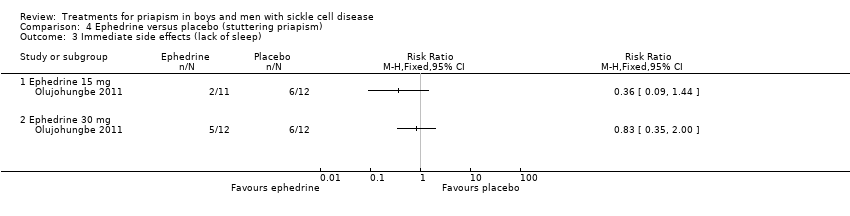 Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep). | ||||
| 3.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking). | ||||
| 4.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.5 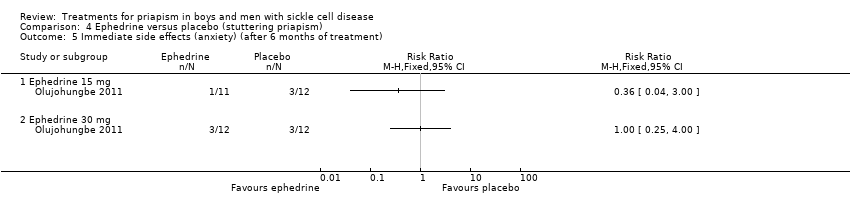 Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment). | ||||
| 5.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.6  Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment). | ||||
| 6.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
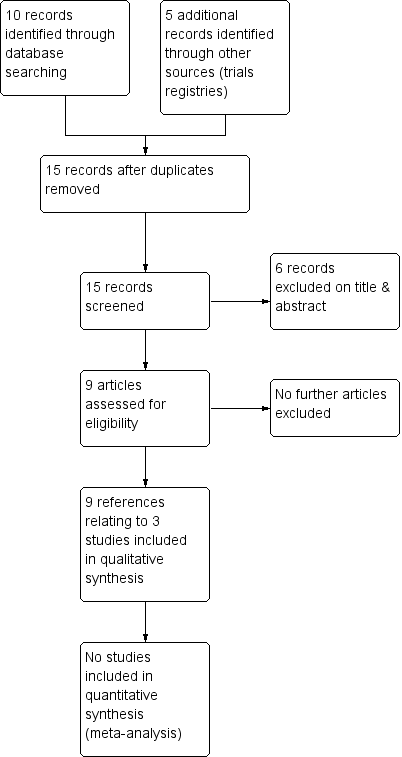
Study flow diagram.
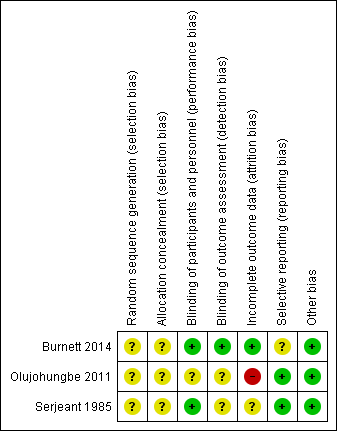
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
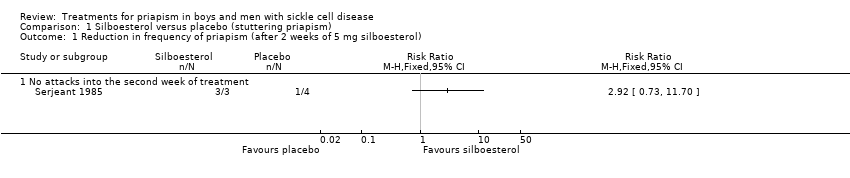
Comparison 1 Silboesterol versus placebo (stuttering priapism), Outcome 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol).
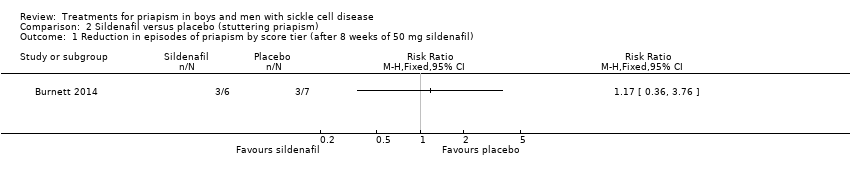
Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil).
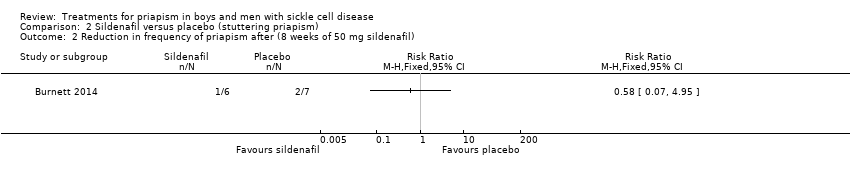
Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil).
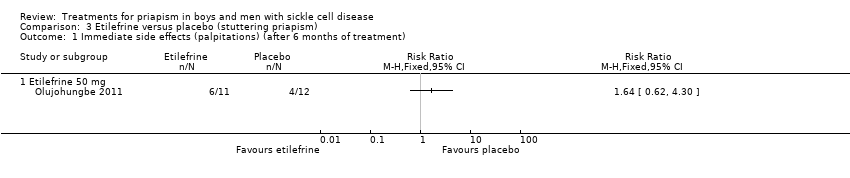
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking).
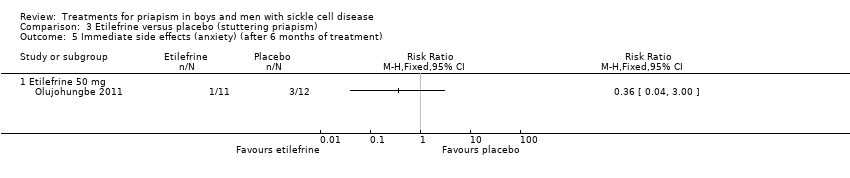
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep).
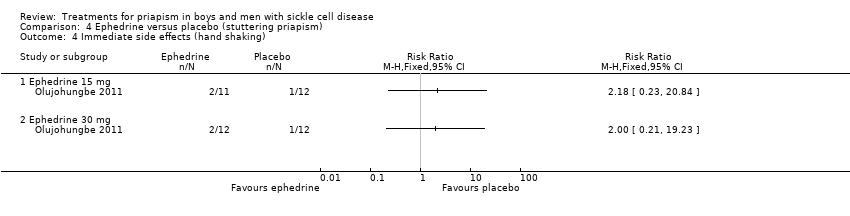
Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment).
| Silboesterol compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: stilboestrol 5 mg Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Stilboestrol 5 mg | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in stuttering priapism Follow‐up: 2 weeks | 250 per 1000 | 730 per 1000 | RR 2.92 (95% CI 0.73 to 11.70) | 7 | ⊕⊝⊝⊝ | |
| Immediate side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to unclear methods of randomisation and allocation. | ||||||
| Sildenafil 50 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: sildenafil 50 mg daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sildenafil 50 mg daily | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 8 weeks | 286 per 1000 | 166 per 1000 (20 to 1000) | RR 0.58 (95% CI 0.07 to 4.95) | 13 (1) | ⊕⊕⊝⊝ | There was also no significant difference between treatments for the reduction in episodes of priapism by score tier: RR 1.17 (95% CI 0.36 to 3.76). |
| Immediate side effects of treatment Follow‐up: 16 weeks | See comment | See comment | N/A | 13 (1) | ⊕⊕⊝⊝ | Side effects of treatment were reported for the whole treatment phase including the open label phase. No significant differences were found between sildenafil and placebo. |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to unclear methods of randomisation and allocation. | ||||||
| Etilefrine 50 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatient Intervention: etilefrine 50mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Etilefrine 50 mg | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months | See comment | See comment | N/A | 23 (1) | ⊕⊝⊝⊝ | No significant difference was found between etilefrine and placebo but there were concerns over the reliability of the published results. |
| Immediate side effects of treatment (palpitations) Follow‐up: 6 months | 333 per 1000 | 546 per 1000 (206 to 1000) | RR 1.64 (95% CI 0.62 to 4.30) | 23 (1) | ⊕⊝⊝⊝ | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Immediate side effects of treatment (tachycardia) Follow‐up: 6 months | 250 per 1000 | 545 per 1000 (178 to 1000) | RR 2.18 (95% CI 0.71 to 6.68) | 23 (1) | ⊕⊝⊝⊝ | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. | ||||||
| Ephedrine 15 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: ephedrine 15 mg/30 mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ephedrine | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months | See comment | See comment | N/A | 24 (1) | ⊕⊝⊝⊝ | No significant difference was found between ephedrine 15mg and placebo or ephedrine 30mg and placebo but there were concerns over the reliability of the published results. |
| Immediate side effects of treatment with 30 mg ephedrine (palpitations) Follow‐up: 6 months | 333 per 1000 | 333 per 1000 (107 to 1000) | RR 1.00 (95% CI 0.32 to 3.10) | 24 (1) | ⊕⊝⊝⊝ | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Immediate side effects of treatment with 30 mg ephedrine (tachycardia) Follow‐up: 6 months | 250 per 1000 | 168 per 1000 (33 to 825) | RR 0.67 (95% CI 0.13 to 3.30) | 24 (1) | ⊕⊝⊝⊝ | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 No attacks into the second week of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate side effects (palpitations) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate side effects (palpitations) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

