Tramadol para el tratamiento del dolor neuropático en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003726.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 junio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neuromuscular
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
For this update, the background and methods were revised by SD, PW, and RAM, based on a template for reviews of drugs for neuropathic pain.
SD, PW, and RAM ran searches and selected studies for inclusion. SD and RAM carried out data extraction, and SD and PW assessed the risk of bias. SD and RAM carried out analyses. All authors were involved in writing the full review.
In the original review, the background was written by RMD and reviewed by J Hollingshead. The description of studies and results were written by J Hollingshead and RMD and reviewed by D Cornblath. The objectives, study criteria, search strategy, and discussion were written jointly by J Hollinghsead and RMD. The entire review was reviewed by D Cornblath.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
RMD: none known
SD: none known
PW: none known
RFB: none known. RFB is a retired specialist pain physician who has managed patients with neuropathic pain.
DA: is a specialist pain physician and manages patients with neuropathic pain. He has received lecture fees from Grünenthal (2014, 2015) and Pfizer (2016).
RAM: RAM has received grant support from Grünenthal relating to individual patient‐level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
Acknowledgements
This updated review was carried out using a template developed in collaboration with Cochrane Musculoskeletal, Cochrane Neuromuscular, and Cochrane Pain, Palliative and Supportive Care. The editorial process was managed by Cochrane Neuromuscular.
Institutional support to review authors was provided by the Oxford Pain Relief Trust.
The National Institute for Health Research (NIHR) is the largest single funder of Cochrane Pain, Palliative and Supportive Care and Cochrane Neuromuscular. Disclaimer: the views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jun 15 | Tramadol for neuropathic pain in adults | Review | Rudolf Martin Duehmke, Sheena Derry, Philip J Wiffen, Rae F Bell, Dominic Aldington, R Andrew Moore | |
| 2006 Jul 19 | Tramadol for neuropathic pain | Review | Rudolf Martin Duehmke, James Hollingshead, David R Cornblath | |
| 2004 Apr 19 | Tramadol for neuropathic pain | Review | Rudolf Martin Dühmke, David D Cornblath, James Hollingshead | |
| 2002 Apr 05 | Tramadol for neuropathic pain | Protocol | Rudolf Martin Duehmke, James Hollingshead, David D Cornblath | |
Differences between protocol and review
The background and methods sections have been updated in line with the current template. The title is changed to emphasise that the review concerns adults only, in line with other, similar, reviews.
We are no longer including quasi‐randomised studies, or studies that were not double‐blind, or comparisons with no treatment (because studies cannot be blinded). We limited the review to adults only. The primary outcome is now substantial or moderate pain relief (50% or more, or 30% or more, or equivalent measures using Patient Global Impression of Change scale).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Aged; Humans; Middle Aged;
PICO

Study flow diagram for the updated search

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Tramadol versus placebo, outcome: 1.1 Participants with ≥ 50% pain intensity reduction.
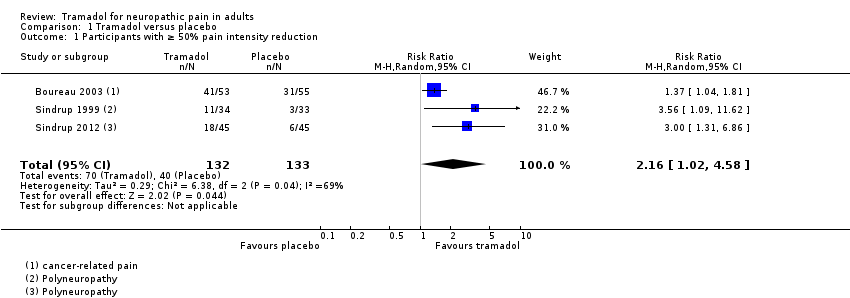
Comparison 1 Tramadol versus placebo, Outcome 1 Participants with ≥ 50% pain intensity reduction.
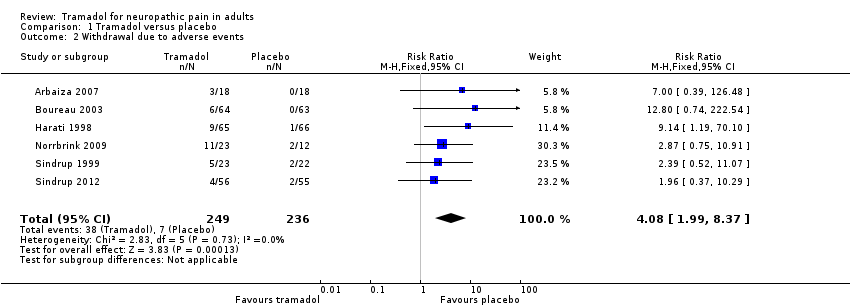
Comparison 1 Tramadol versus placebo, Outcome 2 Withdrawal due to adverse events.
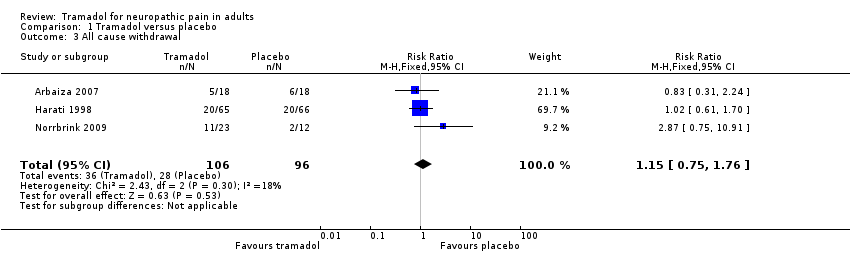
Comparison 1 Tramadol versus placebo, Outcome 3 All cause withdrawal.

Comparison 1 Tramadol versus placebo, Outcome 4 Participants with any adverse event.

Comparison 1 Tramadol versus placebo, Outcome 5 Participants with specific adverse events.
| Tramadol compared with placebo for neuropathic pain | ||||||
| Patient or population: adults with neuropathic pain (any origin) Settings: community Intervention: oral tramadol (typically started at a dose of about 100 mg daily and increased over 1 to 2 weeks to a maximum of 400 mg daily) Comparison: placebo | ||||||
| Outcomes (at trial end) | Probable outcome with | Probable outcome with | Relative effect | No of participants | Quality of the evidence | Comments |
| At least 30% reduction in pain | Not analysed | Not analysed | Not analysed | 157 participants (2 studies) 60 events | Low quality1 | ‐ |
| At least 50% reduction in pain | 530 per 1000 | 300 per 1000 | RR 2.2 (1.02, 4.6) NNT 4.4 (2.9 to 8.8) | 265 participants (3 studies) 110 events | Low quality1 | ‐ |
| PGIC much or very much improved | Not analysed | Not analysed | Not analysed | 35 participants (1 study) 4 events | Very low quality2 | ‐ |
| Withdrawal due to adverse event | 160 per 100 | 30 per 1000 | RR 4.1 (2.0 to 8.4) NNH 8.2 (5.8 to 14) | 485 participants (6 studies) 45 events | Low quality1 | ‐ |
| Participants experiencing any adverse event | 580 per 1000 | 340 per 1000 | RR 1.6 (1.2 to 2.1) NNH 4.2 (2.8 to 8.3) | 266 participants (4 studies) 123 events | Low quality1 | ‐ |
| Serious adverse events | 4 serious adverse events reported in total | Not all studies reported specifically on serious adverse events | Very low quality2 | ‐ | ||
| Death | No data | No data | Not calculated | No data | Very low quality3 | ‐ |
| CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; PGIC: Patient Global Impression of Change; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
| 1Downgraded 2 levels due to small number of studies and participants and relatively few events, and several sources of potential bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain intensity reduction Show forest plot | 3 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.02, 4.58] |
| 2 Withdrawal due to adverse events Show forest plot | 6 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [1.99, 8.37] |
| 3 All cause withdrawal Show forest plot | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.75, 1.76] |
| 4 Participants with any adverse event Show forest plot | 4 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.22, 2.13] |
| 5 Participants with specific adverse events Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Nausea | 6 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [2.23, 5.88] |
| 5.2 Constipation | 5 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.11 [2.36, 7.16] |
| 5.3 Tiredness/fatigue/somnolence | 4 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [1.93, 5.36] |
| 5.4 Dizziness | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.72 [1.94, 7.12] |
| 5.5 Dry mouth | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.35, 4.42] |

