5‐аминосалициловая кислота для поддержания медикаментозной ремиссии при болезни Крона
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double blind, placebo‐controlled multicentre trial | |
| Participants | 248 patients with clinically inactive Crohn's disease at entry (CDAI < 150) ‐ no age or CD location restrictions reported Crohn's disease had to be 'controlled' for at least 1 month prior to entry Controlled described as no steroids or a stable low dose (prednisone 2.5 mg/day or less) | |
| Interventions | Oral 5‐ASA (Mesasal / Claversal, Smith Kline & French company) at a dose of 1.5 g/day (n = 125) Placebo (n = 123) | |
| Outcomes | Primary outcome: relapse at 12 months (CDAI > 150 and an increase in CDAI of 60 points from baseline) Secondary outcomes: adverse events, withdrawal due to adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "predetermined computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | Quote: "Drug supplies were centrally packaged, labelled and randomized in blocks of four according to a predetermined computer‐generated list" |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study Quote: "patients were randomly allocated to treatment with one of the following regiments: 5‐ASA, or matching placebo for 5‐ASA" |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and physician assessed outcomes were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | Although some subgroup analyses were not prespecified these analyses would generally be expected for this type of study |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double blind, placebo‐controlled multicentre trial | |
| Participants | 59 patients with Crohn's disease in continuous remission for at least 6 months ‐ patients could have Crohn's disease of the small bowel, large bowel or both ‐ no age restrictions were reported Remission was defined as a Harvey Bradshaw (Softley‐Clamp modification) index score of < 4 Patients could be treated with only 5‐ASA or sulphasalazine within last 6 months | |
| Interventions | Oral 5‐ASA (Rafassal similar to Salofalk/Claversal, Rafia Laboratory, Israel) at a dose of 1 g/day (n = 28) Placebo (n = 31) | |
| Outcomes | Primary outcome: relapse at 12 months (increase of more than 4 points on the index from baseline) Secondary outcomes: adverse events, withdrawal due to adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Quote: "coding and randomization were performed by a central pharmacy that dispensed the coded medicines to the participating centers" |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study Quote: "Patients were given coded mesalazine or placebo four tablets/day (coated 5‐ASA preparation similar to Salofalk‐Claversal)" |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and physician assessed outcomes were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal Quote: "Ten patients were withdrawn from the trial, four from the placebo group and six from the treatment group. Among these patients, five were withdrawn because of noncompliance, three patients were lost to follow‐up, and one in each group had side effects (headache) leading to withdrawal from the study" |
| Selective reporting (reporting bias) | Unclear risk | Although some subgroup analyses were not prespecified these analyses would generally be expected for this type of study |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double blind, placebo‐controlled multicentre trial | |
| Participants | 202 patients with clinically inactive Crohn's disease | |
| Interventions | Pentasa 1.5g bid (n = 101) Placebo (n = 101) | |
| Outcomes | Primary outcome: relapse at 12 to 18 months Seconadry outcome: adverse events | |
| Notes | Abstract publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "patients were...randomized to active drug or placebo How blinding was achieved was not described in abstract |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Unclear from abstract |
| Methods | Randomised, double‐blind, placebo‐controlled, multicenter trial performed in 17 centres (16 from France and 1 from Switzerland) for one year Data was analysed following an intention‐to‐treat analysis | |
| Participants | Patients (N=132) under the age of 18 and diagnosed with CD before the age of 16, according to clinical, radiological, endoscopic and histological data Specific inclusion criteria: patients in clinical remission within six months of flare‐up treatment started prior to inclusion, with an HB score inferior to 5, ESR superior to 25mm, and normal hepatic and renal function Specific exclusion criteria: patients were excluded if a flare‐up had been treated with mesalazine or immunosuppressants, or if they had a known hypersensitivity to salicylate | |
| Interventions | 50 mg/kg mesalazine or placebo over a 1 year period | |
| Outcomes | Primary outcome: clinical relapse (HB score greater than or equal to 5, confirmed within two weeks) or surgery for acute complication of CD Secondary outcome: treatment failure, defined as relapse, failure of steroid withdrawal, side‐effect intolerance requiring treatment discontinuation, worsening or aggravation of patient's status requiring treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was generated by a third‐party Quote: "the randomization lists were generated by the randomization center" Quote: "patients were randomized by stratum within each center, using randomized blocks of two to four) |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation performed by a third party Quote: "At randomization, a chronological treatment number was assigned to each patient and, accordingly, the treatment allocated to each patient was given to the physician in charge of that patient in numbered bottles, labeled with the protocol identification, center and stratum, patient's initials, treatment number, batch number and expiry date" |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study Intervention and placebo tablets were identical Quote: "Each allocated treatment was sent to the physician in charge of that patient in an individual sealed envelope" |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and physician assessed outcomes were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were similar across intervention groups with similar reasons for withdrawal Low proportion of withdrawals per group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomized, double‐blind, placebo‐controlled, multicentre trial conducted at 22 institutions. Data was analysed following an intention‐to‐treat | |
| Participants | Specific inclusion criteria: patients with an established diagnosis of Crohn's disease localized to the ileum, colon or both with endoscopic, radiological and/or surgical confirmation of disease location within 1 year prior to enrolment; if they had an acute flare‐up of the disease (CDAI > 150 and ≤ 450); if they had achieved remission with a standard steroid‐dose regiment; and if they were between 18 and 70 years of age. Specific exclusion criteria: patients who had oesophageal, gastroduodenal or jejunal localizations of Crohn's disease, clinically significant bowel stenosis, fistulas requiring metronidazole treatment or surgery, or other active extra‐intestinal manifestations requiring steroid treatment; if they had been treated with steroids or immunosuppressants within 3 months of enrolment; if they had clinically significant hepatic, renal or haematological disease; if they were taking H2‐blockers or omeprazole; if they were allergic to salicylates; or if they were pregnant or lactating women | |
| Interventions | Patients were evaluated for inclusion during a flare‐up of Crohn's disease and treated with steroids according to a standard dose regimen (methylprednisolone, 1 mg/kg q.d.s with a maximum of 60 mg q.d.s. given orally for 4‐8 weeks). Those who achieved clinical remission (CDAI <150) were entered into the study. Six tablets, administered in three daily doses, containing either 5‐ASA (Claversal) 500 mg or a placebo. Daily steroid dose was tapered by 0.25 mg/kg every two weeks once this regiment was started | |
| Outcomes | Two outcomes were assessed: i) clinical relapse, defined as an increase of CDAI above 150 and at least 60 points above the value observed at achievement of remission, accompanied by an increase of at least two of the three acute‐phase reactants (ESR, alpha‐1 acid glycoprotein and alpha‐2 globulins) ii) completion of a 24‐month study period without clinical relapse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Procedure used for randomisation not described Quote: "randomization was done centrally in blocks of four" |
| Allocation concealment (selection bias) | Low risk | Central randomisation Quote: "randomization was done centrally in blocks of four" |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, randomised, placebo‐controlled trial Placebo pills were identical to 5‐ASA pills |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal Quote: "Seventeen patients (eight on 5‐ASA and nine on placebo) were withdrawn from the study: 15 of them were lost to follow‐up, one was considered as non‐compliant, one required surgery for ileorectal fistula". |
| Selective reporting (reporting bias) | Unclear risk | Although some subgroup analyses were not prespecified these analyses would generally be expected for this type of study |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicentre study. Described as randomised:Yes Randomisation method described:No Described as double blind:Yes Blind method described:No Follow‐ups described:Yes | |
| Participants | Inclusion criteria: Age greater than 15. CD of the small bowel, colon or both. CD in remission < 24 months. Remission defined as CDAI <150. No steroid use in month before trial. | |
| Interventions | Oral 5‐ASA vs placebo. Allocation: 80 patients allocated to 5‐ASA, and 81 patients allocated to placebo. Name 5‐ASA: Pentasa. Manufacturer: Ferring AS, Vanlose, Denmark. Dose: 2 g per day | |
| Outcomes | Relapse measured at: 24 months. Definition of relapse: Surgery or CDAI >250 or CDAI between 150 and 250 but over the baseline value by >50 points. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "the study followed a randomized, double‐blind, stratified design" Identical Pentasa and placebo tablets used |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and physician assessed outcomes were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | Although some subgroup analyses were not prespecified these subgroup analyses would generally be expected for this type of study |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicentre study. Described as randomised:Yes Randomisation method described:Yes Described as double blind:Yes Blind method described:No Follow‐ups described:Yes | |
| Participants | Inclusion criteria: Age greater than 18. CD of the colitis, ileitis or both. CD in remission for 1 month prior to randomisation. Remission was defined as assessed by investigator and by CDAI <150. No steroid use one month prior to study. | |
| Interventions | Oral 5‐ASA vs placebo. Allocation: 167 patients allocated to 5‐ASA, and 161 patients allocated to placebo. Name 5‐ASA: Olsalazine. Manufacturer: Kabi Pharmacia. Dose: 2 g per day | |
| Outcomes | Relapse measured at: 12 months. Definition of relapse: CDAI > 150 or an increase in the CDAI score by 60 or more from the baseline score at week 0 or clinical relapse (need for additional therapy or surgery). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomization" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "the trial was randomised, double‐blind, parallel study" Quote: "Identical placebos were provided by Kabi Pharmacia" |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and physician assessed outcomes were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "More patients in the olsalazine treated group failed to complete a 52 week treatment period than in the placebo treated group (olsalazine 65.9% v placebo 53.4%)" Quote: "the frequency of intolerable adverse events was higher in the olsalazine than in the placebo treated group (19.8% v 6.2%, respectively)" |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, stratified study conducted in 20 Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID) centres, 19 from France and 1 from Belgium | |
| Participants | Patients (N =129) aged 15 years or older with active CD (CDAI > 200) Specific inclusion criteria: patients with active CD (CDAI > 200); if they were 15 years or older with CD of the small intestine, recto‐colon, or both as documented by barium radiographs and/or colonoscopy within 12 months before inclusion Specific exclusion criteria: imminent need for surgery, purely anorectal CD, contraindication to corticosteroids, previous hypersensitivity to salicylates or intolerance to mesalamine, and liver or kidney insufficiency; patients in whom mesalamine had clearly failed to maintain remissions (i.e., if the attack leading to pre‐inclusion had occurred while they were on mesalamine at a daily dose of > 3 g for more than 2 months) | |
| Interventions | Patients with active CD were pre‐included in the study and were administered oral prednisolone (1 mg/kg once a day) for 3‐7 weeks Patients achieving clinical remission within this time frame were included in the trial and randomly assigned within each centre and stratum to be administered daily either 4 g of mesalamine (Pentasa) or identical placebo tablets Prednisolone was tapered in steps of 10 mg per 10 days to a dose of 0.5 mg/kg/day and then in steps of 5 mg per 10 days to complete discontinuation | |
| Outcomes | Treatment failure defined as a failure to discontinue steroids (secondary steroid resistance, steroid dependence, or surgery because of CD) or a relapse (CDAI > 150 and a 100‐point increase above inclusion value and/or need for surgery) during the 1‐year follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "randomized, double‐blind, stratified design" Quote: "identical tablets containing 500 mg of mesalasmine or placebo were prepared" |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and physician assessed outcomes were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts were balanced across treatment groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicentre study. Described as randomised:Yes Randomisation method described:Yes Described as double blind:Yes Blind method described:Yes Follow‐ups described:Yes | |
| Participants | Inclusion criteria: Aged between 18 and 65. CD of the ileum, colon or both. CD in remission between 3 and 24 months. Remission defined as CDAI<150. No steroid use 3 months prior to study. | |
| Interventions | Oral 5‐ASA vs placebo. Allocation: 64 patients allocated to 5‐ASA, and 61 patients allocated to placebo. Name 5‐ASA: Asacol. Manufacturer: Giuliani, Milan. Dose: 2.4 g / day | |
| Outcomes | Relapse measured at: 12 months. Definition of relapse: CDAI > 150 with an increase of 100 points over the baseline value, confirmed at a second visit 1 week later | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Quote: "study medications were centrally packaged" |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study Quote: "Eligible patients were randomly allocated to receive either 5‐ASA or identical placebo tablets for 12 months" |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and physician assessed outcomes were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Eight patients, 5 on 5‐ASA and 3 on placebo, were withdrawn from the trial because of adverse reactions" Quote: "One patient was lost to follow‐up. Quote: "Two patients in the 5‐ASA group elected to stop treatment" |
| Selective reporting (reporting bias) | Unclear risk | Although some posthoc subgroup analysis performed were not prespecified these subgroup analyses would generally be expected for this type of study |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicentre study. Described as randomised:Yes Randomisation method described:Yes Described as double blind:Yes Blind method described:Yes Follow‐ups described:Yes | |
| Participants | Inclusion criteria: Age greater than 18. CD location restrictions not mentioned. CD in remission for 1 month, but at least 2 flare‐ups within the last 4 years, one within the last 18 months or a recent resection. Remission defined as CDAI<150 at baseline and no symptoms within last 30 days. No steroid use within a month of study. | |
| Interventions | Oral 5‐ASA vs placebo. Allocation: 141 patients allocated to 5‐ASA, and 152 patients allocated to placebo. Name 5‐ASA: microsphere coated with ethylcellulose, Mesalamine. Manufacturer: Not provided by authors. Dose: 3 g per day | |
| Outcomes | Relapse measured at: 12 months. Definition of relapse: 1st occurrence of a CDAI that was >150 as well as the absolute value of at least 60 points higher than baseline or where physician diagnosed a flare‐up of disease but a full diary card was not available for the calculation of the final CDAI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization scheme" |
| Allocation concealment (selection bias) | Low risk | Quote: "medication was packaged by the sponsor and dispensed to each center in coded identical‐appearing boxes |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study Quote: "Medication was dispensed in bottles containing identical appearing capsules of either 250 mg mesalamine or placebo" |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and physician assessed outcomes were blinded Quote: "the statistical analysis was performed using SAS Version 6.04 by a third party" |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal Quote: "Forty‐seven patients were withdrawn within 28 days after entry into the study. Twenty‐five reported a relapse (11 mesalamine‐treated and 14 placebo‐treated patients), and 22 were withdrawn for failure to comply with the protocol (12 mesalmine and 10 placebo‐treated patients" |
| Selective reporting (reporting bias) | Unclear risk | Although some subgroup analyses were not prespecified these analyses would generally be expected for this type of study |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicentre study. Described as randomised:Yes Randomisation method described:Yes Described as double blind:Yes Blind method described:Yes Follow‐ups described:Yes | |
| Participants | Inclusion criteria: Aged between 18 and 70. CD of the ileum, colon or both. CD in remission but had one period of activity within the previous 28 months. Remission defined as CDAI<150. No steroid use within month of study. | |
| Interventions | Oral 5‐ASA vs placebo. Allocation: 102 patients allocated to 5‐ASA, and 105 patients allocated to placebo. Name 5‐ASA: Claversal / Mesasal. Manufacturer: Smith Kline, Beecham. Dose: 1.5 g b.d | |
| Outcomes | Relapse measured at: 12 months. Definition of relapse: CDAI > 150 with at least a 60‐point increase from the baseline index score. | |
| Notes | Originally presented as 2 parts 'Colitis or ileocolitis' and 'Ileitis' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization with Procplan in SAS" |
| Allocation concealment (selection bias) | Low risk | All study medications were supplied in blister packets of six tablets which were indistinguishable from one another |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study Quote: "All study medications (mesalazine and matching placebo) were supplied in blister packets ...." |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and investigator assessed outcomes were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal Quote: "106 patients (51 in the mesalazine group and 55 in the placebo group) were withdrawn from the study due to adverse events |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled study conducted for one year | |
| Participants | Patients (N = 66) with Crohn's disease in remission (CDAI < 150) for at least 3 months without steroids; diagnosis was confirmed by characteristic endoscopy or radiologic findings | |
| Interventions | Study population was randomised to receive mesalazine or placebo in blocks of four patients | |
| Outcomes | Number of relapses was assessed. Adverse reactions were also assessed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind study Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "two patients in the treatment group were noncompliant...data of these patients were not evaluated" |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Patients were not in remission | |
| Not an RCT ‐ alternate allocation | |
| Not an RCT ‐ alternate allocation | |
| Treatment duration < 6 months (4 months) | |
| Not an RCT ‐ systematic review | |
| Study compared sulphasalazine to placebo, not 5‐ASA to placebo or sulphasalazine | |
| Not an RCT ‐ clinical trial | |
| Wrong comparator ‐ study compared sulphasalazine to placebo, not 5‐ASA to placebo or sulphasalazine | |
| Not an RCT: open‐label, compassionate‐use, pre‐marketing clinical trial | |
| This paper comments on the Mahmud 2001 study | |
| Wrong comparator ‐ study compared sulphasalazine to placebo, not 5‐ASA to placebo or sulphasalazine | |
| Wrong comparator: study compared sulphasalazine to placebo, not 5‐ASA to placebo or sulphasalazine | |
| Not an RCT ‐ retrospective cohort study | |
| Wrong comparator ‐ study compared 5‐ASA to 4‐ASA, not 5‐ASA to placebo or sulphasalazine | |
| Wrong comparator: study compared sulphasazline to placebo, not 5‐ASA to placebo or 5‐ASA to sulphasalazine |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 5‐ASA compared to placebo, Outcome 1 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up. | ||||
| 1.1 12 months | 11 | 2014 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.07] |
| 1.2 24 months | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| 1.3 Pediatric | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 2 Sensitivity analysis ‐ Relapse, drop‐outs classed as relapse, grouped by length of follow‐up, random effects Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 5‐ASA compared to placebo, Outcome 2 Sensitivity analysis ‐ Relapse, drop‐outs classed as relapse, grouped by length of follow‐up, random effects. | ||||
| 2.1 12 months | 11 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 2.2 24 months | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.23] |
| 2.3 Pediatric | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.86, 1.33] |
| 3 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 5‐ASA compared to placebo, Outcome 3 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up. | ||||
| 3.1 12 Months | 10 | 1438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.01] |
| 3.2 24 Months | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.29] |
| 3.3 Pediatric | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.31] |
| 4 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up, random effects Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 5‐ASA compared to placebo, Outcome 4 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up, random effects. | ||||
| 4.1 12 Months | 10 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.05] |
| 4.2 24 Months | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.29] |
| 4.3 Pediatric | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.31] |
| 5 Adverse events Show forest plot | 10 | 1814 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.95, 1.17] |
| Analysis 1.5  Comparison 1 5‐ASA compared to placebo, Outcome 5 Adverse events. | ||||
| 6 Withdrawals due to adverse events Show forest plot | 10 | 1833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.88, 1.38] |
| Analysis 1.6  Comparison 1 5‐ASA compared to placebo, Outcome 6 Withdrawals due to adverse events. | ||||
| 7 Serious adverse events Show forest plot | 3 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.24, 8.44] |
| Analysis 1.7  Comparison 1 5‐ASA compared to placebo, Outcome 7 Serious adverse events. | ||||
| 8 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up Show forest plot | 11 | 2014 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.16] |
| Analysis 1.8  Comparison 1 5‐ASA compared to placebo, Outcome 8 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up. | ||||
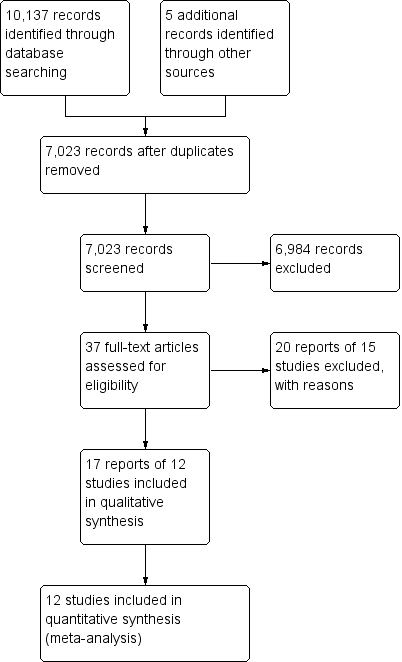
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 5‐ASA compared to placebo, outcome: 1.1 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.
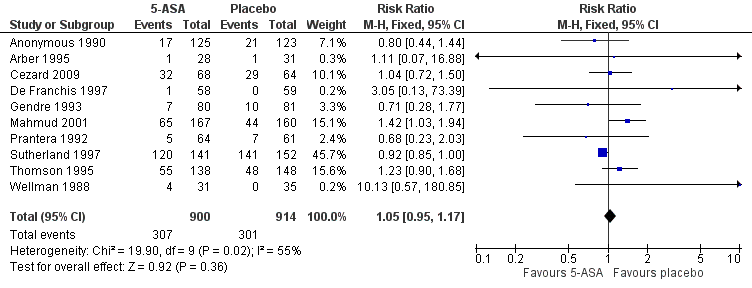
Forest plot of comparison: 1 5‐ASA compared to placebo, outcome: 1.5 Adverse events.

Funnel plot of comparison: 1 5‐ASA compared to placebo, outcome: 1.8 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.

Comparison 1 5‐ASA compared to placebo, Outcome 1 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.
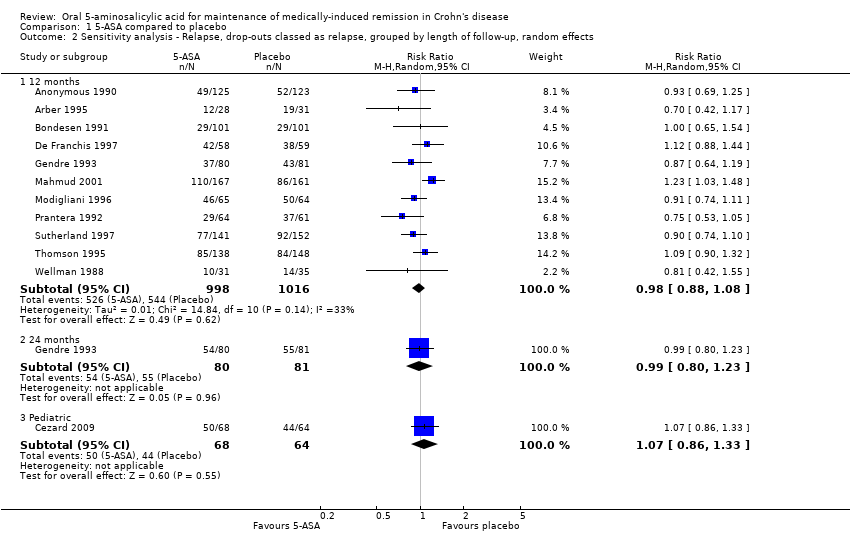
Comparison 1 5‐ASA compared to placebo, Outcome 2 Sensitivity analysis ‐ Relapse, drop‐outs classed as relapse, grouped by length of follow‐up, random effects.

Comparison 1 5‐ASA compared to placebo, Outcome 3 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up.

Comparison 1 5‐ASA compared to placebo, Outcome 4 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up, random effects.

Comparison 1 5‐ASA compared to placebo, Outcome 5 Adverse events.

Comparison 1 5‐ASA compared to placebo, Outcome 6 Withdrawals due to adverse events.

Comparison 1 5‐ASA compared to placebo, Outcome 7 Serious adverse events.

Comparison 1 5‐ASA compared to placebo, Outcome 8 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.
| 5‐ASA compared to placebo for maintenance of medically‐induced remission in Crohn's disease | ||||||
| Patient or population: patients with maintenance of medically‐induced remission in Crohn's disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | 5‐ASA compared to placebo | |||||
| Relapse, drop‐outs classed as relapse, grouped by length of follow‐up ‐ 12 months | 535 per 10001 | 525 per 1000 | RR 0.98 | 2014 | ⊕⊕⊕⊝ | |
| Relapse, drop‐outs classed as relapse, grouped by length of follow‐up ‐ 24 months | 679 per 10003 | 672 per 1000 | RR 0.99 | 161 | ⊕⊕⊝⊝ | |
| Relapse, drop‐outs classed as relapse, grouped by length of follow‐up ‐ Pediatric | 688 per 10003 | 736 per 1000 | RR 1.07 | 132 | ⊕⊕⊕⊝ | |
| Adverse events | 329 per 10001 | 346 per 1000 | RR 1.05 | 1814 | ⊕⊕⊝⊝ | |
| Withdrawals due to adverse events | 130 per 10001 | 144 per 1000 | RR 1.11 | 1833 | ⊕⊕⊝⊝ | |
| Serious adverse events | 7 per 10001 | 10 per 1000 | RR 1.43 | 576 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of meta‐analysis, based on included trials. | ||||||
| Study | Total Unknown ‐ n(%) | 5‐ASA unknown ‐ n(%) | Placebo unknown ‐ n(%) |
| Anonymous 1990 | 55 (22%) | 23 (19%) | 32 (26%) |
| Arber 1995 | 10 (17%) | 6 (21%) | 4 (13%) |
| Cezard 2009 | 30 (23%) | 21 (31%) | 9 (14%) |
| De Franchis 1997 | 17 (14%) | 8 (14%) | 9 (15%) |
| Gendre 1993 (Eng) | 42 (26%) | 23 (29%) | 19 (23%) |
| Mahmud 2001 | 82 (25%) | 55 (33%) | 27 (17%) |
| Modigliani 1996 | 44 (34%) | 17 (26%) | 27 (42%) |
| Prantera 1992 | 15 (12%) | 10 (16%) | 5 (8%) |
| Sutherland 1997 | 92 (31%) | 47 (33%) | 45 (30%) |
| Thompson 1995 | 98 (34%) | 52 (38%) | 46 (31%) |
| Wellman 1988 | 2 (3%) | 2 (6%) | 0 (0%) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 12 months | 11 | 2014 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.07] |
| 1.2 24 months | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| 1.3 Pediatric | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 2 Sensitivity analysis ‐ Relapse, drop‐outs classed as relapse, grouped by length of follow‐up, random effects Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 12 months | 11 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 2.2 24 months | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.23] |
| 2.3 Pediatric | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.86, 1.33] |
| 3 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 12 Months | 10 | 1438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.01] |
| 3.2 24 Months | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.29] |
| 3.3 Pediatric | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.31] |
| 4 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up, random effects Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 12 Months | 10 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.05] |
| 4.2 24 Months | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.29] |
| 4.3 Pediatric | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.31] |
| 5 Adverse events Show forest plot | 10 | 1814 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.95, 1.17] |
| 6 Withdrawals due to adverse events Show forest plot | 10 | 1833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.88, 1.38] |
| 7 Serious adverse events Show forest plot | 3 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.24, 8.44] |
| 8 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up Show forest plot | 11 | 2014 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.16] |

