Treatments for priapism in boys and men with sickle cell disease
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004198.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 19 septiembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
The original review was conceived by the Cochrane Cystic Fibrosis and Genetic Disorders Group, who also conducted searches for relevant trials. Dr Chinegwundoh (FC) designed the original review with constructive comments from Dr Anie (KA).
FC and KA conducted further searches for relevant trials. FC took the lead in the write up of the review, with substantial revisions from KA.
FC is responsible for updating the review and acts as guarantor of the review.
For the 2017 version, Sherie Smith (SS) lead on this update with input from FC and KA.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Francis Chinegwundoh: none known.
Sherie Smith: none known.
Kofi Anie: none known.
Acknowledgements
We are grateful to the following for comments:
Professor Dame Sally C Davies, Imperial College Faculty of Medicine, Department of Haematology, Central Middlesex Hospital, London, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Apr 06 | Treatments for priapism in boys and men with sickle cell disease | Review | Francis I Chinegwundoh, Sherie Smith, Kofi A Anie | |
| 2017 Sep 19 | Treatments for priapism in boys and men with sickle cell disease | Review | Francis I Chinegwundoh, Sherie Smith, Kofi A Anie | |
| 2004 Oct 18 | Treatments for priapism in boys and men with sickle cell disease | Review | Francis I Chinegwundoh, Kofi A Anie | |
| 2003 Apr 22 | Treatments for priapism in boys and men with sickle cell disease | Protocol | Francis I Chinegwundoh, Banji Adeyoju, Kofi A Anie | |
Differences between protocol and review
In a post hoc change, we have added summary of findings tables to the review.
For the 2017, we also searched www.clinicaltrials.gov, Embase for 2016 and the WHO trial portal.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Adrenergic Agents [adverse effects, therapeutic use];
- Anemia, Sickle Cell [*complications];
- Diethylstilbestrol [*therapeutic use];
- Ephedrine [adverse effects, therapeutic use];
- Estrogens, Non-Steroidal [*therapeutic use];
- Etilefrine [adverse effects, therapeutic use];
- Priapism [*drug therapy, etiology];
- Randomized Controlled Trials as Topic;
- Sildenafil Citrate [therapeutic use];
- Tachycardia [chemically induced];
- Vasoconstrictor Agents [adverse effects, *therapeutic use];
Medical Subject Headings Check Words
Humans; Male; Young Adult;
PICO
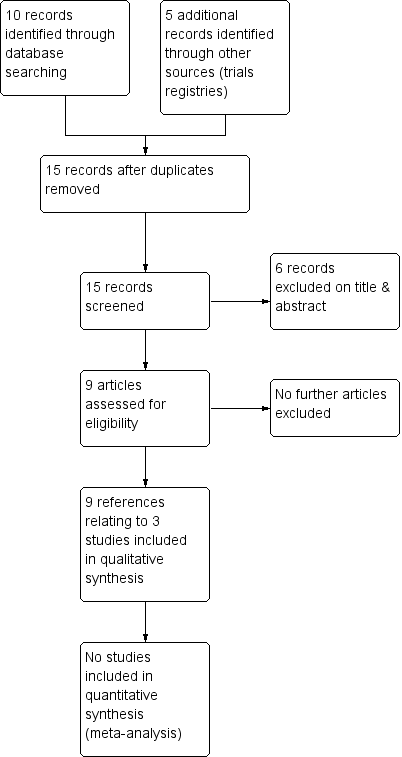
Study flow diagram.
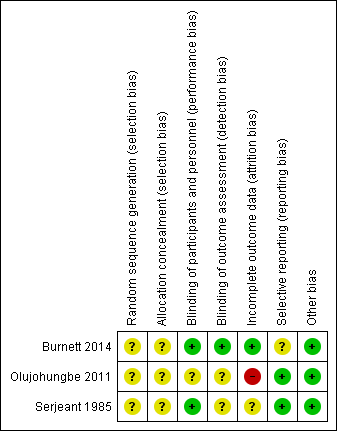
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
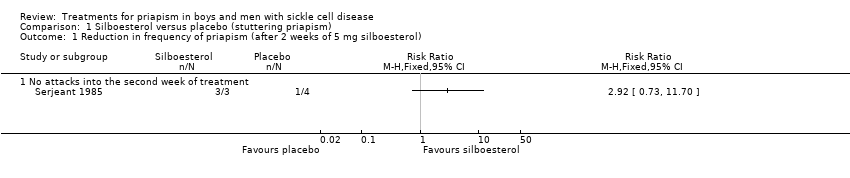
Comparison 1 Silboesterol versus placebo (stuttering priapism), Outcome 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol).
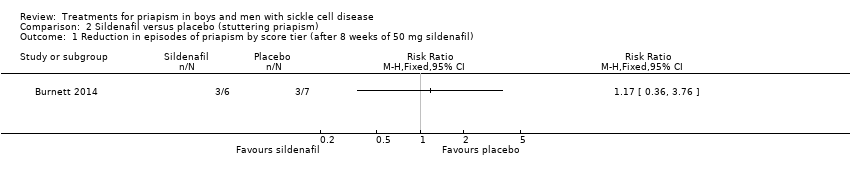
Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil).
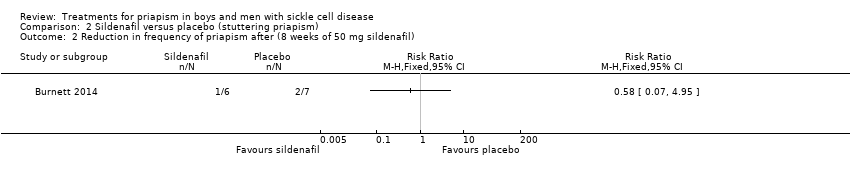
Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil).
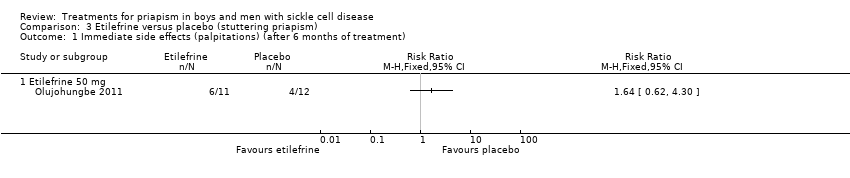
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking).
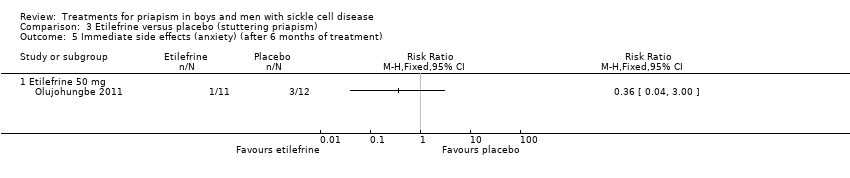
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep).
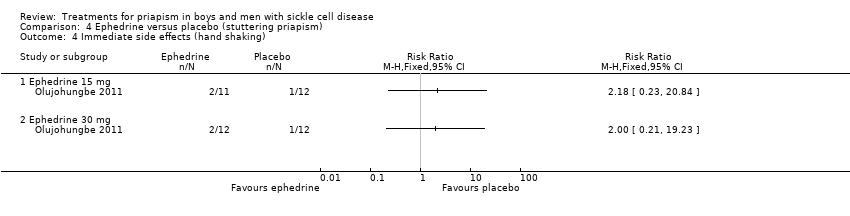
Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment).
| Silboesterol compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: stilboestrol 5 mg Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Stilboestrol 5 mg | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in stuttering priapism Follow‐up: 2 weeks | 250 per 1000 | 730 per 1000 | RR 2.92 (95% CI 0.73 to 11.70) | 7 | ⊕⊝⊝⊝ | |
| Immediate side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to unclear methods of randomisation and allocation. | ||||||
| Sildenafil 50 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: sildenafil 50 mg daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sildenafil 50 mg daily | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 8 weeks | 286 per 1000 | 166 per 1000 (20 to 1000) | RR 0.58 (95% CI 0.07 to 4.95) | 13 (1) | ⊕⊕⊝⊝ | There was also no significant difference between treatments for the reduction in episodes of priapism by score tier: RR 1.17 (95% CI 0.36 to 3.76). |
| Immediate side effects of treatment Follow‐up: 16 weeks | See comment | See comment | N/A | 13 (1) | ⊕⊕⊝⊝ | Side effects of treatment were reported for the whole treatment phase including the open label phase. No significant differences were found between sildenafil and placebo. |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to unclear methods of randomisation and allocation. | ||||||
| Etilefrine 50 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatient Intervention: etilefrine 50mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Etilefrine 50 mg | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months | See comment | See comment | N/A | 23 (1) | ⊕⊝⊝⊝ | No significant difference was found between etilefrine and placebo but there were concerns over the reliability of the published results. |
| Immediate side effects of treatment (palpitations) Follow‐up: 6 months | 333 per 1000 | 546 per 1000 (206 to 1000) | RR 1.64 (95% CI 0.62 to 4.30) | 23 (1) | ⊕⊝⊝⊝ | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Immediate side effects of treatment (tachycardia) Follow‐up: 6 months | 250 per 1000 | 545 per 1000 (178 to 1000) | RR 2.18 (95% CI 0.71 to 6.68) | 23 (1) | ⊕⊝⊝⊝ | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. | ||||||
| Ephedrine 15 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: ephedrine 15 mg/30 mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ephedrine | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months | See comment | See comment | N/A | 24 (1) | ⊕⊝⊝⊝ | No significant difference was found between ephedrine 15mg and placebo or ephedrine 30mg and placebo but there were concerns over the reliability of the published results. |
| Immediate side effects of treatment with 30 mg ephedrine (palpitations) Follow‐up: 6 months | 333 per 1000 | 333 per 1000 (107 to 1000) | RR 1.00 (95% CI 0.32 to 3.10) | 24 (1) | ⊕⊝⊝⊝ | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Immediate side effects of treatment with 30 mg ephedrine (tachycardia) Follow‐up: 6 months | 250 per 1000 | 168 per 1000 (33 to 825) | RR 0.67 (95% CI 0.13 to 3.30) | 24 (1) | ⊕⊝⊝⊝ | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 No attacks into the second week of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate side effects (palpitations) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate side effects (palpitations) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

