Vaginal chlorhexidine during labour for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV)
Abstract
Background
The incidence of chorioamnionitis occurs in between eight and 12 women for every 1000 live births and 96% of cases of chorioamnionitis are due to ascending infection. Following spontaneous vaginal delivery, 1% to 4% of women develop postpartum endometritis. The incidence of neonatal sepsis is 0.5% to 1% of all infants born. Maternal vaginal bacteria are the main agents for these infections. It is reasonable to speculate that prevention of maternal and neonatal infections might be possible by washing the vagina and cervix with an antibacterial agent for all women during labour. Chlorhexidine belongs to the class of compounds known as the bis‐biguanides. Chlorhexidine has antibacterial action against a wide range of aerobic and anaerobic bacteria, including those implicated in peripartal infections.
Objectives
To evaluate the effectiveness and side effects of chlorhexidine vaginal douching during labour in reducing maternal and neonatal infections (excluding group B streptococcal and HIV).
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 June 2014), reference lists of retrieved reports and journal letters and editorials.
Selection criteria
Randomized or quasi‐randomized trials comparing chlorhexidine vaginal douching during labour with placebo or other vaginal disinfectant to prevent (reduce) maternal and neonatal infections (excluding group B streptococcal and HIV).
Data collection and analysis
Two review authors independently assessed trial eligibility and quality, extracted and interpreted the data. A third review author analyzed and interpreted the data. The fourth author also interpreted the data.
Main results
We included three studies (3012 participants). There was no evidence of an effect of vaginal chlorhexidine during labour in preventing maternal and neonatal infections. Although the data suggest a trend in reducing postpartum endometritis, the difference was not statistically significant (three trials, 3012 women, risk ratio 0.83; 95% confidence interval 0.61 to 1.13).
Assessment of the quality of the evidence using GRADE indicated that the levels of evidence for all primary outcomes and one important secondary outcome were low to moderate.
Authors' conclusions
There is no evidence to support the use of vaginal chlorhexidine during labour in preventing maternal and neonatal infections. There is a need for a well‐designed randomized controlled trial using appropriate concentration and volume of vaginal chlorhexidine irrigation solution and with adequate sample size.
PICO
Plain language summary
Vaginal chlorhexidine during labour for preventing maternal and neonatal infections (excluding group B streptococcal and HIV)
Bacteria live in women's vaginas and generally cause no problems. Very occasionally they infect the placenta during labour and can pass to the baby, causing an infection. These infections can occasionally make the baby very ill and very occasionally the baby might die.
The review of three trials (3012 participants) found there was not enough information to say whether the use of chlorhexidine washing of the vagina during labour led to fewer infections for mothers and babies. More research is needed.
Authors' conclusions
Summary of findings
| Chlorhexidine vaginal wash versus placebo for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV) | ||||||
| Population: Pregnant women with a gestational age greater than 28 weeks, considered to be in labour receiving chlorhexidine vaginal douching during labour versus placebo to prevent maternal and neonatal infections (excluding Group B Streptococcal and HIV). | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chlorhexidine vaginal wash versus placebo | |||||
| Chorioamnionitis | Study population | RR 1.1 | 3012 | ⊕⊕⊕⊝ | ||
| 69 per 1000 | 76 per 1000 | |||||
| Moderate | ||||||
| 62 per 1000 | 68 per 1000 | |||||
| Postpartum endometritis | Study population | RR 0.83 | 3012 | ⊕⊕⊕⊝ | ||
| 57 per 1000 | 47 per 1000 | |||||
| Moderate | ||||||
| 72 per 1000 | 60 per 1000 | |||||
| Neonatal sepsis | Study population | RR 0.75 | 2987 | ⊕⊕⊝⊝ low2 | ||
| 3 per 1000 | 2 per 1000 | |||||
| Moderate | ||||||
| 4 per 1000 | 3 per 1000 | |||||
| Blood culture confirming neonatal sepsis | Study population | RR 0.75 | 2077 | ⊕⊕⊝⊝ low2 | ||
| 4 per 1000 | 3 per 1000 | |||||
| Moderate | ||||||
| 4 per 1000 | 3 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Confidence interval crossing the line of no effect. | ||||||
Background
Description of the condition
Chlorioamnionitis is an inflammatory reaction of the placental tissues in response to organism invasion. The incidence of chorioamnionitis occurs in eight to 12 women for every 1000 live births and 96% of the cases of chorioamnionitis are due to an ascending infection (Monif 1993). Following spontaneous vaginal delivery, 1% to 4% of women develop postpartum endometritis (Monif 1993). Although uterine infections are relatively uncommon following uncomplicated vaginal delivery, they continue to be a major problem in women delivered by caesarean section. Vaginal examinations increase the risk of inoculation and colonization of lower uterine incisions and laceration and therefore increase the risk of postpartum endometritis in women delivered by caesarean section (Cunningham 2001).
Bacterial infection is an important cause of neonatal morbidity and mortality. A prospective study from Pakistan reported a prevalence of blood culture proven bacterial sepsis to be 5.6 per 1000 live births (Bhutta 1997). Septicemia accounted for 11% to 30.4% of all neonatal deaths (Boo 1994). Other forms of infection include ophthalmia neonatorum; neonatal pneumonia and neonatal meningitis. Maternal vaginal bacteria are the main agents for these infections.
Description of the intervention
It is reasonable to speculate that prevention of maternal and neonatal infections might be possible by washing the vagina and cervix with an antibacterial agent in women during labour. Vaginal and cervical washing is usually performed by gently introducing a catheter attached to a 50 to 60 mL syringe up to the cervix. The cervical area is flushed with 50 to 60 mL of the solution. The syringe is then refilled without removing the catheter, and a second flushing with the same amount of the solution is performed while slowly withdrawing the catheter (Gaillard 2001). This procedure can be performed within a few minutes and would not interfere with women's labour when they wish to move and adopt a position which they feel is right for them. To be clinically useful, such an agent would need to possess antimicrobial activity against a broad range of bacteria that have been implicated in peripartal infection, be non‐toxic and non‐irritating for mother and fetus/neonate. Ideally, the agent would be inexpensive and commercially available. Chlorhexidine, a widely used medical disinfectant, satisfies these requirements.
How the intervention might work
Chlorhexidine belongs to the class of compounds known as the bis‐biguanides. Because of its high cationic nature, it has a strong affinity for the cell wall of micro‐organisms, to which it binds, disrupting osmotic equilibrium. The disrupted cytoplasmic membrane precipitates intracellularly, preventing repair of the cell wall and eventually resulting in cell death (Davies 1973). These actions endow chlorhexidine with antibacterial action against a wide range of aerobic and anaerobic bacteria, including those implicated in peripartal infections (Emilson 1977; Hennessey 1973). This antibacterial action is achieved at a very low concentration: typical minimum inhibitory concentrations are in microgram (mcg) per millilitre (mL), whereas clinically used concentrations are in milligram (mg) per mL. A randomized controlled trial demonstrated that vaginal douching with chlorhexidine during labour can significantly reduce maternal and early neonatal (including group B streptococcal) infection (Stray‐Pedersen 1999). Finally, complete resistance to chlorhexidine rarely emerges even after long‐term use (Ferretti 1990).
The allergic and toxic potential of chlorhexidine is very low. For many decades, chlorhexidine has been the major medical skin and the mucous membrane disinfectant in use. Despite its widespread use, only individual cases of anaphylactic or even mild allergic reactions in exposed medical personnel have been reported (Bergqvist‐Karlsson 1988). Chlorhexidine tends not to be absorbed by human skin and mucous membrane barrier (Johnsson 1987; Nilsson 1989). A long‐term human oral safety trial did not show any systemic or serious local side effects after two years of continuous use (Johnsson 1987). In contrast to povidone‐iodine, vaginally applied chlorhexidine was not absorbed in measurable amounts into the blood stream (Vorherr 1984).
Why it is important to do this review
Maternal and neonatal infections occur commonly and have serious ramifications for both mothers and newborns. Vaginal chlorhexidine douching may offer a safe, inexpensive, and theoretically sound approach to prevent maternal and neonatal infections. Vaginal disinfection during labour for reducing the risk of mother‐to‐child transmission (MTCT) of HIV infection is addressed in one Cochrane review which indicated that there was no evidence of an effect of vaginal disinfection on MTCT of HIV (Wiysonge 2005). Another Cochrane review also does not support the use of vaginal chlorhexidine during labour to prevent early‐onset neonatal group B streptococcal infection (Stade 2008).
Objectives
To evaluate the effectiveness and side effects of chlorhexidine vaginal douching during labour in reducing maternal and neonatal infections (excluding group B streptococcal and HIV).
Methods
Criteria for considering studies for this review
Types of studies
Randomized, quasi‐randomized or cluster‐randomized trials comparing chlorhexidine vaginal douching during labour with placebo or other vaginal disinfectant to prevent (reduce) maternal and neonatal infections (excluding group B streptococcal and HIV).
Types of participants
All pregnant women with a gestational age greater than 28 weeks, considered to be in labour.
Types of interventions
Chlorhexidine vaginal douching during labour versus placebo or other vaginal disinfectant.
Types of outcome measures
Primary outcomes
1. Maternal outcomes
(a) Chorioamnionitis (variously defined by the authors);
(b) postpartum endometritis (variously defined by the authors).
2. Neonatal outcomes
(a) Neonatal sepsis (variously defined by the authors).
Secondary outcomes
1. Maternal outcomes
(a) Intrapartum fever;
(b) intrapartum treatment with antibiotics;
(c) maternal side effects (vaginal irritation, thrush, antimicrobial resistance);
(d) serious maternal complication of treatment (e.g. anaphylaxis);
(e) laparotomy for infection;
(f) hysterectomy;
(g) maternal death;
(h) satisfaction with care;
(i) length of hospital stay;
(j) postnatal depression;
(k) successful breastfeeding (variously defined by the authors);
(l) costs of care;
(m) antimicrobial resistance.
2. Neonatal outcomes
(a) Ophthalmia neonatorum;
(b) neonatal pneumonia by clinical assessment and/or chest X‐ray;
(c) neonatal meningitis by clinical assessment and/or culture;
(d) blood culture confirming sepsis;
(e) admission to neonatal intensive care unit;
(f) length of hospital stay;
(g) perinatal mortality;
(h) abnormal neurodevelopmental assessment at follow‐up.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 June 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
[For details of additional author searching carried out the previous version of the review (Lumbiganon 2004), please see Appendix 1.]
Searching other resources
We searched cited references from retrieved articles for additional studies. We reviewed abstracts and letters to the editor to identify randomized controlled trials that had not been published and reviewed editorials, indicating expert opinion, to identify and ensure that no key studies were missed for consideration for inclusion in this review.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeLumbiganon 2004.
For this update, two new studies were identified and excluded (Pereira 2011; Saleem 2006). We will use the methods outlined in Appendix 2 for new trials identified at the next update.
We used the following updated methods to assess the risk of bias and assess the quality of already included studies using the GRADE approach. The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Two review authors (PL and JT) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving the third review author (JET).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
We classified blinding as 'high risk of bias' if the blinding status of a trial was unclear or the trial was open.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed the methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
-
unclear risk of bias.
We used a cut‐off point of less than 20% of missing data to assess that a study is adequate.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update, the quality of the evidence was re‐assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison between intervention (chlorhexidine vaginal wash) and control groups.
-
Chorioamnionitis
-
Postpartum endometritis
-
Neonatal sepsis
-
Blood culture confirming sepsis
The GRADEprofiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
Two new studies (Pereira 2011; Saleem 2010) (both excluded) were identified from an updated search of the PCG Trials Register.
Included studies
We included three studies (Eriksen 1997; Rouse 1997; Rouse 2003) involving 3012 women in this review.
Eriksen 1997, from the USA, reported the effectiveness of chlorhexidine vaginal wash during labour to prevent neonatal infection. The data on peripartum infections of this study were reported in the other paper (Sweeten 1997). As they are both reports of the same trial we listed them under Eriksen 1997. Participants at 36 or more weeks' gestation in labour, excluding preterm labour, fetal distress, malpresentation, intra‐amniotic infection, cervical dilatation greater than 6 cm and known allergy to chlorhexidine were eligible for the study. Informed consent was obtained from 1024 women who were eligible. Of these, 77 were excluded from the analysis because of incomplete records (71, 38 in the control and 33 in the study group); three participants were enrolled and subsequently discharged home; the vaginal wash was not given to two women; and there was one infant with anencephaly. Of the remaining 947 participants, 481 were randomized to the study arm and 466 served as controls. A computer software program was used to generate a random block allocation sequence to assign participants to either group. The randomization assignments were contained in sequentially numbered, opaque, sealed packets that were made up independent of the physicians managing the participants. The authors chose not to blind the study because the syringe containing chlorhexidine solution was pink and the investigators could not reproduce the colour artificially in the syringe containing the sterile water. For maternal outcomes, it is not very clear whether the intention‐to‐treat analysis was performed because it was not stated that 77 women were excluded before or after randomization. For neonatal outcomes, intention‐to‐treat analysis was not done because 24 neonatal charts in the chlorhexidine group and 13 in the control group were unavailable for review.
Rouse 1997, from the USA, reported a double‐blinded clinical trial to determine whether chlorhexidine vaginal irrigation prevents maternal peripartal infection. Participants were eligible if they were admitted for delivery at or beyond 24 weeks' gestation. Exclusion criteria included a contra‐indication for cervical digital examination, active genital herpes, chorioamnionitis and known or suspected allergy to chlorhexidine. The chlorhexidine and placebo bottles were randomly ordered with a computer‐generated list and sequentially numbered with a peel‐off study label. The active and placebo solutions were clinically indistinguishable. Among 3234 eligible participants, 1024 were randomized, 508 in chlorhexidine and 516 in placebo groups respectively. Because of incomplete or contradictory data, treatment allocation could not be determined for additional 10 women and these women were not included in the analysis. Trial analysis was restricted to 1024 women and 1030 infants (six sets of twins).
Rouse 2003, from the USA, reported a clinical trial of chlorhexidine vaginal irrigation to prevent peripartal infection in nulliparas. The study was conducted in two hospitals serving predominantly publicly funded patients. Women were eligible if they were nulliparous and admitted for delivery at or beyond 32 weeks' gestation. Exclusion criteria included a contra‐indication to digital cervical examination, active genital herpes, chorioamnionitis and allergy to chlorhexidine. The chlorhexidine and placebo bottles were sequentially numbered (in groups of four) and randomly ordered based on a computer‐generated list (one for each hospital). Each study bottle contained a peel‐off label which, after use, was used to link participants to the correct study group. The chlorhexidine and placebo preparations were clinically indistinguishable. Four women (two women in each group) were enrolled but actually did not undergo irrigation. They are included in the intention‐to‐treat analysis.
Excluded studies
We excluded five studies from an updated search in October 2010 (Cutland 2009; Mushangwe 2006; Pereira 2006; Saleem 2007; Saleem 2010) and two additional studies (Pereira 2011; Saleem 2010) from the most recent updated search. In total, there are nine excluded studies. See the Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 1; Figure 2 for a summary of all 'Risk of bias' assessments.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Details for each trial are in the Characteristics of included studies table.
All included trials have a low risk of bias.
Allocation
All three included trials used computer‐generated allocation sequences and used appropriate random allocation concealment.
Blinding
Two trials (Rouse 1997; Rouse 2003) used indistinguishable placebo controls while one trial (Eriksen 1997) chose not to blind participants and personnel but the physicians managing infants in the nursery were unaware to which arm of the study each participant was randomized.
Incomplete outcome data
Two trials (Rouse 1997; Rouse 2003) obtained the outcomes of all recruited mothers while the other (Eriksen 1997) excluded 7.5% (77/1024) and 11.1% (114/1024) of mothers and neonates from the analysis respectively.
Selective reporting
We did not have the protocols for all three trials and therefore could not evaluate selective reporting.
Other potential sources of bias
There were no other obvious potential sources of biases.
Effects of interventions
We included three studies involving 3012 women in this review.
Chlorhexidine vaginal wash versus placebo
Primary outcomes
Maternal outcomes
Three trials (3012 women) (Eriksen 1997; Rouse 1997; Rouse 2003) reported the incidence of chorioamnionitis, including 1514 and 1498 participants in the chlorhexidine and placebo groups respectively. There was no statistically significant difference between the two groups (risk ratio (RR) 1.10; 95% confidence interval (CI) 0.86 to 1.42) (Analysis 1.1). The same three trials also reported the incidence of postpartum endometritis. Although the data suggest a small reduction in the risk of postpartum endometritis with the use of the chlorhexidine vaginal wash, the difference was not statistically significant (RR 0.83; 95% CI 0.61 to 1.13) (Analysis 1.2).
Neonatal outcomes
Three trials (2987 infants) (Eriksen 1997; Rouse 1997; Rouse 2003) reported on neonatal outcomes, involving 1495 and 1492 neonates in the chlorhexidine and placebo groups respectively. For neonatal sepsis, which was evaluated in the three trials (Eriksen 1997; Rouse 1997; Rouse 2003) involving 1495 and 1492 neonates in the chlorhexidine and placebo groups respectively, there was no significant difference (RR 0.75; 95% CI 0.17 to 3.35) (Analysis 1.7).
Secondary outcomes
Maternal outcomes
There was no report about the other maternal outcomes and side effects of chlorhexidine in these three trials.
Neonatal outcomes
Two trials involving 1038 and 1039 (2077) neonates in the intervention and control groups respectively (Rouse 1997; Rouse 2003) did not find significant difference in blood culture confirming sepsis (RR 0.75; 95% CI 0.17 to 3.35) (Analysis 1.6) and perinatal mortality (RR 1.00, 95% CI 0.17 to 5.79) (2071 infants, Analysis 1.8). One trial with 457 and 453 neonates in the intervention and control group respectively (Eriksen 1997), indicated that there was no significant difference in neonatal pneumonia (RR 0.33; 95% CI 0.01 to 8.09) (910 infants, Analysis 1.4). For neonatal meningitis, one trial with 508 and 516 neonates in the intervention and control groups respectively (Rouse 1997) did not show significant difference (RR 0.34; 95% CI 0.01 to 8.29) (1024 infants, Analysis 1.5). There was a trend that vaginal chlorhexidine during labour might lead to a higher tendency for newborns to receive antibiotics but this association is not statistically significant (RR 1.65; 95% CI 0.73 to 3.74) (one trial, 910 infants, Analysis 1.9). There was no report about the other neonatal outcomes and side effects of chlorhexidine in these three trials.
Discussion
Although all three included trials are of high quality, one trial (Eriksen 1997) used only 20 mL of chlorhexidine or sterile water for vaginal irrigation, while the other two trials (Rouse 1997; Rouse 2003) used 200 mL of chlorhexidine or sterile saline solution. The effectiveness of vaginal chlorhexidine might also depend on the volume of the solution used for irrigation. Since chlorhexidine solution is quite safe, not expensive and vaginal irrigation is not difficult to perform, there is a need for a well‐designed randomized controlled trial with adequate sample size to evaluate this simple intervention. However, the investigators of future trials must use the appropriate concentration and volume of vaginal chlorhexidine irrigation solution. We have identified two ongoing studies from the trial registration website www.clinicaltrials.gov (Madhi 2006; Moss 2006) since the review was first published.
Summary of main results
There was no evidence of an effect of vaginal chlorhexidine during labour in preventing maternal and neonatal infections.
Overall completeness and applicability of evidence
All three included studies were conducted in the USA. This limits the completeness and applicability of evidence to other parts of the world.
Quality of the evidence
All three included studies were of overall low risk of bias; however, only 3012 women were included in total. This review might not have enough power to detect small effect size. Assessment of the quality of the evidence using GRADE indicated that the levels of evidence for all primary outcomes and one important secondary outcome were low to moderate due to wide confidence interval crossing the line of no effect with few events (summary of findings Table for the main comparison).
Potential biases in the review process
We carefully conducted this review following all steps recommended by the Cochrane Pregnancy and Childbirth Review Group. All review authors have no conflict of interest. There should not be any bias in the review process.
Agreements and disagreements with other studies or reviews
One Cochrane review (Stade 2008) has evaluated the effectiveness of vaginal chlorhexidine during labour for preventing early‐onset group B streptococcal infection in newborn infants. Although there was a significant reduction in group B streptococcal colonization in neonates, there were no significant reductions in early‐onset group B streptococcal sepsis, pneumonia, meningitis and mortality. One randomized controlled trial, involving 8011 women and their 8129 newborns, evaluated the effectiveness of chlorhexidine intravaginally and neonatal wipes for preventing early‐onset neonatal sepsis (Cutland 2009). There was no significant reductions in either early‐onset neonatal sepsis nor colonization of group B streptococcal. Another trial, involving 5008 women and their neonates, reported that chlorhexidine vaginal wipes during labour together with neonatal chlorhexidine wipes did not prevent neonatal sepsis, maternal and perinatal mortality (Saleem 2010).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 1 Chorioamnionitis.
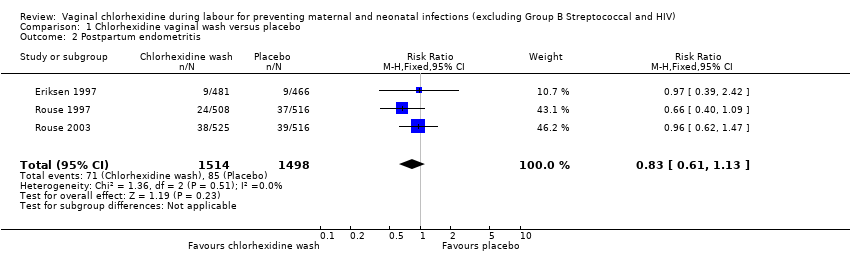
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 2 Postpartum endometritis.

Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 3 Side effects.

Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 4 Neonatal pneumonia.

Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 5 Neonatal meningitis.

Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 6 Blood culture confirming neonatal sepsis.

Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 7 Neonatal sepsis.
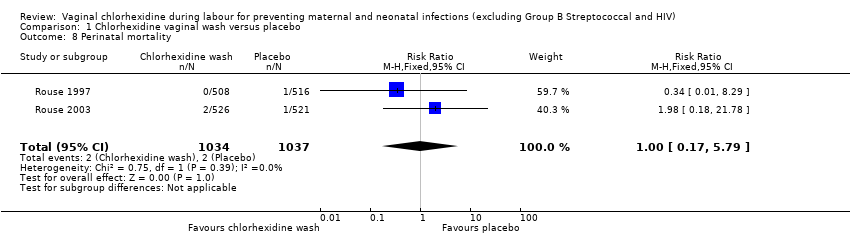
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 8 Perinatal mortality.
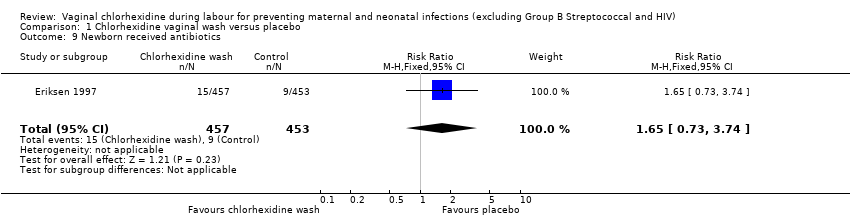
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 9 Newborn received antibiotics.
| Chlorhexidine vaginal wash versus placebo for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV) | ||||||
| Population: Pregnant women with a gestational age greater than 28 weeks, considered to be in labour receiving chlorhexidine vaginal douching during labour versus placebo to prevent maternal and neonatal infections (excluding Group B Streptococcal and HIV). | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chlorhexidine vaginal wash versus placebo | |||||
| Chorioamnionitis | Study population | RR 1.1 | 3012 | ⊕⊕⊕⊝ | ||
| 69 per 1000 | 76 per 1000 | |||||
| Moderate | ||||||
| 62 per 1000 | 68 per 1000 | |||||
| Postpartum endometritis | Study population | RR 0.83 | 3012 | ⊕⊕⊕⊝ | ||
| 57 per 1000 | 47 per 1000 | |||||
| Moderate | ||||||
| 72 per 1000 | 60 per 1000 | |||||
| Neonatal sepsis | Study population | RR 0.75 | 2987 | ⊕⊕⊝⊝ low2 | ||
| 3 per 1000 | 2 per 1000 | |||||
| Moderate | ||||||
| 4 per 1000 | 3 per 1000 | |||||
| Blood culture confirming neonatal sepsis | Study population | RR 0.75 | 2077 | ⊕⊕⊝⊝ low2 | ||
| 4 per 1000 | 3 per 1000 | |||||
| Moderate | ||||||
| 4 per 1000 | 3 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Confidence interval crossing the line of no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Chorioamnionitis Show forest plot | 3 | 3012 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.86, 1.42] |
| 2 Postpartum endometritis Show forest plot | 3 | 3012 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.13] |
| 3 Side effects Show forest plot | 2 | 2065 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Neonatal pneumonia Show forest plot | 1 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Neonatal meningitis Show forest plot | 1 | 1024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.29] |
| 6 Blood culture confirming neonatal sepsis Show forest plot | 2 | 2077 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.35] |
| 7 Neonatal sepsis Show forest plot | 3 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.35] |
| 8 Perinatal mortality Show forest plot | 2 | 2071 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.17, 5.79] |
| 9 Newborn received antibiotics Show forest plot | 1 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.73, 3.74] |

