Les anticoagulants (sur une durée prolongée) pour la prévention de la thromboembolie veineuse après une prothèse totale de hanche ou du genou ou de la réparation d'une fracture de la hanche
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: Randomised, double‐blind, double‐dummy, clinical trial Country: Multi‐Country (21) Setting: Multicentre (160 sites); home and hospital; March 2007 to May 2009 Intention‐to‐treat: Yes, primary analysis on all except those that had no or venograms or uninterpretable venography, also per‐protocol efficacy analysis excluding protocol violations ‐ protocol violations not described ‐ safety data ITT minus those that did not receive at least one dose For the purposes of our meta‐analyses we used a mixture of all randomised and ITT population, as reported appropriate by the study authors | |
| Participants | Number randomised: Total n = 5407 (apixaban n = 2708; enoxaparin n = 2699) Exclusions post randomisation: Total n = 1616 (Apixaban: 363 had no venography, 396 had uninterpretable venograms (efficacy), 35 did not have at least one dose (safety). Enoxaparin: 40 did not receive at least one dose (safety); 364 no venography, 418 uninterpretable venogram (efficacy)) Losses to follow up: No reports of loss to follow up Age mean (range): Apixaban 60.9 (19 ‐ 92); enoxaparin 60.6 (19 ‐ 93) Sex (%F): Apixaban 52.8%; enoxaparin 53.8% Inclusion criteria: Patients scheduled to undergo elective total hip replacement or revision of previously inserted hip replacement Exclusion criteria: Main reasons for exclusion: active bleeding; contraindication to anticoagulant prophylaxis; need for ongoing anticoagulant or antiplatelet treatment | |
| Interventions | Treatment: 2.5 mg apixaban, orally, twice daily, initiated 12 ‐ 24 hours after surgery Control: 40 mg enoxaparin, subcutaneously, once daily, initiated 12 hours before surgery Duration: Prophylaxis continued for 35 days (32 to 38 day range) after surgery; follow‐up evaluations also occurred at 65 and 95 days after surgery | |
| Outcomes | Primary: Composite of asymptomatic or symptomatic DVT, nonfatal PE, or death from any cause; bleeding during treatment period (classed as major, clinically relevant non‐major and minor bleeding, and composite of major and clinically relevant) Secondary: Major VTE: composite of symptomatic or asymptomatic proximal DVT, nonfatal PE or death related to VTE Bleeding definitions: Major bleeding ‐ acute, clinically overt bleeding accompanied by one or more of the following: a decrease in haemoglobin level of 2 g/dL or more over 24 hours; transfusion of 2 or more units of packed red cells; bleeding into the operated joint necessitating reoperation or intervention; intramuscular bleeding; fatal bleeding; Minor bleeding ‐ clinically overt but did not meet the criteria for major or clinically relevant non‐major bleeding; Clinically relevant non‐major bleeding ‐ acute, clinically overt episodes such as wound haematoma, bruising or ecchymosis, gastrointestinal bleeding, haemoptysis, haematuria or epistaxis that did not meet the criteria for major bleeding | |
| Notes | Funding: Bristol‐Myers Squibb and Pfizer Method of VTE evaluation/confirmation: bilateral venography was performed after 35 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using interactive telephone system, randomisation schedule generated at the randomisation centre of Bristol‐Myers Squib with the use of SAS software and was stratified according to study site, with a block size of four |
| Allocation concealment (selection bias) | Low risk | Interactive telephone system |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind, double‐dummy, participants received either placebo tablets or injections based on treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | All VTE, bleeding and adverse events were reviewed by an independent, blinded, adjudication committee |
| Incomplete outcome data (attrition bias) | Low risk | All losses‐to‐follow‐up were recorded per treatment group with adequate reasoning |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Reasons for protocol violations not described; Funded by Bristol‐Myers Squibb and Pfizer; data were collected and monitored by the sponsors, and data and safety monitoring board were given a fee by the sponsors |
| Methods | Study design: Randomised, open‐label, prospective, non‐inferiority, parallel group trial Country: France Setting: Multicentre (17 centres); hospital and home; June 2004 to June 2007 Intention‐to‐treat: No For the purposes of our meta‐analyses we used the per‐protocol population, as reported by the study authors | |
| Participants | Number randomised: Total n = 857 (extended n = 430; short n = 427)* Exclusions post randomisation: Extended: exclusion criteria: 7, not treated: 13; short: exclusion criteria 2, consent withdrawal 4 not treated: 33 Losses to follow up: Extended n = 1; short n = 1 Age mean years (SD): Extended 70.9 (8.1); short 70.1 (8.6) Sex % M: Extended 37.9%; short 35.4% Inclusion criteria: 45 years or older; scheduled for a first total unilateral knee arthroplasty Exclusion criteria: History of confirmed symptomatic venous thromboembolism at any time, stroke or myocardial infarction within the previous month, current active bleed, gastrointestinal bleeding or hemorrhagic stroke within the previous six months, brain, spinal, opthalmological or other major surgery within the previous month, active cancer, renal impairment, hepatic impairment, a contraindication to anticoagulant therapy, hypersensitivity to heparin and patients who required therapeutic anticoagulation | |
| Interventions | Treatment: Anticoagulant treatment was chosen by the investigator and could be: heparin (5000 U, two to three times per day), enoxaparin (4000 IU), dalteparin (5000 IU), tinzaparin (4500 IU), body‐weight adjusted nadroparin, fondaparinux (2.5 mg) Control: No specific control, only short duration prophylaxis Duration: 10 ± 2 days 'short thromboprophylaxis'; 35 ± 5 days 'extended thromboprophylaxis' | |
| Outcomes | Primary: Composite of proximal DVT, symptomatic DVT, non‐fatal symptomatic PE, major bleeding, heparin‐induced thrombocytopenia, all‐cause mortality Secondary: Ultrasonographic distal DVT Bleeding definitions: Major bleeding fatal bleeding, bleeding that was intracranial, intraocular, retroperitoneal, gastrointestinal or intra‐articular, bleeding leading to reoperation, or bleeding requiring cessation of treatment | |
| Notes | Funding: Caen University Hospital with unrestricted grant from the French Health Ministry Method of VTE evaluation/confirmation: All participants were examined for DVT by bilateral whole‐leg ultrasonography on Day 35 ± 5, or earlier if thrombosis was clinically suspected *For the analysis, we only included participants that had negative ultrasonographic scans at discharge, as most comparative studies excluded these participants with positive ultrasonographic scans at discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed randomisation was performed using a centralised telephone system, according to a permuted block design with block size of four, with stratification by centre and by the presence or absence of distal deep‐vein thrombosis on whole leg ultrasonography |
| Allocation concealment (selection bias) | Low risk | "Concealed randomization was performed using a centralized telephone system" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Sonographer was not blinded; Primary outcomes were reviewed by a central, independent, blinded adjudication committee |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | High risk | Safety outcomes reported as "the bleeding risk was very low (0.7%)", no further details provided |
| Other bias | Low risk | Sponsored by the Caen University Hospital with an unrestricted grant from the French Health Ministry ‐ which had no other role in the study |
| Methods | Study design: Randomised, prospective, double‐blind, placebo‐controlled trial Country: Norway Setting: Multicentre, hospital and home, January 1993 ‐ June 1994 Intention‐to‐treat: Yes, after withdrawals removed. For the purposes of our meta‐analyses we used the reported ITT population | |
| Participants | Number randomised: Total n = 265 (extended LMWH n = 134; placebo n = 131) Exclusions post randomisation: Total n = 38 (extended LMWH n = 17; placebo n = 21) withdrawn for reasons other than DVT or PE (21/38 due to adverse events: 10 extended LMWH and 11 placebo) Losses to follow up: No losses to follow‐up all participants received treatment until the day of final visit Age mean years: Extended LMWH 70.98, placebo 71.4 Sex %F: Extended LMWH 68.5%; placebo 73.6% Inclusion criteria: 18 years or older; admitted to hospital for elective primary or secondary hip replacement; obtained written consent form Exclusion criteria: Known renal or liver insufficiency; cerebral bleeding less than three months before surgery or known haemorrhagic diathesis; eye or ear surgery within one month before surgery; severe hypertension; septic endocarditis; threatened arterial circulation in the leg; body weight less than 40 kg; anticoagulant therapy less than one week before surgery; known hypersensitivity to heparin, low‐molecular‐weight heparin, dextran or contrast media; pregnancy or breastfeeding; inability to comply with study protocol; previous surgery within study | |
| Interventions | Treatment: Dalteparin 5000 IU, injections once daily for four weeks Control: Initial treatment with dalteparin 5000 IU followed by placebo (sodium chloride) injections, once daily Duration: 35 days (7 days initial treatment + 28 days continued treatment) | |
| Outcomes | Primary: Verified VTE on days 7 and 35 Secondary: Haematological assessment; safety: reoperation due to bleeding, wound haematoma and local haematoma at injection site | |
| Notes | Funding: Not reported Method of VTE evaluation/confirmation: DVT verified by bilateral venography and PE verified by perfusion ventilation or chest X‐ray | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information given to determine adequate randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given to determine adequate allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blinded and gave placebo subcutaneous injections |
| Blinding of outcome assessment (detection bias) | Low risk | Venograms, X‐rays and V‐Q scans evaluated after the study by a blinded specialist |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, ITT and PP analysis |
| Selective reporting (reporting bias) | High risk | VAS patient acceptability reported but not reported as pre‐planned, as was pain at injection site and serious adverse events, bleeding not reported as outcomes |
| Other bias | Low risk | No evidence of other bias |
| Methods | Study design: Randomised, double‐blind, placebo‐controlled, parallel group, prospective trial Country: Denmark Setting: Multicentre; hospital and home; January to November 1994 Intention‐to‐treat: Yes For the purposes of our meta‐analyses we used the reported ITT population | |
| Participants | Number randomised: Total n = 281 (extended LMWH n = 140; placebo n = 141) Exclusions post randomisation: Total n = 66 (extended LMWH n = 27; placebo n = 39); 39 due to inadequate or missing phlebographies, 14 withdrew consent, 8 adverse events, 2 placebo patients with symptoms of PE had inconclusive lung scans, 2 withdrawn due to reoperation and 1 used other anticoagulant drug Losses to follow up: Not reported Age median years (range): Extended LMWH 68 (30 ‐ 94); placebo 70 (28 ‐ 91) Sex M/F: Extended LMWH 66/74; placebo 62/79 Inclusion criteria: All participants admitted for total hip replacement; age 18 years or older Exclusion criteria: Previous surgery in the study; simultaneous participation in another pharmacological study; informed consent not obtained; high probability for drop‐out; renal insufficiency; hepatic insufficiency; prothrombin < 0.7; platelet count < 100 x 109/L; treatment with oral anticoagulants or heparin within seven days before inclusion; hypersensitivity to heparin, LMWH or contrast media; documented bleeding within three months prior to surgery; intracranial bleeding within three months prior to surgery; eye, ear or CNS surgery within one month prior to surgery; hypertension with diastolic pressure > 120 mmHg; septic endocarditis; body weight < 40 kg; known pregnancy or lactation | |
| Interventions | Treatment: Dalteparin subcutaneous injections (5000 anti‐Xa), once daily Control: Initial treatment with dalteparin (5000 anti‐Xa) followed by placebo (sodium chloride) injections, once daily placebo subcutaneous injections, once daily, started after discharge Duration: 35 days after surgery | |
| Outcomes | Primary: DVT and PE Secondary: Bleeding complications and adverse events; haemological analysis Bleeding definitions: No definition for major bleeding was provided | |
| Notes | Funding: Not reported Method of VTE evaluation/confirmation: Bilateral ascending phlebography at the end of treatment to detect DVT; PE detected by perfusion/ventilation lung scan or pulmonary angiography | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with a separate randomisation list for each centre |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given to determine adequate allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo injections, saline, given to control participants |
| Blinding of outcome assessment (detection bias) | Low risk | All venograms were evaluated by a panel of three radiologists who were unaware of the result of the randomisation |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, similar numbers in groups, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
| Methods | Study design: Randomised, double‐blind, parallel‐group study Country: Multi‐country (16 countries) Setting: Multicentre; hospital and home; September 2005 to February 2006 Intention‐to‐treat: No analysis performed as study stopped prematurely For the purposes of our meta‐analyses we used the per‐protocol population as reported by the authors | |
| Participants | Number randomised: Total n = 1158; 641 completed at time of termination (ximelagatran n = 580; enoxaparin n = 578) Exclusions post randomisation: Total n = 150 (ximelagatran n = 70; enoxaparin n = 80) Not treated: 23 melagatran, 16 enoxaparin; Premature stop: 47 melagatran, 64 enoxaparin Losses to follow up: Study terminated early Age median years (range): Hip replacement: ximelagatran 64.7 (24 ‐ 89); enoxaparin 63.9 (21 ‐ 89); Fracture surgery: ximelagatran 73.1 (44 ‐ 91); enoxaparin 70.7 (26 ‐ 94) Sex M/F: Hip replacement: ximelagatran 229/250; enoxaparin 211/268; Fracture surgery: Ximelagatran 17/60; enoxaparin 23/50 Inclusion criteria: 18 years or older; undergoing primary elective unilateral total hip replacement or surgery for hip fracture Exclusion criteria: "same as in the previously reported phase III studies in orthopaedic surgery on ximelagatran" | |
| Interventions | Treatment 1: 3 mg ximelagatran subcutaneously 4 ‐ 8 hours after surgery and twice daily for up to 2 days post‐op, followed by 24 mg oral ximelagatran twice daily Treatment 2: 40 mg enoxaparin subcutaneous once daily starting the night before surgery or post‐operatively Duration: 32 ‐ 38 days after surgery; Randomised within 5 days before surgery | |
| Outcomes | Primary: Efficacy outcomes: composite of proximal DVT, any clinically suspected and objectively confirmed DVT and/or PE, VTE‐related death or death where VTE could not be ruled out. Safety outcomes: major bleeding events, transfusions of whole blood and packed red blood cells, injection site haematomas > 2 cm, clinically verified and adjudicated myocardial infarction, evidence of hepatic injury Secondary: No distinction between primary and secondary Bleeding definitions: Major bleeding ‐ transfusions of whole blood and packed red blood cells, injection site haematomas > 2 cm, clinically verified and adjudicated myocardial infarction, evidence of hepatic injury | |
| Notes | Funding: AstraZeneca, Sweden; employees of AstraZeneca contributed to study design, interpretation of results and decision to submit paper Method of VTE evaluation/confirmation: bilateral compression ultrasound (CUS) of the legs at the end of treatment period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive web‐based randomisation system; stratified by type of surgery |
| Allocation concealment (selection bias) | Low risk | Used web‐based randomisation system |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes adjudicated by a blinded, independent committee |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT numbers do not add up; insufficient information given |
| Selective reporting (reporting bias) | Unclear risk | No details of the VTE events were provided |
| Other bias | High risk | Terminated early due to safety issues, AstraZeneca supported trial ‐ had influence on study design, interpretation of results and decision to submit paper |
| Methods | Study design: Randomised, double‐blind, clinical trial Country: USA and Canada Setting: Multicentre (18 centres); hospital and home Intention‐to‐treat: Yes, ITT: VTE analysis performed on participants who had successful venography, safety analysis with those who had successful venogram at day six For the purposes of our meta‐analyses we used the reported ITT population | |
| Participants | Number randomised: Total n = 991 but only n = 569 received treatment (pre‐op dalteparin n = 199; post‐op dalteparin n = 190; warfarin/placebo n = 180) Exclusions post randomisation: Total n = 293: 94 refused participation, 40 adverse event in‐hospital, 12 unable to self inject, 42 other reasons, plus 105 no or inadequate venography Losses to follow up: No reports of losses to follow up but could be part of 42 other reasons for no venography Age mean years (SD): Pre‐op dalteparin 62 (12); post‐op dalteparin 63 (12); warfarin/placebo 63 (12) Sex M/F: Pre‐op dalteparin 106/93; post‐op dalteparin 87/103; warfarin/placebo 94/86 Inclusion criteria: Aged 18 years or older; scheduled for elective unilateral total hip replacement; gave informed consent Exclusion criteria: Documented bleeding within three months before surgery; known hypersensitivity to heparin, LMWH, warfarin or contrast media; defective haemostasis; ongoing anticoagulant therapy; pregnancy or breastfeeding; clinically significant hepatic dysfunction; renal insufficiency; severe hypertension; septic endocarditis; weight less than 40 kg; eye, ear or central nervous system surgery within one month before hip surgery; diseases with unfavourable prognosis or concurrent disease making study participation medically complicated; simultaneous participant in another study or receiving any investigation drug 30 days or less before surgery; previous randomisation into this study; use of pneumatic compression stockings during study period | |
| Interventions | Treatment 1: Dalteparin sodium, subcutaneous, 5000 IU once daily, commenced two hours before surgery plus placebo capsules while in hospital Treatment 2: Dalteparin sodium, subcutaneous, 5000 IU once daily, commenced four hours after surgery plus placebo capsules while in hospital Control: Placebo Duration: 35 ± 2 days | |
| Outcomes | Primary: Venogram‐confirmed DVT and proximal DVT Secondary: PE, bleeding complications, death Bleeding definitions: Major bleeding ‐ clinically overt, associated with a decrease in haemoglobin of 20 g/L or more; if it required a blood transfusion of 2 or more units; intracranial, intraocular, intraspinal or intraperitoneal; occurred into a prosthetic joint Minor bleeding ‐ clinically overt but not meeting criteria for major | |
| Notes | Funding: A grant‐in‐aid by Pharmacia & Upjohn to the University of Calgary Method of DVT and PE evaluation/confirmation: Participants with VTE symptoms received objective testing for confirmation; bilateral ascending venography was performed at discharge and at the end of the study period for all participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using computer‐derived treatment schedule, divided into consecutive blocks, stratified by treatment centre |
| Allocation concealment (selection bias) | Low risk | Randomisation by computer‐derived methods |
| Blinding of participants and personnel (performance bias) | Low risk | Used placebo dalteparin injections and placebo warfarin capsules to maintain blind |
| Blinding of outcome assessment (detection bias) | Low risk | Venograms were interpreted by a blinded central reader as well as a local radiologist; disagreements resolved by a second blinded interpretation |
| Incomplete outcome data (attrition bias) | Low risk | All participants were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
| Methods | Study design: Randomised, double‐blind, placebo controlled trial Country: France Setting: Single centre; hospital and home; August 1991 to June 1994 Intention‐to‐treat: Yes, ITT and per‐protocol, although based on second bilateral venography For the purposes of our meta‐analyses we used the reported ITT population | |
| Participants | Number randomised: Total n = 179 (extended LMWH n = 90; placebo n = 89) Exclusions post randomisation: Total n = 6 (extended LMWH n = 5; placebo n = 1), had no second venogram and excluded from efficacy analysis Losses to follow up: No losses to follow up; all participants included in safety analysis Age mean years (SD): Extended LMWH 70 (9.1); placebo 68 (8.2) Sex M/F: Extended LMWH 47/43; placebo 55/34 Inclusion criteria: Underwent primary total hip replacement or conversion/reversion of total hip replacement; received prophylactic treatment with enoxaparin for post‐op VTE; > 45 years of age; body weight 45 ‐ 95 kg; could walk unassisted using crutches; were free of DVT as assessed by bilateral ascending contrast venography performed within five days before discharge; had to be available for a follow‐up visit at day 21 ± 2 days post discharge Exclusion criteria: History of documented DVT or PE within last six months; active cancer; underlying bleeding disorders or haemostasis abnormalities; prothrombin time < 60% or activated partial thromboplastin time > 8 seconds or longer than control subjects; active gastroduodenal ulcer; history of hypersensitivity to heparin or to contrast media; renal or hepatic insufficiency; uncontrolled hypertension; recent stroke; inability to give informed consent | |
| Interventions | Treatment: 40 mg enoxaparin, subcutaneous, daily Control: 40 mg enoxaparin, subcutaneous, daily during hospitalisation (14 ± 1 day) followed by placebo (isotonic saline) injections Duration: 35 days after surgery | |
| Outcomes | Primary: DVT and/or PE Secondary: Onset of proximal or distal DVT; safety: death, major and minor haemorrhages, adverse events Bleeding definitions: Major bleeding ‐ overt and was associated with a decrease in haemoglobin concentration of 2 g/dL or more compared with the last postoperative value, or a need for transfusion of two or more units of packed red blood cells, or if it was retroperitoneal or intracranial. Minor bleeding ‐ overt but did not meet the other criteria for major bleeding | |
| Notes | Funding: Not reported Method of VTE evaluation/confirmation: Clinical evaluation of symptoms and clinical signs of DVT and bilateral venographic evaluation at day 35 post‐surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was balanced in blocks of four by means of a computer‐generated randomization schedule." |
| Allocation concealment (selection bias) | Low risk | Used computer‐generated randomisation schedule |
| Blinding of participants and personnel (performance bias) | Low risk | Open‐label during hospitalisation period when all patients receiving enoxaparin, then double‐blinded after randomisation to extended duration or placebo using saline injections for the placebo group; "The double‐blind conditions (for patients, nurses, attending physicians, and investigators) were maintained until the database was locked." |
| Blinding of outcome assessment (detection bias) | Low risk | Venograms were independently evaluated by two blinded radiologists |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
| Methods | Study design: Randomised, double‐blind, placebo‐controlled trial Country: US Setting: Multicentre (33 centres); hospital and home; November 1994 to November 1997 Intention‐to‐treat: Yes For the purposes of our analyses we used all participants randomised, as reported by the study authors | |
| Participants | Number randomised: Total n = 1195 (Extended LMWH n = 607; Placebo n = 588) Exclusions post randomisation: Unclear Losses to follow up: Unclear Age mean years (SD): Extended LMWH 65 (11); placebo 66 (11) Sex M/F: Extended LMWH 265/342; placebo 275/313 Inclusion criteria: Aged 18 years or older; received elective primary or revision unilateral total hip replacement, primary unilateral or bilateral total knee replacement Exclusion criteria: 'Pregnant, lactating or women of childbearing age; patients with clinical bleeding disorder; uncontrolled hypertension' severely impaired hepatic or renal function; active alcohol or drug abuse; patients who could not comply with home injections or complete a 10‐week post‐op follow‐up; patients receiving warfarin or thrombolytic therapy; had a major surgery within previous seven days; had major orthopaedic surgery involving lower extremities in previous six weeks; history of substantial internal bleeding, active peptic ulcer, myocardial infarction or stroke; intracranial or intraocular surgery in previous eight weeks; patients planning to undergo staged bilateral total knee replacement with anticipated interval of less than 10 weeks; hypersensitivity to heparin, pork products, metabisulphite, methylparaben or propylparaben; weight great than 120 kg; prolonged activated partial thromboplastin time or prothrombin time at baseline; baseline platelet count less than 100 x 109/L; history of VTE; current use of dextran sulphate, desmopressin acetate, other LMWH, oral anticoagulants, thrombolytic agents or external pneumatic compression | |
| Interventions | Treatment: Ardeparin sodium 100 anti‐Xa IU/kg weight, subcutaneous injections, daily, starting within 24 hours after surgery Control: Four to 10 days ardeparin sodium 100 anti‐Xa IU/kg weight (starting within 24 hours after surgery), daily, subcutaneous injection, followed by placebo injections from time of discharge Duration: Six weeks after surgery | |
| Outcomes | Primary: Incidence of symptomatic, objectively documented DVT or PE or death Secondary: The incidence of major and minor bleeding and thrombocytopenia Bleeding definitions: Major bleeding ‐ overt bleeding associated with haemoglobin decrement of at least 20 g/L or transfusion of at least 2 units of blood products, any intracranial, retroperitoneal, intraocular or mediastinal bleeding that occurred after at least one dose of post‐discharge study drug Minor bleeding ‐ overt bleeding not meeting the criteria for major bleeding | |
| Notes | Funding: Wyeth‐Ayerst Research, Philadelphia, US ‐ performed statistical analysis, data interpretation and manuscript preparation done by writing committee, sponsor did not have prior right of approval for final manuscript publication Method of VTE evaluation/confirmation: Compression duplex ultrasonography or venography, ventilation perfusion lung scanning or pulmonary angiography | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by clinical centre, type of surgery and history of VTE; block randomisation derived from a randomisation table |
| Allocation concealment (selection bias) | Low risk | Allocation done in consecutively numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind phase of study achieved by giving placebo injections in identical Tubex cartridges containing 0.5 mL ardeparin sodium or placebo (sodium chloride solution) |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessed by blinded, central adjudication committee |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, although > 15% withdrew in both groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
| Methods | Study design: Randomised, double blind, placebo controlled trial Country: Germany and Czech Republic Setting: Multicentre (13 centres), hospital and home Intention‐to‐treat: Yes and per‐protocol For the purposes of our analyses we used all participants randomised, as reported by the study authors | |
| Participants | Number randomised: Total n = 310 (extended LMWH n = 161; placebo n = 149) Exclusions post randomisation: Total n = 37: protocol violation: 8, adverse events:10, withdrawal of consent: 19 Losses to follow up: None Age mean years (SD): Extended LMWH 78.1 (8.4); placebo 75.8 (8.4) Sex M/F: Extended LMWH 25/136; placebo 29/120 Inclusion criteria: Participants undergoing endoprosthetic joint replacement or osteosynthesis of the lower limb Exclusion criteria: Age under 18 years; hypersensitivity against heparin; clinical conditions with increased risk of bleeding; haemorrhagic diathesis; platelet count < 100.000/ul; concomitant treatment with anticoagulants or platelet inhibitors; renal or hepatic insufficiency; hypertension with systolic values > 200 mmHg and diastolic values > 105 mmHg despite treatment; malignancy; endocarditis lenta; drug abuse; pregnancy; participation in a clinical trial during the last four weeks; thromboembolic complications between start of treatment and randomisation; discontinuation of study medication due to adverse events; withdrawal of consent | |
| Interventions | Treatment: Certoparin 3000 u anti‐Xa Control: Certoparin 3000 u anti‐Xa for 14 days then placebo Duration: 42 days | |
| Outcomes | Primary: Composite of symptomatic or asymptomatic DVT (proximal and/or distal), symptomatic PE and deaths related to VTE Secondary: Coagulation parameters Bleeding definition ‐ not provided | |
| Notes | Funding: Supported by Novartis Pharma, Germany, test kits for fibrin monomers and Ddimer sponsored by Roche Diagnostics Germany, protein C resistance kits sponsored by Dada Behring Germany Method of VTE evaluation/confirmation: DVT was screened for by compression and duplex ultrasonography every week and confirmed by ascending leg and pelvic venography (whenever possible). PE was verified by pulmonary angiography, spiral CT or perfusion lung scanning | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided on allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, participants given placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information provided on assessor blinding |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, although treatment group was not indicated for loss‐to‐follow‐ups |
| Selective reporting (reporting bias) | Low risk | All defined outcomes were reported on |
| Other bias | Low risk | No indication of other bias |
| Methods | Study design: Prospective randomised controlled trial Country: Italy Setting: Single centre; hospital and home; September 1998 to December 2000 Intention‐to‐treat: Yes For the purposes of our meta‐analyses we used the reported ITT population | |
| Participants | Number randomised: Total n = 360 (extended duration n = 184; control n = 176) Exclusions post randomisation: Total n = 6: three protocol violations per group but all were available for clinical follow‐up Losses to follow up: None Age median years (range): Extended duration 68 (48 ‐ 82); control 69 (44 ‐ 87) Sex M/F: Extended duration 83/101; control 79/97 Inclusion criteria: Underwent elective total hip replacement; received warfarin prophylaxis during hospitalisation Exclusion criteria: Previous hip surgery on the same side; history of thromboembolic disorder; needing long‐term anticoagulation; were unavailable for long‐term follow‐up; refused to give written informed consent; developed VTE complications, asymptomatic proximal DVT or major bleeding during hospitalisation period | |
| Interventions | Treatment: 5 mg/d of warfarin sodium prophylaxis starting two days pre‐op; dosage adjusted to increase international normalised ration between 2.0 and 3.0 Control: 5 mg/d of warfarin sodium prophylaxis starting two days pre‐op; dosage adjusted to increase international normalised ration between 2.0 and 3.0, discontinued at discharge Duration: 4 weeks after discharge (Median nine day hospitalisation plus four weeks extended treatment) | |
| Outcomes | Primary: Composite of symptomatic VTE and asymptomatic proximal DVT during first four weeks of follow up, efficacy during complete three months follow up, clinical parameters, also reports on major bleeding, death and other adverse events Secondary: Not specifically defined Bleeding definitions: Major bleeding ‐ clinically overt and associated with either a decrease in haemoglobin of at least 2.0 g/dL or a need of transfusion of two or more units of blood, was intracranial or retroperitoneal, resulted in permanent discontinuation of treatment | |
| Notes | Funding: Not reported Method of VTE evaluation/confirmation: compression ultrasonography for DVT; PE confirmed by ventilation perfusion, spiral CT, abnormal finding on angiography or autopsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Computer‐generated list |
| Blinding of participants and personnel (performance bias) | Unclear risk | No discussion of blinding and no placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Ultrasound was performed by a blinded operator; cause of death adjudicated by a blinded physician and all outcomes evaluated by a blinded committee |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for and no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | High risk | Terminated early "after inclusion of the first 360 patients because a statistically significant and clinically relevant superiority of extended over short‐term thromboprophylaxis was observed" |
| Methods | Study design: Randomised, double‐blind, double‐dummy, non‐inferiority, active‐controlled trial Country: 19 countries in Europe, North America, India, Australia, New Zealand and South Africa Setting: Multicentre (108 locations); hospital and home; March 2008 to May 2009 Intention‐to‐treat: Modified intention‐to‐treat: all participants who had surgery, received treatment and had evaluable venograms For the purposes of our meta‐analyses we used the reported ITT population of participants that underwent surgery | |
| Participants | Number randomised: Total n = 2055 (dabigatran n = 1036; enoxaparin n = 1019) Exclusions post randomisation: Total n = 477 (dabigatran n = 243; enoxaparin n = 234: not treated: dabigatran 25 and enoxaparin 16. Dabigatran 9 and enoxaparin 11 did not have surgery, dabigatran 209 and enoxaparin 207 no or not evaluable venogram Losses to follow up: All participants accounted for Age mean years (SD): Dabigatran 62 (12); enoxaparin 62 (11) Sex F%: Dabigatran 53.6%; enoxaparin 50.0% Inclusion criteria: 18 years or older; scheduled to undergo a primary, unilateral, elective total hip replacement; > 40 kg body weight; gave written informed consent Exclusion criteria: History of bleeding diathesis; excessive risk of bleeding as judged by investigators; major surgery or trauma within three months of enrolment; recent unstable cardiovascular disease; any history of haemorrhagic stroke or any of the following intracranial pathologies: bleeding, neoplasm, atriovenous malformation or aneurysm; ongoing treatment for VTE; clinical relevant bleeding within six months of enrolment; gastric or duodenal ulcer within one year of enrolment; liver disease expected to have any potential impact on survival; active liver disease or liver disease decreasing survival; known severe renal insufficiency; elevated creatinine that contraindicates venography; Treatment with anticoagulants, clopidogrel, ticlopidine, abciximab, aspiring or NSAID within seven days prior to hip replacement or anticipated need of such medication; anticipated required use of intermittent pneumatic compression and electric stimulation of lower leg; active malignant disease or current cytostatic treatment; pre‐menopausal women who are pregnant or nursing, or are of child‐bearing potential and are not practising or do not plan to continue practising acceptable methods of birth control; allergy to radio opaque contrast media, heparins or dabigatran; contraindications to enoxaparin; participation in a clinical trial during the last 30 days; leg amputee; known alcohol or drug abuse which would interfere with study completion; previous participation in this study; history of thrombocytopaenia | |
| Interventions | Treatment 1: 220 mg dabigatran etexilate (2 x 110 mg tablets), orally, once daily, initial dose of 110 mg on the day of surgery plus placebo injection identical to enoxaparin treatment Treatment 2: 40 mg enoxaparin, subcutaneous, once daily, initial dose evening before surgery plus placebo tablets identical to dabigatran treatment Duration: 28 ‐ 35 days | |
| Outcomes | Primary: Composite of total VTE and all‐cause mortality (VTE includes both proximal and distal DVT, symptomatic DVT, PE) Secondary: Major VTE and VTE‐related death, proximal DVT, total DVT, symptomatic DVT, PE, all‐cause mortality, bleeding events, lab parameters and adverse events Bleeding definitions: Major bleeding ‐ fatal, clinically overt associated with loss of haemoglobin greater than or equal to 20 g/L or leading to transfusion of greater than or equal to 2 units of packed cells or whole blood; symptomatic retroperitoneal, intracranial, intraocular or intraspinal; requiring treatment cessation; leading to reoperation. Clinically relevant bleeding ‐ spontaneous skin haematoma greater than or equal to 25 cm2; wound haematoma greater than or equal to 100 cm2; spontaneous nose bleed lasting longer than 5 min; macroscopic haematuria spontaneous or lasting longer than 24 hours if associated with an intervention; spontaneous rectal bleeding (more than a spot on toilet paper); gingival bleeding lasting longer than 5 min; any other bleeding event considered clinically relevant by the investigator Minor bleeding ‐ any other bleeding events that were not classified as major or clinically relevant | |
| Notes | Funding: Boehringer Ingelheim, Sweden Method of VTE evaluation/confirmation: DVT confirmed/detected by bilateral venography or compression ultrasound or autopsy; PE confirmed by pulmonary V‐Q scintigraphy, chest X‐ray, angiography, spiral CT or autopsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by central computer‐generated system, stratified by centre, prepared in blocks of six |
| Allocation concealment (selection bias) | Low risk | Utilised central computer‐generated system |
| Blinding of participants and personnel (performance bias) | Low risk | Both treatment groups received one study drug and one placebo identical in appearance to the other active treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Efficacy outcomes confirmed by blinded, central adjudication committee |
| Incomplete outcome data (attrition bias) | Unclear risk | All participants were accounted for, with similar numbers in each treatment group, but no description was given for excluded participants that did not take study medication |
| Selective reporting (reporting bias) | Low risk | No protocol but all expected outcomes reported |
| Other bias | Unclear risk | Study sponsors were involved in the design and conduct of the trial. The data were collected and analysed by the sponsors of the study |
| Methods | Study design: Randomised, double‐blind, active‐controlled trial Country: Multi‐country (16 countries) in Europe, Australia and South Africa Setting: Multicentre (115 centres); hospital and home; November 2006 to July 2006 Intention‐to‐treat: No, per‐protocol: excluding not treated, no surgery, and inadequate or no venogram For the purposes of our meta‐analyses we used the reported ITT population of participants that underwent surgery | |
| Participants | Number randomised: Total n = 3493 (220 mg dabigatran n = 1157; 150 mg dabigatran n = 1174; enoxaparin n = 1162) Exclusions post randomisation: Total n = 322 (220 mg dabigatran n = 108; 150 mg dabigatran n = 102; enoxaparin n = 112), reason for all: did not complete study Losses to follow up: Not specified Age mean years (SD): 220 mg dabigatran 65 (10); 150 mg dabigatran 63 (11); enoxaparin 64 (11) Sex %F: 220 mg dabigatran 56%; 150 mg dabigatran 57%; enoxaparin 56% Inclusion criteria: 18 years or older; scheduled to undergo a primary, unilateral, elective total hip replacement; > 40 kg body weight; gave written informed consent Exclusion criteria: Patients with an excessive risk of bleeding; active malignant disease or current cytostatic treatment; known severe renal insufficiency; liver disease expected to have any potential impact on survival, or elevated AST or ALT > 2 x upper limit of normal; recent unstable cardiovascular disease or history of myocardial infarction within the last three months; pre‐menopausal women who are pregnant or nursing, or are of child‐bearing potential and are not practising or do not plan to continue practising acceptable methods of birth control; allergy to radio opaque contrast media or iodine, heparins (including heparin‐induced thrombocytopenia) or dabigatran; contraindications to enoxaparin; participation in a clinical trial during the last 30 days | |
| Interventions | Treatment 1*: 150 mg dabigatran etexilate, orally, once daily, starting with half dose on day of surgery plus one placebo pill identical to other treatment and subcutaneous placebo injection Treatment 2*: 220 mg dabigatran etexilate, orally, once daily, starting with half dose on day of surgery plus one placebo pill identical to other treatment subcutaneous placebo injection Control: 40 mg enoxaparin, subcutaneous, once daily plus two placebo pills identical in appearance to active treatments Duration: 28 ‐ 35 days (average 33 days) *For the analyses within this review, the 150 mg and 220 mg treatment groups were combined as both dosages are considered normal therapeutic dosages. | |
| Outcomes | Primary: Composite of total VTE and all‐cause mortality; VTE includes both proximal and distal DVT, symptomatic DVT, PE Secondary: Major VTE and VTE‐related death, proximal DVT, total DVT, symptomatic DVT, PE, all‐cause mortality, bleeding events Bleeding definitions: Major bleeding ‐ fatal, clinically overt associated with loss of haemoglobin greater than or equal to 20g/L or leading to transfusion of greater than or equal to 2 units of packed cells or whole blood; symptomatic retroperitoneal, intracranial, intraocular or intraspinal; requiring treatment cessation; leading to reoperation Clinically relevant bleeding ‐ spontaneous skin haematoma greater than or equal to 25 cm2; wound haematoma greater than or equal to 100 cm2; spontaneous nose bleed lasting longer than 5 min; macroscopic haematuria spontaneous or lasting longer than 24 hours if associated with an intervention; spontaneous rectal bleeding (more than a spot on toilet paper); gingival bleeding lasting longer than 5 min; any other bleeding event considered clinically relevant by the investigator. Minor bleeding ‐ any other bleeding events that were not classified as major or clinically relevant | |
| Notes | Funding: Steering committee and sponsor responsible for study design, data collection and analysis done by sponsor, independent data and safety committee monitored study, steering committee had overall responsibility for all aspects and final responsibility for decision to submit paper Method of VTE evaluation/confirmation: DVT detected and confirmed by bilateral venography; PE confirmed by pulmonary V‐Q scintigraphy, chest X‐ray, angiography, spiral CT or autopsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by central computer‐generated system, stratified by centre, prepared in blocks of six |
| Allocation concealment (selection bias) | Low risk | Utilised central computer‐generated system |
| Blinding of participants and personnel (performance bias) | Low risk | All treatment groups received one study drug and one placebo identical in appearance to the other active treatment as well as a subcutaneous injection |
| Blinding of outcome assessment (detection bias) | Low risk | Efficacy outcomes initially assessed locally, then confirmed by blinded, central adjudication committee |
| Incomplete outcome data (attrition bias) | Low risk | All exclusions were reported with reasons and by study group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
| Methods | Study design: Randomised, double‐blind trial Country: Multi‐country (27 countries) Setting: Multi‐centre; hospital and home; February 2006 to March 2007 Intention‐to‐treat: Yes, modified ITT and per‐protocol analysis For the purposes of our meta‐analyses we used the reported modified ITT population of participants that underwent surgery | |
| Participants | Number randomised: Total n = 4541 (rivaroxaban n = 2266; enoxaparin n = 2275) Exclusions post randomisation: Total n = 1177 (rivaroxaban n = 580; enoxaparin n = 597); 57 rivaroxaban and 51 enoxaparin randomised but not included in safety analysis ‐ did not receive study drug but no further description; excluded from ITT if not treated and did not undergo surgery and had no suitable venograms (additional 523 rivaroxaban, 546 enoxaparin) Losses to follow up: No reports of loss‐to‐follow‐up Age mean years (range): Rivaroxaban 63.1 (18 ‐ 91); enoxaparin 63.3 (18 ‐ 93) Sex F %: Rivaroxaban 55.2%; enoxaparin 55.8% Inclusion criteria: Men and women 18 years or older; scheduled to undergo elective total hip replacement Exclusion criteria: Scheduled to undergo staged, bilateral hip arthroplasty; pregnant or breastfeeding; active bleeding or a high risk of bleeding; contraindication for prophylaxis with enoxaparin; conditions preventing bilateral venography; substantial liver disease; severe renal impairment; concomitant use of protease inhibitors for the treatment of HIV; planned intermittent pneumatic compression; requirement for anticoagulant therapy that could not be stopped | |
| Interventions | Treatment 1: 10 mg rivaroxaban, orally, once daily beginning after surgery Control: 40 mg enoxaparin, subcutaneously, once daily beginning the evening before surgery Duration: 35 days (range 31 ‐ 39); mean duration 33.4 days in rivaroxaban and 33.7 in enoxaparin group ‐ venography took place | |
| Outcomes | Primary: Composite DVT (symptomatic or detected by venography), non‐fatal PE or death from any cause at day 36 Secondary: Major VTE (proximal DVT, non‐fatal PE or death from VTE), major bleeding, DVT, symptomatic VTE, death, lab values and cardiovascular events Bleeding definitions: Major bleeding ‐ fatal, occurred in a critical organ (e.g., retroperitoneal, intracranial, intraocular, and intraspinal bleeding), or required reoperation or extrasurgical‐site bleeding that was clinically overt and was associated with a fall in the haemoglobin level of at least 2 g per decilitre or that required transfusion of 2 or more units of whole blood or packed cells | |
| Notes | Funding: Bayer Healthcare and Johnson & Johnson: data collected and analysed by sponsors, steering committee designed and supervised, all authors contributing had access to all data and analysis and vouch for accuracy and completeness of data reported Method of VTE evaluation/confirmation: Mandatory bilateral venography the day after the last dose of the study drug | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated in permuted blocks and stratification according to centre by a central telephone system with a computer‐generated randomised list |
| Allocation concealment (selection bias) | Low risk | Use of telephone system and computer‐generated randomisation list |
| Blinding of participants and personnel (performance bias) | Low risk | Patients received study medication plus placebo tablets or injection |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessed by a central, blinded adjudication committee |
| Incomplete outcome data (attrition bias) | Unclear risk | 57 rivaroxaban and 51 enoxaparin randomised but not included in safety analysis ‐ did not receive study drug but no reasons given why |
| Selective reporting (reporting bias) | Low risk | No protocol provided but all outcomes reported on |
| Other bias | Unclear risk | Data was collected and analysed by the sponsors: Bayer HealthCare and Johnson & Johnson |
| Methods | Study design: Randomised, double‐blind, controlled trial Country: Multinational (21 countries) Setting: Multicentre (123 centres); hospital and home; February 2006 to April 2007 Intention‐to‐treat: Modified ITT: not‐treated, did not receive surgery, no readable venogram For the purposes of our meta‐analyses we used the reported modified ITT population of participants that underwent surgery | |
| Participants | Number randomised: Total n = 2509 (rivaroxaban n = 1252; enoxaparin n = 1257) Exclusions post randomisation: Total n = 90 (rivaroxaban n = 40; enoxaparin n = 50). Rivaroxaban: 24, enoxaparin: 28 ‐ not taken study medication (no reason why); rivaroxaban 16 and enoxaparin 22 did not receive surgery Losses to follow up: Not reported Age mean years (SD): Rivaroxaban 61.4 (13.2); enoxaparin 61.6 (13.7) Sex %F: Rivaroxaban 54.3% enoxaparin 53.0% Inclusion criteria: Aged 18 or older; scheduled to undergo elective total hip replacement Exclusion criteria: Scheduled to undergo staged, bilateral hip arthroplasty; pregnant or breastfeeding; active bleeding or a high risk of bleeding; contraindication for prophylaxis with enoxaparin; conditions preventing bilateral venography; substantial liver disease; severe renal impairment; concomitant use of protease inhibitors for the treatment of HIV; use of fibrinolytic therapy; planned intermittent pneumatic compression; requirement for anticoagulant therapy that could not be stopped | |
| Interventions | Treatment: 10 mg rivaroxaban, orally, once daily beginning after surgery plus placebo injections for 10 ‐ 14 days Control: 40 mg enoxaparin, subcutaneously, once daily beginning the evening before surgery and continued for 10 ‐ 14 days and received placebo tablets for the entire study period Duration: 31 ‐ 39 days | |
| Outcomes | Primary: Composite of any DVT, nonfatal PE and all‐cause mortality; incidence of major bleeding events Secondary: Major VTE (composite of proximal DVT, non‐fatal PE and VTE‐related death); DVT (proximal and distal), symptomatic VTE, on‐treatment bleeding, death Bleeding definitions: Major bleeding ‐ fatal, occurred in a critical organ (e.g., retroperitoneal, intracranial, intraocular, and intraspinal bleeding), or required reoperation or extrasurgical‐site bleeding that was clinically overt and was associated with a fall in the haemoglobin level of at least 2 g per decilitre or that required transfusion of 2 or more units of whole blood or packed cells | |
| Notes | Funding: Bayer HealthCare AG, Johnson & Johnson Pharmaceutical Research and Development LLC; sponsors involved in design and conduct of trial, data collection and analysis; all authors had full access to data and analyses and vouch for accuracy and completeness of data and were involved in decision to submit the manuscript Method of VTE evaluation/confirmation: DVT confirmed by bilateral venography; PE confirmed by perfusion/ventilation lung scintigraphy, angiography, chest X‐ray or spiral CT or autopsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated in permuted blocks and stratification according to centre by a central telephone system with a computer‐generated randomised list |
| Allocation concealment (selection bias) | Low risk | Use of telephone system and computer‐generated randomisation list |
| Blinding of participants and personnel (performance bias) | Low risk | Patients received study medication plus placebo tablets or injection |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessed by a central, blinded adjudication committee |
| Incomplete outcome data (attrition bias) | Unclear risk | All participants were accounted for, with similar numbers in each treatment group, but no description was given for excluded participants that did not take study medication |
| Selective reporting (reporting bias) | Low risk | No protocol provided but all outcomes reported on |
| Other bias | Unclear risk | Funded by Bayer HealthCare and Johnson & Johnson ‐ study sponsors were involved in the design and conduct of the trial. The data were collected and analysed by the sponsors of the study |
| Methods | Study design: Randomised trial Country: France Setting: Multicentre (65 centres); hospital and home; September 1997 to October 1999 Intention‐to‐treat: Yes, and per‐protocol For the purposes of our meta‐analyses we used the reported ITT population | |
| Participants | Number randomised: Total n = 1289 (LMWH n = 644; anticoagulant n = 645) Exclusions post randomisation: Total n = 10 (LMWH n = 1; anticoagulant n = 9); no treatment 7 (1 reviparin, 6 acenocoumarol); event at randomisation: 3 acenocoumarol Losses to follow up: Not reported Age mean years (SD): LMWH 66 (11); anticoagulant 65 (12) Sex %F: LMWH 51%; anticoagulant 50% Inclusion criteria: 18 years or older; scheduled to undergo elective unilateral primary total hip replacement Exclusion criteria: Femoral neck fracture; current active bleeding or disorders contraindicating anticoagulant therapy; a history of DVT or PE; heparin‐induced thrombocytopaenia, peptic ulcer, allergy to radiopaque contrast medium; use of aspirin or ticlopidine hydrochloride; renal insufficiency; liver failure; acute endocarditis; recent stroke; uncontrolled hypertension; pregnancy; alcoholism; inability to follow instructions | |
| Interventions | Treatment: Fixed‐dose subcutaneous LMWH reviparin sodium, 4200 anti‐Xa IU, beginning 12 hours preoperatively Treatment 2: After initial LMWH treatment as described above, crossed over to adjusted‐dose oral anticoagulant acenocoumarol, international normalised ratio, 2 ‐ 3 Duration: 6 weeks after surgery | |
| Outcomes | Primary: Combined clinical events of symptomatic thromboembolic event, major haemorrhage or death Secondary: Minor bleeding Bleeding definitions: Major bleeding ‐ clinically overt and was associated with a decrease in haemoglobin level of more than 20 g/L or required a transfusion of 2 U or more of packed red blood cells after randomisation or was digestive, intracranial, retroperitoneal or intraocular or was located at the surgical site and required reoperation or, according to the investigator's opinion, led to discontinuation of the treatment. Minor bleeding ‐ clinically overt but not major | |
| Notes | Funding: Supported by Knoll France, Investigators received USD 400 per patient included in the study and PI received final grant of USD 4000 Method of VTE evaluation/confirmation: Suspected DVT confirmed by venography or duplex scanning; suspected PE confirmed by ventilation‐perfusion or angiography | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomized computer‐derived treatment schedule" performed at a central location, stratified by each centre, balanced in blocks of four |
| Allocation concealment (selection bias) | Low risk | Utilised a centralised computer‐derived randomisation schedule |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding of participants or personnel; most likely unblinded as one treatment given subcutaneously and the other orally |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes interpreted by a blinded adjudication committee |
| Incomplete outcome data (attrition bias) | Low risk | Table 1. clearly demonstrates missing participants and reasons, although it should be noted the anticoagulant treatment group had a higher rate of protocol violations (statistical tests not performed) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
| Methods | Study design: Randomised trial Country: China Setting: Single centre; hospital and outpatient clinic; June 2012 and May 2013 Intention‐to‐treat: Yes For the purposes of our analyses we used all participants randomised, as reported by the study authors | |
| Participants | Number randomised: Total n = 40 (extended n = 20; short n = 20) Exclusions post randomisation: Not stated Losses to follow up: None Age mean years (range): Extended 60.1 (37 ‐ 76); short 61.3 (42 ‐ 78) Sex (F/M): Extended 11/9; Short 12/8 Inclusion criteria: All patients admitted to the Center for Bone and Joint Health between June 2012 and May 2013, who were diagnosed with osteonecrosis of the femoral head, scheduled for elective total hip replacement and provided informed consent Exclusion criteria: Had Doppler ultrasound performed 48 hrs prior to the surgery showing DVT; had undergone surgery recently, a history of active major bleeding or a tendency to bleed; had recent use of anticoagulants, antiplatelets or antifibrinogenics; were pregnant or nursing baby or did not take measures to prevent pregnancy; had severe kidney or liver dysfunction or other disease that could interfere with drug metabolism or blood coagulation | |
| Interventions | Treatment: Oral rivaroxaban 10 mg once daily started within 6 ‐ 10 hrs after the surgery for 35 days Control: Oral rivaroxaban 10 mg once daily started within 6 ‐ 10 hrs after the surgery for 7 days followed by no treatment Duration: 35 days post surgery | |
| Outcomes | Primary: Efficacy: haemostatic parameters including thrombin‐antithrombin complexes, prothrombin fragment 1 and 2, D‐dimer and fibrinogen Secondary: DVT Outcomes were recorded pre‐operatively (within 48 hours before surgery) and at 1, 7, and 35 days after surgery | |
| Notes | Funding: Not reported Method of VTE evaluation/confirmation: lower limb ultrasonography | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using random numbers generated by computer prior to the surgery |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, similar numbers in groups, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | All listed outcomes reported on |
| Other bias | Low risk | No indication of other bias |
DVT: deep vein thrombosis
F: female
IU: international unit
LMWH: low‐molecular‐weight heparin
M: male
NSAID: non‐steroidal anti‐inflammatory drug
PE: pulmonary embolism
post‐op: post‐operatively, after operation
pre‐op: prior to operation
VAS: visual analogue scale
V‐Q: ventilation–perfusion
VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Treatment duration only four weeks | |
| Treatment duration only 30 days | |
| Treatment not within scope of review: heparin plus indomethacin versus heparin plus placebo | |
| Treatment duration only 30 days | |
| Treatment duration was extended but not to five weeks | |
| Treatment duration only four weeks | |
| Treatment duration for only one month |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 5 | 2329 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.35, 1.01] |
| Analysis 1.1  Comparison 1 Heparin versus placebo, Outcome 1 Symptomatic VTE (DVT and PE). | ||||
| 1.1 Hip replacement | 4 | 1296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.30] |
| 1.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.92] |
| 1.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.06] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 4 | 2019 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.39, 1.38] |
| Analysis 1.2 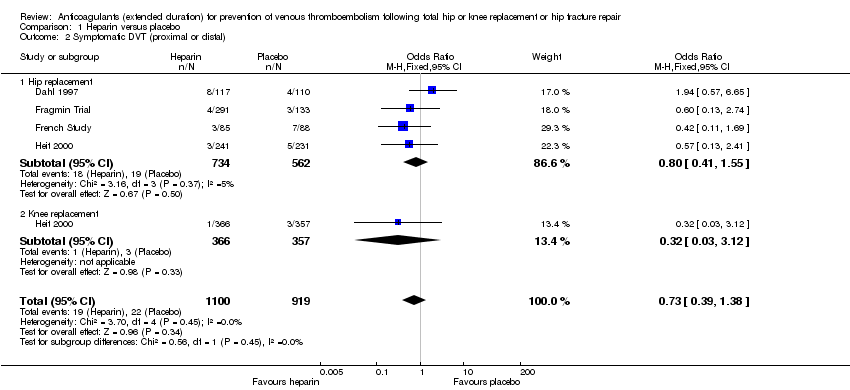 Comparison 1 Heparin versus placebo, Outcome 2 Symptomatic DVT (proximal or distal). | ||||
| 2.1 Hip replacement | 4 | 1296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.55] |
| 2.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.12] |
| 3 Symptomatic PE Show forest plot | 3 | 1595 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.16, 2.33] |
| Analysis 1.3  Comparison 1 Heparin versus placebo, Outcome 3 Symptomatic PE. | ||||
| 3.1 Hip replacement | 3 | 872 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.56] |
| 3.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.24, 8.83] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 6 | 2544 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.28, 0.56] |
| Analysis 1.4  Comparison 1 Heparin versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic). | ||||
| 4.1 Hip replacement | 5 | 1511 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.25, 0.56] |
| 4.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.92] |
| 4.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.90] |
| 5 Asymptomatic DVT Show forest plot | 5 | 1304 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.24, 0.60] |
| Analysis 1.5  Comparison 1 Heparin versus placebo, Outcome 5 Asymptomatic DVT. | ||||
| 5.1 Hip replacement | 4 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.58] |
| 5.2 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.19, 1.52] |
| 6 All‐cause mortality Show forest plot | 5 | 2518 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.31, 3.26] |
| Analysis 1.6  Comparison 1 Heparin versus placebo, Outcome 6 All‐cause mortality. | ||||
| 6.1 Hip replacement | 4 | 1485 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.11, 2.75] |
| 6.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.65] |
| 6.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.69 [0.22, 98.42] |
| 7 Adverse events Show forest plot | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.64] |
| Analysis 1.7  Comparison 1 Heparin versus placebo, Outcome 7 Adverse events. | ||||
| 7.1 Hip replacement | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.64] |
| 8 Bleeding ‐ major Show forest plot | 5 | 2500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.46] |
| Analysis 1.8  Comparison 1 Heparin versus placebo, Outcome 8 Bleeding ‐ major. | ||||
| 8.1 Hip replacement | 4 | 1494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.10] |
| 8.2 Knee replacement | 1 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.06] |
| 8.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Bleeding ‐ minor Show forest plot | 5 | 2500 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.43, 2.81] |
| Analysis 1.9  Comparison 1 Heparin versus placebo, Outcome 9 Bleeding ‐ minor. | ||||
| 9.1 Hip replacement | 4 | 1494 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.53, 3.30] |
| 9.2 Knee replacement | 1 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.58, 2.59] |
| 9.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.11, 69.13] |
| 10 Reoperation Show forest plot | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.10 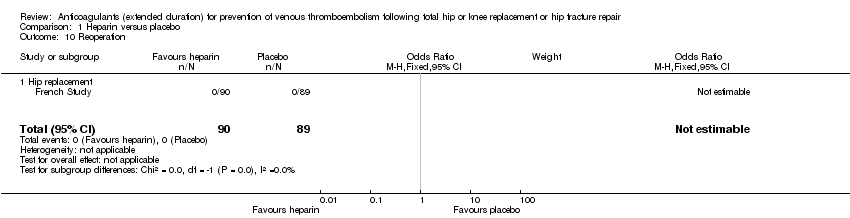 Comparison 1 Heparin versus placebo, Outcome 10 Reoperation. | ||||
| 10.1 Hip replacement | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.94] |
| Analysis 2.1  Comparison 2 Vitamin K antagonists versus placebo, Outcome 1 Symptomatic VTE (DVT and PE). | ||||
| 1.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.94] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| Analysis 2.2  Comparison 2 Vitamin K antagonists versus placebo, Outcome 2 Symptomatic DVT (proximal or distal). | ||||
| 2.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| 3 Symptomatic PE Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.84] |
| Analysis 2.3  Comparison 2 Vitamin K antagonists versus placebo, Outcome 3 Symptomatic PE. | ||||
| 3.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.84] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| Analysis 2.4  Comparison 2 Vitamin K antagonists versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic). | ||||
| 4.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| 5 All‐cause mortality Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.5 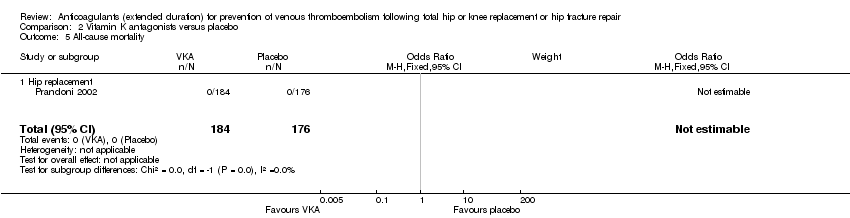 Comparison 2 Vitamin K antagonists versus placebo, Outcome 5 All‐cause mortality. | ||||
| 5.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse events Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.6  Comparison 2 Vitamin K antagonists versus placebo, Outcome 6 Adverse events. | ||||
| 6.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Bleeding ‐ major Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 71.31] |
| Analysis 2.7  Comparison 2 Vitamin K antagonists versus placebo, Outcome 7 Bleeding ‐ major. | ||||
| 7.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 71.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 2419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.68] |
| Analysis 3.1  Comparison 3 DOAC versus placebo, Outcome 1 Symptomatic VTE (DVT and PE). | ||||
| 1.1 Hip replacement | 1 | 2419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.68] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 2 | 2459 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.81] |
| Analysis 3.2  Comparison 3 DOAC versus placebo, Outcome 2 Symptomatic DVT (proximal or distal). | ||||
| 2.1 Hip replacement | 2 | 2459 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.81] |
| 3 Symptomatic PE Show forest plot | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.25] |
| Analysis 3.3  Comparison 3 DOAC versus placebo, Outcome 3 Symptomatic PE. | ||||
| 3.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.25] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.11, 0.33] |
| Analysis 3.4  Comparison 3 DOAC versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic). | ||||
| 4.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.11, 0.33] |
| 5 All‐cause mortality Show forest plot | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.66] |
| Analysis 3.5 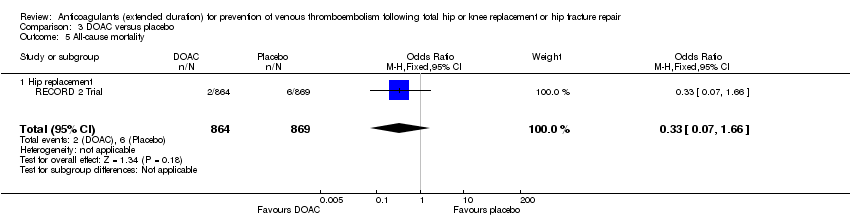 Comparison 3 DOAC versus placebo, Outcome 5 All‐cause mortality. | ||||
| 5.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.66] |
| 6 Adverse events Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| Analysis 3.6  Comparison 3 DOAC versus placebo, Outcome 6 Adverse events. | ||||
| 6.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 7 Bleeding ‐ major Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.02] |
| Analysis 3.7  Comparison 3 DOAC versus placebo, Outcome 7 Bleeding ‐ major. | ||||
| 7.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.02] |
| 8 Bleeding‐ clinically relevant non‐major Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.76, 1.95] |
| Analysis 3.8  Comparison 3 DOAC versus placebo, Outcome 8 Bleeding‐ clinically relevant non‐major. | ||||
| 8.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.76, 1.95] |
| 9 Bleeding ‐ minor Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.74, 1.88] |
| Analysis 3.9  Comparison 3 DOAC versus placebo, Outcome 9 Bleeding ‐ minor. | ||||
| 9.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.74, 1.88] |
| 10 Reoperation Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.10  Comparison 3 DOAC versus placebo, Outcome 10 Reoperation. | ||||
| 10.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Wound infection Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.86] |
| Analysis 3.11  Comparison 3 DOAC versus placebo, Outcome 11 Wound infection. | ||||
| 11.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.74] |
| Analysis 4.1  Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 1 Symptomatic VTE (DVT and PE). | ||||
| 1.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.74] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.21] |
| Analysis 4.2  Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 2 Symptomatic DVT (proximal or distal). | ||||
| 2.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.21] |
| 3 Symptomatic PE Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| Analysis 4.3  Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 3 Symptomatic PE. | ||||
| 3.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| Analysis 4.4  Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic). | ||||
| 4.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 5 Asymptomatic DVT Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| Analysis 4.5 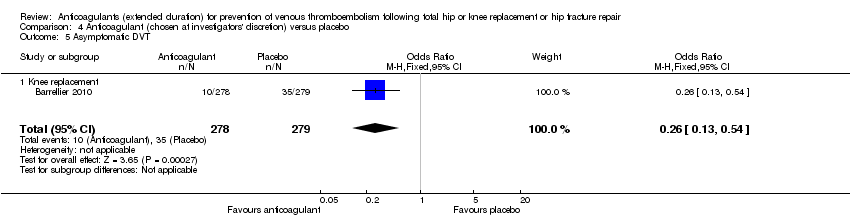 Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 5 Asymptomatic DVT. | ||||
| 5.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 6 Asymptomatic distal DVT Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| Analysis 4.6  Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 6 Asymptomatic distal DVT. | ||||
| 6.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 7 All‐cause mortality Show forest plot | 1 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.7  Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 7 All‐cause mortality. | ||||
| 7.1 Knee replacement | 1 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Bleeding ‐ major Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 105.76] |
| Analysis 4.8  Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 8 Bleeding ‐ major. | ||||
| 8.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 105.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| Analysis 5.1 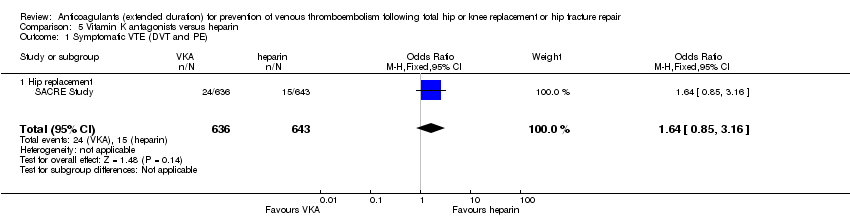 Comparison 5 Vitamin K antagonists versus heparin, Outcome 1 Symptomatic VTE (DVT and PE). | ||||
| 1.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.69, 2.68] |
| Analysis 5.2  Comparison 5 Vitamin K antagonists versus heparin, Outcome 2 Symptomatic DVT (proximal or distal). | ||||
| 2.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.69, 2.68] |
| 3 Symptomatic PE Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.16 [0.49, 170.42] |
| Analysis 5.3 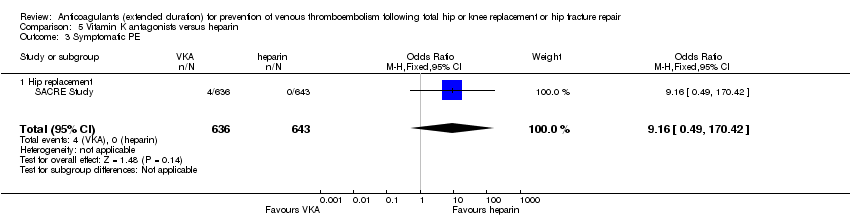 Comparison 5 Vitamin K antagonists versus heparin, Outcome 3 Symptomatic PE. | ||||
| 3.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.16 [0.49, 170.42] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| Analysis 5.4  Comparison 5 Vitamin K antagonists versus heparin, Outcome 4 Total VTE (symptomatic and asymptomatic). | ||||
| 4.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 5 All‐cause mortality Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.24, 105.83] |
| Analysis 5.5  Comparison 5 Vitamin K antagonists versus heparin, Outcome 5 All‐cause mortality. | ||||
| 5.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.24, 105.83] |
| 6 Bleeding ‐ major Show forest plot | 1 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.87 [1.91, 7.85] |
| Analysis 5.6  Comparison 5 Vitamin K antagonists versus heparin, Outcome 6 Bleeding ‐ major. | ||||
| 6.1 Hip replacement | 1 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.87 [1.91, 7.85] |
| 7 Bleeding ‐ minor Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.64, 2.76] |
| Analysis 5.7  Comparison 5 Vitamin K antagonists versus heparin, Outcome 7 Bleeding ‐ minor. | ||||
| 7.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.64, 2.76] |
| 8 Reoperation Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.60 [0.99, 21.38] |
| Analysis 5.8 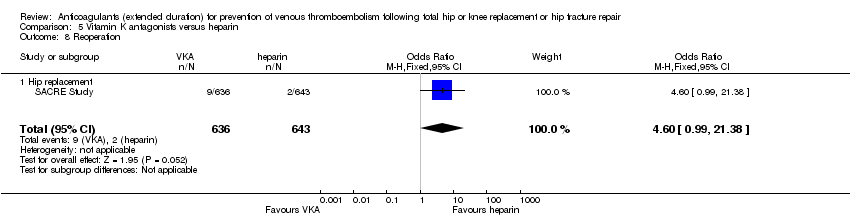 Comparison 5 Vitamin K antagonists versus heparin, Outcome 8 Reoperation. | ||||
| 8.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.60 [0.99, 21.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.28, 1.70] |
| Analysis 6.1  Comparison 6 DOAC versus heparin, Outcome 1 Symptomatic VTE (DVT and PE). | ||||
| 1.1 Hip replacement | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.28, 1.70] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.27] |
| Analysis 6.2  Comparison 6 DOAC versus heparin, Outcome 2 Symptomatic DVT (proximal or distal). | ||||
| 2.1 Hip replacement | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.27] |
| 3 Symptomatic PE Show forest plot | 5 | 14731 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.94] |
| Analysis 6.3  Comparison 6 DOAC versus heparin, Outcome 3 Symptomatic PE. | ||||
| 3.1 Hip replacement | 5 | 14731 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.94] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 4 | 12447 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.97] |
| Analysis 6.4  Comparison 6 DOAC versus heparin, Outcome 4 Total VTE (symptomatic and asymptomatic). | ||||
| 4.1 Hip replacement | 4 | 12447 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.97] |
| 5 Asymptomatic DVT Show forest plot | 2 | 6559 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.59] |
| Analysis 6.5 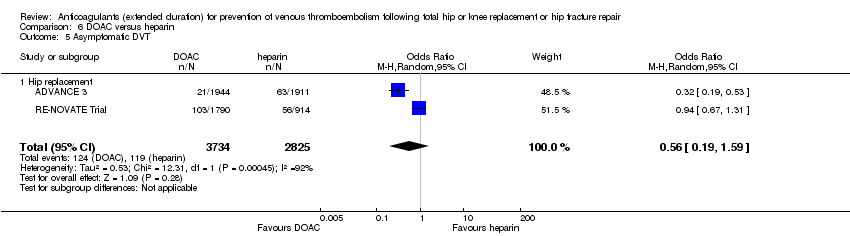 Comparison 6 DOAC versus heparin, Outcome 5 Asymptomatic DVT. | ||||
| 5.1 Hip replacement | 2 | 6559 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.59] |
| 6 Asymptomatic proximal DVT Show forest plot | 1 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.15] |
| Analysis 6.6  Comparison 6 DOAC versus heparin, Outcome 6 Asymptomatic proximal DVT. | ||||
| 6.1 Hip replacement | 1 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.15] |
| 7 Asymptomatic distal DVT Show forest plot | 1 | 2639 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.75, 1.99] |
| Analysis 6.7 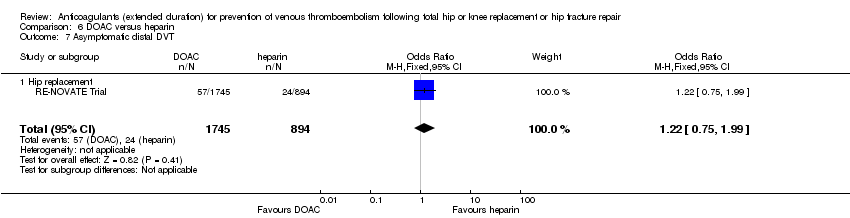 Comparison 6 DOAC versus heparin, Outcome 7 Asymptomatic distal DVT. | ||||
| 7.1 Hip replacement | 1 | 2639 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.75, 1.99] |
| 8 All‐cause mortality Show forest plot | 5 | 14966 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.64, 4.16] |
| Analysis 6.8  Comparison 6 DOAC versus heparin, Outcome 8 All‐cause mortality. | ||||
| 8.1 Hip replacement | 5 | 14966 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.64, 4.16] |
| 9 Adverse events Show forest plot | 3 | 9908 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| Analysis 6.9  Comparison 6 DOAC versus heparin, Outcome 9 Adverse events. | ||||
| 9.1 Hip replacement | 3 | 9908 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 10 Bleeding ‐ major Show forest plot | 5 | 16199 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| Analysis 6.10  Comparison 6 DOAC versus heparin, Outcome 10 Bleeding ‐ major. | ||||
| 10.1 Hip replacement | 5 | 16199 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 11 Bleeding ‐ clinically relevant, non‐major Show forest plot | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.28] |
| Analysis 6.11  Comparison 6 DOAC versus heparin, Outcome 11 Bleeding ‐ clinically relevant, non‐major. | ||||
| 11.1 Hip replacement | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.28] |
| 12 Bleeding ‐ minor Show forest plot | 4 | 11766 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| Analysis 6.12  Comparison 6 DOAC versus heparin, Outcome 12 Bleeding ‐ minor. | ||||
| 12.1 Hip replacement | 4 | 11766 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 13 Reoperation Show forest plot | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.34, 3.24] |
| Analysis 6.13 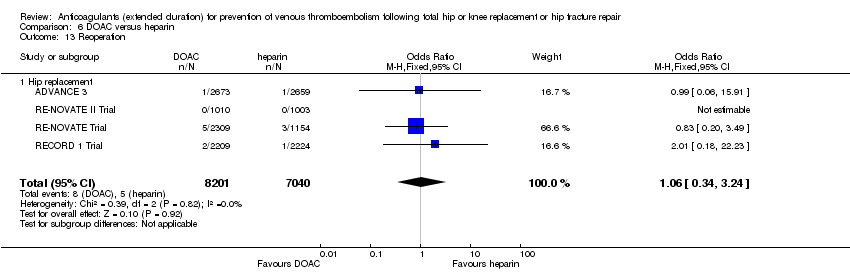 Comparison 6 DOAC versus heparin, Outcome 13 Reoperation. | ||||
| 13.1 Hip replacement | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.34, 3.24] |
| 14 Wound infection Show forest plot | 2 | 6446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.72] |
| Analysis 6.14  Comparison 6 DOAC versus heparin, Outcome 14 Wound infection. | ||||
| 14.1 Hip replacement | 2 | 6446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.72] |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Heparin versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).

Comparison 1 Heparin versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).

Comparison 1 Heparin versus placebo, Outcome 3 Symptomatic PE.
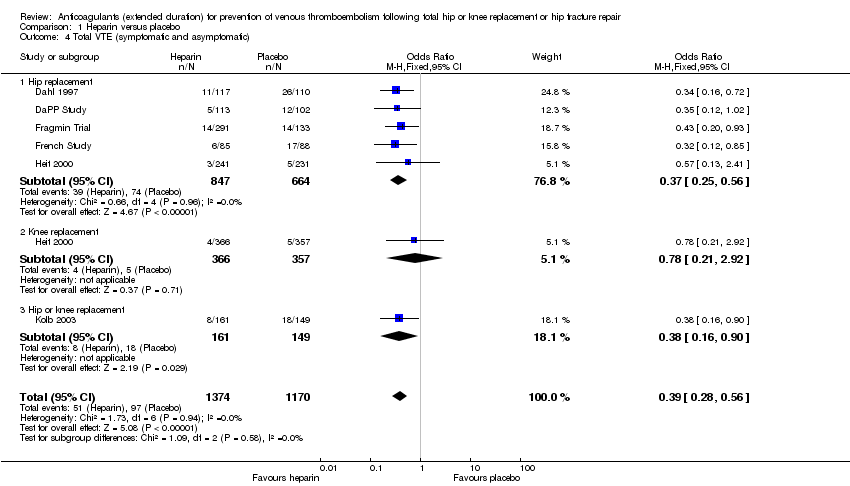
Comparison 1 Heparin versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).

Comparison 1 Heparin versus placebo, Outcome 5 Asymptomatic DVT.
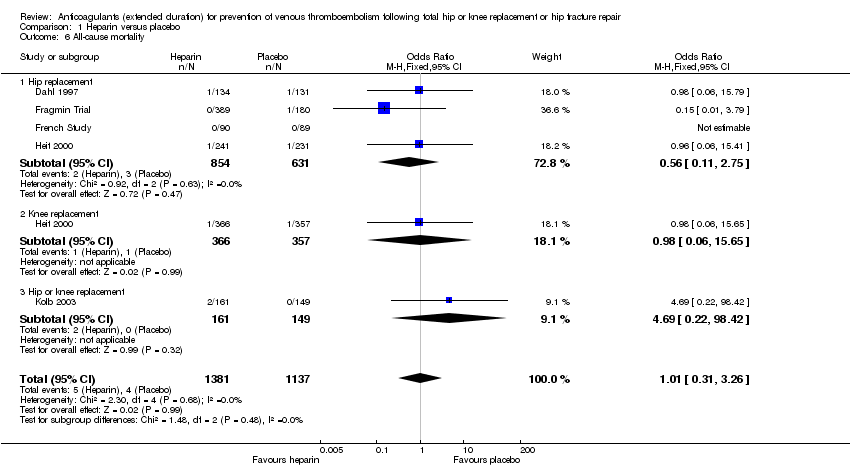
Comparison 1 Heparin versus placebo, Outcome 6 All‐cause mortality.

Comparison 1 Heparin versus placebo, Outcome 7 Adverse events.

Comparison 1 Heparin versus placebo, Outcome 8 Bleeding ‐ major.
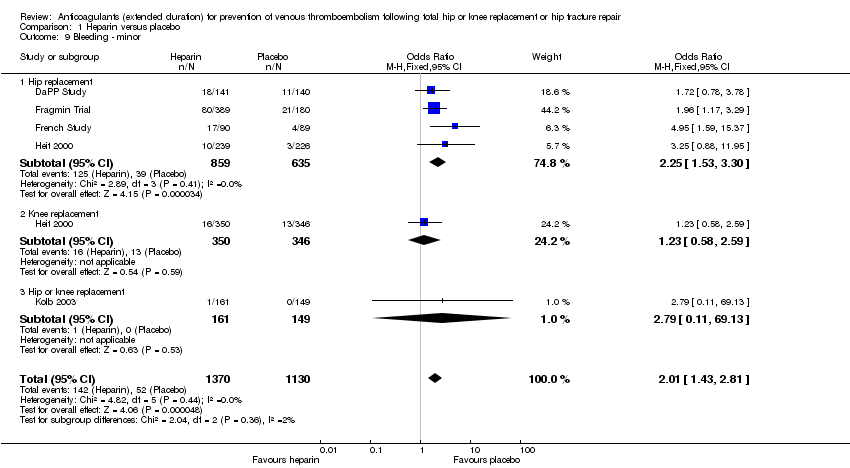
Comparison 1 Heparin versus placebo, Outcome 9 Bleeding ‐ minor.

Comparison 1 Heparin versus placebo, Outcome 10 Reoperation.

Comparison 2 Vitamin K antagonists versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).
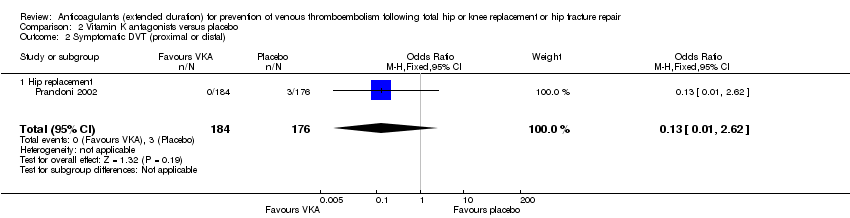
Comparison 2 Vitamin K antagonists versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).
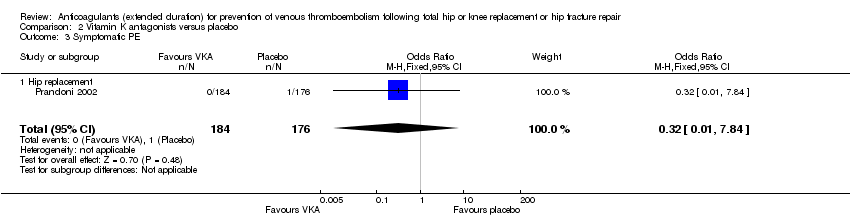
Comparison 2 Vitamin K antagonists versus placebo, Outcome 3 Symptomatic PE.

Comparison 2 Vitamin K antagonists versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).

Comparison 2 Vitamin K antagonists versus placebo, Outcome 5 All‐cause mortality.
Comparison 2 Vitamin K antagonists versus placebo, Outcome 6 Adverse events.

Comparison 2 Vitamin K antagonists versus placebo, Outcome 7 Bleeding ‐ major.

Comparison 3 DOAC versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).
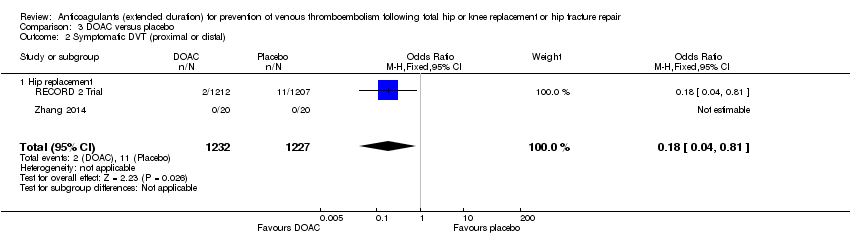
Comparison 3 DOAC versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).
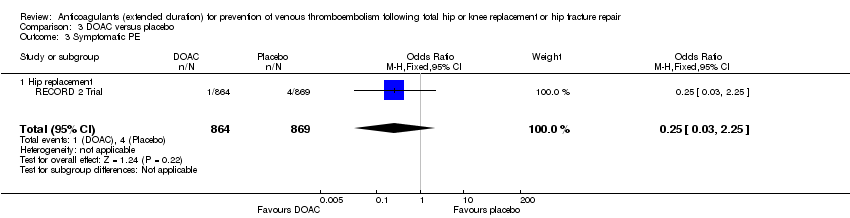
Comparison 3 DOAC versus placebo, Outcome 3 Symptomatic PE.

Comparison 3 DOAC versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).

Comparison 3 DOAC versus placebo, Outcome 5 All‐cause mortality.
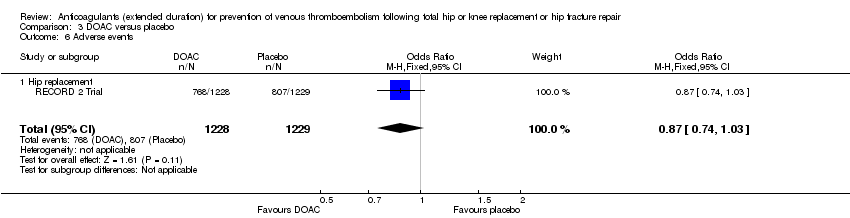
Comparison 3 DOAC versus placebo, Outcome 6 Adverse events.
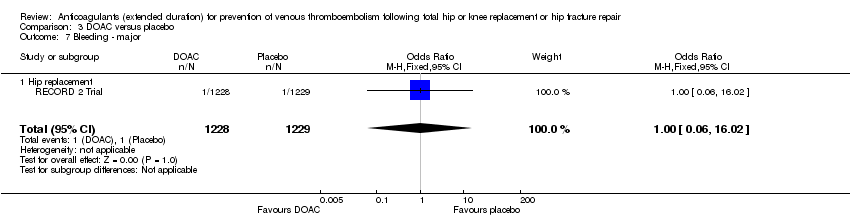
Comparison 3 DOAC versus placebo, Outcome 7 Bleeding ‐ major.

Comparison 3 DOAC versus placebo, Outcome 8 Bleeding‐ clinically relevant non‐major.

Comparison 3 DOAC versus placebo, Outcome 9 Bleeding ‐ minor.
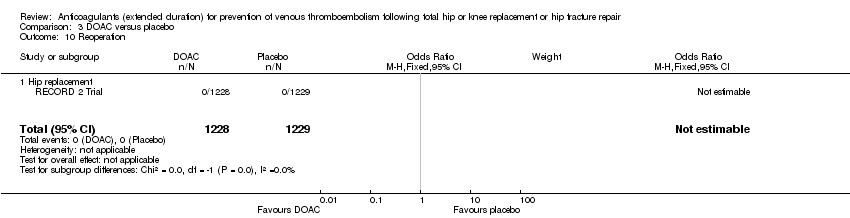
Comparison 3 DOAC versus placebo, Outcome 10 Reoperation.
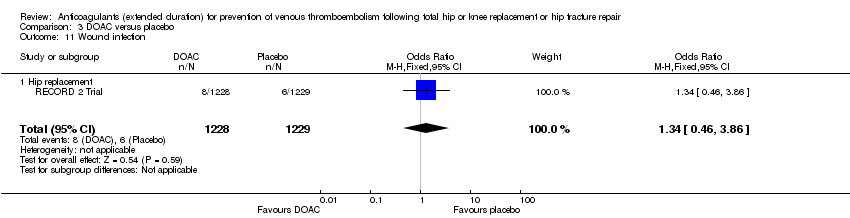
Comparison 3 DOAC versus placebo, Outcome 11 Wound infection.

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 3 Symptomatic PE.

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 5 Asymptomatic DVT.
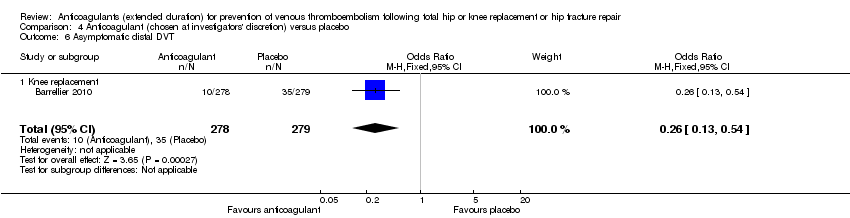
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 6 Asymptomatic distal DVT.

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 7 All‐cause mortality.
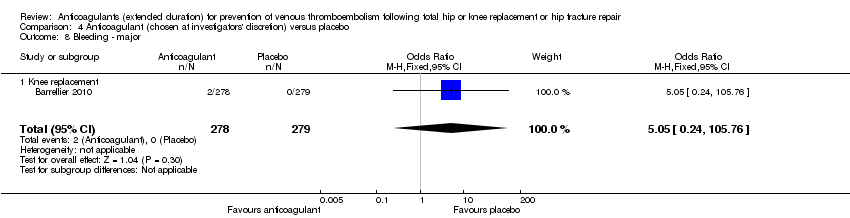
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 8 Bleeding ‐ major.

Comparison 5 Vitamin K antagonists versus heparin, Outcome 1 Symptomatic VTE (DVT and PE).

Comparison 5 Vitamin K antagonists versus heparin, Outcome 2 Symptomatic DVT (proximal or distal).

Comparison 5 Vitamin K antagonists versus heparin, Outcome 3 Symptomatic PE.
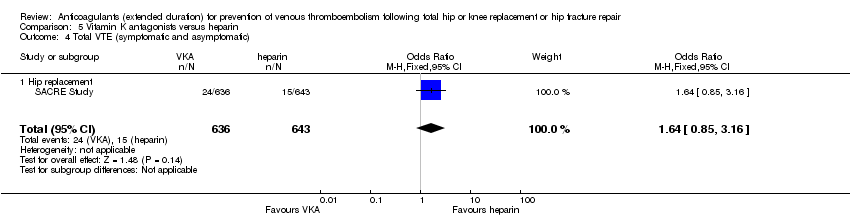
Comparison 5 Vitamin K antagonists versus heparin, Outcome 4 Total VTE (symptomatic and asymptomatic).

Comparison 5 Vitamin K antagonists versus heparin, Outcome 5 All‐cause mortality.

Comparison 5 Vitamin K antagonists versus heparin, Outcome 6 Bleeding ‐ major.
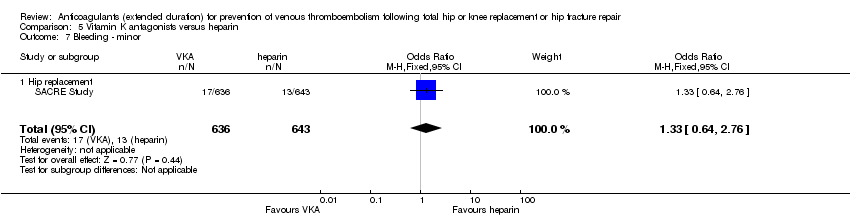
Comparison 5 Vitamin K antagonists versus heparin, Outcome 7 Bleeding ‐ minor.

Comparison 5 Vitamin K antagonists versus heparin, Outcome 8 Reoperation.
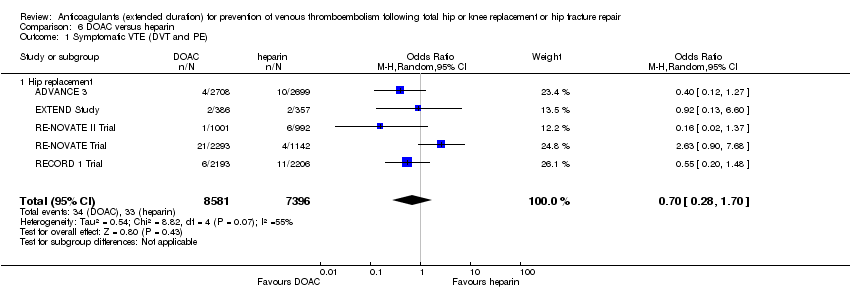
Comparison 6 DOAC versus heparin, Outcome 1 Symptomatic VTE (DVT and PE).

Comparison 6 DOAC versus heparin, Outcome 2 Symptomatic DVT (proximal or distal).

Comparison 6 DOAC versus heparin, Outcome 3 Symptomatic PE.

Comparison 6 DOAC versus heparin, Outcome 4 Total VTE (symptomatic and asymptomatic).

Comparison 6 DOAC versus heparin, Outcome 5 Asymptomatic DVT.

Comparison 6 DOAC versus heparin, Outcome 6 Asymptomatic proximal DVT.
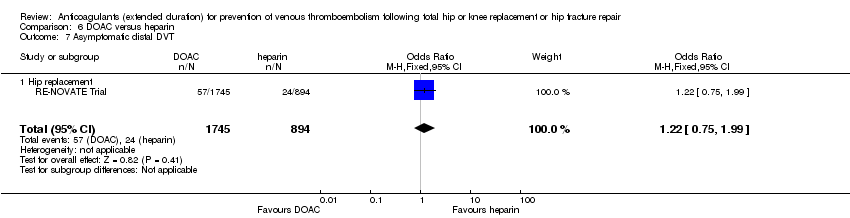
Comparison 6 DOAC versus heparin, Outcome 7 Asymptomatic distal DVT.

Comparison 6 DOAC versus heparin, Outcome 8 All‐cause mortality.

Comparison 6 DOAC versus heparin, Outcome 9 Adverse events.

Comparison 6 DOAC versus heparin, Outcome 10 Bleeding ‐ major.

Comparison 6 DOAC versus heparin, Outcome 11 Bleeding ‐ clinically relevant, non‐major.

Comparison 6 DOAC versus heparin, Outcome 12 Bleeding ‐ minor.

Comparison 6 DOAC versus heparin, Outcome 13 Reoperation.

Comparison 6 DOAC versus heparin, Outcome 14 Wound infection.
| Heparin compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with heparin | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.59 | 2329 | ⊕⊕⊕⊕ | — | |
| 33 per 1000 | 20 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.73 | 2019 | ⊕⊕⊕⊝ | — | |
| 24 per 1000 | 18 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.61 | 1595 | ⊕⊕⊝⊝ | — | |
| 6 per 1000 | 4 per 1000 | |||||
| Bleeding ‐ major | Study population | OR 0.59 | 2500 | ⊕⊕⊕⊝ | — | |
| 4 per 1000 | 2 per 1000 | |||||
| Clinically relevant non‐major bleeding | see comment | — | — | — | not reported | |
| Bleeding ‐ minor | Study population | OR 2.01 | 2500 | ⊕⊕⊕⊕ | — | |
| 46 per 1000 | 88 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, low number of events leading to imprecision of results | ||||||
| Vitamin K antagonists compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with vitamin K antagonists | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.10 | 360 | ⊕⊕⊕⊝ | — | |
| 23 per 1000 | 2 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.13 | 360 | ⊕⊕⊕⊝ | — | |
| 17 per 1000 | 2 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.32 | 360 | ⊕⊕⊕⊝ | — | |
| 6 per 1000 | 2 per 1000 | |||||
| Bleeding ‐ major | see comment | OR 2.89 | 360 | ⊕⊕⊝⊝ | no events recorded in placebo group | |
| Clinically relevant non‐major bleeding | see comment | — | — | — | not reported | |
| Minor bleeding | see comment | — | — | — | not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, results from a single study only so heterogeneity could not be assessed | ||||||
| DOAC compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with DOAC | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.20 | 2419 | ⊕⊕⊕⊝ | — | |
| 12 per 1000 | 3 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.18 | 2459 | ⊕⊕⊕⊕ | — | |
| 9 per 1000 | 2 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.25 | 1733 | ⊕⊕⊝⊝ | — | |
| 5 per 1000 | 1 per 1000 | |||||
| Bleeding ‐ major | Study population | OR 1.00 | 2457 | ⊕⊕⊝⊝ | — | |
| 1 per 1000 | 1 per 1000 | |||||
| Bleeding‐ clinically relevant non‐major | Study population | OR 1.22 | 2457 | ⊕⊕⊕⊝ | — | |
| 27 per 1000 | 33 per 1000 | |||||
| Bleeding ‐ minor | Study population | OR 1.18 | 2457 | ⊕⊕⊕⊝ | — | |
| 28 per 1000 | 32 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, results from a single study so heterogeneity cannot be assessed | ||||||
| Anticoagulants (chosen at investigators' discretion) compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with anticoagulant (chosen at investigators' discretion) | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.50 | 557 | ⊕⊕⊝⊝ | — | |
| 14 per 1000 | 7 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.33 | 557 | ⊕⊕⊝⊝ | — | |
| 11 per 1000 | 4 per 1000 | |||||
| Symptomatic PE | Study population | OR 1.00 | 557 | ⊕⊕⊝⊝ | — | |
| 4 per 1000 | 4 per 1000 | |||||
| Bleeding ‐ major | see comment | OR 5.05 | 557 | ⊕⊕⊝⊝ | no major bleeding recorded in the placebo groups | |
| Clinically relevant non‐major bleeding | see comment | — | — | — | not reported | |
| Minor bleeding | see comment | — | — | — | not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, results from a single study so heterogeneity could not be assessed | ||||||
| Vitamin K antagonists compared to heparin for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with heparin | Risk with vitamin K antagonists | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 1.64 | 1279 | ⊕⊕⊝⊝ | — | |
| 23 per 1000 | 38 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 1.36 | 1279 | ⊕⊕⊝⊝ | — | |
| 23 per 1000 | 31 per 1000 | |||||
| Symptomatic PE | see comment | OR 9.16 | 1279 | ⊕⊕⊝⊝ | no cases of symptomatic PE reported in the heparin study arm | |
| Bleeding ‐ major | Study population | OR 3.87 | 1272 | ⊕⊕⊝⊝ | — | |
| 16 per 1000 | 58 per 1000 | |||||
| Bleeding ‐ clinically indicated non‐major Treatment duration 28 ‐ 42 days | see comment | — | — | — | clinically indicated non‐major bleeding events not reported in single included study in this comparison | |
| Bleeding ‐ minor | Study population | OR 1.33 | 1279 | ⊕⊕⊝⊝ | — | |
| 20 per 1000 | 27 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, single study so heterogeneity could not be assessed | ||||||
| DOAC compared to heparin for people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with heparin | Risk with DOAC | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.70 | 15977 | ⊕⊕⊝⊝ | — | |
| 4 per 1000 | 3 per 1000 | |||||
| Symptomatic DVT (proximal or distal) Treatment duration 28 ‐ 42 days | Study population | OR 0.60 | 15977 | ⊕⊕⊝⊝ | — | |
| 3 per 1000 | 2 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.91 | 14731 | ⊕⊕⊕⊝ | — | |
| 2 per 1000 | 2 per 1000 | |||||
| Bleeding ‐ major Treatment duration 28 ‐ 42 days | Study population | OR 1.11 | 16199 | ⊕⊕⊕⊕ | — | |
| 8 per 1000 | 9 per 1000 | |||||
| Bleeding ‐ clinically relevant, non‐major | Study population | OR 1.08 | 15241 | ⊕⊕⊕⊕ | — | |
| 33 per 1000 | 36 per 1000 | |||||
| Bleeding ‐ minor Treatment duration 28 ‐ 42 days | Study population | OR 0.95 | 11766 | ⊕⊕⊕⊕ | — | |
| 66 per 1000 | 63 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for inconsistency (heterogeneity, I2 = 55%) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 5 | 2329 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.35, 1.01] |
| 1.1 Hip replacement | 4 | 1296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.30] |
| 1.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.92] |
| 1.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.06] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 4 | 2019 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.39, 1.38] |
| 2.1 Hip replacement | 4 | 1296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.55] |
| 2.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.12] |
| 3 Symptomatic PE Show forest plot | 3 | 1595 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.16, 2.33] |
| 3.1 Hip replacement | 3 | 872 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.56] |
| 3.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.24, 8.83] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 6 | 2544 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.28, 0.56] |
| 4.1 Hip replacement | 5 | 1511 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.25, 0.56] |
| 4.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.92] |
| 4.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.90] |
| 5 Asymptomatic DVT Show forest plot | 5 | 1304 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.24, 0.60] |
| 5.1 Hip replacement | 4 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.58] |
| 5.2 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.19, 1.52] |
| 6 All‐cause mortality Show forest plot | 5 | 2518 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.31, 3.26] |
| 6.1 Hip replacement | 4 | 1485 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.11, 2.75] |
| 6.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.65] |
| 6.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.69 [0.22, 98.42] |
| 7 Adverse events Show forest plot | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.64] |
| 7.1 Hip replacement | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.64] |
| 8 Bleeding ‐ major Show forest plot | 5 | 2500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.46] |
| 8.1 Hip replacement | 4 | 1494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.10] |
| 8.2 Knee replacement | 1 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.06] |
| 8.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Bleeding ‐ minor Show forest plot | 5 | 2500 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.43, 2.81] |
| 9.1 Hip replacement | 4 | 1494 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.53, 3.30] |
| 9.2 Knee replacement | 1 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.58, 2.59] |
| 9.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.11, 69.13] |
| 10 Reoperation Show forest plot | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hip replacement | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.94] |
| 1.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.94] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| 2.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| 3 Symptomatic PE Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.84] |
| 3.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.84] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| 4.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| 5 All‐cause mortality Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse events Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Bleeding ‐ major Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 71.31] |
| 7.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 71.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 2419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.68] |
| 1.1 Hip replacement | 1 | 2419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.68] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 2 | 2459 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.81] |
| 2.1 Hip replacement | 2 | 2459 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.81] |
| 3 Symptomatic PE Show forest plot | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.25] |
| 3.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.25] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.11, 0.33] |
| 4.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.11, 0.33] |
| 5 All‐cause mortality Show forest plot | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.66] |
| 5.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.66] |
| 6 Adverse events Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 6.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 7 Bleeding ‐ major Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.02] |
| 7.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.02] |
| 8 Bleeding‐ clinically relevant non‐major Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.76, 1.95] |
| 8.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.76, 1.95] |
| 9 Bleeding ‐ minor Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.74, 1.88] |
| 9.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.74, 1.88] |
| 10 Reoperation Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Wound infection Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.86] |
| 11.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.74] |
| 1.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.74] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.21] |
| 2.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.21] |
| 3 Symptomatic PE Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| 3.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 4.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 5 Asymptomatic DVT Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 5.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 6 Asymptomatic distal DVT Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 6.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 7 All‐cause mortality Show forest plot | 1 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Knee replacement | 1 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Bleeding ‐ major Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 105.76] |
| 8.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 105.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 1.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.69, 2.68] |
| 2.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.69, 2.68] |
| 3 Symptomatic PE Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.16 [0.49, 170.42] |
| 3.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.16 [0.49, 170.42] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 4.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 5 All‐cause mortality Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.24, 105.83] |
| 5.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.24, 105.83] |
| 6 Bleeding ‐ major Show forest plot | 1 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.87 [1.91, 7.85] |
| 6.1 Hip replacement | 1 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.87 [1.91, 7.85] |
| 7 Bleeding ‐ minor Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.64, 2.76] |
| 7.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.64, 2.76] |
| 8 Reoperation Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.60 [0.99, 21.38] |
| 8.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.60 [0.99, 21.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.28, 1.70] |
| 1.1 Hip replacement | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.28, 1.70] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.27] |
| 2.1 Hip replacement | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.27] |
| 3 Symptomatic PE Show forest plot | 5 | 14731 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.94] |
| 3.1 Hip replacement | 5 | 14731 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.94] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 4 | 12447 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.97] |
| 4.1 Hip replacement | 4 | 12447 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.97] |
| 5 Asymptomatic DVT Show forest plot | 2 | 6559 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.59] |
| 5.1 Hip replacement | 2 | 6559 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.59] |
| 6 Asymptomatic proximal DVT Show forest plot | 1 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.15] |
| 6.1 Hip replacement | 1 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.15] |
| 7 Asymptomatic distal DVT Show forest plot | 1 | 2639 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.75, 1.99] |
| 7.1 Hip replacement | 1 | 2639 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.75, 1.99] |
| 8 All‐cause mortality Show forest plot | 5 | 14966 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.64, 4.16] |
| 8.1 Hip replacement | 5 | 14966 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.64, 4.16] |
| 9 Adverse events Show forest plot | 3 | 9908 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 9.1 Hip replacement | 3 | 9908 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 10 Bleeding ‐ major Show forest plot | 5 | 16199 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 10.1 Hip replacement | 5 | 16199 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 11 Bleeding ‐ clinically relevant, non‐major Show forest plot | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.28] |
| 11.1 Hip replacement | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.28] |
| 12 Bleeding ‐ minor Show forest plot | 4 | 11766 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 12.1 Hip replacement | 4 | 11766 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 13 Reoperation Show forest plot | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.34, 3.24] |
| 13.1 Hip replacement | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.34, 3.24] |
| 14 Wound infection Show forest plot | 2 | 6446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.72] |
| 14.1 Hip replacement | 2 | 6446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.72] |

