Tramadol untuk sakit neuropatik dalam kalangan orang dewasa
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised ("matched pair"), double‐blind, parallel‐group, placebo‐controlled Duration: 45 days Assessed at baseline, 15, 30, 45 days | |
| Participants | Cancer‐related or cancer treatment‐related neuropathic pain of ≥ moderate intensity for ≥ 3 months, aged 18‐60 years Exclusion: pain mainly somatic, visceral or sympathetically maintained; scheduled for surgery, radiotherapy, chemotherapy, hormone therapy; use of tricyclic antidepressants, tramadol or any opioid; respiratory failure, chronic obstructive pulmonary disease, intracranial hypertension; Hx psychiatric illness or dependency on alcohol or drugs N = 36 | |
| Interventions | Tramadol 1 mg/kg bodyweight every 6 h; increased to 1.5 mg/kg every 6 h if relief inadequate, n = 18 | |
| Outcomes | PI: 0‐10 NRS | |
| Notes | Peru. Sponsor: Grunenthal Laboratories, Peru | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants with similar pain syndromes were paired, then "randomly assigned using a computer program" |
| Allocation concealment (selection bias) | Unclear risk | Method not described, but effectively, the first of pair was randomised, leaving a possibility of unconcealed allocation |
| Blinding (performance bias and detection bias) | Low risk | Treatments supplied in identical 10 ml bottles, "distinguished only by labels" |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation not mentioned, approximately 30% withdrawals, with different reasons between groups |
| Size | High risk | < 50 participants per treatment arm (18) |
| Methods | Multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled Duration 6 weeks | |
| Participants | Postherpetic neuralgia ≥ 3 months and ≤ 1 year, PI ≥ 40/100, aged 18‐85 years Mean baseline PI: 60/100 | |
| Interventions | Tramadol SR 100 mg taken in evening, n = 64 | |
| Outcomes | PI in last 24 h (daily): 100 mm VAS, 5‐point VRS | |
| Notes | France. Sponsor: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated 4‐block centralised randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) | Low risk | "treatments were identical with regard to appearance" |
| Incomplete outcome data (attrition bias) | High risk | Missing data on VAS and VRS over the 6th week were replaced by data available from the last 7 observations before the final visit (or the visit before premature discontinuation), not including more than 13 days before the end visit. Essentially an LOCF analysis |
| Size | Unclear risk | 50‐199 participants per treatment arm (63, 64) |
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group | |
| Participants | Peripheral diabetic neuropathy (HbA1 < 14%), distal, symmetric, > 3 months, PI moderate without analgesics, aged 18 years and over | |
| Interventions | Tramadol starting at 50 mg/d, increasing to 200 mg/d on day 10, then increased again as required from day 14 to maximum 400 mg/d by day 28, then stable; minimum 100 mg/d from day 14 to end of study, n = 65 Rescue medication: "No pain medications other than the study medications were permitted" | |
| Outcomes | PI at end of study (5‐point scale, 0‐4) Adverse events | |
| Notes | USA. Sponsor: Ortho‐McNeil Pharmaceutical, Raritan, NJ (research grant) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator |
| Allocation concealment (selection bias) | Low risk | Double‐blind code numbers assigned sequentially |
| Blinding (performance bias and detection bias) | Low risk | Identically appearing capsules, indistinguishable |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation not mentioned, approximately 30% withdrawals, with different reasons between groups |
| Size | Unclear risk | 50‐199 participants per treatment arm (65, 66) |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group Duration: 4 weeks Assessed at baseline and week 4 (daily pain diary) | |
| Participants | Spinal cord injury ≥ 12 months, "at or below level of lesion neuropathic pain" ≥ 6 months, PI > 3 (Borg's Category Ratio), aged 18‐70 years Current use of opioids or antidepressants considered on individual basis Worst PI at baseline: 7‐9/10, but general PI 4‐7/10 | |
| Interventions | Tramadol 50 mg x 3 daily, n = 23 | |
| Outcomes | Daily PI: complete relief = 10 | |
| Notes | Sweden. Sponsor: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Low risk | "sealed coded envelopes" provided by third party |
| Blinding (performance bias and detection bias) | Low risk | "identical in appearance" |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis with LOCF |
| Size | High risk | < 50 participants per treatment arm (12, 23) |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over Duration: 2 x 4 weeks with washout of ≥ 1 week between periods Assessed at end of treatment periods | |
| Participants | Polyneuropathy > 6 months, PI without treatment ≥ 4/10, aged 20‐80 years N = 45 (34 provided data for both periods) M 27, F 18 Median age 58 years (range 26‐77) Median baseline PI: 6/10 | |
| Interventions | Tramadol SR, titrated to 100‐200 mg twice daily over at least 1 week (22 participants took tramadol first, 23 placebo first) | |
| Outcomes | Daily PI: NRS 0‐10 (also paraesthesia and touch‐evoked pain) used to calculate median for each week | |
| Notes | Denmark. Sponsor: Grunenthal GmbH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomisation code with a block size of six" |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes", participants numbered consecutively and treated with drugs with corresponding randomisation number |
| Blinding (performance bias and detection bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) | High risk | LOCF imputation, efficacy data only for participants providing data for both phases; reasons for withdrawals per treatment arm not fully reported |
| Size | High risk | < 50 participants per treatment arm (≤ 43, 40) |
| Methods | Multicentre, randomised, double‐blind, active‐ and placebo‐controlled, cross‐over Duration: 3 x 4 weeks with washout of 1‐2 weeks between periods Assessed weekly and at end of each treatment period | |
| Participants | Polyneuropathy (distal, symmetric) > 6 months, PI ≥ 4/10, aged 18‐74 years Baseline PI: 6/10 | |
| Interventions | Tramadol SR 100 mg/d, increasing to 200‐400 mg/d | |
| Outcomes | Daily PI: (NRS 0‐10), then averaged over last 3 days of each period, and for each week | |
| Notes | Denmark, Germany. Sponsor: Grunenthal GmbH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the randomization list was generated via computer" |
| Allocation concealment (selection bias) | Low risk | Each site given "a unique series of numbers which were assigned to each trial patient in ascending order and marked at the corresponding drug packages" |
| Blinding (performance bias and detection bias) | Low risk | The three treatments "had identical appearance and weight and were dosed similarly" |
| Incomplete outcome data (attrition bias) | High risk | Completer analysis used for PP analysis of dichotomous efficacy data. |
| Size | High risk | < 50 participants per treatment arm for efficacy data (maximum 56 for safety data) |
F: female; HbA1c: glycosylated haemoglobin; Hx: history of; LOCF: last observation carried forward; M: male; MAO: monoamine oxidase; N: number of participants in study; n: number of participants in treatment arm; NRS: numerical rating scale; PGIC: Patient Global Impression of Change; PI: pain intensity; PP: per protocol; PR: pain relief; SD: standard deviation; SR: sustained‐release; VAS: visual analogue scale; VRS: verbal rating scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Commentary on Harati 1998 | |
| Review article | |
| Investigates buprenorphine, not tramadol | |
| Short conference abstract with inadequate method description and no usable data | |
| Open‐label study | |
| Correspondence with no new trial data | |
| Follow up to Harati 1998. Not randomised or controlled | |
| Review article | |
| Open‐label study | |
| Correspondence with no new trial data | |
| 7‐day treatment periods, < 10 participants per treatment arm | |
| Open‐label cohort study | |
| Observational study. Not a randomised controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Enriched enrolment, randomised withdrawal design. Single‐blind run‐in phases in which participants were treated with gabapentin then active placebo. Responders were randomised to a double‐blind, 3‐period cross‐over of gabapentin, tramadol, and active placebo Duration: Period A: 1 week; Period B: 2 weeks; double‐blind: 3 x 2‐week periods (1‐week titration, 1‐week stable), each followed by 1‐week washout |
| Participants | Idiopathic small fibre neuropathy ≥ 2 months, self‐reported gabapentin responders (on stable dose 900 ‐ 4800 mg/d), PI > 3 to ≤ 7.5 on medication, aged ≥ 18 years |
| Interventions | Period A: gabapentin at pre‐study dose + matching active placebo (diphenhydramine). Pain scores ≤ 7.5/10 entered period B Treatment period (2‐week test and 1‐week washout in multiple cross‐overs): Rescue medication: 325 mg tablets (probably paracetamol) ‐ limit not specified |
| Outcomes | Daily PI: (NRS 0‐10), averaged over 24 h. If rescue medication or additional gabapentin taken, used score before first rescue dose of the day |
| Notes | USA. Sponsor: Merck Research Laboratories |
F: female; Hx: history of; M: male; N: number of participants in study; NRS: numerical rating scale; PGIC: Patient Global Impression of Change; PI: pain intensity.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain intensity reduction Show forest plot | 3 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.02, 4.58] |
| Analysis 1.1  Comparison 1 Tramadol versus placebo, Outcome 1 Participants with ≥ 50% pain intensity reduction. | ||||
| 2 Withdrawal due to adverse events Show forest plot | 6 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [1.99, 8.37] |
| Analysis 1.2  Comparison 1 Tramadol versus placebo, Outcome 2 Withdrawal due to adverse events. | ||||
| 3 All cause withdrawal Show forest plot | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.75, 1.76] |
| Analysis 1.3  Comparison 1 Tramadol versus placebo, Outcome 3 All cause withdrawal. | ||||
| 4 Participants with any adverse event Show forest plot | 4 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.22, 2.13] |
| Analysis 1.4  Comparison 1 Tramadol versus placebo, Outcome 4 Participants with any adverse event. | ||||
| 5 Participants with specific adverse events Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Tramadol versus placebo, Outcome 5 Participants with specific adverse events. | ||||
| 5.1 Nausea | 6 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [2.23, 5.88] |
| 5.2 Constipation | 5 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.11 [2.36, 7.16] |
| 5.3 Tiredness/fatigue/somnolence | 4 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [1.93, 5.36] |
| 5.4 Dizziness | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.72 [1.94, 7.12] |
| 5.5 Dry mouth | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.35, 4.42] |

Study flow diagram for the updated search

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Tramadol versus placebo, outcome: 1.1 Participants with ≥ 50% pain intensity reduction.
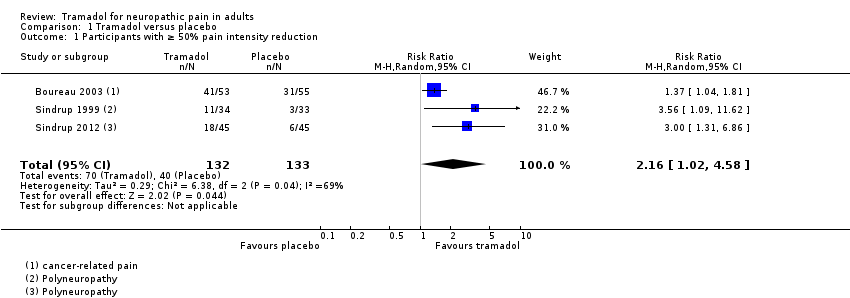
Comparison 1 Tramadol versus placebo, Outcome 1 Participants with ≥ 50% pain intensity reduction.
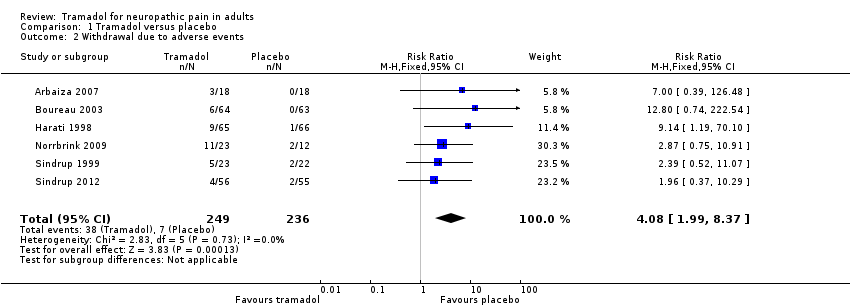
Comparison 1 Tramadol versus placebo, Outcome 2 Withdrawal due to adverse events.
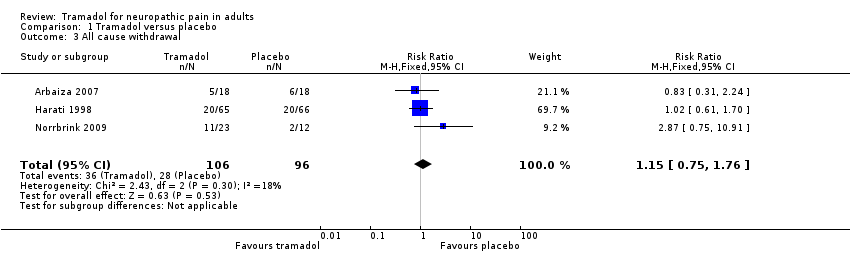
Comparison 1 Tramadol versus placebo, Outcome 3 All cause withdrawal.

Comparison 1 Tramadol versus placebo, Outcome 4 Participants with any adverse event.

Comparison 1 Tramadol versus placebo, Outcome 5 Participants with specific adverse events.
| Tramadol compared with placebo for neuropathic pain | ||||||
| Patient or population: adults with neuropathic pain (any origin) Settings: community Intervention: oral tramadol (typically started at a dose of about 100 mg daily and increased over 1 to 2 weeks to a maximum of 400 mg daily) Comparison: placebo | ||||||
| Outcomes (at trial end) | Probable outcome with | Probable outcome with | Relative effect | No of participants | Quality of the evidence | Comments |
| At least 30% reduction in pain | Not analysed | Not analysed | Not analysed | 157 participants (2 studies) 60 events | Low quality1 | ‐ |
| At least 50% reduction in pain | 530 per 1000 | 300 per 1000 | RR 2.2 (1.02, 4.6) NNT 4.4 (2.9 to 8.8) | 265 participants (3 studies) 110 events | Low quality1 | ‐ |
| PGIC much or very much improved | Not analysed | Not analysed | Not analysed | 35 participants (1 study) 4 events | Very low quality2 | ‐ |
| Withdrawal due to adverse event | 160 per 100 | 30 per 1000 | RR 4.1 (2.0 to 8.4) NNH 8.2 (5.8 to 14) | 485 participants (6 studies) 45 events | Low quality1 | ‐ |
| Participants experiencing any adverse event | 580 per 1000 | 340 per 1000 | RR 1.6 (1.2 to 2.1) NNH 4.2 (2.8 to 8.3) | 266 participants (4 studies) 123 events | Low quality1 | ‐ |
| Serious adverse events | 4 serious adverse events reported in total | Not all studies reported specifically on serious adverse events | Very low quality2 | ‐ | ||
| Death | No data | No data | Not calculated | No data | Very low quality3 | ‐ |
| CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; PGIC: Patient Global Impression of Change; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
| 1Downgraded 2 levels due to small number of studies and participants and relatively few events, and several sources of potential bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain intensity reduction Show forest plot | 3 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.02, 4.58] |
| 2 Withdrawal due to adverse events Show forest plot | 6 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [1.99, 8.37] |
| 3 All cause withdrawal Show forest plot | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.75, 1.76] |
| 4 Participants with any adverse event Show forest plot | 4 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.22, 2.13] |
| 5 Participants with specific adverse events Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Nausea | 6 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [2.23, 5.88] |
| 5.2 Constipation | 5 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.11 [2.36, 7.16] |
| 5.3 Tiredness/fatigue/somnolence | 4 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [1.93, 5.36] |
| 5.4 Dizziness | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.72 [1.94, 7.12] |
| 5.5 Dry mouth | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.35, 4.42] |

