流産予防のためのビタミン補充
アブストラクト
背景
流産はよくみられる妊娠合併症で、さまざまな要因で生じる。食事からのビタミン摂取量が少ないと流産リスクが増加することから、妊娠前または妊娠初期のビタミン補充が流産の予防につながる可能性がある。
目的
本レビューの目的は、自然流産のリスクに対するあらゆるビタミン補充の有効性と安全性を評価することである。
検索戦略
Cochrane Pregnancy and Childbirth Group Trials Register(2015年11月6日)を検索し、得られた研究の文献リストを調べた。
選択基準
妊娠中の1つ以上のビタミン補充と、プラセボ、他のビタミン、ビタミンなし、または他の介入を比較したあらゆるランダム化および準ランダム化比較試験。受胎前、受胎前後、または妊娠初期(妊娠20週未満)に補充を開始した試験を選択した。
データ収集と分析
3名のレビュー著者がそれぞれ選択する試験を評価し、データを抽出し、試験の質を評価した。GRADE法を用いてエビデンスの質を評価した。エビデンスの質は、「知見のまとめ」の表にあるアウトカムの数値結果欄に記載した。
主な結果
妊娠20週までに開始したあらゆるビタミン補充について評価し、少なくとも1つの主要アウトカムを報告した合計40件の試験(女性276,820例、妊娠例278,413例)を、本レビューに適格として選択した。8件の試験はクラスターランダム化試験で、合計で女性217,726例および妊娠例219,267例がデータに寄与した。
ランダム化した順番の作成、および治療群とコントロール群の参加者の適切な隠蔽化について、選択した試験の約半数でバイアスのリスクが低いと評価した。
ビタミンCの補充
ビタミンCとビタミンEの併用群と、プラセボ群またはビタミンC無補充群を比較したところ、以下のリスクについて差はみられなかった。総胎児喪失:リスク比(RR)1.14、95% 信頼区間(CI)0.92 ~ 1.40、7件の試験、女性18,949例、エビデンスの質は高い。早期または後期の流産:RR 0.90、95% CI 0.65 ~ 1.26、4件の試験、女性13,346例、エビデンスの質は中等度。死産:RR 1.31、95% CI 0.97 ~ 1.76、7件の試験、女性21,442例、エビデンスの質は中等度。ビタミン補充に関する有害作用:RR 1.16、95% CI 0.39 ~ 3.41、1件の試験、女性739例、エビデンスの質は中等度。ビタミンCと他のいずれかの併用群と、プラセボ群またはビタミンC無補充群を比較したところ、総胎児喪失や流産のリスクに明白な差はみられなかった。
ビタミンAの補充
ビタミンA+鉄+葉酸の併用群と、プラセボ群またはビタミンA無補充群を比較したところ、以下のリスクに差はみられなかった。総胎児喪失:RR 1.01、95% CI 0.61 ~ 1.66、3件の試験、女性1640例、エビデンスの質は低い。早期または後期の流産:RR 0.86、95% CI 0.46 ~ 1.62、2件の試験、女性1397例、エビデンスの質は低い。死産:RR 1.29、95% CI 0.57 ~ 2.91、3件の試験、女性1640例、エビデンスの質は低い。ビタミンAと他のいずれかの併用群と、プラセボ群またはビタミンA無補充群を比較したところ、総胎児喪失や流産のリスクについて、差を示すエビデンスはなかった。
マルチビタミンの補充
マルチビタミン+鉄+葉酸の併用群と、鉄や葉酸の単独群を比較したところ、死産のリスク低下を示すエビデンスがあった(RR 0.92、95% CI 0.85 ~ 0.99、10件の試験、女性79,851例、エビデンスの質は高い)。以下の女性で総胎児喪失が減少した。葉酸を含まないマルチビタミン群:RR 0.49、95% CI 0.34 ~ 0.70、1件の試験、女性907例。マルチビタミンとビタミンAの併用群およびマルチビタミン単独群:RR 0.60、95% CI 0.39 ~ 0.92、1件の試験、女性1074例。しかし、これらの知見はそれぞれ、少数の女性による1件の試験によるものであった。また、ビタミンAまたはプラセボを補充した女性も対象とした群間比較を行った研究も含むため、解釈には注意を要する。
マルチビタミン+鉄+葉酸の併用群と、鉄や葉酸の単独群を比較したところ、以下のリスクに差はみられなかった。総胎児喪失:RR 0.96、95% CI 0.93 ~ 1.00、10件の試験、女性94,948例、エビデンスの質は高い。早期または後期の流産:RR 0.98、95% CI 0.94 ~ 1.03、10件の試験、女性94,948例、エビデンスの質は中等度。
マルチビタミンと他のいずれかの併用群と、プラセボ群、葉酸群、またはビタミンA群を比較したところ、総胎児喪失や流産のリスクについて、差を示すエビデンスはなかった。
葉酸の補充
葉酸+マルチビタミン+鉄の併用群または葉酸+鉄の併用群または葉酸単独群と、葉酸非補充群を比較したところ、総胎児喪失、早期または後期の流産、死産、先天性奇形のリスクについて、差を示すエビデンスはなかった。
抗酸化ビタミンの補充
抗酸化物質投与群と抗酸化物質低用量群を比較したところ、早期または後期の流産について、差を示すエビデンスはなかった(RR 1.12、95% CI 0.24 ~ 5.29、1件の試験、女性110例)。
著者の結論
妊娠前や妊娠初期のビタミンサプリメントの摂取は、流産を予防しない。しかし、マルチビタミン+鉄+葉酸を補充した女性では死産のリスクが低下することが、エビデンスにより示された。ビタミンのさまざまな併用について、流産や流産に関連するアウトカムへの効果を調べるにはエビデンスが不十分である。
PICO
一般語訳
流産予防のためのビタミン補充
論点
流産は妊婦によくみられるが、原因が不明なこともしばしばある。ビタミン不足の偏った食事は、妊娠初期に胎児を失うリスクを増加させる。妊娠前や妊娠中のビタミン補充は自然流産のリスクを低下させるのか?補充により母親、出生、および乳児のアウトカムが改善するのか?副作用はあるのか?
重要である理由
ビタミン補充は、妊婦や妊娠を計画する女性に広く推奨されている。妊娠前および妊娠中のビタミン補充の普及を考えると、ビタミン補充と早期の妊娠アウトカムの関連性について研究することは重要である。特に、流産の原因が不明であることや、母親の栄養状態が胎児の発達に影響する可能性があることから、研究の重要性が認められる。
どのようなエビデンスが得られたか?
本レビューでは、女性276,820例および妊娠例278,413例を対象とした40件のランダム化比較試験を選択した。いずれかのビタミンを補充しても、流産する女性の人数は減少しない。しかし、マルチビタミン+鉄+葉酸の併用群では、鉄や葉酸の単独群と比較して死産のリスクが低下した。葉酸なしのマルチビタミン群、マルチビタミン群、マルチビタミン+ビタミンA群では総胎児喪失が減少したが、これらの知見はそれぞれ、少数の女性による1件の試験によるものであった。また、ビタミンAまたはプラセボを補充した女性も対象とした群間比較を行った研究も含むため、解釈には注意を要する。
意味するもの
妊娠前や妊娠初期のビタミンサプリメントの摂取は有益な可能性があるが、本レビューでは、ビタミンサプリメントの摂取が流産を予防することを示す十分なエビデンスはみられなかった。
Authors' conclusions
Summary of findings
| Vitamin C plus vitamin E versus control for preventing miscarriage | ||||||
| Population: pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Vitamin C plus vitamin E | |||||
| Total fetal loss | Study population | RR 1.14 | 18,949 | ⊕⊕⊕⊕ | ||
| 17 per 1000 | 20 per 1000 | |||||
| Moderate | ||||||
| 14 per 1000 | 16 per 1000 | |||||
| Early or late miscarriage | Study population | RR 0.90 | 13,346 | ⊕⊕⊕⊝ | ||
| 9 per 1000 | 9 per 1000 | |||||
| Moderate | ||||||
| 8 per 1000 | 8 per 1000 | |||||
| Stillbirth | Study population | RR 1.31 | 21,442 | ⊕⊕⊕⊝ | ||
| 8 per 1000 | 10 per 1000 | |||||
| Moderate | ||||||
| 7 per 1000 | 9 per 1000 | |||||
| Any adverse effects of vitamin supplementation sufficient to stop supplementation | Study population | RR 1.16 | 739 | ⊕⊕⊕⊝ | ||
| 16 per 1000 | 19 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not effective but 95% CI is narrow and precise. 2 Non significant with wide 95% CI. 3 Small sample size. | ||||||
| Vitamin A plus iron plus folate versus iron plus folate for preventing miscarriage | ||||||
| Population: pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Vitamin A | |||||
| Total fetal loss (including miscarriages or combined miscarriages and stillbirths) ‐ Vitamin A + iron + folate versus iron + folate | Study population | RR 1.01 | 1640 | ⊕⊕⊝⊝ | ||
| 37 per 1000 | 37 per 1000 | |||||
| Moderate | ||||||
| 59 per 1,000 | 60 per 1,000 (36 to 98) | |||||
| Early or late miscarriage ‐ Vitamin A + iron + folate versus iron + folate | Study population | RR 0.86 | 1397 | ⊕⊕⊝⊝ | ||
| 31 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| 50 per 1,000 | 43 per 1,000 (23 to 80) | |||||
| Stillbirth ‐ Vitamin A + iron + folate versus iron + folate | Study population | RR 1.29 | 1640 | ⊕⊕⊝⊝ | ||
| 13 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 21 per 1,000 | 27 per 1,000 (12 to 61) | |||||
| Any adverse effects | See comments. | No studies reported this outcome. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High and unclear risk of attrition bias. 2 Wide 95% CI. | ||||||
| Multivitamin plus iron plus folate versus iron plus folate for preventing miscarriage | ||||||
| Population: pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Multivitamin | |||||
| Total fetal loss (including miscarriages or combined miscarriages and stillbirths) ‐ Multivitamins + iron + folic acid versus iron + folic acid | Study population | RR 0.96 | 94,948 | ⊕⊕⊕⊕ | ||
| 136 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 218 per 1,000 | 209 per 1,000 (202 to 218) | |||||
| Early or late miscarriage ‐ Multivitamin + iron + folic acid versus iron + folic acid | Study population | RR 0.98 | 94948 | ⊕⊕⊕⊝ | ||
| 84 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 134 per 1,000 | 132 per 1,000 (126 to 138) | |||||
| Stillbirth ‐ Multivitamin + iron + folic acid versus iron + folic acid | Study population | RR 0.92 | 79,851 | ⊕⊕⊕⊕ | ||
| 29 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| 46 per 1,000 | 43 per 1,000 (39 to 46) | |||||
| Any adverse effects | See comments | No studies reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Publication bias detected by funnel plot. 2 Wide confidence interval crossing the line of no effect. | ||||||
Background
Description of the condition
Miscarriage can be caused by a wide range of factors, and determining the aetiology is often difficult given the variety of underlying mechanisms potentially responsible. Consideration of the timing of the miscarriage is also important, as diverse causes of miscarriage manifest at different periods of gestation. The most common causes include abnormal chromosomal rearrangements, endocrinological disorders and uterine abnormalities (Garrido‐Gimenez 2015). Early miscarriages are mostly associated with chromosomal abnormalities, defective placental development and maternal disease conditions; while late miscarriages are more likely due to structural problems of the uterus and/or cervix such as cervical incompetence. Women experiencing recurrent miscarriage often have an underlying medical condition such as autoimmune disease, i.e. systemic lupus erythematosus and antiphospholipid syndrome, or other blood clotting disorders such as hyperhomocysteinaemia (high levels of homocysteine in the blood) or another thrombophilia (Preston 1996). Other risk factors for miscarriage include higher maternal age at conception, multiple pregnancies and a history of previous miscarriage (Baba 2011; Garcıa‐Enguıdanos 2002; Hure 2012). Behavioural factors including alcohol consumption (Maconochie 2007), smoking (Baba 2011; Hure 2012), use of illicit drugs (Garcıa‐Enguıdanos 2002), and exposure to non‐steroidal anti‐inflammatory drugs (NSAIDs) around the time of conception are also suggested causes of miscarriage (Li 2003; Nielsen 2001). While several factors may promote miscarriage, for a great proportion of women, no cause can be found.
In clinical practice, surgical and non‐surgical interventions are used in the management of miscarriage. Bed rest, commonly prescribed for preventing miscarriage, is lacking proven benefit (Aleman 2005). Similarly, there is currently insufficient evidence on the benefit provided from the use of uterine muscle relaxant drugs (Lede 2005), Chinese herbal medicine (Li 2012), hormones (Devaseelan 2010; Haas 2013; Lim 1013; Morley 2013), and immunotherapy (Wong 2014).
Description of the intervention
Vitamins are essential nutrients required in the body for numerous functions such as to ensure normal metabolism, physical growth and development as well as to prevent diseases. Based on evidence from observational studies, vitamin supplementation has been advocated for the prevention of miscarriage (Hasan 2009; Maconochie 2007), most commonly folate and B vitamins. Due to consistent associations between pregnancy complications and decreased antioxidant defence and infections, it has been suggested that vitamin supplementation during pregnancy might provide protection against adverse pregnancy outcomes and may influence the risk of spontaneous miscarriage in women.
How the intervention might work
Vitamins are either water soluble – such as vitamin C and the B group vitamins (including folate) or fat soluble such as vitamins A, D, E and K. They may be obtained directly from the diet or in the form of dietary supplements of either individual vitamin or multivitamin preparations. Multivitamins contain a range of vitamins and minerals, usually in doses similar to the recommended dietary intakes.
The rationale for vitamin supplementation for the prevention of miscarriage is based on epidemiological studies linking healthy dietary patterns with reduced risk for miscarriage (Hasan 2009; Maconochie 2007). Several studies have reported an association between certain vitamin deficiencies and adverse reproductive outcomes (George 2002; Guerra‐Shinohara 2010; Hübner 2008; Reznikoff‐Etiévant 2002). Nutritional mechanisms underlying this association include homocysteine metabolism and oxidative stress.
Homocysteine is an amino acid that is involved in several key metabolic processes, vital to ongoing cellular activity of the living organism. The metabolism of homocysteine is facilitated by B vitamins and folate. The concentration of homocysteine in the blood is determined by various dietary factors, including folate, vitamin B6 and vitamin B12. Disturbance of maternal and fetal homocysteine metabolism has been associated with various obstetric conditions including miscarriage (Hague 2003; Nelen 2000), and hyperhomocysteinaemia is considered a risk factor for recurrent early pregnancy loss. Therefore, supplementation with B vitamins and folate may influence the risk of spontaneous miscarriage in women with recurrent miscarriage. Moreover, low serum vitamin B12 concentrations have been reported in women with recurrent miscarriage (Hübner 2008). Evidence on the effect of vitamin supplementation, particularly folic acid, on risk of miscarriage is still conflicting; however the few studies that have adjusted for confounding support a protective effect.
Oxidative stress is caused by an imbalance between pro‐oxidants and antioxidants. Pro‐oxidants act either by generating reactive oxygen species (ROS) or by inhibiting antioxidant systems. In living cells, ROS are formed continuously both from biochemical processes occurring in the body and in reaction to external factors. Excessive ROS production may however, overpower the body’s natural antioxidant defence system, creating an environment unsuitable for normal female reproductive processes (Al‐Gubory 2010). A recent review of evidence from experimental and observational studies suggests that oxidative stress is an important cause in spontaneous and recurrent miscarriage (Agarwal 2012b; Al‐Gubory 2010; Gupta 2007). Adequate maternal antioxidant status before and during pregnancy could prevent and control oxidative stress. Therefore, intake of antioxidant vitamins such as vitamin C and vitamin E may be an important factor to reduce the risk of miscarriage. In a population‐based case‐control study, vitamin supplementation (including vitamin C), and eating fresh fruits and vegetables daily were associated with reduced risk of miscarriage (Maconochie 2007). Another observational study demonstrated an association between the risk of spontaneous early miscarriage and dietary factors; poor intake of green vegetables, fruit and dairy products coupled with a high intake of fat was associated with a high risk of spontaneous miscarriage (Di Cintio 2001). There is limited information available about the impact of vitamins on the risk of early versus late miscarriage. However, dietary factors could theoretically influence structural abnormalities such as cervical incompetence. There is a growing body of research investigating the relationship between nutrition and placental development, fetal growth, pregnancy outcomes and adult diseases (McMillen 2008; Meher 2015; Wu 2004). Therefore, adequate maternal nutrition, particularly vitamin intake, may be an important factor in preventing spontaneous miscarriage. Currently, little information is available about the most appropriate vitamin type or combination. Similarly, many commercially available vitamin preparations contain a range of combinations of vitamins. Therefore, this review will cover all vitamin types.
Why it is important to do this review
Vitamin supplementation is frequently recommended for pregnant women and women planning to conceive. The documented benefits of supplementation relate mainly to the lowered risk of congenital anomalies such as neural tube defects (Hovdenak 2012; MRC Vitamin Study Research Group 1991). Given the widespread vitamin supplementation before and during pregnancy, studying the relationship between this common exposure and early pregnancy outcomes is of great value, particularly since the causes of miscarriage are unknown and this exposure is known to affect specific developmental processes.
This is an update of a Cochrane review first published in 2005 and previously updated in 2011. The previously updated review included 28 trials involving 96,674 women (98,267 pregnancies (Rumbold 2011)). Based on available evidence, Rumbold 2011 concluded that taking any vitamin supplements prior to pregnancy or in early pregnancy does not prevent women from experiencing miscarriage or stillbirth. However, there is insufficient evidence to examine the effects of different combinations of vitamins on miscarriage. In the current review, we examined the effect of different vitamin combinations on the risk of miscarriage. The scope of the current update has been restricted to look at miscarriage and miscarriage‐related outcomes.
Objectives
-
To determine the effectiveness and safety of any vitamin supplementation taken by women prior to conception, periconceptionally and in early pregnancy on the risk of spontaneous miscarriage.
-
If vitamins are effective, to determine which of these agents are best and to compare vitamins with other interventions.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials (including individual‐ and cluster‐randomised) and quasi‐randomised trials comparing one or more vitamins with either placebo, other vitamins, no vitamins or other interventions, prior to conception, periconceptionally or in early to mid pregnancy. Cross‐over trials were not included.
Types of participants
Pregnant women (less than 20 weeks' gestation) or women in the reproductive age group planning on becoming pregnant in the near future, regardless of whether they are at low or high risk of having a miscarriage. No restrictions were placed on the age of participants or past obstetric history.
Types of interventions
Comparisons of specific vitamin(s), alone or in combination with other agents with either placebo, other vitamin(s), no vitamin(s) or other interventions for the prevention of miscarriage, either in areas where there is an inadequate dietary intake or where there is a presumed adequate intake of that vitamin(s).
The review authors deemed it important to include any supplementation trials, where supplementation began prior to 20 weeks' gestation, and where at least one miscarriage‐related outcome as specified in the review was reported, even if the intervention was not specifically for the prevention of miscarriage. We excluded trials where the onset of supplementation occurred definitely after 20 weeks' gestation or where it was reported that the majority of women commenced supplementation after 20 weeks' gestation. We included trials where the onset of supplementation occurred both prior to and after 20 weeks' gestation, and when it could not be established whether the majority of the women started supplementation prior to 20 weeks' gestation.
Types of outcome measures
The scope of the current update has been restricted to look at miscarriage and miscarriage‐related outcomes.
Primary outcomes
-
Total fetal loss, defined as the combined numbers of early miscarriage (spontaneous pregnancy loss less than 12 weeks' gestation), late miscarriage (spontaneous pregnancy loss greater than or equal to 12 and less than 24 weeks), and stillbirth (pregnancy loss at greater than or equal to 24 weeks).
-
Early or late miscarriage.
To overcome wide variation in the definitions of miscarriage and stillbirth between studies, we included the combined outcome 'total fetal loss' in the review.
Secondary outcomes
-
Stillbirth.
-
Congenital malformations.
-
Adverse effects of vitamin supplementation sufficient to stop supplementation, such as manifestations of hypervitaminosis, headache, nausea, vomiting, diarrhoea
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (6 November 2015).
The Register is a database containing over 21,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group's Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
[We carried out additional author searching in an earlier version of this review (Rumbold 2005), seeAppendix 1 for details]
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Rumbold 2011.
For this update, the following methods were used for assessing the 90 reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed all the potential studies identified as a result of the search strategy for inclusion. Disagreements were resolved through discussion and, when required, we consulted a third person. We created a study flow diagram to map out the number of records identified, included and excluded.
Data extraction and management
We collected data from the selected studies using a pre‐designed data extraction form. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion. If discrepancies could not be resolved, we consulted a third review author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We describe for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes in the comparisons: 1) vitamin C and vitamin E versus placebo, 2) vitamin A plus iron plus folate versus iron plus folate and 3) multivitamin plus iron plus folate versus iron plus folate.
-
Total fetal loss, defined as the combined numbers of early miscarriage (spontaneous pregnancy loss less than 12 weeks’ gestation), late miscarriage (spontaneous pregnancy loss greater than or equal to 12 and less than 24 weeks), and stillbirth (pregnancy loss at greater than or equal to 24 weeks).
-
Early or late miscarriage.
-
Stillbirth.
-
Adverse effects of vitamin supplementation sufficient to stop supplementation.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence was downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we planned to use the mean difference (MD) if outcomes were measured in the same way between trials and, if appropriate, the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Where trials recruited women prior to becoming pregnant, we reported the denominators for each trial as all women randomised; or where there was accurate information about the number of women in each trial who became pregnant, we reported the denominators as the number of women randomised with confirmed pregnancy.
We included all included trials in the initial analysis which we performed by any vitamin to include all vitamin combinations and then by individual vitamin type.
Cluster‐randomised trials
We included cluster‐randomised trials in the analyses along with individually‐randomised trials. We used the effect estimates and uncertainty range from the cluster trials to perform the meta‐analysis using the generic inverse variance approach for the meta‐analysis of dichotomous outcomes where trials using cluster‐randomisation techniques were included (Alderson 2004).
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We applied tests of heterogeneity between trials to assess the significance of any differences between trials in the analyses. We regarded heterogeneity as substantial if the I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the average of the range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, we have presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses and to consider whether an overall summary was meaningful, and if it was, to use random‐effects analysis to produce it.
In this update, we were not able to carry out the following subgroup analyses:
-
the dose of vitamin(s) (below or above the recommended dietary intake); and the duration of vitamin usage, based on time of trial entry: before pregnancy, < 12 weeks’ gestation, between 12‐20 weeks’ gestation or ’mixed’, which included women enrolled before and after 20 weeks’ gestation;
-
their risk of spontaneous miscarriage (high risk defined as the presence of medical conditions associated with miscarriage such as hyperhomocysteinaemia, thrombophilia, antiphospholipid syndrome, systemic lupus erythematosus; low risk defined as none of the above conditions); their risk of recurrent miscarriage (high risk defined as two or more previous consecutive spontaneous miscarriages, and/or the presence of medical conditions associated with miscarriage such as hyperhomocysteinaemia, thrombophilia, antiphospholipid syndrome, systemic lupus erythematosus; low risk defined as none of the above conditions);
-
low or adequate dietary vitamin intake at trial entry (low intake defined as less than the recommended daily intake for each vitamin in that setting, as measured by dietary questionnaire).
We would have included all outcomes in the subgroup analysis.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014) and to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analysis to explore the effects of trial quality and type of randomisation on the primary outcomes related to fetal loss (total fetal loss and early or late miscarriage). We included only trials with 'adequate' rating on allocation concealment, We considered these trials to be of high quality. We also carried out sensitivity analysis by excluding cluster‐randomised trials and comparing the results of cluster‐randomised trials with the individually‐randomised trials.
Results
Description of studies
See tables Characteristics of included studies and Characteristics of excluded studies for details of individual studies.
Included studies
For the 2016 update, we included a total of 40 trials (involving 276,820 women) assessing supplementation with specific vitamin(s) starting prior to 20 weeks' gestation. Many of the trials assessed interventions not specifically for the prevention of miscarriage, however, the authors included any supplementation trials, where supplementation began prior to 20 weeks' gestation, and where at least one miscarriage‐related outcome as specified in the review was reported.
Participants
The demographic and obstetric characteristics of the women varied widely between the trials (see table Characteristics of included studies). The 40 included trials contributed data for analysis from 276,820 women. Eight of the 40 included studies were cluster‐randomised trials including 217,726 women (Bhutta 2009; Katz 2000; Summit 2008; Sunawang 2009; West 2011; West 2014; Zagre 2007; Zeng 2008). Two of the trials from the previous version of this review (one cluster (Katz 2000), and one small trial (Roberfroid 2008)) included women who were pregnant more than once in the study period; resulting in data contributing to 59,146 pregnancies for the individual trials and 219,267 pregnancies from the cluster trials. Five trials enrolled women prior to conception (Czeizel 1994; Hemmi 2003; ICMR 2000; Kirke 1992; MRC 1991) and asked women to continue taking the supplements up until the second or third missed menstrual period. One trial (Katz 2000) supplemented women from before conception, through pregnancy and postpartum for a total of 3.5 years postpartum. Another eight trials enrolled women in the first trimester (Briscoe 1959; Hans 2010; Rumiris 2006; Tofail 2008; West 2011; West 2014; Wibowo 2012; Zagre 2007), and 24 trials in early to mid pregnancy (Bhutta 2009; Chappell 1999; Fawzi 1998; Fawzi 2007; Fleming 1968; Fleming 1986; Kumwenda 2002; McCance 2010; Osrin 2005; People's League 1942; Poston 2006; Prawirohartono 2011; Roberfroid 2008; Roberts 2010; Rumbold 2006; Rush 1980; Schmidt 2001; Spinnato 2007; Steyn 2003; Sunawang 2009; Van den Broek 2006; Villar 2009; Xu 2010; Zeng 2008). Some of the trials enrolling women in early to mid pregnancy included women enrolled at or after 20 weeks' gestation (Chappell 1999; Fawzi 1998; Fawzi 2007; Fleming 1968; Fleming 1986; Kumwenda 2002; McCance 2010; Osrin 2005; People's League 1942; Roberfroid 2008; Rumbold 2006; Rush 1980; Schmidt 2001; Spinnato 2007; Steyn 2003; Van den Broek 2006; Villar 2009; Zeng 2008). One trial (Summit 2008), enrolled 41,839 women at 'any gestational age', although more than 70% of the women were enrolled in the first or second trimester.
Two trials (Fawzi 1998; Kumwenda 2002) involved vitamin A supplementation in women seropositive for the Human Immunodeficiency Virus (HIV); one trial (Poston 2006) involved only women with clinical risk factors for pre‐eclampsia, while one trial (McCance 2010), limited the eligibility to women with type1 diabetes. Roberts 2010 involved only nulliparous women.
Baseline characteristics of women enrolled in the intervention group and control group were comparable in all the trials except two (Xu 2010; Zagre 2007). In Xu 2010, there was a slightly higher proportion of women with multiple pregnancies in the placebo group; while in Zagre 2007, women in the control group tended to be poorer and less educated, while women in the intervention group had larger households and used more preventive measures against malaria.
The trials were conducted in both resource‐rich and resource‐poor countries including the United States (Briscoe 1959; Roberts 2010; Rush 1980), Canada (Xu 2010), the United Kingdom (Chappell 1999; McCance 2010; People's League 1942; Poston 2006), Hungary (Czeizel 1994), Tanzania (Fawzi 1998; Fawzi 2007), Nigeria (Fleming 1968; Fleming 1986), Burkino Faso (Roberfroid 2008), Japan (Hemmi 2003), India (ICMR 2000), Nepal (Katz 2000; Osrin 2005), the Republic of Ireland (Kirke 1992), Uganda (Hans 2010), Bangladesh (West 2011; West 2014, Tofail 2008), China (Zeng 2008), Niger (Zagre 2007), Pakistan (Bhutta 2009), Australia (Rumbold 2006), Brazil (Spinnato 2007), Mexico (Xu 2010), Malawi (Kumwenda 2002; Van den Broek 2006), Indonesia (Prawirohartono 2011; Rumiris 2006; Schmidt 2001; Sunawang 2009; Summit 2008; Wibowo 2012), and South Africa (Steyn 2003). One trial involved 33 international centres (MRC 1991) and another trial was a multi‐country study involving India, Peru, South Africa and Vietnam (Villar 2009).
Interventions
The 40 trials assessed a range of vitamin supplements, alone or in combination with other supplements. The vitamins included vitamin A, alone or with iron, folic acid, multivitamins, or β‐carotene (Fawzi 1998; Katz 2000; Kumwenda 2002; Prawirohartono 2011; Schmidt 2001; Van den Broek 2006; West 2011); vitamin C with or without multivitamins or vitamin E (Briscoe 1959; Chappell 1999; Hans 2010; Hemmi 2003; McCance 2010; Poston 2006; Roberts 2010; Rumbold 2006; Spinnato 2007; Steyn 2003; Villar 2009; Xu 2010); folic acid with or without multivitamins and/or iron (Czeizel 1994; Fleming 1968; Fleming 1986; ICMR 2000; Kirke 1992; MRC 1991); antioxidant vitamins (Wibowo 2012); multivitamins with/without folic acid, vitamin A, vitamin E or iron and folic acid (Bhutta 2009; Czeizel 1994; Fawzi 1998; Fawzi 2007; ICMR 2000; Kirke 1992; MRC 1991; Osrin 2005; Roberfroid 2008; Rumiris 2006; Rush 1980; Sunawang 2009; Summit 2008; Tofail 2008; West 2014; Zagre 2007; Zeng 2008); and multivitamins alone (People's League 1942; Rush 1980). The doses of vitamins were similar for the vitamin C supplementation trials (range 400 mg to 1000 mg). However, they varied widely between trials for the folic acid (range 0.3 mg to 10 mg), multivitamins and vitamin A trials (range 5000 international units (IU) to 23,300 IU). The components of MMN supplementation were different among the trials but all of them contained iron and folate in the MMN supplements. All supplements were taken orally from the enrolment until delivery or up to 3.5 years postpartum.
Outcomes
Main outcomes
Thirty‐six trials reported pregnancy loss as miscarriage or stillbirth. The outcome 'total fetal loss' included both miscarriage or stillbirth, and overcame problems with different definitions of miscarriage and stillbirth. There was no consistency amongst trials with regards to the definition of miscarriage. For some trials, miscarriage was considered to occur up until 26 or 28 weeks' gestation, while other studies reported miscarriage as pregnancy loss prior to 20 weeks' gestation. Other studies did not specify their definition of miscarriage or stillbirth.
Other outcomes
There was no consistency amongst trials with regards to the definition of stillbirth. In some trials, stillbirth was considered as pregnancy loss greater than or equal to 20 weeks' gestation, while some trials considered stillbirth as pregnancy loss beyond 24 weeks' gestation. Thirty trials reported stillbirth as an outcome. Only one trial (Spinnato 2007) reported on adverse effects of vitamin sufficient to stop supplementation, while congenital malformations was reported in nine trials (Czeizel 1994; Kirke 1992; McCance 2010; MRC 1991; Osrin 2005; Poston 2006; Spinnato 2007; Villar 2009; Xu 2010).
Excluded studies
We excluded 48 trials, of which 16 trials reported no clinically relevant data, or data in a format suitable for inclusion (Christian 2003; Chelchowska 2004; Correia 1982; Hibbard 1969; Laurence 1981; Lira 1989; Meirinho 1987; Mock 2002; Moldenhauer 2002; Semba 2001; Suharno 1993; Tanumihardjo 2002; Taylor 1982; Thauvin 1992; Villamor 2002; Vutyavanich 1995). Seven trials did not clearly report the gestational age when supplementation was started (Biswas 1984; Fletcher 1971; Hampel 1974; Lumeng 1976; Schuster 1984; Trigg 1976; Young 2015), and for two trials, the majority of women were enrolled after 20 weeks and did not report outcomes separately for women starting supplementation prior to 20 weeks (Ferguson 1955; Giles 1971). Thirteen trials (Baumslag 1970; Blot 1981; Chanarin 1968; Colman 1974; Coutsoudis 1999; Dawson 1962; Edelstein 1968; Feyi‐Waboso 2005; Hankin 1966; Kaestel 2005; Marya 1981; Metz 1965; Owen 1966) reported supplementation after 20 weeks' gestation. One trial (Ross 1985) did not specify the contents of the supplements; in five trials all women were given a vitamin supplement (Hekmatdoost 2011; Hunt 1984; Huybregts 2009; Shu 2002; Wehby 2012); one trial was a food intervention (Potdar 2014) and two were non‐randomised (Smithells 1981; Ulrich 1999).
Three other trials (Beazley 2005; Chaudhuri 1969; Rivas‐Echeverria 2000) supplemented women for the prevention of pre‐eclampsia, and did not report any outcomes related to pregnancy loss. These trials are covered in the Cochrane review 'Antioxidants for preventing pre‐eclampsia' (Rumbold 2008).
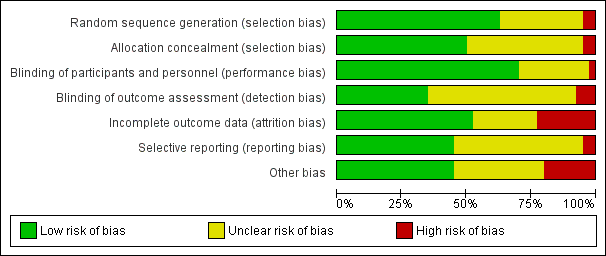
Risk of bias in included studies
Figure 1 and Figure 2 illustrate that the trials were of variable quality. Two trials (Fleming 1968; People's League 1942) used quasi‐random allocation methods involving alternate allocation of participants. Similarly, eight trials (Bhutta 2009; Katz 2000; Summit 2008; Sunawang 2009; West 2011; West 2014; Zagre 2007; Zeng 2008) used cluster randomisation.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation: 25 trials adequately randomised the participants to the intervention and control groups and were judged to be of low risk of bias (Bhutta 2009; Chappell 1999; Hans 2010; Kirke 1992; Kumwenda 2002; McCance 2010; MRC 1991; Osrin 2005; Poston 2006; Prawirohartono 2011; Roberfroid 2008; Roberts 2010; Rumbold 2006; Rumiris 2006; Spinnato 2007; Steyn 2003; Summit 2008; Tofail 2008; Van den Broek 2006; Villar 2009; West 2011; West 2014; Wibowo 2012; Xu 2010; Zeng 2008). The method used for random sequence generation was unclear in 13 trials (Briscoe 1959; Czeizel 1994; Fawzi 1998; Fawzi 2007; Fleming 1986; Hemmi 2003; ICMR 2000; Jauniaux 2004; Katz 2000; Rush 1980; Schmidt 2001; Sunawang 2009; Zagre 2007), because methodological details were not reported or not clearly described. The remaining two trials Fleming 1968 and People's League 1942 used quasi‐randomised methods and were rated as high risk of bias for sequence generation.
Allocation concealment: 20 trials were assessed to have a low risk of bias for adequate concealment of participants to treatment and control groups (Bhutta 2009; Chappell 1999; Fawzi 2007; Kirke 1992; Kumwenda 2002; McCance 2010; MRC 1991; Roberfroid 2008; Rumbold 2006; Rumiris 2006; Spinnato 2007; Steyn 2003; Summit 2008; Tofail 2008; Van den Broek 2006; Villar 2009; West 2011; West 2014; Zagre 2007; Zeng 2008). In 18 trials the method for allocation concealment was not described or not clearly described (Briscoe 1959; Czeizel 1994; Fawzi 1998; Fleming 1986; Hans 2010; Hemmi 2003; ICMR 2000; Jauniaux 2004; Katz 2000; Osrin 2005; Poston 2006; Prawirohartono 2011; Roberts 2010; Rush 1980; Schmidt 2001; Sunawang 2009; Wibowo 2012; Xu 2010). In two trials, allocation was not concealed and therefore judged as high risk of bias (Fleming 1968; People's League 1942).
Blinding
Participants and personnel: 28 trials were assessed as having a low risk of performance bias and reported blinding of participants and personnel to the treatment allocation (Bhutta 2009; Briscoe 1959; Chappell 1999; Fawzi 1998; Fawzi 2007; Fleming 1968; Fleming 1986; Katz 2000; Kirke 1992; McCance 2010; MRC 1991; Poston 2006; Prawirohartono 2011; Roberfroid 2008; Roberts 2010; Rumbold 2006; Rumiris 2006; Spinnato 2007; Summit 2008; Tofail 2008; Van den Broek 2006; Villar 2009; West 2011; West 2014; Wibowo 2012; Xu 2010; Zagre 2007; Zeng 2008). Another 11 trials were judged to have an unclear risk of bias because no or not enough information were provided (Czeizel 1994; Hans 2010; Hemmi 2003; ICMR 2000; Jauniaux 2004; Kumwenda 2002; Osrin 2005; People's League 1942; Rush 1980; Schmidt 2001; Steyn 2003). Sunawang 2009 was rated as high risk of bias as personnel (but not participants) were aware of participants' allocation due to the different appearance of the supplements.
Outcome assessment: blinding of outcome assessors was clearly stated in 14 trials (Bhutta 2009; Chappell 1999; Fawzi 2007; Fleming 1968; Fleming 1986; McCance 2010; Prawirohartono 2011; Roberts 2010; Summit 2008; Tofail 2008; Villar 2009; West 2011; West 2014; Xu 2010) and unclear in 23 trials (Briscoe 1959; Czeizel 1994; Fawzi 1998; Hans 2010; Hemmi 2003; ICMR 2000; Jauniaux 2004; Katz 2000; Kumwenda 2002; MRC 1991; People's League 1942; Poston 2006; Roberfroid 2008; Rumbold 2006; Rumiris 2006; Rush 1980; Schmidt 2001; Spinnato 2007; Steyn 2003; Van den Broek 2006; Wibowo 2012; Zagre 2007; Zeng 2008). In Kirke 1992; Osrin 2005 and Sunawang 2009 outcome assessors were not blinded to the allocation code and the trials were rated as high risk of detection bias.
Incomplete outcome data
Loss to follow‐up ranged from no loss at all in Briscoe 1959; Rumbold 2006; Rumiris 2006; Steyn 2003 to over 20% in Fleming 1968; ICMR 2000; Summit 2008 and Van den Broek 2006. Incomplete outcome data was judged low risk of bias in 21 trials and high in nine trials. Ten trials were rated as unclear risk of bias due to missing information about loss of follow‐up.
Selective reporting
Eighteen trials were considered to have a low risk of reporting bias. Another 20 trials were assessed as unclear risk of bias because of unavailability of trial registration or protocol (Bhutta 2009; Rush 1980), variations between the protocol and the publication (Poston 2006), due to insufficient details provided about methods or selective reporting (Briscoe 1959; Fleming 1968; Hans 2010; Hemmi 2003; ICMR 2000; Kirke 1992; MRC 1991; People's League 1942; Roberfroid 2008; Steyn 2003; Villar 2009; Zagre 2007), variations of information between serial publications (Czeizel 1994; Fawzi 1998; Katz 2000; Schmidt 2001), or the trial was stopped before completion (Jauniaux 2004). The remaining two trials were at high risk of reporting bias; in Fleming 1986, not all outcomes were reported for all treatment groups and in McCance 2010, fewer outcomes were stated in the trial registration compared with the report.
Other potential sources of bias
In 18 trials, other sources of bias were not detected and these trials were rated as low risk of bias. Fourteen trials provided only limited methodological details to exclude other sources of bias and were judged as unclear (Briscoe 1959; Czeizel 1994; Fawzi 1998; Fleming 1968; Fleming 1986; ICMR 2000; Jauniaux 2004; Kumwenda 2002; People's League 1942; Prawirohartono 2011; Rush 1980; Schmidt 2001) as well as Rumiris 2006, were participants in the control and intervention group slightly differed in systolic blood pressure at baseline. Additionally, Zeng 2008 was rated as unclear due to an imbalanced number of excluded clusters across the intervention groups, which may have been due to important baseline differences. The remaining eight trials were at high risk of bias. In Bhutta 2009, the distribution of study participants across the urban and rural areas is unclear from the text and no adjustments were made for cluster design. In Hemmi 2003, no placebo was used in the control group. Two trials (Katz 2000; Roberfroid 2008) used the total number of pregnancies during the study period as dominator and not the total number or randomised women. In Kirke 1992 and MRC 1991, the trials were terminated at an earlier stage and in Steyn 2003 the outcomes resulted from an interim analysis. In Zagre 2007, the participants in the control and interventions groups differed substantially in their baseline characteristics.
Effects of interventions
See: Summary of findings for the main comparison Vitamin C and vitamin E versus placebo for preventing miscarriage; Summary of findings 2 Vitamin A plus iron plus folate versus iron plus folate for preventing miscarriage; Summary of findings 3 Multivitamin plus iron plus folate versus iron plus folate for preventing miscarriage
See summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3 for each of the main comparisons. The quality of evidence is included for numerical results of outcomes included in the 'Summary of findings' tables. We have included 40 trials, involving 276,820 women and 278,413 pregnancies. One trial (Jauniaux 2004) contributed no outcome data because it was stopped early and withdrawn. Thus, 39 trials contributed data to our analyses.
Vitamin C supplementation
The trials involving vitamin C supplementation included the following interventions: vitamin C plus multivitamins versus placebo plus multivitamins (Briscoe 1959; Hans 2010), vitamin C and vitamin E supplementation versus placebo (Chappell 1999; McCance 2010; Poston 2006; Roberts 2010; Rumbold 2006; Spinnato 2007; Xu 2010), and vitamin C alone versus no supplement or placebo (Hemmi 2003; Steyn 2003).
Primary outcomes
There was no difference in the risk of total fetal loss between women receiving:
-
vitamin C with vitamin E (risk ratio (RR) 1.14, 95% confidence interval (CI) 0.92 to 1.40, seven trials, 18,949 women; Analysis 1.1; high‐quality evidence);
-
vitamin C alone (RR 1.28, 95% CI 0.58 to 2.83, two trials, 224 women; Analysis 2.1);
-
vitamin C with multivitamins (RR 1.32, 95% CI 0.63 to 2.77, one trial, 406 women; Analysis 3.1);
compared with placebo or no vitamin C groups.
Similarly, we found no overall difference in the risk for early or late miscarriage between women receiving:
-
vitamin C with vitamin E (RR 0.97, 95% CI 0.70 to 1.34, five trials, 15,882 women; Analysis 1.2; moderate‐quality evidence);
-
vitamin C alone (RR 1.17, 95% CI 0.52 to 2.65, two trials, 224 women; Analysis 2.2);
-
vitamin C with multivitamins (RR 1.19, 95% CI 0.79 to 1.79, two trials, 790 women; Analysis 3.2).
Secondary outcomes
There was no difference in the risk of stillbirth for women receiving:
-
vitamin C with vitamin E (RR 1.24, 95% CI 0.92 to 1.68, seven trials, 18,906 women; Analysis 1.3; moderate‐quality evidence);
-
vitamin C alone (RR 3.00, 95% CI 0.12 to 72.77, one trial, 200 women; Analysis 2.3);
compared with placebo or no vitamin C groups. We found no difference in the risk of congenital malformations (Analysis 1.4) or adverse effects of vitamin supplementation (RR 1.16, 95% CI 0.39 to 3.41, one trial, 739 women; Analysis 1.5; moderate‐quality evidence).
Vitamin A supplementation
The trials involving vitamin A supplementation included the following interventions: vitamin A and/or beta‐carotene versus placebo (Katz 2000; Prawirohartono 2011; West 2011), vitamin A with or without multivitamins versus multivitamins (excluding vitamin A) or placebo (Fawzi 1998), and vitamin A plus iron and folic acid versus iron and folic acid (Kumwenda 2002; Schmidt 2001; Van den Broek 2006).
Primary outcomes
We found no difference in the risk of total fetal loss between women receiving:
-
vitamin A plus iron and folate (RR 1.01, 95% CI 0.61 to 1.66, three trials, 1640 women; Analysis 4.1; low‐quality evidence);
-
vitamin A alone (RR 1.05, 95% CI 0.90 to 1.23, three trials, 52,480 women; Tau² = 0.01, I² = 73% Analysis 5.1);
-
beta‐carotene alone (RR 1.02, 95% CI 0.98 to 1.07, two trials, 51,163 women; Analysis 6.1);
-
vitamin A or beta‐carotene (RR 1.05, 95% CI 0.91 to 1.21, one trial, 17,373 women; Analysis 7.1);
-
vitamin A with or without multivitamin (RR 0.80, 95% CI 0.53 to 1.21, one trial, 1074 women; Analysis 8.1);
compared with placebo or no vitamin A groups.
The heterogeneity in Analysis 5.1 seemed to have been contributed by combining two cluster‐randomised trials and one individually‐randomised trial. Heterogeniety was no longer present when the individually‐randomised trial was excluded. However, this did not change the conclusion of no significant difference between vitamin A and no vitamin A groups.
There was no evidence of a difference in the risk for early or late miscarriage between women receiving:
-
vitamin A plus iron and folate (RR 0.86, 95% CI 0.46 to 1.62, two trials, 1397 women; Analysis 4.2; low‐quality evidence);
-
vitamin A alone (RR 0.98, 95% CI 0.92 to 1.04, one trial, 39,668 women; Analysis 5.2);
-
beta‐carotene alone (RR 1.00, 95% CI 0.94 to 1.06, one trial, 39,860 women; Analysis 6.2);
-
vitamin A with or without multivitamin (RR 0.76, 95% CI 0.37 to 1.55, one trial, 1075 women; Analysis 8.2);
compared with placebo or no vitamin A groups.
Secondary outcomes
There was no difference in the risk for stillbirth for the following supplementation treatments:
-
vitamin A plus iron and folate (RR 1.29, 95% CI 0.57 to 2.91, three trials, 1640 women; Analysis 4.3; low‐quality evidence);
-
vitamin A alone (RR 0.95, 95% CI 0.86 to 1.06, one trial, 39,668 women; Analysis 5.3);
-
beta‐carotene alone (RR 1.09, 95% CI 0.98 to 1.20, one trial, 39,860 women; Analysis 6.3);
-
vitamin A with or without multivitamin (RR 1.04, 95% CI 0.60 to 1.79, one trial, 1075 women; Analysis 8.3);
compared with placebo or no vitamin A groups. Congenital malformations and adverse effects of vitamin supplementation were not reported by trials included in these analyses.
Multivitamin supplementation
The trials involving multivitamin supplementation included the following interventions: multivitamins with or without folic acid versus no multivitamins or folic acid (Czeizel 1994; ICMR 2000; MRC 1991); multivitamins with or without folic acid versus folic acid (Kirke 1992; MRC 1991; Zeng 2008); multivitamins with or without vitamin A versus vitamin A or placebo (Fawzi 1998); multivitamins versus control (People's League 1942); multivitamins with vitamin E versus multivitamins without vitamin E or control (Rush 1980); multivitamins with iron and folic acid versus iron and folic acid (Bhutta 2009; Fawzi 2007; Osrin 2005; Roberfroid 2008; Rumiris 2006; Sunawang 2009; Summit 2008; Tofail 2008; West 2014; Zagre 2007).
Primary outcomes
The risk for total fetal loss was reduced in women supplemented with:
-
multivitamin without folic acid (RR 0.49, 95% CI 0.34 to 0.70, one trial, 907 women; Analysis 10.1);
-
multivitamin with/without vitamin A (RR 0.60, 95% CI 0.39 to 0.92, one trial, 1074 women; Analysis 15.1);
compared with placebo or no multivitamin groups.
There was no difference in the risk of total fetal loss for the following interventions:
-
multivitamin plus iron and folic acid compared iron and folate only groups (RR 0.96, 95% CI 0.93 to 1.00, 10 trials, 94,948 women; Analysis 9.1; high‐quality evidence);
-
multivitamins alone or in combination with other vitamins or micronutrients compared with placebo or no multivitamins groups (Analysis 11.1; Analysis 12.1; Analysis 13.1; Analysis 14.1; Analysis 16.1; Analysis 17.1; Analysis 18.1 (random effects; three trials)).
Similarly, we found no difference in the risk for early or late miscarriage between women receiving the following interventions:
-
multivitamin plus iron and folic acid compared iron and folate only groups (RR 0.98, 95% CI 0.94 to 1.03, 10 trials, 94,948 women; Analysis 9.2; moderate‐quality evidence);
-
multivitamins alone or in combination with other vitamins or micronutrients compared with placebo or no multivitamins groups (Analysis 10.2; Analysis 11.2; Analysis 12.2; Analysis 13.2; Analysis 14.2; Analysis 17.2; Analysis 18.2 (random effects; three trials)).
The heterogeneity in Analysis 18.1 and Analysis 18.2 seemed to have been contributed by ICMR 2000, which included women who had previously given birth to a child with an open neural tube defect. When this trial was excluded, the heterogeneity was no longer present.
Secondary outcomes
There was evidence of a decrease in the risk for stillbirth among women receiving multivitamin plus iron and folic acid compared iron and folate only groups (RR 0.92, 95% CI 0.85 to 0.99, 10 trials, 79,851 women; Analysis 9.3; high‐quality evidence).
There was no difference in the risk of:
-
stillbirth (Analysis 10.3; Analysis 11.3; Analysis 12.3; Analysis 13.3; Analysis 14.3; Analysis 16.2; Analysis 17.3; Analysis 18.3); or
-
congenital malformation (Analysis 10.4; Analysis 11.4; Analysis 12.4; Analysis 13.4; Analysis 14.4; Analysis 18.4) between women receiving multivitamins alone or in combination with other vitamins or micronutrients compared with placebo or no multivitamins groups.
There were no data available to conduct any analysis for adverse effects of vitamin supplementation.
Folic acid supplementation
The trials involving folic acid supplementation included the following interventions: folic acid with or without multivitamins compared with no folic acid or multivitamins (Czeizel 1994; ICMR 2000; MRC 1991); folic acid with or without multivitamins compared with multivitamins (Kirke 1992; MRC 1991); folic acid and iron compared with iron (Fleming 1968); folic acid and iron compared with no iron or folic acid (Fleming 1986).
Primary outcomes
We found no difference in the risk of:
-
total fetal loss (Analysis 19.1 (random effects; three trials); Analysis 20.1; Analysis 21.1; Analysis 22.1; Analysis 23.1; Analysis 24.1; Analysis 25.1; Analysis 26.1); or
-
early or late miscarriage (Analysis 19.2 (random effects; three trials); Analysis 20.2; Analysis 21.2; Analysis 22.2; Analysis 23.2; Analysis 24.2; Analysis 25.2; Analysis 26.2);
between women supplemented with folic acid with or without multivitamins and/or iron compared with no folic acid groups. The heterogeneity found seemed to have been contributed by ICMR 2000, which included women who had previously given birth to a child with an open neural tube defect. Excluding this trial removed the heterogeneity but did not change the conclusion of no difference between the treatment groups.
Secondary outcomes
There was no difference in the risk of:
-
stillbirth (Analysis 19.3; Analysis 20.3; Analysis 21.3; Analysis 22.3 (random effects); Analysis 23.3; Analysis 24.3 (random effects); Analysis 25.3); or
-
congenital malformations (Analysis 19.4; Analysis 20.4; Analysis 21.4; Analysis 22.4 (random effects); Analysis 23.4; Analysis 24.4 (random effects)) between women supplemented with folic acid with or without multivitamins and/or iron;
compared no folic acid groups. There were no data available to conduct any analysis for adverse effects of vitamin supplementation.
Antioxidant vitamins supplementation
The trial involving antioxidant vitamins supplementation included the following interventions: antioxidant with multivitamins compared multivitamins with low antioxidant content (Wibowo 2012).
Primary outcomes
In the one trial involving 110 women (Wibowo 2012), there was no evidence of differences between women given antioxidant with multivitamins compared multivitamins with low antioxidant group on early or late miscarriage (RR 1.12, 95% CI 0.24 to 5.29, one trial, 110 women, Analysis 27.1). No other primary or secondary outcomes were reported by this trial.
Subgroup analyses by dose of vitamins and duration of vitamin usage
Subgroup analyses by dose of vitamin(s) (below or above the recommended dietary intake) were complicated by the limited number of studies in each vitamin group, and by the use of multivitamin supplements. For many of the vitamin types and for those reporting pregnancy loss outcomes, all of the trials supplemented women with amounts that were above the recommended dietary intake. Similarly, the duration of vitamin usage was complicated by the fact that many of the trials had wide recruitment periods, and one trial (Katz 2000) supplemented women up until three years postpartum. We have not performed subgroup analyses based on vitamin dosage or time of trial entry.
Subgroup analyses by women's risk of spontaneous or recurrent miscarriage
Information enabling women to be classified at high or low risk of either spontaneous miscarriage or recurrent miscarriage was not clearly stated in any of the trials included in this update. Based on the inclusion criteria, one trial (Rumbold 2006) included women at low risk of miscarriage. One trial (Briscoe 1959) included women who had experienced recurrent miscarriage as well as women at high risk of miscarriage (more than two previous miscarriages and/or bleeding in the pregnancy) and low‐risk women (two or less previous miscarriages and no bleeding in the pregnancy). After classifying women into these groups, the number of women in the high‐risk group was too small to permit any meaningful comparisons and we have therefore not performed subgroup analyses.
Subgroup analyses by dietary intake of vitamins
Seven trials (Bhutta 2009; Fleming 1968; Kumwenda 2002; People's League 1942; Schmidt 2001; Steyn 2003; West 2011) reported information about women's nutritional status or the percentage of women who were dietary deficient at trial entry for the vitamin of interest. Other trials reported that they were being undertaken in countries where the population was at high risk of multiple micronutrient deficiencies (Osrin 2005; Prawirohartono 2011; Roberfroid 2008; Summit 2008; Villar 2009), or there was a high prevalence of anaemia (Bhutta 2009; Fleming 1986; Sunawang 2009; Zagre 2007; Zeng 2008), but provided no specific information on nutritional status of participants. Two trials (Rumiris 2006; Wibowo 2012) included women with 'low antioxidant status'. There were not enough trials within each vitamin group to assess the role of supplementation in women with dietary deficient intakes of the individual vitamins and results were not reported separately for women with a low dietary vitamin intake; therefore, we could not perform subgroup analyses.
Sensitivity analyses
We carried out sensitivity analysis to explore the effects of trial quality and type of randomisation on the primary outcomes related to fetal loss (total fetal loss and early or late miscarriage). We included only trials with 'adequate' rating on allocation concealment, but found that restricting to only trials with adequate allocation concealment made very little difference to the results for the primary outcomes. Effect of type of randomisation was explored by excluding cluster‐randomised trials and restricting the analyses to individually‐randomised trials. We found no difference between women supplemented with multivitamins compared with controls for total fetal loss or early or late miscarriage when the analyses were restricted to individually‐randomised trials only. These sensitivity analyses indicate that the analyses for the effects of multivitamins on outcomes related to fetal loss and early or late miscarriage are no different when only individually‐randomised trials are included.
Discussion
Summary of main results
The purpose of this review was to determine the effectiveness and safety of any vitamin supplementation taken by women pre‐ or periconceptionally on the risk of miscarriage. In this updated version of the review, we included 40 studies involving 59,094 women from individually‐randomised trials plus a further 217,726 women from eight cluster‐randomised controlled trials. The results did not provide sufficient evidence to support the use of single vitamin supplementation for preventing total fetal loss or early or late miscarriage. However, stillbirth was significantly lower in women given multivitamin supplementation plus iron and folic acid compared to iron and folic acid alone. Although there was evidence of decreased risk for total fetal loss among women receiving multivitamins without folic acid compared with no multivitamin/folic acid and multivitamin supplementation with/without vitamin A compared with vitamin A or placebo; these findings occurred in analyses involving one trial each with small numbers of women involved. Also, they include studies where the comparison groups included women receiving either vitamin A or placebo, and thus require caution in interpretation.
Overall completeness and applicability of evidence
There was considerable consistency in reported total fetal loss (including miscarriages or combined miscarriages and stillbirths) among included studies with no difference in the rates of miscarriage and stillbirth across treatment groups. While this may suggest the true effect of vitamin supplementation on risk of miscarriage, most of the studies included in this review did not originally set out to examine the effect of vitamin supplementation on the risk of miscarriage.
Our review included trials that randomised women prior to conception; however, in some cases, not all women enrolled in these trials fell pregnant during the study period. Some of the trials reported outcomes only for women falling pregnant, whereas other trials did not distinguish between women who were never pregnant and women who may have been pregnant but were lost to follow‐up. The outcomes in this review relating to pregnancy outcomes are not relevant for the women who never became pregnant during the study period. In this review, where trials provided accurate information about the number of women who joined the study and became pregnant in the time period, we included this number in the totals, rather than the number of women who may have been randomised. Where it was not clear about the exact number of women with a confirmed pregnancy, we included all women who had been randomised. This may therefore mean that a certain proportion of women in the denominator were never pregnant during the study period. By including these women who were never pregnant in the totals, the review assumes that if these women had become pregnant, they would not have had a miscarriage, which is unlikely to be entirely correct. Including these women creates the potential to underestimate any treatment effects observed.
Similarly, for one large trial (Katz 2000) and one smaller trial (Roberfroid 2008), some women were pregnant more than once during the study period. In these trials, the denominators reported are the total number of pregnancies during the study period, not the total number of women randomised, which incorrectly assumes that each data point included is independent from the next. This has the potential to either underestimate or overestimate the results, depending on whether the women contributing data for more than one pregnancy may be more or less susceptible to experiencing miscarriage or stillbirth. One way to overcome this may be to summarise the data for each woman so that there is only one set of data points for each woman; however, we were unable to do this for these particular studies.
Quality of the evidence
Some of the trials included in the review were at high risk of bias, either due to poor or unclear allocation concealment or large losses to follow‐up. The data were also complicated by differing definitions of miscarriage. For some trials, miscarriage was considered to occur up until 26 or 28 weeks' gestation, while other studies reported miscarriage as pregnancy loss prior to 20 weeks' gestation, and stillbirth as pregnancy loss greater or equal to 20 weeks' gestation. Other studies did not specify their definition of miscarriage or stillbirth. In addition to the problems with differing definitions, the timing of the onset of vitamin supplementation for some of the included trials occurred in mid‐pregnancy, which may limit the impact of supplementation on the risk of miscarriage. The review attempted to overcome these issues by using the outcome 'total fetal loss', which included either miscarriage or stillbirth.
We assessed the quality of the evidence using GRADE and judged the evidence for vitamin C and vitamin E compared with control as high quality for total fetal loss, and moderate quality for early or late miscarriage, stillbirth, and adverse effects, which was downgraded due to wide 95% confidence intervals (CIs) (summary of findings Table for the main comparison). For vitamin A plus iron plus folate versus iron plus folate trials were judged to have low quality of evidence for total fetal loss, early or late miscarriage, and stillbirth due to design limitation and wide 95% CI (summary of findings Table 2). No studies reported any adverse effects for this comparison. For multivitamin plus iron plus folate versus iron plus folate trials were judged to be high quality for total fetal death and stillbirth, moderate quality for early or late miscarriage, downgraded due to publication bias suspected by funnel plot, or wide CI crossing the line of no effect (summary of findings Table 3). No studies reported any adverse effects for this comparison.
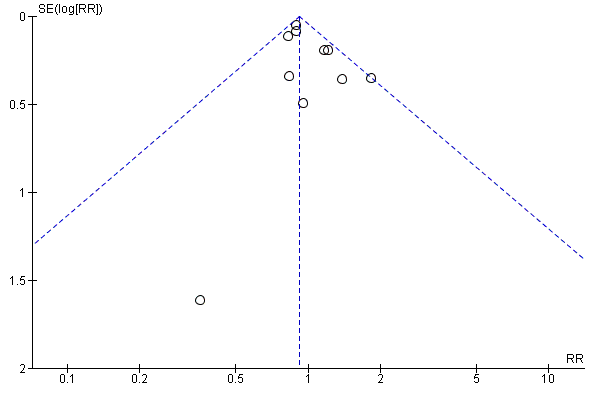
In order to determine the effect of publication bias, we undertook funnel plots for comparisons with 10 or more studies (Figure 3; Figure 4; Figure 5) for the comparisons of multivitamins plus iron and folic acid versus iron and folic acid. Asymmetry was suggested by visual assessment of Figure 4 for early or late miscarriage.

Funnel plot of comparison: 9 Multivitamin plus iron and folic acid versus iron and folic acid, outcome: 9.1 Total fetal loss.

Funnel plot of comparison: 9 Multivitamin plus iron and folic acid versus iron and folic acid, outcome: 9.2 Early or late miscarriage.
Funnel plot of comparison: 9 Multivitamin plus iron and folic acid versus iron and folic acid, outcome: 9.3 Stillbirth.
Potential biases in the review process
We took steps to minimise the introduction of bias during the review process. All relevant trials were identified including published abstracts from conference proceedings, English and non‐English publications. A pro forma translation sheet was used to extract relevant information from non‐English articles. At least two review authors independently assessed each trial, performed data extraction, and assessment of risk of bias for each of the included trials. Our assessment of previously identified ongoing trials that remained unpublished were limited to trial published protocols or the records of the initial communication between our authors and the authors of the unpublished trials.
Agreements and disagreements with other studies or reviews
There are several Cochrane reviews evaluating the effect of single vitamin supplementation during pregnancy on maternal, fetal, neonatal and infant outcomes. Benefits or hazards of vitamin supplementation in pregnancy on total fetal loss and miscarriage have not been or insufficiently investigated. However, our results on secondary outcomes are consistent with finding in the particular publications.
In the analysis by vitamin type, vitamin C supplementation alone or in combination with vitamin E or multivitamins did not show any effect on total fetal loss, miscarriage, or the secondary outcomes stillbirth, congenital malformation and adverse effects. A review focusing on vitamin C supplementation alone or in combination with other separate supplements on pregnancy outcomes, did not observe effects on stillbirth or congenital malformations which is consistent with our results (Rumbold 2015).
Supplementing women with vitamin A alone or in combination with iron and folic acid or multivitamins was not associated with changes in fetal loss or miscarriage as well as stillbirth. These findings are consistent with the Cochrane review 'Vitamin A supplementation during pregnancy for maternal and newborn outcomes' (McCauley 2015), which found no difference in the rate of stillbirth for women receiving vitamin A alone compared with placebo/no treatment or vitamin A with other micronutrients compared with micronutrient supplementation without vitamin A.
In the analysis comparing multivitamin alone or in combination with other vitamins, we found a positive effect of multivitamin supplementation without folic acid compared with no multivitamin/folic acid as well as multivitamin with/without vitamin A compared with vitamin A alone or placebo on total fetal loss. However, these findings resulted from only one study, respectively. Stillbirth was significantly reduced for women receiving multivitamin plus iron and folic acid. This result is consistent with findings in a review assessing the effect of multiple‐micronutrient supplementation during pregnancy on maternal, fetal and infant health outcomes (Haider 2015). Here they also reported a significant reduction in the risk of stillbirth. Miscarriage (loss before 28 weeks) was not effected by this intervention.
Folic acid supplementation with or without multivitamin compared to no folic acid/multivitamin or multivitamin alone did not reduce the risk of total fetal loss, miscarriage, stillbirth or congenital malformations. This in accordance with a review evaluating the effectiveness of oral folic acid supplementation during pregnancy on maternal health and pregnancy outcomes (Lassi 2013). The authors did not observe any effect of folic acid supplementation on stillbirth. Even though miscarriage was included as a secondary outcome, none of the included studies reported on miscarriage. In addition, another review assessed the effects and safety of periconceptional oral folate supplementation for preventing birth defects (De‐Regil 2015). There was no effect of folate versus no intervention, placebo or other micronutrients without folate on miscarriage or stillbirth. They investigated the effect of folate supplementation on several congenital malformations and found a 69% reduction in the risk of neural tube defects.
Antioxidant vitamin supplementation had no effect on early or late miscarriage. The effectiveness and safety of any antioxidant supplementation during pregnancy on the risk of various pregnancy outcomes is explored in the Cochrane review 'Antioxidants for preventing pre‐eclampsia' (Rumbold 2008). Our results are in accordance with the results form this review where any antioxidant supplementation compared to control or placebo had no effect on miscarriage or stillbirth.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
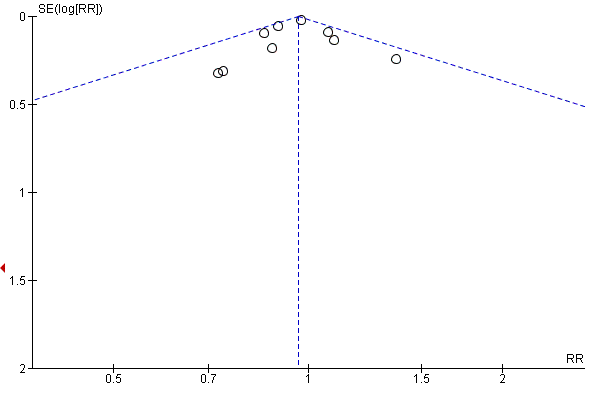
Funnel plot of comparison: 9 Multivitamin plus iron and folic acid versus iron and folic acid, outcome: 9.1 Total fetal loss.
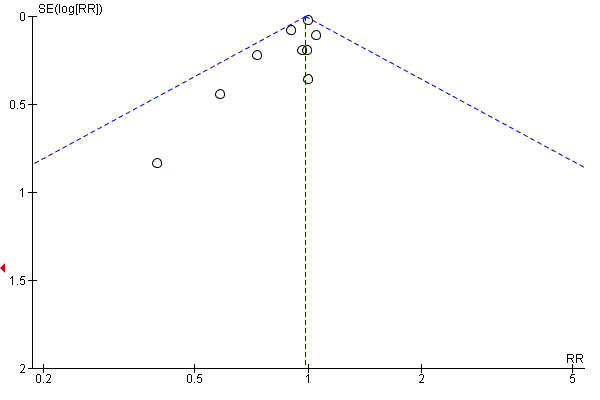
Funnel plot of comparison: 9 Multivitamin plus iron and folic acid versus iron and folic acid, outcome: 9.2 Early or late miscarriage.

Funnel plot of comparison: 9 Multivitamin plus iron and folic acid versus iron and folic acid, outcome: 9.3 Stillbirth.

Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 1 Total fetal loss.
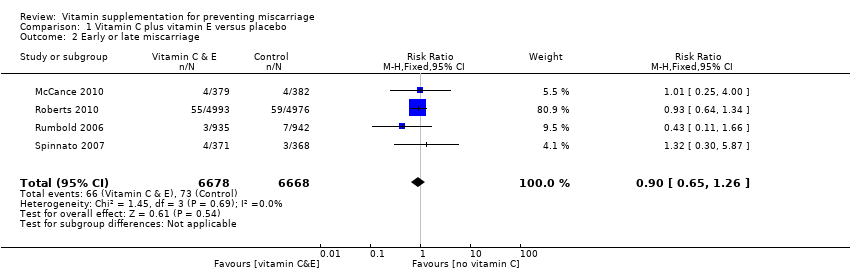
Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 2 Early or late miscarriage.

Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 3 Stillbirth.
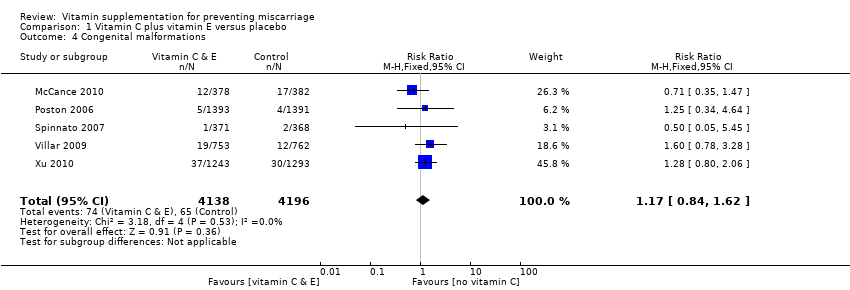
Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 4 Congenital malformations.

Comparison 1 Vitamin C plus vitamin E versus placebo, Outcome 5 Any adverse effects of vitamin supplementation sufficient to stop supplementation.

Comparison 2 Vitamin C versus no supplement/placebo, Outcome 1 Total fetal loss.

Comparison 2 Vitamin C versus no supplement/placebo, Outcome 2 Early or late miscarriage.

Comparison 2 Vitamin C versus no supplement/placebo, Outcome 3 Stillbirth.
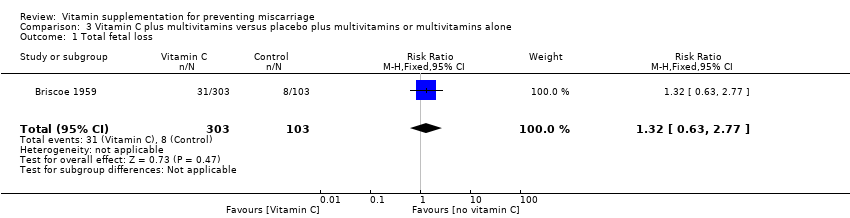
Comparison 3 Vitamin C plus multivitamins versus placebo plus multivitamins or multivitamins alone, Outcome 1 Total fetal loss.
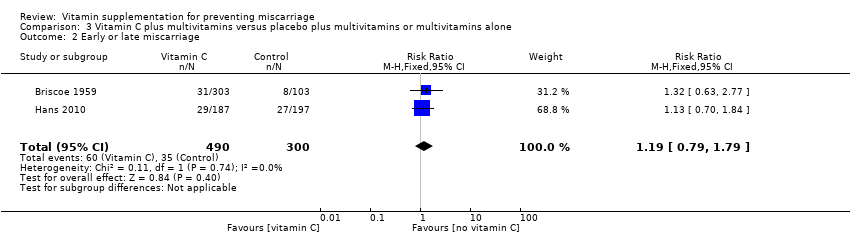
Comparison 3 Vitamin C plus multivitamins versus placebo plus multivitamins or multivitamins alone, Outcome 2 Early or late miscarriage.

Comparison 4 Vitamin A plus iron and folate versus iron and folate, Outcome 1 Total fetal loss.

Comparison 4 Vitamin A plus iron and folate versus iron and folate, Outcome 2 Early or late miscarriage.

Comparison 4 Vitamin A plus iron and folate versus iron and folate, Outcome 3 Stillbirth.
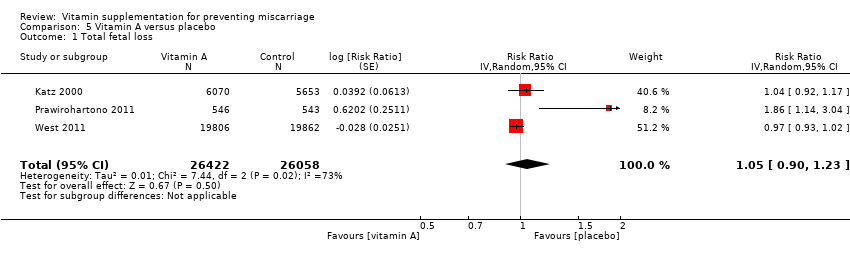
Comparison 5 Vitamin A versus placebo, Outcome 1 Total fetal loss.

Comparison 5 Vitamin A versus placebo, Outcome 2 Early of late miscarriage.

Comparison 5 Vitamin A versus placebo, Outcome 3 Stillbirth.
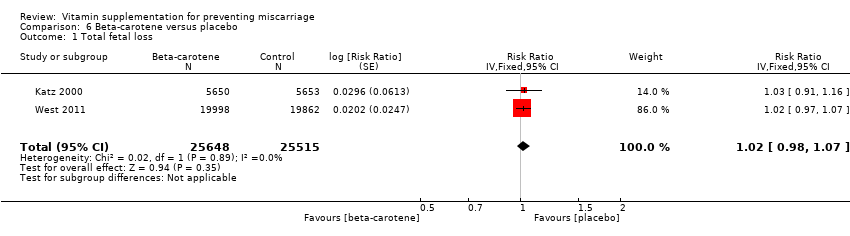
Comparison 6 Beta‐carotene versus placebo, Outcome 1 Total fetal loss.
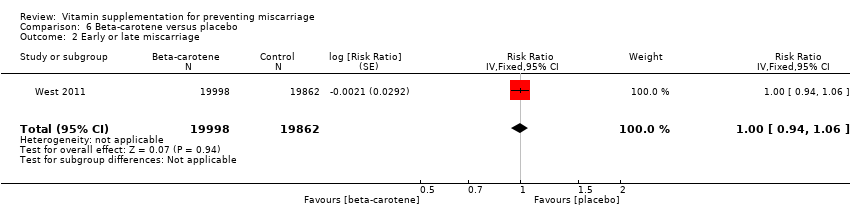
Comparison 6 Beta‐carotene versus placebo, Outcome 2 Early or late miscarriage.

Comparison 6 Beta‐carotene versus placebo, Outcome 3 Stillbirth.
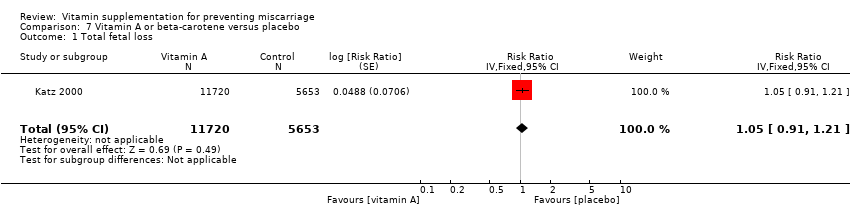
Comparison 7 Vitamin A or beta‐carotene versus placebo, Outcome 1 Total fetal loss.
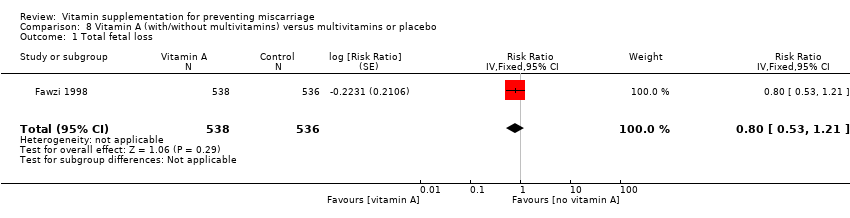
Comparison 8 Vitamin A (with/without multivitamins) versus multivitamins or placebo, Outcome 1 Total fetal loss.

Comparison 8 Vitamin A (with/without multivitamins) versus multivitamins or placebo, Outcome 2 Early or late miscarriage.

Comparison 8 Vitamin A (with/without multivitamins) versus multivitamins or placebo, Outcome 3 Stillbirth.
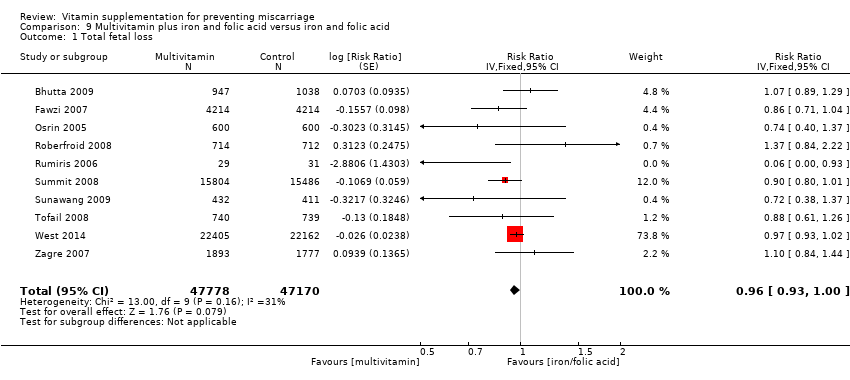
Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 1 Total fetal loss.
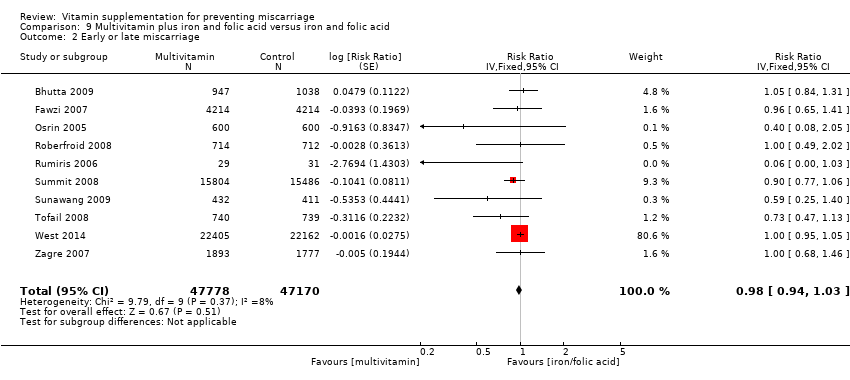
Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 2 Early or late miscarriage.

Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 3 Stillbirth.

Comparison 9 Multivitamin plus iron and folic acid versus iron and folic acid, Outcome 4 Congenital malformation.
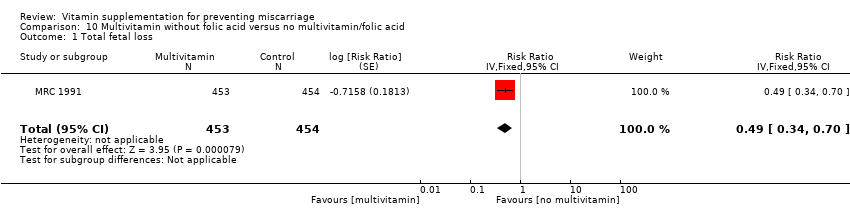
Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 1 Total fetal loss.
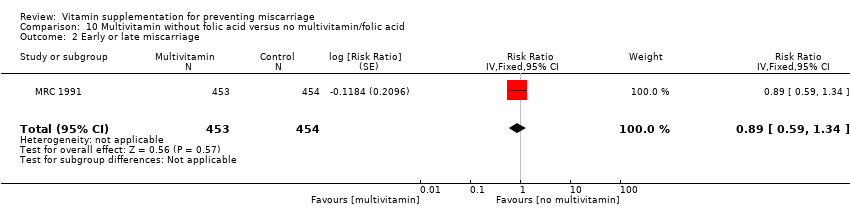
Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 2 Early or late miscarriage.
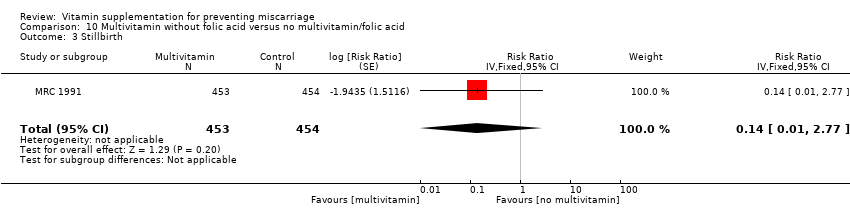
Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 3 Stillbirth.

Comparison 10 Multivitamin without folic acid versus no multivitamin/folic acid, Outcome 4 Congenital malformations.

Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 1 Total fetal loss.
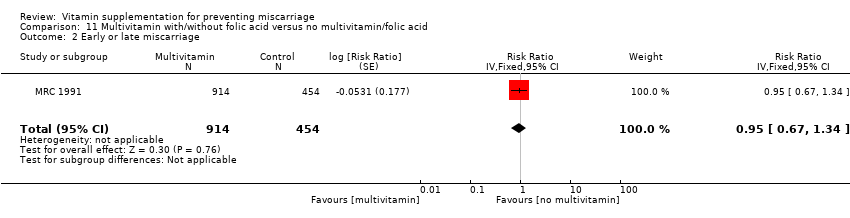
Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 2 Early or late miscarriage.

Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 3 Stillbirth.

Comparison 11 Multivitamin with/without folic acid versus no multivitamin/folic acid, Outcome 4 Congenital malformations.

Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 1 Total fetal loss.

Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 2 Early or late miscarriage.

Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 3 Stillbirth.
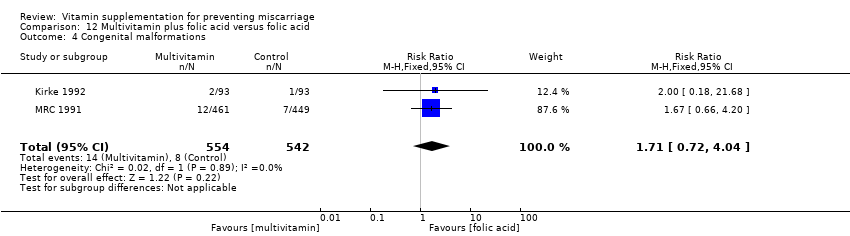
Comparison 12 Multivitamin plus folic acid versus folic acid, Outcome 4 Congenital malformations.

Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 1 Total fetal loss.
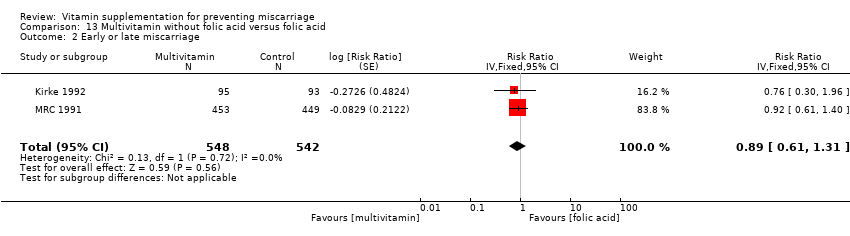
Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 2 Early or late miscarriage.

Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 3 Stillbirth.

Comparison 13 Multivitamin without folic acid versus folic acid, Outcome 4 Congenital malformations.

Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 1 Total fetal loss.
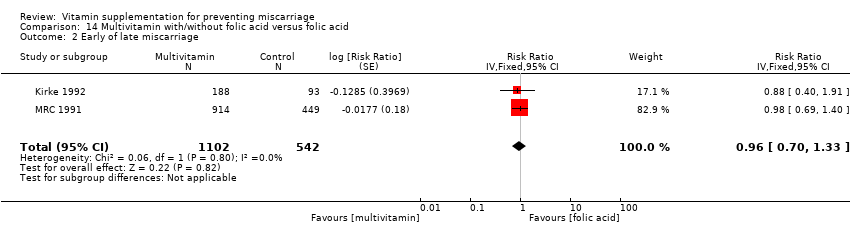
Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 2 Early of late miscarriage.
Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 3 Stillbirth.
Comparison 14 Multivitamin with/without folic acid versus folic acid, Outcome 4 Congenital malformations.

Comparison 15 Multivitamin with/without vitamin A versus vitamin A or placebo, Outcome 1 Total fetal loss.
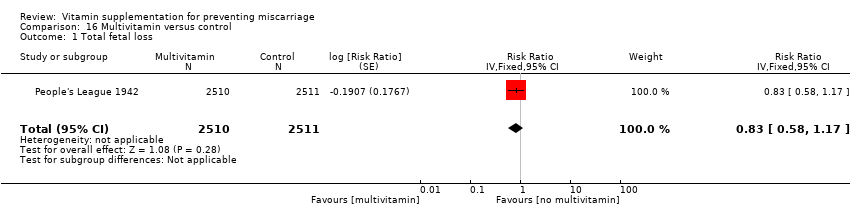
Comparison 16 Multivitamin versus control, Outcome 1 Total fetal loss.

Comparison 16 Multivitamin versus control, Outcome 2 Stillbirth.
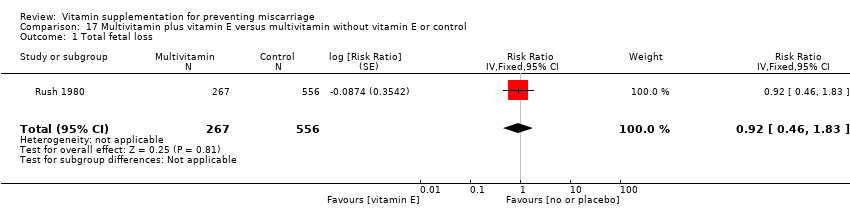
Comparison 17 Multivitamin plus vitamin E versus multivitamin without vitamin E or control, Outcome 1 Total fetal loss.

Comparison 17 Multivitamin plus vitamin E versus multivitamin without vitamin E or control, Outcome 2 Early or late miscarriage.

Comparison 17 Multivitamin plus vitamin E versus multivitamin without vitamin E or control, Outcome 3 Stillbirth.

Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 1 Total fetal loss.
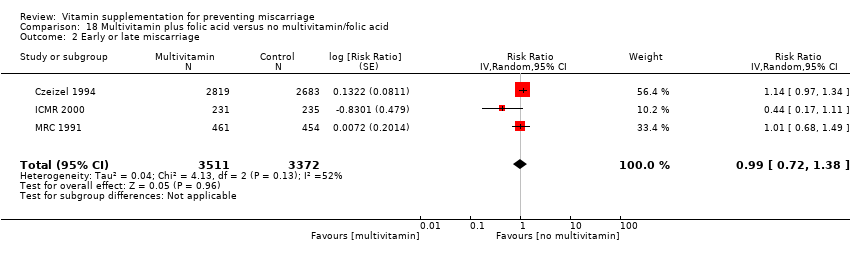
Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 2 Early or late miscarriage.
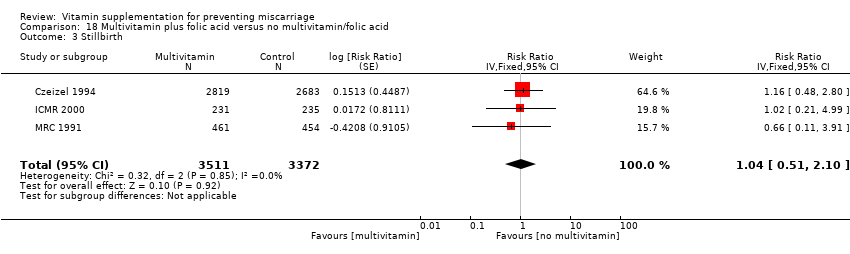
Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 3 Stillbirth.
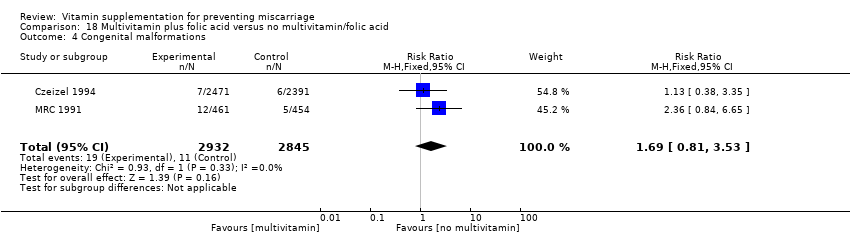
Comparison 18 Multivitamin plus folic acid versus no multivitamin/folic acid, Outcome 4 Congenital malformations.

Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 1 Total fetal loss.

Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 2 Early or late miscarriage.

Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 3 Stillbirth.

Comparison 19 Folic acid plus multivitamin versus no folic acid/multivitamin, Outcome 4 Congenital malformations.
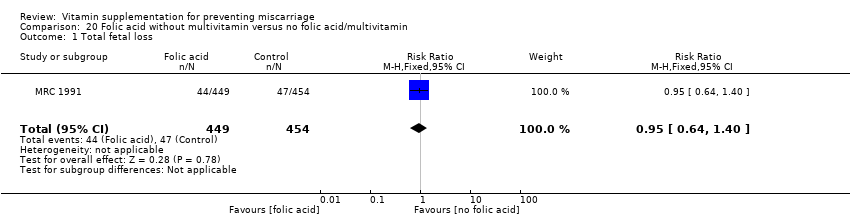
Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 1 Total fetal loss.
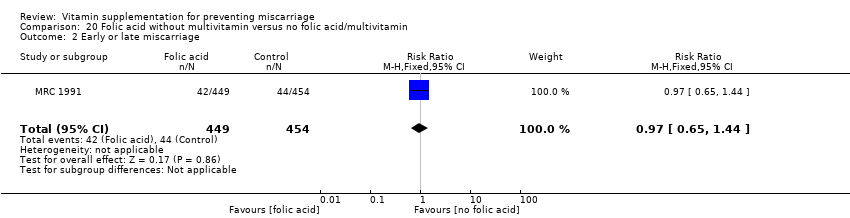
Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 2 Early or late miscarriage.

Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 3 Stillbirth.

Comparison 20 Folic acid without multivitamin versus no folic acid/multivitamin, Outcome 4 Congenital malformations.

Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 1 Total fetal loss.

Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 2 Early or late miscarriage.

Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 3 Stillbirth.

Comparison 21 Folic acid with/without multivitamin versus no folic acid/multivitamin, Outcome 4 Congenital malformations.
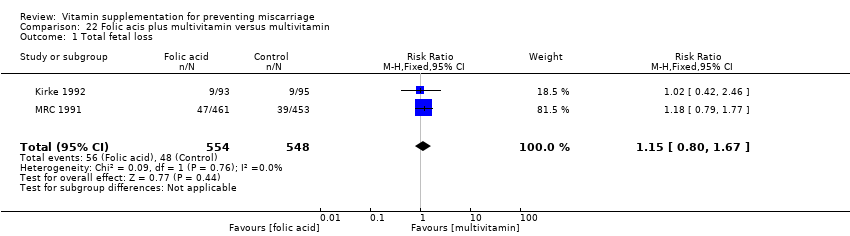
Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 1 Total fetal loss.

Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 2 Early or late miscarriage.

Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 3 Stillbirth.

Comparison 22 Folic acis plus multivitamin versus multivitamin, Outcome 4 Congenital malformations.

Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 1 Total fetal loss.

Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 2 Early or late miscarriage.

Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 3 Stillbirth.

Comparison 23 Folic acid without multivitamin versus multivitamin, Outcome 4 Congenital malformations.

Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 1 Total fetal loss.

Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 2 Early or late miscarriage.
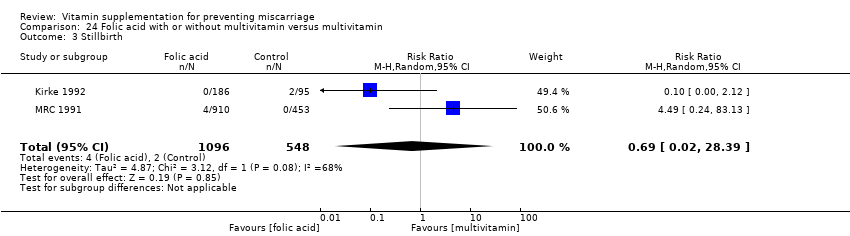
Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 3 Stillbirth.
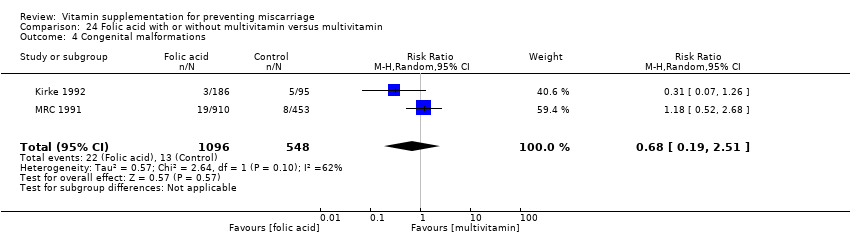
Comparison 24 Folic acid with or without multivitamin versus multivitamin, Outcome 4 Congenital malformations.

Comparison 25 Folic acid plus iron versus iron, Outcome 1 Total fetal loss.
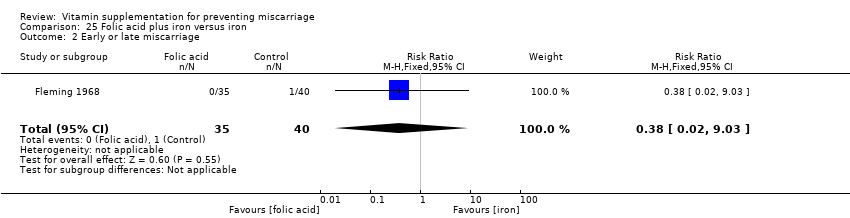
Comparison 25 Folic acid plus iron versus iron, Outcome 2 Early or late miscarriage.

Comparison 25 Folic acid plus iron versus iron, Outcome 3 Stillbirth.
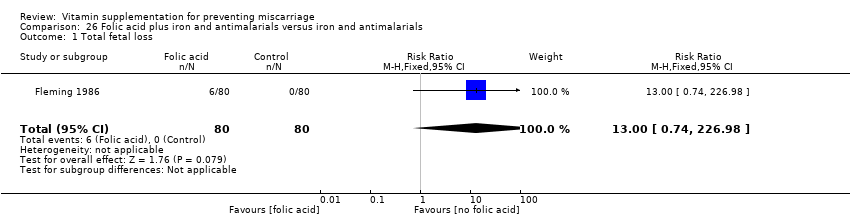
Comparison 26 Folic acid plus iron and antimalarials versus iron and antimalarials, Outcome 1 Total fetal loss.

Comparison 26 Folic acid plus iron and antimalarials versus iron and antimalarials, Outcome 2 Early or late miscarriage.
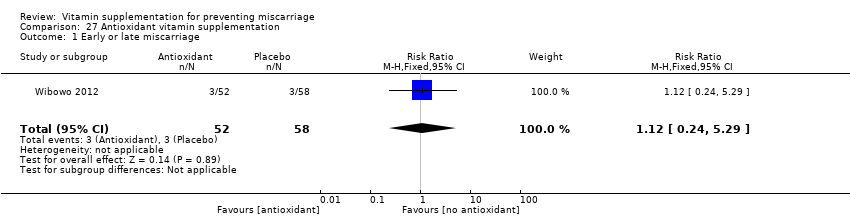
Comparison 27 Antioxidant vitamin supplementation, Outcome 1 Early or late miscarriage.
| Vitamin C plus vitamin E versus control for preventing miscarriage | ||||||
| Population: pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Vitamin C plus vitamin E | |||||
| Total fetal loss | Study population | RR 1.14 | 18,949 | ⊕⊕⊕⊕ | ||
| 17 per 1000 | 20 per 1000 | |||||
| Moderate | ||||||
| 14 per 1000 | 16 per 1000 | |||||
| Early or late miscarriage | Study population | RR 0.90 | 13,346 | ⊕⊕⊕⊝ | ||
| 9 per 1000 | 9 per 1000 | |||||
| Moderate | ||||||
| 8 per 1000 | 8 per 1000 | |||||
| Stillbirth | Study population | RR 1.31 | 21,442 | ⊕⊕⊕⊝ | ||
| 8 per 1000 | 10 per 1000 | |||||
| Moderate | ||||||
| 7 per 1000 | 9 per 1000 | |||||
| Any adverse effects of vitamin supplementation sufficient to stop supplementation | Study population | RR 1.16 | 739 | ⊕⊕⊕⊝ | ||
| 16 per 1000 | 19 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not effective but 95% CI is narrow and precise. 2 Non significant with wide 95% CI. 3 Small sample size. | ||||||
| Vitamin A plus iron plus folate versus iron plus folate for preventing miscarriage | ||||||
| Population: pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Vitamin A | |||||
| Total fetal loss (including miscarriages or combined miscarriages and stillbirths) ‐ Vitamin A + iron + folate versus iron + folate | Study population | RR 1.01 | 1640 | ⊕⊕⊝⊝ | ||
| 37 per 1000 | 37 per 1000 | |||||
| Moderate | ||||||
| 59 per 1,000 | 60 per 1,000 (36 to 98) | |||||
| Early or late miscarriage ‐ Vitamin A + iron + folate versus iron + folate | Study population | RR 0.86 | 1397 | ⊕⊕⊝⊝ | ||
| 31 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| 50 per 1,000 | 43 per 1,000 (23 to 80) | |||||
| Stillbirth ‐ Vitamin A + iron + folate versus iron + folate | Study population | RR 1.29 | 1640 | ⊕⊕⊝⊝ | ||
| 13 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 21 per 1,000 | 27 per 1,000 (12 to 61) | |||||
| Any adverse effects | See comments. | No studies reported this outcome. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High and unclear risk of attrition bias. 2 Wide 95% CI. | ||||||
| Multivitamin plus iron plus folate versus iron plus folate for preventing miscarriage | ||||||
| Population: pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Multivitamin | |||||
| Total fetal loss (including miscarriages or combined miscarriages and stillbirths) ‐ Multivitamins + iron + folic acid versus iron + folic acid | Study population | RR 0.96 | 94,948 | ⊕⊕⊕⊕ | ||
| 136 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 218 per 1,000 | 209 per 1,000 (202 to 218) | |||||
| Early or late miscarriage ‐ Multivitamin + iron + folic acid versus iron + folic acid | Study population | RR 0.98 | 94948 | ⊕⊕⊕⊝ | ||
| 84 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 134 per 1,000 | 132 per 1,000 (126 to 138) | |||||
| Stillbirth ‐ Multivitamin + iron + folic acid versus iron + folic acid | Study population | RR 0.92 | 79,851 | ⊕⊕⊕⊕ | ||
| 29 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| 46 per 1,000 | 43 per 1,000 (39 to 46) | |||||
| Any adverse effects | See comments | No studies reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Publication bias detected by funnel plot. 2 Wide confidence interval crossing the line of no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 7 | 18949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.40] |
| 2 Early or late miscarriage Show forest plot | 4 | 13346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 3 Stillbirth Show forest plot | 7 | 21442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.97, 1.76] |
| 4 Congenital malformations Show forest plot | 5 | 8334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.84, 1.62] |
| 5 Any adverse effects of vitamin supplementation sufficient to stop supplementation Show forest plot | 1 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.58, 2.83] |
| 2 Early or late miscarriage Show forest plot | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.52, 2.65] |
| 3 Stillbirth Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.63, 2.77] |
| 2 Early or late miscarriage Show forest plot | 2 | 790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.79, 1.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 1640 | Risk Ratio (Fixed, 95% CI) | 1.01 [0.61, 1.66] |
| 2 Early or late miscarriage Show forest plot | 2 | 1397 | Risk Ratio (Fixed, 95% CI) | 0.86 [0.46, 1.62] |
| 3 Stillbirth Show forest plot | 3 | 1640 | Risk Ratio (Fixed, 95% CI) | 1.29 [0.57, 2.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 52480 | Risk Ratio (Random, 95% CI) | 1.05 [0.90, 1.23] |
| 2 Early of late miscarriage Show forest plot | 1 | 39668 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.92, 1.04] |
| 3 Stillbirth Show forest plot | 1 | 39668 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 51163 | Risk Ratio (Fixed, 95% CI) | 1.02 [0.98, 1.07] |
| 2 Early or late miscarriage Show forest plot | 1 | 39860 | Risk Ratio (Fixed, 95% CI) | 1.00 [0.94, 1.06] |
| 3 Stillbirth Show forest plot | 1 | 39860 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.98, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 17373 | Risk Ratio (Fixed, 95% CI) | 1.05 [0.91, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1074 | Risk Ratio (Fixed, 95% CI) | 0.80 [0.53, 1.21] |
| 2 Early or late miscarriage Show forest plot | 1 | 1075 | Risk Ratio (Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| 3 Stillbirth Show forest plot | 1 | 1075 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.60, 1.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 10 | 94948 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.93, 1.00] |
| 2 Early or late miscarriage Show forest plot | 10 | 94948 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.94, 1.03] |
| 3 Stillbirth Show forest plot | 10 | 79851 | Risk Ratio (Fixed, 95% CI) | 0.92 [0.85, 0.99] |
| 4 Congenital malformation Show forest plot | 1 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 907 | Risk Ratio (Fixed, 95% CI) | 0.49 [0.34, 0.70] |
| 2 Early or late miscarriage Show forest plot | 1 | 907 | Risk Ratio (Fixed, 95% CI) | 0.89 [0.59, 1.34] |
| 3 Stillbirth Show forest plot | 1 | 907 | Risk Ratio (Fixed, 95% CI) | 0.14 [0.01, 2.77] |
| 4 Congenital malformations Show forest plot | 1 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.53, 4.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1368 | Risk Ratio (Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| 2 Early or late miscarriage Show forest plot | 1 | 1368 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.67, 1.34] |
| 3 Stillbirth Show forest plot | 1 | 1368 | Risk Ratio (Fixed, 95% CI) | 0.33 [0.06, 1.98] |
| 4 Congenital malformations Show forest plot | 1 | 1368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.75, 5.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 5012 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| 2 Early or late miscarriage Show forest plot | 3 | 5012 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.80, 1.18] |
| 3 Stillbirth Show forest plot | 3 | 4316 | Risk Ratio (Fixed, 95% CI) | 1.32 [0.93, 1.88] |
| 4 Congenital malformations Show forest plot | 2 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.72, 4.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1090 | Risk Ratio (Fixed, 95% CI) | 0.90 [0.62, 1.30] |
| 2 Early or late miscarriage Show forest plot | 2 | 1090 | Risk Ratio (Fixed, 95% CI) | 0.89 [0.61, 1.31] |
| 3 Stillbirth Show forest plot | 2 | 1090 | Risk Ratio (Random, 95% CI) | 0.99 [0.04, 22.90] |
| 4 Congenital malformations Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.67, 3.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1644 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.70, 1.33] |
| 2 Early of late miscarriage Show forest plot | 2 | 1644 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.70, 1.33] |
| 3 Stillbirth Show forest plot | 2 | 1644 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.15, 4.10] |
| 4 Congenital malformations Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.76, 3.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1074 | Risk Ratio (Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 5021 | Risk Ratio (Fixed, 95% CI) | 0.83 [0.58, 1.17] |
| 2 Stillbirth Show forest plot | 1 | 5021 | Risk Ratio (Fixed, 95% CI) | 0.83 [0.58, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 823 | Risk Ratio (Fixed, 95% CI) | 0.92 [0.46, 1.83] |
| 2 Early or late miscarriage Show forest plot | 1 | 823 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.26, 4.13] |
| 3 Stillbirth Show forest plot | 1 | 823 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.39, 1.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 6883 | Risk Ratio (Random, 95% CI) | 1.00 [0.75, 1.34] |
| 2 Early or late miscarriage Show forest plot | 3 | 6883 | Risk Ratio (Random, 95% CI) | 0.99 [0.72, 1.38] |
| 3 Stillbirth Show forest plot | 3 | 6883 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.51, 2.10] |
| 4 Congenital malformations Show forest plot | 2 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.81, 3.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 3 | 6883 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.34] |
| 2 Early or late miscarriage Show forest plot | 3 | 6883 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.38] |
| 3 Stillbirth Show forest plot | 3 | 6883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.51, 2.09] |
| 4 Congenital malformations Show forest plot | 2 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.81, 3.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.40] |
| 2 Early or late miscarriage Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.44] |
| 3 Stillbirth Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 4.02] |
| 4 Congenital malformations Show forest plot | 1 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.45, 4.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.69, 1.35] |
| 2 Early or late miscarriage Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.70, 1.39] |
| 3 Stillbirth Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.15, 2.96] |
| 4 Congenital malformations Show forest plot | 1 | 1364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.71, 5.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.67] |
| 2 Early or late miscarriage Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.80, 1.69] |
| 3 Stillbirth Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.04, 22.55] |
| 4 Congenital malformations Show forest plot | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.28, 3.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.77, 1.62] |
| 2 Early or late miscarriage Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.77, 1.64] |
| 3 Stillbirth Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.97 [0.58, 42.29] |
| 4 Congenital malformations Show forest plot | 2 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.26, 1.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.57] |
| 2 Early or late miscarriage Show forest plot | 2 | 1642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
| 3 Stillbirth Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.02, 28.39] |
| 4 Congenital malformations Show forest plot | 2 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.19, 2.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.59] |
| 2 Early or late miscarriage Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.03] |
| 3 Stillbirth Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total fetal loss Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [0.74, 226.98] |
| 2 Early or late miscarriage Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [0.74, 226.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early or late miscarriage Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.24, 5.29] |

