Agonistas alfa‐2 adrenérgicos para prevenir complicações cardíacas em adultos submetidos à cirurgia
Resumo
Introdução
A resposta ao estresse cirúrgico desempenha um papel importante na patogênese das complicações cardíacas peri operatórias. Os agonistas alfa‐2 adrenérgicos atenuam essa resposta e poderiam ajudar a prevenir complicações cardíacas pós‐operatórias.
Objetivos
Avaliar a eficácia e segurança dos agonistas α‐2 adrenérgicos na redução da mortalidade e das complicações cardíacas em adultos submetidos a cirurgias cardíacas e cirurgias não cardíacas.
Métodos de busca
Fizemos buscas nas seguintes bases de dados eletrônicas: CENTRAL (2017, Issue 4), MEDLINE (1950 a abril, semana 4, 2017), Embase (1980 a maio de 2017) e Science Citation Index. Também fizemos buscas em plataformas de registros de ensaios clínicos e revisamos as listas de referências dos estudos incluídos.
Critério de seleção
Incluímos ensaios controlados randomizados (ECR) que compararam agonistas α‐2 adrenérgicos (ou seja, clonidina, dexmedetomidina ou mivazerol) versus placebo ou agonistas adrenérgicos não α‐2. Os estudos incluídos tinham que avaliar a eficácia e a segurança dos agonistas α‐2 adrenérgicos em prevenir a mortalidade peri operatória ou complicações cardíacas (ou ambas), ou medir um ou mais desfechos relevantes (por exemplo, morte, infarto do miocárdio, insuficiência cardíaca, acidente vascular cerebral agudo, taquiarritmia supraventricular e isquemia miocárdica).
Coleta dos dados e análises
Dois autores, trabalhando de forma independente, avaliaram a qualidade dos ECR, extraíram os dados e transferiram os dados para o computador. Contactamos os autores dos estudos primários para obtermos informações adicionais. Também coletamos dados sobre eventos adversos descritos nos estudos. Avaliamos a qualidade dos estudos usando a ferramenta Cochrane 'Risk of bias tool'. Avaliamos a qualidade da evidência do efeito combinado do tratamento usando a metodologia GRADE. Devido à heterogeneidade clínica entre os procedimentos, realizamos análises separadas para cirurgias cardíacas e não cardíacas. Para avaliar o efeito da intervenção, calculamos os riscos relativos (RR) e os intervalos de confiança (IC) de 95%.
Principais resultados
Incluímos 47 ECR com 17.039 participantes. Desses estudos, 24 incluíram apenas participantes submetidos à cirurgias cardíacas, 23 incluíram apenas participantes submetidos à cirurgias não cardíacas e oito incluíram apenas participantes submetidos à cirurgias vasculares. A clonidina foi avaliada em 21 estudos, a dexmedetomidina em 24, e o mivazerol em dois ECR.
Para pacientes submetidos a cirurgias não cardíaca, existe evidência de alta qualidade de que o uso de agonistas α‐2 adrenérgicos, comparado ao controle, não modifica o risco de mortalidade por todas as causas (1,3% no grupo agonistas adrenérgicos α‐2 versus 1,7% no grupo controle; RR 0,80, IC 95% 0,61 a 1,04; participantes = 14.081; estudos = 16). Além disso, o risco de mortalidade cardíaca foi semelhante entre os grupos tratamento versus controle (0,8% versus 1,0%, respectivamente; RR 0,86, IC 95% 0,60 a 1,23; participantes = 12.525; estudos = 5, evidência de alta qualidade). O risco de infarto agudo do miocárdio foi semelhante entre os grupos (RR 0,94; IC 95% 0,69 a 1,27; participantes = 13.907; estudos = 12, evidência de qualidade moderada). Não houve diferença no risco de AVC (RR 0,93; IC 95% 0,55 a 1,56; participantes = 11.542; estudos = 7; evidência de alta qualidade). Por outro lado, existe evidência de qualidade moderada de que os agonistas α‐2 adrenérgicos aumentam os riscos de bradicardia clinicamente significativa (RR 1,59; IC 95% 1,18 a 2,13; participantes = 14.035; estudos = 16) e de hipotensão (RR 1,24; IC 95% 1,03 a 1,48; participantes = 13.738; estudos = 15).
Não há evidência suficiente para avaliar o efeito dos agonistas α‐2 adrenérgicos na mortalidade por todas as causas em cirurgias cardíacas (RR 0,52; IC 95% 0,26 a 1,04; participantes = 1947; estudos = 16, evidência de qualidade moderada) e infarto agudo do miocárdio (RR 1,01; IC 95% 0,43 a 2,40; participantes = 782; estudos = 8, evidência de qualidade moderada). Houve uma morte por complicação cardíaca no grupo que usou clonidina em um estudo com 22 participantes. Existe evidência de baixa qualidade, apoiada em dados muito limitados, de que os agonistas α‐2 adrenérgicos podem reduzir o risco de AVC (RR 0,37; IC 95% 0,15 a 0,93; participantes = 1175; estudos = 7; eventos = 18). Por outro lado, os agonistas α‐2 adrenérgicos aumentam o risco de bradicardia de 6,4% para 12,0% (RR 1,88; IC 95% 1,35 a 2,62; participantes = 1477; estudos = 10; evidência de qualidade moderada). Seu efeito na hipotensão é incerto (RR 1,19; IC 95% 0,87 a 1,64; participantes = 1413; estudos = 9; evidência de baixa qualidade).
As análises de subgrupos e de sensibilidade não modificaram esses resultados, em termos qualitativos.
Conclusão dos autores
Nossa revisão conclui que o uso profilático de agonistas α‐2 adrenérgicos, no geral, não previne a morte peri operatória ou complicações cardíacas graves. Para pacientes submetidos a cirurgias não cardíacas, há evidência de qualidade moderada a alta de que esses medicamentos não previnem morte, infarto agudo do miocárdio ou AVC. Por outro lado, existe evidência de qualidade moderada de que estes medicamentos têm efeitos adversos importantes como aumento dos riscos de hipotensão e bradicardia. Para pacientes submetidos à cirurgias cardíacas, há evidência de moderada qualidade de que os agonistas α‐2 adrenérgicos não modificam os riscos de mortalidade ou infarto agudo do miocárdio e de que eles aumentam o risco de bradicardia. A qualidade das evidências foi inadequada para obter conclusões sobre os efeitos dos agonistas alfa‐2 no acidente vascular cerebral ou na hipotensão durante a cirurgia cardíaca.
PICO
Resumo para leigos
Agonistas alfa 2 adrenérgicos para prevenir complicações cardíacas após cirurgia de grande porte
Pergunta da revisão
As pessoas que recebem agonistas alfa‐2 adrenérgicos (clonidina, dexmedetomidina e mivazerol) perto de quando fazem uma cirurgia têm menos riscos de morrer ou de ter complicações cardíacas?
Resumo
As complicações cardíacas que surgem depois de uma cirurgia podem levar à morte e à longas internações hospitalares. Todos os anos, cerca de 300 milhões de pessoas são submetidas a cirurgias de grande porte e nove milhões delas podem ter complicações cardíacas graves. Essas complicações são devidas, em parte, ao fato de que a cirurgia provoca um grande estresse no coração. Esse estresse pode causar aumento da pressão arterial e da frequência cardíaca durante a cirurgia, duas alterações que não fazem bem para o coração. Os agonistas alfa‐2 adrenérgicos são um grupo de medicamentos que podem prevenir o aumento da pressão arterial e da frequência cardíaca durante a cirurgia. Assim, esses medicamentos também poderiam proteger o coração do estresse cirúrgico. O objetivo desta revisão foi avaliar se a administração destes medicamentos próximo do momento da cirurgia poderia proteger o coração do estresse da cirurgia e assim prevenir complicações cardíacas mais graves.
Características do estudo
Encontramos 47 estudos publicados até maio de 2017. Esses estudos envolveram 17.039 adultos que fizeram cirurgias de grande porte. Vinte e quatro estudos envolveram 2672 adultos submetidos a cirurgia cardíaca. Vinte e três estudos envolveram 14.367 adultos submetidos a outras cirurgias de grande porte. Quarenta estudos compararam agonistas alfa‐2 adrenérgicos versus placebo (substância que parece um remédio de verdade mas não contém nenhum princípio ativo). Os outros sete estudos compararam os alfa‐2 adrenérgicos versus outros medicamentos. Existem vários tipos de agonistas alfa‐2 adrenérgico; 21 estudos testaram a clonidina, 24 testaram a dexmedetomidina e 2 estudos testaram o mivazerol. Os estudos testaram várias formas de administrar os agonistas alfa‐2 adrenérgico, desde uma dose antes da cirurgia até três dias de tratamento. A maioria das pessoas que participaram desses estudos eram homens com idade média de 60 a 70 anos. Catorze estudos relataram ter recebido dinheiro dos fabricantes do medicamento que estava sendo testado. Outros 15 estudos não informaram qual foi a origem da verba necessária para financiar o estudo. O número de participantes por estudo variou de 20 até10.000.. Dezenove estudos incluíram mais de 100 participantes.
Principais resultados
No geral, o uso de agonistas alfa‐2 adrenérgicos não traz benefícios claros para prevenir a morte ou complicações graves pós‐cirurgia. Os agonistas alfa‐2 adrenérgicos não reduzem os riscos de morte, de ter um ataque cardíaco ou um derrame após uma operação de grande porte não cardíaca. Para os pacientes submetidos a cirurgias cardíacas, não existe evidência suficiente de que o uso de alfa‐2 adrenérgicos reduza o risco de morte ou de ter um ataque cardíaco após a operação. Existe alguma evidência, muito limitada, de que esses medicamentos podem prevenir derrames após cirurgias cardíacas. No entanto, para se ter certeza desse benefício, é necessário fazer mais estudos sobre esse assunto. Estes medicamentos também produziram alguns efeitos colaterais importantes. As pessoas que receberam agonistas alfa‐2 adrenérgicos tiveram uma probabilidade muito maior de ter queda da pressão arterial ou da frequência cardíaca durante ou após a cirurgia.
Qualidade da evidência
Avaliamos a qualidade dos resultados de todos os estudos utilizando uma ferramenta especializada chamada GRADE. Em geral,a maior parte das evidências desses estudos era de qualidade moderada ou alta. Assim, com base em nossos resultados, podemos estar razoavelmente certos de que os agonistas alfa‐2 adrenérgicos não são úteis para reduzir o número de mortes ou complicações cardíacas graves que podem ocorrer após uma cirurgia.
Authors' conclusions
Summary of findings
| Alpha‐2 adrenergic agonists compared to control in non‐cardiac surgery | ||||||
| Patient or population: adults undergoing non‐cardiac surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Risk ratio | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
| All‐cause mortality (within 30‐days after surgery: any reported death) | Study population | RR 0.80 | 14,081 | ⊕⊕⊕⊕ | ‐ | |
| 17 per 1000 | 13 per 1000 | |||||
| Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause.) | Study population | RR 0.86 | 12,525 | ⊕⊕⊕⊕ | ‐ | |
| 10 per 1000 | 8 per 1000 | |||||
| Myocardial infarction (within 30‐days after surgery: as detected on an electrocardiogram or trans‐oesophageal echocardiogram) | Study population | RR 0.94 | 13,907 | ⊕⊕⊕⊝ | ‐ | |
| 59 per 1000 | 55 per 1000 | |||||
| Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) | Study population | RR 0.93 | 11,542 | ⊕⊕⊕⊕ | ‐ | |
| 5 per 1000 | 4 per 1000 | |||||
| Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) | Study population | RR 1.59 | 14,035 | ⊕⊕⊕⊝ | ‐ | |
| 75 per 1000 | 119 per 1000 | |||||
| Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) | Study population | RR 1.24 | 13,738 | ⊕⊕⊕⊝ | ‐ | |
| 304 per 1000 | 377 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias was not serious. Although multiple studies lacked proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded. 2Indirectness not serious. Intervention (mivazerol) used in one large study not available for clinical use. Not downgraded. 3Evidence of publication bias in funnel plot of analysis. Downgraded by one level. 4Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded by one level. | ||||||
| Alpha‐2 adrenergic agonists compared to control in cardiac surgery | ||||||
| Patient or population: adults undergoing cardiac surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
| All‐cause mortality (within 30‐days after surgery: any reported death) | Study population | RR 0.52 | 1947 | ⊕⊕⊕⊝ | ‐ | |
| 21 per 1000 | 11 per 1000 | |||||
| Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) | 1 death from 12 participants in clonidine arm, and no deaths in 10 participants in control arm. | Not estimable | 22 (1 RCT) | Not estimable | We did not GRADE evidence for this outcome as accurate estimation of RRs is not possible for such low event rates. | |
| Myocardial infarction (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) | Study population | RR 1.01 | 782 | ⊕⊕⊕⊝ | ‐ | |
| 20 per 1000 | 21 per 1000 | |||||
| Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) | Study population | RR 0.37 | 1175 | ⊕⊕⊝⊝ | Total of 18 acute stokes reported, with 14 in control group and 4 in treatment group. | |
| 24 per 1000 | 9 per 1000 | |||||
| Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) | Study population | RR 1.88 | 1477 | ⊕⊕⊕⊝ | ‐ | |
| 64 per 1000 | 120 per 1000 | |||||
| Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) | Study population | RR 1.19 | 1413 | ⊕⊕⊝⊝ | ‐ | |
| 332 per 1000 | 395 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias was not serious. Although multiple studies lack proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded. 2Serious imprecision, because analysis was below optimal information size and confidence interval includes significant benefit and harm. Downgraded by one level. 3Very serious imprecision, because analysis is below optimal information size and number of events was very small. Downgraded by two levels. 4Serious imprecision, because analysis was below optimal information size. Downgraded by one level 5Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded one level. | ||||||
Background
Description of the condition
Perioperative cardiac complications are a major health concern for the 312 million people who annually undergo major surgery worldwide (Meara 2015). For example, about 3% of people who undergo major non‐cardiac surgery experience perioperative myocardial infarction (MI) (VISION 2014). Major cardiac complications, such as MI, lead to increased mortality, hospital stay and costs (Fleischmann 2003; Force 1990; VISION 2014). The surgical stress response may play an important role in the pathogenesis of these complications. Specifically, surgical stress stimulates the sympathetic nervous system, which in turns leads to increased plasma levels of norepinephrine and epinephrine (Halter 1997). These effects increase blood pressure and heart rate, which can predispose the myocardium to ischaemia, especially in people with decreased coronary blood flow reserve.
Description of the intervention
Alpha‐2 (α‐2) adrenergic agonists selectively bind to presynaptic α‐2 adrenergic receptors to activate a negative feedback mechanism that inhibits central sympathetic outflow (Muzi 1992). These receptors are mainly located in the central nervous system, specifically in the brain stem and locus coeruleus. Activation of these receptors lead to hypotension, bradycardia, central sedation, anxiolysis and analgesia. Three specific α‐2 adrenergic agonists that have been evaluated in people undergoing surgery, namely clonidine, dexmedetomidine and mivazerol. Clonidine and dexmedetomidine are available for clinical use, while the use of mivazerol has been restricted to clinical trials. Clonidine has a half‐life of 12 to 18 hours with excellent bioavailability, lending to its suitability for once daily administration in oral tablet or transdermal patch forms. An intravenous (IV) formulation of clonidine is also available. Dexmedetomidine has a shorter half‐life of only two hours and variable bioavailability, consequently making it more suited for administration as a continuous IV infusion (Flood 2015). Similarly, mivazerol is also administered as a continuous IV infusion (Oliver 1999).
How the intervention might work
As indicated above, α‐2 adrenergic agonists inhibit central sympathetic outflow. Hence, they can attenuate perioperative haemodynamic abnormalities (Ellis 1994; McSPI‐Europe 1997; Talke 1995), and perhaps also prevent cardiac complications. Furthermore, clonidine has the unique ability to reduce sympathetic activity without blunting the baroreflex, which is critical for responding to the fluctuations in circulating blood volume often encountered during surgery (Muzi 1992). Nonetheless, α‐2 adrenergic agonists have important adverse effects, including hypotension and bradycardia (Biccard 2008). These haemodynamic effects may have clinically important consequences for people undergoing surgery. For example, in the Perioperative Ischemic Evaluation ‐ 1 (POISE‐1) randomized controlled trial (RCT), acute perioperative β‐blockade increased risks of bradycardia, hypotension, acute stroke, and death (POISE 2008). Given that α‐2 adrenergic agonists have both potential benefits and adverse effects, a quantitative systematic review may help determine their overall efficacy and safety.
Why it is important to do this review
Previous systematic reviews of perioperative α‐2 adrenergic agonists have been published (Biccard 2008; Nishina 2002; Stevens 2003). However, two of them were restricted to individual α‐2 adrenergic agonists, namely clonidine (Nishina 2002), and dexmedetomidine (Biccard 2008). The other review was restricted to studies published before 2002 (Stevens 2003). A systematic review according to the Cochrane methodology is therefore justified. The current review is an update to a previous Cochrane Review that included studies published before August 2008 (Wijeysundera 2009). This update was deemed necessary, in part, given the publication of the largest RCT to‐date of perioperative α‐2 adrenergic agonists, the Perioperative Ischemic Evaluation ‐ 2 (POISE‐2) trial (Devereaux 2014a).
Objectives
To determine the efficacy and safety of α‐2 adrenergic agonists for reducing mortality and cardiac complications in adults undergoing cardiac surgery and non‐cardiac surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included published RCTs.
Types of participants
We included adults (aged 18 years or older) undergoing surgery under general anaesthesia, neuraxial anaesthesia, or both. We excluded surgery performed under local anaesthesia or peripheral nerve blockade alone because such procedures are generally associated with a very low risk of mortality and morbidity. We also excluded surgery performed on pregnant women, organ transplant recipients, or people with substance withdrawal. Organ transplantation procedures may be associated with a high risk of mortality unrelated to cardiovascular causes, thereby masking any potential benefit from α‐2 adrenergic agonists.
Types of interventions
The experimental intervention must have included clonidine, mivazerol or dexmedetomidine administration before surgery (within 24 hours), during surgery, or after surgery (within 48 hours). The medications must have been administered via IV, intramuscular, oral or transdermal routes. There were no restrictions on the dose, duration or frequency of the intervention.
We permitted active interventions in the comparator group only if the comparator was judged to have minimal to no effect on the primary or secondary outcomes. For example, in a trial where dexmedetomidine was being primarily evaluated for the role of providing postoperative sedation after major surgery, comparison to propofol was judged to be reasonable.
Types of outcome measures
Included trials had to evaluate the efficacy or safety of α‐2 adrenergic agonists in reducing perioperative mortality or cardiac complications, or both. Studies were included if they measured one or more relevant outcomes, which included death, MI, heart failure (HF), acute stroke, supraventricular tachyarrhythmia (SVT) or myocardial ischaemia. In addition, studies with similar objectives to our review were included, even if these same studies did not report any relevant outcome events (i.e. death, MI, HF, acute stroke, SVT, myocardial ischaemia).
Primary outcomes
-
All‐cause mortality within 30 days after surgery: any reported death. The time period for outcome ascertainment in each trial was also documented.
Secondary outcomes
-
Cardiac mortality within 30 days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause. The time period for outcome ascertainment in each trial was also documented.
-
MI within 30 days after surgery: definition as per individual study (specific criteria employed were documented). The time period for outcome ascertainment in each trial was also documented.
-
Myocardial ischaemia within 30 days after surgery: as detected on an electrocardiogram (ECG) or trans‐oesophageal echocardiogram (specific criteria employed were documented). The time period for outcome ascertainment in each trial was also documented.
-
SVT within 30 days after surgery: SVT, atrial fibrillation or atrial flutter. The time period for outcome ascertainment in each trial was also documented.
-
HF within 30 days after surgery: clinical diagnosis of HF or need for postoperative intra‐aortic balloon pump support (applicable only for cardiac surgery). The time period for outcome ascertainment in each trial was also documented.
Adverse effects from treatment
-
Acute stroke within 30 days after surgery: new focal neurological deficit with signs and symptoms lasting longer than 24 hours. The time period for outcome ascertainment in each trial was also documented.
Physiological effects of treatment
-
Bradycardia requiring pharmacological or pacemaker treatment during the period of study drug administration.
-
Hypotension requiring treatment with inotropes or vasopressors during the period of study drug administration.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2017, Issue 4), MEDLINE (1950 to April week 4 2017), Embase (1980 to May 2017), the Science Citation Index and reference lists of articles. The Ovid platform was used for searching the electronic databases.
We searched MEDLINE using the search terms presented in Appendix 1. We then limited the studies to those identified simultaneously by a highly sensitive search strategy for identifying RCTs in MEDLINE (Dickersin 1994). Our search strategies for CENTRAL and Embase are presented in Appendix 1.
Searching other resources
We entered all trials selected for inclusion into the Science Citation Index to identify any additional relevant articles. The bibliographies of all included articles and published reviews were searched to identify any other potentially relevant studies for inclusion. Additionally, we searched clinical trial registries, namely ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), for published studies meeting our inclusion criteria. These additional searches were completed in May 2017.
Data collection and analysis
Selection of studies
Two authors (DD, AS) independently performed literature searches for potentially relevant RCTs. All identified published full papers and abstracts were assessed independently for inclusion by the same two authors. We applied no language restrictions. We documented the reasons for exclusion for all excluded studies. We resolved all disagreements by consensus or involvement of a third author (DNW).
Data extraction and management
Two authors (DD, AS) independently extracted data from the included studies on a predesigned data abstraction form (Appendix 2). These same two authors independently entered all data into Review Manager 5 (RevMan 2014). We were not blinded to study authors, institution or journal when performing data abstraction. Where necessary, we contacted authors of published trials to provide any additional information required for the analyses (see Methods of the review).
Assessment of risk of bias in included studies
Two authors (DD, AS) independently evaluated the quality of all included trials using the criteria recommended by the Cochrane Anaesthesia, Critical and Emergency Care (ACE) Group. These criteria emphasize the adequacy of allocation concealment, randomization, blinding and intention‐to‐treat (ITT) analysis. Each included study was evaluated using the Cochrane 'Risk of bias' tool (Higgins 2011a). We were not blinded to study authors, institution or journal when performing quality assessment.
Measures of treatment effect
We performed all statistical analyses using Review Manager 5 (RevMan 2014). Given that all outcomes and adverse effects were dichotomous, all treatment effects were expressed as pooled risk ratios (RR) with 95% confidence intervals (CI).
Unit of analysis issues
We excluded cross‐over trials and cluster randomized trials in this review. If a study had multiple treatment arms, comparisons were made between α‐2 adrenergic agonist and placebo, or between α‐2 adrenergic agonist and inactive control.
Dealing with missing data
If a study had missing relevant data in the published report, we attempted to contact the study authors up to three times to obtain these data. If data were missing due to participant attrition, and imputation methods were not used in the published report, we employed complete case analysis when importing the data.
Assessment of heterogeneity
We measured heterogeneity using the I2 statistic: the proportion of total variation explained by between‐study variation as opposed to chance (Higgins 2002; Higgins 2003). Higher I2 statistics imply more heterogeneity between studies than would be expected by chance alone.
Assessment of reporting biases
We carried out funnel plot analyses to assess for publication bias (Egger 1997), with formal tests for asymmetry being performed only if meta‐analyses pooled data from 10 or more studies (Higgins 2011b).
Data synthesis
Given the clinical heterogeneity between cardiac and non‐cardiac surgery, we conducted analyses for these two subgroups separately. If an individual study included both cardiac and non‐cardiac surgery procedures, we attempted to obtain subgroup‐specific results from the authors. If such data were not available, and greater than 75% of participants underwent cardiac surgical procedures, the specific study was allocated to the cardiac surgery subgroup. Conversely, the study was allocated to the non‐cardiac surgery subgroup if greater than 75% of participants underwent non‐cardiac surgical procedures. In all other cases, the specific study was excluded from the review. In the presence of low heterogeneity (I2 statistic 25% or less) (Higgins 2003), pooled RRs were calculated using the fixed‐effect model. In the presence of moderate‐to‐significant heterogeneity (I2 statistic greater than 25%) (Higgins 2003), we used the random‐effects model and carried out post‐hoc analyses to attempt to explain the heterogeneity.
Subgroup analysis and investigation of heterogeneity
A priori, we planned several subgroup analyses to determine the potential influence of the surgical procedure, the specific α‐2 adrenergic agonist employed and coexistent therapies on the overall results. Subgroup‐specific results were only calculated if there were two or more studies within the subgroup. These subgroup analyses were as follows.
-
Treatment effects of α‐2 adrenergic agonists on mortality (all‐cause and cardiac‐cause), MI and ischaemia based on the type of non‐cardiac surgical procedure, namely vascular versus non‐vascular non‐cardiac surgery. If a variety of surgical procedures were included in a study, we attempted to obtain subgroup‐specific results from the authors. If such data were not available, and greater than 75% of participants underwent the same class of surgery, the specific study was allocated to that specific subgroup. Failing that, the specific study was excluded from the subgroup analysis based on procedure type. We used statistical tests of interaction to assess for the presence of any subgroup effects.
-
We calculated treatment effects for each of clonidine, mivazerol and dexmedetomidine on mortality (all‐cause and cardiac‐cause), and MI in non‐cardiac surgery. Statistical tests of interaction were used to assess for the presence of any subgroup effects.
Sensitivity analysis
We planned several sensitivity analyses a priori to characterize the influence of study quality and outcome definitions on the overall results.
-
We restricted the meta‐analyses to the subset of studies that clearly reported methods for blinding and allocation concealment.
-
We determined the effect of α‐2 adrenergic agonists on MI in the subset of RCTs that strictly defined MI a priori as either significant new Q waves on an ECG or significant elevations in enzymatic markers of cardiac injury (MB isoenzyme of creatinine kinase, troponin‐I, troponin‐T).
-
We determined the effect of α‐2 adrenergic agonists on myocardial ischaemia in the subset of RCTs that strictly defined ischaemia a priori as ST segment depression or elevation of 0.1 mV or greater for one minute or longer.
In addition, we performed four additional post‐hoc analyses.
-
Significant statistical heterogeneity was identified when calculating the pooled effect of α‐2 adrenergic agonists on hypotension during non‐cardiac surgery. To explore potential explanations for this heterogeneity, we conducted subgroup analyses based on the specific agent (i.e. clonidine, mivazerol or dexmedetomidine) in the included trials. A statistical test of interaction was used to assess for the presence of a subgroup effect.
-
During the course of the review, we identified several very large included RCTs that might have highly influenced the overall pooled estimates. Therefore, we conducted a sensitivity analysis that excluded these very large RCTs.
-
Mivazerol is an experimental α‐2 adrenergic agonist that was studied in several relatively large trials, but never proceeded through the approval process for clinical use. At the request of external peer reviewers of this review, we conducted a sensitivity analysis that excluded trials that evaluated mivazerol.
-
Several relevant studies were conducted prior to 1997, during a period when perioperative practice might not necessarily be generalizable to contemporary practice. At the request of external peer reviewers of this review, we conducted a sensitivity analysis that excluded trials where data were collected prior to 1997.
'Summary of findings' tables and GRADE
To characterize the confidence in the pooled estimated treatment effects better, we used GRADE methodology to assess the quality of evidence (Guyatt 2008). We generated 'Summary of findings' tables that separately presented pooled treatment effect estimates for the subgroups of participants who underwent non‐cardiac surgery and cardiac surgery. To facilitate this process, data from the meta‐analyses in Review Manager 5 (RevMan 2014), were initially exported into GRADEpro. The GRADE approach rates quality of evidence as high, moderate, low or very low (GRADE Handbook 2013). Since all data included in this review were from RCTs, the quality of evidence for each outcome of interest was initially rated as high level, and then potentially downgraded up to three levels based on any deficiencies in the quality of the underlying evidence. The quality of evidence underlying each pooled treatment effect estimate was assessed with respect to the risk of bias, inconsistency, indirectness, imprecision and publication bias (Balshem 2011). The anticipated risk for comparison of each outcome was determined based on the event rate in the control group. Only outcomes judged as critically important, based on their impact on patient health or clinical decision‐making, were chosen for presentation in the 'Summary of findings' tables. In this present review, we included the following outcomes in the 'Summary of findings' tables, provided that relevant estimated pooled treatment effects were present: all‐cause mortality, cardiac mortality, MI, acute stroke, bradycardia and hypotension.
Results
Description of studies
Results of the search
Our search results are presented in Figure 1. The authors identified 3099 separate papers in the literature search and three additional papers from other sources and in total read 389 papers in full.
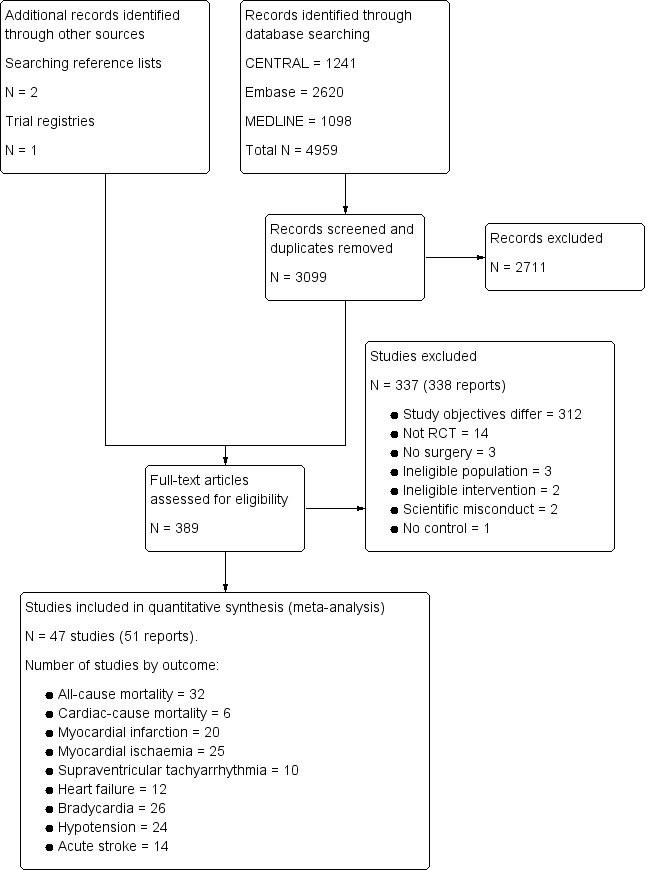
Study flow diagram.
Included studies
We included 47 trials, which encompassed 17,039 participants (Abi‐Jaoude 1993; Ammar 2016; Bergese 2010; Chi 2016; Cho 2016; Corbett 2005; Devereaux 2014a; Djaiani 2016; Dorman 1993; El‐Kerdawy 2004; Ellis 1994; Ghignone 1986; Ghignone 1987; Helbo‐Hansen 1986; Herr 2003; Jalonen 1997; Khalil 2013; Kim 2014a; Lee 2013a; Li 2017; Lipszyc 1991; Liu 2016; Loick 1999; Matot 2000; McSPI‐Europe 1997; Myles 1999; Oliver 1999; Park 2014; Patel 2016; Pawlik 2005; Pluskwa 1991; Quintin 1993; Quintin 1996; Ren 2013; Shehabi 2009; Soliman 2016; Stuhmeier 1996; Su 2016; Talke 1995; Talke 2000; Venn 1999; Venn 2001; Viviano 2012; Wallace 2004; Wijeysundera 2014a; Xu 2014; Yin 2002). These studies are described in detail in the Characteristics of included studies tables.
Twenty four studies with 2672 participants involved cardiac surgery alone (Abi‐Jaoude 1993; Ammar 2016; Chi 2016; Cho 2016; Corbett 2005; Djaiani 2016; Dorman 1993; El‐Kerdawy 2004; Ghignone 1986; Helbo‐Hansen 1986; Herr 2003; Jalonen 1997; Khalil 2013; Kim 2014a; Li 2017; Liu 2016; Loick 1999; Myles 1999; Park 2014; Patel 2016; Quintin 1993; Ren 2013; Shehabi 2009; Venn 1999). In all cases, the procedure involved was coronary artery bypass graft surgery or valve replacement surgery.
Of the 23 studies with 14,367 participants that involved non‐cardiac surgery (Bergese 2010; Devereaux 2014a; Ellis 1994; Ghignone 1987; Lee 2013a; Lipszyc 1991; Matot 2000; McSPI‐Europe 1997; Oliver 1999; Pawlik 2005; Pluskwa 1991; Quintin 1996; Soliman 2016; Stuhmeier 1996; Su 2016; Talke 1995; Talke 2000; Venn 2001; Viviano 2012; Wallace 2004; Wijeysundera 2014a; Xu 2014; Yin 2002), eight involved vascular procedures exclusively (Lipszyc 1991; McSPI‐Europe 1997; Pluskwa 1991; Quintin 1996; Soliman 2016; Stuhmeier 1996; Talke 1995; Talke 2000), and seven involved non‐vascular procedures exclusively (Ghignone 1987; Lee 2013a; Matot 2000; Pawlik 2005; Venn 2001; Viviano 2012; Xu 2014). One non‐cardiac surgery study presented subgroup‐specific results for both vascular and non‐vascular procedures (Oliver 1999).
Sample size
The sample sizes of the included trials ranged from 20 participants to 10,010 participants. Fourteen studies had fewer than 50 participants (Abi‐Jaoude 1993; Dorman 1993; Ghignone 1986; Ghignone 1987; Helbo‐Hansen 1986; Lipszyc 1991; Matot 2000; Pawlik 2005; Pluskwa 1991; Quintin 1993; Quintin 1996; Talke 1995; Talke 2000; Venn 2001), 14 studies had 50 to 100 participants (Ammar 2016; Chi 2016; Corbett 2005; El‐Kerdawy 2004; Ellis 1994; Jalonen 1997; Khalil 2013; Lee 2013a; Liu 2016; Loick 1999; Patel 2016; Viviano 2012; Xu 2014; Yin 2002), and 19 studies had greater than 100 participants (Bergese 2010; Cho 2016; Devereaux 2014a; Djaiani 2016; Herr 2003; Kim 2014a; Li 2017; McSPI‐Europe 1997; Myles 1999; Oliver 1999; Park 2014; Ren 2013; Shehabi 2009; Soliman 2016; Stuhmeier 1996; Su 2016; Venn 1999; Wallace 2004; Wijeysundera 2014a).
Demographics of sample
The mean age of participants in most studies was 60 to 70 years. In addition, the ratio of men to women in the included studies was skewed, with trials generally recruiting disproportionally more men (Characteristics of included studies table).
Intervention and comparators
The number of studies that assessed dexmedetomidine was 24, clonidine was 21 and mivazerol was two. Treatment duration ranged from a single preoperative dose to a 72‐hour course of treatment. With the exception of seven studies (Corbett 2005; Djaiani 2016; Herr 2003; Liu 2016; Park 2014; Shehabi 2009; Venn 2001), all trials compared α‐2 adrenergic agonists against inactive control. Of the four studies with active controls, one compared dexmedetomidine to morphine (Shehabi 2009), whereas the remainder were comparisons of dexmedetomidine versus propofol.
All studies that evaluated dexmedetomidine employed the IV route of administration. Dexmedetomidine was administered intraoperatively in 15 studies, with administration being continued postoperatively in nine of them. The duration of postoperative administration varied across these nine studies, ranging from continuation until arrival to the critical care unit, to continuation for 48 hours. Nine additional studies investigated dexmedetomidine that was administered entirely after surgery in the critical care unit. Both studies of mivazerol administered the drug IV starting from the intraoperative period, with continuation until 72 hours after surgery.
There was considerable variation in the administration regimens used in trials that assessed clonidine. It was administered intraoperatively by the IV route in three studies, with one of these studies also administering an oral loading dose before surgery. A single study used IV clonidine that was administered only preoperatively (i.e. 30 minutes prior to surgery). Four studies employed clonidine administered using the combination of an oral preoperative loading dose, and subsequent maintenance via the transdermal route for 72 hours. Finally, 12 studies administered clonidine orally before surgery, with three of them administering an additional intraoperative dose via the nasogastric route.
Funding
Thirty‐two studies reported their funding sources, whereas 15 did not (Abi‐Jaoude 1993; Chi 2016; Cho 2016; El‐Kerdawy 2004; Ghignone 1986; Ghignone 1987; Lipszyc 1991; Loick 1999; Myles 1999; Park 2014; Pluskwa 1991; Quintin 1993; Ren 2013; Stuhmeier 1996; Viviano 2012). Fourteen studies reported operational funding from pharmaceutical companies (Bergese 2010; Djaiani 2016; Helbo‐Hansen 1986; Herr 2003; Jalonen 1997; Li 2017; McSPI‐Europe 1997; Oliver 1999; Quintin 1996; Su 2016; Talke 1995; Talke 2000; Venn 1999; Venn 2001), and the remaining 18 studies reported that no pharmaceutical funds were used to complete the research (Ammar 2016; Corbett 2005; Devereaux 2014a; Dorman 1993; Ellis 1994; Khalil 2013; Kim 2014a; Lee 2013a; Liu 2016; Matot 2000; Patel 2016; Pawlik 2005; Shehabi 2009; Soliman 2016; Wallace 2004; Wijeysundera 2014a; Xu 2014; Yin 2002). Several studies in the latter group reported that a pharmaceutical company supplied the study drug as in‐kind support, and explicitly stated no further funds were received from the company.
Excluded studies
After the full‐text articles were reviewed, we excluded 337 studies. The reasons for these exclusions are presented in the Characteristics of excluded studies table, as well as the study flow diagram (Figure 1). The most common reason for exclusion was study objectives that differed from this present review (312 excluded studies). In these cases, the focus of these studies was to answer a question unrelated to the efficacy or safety of α‐2 adrenergic agonists for reducing mortality or cardiac complications (e.g. assessing the efficacy of these drugs for providing analgesia). Of the remaining articles, 14 were excluded because the experimental design was not an RCT, three were excluded since participants did not undergoing surgery and three were excluded due to an ineligible population. Two studies could not be classified into either the cardiac or non‐cardiac surgery subgroups, and were therefore excluded (Martin 2003; Triltsch 2002). A further two studies were excluded because the intervention was administered via an ineligible route (Nader 2009; Tzortzopoulou 2009), while one study was excluded due to lack of a control arm (Moghadam 2012). Three reports of two individual studies were excluded due to concerns about scientific misconduct (Boldt 1996; Wahlander 2005). In each of these cases, a lead author was found to have conducted scientific misconduct (Anon 2013; Rasmussen 2011; Wise 2013). Notably, both studies had been included in the previous 2009 version of this review (Wijeysundera 2009), at which point these issues with scientific misconduct had not yet been identified (Boldt 1996; Wahlander 2005).
Studies awaiting classification
No studies are currently awaiting classification.
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
The methodological quality of included studies is shown in the 'Risk of bias' figures (Figure 2; Figure 3). A visual summary of judgements about the quality and risk of bias for each trial is presented in Figure 3. Details explaining the judgements for each domain are presented in the 'Risk of bias' tables (Characteristics of included studies).
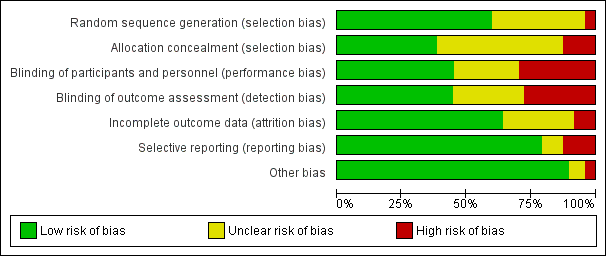
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
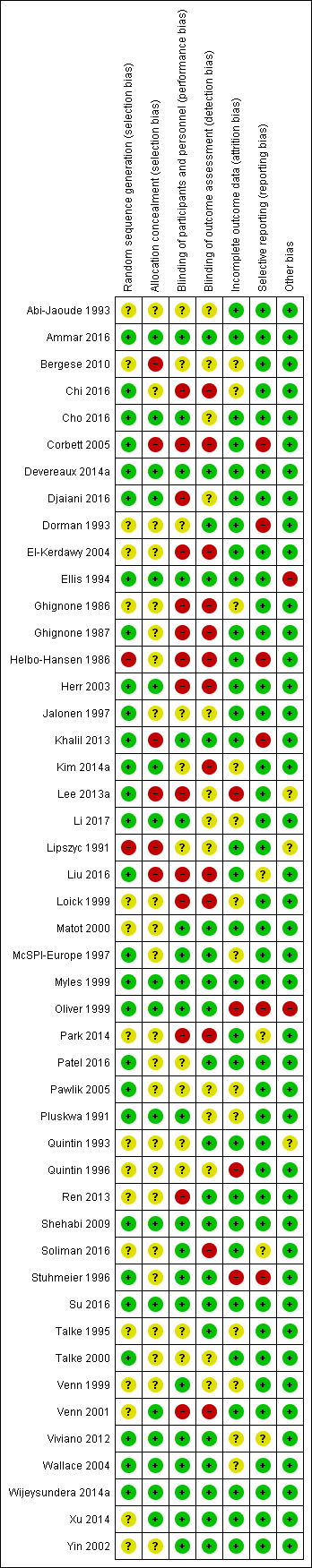
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 47 included trials, only 28 were judged to have adequate methods of generating allocation sequences. Of the remaining 19 studies, two trials used methods likely to produces bias (Helbo‐Hansen 1986; Lipszyc 1991), while the remaining trials were classified as having unclear risk of bias because the methods were not described in adequate detail. Concealment of allocation sequence was generally poor with only 18 studies reporting methods associated with low risk of bias, while six studies described methods associated with a high risk of bias (Bergese 2010; Corbett 2005; Khalil 2013; Lee 2013a; Lipszyc 1991; Liu 2016). Only 16 studies reported adequate allocation sequence generation and allocation concealment.
Blinding
Although 31 studies described themselves as double‐blind, only 21 clearly reported adequate methods for how blinding was achieved. Of the remaining 26 studies, 14 were open‐label and therefore assessed to be high risk of bias, while the others were judged to have an unclear risk of bias. Outcome assessment was blinded in 21 trials, and therefore judged to be at low risk of bias. Only 16 trials demonstrated blinding of participants, personnel and outcome assessors.
Incomplete outcome data
Thirty trials reported no exclusions, exclusions deemed to be appropriate and ITT analysis. For four trials, exclusions (Lee 2013a; Oliver 1999; Quintin 1996; Stuhmeier 1996), were either not reported or judged as being excessive enough to likely cause bias. The remainder either failed to use ITT analysis or adequately account for exclusions. Only 11 studies reported a flow diagram of participants in the trial (Bergese 2010; Chi 2016; Devereaux 2014a; Kim 2014a; Lee 2013a; Li 2017; Liu 2016; Shehabi 2009; Su 2016; Viviano 2012; Wijeysundera 2014a), as is recommended in the CONSORT statement (Schulz 2010).
Selective reporting
Of the 47 trials, 37 demonstrated concordance between outcomes discussed in the methods or protocol and the outcomes reported. Four studies were judged to be of unclear risk of bias because they reported adverse events without discussing any surveillance methods (Liu 2016; Park 2014; Soliman 2016; Viviano 2012). The remaining six studies either failed to report major outcomes, or reported major outcomes not discussed in the relevant methods sections (Corbett 2005; Dorman 1993; Helbo‐Hansen 1986; Khalil 2013; Oliver 1999; Stuhmeier 1996).
Other potential sources of bias
Five trials had other sources of bias classified as unclear risk or high risk. Two of the trials had high risk of bias due to significant changes in their methods during the trial recruitment phase. One trial terminated early (Ellis 1994), while the other changed its selection criteria (Oliver 1999). Three trials were classified as having unclear risk of bias, because two trials (Lipszyc 1991; Quintin 1993), were being published only in abstract form (therefore lacking complete peer‐review), and another lacked reproducible selection criteria (Lee 2013a).
Effects of interventions
See: Summary of findings for the main comparison Alpha‐2 adrenergic agonists compared to control in non‐cardiac surgery; Summary of findings 2 Alpha‐2 adrenergic agonists compared to control in cardiac surgery
Non‐cardiac surgery
Primary outcome
1. All‐cause mortality within 30 days after surgery
Sixteen studies reported all‐cause mortality, with 210 events (1.5%) among 14,081 participants. Alpha‐2 adrenergic agonists had no statistically significant reduction in all‐cause mortality (RR 0.80, 95% CI 0.61 to 1.04, P = 0.10), without any measurable heterogeneity (I2 = 0%) (Analysis 1.1). The quality of this evidence was high (summary of findings Table for the main comparison).
Secondary outcomes
1. Cardiac mortality within 30 days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause
Five studies reported cardiac‐related deaths, with 114 events (0.9%) among 12,525 participants. Alpha‐2 adrenergic agonists did not cause a statistically significant reduction in cardiac‐related mortality (RR 0.86, 95% CI 0.60 to 1.23, P = 0.41) with low measurable heterogeneity (I2 = 16%) (Analysis 1.2). The quality of evidence was high (summary of findings Table for the main comparison).
2. Myocardial infarction within 30 days after surgery (definition as per individual study)
Twelve studies reported MIs, with 835 events (6.0%) among 13,907 participants. Alpha‐2 adrenergic agonists were not associated with any statistically significant difference in the risk of MI (RR 0.94, 95% CI 0.69 to 1.27, P = 0.67) with moderate heterogeneity (I2 = 37%) (Analysis 1.3). The quality of evidence was moderate (summary of findings Table for the main comparison).
3. Myocardial ischaemia within 30 days after surgery: as detected on an electrocardiogram or transoesophageal echocardiogram (definition as per individual study)
Twelve studies reported myocardial ischaemia, with 291 events (21.1%) among 1379 participants. Alpha‐2 adrenergic agonists did not significantly reduced the risk of ischaemia (RR 0.73, 95% CI 0.53 to 1.02, P = 0.06; I2 = 45%) (Analysis 1.4).
4. Supraventricular tachycardia within 30 days after surgery: supraventricular tachycardia, atrial fibrillation or atrial flutter
Two studies reported SVTs, with one event (2.3%) among 44 participants. Both studies evaluated dexmedetomidine. Since there was no events reported in one of the studies (Venn 2001), pooled estimates were not calculated. The remaining trial showed no effect of α‐2 adrenergic agonists on SVT (RR 1.11, 95% CI 0.05 to 24.07) (Analysis 1.5) (Talke 1995).
5. Heart failure within 30 days after surgery: clinical diagnosis of heart failure
Eight studies reported episodes of HF, with 107 events (1.0%) among 10,802 participants. There was no significant reduction in congestive heart failure (CHF) with perioperative α‐2 adrenergic agonist use (RR 1.21, 95% CI 0.83 to 1.75, P = 0.32), with negligible heterogeneity (I2 = 3%) (Analysis 1.6).
Adverse effects from treatment
1. Acute stroke within 30 days after surgery: new focal neurological deficit with signs and symptoms lasting longer than 24 hours
Seven studies reported acute strokes, with 56 strokes (0.5%) among 11,542 participants. Alpha‐2 adrenergic agonists had no significant effect on acute stroke (RR 0.93, 95% CI 0.55 to 1.56 P = 0.79) with no measurable heterogeneity (I2 = 0%) (Analysis 1.7). The quality of evidence for effects on acute stroke was high (summary of findings Table for the main comparison).
Physiological effects of treatment
1. Bradycardia requiring pharmacological or pacemaker treatment
Sixteen studies reported bradycardia, with 1349 events (9.6%) in 14,035 participants. Within these 16 studies, α‐2 adrenergic agonists significantly increased the risk of bradycardia (RR 1.59, 95% CI 1.18 to 2.13, P = 0.002), albeit with substantial heterogeneity (I2 = 53%) (Analysis 1.8). The quality of evidence for treatment effects on bradycardia was moderate (summary of findings Table for the main comparison).
2. Hypotension requiring treatment with inotropes or vasopressors
Fifteen studies reported hypotension, with 4766 events (34.7%) in 13,738 participants. Alpha‐2 adrenergic agonists caused a significant increase in the risk of perioperative hypotension (RR 1.24, 95% CI 1.03 to 1.48, P = 0.02), albeit with substantial heterogeneity (I2 = 54%) (Analysis 1.9). Based on a post‐hoc subgroup analysis, the choice of drug may explain this heterogeneity (Analysis 4.4). Specifically, there was statistically significant evidence of subgroup effects based on whether the studies evaluated clonidine, dexmedetomidine or mivazerol (test of interaction P < 0.001). Clonidine significantly increased the risk of hypotension (RR 1.29, 95% CI 1.23 to 1.35, P < 0.001). Dexmedetomidine was also associated with an increased risk (RR 1.81, 95% CI 1.07 to 3.06, P = 0.03). Conversely, mivazerol did not increase the risk of hypotension (RR 0.95, 95% CI 0.82 to 1.10, P = 0.48). Clonidine and mivazerol subgroup analyses had no measurable heterogeneity (I2 = 0%), whereas the dexmedetomidine subgroup analysis demonstrated significant heterogeneity (I2 = 50%). The quality of evidence for treatment effects on hypotension was moderate (summary of findings Table for the main comparison).
Cardiac surgery
Primary outcome
1. All‐cause mortality within 30 days after surgery
Sixteen studies reported all‐cause mortality, with 29 events (1.5%) among 1949 participants. Alpha‐2 adrenergic agonists did not result in a statistically significant reduction in all‐cause mortality (RR 0.52, 95% CI 0.26 to 1.04, P = 0.06), without any measurable heterogeneity (I2 = 0%) (Analysis 2.1). The quality of this evidence was moderate (summary of findings Table 2).
Secondary outcomes
1. Cardiac mortality within 30 days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause
Only one study reported cardiac mortality, with 1 event among the 12 participants in the clonidine arm and no events among the 10 participants in the control arm (Loick 1999). Thus, no pooled analysis was performed.
2. Myocardial infarction within 30 days after surgery: definition as per individual study
Eight studies reported MIs, with 16 events (2.0%) among 782 participants. Alpha‐2 adrenergic agonists were not associated with reduced risk of MI (RR 1.01, 95% CI 0.43 to 2.40, P = 0.98) in an analysis with no heterogeneity (I2 = 0%) (Analysis 2.2). The quality of evidence was moderate (summary of findings Table 2).
3. Myocardial ischaemia within 30 days after surgery: as detected on an electrocardiogram or transoesophageal echocardiogram (definition as per individual study)
Thirteen studies reported myocardial ischaemia, with 243 events (21.4%) among 1134 participants. Alpha‐2 adrenergic agonists significantly reduced the risk of ischaemia (RR 0.69, 95% CI 0.56 to 0.86, P < 0.001) with no heterogeneity (I2 = 0%) (Analysis 2.3).
4. Supraventricular tachycardia within 30 days after surgery: supraventricular tachycardia, atrial fibrillation or atrial flutter
Six studies reported SVTs, with 79 events (7.7%) among 1044 participants. Alpha‐2 adrenergic agonists had no significant effect on the risk of SVT (RR 0.77, 95% CI 0.50 to 1.16, P = 0.21) with low measurable heterogeneity (I2 = 24%) (Analysis 2.4).
5. Heart failure within 30 days after surgery: clinical diagnosis of heart failure or need for postoperative intra‐aortic balloon pump support
Four studies reported 38 HF events (6.9%) among 549 participants. Alpha‐2 adrenergic agonists had no statistically significant effect on the risk of HF (RR 0.90, 95% CI 0.49 to 1.63, P = 0.72) with no measurable heterogeneity (I2 = 0%) (Analysis 2.5).
Adverse effects from treatment
1. Acute stroke within 30 days after surgery: new focal neurological deficit with signs and symptoms lasting longer than 24 hours
Seven studies reported acute stroke, with 18 events (1.5%) among 1175 participants. Alpha‐2 adrenergic agonists significantly reduced the risk of acute stroke (RR 0.37, 95% CI 0.15 to 0.93, P = 0.03; I2 = 0%) (Analysis 2.6). The quality of evidence was low (summary of findings Table 2).
Physiological effects of treatment
1. Bradycardia requiring pharmacological or pacemaker treatment
Ten studies reported episodes of bradycardia, with 136 events (9.2%) among 1477 participants. Pooled analysis demonstrated that α‐2 adrenergic agonists significantly increased the risk of bradycardia (RR 1.88, 95% CI 1.35 to 2.62, P = 0.0002) with no heterogeneity (I2 = 0%) (Analysis 2.7). The quality of evidence was moderate (summary of findings Table 2).
2. Hypotension requiring treatment with inotropes or vasopressors
Nine studies reported 494 episodes of hypotension (35%) among 1413 participants. Alpha‐2 adrenergic agonists did not significantly increase the risk of hypotension (RR 1.19, 95% CI 0.87 to 1.64, P = 0.28) in an analysis with substantial heterogeneity (I2 = 72%) (Analysis 2.8). The quality of evidence was low (summary of findings Table 2).
Subgroup analyses
Vascular versus non‐vascular non‐cardiac surgery
There was no statistically significant evidence of subgroup effects based on procedure type (i.e. vascular versus non‐vascular procedures) with respect to the outcomes of all‐cause mortality (test of interaction P = 0.17; Analysis 3.1), cardiac mortality (test of interaction P = 0.13; Analysis 3.2), MI (test of interaction P = 0.13; Analysis 3.3), and myocardial ischaemia (test of interaction P = 0.17; Analysis 3.4).
Drug (i.e. clonidine, mivazerol or dexmedetomidine) evaluated in non‐cardiac surgery
There was no statistically significant evidence of subgroup effects based on the specific α‐2 adrenergic agonist evaluated with respect to the outcomes of all‐cause mortality (test of interaction P = 0.50) (Analysis 4.1), and MI (test of interaction P = 0.48) (Analysis 4.3). Conversely, there was a statistically significant subgroup effect with respect to cardiac mortality (test of interaction P = 0.05) (Analysis 4.2). In these subgroup analyses, mivazerol significantly reduced cardiac mortality (RR 0.51, 95% CI 0.27 to 0.98, P = 0.04), whereas clonidine did not (RR 1.12, 95% CI 0.71 to 1.75, P = 0.63). There were insufficient studies that reported myocardial ischaemia as an outcome for dexmedetomidine or mivazerol to facilitate drug‐specific subgroup analysis for the outcome.
Sensitivity analyses
Studies that clearly reported blinding and concealed allocation
The pooled effects of α‐2 adrenergic agonists on all‐cause mortality (RR 0.68, 95% CI 0.41 to 1.11, P = 0.12; participants = 13,066; studies = 7; Analysis 5.1), MI (RR 1.08, 95% CI 0.95 to 1.23, P = 0.26; participants = 13,026; studies = 6; Analysis 5.2), and myocardial ischaemia (RR 0.77, 95% CI 0.40 to 1.48, P = 0.43; participants = 412; studies = 3; Analysis 5.3) were qualitatively similar when analyses were restricted to trials that clearly reported methods for blinding and allocation concealment.
Strict definitions of myocardial infarction and ischaemia
When analyses were restricted to trials that strictly defined MI on ECG or enzymatic criteria, pooled treatment effects in non‐cardiac surgery (RR 0.98, 95% CI 0.70 to 1.36, P = 0.90; participants = 13,003; studies = 8) and cardiac surgery (RR 0.76, 95% CI 0.19 to 2.98, P = 0.69; participants = 275; studies = 3) were qualitatively unchanged (Analysis 6.1). When analyses were restricted to studies that strictly defined events of myocardial ischaemia, the effects in non‐cardiac surgery remained non‐significant (RR 0.76, 95% CI 0.54 to 1.07, P = 0.12; participants = 1175; studies = 9). In cardiac surgery, the sensitivity analysis continued to demonstrate a reduction in the risk of ischaemia (RR 0.71, 95% CI 0.55 to 0.91, P = 0.007; participants = 820; studies = 8) (Analysis 6.2).
Influence of two large trials
The overall results of this review are likely highly influenced by two large RCTs in non‐cardiac surgery, one of which assessed mivazerol (Oliver 1999), while the other assessed clonidine (Devereaux 2014a). Therefore, we performed a post‐hoc sensitivity analysis that excluded these studies. After excluding these two trials, treatment effect on all‐cause mortality became statistically significant (RR 0.45, 95% CI 0.22 to 0.93, P = 0.03; participants = 2174; studies = 14; Analysis 7.1). Conversely, the effect on cardiac mortality (RR 0.47, 95% CI 0.10 to 2.25, P = 0.35; participants = 618; studies = 3; Analysis 7.2), and MI (RR 0.56, 95% CI 0.25 to 1.25, P = 0.16; participants = 2000; studies = 10; Analysis 7.3) were statistically non‐significant, albeit with more optimistic individual point estimates (i.e. pooled treatment effects shifted towards larger risk reductions).
Excluding drugs not introduced into clinical practice (i.e. mivazerol)
In post‐hoc sensitivity analyses excluding the two trials that evaluated mivazerol (McSPI‐Europe 1997; Oliver 1999), there was no change in pooled treatment effects pertaining to all‐cause mortality, cardiac mortality, MI, SVT, HF, stroke, bradycardia, or hypotension (Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.5; Analysis 8.6; Analysis 8.7; Analysis 8.8; Analysis 8.9). Conversely, the pooled treatment effect on ischaemia became statistically significant (RR 0.68, 95% CI 0.48 to 0.97, P = 0.03; participants = 1079; studies = 11; I2 = 40%) (Analysis 8.4), albeit in an analysis with moderate heterogeneity and relatively few participants.
Restricting studies more representative of contemporary perioperative practice
When analyses pertaining to non‐cardiac surgery were restricted to studies that collected data within the previous 20 years, there was no change in the pooled treatment effects pertaining to all‐cause mortality, cardiac mortality, MI, HF or stroke (Analysis 9.1; Analysis 9.2; Analysis 9.3; Analysis 9.5; Analysis 9.6). Nonetheless, exclusion of older studies resulted in a significant reduction in the risk of myocardial ischaemia (RR 0.51, 95% CI 0.28 to 0.93, P = 0.03; participants = 634; studies = 6; I2 = 48%) in an analysis with moderate heterogeneity (Analysis 9.4). In cardiac surgery, exclusion of older studies resulted in no substantive effect on the pooled treatment effects for MI, myocardial ischaemia, SVT, HF or stroke (Analysis 10.2; Analysis 10.3; Analysis 10.4; Analysis 10.5; Analysis 10.6). Conversely, the pooled treatment effect on all‐cause mortality became statistically significant (RR 0.47, 95% CI 0.23 to 0.97; participants = 1782; studies = 13; I2 = 0%) (Analysis 10.1).
Funnel plots
Funnel plots of included studies revealed no obvious publication bias with regard to the outcome of mortality (Figure 4), but some possible bias with regard to MI (Figure 5). Since this analysis pooled results from only nine studies, formal statistical testing for asymmetry was not conducted.
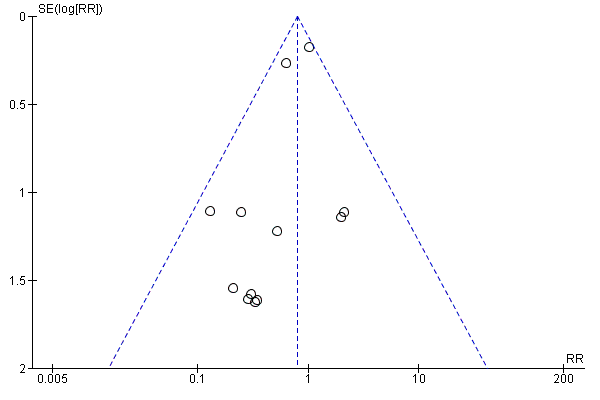
Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.1 All‐cause mortality.
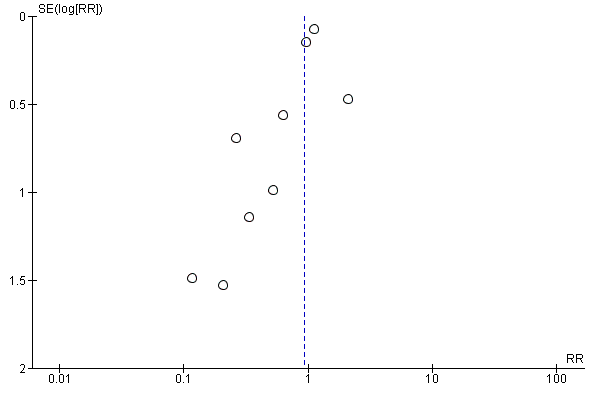
Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.3 Myocardial infarction.
Discussion
Summary of main results
Our present review found high‐quality evidence that perioperative α‐2 adrenergic agonists did not reduce the risk of all‐cause mortality, cardiac mortality or MI in people undergoing non‐cardiac or cardiac surgery (summary of findings Table for the main comparison; summary of findings Table 2). These findings remained stable in sensitivity analyses restricted to studies that either demonstrated low risks of bias or employed strict definitions of MI. Aside from lacking any beneficial effect on these clinical outcomes, α‐2 adrenergic agonists also conferred important risks, specifically increased rates of hypotension and bradycardia. While these haemodynamic effects were not associated with an increased risk of acute stroke, the 95% CIs for this pooled effect were wide (RR 0.93, 95% CI 0.55 to 1.56), thereby not excluding the possibility of a moderate increase in stroke risk with perioperative α‐2 adrenergic agonists.
Overall completeness and applicability of evidence
The 47 RCTs included in this systematic review encompassed 17,039 participants, a wide range of relevant surgical procedures performed in several different countries internationally and clinically relevant dosing regimens of currently available α‐2 adrenergic agonists (i.e. clonidine, dexmedetomidine). Furthermore, a significant number of participants from the included studies underwent surgery within the past decade. Thus, we are confident that the overall findings of our systematic review, namely that α‐2 adrenergic agonists do not significantly reduce risks of cardiovascular complications or mortality when given prophylactically before major non‐cardiac or cardiac surgery, can be reasonably extrapolated to contemporary perioperative practice.
Nonetheless, there were insufficient participants within specific subgroups to conclusively evaluate several potential benefits of α‐2 adrenergic agonists, namely prevention of stroke, myocardial ischaemia and all‐cause mortality after cardiac surgery. The subgroup analysis evaluating effects on stroke during cardiac surgery was small, with only seven included studies that encompassed 1175 participants (Analysis 2.6). The pooled estimate was based on low‐quality data, calculated using very few outcome events (i.e. 18 strokes). Previous research has found that treatment effects are generally overestimated in meta‐analyses that include relatively few outcome events (Thorlund 2011). Consistent with this possibility, the magnitude of the pooled estimate was somewhat implausible (RR 0.37, 95% CI 0.15 to 0.93), in that it suggested a 63% relative reduction in the risk of stroke from a single perioperative intervention. Therefore, further research is needed to determine whether α‐2 adrenergic agonists can truly reduce the risk of acute stroke after cardiac surgery.
Similarly, the statistically significant pooled treatment effect on all‐cause mortality was observed only in a post‐hoc subset analysis restricted to cardiac surgery trials conducted after 1997 (Analysis 10.1). This subset was relatively small (13 studies encompassing 1782 participants), the pooled estimated was calculated using very few outcome events (i.e. 28 deaths) and the magnitude of the pooled estimate was somewhat implausible (RR 0.47, 95% CI 0.23 to 0.97) for a single intervention. More studies are needed to assess the effect of α‐2 adrenergic agonists on all‐cause mortality after cardiac surgery.
In a separate subgroup analysis in cardiac surgery, α‐2 adrenergic agonists also caused a significant reduction in perioperative myocardial ischaemia (Analysis 2.3). Nonetheless, myocardial ischaemia is a surrogate outcome with important associated limitations (Svensson 2013). Especially in the absence of associated reductions in clinical important and patient‐relevant outcomes such as mortality or MI, isolated reductions in perioperative myocardial ischaemia are not sufficient justifications for employing α‐2 adrenergic agonists in clinical practice.
Quality of the evidence
This systematic review was supported by 47 RCTs that recruited 17,039 participants. The sample size of the included RCTs varied greatly, ranging from 20 to over 10,000 participants. Nineteen studies had over 100 participants, with only two studies involving over 1000 participants (Devereaux 2014a; Oliver 1999). The vast majority of these participants (14,367) were recruited into the 23 included trials in non‐cardiac surgery. By comparison, the remaining 24 RCTs in cardiac surgery involved 2672 participants.
Only 16 studies reported adequate methods for random sequence generation and allocation concealment. Furthermore, although 31 studies described themselves as 'double‐blinded,' only 21 studies reported appropriate methods to achieve blinding. Nonetheless, the majority of participants were from well‐designed studies with adequate methods for allocation and blinding, thereby rendering them low risk to be influenced by selection bias, performance bias and detection bias.
The analyses pertaining to the primary and secondary outcomes in people undergoing non‐cardiac surgery were generally robust. These findings were judged as moderate to high quality by GRADE methodology (summary of findings Table for the main comparison). Although many studies failed to report adequate methods to avoid risk of bias, the specific outcomes were unlikely to be influenced and thus no downgrading of quality was necessary. In addition, there was a potential threat of indirectness since the second largest RCT evaluated mivazerol (Oliver 1999), which is not available for clinical use. However, given the similarity of mivazerol to dexmedetomidine (which is available for clinical use), we reasoned that this risk was likely not serious. Conversely, funnel plots suggested that the pooled treatment effects on MI was affected by publication bias (Figure 5). The asymmetry in the funnel plots was produced by two small studies with seemingly unrealistic effect sizes (Ellis 1994; Stuhmeier 1996). While the combined weight of these studies was less than 3% of the pooled analysis, we downgraded the evidence by one level because of suspicion of publication bias for an outcome known to be influenced by performance bias (Analysis 1.3). Finally, the quality of evidence for the physiological effects of bradycardia and hypotension were both downgraded because of substantial heterogeneity (I2 greater than 50%) in the analyses (Analysis 1.8; Analysis 1.9).
The quality of evidence for the effects of α‐2 adrenergic agonists in cardiac surgery was generally lower, largely due to imprecision resulting from significantly fewer participants in the pooled analyses (summary of findings Table 2). The quality of evidence for all outcomes was downgraded because the optimal information size of 2000 participants was not achieved, and the 95% CIs of the pooled estimates did not rule out clinically significant effects (GRADE Handbook 2013). Thus, the quality of evidence for the analyses pertaining to all‐cause mortality and MI was moderate. As there were very few outcomes events in the analysis of acute stroke (i.e. 18 strokes), it was downgraded another level and judged as low quality. Finally, the presence of substantial imprecision in pooled analysis pertaining to hypotension led to the quality of this evidence being downgraded to low (I2 = 72%; Analysis 2.8). Nonetheless, the magnitude of the association between α‐2 adrenergic agonists and hypotension in cardiac surgery (RR 1.19, 95% CI 0.87 to 1.64) was qualitatively very similar to that observed in non‐cardiac surgery (RR 1.24, 95% CI 1.03 to 1.48), where the quality of evidence was moderate.
Potential biases in the review process
There were several limitations to our review process. First, while our search was exhaustive in that it covered all major medical indexes and clinical trial registries, we might have missed some published trials only listed in other less commonly used indices. Nonetheless, we believe it unlikely that our search strategy missed any relevant studies of at least moderate size and quality. Second, we only included studies reporting subgroup‐specific outcome data based on surgical procedure type (i.e. cardiac surgery versus non‐cardiac surgery). Consequently, we excluded any study that did not predominantly include procedures (greater than 75%) from either of these surgical procedure subgroup, unless subgroup‐specific outcome data could be obtained from the authors. Consequently, two otherwise eligible studies could not be included in this systematic review (Martin 2003; Triltsch 2002). In excluding these studies, we balanced the risk of biasing the analyses with the loss of additional data, and chose the latter to maintain the integrity of our analyses.
Agreements and disagreements with other studies or reviews
There are several potential reasons why the theoretically beneficial physiological effects of α‐2 adrenergic agonists did not translate into reduced rates of major postoperative cardiac complications. First, α‐2 adrenergic agonists might not have sufficiently reduced heart rates to mitigate the risks of perioperative MI, as has been previously proposed (Devereaux 2014a). This hypothesis is supported by the observation that, while perioperative β‐blockers caused more significant bradycardia than α‐2 adrenergic agonists, rates of perioperative MI were reduced with β‐blockers but not α‐2 adrenergic agonists (Wijeysundera 2014b). Second, the predominant mechanism underlying perioperative MI in many affected people might not be increases in blood pressure and heart rate induced by the surgical stress response. For example, almost 30% of people with postoperative MI do not have significant obstructive coronary artery disease (Sheth 2015). It is unlikely that limiting perioperative increases in heart rate would help prevent MI in such people. Third, at a population‐level, the beneficial effects of heart rate reduction in some people undergoing surgery might have been offset by equal numbers of people who experienced deleterious effects from significant perioperative hypotension.
Notably, our overall findings with respect to the absence of major reductions in perioperative cardiovascular risk from α‐2 adrenergic agonists was somewhat consistent with results seen with prophylactic therapy with other sympatholytic agents. Specifically, β‐adrenergic blockers have been shown to cause a net harmful effect in non‐cardiac surgery (Wijeysundera 2014b). It remains to be seen whether any strategy of prophylactically attenuating haemodynamic abnormalities with sympatholytic agents can safely reduce perioperative cardiac risk. Indeed, if future RCTs with either alternative regimens of previously evaluated sympatholytic agents (i.e. α‐2 adrenergic agonists, β‐adrenergic blockers) or alternative negative chronotropic agents (e.g. ivabradine) fail to show net overall benefit, the general strategy for perioperative cardiac risk reduction may have to shift from prophylactic therapy to early treatment. Specifically, as opposed to administering these medications to a broad group of people before surgery, clinicians might instead consider targeting treatment in high‐risk people identified on the basis of ischaemic ECG changes or elevated cardiac troponin concentrations early after surgery.
Comparison of our present systematic review on perioperative α‐2 adrenergic agonists with a prior systematic review of perioperative β‐adrenergic blockers in non‐cardiac surgery also provides some potential insights into the mechanisms underlying perioperative stroke (Wijeysundera 2014b). Specifically, α‐2 adrenergic agonists (RR 1.24, 95% CI 1.03 to 1.48) and β‐adrenergic blockers (RR 1.47, 95% CI 1.34 to 1.60) conferred approximately similar risks of perioperative hypotension. Despite this similarity in haemodynamic effects, β‐blockers (RR 1.86, 95% CI 1.09 to 3.16) conferred significantly increased risks of perioperative acute stroke while α‐2 adrenergic agonists did not (RR 0.93, 95% CI 0.55 to 1.56). These contrasting effects are also evident when comparing two large individual perioperative RCTs of these two different drug classes, namely the POISE‐1 trial of metoprolol (POISE 2008) and the POISE‐2 trial of clonidine (Devereaux 2014a). Specifically, while metoprolol (hazard ratio (HR) 1.55, 95% CI 1.38 to 1.74) and clonidine (HR 1.32, 95% CI 1.24 to 1.40) caused qualitatively similar increases in rates of hypotension, metoprolol significantly increased risks of stroke (HR 2.17, 95% CI 1.26 to 3.74) while clonidine did not (HR 1.06, 95% CI 0.54 to 2.05). These findings suggest that, despite the previously observed association between perioperative hypotension and stroke (POISE 2008), the mechanisms underlying perioperative acute stroke are likely more complex than simply a decrease in perfusion pressure. Further research is needed to better delineate these mechanisms, and thereby inform the development of strategies to help prevent this often devastating perioperative complication (POISE 2008).
Importantly, perioperative α‐2 adrenergic agonists do have other potential benefits that may justify their selective use in some people undergoing surgery. Specifically, in a previous systematic review of 30 small RCTs encompassing 1792 participants, these agents decreased both postoperative pain intensity and morphine consumption (Blaudszun 2012). Our present study provided additional data supporting the safety of using α‐2 adrenergic agonists as an adjunct therapy for managing postoperative acute pain, provided that there is adequate attention to the associated risks of hypotension and bradycardia.
Strengths
Our present review had several strengths. The literature search was extensive and encompassed all languages. In addition, our study utilized a substantially larger data set than previous reviews (Biccard 2008; Nishina 2002; Stevens 2003; Wijeysundera 2003; Wijeysundera 2009). Finally, we performed multiple sensitivity analyses to assess the potential influence of study quality, outcome definition and publication bias on our overall conclusions.
Limitations
Our review had several limitations that should be considered. First, the results were heavily influenced by two large trials of mivazerol (Oliver 1999) and clonidine (Devereaux 2014a) in non‐cardiac surgery. While excluding these large trials from the meta‐analysis resulted in somewhat more optimistic point estimates for individual pooled treatment effects, these pooled estimates remained statistically non‐significant. Second, as with any systematic review, our results may have been affected by publication bias. Though funnel plots indicated no obvious bias in the reporting of death (all‐cause and cardiac‐cause), they did suggest reporting bias may be present for MI. Third, our present analysis pooled trials of α‐2 adrenergic agonists with differing selectivity for their target receptors, namely α‐2 adrenoceptors and non‐adrenergic imidazoline receptors (Khan 1999). Specifically, both mivazerol and dexmedetomidine have considerably greater selectivity for these target receptors than clonidine. Furthermore, when comparing the two large individual trials that dominated this meta‐analysis (Devereaux 2014a; Oliver 1999), clonidine had no effect on mortality and increased rates of significant perioperative hypotension, while mivazerol, a more selective α‐2 adrenergic agonist that is not currently available for clinical use, was associated with trends towards reduced mortality and had no effect on rates of hypotension. Importantly, these contrasting findings might have also been due to the different time periods when the studies were conducted, differences in study design, differences in participant characteristics or chance. Nonetheless, the contrasting findings still suggest that any future RCT of α‐2 adrenergic agonists for cardiac risk reduction in people undergoing surgery should focus on agents with higher selectivity for α‐2 adrenoceptors, such as dexmedetomidine or mivazerol.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.1 All‐cause mortality.

Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.3 Myocardial infarction.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 1 All‐cause mortality.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 2 Cardiac mortality.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 3 Myocardial infarction.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 4 Myocardial ischaemia.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 5 Supraventricular tachyarrhythmia.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 6 Heart failure.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 7 Acute stroke.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 8 Bradycardia.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 9 Hypotension.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 1 All‐cause mortality.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 2 Myocardial infarction.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 3 Myocardial ischaemia.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 4 Supraventricular tachyarrhythmia.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 5 Heart failure.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 6 Acute stroke.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 7 Bradycardia.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 8 Hypotension.

Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 1 All‐cause mortality.

Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 2 Cardiac mortality.
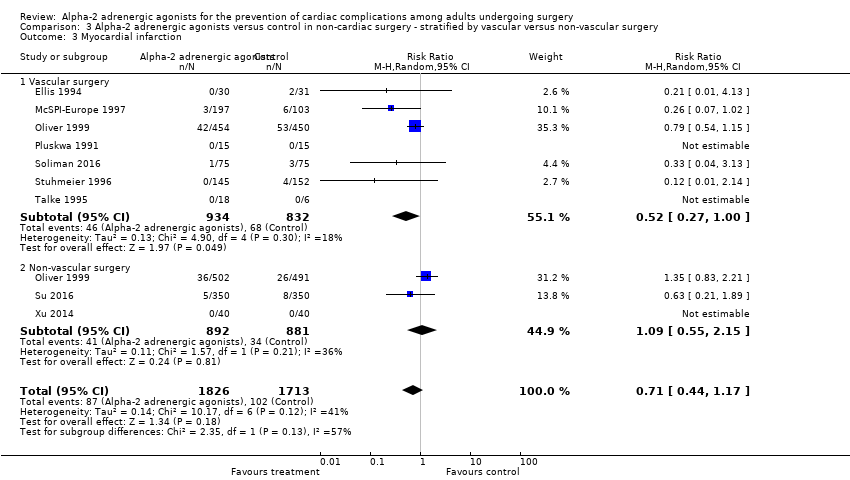
Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 3 Myocardial infarction.

Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 4 Myocardial ischaemia.

Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 1 All‐cause mortality.
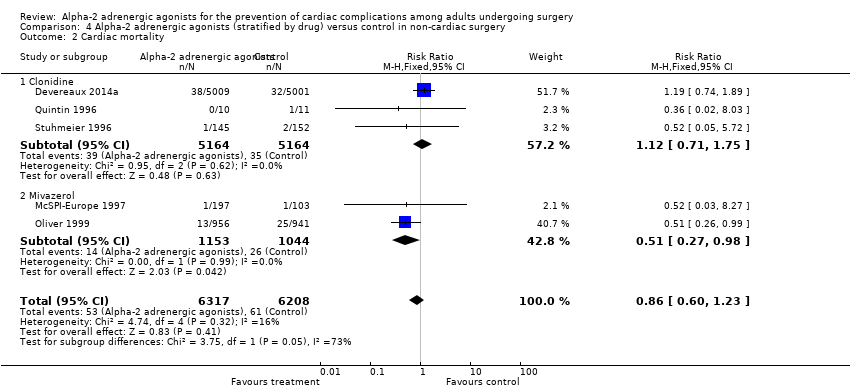
Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 2 Cardiac mortality.

Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 3 Myocardial infarction.

Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 4 Hypotension.

Comparison 5 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation, Outcome 1 All‐cause mortality.

Comparison 5 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation, Outcome 2 Myocardial infarction.

Comparison 5 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation, Outcome 3 Myocardial ischaemia.

Comparison 6 Alpha‐2 adrenergic agonists versus control in studies that used strict definitions of myocardial infarction or ischaemia, Outcome 1 Myocardial infarction.

Comparison 6 Alpha‐2 adrenergic agonists versus control in studies that used strict definitions of myocardial infarction or ischaemia, Outcome 2 Myocardial ischaemia.

Comparison 7 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014), Outcome 1 All‐cause mortality.

Comparison 7 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014), Outcome 2 Cardiac mortality.
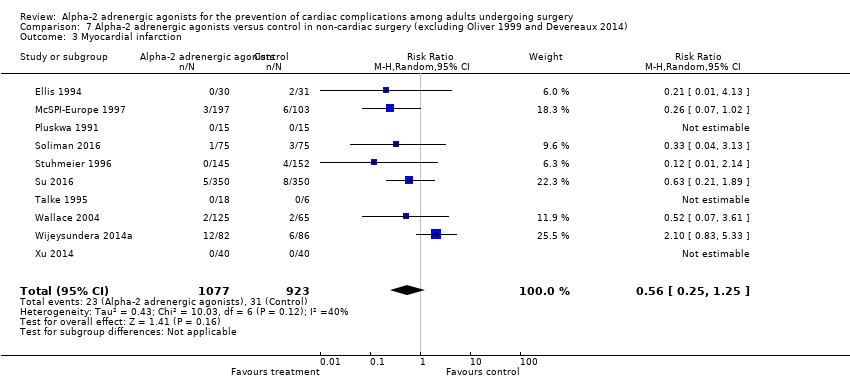
Comparison 7 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014), Outcome 3 Myocardial infarction.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 1 All‐cause mortality.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 2 Cardiac mortality.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 3 Myocardial infarction.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 4 Myocardial ischaemia.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 5 Supraventricular tachyarrhythmia.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 6 Heart failure.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 7 Acute stroke.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 8 Bradycardia.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 9 Hypotension.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 1 All‐cause mortality.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 2 Cardiac mortality.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 3 Myocardial infarction.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 4 Myocardial ischaemia.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 5 Heart failure.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 6 Acute stroke.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 1 All‐cause mortality.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 2 Myocardial infarction.
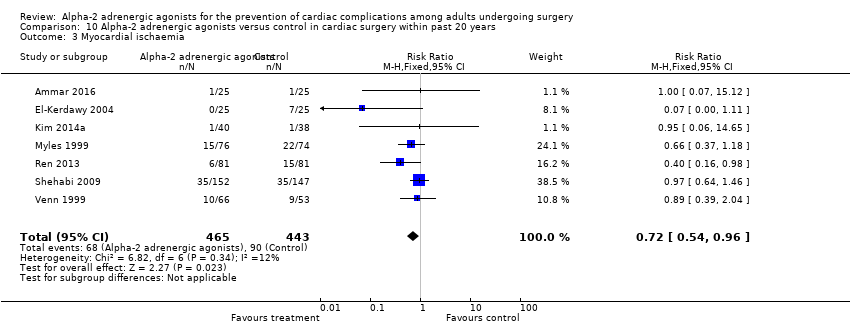
Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 3 Myocardial ischaemia.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 4 Supraventricular tachyarrhythmia.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 5 Heart failure.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 6 Acute stroke.
| Alpha‐2 adrenergic agonists compared to control in non‐cardiac surgery | ||||||
| Patient or population: adults undergoing non‐cardiac surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Risk ratio | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
| All‐cause mortality (within 30‐days after surgery: any reported death) | Study population | RR 0.80 | 14,081 | ⊕⊕⊕⊕ | ‐ | |
| 17 per 1000 | 13 per 1000 | |||||
| Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause.) | Study population | RR 0.86 | 12,525 | ⊕⊕⊕⊕ | ‐ | |
| 10 per 1000 | 8 per 1000 | |||||
| Myocardial infarction (within 30‐days after surgery: as detected on an electrocardiogram or trans‐oesophageal echocardiogram) | Study population | RR 0.94 | 13,907 | ⊕⊕⊕⊝ | ‐ | |
| 59 per 1000 | 55 per 1000 | |||||
| Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) | Study population | RR 0.93 | 11,542 | ⊕⊕⊕⊕ | ‐ | |
| 5 per 1000 | 4 per 1000 | |||||
| Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) | Study population | RR 1.59 | 14,035 | ⊕⊕⊕⊝ | ‐ | |
| 75 per 1000 | 119 per 1000 | |||||
| Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) | Study population | RR 1.24 | 13,738 | ⊕⊕⊕⊝ | ‐ | |
| 304 per 1000 | 377 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias was not serious. Although multiple studies lacked proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded. 2Indirectness not serious. Intervention (mivazerol) used in one large study not available for clinical use. Not downgraded. 3Evidence of publication bias in funnel plot of analysis. Downgraded by one level. 4Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded by one level. | ||||||
| Alpha‐2 adrenergic agonists compared to control in cardiac surgery | ||||||
| Patient or population: adults undergoing cardiac surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
| All‐cause mortality (within 30‐days after surgery: any reported death) | Study population | RR 0.52 | 1947 | ⊕⊕⊕⊝ | ‐ | |
| 21 per 1000 | 11 per 1000 | |||||
| Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) | 1 death from 12 participants in clonidine arm, and no deaths in 10 participants in control arm. | Not estimable | 22 (1 RCT) | Not estimable | We did not GRADE evidence for this outcome as accurate estimation of RRs is not possible for such low event rates. | |
| Myocardial infarction (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) | Study population | RR 1.01 | 782 | ⊕⊕⊕⊝ | ‐ | |
| 20 per 1000 | 21 per 1000 | |||||
| Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) | Study population | RR 0.37 | 1175 | ⊕⊕⊝⊝ | Total of 18 acute stokes reported, with 14 in control group and 4 in treatment group. | |
| 24 per 1000 | 9 per 1000 | |||||
| Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) | Study population | RR 1.88 | 1477 | ⊕⊕⊕⊝ | ‐ | |
| 64 per 1000 | 120 per 1000 | |||||
| Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) | Study population | RR 1.19 | 1413 | ⊕⊕⊝⊝ | ‐ | |
| 332 per 1000 | 395 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias was not serious. Although multiple studies lack proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded. 2Serious imprecision, because analysis was below optimal information size and confidence interval includes significant benefit and harm. Downgraded by one level. 3Very serious imprecision, because analysis is below optimal information size and number of events was very small. Downgraded by two levels. 4Serious imprecision, because analysis was below optimal information size. Downgraded by one level 5Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded one level. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 16 | 14081 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.04] |
| 2 Cardiac mortality Show forest plot | 5 | 12525 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.23] |
| 3 Myocardial infarction Show forest plot | 12 | 13907 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 4 Myocardial ischaemia Show forest plot | 12 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.53, 1.02] |
| 5 Supraventricular tachyarrhythmia Show forest plot | 2 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.05, 24.07] |
| 6 Heart failure Show forest plot | 8 | 10802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.75] |
| 7 Acute stroke Show forest plot | 7 | 11542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.55, 1.56] |
| 8 Bradycardia Show forest plot | 16 | 14035 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.18, 2.13] |
| 9 Hypotension Show forest plot | 15 | 13738 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.03, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 16 | 1947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |
| 2 Myocardial infarction Show forest plot | 8 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.43, 2.40] |
| 3 Myocardial ischaemia Show forest plot | 13 | 1134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.56, 0.86] |
| 4 Supraventricular tachyarrhythmia Show forest plot | 6 | 1044 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.50, 1.16] |
| 5 Heart failure Show forest plot | 4 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.49, 1.63] |
| 6 Acute stroke Show forest plot | 7 | 1175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.93] |
| 7 Bradycardia Show forest plot | 10 | 1477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.35, 2.62] |
| 8 Hypotension Show forest plot | 9 | 1413 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.87, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 13 | 3713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 1.1 Vascular surgery | 8 | 1798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.25, 0.88] |
| 1.2 Non‐vascular surgery | 6 | 1915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.46, 1.67] |
| 2 Cardiac mortality Show forest plot | 4 | 2515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.93] |
| 2.1 Vascular surgery | 4 | 1522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.79] |
| 2.2 Non‐vascular surgery | 1 | 993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.35, 2.77] |
| 3 Myocardial infarction Show forest plot | 9 | 3539 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.44, 1.17] |
| 3.1 Vascular surgery | 7 | 1766 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 1.00] |
| 3.2 Non‐vascular surgery | 3 | 1773 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.55, 2.15] |
| 4 Myocardial ischaemia Show forest plot | 9 | 961 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.17] |
| 4.1 Vascular surgery | 6 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.54, 1.29] |
| 4.2 Non‐vascular surgery | 3 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.04, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 16 | 14081 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.04] |
| 1.1 Clonidine | 7 | 10787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.23] |
| 1.2 Dexmedetomidine | 7 | 1097 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.15, 1.58] |
| 1.3 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.42, 1.15] |
| 2 Cardiac mortality Show forest plot | 5 | 12525 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.23] |
| 2.1 Clonidine | 3 | 10328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.75] |
| 2.2 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.98] |
| 3 Myocardial infarction Show forest plot | 12 | 13907 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 3.1 Clonidine | 6 | 10756 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.57, 1.92] |
| 3.2 Dexmedetomidine | 4 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.49] |
| 3.3 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.08] |
| 4 Hypotension Show forest plot | 15 | 13738 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.03, 1.48] |
| 4.1 Clonidine | 7 | 10485 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.23, 1.35] |
| 4.2 Dexmedetomidine | 6 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.07, 3.06] |
| 4.3 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 7 | 13066 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.41, 1.11] |
| 2 Myocardial infarction Show forest plot | 6 | 13026 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.23] |
| 3 Myocardial ischaemia Show forest plot | 3 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.40, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Non‐cardiac surgery | 8 | 13003 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 1.2 Cardiac surgery | 3 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.19, 2.98] |
| 2 Myocardial ischaemia Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Non‐cardiac surgery | 9 | 1175 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 2.2 Cardiac surgery | 8 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.55, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 14 | 2174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.22, 0.93] |
| 2 Cardiac mortality Show forest plot | 3 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.10, 2.25] |
| 3 Myocardial infarction Show forest plot | 10 | 2000 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 14 | 11884 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.16] |
| 2 Cardiac mortality Show forest plot | 3 | 10328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.75] |
| 3 Myocardial infarction Show forest plot | 10 | 11710 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.59, 1.53] |
| 4 Myocardial ischaemia Show forest plot | 11 | 1079 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.48, 0.97] |
| 5 Supraventricular tachyarrhythmia Show forest plot | 2 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.05, 24.07] |
| 6 Heart failure Show forest plot | 7 | 10502 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.85, 1.84] |
| 7 Acute stroke Show forest plot | 6 | 11242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.49, 1.63] |
| 8 Bradycardia Show forest plot | 14 | 11838 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.17, 2.36] |
| 9 Hypotension Show forest plot | 13 | 11541 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.15, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 11 | 13378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.06] |
| 2 Cardiac mortality Show forest plot | 2 | 11907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.29] |
| 3 Myocardial infarction Show forest plot | 7 | 13195 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.24] |
| 4 Myocardial ischaemia Show forest plot | 6 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.28, 0.93] |
| 5 Heart failure Show forest plot | 5 | 10424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.89, 2.03] |
| 6 Acute stroke Show forest plot | 5 | 11218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.50, 1.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 13 | 1782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.23, 0.97] |
| 2 Myocardial infarction Show forest plot | 4 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.22, 3.03] |
| 3 Myocardial ischaemia Show forest plot | 7 | 908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.54, 0.96] |
| 4 Supraventricular tachyarrhythmia Show forest plot | 5 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.44, 1.19] |
| 5 Heart failure Show forest plot | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.48, 1.77] |
| 6 Acute stroke Show forest plot | 6 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.98] |

