Políticas de lavado para el tratamiento de adultos con sonda urinaria permanente a largo plazo
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: RCT with 4 groups. The study set out to compare a washout with saline versus no washout and also different types of silicone catheters (Silicath and Silastic) Study duration: Six months | |
| Participants | Inclusion criteria: The inclusion criteria stated that participants required a long‐term indwelling catheter for a minimum of six months. Setting: Hospital and home care Country: Finland Health status: Participants were in good general health Number: treatment (N = 20); control (N = 20) Age: Participants age range from 50 to 59 years up to 85 to 99 years
Sex: (m/f): 16/24 Exclusion criteria: Patients not in good general health or unlikely to survive the investigation period were excluded | |
| Interventions | Treatment group 1
Treatment group 2
Control group 1
Control group 2
| |
| Outcomes |
How outcomes were measured: Not provided in translated copy | |
| Notes | Funding source: Not stated Study written in Finnish and translated to English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated random allocation but no description given |
| Allocation concealment (selection bias) | Unclear risk | Stated that 10 participants allocated to each of the 4 groups but no further details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers of participants who did not complete the trial is not clear and numbers do not appear to be consistent throughout the paper. Drop‐outs are given for different outcome measures |
| Selective reporting (reporting bias) | Unclear risk | Information on outcomes not fully reported in methods, therefore uncertainty about reporting bias |
| Other bias | Low risk | Appears to be free from other forms of bias |
| Methods | Study design: 3 centre cross‐over RCT | |
| Participants | Inclusion criteria: Elderly women in long‐term geriatric care with long‐term catheter in situ Setting: 3 geriatric hospitals Country: UK Health status: Not stated Number: 25 entered trial. 11 women lost to follow up (5 died, 3 catheters removed, 2 withdrawn by nursing staff, 1 discharged). 14 women completed full 12 weeks of trial Age: mean age 82 years, range 65 years to 100 years Sex: Female Other relevant Information: Catheter type and material not stated (type patient already wearing used); median duration catheter in situ at start of study: 12 months (range 1 month to 204 months) Exclusion criteria: no exclusion criteria stated | |
| Interventions | Intervention: Cross‐over study of 3 washout treatments Group A: 3 weeks of twice weekly 0.9% sodium chloride washout | |
| Outcomes |
Other outcomes reported (not analysed within this review):
| |
| Notes | Funding source: Not stated
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables used to determine the order of the solutions |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) | Low risk | Stated numbers and reasons for drop‐outs/withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods appear in the results section. Protocol was not reviewed so some uncertainty regarding reporting bias |
| Other bias | Low risk | Appears to be free of other bias |
| Methods | Study design: prospective, randomised, double‐blind, cross‐over, multicentre clinical study, 3 part study Study duration: 4 weeks | |
| Participants | Inclusion criteria: Aged 18 years and older, spinal cord injury or other neurogenic bladder patient requiring a chronic indwelling urinary catheter with a history of 2 episodes of catheter blockage and/or encrustation, and urine pH ≥ 6.5 Setting: Not stated Country: USA Health status: Not stated Number: 7 participants: Part 1 N = 20, Part 2 N = 28, Part 3 N = 19 Age: Age of total group (N = 67 years) mean 46.6 years (range = 21 years to 81 years) Sex: male N = 50 (75%) Other relevant Information: Catheter type: transurethral N = 34 (51%), suprapubic N = 33 (49%) Exclusion criteria: Antibiotics within 7 days, current infections, recent history of autonomic dysreflexia | |
| Interventions |
| |
| Outcomes | Results are given from Part 3 of the study (N = 14 completed) only:
| |
| Notes | Funding source: NovaBay Pharmaceuticals
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Randomisation concealment not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blinded in ClinicalTrials.gov: NCT01243125. As this trial compared one washout versus another, this would have been possible in this trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) | Unclear risk | No details provided although the number of drop‐outs per part of study can be calculated |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods section were reported in the results |
| Other bias | Low risk | Appears to be free of other bias |
| Methods | Study design: Single centre parallel group RCT with 2 groups: Group A ‐ citric acid catheter maintenance solutions (CMS), Group B ‐ planned catheter changes Study duration: 12 weeks | |
| Participants | Inclusion criteria: Community‐based patients with long‐term catheters known to block with encrustation Setting: Community Country: UK Health status: Not stated Number: 11 participants enrolled in trial (number allocated to each group not stated), 7 participants lost to follow‐up (reasons not stated), 4 patients analysed (Group A N = 1, Group B N = 3) Age: Not stated Sex: Not stated Other relevant Information: urethral catheters, material not stated, duration catheter in situ at start of study not stated Exclusion criteria: Not stated | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funding source: Not stated
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding but not possible in a trial of washout versus catheter change |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) | Unclear risk | Stated numbers only (no reasons given) |
| Selective reporting (reporting bias) | High risk | Methods state that symptomatic UTI rates would be monitored. These data were not reported in the results and no reasons were provided |
| Other bias | Low risk | Appears to be free of other biases |
| Methods | Study design: Parallel group RCT, 3 groups: catheter flush with saline vs acidic solution vs standard care (no washout) | |
| Participants | Inclusion criteria: Indwelling catheter in situ longer than 30 days, regular blocker that required catheter changed every 3 weeks or less Setting: Long‐term care setting or received home care Country: Canada Health status: Sufficiently alert according the mini‐mental state examination (MMSE score > 24) Number: 73 enrolled, 53 completed Age: Mean age 66.24 years (Contisol group mean 63.92 years, saline group mean 66.24 years, control group mean 68.56 years) Sex (m/f): 36/37 Exclusion criteria: Symptomatic UTI (individuals were eligible for the study following successful treatment of the UTI after a symptom‐free period of 14 days); urethral erosion allowing continuous bypassing (leakage) around urinary catheter; history of bladder cancer, or radiation or interstitial cystitis; impaired renal function as evidenced by a serum creatinine level of 2.0 mg/dL or higher; gross haematuria; or indwelling catheter that was changed less frequently than every 8 weeks | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funding source: Alberta Heritage Foundation for Medical Research and the Canadian Nurses Foundation
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Group allocation placed in opaque envelope, opened by participant |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of the participants to washout type attempted, not possible to blind the research nurse due to nature of the intervention and the packaging of washouts |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given. Assumed not done |
| Incomplete outcome data (attrition bias) | Low risk | Stated numbers and reasons |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods section were reported in the results |
| Other bias | Low risk | Appears to be free of other biases |
| Methods | Study design: Single centre cross‐over RCT, 2 interventions: Group A: normal saline irrigation, Group B: no irrigation | |
| Participants | Inclusion criteria: Indwelling catheter in situ > 30 days, Females aged 18 years +, were afebrile (temperature ≤ 37.7º) for 7 days, had not received antibiotics for 14 days Setting: Hospital and medical centre Country: USA Health Status: Not stated Number: 44 women entered the trial, 21 women did not complete the full intervention (10 died, 4 discharged, 3 catheter removed, 4 physician request), 23 women completed the 24 week intervention (A first 10, B first 13), 9 women completed at least one phase and five weeks of the second phase of the study Age: mean age 71 years, range 37 years to 88 years, 33 women were aged 65 years or over Sex: Female Other relevant Information: Catheter type: double lumen, 18 F, silicone‐coated latex urethral catheters Exclusion criteria: Patients with malignant bladder neoplasms or patients whose physician insisted on continued bladder irrigation | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funding source: National institute of Aging, National Institutes of Health
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random assignment determined but no further details provided |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding. As a washout solution versus no washout, can assume this was not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) | Low risk | Stated numbers and reasons for drop‐outs/withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported in results |
| Other bias | Low risk | Appears to be free of other biases |
| Methods | Study design: parallel group RCT (double blind but no description given), 3 groups: Group A: normal saline irrigation, Group B: acetic acid irrigation, Group C: neomycin‐polymyxin GU irrigation, groups stratified by sex | |
| Participants | Inclusion criteria: Community residing patients with neurogenic bladder managed by indwelling catheter, at least 6 months post spinal cord injury or onset of other neurological disease, evidence of microscopic bacteriuria and pyuria at time of study enrolment Setting: Community Country: USA Health status: No other details provided Number: 89 participants entered the trial (group A 29, group B 30, group C 30), 37 participants did not complete the full intervention (11 withdrew due to development of symptomatic UTI, 14 withdrew due to other health related reasons, 12 withdrew due to perceived difficulty, inconvenience or unwillingness to perform twice daily irrigations), 52 participants completed the intervention and were analysed (group A 21, group B 9, group C 22) Age: mean age 45.8 years, range 19 years to 82 years Sex (m/f): 49/40 Exclusion criteria: patients with serious UTIs requiring systemic antibiotics or with prior renal function abnormalities, patients who had used an acidifying agent, bladder irrigant or systematic antibiotic in previous 7 days, and patients who were pregnant or unable/unwilling to give informed consent Other relevant Information: no differences in demographic and injury related variables by group at baseline
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funding source: Paralyzed Vetrans of America Spinal Cord Research Foundation
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that participants randomised but no further details provided |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study described as double blinded but further details not provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) | Low risk | Stated numbers and reasons for drop‐outs/withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods reported in results |
| Other bias | Low risk | Appears to be free of other biases |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Primary outcomes of interest to review (i.e. catheter‐associated infection and encrustation) not addressed. Outcomes studied related to cleansing of bladder rather than catheter blockage from pus, fibrin, necrotic tissue and blood clots | |
| Not long‐term catheterisation. RCT of citric acid versus saline to prevent catheter encrustation | |
| Unable to determine duration of catheterisation. RCT (cross‐over design) of four irrigating solutions: saline, 0.25% acetic acid, 0.02% chlorhexidine, 0.25% silver nitrate | |
| Not all patients catheterised for more than 28 days. RCT of chlorhexidine versus saline on urinary bacterial count. 48 patients catheterised for 3 weeks or more. Unable to separate data of patients who met inclusion criteria for this trial | |
| Study methods insufficiently described and insufficient data reported on the effect on bacteruria in treatment and control groups. Thus the study was excluded as it did not contribute information on any of the reviews primary outcome measures, rather it focused on urothelial exfoliations rates and presented these data only graphically. RCT (cross‐over design) of effect of washouts (2.5% noxythiolin or saline) on the urothelium | |
| Unable to determine if patients randomised. Study methods insufficiently described. Insufficient data reported for calculating the effect on bacteruria in treatment and control groups | |
| Not an RCT. Comparison of irrigation with super oxidation water and normal saline in 21 paraplegics (conference abstract at 33rd Annual Meeting of Japan Medical Society of Paraplgia 1998) | |
| Not clear that this is an RCT. Duration of catheterisation at start of study less than 28 days for some patients. Comparison of three methods of irrigation with 0.25% acetic acid (no irrigation, one irrigation a week, two irrigations per day) | |
| Not an RCT. Cross‐over study of saline versus two Uro‐tainer solutions | |
| Not all patients catheterised for more than 28 days. Analysis of long‐term catheterised patients not reported. RCT of nitrofurazone and neomycin/polymyxin for prevention of bacteriuria | |
| Not an RCT. Comparison of effect of mandelic acid on two different bacterial species. There was only a single group of subjects who received a single regimen of 1% mandelic acid | |
| Unable to determine if RCT. Cross‐over comparison of twice daily irrigations with Suby G versus no irrigations | |
| Comparison with intermittent catheterised patients not relevant to review. Comparison of effect of methenamine mandelate and ascorbic acid on bacteriuria between indwelling and intermittent catheterised patients | |
| Not long‐term catheterisation. RCT of neomycin‐polymyxin irrigation versus no irrigation for prevention of UTIs |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Efficacy study of auriclosene irrigation solution on urinary catheter patency |
| Methods | Multicentre, randomised, double blind |
| Participants | Estimated enrolment of 140 participants. Inclusion criteria: aged 18 years and older, history of catheter blockage and/or encrustation. Exclusion criteria: systemic antibiotic use within 14 days of first treatment, current infection |
| Interventions | Experimental group: Auriclosene irrigation solution 0.2%, 8 treatments over 4 weeks Placebo group: Auriclosene Vehicle solution, 8 treatments over 4 weeks |
| Outcomes | Percent flow rate of catheters at time of removal Number of catheters removed due to blockage Number of subjects with serious and non‐serious adverse events |
| Starting date | September 2014 |
| Contact information | |
| Notes | Estimated completion date December 2016 Authors contacted in October 2015 but no results were available at that time |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with symptomatic UTI Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Any washout versus no washout, Outcome 1 Number of participants with symptomatic UTI. | ||||
| 1.1 any washout versus no washout | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 saline washout versus no washout | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 citric acid washout versus no washout | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 weeks to first catheter change Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Any washout versus no washout, Outcome 2 weeks to first catheter change. | ||||
| 2.1 any washout versus no washout | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 saline washout versus no washout | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 citric acid washout versus no washout | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of participants needing catheter replacement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Any washout versus no washout, Outcome 3 Number of participants needing catheter replacement. | ||||
| 3.1 saline washout versus no washout | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.31] |
| 4 Mean number of episodes of high temperature Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Any washout versus no washout, Outcome 4 Mean number of episodes of high temperature. | ||||
| 5 Mean number of episodes of high temperature of poss urinary origin Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Any washout versus no washout, Outcome 5 Mean number of episodes of high temperature of poss urinary origin. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with symptomatic UTI Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 One washout solution versus another, Outcome 1 Number of participants with symptomatic UTI. | ||||
| 1.1 citric acid verus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 weeks to first catheter change Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 One washout solution versus another, Outcome 2 weeks to first catheter change. | ||||
| 2.1 citric acid verus saline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
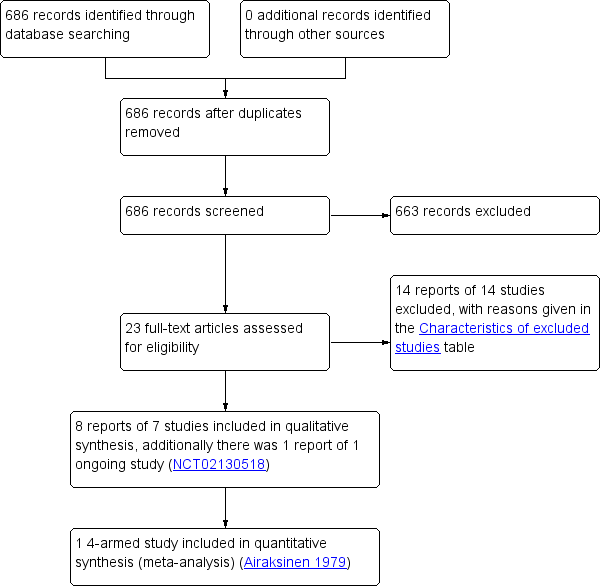
Study flow diagram
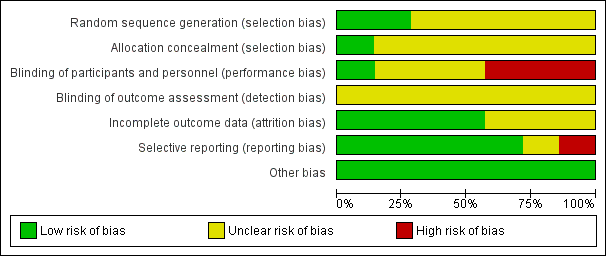
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
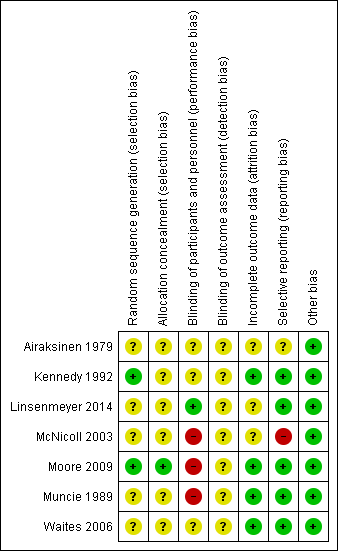
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
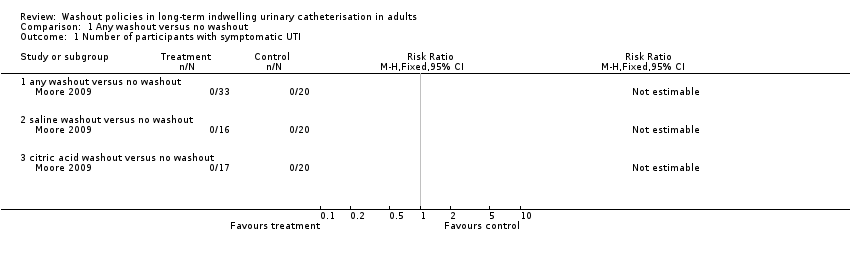
Comparison 1 Any washout versus no washout, Outcome 1 Number of participants with symptomatic UTI.
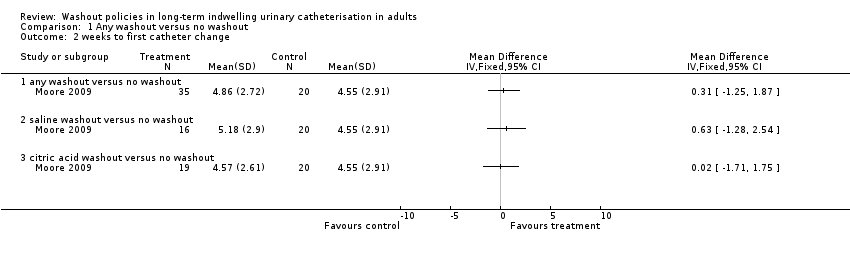
Comparison 1 Any washout versus no washout, Outcome 2 weeks to first catheter change.
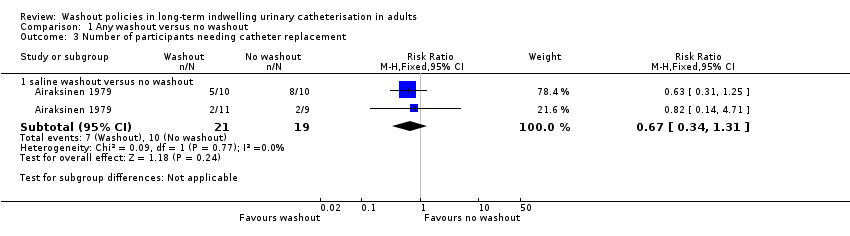
Comparison 1 Any washout versus no washout, Outcome 3 Number of participants needing catheter replacement.
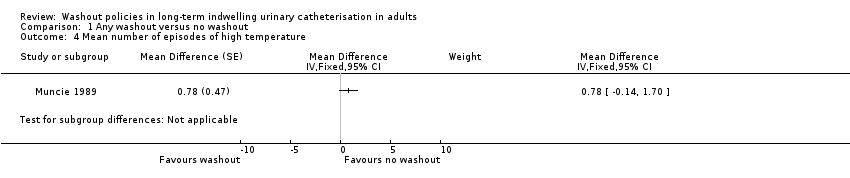
Comparison 1 Any washout versus no washout, Outcome 4 Mean number of episodes of high temperature.
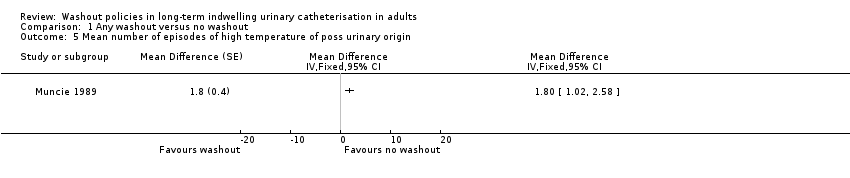
Comparison 1 Any washout versus no washout, Outcome 5 Mean number of episodes of high temperature of poss urinary origin.
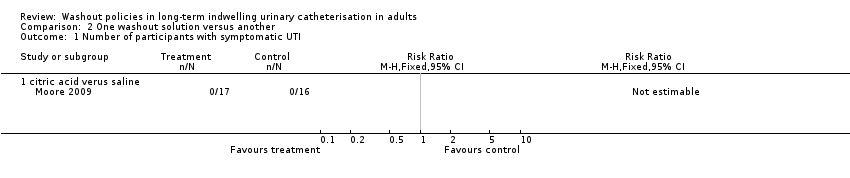
Comparison 2 One washout solution versus another, Outcome 1 Number of participants with symptomatic UTI.
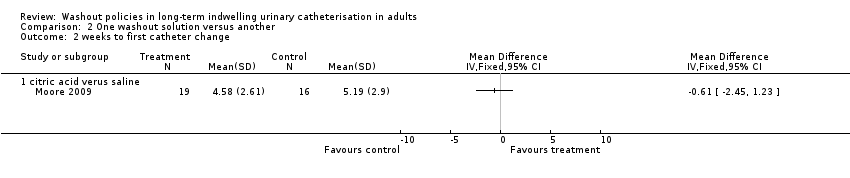
Comparison 2 One washout solution versus another, Outcome 2 weeks to first catheter change.
| Any washout compared to no washout for participants with long‐term indwelling urinary catheterisation | ||||||
| Patient or population: Long‐term indwelling urinary catheterisation in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No washout | Any washout | |||||
| Symptomatic UTI (Number of participants with symptomatic UTI, citric acid or saline washout versus no washout) | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 53 | ⊕⊕⊝⊝ | No participants met the study criteria for symptomatic UTI |
| Symtomatic UTI Mean number of episodes of high temperature (saline washout versus no washout) | ‐ | The mean number of episodes of high temperature (saline washout versus no washout) in the intervention groups was: 0.78 (‐0.14 to 1.70) | Not estimable | 23 | ⊕⊝⊝⊝ | |
| Symptomatic UTI Mean number of episodes of high temperature due to possible urinary origin (saline washout versus no washout) | ‐ | The mean number of episodes of high temperature of possible urinary origin (saline washout versus no washout) in the intervention groups was: 1.80 (1.02 to 2.58) | Not estimable | 23 | ⊕⊝⊝⊝ | |
| Number of catheters used (Number of participants needing catheter replacement, saline washout versus no washout) | 526 per 1000 | 353 per 1000 (179 to 689) | RR 0.67 | 40 | ⊕⊝⊝⊝ | |
| Length of time each catheter was in situ | Not estimable | Not reported | No data available | |||
| Catheter removal rates due to blockage/infection | Not estimable | Not reported | No data available | |||
| Rates of asymptomatic bacteriuria | Not estimable | Not reported | No data available | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels: The sample size was small (N = 53). Personnel not blinded to allocation of treatment. Blinding of outcome assessment not clear. | ||||||
| One washout solution versus another for participants with long‐term indwelling urinary catheterisation | ||||||
| Patient or population: Long‐term indwelling urinary catheterisation in adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | One washout solution versus another | |||||
| Symptomatic UTI Number of participants with symptomatic UTI (citric acid versus saline) | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 33 | ⊕⊕⊝⊝ | No participants met the study criteria for symptomatic UTI |
| Symtomatic UTI Mean number of episodes of high temperature | Not estimable | Not reported | No data available | |||
| Symptomatic UTI Mean number of episodes of high temperature due to possible urinary origin | Not estimable | Not reported | No data available | |||
| Number of catheters used Number of participants needing catheter replacement | Not estimable | Not reported | No data available | |||
| Length of time each catheter was in situ | Not estimable | Not reported | No data available | |||
| Catheter removal rates due to blockage/infection | Not estimable | Not reported | No data available | |||
| Rates of asymptomatic bacteriuria | Not estimable | Not reported | No data available | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels: The sample size was small (N = 33). Personnel not blinded to allocation of treatment. Blinding of outcome assessment not clear. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with symptomatic UTI Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 any washout versus no washout | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 saline washout versus no washout | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 citric acid washout versus no washout | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 weeks to first catheter change Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 any washout versus no washout | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 saline washout versus no washout | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 citric acid washout versus no washout | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of participants needing catheter replacement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 saline washout versus no washout | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.31] |
| 4 Mean number of episodes of high temperature Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5 Mean number of episodes of high temperature of poss urinary origin Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with symptomatic UTI Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 citric acid verus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 weeks to first catheter change Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 citric acid verus saline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

