مقایسه ونتیلاسیون با حجم هدفمند در برابر ونتیلاسیون با فشار محدود در نوزادان
چکیده
پیشینه
آسیبهای ناشی از اتساع بیش از حد ریهها (lung overdistension) (ولوتراما (volutrauma)) در پیشرفت دیسپلازی برونکوپولمونری (bronchopulmonary dysplasia; BPD) دخیل هستند. مدهای (الگوهای) مدرن ونتیلاسیون نوزدان میتوانند مجموعهای از حجم جاری را به عنوان یک جایگزین برای ونتیلاسیون مرسوم با فشار محدود (pressure‐limited ventilation; PLV) با استفاده از فشار بادشدن ثابت (fixed inflation pressure) در نظر بگیرند. هدف از ونتیلاسیون با حجم هدفمند (volume‐targeted ventilation; VTV) تولید حجم جاری پایدارتر به منظور کاهش آسیب ریوی و تثبیت فشار جزئی دیاکسید کربن (pCO2) است.
اهداف
تعیین اینکه VTV در مقایسه با PLV منجر به کاهش نرخ مرگومیر و مرگومیر یا BPD در نوزادان تازه متولد شده میشود یا خیر و اینکه استفاده از VTV پیامدهایی مانند نشت هوا، یافتههای اولتراسوند مغزی و تکامل سیستم عصبی را تحت تاثیر قرار میدهد یا خیر.
روشهای جستوجو
ما از استراتژی جستوجوی استاندارد گروه نوزادان در کاکرین برای جستوجو در پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL؛ شماره 12، 2016)؛ MEDLINE via PubMed (از 1966 تا 13 ژانویه 2017)؛ Embase (از 1980 تا 13 ژانویه 2017) و CINAHL (از 1982 تا 13 ژانویه 2017) استفاده کردیم. همچنین بانکهای اطلاعاتی کارآزماییهای بالینی، مجموعه مقالات کنفرانس و فهرست منابع مقالات بازیابی شده را برای یافتن کارآزماییهای تصادفیسازی و کنترل شده و کارآزماییهای شبه‐تصادفیسازی شده جستوجو کردیم. برای به دست آوردن اطلاعات تکمیلی با محققان اصلی مطالعات تماس گرفتیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و شبه‐تصادفیسازی شده و کنترل شده که به مقایسه VTV در برابر PLV در نوزادان با سن جنینی کمتر از 44 هفته پرداختند و پیامدهای بالینی مرتبط را گزارش کردند.
گردآوری و تجزیهوتحلیل دادهها
با استفاده از متدولوژی کاکرین، خطر سوگیری (bias) را در هر کارآزمایی ارزیابی کردیم. کیفیت شواهد را برای هر پیامد با استفاده از سیستم درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) بررسی کردیم. مورتالیتی، نرخ BPD، پیامدهای بالینی کوتاه‐مدت و پیامدهای طولانیمدت وابسته به تکامل سیستم عصبی را جدولبندی کردیم.
آمار: برای پیامدهای گروهبندی شده، تخمینهای معمولی را با خطرات نسبی (RR)، تفاوتهای خطر (RD) و تعداد افراد مورد نیاز جهت درمان تا حصول یک پیامد مثبت بیشتر (number needed to treat for an additional beneficial outcome; NNTB) محاسبه کردیم. در متغیرهای پیوسته، تخمینهای معمولی را با تفاوتهای میانگین (MD) محاسبه کردیم. از 95% فواصل اطمینان (CI) استفاده کردیم و یک مدل اثر‐ثابت را برای انجام متاآنالیز (meta‐analysis) در نظر گرفتیم.
نتایج اصلی
بیست کارآزمایی تصادفیسازی شده معیارهای ورود ما را داشتند؛ 16 کارآزمایی موازی (977 نوزاد) و چهار کارآزمایی متقاطع (88 نوزاد). در هیچ مطالعهای کورسازی انجام نشد و کیفیت شواهد مربوط به پیامدها از متوسط تا پائین متفاوت ارزیابی شد.
ما تفاوتی را در پیامد اولیه، مرگومیر پیش از ترخیص از بیمارستان، بین مدهای VTV در برابر مدهای PLV نیافتیم (RR معمولی: 0.75؛ 95% CI؛ 0.53 تا 1.07؛ شواهد با کیفیت پائین). با این حال، شواهد با کیفیت متوسطی وجود داشت که نشان داد استفاده از مدهای VTV منجر به کاهش پیامد اولیه، مرگومیر یا BPD در هفته 36 بارداری (RR معمولی: 0.73؛ 95% CI؛ 0.59 تا 0.89؛ NNTB معمولی: 8؛ 95% CI؛ 5 تا 20) و پیامدهای ثانویه زیر شد: نرخ پنوموتوراکس (pneumothorax) (RR معمولی: 0.52؛ 95% CI؛ 0.31 تا 0.87؛ NNTB معمولی: 20؛ 95% CI؛ 11 تا 100)، میانگین روزهای استفاده از ونتیلاسیون مکانیکی (MD: ‐1.35 روز؛ 95% CI؛ 1.83‐ تا 0.86‐)، نرخ هیپوکربی (hypocarbia) (RR معمولی: 0.49؛ 95% CI؛ 0.33 تا 0.72؛ NNTB معمولی: 3؛ 95% CI؛ 2 تا 5)، نرخ هموراژی داخل بطنی درجه 3 یا 4 (RR معمولی: 0.53؛ 95% CI؛ 0.37 تا 0.77؛ NNTB معمولی: 11؛ 95% CI؛ 7 تا 25) و پیامد ترکیبی لوکومالاسی پریونتریکولار (لوکومالاسی پیرامونبطنی) (periventricular leukomalacia) با یا بدون هموراژی داخل بطنی درجه 3 یا 4 (RR معمولی: 0.47؛ 95% CI؛ 0.27 تا 0.80؛ NNTB معمولی: 11؛ 95% CI؛ 7 تا 33). مدهای VTV با افزایش پیامدهای جانبی همراه نبود.
نتیجهگیریهای نویسندگان
نوزادان ونتیله شده با مد VTV، نرخ مرگومیر یا BPD، پنوموتوراکس، هیپوکربی، پاتولوژیهای شدید اولتراسوند کرانیال و مدت زمان استفاده از ونتیلاسیون پائینتری را در مقایسه با نوزادان ونتیله شده با مد PLV داشتند. برای تشخیص اینکه مد VTV باعث بهبود پیامدهای مربوط به تکامل سیستم عصبی میشود یا خیر و همچنین برای مقایسه و اصلاح استراتژیهای VTV، مطالعات بیشتری مورد نیاز هستند.
PICOs
خلاصه به زبان ساده
مقایسه مدهای ونتیلاسیون با حجم هدفمند (volume‐targeted ventilation modes) با مدهای ونتیلاسیون مرسوم با فشار محدود (traditional pressure‐limited ventilation modes) برای نوزادان تازه متولد شده
سوال مطالعه مروری آیا درمان نوزادان استفاده کننده از یک استراتژی هدف قرار دادن حجم بادشدگی به جای فشار بادشدگی منجر به کاهش نرخ مرگومیر یا آسیب ریه (یا هر دو) در میان این نوزادان میشود؟
پیشینه: نوزادان نارس ممکن است برای تنفس نیاز به کمک داشته باشند. خطر بروز مشکلات ریوی با افزایش نارس بودن (نوزادانی که زودهنگام متولد میشوند) افزایش مییابد. برای برخی از نوزادان، ونتیلاتور کمکی (دستگاه تنفس) میتواند منجر به حفظ زندگی شود؛ با این حال، ونتیلاتورها ممکن است به ریههای نارس نوزاد آسیب برسانند. بهطور مرسوم، ونتیلاتورها برای نوزادان در مد ونتیلاسیون با فشار محدود استفاده شده، که این فشار، مقادیر متغیر هوا را وارد ریه میکند. مدهای ونتیلاسیون جدید با حجم هدفمند با هدف کاهش آسیب ریهها از طریق کنترل مقدار هوای وارد شده به ریهها با هر بادشدگی ایجاد شدهاند.
ویژگیهای مطالعه: در یک جستوجو که تا ژانویه 2017 بهروز شده، نویسندگان مرور 20 مطالعه را برای ورود به این مرور شناسایی کردند. شانزده مطالعه (977 نوزاد)، دو گروه جداگانه از نوزادان تحت درمان با مد ونتیلاسیون با حجم هدفمند را در مقایسه با مد ونتیلاسیون با فشار محدود مقایسه کردند. در چهار مطالعه (84 نوزاد)، این نوزادان با هر دو مد ونتیلاسیون در یک طرح متقاطع تحت درمان قرار گرفتند (که در آن نوزادان از یک ونتیلاسیون با یک روش استفاده میکردند و سپس با روش دوم جایگزین میشدند). اکثر این مطالعات دارای کیفیت متوسط تا پائین بودند و هیچ کدام از آنها برای ارزیابی درمان کورسازی نشدند. مهمترین نتایج این مرور بر اساس دادههای به دست آمده از هشت تا 12 مطالعه شامل 584 تا 771 نوزاد بود.
نتایج کلیدی: نوزادانی که با استفاده از مدهای ونتیلاسیون با حجم هدفمند تنفس میکنند، بیشتر احتمال دارد که بقای بدون آسیب ریوی داشته باشند. آنها برای مدت کوتاهی نیاز به ونتیلاتور کمکی داشتند و کمتر احتمال داشت مبتلا به پنوموتوراکس (pneumothorax) شوند (وضعیتی که در آن هوا از ریه به داخل قفسه سینه نفوذ میکند). آنها دارای سطوح پایدارتر دیاکسید کربن در خون بودند، و اختلالات اولتراسوند مغزی کمتری داشتند. هیچ شواهدی وجود نداشت که نشان دهد مدهای با حجم هدفمند نسبت به مدهای مرسوم با فشار محدود، بیشتر احتمال دارد که به نوزاد آسیب برسانند. پژوهشهای بیشتری لازم است تا بدانیم که مدهای با حجم هدفمند منجر به بهبود رشد حرکتی و عقلی نیز میشوند یا خیر. همچنین پژوهشهای بیشتری برای مقایسه تکنیکهای مختلف حجم هدفمند مورد نیاز است.
کیفیت شواهد: کیفیت پائین تا متوسط بود زیرا هیچ یک از مطالعات کورسازی نشده بودند و در رابطه با طراحی برخی از این مطالعات مشکلاتی وجود داشت.
Authors' conclusions
Summary of findings
| Volume‐targeted ventilation compared to pressure‐limited ventilation | ||||||
| Patient or population: neonates up to 44 weeks' postmenstrual age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with PLV | Risk with VTV | |||||
| Death before discharge from hospital | Study population | RR 0.75 | 771 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates. 95% CI < 0.75. | |
| 163 per 1000 | 122 per 1000 | |||||
| Death or BPD (36 weeks) | Study population | RR 0.73 | 584 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 458 per 1000 | 334 per 1000 | |||||
| Duration of positive pressure ventilation (days) | MD of positive pressure ventilation (days); PLV group 0 | MD 1.35 lower | ‐ | 736 | ⊕⊕⊕⊝ | Unblinded studies. |
| Pneumothorax | Study population | RR 0.52 (0.31 to 0.87) | 825 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 88 per 1000 | 46 per 1000 (27 to 77) | |||||
| IVH grade 3‐4 | Study population | RR 0.53 (0.37 to 0.77) | 712 (10 RCTs) | ⊕⊕⊕⊝ | Unblinded studies. | |
| 184 per 1000 | 97 per 1000 (68 to 141) | |||||
| IVH grade 3‐4 or PVL | Study population | RR 0.47 (0.27 to 0.80) | 441 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 164 per 1000 | 77 per 1000 (44 to 131) | |||||
| BPD (supplemental oxygen at 36 weeks) | Study population | RR 0.68 (0.53 to 0.87) | 620 | ⊕⊕⊝⊝ | Unblinded studies. Possible publication bias based on funnel plot. | |
| 346 per 1000 | 235 per 1000 (183 to 301) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPD: bronchopulmonary dysplasia; CI: confidence interval; IVH: intraventricular haemorrhage; MD: mean difference; PLV: pressure‐limited ventilation; PVL: periventricular leukomalacia; RCT: randomised controlled trial; RR: risk ratio; VTV: volume‐targeted ventilation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Volume‐targeted ventilation compared to pressure‐limited ventilation | ||||||
| Patient or population: neonates up to 44 weeks' postmenstrual age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with PLV | Risk with VTV | |||||
| Death or BPD (28 days) | Study population | RR 0.87 (0.64 to 1.18) | 149 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 536 per 1000 | 467 per 1000 (343 to 633) | |||||
| Failure of mode of ventilation | Study population | RR 0.69 | 445 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 242 per 1000 | 167 per 1000 | |||||
| Addition of neuromuscular paralysis where previously not paralysed | Study population | RR 0.32 | 75 | ⊕⊕⊝⊝ | Unblinded studies. Small numbers of participants. | |
| 179 per 1000 | 57 per 1000 | |||||
| Duration of positive pressure ventilation (log data) | The mean duration of IPPV (log data) was 0 | MD 0.08 lower | ‐ | 381 | ⊕⊕⊝⊝ | Unblinded studies. Heterogeneity of study results. |
| Inspired oxygen concentration % (study definition) | The mean difference in inspired oxygen concentration %; PLV group 0 | The mean inspired oxygen concentration % was 0.92 lower (2.08 lower to 0.24 higher) in VTV group | ‐ | 324 | ⊕⊕⊕⊝ | Unblinded studies. |
| Any pH < 7.25 | Study population | RR 0.80 | 98 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 380 per 1000 | 304 per 1000 | |||||
| Hypocarbia pCO2 < 35 mmHg/4.7 kPa | Study population | RR 0.49 (0.33 to 0.72) | 98 | ⊕⊕⊕⊝ | Unblinded studies. Small trials, but large effect and biologically plausible. | |
| 720 per 1000 | 353 per 1000 (238 to 518) | |||||
| Respiratory acidosis pH < 7.25 and pCO2 > 60 mmHg/8 kPa | Study population | RR 0.93 | 98 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 240 per 1000 | 223 per 1000 | |||||
| Either hypocarbia or respiratory acidosis | Study population | RR 0.68 | 37 | ‐ | No quality assessment possible. | |
| 1889 per 1000 | 1000 per 1000 | |||||
| Patent ductus arteriosus | Study population | RR 0.95 | 754 | ⊕⊕⊝⊝ | Unblinded studies. Variable diagnostic practices employed. | |
| 391 per 1000 | 371 per 1000 | |||||
| Air leak (any) | Study population | RR 0.79 | 374 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 117 per 1000 | 92 per 1000 | |||||
| Pulmonary interstitial emphysema | Study population | RR 1.21 | 430 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. | |
| 65 per 1000 | 79 per 1000 | |||||
| Any IVH | Study population | RR 0.82 | 445 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. | |
| 342 per 1000 | 281 per 1000 | |||||
| PVL | Study population | OR 0.43 (0.19 to 0.98) | 508 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 71 per 1000 | 32 per 1000 (14 to 69) | |||||
| Any IVH or PVL | Study population | RR 0.83 | 298 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 308 per 1000 | 256 per 1000 | |||||
| BPD (supplemental oxygen at 28 days) | Study population | RR 0.91 | 206 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. | |
| 354 per 1000 | 322 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPD: bronchopulmonary dysplasia; CI: confidence interval; IPPV: intermittent positive pressure ventilation; IVH: intraventricular haemorrhage; MD: mean difference; pCO2: partial pressure of carbon dioxide; PLV: pressure‐limited ventilation; PVL: periventricular leukomalacia; RCT: randomised controlled trial; RR: risk ratio; VTV: volume‐targeted ventilation. | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Mechanical ventilation remains an essential tool in the care of critically sick and very preterm infants, despite improvements in perinatal care including increased use of antenatal steroids and non‐invasive respiratory support. The Neonatal Research Network in the USA reported for the year 2012 that 82% of infants born before 29 weeks' gestation received conventional mechanical ventilation during their stay in the neonatal intensive care unit (Stoll 2015). The main indications for mechanical ventilation in preterm infants are respiratory distress syndrome (RDS), lung immaturity and poor respiratory drive. Although the respiratory difficulties resolve in most of these infants, studies show that around 40% of surviving infants 28 weeks' or less gestational age (GA) develop bronchopulmonary dysplasia (BPD) with oxygen dependency at 36 weeks' postmenstrual age (Stoll 2015; Stensvold 2017). The resulting burden of BPD includes increased duration of respiratory support and hospital stay, need for home oxygen, impaired neurodevelopmental outcome, more readmissions to hospital and increased mortality.
BPD was first reported in 1967 in a group of preterm infants who developed chronic lung disease after receiving ventilation and high oxygen concentration for RDS (Northway 1967). More recently, BPD has been defined as the requirement for supplemental oxygen at either 28 days' postnatal age (NIH 1979) or at 36 weeks' postmenstrual age (Shennan 1988). The current definition takes into account total duration of oxygen supplementation, need for positive pressure ventilation (PPV) or nasal continuous positive airway pressure (CPAP) and GA, in addition to oxygen dependency at 36 weeks' postmenstrual age (Jobe 2001).
BPD is characterised by the histopathological findings of impaired alveolarisation, altered pulmonary microvasculature and pulmonary fibrosis. The development of BPD has been linked to lung immaturity, intrauterine growth restriction (Bardin 1997; Gortner 1999), infection (Hannaford 1999), oxidant stress (Warner 1998), in‐utero inflammation (Watterberg 1996), and mechanical ventilation (Coalson 1999; Clark 2000). Ventilation strategies have been identified as potentially modifiable cause of BPD, and research has been devoted to developing ventilation strategies which avoid the overdistension, atelectasis and shear stresses that are thought to lead to lung injury and consequently BPD. The fact that lung injury in a preterm lamb model was demonstrated following only six large inflations immediately after birth (Bjorklund 1997), highlights the potential importance of early use of protective ventilation strategies in the neonate.
Description of the intervention
Mechanical ventilation is primarily used because an infant is failing to breathe adequately, leading to CO2 retention. CO2 removal is determined by tidal volume (VT) and respiratory or ventilator rate. Volume‐targeted ventilation (VTV) strategies aim to deliver a consistent VT. TV‐oriented modes have been in use in paediatric and adult practice for many years. However, the technological limitations of older ventilators precluded their use in preterm infants because they were unable to accurately deliver the small VT required when ventilating small preterm infants. Modern microprocessor‐controlled neonatal ventilators with flow sensors permit accurate measurement and delivery of a set VT. Earlier designs included a flow sensor built into the ventilator, however, with this design, VT measurements are affected by the compliance of the ventilator circuit. Newer designs include sensors that can be placed at the Wye piece between the ventilator circuit and the endotracheal tube (ETT). With appropriate software, the ventilators measure and control ventilator parameters to target the delivered VT, and reduce VT variability delivery compared with pressure‐limited ventilation (PLV) modes (Abubakar 2001).
When using a ventilator in a VTV mode, the clinician sets a target VT. Different VTV modes measure inflation VT, expired VT or both to control VT delivery. Expired VT is less affected by ETT leaks, and measuring both inspired and expired VT enables ETT leak to be quantified. There are many different forms of VTV. Depending on the ventilator design and the mode selected, the ventilator adjusts one or more of the peak inflation pressure (PIP), inflation time and inflation flow. Some ventilators offer more than one VTV mode. Thus, there are some differences between the mechanisms or algorithms by which different ventilators control and modify VT, but they all provide a similar volume‐targeted approach to newborn mechanical ventilation.
How the intervention might work
Traditionally, neonatologists treating infants with severe respiratory conditions have employed continuous flow, time‐cycled, PLV. In PLV mode, the assistance provided by the ventilator is controlled in two ways. The magnitude of each inflation is determined by the change in airway pressure (i.e. the difference between PIP and the baseline or positive end‐expiratory pressure (PEEP)). The VT for any inflation depends on both this pressure difference, which drives gas movement, and the lung compliance. Although VT is indirectly determined by the clinician when the PIP and PEEP are set, VT may not be consistent when the infant breathes, cries, splints, is apnoeic or when compliance and resistance change. For example, following administration of artificial surfactant, improved compliance may result in the delivery of increased VT if the PIP is not reduced.
In the past, there was concern about lung damage caused by high pressures ('barotrauma'). However, several studies have indicated that lung collapse and overdistension (or atelectasis and 'volutrauma') are the major instigators of inflammation in the preterm lung (Dreyfuss 1993; Dreyfuss 1998). This is supported by animal studies comparing high PIP in an animal model where a cast was used to reduce chest wall compliance and hence VT (Hernandez 1989). Histological examination demonstrated a significant reduction in lung inflammation in the animals that were protected from high VT. Further support came from a randomised controlled trial (RCT) comparing two ventilation strategies, high VT (12 mL/kg) versus low VT (6 mL/kg), in adults with acute lung injury. This study was stopped prematurely when interim analysis revealed a significant reduction in both mortality and duration of ventilation in low‐VT group (ARDS Network 2000). Lung compliance changes rapidly and substantially during the evolution and treatment of RDS (Hentschel 2002; Wheeler 2009). Ventilation strategies that adapt to these changes may enhance stability and reduce lung injury. Furthermore, avoiding rapid changes in the partial pressure of carbon dioxide in arterial blood (PaCO2) by maintaining stable minute volume ventilation may stabilise cerebral blood perfusion and reduce brain damage.
There is a paucity of information regarding the optimal VT for preterm infants. An observational study of VT values in infants weighing less than 800 g ventilated using VTV during the first three weeks of life reported obtaining acceptable blood gases using target VT of 5 mL/kg to 6 mL/kg with the Drager Babylog 8000plus (Keszler 2009). Other studies have suggested that a VT of 4 mL/kg or less may increase lung inflammation and work of breathing (Lista 2006; Patel 2009; Patel 2010; Chowdhury 2012). When selecting target VT for devices which measure VT at the ventilator (rather than at the Wye piece), allowance must be made for the additional compressible gas volume and compliance of the ventilator circuit (Cannon 2000; Al‐Majed 2004).
Why it is important to do this review
The uptake of VTV varies between countries and continents. Surveys have shown that 5% to 63% of neonatal units in Europe, Australia and New Zealand routinely use VTV modes and perceptions vary as to whether the use of VTV modes leads to improved outcomes (Sharma 2007; Klingenberg 2011; Van Kaam 2010). It is important to understand how outcomes of infants ventilated using VTV modes compare with those of infants ventilated using PLV modes.
Objectives
To determine whether VTV compared with PLV leads to reduced rates of death and death or BPD in newborn infants and to determine whether use of VTV affected outcomes including air leak, cranial ultrasound findings and neurodevelopment.
Methods
Criteria for considering studies for this review
Types of studies
We included only RCTs and quasi‐RCTs.
Types of participants
Participants were intubated newborn infants being mechanically ventilated with PPV at the time of study entry. Infants of all GAs up to 44 weeks' postmenstrual age and both muscle‐relaxed and non‐muscle‐relaxed infants were eligible.
Types of interventions
The review only included studies comparing ventilation using VTV modes versus ventilation using PLV modes.
Types of outcome measures
Primary outcomes
-
Death, defined in two ways:
-
death before discharge from the primary hospital;
-
death before two years' corrected age.
-
-
Death or BPD (BPD defined as need for supplemental oxygen requirement), assessed at two time points:
-
BPD at 28 days or death prior to 28 days;
-
BPD at 36 weeks' postmenstrual age or death prior to 36 weeks' postmenstrual age.
-
Secondary outcomes
-
Failure of mode of ventilation (clinical decision to change to different mode of ventilation).
-
Addition of neuromuscular paralysis where previously not paralysed.
-
Ventilation data:
-
days of PPV;
-
days of non‐invasive respiratory support;
-
total duration of respiratory support in days.
-
-
Markers of gas exchange as shown on arterial or capillary blood gas sampling:
-
any pH less than 7.25;
-
any episode of hypocarbia (partial pressure of carbon dioxide (pCO2) less than 35 mmHg/4.7 kPa);
-
any episode of respiratory acidosis (pH less than 7.25 with pCO2 greater than 60 mmHg/8 kPa).
-
-
Inspired oxygen concentrations (FiO2).
-
Patent ductus arteriosus (PDA).
-
Incidence of air leak:
-
overall incidence of air leak;
-
incidence of pneumothorax;
-
incidence of pulmonary interstitial emphysema (PIE).
-
-
Growth:
-
days to regain birth weight (BW);
-
grams weight gain per week until discharge.
-
-
Intracranial pathology:
-
all cranial ultrasound abnormalities (intraventricular haemorrhage (IVH) and periventricular leukomalacia (PVL)).
-
IVH;
-
cystic PVL.
-
-
Adverse neurosensory sequelae at two years:
-
cerebral palsy;
-
blindness;
-
deafness;
-
moderate to severe developmental delay as assessed on performance in formal neurodevelopmental testing (Bayley score, Wechsler Preschool and Primary Scale of Intelligence (WIPPSI), etc.).
-
-
Surviving infants with BPD:
-
BPD (supplemental oxygen requirement) at 28 days after birth;
-
BPD (supplemental oxygen requirement) at 36 weeks' postmenstrual age.
-
Modifications of these outcome measures (post hoc after viewing the available data):
-
Duration of PPV: measure was calculated in survivors only.
-
Failure of ventilatory mode: was clarified as a change from the assigned mode of ventilation within the study intervention period.
-
Days of non‐invasive respiratory support. The outcome was originally defined as days of CPAP. However, none of the included studies reported this consistently in an extractable fashion. Therefore, this outcome was not analysed.
-
IVH: collected outcomes for both total incidence of IVH and incidence of IVH grade 3 or 4.
-
Cystic PVL: most studies did not specify PVL. We included outcomes for studies reporting any PVL.
-
Data from studies reporting BPD rates were included for all participants when data on survivors were unavailable.
-
BPD at 28 days after birth: only four trials reported BPD at 28 days after birth (Piotrowski 1997; Lista 2004; Piotrowski 2007; Chowdhury 2013). The vast majority of infants included in these four trials were born before 32 weeks' gestation (Characteristics of included studies table). The definition of BPD (Jobe 2001) was based on diagnostic criteria, and it was only recommended to assess an infant for BPD at 28 days of age if the infant was born at or after 32 weeks' gestation. Thus, we did not find it appropriate to report BPD at 28 days in this updated review.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 12) in the Cochrane Library; MEDLINE via PubMed (1966 to 13 January 2017); Embase (1980 to 13 January 2017) and CINAHL (1982 to 13 January 2017) using the following search terms: (ventilation OR ventilator OR artificial respiration OR respiratory support) AND volume, plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization's International Trials Registry and Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry). The previous search was performed using the standard strategy of the Neonatal Review Group of the Cochrane Collaboration. MEDLINE (1966 to January 2010) was searched using the MeSH terms: infant, newborn and respiration, artificial and the text word: volume. These terms were also used in a search of the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, 2015, Issue 4) and CINAHL. there were no language restrictions. A review (1981 to 2015) of abstracts published by the Society for Pediatric Research and the European Society for Pediatric Research completed the literature search. This was combined with cross‐referencing of previous reviews, use of expert informants and newer additional resources such as ClinicalTrials.gov.
Data collection and analysis
We used the standard methods of the Neonatal Review Group of Cochrane. Two review authors (KW and CK) independently performed trial searches, assessments of methodology and extraction of data with comparison and resolution of any differences found at each stage.
For each included study, we collected information regarding method of randomisation, blinding, intervention, stratification and whether the trial was a single‐centre or multi‐centre study. We noted information regarding trial participants, including GA criteria, BW criteria and other inclusion or exclusion criteria. We analysed information on clinical outcomes, including death and BPD and other relevant secondary outcomes. We contacted trial authors to obtain supplementary data and to clarify issues.
Quality of evidence
We used the GRADE approach to assess the quality of evidence, as outlined in the GRADE Handbook (Schünemann 2013). Three review authors (CK, NM and KW) independently assessed the quality of the evidence for each of the primary and secondary outcomes. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create 'Summary of findings' tables to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
-
high: we are very confident that the true effect lies close to that of the estimate of the effect;
-
moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
-
low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect;
-
very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Selection of studies
We included all RCTs and quasi‐RCTs fulfilling the selection criteria. All review authors reviewed results of the search, separately selected studies for inclusion and resolved disagreements by discussion.
Data extraction and management
Two review authors (KW and CK) independently extracted, assessed and coded all data for each study, for studies newly added to this version of the review, using the same categories as in the previous version. We replaced any standard error of the mean reported with the corresponding standard deviation (SD) and resolved disagreements by discussion.
For each study, one review author (KW) entered final data into Review Manager 5 (RevMan 2014), and the other review authors (CK, NM, CM, PGD) checked the data. All review authors reviewed the analysis and draft manuscript.
Assessment of risk of bias in included studies
Three review authors (CK, KW, NM) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane 'Risk of bias' tool (Higgins 2011) for the following eight domains.
-
Random sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Intervention bias (other differences in ventilator management than purely VTV versus PLV).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Any other bias.
We resolved any disagreements by discussion or by discussion with a fourth review author (PGD). See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed meta‐analysis using Review Manager 5 (RevMan 2014). We analysed categorical data using risk ratio (RR), risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH). We analysed continuous data using mean difference (MD). We reported 95% confidence intervals (CIs) on all estimates. We applied the fixed‐effect model.
Unit of analysis issues
We combined individually randomised trials in a single meta‐analysis using the generic inverse variance method. We identified no cluster randomised trials.
Dealing with missing data
We requested supplemental information from authors when data were missing or unclear.
Assessment of heterogeneity
We estimated treatment effects reported by individual trials and examined heterogeneity among trials by inspecting forest plots and quantifying the impact of heterogeneity using the I² statistic. We graded the degree of heterogeneity as less than 25% (no heterogeneity), 25% to 49% (low heterogeneity), 50% to 75% (moderate heterogeneity) or greater than 75% (substantial heterogeneity), as recommended by the Cochrane Neonatal group. When there was evidence of apparent or moderate to substantial heterogeneity (I² greater than 50%), we explored possible causes using sensitivity analysis (e.g. differences between strict and hybrid studies), looking for evidence of bias or methodological differences between trials.
Assessment of reporting biases
Studies included in this review were performed between 1994 and 2014 (published between 1997 and 2016), across the three eras of when prospective trial registrations were not available, was suggested and is now mandatory. We requested possible trial registration number from authors of all studies. In studies where no prospective trial registration was available we defined the risk of reporting bias as unclear.
Data synthesis
We used the Mantel‐Haenszel method for estimates of typical RR, RD, NNTB and NNTH. When we judged meta‐analysis to be inappropriate, we analysed and interpreted outcomes from trials separately.
Subgroup analysis and investigation of heterogeneity
Three subgroup analyses were originally planned based on:
-
mode of VTV: in view of the differences between VTV modes, subgroups were defined according to:
-
volume‐controlled (VC) ventilation;
-
volume‐guaranteed (VG) ventilation;
-
-
age at recruitment into study: in view of possible differences in outcomes according to postnatal age at time of study recruitment, subgroups were defined according to:
-
early recruitment (i.e. commencement of ventilation strategy at birth or within the first four hours of life);
-
late recruitment (i.e. beyond four hours of age). This subgroup included trials in which VTV was tested as a rescue strategy);
-
-
maturity/BW of the infants: in view of the increased risk of BPD in the smallest/most immature infants, subgroups were defined according to:
-
BW, with a cut‐off of 1000 g;
-
GA, with a cut‐off of 30 weeks' gestation.
-
Modifications of these subgroup analyses
Subgroup analysis based on VTV mode was not performed. Since the original protocol was written (McCallion 2002), the range of available VTV modes has changed, and the suggested subgroup classification was not appropriate. We analysed all VTV modes together without attempting to subdivide them into different modes.
Subgroup analysis based on postnatal age at time of study recruitment was not performed as the vast majority of parallel studies all had early recruitment.
Subgroup analysis based on BW was performed.
Sensitivity analysis
For major outcomes, we performed sensitivity analyses by running the meta‐analysis both with strict studies (13 RCTs), in which volume targeting was the only difference in ventilator strategy or ventilator use between groups and hybrid studies (seven RCTs), in which the ventilators used or ventilator triggering modes were different in the two groups. We decided that there was a higher risk of bias (intervention bias) associated with hybrid studies. See further description of strict and hybrid studies under Results and Characteristics of included studies table. In outcomes with moderate to high heterogeneity, we performed analyses both with and without outlying studies as part of a sensitivity analysis.
Results
Description of studies
Results of the search
The previous version of this review identified 12 RCTs. Updated searches revealed 1877 records after duplicates were removed; after screening of 40 full‐text articles/studies, review authors identified eight new RCTs for inclusion in the review (Figure 1).
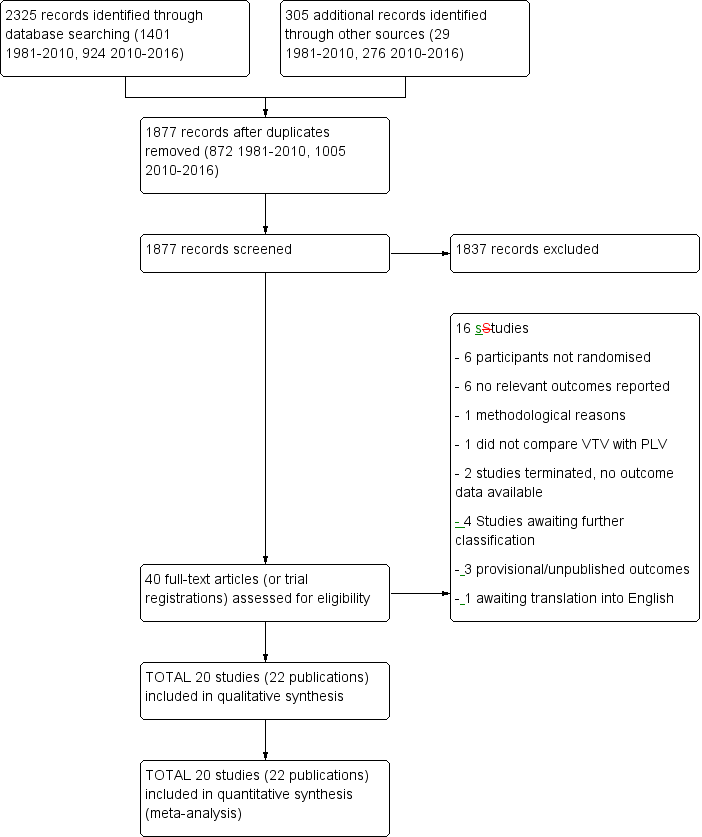
Study flow diagram: review update. PLV: pressure‐limited ventilation; VTV: volume‐targeted ventilation.
Included studies
Twenty RCTs (22 publications) met our inclusion criteria and reported one or more outcomes defined in our protocol. Sixteen were parallel studies, resulting in 18 publications (Piotrowski 1997; Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005; Singh 2006 (including two separate publications of follow‐up data from the inception cohort: Swamy 2008; Singh 2009); Cheema 2007; Piotrowski 2007; Zhou 2007; Liu 2011; Duman 2012; Chowdhury 2013; Guven 2013; Erdemir 2014; Bhat 2016). Four were within‐participant cross‐over studies (Herrera 2002; Hummler 2006; Polimeni 2006; Jain 2016). Details on all 20 included studies are described in the Characteristics of included studies table. In brief, we presented the following general information about the 20 studies.
-
Study sample size: ranging from 15 infants (Hummler 2006) to 212 infants (D'Angio 2005).
-
Inclusion criteria: varied from studies which included only smaller infants (less than 1200 g BW or less than 32 weeks' GA), larger infants (1200 g or greater), any infant less than 2500 g), or infants born at or near term.
-
Time of recruitment: 17 trials recruited infants in the early neonatal period (first three days after birth) (Piotrowski 1997; Sinha 1997; Herrera 2002; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005; Singh 2006; Cheema 2007; Piotrowski 2007; Zhou 2007; Liu 2011; Duman 2012; Chowdhury 2013; Guven 2013; Erdemir 2014; Bhat 2016). Three cross‐over trials enrolled preterm infants with mean postnatal age between 33 and 37 days (Hummler 2006; mean (SD) 33 (13) days of age; Polimeni 2006; mean (SD) 37 (17) days of age; Jain 2016; mean (SD) 33 (22) days of age). All 20 trials studied infants at less than 44 weeks' postmenstrual age.
-
Duration of intervention: for the parallel trials, the duration of intervention ranged from median 95 minutes (Cheema 2007) up to almost the full period of mechanical ventilation. For the cross‐over trials, duration of intervention period ranged from 60 minutes (Herrera 2002) up to 24 hours (Jain 2016).
-
Exclusion criteria: these were similar across trials and included the following: lethal congenital anomalies, muscle relaxation, suspected sepsis, severe IVH, asphyxia, pneumothorax and meconium aspiration. Some studies specified lack of arterial access or treatment with narcotics as additional exclusion criteria. Erdemir 2014 specifically noted that an ETT leak less than 20% as an exclusion criterion. We tried to clarify this but have not been able to verify whether this was an error. Antenatal steroids and surfactant were available in all participating units, although, in the studies by Zhou 2007 and Liu 2011, financial considerations influenced access.
-
Ventilators used in the trials: the VTV group used a range of ventilators, including the VIP Bird and Bird Gold, Siemens Servo 300, Draeger Babylog 8000plus, Stephanie Infant ventilator, AVEA CareFusion and SLE 5000. The ventilation settings were not always well described in each trial. Further details are shown in the Characteristics of included studies table.
We decided post hoc to define some of the studies as 'hybrid studies' (also used in the 2010 version of this review) with increased risk of bias based on the fact that in some trials volume targeting was not the only difference between study groups. The differences detected were:
-
different ventilators: three studies used different ventilators in each groups (Piotrowski 1997; Piotrowski 2007; Liu 2011). This is a potential source of bias;
-
different use of triggering:the use of triggering in one arm of the trial but not in the other is a potential source of bias (Greenough 2008). In the trial of Piotrowski 1997, the PLV group received non‐triggered intermittent mandatory ventilation, whereas the VTV groups received triggered ventilation. Sinha 1997 used an assist‐control (AC) mode in both arms, but the volume control arm used pressure‐triggering and the pressure limited arm used flow‐triggering;
-
different trigger modes: two studies used a mode where all inflations were triggered in the VTV group (pressure‐regulated volume control (PRVC) mode) and synchronised intermittent mandatory ventilation (SIMV) in the PLV group (D'Angio 2005; Piotrowski 2007). This difference in trigger modes is a potential source of bias (Greenough 2008); however, when the inflation rate in the SIMV mode is high (i.e. 50/minute to 60/minute), the difference between the two modes becomes less clinically important;
-
flow termination: in the studies by Nafday 2005 and Erdemir 2014, the VTV group received pressure support ventilation (PSV) with flow termination and the PLV group received SIMV without flow termination.
In view of these differences, we performed a sensitivity analysis of strict studies (both groups initially ventilated with similar modes/ventilators with VTV being to the only difference) versus hybrid studies (other differences between the groups; different ventilators, different use of triggering and different trigger modes).
Supplemental information: we requested raw data and supplemental information to clarify randomisation procedures, outcomes, permit more detailed analysis of duration of ventilation and facilitate subgroup analysis of infants weighing less than 1000 g. The review authors are grateful to the authors for making supplemental information available, details of which are described in the Characteristics of included studies table.
Excluded studies
We excluded 16 studies for the following reasons (see Characteristics of excluded studies table):
-
not randomised (Lista 2000; Abubakar 2001; Wach 2003; Abd El‐Moneim 2005; Shah 2013; Stefanescu 2015);
-
randomised, but did not report any of the outcomes specified in the protocol (Olsen 2002; Dotta 2004; Keszler 2004b; Ramirez‐Del Valle 2006; Colnaghi 2006; Sinha 2008);
-
randomised, but the PIP setting was the same in both arms, which may have interfered with the ventilator's capacity to deliver the set VT and hence affected the outcomes (Cheema 2001);
-
compared two different modes of VTV, and did not include a comparison with PLV (Unal 2014);
-
randomised, but later the studies were terminated (NCT00157989; NCT00295230).
Two studies are awaiting classification (Liu 2016; Miracle 2016; see Characteristics of studies awaiting classification table), and two studies are ongoing (ACTRN12609000986279; Salvia 2006; see Characteristics of ongoing studies table).
Risk of bias in included studies
Details of each study appear in the Characteristics of included studies table and in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation (selection bias): eight studies used block randomisation (Sinha 1997; Nafday 2005; D'Angio 2005; Singh 2006; Cheema 2007 (supplemental data); Piotrowski 2007 (supplemental data); Duman 2012; Guven 2013). Some trials used stratification by BW (D'Angio 2005; Nafday 2005; Singh 2006; Cheema 2007), GA (Lista 2004; Piotrowski 2007), centre (Lista 2004; D'Angio 2005) or a combination of these (D'Angio 2005; Lista 2004). Five trials did not specify the pattern of randomisation (Piotrowski 1997; Keszler 2004a; Zhou 2007; Liu 2011; Jain 2016).
Allocation concealment (selection bias): the studies by Piotrowski 1997; Sinha 1997; Herrera 2002; Keszler 2004a; Nafday 2005; D'Angio 2005; Hummler 2006; Singh 2006; Cheema 2007 (supplemental information); Duman 2012; Chowdhury 2013; Erdemir 2014; and Bhat 2016 used sealed envelopes for blinding of randomisation. Five trials did not specify blinding of randomisation (Lista 2004; Polimeni 2006; Zhou 2007; Liu 2011; Guven 2013).
Blinding
Blinding of participants and personnel (performance bias): none of the studies included in this review attempted to mask the carers to the intervention/group assignment.
Blinding of outcome assessment (detection bias): in the majority of studies, the allocated treatment method of each participant was known to those assessing the trial outcomes. In Sinha 1997, severity of lung disease was assessed by a radiographer blinded to the treatment assignment. In Singh 2006, information regarding masking during interpretation of cranial imaging was not reported. A questionnaire was used to determine neurodevelopmental follow‐up. The questionnaire administrator was masked to the original intervention group. D'Angio 2005 reported neurodevelopment outcomes at six to 18 months as assessed by a paediatric neurologist who was blinded to the treatment assignment (supplemental information).
Intervention bias (strict versus hybrid studies)
Some study designs had potential to be biased as they included comparisons between different ventilator devices and ventilator modes (triggering) in the VTV and the PLV groups. These studies are termed 'hybrid studies' in contrast to studies where this was not a problem ('strict studies'). Sensitivity analyses were performed exploring strict and hybrid studies separately.
Incomplete outcome data
Piotrowski 1997 excluded three out of 60 enrolled infants after randomisation, two who did not fulfil enrolment criteria and one for whom the allocated ventilator was unavailable. Outcome assessment was otherwise complete.
In the study by Lista 2004, there was an uneven distribution of participants between the VTV (30 infants) and PLV (23 infants) groups. Postrandomisation, seven infants were withdrawn because placental histology confirmed chorioamnionitis (supplemental data).
D'Angio 2005 randomised 213 infants, but one infant was erroneously enrolled without consent and immediately withdrawn from the study at the request of the parents. Follow‐up in the hospital was complete for the other 212 infants. However, data on brain ultrasound beyond the first week of life were not available for all infants, and PVL was assessed in only 173 infants. Neurodevelopmental follow‐up data at six to 18 months of age were available in 128 infants (64 from each group). These 128 infants represented 83% of the 154 participants that survived to discharge in one of the two study centres.
Singh 2006 randomised 110 infants, but one infant (randomised to the PLV mode) was excluded postrandomisation following diagnosis of a major congenital anomaly (trisomy 13). Follow‐up in the hospital was complete for the other 109 infants. Of the 94 infants who survived to discharge, mortality and follow‐up data at median age of 22 months were reported on 47/52 (90%) infants in the VTV group and 41/42 (98%) infants in the PLV group.
In the studies by Zhou 2007 and Liu 2011, the denominators for some outcomes were unclear due to inadequately reported completeness of follow‐up (unreported numbers of infant transfers to other hospitals). As the denominators were unclear in these studies, only outcomes reported during the intervention period were included in the meta‐analyses.
Selective reporting
There was only one trial with prospective trial registration (Jain 2016). Four other trials were registered retrospectively in a trial registry (Polimeni 2006; Cheema 2007; Chowdhury 2013; Bhat 2016). No other trials were registered.
Other potential sources of bias
There were imbalances in the study by Piotrowski 2007 in FiO2 in the first six hours of life, and surfactant use. In the published report, Piotrowski 2007 adjusted for this difference, but in this review, we used the unadjusted outcomes.
There were imbalances in the study by Chowdhury 2013 with regard to BW, GA and antenatal steroid use. Participants in the PLV group had lower median GA/BW than participants in the VTV group (median: GA/BW 26 weeks/856 g with PLV versus 28 weeks/1016 g with VTV).
The study by Guven 2013 randomised 90 participants; however, postrandomisation they excluded 15 participants in the PLV group and three participants in the VTV group. After contact with the authors, it seems that randomisation occurred before they had considered exclusion criteria and before parents had given consent.
Weaning strategies: in two trials, both arms were weaned using a PLV mode (Sinha 1997; Singh 2006).
Effects of interventions
See: Summary of findings for the main comparison Volume‐targeted compared to pressure‐limited ventilation: main findings; Summary of findings 2 Volume‐targeted compared to pressure‐limited ventilation: additional findings
The 16 randomised parallel trials recruited 977 infants who were included in the meta‐analysis. Two trials including 74 participants had an intervention period of 24 hours or less (Nafday 2005; Cheema 2007). The mean duration of mechanical ventilation reported by other studies in this review ranged from one to 26 days. We believed that the short duration of intervention in the trials of Cheema 2007 and Nafday 2005 meant that these trials had a reduced ability to detect differences in longer‐term outcomes such as BPD, compared to trials that maintained the two treatment groups for a least 72 hours. Therefore, we only included these two trials in pooled analysis of outcomes that occurred during the intervention period (e.g. blood gas analysis). The study by Bhat 2016 included infants born at or near term and duration of ventilation was short. From this study, we included only duration of ventilation and blood gas data. As noted in the 'Risk of bias' section, for the trials of Zhou 2007 and Liu 2011, we included only outcomes which occurred during the intervention period in the meta‐analysis. The Erdemir 2014 trial primarily investigated VTV versus PLV in the weaning phase. However, as the weaning phase contributed more than 80% of the total duration of ventilation in both groups, we decided to include longer‐term outcomes such as BPD and mortality.
We included outcomes from 11/16 parallel trials (including 771 participants) in meta‐analysis of outcomes beyond the intervention period (Piotrowski 1997; Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Singh 2006; Piotrowski 2007; Duman 2012; Chowdhury 2013; Guven 2013).
Four cross‐over trials recruited 88 infants (Herrera 2002; Hummler 2006; Polimeni 2006; Jain 2016). The only prespecified outcome able to be assessed from these studies was inspired oxygen concentration.
There was no disagreement between assessors regarding inclusion/exclusion of studies, quality assessment or data extraction. We pooled and analysed available data as listed below.
A planned subgroup analysis based on age at enrolment (planned before/after four hours) was not performed as the only study with exclusively early recruitment of all infants only studied infants until their first blood gas (Cheema 2007). Age at enrolment varied in the other trials. In the parallel trials, study enrolment mainly occurred within first 24 hours of life. In the cross‐over trials, Herrera 2002 studied participants at mean (range) five (two to nine) days of age. The other three cross‐over trials enrolled preterm infants with mean postnatal ages between 33 and 37 days (Hummler 2006; Polimeni 2006; Jain 2016).
Piotrowski 1997 and Singh 2006 reported outcomes for subgroup of infants weighing less than 1000 g. Authors of all parallel trials were approached for supplemental data of outcomes in infants weighing less than 1000 g. Data from Keszler 2004a; Lista 2004; and D'Angio 2005 were also included in this subgroup meta‐analysis.
Primary outcomes
Death before discharge from the primary hospital (outcomes 1.1 and 2.1)
Eleven trials (771 participants) provided data for death before discharge from the primary hospital (Sinha 1997; Piotrowski 1997; Keszler 2004a (supplemental data); Lista 2004; D'Angio 2005; Singh 2006; Piotrowski 2007; Duman 2012; Chowdhury 2013 (supplemental data); Guven 2013; Erdemir 2014). No individual study demonstrated a difference in mortality between VTV and PLV groups and the pooled analysis also showed no significant difference (typical RR 0.75, 95% CI 0.53 to 1.07; Analysis 1.1). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates). There was no significant difference in mortality for infants weighing less than 1000 g (typical RR 0.71, 95% CI 0.42 to 1.21; Analysis 2.1).
Death before two years' corrected age
None of the trials formally reported death before two years' corrected age, although Singh 2006 (reported in Singh 2009) reported mortality from discharge to follow‐up at a median age of 22 months. Overall, there were seven (12%) deaths in the VTV group versus 11 (21%) deaths in the PLV group (odds ratio 0.5, 95% CI 0.1 to 1.4; P = 0.13) (calculated by Singh 2009).
Death or bronchopulmonary dysplasia at 28 days age
Only four trials reported BPD at 28 days after birth (Piotrowski 1997; Lista 2004; Piotrowski 2007; Chowdhury 2013). However, none of them reported age of death (days) in those not surviving to discharge. Thus, we cannot report data for this outcome.
Death or bronchopulmonary dysplasia at 36 weeks' postmenstrual age (outcomes 1.2 and 2.2)
Eight trials (584 participants) reported the combined outcome of death or BPD at 36 weeks' postmenstrual age (Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Singh 2006; Duman 2012; Chowdhury 2013 (all supplemental data); Erdemir 2014). The trial by Duman 2012 was an outlier and reported a significant reduction in this outcome in the VTV group. No other individual trial reported a difference between groups. However, pooled meta‐analysis revealed a reduction in the combined outcome (typical RR 0.73, 95% CI 0.59 to 0.89; typical RD ‐0.12, 95% CI ‐0.20 to ‐0.05; typical NNTB 8, 95% CI 5 to 20; Analysis 1.2). There was moderate heterogeneity (I2 = 52%) between hybrid trials, but no heterogeneity between strict trials. Sensitivity analysis revealed that exclusion of the Duman 2012 study reduced heterogeneity between hybrid studies (I2 = 30%), but the overall RR of the remaining seven studies remained in favour of VTV (typical RR 0.77, 95% CI 0.62 to 0.95). We graded the quality of evidence for this outcome as moderate (unblinded intervention).
For infants weighing less than 1000 g, there was no difference between groups (typical RR 0.79, 0.62 to 1.01; Analysis 2.2).
Secondary outcomes
Failure of mode of ventilation (outcome 1.3)
Five trials (445 participants) provided data for failure of ventilatory mode (Sinha 1997; D'Angio 2005; Nafday 2005 (only data from the 24‐hour intervention period); Singh 2006; Chowdhury 2013). Cheema 2007 reported that no infants needed to be rescued with high‐frequency ventilation during the intervention period, but these data were not included in meta‐analysis due to the short intervention period (median 95 minutes, and before a blood gas analysis was available for the treating physician). Overall, there was no difference between groups, but there was a 'trend' towards less failure of primarily assigned ventilatory mode in the VTV group (typical RR 0.69, 95% CI 0.48 to 1.00; Analysis 1.3). Subgroup analysis for infants weighing less than 1000 g could not be performed. We graded the quality of evidence for this outcome as moderate (unblinded intervention).
Addition of neuromuscular paralysis where previously not paralysed (outcome 1.4)
Two trials reported addition of new neuromuscular paralysis (Piotrowski 1997; Keszler 2004a). Overall, there was no difference between groups (Analysis 1.4). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
Duration of positive pressure (endotracheal) ventilation (outcomes 1.5, 1.6, 2.3 and 2.4)
Twelve trials (736 participants) provided data for the duration of positive pressure (endotracheal) ventilation (Sinha 1997; Liu 2011; Guven 2013; and Erdemir 2014 presented data in their publications; supplemental data obtained from Piotrowski 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Singh 2006; Duman 2012; Chowdhury 2013; Bhat 2016).
The trials analysed data in survivors only, with the exception of Sinha 1997 and Liu 2011, where this information was unavailable. In the trial by Sinha 1997, only one participant died in each arm, and the results are likely to be similar. Methods of meta‐analysis assume normally distributed values, but reported data on duration of PPV were skewed. Meta‐analysis performed on the skewed data gave a mathematical MD of ‐1.35 days (95% CI ‐1.83 to ‐0.86) of reduced duration of ventilation using VTV (Analysis 1.5). There was low heterogeneity between studies. The trial by D'Angio 2005 was the only trial that reported a non‐significant increase in mean duration of ventilation in the VTV group. Sensitivity analysis revealed that exclusion of the D'Angio 2005 trial did not change the level of heterogeneity and did not affect the result of the meta‐analysis. We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
Geometrically normally distributed data were achieved by log transformation of supplemental raw data from five trials (381 participants) provided by Piotrowski 1997; Keszler 2004a; Lista 2004; D'Angio 2005; and Singh 2006 (Analysis 1.6). Using this method, after untransforming the log meta‐analysis (MD ‐0.08, 95% CI ‐0.16 to 0), the MD for ventilation with the VTV modes was 0.8 days (95% CI 0.7 to 1.0) shorter. There was evidence of heterogeneity (I2 = 52%) (strict studies 20%, hybrid studies 62%) in the subset of trials where this analysis was possible.
For infants weighing less than 1000 g, meta‐analysis of skewed data from the five trials (199 participants) did not show a statistically significant difference between groups (MD ‐0.82 days, 95% CI ‐4.43 to 2.80 days; Analysis 2.3). In this analysis, there was evidence of heterogeneity, particularly in the hybrid studies (I2 = 78%) (strict 0%, hybrid 92%). Using log transformed data, the MD corresponded to ‐0.01 (95% CI ‐0.12 to 0.10) fewer days of ventilation with VTV (Analysis 2.4; I2 = 83%) (strict 0%, hybrid 94%).
Other ventilation data including abnormal blood gas measurements (1.7 to 1.11 and 2.5 to 2.8)
Inspired oxygen concentration
Three parallel studies (Singh 2006 (reported in Swamy 2008); Cheema 2007; Zhou 2007) and four cross‐over studies (Herrera 2002; Hummler 2006; Polimeni 2006; Jain 2016) representing 332 infants reported inspired oxygen concentration. The oxygen targeting strategies varied; however, no trials reported a difference between groups. The study by Herrera 2002 used the same participant for multiple comparisons (VTV 3.0 mL/kg and 4.5 mL/kg versus PLV). Meta‐analysis was only performed using the measurements from the 4.5 mL/kg group (nine infants), and the eight infants in the 3.0 mL/kg were excluded from meta‐analysis, leaving 324 infants for meta‐analysis. Polimeni 2006 used different groups of participants for the comparisons of VTV 4.5 mL/kg with PLV, and VTV 6.0 mL/kg with PLV. During meta‐analysis, any statistical power gained by using an infant as their own control in a cross‐over trial is lost. Meta‐analysis showed no difference between groups (Analysis 1.7). We graded the quality of evidence for this outcome as moderate (unblinded intervention).
Blood gas data
We requested supplemental blood gas data for four trials (Keszler 2004a; Cheema 2007; Chowdhury 2013; Bhat 2016).
Keszler 2004a reported the frequency of blood gases falling outside the target range using the number of blood gases as the denominator. He found a reduced rate of hypocarbia (pCO2 less than 35 torr = mmHg) in blood gases from the VTV group versus PLV group (16/77 vs 29/80, p < 0.05). Supplemental data were analysed using the participant as the denominator (a participant event was defined as any out of range result).
Cheema 2007 reported the incidence of out of range PaCO2 (PaCO2 less than 5 kPa or PaCO2 greater than 7 kPa) and of hypocarbia (PaCO2 less than 5 kPa) on the first blood gas of 40 enrolled infants. Comparison of all infants showed no statistically significant difference, but for a post hoc subgroup analysis of infants at 26 to 33 weeks of gestation, there was a reported reduction for both outcomes. Supplemental data were analysed to identify the incidence of out of range CO2 by the criteria in this protocol (hypocarbia CO2 less than 35 mmHg, 4.7 kPa, hypercarbia CO2 greater than 60 mmHg, 8 kPa).
Chowdhury 2013 reported the number of episodes of hypocarbia per participant (PaCO2 less than 4.5 kPa) and reported fewer episodes of hypocarbia in the VTV group. Although the definition of hypocarbia used in the review is 4.7 kPa, we have included the data in the meta‐analysis.
Bhat 2016 (40 participants) reported that in the VTV group, there was a 'median of 1.5 (range 0.8 [sic]) episodes of hypocarbia' compared to a median of 4 (range 1 to 13) episodes of hypocarbia in the PLV group (P = 0.005), but data were not extractable for meta‐analysis. We did not receive raw data for meta‐analysis.
Any pH less than 7.25
Three trials (98 participants) reported data for any pH less than 7.25 (Keszler 2004a; Cheema 2007; Chowdhury 2013). Meta‐analysis showed no difference between groups (Analysis 1.8). Likewise, for infants weighing less than 1000 g, there was no difference between groups (Analysis 2.5). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
Hypocarbia (any PaCO2 less than 35 mmHg/4.7 kPa)
Three trials (98 participants) reported extractable data on hypocardia for meta‐analysis (Keszler 2004a; Cheema 2007; Chowdhury 2013). There was a significant reduction in rates of hypocarbia in the VTV group (typical RR 0.49, 95% CI 0.33 to 0.72; typical RD ‐0.38, 95% CI ‐0.54 to ‐0.22; typical NNTB 3, 95% CI 2 to 5; Analysis 1.9). For the subgroup of infants weighing less than 1000 g, there was no statistically significant difference between groups (Analysis 2.6). We graded the quality of evidence for this outcome as moderate (unblinded intervention, small trials but large effect and biologically plausible).
Respiratory acidosis (pH less than 7.25 and pCO2 greater than 60 mmHg/8 kPa)
Three trials (98 participants) reported data on respiratory acidosis (Keszler 2004a; Cheema 2007; Chowdhury 2013). There was no significant difference between groups (Analysis 1.10). Likewise, for the subgroup of infants weighing less than 1000 g, there was no difference between groups (Analysis 2.7). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
Either hypocarbia or respiratory acidosis
Two trials (37 participants) reported data on either hypocarbia or respiratory acidosis (Keszler 2004a; Cheema 2007). There was no difference between groups (Analysis 1.11). Likewise, for infants weighing less than 1000 g, there was no statistically significant difference between groups (Analysis 2.8). There was no quality assessment done as analysis included only 37 participants.
Patent ductus arteriosus (outcomes 1.12 and 2.9)
Ten trials (754 participants) reported data on PDA (Piotrowski 1997; Sinha 1997; Lista 2004; D'Angio 2005; Singh 2006; Piotrowski 2007; Duman 2012; Chowdhury 2013; Guven 2013; Erdemir 2014). The definition of PDA was not consistent, and the reported incidence varied between studies. There was no statistically significant difference in any of the individual trials or the pooled analysis (Analysis 1.12). Likewise, there was no difference for infants weighing less than 1000 g (Analysis 2.9). We graded the quality of evidence for this outcome as low (unblinded intervention, variable and unvalidated diagnostic criteria employed).
Air leak (outcomes 1.13 to 1.15 and 2.10 to 2.12)
Overall incidence of air leak (pneumothorax or pulmonary interstitial emphysema, or both)
Five trials (374 infants) reported data for overall incidence of any air leak (Piotrowski 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005 (supplemental data, only including events during intervention period)). Pooled data showed no statistically significant difference between groups (Analysis 1.13). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates). Likewise, for infants weighing less than 1000 g, there was no difference between groups (Analysis 2.10).
Incidence of pneumothorax
Thirteen trials (575 infants) reported data on incidence of pneumothorax (Piotrowski 1997; Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005 (supplemental data, only including events during intervention period); Singh 2006; Piotrowski 2007; Zhou 2007; Liu 2011; Duman 2012; Chowdhury 2013; Erdemir 2014). There was a significant reduction in infants ventilated using VTV modes (typical RR 0.52, 95% CI 0.31 to 0.87; typical RD ‐0.05, 95% CI ‐0.08 to ‐0.01; typical NNTB 20, 95% CI 12 to 100; Analysis 1.14). There were no concerns regarding heterogeneity between trials. We graded the quality of evidence for this outcome as moderate (unblinded intervention). In the subgroup of infants weighing less than 1000 g, there was no significant difference (Analysis 2.11) (Piotrowski 1997 (supplemental data); Lista 2004 (supplemental data)).
Incidence of pulmonary interstitial emphysema
Six trials (430 infants) reported incidence of PIE (Piotrowski 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005 (supplemental analysis of events occurring during intervention period); Piotrowski 2007). There was no difference for any study or for overall pooled data (Analysis 1.15). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates). Likewise, for infants weighing less than 1000 g, there was no difference between groups (Analysis 2.12).
Growth
None of the studies assessed time taken to regain BW or weight gain.
Intracranial pathology (outcomes 1.16 to 1.20 and 2.13 to 2.17)
Any intraventricular haemorrhage
Six trials (501 participants) reported data on any IVH (Piotrowski 1997; Keszler 2004a (supplemental data); D'Angio 2005; Singh 2006; Piotrowski 2007; Erdemir 2014). Sinha 1997 reported only the combined outcome of large IVH or PVL (or both) and Lista 2004 reported only grade 3 or 4 IVH; the outcomes from these trials are not included in meta‐analysis of 'any IVH' but are included in the relevant meta‐analyses below. None of the individual studies showed any difference between groups and the meta‐analysis did not show a difference in any IVH between groups (Analysis 1.16).
There was moderate heterogeneity (I2 = 53%) between hybrid trials, no heterogeneity between strict trials and low heterogeneity (I2 = 40%) for all trials included. Piotrowski 2007 reported a non‐significant increase in 'any IVH' in the VTV group. However, in this study, participants in the VTV group had increased oxygen requirements at enrolment and increased surfactant use compared with the PLV group. This indicates that infants in the VTV group were unlikely to have been at equal inception risk. Sensitivity analysis revealed that exclusion of the Piotrowski 2007 study reduced heterogeneity between hybrid studies (I2 = 0%). The overall RR of the remaining five trials still showed no difference in 'any IVH' between groups. We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
For infants weighing less than 1000 g, there was no statistically significant difference between groups (Analysis 2.13).
Cystic periventricular leukomalacia
Seven trials (508 participants) reported data on cystic PVL (Keszler 2004a (supplemental data); Lista 2004; D'Angio 2005; Singh 2006; Duman 2012; Chowdhury 2013; Guven 2013). No individual study showed a difference between groups. However, meta‐analysis showed a significant reduction in PVL in the VTV group (typical RR 0.45, 95% CI 0.21 to 0.98; typical RD ‐0.04, 95% CI ‐0.08 to ‐0.00; Analysis 1.17). We graded the quality of evidence for this outcome as moderate (unblinded intervention). For infants weighing less than 1000 g, there was no significant difference between groups (Analysis 2.15).
Severe intraventricular haemorrhage grade 3 or 4
Ten trials (712 participants) reported data on severe IVH grade 3 or 4 (Piotrowski 1997; Keszler 2004a (supplemental data); Lista 2004; D'Angio 2005; Singh 2006; Piotrowski 2007; Liu 2011; Duman 2012; Chowdhury 2013; Guven 2013). No individual study showed a difference between groups. However meta‐analysis showed a significant reduction in IVH grade 3 or 4 in the VTV group (typical RR 0.53, 95% CI 0.37 to 0.77; typical RD ‐0.09, 95% CI ‐0.14 to ‐0.04; typical NNTB 11, 95% CI 7 to 25; Analysis 1.18). In addition, there was evidence of heterogeneity in the 'hybrid studies' (I2 = 49%). As for 'any IVH,' Piotrowski 2007 reported a non‐significant increase in 'IVH grade 3 or 4' in the VTV group, but infants in the VTV group were unlikely to have been at equal inception risk. Sensitivity analysis revealed that exclusion of the Piotrowski 2007 study eliminated heterogeneity, both between hybrid studies and overall (I2 = 0%). The overall RR of the remaining nine trials showed an even stronger reduction in IVH grade 3 or 4 in the VTV group (typical RR 0.42, 95% CI 0.28 to 0.65). We graded the quality of evidence for this outcome as moderate (unblinded intervention).
There was no significant difference for infants weighing less than 1000 g (typical RR 0.53, 95% CI 0.27 to 1.04; Analysis 2.14). There was evidence of heterogeneity in the subgroup of hybrid studies (overall I2 = 10%; strict studies 0%; hybrid studies 57%).
Any intraventricular haemorrhage or periventricular leukomalacia
Three trials (298 participants) reported data on any IVH or PVL (Keszler 2004a (supplemental data); D'Angio 2005; Singh 2006). Pooled analysis showed no statistically significant difference between groups (Analysis 1.19). Likewise, for infants weighing less than 1000 g, there was no difference between groups (Analysis 2.16).
Intraventricular haemorrhage grade 3 or 4 or periventricular leukomalacia
Six trials (441 participants) reported data on IVH grade 3 or 4 or PVL (Sinha 1997; Keszler 2004a (supplemental data); Lista 2004; D'Angio 2005; Singh 2006; Chowdhury 2013 (supplemental data)). No individual study showed a difference between groups. However, meta‐analysis showed a significant reduction in IVH grade 3 or 4 or PVL in the VTV group (typical RR 0.47, 95% CI 0.27 to 0.80; typical RD ‐0.09, 95% CI ‐0.15 to ‐0.03; typical NNTB 11, 95% CI 7 to 33; Analysis 1.20). We graded the quality of evidence for this outcome as moderate (unblinded intervention).
In the subgroup of infants weighing less than 1000 g, there was also a statistically significant reduction in the VTV group (typical RR 0.44, 95% CI 0.20 to 0.99; typical RD ‐0.12, 95% CI ‐0.24 to ‐0.01; typical NNTB 8, 95% CI 4 to 100; Analysis 2.17). For this outcome, there was overall moderate heterogeneity between studies (I2 = 69%). This was primarily caused by infants weighing less than 1000 g from the Lista 2004 study where there was a marked difference in this outcome (VTV group 0/12 infants and PLV group 3/5 infants). Sensitivity analysis revealed that exclusion of the Lista 2004 trial eliminated heterogeneity (I2 = 0%), but also changed the significance level of this outcome in infants weighing less than 1000 g (typical RR 0.56, 95% CI 0.20 to 1.57).
Neurodevelopmental outcome (outcomes 3.1 and 2.2)
No studies reported neurodevelopmental outcome as defined by the review criteria.
Two trials (209 participants) reported neurological follow‐up using their own definition (D'Angio 2005; Singh 2006). We performed a post‐hoc meta‐analysis on these outcomes using the individual study criteria. There was no statistically significant difference between groups (typical RR 0.86, 95% CI 0.47 to 1.59; typical RD ‐0.02, 95% CI ‐0.12 to 0.08; Analysis 3.1).
One study (109 participants) also reported the combined outcome of death or severe disability (Singh 2006). There was no statistically significant difference between groups (typical RR 0.54, 95% CI 0.27 to 1.06; typical RD ‐0.15, 95% CI ‐0.31 to 0.01; Analysis 3.2). This study had unequal postdischarge follow‐up, which could be a potential source of bias.
One study (128 participants) reported gross motor delay (D'Angio 2005). The results from this single trial demonstrated no statistically significant difference between groups (typical RR 1.00, 95% CI 0.47 to 2.14; typical RD 0.00, 95% CI ‐0.13 to 0.13; Analysis 3.3).
Surviving infants with bronchopulmonary dysplasia (outcomes 1.21 and 2.18)
Nine trials (620 participants) reported data on BPD at 36 weeks' postmenstrual age (Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Singh 2006; Duman 2012; Chowdhury 2013; Guven 2013; Erdemir 2014). The trial by Guven 2013 reported a significant reduction in 36 weeks' postmenstrual age in the VTV group. No other individual trial reported a difference between groups. However, pooled meta‐analysis revealed a reduction in BPD at 36 weeks' postmenstrual age (typical RR 0.68, 95% CI 0.53 to 0.87; typical RD ‐0.11, 95% CI ‐0.18 to ‐0.04; typical NNTB 9, 95% CI 6 to 25; Analysis 1.21). We graded the quality of evidence for this outcome as low (unblinded intervention and possible publication bias with asymmetrical funnel plot). Subgroup analysis for infants weighing less than 1000 g showed no difference between groups (Analysis 2.18).
Post hoc analyses on related outcomes (outcomes 3.4 and 3.5)
One trial (203 participants) reported data on postnatal glucocorticoids for treating BPD (D'Angio 2005). There was no statistically significant difference between groups (typical RR 0.93, 95% CI 0.65 to 1.31; typical RD ‐0.03, 95% CI ‐0.16 to 0.10; Analysis 3.4).
Two trials (270 participants) reported data on need for home oxygen (D'Angio 2005; Singh 2006). Neither individual studies nor pooled analysis showed a statistically significant difference between groups (pooled analysis: typical RR 0.64, 95% CI 0.30 to 1.36; typical RD ‐0.04, 95 % CI ‐0.11 to 0.03; Analysis 3.5). Only supplemental data from D'Angio 2005 were available for the subgroup of infants weighing less than 1000 g. There was no statistically significant difference between groups (RR 0.75, 95% CI 0.25 to 2.23; RD ‐0.03, 95% CI ‐0.13 to 0.08; Analysis 3.6).
Subgroup analyses
We reported subgroup analysis in infants weighing less than 1000 g, where possible, alongside the results for primary and secondary outcomes.
Discussion
Summary of main results
Meta‐analyses performed for this updated review showed that use of VTV, compared with PLV, reduced rates of death or BPD, BPD alone, pneumothorax, severe cranial ultrasound pathologies (IVH grade 3 or 4, or PVL, or both), mean duration of mechanical ventilation and hypocarbia. It is plausible that VTV modes, by controlling VT and avoiding volutrauma, may contribute to a reduction in BPD and death. Additionally, by improving the stability of blood gas parameters and reducing hypocarbia, these modes may stabilise cerebral perfusion and reduce neonatal brain injury. Studies to date have not been powered to assess longer‐term neurodevelopmental outcomes. We found no evidence of harm associated with the use of VTV modes.
Overall completeness and applicability of evidence
Most modern ventilators offer VTV modes targeting expired VT. VTV modes have been implemented as a strategy to avoid lung injury due to overinflation or underinflation. However, the target VT set for the whole lung is based on the infant's weight. Regional distribution of VT may vary depending on lung disease. In non‐homogeneous lung disease, using a VTV mode does not eliminate the regional risk of lung injury from local volutrauma or shear stress. Strategies to manage these local variations in lung mechanics may be important.
Quality of the evidence
The overall quality of evidence for outcomes in this review varied from moderate to low. Most researchers and ethics committees agree that neonatal ventilation studies should not be blinded from the clinical personnel. Using the GRADE assessment tool, 'moderate' is the highest quality assessment possible for evidence from well‐conducted randomised but unblinded studies.
Because of the nature of the interventions, blinding of carers to allocated treatment is impossible. There were no major methodological limitations with seven out of 20 studies (Herrera 2002; Keszler 2004a; Hummler 2006; Cheema 2007; Duman 2012; Bhat 2016; Jain 2016). In seven other studies, the ventilators or the triggering modes/devices used (or both) were different in the VTV and the PLV arm, leading to potential intervention bias (defined as hybrid studies) (Piotrowski 1997; Sinha 1997; D'Angio 2005; Nafday 2005; Piotrowski 2007; Liu 2011; Erdemir 2014). However, sensitivity analysis revealed similar results both when we analysed separately studies using the same ventilator and patient triggering modes/device in the VTV and PLV arms, and when we analysed 'hybrid studies.' In the trials by Piotrowski 2007 and Chowdhury 2013, despite randomisation, there was an imbalance in participant characteristics between the VTV and PLV groups. This may have introduced a bias. The studies by Lista 2004 and Guven 2013 excluded a substantial number of randomised participants (Lista 2004: 7/60; Guven 2013: 15/90) after randomisation. The trials by Sinha 1997 and Singh 2006 weaned both arms using PLV mode. Three trials had potential selection bias (Polimeni 2006; Zhou 2007; Liu 2011), and two of these trials also had other biases (Zhou 2007; Liu 2011), thus reducing their quality.
Most trials included were small, single‐ or dual‐centre studies, and some used ventilation modes that are no longer used in modern neonatal ventilators. For some outcomes, with small numbers of participants, there was evidence of imprecision. We did not identify any large multi‐centre studies which were adequately powered to address important long‐term outcomes. Some clinicians will require evidence from such trials before changing practice.
For the vast majority of outcomes, there was no evidence of publication bias. However, the funnel plot for the outcome BPD at 36 weeks' GA in survivors was asymmetrical. We have no evidence to believe that there was publication bias, but based on the funnel plot this cannot be excluded.
A further limitation with this review is that VT delivery in the VTV group was different depending on the ventilators used and additional ventilator modes. Moreover, studies started at different time points after birth and had different weaning approaches. Studies were also conducted in many different countries (England, Germany, Poland, Turkey, the USA and China). However, these factors also represent a strength of this review. Even with a substantial mixture of trial design and inclusion of infants from three different continents, there was little evidence of heterogeneity, the results clearly favour use of VTV and should be generalisable for a wide population of neonates.
We received supplementary data permitting more extensive subgroup analysis of infants weighing less than 1000 g. However, the relatively low numbers of infants weighing less than 1000 g (247) in this subgroup limit the power to identify differences between interventions.
Agreements and disagreements with other studies or reviews
Three previous systematic reviews have compared the efficacy of VTV versus PLV; two previous Cochrane reviews (McCallion 2005; Wheeler 2010), and the review by Peng 2014. The first Cochrane Review included four RCTs with 178 infants (McCallion 2005). There was no significant difference for death by the time of hospital discharge, and no trials reported the combined outcome of death or BPD. However, there were reduced rates of pneumothorax and IVH grade 3 or 4, and there was reduced days of ventilation in the VTV group. The second Cochrane Review included 12 RCTs with 693 infants (Wheeler 2010). There was no significant difference for death by the time of hospital discharge. However, the use of VTV resulted in reduced rates of death or BPD, pneumothorax, days of ventilation, hypocarbia and IVH grade 3 or 4 or PVL. Peng 2014 included 18 RCTs with 954 infants. The authors reported no difference in incidence of death. However, the use of VTV resulted in reduced rates of BPD, duration of ventilation, any IVH, IVH grade 3 or 4, PVL, pneumothorax, failure of primary mode of ventilation, hypocarbia, mean airway pressure and days of supplemental oxygen administration.
In this updated review in 2017, with 20 RCTs and 1065 infants, we confirm the previously reported beneficial effects of VTV with reductions in clinically relevant outcomes. There was no disagreement with previous reports.
Very few observational trials have compared VTV and PLV, and reported relevant clinical outcomes. Stefanescu 2015 studied two cohorts of participants treated with PLV (pressure controlled ventilation) and VTV (PSV with volume guarantee), both modes were provided by the Drager Babylog 8000 plus. Both cohorts included 135 participants. In this trial, the VTV cohort had significantly lower mortality, and lower rates of PIE and hypotension. However, there were no differences in neurodevelopmental impairment at 18 months of age.

Study flow diagram: review update. PLV: pressure‐limited ventilation; VTV: volume‐targeted ventilation.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 1 Death before discharge from hospital.

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 2 Death or bronchopulmonary dysplasia (BPD) (36 weeks).

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 3 Failure of mode of ventilation.
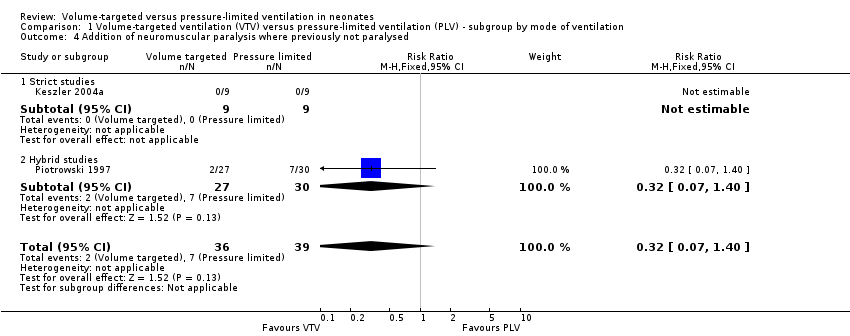
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 4 Addition of neuromuscular paralysis where previously not paralysed.
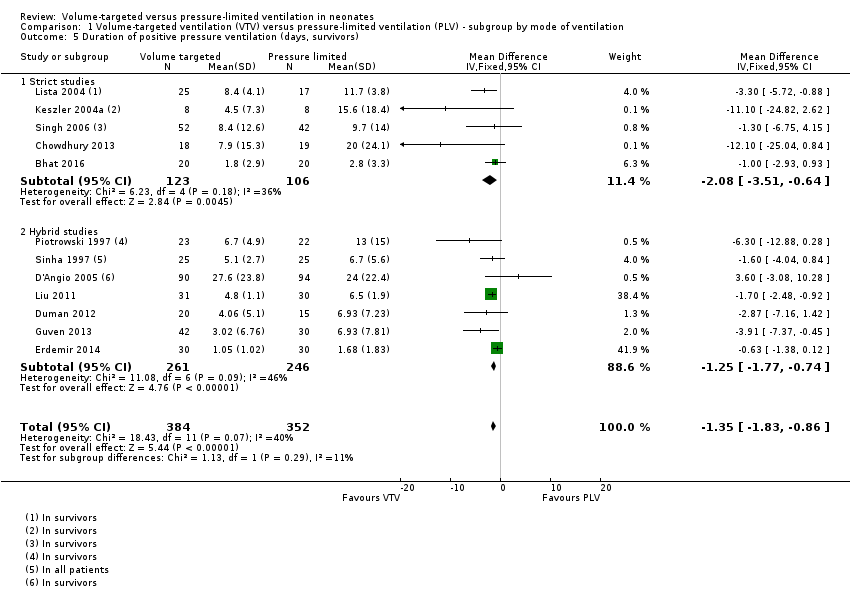
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 5 Duration of positive pressure ventilation (days, survivors).
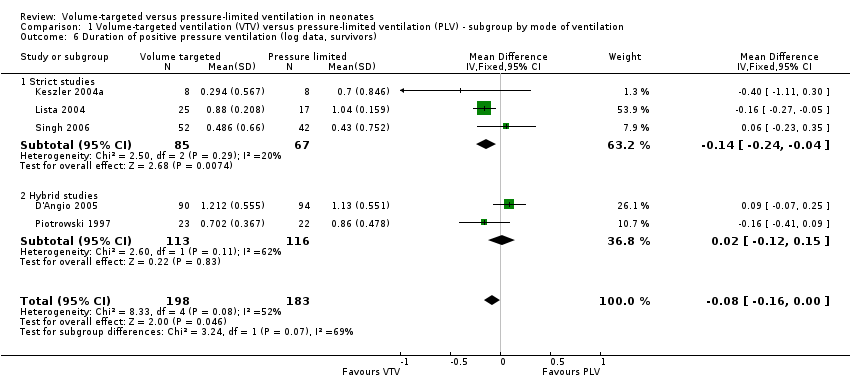
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 6 Duration of positive pressure ventilation (log data, survivors).

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 7 Inspired oxygen concentration % (study definition).
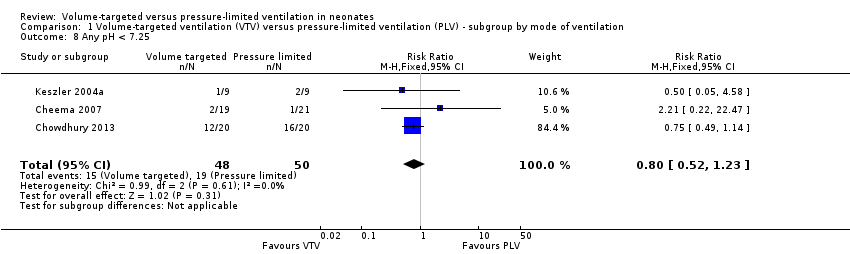
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 8 Any pH < 7.25.
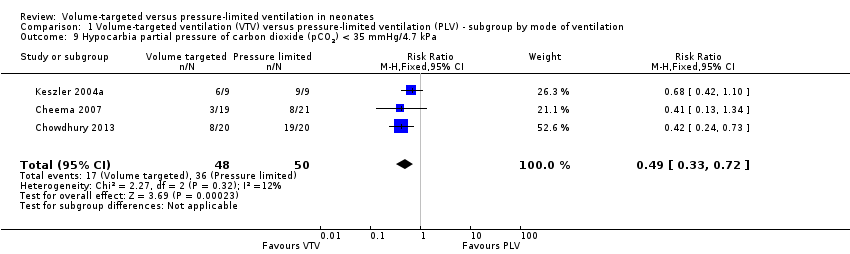
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 9 Hypocarbia partial pressure of carbon dioxide (pCO2) < 35 mmHg/4.7 kPa.
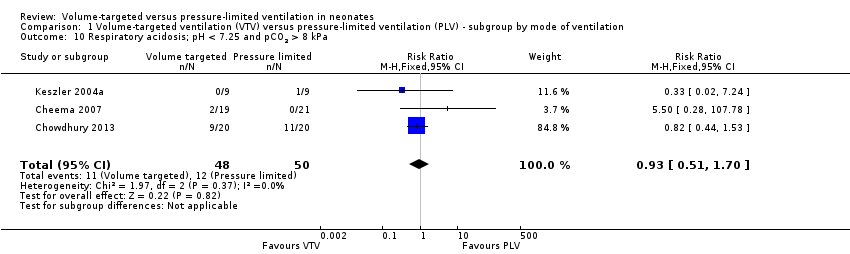
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 10 Respiratory acidosis; pH < 7.25 and pCO2 > 8 kPa.

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 11 Incidence of hypocarbia or respiratory acidosis.
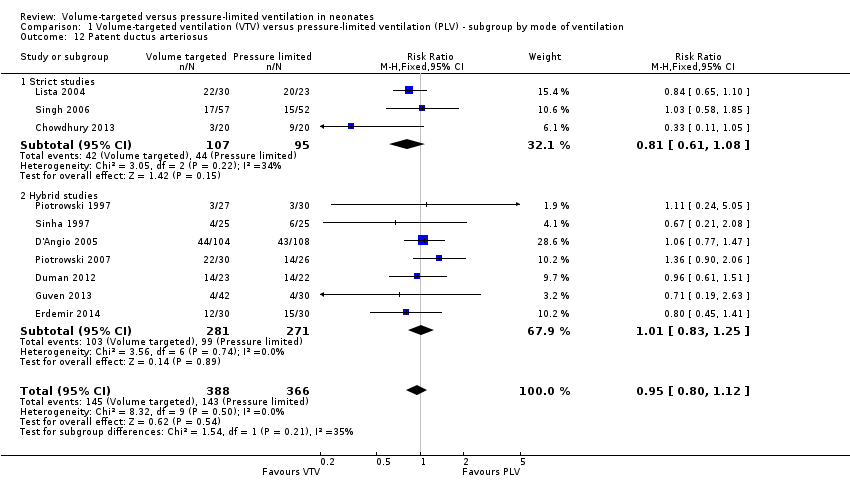
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 12 Patent ductus arteriosus.

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 13 Air leak (any).
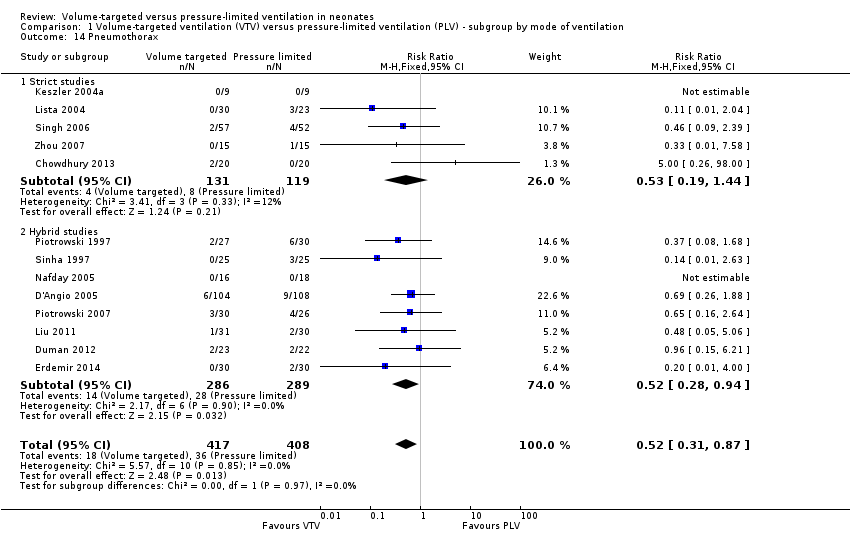
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 14 Pneumothorax.

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 15 Pulmonary interstitial emphysema.
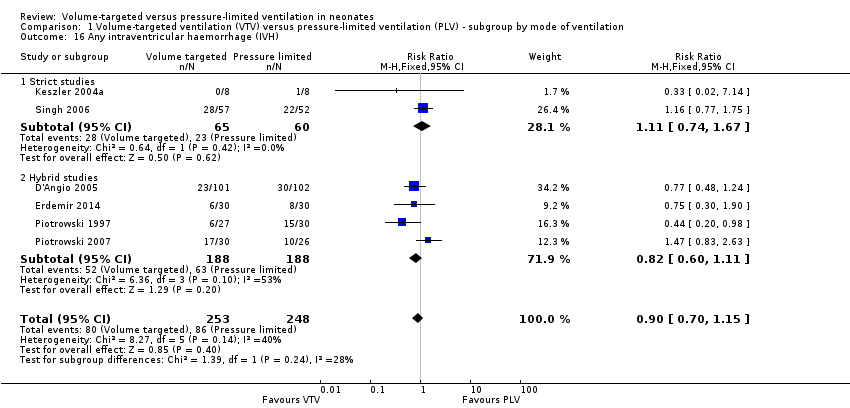
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 16 Any intraventricular haemorrhage (IVH).

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 17 Periventricular leukomalacia (PVL).

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 18 IVH grade 3‐4.

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 19 Any IVH or PVL.

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 20 IVH grade 3‐4 or PVL.
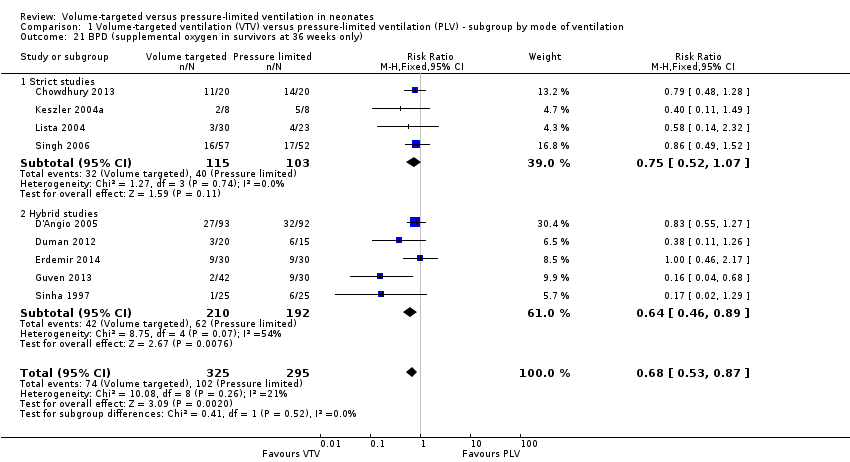
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 21 BPD (supplemental oxygen in survivors at 36 weeks only).

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 1 Death in hospital.

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 2 Death or bronchopulmonary dysplasia (BPD) (36 weeks).

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 3 Duration of positive pressure ventilation (days, survivors).
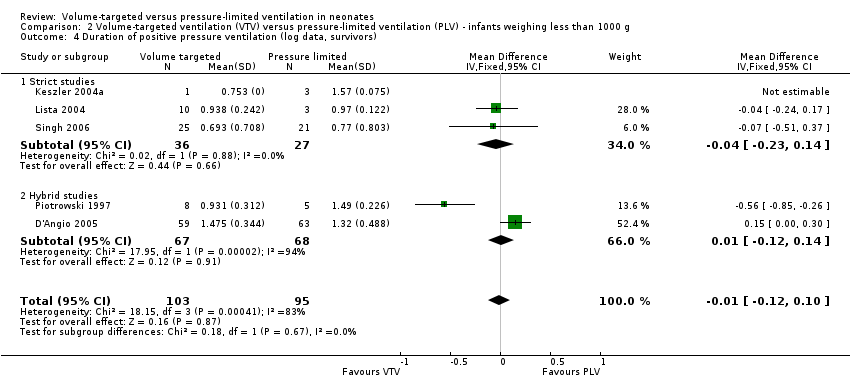
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 4 Duration of positive pressure ventilation (log data, survivors).
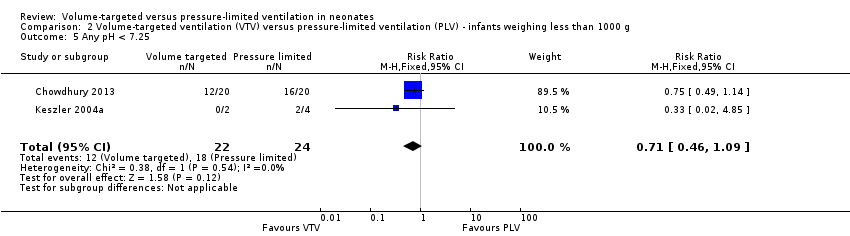
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 5 Any pH < 7.25.
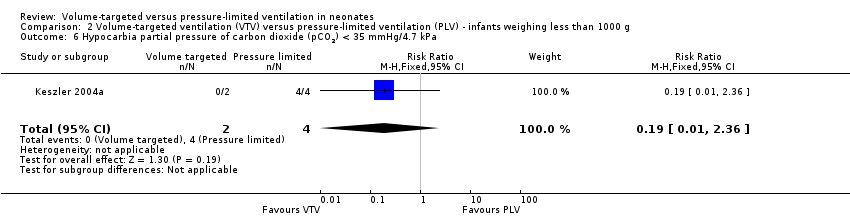
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 6 Hypocarbia partial pressure of carbon dioxide (pCO2) < 35 mmHg/4.7 kPa.

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 7 Respiratory acidosis pH < 7.25 and pCO2 > 60 mmHg/8 kPa.

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 8 Hypocarbia or respiratory acidosis.

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 9 Patent ductus arteriosus.
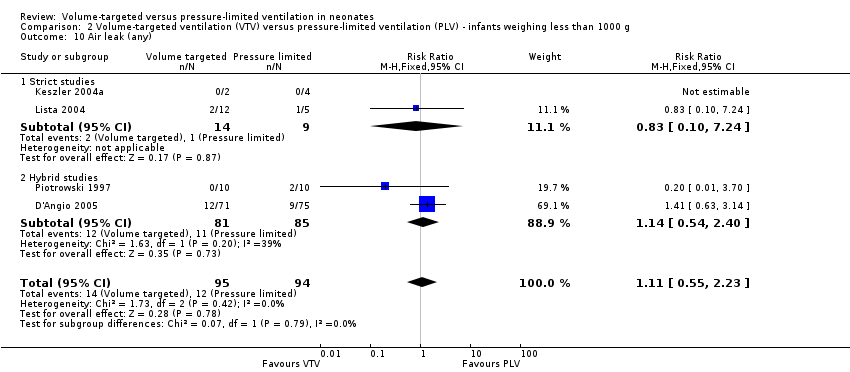
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 10 Air leak (any).
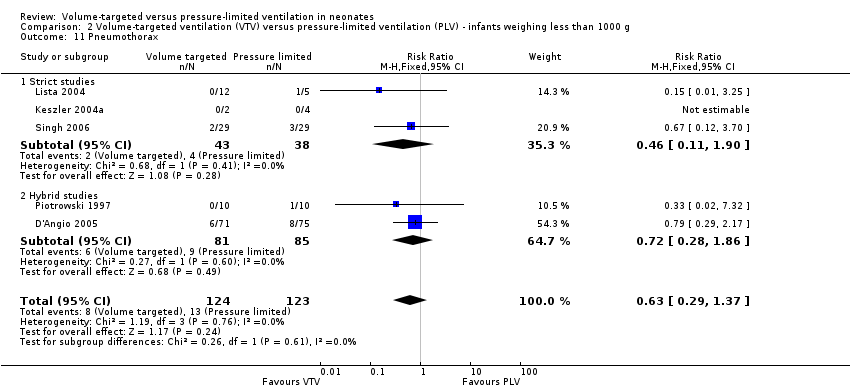
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 11 Pneumothorax.
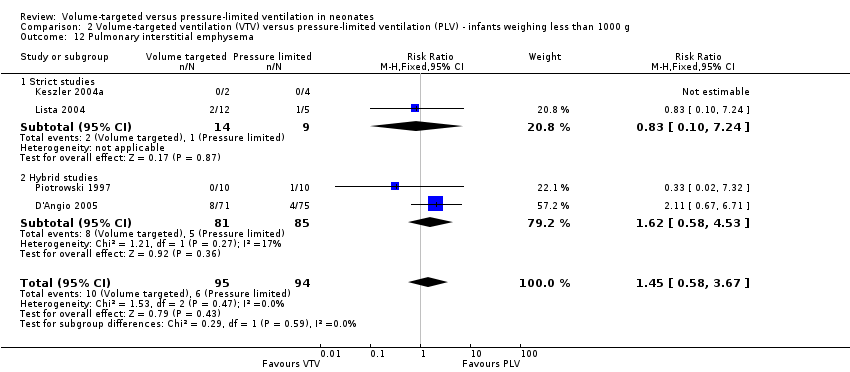
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 12 Pulmonary interstitial emphysema.

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 13 Any intraventricular haemorrhage (IVH).
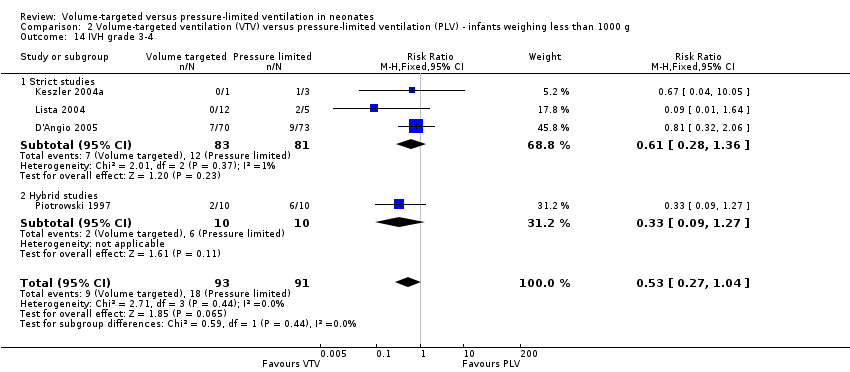
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 14 IVH grade 3‐4.

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 15 Periventricular leukomalacia (PVL).

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 16 Any IVH or PVL.

Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 17 IVH grade 3‐4 or PVL.
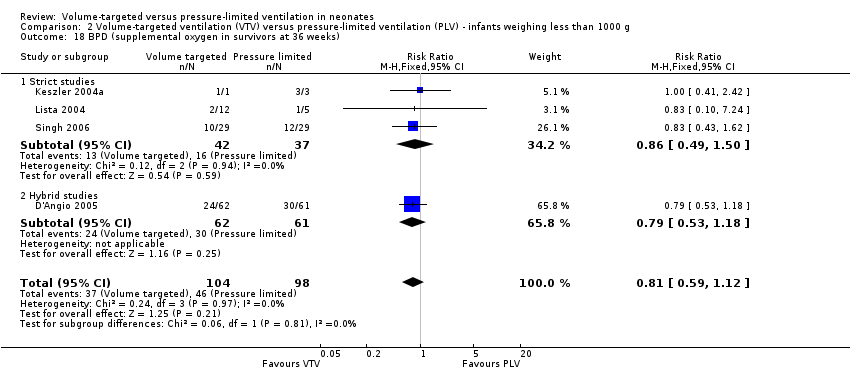
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 18 BPD (supplemental oxygen in survivors at 36 weeks).

Comparison 3 Miscellaneous post hoc analyses, Outcome 1 Severe disability (any definition).
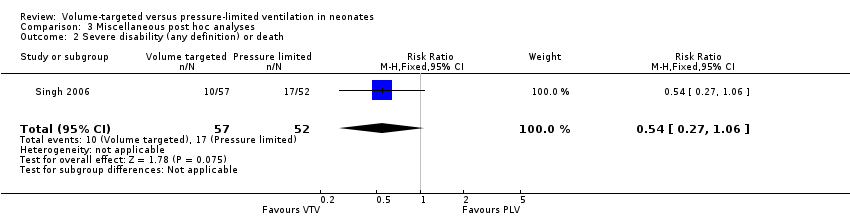
Comparison 3 Miscellaneous post hoc analyses, Outcome 2 Severe disability (any definition) or death.

Comparison 3 Miscellaneous post hoc analyses, Outcome 3 Gross motor developmental issue (any definition).
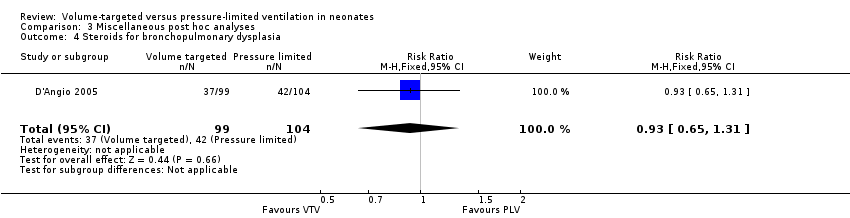
Comparison 3 Miscellaneous post hoc analyses, Outcome 4 Steroids for bronchopulmonary dysplasia.
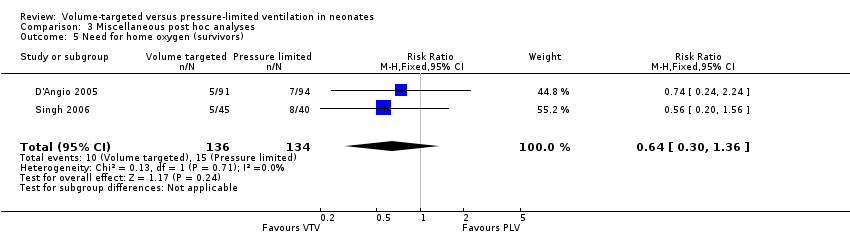
Comparison 3 Miscellaneous post hoc analyses, Outcome 5 Need for home oxygen (survivors).

Comparison 3 Miscellaneous post hoc analyses, Outcome 6 Need for home oxygen (survivors weighing < 1000 g).
| Volume‐targeted ventilation compared to pressure‐limited ventilation | ||||||
| Patient or population: neonates up to 44 weeks' postmenstrual age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with PLV | Risk with VTV | |||||
| Death before discharge from hospital | Study population | RR 0.75 | 771 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates. 95% CI < 0.75. | |
| 163 per 1000 | 122 per 1000 | |||||
| Death or BPD (36 weeks) | Study population | RR 0.73 | 584 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 458 per 1000 | 334 per 1000 | |||||
| Duration of positive pressure ventilation (days) | MD of positive pressure ventilation (days); PLV group 0 | MD 1.35 lower | ‐ | 736 | ⊕⊕⊕⊝ | Unblinded studies. |
| Pneumothorax | Study population | RR 0.52 (0.31 to 0.87) | 825 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 88 per 1000 | 46 per 1000 (27 to 77) | |||||
| IVH grade 3‐4 | Study population | RR 0.53 (0.37 to 0.77) | 712 (10 RCTs) | ⊕⊕⊕⊝ | Unblinded studies. | |
| 184 per 1000 | 97 per 1000 (68 to 141) | |||||
| IVH grade 3‐4 or PVL | Study population | RR 0.47 (0.27 to 0.80) | 441 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 164 per 1000 | 77 per 1000 (44 to 131) | |||||
| BPD (supplemental oxygen at 36 weeks) | Study population | RR 0.68 (0.53 to 0.87) | 620 | ⊕⊕⊝⊝ | Unblinded studies. Possible publication bias based on funnel plot. | |
| 346 per 1000 | 235 per 1000 (183 to 301) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPD: bronchopulmonary dysplasia; CI: confidence interval; IVH: intraventricular haemorrhage; MD: mean difference; PLV: pressure‐limited ventilation; PVL: periventricular leukomalacia; RCT: randomised controlled trial; RR: risk ratio; VTV: volume‐targeted ventilation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Volume‐targeted ventilation compared to pressure‐limited ventilation | ||||||
| Patient or population: neonates up to 44 weeks' postmenstrual age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with PLV | Risk with VTV | |||||
| Death or BPD (28 days) | Study population | RR 0.87 (0.64 to 1.18) | 149 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 536 per 1000 | 467 per 1000 (343 to 633) | |||||
| Failure of mode of ventilation | Study population | RR 0.69 | 445 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 242 per 1000 | 167 per 1000 | |||||
| Addition of neuromuscular paralysis where previously not paralysed | Study population | RR 0.32 | 75 | ⊕⊕⊝⊝ | Unblinded studies. Small numbers of participants. | |
| 179 per 1000 | 57 per 1000 | |||||
| Duration of positive pressure ventilation (log data) | The mean duration of IPPV (log data) was 0 | MD 0.08 lower | ‐ | 381 | ⊕⊕⊝⊝ | Unblinded studies. Heterogeneity of study results. |
| Inspired oxygen concentration % (study definition) | The mean difference in inspired oxygen concentration %; PLV group 0 | The mean inspired oxygen concentration % was 0.92 lower (2.08 lower to 0.24 higher) in VTV group | ‐ | 324 | ⊕⊕⊕⊝ | Unblinded studies. |
| Any pH < 7.25 | Study population | RR 0.80 | 98 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 380 per 1000 | 304 per 1000 | |||||
| Hypocarbia pCO2 < 35 mmHg/4.7 kPa | Study population | RR 0.49 (0.33 to 0.72) | 98 | ⊕⊕⊕⊝ | Unblinded studies. Small trials, but large effect and biologically plausible. | |
| 720 per 1000 | 353 per 1000 (238 to 518) | |||||
| Respiratory acidosis pH < 7.25 and pCO2 > 60 mmHg/8 kPa | Study population | RR 0.93 | 98 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 240 per 1000 | 223 per 1000 | |||||
| Either hypocarbia or respiratory acidosis | Study population | RR 0.68 | 37 | ‐ | No quality assessment possible. | |
| 1889 per 1000 | 1000 per 1000 | |||||
| Patent ductus arteriosus | Study population | RR 0.95 | 754 | ⊕⊕⊝⊝ | Unblinded studies. Variable diagnostic practices employed. | |
| 391 per 1000 | 371 per 1000 | |||||
| Air leak (any) | Study population | RR 0.79 | 374 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 117 per 1000 | 92 per 1000 | |||||
| Pulmonary interstitial emphysema | Study population | RR 1.21 | 430 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. | |
| 65 per 1000 | 79 per 1000 | |||||
| Any IVH | Study population | RR 0.82 | 445 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. | |
| 342 per 1000 | 281 per 1000 | |||||
| PVL | Study population | OR 0.43 (0.19 to 0.98) | 508 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 71 per 1000 | 32 per 1000 (14 to 69) | |||||
| Any IVH or PVL | Study population | RR 0.83 | 298 | ⊕⊕⊕⊝ | Unblinded studies. | |
| 308 per 1000 | 256 per 1000 | |||||
| BPD (supplemental oxygen at 28 days) | Study population | RR 0.91 | 206 | ⊕⊕⊝⊝ | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. | |
| 354 per 1000 | 322 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPD: bronchopulmonary dysplasia; CI: confidence interval; IPPV: intermittent positive pressure ventilation; IVH: intraventricular haemorrhage; MD: mean difference; pCO2: partial pressure of carbon dioxide; PLV: pressure‐limited ventilation; PVL: periventricular leukomalacia; RCT: randomised controlled trial; RR: risk ratio; VTV: volume‐targeted ventilation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death before discharge from hospital Show forest plot | 11 | 771 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.07] |
| 1.1 Strict studies | 4 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.31, 1.15] |
| 1.2 Hybrid studies | 7 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.55, 1.25] |
| 2 Death or bronchopulmonary dysplasia (BPD) (36 weeks) Show forest plot | 8 | 584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.59, 0.89] |
| 2.1 Strict studies | 4 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.94] |
| 2.2 Hybrid studies | 4 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.58, 0.99] |
| 3 Failure of mode of ventilation Show forest plot | 5 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 1.00] |
| 3.1 Strict studies | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.44, 1.72] |
| 3.2 Hybrid studies | 3 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.97] |
| 4 Addition of neuromuscular paralysis where previously not paralysed Show forest plot | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.40] |
| 4.1 Strict studies | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Hybrid studies | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.40] |
| 5 Duration of positive pressure ventilation (days, survivors) Show forest plot | 12 | 736 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐1.83, ‐0.86] |
| 5.1 Strict studies | 5 | 229 | Mean Difference (IV, Fixed, 95% CI) | ‐2.08 [‐3.51, ‐0.64] |
| 5.2 Hybrid studies | 7 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐1.77, ‐0.74] |
| 6 Duration of positive pressure ventilation (log data, survivors) Show forest plot | 5 | 381 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.16, ‐0.00] |
| 6.1 Strict studies | 3 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.24, ‐0.04] |
| 6.2 Hybrid studies | 2 | 229 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.12, 0.15] |
| 7 Inspired oxygen concentration % (study definition) Show forest plot | 7 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐2.08, 0.24] |
| 7.1 Parallel studies | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐3.09, 0.24] |
| 7.2 Cross‐over studies | 4 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐2.05, 1.17] |
| 8 Any pH < 7.25 Show forest plot | 3 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.23] |
| 9 Hypocarbia partial pressure of carbon dioxide (pCO2) < 35 mmHg/4.7 kPa Show forest plot | 3 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.33, 0.72] |
| 10 Respiratory acidosis; pH < 7.25 and pCO2 > 8 kPa Show forest plot | 3 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.70] |
| 11 Incidence of hypocarbia or respiratory acidosis Show forest plot | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.42, 1.12] |
| 12 Patent ductus arteriosus Show forest plot | 10 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.12] |
| 12.1 Strict studies | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.61, 1.08] |
| 12.2 Hybrid studies | 7 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.25] |
| 13 Air leak (any) Show forest plot | 5 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.43] |
| 13.1 Strict studies | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.09, 2.81] |
| 13.2 Hybrid Studies | 3 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.45, 1.58] |
| 14 Pneumothorax Show forest plot | 13 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.31, 0.87] |
| 14.1 Strict studies | 5 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.19, 1.44] |
| 14.2 Hybrid studies | 8 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.94] |
| 15 Pulmonary interstitial emphysema Show forest plot | 6 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.63, 2.30] |
| 15.1 Strict studies | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.12, 5.04] |
| 15.2 Hybrid studies | 4 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.64, 2.57] |
| 16 Any intraventricular haemorrhage (IVH) Show forest plot | 6 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.15] |
| 16.1 Strict studies | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.74, 1.67] |
| 16.2 Hybrid studies | 4 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.11] |
| 17 Periventricular leukomalacia (PVL) Show forest plot | 7 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.98] |
| 17.1 Strict studies | 4 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.65] |
| 17.2 Hybrid studies | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.14, 1.14] |
| 18 IVH grade 3‐4 Show forest plot | 10 | 712 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.14, ‐0.04] |
| 18.1 Strict studies | 4 | 218 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.13, 0.02] |
| 18.2 Hybrid studies | 6 | 494 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.10 [‐0.17, ‐0.04] |
| 19 Any IVH or PVL Show forest plot | 3 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.18] |
| 19.1 Strict studies | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.35] |
| 19.2 Hybrid studies | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.48, 1.39] |
| 20 IVH grade 3‐4 or PVL Show forest plot | 6 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.80] |
| 20.1 Strict studies | 4 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.25, 1.03] |
| 20.2 Hybrid studies | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.96] |
| 21 BPD (supplemental oxygen in survivors at 36 weeks only) Show forest plot | 9 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 21.1 Strict studies | 4 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.52, 1.07] |
| 21.2 Hybrid studies | 5 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death in hospital Show forest plot | 5 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.21] |
| 1.1 Strict studies | 4 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.46, 1.39] |
| 1.2 Hybrid studies | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.86] |
| 2 Death or bronchopulmonary dysplasia (BPD) (36 weeks) Show forest plot | 4 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.01] |
| 2.1 Strict studies | 3 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.52, 1.10] |
| 2.2 Hybrid studies | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.12] |
| 3 Duration of positive pressure ventilation (days, survivors) Show forest plot | 5 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐4.43, 2.80] |
| 3.1 Strict studies | 3 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐4.53, 3.89] |
| 3.2 Hybrid studies | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐9.29, 4.87] |
| 4 Duration of positive pressure ventilation (log data, survivors) Show forest plot | 5 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 4.1 Strict studies | 3 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.23, 0.14] |
| 4.2 Hybrid studies | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.12, 0.14] |
| 5 Any pH < 7.25 Show forest plot | 2 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.46, 1.09] |
| 6 Hypocarbia partial pressure of carbon dioxide (pCO2) < 35 mmHg/4.7 kPa Show forest plot | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 2.36] |
| 7 Respiratory acidosis pH < 7.25 and pCO2 > 60 mmHg/8 kPa Show forest plot | 2 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.43, 1.47] |
| 8 Hypocarbia or respiratory acidosis Show forest plot | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 2.36] |
| 9 Patent ductus arteriosus Show forest plot | 4 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.39] |
| 9.1 Strict studies | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.77, 1.57] |
| 9.2 Hybrid studies | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.50] |
| 10 Air leak (any) Show forest plot | 4 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.23] |
| 10.1 Strict studies | 2 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.10, 7.24] |
| 10.2 Hybrid studies | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.54, 2.40] |
| 11 Pneumothorax Show forest plot | 5 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.29, 1.37] |
| 11.1 Strict studies | 3 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.11, 1.90] |
| 11.2 Hybrid studies | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.28, 1.86] |
| 12 Pulmonary interstitial emphysema Show forest plot | 4 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.58, 3.67] |
| 12.1 Strict studies | 2 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.10, 7.24] |
| 12.2 Hybrid studies | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.58, 4.53] |
| 13 Any intraventricular haemorrhage (IVH) Show forest plot | 4 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.55, 1.16] |
| 13.1 Strict studies | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.62, 2.08] |
| 13.2 Hybrid studies | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.06] |
| 14 IVH grade 3‐4 Show forest plot | 4 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.27, 1.04] |
| 14.1 Strict studies | 3 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.28, 1.36] |
| 14.2 Hybrid studies | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.27] |
| 15 Periventricular leukomalacia (PVL) Show forest plot | 4 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.15, 1.24] |
| 15.1 Strict studies | 3 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.10, 1.53] |
| 15.2 Hybrid studies | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.63] |
| 16 Any IVH or PVL Show forest plot | 3 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.60, 1.35] |
| 16.1 Strict studies | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.61, 1.80] |
| 16.2 Hybrid studies | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.42] |
| 17 IVH grade 3‐4 or PVL Show forest plot | 3 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.15, 0.99] |
| 17.1 Strict studies | 2 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.04] |
| 17.2 Hybrid studies | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.64] |
| 18 BPD (supplemental oxygen in survivors at 36 weeks) Show forest plot | 4 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.12] |
| 18.1 Strict studies | 3 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.49, 1.50] |
| 18.2 Hybrid studies | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severe disability (any definition) Show forest plot | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.47, 1.59] |
| 2 Severe disability (any definition) or death Show forest plot | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.06] |
| 3 Gross motor developmental issue (any definition) Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.14] |
| 4 Steroids for bronchopulmonary dysplasia Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.65, 1.31] |
| 5 Need for home oxygen (survivors) Show forest plot | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.30, 1.36] |
| 6 Need for home oxygen (survivors weighing < 1000 g) Show forest plot | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.25, 2.23] |

