Vitamine als Nahrungsergänzung zur Vorbeugung von Fehlgeburten
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation and allocation concealment: random allocation of the entire population of the urban and rural areas (population, 110,000; 20,400 households) into 28 discrete clusters (16 rural and 12 urban) on the basis of household characteristics, socioeconomic criteria, and geographic location. Each cluster was allocated to a community health worker who distributed the supplements on a cluster‐based allocation strategy of supplements ("either iron–folic acid or multiple micronutrients"). Distribution of the sealed, coded supplement bottles were independently controlled by the pharmacy at Aga Khan University. Blinding of outcome assessment: medical officers, community health workers, social scientists, and data collection team remained blinded to the supplementation allocation. Documentation of exclusion: 373 women (16.5%) were excluded. Use of placebo control: no placebo given, women in the control group were given IFA. | |
| Participants | 2378 women from community settings in urban and rural Sindh (Pakistan) less than 16 weeks of gestation. Eligible women were women with a confirmed pregnancy at less than 16 weeks of gestation. Women who did not have a confirmed pregnancy on ultrasound scanning or women who were clearly advanced beyond 24 weeks of gestation were excluded. | |
| Interventions | Multiple micronutrients comprised 30 mg of iron (ferrous fumarate) and 400 mcg of folic acid along with 800 mcg of retinol (retinyl acetate), 200 IU of vitamin D (ergocalciferol), 10 mg of vitamin E (α‐tocopherol acetate), 70 mg of ascorbic acid, 1.4 mg of vitamin B1 (thiamine mononitrate), 18 mg of niacin (niacinamide) 1.4 mg of vitamin B2, 1.9 mg of vitamin B6 (pyridoxine), 2.6 mcg of vitamin B12 (cyanocobalamin), 15 mg of zinc (zinc gluconate), 2 mg of copper, 65 mcg of selenium, and 150 mcg of iodine. Intervention was timed to start at less than 16 weeks' gestation. Comparison was iron (60 mg) and folic acid (400 mcg). | |
| Outcomes | Maternal outcomes:
Infant outcomes:
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage is unclear. Womens' BMI, Hb, ferritin, zinc, and serum retinol at admission are reported. Sample‐size calculation reported by 2 methods: 1. based on a potential 5% gain in birthweight, 2. based to estimate a difference in birthweight of 150 g between the 2 groups. No intention‐to‐treat analyses performed. Compliance: community health worker performed a tablet count every fourth nightly visit. Proportion of tablets consumed 75.65 in the intervention group and 76.7% in the control group. Location: urban population (Bilal Colony, Karachi) and rural villages (Kot Diji district, rural Sindh), Pakistan. Timeframe: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly allocated the entire population of the urban and rural area." Pg S497. |
| Allocation concealment (selection bias) | Low risk | "cluster‐based allocation strategy of supplements (either iron–folic acid or multiple micronutrients) by the community health workers was implemented. The allocation of either iron–folic acid or multiple micronutrient supplements and the distribution of the sealed, coded supplement bottles were independently controlled by the pharmacy at Aga Khan University, which maintained the allocation codes by individual community health workers." Pg S498. |
| Blinding of participants and personnel (performance bias) | Low risk | All pregnant women were allocated a unique code and a uniquely labelled and numerically coded specific supplement supply for the duration of pregnancy. Blinding is unlikely to have been broken. Pg S498. |
| Blinding of outcome assessment (detection bias) | Low risk | "The field staff (medical officers, community health workers, social scientists, and data collection team) remained completely blinded as to the supplement allocation." Pg S498. |
| Incomplete outcome data (attrition bias) | Low risk | Approximately 16.5% of attrition with balanced number and similar reason for each group. S500 Figure1. |
| Selective reporting (reporting bias) | Unclear risk | No information about trial registration. |
| Other bias | High risk | The distribution of study participants across the urban and rural areas is unclear from the text and no adjustments were made for cluster design. |
| Methods | Randomisation and allocation concealment: unclear, no methodological details given, dubious as the number of women allocated to the treatment group was more than double that allocated to the placebo group. "Unselected patients were each given 200 capsules... these were given a code, unknown to us and contained either an inert powder or 100 mg each of ascorbic acid and hesperidin." Blinding of outcome assessment: women and study investigators did not know the treatment codes. Documentation of exclusion: none reported. Use of placebo control: placebo given; however, all women received an additional multivitamin supplement. | |
| Participants | 406 women were recruited in the study. Eligible women were "unselected patients" in private obstetrics care, who were less than or equal to 10 weeks' pregnant, and were eligible regardless of whether they were currently bleeding or the number of previous pregnancies. Women greater than 10 weeks' gestation were excluded. 406 women were randomised to either vitamin C (n = 303) or placebo (n = 103), no losses to follow‐up were reported. 77 women in the study had more than 2 previous miscarriages and/or bleeding in the pregnancy, and 329 had 2 or fewer miscarriages and no bleeding in the pregnancy. | |
| Interventions | All women were given 200 tablets, containing either 100 mg each of ascorbic acid and hesperidin or placebo (an inert powder). | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage is unclear, as there is no information about concurrent medical conditions or other risk factors for miscarriage. 9 of the 406 women were classified as experiencing recurrent miscarriage. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No methodological details given. |
| Allocation concealment (selection bias) | Unclear risk | No methodological details given. |
| Blinding of participants and personnel (performance bias) | Low risk | Women and study investigators did not know the treatment allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind study, but it is unclear who was blinded and if the code was broken before or after outcome assessment pg289. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Limited information about selection bias, stated that 'unselected patients' were included. |
| Other bias | Unclear risk | Limited methodological details provided including patient compliance. |
| Methods | Randomisation and allocation concealment: a computer‐generated randomisation list using blocks of 10 was given to the hospital pharmacy departments. Researchers allocated the next available number to participants and women collected the trial tablets from the pharmacy department. Blinding of outcome assessment: women, caregivers and researchers were blinded to the treatment allocation until recruitment, data collection and laboratory analyses were complete. Documentation of exclusion: 123 (43.5%) women were excluded, of which 70 women were withdrawn because their second Doppler scan was normal. Pregnancy outcome data were reported for all women randomised. Use of placebo control: placebo control. | |
| Participants | 283 women were recruited into the study. Inclusion criteria: abnormal Doppler waveform in either uterine artery at 18‐22 weeks' gestation or a history in the preceding pregnancy of pre‐eclampsia necessitating delivery before 37 weeks' gestation, eclampsia or the syndrome of HELLP. | |
| Interventions | Women randomised to the vitamin C and E group received tablets containing 1000 mg vitamin C daily and capsules containing 400 IU vitamin E daily. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage is unclear, women were at high risk of pre‐eclampsia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Random number list used blocks of 10 and was held by the pharmacy department. |
| Blinding of participants and personnel (performance bias) | Low risk | Women, caregivers and researchers were blinded until the analyses were completed. |
| Blinding of outcome assessment (detection bias) | Low risk | "The code was revealed to the researchers once recruitment, data collection, and laboratory analyses were complete" pg811. |
| Incomplete outcome data (attrition bias) | Low risk | 123 (43.5%) women were excluded, of which, 70 women were withdrawn because their second Doppler scan was normal. Data were reported for all women randomised. |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes in methods. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomisation and allocation concealment: unclear, "women agreed to their allocation on the basis of a random table". Blinding of outcome assessment: unclear, women were aware of the "blind use of one of two kinds of tablets", but no other details given. Documentation of exclusion: 49 women (1%) were lost to follow‐up and excluded. Use of placebo control: "trace element control" given. | |
| Participants | 7765 women were recruited into the study. Women participating in the HOFPP who volunteered to take part, were not currently pregnant, and who conceived within 12 months of ceasing contraception. In the first 2 years of the HOFPP, women were also required to be aged < 35 years, and not to have had a previous pregnancy except a prior induced abortion. 7905 women were approached, of which 140 refused participation, 7765 were randomised and 5502 women had a confirmed pregnancy and were allocated to either multivitamins (n = 2819) or control (n = 2683). 49 women of the 5502 confirmed pregnancies were lost to follow‐up. | |
| Interventions | Women were provided with multivitamin or trace element 'control' from at least 28 days before conception continuing until at least the second missed menstrual period. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage is unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methodological details unclear. |
| Allocation concealment (selection bias) | Unclear risk | Methodological details unclear, 'women agreed to their allocation on the basis of a random table'. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Women were aware of the 'blind use of one of two kinds of tablets', but no other details given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details are given if outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 49 women (1%) excluded, partial intention‐to‐treat analyses performed. |
| Selective reporting (reporting bias) | Unclear risk | Denominators vary with serial publications. |
| Other bias | Unclear risk | Limited methodological details provided. |
| Methods | Randomisation and allocation concealment: block randomisation using blocks of 20, eligible women were "assigned the next numbered bottle of regimen". The study used a 2 by 2 factorial design and women were randomised to 1 of 4 groups. Tablets were indistinguishable and packaged in identically coded bottles. Blinding of outcome assessment: women and study investigators were unaware of the treatment allocation, no information given about blinding of outcome assessors. Documentation of exclusion: 64 women (6%) were lost to follow‐up and excluded. Use of placebo control: placebo given. | |
| Participants | 1085 women were recruited into the study. Pregnant women between 12 and 27 weeks' gestation who were HIV‐1 infected, living in Dar es Salaam and intended to stay there for at least 1 year were eligible for the study. Women not HIV‐1 positive or moving out of Dar es Salaam were excluded. 13,879 pregnant women consented to be HIV‐1 tested, of which 1806 were positive, and 1085 were randomised. Of these, 3 women were not pregnant and 7 women died before delivery and were excluded from the trial. Of the remaining 1075 women, 54 women (5%) were lost to follow‐up by the time of delivery, leaving birth outcomes reported for 1021 women. Women were randomised to 1 of 4 groups: vitamin A (n = 269), multivitamins excluding vitamin A (n = 269); multivitamins including vitamin A (n = 270) or placebo (n = 267). | |
| Interventions | Women were randomised to 1 of 4 groups:
All women received 400 mg ferrous sulphate and 5 mg folic acid daily, as well as 500 mg chloroquine phosphate weekly. At delivery, all women taking vitamin A were to receive an additional oral dose of 200,000 IU vitamin A and the others an extra dose of a placebo. Pill counts were conducted at each visit and new tablets were given out at each visit. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear, although may be increased due to their HIV‐1 positive status. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation using blocks of 20. |
| Allocation concealment (selection bias) | Unclear risk | Women assigned the 'next numbered bottle of regimen'. |
| Blinding of participants and personnel (performance bias) | Low risk | Women and investigators were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blinded study, but unclear if ocutome assessors were blinded. |
| Incomplete outcome data (attrition bias) | High risk | 64 women (6%) were lost to follow‐up and excluded, intention‐to‐treat analyses performed. |
| Selective reporting (reporting bias) | Unclear risk | Figures change with serial publications, particularly for secondary outcomes, and results are not reported separately for the individual 4 groups. |
| Other bias | Unclear risk | Limited methodological details provided. |
| Methods | Randomisation: unclear about sequence generation. Allocation concealment: states a list was prepared according to the randomisation sequence in blocks of 20, tablets were bottled in identical coded bottles, eligible women were given the next numbered bottle. Blinding of outcome assessment: women and research assistants who assessed the study outcomes were unaware of the intervention groups. Documentation of exclusion: 49 women lost to follow‐up (multivitamin group: 23, placebo group: 26), no post‐randomisation exclusions. Use of placebo control: placebo given. | |
| Participants | 8428 women were randomised in the study. Pregnant women between 12 and 27 weeks who had a negative test for HIV infection and planned to stay in the city until delivery and for 1 year thereafter recruited through antenatal clinics in Dar es Salaam. 8468 women were enrolled, however 40 women were then found to be ineligible. 8428 women were randomly assigned to receive either a multivitamin (n = 4214) or placebo (n = 4214) from the time of enrolment until 6 weeks after delivery. 6 women died before delivery and 43 were lost to follow‐up by the time of delivery. | |
| Interventions | The supplements included 20 mg of vitamin B1, 20 mg of vitamin B2, 25 mg of vitamin B6, 100 mg of niacin, 50 mcg of vitamin B12, 500 mg of vitamin C, 30 mg of vitamin E, and 0.8 mg of folic acid. The active tablets and placebo were similar in shape, size, and colour. All women, irrespective of the assigned study regimen, were given daily doses of iron (60 mg of elemental iron) and folic acid (0.25 mg). They were also given malaria prophylaxis in the form of sulfadoxine‐pyrimethamine tablets at 20 weeks and 30 weeks of gestation. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Women's nutritional status is also unclear. Intention‐to‐treat analyses performed. Compliance: average compliance was 88%, no difference in compliances between the 2 groups. Location: Tanzania. Timeframe: August 2001 and July 2004. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of sequence not reported, except that there were blocks of 20 in the sequence. |
| Allocation concealment (selection bias) | Low risk | Identical coded bottles prepared according to the randomisation list, eligible women were assigned the next numbered bottle. |
| Blinding of participants and personnel (performance bias) | Low risk | Women and outcome assessors were blinded to allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | "Research assistants who assessed the study outcomes were unaware of the intervention groups." pg1424. |
| Incomplete outcome data (attrition bias) | Low risk | 49 (1%) women lost to follow‐up, balanced across groups, analyses by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes appear to be reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomisation and allocation concealment: quasi‐randomised, alternate women were allocated to receive folic acid or placebo according to the order in which they attended antenatal clinic. No other methodological details were given. Blinding of outcome assessment: women and investigators were blinded to the treatment allocation, until after the completion of the trial. Documentation of exclusion: 21 women (28%) excluded from the analysis. Use of placebo control: control tablet containing iron given. | |
| Participants | 75 women were recruited into the trial. Women were eligible if they were primigravida, less than 26 weeks' pregnant (range of gestation 10 to 26 weeks'), with haematocrit value (PCV) 27% or more, and who had not received treatment so far as was known. Women with Hb SC, Hb SS, Hb CC were excluded. Alternate patients were allocated to group A (placebo) or B (folic acid). 75 women were included (40 in group A and 35 in group B) initially; however, only 26 in group A and 28 in group B completed the trial. 16 women (10 in group A and 8 in group B) defaulted from the trial, 3 (2 in group A and 1 in group B) were anaemic on the second visit warranting folic acid treatment, 1 in group A self‐medicated with folic acid and 1 in group A 'aborted'. | |
| Interventions | All women received antimalarials and iron supplements as per the standard antenatal care at the hospital. | |
| Outcomes |
| |
| Notes | Results not reported as intention‐to‐treat; however, where possible, the review authors included data in the review as intention‐to‐treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised, alternate allocation. |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised, alternate allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Women and investigators blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | "The identity of the tablets was not known to investigators until after the completion of the trial." pg426. |
| Incomplete outcome data (attrition bias) | High risk | 21 women (28%) excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Results not reported as intention to treat; however, where possible, the review authors included data in the review as intention to treat. |
| Other bias | Unclear risk | Limited methodological details provided. |
| Methods | Randomisation and allocation concealment: women were "randomly allocated to one of five groups using a random number table", no other details given. Blinding of outcome assessment: women and investigators were blinded to the treatment allocation, until after the completion of the trial. Documentation of exclusion: 18 women (9%) were excluded due to anaemia at enrolment, 'defaulting', or being 'mentally subnormal', these women were replaced by other women chosen by an investigator. A further 42 women were excluded before delivery and another 30 failed to attend the postnatal clinic, birth outcomes were available for 160 women (80%). Use of placebo control: no placebo control. | |
| Participants | 228 women met the eligibility criteria; however 200 pregnant women were recruited into the study. Women were allocated to 1 of 5 groups; 40 women were allocated to each group. Eligible women included:
Women were excluded if they had already taken any antimalarial treatment or haematinics during the pregnancy, or had the following complications: hydatiform mole, Hb SC disease, overt anaemia or proteinuria. The mean gestational age of women at enrolment was 18.5 weeks. | |
| Interventions | Women were allocated to 1 of 5 groups:
| |
| Outcomes | Maternal outcomes
Infant outcomes
Laboratory outcomes
Not all outcomes were reported for each individual treatment group. Miscarriage was reported for the combined groups 4 and 5, therefore for the purpose of this review the groups 4 and 5 are combined (folic acid + iron) and compared with group 2 and group 3 (iron + antimalarials). The authors reported that 8 women had hypertension without other signs, 21 women had pre‐eclampsia and 6 developed eclampsia, with no association between these outcomes and treatment group. No other details were provided, including the breakdown of these outcomes by treatment group. | |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Women were at high risk of anaemia. Information about other nutritional indices was not provided. Intention‐to‐treat analyses not performed, however, where possible, the review authors included data in the review as intention‐to‐treat. Compliance: 72 women (36%) were classed as defaulters. Location: Nigeria. Timeframe: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A random number table was used but no details provided of how it was generated. |
| Allocation concealment (selection bias) | Unclear risk | No details provided about the allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Neither the researchers nor the patients were aware of the treatment allocation until after the completion of the study. |
| Blinding of outcome assessment (detection bias) | Low risk | "Neither the researchers nor the patients were aware of the treatment allocation until after the completion of the study." pg 214. |
| Incomplete outcome data (attrition bias) | High risk | 228 women met the entry criteria, but only 200 were included in the trial. 18 women were excluded and replaced by other women. |
| Selective reporting (reporting bias) | High risk | Not all outcomes are reported by treatment group. In serial publications up to 70% of the data were excluded. |
| Other bias | Unclear risk | Limited methodological details provided. |
| Methods | Randomisation and allocation concealment: women were randomly assigned to receive either 400 mg of vitamin C daily or not in addition to their standard antenatal vitamin. Randomisation was obtained by a computer‐generated, block design sequence to receive vitamin C or not in a 1:1 ratio. No other methodological details given. Blinding of outcome assessment: unclear, no details given. Documentation of exclusion: 16 women (4%) did not complete the trial after randomisation. Use of placebo control: no placebo control. | |
| Participants | 400 women 4 to 12 gestational weeks of pregnancy confirmed serologically by B‐HCG reagent test along with referred last menstrual period not exceeding the past 3 months, aged at least 18. Women with referred pregnancy of more than 3 months by last menstrual period, concomitant HIV infection status, active or recent (< 2 weeks) sexually transmitted disease infection, medical record of any severe organ disease such as heart, liver or renal failure at the time of assessment, diagnosis of pregnancy during inpatient admission for any other reason, recent history of multivitamin supplementation (< 12 weeks) for any reason, except for pregnancy, and patients incapable to read and write. | |
| Interventions | Chewable tablet of synthetic form of L‐ascorbic acid or vitamin C 400 mg administered daily 2 tablet 2 times a day, from first trimester until delivery. Comparison received no vitamin C in addition to their standard antenatal vitamin. All women received ferrous sulphate 200 mg, folic acid 5 mg and vitamin B‐complex 60 mg once daily tablets. Nutritional counselling was provided to all women. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous or recurrent miscarriage is unclear, as is their dietary intake. Sample‐size calculation based on at least 30% of women not hospitalised during pregnancy in the control group and at least 50% of women not hospitalised in the intervention group. Analyses were not based on intention‐to‐treat. Compliance: no details of any compliance assessments were given. Location: Kyeibuza, Uganda. Timeframe: August 2007 and January 2009. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients presented to the health centre, those who met the inclusion criteria were randomly assigned...randomization was obtained by a computer‐generated, block design sequence to receive vitamin C or not in a 1:1 ratio." pg 3. |
| Allocation concealment (selection bias) | Unclear risk | No description for allocation concealment in the text. Probably not done. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on participant or personnel blinding provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on outcome assessor blinding provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐out rates for each group were not clearly described and no information was provided on the reason of exclusion and attrition. |
| Selective reporting (reporting bias) | Unclear risk | Although expected outcomes were reported, the study protocol was not available to see if all prespecified outcomes were reported as planned. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomisation and allocation concealment: unclear, "patients were randomly assigned to the control group or the study group". No other methodological details given. Blinding of outcome assessment: unclear, no details given. Documentation of exclusion: 28 women (19%) in the control group were excluded, no details given for the exclusion. Use of placebo control: no placebo control. | |
| Participants | 150 women were recruited into the study. Women with a luteal phase defect, as described by a peak serum P level < 120 mg/mL in the mid‐luteal phase measured at 3 time points, were eligible and invited to participate. Luteal phase defects were ascertained in 2 consecutive menstrual cycles, and the third cycle was the intervention cycle. Women receiving IVF‐ET treatment were excluded. 313 women were considered for enrolment in the study, 150 (48%) were randomised. 28 women were withdrawn from the control group, leaving 122 women in the study, who were allocated to vitamin C (n = 76) or control (n = 46). 5 women in the control group and 19 women in the vitamin C group became pregnant during the study period. | |
| Interventions | Women in the intervention group took 750 mg vitamin C per day from the first day of the third menstrual cycle until a urinary pregnancy test was positive. Pregnancy rate was checked up until 6 months after the study cycle was started. Women in the control group received no supplementation and no treatment was given in the third cycle. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous or recurrent miscarriage was unclear according to criteria specified in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methodological details unclear. |
| Allocation concealment (selection bias) | Unclear risk | Methodological details unclear. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Methodological details unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No methodological details are given. |
| Incomplete outcome data (attrition bias) | High risk | 28 women (19%) in the control group excluded. |
| Selective reporting (reporting bias) | Unclear risk | No details of exclusion of women in the control group given. |
| Other bias | High risk | No placebo control. |
| Methods | Randomisation and allocation concealment: unclear, "containers of vitamin or placebo capsules were given a random number" and "the key to random numbers was kept at the ICMR Headquarters". No other methodological details were given. Blinding of outcome assessment: "double blind" mentioned in the text, but no details given. Documentation of exclusion: 187 women (40%) were excluded from the analysis. Use of placebo control: placebo control. | |
| Participants | 466 women were recruited into the study. Women who had previously given birth to a child with an open NTD, and planned to have another child were eligible and invited to participate. This was regardless of their parity, number of previous births with an NTD, age, consanguinity, and socioeconomic status. Women who had previously given birth to a child with closed spina bifida, or with a history of diabetes or abnormal fasting and post‐prandial blood sugar, history of epilepsy, congenital anomalies indicative of a genetic syndrome in the previous NTD, history of vitamin intake in the 3 months prior to enrolment, and pregnancy were excluded. 466 women were enrolled and randomised to either vitamin (n = 231) or placebo (n = 235), of these women, 90 were lost to follow‐up immediately and 71 did not conceive until the final follow‐up. Of the remaining 305 women who were known to become pregnant (vitamin n = 152, placebo n = 153), pregnancy outcomes were unknown for 26 women. In the paper, 279 of the initial 466 women were included in the analysis; however, in this review results are presented for main outcomes on an intention‐to‐treat basis (i.e. n = 466). | |
| Interventions | The folic acid containing multivitamin included 120 mg ferrous sulphate, 240 mg calcium phosphate, 4000 IU vitamin A, 400 IU vitamin D, 2.5 mg vitamin B1, 2.5 mg vitamin B2, 2 mg vitamin B6, 15 mg nicotinamide, 40 mg vitamin C, 4 mg folic acid, 10 mg zinc. | |
| Outcomes |
| |
| Notes | The risk profile of women in the trial for spontaneous and recurrent miscarriage is unclear, as is the dietary intake of participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Containers 'given a random number'. |
| Allocation concealment (selection bias) | Unclear risk | 'Key to random numbers were kept at the ICMR headquarters' but no other details given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind mentioned in the text but no details given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear of outcome assessors were unaware of treatment allocation. |
| Incomplete outcome data (attrition bias) | High risk | 187 (40%) women excluded. |
| Selective reporting (reporting bias) | Unclear risk | Difficult to assess given the high losses to follow‐up. |
| Other bias | Unclear risk | Limited methodological details provided. |
| Methods | Randomisation and allocation concealment: randomised controlled trial, but no other information provided. Blinding of outcome assessment: unclear. Documentation of exclusions: unclear, no information provided. Use of placebo: placebo control. | |
| Participants | Women with a history of 2 or more early pregnancy losses, with no identifiable cause for the losses. | |
| Interventions | Vitamin C 1000 mg and vitamin E 400 IU versus placebo. | |
| Outcomes | Miscarriage. | |
| Notes | Updated 27/11/2013: the trial was stopped in October 2009 due to poor recruitment and lack of funding. Women's risk of spontaneous and recurrent miscarriage is unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Could not be assessed because the trial was stopped. |
| Allocation concealment (selection bias) | Unclear risk | Could not be assessed because the trial was stopped. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Could not be assessed because the trial was stopped. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Could not be assessed because the trial was stopped. |
| Incomplete outcome data (attrition bias) | Unclear risk | Could not be assessed because the trial was stopped. |
| Selective reporting (reporting bias) | Unclear risk | Could not be assessed because the trial was stopped. |
| Other bias | Unclear risk | Could not be assessed because the trial was stopped. |
| Methods | Randomisation and allocation concealment: cluster‐randomised. 270 centres in the Salarhi district, Nepal, were involved which included 30 subdistricts each with 9 wards. Each ward was assigned to 1 of 3 treatment groups. "Wards were assigned by a random draw of numbered chits, blocked on subdistrict." Blinding of outcome assessment: women and study investigators were not aware of the treatment codes. Maternal mortality was assessed by study investigators blinded to treatment allocation, no details were given for other outcomes. Documentation of exclusions: 157 (1%) women were lost to follow‐up and excluded. Use of placebo: placebo control. | |
| Participants | 15,832 women were recruited into the study. All married women of child bearing age in the Salarhi district, Nepal, were eligible and invited to participate in the study. Women migrating into the study area, or women that were never pregnant or refused participation, or women who migrated before being pregnant, were excluded from the analysis. Eligible women were identified from census data and marriage registers. 44,646 women were recruited, of which 1136 (2.5%) were excluded as they either emigrated before becoming pregnant, died or refused consent. During the study period 15,832 women identified themselves as being pregnant, and 157 women were lost to follow‐up in the postpartum period. Results are reported for 17,373 pregnancies, allocated to the following groups: vitamin A (n = 6070), beta‐carotene (n = 5650) or placebo (n = 5653). Denominators for the treatment groups vary for the measures of early infant mortality, due to losses to follow‐up after birth. | |
| Interventions | The 3 treatment groups consisted of a weekly single oral supplement of either:
All capsules contained mg dl‐alpha‐tocopherol as an antioxidant. Women took the tablets prior to conception, during pregnancy and postpartum, for a total of 3.5 years. | |
| Outcomes | 1. Fetal loss, defined as any reported miscarriage, stillbirth or maternal death during pregnancy. The outcomes were based on self‐reports, and women who reported to be pregnant for >= 6 weeks but then no longer reported being pregnant were considered to have had a miscarriage. | |
| Notes | Women's risk profile for spontaneous or recurrent miscarriage was unclear, as was their dietary intake of vitamin A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised, unclear how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Each ward was assigned to the treatment groups based on 'a random draw of numbered chits, blocked on subdistrict'. |
| Blinding of participants and personnel (performance bias) | Low risk | Women and investigators blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "This committee and the data analysts were unmasked to the treatment codes, but the codes were made available to study investigators only at the end of the trial." |
| Incomplete outcome data (attrition bias) | Unclear risk | 157 women (1%) were lost to follow‐up and excluded, partial intention‐to‐treat analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Denominators vary in several publications of this trial. |
| Other bias | High risk | Some women were pregnant more than once during the study period, however the denominators reported are the total number of pregnancies during the study period, not the total number of women randomised, which incorrectly assumes that each data point included is independent from the next. |
| Methods | Randomisation and allocation concealment: block randomisation, stratified by hospital, using "consecutively numbered, opaque, sealed envelopes". Blinding of outcome assessment: women and study investigators were initially blinded to the treatment allocation, however the tablet preparations were changed after 55 women were randomised and after this only participants were blinded. Documentation of exclusion: 3 women (1%) were lost to follow‐up and excluded. Use of placebo control: 3 treatment regimens were assessed, no placebo control. | |
| Participants | 354 women were recruited into the study. Women with a previous NTD defined as anencephalus, iniencephalus, encephalocoele, and spina bifida aperta, who were not pregnant when contacted but were planning a future pregnancy, were eligible and invited to participate. Women were identified from case registers at the participating hospitals. Women with conditions likely to result in impaired absorption from the gastrointestinal tract were excluded. | |
| Interventions | Indistinguishable trial tablets were initially made by Beecham and Glaxo, however Beecham withdrew their support after 55 women had been randomised. After this time a commercially available pregnavite Forte F was used (MF tablet) and Antigen Pharmaceuticals produced a white multivitamin tablet without folic acid. This was associated with a loss of blinding. Women were randomised to 1 of 3 treatments:
The F and MF resulted in a daily dose of 0.3 mg folic acid. The MF and MV resulted in a daily dose of 4000 IU vitamin A, 400 IU calciferol, 1.5 mg thiamine hydrochloride, 1.5 mg riboflavine, 1 mg pyridoxine hydrochloride, 15 mg nicotinamide, 40 mg ascorbic acid, 480 mg calcium phosphate, and 252 mg ferrous sulphate. Women took the tablets for at least 2 months prior to conception and until the date of the 3rd missed period. | |
| Outcomes |
| |
| Notes | The trial was stopped after there were poor recruitment rates and birth rates. A sample‐size calculation required 462 women to show a reduction in NTDs from 5% to 1%. Data from 106 women who were already pregnant at time of recruitment are also included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation stratified by hospital site. |
| Allocation concealment (selection bias) | Low risk | Consequtively numbered, opaque sealed envelopes used. |
| Blinding of participants and personnel (performance bias) | Low risk | Only participants were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Only participants were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 3 women (1%) lost to follow‐up and excluded. Intention‐to‐treat analyses performed. |
| Selective reporting (reporting bias) | Unclear risk | Compliance data not reported. |
| Other bias | High risk | The trial was stopped after there were poor recruitment rates and birth rates. |
| Methods | Randomised controlled trial of vitamin A, iron and folic acid supplementation versus iron and folic acid only, during pregnancy, to improve infant outcomes born to women infected with HIV in Malawi. Randomisation and allocation concealment: "treatment assignment was determined by use of a computer's random‐number generator" and "mothers were assigned an original study identification number at enrolment and were given the next sequentially numbered opaque bottle with supplements". "Treatment assignment was concealed by pre packing study supplements in sequentially numbered series assigned to study identification numbers." Blinding of outcome assessment: unclear, not specifically stated, but participants were blind to their treatment allocation. Documentation of exclusion: 63 (9%) women were lost to follow‐up and 14 (2%) pairs of twins were excluded. Use of placebo control: control tablets containing iron and folic acid were given. | |
| Participants | Pregnant women between 18 and 29 weeks' gestation and infected with HIV. The average gestation of participants was 23 weeks. 693 women were enrolled and allocated to either vitamin A (n = 340) or control (n = 357), of which pregnancy outcomes were known for 623 women. 63 women were lost to follow‐up and 14 sets of twins were excluded due to their higher risk of low birthweight and infant mortality. | |
| Interventions | All women received orally administered daily doses of 30 mg iron and 400 mcg folic acid during the study. Women in the intervention group received 10,000 IU vitamin A (3 mg retinol equivalent) orally, in addition to the iron and folic acid supplements. Women were asked to take the tablets from enrolments until delivery. Tablet counts were conducted every 4 weeks. All women received 30 mg retinol equivalents at 6 weeks postpartum, according to standard postpartum care in Malawi. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage is unclear, although may be increased due to their HIV status. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Sequentially number opaque bottles used. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specifically stated but women were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear of outcome assessors were unaware of treatment allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | 63 women (9%) lost to follow‐up and 14 pairs of twins (2%) excluded. No intention‐to‐treat analyses performed. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes appear to be reported. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of other bias exists. |
| Methods | Randomisation and allocation concealment: participants were randomly allocated in a 1:1 ratio to receive 1000 mg vitamin C and 400 IU vitamin E. A randomisation sequence generated in advance by Victoria Pharmaceuticals using PRISYM ID software (version1.0009) was used. The randomisation sequence was stratified by centre with balanced blocks of 8 patients and was held by Victoria Pharmaceuticals. Individual sealed envelopes containing treatment allocations were given to trial pharmacists in every centre allowing treatment groups to be revealed in a clinical emergency. Blinding of outcome assessment: diagnosis was independently confirmed by 3 senior clinicians, who were unaware of treatment allocation. Documentation of exclusion: only 1 loss to follow‐up was reported. Use of placebo control: matched placebo control. | |
| Participants | 762 pregnant women between 8 and 22 weeks' gestation with type‐1 diabetes attending 25 antenatal metabolic clinics across Northern Ireland, Scotland, and northwest England. Participants were women with type 1 diabetes preceding pregnancy, presentation between 8 weeks' and 22 weeks' gestation, singleton pregnancy, and age 16 years or older. Women with chronic hypertension were included in the trial. Women were excluded if they did not give consent, were enrolled in another research study, were being treated with warfarin, or were known to misuse drugs Women taking vitamin supplements were excluded only if these contained 500 mg or more vitamin C or 200 IU or more vitamin E daily. | |
| Interventions | 1000 mg vitamin C and 400 IU vitamin E versus matched placebo started between 8 and 22 weeks' gestation and taken until delivery. | |
| Outcomes |
| |
| Notes | Women's risk profile for spontaneous and recurrent miscarriage: women with chronic hypertension were included. Multivitamin supplementation at randomisation was reported at trial entry. Other information about nutrition status are not provided. Sample‐size calculation: based on 40% reduction in pre‐eclampsia. Modified intention to treat was used for analysis of the primary endpoint. Compliance: unused tablets and capsules were collected during delivery admission or at the 6‐week postnatal trial visit, or were returned in postage prepaid envelopes. Location: Northern Ireland, Scotland, northwest England. Tiimeframe: April 2003 to June 2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisations by Victoria Pharmaceuticals using PRISYM ID software (version1.0009). |
| Allocation concealment (selection bias) | Low risk | Supplements were identical in appearance. The randomisation sequence was stratified by centre with balanced blocks of 8 patients, and was held by Victoria Pharmaceuticals. |
| Blinding of participants and personnel (performance bias) | Low risk | Treatment allocation was masked from all trial personnel and participants until trial completion. |
| Blinding of outcome assessment (detection bias) | Low risk | Diagnosis were independently confirmed by 3 senior clinicians, who were unaware of treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 loss to follow‐up in placebo group, but the reason is unclear. |
| Selective reporting (reporting bias) | High risk | Fewer outcomes were stated in the trial registration. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomisation and allocation concealment: third party randomisation, "randomisation was carried out through the Clinical Trials Service Unit in Oxford". Randomisation was stratified by centre. Blinding of outcome assessment: women, caregivers and study investigators were blinded to the treatment allocation. Documentation of exclusion: 164 women (9%) excluded. Use of placebo control: placebo control. | |
| Participants | 1817 women were recruited into the study. Women who had a previous pregnancy affected by a NTD, and were planning another pregnancy and not already taking supplements were eligible for the study. Women whose affected child had Meckel's syndrome and those women with epilepsy were excluded. 1817 women were randomised to either F (n = 449), MV (n = 453), MF (n = 461) or P (n = 454), of which, 1195 were informative pregnancies that is, where the outcome of NTD or not was definitely known (F n = 298, MV n = 302, MF n = 295, P n = 300). Results for pregnancy loss are reported for both informative and not informative pregnancies. 164 women were excluded as they may have been pregnant at the time of randomisation. | |
| Interventions | Women were randomised into 1 of 4 groups:
Women took the tablets prior to conception and attended the site every 3 months to collect additional supplies and again during the 12th week of pregnancy. No special dietary advice was given to women. | |
| Outcomes |
| |
| Notes | The trial was stopped early after there were 1195 informative pregnancies, according to prespecified stopping rules. The aim of the study was to obtain information on at least 2000 informative pregnancies unless a sufficiently clear result emerged sooner. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Third party randomisation, "randomisation was carried out through the Clinical Trials Service Unit in Oxford". |
| Allocation concealment (selection bias) | Low risk | Third party randomisation, "randomisation was carried out through the Clinical Trials Service Unit in Oxford". |
| Blinding of participants and personnel (performance bias) | Low risk | Women, caregivers and investigators blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details are given if outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 164 women (9%) excluded, intention‐to‐treat analyses performed. |
| Selective reporting (reporting bias) | Unclear risk | No information provided about any women randomised that did not become pregnant in the study period. |
| Other bias | High risk | The trial was stopped early after there were 1195 informative pregnancies, according to prespecified stopping rules. |
| Methods | Randomisation and allocation concealment: 1 of the authors 'randomly allocated 1200 participant numbers by computer into 2 groups in permuted blocks of 50'. Every identification number was allocated a supplement container, which was then packed by a team member not otherwise involved in the trial. After enrolment, another author allocated participants sequential identification numbers with the corresponding supplement containers. Blinding of outcome assessment: double‐blind stated but no other details given. Documentation of exclusion: 61 women (5%) withdrew or were lost to follow‐up, however data on miscarriage were reported for those who withdrew due to miscarriage. Use of placebo control: control of iron and folic acid supplements given which looked identical to the intervention supplements. | |
| Participants | 1200 women were recruited into the study. Women were eligible if they were: less than 20 completed weeks, had a singleton pregnancy, no notable fetal abnormality, no existing maternal illness of a severity that could compromise the outcome of pregnancy, and lived in an area of Dhanusha or the adjoining district of Mahottari accessible for home visits. Maternal illnesses that led to exclusion were: recently treated recurrent cysticercosis, need for chlorpromazine or anticoagulant drugs with changing doses, and symptomatic mitral stenosis or multivalvular heart disease. Fetal exclusions were: twin pregnancies, anencephaly, occipital meningocele, encephalocele, duodenal atresia and a grossly dilated pelvicalyceal system. | |
| Interventions | Intervention group: vitamin A 800 mcg, vitamin E 10 mg, vitamin D 5 mcg, vitamin B1 1.4 mg, vitamin B2 1.4 mg, niacin 18 mg, vitamin B6 1.9 mg, vitamin B12 2.6 mcg, folic acid 400 mcg, vitamin C 70 mg, iron 30 mg, zinc 15 mg, copper 2 mg, selenium 65 mcg, and iodine 150 mcg. Control group: iron 60 mg and folic acid 400 mcg. Supplementation began at a minimum of 12 weeks’ gestation and continued until delivery. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Women's nutritional status is also unclear, however, women are presumable at high risk of under‐nutrition as the paper states that in Nepal 'deficiencies of several micronutrients have been well described in individual studies and in a national sample'. Intention‐to‐treat analyses performed. Compliance: median 'adherence' was 98% in the control group and 97% in the intervention group. Location: Nepal. Timeframe: August 2002 to October 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated in permuted blocks of 50. |
| Allocation concealment (selection bias) | Unclear risk | 1 of the authors allocated participants with sequential identification numbers, but unclear if this person was involved in the recruitment of participants. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind stated in the text but no other details given. |
| Blinding of outcome assessment (detection bias) | High risk | The allocation code was broken for the analysis. Pg 956. |
| Incomplete outcome data (attrition bias) | Low risk | 61 women (5%) withdrew or were lost to follow‐up, however data on miscarriage were reported for those who withdrew due to miscarriage. Intention‐to‐treat analyses performed. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes appear to be reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomisation and allocation concealment: "women enrolled at the antenatal clinic were divided into two main groups by placing them alternatively on separate lists". Blinding of outcome assessment: unclear, no information given on blinding of participants, carers or outcome assessors. Documentation of exclusion: 622 women (11%) were excluded. Use of placebo control: no placebo given. | |
| Participants | 5644 women were recruited into the study. All women attending the antenatal clinics and who were less than or equal to 24 weeks' gestation and who were in 'good health' were eligible for the study. Women who were more than 24 weeks' gestation and women who suffered from any disease or physical abnormality were excluded from the study. After enrolment, women who had twin births and who miscarried at an early stage were also excluded. | |
| Interventions | Women allocated to the treatment group were given daily vitamin C 100 mg, ferrous iron 0.26 g, calcium 0.26 g, minute quantities of iodine, manganese and copper, adsorbate of vitamin B1 containing all factors of the B complex and halibut liver oil 0.36 g containing vitamin A (52,000 IU per g) and vitamin D (2500 IU per g). | |
| Outcomes |
| |
| Notes | Women risk status for spontaneous and recurrent miscarriage is unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomistion using alternate separate lists. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information about blinding provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about blinding provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | 622 women (11%) excluded, intention‐to‐treat analyses not performed. |
| Selective reporting (reporting bias) | Unclear risk | Limited methodological details provided. |
| Other bias | Unclear risk | Limited methodological details provided. |
| Methods | Randomisation and allocation concealment: the randomisation (computer‐generated sequence) was blocked—i.e., balanced—by centre in groups of 2 to 10 individuals. Blinding of outcome assessment: none of the trial staff or any other person involved in the trial knew the allocated treatment of any woman until after completion of the study. Documentation of exclusion: 9 (0.4%) women were excluded. Use of placebo control: placebo control. | |
| Participants | 2404 women with clinical risk factors for pre‐eclampsia Inclusion criteria: gestational age 14+⁰–21+⁶ weeks; one or more of the following risk factors: pre‐eclampsia in the pregnancy preceding the index pregnancy, requiring delivery before 37 completed weeks’ gestation, diagnosis of HELLP syndrome in any previous pregnancy, eclampsia in any previous pregnancy; essential hypertension requiring medication, type 1 or type 2 diabetes, multiple pregnancy; abnormal uterine artery doppler waveform primiparity with BMI at first antenatal appointment of 30 kg/m² or more. Exclusion criteria: women unable or unwilling to give written informed consent or women who were being treated with warfarin. Women taking vitamin supplements that contained doses of vitamin C of 200 mg or more or of vitamin E of 40 IU or more daily were excluded. | |
| Interventions | Women were assigned to 1000 mg vitamin C and 400 IU vitamin E (RRR α tocopherol; n = 1199) or matched placebo (n = 1205) daily from the second trimester of pregnancy until delivery. | |
| Outcomes | Primary outcomes
Secondary outcomes:
| |
| Notes | Women risk status for spontaneous and recurrent miscarriage: history of chronic hypertension, BMI, pre‐eclampsia, multiple pregnancy, diabetes, and other risk factors reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence Pg 1146 |
| Allocation concealment (selection bias) | Unclear risk | Although paper states that supplementation and placebo looked and tasted the same, (Pg 1146) there is no clear description of how women were allocated to treatment group |
| Blinding of participants and personnel (performance bias) | Low risk | “none of the trial staff or any other person involved in the trial knew the allocated treatment of any woman until after completion of the study” Pg 1146 |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear from text if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Very low loss to follow‐up rates. In the supplementation group 3 (0.25%) losses to follow‐up and in the placebo group 6 (0.5%). |
| Selective reporting (reporting bias) | Unclear risk | A large number of outcomes reported in the publication, but not pre‐specified in the registered trial. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomisation and allocation concealment: community‐based, individually‐randomised, placebo‐controlled and double‐blinded study. Pregnat women were randomly allocated in a 1:1:1:1 ratio in blocks of 12 based on a list of treatment numbers derived from a pseudo‐random number generated with SAS software. Blinding of outcome assessment: all investigators, field and laboratory staff and participants were blinded to the treatment code until all field data had been collected and preliminary data analysis by coded groups had been completed. Documentation of exclusions: 75 women (3.5%) were excluded. Use of placebo control: placebo control. | |
| Participants | 2173 women at a gestational age of ,17 weeks were included in the study. Women at a gestational age of >=17 weeks were not eligible. | |
| Interventions | Women were allocated to one of the three intervention groups:
Comparison group: placebo. All capsules also contained 2mg dl‐alpha‐tocopherol as antioxidant and 350 mg of soyabean oil, 20 mg of beeswax and 8 mg of lecithin as capsule filler. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Women's nutritional status is unclear Intention‐to‐treat analyses not performed. Sample‐size calculation not performed Compliance: consumption of 70% of supplements. Location: Indonesia. Timeframe: September 1995 to December 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pregnant women were randomly allocated in a 1:1:1:1 ratio in blocks of 12 based on a list of treatment numbers derived from a computer‐generated pseudo‐random number |
| Allocation concealment (selection bias) | Unclear risk | Treatment allocations was prepared and held at the University of Newcastle ....Supplements were coded with treatment numbers and women were assigned a treatment number in sequence based on date they consented to participate in the study. However, supplements were packed in plastic strips in identical opaque capsules..page 16 ‐ 17. |
| Blinding of participants and personnel (performance bias) | Low risk | All investigators, field and laboratory staff and participants were blinded to the treatment code until all field data had been collected and preliminary data analysis by coded groups had been completed page 17 |
| Blinding of outcome assessment (detection bias) | Low risk | All investigators, field and laboratory staff and participants were blinded to the treatment code until all field data had been collected and preliminary data analysis by coded groups had been completed page 17 |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out rate appears balanced between groups and reasons for loss to follow‐up were provided. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable but outcomes predefined outcomes were reported |
| Other bias | Unclear risk | Overall, 70% of supplements were consumed, but it is unclear in text where there was more or less compliance. |
| Methods | Randomisation and allocation concealment: the randomisation scheme was generated by a computer program in permuted blocks of 4. Randomisation numbers were sealed in opaque envelopes. At each inclusion, the consulting physician opened the next sealed envelope and transmitted the randomisation number to a pharmacist managing the allocation sequence and the packaging of drugs at a central location. Blinding of outcome assessment: the consulting physicians, pharmacist and women were blinded to allocation. Documentation of exclusions: 107 women were lost to follow‐up (however their pregnancy outcome was reported). Post randomisation 26 twins were excluded (multivitamin group: 15; iron/folic acid group: 11 twins (including 1 set of triplets). Only singleton pregnancies were included in the analysis because fetal loss and anthropometric measures at birth in multiple pregnancies are not primarily nutrition‐related. 3 women died before delivery and 1 woman underwent a therapeutic abortion. Use of placebo control: no placebo. | |
| Participants | 1374 women were recruited to participate, however 52 women were randomly assigned twice for consecutive pregnancies, resulting in data for 1426 pregnancies. Women had a pregnancy confirmed by urine testing and were randomly assigned to receive either IFA (n = 712) or UNIMMAP (n = 714) daily until 3 months after delivery. Women were recruited between 5 to 36 weeks’ gestation; 34.6% (n = 493) of the participants were recruited in the first trimester of pregnancy, mean gestational age at enrolment was 17.3 weeks (SD 7.8 weeks). | |
| Interventions | UNIMMAP: vitamin A 800 mcg, vitamin D 200 IU, vitamin E 10 mg, vitamin B‐1 1.4 mg, vitamin B‐2 1.4 mg, niacin 18 mg, folic acid 400 mcg, vitamin B‐6 1.9 mg, vitamin B‐12 2.6 mcg, vitamin C 70 mg, zinc 15 mg, iron 30 mg, copper 2 mg, selenium 65 mcg, iodine 150 mcg. IFA (control): folic acid 400 mcg, Iron 60 mg. In a case of maternal illness, appropriate treatments were provided according to national guidelines. Severely anaemic women (Hb < 70 g/L, without dyspnoea) received ferrous sulphate (200 mg) + folic acid (0.25 mg) twice daily, for 3 months, regardless of their allocation group. All participants also received 400 mg albendazole in the second and third trimesters. If malaria occurred despite chemoprophylaxis, quinine (300 mg, 3 times/day) was given for 5 days. Vitamin A (200,000 IU) was given to all women after delivery, in accordance with national recommendations. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. 18% of women in each group had experienced a previous fetal loss. Women's nutritional status is unclear, although women are presumable at risk as the purpose of the trial is to correct MMN deficiencies. Intention‐to‐treat analyses not performed, however the review included details of losses to follow‐up where the outcome was known. Compliance: unclear, states that there was no difference in compliance between the 2 groups. Location: Burkino Faso. Timeframe: March 2004 to October 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated with permuted blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Randomisation numbers were kept in sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Consulting physicians, pharmacist and women were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Authors claim the study had double‐blind design, but it is unclear if the assessors were blinded. |
| Incomplete outcome data (attrition bias) | High risk | Data were reported for singletons only. |
| Selective reporting (reporting bias) | Unclear risk | As above ‐ data only reported for singletons. |
| Other bias | High risk | Some women were pregnant more than once during the study period, however the denominators reported are the total number of pregnancies during the study period, not the total number of women randomised, which incorrectly assumes that each data point included is independent from the next. |
| Methods | Randomisation and allocation concealment: women were randomly assigned to receive capsules containing a combination of 1000 mg of vitamin C (ascorbic acid) and 400 IU of vitamin E (RRR‐alpha‐tocopherol acetate) or matching placebo (mineral oil). The simple urn method, with stratification according to clinical centre, was used by the data co‐ordinating centre to create a randomisation sequence. No further methodological details are provided. Blinding of outcome assessment: medical charts were reviewed by at least 3 reviewers who were unaware of the treatment assignments. Documentation of exclusions: 183 women (1.8%) were lost to follow‐up and for 2 women had been removed after randomisation. Use of placebo control: placebo given. | |
| Participants | 10,154 pregnant women who had a singleton fetus with a gestational age of less than 16 weeks 0 days at the time of screening attending 16 clinical centres and the independent data coordinating centre of the MFMU Network. Women were eligible for inclusion if they had not had a previous pregnancy that lasted beyond 19 weeks 6 days. Women were not eligible if they had elevated systolic or diastolic blood pressure, proteinuria, were taking or had taken antihypertensive medication, or were taking more than 150 mg of vitamin C or more than 75 IU of vitamin E daily. Other exclusion criteria were diabetes that was present before the pregnancy, treatment with antiplatelet drugs or non‐steroidal anti‐inflammatory agents, uterine bleeding within the week before recruitment, uterine malformation, serious medical condition, known fetal anomaly or aneuploidy, in vitro fertilisation resulting in the current pregnancy, or abuse of illicit drugs or alcohol. | |
| Interventions | A combination of 1000 mg of vitamin C (ascorbic acid) and 400 IU of vitamin E daily administered from enrolment until delivery. The control group received placebo. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Use of prenatal vitamins or multivitamins, daily dose of vitamin C and E were reported at trial entry. Sample size calculation was based on a 30% reduction in the rate of the primary outcome. Intention‐to‐treat analysis was performed. Compliance: monthly, participants returned unused study drugs from the previous month. Location: UK. Timeframe: July 2003 to February 2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The simple urn method, with stratification according to clinical center, was used by the data coordinating center to create a randomization sequence." pg 1283, last pgh. |
| Allocation concealment (selection bias) | Unclear risk | Text says participants "were randomly assigned to receive capsules containing...". No details on how participants were allocated to groups. pg 1283 last pgh. |
| Blinding of participants and personnel (performance bias) | Low risk | "Neither the participants nor the investigators were aware of the treatment assignments." pg 1283, last pgh. |
| Blinding of outcome assessment (detection bias) | Low risk | "Deidentified medical charts of all women with pregnancy‐associated hypertension were reviewed centrally by at least three reviewers who were unaware of the treatment assignments." pg 1284. |
| Incomplete outcome data (attrition bias) | Low risk | Vitamin C and E = 95/5088 missing and in placebo = 90/5066 missing. Reasons for missing similar between groups. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported as pre‐specified in the protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomisation and allocation concealment: computer‐generated random number list with balanced variable blocks and stratification for collaborating centre and gestational age (< 18 weeks versus 18 weeks or more), allocation occurred via a central telephone randomisation service. The treatment packs contained 4 sealed, opaque, white plastic bottles of either the antioxidants vitamin C and vitamin E or the placebo and were prepared by a researcher not involved in recruitment or clinical care. Blinding of outcome assessment: women, caregivers and investigators were blinded to allocation. Documentation of exclusion: no losses to follow‐up. Use of placebo control: placebo given. | |
| Participants | 1877 women were recruited into the study. Eligible women included those: with a nulliparous singleton pregnancy, between 14 and 22 weeks of gestation and with normal blood pressure at the first measurement in pregnancy and again at trial entry. Women who had any of the following were excluded: known multiple pregnancy, known potentially lethal fetal anomaly, known thrombophilia, chronic renal failure, antihypertensive therapy, or specific contraindications to vitamin C or E therapy such as haemochromatosis or anticoagulant therapy. Women were allocated to the vitamin C and E group (n = 935) or placebo (n = 935). | |
| Interventions | Women allocated to the vitamin C and E group took 4 coated tablets of a combination of 250 mg of vitamin C (as ascorbic acid) and 100 IU of vitamin E (as d‐alpha‐tocopherol succinate) each day from trial entry until delivery (total daily dose of vitamin C: 1000 mg; vitamin E: 400 IU). Women were advised not to take any other antioxidant supplements, although a multivitamin preparation that provided a daily intake of no more than 200 mg of vitamin C or 50 IU of vitamin E was permitted. | |
| Outcomes |
| |
| Notes | Women were at low risk of spontaneous and recurrent miscarriage based on the review criteria. The majority of women participating had a baseline dietary intake of vitamin C and E above the Australian recommended daily amount. Intention‐to‐treat analyses performed. Compliance: there was no difference in compliance between the vitamin group (67%) and the placebo group (70%). Location: Australia. Timeframe: December 2001 and January 2005. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Allocation occurred via a central telephone randomisation service. Tablets were provided in sealed opaque bottles. |
| Blinding of participants and personnel (performance bias) | Low risk | Women, caregivers and investigators were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Generation of random number sequence: a computer‐generated random number sequence. Randomisation and allocation concealment: central allocation (randomisation by an independent third party who had no conflict of interest in the study). Blinding of outcome assessment: treatment allocations were blinded to both the investigator and the patient until the study was finished. Documentation of exclusion: none reported. Use of placebo control: no, comparisons were between antioxidants versus iron and folic acid. | |
| Participants | 60 women between 8 and 12 weeks' gestation were eligible for randomisation (supplementation group: n = 29; folic acid group: n = 31). Setting: at the antenatal clinic of the Department of Obstetrics and Gynecology, University of Indonesia between March 2003 and June 2004. Eligibility criteria: pregnant women with low antioxidant status. Exclusion criteria:
| |
| Interventions | Supplementation group: received antioxidant supplements daily ‐ vitamins A (1000 IU), B6 (2.2 mg), B12 (2.2 mcg), C (200 mg), E (400 IU), folic acid (400 mcg), N‐acetylcysteine (200 mg), Cu (2 mg), Zn (15 mg), Mn (0.5 mg), Fe (30 mg), calcium (800 mg), and selenium (100 mcg). Folic acid group: received Fe 30 mg and folic acid 400 mcg daily. Timing of the intervention: early pregnancy (8 to 12 weeks). | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Participating women had low antioxidant status at enrolment, as defined as superoxide dismutase level below 164U/mL. No nutritional information provided. Intention‐to‐treat analyses performed. Compliance: unclear, no information reported. Location: Indonesia. Timeframe: March 2003 and June 2004. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Central allocation (randomisation by an independent third party who had no conflict of interest in the study). |
| Blinding of participants and personnel (performance bias) | Low risk | Treatment allocations were blinded to both the investigator and the patient until the study was finished. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Authors claim the study had a double‐blind design, but it is unclear if the assessors were blinded. Treatment allocations were blinded to both the investigator and the patient until the study was finished. |
| Incomplete outcome data (attrition bias) | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported, no apparent evidence of selective reporting. |
| Other bias | Unclear risk | At baseline, the control group appears to have a 2 mmHg higher systolic blood pressure than the intervention group, this figure was of borderline statistical significance, P = 0.059. |
| Methods | Randomisation and allocation concealment: unclear, women were allocated to groups based on "random assignment". Randomisation was stratified on pre‐pregnancy weight, weight gain during pregnancy, previous low birthweight infant and protein intake. No other methodological details given. Blinding of outcome assessment: unclear, women were allocated to 2 forms of treatment or control, where both treatments were given as a canned beverage and the control group were given standard oral multivitamins. No information is given on blinding of outcome assessors. Documentation of exclusion: 237 women (22%) were excluded. Use of placebo control: no placebo, the control group received standard prenatal multivitamin supplements. | |
| Participants | 1051 women were recruited into the study. Women eligible were black, English speaking, and not greater than 30 weeks' gestation. They also had 1 of the following criteria: low pre‐pregnant weight (under 110 pounds at conception); low weight gain at the time of recruitment; at least 1 previous low birthweight infant; a history of protein intake of less than 50 g in the 24 hours preceding recruitment. Women were not eligible if they were known to be seeking a termination, had specific chronic health disorders, if they admitted to recent use of narcotics or heavy use of alcohol, or weighed >= 140 pounds at conception. | |
| Interventions | Women were randomised to 1 of 3 groups:
Women received the high protein or balanced protein‐energy supplements in the format of a drink. Women in the control group received a standard oral prenatal multivitamin supplement. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage is unclear, as there is no information about concurrent medical conditions or other risk factors for miscarriage. Women in the trial had a low caloric intake at trial entry, and unexpectedly, an adequate protein intake. No other specific nutritional information is reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No methodological details given beyond reporting of 'random assignment'. |
| Allocation concealment (selection bias) | Unclear risk | No methodological details given beyond reporting of 'random assignment'. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided about blinding of participants and personnel. Unlikely as participants were given canned beverages or multivitamins. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | 237 women (22%) excluded, no intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all pre‐specified outcomes reported. |
| Other bias | Unclear risk | Limited methodological details provided. |
| Methods | Randomisation and allocation concealment: unclear, women were "randomly assigned on an individual basis, to double‐blind, weekly supplementation until delivery". Blinding of outcome assessment: unclear, double‐blind stated in text but no details given. Documentation of exclusion: 42 women (17%) were lost to follow‐up and excluded. Use of placebo control: control tablets containing iron and folic acid were given. | |
| Participants | 243 women were recruited into this study. Pregnant women between 16 and 20 weeks' gestation, aged between 17 and 35 years old, with a parity < 6 and Hb level between 80‐140 g/L, were eligible for this study. Women were randomised to receive either vitamin A plus iron and folic acid (n = 122) or iron and folic acid only (n = 121). Of these 22 (18%) and 20 (17%) women in vitamin A plus iron and folic acid and the iron and folic acid groups respectively, dropped out between enrolment and the follow‐up at 4 months. | |
| Interventions | Women were randomised to a weekly supplementation with 120 mg ferrous sulfate and 500 mcg folic acid, with or without vitamin A (2400 retinol equivalents). Women were asked to take the trial tablets from between 16 and 20 weeks' gestation until birth. | |
| Outcomes | 1. Stillbirth. | |
| Notes | Women risk status for spontaneous and recurrent miscarriage is unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Women were 'randomly assigned on an individual basis' but no other details given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind used in the text but no details provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Authors claim the study had double‐blind design, but it is unclear who were blinded. No further information was available. |
| Incomplete outcome data (attrition bias) | Unclear risk | 42 women (17%) were lost to follow‐up and excluded, no intention‐to‐treat analyses performed. |
| Selective reporting (reporting bias) | Unclear risk | Serial publications of this study report different denominators. |
| Other bias | Unclear risk | Limited methodological details provided. |
| Methods | Generation of random number sequence: the randomisation sequence was constructed by the data co‐ordinating centre (DCC) as permuted blocks of random size, stratified by clinical centre, and implemented via a program residing on the clinical centres study computer. Randomisation and allocation concealment: central allocation. Blinding of outcome assessment: all clinicians and clinical investigators were blinded to group assignment. Documentation of exclusion: none reported. Use of placebo control: placebo control. | |
| Participants | 739 eligible women between 120/7 and 196/7 weeks of gestation were enrolled in the study (treatment: 371; placebo: 368). Setting: 4 Brazilian clinical centres: 1 primary clinical centre (Recife) and 3 additional clinical sites (Campinas, Botucatu, and Porto Alegre); each site’s major teaching hospital serves a primarily urban low‐income population. Eligibility criteria: women between 120/7 and 196/7 weeks of gestation and diagnosed with nonproteinuric chronic hypertension or a prior history of pre‐eclampsia in their most recent pregnancy that progressed beyond 20 weeks' gestation. Exclusion criteria:multifetal gestation, allergy to vitamin C or vitamin E, requirement for aspirin or anticoagulant medication, 24‐hour urinary protein ≥ 300 mg, pre‐pregnancy diabetes mellitus, known fetal anomaly incompatible with life. Loss to follow‐up: 32 women (treatment 16; placebo 16). | |
| Interventions | Intervention group: daily treatment with both vitamin C (1000 mg) and E (400 IU) until delivery or until the diagnosis of pre‐eclampsia. Control group: daily placebo until delivery or until the diagnosis of pre‐eclampsia. Timing of the intervention: between 120/7 and 196/7 weeks of gestation. | |
| Outcomes |
| |
| Notes | 25 inclusion/exclusion criteria violations (23 enrolled outside 12‐19 weeks’ gestation; 2 twin gestations ‐ 1 lost to spontaneous abortions, 1 delivered liveborn in treatment group); all 25 women remained in their assigned study groups. 26 women had early treatment terminations (treatment 19; placebo 7), but remained in follow‐up. Women's risk of spontaneous and recurrent miscarriage was unclear. Women's nutritional status is also unclear. Intention‐to‐treat analyses performed. Compliance: average compliance was 85%, and similar between treatment groups. Location: Brazil. Timeframe: July 2, 2003 and November 23, 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence number. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | All clinicians and clinical investigators were blinded to group assignment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Authors claim the study had double‐blind design, but it is unclear who was blinded. No further information was available. |
| Incomplete outcome data (attrition bias) | Low risk | Small numbers of missing data, balanced across groups (32 women; treatment 16; placebo 16). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported, no apparent evidence of selective reporting. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomisation and allocation concealment: "randomisation was undertaken by computer‐generated numbers". Roche Pharmaceutical supplied numbered containers with either vitamin C or matching placebo, and they retained the study code until completion of the study. No other methodological details given. Blinding of outcome assessment: "double blind" stated, Roche Pharmaceuticals retained the code until completion of the study. Documentation of exclusion: none reported. Use of placebo control: placebo control. | |
| Participants | 200 women were recruited into the study. Women with a history of a previous mid‐trimester abortion (defined as spontaneous expulsion of the uterine contents between 13 and 26 weeks' gestational age), or previous preterm labour, and less than 26 weeks' gestation were eligible and invited to participate. Women with iatrogenic causes of their previous preterm labour such as previous induction of labour before term for severe pre‐eclampsia, were excluded. 203 consecutive women were approached, of which 200 (98.5%) consented and were randomised to either vitamin C (n = 100) or placebo (n = 100). No losses to follow‐up were reported. | |
| Interventions | Twice daily tablet of either 250 mg vitamin C or placebo, from trial entry until 34 weeks' gestation. All women were tested for bacterial vaginosis and all women with positive cultures for Mycoplasma hominis (and between 22 and 32 weeks' gestation) were treated with erythromycin for 7 days. | |
| Outcomes |
The age of fetal viability was considered to be 28 weeks' gestation. | |
| Notes | Results are from an interim analysis performed when 100 participants were recruited into each arm. Recruitment was stopped after the interim analysis revealed few differences between the 2 groups. Unclear if there was a sample‐size calculation performed. Women's risk profile spontaneous and recurrent miscarriage is unclear, although they are clearly at high risk of preterm birth. It is also unclear if multiple births were included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence number. |
| Allocation concealment (selection bias) | Low risk | Roche pharmaceuticals supplied numbered study containers and kept the study code until completion of the study. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Authors claim the study had double‐blind design, but it is unclear who was blinded. Allocation was double‐blind and Roche Pharmaceuticals retained the code until completion of the study. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Authors claim the study had double‐blind design, but it is unclear who was blinded. allocation was double‐blind and Roche Pharmaceuticals retained the code until completion of the study. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Recruitment stopped after an interim analysis. |
| Other bias | High risk | Results are from an interim analysis performed when 100 participants were recruited into each arm. |
| Methods | Generation of random number sequence: computer‐generated number; 262 clustered unit of randomisation (all pregnant women served by the same midwife received supplements with the same midwife identification number). Randomisation and allocation concealment: central allocation. Blinding of outcome assessment: all study scientists and personnel, government staff, and enrollees were unaware of the allocation. Documentation of exclusion: 1748 loss to follow‐up before delivery (IFA: 853; MMN: 895); 1128 loss to follow‐up after delivery (IFA: 553; MMN: 575). 10,549 pregnant women excluded post‐randomisation because of trial termination (IFA group: 5057; MMN group: 5492). Use of placebo control: no placebo, comparisons were between multiple micronutrients and iron and folic acid. | |
| Participants | 41,839 pregnant women of any gestational age living on Lombok, Nusa Tenggara Barat Province, Indonesia. Women were allocated to IFA (n = 20,543) or MMN (n = 21,296). | |
| Interventions | MMN group: the MMN was the UNIMMAP formulation containing 30 mg iron (ferrous fumarate) and 400 mcg folic acid along with 800 mcg retinol (retinyl acetate), 200 IU vitamin D (ergocalciferol), 10 mg vitamin E (alpha tocopherol acetate), 70 mg ascorbic acid, 1.4 mg vitamin B1 (thiamine mononitrate), 18 mg niacin (niacinanide), 1.9 mg vitamin B6 (pyridoxine), 2.6 mcg vitamin B12 (cyanocobalamin), 15 mg zinc (zinc gluconate), 2 mg copper, 65 mcg selenium, and 150 mcg iodine ‐ 1 capsule daily up to 3 months after birth. IFA group: the IFA contained 30 mg iron (ferrous fumarate) and 400 mcg folic acid ‐ 1 capsule daily up to 3 months after birth. Timing of the intervention: any time during pregnancy. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Women's nutritional status is also unclear. However, 30% of women in each group had an mid upper arm circumference < 23.5cm, which was used as an indicator of women being undernourished. Intention‐to‐treat analyses performed. Compliance: median compliance was 85%, there was no difference between treatment groups in compliance. Location: Indonesia. Timeframe: July 1, 2001 to April 1, 2004. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | All study scientists and personnel, government staff, and enrollees were unaware of the allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | The code to indicate which strip was IFA or MMN was known only by the manufacturing production manager and a quality control officer from UNICEF. All study scientists and personnel. government staff, were unaware of the allocation. Blinding is unlikely to be broken. |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up: 1748 loss to follow‐up before delivery (IFA: 853; MMN: 895); 1128 loss to follow‐up after delivery (IFA: 553; MMN: 575). Post‐randomisation exclusion: 10,549 pregnant women excluded because of trial termination (IFA group: 5057; MMN group: 5492). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported, no apparent evidence of selective reporting. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomisation and allocation concealment: 160 clusters were randomly assigned to 4 blocks of 40 clusters each. 2 of the 4 blocks received either MMN of IFA. No details of how blocks were selected. 432 women in 40 clusters were allocated to the group receiving multiple micronutrients and 411 in the other 40 clusters were allocated to the iron–folic acid group. No further details on how allocation was done. Blinding of outcome assessment: single‐blind design, no further details provided. Documentation of exclusion: 93 women (11%) were excluded. Use of placebo control: no placebo, the control group received iron‐folic acid supplementation. | |
| Participants | 843 women residing in Indramayu District in the West Java Province of Indonesia who were between 12 to 20 weeks of gestation. Only women intending to remain in the study location until giving birth were recruited. Women suffering from potentially confounding illnesses, including diabetes, coronary heart disease, and tuberculosis, were excluded from the study. | |
| Interventions | MMN supplements containing 15 micronutrients vs IFA (60 mg of elemental iron as ferrous sulfate and 0.25 mg of folic acid). Supplementation was from time of enrolment at 12 to 20 weeks of gestation and continued to 30 days postpartum. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage is unclear. Women's nutritional status is also unclear. Sample size calculation not done. Intention‐to‐treat analysis performed. Compliance: Data on compliance was collected from weekly visits. Mean adherence in the intervention group 68% and control group 71%. Location: Indonesia. Timeframe: May 2001 to December 2002. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 160 clusters were randomly assigned to 4 blocks of 40 clusters each. 2 of the 4 blocks received either MMN of IFA. No details of how blocks were selected. pg s489 pgh 5. |
| Allocation concealment (selection bias) | Unclear risk | 432 women in 40 clusters were allocated to the group receiving multiple micronutrients and 411 in the other 40 clusters were allocated to the iron–folic acid group. No further details on how allocation was done. pg s489, pgh 7. |
| Blinding of participants and personnel (performance bias) | High risk | Authors claim the study had a single‐blind design, but it is unclear who was blinded. Probably not done. pg s489 pgh 8. |
| Blinding of outcome assessment (detection bias) | High risk | No details of blinded outcome assessment provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | 23/432 missing in MMN and 33/411 missing in IFA. Reasons for drop‐out are similar, but IFA had 5 twin births while MMN cluster had none. Fig. 1. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported as described. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Generation of random number sequence: a computer‐generated register was used for randomisation. Randomisation was performed in blocks of 12 food supplementation was randomly allocated but not blinded. micronutrient capsules looked identical and women were allocated to 1 of 3 types of micronutrient supplements in a 2 by 3 design. Blinding of outcome assessment: allocation code was not broken until after the analysis. Documentation of exclusion: 845 losses to follow‐up (19%). Use of placebo control: no placebo, the control group received IFA (Fe30Fol) supplementation. | |
| Participants | 4436 pregnant women from rural Bangladesh within 6‐8 weeks of conception (not more than 14 weeks). Women with Hb < 80 g/L were ineligible for participation. | |
| Interventions | MMNs containing 15 recommended micronutrients (30 mg of iron, 400 μg of folic acid). The micronutrient supplements were offered to the enrolled pregnant women at the 14weeks’ clinic visit up to 3 months after delivery. Intervention was divided into early or late food supplementation. Early or usual food supplementation plus 1, 2 or 3: 1) 30 mg iron(fumarate) + 400 mcg folic acid (Fe30Fol) 2) 60 mg iron (fumarate) + 400 mcg folic acid (Fe60Fol) 3) 30 mg iron (fumarate), 400 mcg folic acid, 800 mcg RE vitamin A (retinyl acetate), 5 mcg vitamin D (cholecalciferol), 10 mg vitamin E (a‐tocopherol acetate), 70 mg vitamin C, 1.4 mg thiamine (mononitrate), 1.4 mg riboflavin, 18 mg niacin, 1.9 mg vitamin B‐6 (pyridoxine hydrochloride), 2.6 mcg vitamin B‐12 (cyanocobalamin), 15 mg zinc (sulfate), 2 mg copper (sulfate), 65 mcg selenium (sodium selenite), and 150 mcg iodine (potassium iodide) (MMNs). All women received food supplement which consisted of roasted rice powder, roasted pulse powder, molasses, and soybean oil and had a total energy content of 2500 kJ. Early (immediately after detection of pregnancy, around 9 weeks) enrolment in food supplementation or usual (at the time of their choosing, around 20 weeks). | |
| Outcomes | Infant outcomes:
Maternal outcome:
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Women's nutritional status is also unclear. Sample size calculation: not done. Intention‐to‐treat analysis performed. Compliance: the number of micronutrient pills taken in the first 30 weeks of pregnancy was 79+/‐34 in the Fe30 group, 78+/‐34 in the Fe60 group, and 75+/‐33 in the MM group. Location: Bangladesh. Timeframe: November 2001 to June 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised tracking system assigned women to 1 of the 6 groups in blocks of 12 women were randomly allocated. "Computer generated register of study identity numbers with random assignment of food groups (“E” or “U”) and micronutrient groups (from 12 possible pill bottle number codes) was used for randomization.” Randomisation was probably done. Eneroth 2011 pg 221. |
| Allocation concealment (selection bias) | Low risk | Micronutrient capsules looked identical. |
| Blinding of participants and personnel (performance bias) | Low risk | Each micronutrient group had been given 4 different number codes to decrease the risk of unblinding. Pg 2054. |
| Blinding of outcome assessment (detection bias) | Low risk | Randomisation codes were safely kept at the administrative office of ICDDR,B and were not broken until after performing the intention‐to‐treat analyses. Pg 2054. |
| Incomplete outcome data (attrition bias) | Low risk | 845 of 4436 losses to follow‐up (19%) before birth; reasons were available. Numbers of women lost to follow‐up did not differ among the treatment groups. |
| Selective reporting (reporting bias) | Low risk | All the study's pre‐specified outcomes have been reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Generation of random number sequence: using a random‐generation procedure. Randomisation and allocation concealment: the supplements in vitamin A and placebo treatments allocated were prepared in identical capsules and were packaged in bottles according to the randomisation schedule (sealed envelopes) by midwives who were not involved in the trial conduct. Blinding of outcome assessment: neither the women nor the midwives involved in treatment allocation revealed the randomisation schedule to anyone involved in the conduct of the trial. Documentation of exclusion: 77 loss to follow‐up before assessment at 26‐28 weeks (5000 IU vitamin A: 26; 10,000 IU vitamin A: 26; placebo: 25). Additional 93 loss to follow‐up before assessment at 36‐38 weeks (5000 IU vitamin A: 34; 10,000 IU vitamin A: 28; placebo: 31). Use of placebo control: placebo control. | |
| Participants | 700 women with singleton pregnancies at 12‐24 weeks' gestation measured by ultrasound scan (5000 IU vitamin A: 234; 10,000 IU vitamin A: 234; placebo: 232). Setting: the antenatal clinic at the Namitambo rural Health Centre in southern Malawi, central Africa. Eligibility criteria: (Hb) < 11.0 g/dL by HemoCue screening method at first antenatal visit, singleton pregnancy with gestational age > 12 weeks and ≤ 24 weeks measured by ultrasound scan, no fetal abnormality detectable by ultrasound at time of booking, residing in the catchment area of the health centre. Exclusion criteria: women > 24 weeks' gestation, or twin pregnancy. | |
| Interventions |
Timing of the intervention: supplementation started as early as possible after 12 weeks of pregnancy. All women received iron tablets daily (60 mg elemental iron as ferrous sulphate with 0.25 mg folic acid). | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Women's nutritional status is also unclear. Intention‐to‐treat analyses performed. Compliance: unclear, no information provided. Location: Malawi. Timeframe: April 1997 and July 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐generation procedure used. |
| Allocation concealment (selection bias) | Low risk | The vitamin A and placebo treatments allocated were prepared in identical capsules and packaged in bottles according to the randomisation schedule (sealed envelopes) by midwives who were not involved in the trial conduct. |
| Blinding of participants and personnel (performance bias) | Low risk | Neither the women nor the midwives involved in treatment allocation revealed the randomisation schedule to anyone involved in the conduct of the trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | High risk | 77 loss to follow‐up before assessment at 26‐28 weeks (5000 IU vitamin A: 26; 10,000 IU vitamin A: 26; placebo: 25). Additional 93 loss to follow‐up before assessment at 36‐38 weeks (5000 IU vitamin A: 34; 10,000 IU vitamin A: 28; placebo: 31). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported, no apparent evidence of selective reporting. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Generation of random number sequence: no sequence generation details available. Randomisation and allocation concealment: central allocation (randomisation was performed by the statisticians of the British VIP Trial). Blinding of outcome assessment: "double blind" stated. Documentation of exclusion: 10 women (treatment 6; placebo 4), and 29 infants (treatment 13, placebo 16) were lost to follow‐up. Use of placebo control: placebo control. | |
| Participants | 1365 women between 14‐22 gestational age agreed to participate and were randomised (vitamins group: 687; placebo group: 678). Setting: antenatal clinics located in Nagpur, India; Lima and Trujillo, Peru; Cape Town, South Africa; and Ho Chi Minh City, Viet Nam which served populations with low social‐economic status and had evidence of overall low nutritional status, between October 2004 and December 2006. Eligibility criteria: pregnant women considered high risk for pre‐eclampsia (chronic hypertension, renal disease, pre‐eclampsia‐eclampsia in the pregnancy preceding the index pregnancy requiring delivery before 37 weeks’ gestation, HELLP syndrome in any previous pregnancy, pre‐gestational diabetes, primiparous with a BMI > 30 kg/m2, history of medically‐indicated preterm delivery, abnormal uterine artery Doppler waveforms and women with antiphospholipid syndrome), multifetal gestation. Women ingesting medications with aspirin‐like compounds were not excluded. Exclusion criteria: women ingesting vitamin supplements that contained ≥ 200 mg of vitamin C and/or ≥ 50 IU of vitamin E and women receiving warfarin. | |
| Interventions | Intervention group: received 1000 mg vitamin C and 400 IU of vitamin E daily until delivery. Comparison group: received placebo daily until delivery. Timing of the intervention: between 14 and 22 weeks' gestation. | |
| Outcomes |
Pre‐eclampsia information was unavailable for 14 women in the vitamins and 9 in the placebo group. There were data from 81 supplemented (11.8%) and 100 placebo‐treated (14.7%) women with multiple pregnancies, for whom newborn outcomes were considered separately. | |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Women at high risk of pre‐eclampsia were included but data on fetal loss was not reported separately for this group. No specific information on women's nutritional status is included; however, the paper states that the trial was conducted in populations with 'documented low nutritional status'. Intention‐to‐treat analyses performed. Compliance: median compliance was 87%, and was similar between the treatment groups. Location: Antenatal clinics in India, Peru, South Africa and Vietnam. Timeframe: October 2004 and December 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence blocked by centre in groups of 2 to 10 individuals. |
| Allocation concealment (selection bias) | Low risk | Central allocation (randomisation was performed by the statisticians of the British VIP Trial). |
| Blinding of participants and personnel (performance bias) | Low risk | Women and investigators blinded to allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Identifying characteristics and randomisation code were kept in a secure and separate database in the server unavailable to the research team, until all data analyses were completed. |
| Incomplete outcome data (attrition bias) | Low risk | Small numbers of missing data, balanced across groups. Women: 10 (treatment 6; placebo 4). Infants: 29 (treatment 13; placebo 16). |
| Selective reporting (reporting bias) | Unclear risk | Perinatal death was reported instead of pre‐specified neonatal death. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Generation of random number sequence: sectors were used as the unit of randomisations for supplement allocation, 3 sets of 3 identical coins on which the numbers 1, 2 or 3 were written were placed into a container, mixed and removed randomly, without replacement, and the 3‐digit code of each sector was read aloud sequentially. Study supplements were identical in colour, taste and external appearance. Treatment codes were assigned by the Nutrilite Health Institute and were kept in a sealed envelope in a locked cabinet at both Nutrilite and Johns Hopkins University. Masking was further ensured by having a senior administrative staff (not involved in field activities) recode the supplement bottles with sector‐specific permanent stickers bearing codes from 001 to 596. Blinding of outcome assessment: investigators were all blinded to the allocation codes until the end of the parent trial. Documentation of exclusion: 628 women (1%) were excluded. Use of placebo control: placebo control. | |
| Participants | 102,769 women from 596 sectors (60,294 pregnancy identified and 59,666 pregnancies included) 19 rural unions in the northwest Districts of Gaibandha and Rangpur in rural Bangladesh. Women were recruited from the first trimester of pregnancy through 12 weeks (84th day) after pregnancy termination. Eligible women included pregnant or postpartum, lactationally amenorrhoeic women were placed on a 'waiting list', and only became eligible for pregnancy surveillance once their menses resumed. Married women, women entering the study area within 4 months of marriage were also eligible to join the cohort under pregnancy surveillance. And when identified as pregnant, enrolled. Women who never got pregnant during the study period, permanently moved from the study area, were sterilised, reported menopause, divorce or death of husband, refused to participate or participation status unknown, died before detecting a pregnancy , had a pregnancy outcome after October 12, 2006, reported a last menstrual period after January 5, 2006 or had an unknown date of a last menstrual cycle were excluded. | |
| Interventions | 1. Vitamin A (consisting of 7000 mcg retinol equivalents, or 23,300 IU, of VA palmitate in soybean oil with a small amount of vitamin E as an antioxidant). 2. β‐carotene at 42 mg—an amount equivalent to 7000 mcg REs administered weekly from the time of pregnancy enrolment until 3 months postpartum. 3. Control group received placebo (consisting of soybean oil with a small amount of vitamin E as an antioxidant). All 3 supplements contained 5 IU vitamin E in oil. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage was unclear. Women's nutritional status: a 1‐week history of diet at trial entry and 12 weeks after pregnancy was reported. Sample size estimation: based on a 35% or greater reduction in all‐cause mortality. Primary outcomes were compared on an intent‐to‐treat basis. Compliance: adherence to supplementation was comparable across groups, with approximately 80% of women having directly consumed (under staff supervision) at least 64% of eligible supplements. Location: Bangladesh. Timeframe: August 2001 to January 2007. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sectors were randomised in blocks of 9. 3 sets of 3 identical coins on which the numbers 1, 2 or 3 were written were placed into a container, mixed and removed randomly, without replacement, and the 3‐digit code of each sector was read aloud sequentially. Pg 7 (Labrique). |
| Allocation concealment (selection bias) | Low risk | Study supplements were identical in colour, taste and external appearance. Supplements were originally shipped in identically‐labelled, 100‐count white opaque plastic bottles distinguished only by the code number‐1, 2 or 3‐listed on the label. A senior administrative staff (not involved in field activities) recode the supplement bottles with sector‐specific permanent stickers bearing codes from 001 to 596. Treatment codes were assigned by the Nutrilite Health Institute and were kept in a sealed envelope in a locked cabinet at both Nutrilite and Johns Hopkins University. Pg 7‐8 (Labrique). |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants, interviewers, field supervisors and investigators remained masked to treatment assignments until the end of the trial. Pg 8 (Labrique). |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators remained masked to treatment assignments until the end of the trial. Pg 8 (Labrique). |
| Incomplete outcome data (attrition bias) | Low risk | Number of missing data among each group are small and balanced with detailed reason. |
| Selective reporting (reporting bias) | Low risk | Registration of trial and outcomes reported as described previously. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Generation of random number sequence: 596 sectors of comparable size were used as units of randomisations. "We stratified the contiguous list of 596 sectors into 74 blocks of 8 each (for the first 592 sectors), plus a 75th block of 4 sectors. A sector‐supplement code key with A or B was created by flip of a coin, reflecting assignment to iron–folic acid or MM supplementation, and duplicated, and each of 2 copies was sealed into an envelope by an uninvolved colleague at Johns Hopkins. Computer program randomised sectors within blocks to 1 of 2 codes such that each permutation had an equal probability of being chosen. The resulting 2 lists of sectors were securely transmitted to field headquarters. 1 envelope with the code key was securely transmitted to the supplement producer and the other sealed in an envelope and secured at Johns Hopkins. At no time during the trial did study investigators or field or data management staff have access to the key." Blinding of outcome assessment: outcome assessors were blinded to the treatment allocation. Documentation of exclusion: 1351 pregnant women (3%) were excluded. Use of placebo control: no placebo, the control group received IFA supplementation. | |
| Participants | Participants in the study included 44,567 pregnant women and 28,518 infants in rural setting in Bangladesh. Pregnant women were recruited around ˜ 10 weeks' gestation. All women under 45 years of age who are married and living with their husbands as residents of the JiVitA study area at the time of an initial population enumeration, and those who enter the cohort as newlyweds, were eligible for pregnancy surveillance and, when identified as pregnant, enrolled. Women who permanently moved, were sterilised or menopausal, died, and did not get pregnant were excluded. | |
| Interventions | Weekly supplementation from enrolment (early pregnancy) to 3 months postpartum. Multiple‐micronutrient: vitamin A (770 mcg retinol equivalents), vitamin D (5 mcg), vitamin E (15 mg), thiamin (1.4 mg), riboflavin (1.4 mg), niacin (1.4 mg), vitamin B12 (2.5 mg), vitamin B6 (1.9 mg), vitamin C (85 mg), zinc (12 mg), iodine (220 mcg), copper (1000 mcg), and selenium (60 mcg). The control group received IFA supplement (standard care). | |
| Outcomes | 1. Infant survival: determine the efficacy of a standard MMN supplement given to women daily during pregnancy through 12 weeks postpartum in lowering neonatal and infant mortality through 6 months of age by > 15% compared to the mortality of infants whose mothers receive daily iron + folic acid. 2. Fetal and newborn outcomes: assess the efficacy of the MM intervention in reducing the: a) stillbirth rate by 23% or more, from an expected 30 to 24 or fewer stillbirths per 1000 births (i.e. live + still births); b) rate of preterm birth (live birth delivered < 37 weeks’ gestation) by 10% or more, from an expected 20% to 18% or fewer of all live births; c) prevalence of low birthweight (< 2500 g) by 5% or more, from an expected 40% to 38% or fewer of all live births; and d) neonatal morbidity related to sepsis, birth asphyxia, hypothermia and diarrhoea. 3. Other infant outcomes (to 6 months of age): assess the efficacy of the MM supplement intervention in improving: a) linear and ponderal growth, including its ability to protect lean body mass, that could lead to reduced prevalences of stunting, wasting and underweight status in infancy; b) infant morbidity, including diarrhoea and acute lower respiratory infection, in the first 3 months of life; c) micronutrient intake, represented by measured breast milk micronutrient concentrations; d) micronutrient status, and reducing prevalences of multiple deficiencies. 4. Maternal outcomes: assess the efficacy of either MM supplement intervention in influencing among mothers the: a) prevalence of infectious morbidity, based on history, testing and signs, during pregnancy and in the 1st 6 months postpartum; b) rates of potentially fatal (“near miss”) obstetric complications. | |
| Notes | Women's risk of spontaneous and recurrent miscarriage: previous fetal loss and infant death reported. Women's nutritional status is unclear. Sample size estimation: live‐born infants based on a 15% or greater reduction in 6‐month mortality; pregnancies based on a 30% loss from induced abortion, miscarriage, and stillbirth. Intent‐to‐treat analysis of all outcomes. Compliance: adherence was high, with half the women in both groups estimated to consume a median of approximately 95% of all distributed supplements; 80% in both groups consumed more than 80% of their intended tablets. Location: Bangladesh. Timeframe: 2008 to 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used an in‐house program (VBScript, Microsoft) that recognized 70 possible permutations for n = 8 sectors and k = 2 supplement allocations and 6 for the last block of n = 4sectors. Using this program, we randomised sectors within blocks to 1 of 2 codes such that each permutation had an equal probability of being chosen". Pg 2650. |
| Allocation concealment (selection bias) | Low risk | "Supplements were identical in appearance. Tablets were packed into opaque plastic bottles, affixed with codes AorB representing supplement content, and shipped to the field where logistics staff, uninvolved in the study, relabeled bottles with sector numbers(001‐596) according to the random allocation list." Pg 2652. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded and blinding of the participants is unlikely to have been broken. |
| Blinding of outcome assessment (detection bias) | Low risk | At no time during the trial did study investigators or field or data management staff have access to the key. Pg 2651. |
| Incomplete outcome data (attrition bias) | Low risk | Numbers of missing outcome data in both group are small and balanced with similar reasons. Pg 2651, fig 1. |
| Selective reporting (reporting bias) | Low risk | Study protocol was available and all of the study's pre‐specified outcomes were reported in studies. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Generation of random number sequence: participants were randomised according to a computer‐generated random number sequence. "Participants were assigned on an individual basis to antioxidant or placebo supplementation and remained in the same allocation group throughout the pregnancy and 2 weeks postpartum." No details about how allocation concealment was done. Blinding of outcome assessment: unclear. Documentation of exclusion: 6 women (5.5%) were excluded. Use of placebo control: no placebo, the control group received non‐antioxidant multiple micronutrients. | |
| Participants | 110 women residing in Cipto Mangunkusumo National Hospital, Jakarta, Indonesia between 8 and 12 weeks of gestation. To be eligible, women had to have normal blood pressure at their first visit in pregnancy and again at trial entry. Women with multiple pregnancy, fetal anomaly, thrombophilia, infections, mola hydatidosa, chronic renal failure, uncontrolled hypertension, placental abnormalities, documented uterine bleeding within a week of screening, uterine malformation and history of medical and metabolic complication, such as heart disease or diabetes were excluded. | |
| Interventions | Antioxidant multiple micronutrient (MMN) supplement mixed into milk administered from trial entry until 2 weeks postpartum. Control received non‐antioxidant (MMN) supplement and all women received 40 g of milk powder. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage: history of pre‐eclampsia reported. Women's nutritional status is unclear. Sample size calculation: based on an expected incidence of pre‐eclampsia in the control group of at least 29% and in the treatment group of at least 30%. Analyses were performed on an intention‐to‐treat basis. Compliance: unclear, no information reported. Location: Jakarta, Indonesia. Timeframe: June 2001 to December 2009. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were "..randomized according to a computer‐generated random number sequence" pg 1153, pgh 2. |
| Allocation concealment (selection bias) | Unclear risk | "Participants were assigned on an individual basis to antioxidant or placebo supplementation." No details of how allocation was done. pg 1153, pgh 2. |
| Blinding of participants and personnel (performance bias) | Low risk | "Treatment allocations were blinded to both investigator and the patient..." pg 1153, pgh 2. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Investigators blinded to the sample background" but not sure if they blinded outcomes of interest in current review. |
| Incomplete outcome data (attrition bias) | Low risk | 3/52 drop‐out from supplementation group and 3/58 drop‐out from control group. drop‐out rate balanced in both groups. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported as planned in the protocol. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Generation of random number sequence: double‐blinded, multicentre trial. Randomisation was performed through an electronic data management platform, which enabled randomisation and data entry over the Internet. They were randomly allocated at a ratio of 1:1 to antioxidant supplementation (vitamins C and E) group or to placebo group through an electronic data management platform. Blinding of outcome assessment: outcome assessors were blinded to the treatment allocation. Documentation of exclusion: 277 women (10.5%) were excluded. Use of placebo control: placebo control. | |
| Participants | 2460 women participated in the study. Women were eligible for the trial if they were between 12 and 18 completed weeks of pregnancy on the basis of last menstrual period and confirmed by early ultrasound examination. The exclusion criteria were: (1) women who regularly consumed supplements200 mg/day for vitamin C and/or 50 IU/day for vitamin E; (2) women who took warfarin; (3) women who had known fetal abnormalities, (4) women who had a history of medical complications, (5) women with repeated spontaneous abortion, (6) women who used an illicit drug during the current pregnancy. | |
| Interventions | Women were provided either with vitamins C and E or placebo. Total daily dose of vitamin C was 1000 mg, and that of vitamin E was 400 IU. | |
| Outcomes | Primary outcomes:
Other maternal outcomes:
Fetal and neonatal outcomes:
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage: history of pre‐eclampsia and gestational hypertension reported as well as obstetric history (abortion, stillbirth, low birthweight, preterm birth). Women's nutritional status: use of supplements reported . Sample size calculation: based on an expected 4% and 15% incidence of the primary outcome in the low‐ and high‐risk strata, respectively. Analyses were performed on an intention‐to‐treat basis. Compliance: 85.5% in the vitamin group, 86.5% in the placebo group. Location: multicentre trial in Canada (17 centres) and Mexico (10 centres). Timeframe: January 2004 to March 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They [women] were randomly allocated at a ratio of 1:1 to antioxidant supplementation (vitamins C and E) group or to placebo group through an electronic data management platform.”Pg 239.e3 |
| Allocation concealment (selection bias) | Unclear risk | Although paper states that "Women in the placebo group were advised to take capsules that were identical in appearance to the active treatment capsules", no details were provided on how they were allocated to treatment groups |
| Blinding of participants and personnel (performance bias) | Low risk | None of the trial staff or any other person involved in the trial knew the treatment allocation for any women until after completion of the trial analysis.” Pg 239.e3 and 4 |
| Blinding of outcome assessment (detection bias) | Low risk | “None of the trial staff or any other person involved in the trial knew the treatment allocation for any women until after completion of the trial analysis.”Pg 239.e4 |
| Incomplete outcome data (attrition bias) | Unclear risk | A higher loss to follow‐up was seen in the Mexican centres although the loss was balanced between treatment and placebo groups |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to the trial registration. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Generation of random number sequence: cluster‐randomised study wherin villages‐not individuals‐were randomly assigned to 1 treatment group or the other. The 2 supplements looked different but a coding system was adopted. “…packaged the supplements in boxes with identical labelling except for the supplement code”… "the code letter did not distinguish which supplement was used". Blinding of outcome assessment: unclear if outcome assessors were unaware of participants' allocation to treatment or control group. Documentation of exclusion: 768 women (20.9%) were excluded. Use of placebo control: no placebo, the control group received iron‐folic acid supplementation. | |
| Participants | 3670 women from Maradi, rural Niger. Women who lived in 1 of the selected villages and who had experienced amenorrhoea for less than 12 weeks were eligible for participation. Exclusion criteria included women with night blindness and/or clinical signs of severe anaemia. | |
| Interventions | Daily multiple micronutrients consisting vitamin A 800 mcg,vitamin D 200 IU, vitamin E 10 mg, vitamin C 70 mg, vitamin B1 1.4 mg, vitamin B2 1.4 mg, vitamin B3 18 mg, vitamin B6 1.9, vitamin B12 2.6 mg, folic acid 400 mcg, iron 30 mg, zinc 15 mg, copper 2 mg, selenium 65 mcg, and iodine 150 mcg from enrolment until delivery. The control group received iron/folic acid. | |
| Outcomes | Maternal outcomes:
Infant outcome:
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage is unclear. Women's nutritional status is unclear. Sample size calculation: based on reduction of 25% in low birthweight. Intention‐to‐treat analysis not performed. Compliance: at subsequent visits, the remaining capsules were counted, and the number of missing capsules was replenished for another month and noted in the particular booklet. Compliance with treatment in the intervention group was 79.2% ± 18.1% and in the control group 78.4% ± 18.5%. Location: Maradi, Niger. Timeframe: January 2004 to March 2005. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Villages‐not individuals‐were randomly assigned to one treatment group or the other." No details on how randomisation was done. |
| Allocation concealment (selection bias) | Low risk | "Packaged the supplements in boxes with identical labeling except for the supplement code." |
| Blinding of participants and personnel (performance bias) | Low risk | Health workers and midwives did not distinguish which supplement was used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Data collectors were informed that each supplement came in two sizes and colors, so that the code letter did not distinguish which supplement was used." |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of women lost to follow‐up was significantly different across groups 335/1777 in IFA and 290/L ,893 in MMN. Detailed reasons for missing data not provided. |
| Selective reporting (reporting bias) | Unclear risk | Many outcomes were assessed but report was limited to only 2 outcomes. |
| Other bias | High risk | There were differences in the baseline characteristics of participants. Women in control group tended to be poorer and less educated and more women in intervention group used more preventive measures against malaria and had larger households. |
| Methods | Generation of random number sequence: randomisation of villages was stratified by county with a fixed ratio of treatments (1:1:1) and blocking of 15. The randomisation schedule was generated off site with a pseudo‐random number generator. Allocation concealment was described “a treatment colour code was assigned to each village based on the treatment allocation schedule. The treatment codes were opened only once all data had been collected”. Blinding of outcome assessment: unclear. Documentation of exclusion: 133 women (2.3%) were lost to follow‐up, 279 women (4.8%) stopped taking the supplement and refused to continue to participate, and 601 women (10.3%) experienced fetal loss. Use of placebo control: no placebo, the control group received folic acid alone. | |
| Participants | 5828 women from Shaanxi Province of north west China between < 12 – 28 weeks' gestational age. Eligibility included all women resident in the counties who became pregnant between August 2002 and January 2006. Women were ineligible if they: 1. were already taking supplements; 2. had a serious illness; 3. had an abnormal reproductive history; 4. were planning; 5. to work outside the area; or were more than 28 weeks pregnant. | |
| Interventions | Supplementation with MMN; IFA (60 mg of iron and 400 mcg of folic acid) or folic acid alone (400 mcg of folic acid) from enrolment until delivery. | |
| Outcomes |
| |
| Notes | Women's risk of spontaneous and recurrent miscarriage is unclear. Women's nutritional status is unclear. Sample size calculation: based on a 25% reduction in low birthweight between either iron‐folic acid or multiple micronutrient and folic acid (control) groups. Compliance: the number of remaining capsules was reported by the village doctor who visited the women every 2 weeks. The level of compliance with the supplementation was high in all treatment groups. Location: Shaanxi Province, China. Timeframe: August 2002 to January 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation of villages was stratified by county with a fixed ratio of treatments (1:1:1) and blocking of 15 ....... The randomisation schedule was generated off site with a pseudo‐random number generator....." pg 2, pgh 3. |
| Allocation concealment (selection bias) | Low risk | "A treatment colour code was assigned to each village based on the treatment allocation schedule. The treatment codes were opened only once all data had been collected." pg 2, pgh 3. |
| Blinding of participants and personnel (performance bias) | Low risk | "A treatment colour code was assigned to each village based on the treatment allocation schedule. The treatment codes were opened only once all data had been collected." pg 2, pgh 3. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | High risk | 121/1666 missing in folic acid, 67/1537 missing in iron‐folic acid, 88/1494 missing in multiple micronutrients in birthweight analysis. No reasons for missing data. Amount of missing data from other outcomes unknown. Fig 1; table 4. |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and all primary outcomes were reported as described in the protocol. |
| Other bias | Unclear risk | From the 531 clusters, 13 clusters were excluded due to no birth outcomes in the excluded clusters. The number of clusters excluded was unbalanced across the intervention groups which may have been due to important baseline differences. |
B‐HCG: Beta human chorionic gonadotropin
BMI: body mass index
F: folic acid
Hb: haemoglobin
HbCC: haemoglobin C disease
HbSC:haemoglobin SC disease
HbSS: haemoglobin sickle cell disease
HELLP syndrome: haemolysis, elevated liver enzymes, low platelet count syndrome
HIV‐1: Human Immunodeficiency Virus‐1
HOFPP: Hungarian Optimal Family Planning Programme
IQR: interquartile range
IFA: iron and folic acid
IU: international units
IVF‐ET: in vitro fertilization and embryo transfer
mcg: micrograms
mg/mL: milligrams per millilitre
MF: multivitamins with folic acid
mg: milligrams
MMN: multiple micronutrient
MRDR: modified relative dose‐response
MV: multivitamins without folic acid
MRC: Medical Research Council
NTD: neural tube defect
P: progesterone
PAI‐1: plasminogen activator inhibitor‐1
PAI‐2: plasminogen activator inhibitor‐2
PCV: packed cell volume
sTfR: Soluble transferrin receptor
UK: United Kingdom
UNIMMAP: United Nations International Multiple Micronutrient Preparation
USA: United States of America
WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Onset of supplementation was > 20 weeks' gestation. | |
| Unclear of the gestational age at which women entered the trial. | |
| Onset of supplementation was > 20 weeks' gestation. | |
| Onset of supplementation was > 20 weeks' gestation. | |
| No relevant clinical outcomes, reports biochemical markers of antioxidant status only. | |
| Data for main outcomes of interest were not reported in a form that could be included in the analysis. Perinatal death was reported which included stillbirths (gestational age >= 28 weeks) and death among infants in the first 7 days of life. Miscarriage data (defined as fetal loss < 28 weeks' gestation age) was not provided. | |
| Onset of supplementation was > 20 weeks' gestation. | |
| No main outcomes of interest reported. Outcomes presented in the study were fetal weight (birthweight) and placental weight. Women's risk of spontaneous and recurrent miscarriage is unclear. | |
| Onset of supplementation was > 20 weeks' gestation. | |
| Onset of supplementation was > 20 weeks' gestation. | |
| Onset of supplementation was > 20 weeks' gestation. | |
| Only 24 (9%) of the 269 women in the trial began to participate before 15 weeks' gestation and outcomes not reported separately according to gestation at enrolment. | |
| Onset of supplementation was 20 or more weeks' gestation. | |
| No inclusion/exclusion criteria reported, unclear of gestational age at enrolment to the study, reports combined outcomes for "antepartum and threatened or complete abortion" and "stillbirth or neonatal death or congenital malformation" (not reported separately). | |
| Onset of supplementation was > 20 weeks' gestation for a large proportion of the participants. | |
| Unclear of the gestational age at which women entered the trial. | |
| No main outcomes reported. | |
| The intervention assessed the effectiveness of different forms of the same vitamin ‐ folate (folic acid vs 5‐methylenetetrahydrofolate (MTHF)) against each other. There was no placebo or control group. | |
| No main outcomes reported. | |
| All women received a multivitamin in addition to the zinc supplement or placebo. | |
| Both groups received a multivitamin supplement (same vitamin content in each group). | |
| Women were recruited until late pregnancy and onset of supplementation occurred after 20 weeks. | |
| No main outcomes or pregnancy loss outcomes reported. Miscarriage reported in those women where there was a neural tube defect, but not in all women according to treatment group. | |
| Intervention assessed effect of nutritional supplement besides vitamins. No main outcomes reported. | |
| No main outcomes reported. | |
| Unclear gestational age at enrolment, 5 women were excluded due to abortion, premature labour, inadequate dietary records or missing more than 3 prenatal visits. Exclusions were not reported by group allocation. Outcomes related to maternal and fetal plasma levels of pyridoxal 5'‐phosphate and coenzyme saturation of aspartate aminotransferase and alanine aminotransferase in maternal erthrocytes were reported. | |
| Onset of supplementation was > 20 weeks' gestation. | |
| No clinical outcomes reported. | |
| Onset of supplementation was > 20 weeks' gestation. | |
| No main outcomes reported. | |
| No main outcomes reported. | |
| Onset of supplementation was > 20 weeks' gestation. | |
| Study was a food intervention trial. | |
| Unclear about content of vitamin supplements. | |
| Unclear of gestation at enrolment to the trial. | |
| No main outcomes reported. | |
| Both groups received a multivitamin (same vitamin content in both groups). | |
| Non‐randomised study of periconceptional multivitamin supplementation for the prevention of neural tube defects. | |
| No main outcomes reported. | |
| No main outcomes reported. | |
| No main outcomes reported. Haematological features and serum ferritin were determined. No reports on pregnancy loss. | |
| No main outcomes reported. | |
| Unclear of gestation at enrolment to the trial. | |
| Non‐randomised study. | |
| No main outcomes reported. | |
| No main outcomes reported. | |
| This trial involved only one vitamin compared at different doses. | |
| No relevant outcomes were reported in this study. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Partially double‐blind, individually‐randomised controlled trial. |
| Participants | 1320 pregnant women around 20 gestational weeks. |
| Interventions | Women received 1 of 3 supplements daily until delivery: 60 mg Fe + 400 μg folic acid (IFA), or 1‐2 recommended dietary allowances of 18 micronutrients including 20 mg Fe (MMN), or SQ‐LNS with the same nutrient levels as in MMN, plus 4 additional minerals as well as macronutrients contributing 118 kcal (LNS). |
| Outcomes | Haemoglobin concentration (g/L) and 2 markers of iron status, zinc protoporphyrin (ZPP, μmol/mol heme) and transferrin receptor (TfR, mg/L) were assessed. |
| Notes | Only study abstract available and the composition of the supplement is not clear. Need to see full text. |
| Methods | Individually‐randomised controlled trial. |
| Participants | 50 women of recurrent of abortions (more than 2). |
| Interventions | Women received Vitamin B12 1500 mcg, Vitamin B6 10 mg and folic acid 5 mg daily throughout pregnancy. |
| Outcomes |
|
| Notes | Only study abstract available, comparison group also received same intervention but duration is different. |
| Methods | Unclear. |
| Participants | Unclear. |
| Interventions | Unclear. |
| Outcomes | Unclear. |
| Notes | A copy of the paper could not be located. |
| Methods | Randomised cohorts. |
| Participants | Pregnant women and infants. |
| Interventions | Various formulations of lipid‐based nutrient supplements (LNS) during pregnancy and infancy or infancy alone. |
| Outcomes |
|
| Notes | Abstract only, no specification what LNS is in abstract. |
IFA: iron and folic acid
IUGR: Intrauterine growth restriction
MMN: multiple micronutrient
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The effect of antioxidant supplementation on women with threatened miscarriage. |
| Methods | Randomised controlled trial. |
| Participants | 580 women who present with first trimester bleeding. |
| Interventions | Vitamin C 1000 mg and Vitamin E 400 IU versus placebo. |
| Outcomes |
|
| Starting date | 01/03/2004. |
| Contact information | Dr Jemma Johns UCLH/UCL Research & Development Governance Committee |
| Notes | Listed as completed. |
IU: internation unit(s)
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 7 | 18949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.40] |
| Analysis 1.1  Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 4 | 13346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| Analysis 1.2 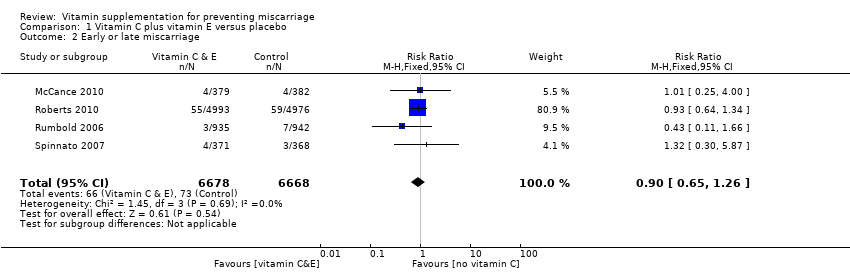 Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 7 | 21442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.97, 1.76] |
| Analysis 1.3  Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 5 | 8334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.84, 1.62] |
| Analysis 1.4  Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 4 Congenital malformations. | ||||
| 5 Any adverse effects of vitamin supplementation sufficient to stop supplementation Show forest plot | 1 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.41] |
| Analysis 1.5 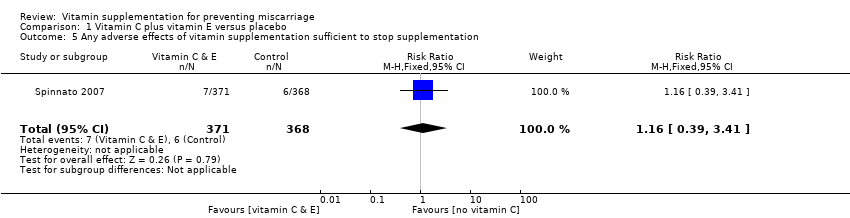 Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 5 Any adverse effects of vitamin supplementation sufficient to stop supplementation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.58, 2.83] |
| Analysis 2.1  Comparison 2 Vitamin C versus no supplement/placebo, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.52, 2.65] |
| Analysis 2.2  Comparison 2 Vitamin C versus no supplement/placebo, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| Analysis 2.3 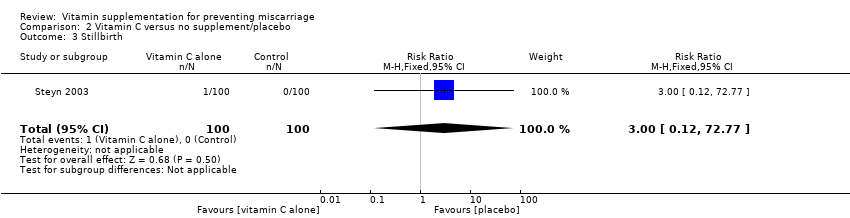 Comparison 2 Vitamin C versus no supplement/placebo, Outcome 3 Stillbirth. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.63, 2.77] |
| Analysis 3.1 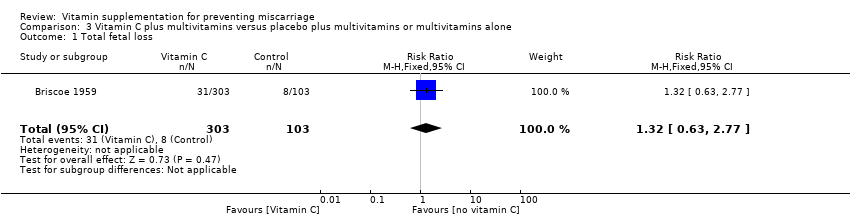 Comparison 3 Vitamin C plus multivitamins versus placebo plus multivitamins or multivitamins alone, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 2 | 790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.79, 1.79] |
| Analysis 3.2  Comparison 3 Vitamin C plus multivitamins versus placebo plus multivitamins or multivitamins alone, Outcome 2 Early or late miscarriage. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 1640 | Risk Ratio (Fixed, 95% CI) | 1.01 [0.61, 1.66] |
| Analysis 4.1 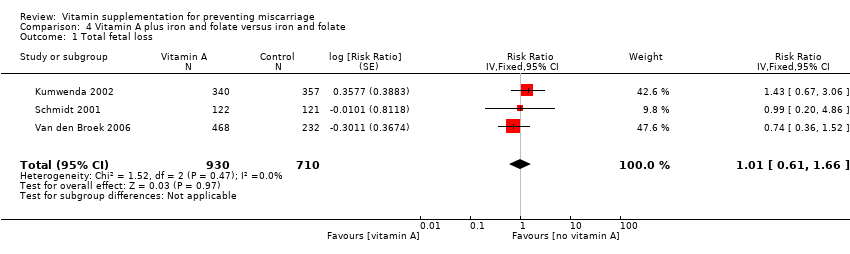 Comparison 4 Vitamin A plus iron and folate versus iron and folate, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 2 | 1397 | Risk Ratio (Fixed, 95% CI) | 0.86 [0.46, 1.62] |
| Analysis 4.2  Comparison 4 Vitamin A plus iron and folate versus iron and folate, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 3 | 1640 | Risk Ratio (Fixed, 95% CI) | 1.29 [0.57, 2.91] |
| Analysis 4.3  Comparison 4 Vitamin A plus iron and folate versus iron and folate, Outcome 3 Stillbirth. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 52480 | Risk Ratio (Random, 95% CI) | 1.05 [0.90, 1.23] |
| Analysis 5.1  Comparison 5 Vitamin A versus placebo, Outcome 1 Total fetal loss. | ||||
| 2 Early of late miscarriage Show forest plot | 1 | 39668 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.92, 1.04] |
| Analysis 5.2 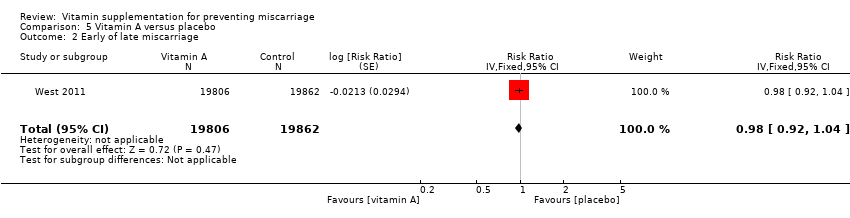 Comparison 5 Vitamin A versus placebo, Outcome 2 Early of late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 39668 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| Analysis 5.3  Comparison 5 Vitamin A versus placebo, Outcome 3 Stillbirth. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 51163 | Risk Ratio (Fixed, 95% CI) | 1.02 [0.98, 1.07] |
| Analysis 6.1  Comparison 6 Beta‐carotene versus placebo, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 1 | 39860 | Risk Ratio (Fixed, 95% CI) | 1.00 [0.94, 1.06] |
| Analysis 6.2  Comparison 6 Beta‐carotene versus placebo, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 39860 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.98, 1.20] |
| Analysis 6.3  Comparison 6 Beta‐carotene versus placebo, Outcome 3 Stillbirth. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 17373 | Risk Ratio (Fixed, 95% CI) | 1.05 [0.91, 1.21] |
| Analysis 7.1  Comparison 7 Vitamin A or beta‐carotene versus placebo, Outcome 1 Total fetal loss. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1074 | Risk Ratio (Fixed, 95% CI) | 0.80 [0.53, 1.21] |
| Analysis 8.1  Comparison 8 Vitamin A (with/without multivitamins) versus multivitamins or placebo, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 1 | 1075 | Risk Ratio (Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| Analysis 8.2  Comparison 8 Vitamin A (with/without multivitamins) versus multivitamins or placebo, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 1075 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.60, 1.79] |
| Analysis 8.3  Comparison 8 Vitamin A (with/without multivitamins) versus multivitamins or placebo, Outcome 3 Stillbirth. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 10 | 94948 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.93, 1.00] |
| Analysis 9.1  Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 10 | 94948 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.94, 1.03] |
| Analysis 9.2 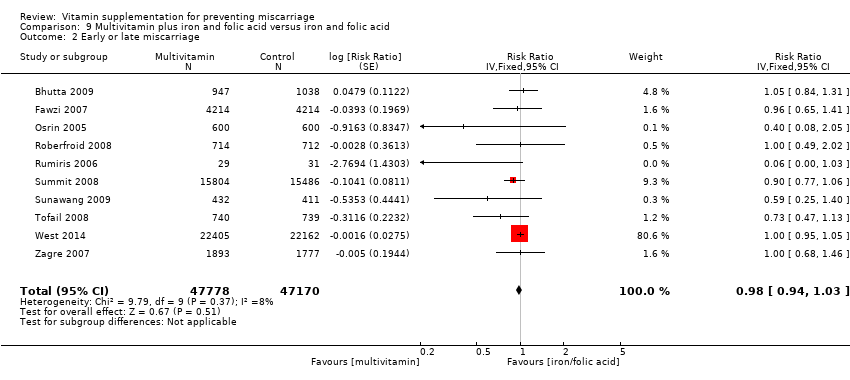 Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 10 | 79851 | Risk Ratio (Fixed, 95% CI) | 0.92 [0.85, 0.99] |
| Analysis 9.3  Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformation Show forest plot | 1 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.08] |
| Analysis 9.4  Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 4 Congenital malformation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 907 | Risk Ratio (Fixed, 95% CI) | 0.49 [0.34, 0.70] |
| Analysis 10.1 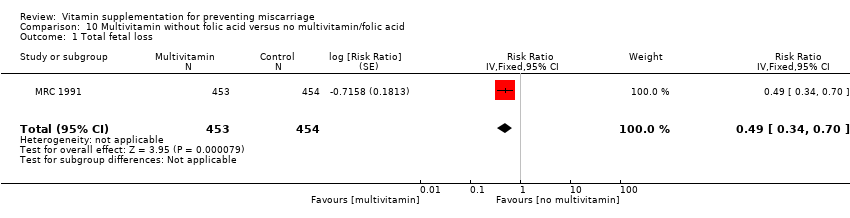 Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 1 | 907 | Risk Ratio (Fixed, 95% CI) | 0.89 [0.59, 1.34] |
| Analysis 10.2  Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 907 | Risk Ratio (Fixed, 95% CI) | 0.14 [0.01, 2.77] |
| Analysis 10.3 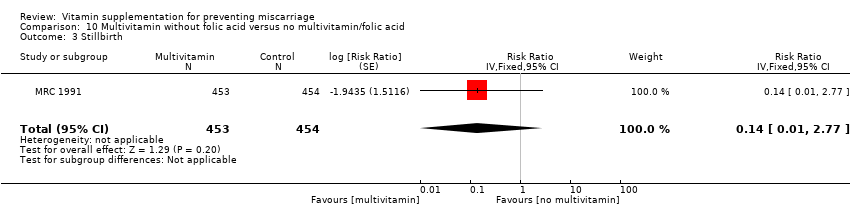 Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 1 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.53, 4.86] |
| Analysis 10.4 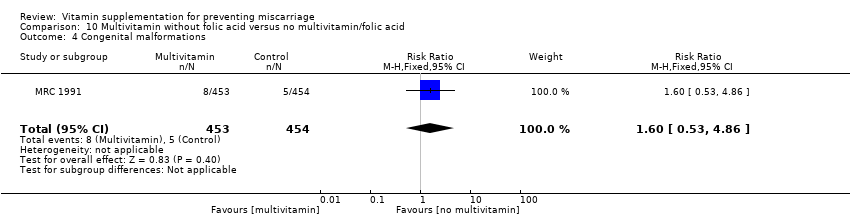 Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1368 | Risk Ratio (Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| Analysis 11.1 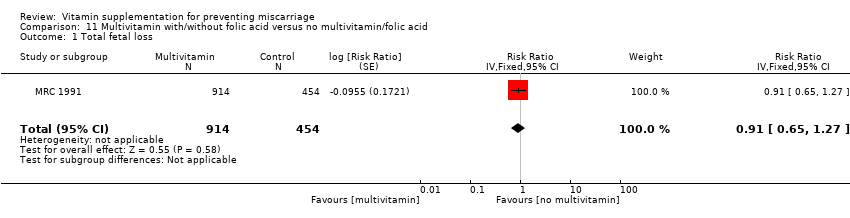 Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 1 | 1368 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.67, 1.34] |
| Analysis 11.2  Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 1368 | Risk Ratio (Fixed, 95% CI) | 0.33 [0.06, 1.98] |
| Analysis 11.3 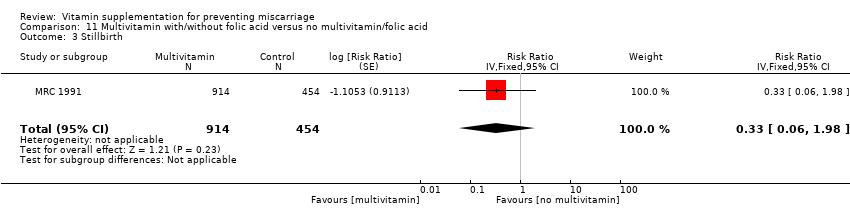 Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 1 | 1368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.75, 5.26] |
| Analysis 11.4 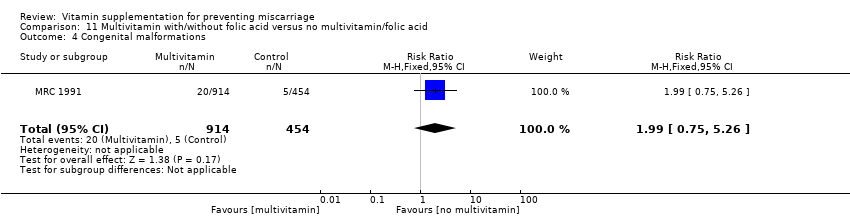 Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 5012 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| Analysis 12.1  Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 3 | 5012 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.80, 1.18] |
| Analysis 12.2  Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 3 | 4316 | Risk Ratio (Fixed, 95% CI) | 1.32 [0.93, 1.88] |
| Analysis 12.3  Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 2 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.72, 4.04] |
| Analysis 12.4  Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1090 | Risk Ratio (Fixed, 95% CI) | 0.90 [0.62, 1.30] |
| Analysis 13.1  Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 2 | 1090 | Risk Ratio (Fixed, 95% CI) | 0.89 [0.61, 1.31] |
| Analysis 13.2  Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 2 | 1090 | Risk Ratio (Random, 95% CI) | 0.99 [0.04, 22.90] |
| Analysis 13.3  Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.67, 3.85] |
| Analysis 13.4 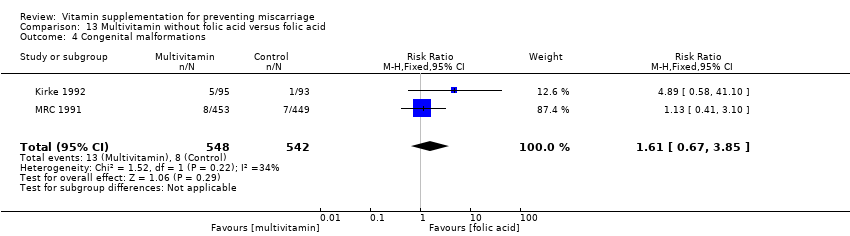 Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1644 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.70, 1.33] |
| Analysis 14.1 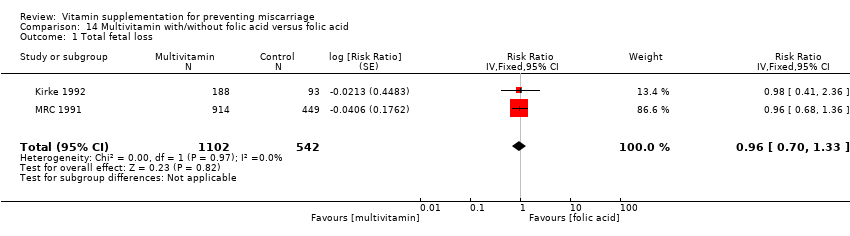 Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 1 Total fetal loss. | ||||
| 2 Early of late miscarriage Show forest plot | 2 | 1644 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.70, 1.33] |
| Analysis 14.2  Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 2 Early of late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 2 | 1644 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.15, 4.10] |
| Analysis 14.3  Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.76, 3.63] |
| Analysis 14.4  Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1074 | Risk Ratio (Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| Analysis 15.1 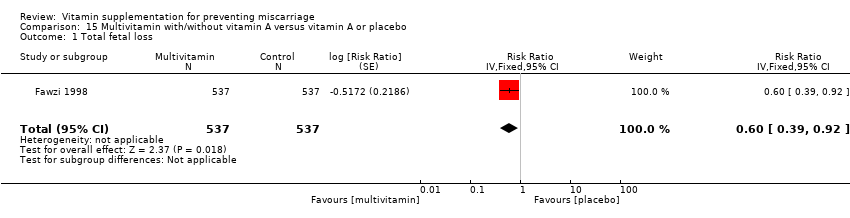 Comparison 15 Multivitamin with/without vitamin A versus vitamin A or placebo, Outcome 1 Total fetal loss. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 5021 | Risk Ratio (Fixed, 95% CI) | 0.83 [0.58, 1.17] |
| Analysis 16.1  Comparison 16 Multivitamin versus control, Outcome 1 Total fetal loss. | ||||
| 2 Stillbirth Show forest plot | 1 | 5021 | Risk Ratio (Fixed, 95% CI) | 0.83 [0.58, 1.17] |
| Analysis 16.2  Comparison 16 Multivitamin versus control, Outcome 2 Stillbirth. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 823 | Risk Ratio (Fixed, 95% CI) | 0.92 [0.46, 1.83] |
| Analysis 17.1 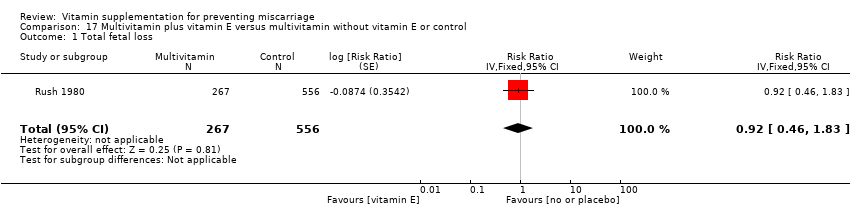 Comparison 17 Multivitamin plus vitamin E versus multivitamin without vitamin E or control, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 1 | 823 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.26, 4.13] |
| Analysis 17.2  Comparison 17 Multivitamin plus vitamin E versus multivitamin without vitamin E or control, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 823 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.39, 1.98] |
| Analysis 17.3 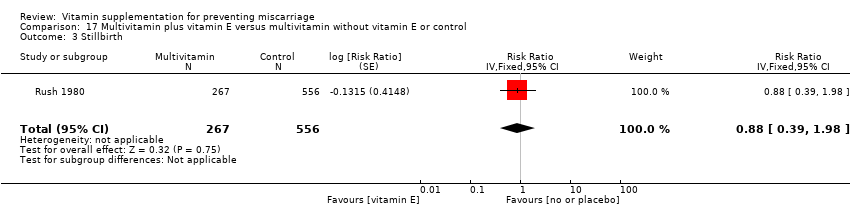 Comparison 17 Multivitamin plus vitamin E versus multivitamin without vitamin E or control, Outcome 3 Stillbirth. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 6883 | Risk Ratio (Random, 95% CI) | 1.00 [0.75, 1.34] |
| Analysis 18.1  Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 3 | 6883 | Risk Ratio (Random, 95% CI) | 0.99 [0.72, 1.38] |
| Analysis 18.2 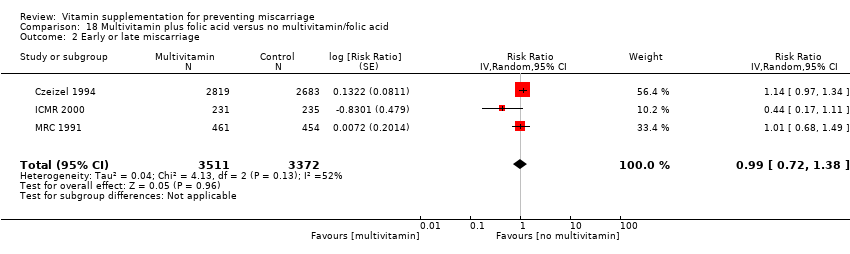 Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 3 | 6883 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.51, 2.10] |
| Analysis 18.3  Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 2 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.81, 3.53] |
| Analysis 18.4  Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 6883 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.34] |
| Analysis 19.1  Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 3 | 6883 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.38] |
| Analysis 19.2  Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 3 | 6883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.51, 2.09] |
| Analysis 19.3 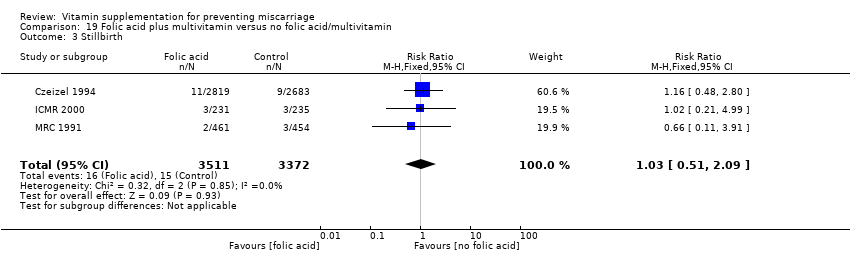 Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 2 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.81, 3.53] |
| Analysis 19.4 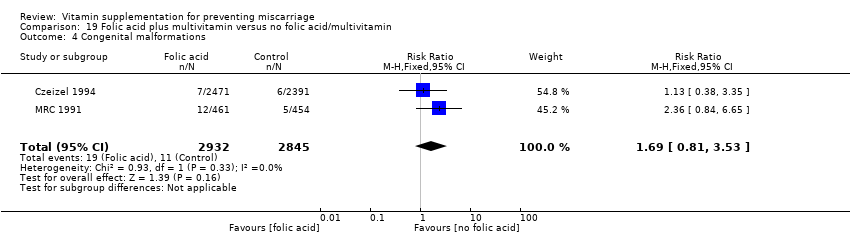 Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.40] |
| Analysis 20.1  Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.44] |
| Analysis 20.2  Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 4.02] |
| Analysis 20.3  Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.45, 4.43] |
| Analysis 20.4 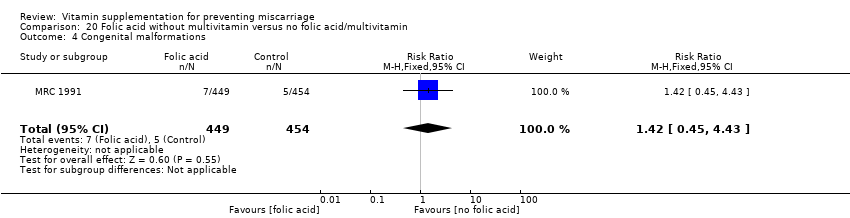 Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.69, 1.35] |
| Analysis 21.1 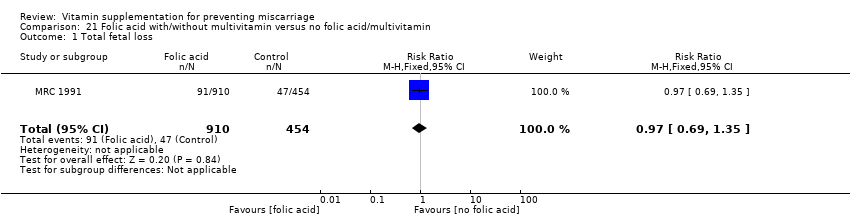 Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.70, 1.39] |
| Analysis 21.2  Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.15, 2.96] |
| Analysis 21.3 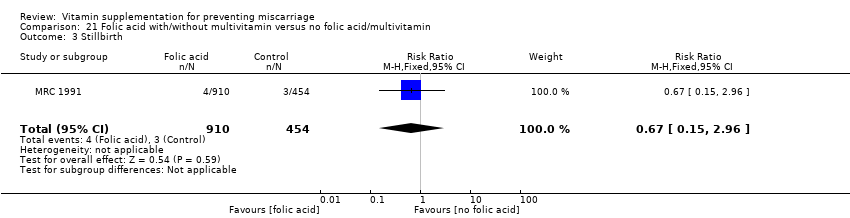 Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.71, 5.04] |
| Analysis 21.4 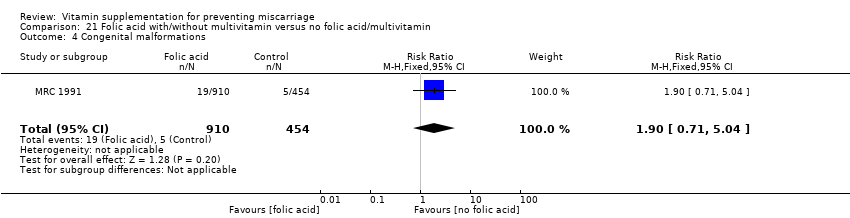 Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.67] |
| Analysis 22.1  Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.80, 1.69] |
| Analysis 22.2 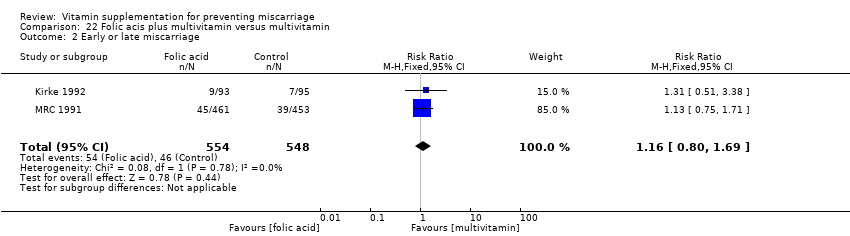 Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.04, 22.55] |
| Analysis 22.3 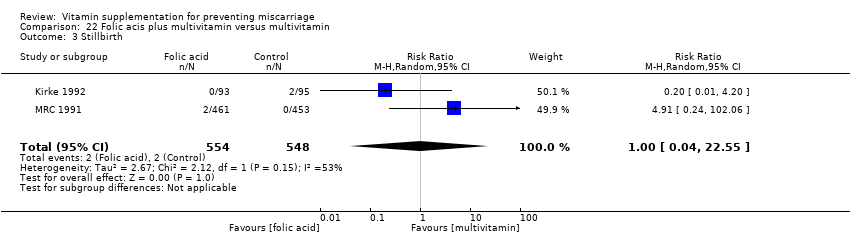 Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.28, 3.12] |
| Analysis 22.4  Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.77, 1.62] |
| Analysis 23.1  Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.77, 1.64] |
| Analysis 23.2  Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.97 [0.58, 42.29] |
| Analysis 23.3 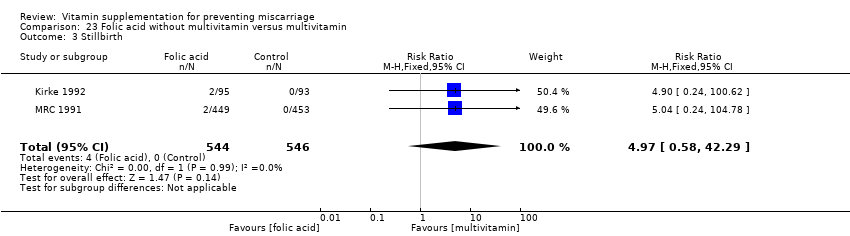 Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.26, 1.49] |
| Analysis 23.4 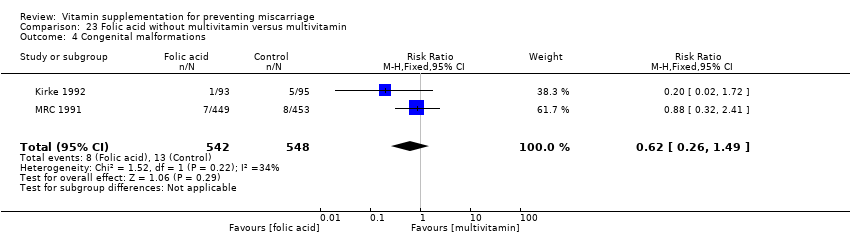 Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.57] |
| Analysis 24.1 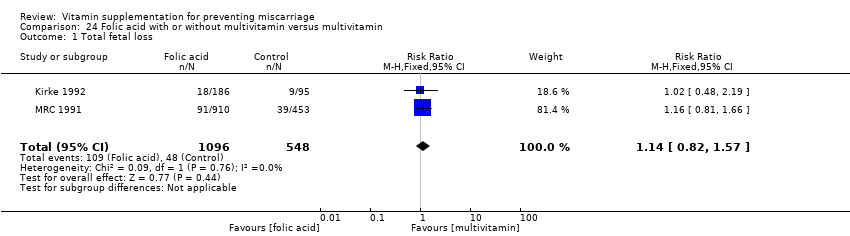 Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 2 | 1642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
| Analysis 24.2  Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.02, 28.39] |
| Analysis 24.3 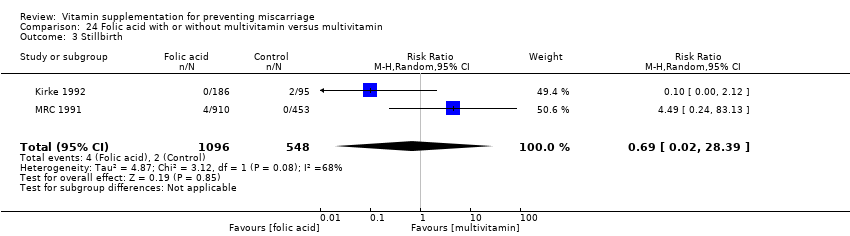 Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 3 Stillbirth. | ||||
| 4 Congenital malformations Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.19, 2.51] |
| Analysis 24.4 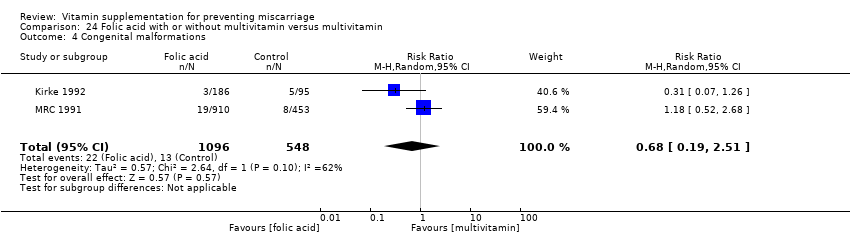 Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 4 Congenital malformations. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.59] |
| Analysis 25.1  Comparison 25 Folic acid plus iron versus iron, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.03] |
| Analysis 25.2  Comparison 25 Folic acid plus iron versus iron, Outcome 2 Early or late miscarriage. | ||||
| 3 Stillbirth Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.03] |
| Analysis 25.3  Comparison 25 Folic acid plus iron versus iron, Outcome 3 Stillbirth. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [0.74, 226.98] |
| Analysis 26.1  Comparison 26 Folic acid plus iron and antimalarials versus iron and antimalarials, Outcome 1 Total fetal loss. | ||||
| 2 Early or late miscarriage Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [0.74, 226.98] |
| Analysis 26.2  Comparison 26 Folic acid plus iron and antimalarials versus iron and antimalarials, Outcome 2 Early or late miscarriage. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early or late miscarriage Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.24, 5.29] |
| Analysis 27.1  Comparison 27 Antioxidant vitamin supplementation, Outcome 1 Early or late miscarriage. | ||||

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
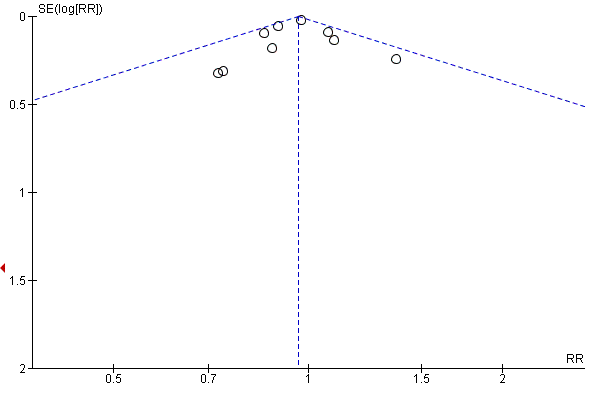
Funnel plot of comparison: 9 Multivitamin plus iron and folic acid versus iron and folic acid, outcome: 9.1 Total fetal loss.
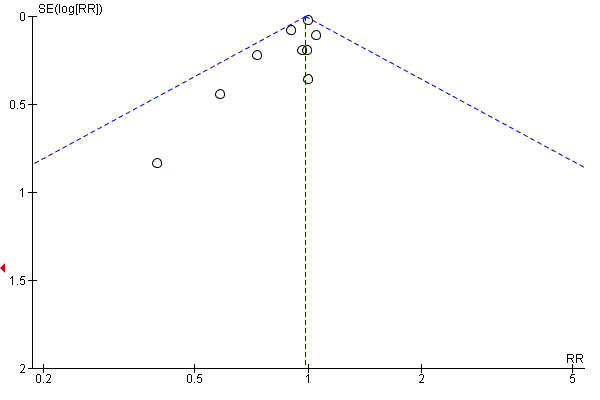
Funnel plot of comparison: 9 Multivitamin plus iron and folic acid versus iron and folic acid, outcome: 9.2 Early or late miscarriage.

Funnel plot of comparison: 9 Multivitamin plus iron and folic acid versus iron and folic acid, outcome: 9.3 Stillbirth.

Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 1 Total fetal loss.
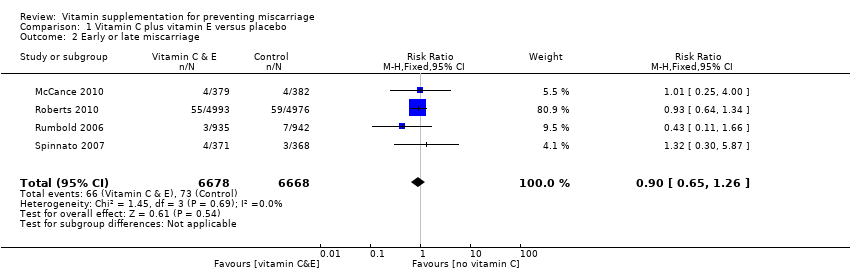
Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 2 Early or late miscarriage.

Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 3 Stillbirth.
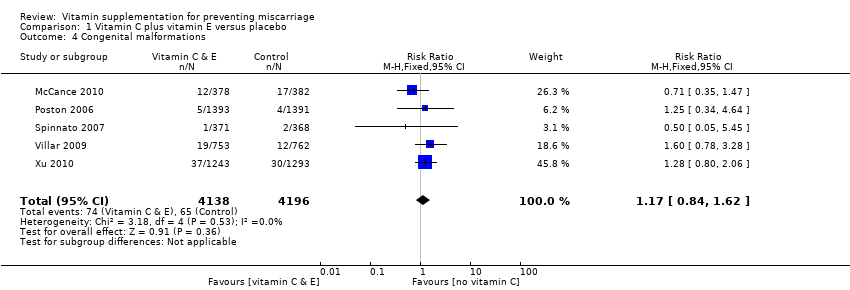
Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 4 Congenital malformations.

Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 5 Any adverse effects of vitamin supplementation sufficient to stop supplementation.

Comparison 2 Vitamin C versus no supplement/placebo, Outcome 1 Total fetal loss.

Comparison 2 Vitamin C versus no supplement/placebo, Outcome 2 Early or late miscarriage.

Comparison 2 Vitamin C versus no supplement/placebo, Outcome 3 Stillbirth.
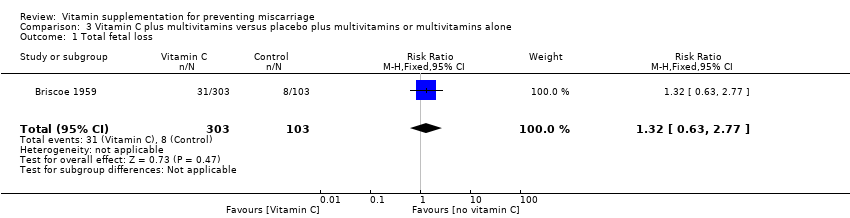
Comparison 3 Vitamin C plus multivitamins versus placebo plus multivitamins or multivitamins alone, Outcome 1 Total fetal loss.
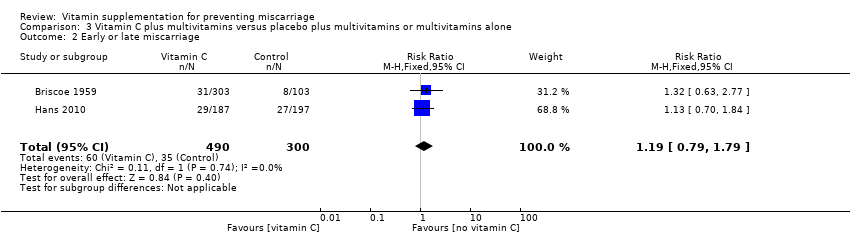
Comparison 3 Vitamin C plus multivitamins versus placebo plus multivitamins or multivitamins alone, Outcome 2 Early or late miscarriage.

Comparison 4 Vitamin A plus iron and folate versus iron and folate, Outcome 1 Total fetal loss.

Comparison 4 Vitamin A plus iron and folate versus iron and folate, Outcome 2 Early or late miscarriage.

Comparison 4 Vitamin A plus iron and folate versus iron and folate, Outcome 3 Stillbirth.
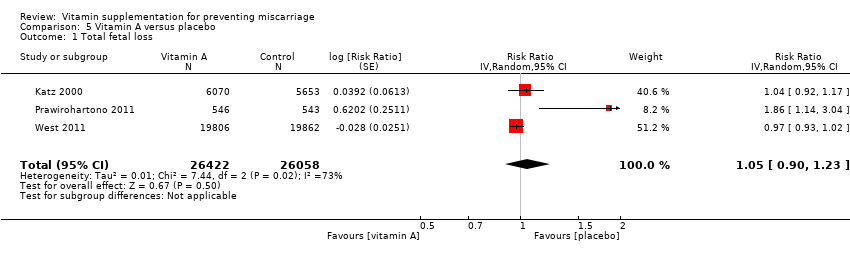
Comparison 5 Vitamin A versus placebo, Outcome 1 Total fetal loss.

Comparison 5 Vitamin A versus placebo, Outcome 2 Early of late miscarriage.

Comparison 5 Vitamin A versus placebo, Outcome 3 Stillbirth.
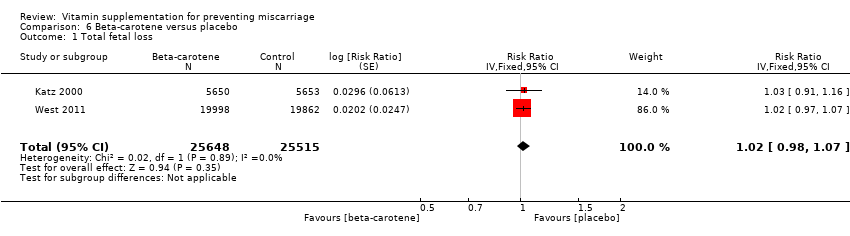
Comparison 6 Beta‐carotene versus placebo, Outcome 1 Total fetal loss.
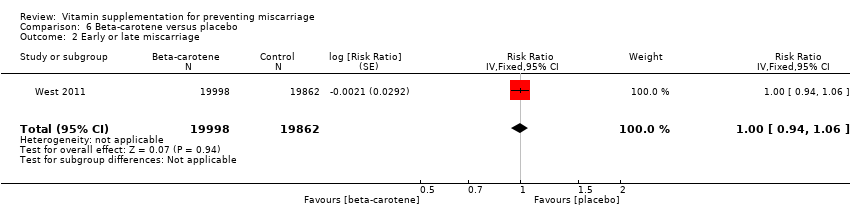
Comparison 6 Beta‐carotene versus placebo, Outcome 2 Early or late miscarriage.

Comparison 6 Beta‐carotene versus placebo, Outcome 3 Stillbirth.
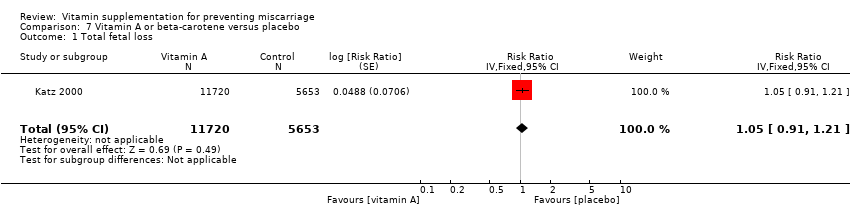
Comparison 7 Vitamin A or beta‐carotene versus placebo, Outcome 1 Total fetal loss.
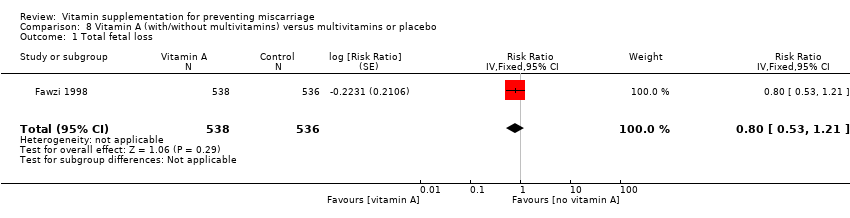
Comparison 8 Vitamin A (with/without multivitamins) versus multivitamins or placebo, Outcome 1 Total fetal loss.

Comparison 8 Vitamin A (with/without multivitamins) versus multivitamins or placebo, Outcome 2 Early or late miscarriage.

Comparison 8 Vitamin A (with/without multivitamins) versus multivitamins or placebo, Outcome 3 Stillbirth.
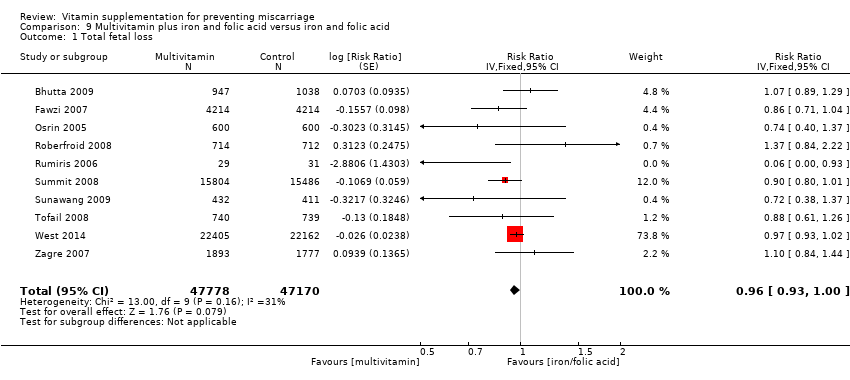
Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 1 Total fetal loss.
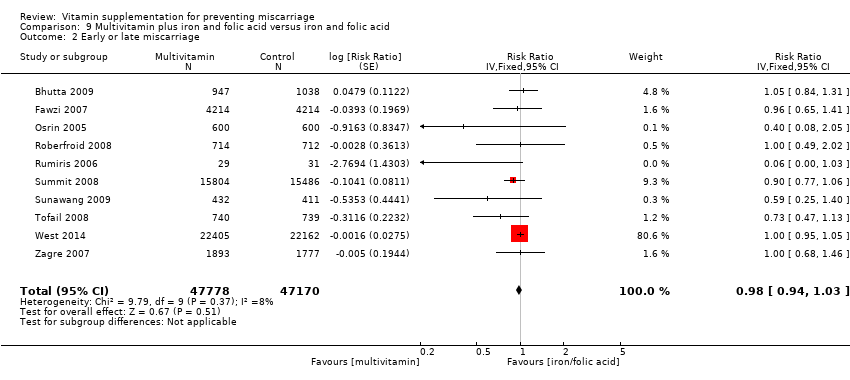
Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 2 Early or late miscarriage.

Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 3 Stillbirth.

Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 4 Congenital malformation.
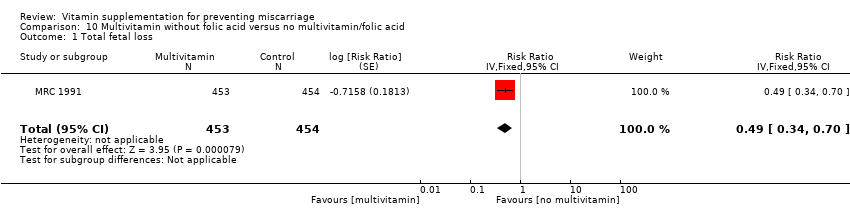
Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 1 Total fetal loss.
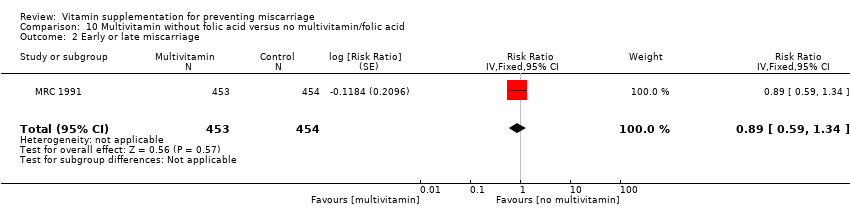
Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 2 Early or late miscarriage.
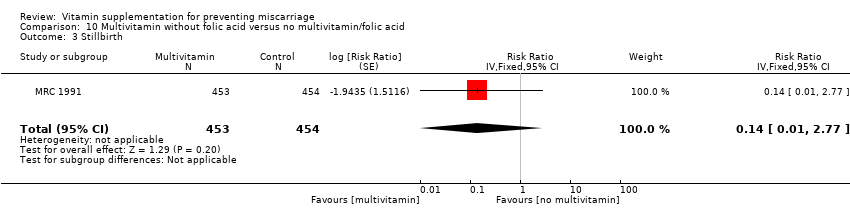
Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 3 Stillbirth.

Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 4 Congenital malformations.

Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 1 Total fetal loss.
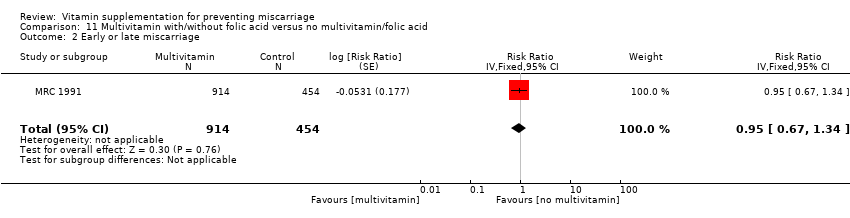
Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 2 Early or late miscarriage.

Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 3 Stillbirth.

Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 4 Congenital malformations.

Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 1 Total fetal loss.

Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 2 Early or late miscarriage.

Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 3 Stillbirth.
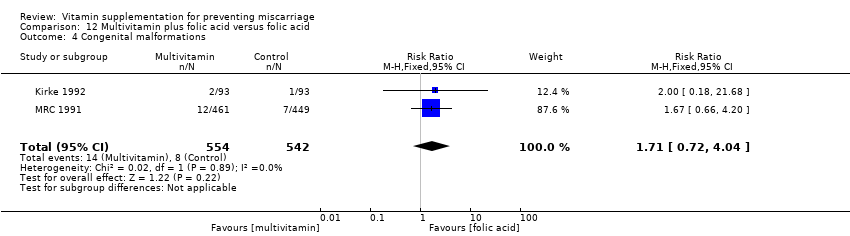
Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 4 Congenital malformations.

Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 1 Total fetal loss.
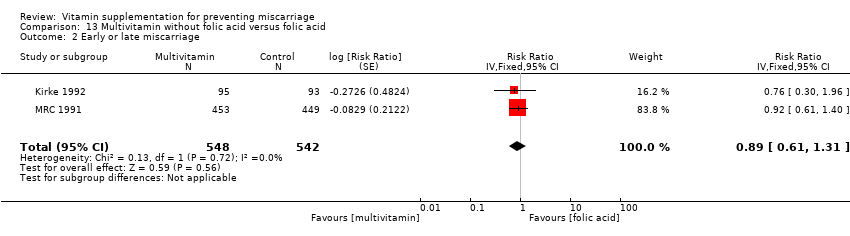
Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 2 Early or late miscarriage.

Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 3 Stillbirth.

Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 4 Congenital malformations.

Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 1 Total fetal loss.
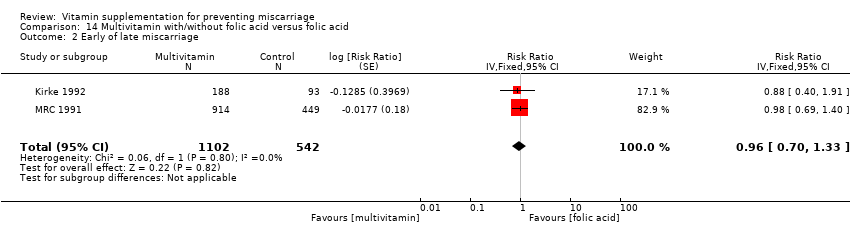
Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 2 Early of late miscarriage.
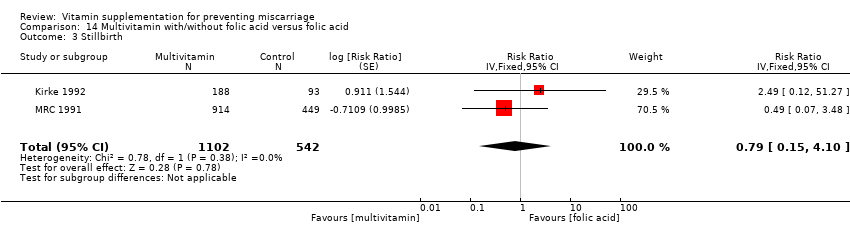
Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 3 Stillbirth.
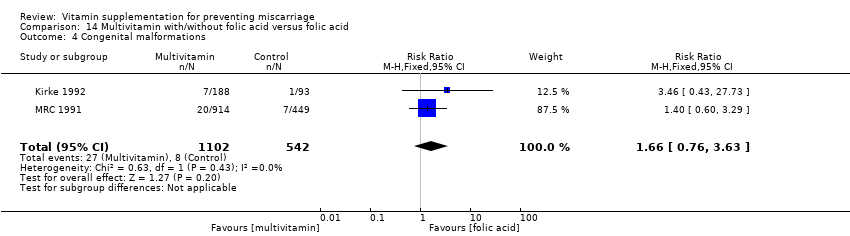
Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 4 Congenital malformations.

Comparison 15 Multivitamin with/without vitamin A versus vitamin A or placebo, Outcome 1 Total fetal loss.
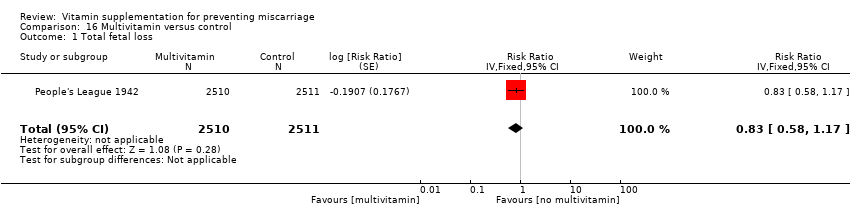
Comparison 16 Multivitamin versus control, Outcome 1 Total fetal loss.

Comparison 16 Multivitamin versus control, Outcome 2 Stillbirth.
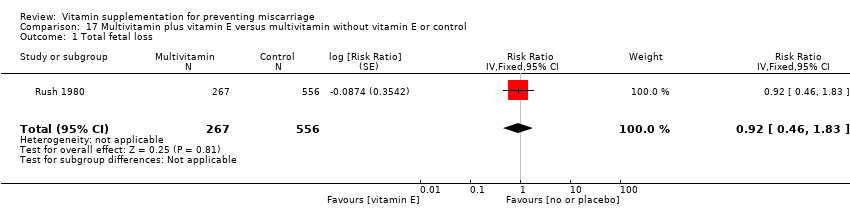
Comparison 17 Multivitamin plus vitamin E versus multivitamin without vitamin E or control, Outcome 1 Total fetal loss.

Comparison 17 Multivitamin plus vitamin E versus multivitamin without vitamin E or control, Outcome 2 Early or late miscarriage.

Comparison 17 Multivitamin plus vitamin E versus multivitamin without vitamin E or control, Outcome 3 Stillbirth.

Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 1 Total fetal loss.
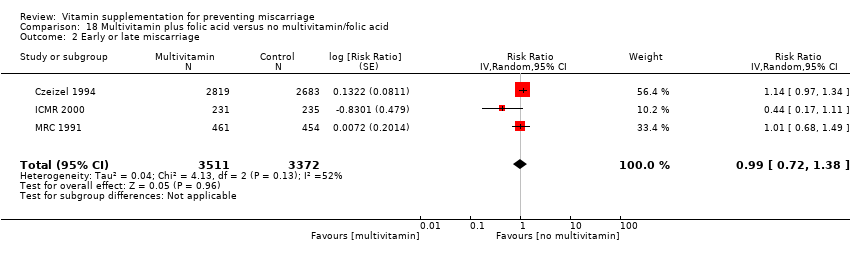
Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 2 Early or late miscarriage.
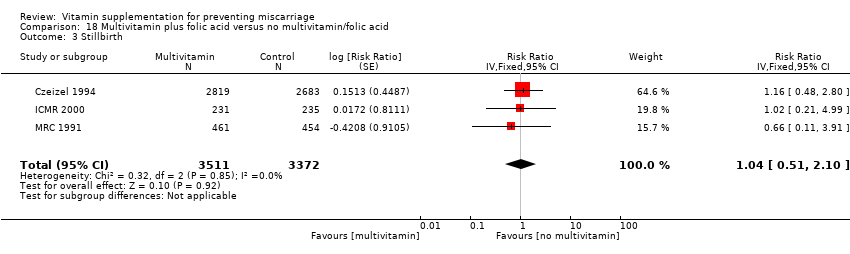
Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 3 Stillbirth.
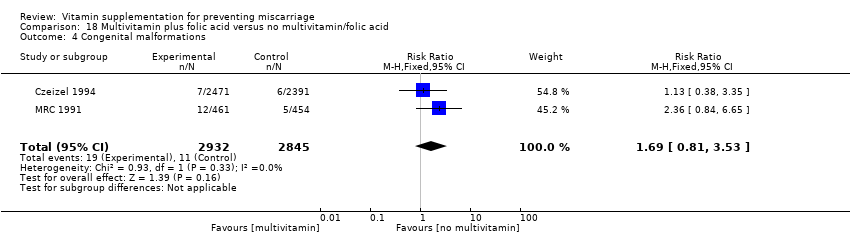
Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 4 Congenital malformations.

Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 1 Total fetal loss.

Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 2 Early or late miscarriage.

Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 3 Stillbirth.

Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 4 Congenital malformations.
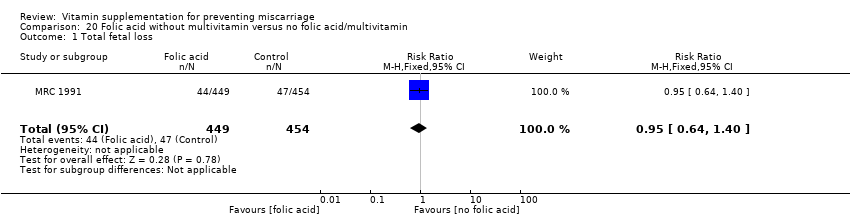
Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 1 Total fetal loss.
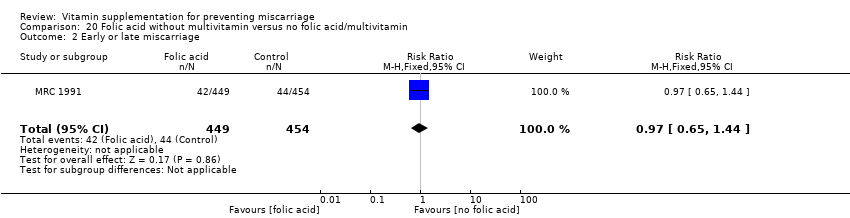
Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 2 Early or late miscarriage.

Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 3 Stillbirth.

Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 4 Congenital malformations.

Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 1 Total fetal loss.

Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 2 Early or late miscarriage.

Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 3 Stillbirth.

Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 4 Congenital malformations.
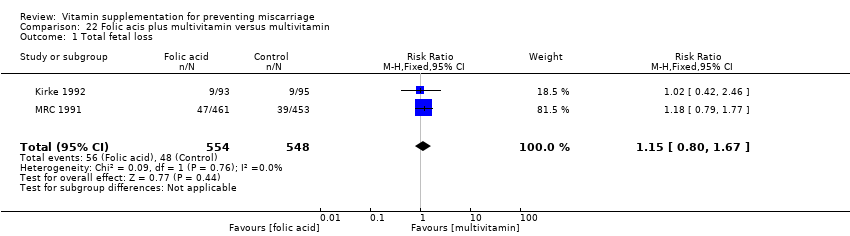
Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 1 Total fetal loss.

Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 2 Early or late miscarriage.

Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 3 Stillbirth.

Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 4 Congenital malformations.

Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 1 Total fetal loss.

Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 2 Early or late miscarriage.

Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 3 Stillbirth.

Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 4 Congenital malformations.

Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 1 Total fetal loss.

Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 2 Early or late miscarriage.
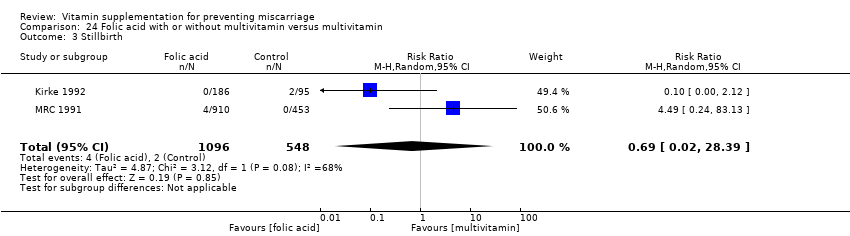
Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 3 Stillbirth.
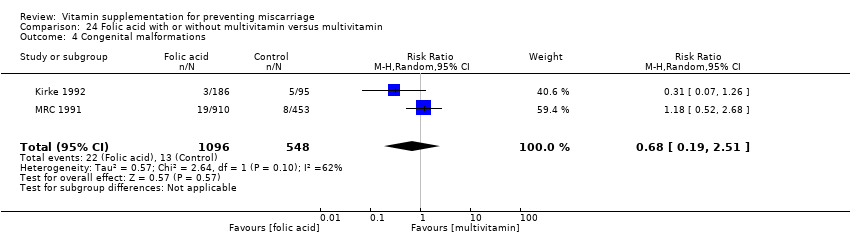
Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 4 Congenital malformations.

Comparison 25 Folic acid plus iron versus iron, Outcome 1 Total fetal loss.
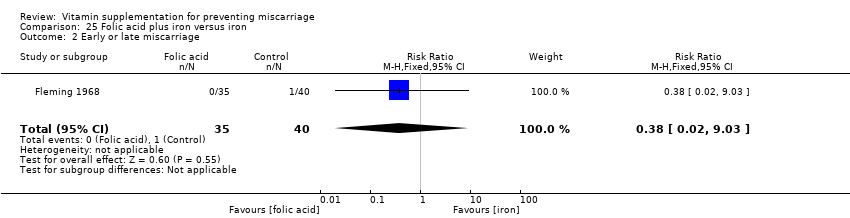
Comparison 25 Folic acid plus iron versus iron, Outcome 2 Early or late miscarriage.

Comparison 25 Folic acid plus iron versus iron, Outcome 3 Stillbirth.
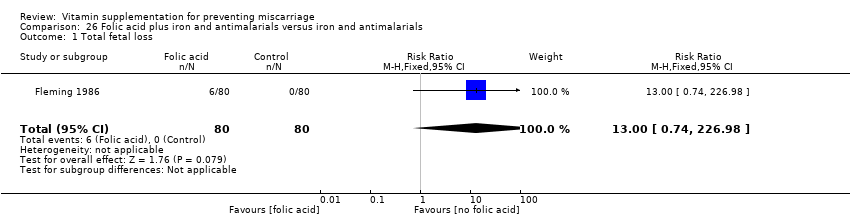
Comparison 26 Folic acid plus iron and antimalarials versus iron and antimalarials, Outcome 1 Total fetal loss.

Comparison 26 Folic acid plus iron and antimalarials versus iron and antimalarials, Outcome 2 Early or late miscarriage.
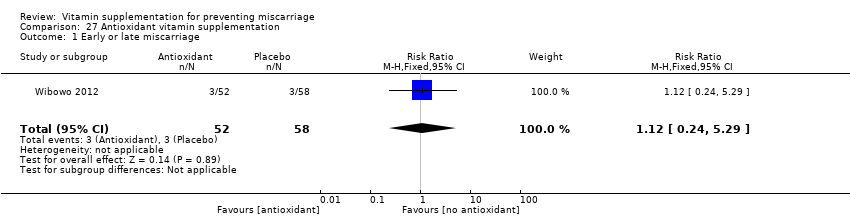
Comparison 27 Antioxidant vitamin supplementation, Outcome 1 Early or late miscarriage.
| Vitamin C plus vitamin E versus control for preventing miscarriage | ||||||
| Population: pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Vitamin C plus vitamin E | |||||
| Total fetal loss | Study population | RR 1.14 | 18,949 | ⊕⊕⊕⊕ | ||
| 17 per 1000 | 20 per 1000 | |||||
| Moderate | ||||||
| 14 per 1000 | 16 per 1000 | |||||
| Early or late miscarriage | Study population | RR 0.90 | 13,346 | ⊕⊕⊕⊝ | ||
| 9 per 1000 | 9 per 1000 | |||||
| Moderate | ||||||
| 8 per 1000 | 8 per 1000 | |||||
| Stillbirth | Study population | RR 1.31 | 21,442 | ⊕⊕⊕⊝ | ||
| 8 per 1000 | 10 per 1000 | |||||
| Moderate | ||||||
| 7 per 1000 | 9 per 1000 | |||||
| Any adverse effects of vitamin supplementation sufficient to stop supplementation | Study population | RR 1.16 | 739 | ⊕⊕⊕⊝ | ||
| 16 per 1000 | 19 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not effective but 95% CI is narrow and precise. 2 Non significant with wide 95% CI. 3 Small sample size. | ||||||
| Vitamin A plus iron plus folate versus iron plus folate for preventing miscarriage | ||||||
| Population: pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Vitamin A | |||||
| Total fetal loss (including miscarriages or combined miscarriages and stillbirths) ‐ Vitamin A + iron + folate versus iron + folate | Study population | RR 1.01 | 1640 | ⊕⊕⊝⊝ | ||
| 37 per 1000 | 37 per 1000 | |||||
| Moderate | ||||||
| 59 per 1,000 | 60 per 1,000 (36 to 98) | |||||
| Early or late miscarriage ‐ Vitamin A + iron + folate versus iron + folate | Study population | RR 0.86 | 1397 | ⊕⊕⊝⊝ | ||
| 31 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| 50 per 1,000 | 43 per 1,000 (23 to 80) | |||||
| Stillbirth ‐ Vitamin A + iron + folate versus iron + folate | Study population | RR 1.29 | 1640 | ⊕⊕⊝⊝ | ||
| 13 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 21 per 1,000 | 27 per 1,000 (12 to 61) | |||||
| Any adverse effects | See comments. | No studies reported this outcome. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High and unclear risk of attrition bias. 2 Wide 95% CI. | ||||||
| Multivitamin plus iron plus folate versus iron plus folate for preventing miscarriage | ||||||
| Population: pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Multivitamin | |||||
| Total fetal loss (including miscarriages or combined miscarriages and stillbirths) ‐ Multivitamins + iron + folic acid versus iron + folic acid | Study population | RR 0.96 | 94,948 | ⊕⊕⊕⊕ | ||
| 136 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 218 per 1,000 | 209 per 1,000 (202 to 218) | |||||
| Early or late miscarriage ‐ Multivitamin + iron + folic acid versus iron + folic acid | Study population | RR 0.98 | 94948 | ⊕⊕⊕⊝ | ||
| 84 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 134 per 1,000 | 132 per 1,000 (126 to 138) | |||||
| Stillbirth ‐ Multivitamin + iron + folic acid versus iron + folic acid | Study population | RR 0.92 | 79,851 | ⊕⊕⊕⊕ | ||
| 29 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| 46 per 1,000 | 43 per 1,000 (39 to 46) | |||||
| Any adverse effects | See comments | No studies reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Publication bias detected by funnel plot. 2 Wide confidence interval crossing the line of no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 7 | 18949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.40] |
| 2 Early or late miscarriage Show forest plot | 4 | 13346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 3 Stillbirth Show forest plot | 7 | 21442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.97, 1.76] |
| 4 Congenital malformations Show forest plot | 5 | 8334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.84, 1.62] |
| 5 Any adverse effects of vitamin supplementation sufficient to stop supplementation Show forest plot | 1 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.58, 2.83] |
| 2 Early or late miscarriage Show forest plot | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.52, 2.65] |
| 3 Stillbirth Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.63, 2.77] |
| 2 Early or late miscarriage Show forest plot | 2 | 790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.79, 1.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 1640 | Risk Ratio (Fixed, 95% CI) | 1.01 [0.61, 1.66] |
| 2 Early or late miscarriage Show forest plot | 2 | 1397 | Risk Ratio (Fixed, 95% CI) | 0.86 [0.46, 1.62] |
| 3 Stillbirth Show forest plot | 3 | 1640 | Risk Ratio (Fixed, 95% CI) | 1.29 [0.57, 2.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 52480 | Risk Ratio (Random, 95% CI) | 1.05 [0.90, 1.23] |
| 2 Early of late miscarriage Show forest plot | 1 | 39668 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.92, 1.04] |
| 3 Stillbirth Show forest plot | 1 | 39668 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 51163 | Risk Ratio (Fixed, 95% CI) | 1.02 [0.98, 1.07] |
| 2 Early or late miscarriage Show forest plot | 1 | 39860 | Risk Ratio (Fixed, 95% CI) | 1.00 [0.94, 1.06] |
| 3 Stillbirth Show forest plot | 1 | 39860 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.98, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 17373 | Risk Ratio (Fixed, 95% CI) | 1.05 [0.91, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1074 | Risk Ratio (Fixed, 95% CI) | 0.80 [0.53, 1.21] |
| 2 Early or late miscarriage Show forest plot | 1 | 1075 | Risk Ratio (Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| 3 Stillbirth Show forest plot | 1 | 1075 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.60, 1.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 10 | 94948 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.93, 1.00] |
| 2 Early or late miscarriage Show forest plot | 10 | 94948 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.94, 1.03] |
| 3 Stillbirth Show forest plot | 10 | 79851 | Risk Ratio (Fixed, 95% CI) | 0.92 [0.85, 0.99] |
| 4 Congenital malformation Show forest plot | 1 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 907 | Risk Ratio (Fixed, 95% CI) | 0.49 [0.34, 0.70] |
| 2 Early or late miscarriage Show forest plot | 1 | 907 | Risk Ratio (Fixed, 95% CI) | 0.89 [0.59, 1.34] |
| 3 Stillbirth Show forest plot | 1 | 907 | Risk Ratio (Fixed, 95% CI) | 0.14 [0.01, 2.77] |
| 4 Congenital malformations Show forest plot | 1 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.53, 4.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1368 | Risk Ratio (Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| 2 Early or late miscarriage Show forest plot | 1 | 1368 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.67, 1.34] |
| 3 Stillbirth Show forest plot | 1 | 1368 | Risk Ratio (Fixed, 95% CI) | 0.33 [0.06, 1.98] |
| 4 Congenital malformations Show forest plot | 1 | 1368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.75, 5.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 5012 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| 2 Early or late miscarriage Show forest plot | 3 | 5012 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.80, 1.18] |
| 3 Stillbirth Show forest plot | 3 | 4316 | Risk Ratio (Fixed, 95% CI) | 1.32 [0.93, 1.88] |
| 4 Congenital malformations Show forest plot | 2 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.72, 4.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1090 | Risk Ratio (Fixed, 95% CI) | 0.90 [0.62, 1.30] |
| 2 Early or late miscarriage Show forest plot | 2 | 1090 | Risk Ratio (Fixed, 95% CI) | 0.89 [0.61, 1.31] |
| 3 Stillbirth Show forest plot | 2 | 1090 | Risk Ratio (Random, 95% CI) | 0.99 [0.04, 22.90] |
| 4 Congenital malformations Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.67, 3.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1644 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.70, 1.33] |
| 2 Early of late miscarriage Show forest plot | 2 | 1644 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.70, 1.33] |
| 3 Stillbirth Show forest plot | 2 | 1644 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.15, 4.10] |
| 4 Congenital malformations Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.76, 3.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1074 | Risk Ratio (Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 5021 | Risk Ratio (Fixed, 95% CI) | 0.83 [0.58, 1.17] |
| 2 Stillbirth Show forest plot | 1 | 5021 | Risk Ratio (Fixed, 95% CI) | 0.83 [0.58, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 823 | Risk Ratio (Fixed, 95% CI) | 0.92 [0.46, 1.83] |
| 2 Early or late miscarriage Show forest plot | 1 | 823 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.26, 4.13] |
| 3 Stillbirth Show forest plot | 1 | 823 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.39, 1.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 6883 | Risk Ratio (Random, 95% CI) | 1.00 [0.75, 1.34] |
| 2 Early or late miscarriage Show forest plot | 3 | 6883 | Risk Ratio (Random, 95% CI) | 0.99 [0.72, 1.38] |
| 3 Stillbirth Show forest plot | 3 | 6883 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.51, 2.10] |
| 4 Congenital malformations Show forest plot | 2 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.81, 3.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 6883 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.34] |
| 2 Early or late miscarriage Show forest plot | 3 | 6883 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.38] |
| 3 Stillbirth Show forest plot | 3 | 6883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.51, 2.09] |
| 4 Congenital malformations Show forest plot | 2 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.81, 3.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.40] |
| 2 Early or late miscarriage Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.44] |
| 3 Stillbirth Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 4.02] |
| 4 Congenital malformations Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.45, 4.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.69, 1.35] |
| 2 Early or late miscarriage Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.70, 1.39] |
| 3 Stillbirth Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.15, 2.96] |
| 4 Congenital malformations Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.71, 5.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.67] |
| 2 Early or late miscarriage Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.80, 1.69] |
| 3 Stillbirth Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.04, 22.55] |
| 4 Congenital malformations Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.28, 3.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.77, 1.62] |
| 2 Early or late miscarriage Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.77, 1.64] |
| 3 Stillbirth Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.97 [0.58, 42.29] |
| 4 Congenital malformations Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.26, 1.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.57] |
| 2 Early or late miscarriage Show forest plot | 2 | 1642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
| 3 Stillbirth Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.02, 28.39] |
| 4 Congenital malformations Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.19, 2.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.59] |
| 2 Early or late miscarriage Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.03] |
| 3 Stillbirth Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [0.74, 226.98] |
| 2 Early or late miscarriage Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [0.74, 226.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early or late miscarriage Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.24, 5.29] |

