نقش ونتیلاسیون غیر‐تهاجمی در کنترل نارسایی تنفسی هیپرکپنیک حاد ناشی از تشدید بیماری مزمن انسدادی ریه
چکیده
پیشینه
ونتیلاسیون غیر‐تهاجمی (non‐invasive ventilation; NIV) با فشار مثبت دومرحلهای راه هوایی (bi‐level positive airway pressure; BiPAP) معمولا برای درمان بیمارانی استفاده میشود که با نارسایی تنفسی هیپرکپنیک حاد (acute hypercapnic respiratory failure; AHRF) ثانویه به حمله حاد بیماری مزمن انسدادی ریه (acute exacerbation of chronic obstructive pulmonary disease; AECOPD) در بیمارستان بستری میشوند.
اهداف
مقایسه اثربخشی NIV همراه با مراقبت معمول در مقایسه با مراقبت معمول شامل عدم استفاده از ونتیلاسیون مکانیکی بهتنهایی در بزرگسالان مبتلا به AHRF ناشی از AECOPD. هدف این مرور، بهروز کردن پایگاه شواهد به منظور حمایت از عملکرد بالینی و ارائه توصیههایی برای ارزیابی و تحقیق در آینده است.
روشهای جستوجو
کارآزماییها را از طریق جستوجوی دستی در مجلات تنفسی و خلاصه نشستها و جستوجو در پایگاه ثبت تخصصی گروه راههای هوایی در کاکرین (CAGR) شناسایی کردیم، که از جستوجوهای سیستماتیک در بانکهای اطلاعاتی کتابشناختی زیر مشتق شده است: پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL)؛ MEDLINE؛ Embase؛ Cumulative Index to Nursing and Allied Health Literature (CINAHL)، پایگاه اطلاعات طب مکمل و وابسته (AMED)، و PsycINFO. این نسخه بهروز از مرور اصیل شامل نتایج جستوجوی بانک اطلاعاتی تا ژانویه 2017 است.
معیارهای انتخاب
تمام کارآزماییهای تصادفیسازی و کنترل شده واجد شرایط برای ورود بودند که به مقایسه مراقبت معمول همراه با NIV؛ (BiPAP) با مراقبت معمول بهتنهایی در شرایط حاد بیمارستانی برای بیماران مبتلا به AECOPD ناشی از AHRF پرداخته بودند. AHRF با میانگین pH < 7.35 در هنگام بستری و میانگین فشار نسبی دیاکسید کربن (PaCO2) > 45 میلیمتر جیوه (6 kPa) تعریف شد. پیامدهای اولیه این مرور میزان مرگومیر حین بستری در بیمارستان و نیاز به لولهگذاری (انتوباسیون) تراشه بودند. پیامدهای ثانویه شامل طول مدت بستری در بیمارستان، عدم تحمل درمان، عوارض، تغییرات نشانهها و تغییرات در گازهای خون شریانی بود.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم، با بهکارگیری معیارهای انتخاب برای تعیین واجد شرایط بودن مطالعات، استخراج دادهها و تعیین خطر سوگیری (bias) مطابق دستورالعملهای کاکرین پرداختند. نویسندگان مرور برای دادههایی که به هر دو صورت بالینی و آماری هموژن بودند؛ متاآنالیز انجام دادند، و دادهها را به صورت یک نمونه کلی تجمعی و با توجه به دو زیر‐گروه از پیش تعریف شده مربوط به شدت حمله (pH هنگام بستری بین 7.35 و 7.30 در مقایسه با پائینتر از 7.30) و تنظیمات درمانی NIV (واحد مراقبتهای ویژه در مقایسه با بخش) مورد تجزیهوتحلیل قرار دادند. نتایج مربوط به مرگومیر، نیاز به لولهگذاری تراشه و طول مدت بستری در بیمارستان را در یک جدول «خلاصه یافتهها» گزارش کرده و کیفیت آنها را بر اساس معیارهای درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) ارزیابی کردیم.
نتایج اصلی
در این مرور 17 کارآزمایی تصادفیسازی و کنترل شده شامل 1264 شرکتکننده وارد شده است. دادههای موجود نشان میدهد که میانگین سنی در هنگام ورود به مطالعه 66.8 سال (محدوده 57.7 تا 70.5 سال) بوده و اکثر شرکتکنندگان (65%) مرد بودند. بیشتر مطالعات (12/17) در معرض خطر سوگیری (bias) عملکرد قرار داشتند و در اکثر آنها (14/17) خطر سوگیری تشخیص نامطمئن بود. این خطرات ممکن است به ترتیب تحت تاثیر پیامدهای غیر‐عینی اندازهگیری شده توسط خود بیمار (مانند تنگی نفس) و پیامدهای ثانویه مرور قرار گیرند.
استفاده از NIV خطر مرگومیر را 46% کاهش داد (خطر نسبی (RR): 0.54؛ 95% فاصله اطمینان (CI): 0.38 تا 0.76؛ 12 مطالعه؛ تعداد افراد مورد نیاز جهت درمان تا حصول یک پیامد مثبت بیشتر (number needed to treat for an additional beneficial outcome; NNTB): 12؛ 95% CI؛ 9 تا 23) و خطر نیاز به لولهگذاری تراشه را 65% کاهش داد (RR: 0.36؛ 95% CI؛ 0.28 تا 0.46؛ 17 مطالعه؛ NNTB: 5؛ 95% CI؛ 5 تا 6). ما کیفیت هر دو پیامد را به دلیل عدم قطعیت در مورد خطر سوگیری برای چندین مطالعه «متوسط» برآورد کردیم. نمودار قیفی (funnel plot) مربوط به نیاز به لولهگذاری داخل تراشه، احتمال برخی از سوگیریهای انتشار مربوط به این پیامد را افزایش داد. استفاده از NIV همچنین با کاهش طول مدت بستری در بیمارستان (تفاوت میانگین (MD): 3.39‐ روز؛ 95% CI؛ 5.93‐ تا 0.85‐؛ 10 مطالعه)، کاهش بروز عوارض (غیر‐مرتبط با NIV) (RR: 0.26؛ 95% CI؛ 0.13 تا 0.53؛ 2 مطالعه) و بهبود pH؛ (MD: 0.05؛ 95% CI؛ 0.02 تا 0.07؛ 8 مطالعه) و بهبود فشار نسبی اکسیژن (PaO2)؛ (MD: 7.47 میلیمتر جیوه؛ 95% CI؛ 0.78 تا 14.16 میلیمتر جیوه؛ 8 مطالعه) در یک ساعت همراه بود. روند بهبودی در PaCO2 مشاهده شد، اما این یافته دارای اهمیت آماری نبود (MD: ‐4.62 میلیمتر جیوه؛ 95% CI؛ 11.05‐ میلیمتر جیوه تا 1.80 میلیمتر جیوه؛ N = 8 مطالعه). تجزیهوتحلیل post‐hoc نشان داد که بخشی از این عدم فایده ناشی از این واقعیت است که دادههای مربوط به دو مطالعه که در معرض خطر بالایی از سوگیری قرار داشتند، عدم تعادل پایه را برای این پیامد نشان دادند (در گروه NIV بدتر از گروه مراقبت معمول بود). تجزیهوتحلیل مربوط به حساسیت حاکی از آن بود که حذف این دو مطالعه منجر به تاثیرات مثبت NIV با اهمیت آماری روی PaCO2 میشود. عدم تحمل درمان بهطور قابل ملاحظهای در گروه NIV بیشتر از گروه مراقبت معمول بود (تفاوت خطر (RD): 11.0؛ 95% CI؛ 0.04 تا 0.17؛ 6 مطالعه). تجزیهوتحلیل نتایج نشان دهنده روند غیر‐قابل توجهی در کاهش تنگی نفس با NIV در مقایسه با مراقبت معمول بود (تفاوت میانگین استاندارد شده (SMD): 0.16‐؛ 95% CI؛ 0.34‐ تا 0.02؛ 4 مطالعه). تجزیهوتحلیل زیر‐گروه تفاوت معنیداری را میان گروهها نشان نداد.
نتیجهگیریهای نویسندگان
دادههای حاصل از کارآزماییهای تصادفیسازی و کنترل شده با کیفیت خوب نشان میدهد که NIV به عنوان مداخله اول در همراهی با مراقبت معمول برای کاهش احتمال مرگومیر و لولهگذاری تراشه در بیماران بستری شده با نارسایی تنفسی هیپرکپنیک حاد ثانویه به حمله حاد بیماری مزمن انسدادی ریه (COPD) مفید است. به نظر میرسد مزیت این پیامدها برای بیماران مبتلا به اسیدوز خفیف (pH؛ 7.30 تا 7.35) در برابر اسیدوز شدید (pH < 7.30) و زمانی که NIV در بخش مراقبتهای ویژه (ICU) یا در بخش عمومی تنظیم میشود، مشابه است.
PICOs
خلاصه به زبان ساده
ونتیلاسیون غیر‐تهاجمی برای افراد مبتلا به نارسایی تنفسی به علت تشدید بیماری مزمن انسدادی ریه (COPD)
چرا این سوال مهم است؟
هنگامی که افراد دچار حمله شدید COPD میشوند، تنفس آنها بسیار دشوار میشود. این مسئله میتواند تبدیل به نارسایی تنفسی (نارسایی تنفسی هیپرکپنیک حاد (acute hypercapnic respiratory failure; AHRF)) شود که اغلب به مراقبت فوری پزشکی در بیمارستان نیاز دارد. یکی از درمانهایی که ممکن است برای فرد ارائه شود حمایت تنفسی (لولهگذاری و ونتیلاسیون مکانیکی) است. این روش، شامل تحویل هوا و/یا اکسیژن از طریق یک ونتیلاتور متصل به یک لوله است که داخل گلو و ریهها قرار داده میشود. این روش، بدون شوک یک پروسیجر نجات برای بیمارانی است که دچار حملات شدید تهدید کننده حیات COPD شدهاند؛ اما با چندین عوارض جانبی ناخواسته همراه است.
ونتیلاسیون غیر‐تهاجمی (non‐invasive ventilation; NIV) از طریق یک ونتیلاتور متصل به یک ماسک بینی یا ماسک صورت، حمایت تنفسی ایجاد میکند. امروزه برای کمک به این بیماران در بسیاری از بیمارستانها از NIV بیشتر استفاده میشود. این مرور با هدف تعیین اثربخشی افزودن NIV به مراقبت معمول در این بیماران انجام شده است.
ما چگونه به این سوال پاسخ دادیم؟
همه شواهد موجود را در مورد تاثیرات NIV همراه با مراقبت معمول در مقایسه با مراقبت معمول (بدون ونتیلاسیون) تا ژانویه 2017 مرور کردیم. از آنجایی که تا 20% از افرادی که مبتلا به COPD هستند و نارسایی تنفسی دارند، ممکن است در اثر این بیماری بمیرند، ما تعداد مرگومیر را به عنوان پیامد اولیه در نظر گرفتیم. همچنین نیاز به لولهگذاری و زمان صرف شده را در بیمارستان مورد بررسی قرار دادیم.
چه چیزی را پیدا کردیم؟
ما اطلاعات حاصل از 17 کارآزمایی بالینی را با مجموع 1264 بیمار وارد کردیم. در مقایسه با مراقبت معمول در این گروه بیماران، ما دریافتیم که NIV برای کاهش موارد مرگومیر و تعداد بیمارانی که نیاز به لولهگذاری دارند، مفیدتر است. بهطور میانگین، خطر مرگ به میزان 46% و خطر نیاز به لولهگذاری به میزان 65% کاهش یافت. داوران (با استفاده از معیارهای درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE)) کیفیت شواهد هر دوی این یافتهها را «متوسط» ارزیابی کردند. افرادی که NIV داشتند بهطور میانگین سه روز و نیم کمتر از بیمارانی که NIV نداشتند، در بیمارستان بستری بودند.
نتیجهگیری
این مرور شواهد قانع کنندهای را برای حمایت از استفاده از NIV به عنوان یک استراتژی درمانی موثر برای بیمارانی فراهم میکند که به علت حملات حاد COPD و نارسایی تنفسی در بیمارستان بستری میشوند.
Authors' conclusions
Summary of findings
| Non‐invasive ventilation versus usual medical care for management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease (overall effects) | ||||||
| Patient or population: Patients admitted to hospital with acute hypercapnic respiratory failure due to an exacerbation of chronic obstructive pulmonary disease (COPD) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with usual care ‐ Overall | Risk with NIV | |||||
| Mortality | 183 per 1000 | 99 per 1000 | RR 0.54 | 854 | ⊕⊕⊕⊝ | Downgraded owing to risk of bias for some included studies |
| Need for endotracheal intubation | 341 per 1000 | 123 per 1000 | RR 0.36 | 1105 | ⊕⊕⊕⊝ | Downgraded owing to risk of bias for some included studies |
| Length of hospital stay (days) | Mean length of hospital stay (days) was 17.5 | MD 3.39 lower | ‐ | 888 | ⊕⊕⊕⊝ | Downgraded owing to risk of bias and inconsistency of findings for some included studies |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSeveral risk of bias items rated 'unclear' bOne study reported an effect estimate that favoured usual medical care (non‐significant); significant statistical heterogeneity identified within the intensive care unit subgroup was unable to be resolved | ||||||
Background
Individuals with chronic obstructive pulmonary disease (COPD), particularly those with more severe disease, are prone to exacerbations that frequently result in admission to hospital. Severe acute exacerbations of COPD (AECOPDs) are commonly characterised by development of acute respiratory acidaemia due to prolonged hypercapnia (elevated levels of carbon dioxide). This clinical state is known as acute hypercapnic respiratory failure (AHRF). Between one fifth and one third of patients with COPD admitted to hospital with AHRF die in hospital despite the use of mechanical ventilation support strategies (Ambrosino 1995; Bott 1993; Brochard 1995; Foglio 1992; Jeffrey 1992; Roberts 2011).
Description of the condition
In severe COPD, hyperinflation places the respiratory muscles at a mechanical disadvantage, and they function close to their maximum capacity (Macklem 1984; Tobin 1986). During acute exacerbations, elastic and resistive loads on the respiratory muscles increase, and this may lead to ventilatory failure. Ensuing tissue acidosis further impairs ventilatory muscle function, which leads to the downward spiral of ventilatory failure (Jaun 1984).
Various methods of ventilatory support are available for the compromised patient. Conventional therapy aims to facilitate adequate oxygenation while treating the cause of the exacerbation. This is usually achieved with the use of bronchodilators, corticosteroids, antibiotics, and controlled oxygen. Traditionally, patients who do not respond to conventional treatment would receive invasive mechanical ventilation. This mode of ventilation involves sedation, intubation (insertion of a tube into the airway for breathing), attachment to a mechanical ventilator, and transfer to an intensive care unit (ICU). This treatment strategy has been commonly used in clinical practice for some years and is associated with successful reversal of hypercapnic acidaemia and recovery of breathing function in some individuals. However, it is also associated with significant risks. The intubation process may cause damage to local tissue structures, and the course of ventilation may be complicated by factors such as ventilator‐associated pneumonia and sinusitis (Fagon 1993; Koenig 2006; Waters 2015). Invasive mechanical ventilation in patients with COPD is also associated with high morbidity and difficulty weaning from ventilatory support (Brochard 1994; Esteban 1995). Prolonged length of ICU stay is therefore not uncommon for this patient group.
Description of the intervention
Non‐invasive ventilation (NIV) is an alternative management option for AHRF secondary to AECOPD (Bott 1993; Fagon 1993; Kramer 1995; Meduri 1989). NIV allows provision of positive pressure ventilation; however unlike invasive ventilation, NIV is performed without the need for sedation and intubation. Instead, ventilatory support is provided by a flow generator connected to NIV via a full face or nasal mask. Advantages of NIV over invasive ventilation include the ability to apply it for short, intermittent periods (which may be sufficient to reverse ventilatory failure);lack of sedation and its potential adverse secondary effects (e.g. ventilatory suppression); maintenance of the ability to eat, drink, and converse; and the consequent opportunity for individuals to have continued involvement in decisions regarding their care. It is important to note that the incidence of nosocomial pneumonia observed with NIV use is less than that seen among intubated patients (Guerin 1997; Kramer 1999; Nourdine 1999). NIV is increasingly used as adjunctive therapy in the management of acute exacerbations of COPD. Therefore, it is essential that the effectiveness of NIV as a primary management option is accurately determined to verify its use in patients with AECOPD previously characterised by greater reliance on invasive ventilation.
How the intervention might work
The mechanisms underpinning effects of NIV among patients with AHRF are fundamentally similar to those supporting mechanical ventilation, that is, NIV works to enhance ventilation by providing pressure‐supported airflow to unload fatigued ventilatory muscles. This enables recovery of function of respiratory muscles of ventilation and facilitates normalisation of, or improvement in, lung volumes and lung mechanics to reverse acidaemia (Appendi 1994). Clinical improvement is most commonly determined via analysis of arterial blood gas samples and overall clinical state. NIV is used increasingly in clinical practice and is an established form of treatment for patients with a variety of chronic hypoventilatory syndromes (Moloney 1999).
Why it is important to do this review
Use of NIV in AHRF due to AECOPD has been supported by a number of case series (Brochard 1990; Foglio 1992; Meduri 1989) and randomised controlled trials (RCTs; Bott 1993; Celikel 1998, Plant 2001). Despite this fact, NIV is not more successful than usual care in all cases of AHRF due to AECOPD (Barbe 1996), and failure rates of between 9% and 50% have been reported (Kramer 1995; Soo Hoo 1994). Factors that may relate to this include patient‐ventilator dyssynchrony, the impact of coadjuvant polypharmacy for AECOPDs such as anxiolytics or respiratory suppressants, and factors related to staff (e.g. time, expertise) and individual patients (e.g. claustrophobia). A matter of concern is that NIV, particularly when applied unsuccessfully, may delay the start of endotracheal intubation and mechanical ventilation, thereby potentially resulting in poorer health outcomes (Ambrosino 1996; Wood 1998). This may be influenced by the common clinical situation whereby patients with AECOPD find tight‐fitting NIV masks (whether nasal or full face) uncomfortable or claustrophobic. Intolerance may result in poor treatment adherence and, ultimately, in NIV ineffectiveness. This is an update of a Cochrane Review (Ram 2004).
Objectives
To compare the efficacy of NIV applied in conjunction with usual care versus usual care involving no mechanical ventilation alone in adults with AHRF due to AECOPD. The aim of this review is to update the evidence base with the goals of supporting clinical practice and providing recommendations for future evaluation and research.
Methods
Criteria for considering studies for this review
Types of studies
We considered only RCTs for inclusion in this review. We did not exclude studies described as 'randomised' but lacking sufficient information to reveal the adequacy of such methods. Cross‐over studies were not eligible for inclusion in the review.
Types of participants
Studies must have been conducted on adult patients admitted to hospital with AHRF due to AECOPD. Studies of patients who commenced NIV before hospital admission were not eligible for inclusion. We defined AHRF by a mean admission pH < 7.35 and mean baseline admission partial pressure of carbon dioxide (PaCO2) greater than 45 mmHg (6 kPa). If we could not verify mean baseline pH data, we accepted studies if investigators stated within their inclusion criteria that participants needed to have had an admission pH < 7.35. Studies of participants with a primary diagnosis of pneumonia and of those with other underlying pathologies were not eligible for inclusion. Studies involving a mixed group of participant pathologies (e.g. some with COPD, some with congestive cardiac failure (CCF)) were eligible if data specifically pertaining to those with COPD were available or could be obtained. We excluded no studies on the basis of the presence of concurrent respiratory comorbidities such as obesity, obstructive sleep apnoea, obesity, and hypoventilation syndrome.
Types of interventions
Studies must have compared effects of NIV versus usual care. Non‐invasive ventilation was defined as delivery of BiPAP when inspiratory positive airway pressure (IPAP) was greater than expiratory positive airway pressure (EPAP). NIV may have been delivered via any type of interface (e.g. full face mask, nasal mask, helmet). Usual care was defined according to trial authors' definitions, which typically involveda combination of supplemental oxygen, antibiotics, bronchodilators, steroids, respiratory stimulants, and/or other suitable medical interventions (e.g. diuretics, methylxanthines). However, usual care could not include any form of 'usual' NIV or invasive ventilation. Studies that involved participants who had already received a form of invasive or non‐invasive ventilation (including continuous positive airway pressure (CPAP)) before enrolment, including studies of NIV weaning, were not eligible for inclusion.
Types of outcome measures
Primary outcomes
-
Mortality during hospital episode of respiratory failure
-
Need for endotracheal intubation (qualification for intubation and mechanical ventilation criteria, as defined by study investigators. If criteria regarding the need for endotracheal intubation were not specified or could not be accurately evaluated, actual incidence of intubation was accepted)
Secondary outcomes
-
Length of hospital stay
-
Length of ICU stay
-
Symptom scores (e.g. ratings of dyspnoea)
-
Treatment intolerance (e.g. participant unable or unwilling to adhere to treatment owing to undesirable treatment effects)
-
Complications (NIV‐related and those not related to NIV)
-
Arterial blood gas tensions one hour following commencement of NIV (pH, PaCO2, partial pressure of oxygen (PaO2))
Search methods for identification of studies
Electronic searches
For this review update, we identified trials from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
-
Weekly searches of MEDLINE Ovid SP 1946 to date.
-
Weekly searches of Embase Ovid SP 1974 to date.
-
Monthly searches of PsycINFO Ovid SP.
-
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature).
-
Monthly searches of AMED EBSCO (Allied and Complementary Medicine).
-
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of the Cochrane Airways Review Group. Details of these strategies, as well as a list of handsearched conference proceedings, can be found in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We conducted searches with no restriction on language or type of publication. This review update included searches conducted in November 2013, July 2015, and January 2017. We performed additional searches of three online clinical trials registries: ClinicalTrials.gov (www.ClinicalTrials.gov), controlled‐trials (www.controlled‐trials.com), and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) (refer Appendix 3). For search methods used before 2004, see Appendix 4.
Searching other resources
We searched the reference lists of included RCTs for additional papers that might be eligible for inclusion in the review. We contacted authors of included RCTs to ask about other published and unpublished studies.
Data collection and analysis
Selection of studies
Two review authors independently assessed studies yielded by electronic searches for inclusion in the review. We coded studies as include, unclear, or exclude, according to the following criteria.
-
INCLUDE: Study clearly met all review criteria.
-
UNCLEAR: Study met some review criteria but available information is insufficient to confirm eligibility.
-
EXCLUDE: Study clearly did not meet review criteria.
We determined final study inclusion by obtaining consensus of two review authors using full‐text copies of studies identified as INCLUDE and UNCLEAR. We resolved discordance between review authors through consultation with a third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standardised template designed specifically for this review. When data were missing, or when we were uncertain about data presented in included studies, we contacted original authors by email to attempt to obtain data or resolve uncertainty. We included these data only if we obtained confirmation from trial authors. Two review authors entered data into Revman 5.3.5 and randomly checked accuracy. No review authors handled data from clinical trials on which they were a named investigator.
Assessment of risk of bias in included studies
We assessed risk of bias of included studies using the Cochrane 'Risk of bias' tool (Higgins 2008). This tool evaluates potential for study bias according to six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and 'other issues'). Within this approach, we specified the following four additional items considered relevant to the context of the present review: imbalance among outcome measures at baseline, comparability of intervention and control group characteristics at baseline, protection against contamination, and selective recruitment of participants. We rated risk of bias as low, high, or unclear for all domains and presented our assessment in a 'Risk of bias' table within the review.
Measures of treatment effect
We pooled for meta‐analysis outcome data that were clinically homogenous. For continuous variables, we calculated mean differences (MDs) or standardised mean differences (SMDs) and 95% confidence intervals (95% CIs). For dichotomous variables, we calculated risk ratios (RRs) with 95% CIs, as well as the number needed to treat for an additional beneficial outcome (NNTB) using the formula NNTB = 1/ [CER * (1 ‐ RR)] (where CER = control event rate and RR = risk ratio).
Unit of analysis issues
We analysed mortality, need for endotracheal intubation, treatment intolerance, and complications as dichotomous data. We reported all other variables as continuous data. We analysed measures of blood gas tensions for PaCO2 and PaO2 as mmHg, and we converted data presented as kPa using the formula: mmHg = kPa*7.5. As we anticipated the risk of treatment intolerance to be very low in the usual care group, we evaluated data related to this outcome as risk differences.
Dealing with missing data
We attempted to contact authors of included studies if data were not readily available for analysis. We reported unpublished data obtained from study authors in characteristics of studies tables. We included only data on participants with COPD from studies that comprised mixed patient conditions (e.g. COPD and heart failure), if we could obtain the data.
Assessment of heterogeneity
We performed meta‐analyses using a fixed‐effect model when possible. When outcome data demonstrated a 'greater than moderate' risk of statistical heterogeneity, indicated by an I2 statistic > 60% (Higgins 2008), we undertook analysis using a random‐effects model.
Assessment of reporting biases
We explored the potential for publication bias in the meta‐analysis by generating funnel plots for the outcomes of mortality, need for endotracheal intubation, and hospital length of stay, assuming that five or more studies were included.
Data synthesis
We collated and analysed data from all trials using Review Manager 5.3.5. We evaluated data related to primary review outcomes as well as hospital length of stay according to the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) and presented this information in a 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
We investigated the cause of any significant statistical heterogeneity (I2 > 60%) for any outcome on the basis of duration of NIV, type of mask used to administer NIV, and risk of bias.
As management of AHRF may differ according to the severity of the presenting condition and the hospital setting in which treatment is provided, we specified the following two subgroup analyses a priori and conducted these analyses for the primary outcomes of mortality and need for endotracheal intubation.
-
pH: We compared studies of participants with initial mean presentation pH < 7.30 (i.e. worse) versus studies of participants with initial mean presentation pH between 7.30 and 7.35 (i.e. better); and
-
Hospital setting for delivery of intervention: We compared studies that applied NIV on a general ward (or in an emergency department (ED)) versus studies that applied NIV in the ICU. We defined hospital location in accordance with study author descriptions (i.e. we employed no review‐specific operationalised definitions).
Sensitivity analysis
We performed a sensitivity analysis for our primary review outcomes to evaluate the impact of studies that did not report outcome data on an intention‐to‐treat basis. We believed this was necessary, as anecdotal evidence suggests that some patients drop out or withdraw from studies of NIV after randomisation and/or upon initiation of NIV owing to discomfort.
Results
Description of studies
Refer to Characteristics of included studies and Characteristics of excluded studies for complete details of studies included or excluded from the review. This is an update of a Cochrane Review (Ram 2004).
Results of the search
Refer to Figure 1 for the PRISMA flow chart.
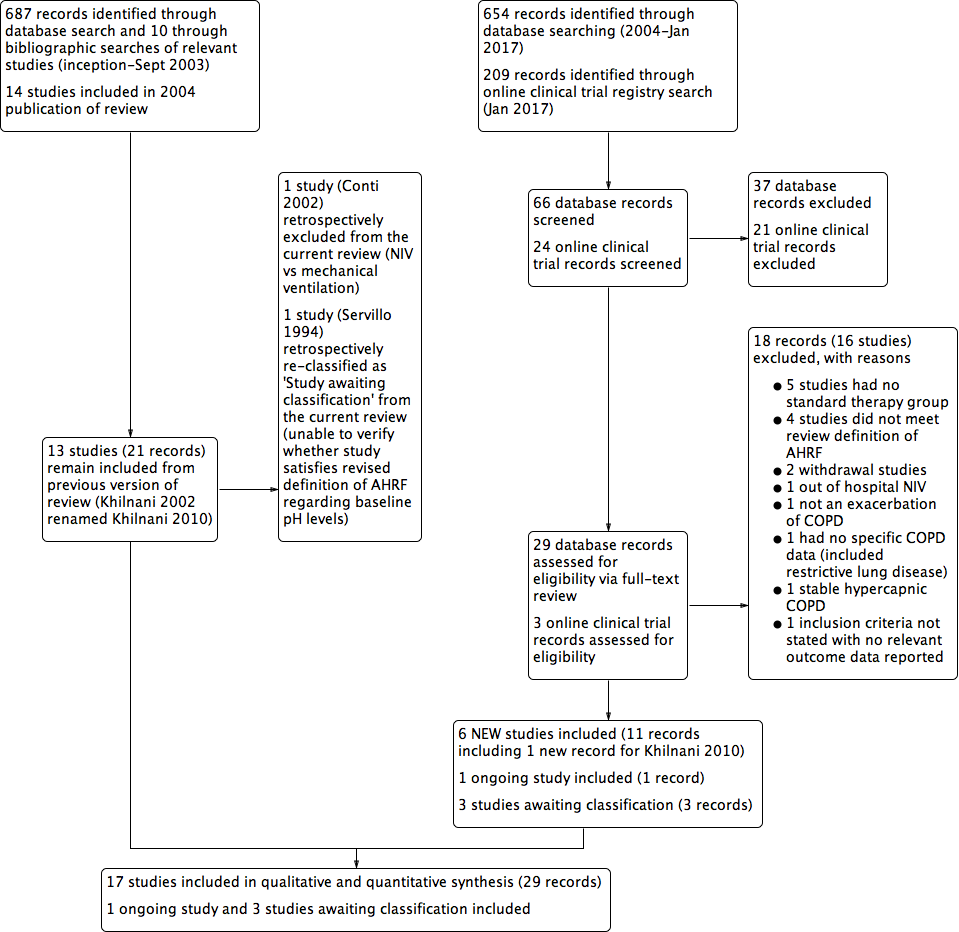
Study flow diagram for 2004‐2017 literature searches.
An electronic search conducted in September 2003 yielded 697 citations: 602 from the Cochrane Airways Trials Register and 85 from Embase, MEDLINE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and online respiratory journal databases. We obtained 10 additional references through bibliographic searching of relevant articles. On the basis of review of 697 abstracts, we identified 160 studies as potentially suitable for inclusion. Full‐text review resulted in exclusion of 138 studies and preparation of a complete list of reasons for exclusion provided under Characteristics of excluded studies. We included the remaining 22 records from 14 original studies after identifying duplicate records for Brochard 1995 (single), Dikensoy 2002 (single), and Plant 2001 (six duplicate records).
We updated the review in April 2004 with exclusion of one further study (Potena 2003).
An updated search conducted in September 2013 resulted in identification of six additional appropriate studies for inclusion (Carrera 2009; Collaborative 2005; Khilnani 2010; Liu 2005; Matuska 2006; Samaria 2009), one of which was a more complete version of the original abstract study of Khilnani 2002 (study ID changed to Khilnani 2010). We identified three Studies awaiting classification because of uncertainty regarding randomisation (Samaria 2013) and baseline pH status required to confirm the presence of AHRF (Liao 2004; Servillo 1994). We attempted to contact authors of these studies for clarification, without reply. We identified one ongoing study (Ongoing studies) via the clinical trials registry search (Duan 2011). At this time, we removed a posteriori from the review one study (Conti 2002) because we noted that it clearly failed to meet one eligibility criterion (comparison of NIV vs mechanical ventilation). The most recent updates (July 2015 and January 2017) yielded no additional included studies but one excluded study (Kong 2015).
Included studies
Seventeen studies met review inclusion criteria: Avdeev 1998; Barbe 1996; Bott 1993; Brochard 1995; Carrera 2009; Celikel 1998; Collaborative 2005; del Castillo 2003; Dikensoy 2002; Khilnani 2010; Kramer 1995; Liu 2005; Matuska 2006; Plant 2001; Samaria 2009; Thys 2002; Zhou 2001. We provide full methodological details of these studies under Characteristics of included studies and summary details below.
Design
All studies were RCTs using a parallel‐group design. We found no cross‐over studies. Some studies reported on participants crossing from the control group to receive the NIV intervention as 'rescue therapy', but we did not include such data in meta‐analyses.
Population
The included studies spanned various regions of the world including Belgium (Thys 2002), China (Collaborative 2005; Liu 2005; Zhou 2001), Czech Republic (Matuska 2006), France (Brochard 1995), India (Khilnani 2010; Samaria 2009), Italy (Brochard 1995), Russia (Avdeev 1998), Spain (Barbe 1996; Brochard 1995; Carrera 2009; del Castillo 2003), Turkey (Celikel 1998; Dikensoy 2002), the United Kingdom (Bott 1993; Plant 2001), and the United States of America (Kramer 1995). Six of the included studies were multi‐centric, including Brochard 1995 (the only international multi‐centric study, conducted in France, Spain, and Italy), Carrera 2009 (conducted in seven hospitals in Spain), Kramer 1995 (conducted in two hospitals in the USA), Bott 1993 (conducted in three centres in the UK), Plant 2001 (conducted in 14 centres in the UK), and Collaborative 2005 (conducted across 19 hospitals in China). All trials included patients who had AHRF due to AECOPD, but two studies also included patients with other diagnoses. Kramer 1995 included patients with AECOPD, heart failure, pneumonia, asthma, and pulmonary embolus, and Thys 2002 included patients with acute respiratory failure due to AECOPD and acute pulmonary oedema. For both studies, we included in the review only data related to patients with AECOPD. It is likely that participants in included studies did not represent the full spectrum of patients with AHRF due to AECOPD observed in clinical practice, as those requiring immediate intubation were typically ineligible for inclusion in the clinical trials of this review.
The number of participants in each included study ranged from 20 to 342 (median 41), with an aggregate total of 1264 (at the time of randomisation) in the review. We could not determine the precise number of participants who completed clinical trials. All trials recruited similar numbers of patients for both study groups. Available data show that mean age at recruitment was 66.8 (range 57.7 to 70.5) years, and males and females accounted for 65% and 35% of participants, respectively.
Interventions
All included studies compared NIV plus usual care versus usual care alone. The precise nature of usual care varied slightly between studies, but it typically included combinations of pharmacological therapies such as oxygen therapy, bronchodilators, corticosteroids, theophylline, antibiotics, mucolytics, doxapram, diuretics, and heparin. Variability in care was most likely attributable to differences in the years studies were conducted and/or local practices within specific regions or hospitals. Six trials were conducted in hospital respiratory/medical wards (Barbe 1996; Bott 1993; Carrera 2009; del Castillo 2003; Dikensoy 2002; Plant 2001), seven in ICU or critical care settings (Brochard 1995; Celikel 1998; Khilnani 2010; Kramer 1995; Liu 2005; Matuska 2006; Samaria 2009), one on an 'intermediate care ward' (Avdeev 1998), and one (Thys 2002) primarily in a hospital ED. We did not include in location subgroup analyses data from this latter study (Thys 2002), and we could not determine the setting for two studies (Collaborative 2005; Zhou 2001).
Investigators most commonly delivered NIV via pressure‐cycled ventilation (N = 21 studies). One study (Bott 1993) used volume‐cycled nasal NIV. Mean (range) inspiratory positive airway pressure (IPAP) values used when NIV was commenced were 10.7 (3 to 20) cmH2O, but IPAP levels were frequently titrated during early phases, according to the maximum level tolerated by the patient or a target respiratory rate. The mean (range) expiratory positive airway pressure (EPAP) value used upon NIV initiation was 4 (0 to 5) cmH2O.
Nine studies delivered NIV via a face mask interface (Brochard 1995; Carrera 2009; Celikel 1998; Collaborative 2005; del Castillo 2003; Dikensoy 2002; Liu 2005; Matuska 2006; Thys 2002), and three used nasal masks only (Barbe 1996; Bott 1993; Khilnani 2010). Four studies allowed optional use of a face mask and/or a nasal mask (Avdeev 1998; Kramer 1995; Plant 2001; Zhou 2001). We could not determine the type of interface used in the remaining included studies.
The duration of total NIV use was highly variable across included studies. Studies typically implemented NIV according to protocols that aimed to achieve a target number of hours of NIV use per day (reduced from early to late admission), but the total number of hours of NIV use was almost always individualised according to the time needed for AHRF to resolve.
Outcomes
The most commonly reported outcomes of relevance for this review were mortality (N = 12 studies), need for endotracheal intubation (N = 17 studies), and hospital length of stay (N = 10 studies). The outcome of treatment intolerance rarely included adverse events in the control group, hence results clearly appeared to favour usual care over NIV. One should consider this when interpreting the quantitative findings derived from this analysis. The extent of outcome data retrieved upon request from study authors is provided under Characteristics of included studies.
Excluded studies
We have provided a full list of reasons for study exclusion under Characteristics of excluded studies. The most common reasons for exclusion were lack of a suitable control group (N = 5 studies) and failure to meet the review definition of AHRF (N = 4 studies).
Risk of bias in included studies
We have provided in Figure 2 a summary of risk of bias for all included studies.
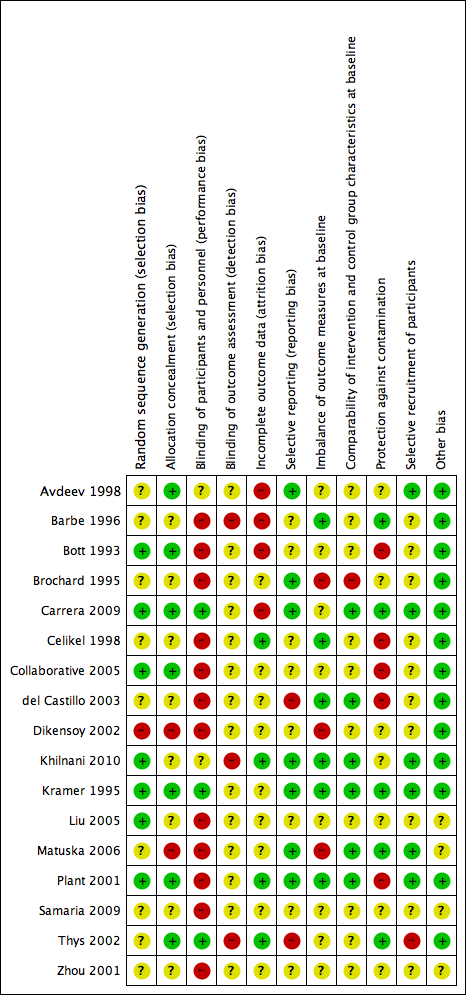
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Risk of bias due to selection procedures (random sequence generation and/or allocation concealment) was low or unclear for most studies, and we rated only Dikensoy 2002 and Matuska 2006 as high risk.
Blinding
Adequate blinding of participants to reduce knowledge of the received intervention was rare and occurred in only three studies (Carrera 2009; Kramer 1995; Thys 2002). Adequate blinding is inherently difficult to achieve in clinical trials of NIV interventions, as delivery of placebo care is challenging, and differences between active and inactive treatments are easily detectable. Knowledge of the intervention group may have affected subjective patient‐reported outcome measures such as ratings of dyspnoea, but it is likely that most other outcomes were not affected. Much uncertainty surrounds the adequacy of assessor blinding across included studies. Lack of outcome assessor blinding may have affected results related to several of the secondary review outcomes.
Incomplete outcome data
Four studies had low risk of bias owing to adequate completeness of outcome data (Celikel 1998; Khilnani 2010; Plant 2001; Thys 2002). Four studies demonstrated high risk of bias for this item owing to attrition related to primary or secondary outcomes and/or failure to adopt an intention‐to‐treat approach for analysis (Avdeev 1998; Barbe 1996; Bott 1993; Carrera 2009).
Selective reporting
For many studies (N = 10), risk of bias due to selective reporting of outcome data was unclear. We rated two studies as having high risk of bias (del Castillo 2003; Thys 2002) and the rest as having low risk.
Other potential sources of bias
We specified additional risk of bias items related to (a) imbalance of outcome measures at baseline; (b) comparability of group characteristics at baseline; (c) protection against contamination; and (d) selective recruitment of participants, owing to their potential to impact outcomes in the context of this review question. Studies at high risk of bias for these items, respectively, were (a) Brochard 1995; Dikensoy 2002; and Matuska 2006; (b) Brochard 1995; (c) Bott 1993; Celikel 1998; Collaborative 2005; del Castillo 2003; and Plant 2001; and (d) Thys 2002.
For most studies, we identified no other sources of bias and therefore determined that they were at low risk of other bias. The risk of other sources of bias was uncertain for the remaining four studies owing to insufficient information by which to judge this (Liu 2005; Matuska 2006; Samaria 2009; Zhou 2001).
Effects of interventions
Mortality during the hospital episode of respiratory failure
Twelve studies including 854 participants (Avdeev 1998; Barbe 1996; Brochard 1995; Celikel 1998; Collaborative 2005; Dikensoy 2002; Khilnani 2010; Liu 2005; Matuska 2006; Plant 2001; Samaria 2009; Thys 2002) contributed data towards this outcome. The overall pooled analysis shows a significantly lower incidence of mortality among participants who received NIV compared with those who received usual care. Investigators observed a 46% risk reduction (RR 0.54, 95% CI 0.38 to 0.76; participants = 854; studies = 12; I2 = 0%) (Analysis 1.1; Figure 3), yielding an NNTB of 12 (95% CI 9 to 23; Figure 4). No publication bias was evident in the funnel plot (Figure 5). One study (Barbe 1996) reported no events in either group. Another study (Bott 1993) reported mortality incidence as 3/30 for the NIV group and 9/30 for the usual care group (not significant for between‐group analysis); these data refer to 30‐day mortality. One study (Khilnani 2010) demonstrated an effect estimate that tended to favour usual care (not statistically significant); this study was unusual as participants were characterised by very severe hypercapnia upon presentation to hospital (PaCO2 > 80 mmHg in both groups). For this outcome, we included from Collaborative 2005 only data related to subgroups with pH < 7.35. We rated findings for this outcome as showing 'moderate' quality according to GRADE owing to an 'unclear' risk of bias rating for several items (summary of findings Table for the main comparison).

NIV vs usual care (overall) ‐ Mortality
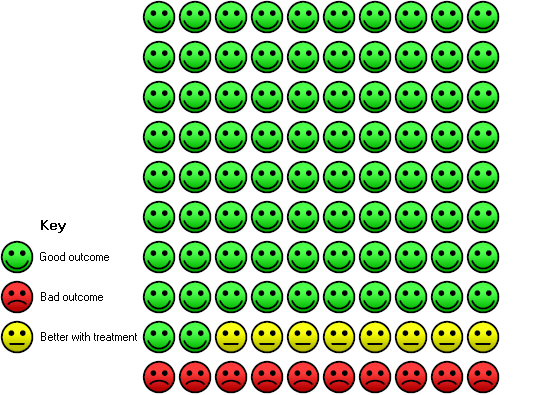
Cates plot Analysis 1.1 (mortality), NIV group: In the usual care group, 18 of 100 people died during the period of hospitalisation, compared with 10 (95% CI 7 to 14) of 100 in the NIV group.

Funnel plot of comparison: 1 NIV vs usual care ‐ Overall, outcome: 1.1 Mortality.
Admission pH subgroups
Results for admission pH subgroups ranging from 7.35 to 7.30 (Barbe 1996; Collaborative 2005; Liu 2005; Plant 2001; Samaria 2009) and below 7.30 (Avdeev 1998; Brochard 1995; Celikel 1998; Collaborative 2005; Dikensoy 2002; Khilnani 2010; Matuska 2006; Thys 2002) were significantly lower with NIV use (RR 0.50, 95% CI 0.30 to 0.84; participants = 454; studies = 5; I2 = 0%; and RR 0.57, 95% CI 0.35 to 0.90; participants = 400; studies = 8; I2 = 0%, respectively). Differences between the two pH subgroups were not statistically significant according to the test for subgroup differences (Analysis 2.1).
Study location subgroups
Mortality was significantly reduced in the NIV group based on use of a ward setting (Avdeev 1998; Bott 1993; Collaborative 2005; Dikensoy 2002; Plant 2001) (RR 0.48, 95% CI 0.29 to 0.78; participants = 543; studies = 5; I2 = 0%). Barbe 1996 (ward‐based) reported no events in either group. Data from studies conducted in the ICU (Brochard 1995; Celikel 1998; Khilnani 2010; Liu 2005; Matuska 2006) showed a trend towards reduced mortality that did not reach statistical significance (RR 0.60, 95% CI 0.34 to 1.07; participants = 251; studies = 5; I2 = 1%). Results showed no significant differences between the two locations (ICU vs ward) for the outcome of mortality (Analysis 3.1).
Need for endotracheal intubation
A total of 17 studies including 1105 participants (Avdeev 1998; Barbe 1996; Bott 1993; Brochard 1995; Carrera 2009; Celikel 1998; Collaborative 2005; del Castillo 2003; Dikensoy 2002; Khilnani 2010; Kramer 1995; Liu 2005; Matuska 2006; Plant 2001; Samaria 2009; Thys 2002; Zhou 2001) contributed data towards this outcome. Results showed a significant reduction in the risk of intubation of approximately two‐thirds (64%) in the NIV group compared with the usual care group (RR 0.36, 95% CI 0.28 to 0.46; participants = 1105; studies = 17; I2 = 0%; Analysis 1.2; Figure 6) with an NNTB of 5 (95% CI 5 to 6; Figure 7). Visual inspection of the funnel plot for this outcome raised some potential for publication bias, evident by a relative lack of study data in the lower right‐hand quadrant of the plot (area signifying lack of clinical benefit in studies of small sample sizes) (Figure 8). Barbe 1996 reported no events in either group. For this outcome, we included from Collaborative 2005 only data related to subgroups with a pH < 7.35. We rated evidence as showing 'moderate' quality according to GRADE owing to ratings of 'unclear' risk of bias for several items (summary of findings Table for the main comparison).

NIV vs usual care (overall) ‐ Need for endotracheal intubation

Cates plot Analysis 1.2 (need for endotracheal intubation), NIV group: In the usual care group, 34 of 100 people experienced the need for endotracheal intubation during the period of hospitalisation, compared with 12 (95% CI 10 to 16) of 100 in the NIV group.
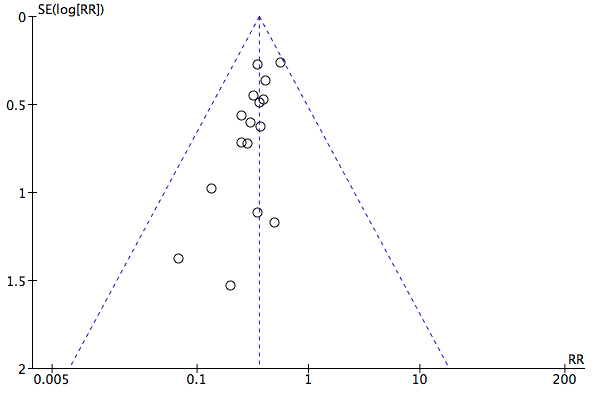
Funnel plot of comparison: 1 NIV vs usual care ‐ Overall, outcome: 1.2 Need for endotracheal intubation.
Admission pH subgroups
Need for intubation among admission subgroups with pH between 7.35 and 7.30 (Bott 1993; Carrera 2009; Collaborative 2005; Liu 2005; Plant 2001; Samaria 2009) and below 7.30 (Avdeev 1998; Brochard 1995; Celikel 1998; del Castillo 2003; Dikensoy 2002; Khilnani 2010; Kramer 1995; Matuska 2006; Thys 2002; Zhou 2001) was significantly less with NIV use (RR 0.44, 95% CI 0.30 to 0.63; participants = 589; studies = 7; I2 = 0%; and RR 0.31, 95% CI 0.22 to 0.42; participants = 516; studies = 11; I2 = 0%; test for subgroup differences; P = 0.16; Analysis 2.2). Barbe 1996 (pH > 7.30) reported no events in either group. Collaborative 2005 is represented in both subgroups, as researchers reported specific data separately for each pH cutoff threshold.
Study location subgroups
Among location subgroups, risk of intubation was significantly reduced by NIV in both ICU‐based (Brochard 1995; Carrera 2009; Celikel 1998; Khilnani 2010; Kramer 1995; Liu 2005; Matuska 2006; Samaria 2009; Thys 2002) and ward‐based subgroups (Barbe 1996; Bott 1993; Carrera 2009; Collaborative 2005; del Castillo 2003; Dikensoy 2002; Plant 2001; Zhou 2001) (RR 0.30, 95% CI 0.21 to 0.43; participants = 401; studies = 9; I2 = 0%; and RR 0.43, 95% CI 0.31 to 0.60; participants = 721; studies = 8; I2 = 0%, respectively). No significant differences were noted between the two subgroups (studies based in the ICU or on the ward) regarding need for intubation (test for subgroup differences; P = 0.15; Analysis 3.2). Barbe 1996 (ward‐based study) reported no events in either group. Samaria 2009 involved delivery of NIV in the ICU setting but usual care on the ward.
Length of hospital stay
Ten studies involving 888 participants (Avdeev 1998; Barbe 1996; Brochard 1995; Celikel 1998; Collaborative 2005; Dikensoy 2002; Khilnani 2010; Kramer 1995; Plant 2001; Thys 2002) revealed length of hospital stay to be significantly shorter for participants who received NIV compared with those who did not (MD ‐3.39, 95% CI ‐5.93 to ‐0.85; participants = 888; studies = 10; I2 = 84%). We used a random‐effects model for this analysis owing to significant statistical heterogeneity. Step‐by‐step removal of studies suggested that these results were most heavily affected by data from Collaborative 2005 (which included data pertaining to participants with admission pH ≥ 7.35) and Khilnani 2010. Additionally, Bott 1993 reported the same median length of stay for both groups (nine days). Despite several 'unclear' ratings of items for this outcome and modest inconsistency of findings related to Collaborative 2005 (which tended to favour usual care, albeit non‐significant), we believe the impact of these factors in the large review sample equated to downgrading of only one level according to GRADE criteria, resulting in an overall evidence rating of 'moderate' quality (summary of findings Table for the main comparison).
Length of ICU stay
One study (Thys 2002) involving 20 participants provided data for length of ICU stay. Although a non‐significant effect favoured a reduction in ICU length of stay in the NIV group (MD ‐2.70 days, 95% CI ‐6.79 to 1.39), this finding should be interpreted with caution, as data were skewed but non‐parametric data were not available for analysis.
Symptom scores
Four studies measured dyspnoea via three different metrics (Borg scale used by Avdeev 1998 and Barbe 1996; visual analogue scale used by Bott 1993; and custom scale used by Collaborative 2005). Data from Avdeev 1998 and Collaborative 2005 represent endpoint dyspnoea ratings (at 1 and 24 hours, respectively), and data from Bott 1993 represent median symptoms over the first three days of admission (not included within the meta‐analysis). Data from Barbe 1996 represent the magnitude of symptom change over time. Pooled meta‐analysis of these data via SMD revealed a non‐significant trend towards favourable reductions in dyspnoea with NIV compared with usual care (SMD ‐0.16, 95% CI ‐0.34 to 0.02; participants = 484; studies = 4; I2 = 70%). This finding was heavily influenced by Collaborative 2005 (71.8% weighting). Plant 2001 reported a statistically significant reduction in time to resolution of dyspnoea (median time 4 days in NIV group vs 7 days in control group; P = 0.025); however these data were not suitable for inclusion in the meta‐analysis.
Treatment intolerance
Six studies involving 346 participants (Avdeev 1998; Barbe 1996; Dikensoy 2002; Khilnani 2010; Liu 2005; Matuska 2006) demonstrated significantly greater (11%) risk of treatment intolerance in the NIV group compared with the usual care group (risk difference (RD) 0.11, 95% CI 0.04 to 0.17; participants = 252; studies = 6; I2 = 0%; Analysis 1.6). Plant 2001 noted that some participants were intolerant of NIV treatment, but we could not ascertain the magnitude of this estimate nor the direction of effect relative to participants in the usual care group. Owing to the clear difference between the nature of NIV and that of usual care interventions, we expected treatment intolerance to be higher in the NIV group than in the usual care group.
Complications of treatment
Six studies involving 567 participants (Brochard 1995; Celikel 1998; Collaborative 2005; Dikensoy 2002; Khilnani 2010; Liu 2005) contributed data towards this outcome. Analysis showed that usual care had a significantly lower risk of NIV‐related treatment complications compared with NIV (RR 29.60, 95%CI 9.47, 92.51; participants = 567; studies = 6; I2 =24%). Owing to the nature of this outcome, this result is to be expected. Two studies evaluated effects of interventions on treatment complications unrelated to NIV and found significantly lower risks of complications with NIV compared with usual care (RR 0.26, 95%CI 0.13 to 0.53; participants = 125; studies = 2; I2 =29%).
Arterial blood gas tensions one hour following commencement of NIV
pH one hour post intervention
Eight studies involving 585 participants provided pH data one hour after initiation of treatment (Avdeev 1998; Brochard 1995; Carrera 2009; Celikel 1998; Dikensoy 2002; Khilnani 2010; Matuska 2006; Plant 2001). Data revealed a significant improvement in pH with NIV compared with usual care (MD 0.05, 95% CI 0.02 to 0.07; participants = 585; studies = 8; I2 = 73%). As we detected significant statistical heterogeneity, we performed step‐by‐step elimination of each study, which revealed that Avdeev 1998 contributed the most to this heterogeneity. Exclusion of this study from the analysis reduced heterogeneity but did not meaningfully alter the pooled effect estimate. Data from Dikensoy 2002 were associated with very large confidence intervals for reasons that were not clear from the original article. Caution is recommended regarding interpretation of the data from this particular study.
PaCO2 one hour post intervention (mmHg)
Eight studies involving 585 participants provided data on PaCO2 one hour after the start of treatment (Avdeev 1998; Brochard 1995; Carrera 2009; Celikel 1998; Dikensoy 2002; Khilnani 2010; Matuska 2006; Plant 2001). The overall result tended to favour use of NIV, but this difference was not statistically significant and statistical heterogeneity was high (MD ‐4.62, 95% CI ‐11.05 to 1.08; participants = 585; studies = 8; I2 = 84%). Neither use of a random‐effects model nor previously defined criteria resolved the heterogeneity. Step‐by‐step elimination of studies from the meta‐analysis revealed that Avdeev 1998 was making the greatest contribution to this heterogeneity. Removal of this study reduced the I2 statistic to 60% but did not fundamentally affect the pooled effect estimate. We observed statistically significant improvement in the magnitude of change in PaCO2 in Bott 1993 (MD 9.0, 95% CI 3.38 to 15.23; P < 0.01), but insufficient study information was available for incorporation into the meta‐analysis. Post hoc examination of findings related to this outcome revealed that two studies (Dikensoy 2002 and Matuska 2006) had inconsistent overall mean effect estimates relative to the others, and that each study was at high risk of bias owing to imbalance of outcome measures related specifically to this outcome. Exploratory (unplanned) sensitivity analysis involving removal of these two studies from the meta‐analysis resulted in an overall effect estimate that became statistically significant in favour of NIV use (MD ‐8.35 units, 95% CI ‐14.84 to ‐1.86; participants = 491; studies = 6; I2 = 81%). We have provided in the Discussion section of this review additional details regarding these studies.
PaO2 one hour post intervention (mmHg)
Eight studies involving 585 participants provided data on PaO2 one hour after the start of treatment (Avdeev 1998; Brochard 1995; Carrera 2009; Celikel 1998; Dikensoy 2002; Khilnani 2010; Matuska 2006; Plant 2001). The overall result revealed a statistically significant improvement in PaO2 favouring NIV compared with usual care, but significant statistical heterogeneity was present (MD 7.47, 95% CI 0.78 to 14.16; participants = 585; studies = 8; I2 = 80%). Removal of Avdeev 1998 resolved this issue but resulted in loss of statistical significance for the final pooled effect estimate (MD 4.71, 95% CI ‐0.25 to 9.66; participants = 527; studies = 7; I2 = 50%).
Sensitivity analysis
We identified four studies as being at high risk of bias owing to attrition and/or failure to adopt an intention‐to‐treat approach for analysis (Avdeev 1998; Barbe 1996; Bott 1993; Carrera 2009). As described earlier, we found that Avdeev 1998 influenced the extent of observed statistical heterogeneity in several analyses. Removal of these studies had little effect on most of the primary outcomes, but removal of Avdeev 1998 resulted in loss of statistical significance for the outcome of mortality in the pH < 7.30 subgroup, with a revised risk estimate of RR 0.63, 95% CI 0.38 to 1.06. Barbe 1996 delayed initiation of NIV for 12 to 48 hours, and other studies started NIV as soon as possible. Removal of this study from relevant meta‐analyses had a negligible effect on outcome effect estimates.
Discussion
Summary of main results
Addition of non‐invasive ventilation (NIV) to usual care for management of acute hypercapnic respiratory failure due to acute exacerbation of chronic obstructive pulmonary disease (COPD) significantly reduces risks of mortality and endotracheal intubation. Treatment with NIV is associated with a significant reduction in hospital length of stay on average, but this finding relates most often to patients with prolonged admissions. NIV appears to improve acidosis within one hour of initiation. Results appear generally consistent across both intensive care unit (ICU) and ward settings, and for patients admitted with more severe (pH < 7.30) or less severe (7.30 to 7.35) acidaemia.
Interpretation of main findings
We included in the review 17 randomised controlled trials (RCTs) involving 1264 participants. This represents a substantial increase from the original review in the number of studies and number of participants included. Trial results demonstrate clear benefits associated with use of NIV as adjunctive therapy to usual care (compared with usual care alone) for treatment of patients admitted to hospital with acute hypercapnic respiratory failure (AHRF) secondary to acute exacerbation of COPD (AECOPD). Benefits were consistent across a range of outcomes considered to be clinically important. Compared with usual care, the overall pooled effect of NIV across included studies included significant reductions in risk of mortality and need for endotracheal intubation, with the average number of patients required to be treated to derive benefit in the magnitude of 12 and 5, respectively. Although no clinical consensus has been reached regarding the most acceptable number needed to treat for an additional beneficial outcome (NNTB) for such outcomes, this approach appears to represent good return upon investment with respect to the importance of these clinical outcomes and the generally low incidence of adverse events reported in studies included in this review. Effects of NIV + usual care versus usual care alone across secondary outcomes were derived from a significantly smaller pool than derived across primary outcomes and were less consistent. The magnitude of effect observed among subgroups defined on the basis of admission pH (< 7.30 or from 7.35 to 7.30) or clinical setting (ICU vs ward) did not significantly differ for most outcomes, with the exception of hospital length of stay, which demonstrated significantly greater benefit for those with more severe acidosis (pH < 7.30) than for those with milder acidosis (pH 7.35 to 7.30). This suggests that benefits derived from NIV use are likely to extend across a range of differing clinical scenarios.
Researchers have reported a significant 46% relative reduction in risk of mortality with NIV compared with usual care. This equates to potential avoidance of one death for every 12 patients treated with NIV (a slight increase from the original review (NNTB = 10)). This mortality benefit across the large number of included studies is of considerable clinical importance. Debate has surrounded the issue of whether NIV would delay necessary endotracheal intubation and therefore increase mortality. However, this position is not supported by the findings of this review. Mortality with NIV was reduced overall and across almost all of the pH and location subgroups, inferring generally similar responses irrespective of such factors. The only subgroup that did not reach statistical significance for this outcome was the ICU subgroup (risk ratio (RR) 0.60, 95% confidence interval (CI) 0.34 to 1.07). It is noteworthy to mention that this subgroup included the Khilnani 2010 study, which was unique compared with any other included study, as the mean admission partial pressure of carbon dioxide (PaCO2) across both groups was in excess of 80 mmHg. This would be considered an indicator for intubation at many hospitals. Although this study demonstrated significant improvement in arterial blood gases and need for intubation, the mortality effect was small and non‐significant (two deaths in NIV group due to septicaemia and acute coronary event vs one death in the control group due to septicaemia). In translating findings related to this outcome into clinical practice, it may be worth considering that the potential for mortality in clinical practice could be greater than that observed in clinical trials owing to factors related to patient suitability for mechanical ventilation. Individuals who are poor candidates for intubation and ventilation, including those with a very poor prognosis or a low likelihood of satisfactory quality of life or prespecified end‐of‐life choices (e.g. not for resuscitation/intubation wishes), are unlikely to feature in clinical trials such as those included within this review, yet may be appropriate candidates for NIV.
Need for endotracheal intubation was reduced by approximately two‐thirds (64%) relative to usual care, equating to just five patients needing to be treated with NIV to potentially avoid intubation of one patient. The magnitude of benefit was clear and statistically significant for all subgroups, and no significant differences were observed for this outcome across subgroups related to admission pH or treatment location. These data further demonstrate the clearly important clinical benefits associated with NIV. It is worth noting that findings related to this outcome could be considered a conservative underestimation of the true effect of NIV due to inherent challenges in evaluating and reporting this outcome in clinical trials. Whereas failure of treatment for patients enrolled into an NIV treatment arm commonly results in progression to intubation and mechanical ventilation or withdrawal of treatment, failure in a usual care arm typically results in escalation of medical management to 'off‐protocol' NIV, followed by potential subsequent intubation and mechanical ventilation (or treatment withdrawal). The precise incidence of 'actual intubation' therefore is likely to be less than the 'need for intubation' in usual care groups. Additionally, actual intubation rates may be influenced by the availability of beds in ICU settings. Therefore we considered the need for intubation as our principal definition for this outcome, as we believed this more accurately evaluated treatment 'success' versus 'failure'. Studies that reported only actual intubation rates were not rated as having high risk of bias due to data contamination, as we believed this phenomenon was representative of clinical care and was unlikely to overinflate effect estimates related to this outcome.
Need for endotracheal intubation is not always considered a 'negative' outcome, particularly in the context of treatment failure and concerns regarding the timeliness of 'essential' intubation and mechanical ventilation. Although this review did not set out to answer specific questions related to such examples, some inferences may be drawn from the present data (with due caution related to indirectness of the data for answering such questions) on the basis of lack of significant differences in beneficial effect estimates for the need for intubation (and mortality) in subgroups defined according to baseline pH levels. Although time to intubation was not specifically examined within this review, the data should alleviate some concerns regarding the safety of NIV as a first‐line therapy option (or trial of therapy for some) for patients who may present later in the course of their AHRF (when concerns regarding timeliness of invasive ventilation may be greater).
NIV use was not associated with exclusively positive outcomes. Data from our review demonstrate the need for intubation criteria was met by 66 of the 559 participants in the intervention group (12% incidence; Analysis 1.2). Rapid access to teams and resources capable of delivering invasive ventilation would therefore appear advisable for individuals considered appropriate for escalation of care when NIV is used. However, it is essential that evaluations and judgements regarding end‐of‐life decisions are made for all patients with severe AECOPDs characterised by AHRF on an individual needs basis. Although some patients with COPD may not be candidates for invasive ventilation, a considerable proportion of those who present to hospital with severe exacerbations requiring NIV have greater underlying disease severity and, in the setting of NIV treatment failure, may be more appropriate for attempted continuation/titration of NIV, conservative management, or treatment withdrawal. For example, patients who may have received prolonged (> 7 days) intubation/ventilation in the past owing to respiratory muscle atrophy may be characterised by reduced ventilatory reserve and impaired capacity to clear pulmonary secretions ‐ features that are likely to recur during subsequent exacerbations (Coakley 1992, Helliwell 1991; Le Bourdelles 1994). Other risks associated with invasive positive pressure ventilation such as barotrauma, cardiac output impairment, increased work of breathing related to dead space ventilation (due to length of the endotracheal tube), and the potential for prolonged or difficult weaning (Shapiro 1986) are relevant factors for consideration when treatment plans for such patients are determined.
The findings of this review are intended to be interpreted with respect to initial management of AHRF secondary to AECOPD. Several studies have been conducted to examine the effectiveness of NIV in patients who are weaning from invasive ventilation; we explicitly excluded these from this review and believe that our results should not be extrapolated to such contexts.
It is noteworthy to reflect upon Barbe 1996, which was one of the only studies to conclude that addition of NIV to usual care was not beneficial. This trial adopted a less common approach to delivery of NIV, as investigators delayed initiation of nasal NIV by 12 to 48 hours after hospital admission (a period longer than most other included studies) and administered it in two fixed sessions (three hours per day). Most other clinical trials in this field, however, adopted flexible prescription practices, allowing quicker initiation and longer duration of treatment in accordance with participant responses (e.g. change in clinical condition). This latter approach is more likely to reflect current clinical practice in many countries where NIV is common. Removal of this study from analyses did not meaningfully impact results, most likely because of the relatively low weighting attributed to this (or any) study, in the light of the large quantity of pooled data for most review outcomes. This small study (N = 24) was also characterised by a mild baseline level of acidosis (mean admission pH of 7.33) at which significant mortality may not be expected to occur.
NIV significantly reduced length of hospital stay by more than three days. It should be noted that the (weighted) mean length of hospital stay for the usual care group was very high (17.5 days). This duration of admission is far in excess of that commonly observed in clinical practice in many countries (Chandra 2012). It remains to be seen whether the magnitude of benefit would be the same for patients admitted to clinical settings in which shorter admissions are more common. This could be an important area of future research. Only one study (Thys 2002) contributed data to the outcome of ICU length of stay, limiting wider applicability of this non‐significant finding that tended to favour use of NIV.
The high incidence of intolerance in the NIV group was not surprising, given that this finding is clearly related to the potential discomfort of NIV in the NIV group and the absence of such discomfort in the usual care group. Although this finding indicates that NIV is not well tolerated by all patients with AHRF due to AECOPD, care should be taken not to interpret this finding as indicative of harm or a reason to deny a patient the opportunity to receive NIV when indicated. When review authors explored the incidence of complications, it became clear that most complications were related to delivery of NIV (e.g. mask‐related facial pressure areas, bloating) but were of a generally mild nature with little long‐term clinical consequence (Analysis 1.7). The two studies that reported data unrelated to NIV use described significant benefits favouring NIV use; however, additional confirmatory data appear necessary to verify this finding.
Four studies provided data regarding patient‐reported ratings of perceived breathlessness. Investigators used three different measurement instruments (Visual Analogue Scale (VAS), Borg score, and verbal rating score) at various times after admission. Therefore, pooling of these data was difficult. Although we noted statistical significance for the single study (N = 60) using VAS, we believe this does not represent any preferable sensitivity to detect changes associated with NIV use. It makes clinical sense to expect that resolution of AHRF would be associated with reduced perception of dyspnoea. Hence, although data for this outcome appear limited in the extent to which they may apply beyond this review, we believe that additional research is not strongly indicated to vigorously pursue data collection for this outcome.
Acidosis has been shown to be an important prognostic factor of survival from respiratory failure in COPD; thus early correction of acidosis is an essential goal of therapy (Jeffrey 1992). This review has shown that NIV (compared with usual care) achieves more rapid correction of acidosis within the first hour. The collective observed benefits of NIV for pH, PaO2, PaCO2 (not statistically significant), and symptoms of dyspnoea suggest a picture of overall clinical improvement in respiratory failure status. Although data presently available are not confirmatory, it is intuitive to hypothesise that this clinical improvement may be a logical mechanism underpinning observed beneficial effects of NIV on need for endotracheal intubation and mortality. A previous study using NIV in respiratory failure secondary to exacerbations of COPD (Brochard 1990), which was not included in this review (not an RCT), reported reductions in respiratory rate and transdiaphragmatic activity with increases in tidal volume and minute ventilation. These findings support the mechanism that NIV not only rapidly improves gas exchange but also allows respiratory muscles to rest, thereby reducing respiratory muscle work in respiratory failure. NIV appears to optimise the window of opportunity for respiratory muscle recovery and for other conventional treatments (bronchodilators, oxygen, corticosteroids, antibiotics, etc.) to take maximal effect. It is interesting to note that in the current review, the outcome for PaCO2 did not reach statistical significance; however, our post hoc exploration of this finding showed that it was heavily influenced by data derived from Dikensoy 2002 and Matuska 2006, both of which were rated as having high risk of bias owing to imbalance of outcomes at baseline. In each instance, participants in the NIV group happened to have statistically significantly worse baseline PaCO2 levels before commencing the intervention (despite randomisation). Endpoint data recorded at one hour were used for meta‐analysis, yet these values appeared numerically worse in the NIV group than in the usual care group. Inspection of the degree of change in both studies showed NIV to be superior to usual care in both instances (NIV group improved and usual care group deteriorated in both studies). This finding may need to be interpreted with due diligence.
A specific point to consider regarding observed changes in blood acidity levels relates to the unit of measurement for the pH outcome. It is perhaps ideal to express changes in this outcome in units of hydrogen ion concentrations (H+) rather than pH; however the latter clearly is used more widely and is easier to interpret in the clinical domain than the former. pH is the negative log of the hydrogen ion concentration; because of the logarithmic nature of pH, one cannot assume that differences in hydrogen ion concentrations between (for example) a pH of 7.26 versus 7.27 compared with 7.27 versus 7.28 are linear. Descriptions of mean changes in acidity of 0.01 of a pH unit can therefore risk becoming meaningless. As reporting of pH in clinical trials and clinical practice regarding NIV use is far more common than reporting of use of H+, we opted to report data in this more conventional manner and do not advocate change. Rather, we encourage appropriate due diligence in implementation of findings related to this outcome, especially when stratification according to acidosis severity may be pertinent.
Overall completeness and applicability of evidence
We attempted to contact the authors of all included studies to verify study quality and/or obtain additional data (as required). Authors of five studies (Avdeev 1998; Bott 1993; Kramer 1995; Plant 2001; Thys 2002) supplied requested information.
Quality of the evidence
We rated evidence for the primary review outcomes of mortality and need for endotracheal intubation as showing 'moderate' quality according to GRADE criteria. This rating was largely due to uncertainty regarding risk of bias judgements for several included studies. In the light of the relatively small weighting of any individual trial in this large meta‐analysis, the impact of such issues could be considered potentially small, especially given the nature of robust outcomes such as mortality, for which the threat of issues such as performance or detection bias could be considered less. Further research may impact the magnitude and our confidence in treatment effect estimates, particularly for several of the secondary review outcomes.
Potential biases in the review process
One should consider some important factors when interpreting the findings of this review. One issue affecting data across numerous secondary outcomes is the systematic bias associated with treatment success/failure in favour of the intervention. Treatment failure typically would result in loss of data collection for some outcomes (e.g. arterial blood gas parameters measured after the first hour of treatment), hence it is reasonable to suspect that observed effect estimates may overestimate the true effectiveness of NIV in management of respiratory failure for some outcomes.
It is also well accepted that no definition of an intensive care unit has been universally accepted, and this has resulted in widespread variability in the scope and context of such settings across the world. The applicability of our subgroup analyses comparing effectiveness of NIV in ICU and ward environments was therefore constrained by the definitions proposed by study authors. We believe this is the most appropriate approach for handling this issue.
Many of the studies included in the pH subgroup < 7.30 (indicative of more severe acidaemia) examined NIV delivered within the ICU environment. Similarly, most studies conducted in the pH subgroup 7.30 to 7.35 took place in the ward environment. Risk ratios for primary outcomes for subgroup analyses were not significantly different. Therefore it is challenging to distinguish between the relative impact of the severity of presenting acidaemia versus NIV hospital setting on these outcomes. One could speculate the null hypothesis that little difference would likely exist between subgroups, and that ICU‐based studies and studies with lower mean admission pH would do better with NIV.
Potentially relevant research findings might always be presented in works that are not available for inclusion within a review or have not been published owing to factors such as 'negative' outcomes. We believe our comprehensive search strategy was sufficient for this purpose, as it included electronic searching of databases regularly updated through the Cochrane Airways Group Specialised Register of trials (CAGR), including handsearching of respiratory journals and meeting abstracts. Selection bias from the review team was minimised by systematic extraction of data by two independent members of the review team using standardised templates.
The extent to which bias related to clinical decision making for study participants influenced clinical outcomes is difficult to accurately ascertain. No study in this review could be described as 'double‐blinded' owing to the practical challenges associated with such procedures; however Thys 2002 employed 'sham' NIV. Other studies ensured that research personnel responsible for making clinical management decisions were unaware of which treatment arm a participant was assigned to, until NIV had commenced (Bott 1993; Brochard 1995), or were not involved in decisions to intubate (Celikel 1998; Kramer 1995), or employed a priori criteria for decisions regarding intubation (del Castillo 2003; Plant 2001; Thys 2002) or treatment failure (Dikensoy 2002; Plant 2001). It is clearly important that interventions associated with a real potential for serious adverse events (such as NIV) should have sufficient monitoring in place and should provide appropriate rescue therapy in the event of clinical deterioration. Although we would like to assume that all patients who 'fail' NIV would be offered immediate alternative management (e.g. intubation and mechanical ventilation), it remains possible that, in an unblinded trial, management could be delayed to prolong the window of opportunity for clinical benefit associated with NIV. Variability in the criteria used to define such treatment failure therefore has significant potential to influence such outcomes. For example, Khilnani 2010 enrolled patients presenting to hospital with severe AHRF characterised by PaCO2 levels over 80 mmHg in both groups. This would warrant immediate intubation at many hospitals.
Finally, NIV itself has limitations. Some 13% to 29% of patients are unable to tolerate the mask (Foglio 1992; Wood 1998), and facial skin ulcers can be caused by mask pressure (Stauffer 1982). Other limitations include lack of direct access to the airways, which could promote mucus plugging and/or atelectasis in patients with excessive secretions, thereby increasing the risk of aspiration. NIV initiation requires a conscious and co‐operative patient and cannot be done in patients with haemodynamic instability or life‐threatening hypoxaemia. No conclusions can be drawn regarding NIV as an alternative to intubation owing to eligibility requirements of this review.
Agreements and disagreements with other studies or reviews
A previous review evaluated effects of NIV added to standard treatment for management of acute respiratory failure (Keenan 1997). The Keenan 1997 review, which analysed participants with COPD separately, showed a strong survival benefit (odds ratio (OR) = 0.29) and a reduced need for intubation (OR = 0.12) in favour of NIV. Although our systematic review derived similar conclusions as those presented by Keenan and coworkers, some important limitations of this previous review led to the need for the present review. First, our review included 17 RCTs, but we included only three of the seven studies included in the Keenan 1997 review and excluded the remaining four studies for various reasons. Ahmed 1992 did not compare NIV with usual care but compared NIV with doxapram; Daskalopoulou 1993 used an inadequate method for randomisation (alternation); Martin 1995 included only a subgroup of participants with COPD and not all participants met the inclusion criterion of PaCO2 > 45 mmHg; and Wysocki 1995 excluded all patients who had COPD.
Second, we sought to implement a more comprehensive search strategy compared with the one used by Keenan 1997, as indexing of MEDLINE alone is likely to miss potentially relevant studies. The strategy used in this review included electronic database searches, handsearching, and inclusion of non‐English studies.
Our current review includes only studies that were primarily set up to compare NIV with usual care for management of respiratory failure secondary to an acute exacerbation of COPD. The Keenan 1997 review set out to investigate NIV versus usual care in acute respiratory failure due to various causes, including cor pulmonale, respiratory distress, COPD, and pneumonia. The Keenan 1997 review conducted a post hoc analysis of the COPD subgroup.

Study flow diagram for 2004‐2017 literature searches.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

NIV vs usual care (overall) ‐ Mortality

Cates plot Analysis 1.1 (mortality), NIV group: In the usual care group, 18 of 100 people died during the period of hospitalisation, compared with 10 (95% CI 7 to 14) of 100 in the NIV group.

Funnel plot of comparison: 1 NIV vs usual care ‐ Overall, outcome: 1.1 Mortality.

NIV vs usual care (overall) ‐ Need for endotracheal intubation

Cates plot Analysis 1.2 (need for endotracheal intubation), NIV group: In the usual care group, 34 of 100 people experienced the need for endotracheal intubation during the period of hospitalisation, compared with 12 (95% CI 10 to 16) of 100 in the NIV group.

Funnel plot of comparison: 1 NIV vs usual care ‐ Overall, outcome: 1.2 Need for endotracheal intubation.

Comparison 1 NIV vs usual care ‐ Overall, Outcome 1 Mortality.

Comparison 1 NIV vs usual care ‐ Overall, Outcome 2 Need for endotracheal intubation.

Comparison 1 NIV vs usual care ‐ Overall, Outcome 3 Length of hospital stay (days).

Comparison 1 NIV vs usual care ‐ Overall, Outcome 4 Length of ICU stay (days).

Comparison 1 NIV vs usual care ‐ Overall, Outcome 5 Symptom scores (higher score means more dyspnoea).

Comparison 1 NIV vs usual care ‐ Overall, Outcome 6 Treatment intolerance.

Comparison 1 NIV vs usual care ‐ Overall, Outcome 7 Complications of treatment.

Comparison 1 NIV vs usual care ‐ Overall, Outcome 8 pH 1 hour post intervention.

Comparison 1 NIV vs usual care ‐ Overall, Outcome 9 PaCO2 mmHg ‐ 1 hour post intervention.

Comparison 1 NIV vs usual care ‐ Overall, Outcome 10 PaO2 mmHg ‐ 1 hour post intervention.

Comparison 2 NIV vs UMC ‐ Admission pH subgroups, Outcome 1 Mortality.

Comparison 2 NIV vs UMC ‐ Admission pH subgroups, Outcome 2 Need for endotracheal intubation.

Comparison 3 NIV vs UMC ‐ Trial location subgroups, Outcome 1 Mortality.

Comparison 3 NIV vs UMC ‐ Trial location subgroups, Outcome 2 Need for endotracheal intubation.
| Non‐invasive ventilation versus usual medical care for management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease (overall effects) | ||||||
| Patient or population: Patients admitted to hospital with acute hypercapnic respiratory failure due to an exacerbation of chronic obstructive pulmonary disease (COPD) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with usual care ‐ Overall | Risk with NIV | |||||
| Mortality | 183 per 1000 | 99 per 1000 | RR 0.54 | 854 | ⊕⊕⊕⊝ | Downgraded owing to risk of bias for some included studies |
| Need for endotracheal intubation | 341 per 1000 | 123 per 1000 | RR 0.36 | 1105 | ⊕⊕⊕⊝ | Downgraded owing to risk of bias for some included studies |
| Length of hospital stay (days) | Mean length of hospital stay (days) was 17.5 | MD 3.39 lower | ‐ | 888 | ⊕⊕⊕⊝ | Downgraded owing to risk of bias and inconsistency of findings for some included studies |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSeveral risk of bias items rated 'unclear' bOne study reported an effect estimate that favoured usual medical care (non‐significant); significant statistical heterogeneity identified within the intensive care unit subgroup was unable to be resolved | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 12 | 854 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.38, 0.76] |
| 2 Need for endotracheal intubation Show forest plot | 17 | 1105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.28, 0.46] |
| 3 Length of hospital stay (days) Show forest plot | 10 | 888 | Mean Difference (IV, Random, 95% CI) | ‐3.39 [‐5.93, ‐0.85] |
| 4 Length of ICU stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Symptom scores (higher score means more dyspnoea) Show forest plot | 4 | 484 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.34, 0.02] |
| 5.1 Borg score | 2 | 82 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.79, 0.08] |
| 5.2 Visual analogue scale | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.40, ‐0.34] |
| 5.3 Dyspnoea score at 24 hours | 1 | 342 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.21, 0.21] |
| 6 Treatment intolerance Show forest plot | 6 | 252 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.17] |
| 7 Complications of treatment Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 NIV related | 6 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 29.60 [9.47, 92.51] |
| 7.2 Non‐NIV related | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.53] |
| 8 pH 1 hour post intervention Show forest plot | 8 | 585 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.02, 0.07] |
| 9 PaCO2 mmHg ‐ 1 hour post intervention Show forest plot | 8 | 585 | Mean Difference (IV, Random, 95% CI) | ‐4.62 [‐11.05, 1.80] |
| 10 PaO2 mmHg ‐ 1 hour post intervention Show forest plot | 8 | 585 | Mean Difference (IV, Random, 95% CI) | 7.47 [0.78, 14.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Admission pH > 7.30 | 5 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.30, 0.84] |
| 1.2 Admission pH < 7.30 | 8 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.35, 0.90] |
| 2 Need for endotracheal intubation Show forest plot | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Admission pH > 7.30 | 7 | 589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.63] |
| 2.2 Admission pH < 7.30 | 11 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.22, 0.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Intensive care unit | 5 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.07] |
| 1.2 Ward | 5 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.78] |
| 2 Need for endotracheal intubation Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Intensive care unit | 9 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.21, 0.43] |
| 2.2 Ward | 8 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.31, 0.60] |

