Eradication therapy for peptic ulcer disease in Helicobacter pylori‐positive people
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multi‐centre RCT | |
| Participants | Finland | |
| Interventions | Bi quadruple therapy (2 weeks colloidal bismuth subcitrate 120 mg qds, lansoprazole 30 mg bd, tetracycline 500 mg qds, and metronidazole 400 mg qds) PPI triple therapy (2 weeks lansoprazole 30 mg bd, amoxicillin 500 mg qds, and clarithromycin 500 mg tds) PPI dual therapy (lansoprazole 30 mg bd and amoxicillin 500 mg qds) versus PPI (lansoprazole 30 mg bd for 2 weeks, then 30 mg od for 2 weeks) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment groups were determined by a list of random numbers generated by computer |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | Endoscopists were blinded for the treatment |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Collected serology for H. pylori status but did not record these data |
| Other bias | High risk | Participants in placebo arm had H. pylori eradication at 8 weeks so 12 month follow‐up not randomised |
| Methods | Multi‐centre RCT | |
| Participants | Japan | |
| Interventions | PPI triple therapy (5 weeks (DU)/7 weeks (GU) lansoprazole 30 mg bd, 1 week amoxicillin 750 mg bd and clarithromycin 200 mg/400 mg bd) versus PPI (5 weeks (DU)/7 weeks (GU) lansoprazole 30 mg bd) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | Double blind |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Turkey | |
| Interventions | Bi triple therapy (4 weeks colloidal bismuth subcitrate 120 mg qds, 2 weeks tetracycline 250 mg qds and metronidazole 250 mg tds) versus PPI (8 weeks omeprazole 40 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "Endoscopies were performed by one of the authors, who was blinded to the clinical data, bacteriological findings and treatment regimen" |
| Incomplete outcome data (attrition bias) | Low risk | 20/45 (44%) lost to follow up |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported in sufficient detail |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | UK and Republic of Ireland | |
| Interventions | PPI dual therapy (8 weeks omeprazole 40mg od and 2 weeks amoxicillin 750 mg bd) versus PPI (8 weeks omeprazole 40 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation... From a computer generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind, double dummy design" |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Multi‐national | |
| Interventions | RBC dual therapy (2 weeks RBC 400 mg/800 mg bd and clarithromycin 250 mg qds, then 2 weeks RBC 400 mg bd) versus RBC (4 weeks RBC 400 mg bd) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind,double dummy" |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Germany | |
| Interventions | PPI dual therapy (10 days omeprazole 40 mg bd and amoxicillin 1 g bd, then 4 1/2 weeks omeprazole 20 mg od) versus PPI (10 days omeprazole 40 mg bd then 4 1/2 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "investigator blinded clinical trial" |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Endoscopy performed if symptoms recurred but this data not given |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Germany | |
| Interventions | PPI dual therapy (2 weeks omeprazole 40 mg tds and amoxicillin 750 mg tds, then 4 weeks omeprazole 20 mg od) versus PPI (2 weeks omeprazole 40 mg tds then 4 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind" "placebo treatment" |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Highlighted differences in pretreatment with omeprazole but it is hard to believe that this was the only subgroup analysed |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Germany | |
| Interventions | Bi triple therapy (8 weeks bismuth subsalicylate 600 mg tds, 10 days amoxicillin 500 mg bd and tinidazole 1 g bd) versus PPI (8 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Low risk | "Randomisation was carried out by a central study secretariat" |
| Blinding (performance bias and detection bias) | Low risk | "investigator blinded" |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Sweden | |
| Interventions | PPI triple therapy (1 week omeprazole 20 mg bd, metronidazole 400 mg bd, clarithromycin 250 mg bd) versus PPI (1 week omeprazole 20 mg bd then 3 weeks 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind? Using placebo of the same size and appearance as conventional metronidazole and clarithromycin tablets" |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported in sufficient detail |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Italy | |
| Interventions | Bi triple therapy (4 weeks colloidal bismuth subcitrate 120 mg qds, 1 week amoxicillin 1 g tds and tinidazole 500 mg bd) versus sucralfate (4 weeks 1 g qds) | |
| Outcomes | Ulcer healing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "allocated, according to a randomised list" |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Unclear risk | This information was not available |
| Incomplete outcome data (attrition bias) | Low risk | Reported according to H. pylori status for 12 month data rather than randomised groups |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | High risk | Reported data according to H. pylori status rather than ITT |
| Methods | Single‐centre RCT | |
| Participants | Italy | |
| Interventions | PPI triple therapy (4 weeks omeprazole 20 mg od, 2 weeks metronidazole 250 mg qds and amoxicillin 1 g tds) versus PPI (4 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing Ulcer recurrence at one year | |
| Notes | If the ulcer did not heal, participants crossed over to other therapy, therefore we were unable to extract eradication rates | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Spain | |
| Interventions | Bi triple therapy (6 weeks colloidal bismuth subcitrate 120 mg qds, 12 days amoxicillin 500 mg tds and metronidazole 500 mg bd) or H2RA triple therapy (6 weeks ranitidine 300 mg qds, 12 days amoxicillin 500 mg tds and metronidazole 500 mg bd) versus H2RA (6 weeks ranitidine 300 mg qds) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "treatment assignments were determined by a list of random numbers generated by computer" |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | Data only on group A 39/44, Group B 38/40 and group C 34/38 at 12 months |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Taiwan | |
| Interventions | Bi triple therapy (1 or 2 weeks colloidal bismuth subcitrate 120 mg qds, amoxicillin 500 mg tds and metronidazole 500 mg tds) versus no treatment | |
| Outcomes | Ulcer recurrence at 1 year | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Dyspepsia symptoms obtained but not reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | China | |
| Interventions | PPI triple therapy (10 days lansoprazole 30 mg qds, clarithromycin 250 mg bd, amoxicillin 500 mg bd) versus 'killing' quadruple therapy (10 days lansoprazole 30 mg qds, clarithromycin 250 mg bd, amoxicillin 500 mg bd and 4 weeks H. pylori 'killing' capsule 6 bd) versus placebo | |
| Outcomes | Ulcer healing at 4 weeks | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind", The medicine, starch or placebo (gastropine) was packed in gelatin capsules of similar appearance. The investigators did not know what medicines were given to patients, and the patients did not know what medicines they had taken" |
| Incomplete outcome data (attrition bias) | High risk | Data from 5 participants not reported on at one year |
| Selective reporting (reporting bias) | High risk | Dyspepsia symptoms data obtained but not reported (only that upper abdominal pain was significantly less (P < 0.05) in group B) |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Chile | |
| Interventions | Bi quadruple therapy (4 weeks omeprazole 20 mg qds, bismuth subsalicylate 524 mg qds, amoxicillin 500 mg tds and metronidazole 250 mg tds) versus PPI (4 weeks omeprazole 20nmg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "single blind" |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Japan | |
| Interventions | PPI dual therapy (8 weeks lansoprazole 30 mg od and 2 weeks clarithromycin 200 mg tds) versus PPI (8 weeks omeprazole 20 mg od or lansoprazole 30 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | Unclear risk | This information was not available |
| Selective reporting (reporting bias) | Unclear risk | This information was not available |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Japan | |
| Interventions | PPI dual therapy (8 weeks lansoprazole 30 mg qds and 2 weeks clarithromycin 200 mg tds/amoxicillin 500 mg tds) versus PPI (8 weeks omeprazole 20 mg qds or lansoprazole 30 mg qds) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "endoscopy was performed at 3 month intervals by a gastroenterologist who was kept uninformed of the details of the patients' past medical histories" |
| Incomplete outcome data (attrition bias) | High risk | Data from 2 participants missing |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Japan | |
| Interventions | PPI dual therapy (6 weeks lansoprazole 30 mg qds and 2 weeks amoxicillin 1‐2 g qds) versus PPI (6 weeks lansoprazole 30 mg qds) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers not given at end of follow‐up just percentages |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | USA | |
| Interventions | Bi triple therapy (2 weeks bismuth subsalicylate 300 mg qds/150 mg tds + 300 mg nocte, tetracycline 500 mg qds and metronidazole 250 mg tds) versus H2RA (16 weeks ranitidine 300 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "results were not shared with the endoscopist" |
| Incomplete outcome data (attrition bias) | Unclear risk | Percentages rather than numbers given for follow‐up data (6 lost to follow up in the eradication group) |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | USA | |
| Interventions | Bi triple therapy | |
| Outcomes | Ulcer recurrence at 1 year | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "the endoscopist was blinded to the treatment status of the patient" |
| Incomplete outcome data (attrition bias) | Low risk | 83/112 (74%) with ulcer healing agreed to enter follow‐up part of study |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | USA and Puerto Rico | |
| Interventions | RBC dual therapy (4 weeks RBC 400 mg bd, 2 weeks amoxicillin 500 mg qds) versus Bi (4 weeks RBC 400 mg bd) and placebo | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind" placebo |
| Incomplete outcome data (attrition bias) | Unclear risk | 24‐week data reported as percentage not absolute numbers |
| Selective reporting (reporting bias) | High risk | 24‐week data according to H. pylori status not randomised groups |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | USA | |
| Interventions | PPI dual therapy (2 weeks lansoprazole 30 mg bd/tds and amoxicillin 1 g tds) versus PPI (2 weeks lansoprazole 30 mg tds) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "matching placebos were supplied to maintain the double‐blind nature of the study" |
| Incomplete outcome data (attrition bias) | Low risk | Ulcer data missing on 50/262 (19%) subjects |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Two centre RCT | |
| Participants | Austria | |
| Interventions | H2RA triple therapy (6 weeks ranitidine 300 mg od, 12 days amoxicillin 750 mg tds and metronidazole 500 mg tds) versus H2RA (6 weeks ranitidine 300 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "identical appearing placebos" |
| Incomplete outcome data (attrition bias) | High risk | 1/104 dropped out (from eradication group) |
| Selective reporting (reporting bias) | High risk | Histology taken but not reported |
| Other bias | Low risk | There was no other bias |
| Methods | Two‐centre RCT | |
| Participants | Japan | |
| Interventions | PPI triple therapy (1 week lansoprazole 30 mg od or rabeprazole 20 mg od plus amoxicillin 1.5 g od and clarithromycin 800 mg od) versus PPI (lansoprazole 30 mg od or rabeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a table of random numbers was used to generate the randomisation sequence" |
| Allocation concealment (selection bias) | Low risk | "using sealed opaque envelopes numbered sequentially and containing the assignment" |
| Blinding (performance bias and detection bias) | Low risk | "Patients and their physicians were aware of the treatment assignment, but endoscopists and pathologists were not" |
| Incomplete outcome data (attrition bias) | High risk | 109/120 (91%) completed trial |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Hong Kong | |
| Interventions | Bi quadruple therapy (4 weeks omeprazole 40 mg qds, 1 week colloidal bismuth subcitrate 120 mg qds, tetracycline 500 mg qds and metronidazole 400 mg qds) versus PPI (4 weeks omeprazole 40 mg qds) | |
| Outcomes | Ulcer healing Ulcer recurrence at 1 year | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised by instructions" |
| Allocation concealment (selection bias) | Low risk | "randomised in consecutively numbered sealed opaque envelopes" |
| Blinding (performance bias and detection bias) | Low risk | "staff performing the endoscopic and bacteriologic assessments were unaware of the drugs the patient had been taking" |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Japan | |
| Interventions | PPI dual therapy (6 weeks (DU)/8 weeks (GU) lansoprazole 30 mg od and 2 weeks amoxicillin 500 mg qds) versus PPI (6 weeks (DU)/8 weeks (GU) lansoprazole 30 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | Low risk | 99/119 (83%) completed 1 year follow up |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Japan | |
| Interventions | PPI dual therapy (6 weeks (DU)/8 weeks (GU) lansoprazole 30 mg od and 2 weeks amoxicillin 500 mg qds) versus PPI (6 weeks (DU)/8 weeks (GU) lansoprazole 30mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | Low risk | There were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Turkey | |
| Interventions | PPI triple therapy (1 week omeprazole 20 mg bd, amoxicillin 1 g bd and metronidazole 500 mg tds, then 3 weeks omeprazole 20 mg od) versus PPI (1 week omeprazole 20 mg bd then 3 weeks 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | South Korea | |
| Interventions | PPI triple therapy (1 week omeprazole 20 mg bd, amoxicillin 1 g bd and clarithromycin 500 mg bd) versus no treatment | |
| Outcomes | Ulcer recurrence at 30 months | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised?.using a computer generated list" |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "two experienced endoscopists, who were blind to the clinical data" |
| Incomplete outcome data (attrition bias) | High risk | "no patient was lost to follow up" |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Hong Kong | |
| Interventions | Clarithromycin monotherapy (2 weeks clarithromycin 250 mg qds) versus placebo | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "placebo capsules were identical in appearance and taste" |
| Incomplete outcome data (attrition bias) | Low risk | 81/97 (83%) completed the trial |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Italy | |
| Interventions | PPI dual therapy (4 weeks omeprazole 20 mg bd and 2 weeks amoxicillin 1 g tds) versus PPI (4 weeks omeprazole 20 mg bd) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "allocated according to a computer generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind" placebo |
| Incomplete outcome data (attrition bias) | Low risk | 15/59 (25%) lost to follow up |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported in sufficient detail |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Taiwan | |
| Interventions | Bi triple therapy (4 weeks colloidal bismuth subcitrate 120 mg qds, 1 week metronidazole 250 mg qds and amoxicillin 500 mg qds) versus H2RA (4 weeks famotidine 20 mg bd) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | 3/42 (7%) lost to follow up at 12 months |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | UK | |
| Interventions | PPI dual therapy (4 weeks omeprazole 40 mg od and 2 weeks clarithromycin 500 mg tds) versus PPI (4 weeks omeprazole 40 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "identically appearing placebo" |
| Incomplete outcome data (attrition bias) | Low risk | "17 clarithromycin treated patients lost to follow up" |
| Selective reporting (reporting bias) | High risk | Symptom data not reported at one year |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Germany, Hungary and Poland | |
| Interventions | PPI triple therapy (1 week omeprazole 20 mg bd, amoxicillin 1 g bd and clarithromycin 500 mg bd or 1 week omeprazole 20 mg bd, metronidazole 400 mg bd and clarithromycin 250 mg bd) versus PPI (1 week omeprazole 20 mg bd) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "identical tablets/capsules containing active drug or placebo" |
| Incomplete outcome data (attrition bias) | Unclear risk | Number lost to follow up at 6 months not stated |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Greece | |
| Interventions | Bi triple therapy (8 weeks colloidal bismuth subcitrate 120 mg qds, 2 weeks tetracycline 500 mg qds and metronidazole 500 mg tds) versus Bi (8 weeks colloidal bismuth subcitrate 120 mg qds) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "Endoscopies were all performed by the same physician who was unaware of the patient's treatment category" |
| Incomplete outcome data (attrition bias) | Low risk | 3/12 in H. pylori eradication arm withdrew because of side effects |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported in sufficient detail |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Germany | |
| Interventions | PPI dual therapy (2 weeks omeprazole 40 mg bd and amoxicillin 750 mg tds then 2 weeks omeprazole 20 mg od) versus PPI (2 weeks omeprazole 40 mg bd then 2 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each centre had its own randomisation list" |
| Allocation concealment (selection bias) | Low risk | "Randomisation was carried out by a central study secretariat" |
| Blinding (performance bias and detection bias) | Low risk | "amoxicillin‐placebo" "double‐blind trial" |
| Incomplete outcome data (attrition bias) | Low risk | 23/185 (12%) missed follow up |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Spain | |
| Interventions | PPI triple therapy (1 week omeprazole 20 mg bd, amoxicillin 1 g bd and clarithromycin 500 mg bd then 3 weeks omeprazole 20 mg od) versus PPI (1 week omeprazole 20 mg bd then 3 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a computerized randomisation program" |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind" "antibiotic matching placebo" |
| Incomplete outcome data (attrition bias) | Unclear risk | This information was not available |
| Selective reporting (reporting bias) | High risk | Dyspepsia symptoms obtained but not reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Republic of Ireland, Germany and New Zealand | |
| Interventions | PPI dual therapy (2 weeks omeprazole 40 mg od and clarithromycin 500 mg tds, then 2 weeks omeprazole 20 mg od) versus PPI (2 weeks omeprazole 40 mg od then 2 weeks 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double‐blind" "identically appearing placebo" |
| Incomplete outcome data (attrition bias) | Low risk | 33/208 (16%) did not have follow up endoscopy |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Italy | |
| Interventions | PPI dual therapy (4 weeks lansoprazole 30 mg bd and 2 weeks amoxicillin 1 g tds) and Bi quadruple therapy (4 weeks lansoprazole 30 mg od, 2 weeks bismuth 240 mg bd, amoxicillin 1 g tds and tinidazole 500 mg bd) versus PPI (4 weeks lansoprazole 30 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Venezuela | |
| Interventions | Bi triple therapy (2 weeks colloidal bismuth subcitrate 120 mg qds, amoxicillin 500 mg tds and metronidazole 500 mg tds) versus PPI (4 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | This information was not available |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Multi‐national | |
| Interventions | RBC dual therapy (2 weeks RBC 400 mg/800 mg bd and clarithromycin 250 mg qds, then 2 weeks RBC 400 mg bd) versus RBC (4 weeks 400 mg bd) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "blinded study" "placebo capsules" |
| Incomplete outcome data (attrition bias) | High risk | 10/95 (11%) lost to follow up |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Netherlands | |
| Interventions | Bi triple therapy (4 weeks colloidal bismuth subcitrate 120 mg qds and amoxicillin 375 mg tds, 10 days metronidazole 500 mg tds) versus Bi (4 weeks colloidal bismuth subcitrate 120 mg qds) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | "open study" |
| Incomplete outcome data (attrition bias) | High risk | 5/24 on triple therapy withdrew due to side effects, 11 others lost to follow up |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | USA | |
| Interventions | PPI dual therapy (2 weeks lansoprazole 30 mg bd and clarithromycin 500 mg bd/tds or 2 weeks lansoprazole 30 mg bd/tds and amoxicillin 1 g tds) and triple therapy (2 weeks lansoprazole 30 mg bd, amoxicillin 1 g bd and clarithromycin 500 mg bd) versus PPI (2 weeks lansoprazole 30 mg tds) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "all study medication was matched with placebo to maintain the double‐blind nature of the study" |
| Incomplete outcome data (attrition bias) | Unclear risk | This information was not available |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Japan | |
| Interventions | H2RA triple therapy (6 weeks cimetidine 400 mg bd, 2 weeks amoxicillin 300 mg tds and metronidazole 250 mg tds) versus H2RA (6 weeks cimetidine 400 mg bd) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "endoscopic examinations were ultimately judged by an experienced endoscopist who was also not informed of the treatment" |
| Incomplete outcome data (attrition bias) | Low risk | 8/50 (16%) were lost to follow up |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | France | |
| Interventions | H2RA triple therapy (6 weeks famotidine 40 mg od, 1 week amoxicillin 500 mg qds and tinidazole 500 mg tds) versus H2RA (6 weeks famotidine 40 mg od then 20 weeks 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind, double dummy" |
| Incomplete outcome data (attrition bias) | High risk | 9/97 (9%) of healed ulcer participants were lost to follow up over 6 months |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Italy | |
| Interventions | PPI dual therapy (4 weeks omeprazole 20 mg od, 2 weeks amoxicillin 1 g bd) versus PPI (4 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | 3/53 (6%) dropped out |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Cuba | |
| Interventions | Bi triple therapy (6 weeks colloidal bismuth subcitrate 240 mg bd, 10 days metronidazole 500 mg tds and tetracycline 500 mg tds/amoxicillin 750 mg bd) versus Bi (6 weeks colloidal bismuth subcitrate 240 mg bd) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | Low risk | 7/60 (12%) drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Hong Kong | |
| Interventions | Bi triple therapy (1 week colloidal bismuth subcitrate 120 mg qds, tetracycline 500 mg qds and metronidazole 400 mg qds) versus PPI (4 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | treatment assignments were determined with a list of random numbers generated by computer |
| Allocation concealment (selection bias) | Low risk | "randomly assigned to one of two treatment groups with the use of sealed envelopes" |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | 85/100 (85%) completed the trial |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported in sufficient detail |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Japan | |
| Interventions | PPI triple therapy (6 weeks (DU) / 8 weeks (GU) lansoprazole 30 mg od or omeprazole 20 mg od, 2 weeks amoxicillin 1.5 g od and clarithromycin 400 mg od) versus PPI (6 weeks (DU) / 8 weeks (GU) lansoprazole 30 mg od or omeprazole 20 mg od) or H2RA (6 weeks (DU) / 8 weeks (GU) famotidine 40 mg od or cimetidine 800 mg od) | |
| Outcomes | Ulcer recurrence at 5 years | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | 32/445 (7%) loss to follow up |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT Double‐blinded | |
| Participants | Bulgaria, Czech Republic, Germany, Hong Kong, Hungary, Philippines, Poland, Romania, and Slovakia 402 people with gastric ulcer | |
| Interventions | PPI triple therapy (1 week esomeprazole 20 mg bd, amoxicillin 1 g bd, clarithromycin 500 mg bd followed by either 3 weeks of esomeprazole 20 mg od or placebo) versus PPI (1 week of esomeprazole 20 mg bd followed by 3 weeks of esomeprazole 20 mg od) | |
| Outcomes | Ulcer healing Ulcer recurrence at 12 months H. pylori eradication rates | |
| Notes | Eradication rates: PPI triple therapy 79.2% PPI 9.5% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised according to a computer‐generated list" |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "to maintain blinding, the active and placebo tablets were identical in terms of appearance, taste and smell, as well as packaging and labelling" |
| Incomplete outcome data (attrition bias) | High risk | 14/480 (3%) no primary end point data |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Sweden | |
| Interventions | PPI dual therapy (4 weeks omeprazole 40 mg od and 2 weeks amoxicillin 750 mg bd) versus PPI (4 weeks omeprazole 40 mg od) | |
| Outcomes | Ulcer recurrence at 6 months | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "double blind and used a single placebo technique" |
| Incomplete outcome data (attrition bias) | Unclear risk | This information was not available |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Multi‐centre RCT | |
| Participants | Canada | |
| Interventions | PPI triple therapy (1 week omeprazole 20 mg bd, amoxicillin 1 g bd and clarithromycin 500 mg bd or 1 week omeprazole 20 mg bd, metronidazole 400 mg bd and clarithromycin 250 mg bd then 3 weeks omeprazole 20 mg od) versus PPI (4 weeks omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | Blinding was performed |
| Incomplete outcome data (attrition bias) | High risk | 17/146 (12%) loss to follow up (n = 9) or not included in ulcer relapse analysis (n = 8) |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Taiwan | |
| Interventions | Bi triple therapy (4 weeks colloidal bismuth subcitrate 120 mg qds, 2 weeks tetracycline 500 mg qds and metronidazole 250 mg qds) versus H2RA (4 weeks ranitidine 150 mg bd) and Bi (4 weeks colloidal bismuth subcitrate 120 mg qds) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | H2RA given until ulcers healed but these data were not given |
| Other bias | Low risk | There was no other bias |
| Methods | Single‐centre RCT | |
| Participants | Taiwan | |
| Interventions | Bi triple therapy (4 weeks colloidal bismuth subcitrate 300 mg qds, 1 week amoxicillin 750 mg bd and metronidazole 500 mg tds) and PPI dual therapy (4 weeks omeprazole 20 mg bd/qds and 10 days amoxicillin 750 mg bd) versus PPI (4 weeks omeprazole 20 mg qds) and H2RA (4 weeks nizatidine/ranitidine 150 mg bd) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | High risk | Blinding was not performed |
| Incomplete outcome data (attrition bias) | High risk | There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Collected data was reported |
| Other bias | High risk | Reported according to H. pylori status and not randomised groups |
| Methods | Single‐centre RCT | |
| Participants | Hong Kong | |
| Interventions | Clarithromycin monotherapy (2 weeks 250 mg qds) versus PPI (1 year omeprazole 20 mg od) | |
| Outcomes | Ulcer healing | |
| Notes | Eradication rates: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available |
| Allocation concealment (selection bias) | Unclear risk | This information was not available |
| Blinding (performance bias and detection bias) | Low risk | "the endoscopists were blinded to the treatment type and any clinical information related to the patients" |
| Incomplete outcome data (attrition bias) | Low risk | 15/114 (13%) drop‐outs |
| Selective reporting (reporting bias) | High risk | Symptom data collected but not reported |
| Other bias | Low risk | There was no other bias |
bd: twice per day
od: once per day
qds: four times per day
tds: three times per day
Bi quadruple therapy: Bismuth quadruple therapy
PPI: proton pump inhibitor
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| No ulcer healing or recurrence data | |
| Not all participants were H. pylori positive, and no way of extracting data for just the H. pylori‐positive participants | |
| Control arm of the trial were all H. pylori negative | |
| Not participants with peptic ulcer disease | |
| No ulcer healing or recurrence data | |
| No comparative intervention | |
| Not truly randomised | |
| No ulcer healing or recurrence data | |
| No ulcer healing or recurrence data | |
| No ulcer healing or recurrence data | |
| No ulcer healing or recurrence data | |
| Not truly randomised | |
| Not truly randomised | |
| Not truly randomised | |
| Not all participants were H. pylori‐positive, and no way of extracting data for just the H. pylori‐positive participants | |
| Not all participants had documented peptic ulcer disease | |
| Not a recognised eradication regimen | |
| Not truly randomised | |
| No ulcer healing or recurrence data | |
| No ulcer healing or recurrence data | |
| Not truly randomised | |
| No ulcer healing or recurrence data | |
| Not participants with peptic ulcer disease | |
| Not all participants had documented peptic ulcer disease | |
| Not truly randomised |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 34 | 3910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.58, 0.76] |
| Analysis 1.1  Comparison 1 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone: duodenal ulcer acute healing, Outcome 1 Proportion not healed. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.26, 0.53] |
| Analysis 2.1  Comparison 2 H. pylori eradication vs. no treatment/placebo: duodenal ulcer acute healing, Outcome 1 Proportion not healed. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 15 | 1974 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.90, 1.68] |
| Analysis 3.1  Comparison 3 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone: gastric ulcer acute healing, Outcome 1 Proportion not healed. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.31, 0.85] |
| Analysis 4.1  Comparison 4 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone: peptic ulcer acute healing, Outcome 1 Proportion not healed. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 H. pylori eradication vs. no treatment/placebo: peptic ulcer acute healing, Outcome 1 Proportion not healed. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion recurred Show forest plot | 4 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.42, 1.25] |
| Analysis 6.1  Comparison 6 H. pylori eradication vs. ulcer healing drug alone (after initial ulcer healing): duodenal ulcer recurrence, Outcome 1 Proportion recurred. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion recurred Show forest plot | 27 | 2509 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.15, 0.26] |
| Analysis 7.1  Comparison 7 H. pylori eradication vs. no treatment (after initial ulcer healing): duodenal ulcer recurrence, Outcome 1 Proportion recurred. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion recurred Show forest plot | 12 | 1476 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.22, 0.45] |
| Analysis 8.1  Comparison 8 H. pylori eradication vs. no treatment (after initial ulcer healing): gastric ulcer recurrence, Outcome 1 Proportion recurred. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion recurred Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 H. pylori eradication vs. no treatment (after initial ulcer healing): peptic ulcer recurrence, Outcome 1 Proportion recurred. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone Show forest plot | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.74] |
| Analysis 10.1  Comparison 10 Global symptoms persisting, Outcome 1 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone. | ||||
| 2 H. pylori eradication vs. no treatment Show forest plot | 2 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.83, 1.93] |
| Analysis 10.2  Comparison 10 Global symptoms persisting, Outcome 2 H. pylori eradication vs. no treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall, proportion occurred Show forest plot | 43 | 6093 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.77, 2.99] |
| Analysis 11.1  Comparison 11 Adverse events, Outcome 1 Overall, proportion occurred. | ||||
| 2 Diarrhoea, proportion occurred Show forest plot | 30 | 4590 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [2.11, 3.88] |
| Analysis 11.2  Comparison 11 Adverse events, Outcome 2 Diarrhoea, proportion occurred. | ||||
| 3 Nausea/vomiting, proportion occurred Show forest plot | 15 | 1533 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.76 [1.91, 7.37] |
| Analysis 11.3  Comparison 11 Adverse events, Outcome 3 Nausea/vomiting, proportion occurred. | ||||
| 4 Skin rash, proportion occurred Show forest plot | 18 | 2385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.78, 2.37] |
| Analysis 11.4  Comparison 11 Adverse events, Outcome 4 Skin rash, proportion occurred. | ||||
| 5 Headache, proportion occurred Show forest plot | 14 | 2292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.70, 1.75] |
| Analysis 11.5  Comparison 11 Adverse events, Outcome 5 Headache, proportion occurred. | ||||
| 6 Epigastric pain, proportion occurred Show forest plot | 11 | 1491 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [1.90, 8.82] |
| Analysis 11.6  Comparison 11 Adverse events, Outcome 6 Epigastric pain, proportion occurred. | ||||
| 7 Altered taste, proportion occurred Show forest plot | 13 | 2299 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.85 [4.38, 17.90] |
| Analysis 11.7  Comparison 11 Adverse events, Outcome 7 Altered taste, proportion occurred. | ||||
| 8 Stomatitis, proportion occurred versus not occurred Show forest plot | 8 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.94, 7.48] |
| Analysis 11.8  Comparison 11 Adverse events, Outcome 8 Stomatitis, proportion occurred versus not occurred. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 H. pylori eradication + ulcer‐healing drug vs. ulcer‐healing drug alone: duodenal ulcer acute healing, outcome: 1.1 Proportion not healed.
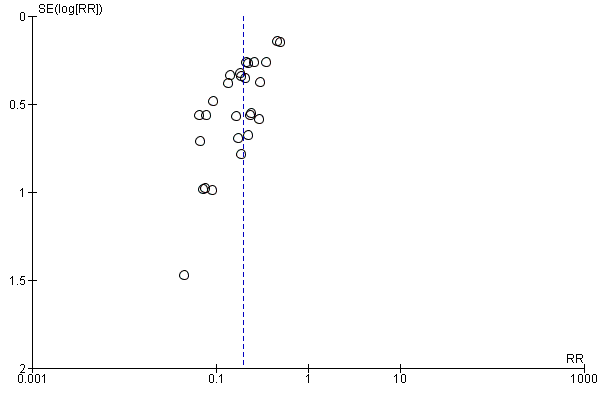
Funnel plot of comparison: 7 H. pylori eradication vs. no treatment (after initial ulcer healing): duodenal ulcer recurrence, outcome: 7.1 Proportion recurred.

Funnel plot of comparison: 8 Gastric ulcer recurrence with H. pylori eradication vs. no treatment (after initial ulcer healing), outcome: 8.1 Proportion recurred.

Comparison 1 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone: duodenal ulcer acute healing, Outcome 1 Proportion not healed.
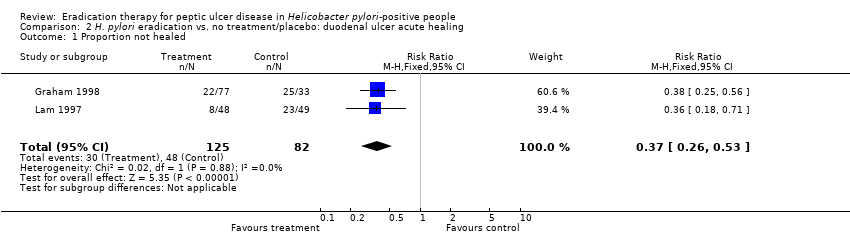
Comparison 2 H. pylori eradication vs. no treatment/placebo: duodenal ulcer acute healing, Outcome 1 Proportion not healed.

Comparison 3 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone: gastric ulcer acute healing, Outcome 1 Proportion not healed.

Comparison 4 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone: peptic ulcer acute healing, Outcome 1 Proportion not healed.

Comparison 5 H. pylori eradication vs. no treatment/placebo: peptic ulcer acute healing, Outcome 1 Proportion not healed.

Comparison 6 H. pylori eradication vs. ulcer healing drug alone (after initial ulcer healing): duodenal ulcer recurrence, Outcome 1 Proportion recurred.
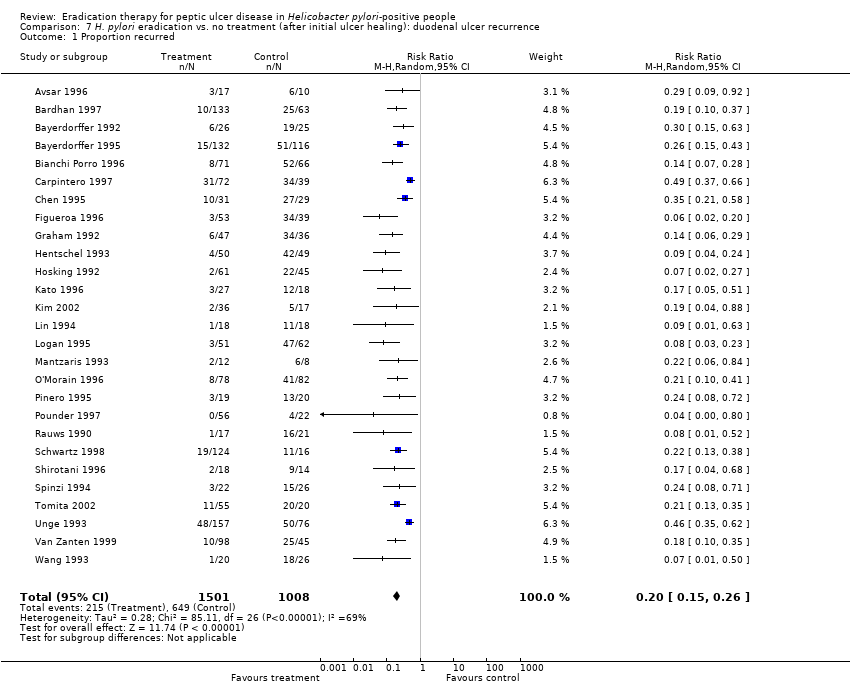
Comparison 7 H. pylori eradication vs. no treatment (after initial ulcer healing): duodenal ulcer recurrence, Outcome 1 Proportion recurred.

Comparison 8 H. pylori eradication vs. no treatment (after initial ulcer healing): gastric ulcer recurrence, Outcome 1 Proportion recurred.
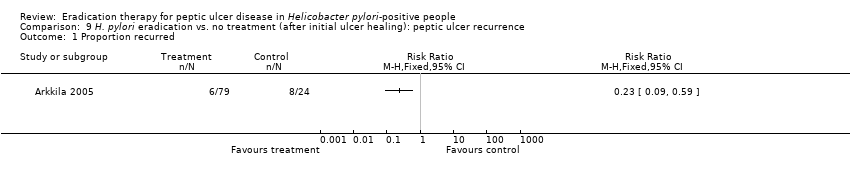
Comparison 9 H. pylori eradication vs. no treatment (after initial ulcer healing): peptic ulcer recurrence, Outcome 1 Proportion recurred.

Comparison 10 Global symptoms persisting, Outcome 1 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone.

Comparison 10 Global symptoms persisting, Outcome 2 H. pylori eradication vs. no treatment.
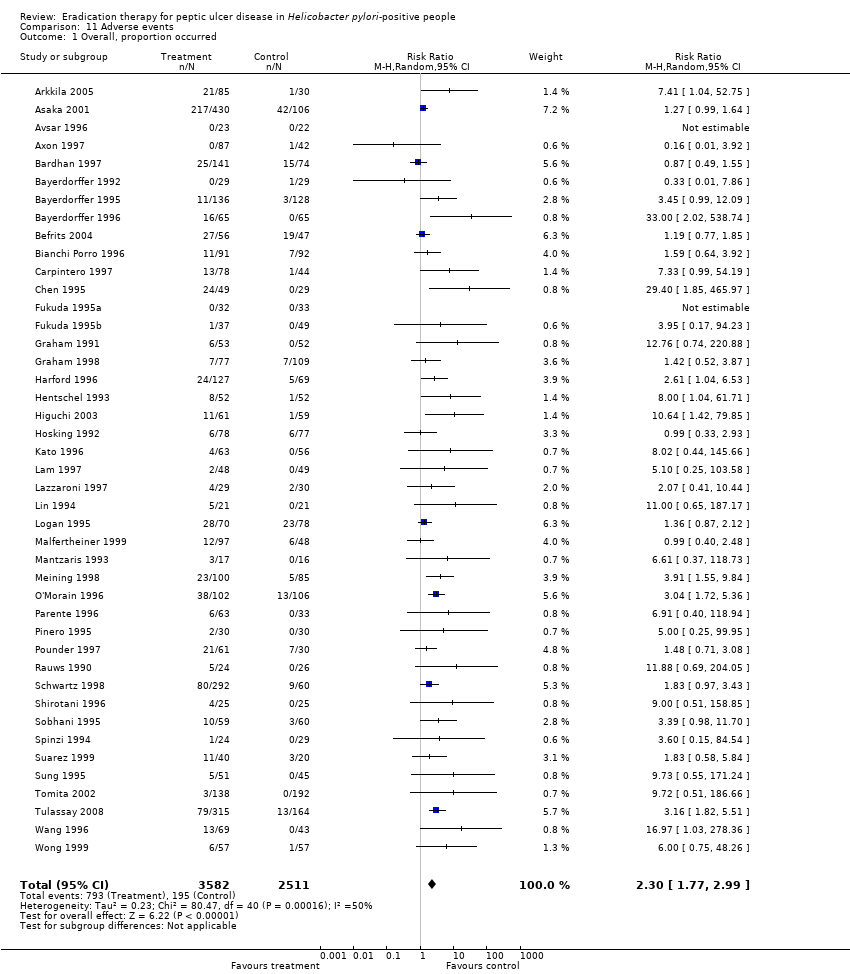
Comparison 11 Adverse events, Outcome 1 Overall, proportion occurred.
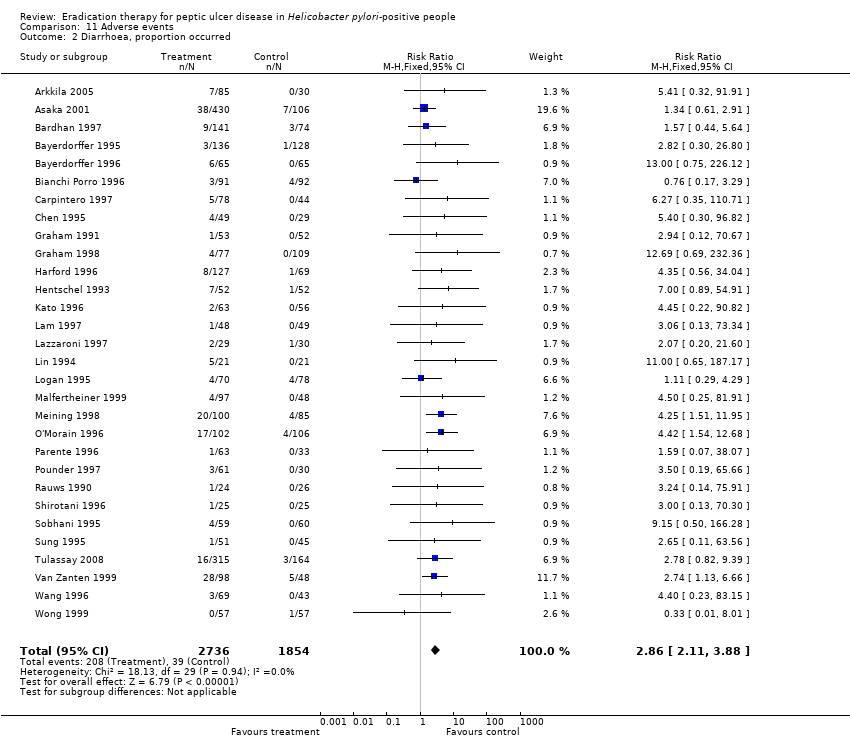
Comparison 11 Adverse events, Outcome 2 Diarrhoea, proportion occurred.

Comparison 11 Adverse events, Outcome 3 Nausea/vomiting, proportion occurred.

Comparison 11 Adverse events, Outcome 4 Skin rash, proportion occurred.
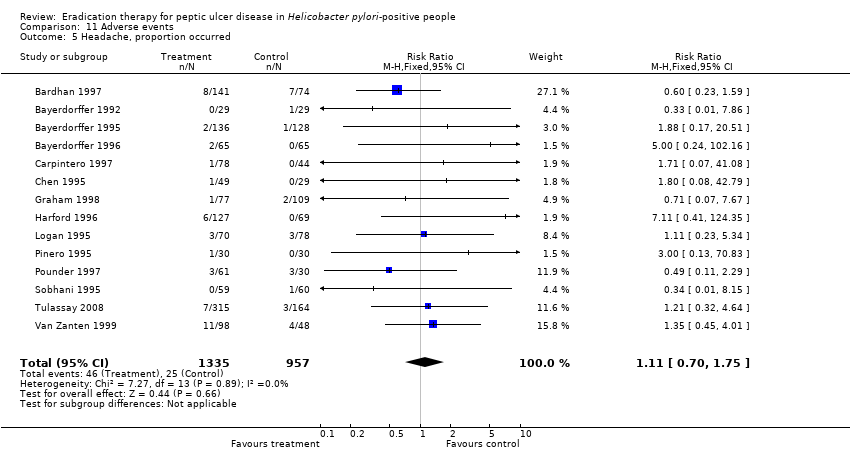
Comparison 11 Adverse events, Outcome 5 Headache, proportion occurred.
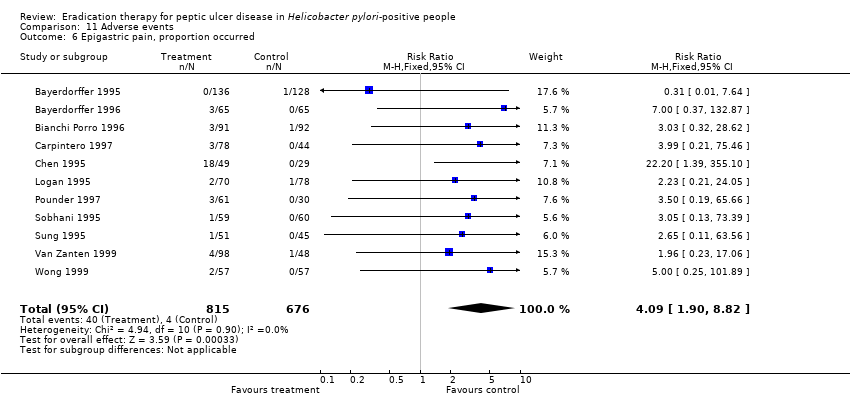
Comparison 11 Adverse events, Outcome 6 Epigastric pain, proportion occurred.
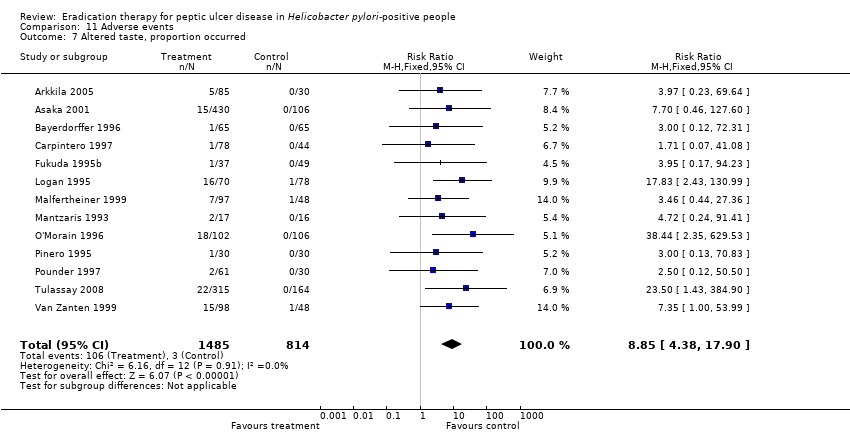
Comparison 11 Adverse events, Outcome 7 Altered taste, proportion occurred.

Comparison 11 Adverse events, Outcome 8 Stomatitis, proportion occurred versus not occurred.
| Additional Helicobacter pylori eradication therapy for acute ulcer healing in people with Helicobacter pylori‐positive peptic ulcer | |||||
| Patient or population: people with Helicobacter pylori‐positive peptic ulcer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Additional Helicobacter pylori eradication therapy | ||||
| Proportion of people with duodenal ulcer not healed after initial therapy | |||||
| H. pylori eradication therapy plus ulcer healing drug versus ulcer healing drug alone | 187 per 1000 | 124 per 1000 | RR 0.66 | 3910 | ⊕⊕⊝⊝ |
| H. pylori eradication therapy versus no active treatment | 585 per 1000 | 217 per 1000 | RR 0.37 | 207 | ⊕⊕⊝⊝ |
| Proportion of people with gastric ulcer not healed after initial therapy | |||||
| H. pylori eradication therapy plus ulcer healing drug versus ulcer healing drug alone | 130 per 1000 | 160 per 1000 | RR 1.23 | 1974 | ⊕⊝⊝⊝ |
| Proportion of people with peptic ulcer (gastric or duodenal ulcer) not healed after initial therapy | |||||
| H. pylori eradication therapy plus ulcer healing drug versus ulcer healing drug alone | 247 per 1000 | 129 per 1000 | RR 0.52 | 287 | ⊕⊕⊝⊝ |
| H. pylori eradication therapy versus no active treatment | 800 per 1000 | 120 per 1000 | RR 0.15 | 40 | ⊕⊕⊝⊝ |
| None of the trials reported proportion of people with gastric ulcer not healed after initial therapy between H.pylori eradication therapy and no active treatment. | |||||
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The risk of bias in trial(s) was high. | |||||
| Additional Helicobacter pylori eradication therapy for prevention of recurrence in people with Helicobacter‐positive peptic ulcer | |||||
| Patient or population: prevention of recurrence in people with Helicobacter pylori‐positive peptic ulcer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Additional Helicobacter pylori eradication therapy | ||||
| Proportion of people with duodenal ulcer with recurrence after maintenance therapy | |||||
| H. pylori eradication therapy versus ulcer healing drug alone | 162 per 1000 | 119 per 1000 | RR 0.73 | 319 | ⊕⊝⊝⊝ |
| H. pylori eradication therapy versus no active treatment | 644 per 1000 | 129 per 1000 | RR 0.2 | 2509 | ⊕⊝⊝⊝ |
| Proportion of people with gastric ulcer with recurrence after maintenance therapy | |||||
| H. pylori eradication therapy versus no active treatment | 524 per 1000 | 163 per 1000 | RR 0.31 | 1476 | ⊕⊝⊝⊝ |
| Proportion of people with peptic ulcer (gastric or duodenal ulcer) with recurrence after maintenance therapy | |||||
| H. pylori eradication therapy versus no active treatment | 333 per 1000 | 77 per 1000 | RR 0.23 | 103 | ⊕⊕⊝⊝ |
| None of the trials reported proportion of people with recurrent gastric ulcer or peptic ulcers during maintenance therapy between H.pylori eradication therapy and ulcer‐healing drug therapy. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The risk of bias in trial(s) was high. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 34 | 3910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.58, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.26, 0.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 15 | 1974 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.90, 1.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.31, 0.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion not healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion recurred Show forest plot | 4 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.42, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion recurred Show forest plot | 27 | 2509 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.15, 0.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion recurred Show forest plot | 12 | 1476 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.22, 0.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion recurred Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 H. pylori eradication + ulcer healing drug vs. ulcer healing drug alone Show forest plot | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.74] |
| 2 H. pylori eradication vs. no treatment Show forest plot | 2 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.83, 1.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall, proportion occurred Show forest plot | 43 | 6093 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.77, 2.99] |
| 2 Diarrhoea, proportion occurred Show forest plot | 30 | 4590 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [2.11, 3.88] |
| 3 Nausea/vomiting, proportion occurred Show forest plot | 15 | 1533 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.76 [1.91, 7.37] |
| 4 Skin rash, proportion occurred Show forest plot | 18 | 2385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.78, 2.37] |
| 5 Headache, proportion occurred Show forest plot | 14 | 2292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.70, 1.75] |
| 6 Epigastric pain, proportion occurred Show forest plot | 11 | 1491 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [1.90, 8.82] |
| 7 Altered taste, proportion occurred Show forest plot | 13 | 2299 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.85 [4.38, 17.90] |
| 8 Stomatitis, proportion occurred versus not occurred Show forest plot | 8 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.94, 7.48] |

