Contraceptifs combinés : effets sur le poids
Résumé scientifique
Contexte
La prise de poids est souvent considérée comme un effet secondaire des contraceptifs hormonaux combinés et nombre de femmes et de praticiens croient qu'il existe un lien entre les deux. La crainte d’une prise de poids peut limiter le recours à cette méthode contraceptive très efficace en dissuadant la femme de commencer à l'utiliser et en provoquant son abandon précoce par les utilisatrices. Toutefois, aucun lien de cause à effet entre les contraceptifs combinés et une prise de poids n'a été établi.
Objectifs
L'objectif de cette revue était d'évaluer le rapport potentiel entre l'utilisation de contraceptifs combinés et les variations pondérales.
Stratégie de recherche documentaire
En novembre 2013, nous avons consultés les bases de données informatisées CENTRAL (Bibliothèque Cochrane), MEDLINE, POPLINE, EMBASE, et LILACS pour les études sur les contraceptifs combinés, ainsi que ClinicalTrials.gov et le Système d'enregistrement international des essais cliniques de l'OMS (ICTRP). Pour la revue initiale, nous avions également écrit à des chercheurs et fabricants de renom pour solliciter des informations concernant d'autres essais, publiés ou non, que nous n’aurions pas trouvés au cours de nos recherches.
Critères de sélection
Tous les essais contrôlés randomisés en langue anglaise étaient éligibles s'ils comprenaient au moins trois cycles de traitement et comparaient des contraceptifs combinés à un placebo ou à un contraceptif combiné différent du point de vue de la substance active, du dosage, de la posologie ou de la durée de l'étude.
Recueil et analyse des données
Nous avons évalué tous les titres et résumés disponibles dans les recherches de littérature. Les données ont été saisies et analysées par le logiciel RevMan. Un second auteur a vérifié les données saisies. Pour les données continues, nous avons calculé la différence moyenne et l'intervalle de confiance (IC) à 95 % pour la variation pondérale moyenne entre les mesures de base et les mesures post‐traitement en utilisant un modèle à effets fixes. Quant aux données catégorielles, telles que la proportion de femmes qui ont pris ou perdu plus qu'un certain poids, nous avons calculé le rapport de cotes avec un IC à 95 %.
Résultats principaux
Nous avons trouvé 49 essais qui répondaient à nos critères d'inclusion. Les essais incluaient 85 comparaisons de la variation pondérale pour 52 paires de contraceptifs différentes (ou contraceptif et placebo). Les quatre essais avec un placebo ou un groupe de non‐intervention n'ont fait apparaître aucune preuve à l’appui d’un lien de causalité entre les contraceptifs oraux combinés ou un timbre transdermique combiné et les variations pondérales. La plupart des comparaisons entre les divers contraceptifs combinés n'ont pas mis en évidence de différence importante de poids. En outre, l'abandon des contraceptifs combinés à cause de variations pondérales ne différait pas entre les groupes dans les études examinant cette question.
Conclusions des auteurs
Les preuves disponibles étaient insuffisantes pour déterminer l'effet des contraceptifs combinés sur le poids, mais aucun effet important n'a été relevé. Les essais visant à évaluer le lien entre les contraceptifs combinés et le poids requièrent un groupe placebo ou un groupe non hormonal pour contrôler les autres facteurs, notamment les variations pondérales au fil du temps.
PICO
Résumé simplifié
Effet des pilules contraceptives et des timbres transdermiques sur le poids
La prise de poids est considérée comme un effet secondaire des méthodes contraceptives. De nombreuses femmes et professionnels de la santé pensent que les pilules et les timbres provoquent une prise de poids. La crainte de prendre du poids peut limiter l'utilisation de ces méthodes contraceptives efficaces. La crainte de prendre du poids empêche certaines femmes de prendre la pilule ou d'utiliser un timbre transdermique. Les femmes peuvent cesser de prendre la pilule car elles pensent que celle‐ci a été à l'origine d'une prise de poids. Cette revue a étudié les essais concernant les pilules contraceptives ou les timbres dans lesquels le poids des femmes a été contrôlé.
En novembre 2013, nous avons effectué une recherche informatisée d’études relatives aux pilules ou timbres contenant deux types d'hormones. Pour la revue initiale, nous avions également écrit aux chercheurs et aux fabricants pour obtenir d’autres essais. Nous avons inclus les essais randomisés en anglais s'ils comprenaient au moins trois cycles de traitement. Les études devaient également comparer deux types de méthodes contraceptives ou un type avec une méthode dite factice.
Nous avons trouvé 49Â essais. Ces essais comparaient 52 paires différentes de méthodes contraceptives, ou une méthode contraceptive et une méthode dite factice. Les quatre essais, suivant une méthode avec ou sans groupe factice, n'ont pas démontré que ces pilules ou timbres provoquaient une variation pondérale. La plupart des études sur les diverses méthodes contraceptives n'ont mis en évidence aucune différence significative de poids. De même, les femmes ne cessaient pas d'utiliser la pilule ou le timbre à cause d'une variation pondérale. Les preuves n'étaient pas assez solides pour affirmer que ces méthodes ne provoquaient pas une certaine variation pondérale. Nous n'avons cependant pas relevé d'effet important sur le poids. Pour observer le lien entre les méthodes de contraception et la variation pondérale, les études devraient comporter un groupe factice ou un groupe n'utilisant pas d’hormones. Ce type de groupe de contrôle permettrait de supprimer d'autres facteurs, tels que la variation pondérale au fil du temps.
Authors' conclusions
Background
Weight gain is often considered a side effect of using combination contraceptives (that is, an estrogen plus a progestin) (IOM 1996; Nelson 2007), and many women and clinicians believe that an association exists. Almost three‐quarters of women in a random survey conducted in the United Kingdom reported believing that weight gain was related to oral contraceptive use (Turner 1994). In a Canadian survey of women filling an oral contraceptive (OC) prescription (Gaudet 2004), 68% had counseling from their physician on weight gain and the pill. Of those who had counseling, 36% said their weight would stay the same while on the pill compared to 50% of those who had no counseling. In the United States, 45% of adolescents starting OC use believed that oral contraceptive use increased the risk of weight gain (Emans 1987). Also, in a large German convenience sample, about 27% of ever users reported gaining weight from oral contraceptive use (Oddens 1999). In a representative sample of 3600 females in France, aged 15 to 45 years, 1665 were taking OCs (Le 2003). Of these women using the pill, 30% claimed to have gained weight on their most recent pill.
Concern about weight gain can deter the initiation of combination contraceptives and cause early discontinuation among users. Weight gain was the most frequently cited reason for oral contraceptive discontinuation in a national study of adult women in the United States (Rosenberg 1998). A second survey found that about 20% of women claimed that weight gain was a reason for oral contraceptive discontinuation or failure to initiate use (Wysocki 2000). In a convenience sample of oral contraceptive users in five European nations, women who reported weight gain had a relative risk of 1.4 (95% CI 1.2 to 1.6) of method discontinuation before two years of use compared to those who did not report a gain in weight (Rosenberg 1995). Furthermore, even the perception of weight gain can lead to contraceptive discontinuation. A United States study found that women who stopped using OCs were more likely to report weight gain than those who continued using the method, even with no significant difference in measured weight gain between the two groups (Emans 1987). Thus, concern about weight gain limits the use of a highly effective method of contraception.
Nevertheless, a causal relationship between combination contraceptives and weight gain has not been established. Several mechanisms by which combination contraceptives could lead to weight gain have been hypothesized. In general, weight gain is due to an increase in one or more factors of fluid retention, muscle mass, and fat deposition. Fluid retention could be induced by the mineralocorticoid activity that occurs when ethinyl estradiol, the estrogen in combination oral contraceptives, enters the renin‐angiotensin‐aldosterone system (Corvol 1983). Estrogen has been associated with increased subcutaneous fat, especially in the breasts, hips, and thighs (Nelson 2007). The anabolic properties of combination contraceptives could result in increased food intake through a physiological effect on satiety and appetite. Androgens may stimulate nitrogen retention and increase muscle mass, although it is unlikely that oral contraceptives would cause such weight gain (Nelson 2007).
The possible causal association between combination contraceptives and weight gain is difficult to study for several reasons. During adolescence, some weight gain is developmentally normal and appropriate. Also, women tend to gain weight over time (Flegal 2000). A contemporaneous control group is needed, but a randomized controlled trial comparing a combination contraceptive method with a placebo or non‐hormonal method for contraception raises ethical issues. Few such studies have been conducted. Comparing combination contraceptive products is complicated by the variety of formulations and regimens. In addition, most combination contraceptive studies have been of short duration (that is, six cycles or fewer); more time might be required for the weight gain to become evident. Finally, no consensus exists regarding what constitutes excessive weight gain. Ideally, studies would set an a priori definition of clinically important weight gain, but this is rarely specified, perhaps because weight change is not a primary outcome in most comparison trials of combination contraception. Most studies that present a dichotomous classification for weight gain selected either 2.0 or 2.3 kilograms as the cut point; however, the justification for this decision is not apparent. Even if clinically important weight gain was well specified, any gain in weight could still be relevant since the mere perception of weight gain is associated with discontinuation of oral contraception.
Objectives
The aim of the review was to evaluate the potential association between combination contraceptive use and changes in weight. The primary hypothesis was that combination contraceptives do not result in weight changes greater than that of a placebo. The secondary hypothesis was that different formulations and regimens of combination contraceptives are not associated with differences in weight changes.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials reported in English (Juni 2002; Moher 2000) that compared a combination contraceptive to a placebo, no intervention, or a combination contraceptive that differed in drug, dosage, regimen, or study length.
Types of participants
Women of reproductive age without medical contraindications to combination contraceptives.
Types of interventions
Any combination contraceptive compared to a placebo, no intervention, or another combination contraceptive. Trial drug interventions must have included at least three consecutive cycles to be eligible.
Types of outcome measures
Trials must have collected data on change in body weight to be eligible for inclusion. Weight change could have been measured as either the change in the study group's mean weight or as the proportion of the study group who lost or gained more than a specified amount.
Search methods for identification of studies
Electronic searches
In November 2013, we searched the computerized databases Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE, POPLINE, EMBASE, and LILACS for studies of combination contraceptives. We also searched for trials via ClinicalTrials.gov and the search portal of the International Clinical Trials Registry Platform (ICTRP). The strategies are given below.
Searching other resources
For the initial review, we wrote to known investigators and manufacturers to request information about other published or unpublished trials not discovered in our search.
Data collection and analysis
Selection of studies
All titles and abstracts identified during the literature searches were assessed for inclusion, and all potentially relevant articles were photocopied. For the initial review, we wrote to the manufacturers of combination contraception and authors of the included trials to seek other published or unpublished trials.
Data extraction and management
The abstracted data were entered into RevMan and were double‐checked by a second author. The analysis depended on the data available. For the mean change in weight between baseline and post‐treatment measurements, the mean difference (MD) with 95% confidence interval (CI) was calculated using a fixed‐effect model. Alternatively, the Peto odds ratio (OR) with 95% CI was calculated using the proportion of women who gained or lost more than a specified amount of weight.
Significant weight change could be considered a negative side effect of contraceptive use. We used a consistent direction for the graph labels even though the outcomes differed. Therefore, the intervention ‘favors treatment’ if the change is greater in the control group. The intervention ‘favors control’ if the change is greater in the treatment group.
Assessment of risk of bias in included studies
The validity of trials was critically appraised by assessing potential biases; however, summary quality scores were not calculated since the available evidence does not support their use (Higgins 2011). The appraisal of potential biases concentrated on the study design, blinding, randomization method, group allocation concealment, and loss to follow up and early discontinuation.
Measures of treatment effect
Despite some trials reporting weight change data for multiple cycles, only one weight change measure was abstracted for each intervention group. We chose the cycle 12, cycle 6, or last treatment cycle data (in this order of preference) to facilitate comparisons between trials. For trials that included more than two interventions, comparisons were made between the control group and the other groups. If the authors did not identify a control group, all possible combinations were included in the review. We did not use any technique to control for multiple testing.
Significance testing has been criticized for forcing the decision to recognize a difference between two interventions to rest on an arbitrary alpha‐level. Rather than determine differences solely based on a dichotomous classification of the P value, analyses using interval estimation consider both the location of the point estimate and the spread of the CI (Rothman 1998). While this process introduces more subjectivity, borderline 'statistically significant' results are not overlooked and clinically insignificant results are not ascribed undue importance. Although interval estimation is a preferable method, given the reliance on significance testing, we presented results based on both considerations.
Dealing with missing data
For the initial review, we wrote to the authors of 94 trials that either did not report weight data or reported insufficient details. At that point we revised and broadened the search strategy and decided that continuing to contact the authors of trials that appeared to be eligible except for a lack of weight data was no longer feasible. The 14 trials with additional, unpublished data supplied by the authors contacted before the protocol change were included in the present review.
Since point estimates that are not accompanied by a measure of sampling variation have limited interpretation, trial reports that did not include the standard error, standard deviation, or CI for the mean change in weight were not included in the review. For trials that did not report the denominators used to calculate the mean weight difference, or the percentage of women experiencing weight change, we estimated these numbers based on denominators used for other outcomes or the number of women who completed the trial.
Data synthesis
Studies were combined for a meta‐analysis only when identical drugs, dosages, regimens, and delivery systems were compared. No sensitivity analyses were planned since few trials were anticipated to be eligible for meta‐analysis. The data abstracted for the review were dependent on the analytic method used in the trial report (for example, intent to treat, per protocol, or a modification of either type).
Results
Description of studies
Results of the search
Initial review
The initial search strategy in 2002 yielded 570 reports of randomized controlled trials that compared a combination contraceptive to a placebo or to another combination contraceptive. Of those, 476 articles were not eligible for inclusion due to a study length less than three treatment cycles in duration or a lack of reported weight change. An additional 53 articles with weight data were excluded since they lacked an estimate of the sampling variability for the mean difference in weight. The 41 eligible articles, including the 14 articles with additional unpublished data from the authors, reported on 44 trials. One article (Oelkers 2000) described two trials, and two articles (Coney 2001; Kaunitz 2000) each reported pooled results from two eligible trials with similar or identical protocols. Since they could not be disaggregated, we treated the pooled results as if they were from one larger trial, for a total of 42 trials.
Updates
-
2005: 12 new RCTs of combination contraceptives also included a weight measurement. Only two trials were of sufficient duration and had reported weight change data with sampling variability, which yielded 44 trials.
-
2008: 13 trials had weight measurements, but only three had sufficient data for inclusion, for a new total of 47 trials.
-
2011: Of five possible trials, two met our inclusion criteria. The new total was 49 trials.
-
2013: The search yielded 134 unduplicated citations from the electronic databases. We reviewed the full text of eight articles; none met our inclusion criteria. In addition, we found 32 unduplicated listings in ClinicalTrials.gov and ICTRP. We identified one ongoing trial that was relevant (Mahidol 2013).
Included studies
Of the 49 eligible trials, four included a placebo group or no hormonal method. Three of these trials evaluated oral contraceptives (Coney 2001; Goldzieher 1971; Procter‐Gray 2008) and one studied a contraceptive skin patch (Sibai 2001). The products evaluated in the 49 trials included 18 progestins and three estrogens. Trials examined combination oral contraceptives except for the following comparisons: two combination injectables (Sang 1995); two combination vaginal rings (Weisberg 1999); a combination skin patch (Sibai 2001; Stewart 2005); and a combination ring with an oral contraceptive (Milsom 2006; Oddsson 2005). Seven trials included more than two intervention groups; three of these trials did not specify a control group.
The sample sizes for the trials ranged from 20 to 5654 randomized participants with a median of 196 participants. The study location was not described for 13 trials; the other studies were conducted in locations worldwide. The number of trial sites ranged from a single site (12 trials) to 131 sites, except for 10 trials that did not specify the number of sites. The duration of the trials ranged from 3 to 24 treatment cycles with most trials designed to be either 6 or 12 treatment cycles in length. The eligibility criteria for the participants varied among the trials with most trials recruiting healthy women of reproductive age without contraindications to hormonal contraceptive use. However, six of the articles did not describe any inclusion or exclusion criteria.
Risk of bias in included studies
The quality of the reporting of the trials on this topic was generally poor, and poor quality is associated with empirical evidence of bias (Schulz 1995).
Allocation
The method of generating the randomization sequence was not reported for 31 trials. The remaining 18 trials included at least some detail of the process (for example, use of a random numbers table, pre‐distributed lists, or computer‐generated sequence). Most trial reports (N = 45) did not describe a method of allocation concealment (Schulz 2002a). Cachrimanidou 1993 and Kashanian 2010 reported the use of sealed envelopes but did not provide details on whether the envelopes were impervious to deciphering (for example, use of opaque, sequentially‐numbered envelopes). Only three articles reported adequate allocation concealment: Miller 2001 used sealed, sequentially‐numbered, opaque envelopes containing carbon paper, which allowed the participant to sign the allocation card before study staff opened the envelope and learned the group assignment; Milsom 2006 and Oddsson 2005 had interactive voice response systems for the randomization process.
Only four articles reported the number of women recruited for the trial (Cachrimanidou 1993; Kashanian 2010; Oddsson 2005; Wiegratz 2002). One article stated that not all randomized women were included in the study results but did not specify the number of randomized women (Worsley 1980). A second trial report included sample sizes for the weight outcome but did not state whether these data included all randomized women (Sibai 2001). Although they reported the total number of randomized women, seven trial reports did not provide the number of randomized women stratified by study group.
Blinding
About half of the eligible trials were open; two were single‐blinded, 10 were double‐blinded, and one was triple‐blinded. Blinding was not mentioned in 15 trials. Participants, investigators, and outcome assessors were blinded as to group assignment in the triple‐blinded study (Oelkers 1995), and participants appeared to have been blinded in two of the double‐blinded trials (Coney 2001; Goldzieher 1971) using active and placebo pills that were identical in appearance. Since the Goldzieher 1971 trial reported that the randomization code was not broken during the study, the blinding of the investigators can be inferred. Two trials did not inform the assessors of group assignment, but the investigators and participants were not blinded (Kashanian 2010; Procter‐Gray 2008). The remaining blinded trials were unclear about who was blinded, and none of the trials included details regarding whether blinding appeared to have been implemented successfully (Schulz 2002b).
Incomplete outcome data
Eleven trials did not report the denominators used to derive the mean weight difference (Coenen 1996) or the percentage of women with weight change greater than a specified amount (Brill 1991; Dionne 1974; Endrikat 1999; Endrikat 2001a; Goldzieher 1971; Halbe 1998; Koetsawang 1995; Lachnit‐Fixson 1984; Oelkers 1995; Rosenbaum 2000). Discrepancies existed in at least 13 trials, since more women were missing in the weight estimate than could be explained with the possible reasons in the article (for example, non‐starters, early discontinuation, those lost to follow up, or exclusion).
Deducing which participants were included in the reported weight change estimate was hindered for most trials due to the lack of details regarding the method of analysis. An intention‐to‐treat analysis was described for three trials (Oddsson 2005; Procter‐Gray 2008; Wiegratz 2002); a per‐protocol analysis (also called valid‐case) was reported for three trials (Endrikat 1999; Serfaty 1998; Winkler 1996); and an analysis based on all participants who started treatment was reported for five trials (Coney 2001; Endrikat 2001b; Gruber 2006; Kaunitz 2000; Milsom 2006). Two trials (Endrikat 2001a; Stewart 2005) described intention‐to‐treat or valid‐case analyses but did not specify which was used for the weight change data. Two trials (Spellacy 1970; Van der Does 1995) had complete study participation and based the weight estimates on measurements from all participants. Kashanian 2010 excluded from the analysis two women who discontinued the intervention. The remaining 33 trials did not specify the analytic method used for the weight change data.
Other potential sources of bias
Change in body weight was a primary outcome for only one trial (Sibai 2001). Most trials either recorded weight at the baseline and follow‐up clinic visits or did not describe the method used for measuring weight. Liukko 1987 reported that the clinic visits, in which weight was measured, were scheduled around day 22 of the cycle. Milsom 2006 and Procter‐Gray 2008 described the methods used to standardize weight measurements. Four trials instructed participants to weigh and record their weight at home (Rosenbaum 2000), at home without clothing every second day (Oelkers 2000, Study 2) and in a fasting state (Oelkers 1995), or at home without clothing on a weekly basis (Oelkers 2000, Study 3).
Most trial reports either did not include data on loss to follow up (19 trials) or reported it combined with early discontinuation (nine trials). Loss to follow up ranged from zero to 17% for the 20 trials with loss to follow up reported separately from early discontinuation and from 10% to 35% for those that only reported losses from all causes. Early discontinuation ranged from zero to 39% for the 31 trials that reported this study factor separately. Eight trial reports did not include information on early discontinuation. Furthermore, 18 trial reports described excluding randomized women from study participation or analysis. Exclusions after randomization are not consistent with an intention‐to‐treat analysis and could have led to biased results (Weiss 1998).
Effects of interventions
The trials included 85 weight change comparisons for 52 distinct contraceptive pairs (or a placebo) that were eligible for the present review. We combined data from two or more trials for four of the 84 comparisons since they were identical in drug, dosage, regimen, and study length. The comparisons of a combination contraceptive with a placebo or no hormonal method showed no significant differences in weight change. These included five comparisons between an oral contraceptive and placebo (Coney 2001; Goldzieher 1971) or no intervention (Procter‐Gray 2008) and one comparison between a combination skin patch and placebo (Sibai 2001).
The study groups differed significantly in six comparisons of two pills and one comparison of the vaginal ring with an OC.
-
Three studies showed differences in the numbers of women with a weight change of more than 2 kg.
-
For gaining more than 2 kg, the OR was 3.29 (95% CI 1.84 to 5.88) for women assigned to oral desogestrel 150 μg and ethinyl estradiol (EE) 30 μg compared to those with oral levonorgestrel 50‐75‐125 μg and EE 30‐40‐30 μg (Lachnit‐Fixson 1984) (Analysis 11.1).
-
For losing more than 2 kg, the OR was 9.22 (95% CI 1.79 to 55.04) for oral drospirenone 3 mg and EE 15 μg compared to oral levonorgestrel 150 μg and EE 30 μg (Oelkers 1995) (Analysis 20.2).
-
The OR for losing more than 2 kg was 1.65 (95% CI 1.13 to 2.41) for the oral desogestrel 150 μg and EE 20 μg group compared to the oral gestodene 75 μg and EE 20 μg group (Serfaty 1998) (Analysis 9.2).
-
-
Four studies showed differences in the mean weight change between groups. One group had a slight increase while the other group showed a small decrease or no change.
-
The mean difference in Kaunitz 2000 was 0.26 kg (95% CI 0.12 to 0.40) (Analysis 46.1). Women assigned to oral norethindrone 500‐750‐1000 μg and EE 35 μg group had a slight mean increase, and those in the oral desogestrel 100‐125‐150 μg and EE 25 μg group had a slight decrease.
-
In Loudon 1990, the mean difference was 0.70 kg (95% CI 0.14 to 1.26) (Analysis 35.1). The group with oral levonorgestrel 150 μg and EE 30 μg gained, on average, while those with oral gestodene 75 μg and EE 30 μg lost a little weight.
-
The mean difference was ‐0.67 kg (95% CI ‐1.16 to ‐0.18) in Gruber 2006 (Analysis 23.1). On average, the women assigned to drospirenone 3 mg and EE 20 μg had a slight decrease, and the group with desogestrel 150 μg and EE 20 μg had a small increase.
-
Tn Milsom 2006, the mean difference was 0.40 kg (95% CI 0.03 to 0.77) (Analysis 52.1). Women assigned to the vaginal ring gained on average while the group with the combination oral contraceptive (COC) containing drospirenone 3 mg and EE 30 µg had no change overall.
-
Six additional comparisons had a point estimate and 95% CI that were consistent with at least a minimal difference between the two groups. Odds ratios for three of the six studies were as follows:
-
OR 1.54 (95% CI 0.92 to 2.60) for gaining more than 2 kg with oral gestodene 75 μg and EE 30 μg versus oral norgestimate 250 μg and EE 35 μg (Brill 1991) (Analysis 29.1);
-
OR 1.75 (95% CI 0.98 to 3.11) for gaining more than 2.5 kg with oral lynestrenol 2 mg and EE 40 μg versus oral lynestrenol 1 mg and EE 40 μg (Koetsawang 1977) (Analysis 42.1);
-
OR 1.69 (95% CI 0.89 to 3.20) of losing more than 2 kg with oral norethisterone 500 μg and EE 20 μg versus oral levonorgestrel 150 μg and EE 30 μg (Endrikat 2001b) (Analysis 45.2).
Mean differences in kg were as follows for the other three studies:
-
MD 1.30 (95% CI ‐0.32 to 2.92) between oral levonorgestrel 50, 75, 125 μg and EE 30, 40, 30 μg group and oral desogestrel 50‐100‐150 μg and EE 35‐30‐30 μg group (Van der Does 1995) (Analysis 38.2);
-
MD 1.80 (95% CI ‐0.73 to 4.33) between a standard versus prolonged oral norgestrel and EE regimen (Miller 2001) (Analysis 48.1);
-
MD 1.14 (95% CI ‐0.54 to 2.82) between oral dl‐norgestrel 500 μg and EE 50 μg group and oral norethisterone acetate 1 mg and EE 50 μg group (Worsley 1980) (Analysis 18.1).
Twenty‐one trial reports provided quantitative data on the primary reasons for early discontinuation. Ten of these trial reports included weight change as a distinct category for early discontinuation from trial participation (Table 1). The proportions of women who discontinued due to weight changes were small (zero to 5%) and did not differ between the study groups. Sang 1995 included the mean weight gain for the women who cited weight gain as the primary or secondary reason for early discontinuation. In the group with injectable medroxyprogesterone acetate 25 mg and estradiol cypionate 5 mg, the 18 women who attributed weight gain to the contraceptive had a mean gain of 2.8 kg compared to 3.1 kg for the 16 women in the group assigned to the injectable norethisterone enanthate 50 mg and estradiol valerate 5 mg (Sang 1995).
| Study ID | Intervention group | n | N (randomized women) |
| Prolonged desogestrel / ethinyl estradiol (EE) regimen | 10 | 200 | |
| Standard desogestrel / EE regimen | 1 | 100 | |
| Norgestimate 250 μg / EE 35 μg | 1 | 25 | |
| Gestodene 75 μg / EE 30 μg | 0 | 25 | |
| Desogestrel 150 μg / EE 30 μg | 0 | 25 | |
| Desogestrel 150 μg / EE 20 μg | 0 | 25 | |
| Levonorgestrel 100 μg / EE 20 μg | 2 | 359 | |
| Placebo | 0 | 362 | |
| Levonorgestrel 250 μg / EE 50 μg | 3 | 73 | |
| Levonorgestrel 150 μg / EE 30 μg | 1 | 77 | |
| Gestodene 75 μg / EE 30 μg | 4 | 279 | |
| Desogestrel 150 μg / EE 30 μg | 0 | 316 | |
| Gestodene 75 μg / EE 30 μg | 4 | 505 | |
| Desogestrel 150 μg / EE 20 μg | 2 | 501 | |
| Standard norgestrel / EE regimen | 0 | 44 | |
| Prolonged norgestrel / EE regimen | 1 | 46 | |
| Vaginal ring etonogestrel 120 µg / EE 15 µg | 2 | 512 | |
| Levonorgestrel 150 µg / EE 30 µg | 6 | 518 | |
| Injectable medroxyprogesterone acetate 25 mg / estradiol cypionatge (EC) 5 mg | 14 | 1955 | |
| Injectable norethisterone enanthate 50 mg / estradiol valerate (EV) 5 mg | 10 | 1960 | |
| Norethisterone 500‐1000 μg / EE 35 μg | 3 | 100 | |
| Levonorgestrel 50‐75‐125 μg / EE 30‐40 μg | 1 | 96 |
Discussion
Summary of main results
The four trials that included a placebo group or 'no intervention' group (Coney 2001; Goldzieher 1971; Procter‐Gray 2008; Sibai 2001) did not find evidence supporting the putative association between combination contraceptive use and weight change. The lack of an association in those trials could be due to the limited number of contraceptives evaluated. Goldzieher 1971, conducted more than three decades ago, studied three high‐estrogen dose oral contraceptives. Coney 2001 and Procter‐Gray 2008 evaluated one oral contraceptive each. Sibai 2001 studied one skin patch. Given the numerous combination contraceptive drugs, doses, and regimens, the possibility that one or more combination contraceptives could cause weight change cannot be eliminated with the data from the four placebo‐controlled randomized trials conducted to date.
Of the 79 weight change comparisons evaluating two combination contraceptives, seven showed a difference in the mean weight change or the proportion of women gaining or losing more than a set amount of weight. Even if no association existed between combination contraceptives and weight, one would expect several significant results (Type I errors) since numerous comparisons were made. Regardless of statistical significance, the clinical significance seems negligible. The point estimates for the mean difference in weight between the comparison groups were small. The largest notable difference was 1.80 kg (95% CI ‐0.73 to 4.33) after 12 treatment cycles (Miller 2001). The ORs for the proportions of women who gained or lost more than a set amount were generally either weak or too imprecise to convey much meaning. The CI from Oelkers 1995 was very wide since no one in the levonorgestrel and EE group lost more than the specified 2 kg.
If a mechanism for weight gain were estrogen‐dependent, two contraceptives containing the same progestin and estrogen types but different hormone doses might show more weight gain with the higher‐estrogen contraceptive. In this review, 11 of the 51 comparison pairs included two oral contraceptives with identical progestin and estrogen types but different hormone doses or regimens. Only Miller 2001 detected a possible difference in weight change between the groups, and the higher weight gain was for the group assigned less estrogen. While a dose‐related effect would have supported the hypothesized causal link between estrogen and weight gain, the lack of this finding does not disprove the possible association. The studies could have been underpowered to detect a dose‐gradient response between the estrogen content and weight gain.
Overall completeness and applicability of evidence
Only one trial examined weight change as a primary outcome, so most trials did not use rigorous methods for measuring weight. The reliability of the measurements could be affected by numerous factors, such as the use of calibrated scales, the time of day and cycle when measurements were collected, the use of a fasting state, and the amount of clothing on the participant. The degree of error in measuring weight change is likely to be similar between study groups and to dilute the effect estimate toward the null value of no difference.
The trials also could have failed to detect differences in weight if the rates for early discontinuation or loss to follow up had systematically differed between intervention groups for women who gained or lost weight. Ten trials reported the proportion of women for whom weight change was the primary reason for early discontinuation, and did not find differences by study group. Also, the one trial that reported the mean weight gain for the women who discontinued early for this reason found similar mean weight changes for the two combination injectable groups. The interpretation of the trial results would have been strengthened by including weight change data for women who did not complete the trial.
Quality of the evidence
More than 25% of the trials had high risk of bias due to lack of blinding or incomplete outcome data (Figure 1). The majority of studies had unclear risk of bias due to missing information on randomization sequence generation or allocation concealment. However, most of those trials were published before CONSORT (Moher 2001; CONSORT 2009) (Figure 2).
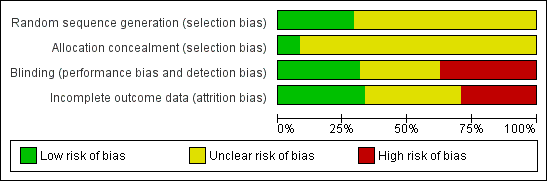
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
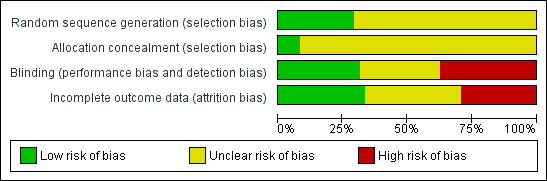
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
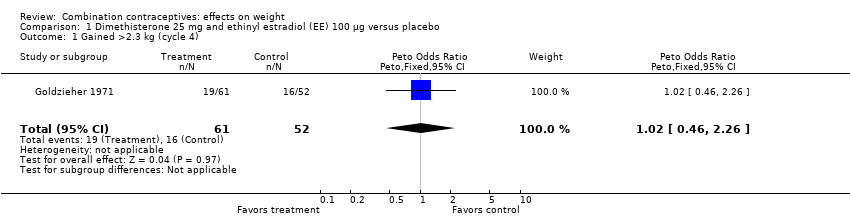
Comparison 1 Dimethisterone 25 mg and ethinyl estradiol (EE) 100 µg versus placebo, Outcome 1 Gained >2.3 kg (cycle 4).
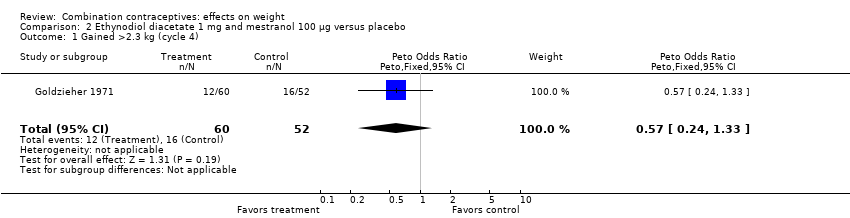
Comparison 2 Ethynodiol diacetate 1 mg and mestranol 100 µg versus placebo, Outcome 1 Gained >2.3 kg (cycle 4).

Comparison 3 Levonorgestrel 100 µg and EE 20 µg versus placebo, Outcome 1 Mean weight change in kg (cycle 6).

Comparison 4 Norgestrel 300 µg and EE 30 µg versus no intervention, Outcome 1 Mean weight change in kg per year.
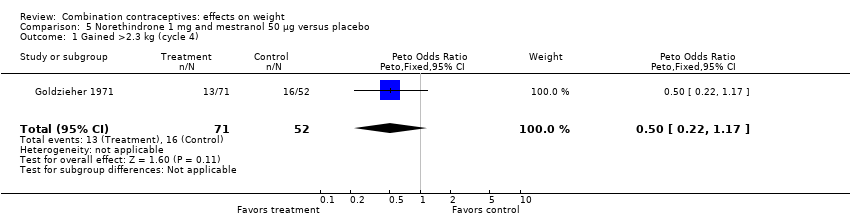
Comparison 5 Norethindrone 1 mg and mestranol 50 µg versus placebo, Outcome 1 Gained >2.3 kg (cycle 4).
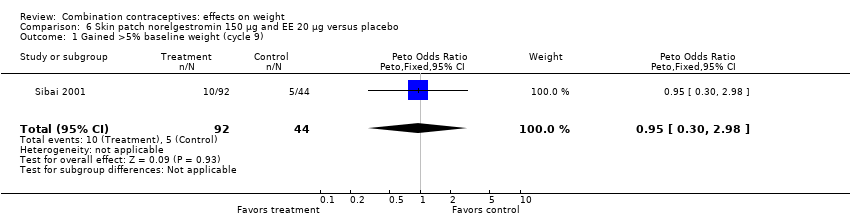
Comparison 6 Skin patch norelgestromin 150 µg and EE 20 µg versus placebo, Outcome 1 Gained >5% baseline weight (cycle 9).

Comparison 6 Skin patch norelgestromin 150 µg and EE 20 µg versus placebo, Outcome 2 Lost >5% baseline weight (cycle 9).
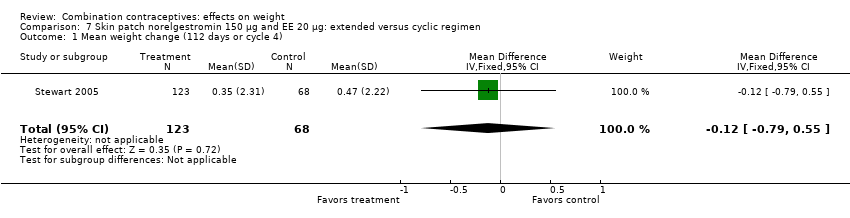
Comparison 7 Skin patch norelgestromin 150 µg and EE 20 µg: extended versus cyclic regimen, Outcome 1 Mean weight change (112 days or cycle 4).

Comparison 8 Desogestrel 150 µg and EE 20 µg versus desogestrel 150 µg and EE 30 µg, Outcome 1 Mean body mass percentage change (cycle 6).

Comparison 9 Desogestrel 150 µg and EE 20 µg versus gestodene 75 µg and EE 20 µg, Outcome 1 Gained >2 kg (cycle 6).

Comparison 9 Desogestrel 150 µg and EE 20 µg versus gestodene 75 µg and EE 20 µg, Outcome 2 Lost >2 kg (cycle 6).

Comparison 9 Desogestrel 150 µg and EE 20 µg versus gestodene 75 µg and EE 20 µg, Outcome 3 Gained >2 kg (cycle 12).
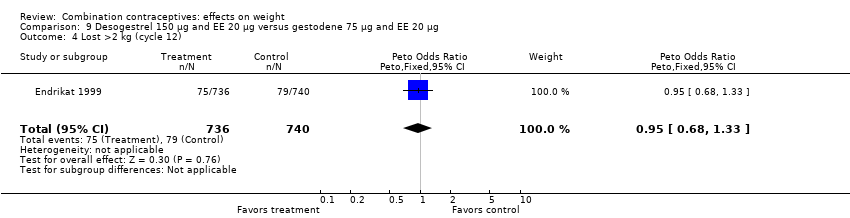
Comparison 9 Desogestrel 150 µg and EE 20 µg versus gestodene 75 µg and EE 20 µg, Outcome 4 Lost >2 kg (cycle 12).
Comparison 10 Desogestrel 150 µg and EE 20 µg versus norgestimate 250 µg and EE 35 µg, Outcome 1 Mean body mass percentage change (cycle 6).
Comparison 11 Desogestrel 150 µg and EE 30 µg versus levonorgestrel 50‐75‐125 µg and EE 30‐40‐30 µg, Outcome 1 Gained >2 kg (cycle 6).

Comparison 12 Standard desogestrel and EE regimen versus prolonged gestodene and EE regimen, Outcome 1 Gained >2 kg (cycle 7).
Comparison 13 Prolonged desogestrel and EE regimen versus standard desogestrel and EE regimen, Outcome 1 Mean weight change in kg (cycle 12).

Comparison 14 Dienogest 2 mg, EE 10 µg and estradiol valerate 2 mg versus levonorgestrel 100 µg and EE 20 µg, Outcome 1 Gained >5% baseline weight (cycle 3).
Comparison 14 Dienogest 2 mg, EE 10 µg and estradiol valerate 2 mg versus levonorgestrel 100 µg and EE 20 µg, Outcome 2 Lost >5% baseline weight (cycle 3).
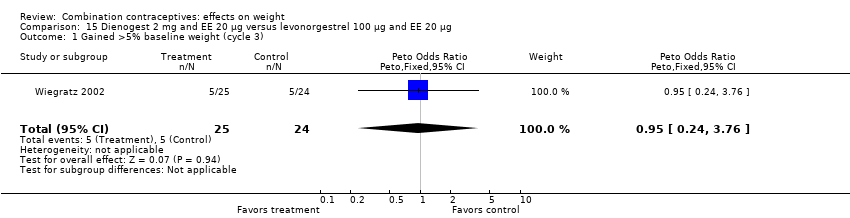
Comparison 15 Dienogest 2 mg and EE 20 µg versus levonorgestrel 100 µg and EE 20 µg, Outcome 1 Gained >5% baseline weight (cycle 3).
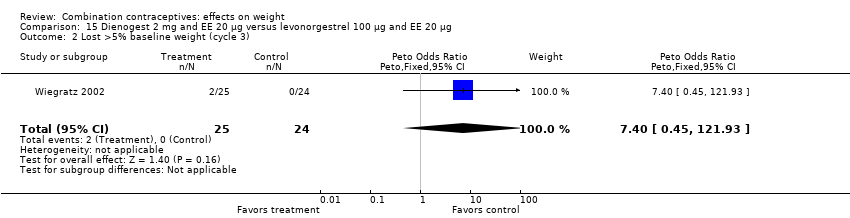
Comparison 15 Dienogest 2 mg and EE 20 µg versus levonorgestrel 100 µg and EE 20 µg, Outcome 2 Lost >5% baseline weight (cycle 3).
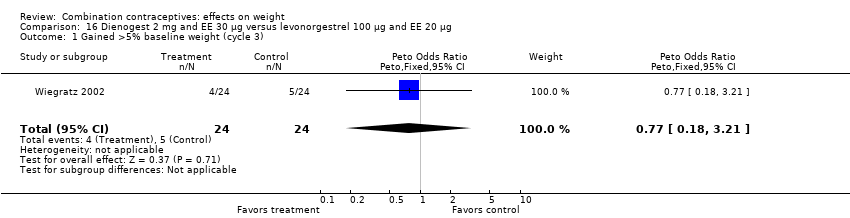
Comparison 16 Dienogest 2 mg and EE 30 µg versus levonorgestrel 100 µg and EE 20 µg, Outcome 1 Gained >5% baseline weight (cycle 3).
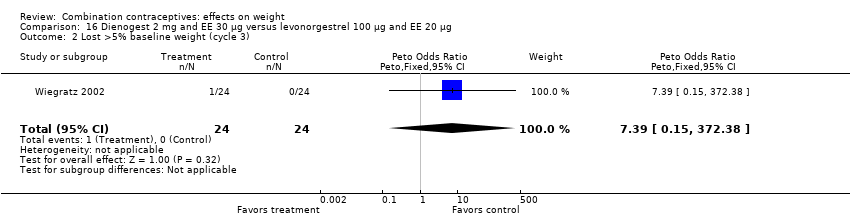
Comparison 16 Dienogest 2 mg and EE 30 µg versus levonorgestrel 100 µg and EE 20 µg, Outcome 2 Lost >5% baseline weight (cycle 3).

Comparison 17 Dl‐norgestrel 500 µg and EE 50 µg versus levonorgestrel 250 µg and EE 50 µg, Outcome 1 Mean weight change in kg (cycle 3).
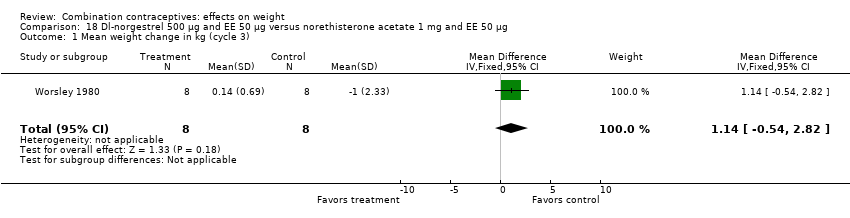
Comparison 18 Dl‐norgestrel 500 µg and EE 50 µg versus norethisterone acetate 1 mg and EE 50 µg, Outcome 1 Mean weight change in kg (cycle 3).

Comparison 19 Drospirenone 2 mg and EE 30 µg versus drospirenone 3 mg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 3).
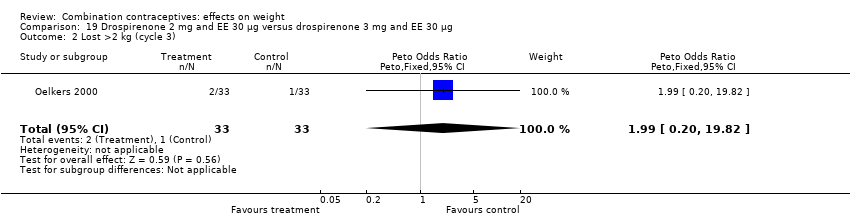
Comparison 19 Drospirenone 2 mg and EE 30 µg versus drospirenone 3 mg and EE 30 µg, Outcome 2 Lost >2 kg (cycle 3).

Comparison 20 Drospirenone 3 mg and EE 15 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 6).
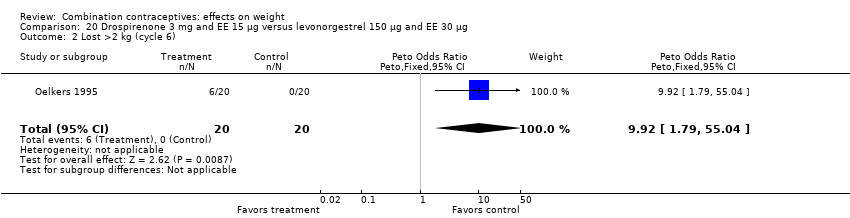
Comparison 20 Drospirenone 3 mg and EE 15 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 2 Lost >2 kg (cycle 6).
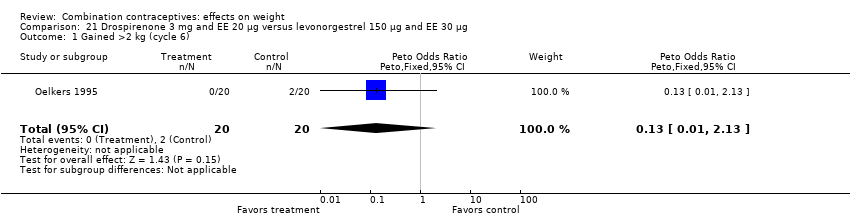
Comparison 21 Drospirenone 3 mg and EE 20 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 6).

Comparison 21 Drospirenone 3 mg and EE 20 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 2 Lost >2 kg (cycle 6).
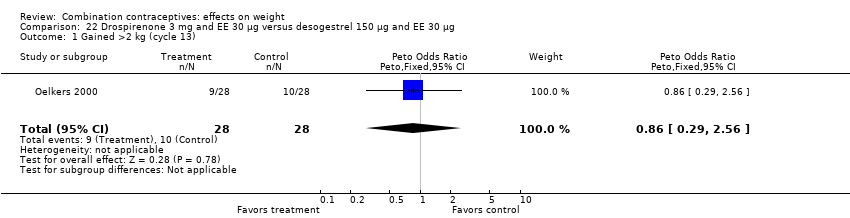
Comparison 22 Drospirenone 3 mg and EE 30 µg versus desogestrel 150 µg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 13).

Comparison 22 Drospirenone 3 mg and EE 30 µg versus desogestrel 150 µg and EE 30 µg, Outcome 2 Lost >2 kg (cycle 13).

Comparison 23 Drospirenone 3 mg and EE 20 μg versus desogestrel 150 μg and EE 20 μg, Outcome 1 Mean weight change in kg (cycle 7).
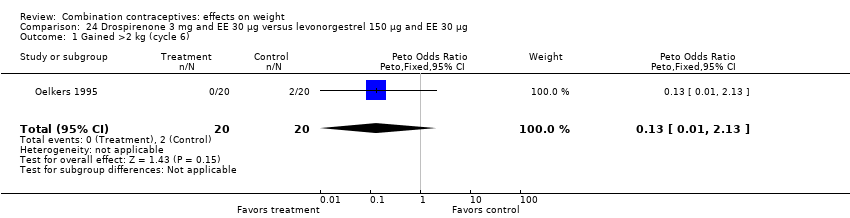
Comparison 24 Drospirenone 3 mg and EE 30 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 6).

Comparison 24 Drospirenone 3 mg and EE 30 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 2 Lost >2 kg (cycle 6).
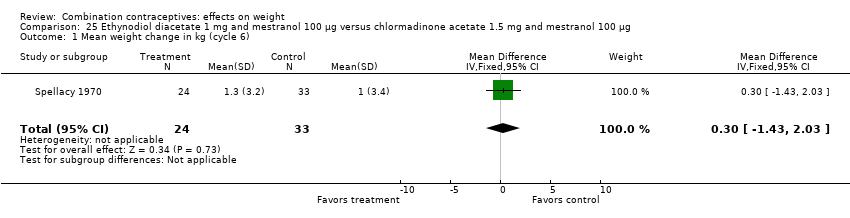
Comparison 25 Ethynodiol diacetate 1 mg and mestranol 100 µg versus chlormadinone acetate 1.5 mg and mestranol 100 µg, Outcome 1 Mean weight change in kg (cycle 6).
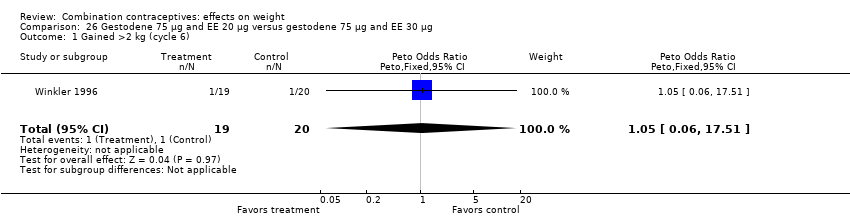
Comparison 26 Gestodene 75 µg and EE 20 µg versus gestodene 75 µg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 6).
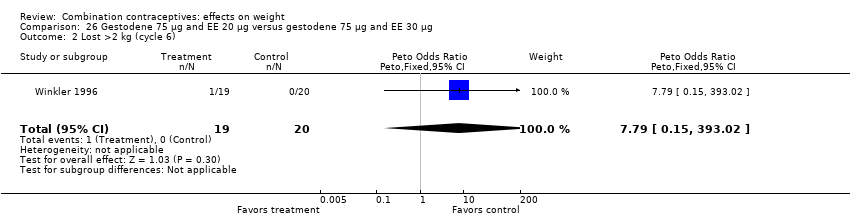
Comparison 26 Gestodene 75 µg and EE 20 µg versus gestodene 75 µg and EE 30 µg, Outcome 2 Lost >2 kg (cycle 6).
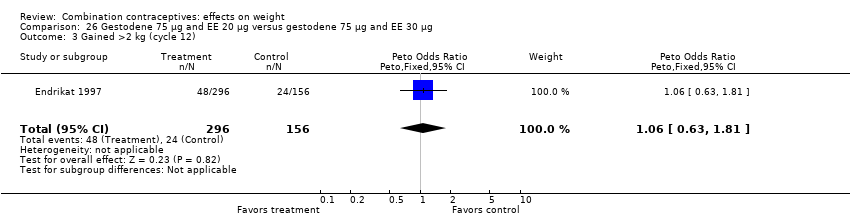
Comparison 26 Gestodene 75 µg and EE 20 µg versus gestodene 75 µg and EE 30 µg, Outcome 3 Gained >2 kg (cycle 12).
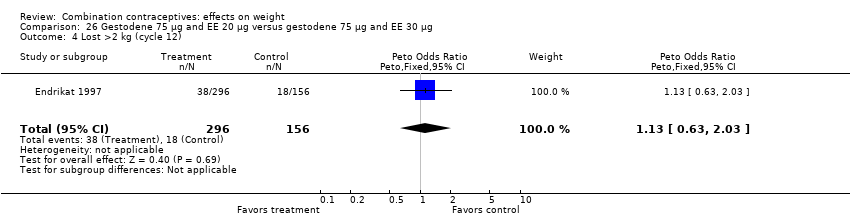
Comparison 26 Gestodene 75 µg and EE 20 µg versus gestodene 75 µg and EE 30 µg, Outcome 4 Lost >2 kg (cycle 12).

Comparison 26 Gestodene 75 µg and EE 20 µg versus gestodene 75 µg and EE 30 µg, Outcome 5 Gained >2 kg (cycle 13).
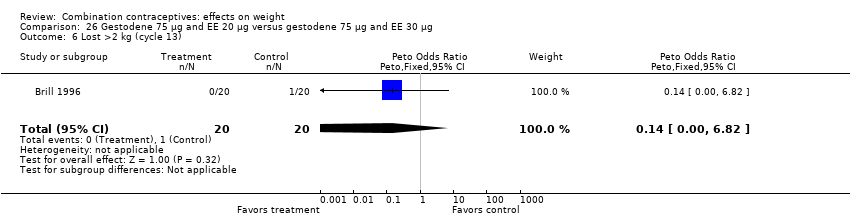
Comparison 26 Gestodene 75 µg and EE 20 µg versus gestodene 75 µg and EE 30 µg, Outcome 6 Lost >2 kg (cycle 13).
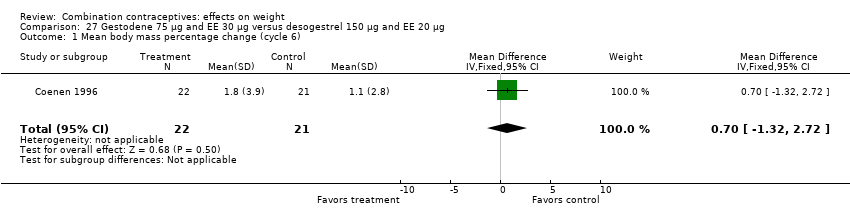
Comparison 27 Gestodene 75 µg and EE 30 µg versus desogestrel 150 µg and EE 20 µg, Outcome 1 Mean body mass percentage change (cycle 6).

Comparison 27 Gestodene 75 µg and EE 30 µg versus desogestrel 150 µg and EE 20 µg, Outcome 2 Mean weight change in kg (cycle 6).

Comparison 27 Gestodene 75 µg and EE 30 µg versus desogestrel 150 µg and EE 20 µg, Outcome 3 Mean weight change in kg (cycle 12).

Comparison 28 Gestodene 75 µg and EE 30 µg versus desogestrel 150 µg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 6).
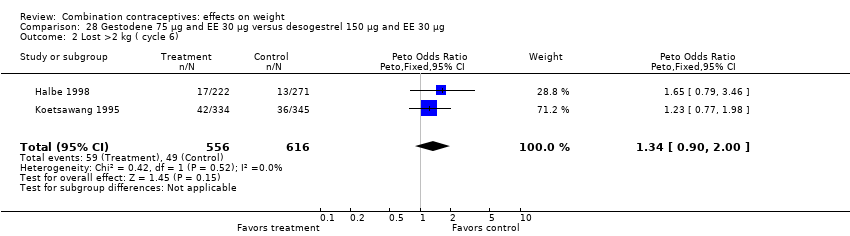
Comparison 28 Gestodene 75 µg and EE 30 µg versus desogestrel 150 µg and EE 30 µg, Outcome 2 Lost >2 kg ( cycle 6).

Comparison 28 Gestodene 75 µg and EE 30 µg versus desogestrel 150 µg and EE 30 µg, Outcome 3 Mean body mass percentage change (cycle 6).

Comparison 29 Gestodene 75 µg and EE 30 µg versus norgestimate 250 µg and EE 35 µg, Outcome 1 Gained >2 kg (cycle 6).

Comparison 29 Gestodene 75 µg and EE 30 µg versus norgestimate 250 µg and EE 35 µg, Outcome 2 Mean body mass percentage change (cycle 6).
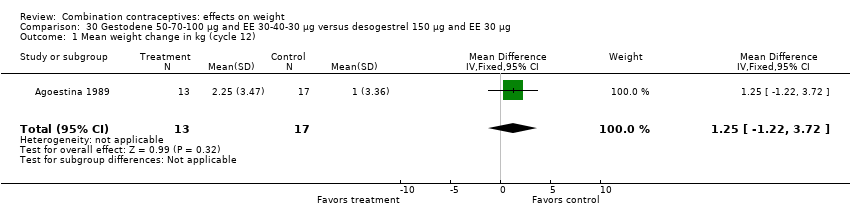
Comparison 30 Gestodene 50‐70‐100 µg and EE 30‐40‐30 µg versus desogestrel 150 µg and EE 30 µg, Outcome 1 Mean weight change in kg (cycle 12).

Comparison 31 Gestodene 50‐70‐100 µg and EE 30‐40‐30 µg versus norgestimate 250 µg and EE 35 µg, Outcome 1 Mean weight change in kg (cycle 12).
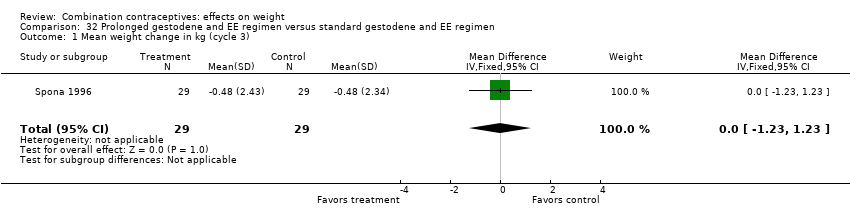
Comparison 32 Prolonged gestodene and EE regimen versus standard gestodene and EE regimen, Outcome 1 Mean weight change in kg (cycle 3).
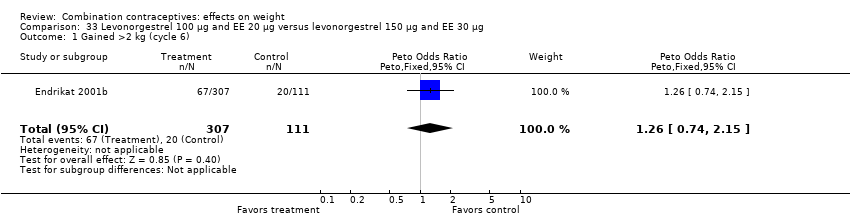
Comparison 33 Levonorgestrel 100 µg and EE 20 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 6).
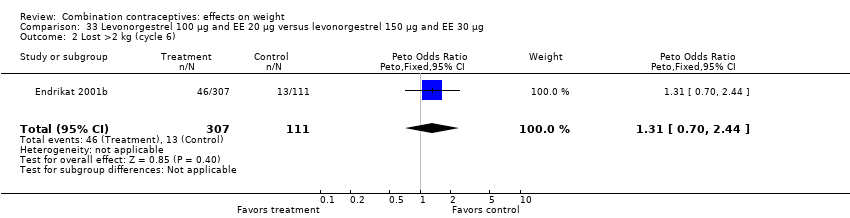
Comparison 33 Levonorgestrel 100 µg and EE 20 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 2 Lost >2 kg (cycle 6).
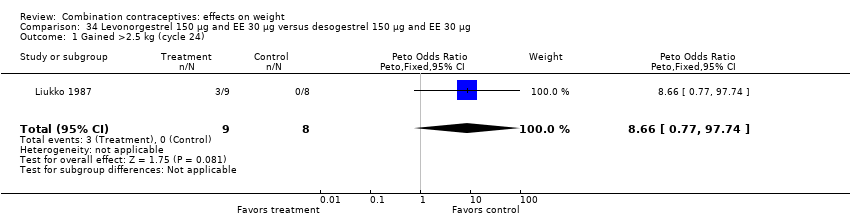
Comparison 34 Levonorgestrel 150 µg and EE 30 µg versus desogestrel 150 µg and EE 30 µg, Outcome 1 Gained >2.5 kg (cycle 24).
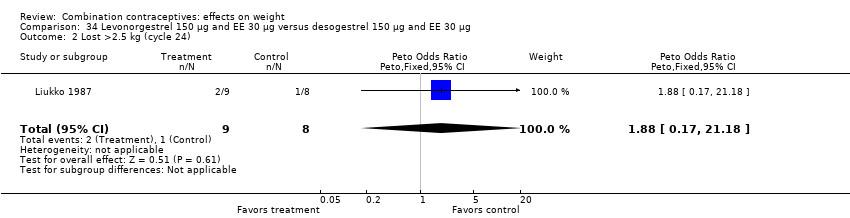
Comparison 34 Levonorgestrel 150 µg and EE 30 µg versus desogestrel 150 µg and EE 30 µg, Outcome 2 Lost >2.5 kg (cycle 24).
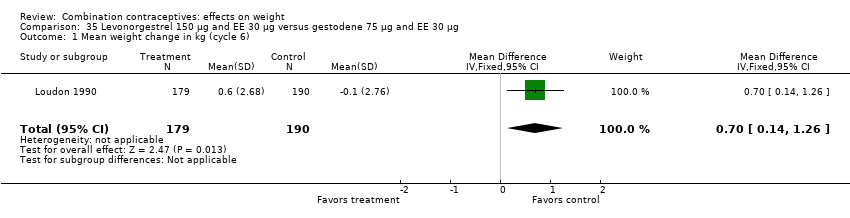
Comparison 35 Levonorgestrel 150 µg and EE 30 µg versus gestodene 75 µg and EE 30 µg, Outcome 1 Mean weight change in kg (cycle 6).

Comparison 36 Levonorgestrel 250 µg and EE 50 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 1 Gained >2.7 kg (cycle 6).
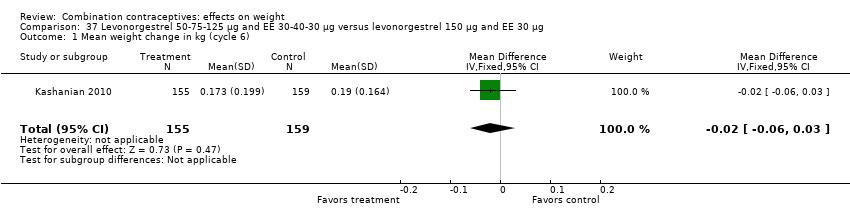
Comparison 37 Levonorgestrel 50‐75‐125 µg and EE 30‐40‐30 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 1 Mean weight change in kg (cycle 6).
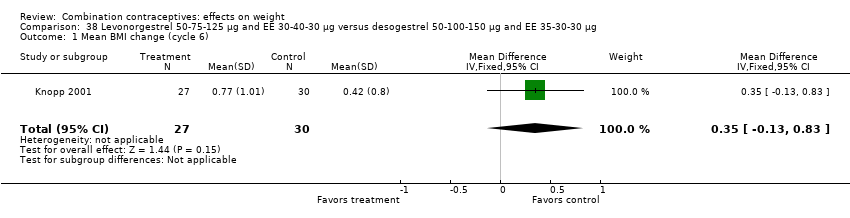
Comparison 38 Levonorgestrel 50‐75‐125 µg and EE 30‐40‐30 µg versus desogestrel 50‐100‐150 µg and EE 35‐30‐30 µg, Outcome 1 Mean BMI change (cycle 6).

Comparison 38 Levonorgestrel 50‐75‐125 µg and EE 30‐40‐30 µg versus desogestrel 50‐100‐150 µg and EE 35‐30‐30 µg, Outcome 2 Mean weight change in kg (cycle 6).
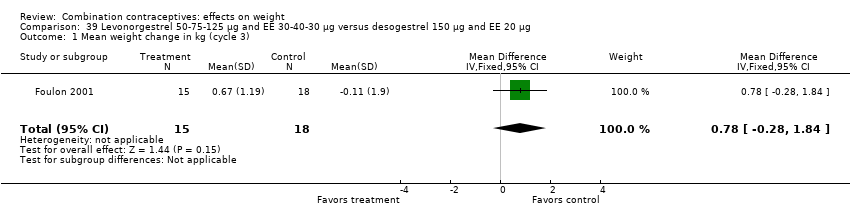
Comparison 39 Levonorgestrel 50‐75‐125 µg and EE 30‐40‐30 µg versus desogestrel 150 µg and EE 20 µg, Outcome 1 Mean weight change in kg (cycle 3).

Comparison 40 Levonorgestrel 250 µg and EE 50 µg versus norethisterone acetate 1 mg and EE 50 µg, Outcome 1 Mean weight change in kg (cycle 3).

Comparison 41 Levonorgestrel and EE 6‐6‐9 day regimen versus levonorgestrel 6‐5‐10 day regimen, Outcome 1 Mean weight change in kg (cycle 3).
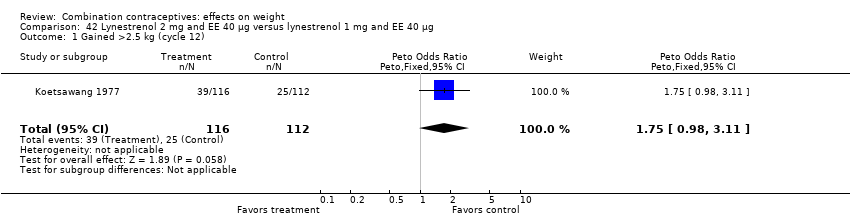
Comparison 42 Lynestrenol 2 mg and EE 40 µg versus lynestrenol 1 mg and EE 40 µg, Outcome 1 Gained >2.5 kg (cycle 12).

Comparison 42 Lynestrenol 2 mg and EE 40 µg versus lynestrenol 1 mg and EE 40 µg, Outcome 2 Lost >2.5 kg (cycle 12).
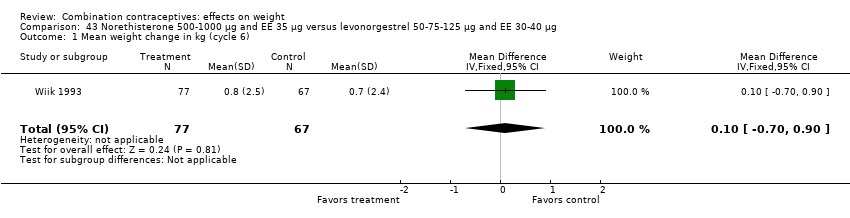
Comparison 43 Norethisterone 500‐1000 µg and EE 35 µg versus levonorgestrel 50‐75‐125 µg and EE 30‐40 µg, Outcome 1 Mean weight change in kg (cycle 6).

Comparison 44 Norgestimate 250 µg and EE 35 µg versus desogestrel 150 µg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 6).
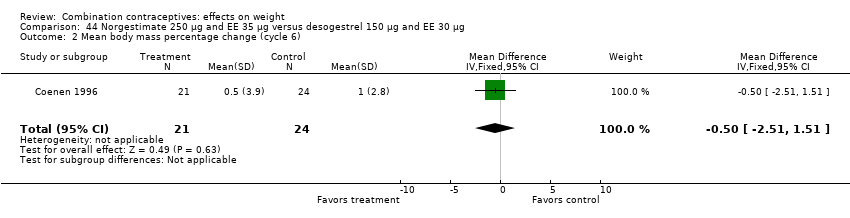
Comparison 44 Norgestimate 250 µg and EE 35 µg versus desogestrel 150 µg and EE 30 µg, Outcome 2 Mean body mass percentage change (cycle 6).

Comparison 45 Norethisterone 500 µg and EE 20 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 1 Gained >2 kg (cycle 6).
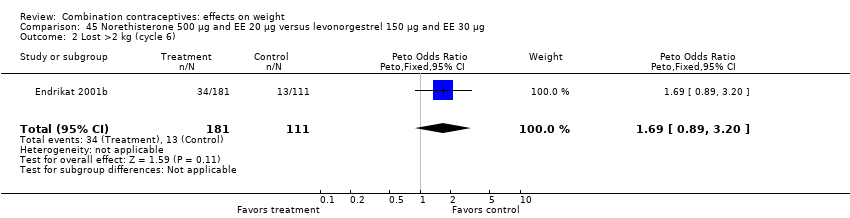
Comparison 45 Norethisterone 500 µg and EE 20 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 2 Lost >2 kg (cycle 6).
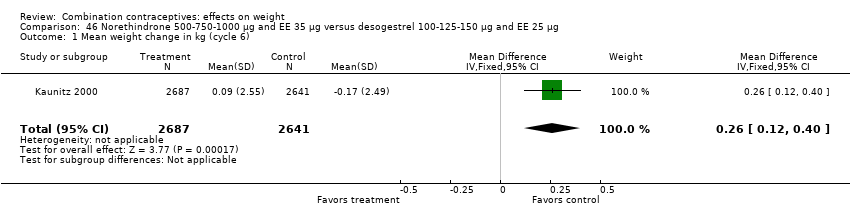
Comparison 46 Norethindrone 500‐750‐1000 µg and EE 35 µg versus desogestrel 100‐125‐150 µg and EE 25 µg, Outcome 1 Mean weight change in kg (cycle 6).
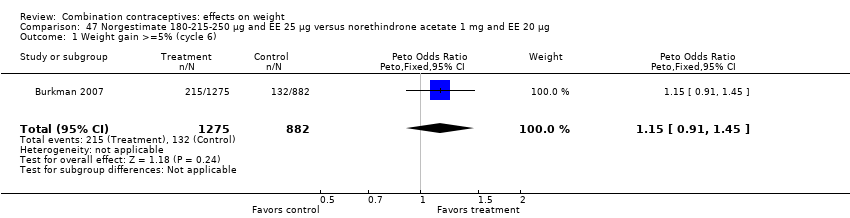
Comparison 47 Norgestimate 180‐215‐250 µg and EE 25 µg versus norethindrone acetate 1 mg and EE 20 µg, Outcome 1 Weight gain >=5% (cycle 6).

Comparison 47 Norgestimate 180‐215‐250 µg and EE 25 µg versus norethindrone acetate 1 mg and EE 20 µg, Outcome 2 Weight gain >=5% (cycle 13).

Comparison 47 Norgestimate 180‐215‐250 µg and EE 25 µg versus norethindrone acetate 1 mg and EE 20 µg, Outcome 3 Weight loss >=5% (cycle 6).

Comparison 47 Norgestimate 180‐215‐250 µg and EE 25 µg versus norethindrone acetate 1 mg and EE 20 µg, Outcome 4 Weight loss >=5% (cycle 13).

Comparison 48 Standard norgestrel and EE regimen versus prolonged norgestrel and EE regimen, Outcome 1 Mean weight change in kg (cycle 12).

Comparison 49 Injectable medroxyprogesterone acetate 25 mg and EC 5 mg versus norethisterone enanthate 50 mg and EV 5 mg, Outcome 1 Mean weight change in kg (cycle 12).
Comparison 50 Vaginal ring with norethindrone acetate 1 mg and EE 15 µg versus norethindrone acetate 1 mg and EE 20 µg, Outcome 1 Gain >2 kg (cycle 4).

Comparison 50 Vaginal ring with norethindrone acetate 1 mg and EE 15 µg versus norethindrone acetate 1 mg and EE 20 µg, Outcome 2 Lost >2 kg (cycle 4).

Comparison 51 Vaginal ring etonogestrel 120 µg and EE 15 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 1 Gain >=7% body weight (cycle 13).
Comparison 51 Vaginal ring etonogestrel 120 µg and EE 15 µg versus levonorgestrel 150 µg and EE 30 µg, Outcome 2 Lost >=7% body weight (cycle 13).

Comparison 52 Vaginal ring etonogestrel 120 µg and EE 15 µg versus drospirenone 3 mg and EE 30 µg, Outcome 1 Mean weight change in kg (cycle 13 or last assessment).
| Study ID | Intervention group | n | N (randomized women) |
| Prolonged desogestrel / ethinyl estradiol (EE) regimen | 10 | 200 | |
| Standard desogestrel / EE regimen | 1 | 100 | |
| Norgestimate 250 μg / EE 35 μg | 1 | 25 | |
| Gestodene 75 μg / EE 30 μg | 0 | 25 | |
| Desogestrel 150 μg / EE 30 μg | 0 | 25 | |
| Desogestrel 150 μg / EE 20 μg | 0 | 25 | |
| Levonorgestrel 100 μg / EE 20 μg | 2 | 359 | |
| Placebo | 0 | 362 | |
| Levonorgestrel 250 μg / EE 50 μg | 3 | 73 | |
| Levonorgestrel 150 μg / EE 30 μg | 1 | 77 | |
| Gestodene 75 μg / EE 30 μg | 4 | 279 | |
| Desogestrel 150 μg / EE 30 μg | 0 | 316 | |
| Gestodene 75 μg / EE 30 μg | 4 | 505 | |
| Desogestrel 150 μg / EE 20 μg | 2 | 501 | |
| Standard norgestrel / EE regimen | 0 | 44 | |
| Prolonged norgestrel / EE regimen | 1 | 46 | |
| Vaginal ring etonogestrel 120 µg / EE 15 µg | 2 | 512 | |
| Levonorgestrel 150 µg / EE 30 µg | 6 | 518 | |
| Injectable medroxyprogesterone acetate 25 mg / estradiol cypionatge (EC) 5 mg | 14 | 1955 | |
| Injectable norethisterone enanthate 50 mg / estradiol valerate (EV) 5 mg | 10 | 1960 | |
| Norethisterone 500‐1000 μg / EE 35 μg | 3 | 100 | |
| Levonorgestrel 50‐75‐125 μg / EE 30‐40 μg | 1 | 96 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2.3 kg (cycle 4) Show forest plot | 1 | 113 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.46, 2.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2.3 kg (cycle 4) Show forest plot | 1 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.24, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 6) Show forest plot | 1 | 473 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.23, 0.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg per year Show forest plot | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.39, 0.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2.3 kg (cycle 4) Show forest plot | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.22, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >5% baseline weight (cycle 9) Show forest plot | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.30, 2.98] |
| 2 Lost >5% baseline weight (cycle 9) Show forest plot | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.04, 1.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change (112 days or cycle 4) Show forest plot | 1 | 191 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.79, 0.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean body mass percentage change (cycle 6) Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.54, 1.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 801 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.58, 1.22] |
| 2 Lost >2 kg (cycle 6) Show forest plot | 1 | 801 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.13, 2.41] |
| 3 Gained >2 kg (cycle 12) Show forest plot | 1 | 1476 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.85, 1.49] |
| 4 Lost >2 kg (cycle 12) Show forest plot | 1 | 1476 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean body mass percentage change (cycle 6) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.45, 2.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 469 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.29 [1.84, 5.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 7) Show forest plot | 1 | 890 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.86, 1.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 12) Show forest plot | 1 | 196 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐0.42, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >5% baseline weight (cycle 3) Show forest plot | 1 | 49 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.32, 4.51] |
| 2 Lost >5% baseline weight (cycle 3) Show forest plot | 1 | 49 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.10 [0.14, 358.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >5% baseline weight (cycle 3) Show forest plot | 1 | 49 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.24, 3.76] |
| 2 Lost >5% baseline weight (cycle 3) Show forest plot | 1 | 49 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.40 [0.45, 121.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >5% baseline weight (cycle 3) Show forest plot | 1 | 48 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.18, 3.21] |
| 2 Lost >5% baseline weight (cycle 3) Show forest plot | 1 | 48 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 3) Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐1.30, 1.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 3) Show forest plot | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 1.14 [‐0.54, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 3) Show forest plot | 2 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 2 Lost >2 kg (cycle 3) Show forest plot | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.99 [0.20, 19.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.05, 5.06] |
| 2 Lost >2 kg (cycle 6) Show forest plot | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.92 [1.79, 55.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.13] |
| 2 Lost >2 kg (cycle 6) Show forest plot | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 13) Show forest plot | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.29, 2.56] |
| 2 Lost >2 kg (cycle 13) Show forest plot | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.29, 6.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 7) Show forest plot | 1 | 441 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.16, ‐0.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.13] |
| 2 Lost >2 kg (cycle 6) Show forest plot | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 6) Show forest plot | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.43, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 39 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.06, 17.51] |
| 2 Lost >2 kg (cycle 6) Show forest plot | 1 | 39 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.79 [0.15, 393.02] |
| 3 Gained >2 kg (cycle 12) Show forest plot | 1 | 452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.63, 1.81] |
| 4 Lost >2 kg (cycle 12) Show forest plot | 1 | 452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.63, 2.03] |
| 5 Gained >2 kg (cycle 13) Show forest plot | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.14, 3.57] |
| 6 Lost >2 kg (cycle 13) Show forest plot | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean body mass percentage change (cycle 6) Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.7 [‐1.32, 2.72] |
| 2 Mean weight change in kg (cycle 6) Show forest plot | 1 | 805 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.00, 0.40] |
| 3 Mean weight change in kg (cycle 12) Show forest plot | 2 | 462 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.50, 0.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 3 | 1524 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.87, 1.60] |
| 2 Lost >2 kg ( cycle 6) Show forest plot | 2 | 1172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.90, 2.00] |
| 3 Mean body mass percentage change (cycle 6) Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐1.18, 2.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 357 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.92, 2.60] |
| 2 Mean body mass percentage change (cycle 6) Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 1.3 [‐1.03, 3.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 12) Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐1.22, 3.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 12) Show forest plot | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.09, 0.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 3) Show forest plot | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.23, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 418 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.74, 2.15] |
| 2 Lost >2 kg (cycle 6) Show forest plot | 1 | 418 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.70, 2.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2.5 kg (cycle 24) Show forest plot | 1 | 17 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.66 [0.77, 97.74] |
| 2 Lost >2.5 kg (cycle 24) Show forest plot | 1 | 17 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.88 [0.17, 21.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 6) Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.7 [0.14, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2.7 kg (cycle 6) Show forest plot | 1 | 109 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [0.74, 4.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 6) Show forest plot | 1 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BMI change (cycle 6) Show forest plot | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.13, 0.83] |
| 2 Mean weight change in kg (cycle 6) Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.32, 2.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 3) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.78 [‐0.28, 1.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 3) Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 0.92 [‐1.25, 3.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 3) Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐1.15, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2.5 kg (cycle 12) Show forest plot | 1 | 228 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.75 [0.98, 3.11] |
| 2 Lost >2.5 kg (cycle 12) Show forest plot | 1 | 228 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.25, 1.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 6) Show forest plot | 1 | 144 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.70, 0.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.65, 2.06] |
| 2 Mean body mass percentage change (cycle 6) Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.51, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gained >2 kg (cycle 6) Show forest plot | 1 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.60, 1.99] |
| 2 Lost >2 kg (cycle 6) Show forest plot | 1 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [0.89, 3.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 6) Show forest plot | 1 | 5328 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.12, 0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight gain >=5% (cycle 6) Show forest plot | 1 | 2157 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.91, 1.45] |
| 2 Weight gain >=5% (cycle 13) Show forest plot | 1 | 453 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.69, 1.74] |
| 3 Weight loss >=5% (cycle 6) Show forest plot | 1 | 2157 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.72, 1.37] |
| 4 Weight loss >=5% (cycle 13) Show forest plot | 1 | 453 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.74, 2.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 12) Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 1.8 [‐0.73, 4.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 12) Show forest plot | 1 | 3029 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.04, 0.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gain >2 kg (cycle 4) Show forest plot | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.13, 3.58] |
| 2 Lost >2 kg (cycle 4) Show forest plot | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [0.35, 8.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gain >=7% body weight (cycle 13) Show forest plot | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.55, 1.28] |
| 2 Lost >=7% body weight (cycle 13) Show forest plot | 1 | 1030 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.83, 2.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean weight change in kg (cycle 13 or last assessment) Show forest plot | 1 | 937 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [0.03, 0.77] |

