Suplementos de selenio en adultos gravemente enfermos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Method of randomization: states stratified randomization only | |
| Participants | Location: intensive care unit, Klinikum Innenstadt, University of Munich, Germany | |
| Interventions | Timing of intervention: from day of admission to intensive care for additional supplementation for nine days | |
| Outcomes | Length of follow up: until discharge | |
| Notes | Request for further details of interventions sent 24th October 2003, reply received 17th November 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Method of randomisation: states randomised but no further details | |
| Participants | Location: 11 independent German intensive care units | |
| Interventions | Timing of intervention: admission into study after diagnosis within 24 hours, study treatment beginning within 1 hour after inclusion | |
| Outcomes | Length of follow up: 28 days | |
| Notes | Emailed 8th August 2006 asking for further details on participants with all infections, length of ventilation, total numbers randomised to each group, and further details of the randomisation process. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Method of randomization: concealed | |
| Participants | Location: surgical intensive care unit (ICU) of the Central Hospitalier Universitaire Vaudois, Lausanne, Switzerland | |
| Interventions | Timing of intervention: from day of admission for five days | |
| Outcomes | Length of follow up: appears followed up until died or left hospital, maximum length of stay 249 days | |
| Notes | Intention to treat data taken from paper in Nutrition Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Method of randomization: states randomized but no further details | |
| Participants | Location: hospital, Rostock, Germany | |
| Interventions | Timing of intervention: unclear ?8 days | |
| Outcomes | Length of follow up: | |
| Notes | Request for further details on dose of selenium given sent 20th October 2003. Letter returned as author no longer at address in publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Method of randomisation: states randomised but no further details | |
| Participants | Location: medical centre, Chemnitz, Germany | |
| Interventions | Timing of intervention: start unclear, given until discharged | |
| Outcomes | Length of follow up: until discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Method of randomisation: states randomised but no further details | |
| Participants | Location: intensive care unit, Liverpool, UK | |
| Interventions | Timing of intervention: within 24 hours of admission to intensive care and within 72 hours since diagnosis of sepsis, given until discharged | |
| Outcomes | Length of follow up: 28 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Method of randomization: states randomized list but no further details | |
| Participants | Location: 28 Japanese neurosurgical and neurological units | |
| Interventions | Timing of intervention: started within 12 hours of middle cerebral artery occlusion, given for 14 days | |
| Outcomes | Length of follow up: | |
| Notes | Request for further details of denominators and infections sent October 22nd 2003, no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Method of randomization: states randomized lists but no further details | |
| Participants | Location: 84 Japanese neurosurgical units | |
| Interventions | Timing of intervention: started within 96 hours of subarachnoid haemorrhage, given for 14 days | |
| Outcomes | Length of follow up: 3 months | |
| Notes | Request for further details of unpublished trial mentioned in main trial report, and numbers of patients with infections sent 21st October 2003, no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Method of randomization: states randomized lists but no further details | |
| Participants | Location: 68 Japanese neurological and neurosurgical units | |
| Interventions | Timing of intervention: started within 48 hours of stroke, given for 14 days | |
| Outcomes | Length of follow up: 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Method of randomization: states randomized but no further details | |
| Participants | Location: university hospital, Dresden, Germany | |
| Interventions | Timing of intervention: start unclear, given for 28 days | |
| Outcomes | Length of follow up: 28 days | |
| Notes | Request for details of denominators and infections sent 21st October 2003, no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Randomized trial in adults with burns; selenium part of trace element supplement evaluated, which also contained copper and zinc | |
| Randomized trial in adults with burns; selenium part of trace element supplement evaluated, which also contained copper and zinc | |
| Randomized trial in adults with cardiac surgery; selenium part of antioxidant supplement evaluated. | |
| Combined results of Berger 2004 and Berger 1998 | |
| Randomized trial in adults with cardiac surgery, myocardial infarction, trauma or subarachnoid haemorrhage; selenium part of antioxidant supplement evaluated. | |
| Not randomized trial, not adults | |
| Randomized trial of antioxidant therapy (including selenium) versus placebo in trauma patients | |
| Not randomized trial, not critical care | |
| Randomized, crossover trial of antioxidant therapy (including selenium) versus placebo in the prevention of recurrence of pancreatitis | |
| Randomized trial of micronutrients (including selenium) versus placebo in patients undergoing elective aneurysmectomy | |
| Not randomized trial, selenium supplementation in acute pancreatitis |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | SIGNET trial (Scottish multicentre trial of glutamine and selenium supplemented parenteral nutrition for critically ill patients) |
| Methods | |
| Participants | 500 patients, on intensive care units, requiring at least half nutritional requirements by parenteral route, aged 16 years and over |
| Interventions | Factorial design with glutamine containing versus non‐glutamine containing parenteral nutrition for 7 days, with or without 500 mcg/d selenium as sodium selenite for 7 days |
| Outcomes | Participants with new infections, length of stay in intensive care, mortality, infections, days of antibiotic use, duration of parenteral nutrition, alive ventilator‐free days, acute hospital length of stay, quality of life, economic evaluation |
| Starting date | June 2004, recruitment due to finish August 2008 |
| Contact information | Dr Peter Andrews; Anaesthetics, Intensive Care and Pain Medicine; University of Edinburgh, Western General Hospital, Crewe Road, Edinburgh, EH24 2XU, UK |
| Notes |
| Trial name or title | Forty centre, double‐blind, placebo‐controlled trial |
| Methods | |
| Participants | 394 patients with acute non‐lacunar stroke (cardio‐embolic or atherothrombotic infarction < 24 hours) |
| Interventions | Ebselen 150 mg twice daily or placebo started within 24 hours of onset for 14 days |
| Outcomes | Glasgow Outcome Scale three months post stroke, National Institute of Health Stroke Scale and Barthel Index scores at one and three months |
| Starting date | March 2000, recruitment finished September 2002 |
| Contact information | Takanori Yamaguchi, for the Ebselen Study Group, National Cardiovascular Center, Osaka, Japan |
| Notes | Letter requesting further details sent 20th October 2003. Reply received 3rd November 2003, giving further details of inclusion criteria and trial intervention from Dr T Motohashi, Daiichi Pharmaceutical Co Ltd. Email sent to Dr Motohashi requesting results of trial 12th June 2006, reply received 19th June 2006 stating that publication is still planned. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by duration (sodium selenite) Show forest plot | 7 | 476 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.53, 1.06] |
| Analysis 1.1  Comparison 1 Selenium versus no selenium, Outcome 1 Mortality by duration (sodium selenite). | ||||
| 1.1 Selenium 28 day | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.43, 1.17] |
| 1.2 Selenium 90 day | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.35, 2.14] |
| 2 Selenium mortality ICU and pancreatitis (sodium selenite) Show forest plot | 7 | 476 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.53, 1.06] |
| Analysis 1.2  Comparison 1 Selenium versus no selenium, Outcome 2 Selenium mortality ICU and pancreatitis (sodium selenite). | ||||
| 2.1 General intensive care patients | 5 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.96] |
| 2.2 Acute pancreatitis | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.01, 12.30] |
| 3 Mortality by duration (ebselen) Show forest plot | 3 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.35] |
| Analysis 1.3  Comparison 1 Selenium versus no selenium, Outcome 3 Mortality by duration (ebselen). | ||||
| 3.1 Ebselen 30 day | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.53, 5.95] |
| 3.2 Ebselen 3 month | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of infected participants Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Selenium versus no selenium, Outcome 1 Number of infected participants. | ||||
| 1.1 Selenium | 3 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.23] |
| 1.2 Ebselen | 3 | 685 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with adverse event Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Selenium versus no selenium, Outcome 1 Number of participants with adverse event. | ||||
| 1.1 Selenium | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.40, 1.43] |
| 1.2 Ebselen | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.40, 3.36] |
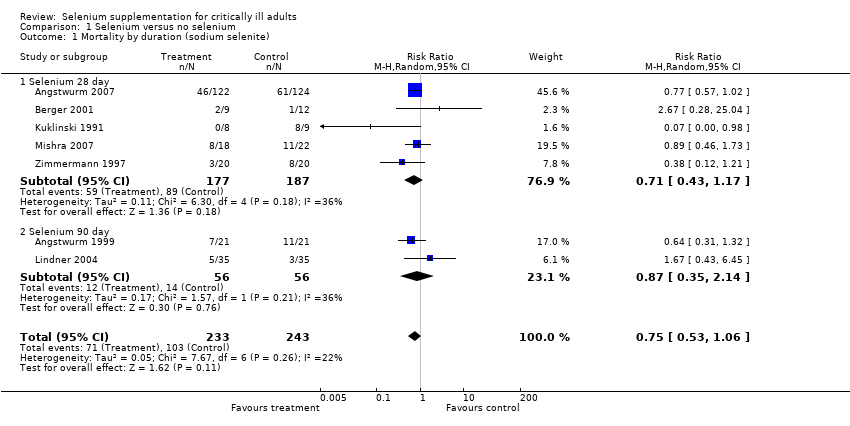
Comparison 1 Selenium versus no selenium, Outcome 1 Mortality by duration (sodium selenite).

Comparison 1 Selenium versus no selenium, Outcome 2 Selenium mortality ICU and pancreatitis (sodium selenite).

Comparison 1 Selenium versus no selenium, Outcome 3 Mortality by duration (ebselen).

Comparison 2 Selenium versus no selenium, Outcome 1 Number of infected participants.
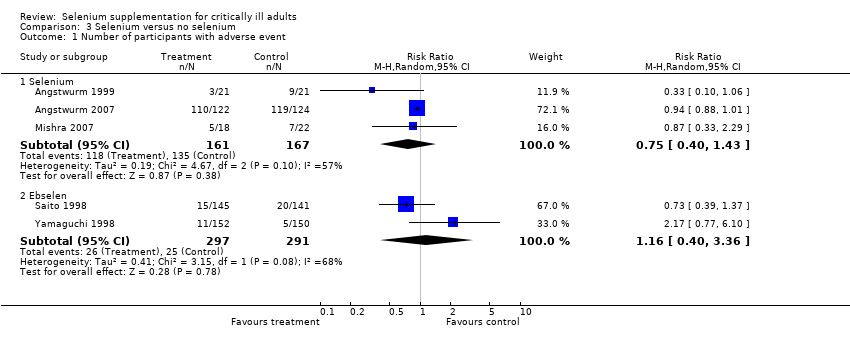
Comparison 3 Selenium versus no selenium, Outcome 1 Number of participants with adverse event.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by duration (sodium selenite) Show forest plot | 7 | 476 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.53, 1.06] |
| 1.1 Selenium 28 day | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.43, 1.17] |
| 1.2 Selenium 90 day | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.35, 2.14] |
| 2 Selenium mortality ICU and pancreatitis (sodium selenite) Show forest plot | 7 | 476 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.53, 1.06] |
| 2.1 General intensive care patients | 5 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.96] |
| 2.2 Acute pancreatitis | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.01, 12.30] |
| 3 Mortality by duration (ebselen) Show forest plot | 3 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.35] |
| 3.1 Ebselen 30 day | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.53, 5.95] |
| 3.2 Ebselen 3 month | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of infected participants Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Selenium | 3 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.23] |
| 1.2 Ebselen | 3 | 685 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with adverse event Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Selenium | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.40, 1.43] |
| 1.2 Ebselen | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.40, 3.36] |

