ترویج شستوشوی صورت برای پیشگیری از ابتلا به تراخم فعال
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: cluster‐RCT Number randomized: 36 communities (1143 children) Exclusions after randomization: none reported Unit of analysis: individual children Number analyzed: 1143 children total; 374 in eye drops group; 246 in eye washing group; 312 in combined group; 211 in non‐treatment group Losses to follow‐up: 128 (11.2%) children total; 34 in eye drops group; 44 in eye washing group; 28 in combined group; 22 in non‐treatment group How was missing data handled?: "Children which the trachoma workers could not follow‐up were assumed to have follicular trachoma and were included in the analysis." Reported power calculation: sample size of 1500; power 80% Unusual study design: "For each trachoma worker communities were allocated to all four groups and the allocation was done in stages…" | |
| Participants | Country: Northern Territory of Australia Age: 5 to 14 years; also included school children older than 14 years and pre‐school children (ages not specified) Gender: boys and girls Inclusion criteria for community: Aboriginal communities in the Northern Territory; population of about 100 or more Inclusion criteria for children: Aboriginal children with follicular trachoma Exclusion criteria for community: communities where “the yield of trachoma cases was expected to be small (≤6)" Equivalence of baseline characteristics: no; "… more children aged 10 years old and above in the control group than in the other treatment groups …" | |
| Interventions | Intervention 1: oily tetracycline eye drops daily for one week every month Intervention 2: daily (every school day) eye washing Intervention 3: oily tetracycline eye drops daily (every school day) for one week every month plus daily (every school day) eye washing Intervention 4: no treatment Length of follow‐up: Planned: 3 months | |
| Outcomes | Primary outcome, as defined in trial reports: follicular trachoma status (present or absent) Intervals at which outcomes assessed: baseline and 3 months | |
| Notes | Type of study: published Funding sources: "The study was funded entirely by the Northern Territory Trachoma and Eye Health Committee Inc." Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: yes (trachoma worker, geographical zone); "None of the differences between the trachoma workers in the results they obtained for any of the treatment programmes was statistically significant"; "The reduction in the number of children with follicular trachoma after the combined programme was significantly greater in communities in zones 2 and 8 than in zone 1." Trial investigators contacted?: yes; trial investigator contacted and provided information for previous versions of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used to allocate communities to one of four treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking outcome assessors | Low risk | "Trachoma workers did not know what treatment programme, if any, had been allocated to a particular community." "For each trachoma worker communities were allocated to all four groups and the allocation was done in stages to control for observer variation and a possible learning effect by the trachoma workers". |
| Incomplete outcome data (attrition bias) | Low risk | 128 (11.2%) children total (34 in eye drops group, 44 in eye washing group, 28 in combined group, 22 in non‐treatment group) lost to follow‐up; "The children whom the trachoma workers were unable to follow‐up were assumed to have follicular trachoma and were included in the analysis… The data were re‐analyzed after having excluded the missing cases and the overall results were similar"; "Level of missingness differed by treatment group – eye washing group had the highest % of missing cases". |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | This was a cluster‐RCT; however, data were analyzed at the individual participant level without accounting for the cluster design; sensitivity and specificity of aboriginal trachoma workers in diagnosing follicular trachoma varied. |
| Methods | Study design: cluster‐RCT Number randomized: 6 villages (1417 children); villages paired so that one village was assigned to the intervention and the other assigned to control; Pair 1: 178 children in intervention village and 231 in control village; Pair 2: 248 children in intervention village and 247 in control village; Pair 3: 254 children in intervention village and 259 in control village; Total: 680 children in intervention group and 737 in control group Exclusions after randomization: none reported Unit of analysis: villages (overall and by pairs) Number analyzed: 6 villages Losses to follow‐up: 113 children (8% of 1417); "reasons for loss to follow‐up were that the child died (2%) or that the family moved out of the village or out of the study area (6%)" How was missing data handled?: not reported Reported power calculation: none Unusual study design?: pairs of villages matched for baseline characteristics; "We randomized three pairs of villages‐one of each pair would receive mass treatment followed by the health education campaign, and the other would receive mass treatment alone. The pairs of villages were matched for maternal education (years of formal education), baseline prevalence of clean faces in young children, and trachoma status (based on clinical observation at enrolment). Within each village, a complete census was taken by trained field‐workers. 250 eligible households containing at least 1 child aged 1‐7 years were randomly selected in each village. Within the household, 1 child was randomly selected to take part in the study." | |
| Participants | Country: Kongwa, Tanzania Age: 1 to 7 years Gender: boys and girls Inclusion criteria: 1) children aged 1 to 7 years in the area where trachoma was endemic; 2) face washing campaign could be carried out at village level; 3) households containing at least 1 child Exclusion criteria: none reported Equivalence of baseline characteristics: no; pairs of villages were matched for maternal education, and baseline prevalence of clean faces in young children, and trachoma status based on clinical observation at enrolment; however, the prevalence of trachoma may have been higher in the intervention villages based on photographic evidence of trachoma | |
| Interventions | Intervention 1: 30‐day mass treatment campaign with topical tetracycline ointment once daily followed by intensive 1‐month community‐based participatory hygiene intervention, including neighborhood meetings and several reinforcement activities to improve face washing of the young children, during and after mass treatment Intervention 2: 30‐day mass treatment campaign with topical tetracycline ointment once daily alone Length of follow‐up: Planned: 12 months Actual: 12 months | |
| Outcomes | Outcomes, as defined in trial reports: facial cleanliness based on the presence or absence of nasal discharge, ocular discharge, and flies on the face; trachoma status evaluated by photographs of the right eye of each index child graded on the WHO simplified grading scheme Adverse events: not reported Intervals at which outcomes assessed: baseline, month 2 (1 month after the end of the mass treatment campaign), 6 and 12 months | |
| Notes | Type of study: published Funding sources: "This work was supported by the Edna McDonnell Clark Foundation and the Central Eye Health Foundation." Disclosures of interest: one author (Dr. Sheila West) is a Research to Prevent Blindness senior scientific investigator Study period: not reported Reported subgroup analyses: yes (trachoma status at baseline and prevalence of clean faces at baseline, 2 months, 6 months and 12 months) Trial investigators contacted?: yes; trial investigator contacted and provided information for previous versions of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking outcome assessors | Low risk | "Records of facial cleanliness were made by trained observers who were not part of the hygiene intervention team." "At each time, the tarsal plate of the right eye of each index child was photographed. The photographs were graded on the WHO simplified grading scheme by one examiner who was unaware of the randomization status of the village and the time of the photograph." |
| Incomplete outcome data (attrition bias) | Low risk | "92% were followed up for 1 year. The main reasons for loss to follow‐up were that the child died (2%) or that the family moved out of the village or out of the study area (6%)." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No other sources of bias identified. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Face washing was not part of the study intervention. Communities were randomly assessed to different health education programs:
| |
| Participants were not randomized to a face‐washing program. Houses were randomly selected in communities where the face‐washing "F" and education "E" components of the trachoma initiative were implemented. | |
| Unable to separate the effect of face washing from environmental sanitation interventions as both were indirectly examined as "one intervention". | |
| Study aim was to develop standardized definition for a clean face in trachoma prevention. | |
| Study intervention is health education promotion of face washing; face washing was not part of the study intervention. The educational program had a primary and five secondary messages. The primary message is: "Eye disease can be prevented by washing children's faces with soap and water at least once each day." The secondary messages are:
| |
| Not a RCT; communities were not randomly assigned care groups. Care groups are villages who are involved in prevention and treatment of trachoma. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study design: cluster‐RCT Unit of randomization: villages. "We will randomly select six villages to be the "intervention" villages and six villages to be the "control" villages. We will aim to include 360 children, randomly selected from the 6 "intervention" villages (one per mother for a total of up to 60 in each village) and 360 children randomly selected from 6 "control" villages." |
| Participants | Country: Niger Age: 6 months to 5 years and five months Gender: boys and girls Inclusion criteria: villages of size between 900 to 2100 residents as of 1995 census in Kornaka West district of Niger; village leadership approval of entry of village in the study; sentinel children ages 6 months to 5 years and five months Exclusion criteria: village already has health education program for hygiene; village within 5 km of a well; child already has a sibling in the study population |
| Interventions | Intervention 1: water and health education program to improve hygiene; "World Vision plans water wells to serve a population of about 300, in villages of about 300‐5,000 persons. Thus each village has around 1‐17 wells. The goal is to provide water within 500 meters with a wait time of less than 15 minutes. Health education on use of water and hygiene practices is also part of services delivery. A World Vision Area Development Program officer establishes and trains a water and sanitation committee to provide health education for their village." Intervention 2: "control" villages where services not available immediately; "villages where the planning process has just started and wells would not be drilled for over two years" Length of follow‐up: Planned: 3 years |
| Outcomes | Primary outcome, as defined in trial: trachoma (ocular C. trachomatis infection) Intervals at which outcomes assessed: baseline, 1, 2, and 3 years from baseline |
| Notes | Type of study: unpublished Funding sources: Johns Hopkins University, World Vision, CONRAD Disclosures of interest: not reported Study period: not reported Planned subgroup analyses: none reported |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Presence of follicular trachoma Show forest plot | 1 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.11] |
| Analysis 1.1 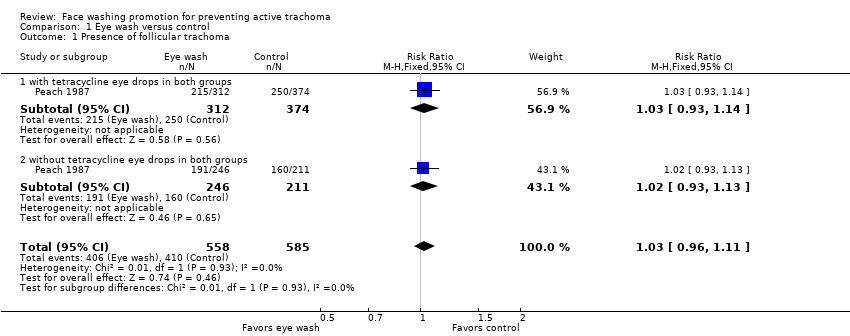 Comparison 1 Eye wash versus control, Outcome 1 Presence of follicular trachoma. | ||||
| 1.1 with tetracycline eye drops in both groups | 1 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 1.2 without tetracycline eye drops in both groups | 1 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.13] |
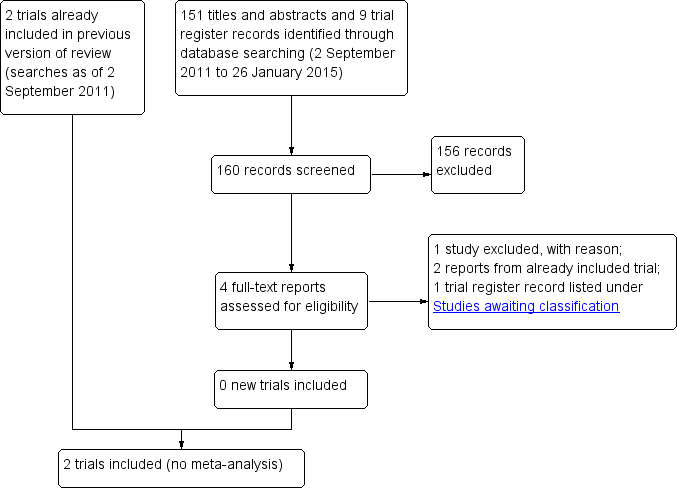
Study flow diagram.
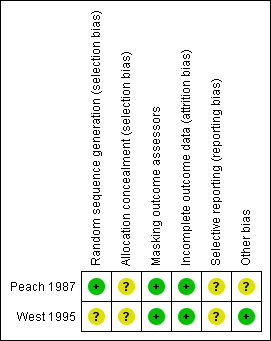
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Eye wash versus control, Outcome 1 Presence of follicular trachoma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Presence of follicular trachoma Show forest plot | 1 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.11] |
| 1.1 with tetracycline eye drops in both groups | 1 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 1.2 without tetracycline eye drops in both groups | 1 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.13] |

