نقش داروهای غیر‐استروئیدی ضد‐التهابی برای القای بازگشت و پیشگیری از پیشرفت نئوپلازی داخل اپیتلیالی دهانه رحم
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | True randomisation with allocation by computer‐generated blocks. Parallel design without cross‐over. Patient, provider, pathologist blinded. All pathologic specimens were reviewed by a blinded central pathologist. Enrollment June 2002 to October 2003. Analysis was by ITT | |
| Participants | 25 women 18 years or older with biopsy proven CIN 2 or CIN 3. | |
| Interventions | Celecoxib 200 mg or placebo by mouth twice daily with meals for six months or until progression of dysplasia. All patients underwent initial colposcopy, thin‐prep liquid‐based cervical cytology, reflex HPV testing, and colpography of lesion with specimens reviewed by a blinded pathologist. Patients were seen at eight‐week intervals for six months with thin‐prep liquid‐based cervical cytology, colposcopy, photography of the cervix and biopsy. If colposcopy was normal, representative biopsy was taken from the area of previous dysplastic abnormality. Participants were removed from the study and underwent loop electrosurgical excision procedure (LEEP) for any increase in severity of CIN on cytology or histology. After six months, persistent CIN 3 required removal from study and treatment with LEEP. Participants missing an eight‐week follow‐up were contacted and asked to return for evaluation as soon as possible. Anyone not attending follow‐up was removed from the study and treated with LEEP | |
| Outcomes | Primary objectives were to determine response rate to treatment and toxicity of treatment. Toxicity was assessed at each eight‐week follow‐up visit by the treating pharmacist by direct questioning about the most common side effects of celecoxib. No toxicity was reported by participants. Response was defined as any decrease in severity of CIN on histology. A complete response was defined as a complete visual resolution of the lesion on colposcopy and normal thin‐prep liquid‐based cytology and tissue histology. Partial response was defined as a decrease in severity of CIN on histology with no cytologic change in severity of CIN. Stable disease defined as no change in histologic degree of CIN from entry cervical biopsy. Progression was defined as any increase in severity of CIN on cytology or histology. Of the 25 participants 13 were randomised to the placebo and 12 to the treatment arm. Five participants (two in the treatment and three in the placebo arm) discontinued the study early because they desired definitive treatment. Overall response was N = 4 (31%) placebo versus N = 9 (75%) treatment. Mean time to response was 72 (+/‐ 38) days in the placebo arm and 73 (+/‐ 26) days in the treatment arm (P value 0.39). Complete response N = 2 (15%) placebo versus N = 4 (33%) treatment. Partial response was N = 2 (15%) placebo versus N = 5 (42%) treatment. Progression to higher degree of CIN was N = 2 (15%) placebo and N = 1 (8%) treatment arm with a mean time to progression of 65 days. No patients progressed to invasive carcinoma. The treatment arm was 2.5 x more likely to have regression of their cervical dysplasia | |
| Notes | 100% of treatment arm women were positive for high‐risk types of HPV by Hybrid Capture II in comparison with 85% in the placebo arm. Eleven of 12 (92%) women in the treatment arm and 11 of 13 (85%) in the placebo arm had CIN 2 with 1 of 12 (8%) in the treatment arm and 2 of 13 (15%) in the placebo arm having CIN 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer generator Random Allocation Software, which randomised each participant to a single treatment, by using the method of randomly permuted blocks |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was accomplished by the Department of Pharmacy. All physicians who participated in the implementation of the treatments, medication or placebo, were blinded to the randomisation process. Only the Pharmacy co‐ordinator (E.G.) was aware of the allocation of the medications. Participants obtained their medications from E.G. in a separate encounter to their clinical follow‐up |
| Blinding of participants and personnel (performance bias) | Low risk | Both the patients and the examining physicians were blinded to the treatment option to which the patients were randomised |
| Blinding of outcome assessment (detection bias) | Low risk | Both the patients and the examining physicians were blinded to the treatment option to which the patients were randomised |
| Incomplete outcome data (attrition bias) | High risk | Five patients (20%) discontinued the study early but were included in the final statistical analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | True randomisation with allocation by computer‐generated blocks. Parallel design without cross‐over. Patient and physicians were blinded. No mention of pathologist. Analysis by ITT. No mention of toxicity assessment. Enrollment May to October 2005. Study was closed after 87 days due to withdrawal of rofecoxib from the market. Randomisation code broken at this point | |
| Participants | 16 women with histological evidence of CIN 2 or 3, a fully visible transformation zone and lesion margin, compliant patient, and safe contraception. Age: range 24 to 36 years, mean 27.1 years for treatment arm and 31.2 years for placebo arm | |
| Interventions | Rofecoxib 25 mg or placebo, by mouth, daily for three months. After the screening visit a physical examination was performed, a questionnaire was answered and study medication was distributed. At initial visit all patients meeting inclusion criteria were evaluated for HPV status via Hybrid Capture (Digene Corp., Gaithersburg, MD, USA). Follow‐up examinations performed at three and six months and included gynaecological examination with ecto‐ and endocervical cytology smears, colposcopy and biopsy, HPV test and pregnancy test. These modalities were used for the evaluation of regression, persistence, or progression of cervical dysplasia | |
| Outcomes | Primary outcomes assessed were regression and remission of CIN over a 3‐month treatment period, and secondary were safety and side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a random number sequence with permuted block size of 12 |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Randomisation code broken when rofecoxib withdrawn from the market. Statistician and pathologist blinded at all times |
| Blinding of outcome assessment (detection bias) | Unclear risk | Randomisation code broken when rofecoxib withdrawn from the market. Statistician and pathologist blinded at all times |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Early closure of study. Insufficient information to assess whether an important risk of bias exists |
| Methods | Randomised 1:1 with stratification by lesion size (coverage of ≤ 50% of cervix or >50%) and severity (CIN 3 versus CIN 2/3). Enrollment June 2005 through April 2012. | |
| Participants | 130 woman 18 years or older with CIN 3 verified on central pathology review and a visible lesion present by colposcopy after initial biopsy. Exclusion criteria included adenocarcinoma in situ, pregnancy, allergy to sulphonamides, history of cardiovascular disease or uncontrolled hypertension, and renal or hepatic disorders. Multicentre trial. | |
| Interventions | Celecoxib 400 mg daily or placebo. Baseline exam with colposcopy, serum sample, and cervical swabs were obtained. Interval colposcopy repeated at 8 weeks. Treatment continued 14‐18 weeks. | |
| Outcomes | Primary endpoints were regression to CIN 1 or less and estimation of toxicity. Complete response was regression to normal tissue or squamous metaplasia. Partial response was regression to CIN 1. Progressive disease was development of squamous carcinoma and persistent disease was presence of CIN 2 or 3 or squamous carcinoma in‐situ after treatment. 67 patients were randomised to celecoxib with 63 receiving treatment and 50 having evaluable tissue. 63 were randomised to placebo with 58 receiving treatment and 41 having evaluable tissue. One grade 3 gastrointestinal adverse event was noted in the celecoxib group; otherwise, toxicity was similar between arms. Histological responses were present in 20/50 (40%) of the celecoxib arm versus 14/41 (34.1%) in the placebo group. With an ITT analysis, response rates were 20/67 (29.9%) and 14/63 (22.2%) for treatment and placebo. 31/81 (38%) response was noted if the lesion was less than 50% of the cervix versus 3/10 (30%) if greater than 50%. Response rates were similar if HPV‐16 was present or absent at 36.2% and 38.5%. Patients with high serum VEGF levels were more like to response to celecoxib versus placebo at 47.4% and 14.3%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised in 1:1 fashion. |
| Allocation concealment (selection bias) | Low risk | Celecoxib and placebo supplied by Biologics Inc. |
| Blinding of participants and personnel (performance bias) | Low risk | Both physicians and patients blinded to treatment group. Translational biological sample analysis was also blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Both physicians and patients blinded to treatment group. Translational biological sample analysis was also blinded. |
| Incomplete outcome data (attrition bias) | Low risk | There was a loss of 30% of the patients; however, the larger number of women enrolled reduces the risk for attrition bias. Data were analysed both on patients completing the study and as ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
ALT: alanine aminotransferase
AST: aspartate aminotransferase
CIN: cervical intraepithelial neoplasia
HPV: human papillomavirus
ITT: intention‐to‐treat
LEEP: loop electrosurgical excision procedure
NSAID: non‐steroidal anti‐inflammatory agent
VEGF: vascular endothelial growth factor
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Laboratory study of celecoxib in cervical cancer cell lines | |
| Phase I‐II study of celecoxib with chemoradiation in patients with cervical cancer | |
| Review of chemoprevention trials and surrogate end point biomarkers in the cervix | |
| Review of biomarkers and their use in cervical cancer chemoprevention |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression of CIN to higher grade of CIN Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.06, 5.24] |
| Analysis 1.1  Comparison 1 Progression of CIN, Outcome 1 Progression of CIN to higher grade of CIN. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Partial or complete regression of CIN 2 or CIN 3 Show forest plot | 3 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.93, 2.27] |
| Analysis 2.1 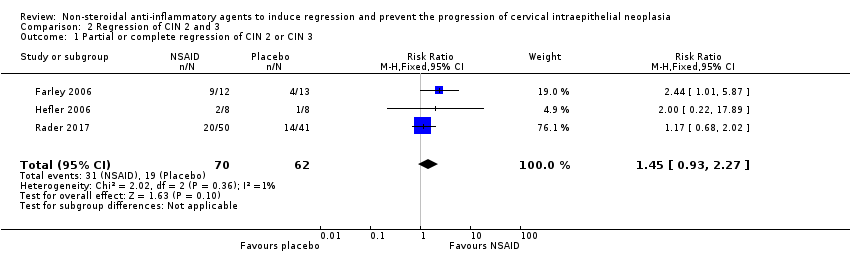 Comparison 2 Regression of CIN 2 and 3, Outcome 1 Partial or complete regression of CIN 2 or CIN 3. | ||||
| 2 Complete regression of CIN 2 or CIN 3 Show forest plot | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.65, 2.67] |
| Analysis 2.2  Comparison 2 Regression of CIN 2 and 3, Outcome 2 Complete regression of CIN 2 or CIN 3. | ||||
| 3 Partial regression of CIN 2 or CIN 3 Show forest plot | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.72, 3.40] |
| Analysis 2.3  Comparison 2 Regression of CIN 2 and 3, Outcome 3 Partial regression of CIN 2 or CIN 3. | ||||

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Progression of CIN, Outcome 1 Progression of CIN to higher grade of CIN.

Comparison 2 Regression of CIN 2 and 3, Outcome 1 Partial or complete regression of CIN 2 or CIN 3.

Comparison 2 Regression of CIN 2 and 3, Outcome 2 Complete regression of CIN 2 or CIN 3.

Comparison 2 Regression of CIN 2 and 3, Outcome 3 Partial regression of CIN 2 or CIN 3.
| Non‐steroidal anti‐inflammatory agents (NSAIDs) compared with placebo for CIN 2 or CIN 3 | ||||||
| Patient or population: women with CIN 2 or CIN 3 Settings: outpatient Intervention: celecoxib 400 mg by mouth daily for 14‐18 weeks, celecoxib 200 mg by mouth twice daily for six months or rofecoxib 40 mg by mouth daily for three months Comparison: placebo tablet by mouth, daily for three to six months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Progression of CIN to a higher grade of CIN | 77 per 10001 | 42 per 1000 | RR 0.54 (0.06 to 5.24) | 25 (one study) | ⊕⊝⊝⊝3 | |
| Partial or complete regression of CIN 2 or CIN 3 | 308 per 10002 | 447 per 1000 | RR 1.45 (0.93 to 2.27) | 132 (three studies) | ⊕⊕⊕⊝3 | |
| Complete regression of CIN 2 or CIN 3 | 174 per 10001 | 228 per 1000 | RR 1.31 (0.65 to 2.67) | 116 (two studies) | ⊕⊕⊕⊝3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The basis for the assumed risk is from the spontaneous complete regression rate in the placebo arm of Farley 2006 and Rader 2017 2 The basis for the assumed risk is from the combined spontaneous partial or complete regression rates in the placebo arms of Farley 2006; Hefler 2006; Rader 2017 3Given the increased sample size with the addition of Rader 2017, we have upgraded the certainty to high other than the Progression analysis as it is based on one small study and thus remained very low certainty. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression of CIN to higher grade of CIN Show forest plot | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.06, 5.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Partial or complete regression of CIN 2 or CIN 3 Show forest plot | 3 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.93, 2.27] |
| 2 Complete regression of CIN 2 or CIN 3 Show forest plot | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.65, 2.67] |
| 3 Partial regression of CIN 2 or CIN 3 Show forest plot | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.72, 3.40] |

