Effets comparés des régimes hyposodés et à teneur en sodium élevée sur la tension artérielle, la rénine, l’aldostérone, les catécholamines, le cholestérol et les triglycérides
Résumé scientifique
Contexte
Bien que l’on cherche, depuis plus de 100 ans, à savoir si une réduction de la consommation de sodium améliore la santé, la question n’est toujours pas tranchée.
Objectifs
Estimer les effets d’un apport faible en sodium comparé à un apport riche en sodium sur la tension artérielle systolique et diastolique (TAS et TAD) ainsi que sur les niveaux de rénine, d’aldostérone, de catécholamines, de cholestérol, de lipoprotéine de haute densité (HDL), de lipoprotéine de faible densité (LDL) et de triglycérides dans le sérum ou le plasma.
Stratégie de recherche documentaire
Le Spécialiste de l’information du Groupe Cochrane sur l’hypertension a effectué des recherches dans les bases de données suivantes pour trouver des essais contrôlés randomisés jusqu’à mars 2016 : registre spécialisé du groupe Cochrane sur l’hypertension artérielle, registre Cochrane des essais contrôlés (CENTRAL) (2016, numéro 3), MEDLINE (à partir de 1946), Embase (à partir de 1974), Système d’enregistrement international des essais cliniques de l’OMS (ICTRP) et ClinicalTrials.gov. Nous avons également effectué des recherches dans les listes bibliographiques des articles pertinents.
Critères de sélection
Des études randomisant des sujets entre des régimes à apports réduits et élevés de sodium ont été incluses si elles évaluaient au moins un des paramètres de résultats ci‐dessus.
Recueil et analyse des données
Les données ont été recueillies de manière indépendante par deux auteurs de la revue et analysées à l’aide de Review Manager 5.3.
Résultats principaux
Au total, 185 études ont été incluses. Les apports moyens de sodium ont été réduits de 201 mmol/jour (ce qui correspond aux apports élevés habituels) à 66 mmol/jour (ce qui correspond aux apports recommandés).
L’effet de la réduction du sodium sur la tension artérielle (TA) était le suivant : Caucasiens normotendus : TAS : différence moyenne (DM) de ‐1,09 mmHg (intervalle de confiance (IC) à 95 % de ‐1,63 à ‐0,56 ; P = 0,0001) ; 89 études, 8569 participants ; TAD : DM +0,03 mmHg (IC à 95 % de ‐0,37 à 0,43 ; P = 0,89) ; 90 études, 8833 participants. Données probantes de bonne qualité.
Noirs normotendus : TAS : DM de ‐4,02 mmHg (IC à 95 % de ‐7,37 à ‐0,68 ; P = 0,002) ; sept études, 506 participants ; TAD : DM de ‐2,01 mmHg (IC à 95 % de ‐4,37 à 0,35 ; P = 0,09) ; sept études, 506 participants. Données de qualité moyenne.
Asiatiques normotendus : TAS : DM de ‐0,72 mmHg (IC à 95 % de ‐3,86 à 2,41 ; P = 0,65) ; TAD : DM de ‐1,63 mmHg (IC à 95 % de ‐3,35 à 0,08 ; P = 0,06) ; trois études, 393 participants. Données de qualité moyenne.
Caucasiens hypertendus : TAS : DM de ‐5,51 mmHg (IC à 95 % de ‐6,45 à ‐4,57 ; P < 0,00001) ; 84 études, 5925 participants ; TAD : DM de ‐2,88 mmHg (IC à 95 % de ‐3,44 à ‐2,32 ; P < 0,00001) ; 85 études, 6001 participants. Données probantes de bonne qualité.
Noirs hypertendus : TAS : DM de ‐6,64 mmHg (IC à 95 % de ‐9,00 à ‐4,27 ; P = 0,00001) ; huit études, 619 participants ; TAD de ‐2,91 mmHg (IC à 95 % de ‐4,52 à ‐1,30 ; P = 0,0004) ; huit études, 619 participants. Données de qualité moyenne.
Asiatiques hypertendus : TAS : DM de ‐7,75 mmHg (IC à 95 % de ‐11,44 à ‐4,07 ; P < 0,0001) neuf études, 501 participants ; TAD : DM de ‐2,68 mmHg (IC à 95 % de ‐4,21 à ‐1,15 ; P = 0,0006). Données de qualité moyenne.
Dans le plasma ou le sérum, on constate une augmentation significative de la rénine (P < 0,00001), de l’aldostérone (P < 0,00001), de la noradrénaline (P < 0,00001), de l’adrénaline (P < 0,03), du cholestérol (P < 0,0005) et des triglycérides (P < 0,0006) avec une consommation réduite de sodium par rapport à une forte consommation de sodium. Tous ces effets étaient stables dans 125 populations d’étude ayant reçu des apports de sodium inférieurs à 250 mmol/jour, dans le cadre d’une intervention de réduction du sodium ayant duré au moins une semaine.
Conclusions des auteurs
La réduction des apports de sodium moyens du niveau élevé habituel (201 mmol/jour) à un apport de 66 mmol/jour, qui est inférieur à la limite haute recommandée de 100 mmol/jour (5,8 g de sel), a entraîné une baisse de la TAS et de la TAD de 1/0 mmHg chez les participants caucasiens normotendus et une baisse de la TAS/TAD de 5,5/2,9 mmHg chez les participants caucasiens hypertendus. Quelques études ont montré que ces effets étaient plus prononcés dans les populations d’origine africaine et asiatique. Les effets sur les hormones et les lipides étaient similaires chez les sujets normotendus et hypertendus. La rénine a augmenté de 1,60 ng/ml/heure (55 %), l’aldostérone de 97,81 pg/ml (127 %), l’adrénaline de 7,55 pg/ml (14 %), la noradrénaline de 63,56 pg/ml (27 %), le cholestérol de 5,59 mg/dl (2,9 %) et les triglycérides de 7,04 mg/dl (6,3 %).
PICO
Résumé simplifié
Effet d’un régime alimentaire pauvre en sel sur la tension artérielle, certaines hormones et certains lipides chez les personnes normotendues et hypertendues
Question de la revue
Des études dans lesquelles les participants étaient répartis au hasard dans des groupes recevant des apports de sel élevés et réduits ont été analysées afin d’étudier l’effet de la réduction de la consommation de sel sur la tension artérielle (PA) et les effets secondaires potentiels de la réduction du sodium sur certaines hormones et certains lipides.
Contexte
Comme une diminution de la consommation de sel fait baisser la tension artérielle (TA) des sujets hypertendus, on entend souvent conseiller de réduire la consommation de sel. Cependant, l’effet de la réduction du sel sur la tension artérielle des personnes normotendues a été remis en cause. En outre, plusieurs études ont montré que la réduction du sel activait le système hormonal convervateur du sodium (rénine et aldostérone), les hormones de stress (adrénaline et noradrénaline) et augmentait les lipides (cholestérol et triglycérides dans le sang).
Date de la recherche
Les données probantes actuelles sont à jour à la date d’avril 2016.
Caractéristiques de l'étude
Cent quatre‐vingt‐cinq études interventionnelles portant sur 12 210 sujets et d’une durée de 4 à 1100 jours, évaluant au moins une mesure de l’effet, ont été incluses. Les participants étaient en bonne santé ou avaient une tension artérielle élevée. Les études longitudinales ont montré que l’effet de la réduction de la consommation de sel sur la TA était stable après 7 jours au maximum et les études de population ont montré que très peu de patients consommaient plus de 14,5 g de sel par jour. Par conséquent, nous avons également réalisé des sous‐analyses de sous‐groupes de 125 études, d’une durée d’au moins sept jours et comportant un apport en sel de 14,5 g au maximum.
Sources de financement des études
Quarante‐quatre études ne mentionnaient pas de soutien. Cent vingt‐deux études étaient soutenues par des fonds publics. Douze études ont été financées par l’industrie pharmaceutique et une étude par une société d’électronique. Six études ont été financées par des organisations de l’industrie alimentaire.
Principaux résultats
L’apport moyen en sodium alimentaire a été réduit de 11,5 g par jour à 3,8 g par jour. La réduction de la TAS/TAD chez les sujets normotendus était d’environ 1/0 mmHg, et chez les sujets hypertendus d’environ 5,5/2,9 mmHg. En revanche, l’effet sur les hormones et les lipides était similaire chez les sujets normotendus et hypertendus. La rénine a augmenté de 1,60 ng/ml/h (55 %), l’aldostérone de 97,81 pg/ml (127 %), l’adrénaline de 7,55 pg/ml (14 %), la noradrénaline de 63,56 pg/ml (27 %), le cholestérol de 5,59 mg/dl (2,9 %) et les triglycérides de 7,04 mg/dl (6,3 %).
Qualité des données probantes
Seuls les essais contrôlés randomisés ont été inclus et le niveau de données probantes de base était donc considéré comme élevé, bien qu’il ait été rabaissé dans certaines analyses plus petites. En général, la description de la procédure de randomisation était insuffisante, ce qui introduisait un biais pouvant exagérer les effets, mais de nombreuses études ont été publiées à une époque où il n’était pas habituel de rapporter ces éléments. La majorité des études étaient ouvertes, mais leurs résultats n’étaient pas différents de ceux des études en double aveugle. Presque toutes les études chez des participants ayant une tension artérielle (TA) normale ne montrent aucun effet significatif de la réduction du sodium sur la TA, alors qu’un grand nombre d’études chez des sujets souffrant d’hypertension ont bien montré un effet significatif. Il y a donc un niveau élevé de cohérence entre les résultats des différentes études et ceux des méta‐analyses. Les analyses de sensibilité des études d’une durée d’au moins une semaine (le délai d’efficacité maximale) confirment les analyses principales. Enfin, l’impact des intérêts commerciaux sur les critères de jugement était négligeable.
Authors' conclusions
Summary of findings
| Low sodium intake compared with high sodium intake for blood pressure | ||||
| Patient or population: White population with normal or elevated blood pressure, but otherwise healthy Settings: Hospitals units in Europe and North America Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| White population, normotensive, SBP mmHg | ‐1.09 (‐1.63 to ‐0.56) | 8569 | ⊕⊕⊕⊕ | |
| White population, normotensive, DBP mmHg | 0.03 (‐0.37 to 0.43) | 8833 | ⊕⊕⊕⊕ | |
| White population, hypertensive, SBP mmHg | ‐5.51 (‐6.45 to ‐4.57) | 5925 | ⊕⊕⊕⊕ | |
| White population, hypertensive, DBP mmHg | ‐2.88 (‐3.44 to ‐2.32) | 6001 | ⊕⊕⊕⊕ | |
| GRADE Working Group grades of evidence DBP: diastolic blood pressure; SBP: systolic blood pressure | ||||
| Low sodium intake compared with high sodium intake for blood pressure | ||||
| Patient or population: Black population with normal or elevated blood pressure, but otherwise healthy Settings: Hospital units in North America, UK and Africa Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Mean difference | No of Participants | Quality of the evidence | Comments |
| Black population, normotensive, SBP mmHg | ‐4.02 (‐7.37 to ‐0.68) | 506 | ⊕⊕⊕⊝ | |
| Black population, normotensive, DBP mmHg | ‐2.01 (‐4.37 to 0.35) | 506 | ⊕⊕⊕⊝ | |
| Black population, hypertensive, SBP mmHg | ‐6.64 (‐9.00 to ‐4.27) | 619 | ⊕⊕⊕⊝ | |
| Black population, hypertensive, DBP mmHg | ‐2.91 (‐4.52 to ‐1.30) | 619 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence DBP: diastolic blood pressure; SBP: systolic blood pressure | ||||
| 1. Downgraded due to the wide confidence intervals | ||||
| Low sodium intake compared with high sodium intake for blood pressure | ||||
| Patient or population: Asian population with normal or elevated blood pressure, but otherwise healthy Settings: Hospital units in Japan and China Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Mean difference | No of Participants | Quality of the evidence | Comments |
| Asian population, normotensive, SBP mmHg | ‐0.72 (‐3.86 to 2.41) | 393 | ⊕⊕⊕⊝ | |
| Asian population, normotensive, DBP mmHg | ‐1.63 (‐3.35 to 0.08) | 393 | ⊕⊕⊕⊝ | |
| Asian population, hypertensive, SBP mmHg | ‐7.75 (‐11.44 to ‐4.07) | 501 | ⊕⊕⊕⊝ | |
| Asian population, hypertensive, DBP mmHg | ‐2.68 (‐4.21 to ‐1.15) | 501 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence DBP: diastolic blood pressure; SBP: systolic blood pressure | ||||
| 1. Downgraded due to the wide confidence intervals | ||||
| Low sodium intake compared with high sodium intake for hormones | ||||
| Patient or population: Participants with normal or elevated blood pressure, but otherwise healthy Settings: Hospital units Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Mean difference | No of Participants | Quality of the evidence | Comments |
| Renin SMD | 1.22 (1.07 to 1.37) N*: 1.44 (1.24 to 1.65) H*: 0.91 (0.71 to 1.10) | 5498 | ⊕⊕⊕⊕ | |
| Aldosterone pg/mL | 97.81 (82.56 to 113.05) N*: 115.83 (91.74 to 139.91) H*: 73.02 (55.94 to 90.09) | 4884 | ⊕⊕⊕⊕ | |
| Noradrenaline pg/mL | 63.56 (42.66 to 84.46) N*: 66.50 (41.72 to 91.29) H*: 57.36 (14.10 to 100.61) | 1736 | ⊕⊕⊕⊕ | |
| Adrenaline pg/mL | 7.55 (0.85 to 14.26) N*:4.45 (3.43 to 12.33) H*:13.45 (1.25 to 25.66) | 662 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence Very low quality: We are very uncertain about the estimate. SMD: standardised mean difference | ||||
| N*: Study populations with mean SBP < 140 mmHg H*:Study populations with mean SBP > 140 mmHg 1. Downgraded due to the wide confidence interval | ||||
| Low sodium intake compared with high sodium intake for lipids | ||||
| Patient or population: Participants with normal or elevated blood pressure, but otherwise healthy Settings: Hospital units Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Mean difference | No of Participants | Quality of the evidence | Comments |
| Cholesterol mg/dL | 5.64 (2.46, 8.82) N*:7.46 (3.65, 11.28) H*:2.55 (‐2.69, 7.80) | 1800 | ⊕⊕⊕⊝ | |
| Trigyceride mg/dL | 7.04 (3.04, 11.05) N*: 6.88 (1.18, 12.59) H*: 7.19 (1.57, 12.81) | 1390 | ⊕⊕⊕⊝ | |
| High‐density lipoprotein (HDL) mg/dL | ‐0.29 (‐1.66, 1.08) | 1442 | ⊕⊕⊕⊝ moderate1 | |
| Low‐density lipoprotein (LDL) mg/dL | 3.12 (‐0.41, 6.64) | 1358 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence | ||||
| Downgraded due to the wide confidence intervals. | ||||
Background
Description of the condition
Some health institutions (WHO 2012), and dietary recommendations (ADG 2015), assume that reduction in salt intake from "high" to "low" levels is associated with reduction in systolic and diastolic blood pressure (SBP and DBP), which might result in a decrease in mortality. However, the definitions of “high”, “normal” and “low” sodium intake are unclear. The present usual sodium intake indicates that an intake in the interval 109 mmol/day to 209 mmol/day (McCarron 2013; Powles 2013, Table 1) would be “normal”, a high sodium intake would be above 209 mmol/day and a low sodium intake would be below 109 mmol/day, but according to the health institutions a “normal” sodium intake is below 100 mmol/day (ADG 2015), or below 87 mmol/day (WHO 2012), and a sodium intake above 100 mmol/day is “high”, whereas a “low” sodium intake is not defined. The confusion is strengthened by the use of different terms to describe salt (salt (sodium chloride) and sodium) and different units for salt/sodium intake (mg/day or mmol/day). To reduce the confusion we have shown the different definitions and units for salt and sodium intake in Table 1. In the present review, which represents a third update of the first meta‐analysis that includes an analysis of hormones and lipids in addition to blood pressure (Graudal 1998), updated in 2003 (Jürgens 2003) and 2011 (Graudal 2011), we use the term "sodium" and the unit "mmol".
| Reference | Recommended upper level* | World, lower range* | World, lower 2.5%* | World, mean* | World, Upper 97.5%* | World, upper range* |
| 100 (2300) (5800) | ||||||
| 87 (2000) (5046) | ||||||
| 90 (2070) (5220) | 109 (2500) (6320) | 159 (3660) (9220) | 209 (4810) (12120) | 248 (5700) (14400) | ||
| 95 (2200) (5510) | 172 (3950) (10000) | 240 (5520) (13920) |
1. number: mmol; 2. number: mg sodium; 3. number: mg sodium chloride
Blood pressure is associated with mortality (Collins 1990).The hypothesis that a reduced sodium intake (sodium reduction) will reduce blood pressure (BP) and subsequently reduce morbidity and mortality was raised in 1904 on the basis of individual patient cases (Ambard 1904). Subsequently in 1907, these results were opposed (Löwenstein 1907). The clinical and physiological effects of salt published in studies during the first half of the 20th century were reviewed in 1949 (Chapman 1949). Consequently, scientific studies have been performed for almost 70 years before modern standard scientific randomised controlled trials (RCTs) (1000 Parijs 1973) and observational studies (Kagan 1985) were performed in humans. However, these scientific studies are interpreted differently (Taubes 1998, Graudal 2005, Bayer 2012). While health institutions (ADG 2015, WHO 2012) support sodium reduction below 100 mmol/day sceptics have claimed that this recommended upper limit (UL) for sodium intake is based on a biased selection of evidence (Folkow 2011), and is inconsistent with Institute of Medicine’s definition of an adequate nutrient intake, which is “the approximate intake found in apparently healthy populations" (IOM 2006; Heaney 2013). For sodium "the approximate intake in apparently healthy populations" is between 90 mmol/day and 248 mmol/day (Table 1).
The present Cochrane review is based on a meta‐analysis published in 1998 (Graudal 1998). In 1998, the usual sodium intake was known in some populations, but it was not well‐defined worldwide until recently (Table 1). The present upper level of 100 mmol/day was defined in 2005 (IOM 2005). Furthermore, the significance of the duration of sodium reduction was not established. In 1998, we therefore included all available randomised studies, irrespective of sodium intake and duration of intervention, assuming that the average values of multiple studies would be relevant for the general population. We separated study populations in a group of populations with normal BP to investigate the potential effect of sodium reduction in the general population and in a group of hypertensive populations to investigate the potential effect of sodium reduction as a treatment for hypertensive individuals. In a cross‐sectional multiple regression analysis including many covariates we found that the duration of the sodium reduction intervention had no impact on the effect of sodium reduction on BP (Graudal 1998). In addition to this cross‐sectional meta‐regression analysis, a recent meta‐analysis of longitudinal studies measuring the BP‐effect of sodium reduction several times during the observation period showed that there was no difference in SBP effect or DBP effect between week one and week six, thus estimating the time point for maximal efficacy to be at maximum at one week (Graudal 2015). These results are shown in Table 2. In the Graudal 1998 analysis, the average sodium intake in the non‐reduced group was 203 mmol/day and in the reduced group it was 62 mmol/day. In the two following updates of the review, the corresponding sodium reductions were from 205 mmol/day to 64 mmol/day (Jürgens 2003) and from 202 mmol/day to 67 mmol/day (Graudal 2011). We now know (McCarron 2013; Powles 2013) that this reduction corresponds to a reduction from a high usual level to the present recommended levels (defined in 2005 (IOM 2005) and in 2012 (WHO 2012) i.e. the present review is relevant in the context of evaluating the consequences of the present recommendations to reduce sodium intake to a level below 100 mmol/day.
Data from Graudal 2015
Description of the intervention
As in the previous meta‐analyses, RCTs are included, which allocate participants to two diets with a different content of salt (sodium chloride) or to either salt tablets or placebo tablets. The compliance in the RCTs is ensured by measurement of sodium excretion in the urine, which is accepted to be a reliable surrogate for the measuring of sodium intake. The sodium content of the “high” and “low” sodium diets were not defined according to the recommendations or the usual sodium intake, but just to describe the relative content of the two randomised study populations.
How the intervention might work
Extracellular fluid volume (ECFV) is determined by the balance between sodium intake and renal excretion of sodium. A steady state exists whereby sodium intake equals output, while ECFV is expanded during salt loads and shrunken during salt restriction (Palmer 2008). Thus, the idea behind sodium reduction is to shrink ECFV in order to decrease BP. The precondition for this idea is that the smaller ECFV associated with the decrease in BP has no counteracting effects on health outcomes that could outweigh the BP‐effect.
Why it is important to do this review
A verification of the hypothetical sodium‐BP relationship would support continuous attempts to lower sodium intake in order to reduce mortality. In this context it is important to define the correct UL for a healthy sodium intake, which would have a significant impact on the strategy to lower sodium intake. For instance if 100 mmol/day is the correct UL, more than 95% of the World’s populations should reduce sodium intake, but if the UL is 250 mmol/day, only about 5% should reduce sodium intake. In the latter case, a strategy to lower sodium intake in the general population would not be necessary, which would save significant efforts and costs. The same would be the case if the sodium‐BP relationship could be denied, as indicated by many RCTs of participants with normal BP (Graudal 2011). Worst case scenario is that sodium reduction could lead to side effects, which might trump the potential BP effect and result in increased mortality, as indicated by longitudinal observational studies (Alderman 2010, Pfister 2014, O'Donnell 2014, Graudal 2014; Mente 2016). Consequently, it is important to investigate the effect of sodium reduction not only on BP, but also on potential surrogate markers for clinical side effects.
Objectives
The purpose of the present review was to estimate the influence of low‐ versus high‐dietary sodium intake on systolic blood pressure (SBP) and diastolic blood pressure (DBP), and blood concentrations of renin, aldosterone, catecholamines, cholesterol, high‐density lipoprotein (HDL), low‐density lipoprotein (LDL) and triglyceride to contribute to the evaluation of the possible suitability of sodium reduction as a prophylaxis initiative and treatment of hypertension.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) allocating participants to diets with different sodium contents, the lowest defined as “low” and the highest defined as “high”, and in which the sodium intake was estimated by the 24‐hour urinary sodium excretion (either measured on the basis of a 24‐hour urine collection, or estimated from a sample of at least eight hours).
Types of participants
Persons with normal or elevated blood pressure irrespective of race and age were included. Studies systematically investigating unhealthy patients with other diseases than elevated blood pressure, for instance diabetes or heart failure, were excluded.
Types of interventions
The intervention was a change in sodium intake, the study populations randomly being divided into a group eating a “low” sodium diet or a "high" sodium diet. As "low" and "high" were not specifically defined in relation to the usual intake or the definitions of the health institutions (Table 1), both diets could contain any amount of sodium, the assumption being that in most studies a "low" sodium diet would contain sodium within the low range (< 100 mmol)/day or usual range (100 mmol to 250 mmol/day) and the “high” sodium diet would contain sodium within the usual range (100 mmol to 250 mmol/day) or above the usual range (≥ 250 mmol/day). Confounding was not allowed, i.e. studies treating persons with a concomitant intervention such as an antihypertensive medication, potassium supplementation or weight reduction were only included if the concomitant intervention was identical during the low and the high‐sodium diet.
Types of outcome measures
Outcome measures were effects on SBP, DBP, renin, aldosterone, adrenaline, noradrenaline, triglyceride, cholesterol, LDL and HDL. In studies reporting BP only as mean arterial pressure (MAP), SBP was estimated from SBP = 1.3 MAP + 1.4, and DBP was estimated from DBP = 0.83 MAP – 0.7 (Tozawa 2002). Separate meta‐analyses were performed for each outcome measure. Concerning blood pressure, participants were stratified according to ethnicity (Whites, Blacks and Asians) and according to level of blood pressure (hypertension or normotension). Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg. Study populations in which participants were treated with antihypertensive treatment were defined as hypertensive irrespective of baseline BP. In studies that investigated different ethnicities and different BP levels, the first priority was to separate these subgroups. If separate data were not given, the study data would be analysed according to the biggest subgroup. Concerning all other outcome variables, no stratifications were performed.
Primary outcomes
All outcomes were considered primary outcomes.
Secondary outcomes
None.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches in the following databases for randomised controlled trials without language, publication year or publication status restrictions:
-
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (searched 7 March 2016);
-
the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 3) via the Cochrane Register of Studies Online (CRSO) (searched 7 March 2016);
-
MEDLINE Ovid (from 1946 onwards), and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 7 March 2016);
-
Embase Ovid (searched 7 March 2016);
-
ClinicalTrials.gov (www.clinicaltrials.gov) searched 7 March 2016).
The Hypertension Group Specialised Register includes controlled trials from searches of CAB Abstracts & Global Health, CINAHL, Cochrane Central Register of Controlled Trials, Embase, MEDLINE, ProQuest Dissertations & Theses, PsycINFO, Web of Science and the WHO International Clinical Trials Registry Platform (ICTRP).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the sensitivity and precision‐maximising search strategy designed by Cochrane for identifying randomised controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.d. (Handbook 2011)). Search strategies for major databases are provided in Appendix 1.
Searching other resources
-
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE for systematic reviews) to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
-
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
-
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
Searches carried out for previous versions of this review
Trial search: Parijs and colleagues published the first RCT of the effect of sodium reduction on BP in 1973 (1000 Parijs 1973). In our first meta‐analysis (Graudal 1998), a literature search in MEDLINE (1966‐through December 1997) was performed using the following combinations of search terms: 1) salt or sodium, 2) restriction or dietary, 3) blood pressure or hypertension, 4) randomized or random. We combined 1, 2, 3 and 4 and found 291 references. Of these, 76 randomised trials from 60 references met the inclusion criteria. From the reference lists of these articles and from four previous meta‐analyses (Grobbee 1986, Law 1991, Cutler 1991, Midgley 1996), an additional 23 references reporting on 39 trials were identified, resulting in a total of 83 references.
Similar searches were made for hormones and lipids changing the third search term (blood pressure or hypertension) with the hormone or lipid term resulting in additional five sub‐studies dealing with hormones and lipids (Jula‐Karanko 1992, Jula‐Mäki 19921026 Koolen 1984(2), 1104 Overlack 1993, Ruppert 1994). Of these 88 references, three dealing exclusively with diabetes patients were excluded in the 2003 update (Dodson 1989, Mühlhauser 1996, Miller 1997).
In January 2002, a repeated search was performed through December 2001, revealing an additional 12 references, of which one was excluded because it only included patients with diabetes (Imanishi 2001). Accordingly, the 2004 updated review included a total of 96 references.
In December 2009, a literature search for the 2011 update was performed from 1950 through December 2009. This search revealed a total of 511 references in Ovid MEDLINE, 282 in Ovid EMBASE and 1428 in Cochrane CENTRAL. Headlines and abstracts were read and 44 articles from MEDLINE (26 included), eight from Embase (one included) and 129 from CENTRAL (45 included) were retrieved as full‐text papers for further review. A total of 72 new references investigating at least one of the effect variables met the inclusion criteria for this review. The search was not limited to English language studies. Two studies in Italian were identified and included. During the present revision, we discovered that in a few of the previously included studies, some subgroup data were published in two papers. To avoid duplication due to including subgroup data from several papers, we included them from the main paper only. As a result, three previously included references were excluded (Steegers 1991, Ruppert 1991, Ruppert 1994). The most recent search was performed on July 21, 2011, revealing 293 additional references. After screening of titles and abstracts, four full‐text papers were retrieved, of which two contained data to be included. Consequently a total of 167 studies were supposed to be included in the 2011 updated version of this systematic review. However, in connection with the present update, a recount revealed a counting error, as the number of references in reality was 166.
Data collection and analysis
Selection of studies
See Search methods for identification of studies.
Review author NG performed the study selection for the 1998 version (Graudal 1998) and the 2003 version (Jürgens 2003). Review authors NG and GJ independently performed the supplementary study selection for the 2011 version (Graudal 2011. NG and THG independently performed the supplementary study selection for the current 2016 version. Discrepancies were resolved by agreement.
Data extraction and management
Two authors independently recorded the following data from each trial:
-
the sample size (N);
-
the mean age of participants;
-
the fraction of females, males; Whites, Blacks and Asians;
-
the duration of the intervention;
-
the sodium reduction measured as the difference between 24‐hour urinary sodium excretion during low‐sodium and high‐sodium diets and standard deviation (SD);
-
SBP (SD) and DBP (SD) before and after intervention;
-
difference between changes in SBP and DBP obtained during low‐sodium and high‐sodium diets and the SD of these differences;
-
for cross‐over studies, when possible, the overall effect estimate and standard error (SE);
-
levels of hormones and lipids in the blood and their standard deviations during low‐sodium and high‐sodium diets. Concerning lipids, cholesterol units of mmol/L were transformed to mg/dL by means of the factor 38.6 and triglyceride units of mmol/L were transformed to mg/dL by means of the factor 88.4. Other renin units than ng/mL/hour were when possible transformed to ng/mL/hour, and units of aldosterone, noradrenalin and adrenalin other than pg/mL were transformed to pg/mL by means of the molecular weights.
If there were discrepancies between review authors they looked at the data together and came to an agreement.
Assessment of risk of bias in included studies
This was performed using the Cochrane 'Risk of bias' tool, including recording of allocation, blinding, incomplete outcome data and selective reporting. Subgroup analyses of the primary analysis of SBP were performed for contrasting sources of bias appearing from the 'Risk of bias' analysis.
Measures of treatment effect
This was defined as the mean difference (MD) between the changes from baseline to end of treatment during low‐ and high‐sodium diets. When units within an analysis were different the standardised mean difference (SMD) was used.
Unit of analysis issues
Blood pressure (BP)
Combined analyses were performed including both parallel and cross‐over studies. The generic inverse variance data type was used to analyse the effect in order to ensure that the weight of the cross‐over studies was not underestimated compared with the parallel studies. For parallel studies, the SE was calculated in the usual way as follows: SE (diff) = sqrt SE12 + SE22. For cross‐over studies the given SE (difference) was used. A linear regression equation linking the given SE to the calculated SE (sqrt SE12 + SE22) was calculated by means of the studies which reported both SE (difference) and SE on BP during both intervention periods. This equation was used to transform all calculated SEs to estimated “true” SEs (difference) in cross‐over studies that did not report SE (difference). In this way, it was ensured that cross‐over studies were attributed proper weight compared with the parallel studies. There were not enough studies to calculate separate equations for Black and Asian populations and therefore the equations calculated in the white populations were used to transform these SEs when necessary.
Hormones and lipids
The very few parallel studies were excluded and the large fraction of cross‐over studies were analysed separately. As the large majority of cross‐over studies reported separate data for each intervention period instead of overall estimates of effect, the continuous data type was used in the separate analyses of the cross‐over studies.
Dealing with missing data
If the SD was not reported it was calculated from a given SE, 95% confidence interval (CI), P value or t value, estimated from a figure or imputed from the formula SD (change) = sq root (SD1sq + SD2sq), SD1 is SD on blood pressure before intervention and SD2 is SD on blood pressure after intervention.
Assessment of heterogeneity
A Chi2 test included in the forest plot was used to assess whether observed differences in results are compatible with chance alone. A low P value (or a large Chi2 statistic relative to its degree of freedom) provides evidence of heterogeneity of intervention effects (variation in effect estimates beyond chance).
Assessment of reporting biases
Funnel plots were assessed for asymmetry. Selective reporting of SBP and DBP was recorded
Data synthesis
Individual study subgroup data defined before randomisation based on ethnicity and state of hypertension were included in the meta‐analysis as subgroups, whereas sodium sensitivity subgroups, which were defined by the authors of the individual studies after they had analysed the data, were combined by the present authors and subsequently the combined data were included in the meta‐analyses.
The mean difference (MD) was calculated for outcome measures with identical units in the included studies (BP without transformation of data (all measured as mmHg), adrenaline, aldosterone, noradrenalin and lipids, after transformation). The standardised mean difference (SMD) was calculated for outcome measures with different units (renin), but a separate calculation of MD for the majority of renin studies with identical unit (ng/mL/hour) was also performed. With the MD method, the difference in effect between two treatments is divided by the SD of the measurements. By that transformation, the effect measures become dimensionless and the outcomes from trials, which have used different units, can consequently be combined. As we accumulated data from a series of studies that had been performed by researchers operating independently, and as the goal of the analysis was to extrapolate to other populations, we used a random‐effects model in our primary analysis to estimate the summary measure as the mean of a distribution of effects.
Level of significance: In case of multiple independent comparisons, it is important to avoid coincidental significance. Ten meta‐analyses were performed. However, the SBP and DBP comparisons are not independent of each other and BP depends on renin and aldosterone as well as catecholamines. Concerning lipids, these are mutually dependent, whereas the dependency on BP and hormones is not obvious. Consequently, the 10 meta‐analyses could be sub‐classified into a group of meta‐analyses of mutually dependent BP and hormones and an independent group of meta‐analyses of mutually dependent lipid fractions. Consequently, the level of significance was reduced by means of the formula 1‐0.95 x 1/N = 1‐0.95 x 1/2 = 0.025, (N = number of independent investigations = 2).
Subgroup analysis and investigation of heterogeneity
Since the previous version of this review, we now have reasonable evidence to determine the time of maximal efficacy to be one week (Table 2). Therfore, there is a risk that studies lasting for less than one week may underestimate the effect of sodium reduction. Furthermore, evidence has appeared to indicate that all of the world’s populations have a mean sodium intake below 250 mmol/day (Table 1), and as dose‐response studies have indicated that sodium reductions from very high levels have bigger effects than reductions from usual levels (Graudal 2015), such studies may contribute to overestimate the effect. We therefore performed a subgroup analysis intending to eliminate these potential biases on SBP and DBP (stratified according to normal BP or hypertension) and renin, aldosterone, noradrenalin, adrenalin, cholesterol triglyceride, HDL and LDL by exclusion of studies with a duration of less than seven days and sodium intake above 250 mmol/day.
Sources of bias: subgroup analyses were performed for contrasting sources of bias appearing from the 'Risk of bias' analysis.
Sensitivity analysis
Sensitivity analyses were performed excluding studies giving rise to asymmetry in the funnel plots.
Results
Description of studies
Results of the search
During this 2016 update, we identified two studies with duplicate data, which were subsequently excluded (Jula‐Karanko 1992;Jula‐Mäki 1992), as all data could be extracted from a later paper (1110 Jula 1994).
In September 2014, a literature search for the present update was performed as described in "Search methods for identification of studies". The de‐duplicated results from the searches revealed 626 articles. On the basis of titles, 549 were excluded. Seventy‐seven abstracts were read and 27 full‐text articles obtained, of which, nine fulfilled the inclusion criteria. In a supplementary search in April 15 2015, an additional 102 references were identified. Six articles were obtained, of which three fulfilled the inclusion criteria.The last updated search was performed on 7 March 2016. The de‐duplicated results from the searches revealed 994 articles. During the primary screening, 687 were excluded and on the basis of titles and abstracts, a further 236 articles were eliminated. Seventy‐one abstracts were read in detail and 29 full‐text articles obtained, of which, seven fulfilled the inclusion criteria. Additionally, two articles were identified from a reference list of a review article. A WHO International Clinical Trials Registry Platform search using the search term “diet and sodium” revealed 141 trials, but none were included.
A total of 185 references (164 from the 2011 review plus 9 + 3 + 9 new references) were thus included in the present updated 2016 version.
Included studies
See Characteristics of included studies.
One hundred and eighty‐five references were included in the review. Eight included only data on hormones and lipids, whereas 177 included BP data, as well as hormone and lipid data in a significant number of these. The total number of study populations with BP outcomes included in the primary analysis was 206. The median of the mean ages was 44 years (range: 12 to 73), which is a little higher than the median age of most populations (typically 35 years) and the mean sodium intake in the high‐sodium group was 201 mmol/day (SD: 69) and in the low‐sodium group was 66 mmol/day (SD: 47), corresponding to a mean sodium reduction of 135 mmol/day.The median of the mean ages of the study's 125 white populations included in the subgroup analysis (duration of at least seven days, a sodium intake of less than 250 mmol/day) was 45.4 years (range: 13 to 73) the mean sodium intake in the high‐sodium group was 177 mmol/day (SD: 35) and in the low‐sodium group was 68 mmol/day (SD: 36), corresponding to a mean sodium reduction of 109 mmol/day. The mean BP in the normotensive study populations was 119/71 mmHg, which is close to the population mean of the USA population (119/71 mmHg) (Wright 2011), and a little higher than the mean of the normotensive fraction of the USA population (115/70 mmHg) (Wright 2011). The mean BP in the untreated hypertensive study populations was 151/93 mmHg and in the treated hypertensive study populations was 144/88 mmHg, both of which are higher than corresponding pressures in the USA population (146/84 mmHg and 131/72 mmHg) (Wright 2011).
In 83 studies including 7729 participants, there was information of the baseline 24‐hour sodium excretion, not influenced by diets. This was 159 mmol/24‐hour (range: 90‐274 mmol) (10‐90 percentiles: 123‐194 mmol).
Excluded studies
Risk of bias in included studies
See Characteristics of included studies and Figure 1

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
The obligatory trial quality criterion was randomisation. Double‐blind, single‐blind or open studies with a parallel or a cross‐over design were accepted. A study was defined as single‐blind if an investigator measured BP without knowledge of the diet or by a computerised manometer, and as open if precautions to decrease observer bias were not mentioned.
We found two important contrasts: general blinding and blinding of outcome detection (Figure 1). We performed subgroup analyses of BP in both normotensive and hypertensive white populations, but not in the black and Asian populations due to the small numbers of trials. We did not perform subgroup analyses on the biochemical outcomes (hormones and lipids) as they are supposed to be performed blindly in 100% of cases.
Allocation
Only 14 studies (1034 Watt 1985; 1078 Egan 1991; 1081 TOHP I 1992; 1107 MacFadyen 1994;1135 TOHP II 1997; 1136 van Buul 1997;1142 Knuist 1998; 1195 Jessani 2008; 1197 Dickinson 2009; 1198 He 2009; 1206 Graffe 2012; 1208 Todd 2012; 1217 Markota 2015; 1225 Gijsbers 2015), either partly or sufficiently explained the allocation sequence generation and concealment. Consequently, there is a general significant risk that allocation was not unbiased.
Blinding
Fifty‐six studies were reported to be double‐blind and in 115 studies, the risk of detection bias was estimated to be low (Figure 1). Separate analyses were performed on studies with low and high risks of general blinding and outcome detection.
Incomplete outcome data
Based on the information given in the individual articles, incomplete outcome data generally was a small problem (Figure 1). However, only a few studies showed flow charts of the fate of the participants. Therefore, this bias may be significant.
Selective reporting
Based on the information given in the individual articles, reporting bias was small (Figure 1). However, as protocols did not exist for the vast majority of studies, this evaluation may be imprecise.
Other potential sources of bias
The effect of an intervention on BP may depend on factors such as baseline BP and ethnicity. Therefore, a biased distribution of such factors in the included study populations compared with the general population may bias the effect of the intervention found in the meta‐analysis to be different from the potential effect in the general population. We therefore performed separate analyses for hypertensive and normotensive individuals and for different ethnicities.
Effects of interventions
See: Summary of findings for the main comparison ; Summary of findings 2 ; Summary of findings 3 ; Summary of findings 4 ; Summary of findings 5
See Data and analyses.
Blood pressure in white participants
See summary of findings Table for the main comparison
In the meta‐analyses of trials of white participants with normal blood pressure (BP), the mean difference (MD) was a change in systolic blood pressure (SBP) of ‐1.09 mmHg (95% CI: ‐1.63 to ‐0.56) (P = 0.0001) (89 trials, 8569 trials) (Analysis 1.1; Figure 2), and in diastolic blood pressure (DBP) of + 0.03 mmHg (95% CI: ‐0.37 to 0.43) (P = 0.89) (90 trials, (8833 participants) (Analysis 1.2; Figure 3) (high‐quality evidence).

Forest plot of comparison: 1 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in whites, outcome: 1.2 Whites, normotensive, SBP.

Forest plot of comparison: 1 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in whites, outcome: 1.2 Whites, normotensive, DBP.
In subgroup meta‐analyses of trials with a duration of at least one week and a sodium intake of a maximal 250 mmol/day, the MD showed a decrease in SBP of ‐1.31 mmHg (‐1.83 to ‐0.80) (P = 0.00001) (59 trials, 7125 participants) (Analysis 4.1) and in DBP of ‐0.36 mmHg (95% CI: ‐0.79, 0.07) (P = 0.10) (61 trials) (Analysis 4.2). A further elimination of five studies, which, although the mean BP was normal, did include individuals with hypertension, reduced the SBP/DBP effect to ‐1.08/‐0.24 mmHg.
In the trials of white people with elevated BP, MD showed a decrease in SBP of ‐5.51 mmHg (95% CI: ‐6.45 to ‐4.57) (P < 0.00001) (84 trials, 5925 participants) (Analysis 1.3), and in DBP of ‐2.88 mmHg (95% CI: ‐3.44 to ‐2.32) (P < 0.00001) (85 trials, 6001 participants) (Analysis 1.4) (high‐quality evidence).
In subgroup meta‐analyses of trials with a duration of at least one week and a sodium intake of a maximal 250 mmol/day, MD showed a decrease in SBP of ‐5.02 mmHg (‐6.00 to ‐4.05) (P < 0.00001) (63 trials) ( Analysis 4.3) and in DBP of ‐2.78 mmHg (95% CI: ‐3.42 to ‐2.14) (P < 0.00001) (64 trials ) (Analysis 4.4).
Blood pressure in black participants
See summary of findings Table 2
In the meta‐analyses of seven trials involving 506 black participants with normal BP, MD showed a decrease in SBP of ‐4.02 mmHg (95% CI:‐7.37 to ‐0.68) (P = 0.02) (Analysis 2.1) and in DBP of ‐2.01 mmHg (95% CI:‐4.37, 0.35) (P = 0.09) (Analysis 2.2) (moderate‐quality evidence).
In the meta‐analyses of eight trials of 619 black participants with elevated BP, MD showed a decrease in SBP of ‐6.64 mmHg (95% CI:‐9.00, ‐4.27)
(P = 0.00001) (Analysis 2.3) and in DBP of ‐2.91 mmHg (95% CI:‐4.52, ‐1.30) (P = 0.0004) (Analysis 2.4) (moderate‐quality evidence).
Blood pressure in Asian participants
See summary of findings Table 3
In the meta‐analyses of three trials involving 393 Asian participants with normal BP, MD showed a decrease in SBP of ‐0.72 mmHg (95% CI: ‐3.86, 2.41) (P = 0.65) (Analysis 3.1) and in DBP of ‐1.63 mmHg (95% CI:‐3.35 to 0.08) (P= 0.06) (Analysis 3.2) (moderate‐quality evidence).
In the meta‐analyses of nine trials involving 501 Asian participants with elevated BP, MD showed a decrease in SBP of of ‐7.75 mmHg (95% CI:‐11.44, ‐4.07) (P < 0.0001) (Analysis 3.3) and in DBP of ‐2.68 mmHg (95% CI: ‐4.21 to ‐1.15)(P = 0.0006) (Analysis 3.4) (moderate‐quality evidence).
Renin
See summary of findings Table 4
Two parallel trials were excluded (1110 Jula 1994; 1155 Heer 2000).
In the remaining 82 cross‐over trials (5498 participants) of measurement of renin (including 88 comparisons reported in the Data & analyses), the standardised mean difference (SMD) of sodium reduction was 1.22 standardized units (95% CI: 1.07 to 1.37) (Z= 15.68, P < 0.00001) (Analysis 5.1) (high‐quality evidence). In 73 comparisons, which all had the same unit (ng/mL/hour), either directly or after transformation, the MD was 1.60 ng/mL/hour (95% CI: 1.40 to 1.79) (Z= 16.04, P < 0.00001).
In comparisons with a duration of at least seven days and a sodium intake of less than 250 mmol/day (44 trials, 3470 participants), the SMD was 1.05 standardized units (95% CI: 0.85 to 1.24), (Z= 10.35, P < 0.00001) (Analysis 6.1) In 39 comparisons using ng/mL/hour as the unit, the corresponding MD was 1.30 ng/mL/hour (95% CI: 1.06 to 1.53), (Z= 10.65, P < 0.00001). The effect in normotensive participants was significantly higher than in hypertensive participants (summary of findings Table 4).
Aldosterone
See summary of findings Table 4
Three parallel trials were excluded (1110 Jula 1994; 1111 Howe 1994; 1155 Heer 2000).
In the remaining 65 cross‐over trials (4884 participants) of measurement of aldosterone, MD was 97.81 pg/mL (95% CI: 82.56 to 113.05) (Z = 12.58, P < 0.00001) (Figure 4, Analysis 5.2) (high‐quality evidence). In comparisons with duration of at least one week and sodium intake of less than 250 mmol/day (34 trials, 3128 participants), MD was 95.59 pg/mL (95% CI: 74.12 to 117.05), P = 0.00001 (Analysis 6.2).The effect in normotensive participants was significantly higher than in hypertensive participants (summary of findings Table 4).

Forest plot of comparison: 5 Effect of salt reduction on hormones, outcome: 5.2 Aldosterone (pg/mL).
Noradrenaline
See summary of findings Table 4
One parallel trial was excluded (1110 Jula 1994).
In the remaining 34 cross‐over trials (1736 participants) of measurement of noradrenaline (including 36 comparisons reported in the Data & analyses), MD was 63.56 pg/mL (95% CI: 42.66 to 84.46), (z = 5.96, P = 0.00001) (Figure 5, Analysis 5.3) (high‐quality evidence). In comparisons with duration of at least one week and a sodium intake of less than 250 mmol/day (23 studies, 964 participants) MD was 48.66 pg/mL (95% CI: 28.88 to 68.44), P = 0.00001 (Analysis 6.3). There was no difference between normotensive participants and hypertensive participants (summary of findings Table 4).

Forest plot of comparison: 5 Effect of salt reduction on hormones, outcome: 5.3 Noradrenaline (pg/mL).
Adrenaline
See summary of findings Table 4
One parallel trial was excluded (1110 Jula 1994).
In the remaining 15 cross‐over trials (662 participants) of measurement of adrenaline (including 16 comparisons reported in the Data & analyses), MD was 7.55 pg/mL (95% CI: 0.85 to 14.26), (z = 2.21, P = 0.03) (Analysis 5.4) (moderate‐quality evidence). In comparisons with duration of at least one week and sodium intake of less than 250 mmol/day (12 studies, 486 participants) MD was 7.79 pg/mL (95% CI: 0.31 to 15.28), P = 0.04 (Analysis 6.4). There was no difference between normotensive participants and hypertensive participants (summary of findings Table 4).
Cholesterol
See summary of findings Table 5
Three parallel trials were excluded (1015 Bulpitt 1984; 1085 Sciarrone 1992; 1199 Meland 2009). In the remaining 26 cross‐over trials (1800 participants) of measurement of cholesterol (including 27 comparisons reported in the Data & analyses), MD showed an increase of 5.64 mg/dL (95% CI: 2.46 to 8.82), P = 0.0005 (Figure 6, Analysis 7.1) (moderate‐quality evidence). In comparisons with duration of at least one week and sodium intake of less than 250 mmol/day (20 trials, 1180 participants) MD was 4.88 mg/dL (95% CI: 1.19 to 8.56), P = 0.01 (Analysis 8.1). The effect in normotensive participants was significantly higher than in hypertensive participants (summary of findings Table 5)

Forest plot of comparison: 6 Effect of salt reduction on lipids, outcome: 6.1 Cholesterol.
Triglyceride
See summary of findings Table 5
Two parallel trials were excluded (1085 Sciarrone 1992; 1199 Meland 2009) .
In the remaining 19 cross‐over trials (1390 participants) of measurement of triglyceride, MD showed an increase of 7.04 mg/dL (95% CI: 3.04 to 11.05), P = 0.0006 (Analysis 7.2) (moderate‐quality evidence). In comparisons with duration of at least one week and sodium intake of less than 250 mmol/day (12 trials, 770 participants) the effect was 6.92 (mg/dL [95% CI: 1.82 to 12.02), P = 0.008 (Analysis 8.2). There was no difference between normotensive participants and hypertensive participants (summary of findings Table 5)
High‐density lipoprotein (HDL)
See summary of findings Table 5
Two parallel trials were excluded (1085 Sciarrone 1992; 1199 Meland 2009).
In the remaining 19 cross‐over trials (1442 participants) of measurement of HDL, there was no effect of sodium reduction on serum HDL: MD: ‐0.29 mg/dL (95% CI: ‐1.66 to 1.08) P = 0.68 (Analysis 7.3) (moderate‐quality evidence). This result did not change in comparisons with duration of at least one week and sodium intake of less than 250 mmol/day (‐0.67 mg/dL (‐2.18 to 0.83), P = 0.38 (14 trials, 948 participants)) (Analysis 8.3).
Low‐density lipoprotein (LDL)
See summary of findings Table 5
One parallel trial was excluded (1085 Sciarrone 1992).
In the remaining 17 cross‐over trials (1358 participants) of measurement of LDL, MD showed a non‐significant increase of 3.12 mgdL (95% CI: ‐0.41, to, 6.64), P = 0.08 (Analysis 7.4). In comparisons with duration of at least one week and sodium intake of less than 250 mmol/day (12 trials, 864 participants), MD was 3.63 mgdL (95% CI: ‐0.44 to 7.69), P = 0.08 (Analysis 8.4).
Bias analyses
Bias analysis: Comparing low bias risk versus high bias risk of general blinding and blinding of outcome detection for SBP‐outcomes in white people with normotension and hypertension showed no important differences. See Data and analyses: 9 Bias analyses.
Additional subgroup analyses
Hormones and lipids
Inclusion of the few parallel studies did not change any of the results (data not shown).
Sensitivity analyses
The funnel plots of all analyses were investigated. For each funnel plot, all studies giving rise to asymmetry were eliminated. The resulting effect was compared with the original analysis. All these analyses showed only marginal effects without significance (not shown).
Discussion
Summary of main results
The effect of sodium reduction from an average high usual intake (201 mmol/day) to the recommended level (66 mmol/day) was small in study populations with normal blood pressure (BP) (‐1.09/+0.03 mmHg) corresponding to a mean arterial pressure effect of only ‐0.3 mmHg . In hypertensive study populations the effect was (‐5.51/‐2.88 mmHg). In a subgroup analysis intending to eliminate the potential bias of a very short intervention duration (< seven days) and very high sodium intake (> 250 mmol/day), the decrease in BP in study populations with a normal BP (‐1.31/‐0.36 mmHg) and hypertension (‐5.02/‐2.78 mmHg) was also small. The effect of sodium reduction on hormones and on lipids showed statistically significant increases in renin, aldosterone, noradrenalin, cholesterol and triglyceride in the primary analysis, as well as in the subgroup analysis, whereas the increase in adrenalin was borderline significant (P < 0.03). The increase in cholesterol in the low‐salt group seemed mainly to be due to an increase in low‐density lipoprotein (LDL), which was borderline significant. The slight decrease in high‐density lipoprotein (HDL) in the low‐salt group was not significant.
The analysis of black populations showed that the effect of sodium reduction in black people with normotension corresponded to the one found in black people with hypertension. This was in contrast to the analyses of white and Asian populations in whom the effect was smaller in those who were normotensive than in those who were hypertensive. However, compared with previous analyses (Graudal 1998; Jürgens 2003), the diverging results within the black populations and between the black and white populations are smaller. In a recent detailed analysis, we found that a significant fraction of the differences between the three ethnicity groups could be ascribed to differences in baseline BP, age, and amount of sodium reduction. Furthermore there was no difference in BP outcome between ethnicity groups investigated in the same study (Graudal 2015b) indicating that the differences found in the present meta‐analysis mainly may be due to confounders rather than ethnical differences.
Overall completeness and applicability of evidence
In the primary analysis, population samples from the whole BP distribution of the populations were included. In this analysis, the intake of sodium in the “high” sodium group was in the interval 100 mmol/day to 795 mmol/day in 205 comparisons (99%), and below 100 mmol/day in one comparison, the mean level being 201 mmol/day. The intake of sodium in the low‐sodium group was below 100 mmol/day in 168 comparisons (82%) and above 100 mmol/day in 38 comparisons, the mean level being 66 mmol/day. In the subgroup analysis, the intake of sodium in the “high” sodium group was in the interval 109 mmol/day to 248 mmol/day in 143 comparisons (99%), and below 100 mmol/day in one comparison, the mean level being 167 mmol/day. The intake of sodium in the low‐sodium group was below 100 mmol/day in 114 comparisons (80%) and above 100 mmol/day in 30 comparisons, the mean level being 60 mmol/day. Consequently, this meta‐analysis in general compares the effects of a dietary sodium intake, which is lower than usual and in accordance with the recommendations to reduce sodium below 100 mmol/day with a sodium intake, which is within the present world‐wide usual range of sodium intake, the level in the primary analysis being in the high end of the usual intake and the level in the subgroup analysis being close to the world mean of 159 mmol/day (Table 1). The mean and the range of the baseline 24‐hour sodium excretion of the included populations before diet manipulation (159 mmol/24 hours (10 to 90 percentile: 123 to 194)) were almost identical with the usual range of sodium intake in the world's populations (McCarron 2013; Powles 2013). Thus, the present review shows the consequences of the recommendations of the health institutions, which is to reduce the usual sodium intake of the world's populations (90 mmol/day to 250 mmol/day) to a level below 87 mmol/day to 100 mmol/day.
Quality of the evidence
The study populations included in the present meta‐analysis were in general very heterogeneous with large variations in baseline BP, age, sodium intake and degree of sodium reduction. The analyses of BP and hormones were generally very heterogeneous. Especially, the I2 values for renin and aldosterone were very high, but as renin and aldosterone rise sharply with sodium reduction below 2.3 g sodium, but only moderately above 2.3 g sodium (Brunner 1972; Graudal 1998), the extreme heterogeneity of the outcomes of these variables is not surprising. In spite of the clinical heterogeneity, no heterogeneity was detected in the meta‐analyses of lipid outcomes implying that these outcomes are robust. The number of studies included in the BP analyses (n = 206) is substantial as is the number of participants (more than 6000). This should allow robust conclusions. The fundamental quality criterion was randomisation, but a weakness is that very few of the studies described the sequence generation and the random allocation procedures leaving a substantial bias risk of insufficient randomisation, which could not be explored in a meaningful way due to the lack of contrasts between the number of studies with low and high risk of these biases (Figure 1). Another weakness was that a large number of studies were not double‐blind. However, concerning this source of bias, there were no obvious trends towards different effects in the low‐risk blinded groups compared with the high‐risk open groups (Analysis 9.1; Analysis 9.2; Analysis 9.3; Analysis 9.4; Analysis 9.5; Analysis 9.6; Analysis 9.7; Analysis 9.8).
Potential biases in the review process
The present review is the largest of the many existing meta‐analyses on sodium reduction, and other meta‐analyses have not identified studies, which were not identified by our search. Our analysis is the largest partly because our selection criteria were less restrict. Therefore, a fraction of the included studies had an experimental character investigating a sodium intake far beyond the sodium intake in the general population for only four to six days, which may not be relevant for the general population on long‐term sodium reduction. The fact that the subgroup analysis eliminating the potential short‐term intervention bias and very high sodium intake bias showed similar results as the primary analysis indicates that the inclusion of extreme studies had a minor impact on the mean of the outcome effects. Other meta‐analyses have extracted almost identical data in the individual studies indicating that our data extraction is unbiased. Finally, elimination of studies giving rise to asymmetry in the funnel plots did not change the results indicating a low risk of publication bias.
Agreements and disagreements with other studies or reviews
The scientific evidence behind the sodium reduction recommendations is a series of studies and meta‐analyses, which are biased by high baseline blood pressure, high age and overweight (Graudal (3) 2016). The most prominent of these studies (DASH 2001), was additionally biased by a control group diet, which was designed to contain only half of the normal amount of potassium. Despite these studies are irrelevant as evidence for pubic health recommendations, the Food and Drug Administration (FDA) has released draft proposed voluntary guideline to encourage companies to steadily reduce sodium in processed foods (Frieden TR 2016), the main argument being a dose‐response meta‐regression analysis of mixed normotensive and hypertensive study populations, which was biased because it included mainly studies with high blood pressure and inappropriately forced the dose‐response relationship through zero and thereby further doubled the postulated effect. In contrast, previous meta‐analyses of randomised controlled trials (RCTs) have shown similar results of sodium reduction on BP. In 1986, Grobbee and Hofman combined 13 studies of persons with normal and elevated BP in a meta‐analysis and found a significant hypotensive effect of reduced sodium intake on SBP of ‐3.6 mmHg and a non‐significant effect on DBP of ‐2.0 mmHg (Grobbee 1986). In 1991, a second meta‐analysis of 24 RCTs showed an effect of ‐4.0/‐2.5 mmHg for persons with elevated BP and ‐1.0/‐0.2 for persons with normal BP (Cutler 1991). This was verified in an update from 1997 (Cutler 1997). In 1996, a meta‐analysis of 53 RCTs showed an effect of ‐3.7/‐0.9 mmHg in persons with elevated BP and ‐1.0/‐0.1 in persons with normal BP (Midgley 1996). In an analysis of eight RCTs lasting for at least six months, the effect was ‐2.9/‐2.1 mmHg for persons with elevated BP and ‐1.3/‐ 0.8 mmHg for persons with normal BP (Ebrahim 1998). These results were confirmed in an update (Hooper 2002). All these similar results confirm that selection of RCTs based on magnitude of sodium difference or duration of the intervention does not significantly change the overall effect size estimate. These meta‐analyses indicate that major disagreements about this effect size no longer seem to exist. However, there is still significant disagreement regarding the relevance of the effect size and the relevance of potential side effects (Taubes 1998).
The effect of sodium reduction on BP in hypertensive and normotensive study populations in the present review matches the effects found in most of these previous reviews, although the effect of sodium reduction on BP in normotensives is marginally lower than in the meta‐analysis, which supports the WHO recommendations (Aburto 2013). In hypertensive study populations, there was no differences between the WHO review and our review. In normotensive study populations, the difference was small, the BP effect in the WHO review being ‐1.38/‐0.58 mmHg and in ours being ‐1.09/0.03 (‐1.31/‐0.36 in the subgroup analysis).This study differed from ours as it only included studies lasting at least four weeks. However, as duration has no impact on the BP effect (Table 2), a more reliable explanation for the difference between the WHO review and our review is that the study populations with normal BP in the WHO review generally have a high baseline BP in the upper 50% percentile of the population.
According to WHO, the small effect in normotensive study populations is sufficient to recommend sodium reduction for the whole population, the assumption being that the association between BP and mortality is consistent. This, however, may not be the case. For instance, beta‐blockers reduce BP in hypertensive individuals, but not mortality (Wiysonge 2012), and a recent meta‐analysis of patients with diabetes showed that antihypertensive treatment reduces the risk of mortality and cardiovascular morbidity in diabetes patients with SBP higher than 140 mm Hg, but if SBP is less than140 mm Hg further treatment is associated with an increased risk of cardiovascular death, with no observed benefit (Brunström 2016). Such studies indicate that it is not possible to extend the general association of BP with mortality (Collins 1990) to the effect of a BP‐reducing intervention on mortality. The reason for this inconsistency may be side effects of the intervention. However, while short duration has been suggested to underestimate the BP effect, it has concomitantly been suggested to overestimate possible adverse effects on hormones and lipids. This idea that the duration of the intervention tends to underestimate some physiological outcomes and overestimate others has not been documented, but still has been used to disregard side effects shown in studies lasting less than four weeks. Very few studies lasting more than for weeks have investigated side effects, and further more these studies do not reduce sodium to the recommended level, but to levels above 87 mmol/day, and therefore the side effects in these few studies may not be fully disclosed. In contrast, the present analysis shows that the adverse effects on hormones and lipids are significant, when the sodium intake is lowered from a high usual sodium intake to a level in accordance with the recommendations of the health institutions. In addition, we have just shown that sodium reduction results in an increase in heart rate of 2.4% (Graudal (2) 2016). This may be an important side effect as resting heart rate is directly associated with mortality (Ho 2014; Jensen 2012). The assumption that at least some of these effects may be persistent and not just temporary has been indicated in observational studies. Yanomamo Indians, who persistently ingest very small amounts of sodium, have a three times higher level of renin in the blood and a 10 times higher excretion of aldosterone in the urine than normal controls (Oliver 1975). Furthermore, renin and aldosterone rise slowly as long as the intake is above 100 mmol/day, but exponentially, when sodium intake is reduced to levels below 100 mmol/day (Brunner 1972). Thus, the present meta‐analysis provides a possible explanation for the small effect of reduced sodium intake on blood pressure: compensatory activation of the renin‐aldosterone system is proportional to the degree of sodium reduction. Furthermore, the increases in noradrenaline and adrenaline may contribute to this counter‐regulation (Warren 1980) and contribute to an increase in heart rate.
The very small effect of sodium reduction on BP in healthy individuals shown in the present review and other reviews including the WHO review, the risk of significant side effects shown in this review, and the possibility that an intervention to reduce BP may not reduce mortality (Wiysonge 2012), and even may increase mortality in some population groups with a normal BP (Brunström 2016) indicate that the BP‐effect is not sufficient as a basis for recommendations in the general population, but should be verified in studies directly relating sodium intake with morbidity and mortality. Unfortunately, RCTs of the effect of sodium reduction below 100 mmol/day on mortality in healthy individuals do not exist (Graudal (1) 2016). A recently updated meta‐analysis of eight RCTs with follow‐up data on morbidity and mortality found a non‐significant trend versus reduced cardiovascular (CV) morbidity, but could not demonstrate reduced all‐cause mortality in the low‐sodium group (Adler 2014). These trials were performed in overweight pre‐hypertensive or hypertensive individuals and the sodium reduction was not below 100 mmol/day, but down to 100 mmol/day.
The sodium‐mortality relationship has also been estimated by means of 27 observational studies (Alderman 2010; Mente 2016; O'Donnell 2014; Pfister 2014), which directly asses the relationship between sodium intake in the individual and mortality. Most of these studies were evaluated in an IOM report (IOM 2013). This IOM report did not confirm the 100 mmol/day upper level for sodium intake, which was defined in a previous IOM report (IOM 2005), but concluded that “Science was insufficient and inadequate to establish whether reducing sodium intake below 2300 mg/d (100 mmol) either decreases or increases CVD risk in the general population”. A later meta‐analysis of these population studies found that a sodium intake below 114 mmol/day was associated with increased mortality, as was a sodium intake above 214 mmol/day (Graudal 2014). Increased mortality with high sodium intake has also been shown in another meta‐analysis, which, however, did not investigate the effect of a low sodium intake (Strazzulo 2009). This U‐shaped relation between sodium intake and mortality has been identified in several individual population studies (O'Donnell 2011; O'Donnell 2014; Pfister 2014; Thomas 2011). The health institutions, however, generally do not accept this evidence from the observational studies (Gunn 2013; Whelton 2012; WHO 2012). In a recent paper, which discusses methodological issues of observational studies, representatives of the American Heart Association state that the association of low sodium intake with increased mortality observed in observational studies may reflect that sick people have a low sodium intake (reverse causality: sick people with a high mortality have a low sodium intake, it is not the low sodium intake, which increases the mortality) (Cobb 2014). This hypothesis is not directly supported by the observational studies, as the outcomes generally are adjusted for confounders such as cardiovascular and renal diseases and diabetes and show that the mortality associated with a low sodium intake is higher in healthy populations than in populations including sick individuals (Graudal 2014; O'Donnell 2014). Table 3 shows a meta‐analysis of the risk of all‐cause mortality in Study populations within the usual sodium intake range versus a low sodium intake below 114 mmol/day (Graudal 2014) or below 130 mmol/day (O'Donnell 2014). The analysis is confined to include samples of individuals representative of the general populations and all individual study analyses are adjusted for multiple confounders such as cardiovascular disease, hypertension and diabetes. To further reduce the risk of reverse causality, the most healthy subgroup was included in the analysis, when results were given for subgroups, The possibility of reverse causality can never be completely excluded, but as a minimum there is no indication in population studies that sodium intake below 100 mmol/day has beneficial health effects in healthy individuals. In the NHANES I and III studies this was demonstrated by independent groups (Alderman 1998; Cohen 2008; He 1999; Yang 2011).
| Study | Multiple adjustment* | Exclusion | N (LS) | N (US) | RR/OR (95% CI) |
| Alderman 1998 (NHANES I) | Yes | None | 2837 | 8509 | 0.88 (0.80, to, 0.97) |
| He 1999 (NHANES I) | Yes | Overweight (BMI > 27.3) | 1699 | 5098 | 0.98 (0.88 to 1.09) |
| Yes | Males** | 634 | 311 | 0.91 (0.56 to 1.48) | |
| Cohen 2006 (NHANES II) | Yes | None | 3711 | 3443 | 0.78 (0.67 to 0.91) |
| Yes | CVD and HT | 392 | 392 | 1.12 (0.86 to 1.46) | |
| Cohen 2008 (NHANES III) | Yes | None | 2175 | 4350 | 0.83 (0.73 to 0.94) |
| Yang 2011 (NHANES III) | Yes | Overweight (BMI > 25) | 3067 | 6133 | 0.93 (0.73 to 1.18) |
| Yes | None | 1250 | 1220 | 0.82 (0.62 to 1.08) | |
| Yes | None | 1138 | 961 | 0.89 (0.74 to 1.07) | |
| Pfister 2014 (Norfolk) | Yes | 0‐2 year events | 3070 | 9249 | 0.92 (0.82 to 1.02) |
| O'Donnell 2014 (PURE) | Yes | CVD, Cancer, DM, smokers | 6162 | 38643 | 0.62 (0.54 to 0.71)] |
| Total (95% CI)# | 21369 | 67078 | 0.84 (0.76 to 0.93) | ||
| Total (95% CI)## | 21123 | 65450 | 0.87 (0.76 to 0.98) |
Only studies, which were representative for the general population and which adjusted for confounders were included.
If subgroup results were given, the results of the most healthy subgroup was used in the analysis to reduce
the possibility of reverse causation
#With primary NHANES analyses (Alderman 1998, Cohen 2008)
## With NHANES re‐analyses (He 1999, Yang 2011)
* Studies were generally adjusted for at least sex, age and CVD risk factors
** In the male group a low salt intake group could not be identified, as the salt intake
in the lowest salt intake quartile was up to 159 mmol.
BMI: body mass index; CVD: cardiovascular disease; DM: diabetes mellitus; HT: hypertension
The BP effect of reduced sodium intake has been related to age. Freedman and Petitti analysed data from Intersalt (Intersalt 1988) and found the paradox that along with the significant association between increase in blood pressure with age and the salt excretion in urine, there was an inverse relationship between estimated BP and salt excretion in urine at age 20. Freedman stated that unless you preferred to conclude that salt should be eaten in high doses by youngsters and in reduced amounts by the elderly, the findings were probably due to uncontrolled confounding, not to variation in salt intake (Freedman 2001). Furthermore, it is now clear that the BP of different age cohorts in a cross‐sectional study like Intersalt is not representative of each other, verified by a study showing that recent birth cohorts attained lower BP than did earlier birth cohorts in the period 1887 to 1994 (Goff 2001). According to this study, based on data from more than 50,000 persons, it can be estimated that the median BP is about 15 mmHg lower in a 50‐year old person from a recent birth cohort compared with a 50‐year old from a birth cohort from the late 19th century. Consequently, there has been a dramatic fall in BP during the 20th century. In this context, the possible mean arterial pressure effect of sodium reduction of ‐0.3 mmHg in normotensive persons seems negligible. Finally, it has been difficult to maintain a significant sodium reduction in longer‐term studies, which should be taken into consideration, when recommending sodium reduction. One reason for this could be that the sodium intake is regulated by neuro‐physiological and hormonal mechanisms (Geerling 2008), and therefore difficult to diverge from.
The hypothetical consequences of the present findings are that people with normotension would have no benefit from sodium reduction, but may suffer from harms, because sodium reduction has a negligible effect on BP, but results in significant side effects. People with hypertension may benefit due to the effect on BP, but may also suffer from harms due to the side effects. This is exactly what was found in the most recent meta‐analysis of four population studies (133,000 individuals) in which the authors had access to individual participant data (Mente 2016). The conclusion was "Compared with moderate sodium intake, high sodium intake is associated with an increased risk of cardiovascular events and death in hypertensive populations (no association in normotensive population), while the association of low sodium intake with increased risk of cardiovascular events and death is observed in those with or without hypertension. These data suggest that lowering sodium intake is best targeted at populations with hypertension who consume high sodium diets", a conclusion, which matches perfectly with the results of the present meta‐analysis.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in whites, outcome: 1.2 Whites, normotensive, SBP.

Forest plot of comparison: 1 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in whites, outcome: 1.2 Whites, normotensive, DBP.

Forest plot of comparison: 5 Effect of salt reduction on hormones, outcome: 5.2 Aldosterone (pg/mL).

Forest plot of comparison: 5 Effect of salt reduction on hormones, outcome: 5.3 Noradrenaline (pg/mL).

Forest plot of comparison: 6 Effect of salt reduction on lipids, outcome: 6.1 Cholesterol.

Comparison 1 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Whites, Outcome 1 White population, normotensive, SBP.

Comparison 1 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Whites, Outcome 2 White population, normotensive, DBP.

Comparison 1 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Whites, Outcome 3 White population, hypertensive, SBP.

Comparison 1 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Whites, Outcome 4 White population, hypertensive, DBP.

Comparison 2 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Blacks, Outcome 1 Black population, normotensive, SBP.

Comparison 2 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Blacks, Outcome 2 Black population, normotensive, DBP.

Comparison 2 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Blacks, Outcome 3 Black population, hypertensive, SBP.

Comparison 2 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Blacks, Outcome 4 Black population, hypertensive, DBP.

Comparison 3 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Asians, Outcome 1 Asians population normotensive, SBP.

Comparison 3 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Asians, Outcome 2 Asian population, normotensive, DBP.

Comparison 3 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Asians, Outcome 3 Asian population, hypertensive, SBP.

Comparison 3 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Asians, Outcome 4 Asian population, hypertensive, DBP.

Comparison 4 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Whites, subgroup analysis, Outcome 1 White population, normotensive, SBP.

Comparison 4 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Whites, subgroup analysis, Outcome 2 White population, normotensive, DBP.

Comparison 4 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Whites, subgroup analysis, Outcome 3 White population, hypertensive, SBP.

Comparison 4 Effect of salt reduction on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in Whites, subgroup analysis, Outcome 4 White population, hypertensive, DBP.

Comparison 5 Effect of salt reduction on hormones, Outcome 1 Renin (ng/mL/hour).

Comparison 5 Effect of salt reduction on hormones, Outcome 2 Aldosterone (pg/mL).
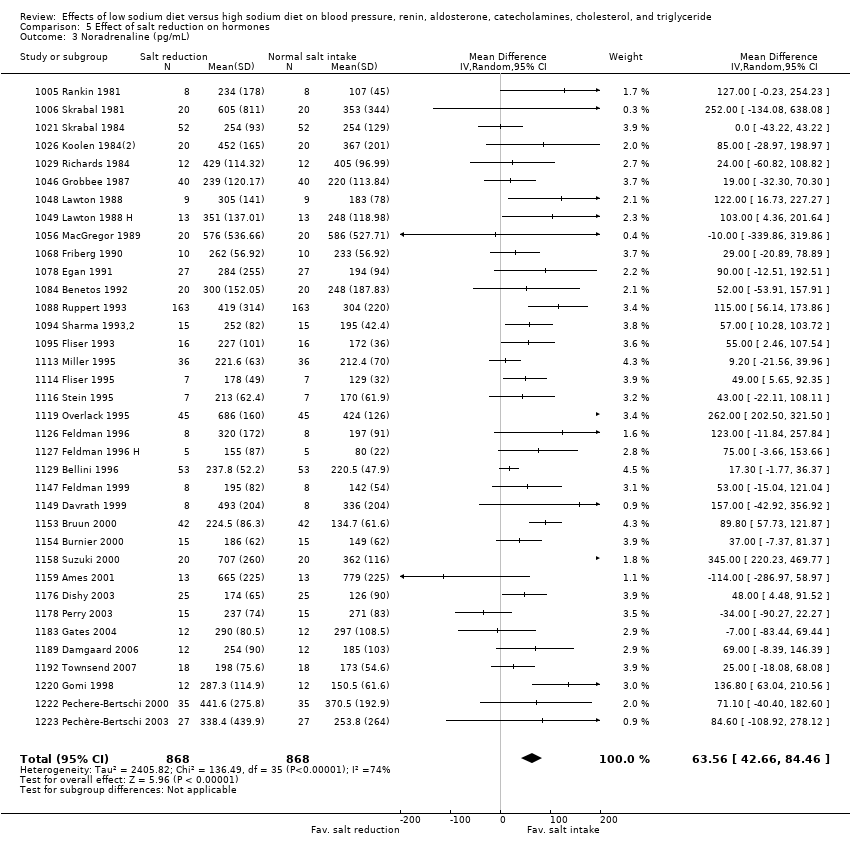
Comparison 5 Effect of salt reduction on hormones, Outcome 3 Noradrenaline (pg/mL).

Comparison 5 Effect of salt reduction on hormones, Outcome 4 Adrenaline (pg/mL).

Comparison 6 Effect of salt reduction on hormones, subgroup analysis, Outcome 1 Renin (ng/mL/hour).

Comparison 6 Effect of salt reduction on hormones, subgroup analysis, Outcome 2 Aldosterone (pg/mL).

Comparison 6 Effect of salt reduction on hormones, subgroup analysis, Outcome 3 Noradrenaline (pg/mL).

Comparison 6 Effect of salt reduction on hormones, subgroup analysis, Outcome 4 Adrenaline (pg/mL).

Comparison 7 Effect of salt reduction on lipids, Outcome 1 Cholesterol (mg/dL).
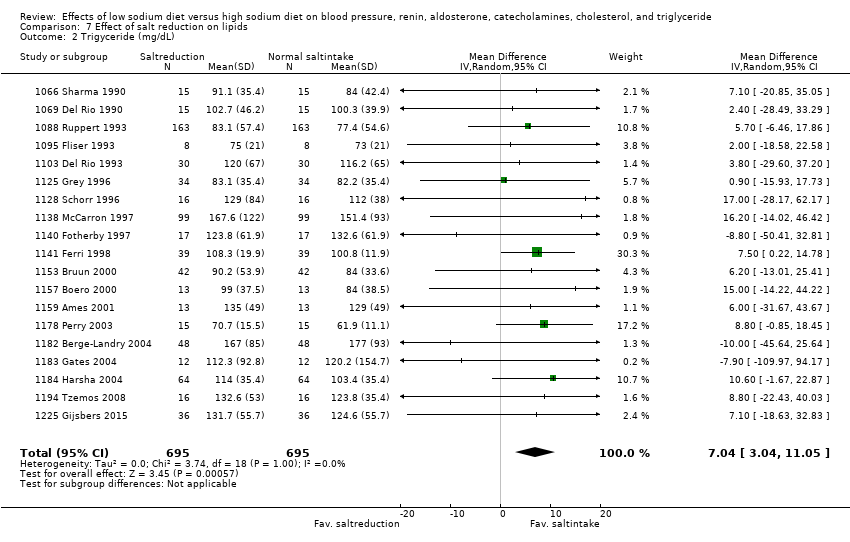
Comparison 7 Effect of salt reduction on lipids, Outcome 2 Trigyceride (mg/dL).

Comparison 7 Effect of salt reduction on lipids, Outcome 3 High density lipoprotein, HDL (mg/dL).

Comparison 7 Effect of salt reduction on lipids, Outcome 4 Low density lipoprotein, LDL (mg/dL).

Comparison 8 Effect of salt reduction on lipids, subgroup analysis, Outcome 1 Cholesterol (mg/dL).

Comparison 8 Effect of salt reduction on lipids, subgroup analysis, Outcome 2 Trigyceride (mg/dL).

Comparison 8 Effect of salt reduction on lipids, subgroup analysis, Outcome 3 High density lipoprotein, HDL (mg/dL).

Comparison 8 Effect of salt reduction on lipids, subgroup analysis, Outcome 4 Low density lipoprotein, LDL (mg/dL).

Comparison 9 Bias analyses, Outcome 1 White population, normotensive, SBP blinding‐high.

Comparison 9 Bias analyses, Outcome 2 White population, normotensive, SBP blinding‐low.

Comparison 9 Bias analyses, Outcome 3 White population, normotensive, SBP outcome‐assesed‐high.

Comparison 9 Bias analyses, Outcome 4 White population, normotensive, SBP outcome‐assesed‐low.

Comparison 9 Bias analyses, Outcome 5 White population, hypertensive, SBP blinding‐high.

Comparison 9 Bias analyses, Outcome 6 White population, hypertensive, SBP blinding‐low.

Comparison 9 Bias analyses, Outcome 7 White population, hypertensive, SBP outcome‐assesed‐high.

Comparison 9 Bias analyses, Outcome 8 White population, hypertensive, SBP outcome‐assesed‐low.
| Low sodium intake compared with high sodium intake for blood pressure | ||||
| Patient or population: White population with normal or elevated blood pressure, but otherwise healthy Settings: Hospitals units in Europe and North America Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| White population, normotensive, SBP mmHg | ‐1.09 (‐1.63 to ‐0.56) | 8569 | ⊕⊕⊕⊕ | |
| White population, normotensive, DBP mmHg | 0.03 (‐0.37 to 0.43) | 8833 | ⊕⊕⊕⊕ | |
| White population, hypertensive, SBP mmHg | ‐5.51 (‐6.45 to ‐4.57) | 5925 | ⊕⊕⊕⊕ | |
| White population, hypertensive, DBP mmHg | ‐2.88 (‐3.44 to ‐2.32) | 6001 | ⊕⊕⊕⊕ | |
| GRADE Working Group grades of evidence DBP: diastolic blood pressure; SBP: systolic blood pressure | ||||
| Low sodium intake compared with high sodium intake for blood pressure | ||||
| Patient or population: Black population with normal or elevated blood pressure, but otherwise healthy Settings: Hospital units in North America, UK and Africa Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Mean difference | No of Participants | Quality of the evidence | Comments |
| Black population, normotensive, SBP mmHg | ‐4.02 (‐7.37 to ‐0.68) | 506 | ⊕⊕⊕⊝ | |
| Black population, normotensive, DBP mmHg | ‐2.01 (‐4.37 to 0.35) | 506 | ⊕⊕⊕⊝ | |
| Black population, hypertensive, SBP mmHg | ‐6.64 (‐9.00 to ‐4.27) | 619 | ⊕⊕⊕⊝ | |
| Black population, hypertensive, DBP mmHg | ‐2.91 (‐4.52 to ‐1.30) | 619 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence DBP: diastolic blood pressure; SBP: systolic blood pressure | ||||
| 1. Downgraded due to the wide confidence intervals | ||||
| Low sodium intake compared with high sodium intake for blood pressure | ||||
| Patient or population: Asian population with normal or elevated blood pressure, but otherwise healthy Settings: Hospital units in Japan and China Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Mean difference | No of Participants | Quality of the evidence | Comments |
| Asian population, normotensive, SBP mmHg | ‐0.72 (‐3.86 to 2.41) | 393 | ⊕⊕⊕⊝ | |
| Asian population, normotensive, DBP mmHg | ‐1.63 (‐3.35 to 0.08) | 393 | ⊕⊕⊕⊝ | |
| Asian population, hypertensive, SBP mmHg | ‐7.75 (‐11.44 to ‐4.07) | 501 | ⊕⊕⊕⊝ | |
| Asian population, hypertensive, DBP mmHg | ‐2.68 (‐4.21 to ‐1.15) | 501 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence DBP: diastolic blood pressure; SBP: systolic blood pressure | ||||
| 1. Downgraded due to the wide confidence intervals | ||||
| Low sodium intake compared with high sodium intake for hormones | ||||
| Patient or population: Participants with normal or elevated blood pressure, but otherwise healthy Settings: Hospital units Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Mean difference | No of Participants | Quality of the evidence | Comments |
| Renin SMD | 1.22 (1.07 to 1.37) N*: 1.44 (1.24 to 1.65) H*: 0.91 (0.71 to 1.10) | 5498 | ⊕⊕⊕⊕ | |
| Aldosterone pg/mL | 97.81 (82.56 to 113.05) N*: 115.83 (91.74 to 139.91) H*: 73.02 (55.94 to 90.09) | 4884 | ⊕⊕⊕⊕ | |
| Noradrenaline pg/mL | 63.56 (42.66 to 84.46) N*: 66.50 (41.72 to 91.29) H*: 57.36 (14.10 to 100.61) | 1736 | ⊕⊕⊕⊕ | |
| Adrenaline pg/mL | 7.55 (0.85 to 14.26) N*:4.45 (3.43 to 12.33) H*:13.45 (1.25 to 25.66) | 662 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence Very low quality: We are very uncertain about the estimate. SMD: standardised mean difference | ||||
| N*: Study populations with mean SBP < 140 mmHg H*:Study populations with mean SBP > 140 mmHg 1. Downgraded due to the wide confidence interval | ||||
| Low sodium intake compared with high sodium intake for lipids | ||||
| Patient or population: Participants with normal or elevated blood pressure, but otherwise healthy Settings: Hospital units Intervention: Low sodium intake Comparison: High sodium intake | ||||
| Outcomes | Mean difference | No of Participants | Quality of the evidence | Comments |
| Cholesterol mg/dL | 5.64 (2.46, 8.82) N*:7.46 (3.65, 11.28) H*:2.55 (‐2.69, 7.80) | 1800 | ⊕⊕⊕⊝ | |
| Trigyceride mg/dL | 7.04 (3.04, 11.05) N*: 6.88 (1.18, 12.59) H*: 7.19 (1.57, 12.81) | 1390 | ⊕⊕⊕⊝ | |
| High‐density lipoprotein (HDL) mg/dL | ‐0.29 (‐1.66, 1.08) | 1442 | ⊕⊕⊕⊝ moderate1 | |
| Low‐density lipoprotein (LDL) mg/dL | 3.12 (‐0.41, 6.64) | 1358 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence | ||||
| Downgraded due to the wide confidence intervals. | ||||
| Reference | Recommended upper level* | World, lower range* | World, lower 2.5%* | World, mean* | World, Upper 97.5%* | World, upper range* |
| 100 (2300) (5800) | ||||||
| 87 (2000) (5046) | ||||||
| 90 (2070) (5220) | 109 (2500) (6320) | 159 (3660) (9220) | 209 (4810) (12120) | 248 (5700) (14400) | ||
| 95 (2200) (5510) | 172 (3950) (10000) | 240 (5520) (13920) | ||||
| 1. number: mmol; 2. number: mg sodium; 3. number: mg sodium chloride | ||||||
| Study | Multiple adjustment* | Exclusion | N (LS) | N (US) | RR/OR (95% CI) |
| Alderman 1998 (NHANES I) | Yes | None | 2837 | 8509 | 0.88 (0.80, to, 0.97) |
| He 1999 (NHANES I) | Yes | Overweight (BMI > 27.3) | 1699 | 5098 | 0.98 (0.88 to 1.09) |
| Yes | Males** | 634 | 311 | 0.91 (0.56 to 1.48) | |
| Cohen 2006 (NHANES II) | Yes | None | 3711 | 3443 | 0.78 (0.67 to 0.91) |
| Yes | CVD and HT | 392 | 392 | 1.12 (0.86 to 1.46) | |
| Cohen 2008 (NHANES III) | Yes | None | 2175 | 4350 | 0.83 (0.73 to 0.94) |
| Yang 2011 (NHANES III) | Yes | Overweight (BMI > 25) | 3067 | 6133 | 0.93 (0.73 to 1.18) |
| Yes | None | 1250 | 1220 | 0.82 (0.62 to 1.08) | |
| Yes | None | 1138 | 961 | 0.89 (0.74 to 1.07) | |
| Pfister 2014 (Norfolk) | Yes | 0‐2 year events | 3070 | 9249 | 0.92 (0.82 to 1.02) |
| O'Donnell 2014 (PURE) | Yes | CVD, Cancer, DM, smokers | 6162 | 38643 | 0.62 (0.54 to 0.71)] |
| Total (95% CI)# | 21369 | 67078 | 0.84 (0.76 to 0.93) | ||
| Total (95% CI)## | 21123 | 65450 | 0.87 (0.76 to 0.98) | ||
| Only studies, which were representative for the general population and which adjusted for confounders were included. If subgroup results were given, the results of the most healthy subgroup was used in the analysis to reduce the possibility of reverse causation #With primary NHANES analyses (Alderman 1998, Cohen 2008) ## With NHANES re‐analyses (He 1999, Yang 2011) * Studies were generally adjusted for at least sex, age and CVD risk factors ** In the male group a low salt intake group could not be identified, as the salt intake in the lowest salt intake quartile was up to 159 mmol. BMI: body mass index; CVD: cardiovascular disease; DM: diabetes mellitus; HT: hypertension | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 White population, normotensive, SBP Show forest plot | 89 | 8569 | Mean Difference (Random, 95% CI) | ‐1.09 [‐1.63, ‐0.56] |
| 2 White population, normotensive, DBP Show forest plot | 90 | 8833 | Mean Difference (Random, 95% CI) | 0.03 [‐0.37, 0.43] |
| 3 White population, hypertensive, SBP Show forest plot | 84 | 5925 | Mean Difference (Random, 95% CI) | ‐5.51 [‐6.45, ‐4.57] |
| 4 White population, hypertensive, DBP Show forest plot | 85 | 6001 | Mean Difference (Random, 95% CI) | ‐2.88 [‐3.44, ‐2.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Black population, normotensive, SBP Show forest plot | 7 | 506 | Mean Difference (Random, 95% CI) | ‐4.02 [‐7.37, ‐0.68] |
| 2 Black population, normotensive, DBP Show forest plot | 7 | 506 | Mean Difference (Random, 95% CI) | ‐2.01 [‐4.37, 0.35] |
| 3 Black population, hypertensive, SBP Show forest plot | 8 | 619 | Mean Difference (Random, 95% CI) | ‐6.64 [‐9.00, ‐4.27] |
| 4 Black population, hypertensive, DBP Show forest plot | 8 | 619 | Mean Difference (Random, 95% CI) | ‐2.91 [‐4.52, ‐1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Asians population normotensive, SBP Show forest plot | 3 | 393 | Mean Difference (Random, 95% CI) | ‐0.72 [‐3.86, 2.41] |
| 2 Asian population, normotensive, DBP Show forest plot | 3 | 393 | Mean Difference (Random, 95% CI) | ‐1.63 [‐3.35, 0.08] |
| 3 Asian population, hypertensive, SBP Show forest plot | 8 | 501 | Mean Difference (Random, 95% CI) | ‐7.75 [‐11.44, ‐4.07] |
| 4 Asian population, hypertensive, DBP Show forest plot | 8 | 501 | Mean Difference (Random, 95% CI) | ‐2.68 [‐4.21, ‐1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 White population, normotensive, SBP Show forest plot | 59 | 7125 | Mean Difference (Random, 95% CI) | ‐1.31 [‐1.83, ‐0.80] |
| 2 White population, normotensive, DBP Show forest plot | 61 | Mean Difference (Random, 95% CI) | ‐0.36 [‐0.79, 0.07] | |
| 3 White population, hypertensive, SBP Show forest plot | 63 | Mean Difference (Random, 95% CI) | ‐5.02 [‐4.00, ‐4.05] | |
| 4 White population, hypertensive, DBP Show forest plot | 64 | Mean Difference (Random, 95% CI) | ‐2.78 [‐3.42, ‐2.14] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Renin (ng/mL/hour) Show forest plot | 88 | 5498 | Std. Mean Difference (IV, Random, 95% CI) | 1.22 [1.07, 1.37] |
| 2 Aldosterone (pg/mL) Show forest plot | 65 | 4884 | Mean Difference (IV, Random, 95% CI) | 97.81 [82.56, 113.05] |
| 3 Noradrenaline (pg/mL) Show forest plot | 36 | 1736 | Mean Difference (IV, Random, 95% CI) | 63.56 [42.66, 84.46] |
| 4 Adrenaline (pg/mL) Show forest plot | 16 | 662 | Mean Difference (IV, Random, 95% CI) | 7.55 [0.85, 14.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Renin (ng/mL/hour) Show forest plot | 44 | 3470 | Std. Mean Difference (IV, Random, 95% CI) | 1.05 [0.85, 1.24] |
| 2 Aldosterone (pg/mL) Show forest plot | 34 | 3128 | Mean Difference (IV, Random, 95% CI) | 95.59 [74.12, 117.05] |
| 3 Noradrenaline (pg/mL) Show forest plot | 23 | 964 | Mean Difference (IV, Random, 95% CI) | 48.66 [28.88, 68.44] |
| 4 Adrenaline (pg/mL) Show forest plot | 12 | 486 | Mean Difference (IV, Random, 95% CI) | 7.79 [0.31, 15.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cholesterol (mg/dL) Show forest plot | 27 | 1800 | Mean Difference (IV, Random, 95% CI) | 5.64 [2.46, 8.82] |
| 2 Trigyceride (mg/dL) Show forest plot | 19 | 1390 | Mean Difference (IV, Random, 95% CI) | 7.04 [3.04, 11.05] |
| 3 High density lipoprotein, HDL (mg/dL) Show forest plot | 19 | 1442 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐1.66, 1.08] |
| 4 Low density lipoprotein, LDL (mg/dL) Show forest plot | 17 | 1358 | Mean Difference (IV, Fixed, 95% CI) | 3.12 [‐0.41, 6.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cholesterol (mg/dL) Show forest plot | 20 | 1180 | Mean Difference (IV, Random, 95% CI) | 4.88 [1.19, 8.56] |
| 2 Trigyceride (mg/dL) Show forest plot | 12 | 770 | Mean Difference (IV, Fixed, 95% CI) | 6.92 [1.82, 12.02] |
| 3 High density lipoprotein, HDL (mg/dL) Show forest plot | 14 | 948 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐2.18, 0.83] |
| 4 Low density lipoprotein, LDL (mg/dL) Show forest plot | 12 | 864 | Mean Difference (IV, Fixed, 95% CI) | 3.63 [‐0.44, 7.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 White population, normotensive, SBP blinding‐high Show forest plot | 66 | 7100 | Mean Difference (Fixed, 95% CI) | ‐0.91 [‐1.19, ‐0.63] |
| 2 White population, normotensive, SBP blinding‐low Show forest plot | 24 | 1193 | Mean Difference (Fixed, 95% CI) | ‐1.05 [‐1.61, ‐0.50] |
| 3 White population, normotensive, SBP outcome‐assesed‐high Show forest plot | 36 | 2771 | Mean Difference (Fixed, 95% CI) | ‐1.12 [‐1.60, ‐0.65] |
| 4 White population, normotensive, SBP outcome‐assesed‐low Show forest plot | 56 | 5768 | Mean Difference (Fixed, 95% CI) | ‐0.89 [‐1.18, ‐0.60] |
| 5 White population, hypertensive, SBP blinding‐high Show forest plot | 45 | 3814 | Mean Difference (Fixed, 95% CI) | ‐6.03 [‐6.64, ‐5.41] |
| 6 White population, hypertensive, SBP blinding‐low Show forest plot | 36 | 1911 | Mean Difference (Fixed, 95% CI) | ‐5.78 [‐6.39, ‐5.17] |
| 7 White population, hypertensive, SBP outcome‐assesed‐high Show forest plot | 27 | 2470 | Mean Difference (Fixed, 95% CI) | ‐6.07 [‐6.95, ‐5.19] |
| 8 White population, hypertensive, SBP outcome‐assesed‐low Show forest plot | 55 | 3433 | Mean Difference (Fixed, 95% CI) | ‐5.71 [‐6.23, ‐5.19] |

