Tratamientos psicosociales combinados con mantenimiento con agonistas versus tratamientos de mantenimiento con agonistas solos para la dependencia de opiáceos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial. Patients recruited from consecutive admissions to a large urban drug treatment centre; | |
| Participants | 180 opiate dependent (DSM‐III‐R), residing stably in USA, age 18 or older, eligible for MMT according FDA requirements. Exclusion Criteria: Acute psychosis, pregnancy, discharge from treatment at the centre within the past 6 months, grosses cognitive impairment. Analysis on 166 (1) N 103, (2) N 63. Average age 37; 69% men; 79% Hispanic; mean use of heroin 11.73 years, mean use of cocaine 1.43 years, mean problematic alcohol use 4.13 years; 27% married, 39% widowed, divorced or separated, 34% single; average years of educational level 11.51, 33% < high school, 51% high school, 16% > high school; 55% employed, 31% unemployed, 14% not employed due to recent incarceration; 23% referred by probation. | |
| Interventions | All MMT
Duration 8 months + 6 months follow‐up. | |
| Outcomes | Retention in treatment assessed using a survival analysis. No data on the groups. Use of primary substance of abuse as % of opiate negative urine samples and as number of participants opiate negative for three consecutive weeks. Severity of dependence as ASI (mean composite scores) and as Risk Assessment Battery | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to one of the three treatment groups by a permuted block design. Bloks were formed by five dichotomized control factors: gender, ethnicity, ASI drug abuse, ASI psychiatric severity, admission mandated by criminal justice system. |
| Allocation concealment (selection bias) | Unclear risk | Participants randomised within these blocks to balance the factors between treatment groups. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Research assistant, blind to treatment assignment, administered all assessment instruments. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Research assistant, blind to treatment assignment, administered all assessment instruments. |
| Incomplete outcome data (attrition bias) | Unclear risk | Of the 180 subjects who were randomly assigned, 166 were engaged in treatment. COMMENT: reason not given, not specified the number of subjects withdrawn from each group; the treatment outcome results are reported for the group which were followed for 6 months (91%). |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Patients recruited in a Methadone Maintenance Unit. Groups similar for drug use and demographic data. | |
| Participants | 14 opiate dependent in MMT Average age 28; 87% men; 47% African‐American, 53% White, mean number of previous treatments 2,5; mean use of drugs/alcohol 8 years; months of methadone maintenance treatment 9; 29% married; 64% high school; 100% history of criminal charges. | |
| Interventions | All MMT, no information on doses
| |
| Outcomes | Use of primary substance as % of contaminated samples. Compliance as group attendance (scores). Psychiatric symptoms/psychological distress as: Internal‐External Locus of Control, Interpersonal Trust, State‐Trait Anxiety, Social Desirability, Depression, Assertion, Pleasant Events (all scores). Quality of life as % employed and/or academically involved. | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants who completed the baseline test materials were randomly assigned to either the CB group or a nondirective methadone maintenance group. |
| Allocation concealment (selection bias) | Unclear risk | Participants who completed the baseline test materials were randomly assigned to either the CB group or a nondirective methadone maintenance group. |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawal from the study |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled study. Recruitment modality: patients who agreed to participate from 251 eligible consecutive admission to a inner city methadone maintenance programs | |
| Participants | 220 opiate dependent seeking methadone treatment Incl criteria: at least 18 years old, injecting drug users, not active suicidal, homicidal or psychotic. 24% of the sample first time in methadone treatment. Mean Age (1)37.8 (2)36.0 years; Males (1)67% (2)70%; Race: White (1)66.7% (2)65.2%, African American (1)16.7% (2)14.3%, Hispanic (1)16.7% (2) 18.8%, Other minority (2)9%; Mean years of education (1)11.9 (2) 11.7; Employed full time (1)13.9% (2)18.8%; Mean years of opiate use (1)12.8 (2)12.3; cocaine users (1)79% (2)72%; Mean years of cocaine use (1)12.1 (2)11.5. | |
| Interventions | All MMT average dose 85.5 mg/day;
Duration 12 weeks. | |
| Outcomes | Retention in treatment, use of opiates as at least three weeks with drug free urine, compliance | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to one of two treatment conditions using a computerized randomisation program |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned to one of two treatment conditions using a computerized randomisation program |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | All primary outcome analysis were conducted on the intention to treat sample defined as patients who attended at least one treatment session |
| Selective reporting (reporting bias) | Low risk | |
| Methods | randomised controlled trial | |
| Participants | 135 volunteer outpatients who met the DSM‐IV criteria for opioid dependence; mean age: 29 years; male: 56% | |
| Interventions | Buprenorphine maintenance for all patients: maintenance dose of either 6, 12, or 18
Duration of the intervention: 23 weeks | |
| Outcomes | retention in treatment, opioid and cocaine abstinence | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: patients were randomly assigned to one of the three maintenance treatments using a computer generated stratified randomisation procedure" |
| Allocation concealment (selection bias) | Unclear risk | information not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | drop out did not significantly differed across conditions |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: "study participants were opioid‐dependent patients new admitted to an outpatient treatment program in Baltimore | |
| Participants | 127 opioid dependent (DSM III‐R); Mean age 38.2 years, males 46%, White 37%, African‐American 63%; Current married 11%; average educations 11.4 years; unemployed 75%; almost 50% current diagnosis of cocaine dependence | |
| Interventions | For all MMT mean dose across the study 60 mg/day
Duration 6 weeks | |
| Outcomes | Treatment response, counselling attendance; use of substances | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to the MSC or the SSC control condition, after being stratified on baseline rates of cocaine positive urine specimens and lifetime psychiatric comorbidity status |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned to the MSC or the SSC control condition, after being stratified on baseline rates of cocaine positive urine specimens and lifetime psychiatric comorbidity status |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information on drop out from the study not given |
| Selective reporting (reporting bias) | High risk | Data on retention in treatment not reported |
| Methods | Randomised controlled trial. Recruitment modality: not described | |
| Participants | 24 opioid dependent (DSM IV) with positive urine toxicologic test, aged 18‐65 years. Excl Cr: Alcohol or benzodiazepines dependence, greater that three times normal liver enzymes, current suicide/homicide risk, current psychotic disorder, major depression, life‐threatening or unstable medical problem. | |
| Interventions | For all BMT range 12‐16 mg/day.
Duration 10 weeks. | |
| Outcomes | Retention in treatment; use of opiates as long period of abstinence in weeks | |
| Notes | Country of origin: Malaysia. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using a computer‐generated simple randomisation procedure to either standard services or enhanced services |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned using a computer‐generated simple randomisation procedure to either standard services or enhanced services |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | Only one participant in the standard service group drop out from study |
| Selective reporting (reporting bias) | Low risk | |
| Methods | randomised controlled trial | |
| Participants | 37 heroin dependents patients enrolling in two MMT clinics; mean age: 36.5; male: 81% | |
| Interventions | for all MMT 45 mg daily
duration of intervention: three months | |
| Outcomes | retention in treatment, use of substance of abuse | |
| Notes | Country of origin: China, Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: " patients were randomly assigned to condition using a simple randomisation procedure: a computer generated randomisation list in the US was used and randomisation codes were provided to the research personnel in China on the day of randomisation" |
| Allocation concealment (selection bias) | Low risk | quote: "a computer generated randomisation list in the US was used and randomisation codes were provided to the research personnel in China on the day of randomisation" |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Unclear risk | no significant difference in drop out rate between groups |
| Selective reporting (reporting bias) | Low risk | |
| Methods | randomised controlled trial | |
| Participants | 120 opioid dependent patients; mean age: 31 years, male: 58%, | |
| Interventions | For all buprenorphine maintenance . doses of 12‐18 mg daily
duration of the intervention: 12 weeks | |
| Outcomes | retention in treatment;continuous week of abstinence | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: " participants were randomly assigned to one of three treatment grups using minimum likelihood allocation (Aickin 1982). This method of permutation has been shown to achieve balance between treatment groups on patients characteristics likely to influence treatment outcome. Three characteristics were used to stratify patients: stabilization dose of buprenorphine, cocaine use in the past month, distance from the clinics |
| Allocation concealment (selection bias) | Unclear risk | information not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | High risk | "the proportions of patients completing the trial was significantly different among groups; |
| Selective reporting (reporting bias) | Low risk | |
| Methods | randomised controlled trial | |
| Participants | 82 opioid dependent patients admitted to a private, for profit methadone maintenance clinic; mean age: 40 years; male: 70%; African American: 16%, Hispanic: 63%, white: 21% | |
| Interventions | For all methadone maintenance;
| |
| Outcomes | opiate use | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quote: " patients were randomly assigned to condition" |
| Allocation concealment (selection bias) | Unclear risk | information not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Unclear risk | information not reported |
| Selective reporting (reporting bias) | High risk | no information on retention in treatment |
| Methods | randomised controlled trial | |
| Participants | 252 heroin and cocaine abusing patients; mean age: 38 years, male: 48%, African American: 66% | |
| Interventions |
Duration of the intervention: 12 weeks | |
| Outcomes | retention in treatment, use of substance | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: " participants were first randomised to contingency management condition by a study technician who used a Microsoft Excel macro that stratified randomisation by race, sex, employment status, probation status, frequency of opiate and cocaine positive urine during baseline. Participants were then randomised to a dose condition using a similar macro with identical stratification variables" |
| Allocation concealment (selection bias) | Low risk | quote "randomisation was done by an investigator who had no contact with participants. Dose assignment were known only to her and to a in‐house pharmacy staff who also had no contact with participants" |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | no significant difference in drop out rate among conditions |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: not described | |
| Participants | 166 opioid dependent (DSM IV) that met criteria for opioid agonist maintenance treatment. Excl Cr:dependent on alcohol, benzodiazepines, sedatives; dangerous to themselves or others; psychotic or with major depression; unable to comprehend English; life‐threatening medical problem; women of childbearing age agreed to use contraception and undergo monthly pregnancy monitoring Age (1) 35.1(2)36.4; White 127; full employed 79; high school graduate 134; never married 95. | |
| Interventions | For all BMT+naloxone combination (tablets which include buprenorphine and naloxone in a 4:1 ratio, average dose of buprenorphine 16 mg/day.
Duration 24 weeks | |
| Outcomes | Retention in treatment, use of opiate, use of cocaine, patient satisfaction, adherence to pharmacological treatment | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An urn randomisation procedure were used to ensure that the groups were similar with regard to sex ratio, employment status, presence of cocaine abuse and presence of personality disorders |
| Allocation concealment (selection bias) | Unclear risk | An urn randomisation procedure were used to ensure that the groups were similar with regard to sex ratio, employment status, presence of cocaine abuse and presence of personality disorders |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | High drop out rate. reason for drop out given for each group. drop out patients balanced among groups |
| Selective reporting (reporting bias) | Low risk | |
| Methods | randomised controlled trial | |
| Participants | 116 heroin and cocaine users admitted to methadone maintenance treatment.Mean age: 37 years, male 56%; African American : 47% | |
| Interventions | for all methadone maintenance ; daily maintenance dose ranged from 70 to 100 mg, adjusted based on feedback from the
duration: 25 weeks | |
| Outcomes | retention in treatment | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quote "subjects were randomly assigned to experimental or control group: Randomisation was stratified by race, sex, employment status, probation status, frequency of opiate and cocaine positive urine at baseline" |
| Allocation concealment (selection bias) | Unclear risk | information not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | analysis done by the intention to treat principle |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial . Recruitment modality: "patients were recruited via a variety of advertisements" | |
| Participants | 60 opioid dependent (DSM‐UIV), 18 years or older, in good health, met criteria for agonist maintenance treatment. Exc Cr: evidence of acute psychosis or serious medical illness; pregnancy. Males 33; Mean age 32.5 years; White 90% | |
| Interventions | For all BMT 8‐16 mg/day;
Duration 12 weeks | |
| Outcomes | Retention in treatment; use of opiates as weeks of continuous abstinence, use of cocaine, ASI scores | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to one of three treatments groups using minimum‐likelihood allocation. This method was designed to achieve balance between treatment groups on patients characteristics likely to influence outcomes. Five characteristics were used to stratify patients: buprenorphine maintenance dose, history of injection use, gender, prior history of buprenorphine treatment, presence or not of cocaine use |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned to one of three treatments groups using minimum‐likelihood allocation. This method was designed to achieve balance between treatment groups on patients characteristics likely to influence outcomes. Five characteristics were used to stratify patients: buprenorphine maintenance dose, history of injection use, gender, prior history of buprenorphine treatment, presence or not of cocaine use |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | 20% of drop out. Reason for withdrawn not given. "there were no significant difference in the percentages of patients who completed the trial (X2: 1.6; p= 0.49) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: patients who received methadone for at least 60 days and who had used opiates during that time were recruited from one of three community‐based methadone clinics. | |
| Participants | 124 opioid dependent (DSM IV), who had received MMT for at least 60 days and who had used opiates during that time. Exc Cr: current DSM IV diagnosis of schizophrenia, schizoaffective disorder, psychosis not other specified, bipolar affective disorder, imminent criminal justice proceedings that might result in incarceration during the treatment. Males 49%; ethnic minorities 13%; on average 42.2 years old; single 72%; unemployed 60%; mood disorder 40%; anxiety disorder 42%; dependent on alcohol 35%; cocaine 46%; other drugs 35%. | |
| Interventions | For all MMTdoses not reported;
Duration 16 weeks | |
| Outcomes | Retention in treatment, use of opiates as number of subjects with urine negative at the end of treatment and at follow up; psychiatric symptoms/psychological distress measured with Beck Depression InventorY and with Symptom Check List‐90 | |
| Notes | Country of origin: USA. Setting: outpatients The participants in the Standard care Group (n. 38) are considered both in arm a and in arm b | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly assigned in sequential waves of three to MM, ACT, ITSF |
| Allocation concealment (selection bias) | High risk | Participants were randomly assigned in sequential waves of three to MM, ACT, ITSF |
| Blinding of outcome assessment (detection bias) | Low risk | All assessment were carried out by a team of assessors blind to the treatment condition of participants |
| Blinding of outcome assessment (detection bias) | Low risk | All assessment were carried out by a team of assessors blind to the treatment condition of participants |
| Incomplete outcome data (attrition bias) | Low risk | At post, outcome data were available for 57%, 59% and 74% of each group, a non significant difference (Pearson X2 2.76, p:ns). At follow up, outcome data were available for 43%, 57% and 68% of each group, which is also non significant, but barely so (Pearson X2 5.49, p:<0.07) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality: all patients admitted at the methadone maintenance treatment at a clinic. Groups similar for drug use and demographic data. | |
| Participants | 103 opiate dependent at least 1 year of opiate use. Ex C: Significant medical condition, symptoms of active psychosis, involved in drug treatment within the past month. Average age 36; 63% men; 85% White, 12% African‐American; 3% Hispanic; mean use of heroin 5.8 years; 33% married, 17% widowed, divorced or separated, 50% single; average years of educational level 11.4; 34% employed full time, 5% employed part time, 57% unemployed, 1% home workers, 1% retired, 2% disabled. | |
| Interventions | For all MMT, subjects stabilized for 6 weeks on methadone than randomised, (no information on doses), plus regularly scheduled individual counselling sessions along with a system of privilege levels for determining take home medication eligibility
Duration 12 weeks plus 12 weeks follow‐up. | |
| Outcomes | Results at follow‐up as % of dropouts at the end of follow‐up. Use of primary substance of abuse as % of drug‐free urine samples. Compliance as clinic Attendance (mean number of counselling sessions attended). | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to one of the three treatment protocol |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned to one of the three treatment protocol |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | High risk | Total attrition at the end of the study was 22%9 for the STD group and 33.3% for the UA group. COMMENT: high rate of drop out and unbalanced between group |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality: drawn from the outpatient methadone clinic. | |
| Participants | 37 opiate dependent, receiving maintenance doses of methadone for no more than 2 weeks. Average age 29.5; 100% men; 43% not White; mean use of heroin 7.7 years; 65% high school; 32% employed. | |
| Interventions | For all MMT, mean dose 39.5 mg/day plus routine clinic counselling and ancillary therapies.
Duration 15 sessions + 1 month follow‐up | |
| Outcomes | Retention in treatment as % of participants that completed all 15 sessions of treatment. Results at follow‐up as no. of participants relapsed at 1 month only on participants that completed the 15 session, and as no. of participants in MMT at 1 month. Psychiatric symptoms/psychological distress as scores. | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Those interested in participating were randomly assigned to either an experimental group or a control group |
| Allocation concealment (selection bias) | Unclear risk | Those interested in participating were randomly assigned to either an experimental group or a control group |
| Blinding of outcome assessment (detection bias) | Low risk | The double blind design ensured that neither the patients nor those running the study were aware of subject's experimental status |
| Blinding of outcome assessment (detection bias) | Low risk | The double blind design ensured that neither the patients nor those running the study were aware of subject's experimental status |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop out was high in both group: 54% in the experimental condition and 38% in the control condition but difference was not significant P:0.3. COMMENT: small sample, perhaps the test had low power to detect significant difference |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality: "cocaine abusing opiate dependent patients seeking opiate maintenance recruited from the general Greater New haven area" | |
| Participants | 80 opioid and cocaine dependent (DSM IV). Exc Cr: pregnancy, cardiac conduction problems, acute hepatitis, current suicidality or psychosis, inability to read or understand symptom check list, current alcohol or sedative dependence, use of medications that interact with study medication; women of childbearing age agreed to use contraception and undergo monthly pregnancy monitoring. Males 51; aged 21‐65; Withe 39, African American 30, Other 11; High school 54 | |
| Interventions | For all BMT 8‐12 mg/day plus desipramine 150 mg/day
Duration 6 weeks | |
| Outcomes | Retention in treatment | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation to one of four treatment conditions |
| Allocation concealment (selection bias) | Unclear risk | Simple randomisation to one of four treatment conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified |
| Incomplete outcome data (attrition bias) | Low risk | High rate of drop out. reason for drop out given. "49% of patients completed the trial, which did not differ among the four treatment groups ( Wilcoxon 0.4; p: ns) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: "patients recruited at three methadone clinics. Recruitment occurred via several means including referral from counsellors, visits made by research assistant to ongoing drug counselling groups and medication lines, referrals from mothers who had already participated in the study" | |
| Participants | 61 heroin addicted mothers. mothers had to have children less than 16 years of age and report subjective experience of problems with parenting. Exc Cr: cognitive deficit, psychotic thought process, suicidality. Single (1)63%, (2)70%; Caucasian (1)78%, (2)65%; African American (1)10%, (2)30%; Hispanic (1)12%, (2)5%. | |
| Interventions | For all MMT, doses not reported;
Duration 6 months | |
| Outcomes | Retention in treatment | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mothers were randomised to either the RPMG or comparison group |
| Allocation concealment (selection bias) | Unclear risk | Mothers were randomised to either the RPMG or comparison group |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | 15% of drop out. Reason for withdrawal given. Drop out balanced between groups |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: "patients who applied to the study were either referred by their primary methadone treatment counsellors (about 85%) or were self referred (about 15%). | |
| Participants | 168 opiate dependents. Incl Cr: Unemployed, stabilized on appropriate methadone dose, opiate negative urine, absence of any condition that would preclude any work at all (i.e. severe mental illness, severe physical problem, willingness to enter in the study. Males 58%; Minority group 75%; average age 45 years; in MMT on average of 5 years; never married 47% | |
| Interventions | For all MMT doses not reported;
Duration 12 months; Follow up 12 months | |
| Outcomes | Competitive and or informal employment, 6 and 12 months; any paid employment 6 and 12 months | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A total of 213 patients were randomised during the study period". "Consenting subjects were randomised to either the CES Vocational model or the clinic standard vocational counselling |
| Allocation concealment (selection bias) | Unclear risk | A total of 213 patients were randomised during the study period". "Consenting subjects were randomised to either the CES Vocational model or the clinic standard vocational counselling |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Unclear risk | 45 patients out of 213 randomised drop out for reason explained in the text; but it is not specified how many participants dropped out from each group |
| Selective reporting (reporting bias) | High risk | Retention in treatment, a measure usually utilized in drug addiction trial, not reported for each group |
| Methods | cluster randomised controlled trial; randomisation by pharmacy | |
| Participants | 77 pharmacists and 542 opioid dependent patients ; mean age 32.5 years, male: 64 % | |
| Interventions | motivational intervention delivered by pharmacists: 40 pharmacies, 295 participants control: no intervention: 36 pharmacies, 247 participants | |
| Outcomes | retention in treatment, substance use, psychological and physical health | |
| Notes | Country of origin: Scoltland; setting: outpatients; the full text of the study was obtained trough a correspondence with the first author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quote: " Pharmacies were then randomised to intervention or control groups by the Health Services Research Unit in the University of Aberdeen (independent of the study team)." |
| Allocation concealment (selection bias) | High risk | quote: " Pharmacies were then randomised to intervention or control groups by the Health Services Research Unit in the University of Aberdeen(independent of the study team)." But " Following randomisation, three pharmacies in the control group (each with one pharmacist) said they would only take part in the intervention group and four pharmacies (each with one pharmacist) in the intervention arm said they would only take part as controls (because they could not attend training)." |
| Blinding of outcome assessment (detection bias) | Low risk | outcome assessed by the researcher. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | outcome assessed by the researcher. |
| Incomplete outcome data (attrition bias) | Low risk | Drop out balanced between groups. 38% of participants did not completed the follow up questionnaire in each group |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: sample drawn from patients admitted to a methadone maintenance clinic. | |
| Participants | 92 opiate dependent. Ex C: Need for medical or psychiatric hospitalisation at the time of admission, plan for an imminent move from the Philadelphia area .Average age 41; 100% men; 74% African‐American; 27% married; average years of educational level 12; 47% employed; mean use of heroin 11 years, mean use of cocaine 3 years, mean problematic alcohol use 7 years. | |
| Interventions | For all MMT, 60 to 90 mg/day.
Duration 24 weeks. | |
| Outcomes | Use of primary substance of abuse as % of opiate positive urine samples and as % of subjects with opiate free urine samples per 8, 12, 16 consecutive weeks. Use of other drugs as % of cocaine positive urine samples. | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Following the orientation period, patients were randomly assigned to one of the three intervention |
| Allocation concealment (selection bias) | Unclear risk | Following the orientation period, patients were randomly assigned to one of the three intervention |
| Blinding of outcome assessment (detection bias) | Low risk | The pretreatment and post treatment evaluation were performed by project technician who were independent of the treatment process |
| Blinding of outcome assessment (detection bias) | Low risk | The pretreatment and post treatment evaluation were performed by project technician who were independent of the treatment process |
| Incomplete outcome data (attrition bias) | Low risk | 10% of the all sample drop out; withdrawn from the study balanced between groups |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: patients on methadone maintenance treatment | |
| Participants | 74 opiate dependent in program for 90 days and had verifiable narcotic addiction history of 2 years. Age range between 21‐54; 82% men; 48% White; 52% African‐American. | |
| Interventions | For all: MMT, no information on doses
Duration 7 weeks, follow‐up at 2 months. | |
| Outcomes | Retention in treatment as participants attended 14 consecutive weeks (7 before and then the contingency). Use of primary substance of abuse as % of opiate negative urine samples and as number of patients who met 7 consecutive clean urine samples before and after contingency. | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to group I or II by a coin toss |
| Allocation concealment (selection bias) | High risk | In four cases a husband and wife were randomly assigned as a unit rather than individually |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified..COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawn from the study |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality: "patients recruited from the Addiction Treatment Services program" | |
| Participants | 100 opioid dependent and Antisocial Personality Disorder (DSM IV). Exc Cr: pregnancy, bipolar disorder, schizophrenia. Male 77%; Mean age 39; Caucasian 40%; Married 12%; Average years of education 10.7; Employed 34% | |
| Interventions | For all MMT mean 55 mg/day;
The contingent intervention was highly structured designed to reinforce abstinence and adherence to scheduled counselling sessions. The protocol incorporated 9 steps to provide rapid delivery of predictable and increasingly positive consequences for attendance and abstinence (step +1 to +4) and increasingly negative consequences for missed counselling sessions and ongoing drug use (step ‐1 to ‐4). Duration 14 weeks | |
| Outcomes | Retention in treatment, compliance, use of substances, ASI | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were stratified on race, gender, baseline urine results, presence of other psychiatric diagnoses and therapist assignment and were randomised to one of two treatment conditions. COMMNENT: authors state that they stratified patients but do not described how they randomised people within each strata |
| Allocation concealment (selection bias) | Unclear risk | Participants were stratified on race, gender, baseline urine results, presence of other psychiatric diagnoses and therapist assignment and were randomised to one of two treatment conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor not blinded. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessor not blinded |
| Incomplete outcome data (attrition bias) | Low risk | High drop out rate. Reason for withdrawn from the study not given. Drop out rate balanced between group (55% vs 43%; OR: 1.62, CI95% 0.74‐3.58, p: ns) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality: "cocaine abusing opioid dependent patients seeking opioid maintenance treatment were recruited from the greater New haven area" | |
| Participants | 140 opioid and cocaine dependents (DSM IV). Exc Cr: pregnancy, respiratory conditions such as asthma, abnormal liver enzyme level, use of other drugs that interact with LAAM, current diagnosis of other drugs dependence, history of major psychiatric disorders (psychosis, schizophrenia, bipolar), current suicidality and inability to read and understand the consent form; women of childbearing age agreed to use contraception and undergo monthly pregnancy monitoring. Age 21‐55; females 45; Africa American 39%, Hispanic 10%, Caucasian 91% | |
| Interventions | For all LAAM maintenance (range 30‐130 mg/three times a week), N= 70 Contingency Management (voucher for opiate and cocaine free urine); N= 70 Standard treatment. Duration 12 weeks | |
| Outcomes | Retention in treatment, use of substances, withdrawal and depression symptoms | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised by sex to one of the four treatment condition |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised by sex to one of the four treatment condition |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified..COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified.. |
| Incomplete outcome data (attrition bias) | Low risk | The intent to treat sample of 140 participants were used for the statistical analysis. In any case reason for premature termination of the studies are given and group are balanced for drop out rate ( log rank: 2.77;p:<0.44) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality: "patients were recruited from six methadone maintenance community treatment programs that were members of the Clincal Trial Network" | |
| Participants | 388 stimulant abusing patients enrolled in MMT for at least one month and no more than three years. Exc Cr: recovery for gambling problems (because the potential similarity between gambling and the prize draw incentive procedure). Participants were enrolled from 6 MMT sites and their characteristics are described for each site. | |
| Interventions | For all MMT from 67.9 to 108 mg/day; N= 190 Contingency Management (chance to win prizes for free urine); N= 108 Standard care Duration 12 weeks; follow up 6 months | |
| Outcomes | retention, drug use, incentives earned | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Results of the first urine sample were used to stratify patients according to two variables: presence or absence of a stimulant drug, presence or absence of opioids. Stratification and random assignment were conducted independently at each site and accomplished by a computer program using a dynamic balanced randomisation procedure. |
| Allocation concealment (selection bias) | Low risk | Research staff did not know the randomisation sequence, but were aware of individual group assignment |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified..COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | High drop out rate. Reason for withdrawn not given. "the decline in study retention over time was virtually identical for the two groups" |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial: Recruitment modality: not described | |
| Participants | 77 cocaine dependents in MMT treatment (DSM IV) on stable dose of methadone and English speaking. Exc Cr: severe dementia, active uncontrolled psychosis or bipolar disorder, recovery for pathological gambling (because the potential similarity between gambling and the prize draw incentive procedure). Men (1)27.5%, (2) 27%; mean age (1)40, (2) 39; mean years of education (1)10.5, (2) 10.9; Hispanic (1)47.5%, (2)43.2%; African America (1)37.5%, (2) 32.4%; Cucasian (1)15%, (2)24.3%; full employed (1)27.5%, (2)21.6%; never married (1)62.5%, (2)73%. | |
| Interventions | For all MMT (1)71.5 mg/day, (2) 78.4 mg/day;
Duration 12 weeks | |
| Outcomes | Retention in treatment, compliance as N. of therapy sessions attended | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimum likelihood allocation was used to randomise patients to condition. Group were allocated on the following variables: gender, race, age (less than 35), presence of cocaine negative samples in three months prior the study initiation, attendance at more than three groups in the three months prior the study initiation |
| Allocation concealment (selection bias) | Unclear risk | Minimum likelihood allocation was used to randomise patients to condition. Group were allocated on the following variables: gender, race, age (less than 35), presence of cocaine negative samples in three months prior the study initiation, attendance at more than three groups in the three months prior the study initiation |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified.. |
| Incomplete outcome data (attrition bias) | Low risk | Few drop out from the study. reason for withdrawn given. Drop out balanced between groups |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality: not described | |
| Participants | 74 opiate and cocaine dependents (DSM IV) in MMT, 18 years or older, spoke English Exc Cr: psychotic disorder (schizophrenia, bipolar), current suicidal, recovery for pathological gambling (because the potential similarity between gambling and the prize draw incentive procedure). Male 52; average age of education 12%; current married 2%; employed full or part time 11% European American 16%; African American 34%; Hispanic American 23%; Other 1%.; | |
| Interventions |
Duration 12 weeks | |
| Outcomes | Retention in treatment, use of drugs, adverse events | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned to one of the three conditions. A computer urn randomisation procedure balanced groups on gender, ethnicity, employment status and baseline cocaine results |
| Allocation concealment (selection bias) | Unclear risk | Participants randomly assigned to one of the three conditions. A computer urn randomisation procedure balanced groups on gender, ethnicity, employment status and baseline cocaine results" |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified..COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified.. |
| Incomplete outcome data (attrition bias) | Low risk | Data analysis were conducted on an intent to treat basis. The proportion of patients drop out from treatment were not significantly different among groups ( X2: 2.55; p: 0.28) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recrutiment modality: subjects selected from 285 patients consecutively admitted to a methadone maintenance program . Factorial design | |
| Participants | 120 opiate dependent Ex C: Current major psychiatric illness, unstable serious medical illness, current physical dependence on alcohol or benzodiazepines. Age between 18 and 65 years, eligible for MMT according to FDA requirements. (1)29 (2)31 (3)32 (4)28. Average age 37.6; 67.5% men; 42% African‐American, 58% White; mean use of heroin 12.6 years; 17% married, 41.5% widowed, divorced or separated, 41.5% never married; 28% employed full time, 14% employed part time 58% unemployed. | |
| Interventions | For all MMT, 50 mg/day dose constant plus 1 session of counselling per week.
Duration, 13 weeks (5 weeks baseline + 8 weeks intervention). | |
| Outcomes | Retention in treatment as number of participants completing 8 week intervention. Use of primary substance of abuse as % of opiate negative urine, % of patient abstinent on 39 successive urine test as graph, number of consecutive opiate negative urine samples, self‐ reported opiate use as mean frequency per day. Craving as self‐reported opiate craving as scores. Quality of life as positive lifestyle changes and criminal activity (scores). Use of other drugs as graph. | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Two studies were conducted concurrently: one on cocaine dependent patients and one on heroin dependent. Patients who met the criteria for both studies were randomised to one of them by a coin toss. This was followed by assignment to contingent or non contingent; the first 10 patients were assigned to contingent vouchers yo allow for yoking of non contingent patients. Thereafter, patients were assigned to a voucher condition by coin flip. Dose randomisation was then conducted separately for contingent and non contingent group by the study pharmacist, using a random number table |
| Allocation concealment (selection bias) | High risk | The first 10 patients were assigned to contingent vouchers yo allow for yoking of non contingent patients. Thereafter, patients were assigned to a voucher condition by coin flip. Dose randomisation was then conducted separately for contingent and non contingent group by the study pharmacist, using a random number table. COMMENT: not concealed for contingent, non contingent assignment, concealed for dose increase, not dose increase assignment. Because the objective of the review is to assess the effect of psychosocial intervention (i.e. contingent vs non contingent) the study has been considered with high risk of bias for this comparison |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified..COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified.. |
| Incomplete outcome data (attrition bias) | Low risk | "93.3% of participants completed the intervention. No significant between group differences were noted in retention" |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: patients members of the New have methadone maintenance program. Groups significantly different for race. | |
| Participants | 72 methadone maintained opiate addicts, in treatment for a minimum of 6 weeks, Exc Cr: Schizophrenic and manic patients Current psychiatric disorder or a personality disorder.57%; age over 27; men 61%; White 58%; single, divorced or separated 61%; high school 95%; employed full time 50%. . | |
| Interventions | For all MMT, no information on doses, daily contact with the clinic, monitoring of urine for illicit substance use and mandatory weekly 90‐min group psychotherapy sessions as minimal core components of the treatment plan.
Duration 6 months. | |
| Outcomes | Retention in treatment as number of voluntary and of symptomatic failure drop outs, number completed. Use of primary substance of abuse as number of urine positive samples. Psychiatric symptoms/psychological distress as scores. | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to one of the two treatment |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned to one of the two treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Clinical evaluators were blind to the treatment the subjects were receiving and the patients were instructed not to inform the raters of the treatment received |
| Blinding of outcome assessment (detection bias) | Low risk | Clinical evaluators were blind to the treatment the subjects were receiving and the patients were instructed not to inform the raters of the treatment received |
| Incomplete outcome data (attrition bias) | Low risk | High drop out rate in both group. But reason for attrition clearly described. Because of the large number of early drop out, it was possible that the two groups were no longer comparable. To evaluate this, demographic characteristics, diagnosis and a range of variables that might predispose to terminate early(symptoms, drug use, legal history) were compared between the two remaining group. The two group remained similar except for ethnic composition |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality:"subjects recruited from individuals seeking MMT ay the urban centre for the assignment of heroin addicts to various MMT clinics and general practitioners" | |
| Participants | 73 opiate addicts Male 53; mean age 30 years; duration of dependence mean 7 years; additional psychiatric disorder 71%; additional current addiction or poly drug dependence 83%; employed 66%; single 22%. | |
| Interventions | For all MMT
Duration 6 months and 6 months follow up | |
| Outcomes | Retention in treatment | |
| Notes | Country of origin: Germany. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant was randomly allocated to one of two group by flipping a coin |
| Allocation concealment (selection bias) | Unclear risk | Each participant was randomly allocated to one of two group by flipping a coin |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified. COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | The analysis was made according to the intention to treat principle (ITT), meaning that the data of all included studies were analysed, whether they had completed the study or not. Missing data at months 6 and 12 were substituted by the last available urine samples |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: "patients selected from newly admitted patients to a methadone treatment program" | |
| Participants | 78 opiate dependents, 18‐50 years old Excl Cr: pregnant women, serious psychiatric illness Mean age (1)39.3, (2)40.9; Men (1)50%, (2)65%; African American (1)71%, (2)69%; White (1)29%, (2)31%; 12 years of education (1)46%, (2)38%; married (1)15%, (2)15%; employed (1)15%, (2)23% | |
| Interventions | For all MMT mean 60 mg/day;
Duration 24 weeks | |
| Outcomes | Retention in treatment, use of opiates as longest duration of abstinence, compliance as mean hours of counselling received | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified for five variables: urine sample positive for cocaine, urine sample positive for opiates, criteria for antisocial personality disorders, full time employment, race . A computerized random number generator accomplished random assignment |
| Allocation concealment (selection bias) | High risk | The same staff person identified potential participants as eligible, stratified and randomly assigned them and introduced them to their study condition |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified.COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | Low risk | High drop out rate. Reason for withdrawn given. "There were no significant difference among groups in study retention" |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. recruitment modality: all patients admitted to methadone maintenance treatment during the recruitment period and eligible. | |
| Participants | 53 opiate dependent eligible for MMT. Average age 34; 72% men; 66% White; mean use of heroin 15 years; 23% married; 34% employed; 77% high school; 40% legally free, 38% on probation, 22% pending trial. Ex C: Psychiatric and behavioural problem. | |
| Interventions | For all MMT, mean dose 51.4 mg/day, all stabilized for 12 weeks and then randomised plus counselling session 1 per week.
Duration 6 months. | |
| Outcomes | Retention in treatment as n. of drop‐outs. Use of primary substance of abuse as % of positive urine and as % of subjects with at least 12 consecutive free urine. | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were stratified for gender and race and than randomly assigned to one of two condition. COMMNENT: authors state that they stratified patients but do not described how they randomised people within each strata |
| Allocation concealment (selection bias) | Unclear risk | Participants were stratified for gender and race and than randomly assigned to one of two condition |
| Blinding of outcome assessment (detection bias) | Low risk | Blindness of outcome assessor not specified.COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blindness of outcome assessor not specified. |
| Incomplete outcome data (attrition bias) | High risk | Final attrition included 38% of patients from the contingent group and 26% form the non‐contingent group. COMMENT: reason from drop out not given. High rate of drop out. Unbalanced between group |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality:not described. Groups similar for drug use and demographic data. | |
| Participants | 47 opiate dependent in MMT. Average age 38; 100% men; 34% Hispanic, 21% White, 45% African‐American; 47% participated in previous treatment program. | |
| Interventions | All MMT.
Duration 6 weeks. | |
| Outcomes | Retention in treatment as no. of drop‐outs. Use of primary substance of abuse as chi square results on data and as no. of participants opiate‐free at 2,3 weeks. Psychiatric symptoms/psychological distress as positive dream feelings (chi square results) and as results to a questionnaire designed by the authors to tap their own assessment of changes (scores and chi square results). Results at follow‐up as no. of participants with negative urine samples at 2 and 3 weeks post‐experimental period presented as chi square statistical analysis results. | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were assigned to the experimental or control group n a random fashion, except for an attempt to keep relatively balanced the average age and racial distribution of the two group |
| Allocation concealment (selection bias) | High risk | Participants were assigned to the experimental or control group on a random fashion, except for an attempt to keep relatively balanced the average age and racial distribution of the two group |
| Blinding of outcome assessment (detection bias) | Low risk | In a large number of prior studies under the same conditions, no subjects could recognize the content of the stimulus. Since the experimenters were also blind , the current study can be said to have been carried out under double blind conditions |
| Blinding of outcome assessment (detection bias) | Low risk | In a large number of prior studies under the same conditions, no subjects could recognize the content of the stimulus. Since the experimenters were also blind , the current study can be said to have been carried out under double blind conditions |
| Incomplete outcome data (attrition bias) | Low risk | Of the 58 participants, 11 dropped out during the course of the study (6 control and 5 experimental) for reason ranging from feeling of disinterest to absenteeism from the clinic. The remaining members were comparable for demographic and background characteristics. COMMENT: reasons described; drop out balanced |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Randomised controlled trial. Recruitment modality:patients recruited by their counsellor or by the author of the study from patients receiving methadone for at least two weeks but no more than six month. | |
| Participants | 110 opiate dependent, age between 18 and 55 years, met the FDA requirements for MMT, and had been receiving methadone for at least 2 weeks but no more than 6 months. Average age 32.5; 100% men; 39% White, 61% African‐American; mean use of heroin 9.4 years; mean prior treatment 3.6; 34% married, 34% divorced or separated, 32% never married; average years of educational level 12.3; criminal convictions 3. Ex C: Psychosis, persistent or clinically significant organic brain syndrome. | |
| Interventions | For all MMT.
Duration: 7 months plus 12 months follow‐up. | |
| Outcomes | Use of primary substance of abuse as urinalysis results as value of F. Psychiatric symptoms/psychological distress as scores. Severity of dependence as mean methadone dose (graph), % of participants receiving ancillary medications (graph), ASI (scores). Results at follow‐up as number still in treatment, number of lost and number of abstained. | |
| Notes | Country of origin: USA. Setting: outpatients The participants in the Standard Drug Counseling (n. 39) are considered both in arm a and in arm b | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to three treatment conditions |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned to three treatment conditions |
| Blinding of outcome assessment (detection bias) | Low risk | The interviews were done by independent technicians who were not part of the treatment staff and were not aware of patients group assignments |
| Blinding of outcome assessment (detection bias) | Low risk | The interviews were done by independent technicians who were not part of the treatment staff and were not aware of patients group assignments |
| Incomplete outcome data (attrition bias) | Low risk | Participants randomised were required to complete three appointments with their counsellors or therapists. If they failed to complete the appointments they were considered not engaged and their were dropped from the study. Approximatively 80% of patients keep these initial appointments. There were no significant differences (p>0.1) between groups in the proportion of patients who completed initial appointments. All patients who completed these initial appointments underwent subsequent evaluation and were include in the analysis regardless of their subsequent attendance |
| Selective reporting (reporting bias) | High risk | Retention in treatment, a measure usually utilized in drug addiction trial, not reported |
| Methods | Randomised controlled trial. recruitment modality: not described | |
| Participants | 93 opiate dependent age between 18 and 55 years, met the FDA requirements for MMT, had been receiving methadone for at least 2 weeks but no more than 6 months. Average age 41; 100% men; 60 % White, 57% African‐American, 43% Caucasian; average years of educational level 12; 36% employed; 46% had been incarcerated. 13% on probation. Ex C: Psychosis, persistent or clinically significant organic brain syndrome. | |
| Interventions | For all MMT, no information on doses
Duration: 24 weeks, follow‐up at 1 and 6 months. | |
| Outcomes | Retention in treatment as n. of retained. Use of primary substance of abuse as % of opiate positive urine samples by graph and as % of participants with positive urine samples. Use of other drugs as % of cocaine positive UA and as no. participants with positive UA for other drugs. | |
| Notes | Country of origin: USA. Setting: outpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to supportive expressive therapy or drug counselling |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned to supportive expressive therapy or drug counselling |
| Blinding of outcome assessment (detection bias) | Low risk | All measures were completed by independent research technicians who were not part od the treatment programs or the therapy |
| Blinding of outcome assessment (detection bias) | Low risk | All measures were completed by independent research technicians who were not part od the treatment programs or the therapy |
| Incomplete outcome data (attrition bias) | Low risk | Participants were required to complete three appointments with their counsellor in order to be considered engaged.76% of the psychotherapy group and 76% of the counsellor group became engaged. 92% of psychotherapy group patients and 87% of counselling group were contacted at follow up. COMMENT: reason for drop out given; drop out balanced between group |
| Selective reporting (reporting bias) | Low risk | |
ASI scores: Addiction Severity Index scores
BMT: Buprenorphine Maintenance Treatment
CRA: Community Reinforcement Approach
DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, American Psychiatric Association Washington DC
Ex Cr: Exclusion Criteria
FDA: Food and Drug Administration
MMT: Methadone Maintenance Treatment
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| excluded as outcome measures not in the inclusion criteria | |
| excluded as participants not in the inclusion criteria: only 19% of participants were opioid dependents | |
| excluded as outcome measures not in the inclusion criteria | |
| Excluded as type of intervention not in the inclusion criteria: there is not a group with pharmacological alone | |
| Excluded as type of intervention not in the inclusion criteria: there is not a group with pharmacological alone | |
| Excluded as study design not in the inclusion criteria: it is impossible to evaluate the effects of the single interventions not knowing the number of participants for each group | |
| excluded as : intervention (both group received psychosocial intervention) and outcome (cocaine use) not in the inclusion criteria | |
| excluded as participants not in the inclusion criteria: only 5% of participants were opioid dependents | |
| Excluded as the type of participants not in the inclusion criteria: females dependent/abusing alcohol, prescription drugs or both. | |
| Excluded as type of intervention not in the inclusion criteria: no group with pharmacological alone | |
| excluded as the intervention is nor in the inclusion criteria: both groups received psychosocial intervention | |
| Excluded as type of participants and intervention not in the inclusion criteria: participants were drug dependent (any drug) and the treatments compared were both psychosocial without pharmacological intervention | |
| Excluded as the type of intervention not in the inclusion criteria: aim is to address cognitive deficits that may impede substance abuse treatment within the criminal justice system | |
| Excluded as type of outcomes not in the inclusion criteria: cocaine negative urine | |
| Excluded as the type of participants not in the inclusion criteria: substances abusers (any drug) | |
| Excluded as type of intervention not in the inclusion criteria: no group with pharmacological alone | |
| Excluded as the study design not in the inclusion criteria: review article. | |
| Excluded as type of intervention not in the inclusion criteria: two groups, one only network therapy and the other only medication management (buprenorphine) | |
| excluded as study design not in the inclusion criteria: cross sectional survey | |
| excluded as outcome measures not in the inclusion criteria | |
| Excluded as the study design not in the inclusion criteria: overview | |
| excluded as outcome measures not in the inclusion criteria | |
| Excluded as the outcomes not in the inclusion criteria: effects of regulation of dosage and increased number of take‐home doses to decrease the methadone dose | |
| excluded as the intervention not in the inclusion criteria: engagement in a maintenance treatment is the outcome | |
| Excluded as the type of participants not in the inclusion criteria: not only opiate addicts in the final stages of their residential drug treatment program | |
| Excluded as the type of intervention not in the inclusion criteria: no pharmacological intervention alone | |
| Excluded as the type of participants not in the inclusion criteria: residents of an alcohol and drug treatment centre. | |
| Excludes as study design not in the inclusion criteria: cohort study | |
| Excluded as outcomes not in the inclusion criteria: results only on sub group of participants, likely to be selected | |
| Excluded as type of intervention not in the inclusion criteria: two groups, (1) methadone (2) stepped treatment initiated with buprenorphine/naloxone and escalated to methadone if needed | |
| Excluded as type of intervention not in the inclusion criteria: no pharmacological intervention alone | |
| Excluded as type of outcomes not in the inclusion criteria: rates of cannabis use and the effectiveness of an adaptive stepped care intervention for reducing cannabis use | |
| excluded as type of intervention not in the inclusion criteria: no maintenance treatment | |
| Excluded as type of intervention not in the inclusion criteria: no group with pharmacological treatment alone | |
| excluded as type of intervention not in the inclusion criteria: all groups receive psychosocial intervention | |
| Excluded as type of intervention not in the inclusion criteria: cocaine abstinence | |
| Excluded as study design not in the inclusion criteria: no randomisation for allocate participants in the groups | |
| Excluded as outcomes not in the inclusion criteria: effect of Contingency Management on motivation to change substance use | |
| Excluded as study design not in the inclusion criteria: performance analysis through benchmark comparison | |
| Excluded as type of participants not in the inclusion criteria: participants were dependent on alcohol, drugs (any) or both. | |
| Excluded as study design not in the inclusion criteria: no randomisation for psychosocial interventions | |
| Excluded as the type of participants not in the inclusion criteria: substance abusers (any drug) | |
| excluded as type of intervention not in the inclusio criteria: not all patients receive maintenance treatment | |
| Excluded as outcomes not in the inclusion criteria: responses on an interview contained 15 agree/disagree questions tapping orientations to locus‐of‐control beliefs about drug misuse | |
| excluded ad outcome measures not in the inclusion criteria | |
| Excluded as the type of participants not in the inclusion criteria: participants were drug dependent (any drug) | |
| Excluded as type of intervention not in the inclusion criteria: no pharmacological alone | |
| Excluded as type of participants not in the inclusion criteria: opiate or cocaine abusers, analysis not separated | |
| excluded as study design not in the inclusion criteria: secondary analysis of already included or excluded studies | |
| Excluded as type of intervention not in the inclusion criteria: no group with pharmacological alone | |
| excluded as : intervention not in the inclusion criteria: both groups received psychosocial treatment | |
| Excluded as type of intervention not in the inclusion criteria: no group with pharmacological alone | |
| Excluded as type of intervention and outcomes not in the inclusion criteria: counselling on cocaine use and cocaine use as outcome | |
| Excluded as type of intervention not in the inclusion criteria: no information available on pharmacological intervention | |
| Excluded as type of intervention not in the inclusion criteria:no pharmacological alone | |
| Excluded as type of intervention not in the inclusion criteria:no pharmacological alone | |
| Excluded as type of outcome not in the inclusion criteria: HIV risk behaviours | |
| Excluded as type of outcomes not in the inclusion criteria: cocaine negative urine | |
| Excluded as the type of intervention: (1) abstinence‐and‐work and (2) work‐only | |
| Excluded as type of intervention not in the inclusion criteria: contingency management intervention designed to improve medication adherence | |
| Excluded as type of intervention not in the inclusion criteria: 3 groups, (1) $ 15.00 cash, (2) 2 methadone take‐home doses, (3) the opportunity of self‐regulate methadone doses. |
MMT= Methadone Maintenance Treatment
CC: contingency contracting
NC: no contingencies
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 27 | 3124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.98, 1.07] |
| Analysis 1.1  Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 1 Retention in treatment. | ||||
| 2 Opioid abstinence Show forest plot | 8 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.92, 1.37] |
| Analysis 1.2  Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 2 Opioid abstinence. | ||||
| 3 Number of participants still in treatment at the end of follow‐up Show forest plot | 3 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.07] |
| Analysis 1.3  Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 3 Number of participants still in treatment at the end of follow‐up. | ||||
| 4 Number of participants abstinent at the end of follow‐up Show forest plot | 3 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.98, 1.36] |
| Analysis 1.4  Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 4 Number of participants abstinent at the end of follow‐up. | ||||
| 5 Compliance Show forest plot | 3 | 685 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.05, 0.92] |
| Analysis 1.5  Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 5 Compliance. | ||||
| 6 Psychiatric symptoms SCL‐90 Show forest plot | 3 | 279 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.28, 0.31] |
| Analysis 1.6  Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 6 Psychiatric symptoms SCL‐90. | ||||
| 7 Depression (BDI) Show forest plot | 3 | 279 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.91, 0.51] |
| Analysis 1.7  Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 7 Depression (BDI). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 1 Retention in treatment. | ||||
| 1.1 Any behavioural plus pharm versus pharm standard | 19 | 2065 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.06] |
| 1.2 Contingency management plus pharm versus pharm standard | 14 | 1616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.96, 1.08] |
| 2 Opioid abstinence Show forest plot | 4 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| Analysis 2.2  Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 2 Opioid abstinence. | ||||
| 3 Continuous weeks of abstinence Show forest plot | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [0.20, 3.62] |
| Analysis 2.3  Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 3 Continuous weeks of abstinence. | ||||
| 4 Number of participants still in treatment at the end of follow‐up Show forest plot | 3 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| Analysis 2.4  Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 4 Number of participants still in treatment at the end of follow‐up. | ||||
| 5 Number of participants abstinent at the end of follow‐up Show forest plot | 3 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.98, 1.41] |
| Analysis 2.5  Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 5 Number of participants abstinent at the end of follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.07] |
| Analysis 3.1  Comparison 3 Psychoanalytic oriented treatments plus pharm versus pharm standard, Outcome 1 Retention in treatment. | ||||
| 2 Opioid abstinence Show forest plot | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.82, 1.78] |
| Analysis 3.2  Comparison 3 Psychoanalytic oriented treatments plus pharm versus pharm standard, Outcome 2 Opioid abstinence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 retention in treatment Show forest plot | 4 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.98, 1.15] |
| Analysis 4.1  Comparison 4 Counselling plus pharm versus pharm standard, Outcome 1 retention in treatment. | ||||
| 2 opioid abstinence Show forest plot | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.14] |
| Analysis 4.2  Comparison 4 Counselling plus pharm versus pharm standard, Outcome 2 opioid abstinence. | ||||
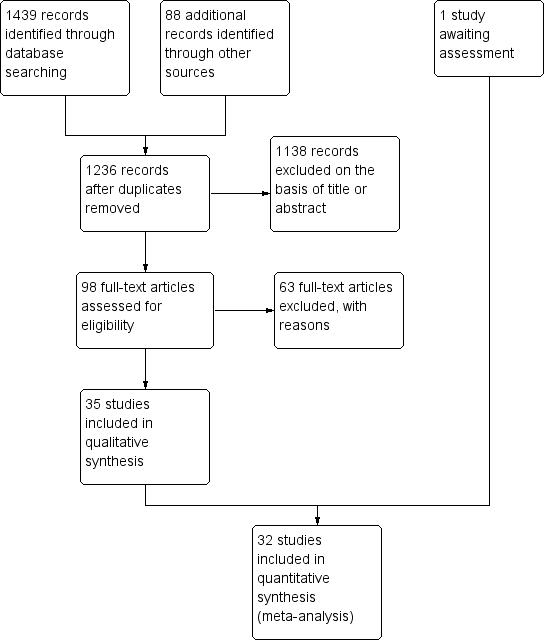
Study flow diagram.
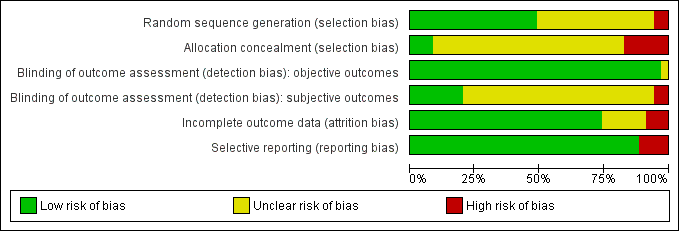
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
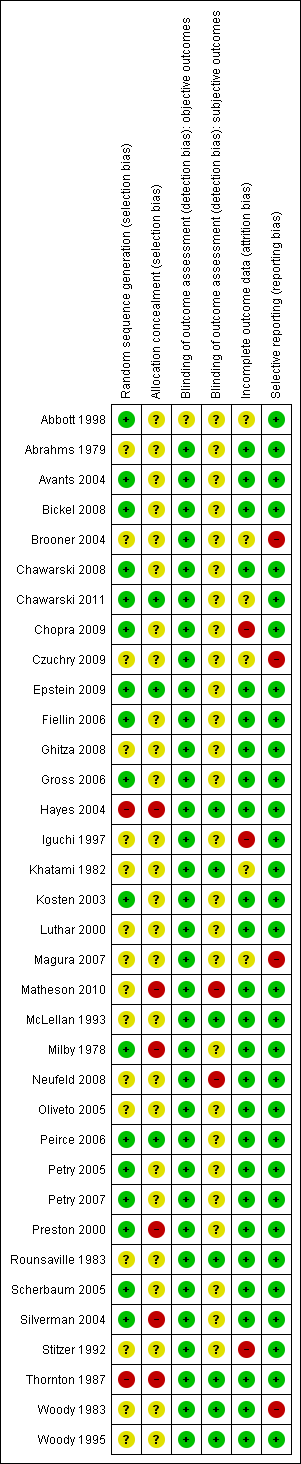
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.

Forest plot of comparison: 1 Any Psychosocial intervention plus pharm versus pharm standard, outcome: 1.1 Retention in treatment.
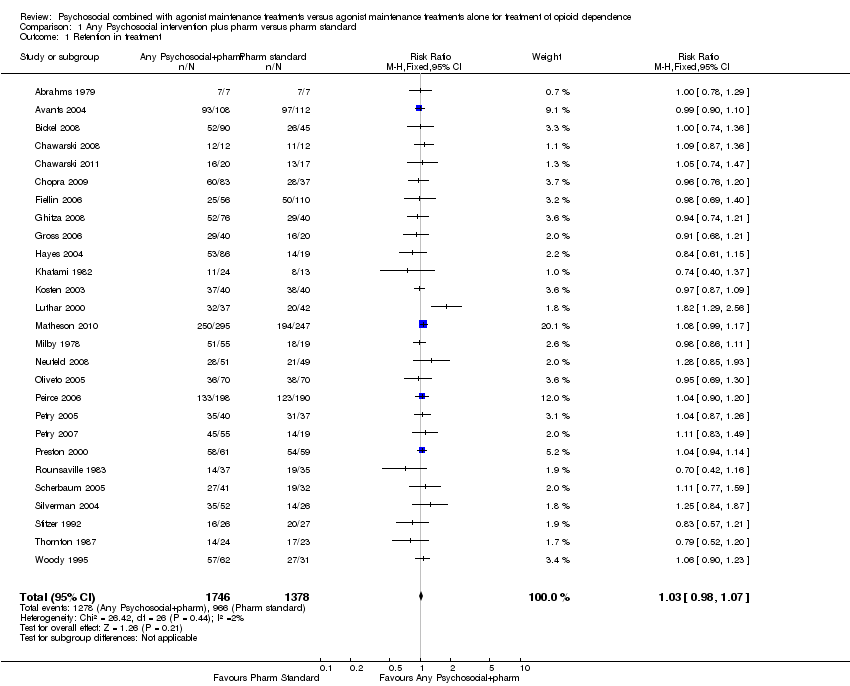
Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 1 Retention in treatment.

Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 2 Opioid abstinence.
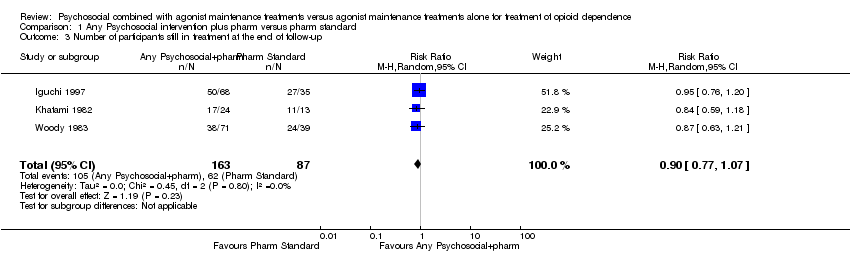
Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 3 Number of participants still in treatment at the end of follow‐up.

Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 4 Number of participants abstinent at the end of follow‐up.
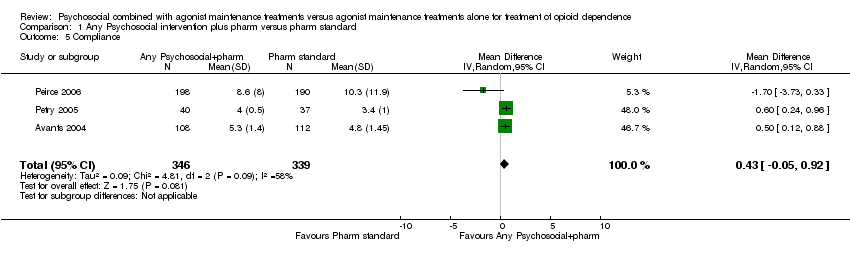
Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 5 Compliance.

Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 6 Psychiatric symptoms SCL‐90.
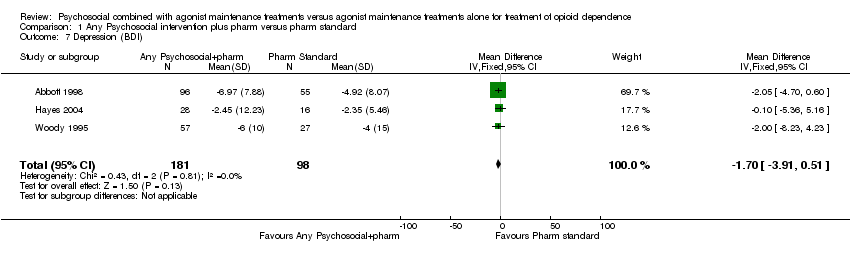
Comparison 1 Any Psychosocial intervention plus pharm versus pharm standard, Outcome 7 Depression (BDI).
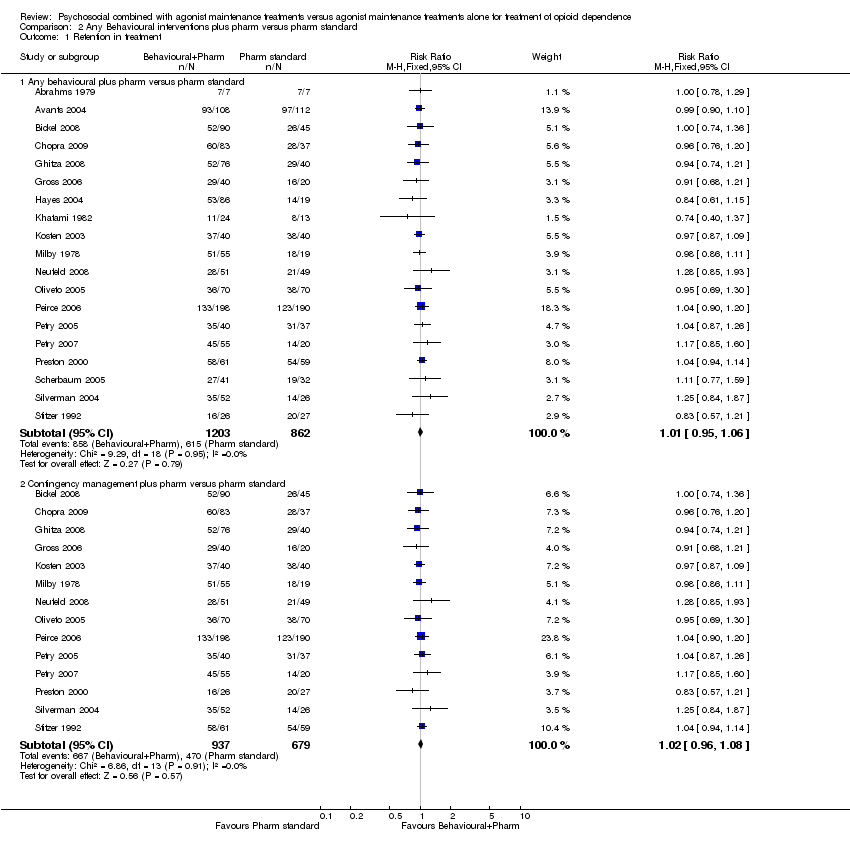
Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 1 Retention in treatment.

Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 2 Opioid abstinence.

Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 3 Continuous weeks of abstinence.

Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 4 Number of participants still in treatment at the end of follow‐up.

Comparison 2 Any Behavioural interventions plus pharm versus pharm standard, Outcome 5 Number of participants abstinent at the end of follow‐up.

Comparison 3 Psychoanalytic oriented treatments plus pharm versus pharm standard, Outcome 1 Retention in treatment.

Comparison 3 Psychoanalytic oriented treatments plus pharm versus pharm standard, Outcome 2 Opioid abstinence.

Comparison 4 Counselling plus pharm versus pharm standard, Outcome 1 retention in treatment.

Comparison 4 Counselling plus pharm versus pharm standard, Outcome 2 opioid abstinence.
| Any Psychosocial intervention plus pharm versus pharm standard for treatment of opioid dependence | ||||||
| Patient or population: patients with treatment of opioid dependence | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any Psychosocial intervention plus pharm versus pharm standard | |||||
| Retention in treatment | Study population | RR 1.02 | 2582 | ⊕⊕⊕⊕ | ||
| 683 per 1000 | 696 per 1000 | |||||
| Moderate | ||||||
| 738 per 1000 | 753 per 1000 | |||||
| Opioid abstinence | Study population | RR 1.19 | 667 | ⊕⊕⊕⊕ | ||
| 502 per 1000 | 597 per 1000 | |||||
| Moderate | ||||||
| 527 per 1000 | 627 per 1000 | |||||
| Number of participants still in treatment at the end of follow‐up | Study population | RR 0.9 | 250 | ⊕⊕⊕⊕ | ||
| 713 per 1000 | 641 per 1000 | |||||
| Moderate | ||||||
| 771 per 1000 | 694 per 1000 | |||||
| Number of participants abstinent at the end of follow‐up | Study population | RR 1.15 | 181 | ⊕⊕⊕⊕ | ||
| 724 per 1000 | 833 per 1000 | |||||
| Moderate | ||||||
| 429 per 1000 | 493 per 1000 | |||||
| Compliance | The mean compliance in the intervention groups was | 685 | ⊕⊕⊕⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 studies were judged at unclear risk of detection bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 27 | 3124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.98, 1.07] |
| 2 Opioid abstinence Show forest plot | 8 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.92, 1.37] |
| 3 Number of participants still in treatment at the end of follow‐up Show forest plot | 3 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.07] |
| 4 Number of participants abstinent at the end of follow‐up Show forest plot | 3 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.98, 1.36] |
| 5 Compliance Show forest plot | 3 | 685 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.05, 0.92] |
| 6 Psychiatric symptoms SCL‐90 Show forest plot | 3 | 279 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.28, 0.31] |
| 7 Depression (BDI) Show forest plot | 3 | 279 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.91, 0.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Any behavioural plus pharm versus pharm standard | 19 | 2065 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.06] |
| 1.2 Contingency management plus pharm versus pharm standard | 14 | 1616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.96, 1.08] |
| 2 Opioid abstinence Show forest plot | 4 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| 3 Continuous weeks of abstinence Show forest plot | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [0.20, 3.62] |
| 4 Number of participants still in treatment at the end of follow‐up Show forest plot | 3 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 5 Number of participants abstinent at the end of follow‐up Show forest plot | 3 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.98, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.07] |
| 2 Opioid abstinence Show forest plot | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.82, 1.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 retention in treatment Show forest plot | 4 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.98, 1.15] |
| 2 opioid abstinence Show forest plot | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.14] |

