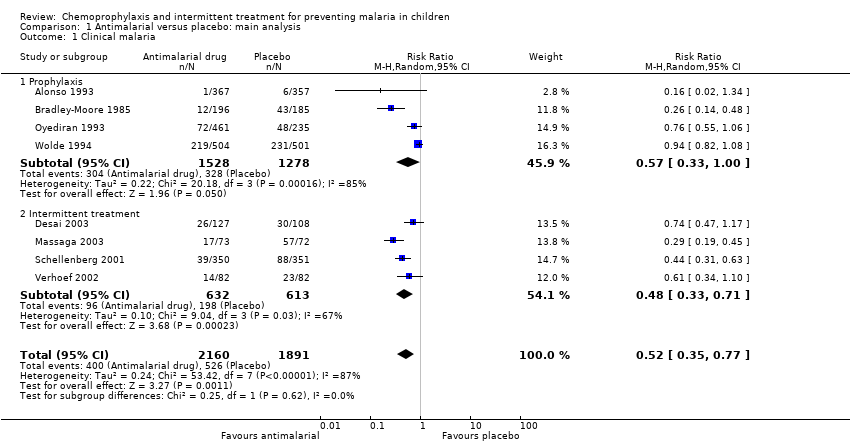
| 1 Clinical malaria Show forest plot | 8 | 4051 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.77] |
|
| 1.1 Prophylaxis | 4 | 2806 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.33, 1.00] |
| 1.2 Intermittent treatment | 4 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.33, 0.71] |
| 2 Severe anaemia Show forest plot | 8 | 2727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.42, 0.68] |
|
| 2.1 Prophylaxis | 4 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.37, 0.69] |
| 2.2 Intermittent treatment | 4 | 1218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.40, 0.84] |
| 3 Death from any cause Show forest plot | 11 | 7929 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.04] |
|
| 3.1 Prophylaxis | 7 | 6685 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.03] |
| 3.2 Intermittent treatment | 4 | 1244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.49, 1.99] |
| 4 Hospital admission for any cause Show forest plot | 3 | 1149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.52, 0.68] |
|
| 4.1 Prophylaxis | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.40, 0.60] |
| 4.2 Intermittent treatment | 2 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.56, 0.79] |
| 5 Parasitaemia Show forest plot | 6 | 1785 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.79] |
|
| 5.1 Prophylaxis | 4 | 1426 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.13, 0.45] |
| 5.2 Intermittent treatment | 2 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 6 Enlarged spleen Show forest plot | 4 | 1589 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.56] |
|
| 6.1 Prophylaxis | 4 | 1589 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.56] |
| 7 Mean haematocrit Show forest plot | 3 | 794 | Mean Difference (IV, Fixed, 95% CI) | 2.12 [1.47, 2.77] |
|
| 7.1 Prophylaxis | 3 | 794 | Mean Difference (IV, Fixed, 95% CI) | 2.12 [1.47, 2.77] |
| 8 Adverse events Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 8.1 Vomiting (prophylaxis) | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [0.31, 28.47] |
| 8.2 Hyperpigmented macules (prophylaxis) | 1 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 67.08 [4.12, 1092.11] |