Liječenje prijapizma u dječaka s anemijom srpastih stanica
Abstract
Background
Sickle cell disease comprises a group of genetic haemoglobin disorders. The predominant symptom associated with sickle cell disease is pain resulting from the occlusion of small blood vessels by abnormally 'sickle‐shaped' red blood cells. There are other complications, including chronic organ damage and prolonged painful erection of the penis, known as priapism. Severity of sickle cell disease is variable, and treatment is usually symptomatic. Priapism affects up to half of all men with sickle cell disease, however, there is no consistency in treatment. We therefore need to know the best way of treating this complication in order to offer an effective interventional approach to all affected individuals.
Objectives
To assess the benefits and risks of different treatments for stuttering (repeated short episodes) and fulminant (lasting for six hours or more) priapism in sickle cell disease.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Haemoglobinopathies Trials Register, which comprises references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. We also searched trial registries.
Date of the most recent search of the Group's Haemoglobinopathies Trials Register: 15 September 2017.
Date of most recent search of trial registries and of Embase: 12 December 2016.
Selection criteria
All randomised or quasi‐randomised controlled trials comparing non‐surgical or surgical treatment with placebo or no treatment, or with another intervention for stuttering or fulminant priapism.
Data collection and analysis
The authors independently extracted data and assessed the risk of bias of the trials.
Main results
Three trials with 102 participants were identified and met the criteria for inclusion in this review. These trials compared stilboestrol to placebo, sildenafil to placebo and ephedrine or etilefrine to placebo and ranged in duration from two weeks to six months. All of the trials were conducted in an outpatient setting in Jamaica, Nigeria and the UK. None of the trials measured our first primary outcome, detumescence but all three trials reported on the reduction in frequency of stuttering priapism, our second primary outcome. No significant effect of any of the treatments was seen compared to placebo. Immediate side effects were not found to be significantly different from placebo in the two trials where this information was reported. We considered the quality of evidence to be low to very low as all of the trials were at risk of bias and all had low participant numbers.
Authors' conclusions
There is a lack of evidence for the benefits or risks of the different treatments for both stuttering and fulminant priapism in sickle cell disease. This systematic review has clearly identified the need for well‐designed, adequately‐powered, multicentre randomised controlled trials assessing the effectiveness of specific interventions for priapism in sickle cell disease.
PICO
Laički sažetak
Liječenje dugotrajne i bolne erekcije penisa (prijapizam) u dječaka s anemijom srpastih stanica
Istraživačko pitanje
Koje su koristi i rizici različitih postupaka liječenja kratkog i ponavljanog (koje se ponavlja u kratkim epizodama) i fulminantnog (u trajanju od šest sati ili više) prijapizma kod anemije srpastih stanica?
Dosadašnje spoznaje
Prijapizam (produljena bolna erekcija penisa) je čest u muškaraca s anemijom srpastih stanica. Trajanje prijapizma razlikuje se kod različitih tipova poremećaja, te stoga postoji i više postupaka liječenja. Pristupi samopomoći mogu biti korisni. Tražili smo randomizirana kontrolirana ispitivanja različitih postupaka liječenja kako bi pronašli najbolju opciju.
Datum pretraživanja
Zadnje pretraživanje literature obavljeno je 15 rujna 2017.
Obilježja uključenih istraživanja
Pronašli smo tri ispitivanja provedena u Jamajci, Nigeriji i Velikoj Britaniji koja su uključivala ukupno 102 osobe.
Ključni rezultati
U kliničkim ispitivanjima, četiri različita lijeka (stilboestrol, sildenafil, efedrin i etilefrine) bila su uspoređivana s placebom. Sva su ispitivanja istraživala da li postupci liječenja smanjuju učestalost napadaja prijapizma koja se događaju. Nije bilo razlike između bilo kojeg postupka liječenja u usporedbi s placebom. Zbog nedostatka dokaza, nismo u mogućnosti zaključiti koji je najbolji postupak liječenja prijapizma kod anemije srpastih stanica. Potrebno je obaviti dodatna istraživanja.
Kvaliteta dokaza
Zaključili smo da je kvaliteta dokaza niska do vrlo niska jer je kod svih ispitivanja uočen rizik od pristranosti i sva su imala mali broj sudionika.
Authors' conclusions
Summary of findings
| Silboesterol compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: stilboestrol 5 mg Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Stilboestrol 5 mg | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in stuttering priapism Follow‐up: 2 weeks | 250 per 1000 | 730 per 1000 | RR 2.92 (95% CI 0.73 to 11.70) | 7 | ⊕⊝⊝⊝ | |
| Immediate side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to unclear methods of randomisation and allocation. | ||||||
| Sildenafil 50 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: sildenafil 50 mg daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sildenafil 50 mg daily | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 8 weeks | 286 per 1000 | 166 per 1000 (20 to 1000) | RR 0.58 (95% CI 0.07 to 4.95) | 13 (1) | ⊕⊕⊝⊝ | There was also no significant difference between treatments for the reduction in episodes of priapism by score tier: RR 1.17 (95% CI 0.36 to 3.76). |
| Immediate side effects of treatment Follow‐up: 16 weeks | See comment | See comment | N/A | 13 (1) | ⊕⊕⊝⊝ | Side effects of treatment were reported for the whole treatment phase including the open label phase. No significant differences were found between sildenafil and placebo. |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to unclear methods of randomisation and allocation. | ||||||
| Etilefrine 50 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatient Intervention: etilefrine 50mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Etilefrine 50 mg | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months | See comment | See comment | N/A | 23 (1) | ⊕⊝⊝⊝ | No significant difference was found between etilefrine and placebo but there were concerns over the reliability of the published results. |
| Immediate side effects of treatment (palpitations) Follow‐up: 6 months | 333 per 1000 | 546 per 1000 (206 to 1000) | RR 1.64 (95% CI 0.62 to 4.30) | 23 (1) | ⊕⊝⊝⊝ | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Immediate side effects of treatment (tachycardia) Follow‐up: 6 months | 250 per 1000 | 545 per 1000 (178 to 1000) | RR 2.18 (95% CI 0.71 to 6.68) | 23 (1) | ⊕⊝⊝⊝ | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. | ||||||
| Ephedrine 15 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: ephedrine 15 mg/30 mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ephedrine | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months | See comment | See comment | N/A | 24 (1) | ⊕⊝⊝⊝ | No significant difference was found between ephedrine 15mg and placebo or ephedrine 30mg and placebo but there were concerns over the reliability of the published results. |
| Immediate side effects of treatment with 30 mg ephedrine (palpitations) Follow‐up: 6 months | 333 per 1000 | 333 per 1000 (107 to 1000) | RR 1.00 (95% CI 0.32 to 3.10) | 24 (1) | ⊕⊝⊝⊝ | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Immediate side effects of treatment with 30 mg ephedrine (tachycardia) Follow‐up: 6 months | 250 per 1000 | 168 per 1000 (33 to 825) | RR 0.67 (95% CI 0.13 to 3.30) | 24 (1) | ⊕⊝⊝⊝ | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. | ||||||
Background
Description of the condition
Sickle cell disease (SCD) is a genetic haemoglobin disorder, caused by inheritance from both parents of an altered beta‐globin chain gene of which one at least is beta S. SCD affects people originating from Sub‐Saharan Africa, the African diaspora, Arab countries, the Mediterranean and South America (Davies 1997). Approximately 75,000 people in the USA (mostly African‐Americans) (NHLBI 2002), and about one in 60 West Africans (WHO 1994) are affected by SCD. There are on average 160 children born with SCD in England each year (Hickman 1999).
The pathophysiology of SCD is caused by the abnormal haemoglobin within red blood cells causing deformation or sickling of the cells when they give up oxygen. These abnormal cells block blood vessels causing vaso‐occlusion, resulting in symptoms and organ damage.
Priapism is the persistence of erection that does not result from sexual desire and fails to subside despite orgasm. There are two kinds of priapism: low‐flow ischaemic or veno‐occlusive priapism, which is the form seen in SCD and high‐flow priapism or non‐ischaemic, which is associated with external trauma causing damage to the cavernosal artery. Priapism is usually accompanied by pain and tenderness. It can occur in all age groups, with a reported peak incidence in SCD between the ages of 5 to 10 years and 20 to 50 years (Lue 1998). However, work from Jamaica suggests that the onset of priapism can be anytime between the ages of 5 and 45 years, with the peak incidence among young adults (aged 20 years to 25 years) (Emond 1980). Typically, priapism only affects the corpora cavernosa (very rarely the corpus spongiosum may be affected) (Lue 1998), thus surgical treatments to relieve priapism are designed to remove blood from the corpora cavernosa. Low‐flow priapism may result in impotence or erectile dysfunction secondary to corporeal fibrosis.
There is no clear demarcation between a prolonged erection and priapism. However, many would call an erection lasting over six hours fulminant or prolonged priapism. This is because in the low‐flow type (as occurs in the priapism of SCD) penile ischaemia and acidosis occur after six hours (Broderick 1994). Others, however, call an erection in males with SCD a priapic episode when it is in excess of one hour, or when it is painful.
Priapism is a common occurrence in SCD and may occur as acute episodes or the so‐called stuttering priapism, defined as recurrent episodes of prolonged erections. These can last from a few minutes to three hours. Stuttering priapism by repetitive vaso‐occlusion can lead to erectile dysfunction, and may develop into full‐blown priapism. Treatment is aimed at preventing such an occurrence. In one study of men with homozygous SCD (SS) in a Jamaican clinic, stuttering nocturnal attacks of priapism lasting two to six hours affected 42 per cent of participants (Serjeant 1985). A more recent questionnaire study from the USA reported an actuarial probability of experiencing priapism by the age of 20 years as 89% (+/‐ 9%) (Mantadakis 1999).
Red cell sickling and later sludging of blood occur in the corpora cavernosa, perhaps as a result of abnormal endothelial adherence, the relatively acidic state of the corpora during erection, mild acidosis accompanying hypoventilation during sleep, or mild trauma with masturbation and intercourse. When the venous channels are maximally compressed during nocturnal penile tumescence, the sludged red cells can block the microscopic subtunical venules and trigger diffuse veno‐occlusion (Lue 1998). More recent evidence has shown that priapism results from deficient erection control mechanisms at a molecular level (Bivalacqua 2006).
Description of the intervention
Interventions for priapism fall into two categories of preventing or reducing the frequency of priapic attacks, and treating fulminant priapic episodes. Several treatments have been described including: analgesia; hydration; blood alkalinisation; red cell transfusion; partial exchange transfusion to lower haemoglobin S; pharmaceutical agents and surgical procedures such as washouts. Surgical shunt procedures and even penile implantation have been described for intractable cases (Upadhyay 1998). Current treatments focus on resolving acute attacks and are often administered too late during an episode. With improved understanding of the true pathophysiology of priapism in SCD, newer treatments aim to target disease specific molecular abnormalities. Recent research suggests that there is abnormal signalling of the endothelium‐derived nitric oxide and phosphodiesterase type 5 (PDE5) signal transduction pathway in the penis (Bivalacqua 2012).
How the intervention might work
With a better understanding of the underlying pathophysiology of priapism in men with SCD, interventions can be better targeted at the underlying cause. Interventions to treat an acute attack aim to abort the attack and restore normal vascular homeostasis. It is thought that the nitric oxide imbalance leads to PDE5 dysregulation which then has a physiological impact on cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphatecAMP activity. Treatments that interrupt abnormal mechanisms may be effective in reducing the frequency of attacks.
Why it is important to do this review
Priapism is associated with with long‐term erectile dysfunction and psychological consequences in often young men with SCD. It is important to review the evidence for interventions to treat acute episodes of priapism but also to prevent or reduce episodes in the longer term. Treatment of acute and stuttering priapism often depends on the type of clinician or institution to whom the male with SCD presents. With an increasing understanding of the underlying pathophysiology of the condition, new treatments such as etilefrine, an α adrenergic stimulator, and sildenafil, are showing encouraging results for preventing priapism (Bachir 1996; Okpala 2002; Virag 1996). This review seeks to determine safe, effective interventions for preventing or treating (or both) priapism.
Objectives
To assess the benefits and risks of the different treatments for both stuttering and fulminant priapism in SCD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised trials, whether published or unpublished.
Types of participants
Males with priapism, either fulminant or stuttering with any type of SCD including: homozygous SCD, i.e. sickle cell anaemia (SS); haemoglobin SC disease (SC); sickle beta thalassaemia (Sβº and Sβ⁺), irrespective of age, race, ethnic origin and setting.
Types of interventions
Any treatment for priapism in SCD, whether fulminant or stuttering, non‐surgical or surgical, compared with placebo or no treatment, or against another intervention.
Types of outcome measures
Primary outcomes
-
Detumescence or resolution of priapism and return of penis to non‐erect state (immediate or after one, two or four hours)
-
Reduction in frequency of stuttering priapism
Secondary outcomes
-
Return of priapism
-
Immediate side effects, e.g. pain, bleeding
-
Later sexual function
-
Other untoward systemic side effects, e.g. gynaecomastia, liver problems
-
Efficacy of a prevention strategy for stuttering priapism (and thus preventing fulminant priapism)
Search methods for identification of studies
There were no restrictions regarding language or publication status.
Electronic searches
Relevant trials were identified from the Group's Haemoglobinopathies Trials Register using the terms: (sickle cell OR (haemoglobinopathies AND general)) AND (priap* OR erection).
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Group's Haemoglobinopathies Trials Register: 15 September 2017.
We also searched Embase separately (1974 to 2003) (Appendix 1) and the Internet (December 2003) (Appendix 2) for the last update of this review. For this update Embase is included in the CENTRAL search but there may be a delay before references appear in CENTRAL. We therefore searched Embase for the six months prior to the main search being carried out (16 August 2016) to current (12 December 2016) (Appendix 3).
Date of most current Embase search: 12 December 2016.
We searched the clinical trial registries ClinicalTrials.gov (http://www.clinicaltrials.gov) and the World Health Organisation International Clinical Trials Registry Platform (http://www.who.int/ictrp/search/en/) for any ongoing and or unpublished trials using the terms " sickle cell" and "priapi*" (Appendix 4).
Date of most recent search 12 December 2016.
Searching other resources
Reference lists
We searched reference list of all included trials to identify any further relevant trials.
Date of the latest search: 12 December 2016.
Correspondence
Attempts were also made to identify any unpublished trials through contact with experts in the field and personal communication with known authors.
Data collection and analysis
Selection of studies
Each author independently identified potentially relevant trials from the search results. No disagreement occurred, so no other expert in the field was invited to make an independent assessment based on the selection criteria.
Data extraction and management
Two authors independently extracted data using standard data acquisition forms. Where there was disagreement or uncertainty, a third author arbitrated.
Outcome data were to be grouped into those measured at 1, 3, 6 and 12 months and annually thereafter. This was to gauge the long‐term benefit or risk from an intervention. Trials reporting on time periods not listed above, were considered as well and described within the review.
Assessment of risk of bias in included studies
Two review authors independently assessed trials following the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The assessments were compared and any inconsistencies between the review authors were discussed and resolved.
Assessment was made of the following domains, each being assessed as having either a low, unclear or high risk of bias:
-
generation of the allocation sequence
-
concealment of allocation
-
blinding (of participants, personnel and outcome assessors)
-
incomplete outcome data
-
record of participants lost to follow‐up or subsequently excluded from the trial
-
whether an intention‐to‐treat analyses has been performed
-
-
selective reporting
-
other bias
Measures of treatment effect
For binary outcome measures we calculated a pooled estimate of the treatment effect for each outcome across trials using the risk ratio (RR) with 95% confidence intervals (CIs), where appropriate.
For continuous outcomes, we planned to record either mean change from baseline for each group or mean post‐treatment or post‐intervention values and standard deviation for each group. We also intended to compute a pooled estimate of treatment effect by calculating the mean difference (MD) with 95% CIs; however; we were unable to do this because of insufficient data.
Unit of analysis issues
Elbourne discusses methods for meta‐analysing cross‐over trials (Elbourne 2002). The methods discussed rely on the data that is reported within the primary paper. In this review, we have adopted the method that uses the data from the first period only for the included cross‐over trial, as this is currently the only data available from the trial (Serjeant 1985). However, for future updates, we plan to use the most appropriate method that is available in RevMan to analyse cross‐over data (Review Manager 2014).
Dealing with missing data
We contacted authors, where further clarification was needed.
In order to allow an intention‐to‐treat analysis, we sought data on the number of participants with each outcome event, by allocated treated group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up.
Assessment of heterogeneity
We could not test for heterogeneity between trial results using a standard Chi² test as intended as the included trials reported different outcomes and could not be combined. However, for future updates, when more trials are included we plan to test for heterogeneity using the Chi² test.
Assessment of reporting biases
We planned to generate a funnel plot to attempt to identify any publication bias in the included trials (Higgins 2011), however, there were less than 10 included trials and we were therefore unable to conduct this analysis. We attempted to identify any selective reporting in the included publications, by comparing the trial protocols or trial registry, where available, with the final papers and by examination of the trial publications. We considered reporting of both positive and negative effects of the intervention. Where trial protocols weren't available, we compared the outcomes reported in the results section against the methods section of the paper. We extracted information on the sponsors, sources of funding and competing interests of the authors to rule out external bias.
Data synthesis
We generated forest plots using the Review Manager software (Review Manager 2014) from the trial data but were unable to combine the results into a meta‐analysis due to the included trials reporting different interventions and outcomes. We carried out separate analyses for each of the interventions.
We have used the fixed‐effect approach. If further trials are included, in the absence of homogeneity of treatment effects, we will use a random‐effect approach, otherwise, we will use the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We were unable to carry out subgroup analyses as there were insufficient trials with comparable outcomes. The management of stuttering priapism in people with SCD is about prevention, and therefore differs from the management of fulminant priapism. Thus, future analyses will not only be by group but also by acute management versus prevention.
Sensitivity analysis
We planned to carry out sensitivity analyses to look at the effect of the risk of bias findings and to look at the effect of adding in and taking out trials where there was high risk of bias. This was not possible due to there not being any trials included in a meta‐analysis.
Summary of findings tables and quality of the evidence (GRADE)
In a post hoc change in line with current Cochrane guidance, at the 2017 update we added a summary of findings table for each comparison presented in the review (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4). We selected the following outcomes to report (chosen based on relevance to clinicians and consumers).
-
Detumescence
-
Reduction in stuttering priapism
-
Immediate side effects of treatment (up to two specific effects)
-
Effect on later sexual function
-
Other untoward side effects of treatment
-
Efficacy of a prevention strategy
We determined the quality of the evidence using the GRADE approach; and downgraded evidence in the presence of a high risk of bias in at least one trial, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We downgraded evidence by one level if they considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
The previous version of the review included one trial (two references) (Serjeant 1985). The 2017 searches identified eight new references, one of which was not eligible for any section of the review. The remaining seven new references were to two new eligible trials (Burnett 2014; Olujohungbe 2011). One of these references was to an ongoing trial that appeared in the previous version of this review but the full paper has now been published and we can include it (Olujohungbe 2011). The results of the search are shown in a PRISMA flow diagram (Figure 1).

Study flow diagram.
Included studies
Nine references to three trials (with a total of 102 participants) on the treatment of stuttering priapism, were identified as eligible for inclusion in the review (Burnett 2014; Olujohungbe 2011; Serjeant 1985) (Characteristics of included studies). The two more recent trials are both double‐blind, placebo‐controlled parallel trials with treatment periods ranging from eight weeks (Burnett 2014) to 26 weeks (Olujohungbe 2011). The third trial is a double‐blind, placebo controlled cross‐over trial, in which participants who did not respond to the first arm of the trial were entered into the second arm of the trial with no washout period described (Serjeant 1985). There were no randomised trials of treatments for fulminant priapism. The literature reveals several case reports and case series of well‐established therapies and experimental therapies for stuttering and fulminant priapism.
Participants and setting
The 102 participants across the three trials were all outpatients with confirmed SCD and stuttering priapism from clinics in the USA, Nigeria, the UK and Jamaica. Olujohungbe recruited from clinics in the middle east for a short period due to poor recruitment in the UK (Olujohungbe 2011). The inclusion criteria for the Burnett and Serjeant trials stated that participants had to have at least two stuttering priapism episodes per week (Burnett 2014; Serjeant 1985). The Olujohungbe trial did not specify the number of episodes of stuttering priapism per week, but did exclude participants who had an attack which lasted longer than four hours or required hospitalisation (Olujohungbe 2011).
The mean (SD) age of participants ranged from 21.7 (5.3) years (Burnett 2014 Sildenafil group) to 24.3 (3.1) years (Serjeant 1985). Participant numbers were low in two of the trials with Burnett including 13 participants and Serjeant including only 11 (Burnett 2014; Serjeant 1985). The Olujohungbe trial required 320 participants to give 80% power to detect differences of 1.5 attacks per month between groups and did include larger numbers of participants but they only enrolled 131 of the required 320 and only 78 were eligible to be randomised to the intervention phase. Only 46 of these participants were included in the analysis and only 25 completed the treatment period (Olujohungbe 2011).
Type of intervention
All three trials report on different treatments from each other but they all compare to a placebo. The Serjeant trial looks at the preventive effects of 5 mg of stilboestrol on stuttering priapism (Serjeant 1985). Burnett looks at the effect of sildenafil 50 mg, a phosphodiesterase type 5 inhibitor, given once daily over eight weeks on preventing attacks of priapism (Burnett 2014). Olujohungbe looks at the effect of two oral alpha‐adrenergic agonists against placebo, etilefrine 50 mg, and two different doses of ephedrine (15 mg and 30 mg) (Olujohungbe 2011). In this trial the participants completed diaries in an observation phase for three to thirteen weeks before taking part in the randomised intervention phase. During the intervention phase participants were asked to take either 15 mg ephedrine, 30 mg ephedrine, 50 mg etilefrine or a placebo once daily, at bedtime for six months.
Type of outcomes
None of the included trials looked at our main primary outcome of detumescence or resolution of a priapism attack but all three trials measured the effect of treatment on the frequency of attacks. Burnett used a scoring system to record the frequency of attacks where 0 = no episodes, 1 = one to two episodes, 2 = three to four episodes, 3 = five to eight episodes and 4 = nine to sixteen episodes and also reported on the duration of episodes (Burnett 2014).The trial of stilboestrol also reported on the return of priapism after a period of no attacks (Serjeant 1985). The two more recent trials reported on immediate side effects of treatment but the specific side effects recorded were different in the two trials due to the actions of the medications being different. Burnett reported on headache, flushing, nasal congestion, abnormal vision, dyspepsia, myalgia whilst Olujohungbe reported on palpitations, tachycardia, lack of sleep, hand shaking, anxiety and dry mouth (Burnett 2014; Olujohungbe 2011). The ephedrine and etilefrine trial was the only trial to look at longer term effects by recording the effect on systolic and diastolic blood pressure but this was as a safety mechanism. Where participants were found to have an increase in systolic blood pressure of 20 mmHg and an increase in diastolic pressure of 10 mmHg the trial medication was discontinued (Olujohungbe 2011).
Excluded studies
There were no excluded studies.
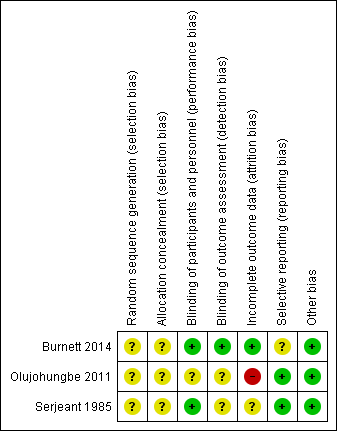
Risk of bias in included studies
The risk of bias was assessed by both authors for all trials included in the review and the details are given in the table Characteristics of included studies. This has been summarised in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of randomisation was unclear and the concealment of allocation was not described in any of the three trials, therefore, we assessed each of these domains as having an unclear risk of bias for all three trials.
Blinding
All three trials were described as double‐blind (physician and participant) and we therefore assessed this domain as low risk. In the Olujohungbe trial, blinding of the trial drugs was done by an external clinical trial supplies company. In the Burnett trial, adverse events were reported for the whole of the treatment period (confirmed by the author team) including the open label phase and we therefore have not entered the data into our analysis as this would bias the results (Burnett 2014).
Incomplete outcome data
The Burnett trial included 13 participants and analysed the data both on an intention‐to‐treat basis and on a per protocol basis. All 13 participants' data were included in the analysis and the trial was deemed to be at low risk of bias for this domain (Burnett 2014).
Although the trial of ephedrine and etilefrine had higher numbers of participants, there was a high rate of attrition at each phase of the trial. The authors stated that 320 participants were needed to give 80% power to detect differences of 1.5 attacks per month between groups. The number enrolled was much less than this (131) and only a proportion completed the observation phase thus enabling them to be randomised to the treatment phase. Of the 78 participants that were randomised to the intervention phase, data was only available for 46 of them. The remainder were lost to follow up. Just under half of the participants analysed completed the full 26 weeks of treatment. We deemed this trial to be at a high risk of bias due to attrition (Olujohungbe 2011).
In the cross‐over trial one participant defaulted, and in two participants the attacks of stuttering priapism ceased spontaneously during the baseline observation period. There was a fourth participant who initially did not take the placebo because a painful crisis aborted the stuttering priapism prior to taking the assigned tablet. In this participant (identified as patient nine) attacks of priapism recurred two weeks later and data on a second baseline period were collected. The paper did not discuss an intention‐to‐treat analysis and we therefore assessed this paper as being at an unclear risk of bias for this domain (Serjeant 1985).
Selective reporting
We deemed two of the trials to be at low risk of bias for selective reporting (Olujohungbe 2011; Serjeant 1985) although we were unable to find a published protocol for any of the trials. The Burnett trial stated that there were no significant differences between groups for decreased median weekly change in priapism episode score but no data were shown (Burnett 2014). The same paper reported adverse effects in the published paper but did not include this as an outcome in either the methods section or in the trial registration document. We deemed this trial to be at an unclear risk of bias for this domain.
Other potential sources of bias
We did not find any other potential sources of bias.
Effects of interventions
See: Summary of findings for the main comparison Silboesterol versus placebo; Summary of findings 2 Sildenafil versus placebo; Summary of findings 3 Etilefrine versus placebo; Summary of findings 4 Ephedrine versus placebo
Silboesterol versus placebo
One trial with 11 participants reported on the effect of 5 mg stilboestrol versus placebo (Serjeant 1985).
Primary outcomes
1. Detumescence (immediate or after one, two or four hours)
This outcome was not assessed in this trial.
2. Reduction in frequency of stuttering priapism
The trial of stilboestrol reported that in four out of the five participants randomised initially to placebo, the attacks of stuttering priapism continued unchanged. In the remaining one participant (participant seven) the attacks ceased immediately on placebo, but he developed a painful crisis six days later. Attacks of priapism recurred one month later and data for a second baseline period were collected before being started on stilboestrol. In all these five participants, switching over to stilboestrol led to the cessation of attacks, immediately in four and gradually in one.
Analysis of the first treatment period (in which no tablet was taken by four participants) showed no significant treatment effect, RR 2.92 (95% CI 0.73 to 11.70) (P = 0.13) (very low quality evidence) (Analysis 1.1). However, the fact that four participants did not take a tablet strongly biases the result (Serjeant 1985).
Secondary outcomes
1. Return of priapism
Return of priapism was addressed in the Serjeant trial but not in a randomised manner (Serjeant 1985).
2. Immediate side effects, e.g. pain, bleeding
This outcome was not assessed (Serjeant 1985).
3. Later sexual function
This outcome was not assessed (Serjeant 1985).
4. Other untoward systemic side effects, e.g. gynaecomastia, liver problems
This outcome was not assessed (Serjeant 1985).
5. Efficacy of a prevention strategy for stuttering priapism (and thus preventing fulminant priapism)
This outcome was not assessed (Serjeant 1985).
Sildenafil versus placebo
One trial with 13 participants reported the effects of sildenafil 50 mg versus placebo (Burnett 2014).
Primary outcomes
1. Detumescence (immediate or after one, two or four hours)
This outcome was not assessed in this trial (Burnett 2014).
2. Reduction in frequency of stuttering priapism
The Burnett trial reported that there was no significant difference between sildenafil and placebo regarding decreases in episodes of priapism by score tier, RR 1.17 (95% CI 0.36 to 3.76) (low quality evidence) (Analysis 2.1), or decrease in frequency, RR 0.58 (95% CI 0.07 to 4.95) (low quality evidence) (Analysis 2.2) (Burnett 2014). One out of six participants taking sildenafil showed a reduction in frequency of episodes whilst two out of seven participants taking placebo showed a decrease in frequency (Burnett 2014).
Secondary outcomes
1. Return of priapism
This outcome was not assessed in this trial (Burnett 2014).
2. Immediate side effects, e.g. pain, bleeding
The Burnett trial reports adverse effects for the total treatment period including the open label phase (Burnett 2014). Burnett found no significant differences between sildenafil and placebo regarding headache (P = 1.0), flushing (P = 0.19), nasal congestion (P = 1), abnormal vision (P = 0.46), dyspepsia (P = 0.46), myalgia (P = 1.0) or hypotension (P = 1.0). Seven out of the 13 participants were hospitalised 14 times over the course of the trial (53.8%). Of these hospitalisations, 10 visits made by six participants were due to major priapism episodes. Two of these episodes occurred in participants who were taking sildenafil (20%) and the remaining eight occurred in participants who were taking placebo treatment (80%) (Burnett 2014).
3. Later sexual function
This outcome was not assessed in this trial (Burnett 2014).
4. Other untoward systemic side effects, e.g. gynaecomastia, liver problems
This outcome was not assessed in this trial (Burnett 2014).
5. Efficacy of a prevention strategy for stuttering priapism (and thus preventing fulminant priapism)
This outcome was not assessed in this trial (Burnett 2014).
Etilefrine or ephedrine versus placebo
One trial with 78 participants assessed the effect of etilefrine 50 mg versus placebo, ephedrine 15 mg versus placebo or ephedrine 30 mg within a four‐armed trial (Olujohungbe 2011).
Primary outcomes
1. Detumescence (immediate or after one, two or four hours)
This outcome was not assessed in this trial (Olujohungbe 2011).
2. Reduction in frequency of stuttering priapism
The results of the trial showed no significant differences between the four treatment groups in the weekly total number of attacks during the trial (Phase B) compared to a baseline pre‐randomisation period (Phase A). We do not quote any numerical results in this review due to concerns over the reliability of published results; the trial was substantially underpowered with only 46 out of a target 320 participants included in final analysis (14%) and the authors comment on poor compliance in the completion of symptom diaries during both Phase A and Phase B of the trial (Olujohungbe 2011).
Secondary outcomes
1. Return of priapism
This outcome was not assessed in this trial (Olujohungbe 2011).
2. Immediate side effects, e.g. pain, bleeding
Olujohungbe reported side effects of treatment in the blinded phase of the trial for etilefrine 50 mg, ephedrine 15 mg or ephedrine 30 mg (Olujohungbe 2011). Many of the participants experienced tachycardia but there was no significant difference between any of the treatments and placebo for any of the immediate side effects measured (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6) (very low quality evidence). The three adverse events reported were three hospital admissions due to painful vaso‐occlusive crises in the same individual and were not thought to be related to the trial treatment.
3. Later sexual function
This outcome was not assessed in this trial (Olujohungbe 2011).
4. Other untoward systemic side effects, e.g. gynaecomastia, liver problems
This outcome was not assessed in this trial (Olujohungbe 2011).
5. Efficacy of a prevention strategy for stuttering priapism (and thus preventing fulminant priapism)
This outcome was not assessed in this trial (Olujohungbe 2011).
Discussion
Summary of main results
Since the last update we have found two further trials of treatments for priapism in boys and men with sickle cell disease bringing the number of included trials to three (Burnett 2014; Olujohungbe 2011; Serjeant 1985). The three trials included small numbers of participants, had different trial designs and assessed four different drug treatments. None of the trials primarily looked at fulminant priapism and so no trials reported on detumescence, one of our primary outcomes. No significant effect of any of the treatments was seen in relation to our second primary outcome of reduction in frequency of stuttering priapism. The only secondary outcome reported was immediate side effects which were reported in two of the trials but in one of the trials, the reporting of side effects was done for the whole treatment period including the open label phase which may have biased the results. A summary of the results is presented in the summary of findings tables (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4)
Overall completeness and applicability of evidence
We are confident that the searches were thorough and have identified all the available trials. The included trials were generally complete although we needed to clarify a couple of issues with the author team. However, the small number of participants and the high attrition rate limit the applicability of the results to the wider population of males with priapism related to sickle cell disease.
Quality of the evidence
The evidence provided by this review is low to very low quality, limited by the quantity and quality of the trials. All of the trials were randomised but the method of randomisation and allocation concealment were unclear. The risk of bias for blinding was deemed to be low as all three of the trials were double blind. The Burnett trial, however, reported on an open label phase for a period of time after the double‐blind phase of the trial. Immediate side effects of treatment were reported for the whole period, so there was the possibility that participants under or over reported any side effects due to the knowledge of the treatment they were on (Burnett 2014).
Poor recruitment and attrition bias were problems in the Olujohungbe trial where large numbers were required to show a significant effect. In stuttering priapism young males may be reluctant to report this complication of sickle cell disease and may also be reluctant to participate in research. Similarly, the nature of the disease may have been the reason for the number of men lost to follow‐up. Without information on why the participants failed to complete the trial it is not possible to comment on the evidence provided by those that did complete the trial (Olujohungbe 2011).
Potential biases in the review process
One of the co‐authors of this review, KA is also an author on one of the included trials (Olujohungbe 2011).
Agreements and disagreements with other studies or reviews
There are no similar reviews of treatment in this area with which we can compare our findings.
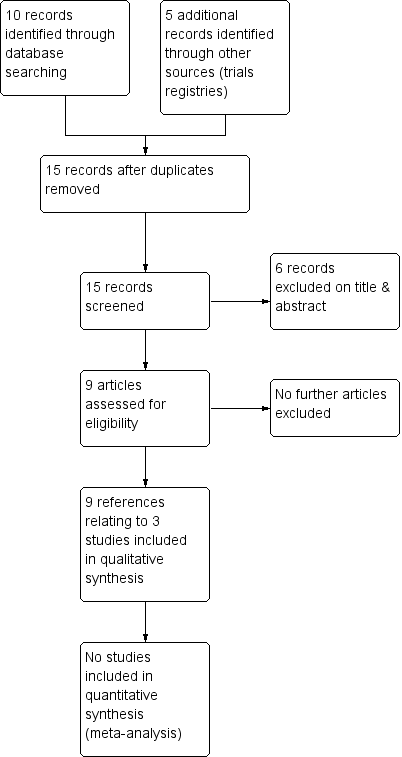
Study flow diagram.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
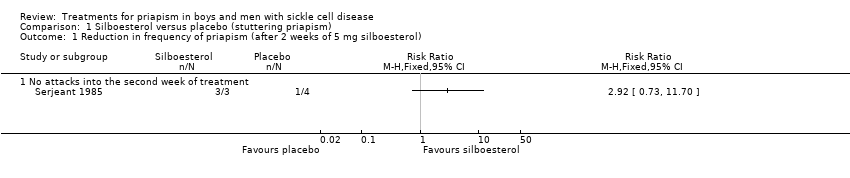
Comparison 1 Silboesterol versus placebo (stuttering priapism), Outcome 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol).
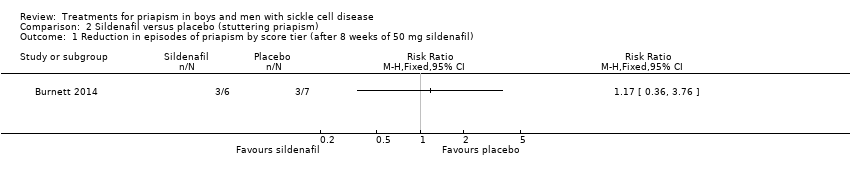
Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil).
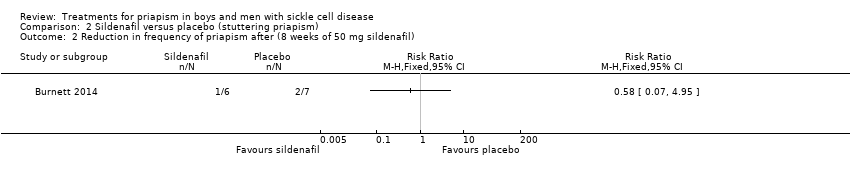
Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil).
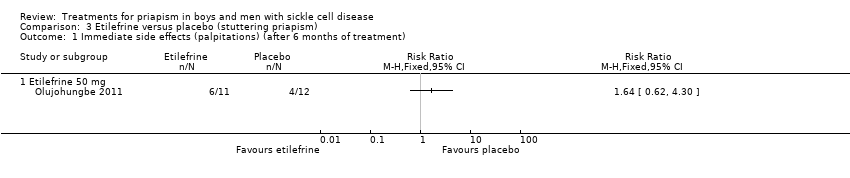
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking).
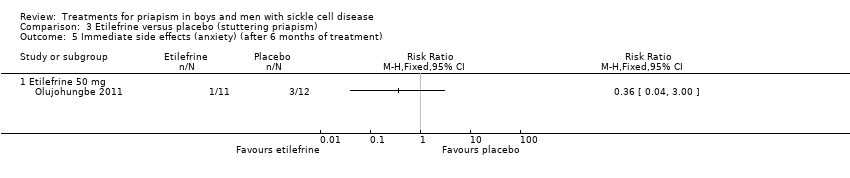
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment).

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep).
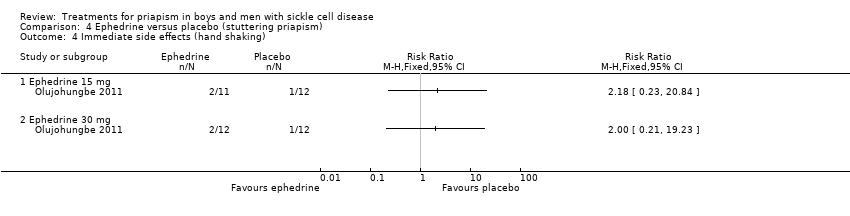
Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment).

Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment).
| Silboesterol compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: stilboestrol 5 mg Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Stilboestrol 5 mg | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in stuttering priapism Follow‐up: 2 weeks | 250 per 1000 | 730 per 1000 | RR 2.92 (95% CI 0.73 to 11.70) | 7 | ⊕⊝⊝⊝ | |
| Immediate side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to unclear methods of randomisation and allocation. | ||||||
| Sildenafil 50 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: sildenafil 50 mg daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sildenafil 50 mg daily | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 8 weeks | 286 per 1000 | 166 per 1000 (20 to 1000) | RR 0.58 (95% CI 0.07 to 4.95) | 13 (1) | ⊕⊕⊝⊝ | There was also no significant difference between treatments for the reduction in episodes of priapism by score tier: RR 1.17 (95% CI 0.36 to 3.76). |
| Immediate side effects of treatment Follow‐up: 16 weeks | See comment | See comment | N/A | 13 (1) | ⊕⊕⊝⊝ | Side effects of treatment were reported for the whole treatment phase including the open label phase. No significant differences were found between sildenafil and placebo. |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to unclear methods of randomisation and allocation. | ||||||
| Etilefrine 50 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatient Intervention: etilefrine 50mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Etilefrine 50 mg | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months | See comment | See comment | N/A | 23 (1) | ⊕⊝⊝⊝ | No significant difference was found between etilefrine and placebo but there were concerns over the reliability of the published results. |
| Immediate side effects of treatment (palpitations) Follow‐up: 6 months | 333 per 1000 | 546 per 1000 (206 to 1000) | RR 1.64 (95% CI 0.62 to 4.30) | 23 (1) | ⊕⊝⊝⊝ | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Immediate side effects of treatment (tachycardia) Follow‐up: 6 months | 250 per 1000 | 545 per 1000 (178 to 1000) | RR 2.18 (95% CI 0.71 to 6.68) | 23 (1) | ⊕⊝⊝⊝ | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. | ||||||
| Ephedrine 15 mg compared with placebo for stuttering priapism | ||||||
| Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: ephedrine 15 mg/30 mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ephedrine | |||||
| Detumescence Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months | See comment | See comment | N/A | 24 (1) | ⊕⊝⊝⊝ | No significant difference was found between ephedrine 15mg and placebo or ephedrine 30mg and placebo but there were concerns over the reliability of the published results. |
| Immediate side effects of treatment with 30 mg ephedrine (palpitations) Follow‐up: 6 months | 333 per 1000 | 333 per 1000 (107 to 1000) | RR 1.00 (95% CI 0.32 to 3.10) | 24 (1) | ⊕⊝⊝⊝ | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Immediate side effects of treatment with 30 mg ephedrine (tachycardia) Follow‐up: 6 months | 250 per 1000 | 168 per 1000 (33 to 825) | RR 0.67 (95% CI 0.13 to 3.30) | 24 (1) | ⊕⊝⊝⊝ | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A | See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 No attacks into the second week of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate side effects (palpitations) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate side effects (palpitations) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

