Administración de suplementos de vitamina A para reducir la transmisión del VIH de madre a hijo
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003648.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 07 septiembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades infecciosas
- Clasificada:
-
- Actualizada
All studies incorporated from most recent search
Updated review: all eligible published studies found in the last search (25 Aug, 2017) were includedEvaluada: 2 April 2019
- Actualizada
- Copyright:
-
- Copyright © 2017 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
- This is an open access article under the terms of the Creative Commons Attribution‐Non‐Commercial Licence, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Charles S Wiysonge led the conduct and writing of this update of the Cochrane Review, with substantial intellectual contributions from Valantine N Ndze, Eugene J Kongnyuy, and Muki S Shey. All review authors approved the final version of the review for submission.
Sources of support
Internal sources
-
South African Medical Research Council, South Africa.
-
Liverpool School of Tropical Medicine, UK.
External sources
-
Effective Health Care Research Consortium, UK.
Grant: 5242
-
National Research Foundation of South Africa, South Africa.
Grant: 108571
Declarations of interest
Charles S Wiysonge has no known conflicts of interest.
Valantine N Ndze has no known conflicts of interest.
Eugene J Kongnyuy has no known conflicts of interest.
Muki S Shey has no known conflicts of interest.
Acknowledgements
We thank Paul Garner, George Rutherford, and Harshi Sachdev for comments on earlier versions of this edition of the Cochrane Review, which helped to substantially improve the quality of the conduct and reporting of the review. We acknowledge the substantial contributions of Jonathan Sterne and Peter Brocklehurst to previous editions of this Cochrane Review. We are grateful to Henrik Friis and Perpetual Chikobvu for sharing unpublished trial data with us.
Charles S Wiysonge's work is supported by the South African Medical Research Council, the National Research Foundation of South Africa (Grant: 108571), and the Effective Health Care Research Consortium. The Effective Health Care Research Consortium and the CIDG editorial base are funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Sep 07 | Vitamin A supplements for reducing mother‐to‐child HIV transmission | Review | Charles S Wiysonge, Valantine N Ndze, Eugene J Kongnyuy, Muki S Shey | |
| 2011 Jan 19 | Vitamin A supplementation for reducing the risk of mother‐to‐child transmission of HIV infection | Review | Charles Shey Wiysonge, Muki Shey, Eugene J Kongnyuy, Jonathan AC Sterne, Peter Brocklehurst | |
| 2005 Oct 19 | Vitamin A supplementation for reducing the risk of mother‐to‐child transmission of HIV infection | Review | Charles Shey Wiysonge, Muki Shey, Eugene J Kongnyuy, Jonathan AC Sterne, Peter Brocklehurst | |
| 2002 Apr 22 | Vitamin A supplementation for reducing the risk of mother‐to‐child transmission of HIV infection | Review | Charles U. Shey Wiysonge, Peter Brocklehurst, Jonathan AC Sterne | |
Differences between protocol and review
Differences between 2011 review and this review update
Authorship
The 2011 review had five authors (Wiysonge CS, Shey MS, Kongnyuy EJ, Sterne JA, and Brocklehurst P), but the current update has four authors (Wiysonge CS, Ndze VN, Kongnyuy EJ, and Shey MS).
Primary outcome
There are no differences between the two versions of the review, as both have an identical primary outcome (HIV infection status of the child).
Secondary outcomes
The 2011 review had 12 secondary outcomes linked to the child (infant death, stillbirth, neonatal sepsis, neonatal admission to neonatal unit, death by 24 months of age, side effects in the child, preterm delivery, very preterm delivery, birth weight, low birth weight, very low birthweight, and long‐term side effects in survivors) and five maternal secondary outcomes (maternal death, postpartum infection, side effects in the mother, cost of the intervention, and acceptability of the intervention). The current update has five child‐related secondary outcomes (mean birthweight, low birthweight, child death by two years of age, preterm delivery, and stillbirth) and two secondary outcomes linked to the mother (maternal death and postpartum CD4 count).
Methods
In Wiysonge 2011, we used a fixed‐effect method as our default method for meta‐analysis, and only used a random‐effects model when there was substantial statistical heterogeneity (P < 0.1). However, due to clinical heterogeneity, we used the random‐effects method for all meta‐analyses in this review update.
Included studies
We included five studies in the 2011 review (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Friis 2004; Humphrey 2006), and five reviews in the current update (Coutsoudis 1999; Chikobvu 2000; Fawzi 2002; Kumwenda 2002; Humphrey 2006). We included Friis 2004 in the 2011 review but excluded it from this review update because further assessment revealed that the study did not meet our inclusion criteria. In addition, we included a new study in this update (Chikobvu 2000).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Pregnancy Complications, Infectious;
- HIV Infections [mortality, prevention & control, *transmission];
- Infectious Disease Transmission, Vertical [*prevention & control];
- Randomized Controlled Trials as Topic;
- Treatment Outcome;
- Vitamin A [*administration & dosage];
- Vitamin A Deficiency [*complications, drug therapy];
- Vitamins [*administration & dosage];
Medical Subject Headings Check Words
Female; Humans; Infant, Newborn; Pregnancy;
PICO

PRISMA flow diagram

ʽRisk of bias' graph: review authors' judgements about each ʽRisk of bias' item presented as percentages across all included trials

ʽRisk of bias' summary: review authors' judgements about each ʽRisk of bias' item for each included trial

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 1 HIV infection status of the child.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 2 Mean birthweight.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 3 Low birthweight.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 4 Child death by two years of age.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 5 Preterm delivery.
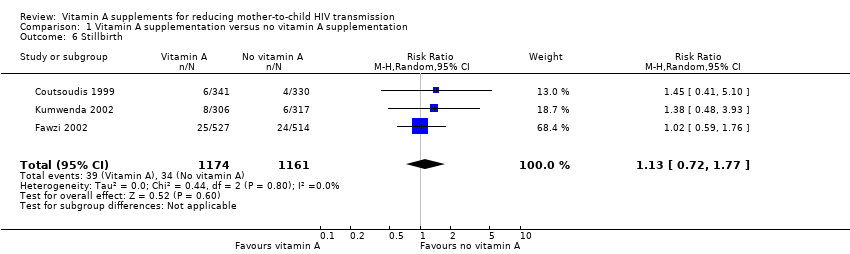
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 6 Stillbirth.
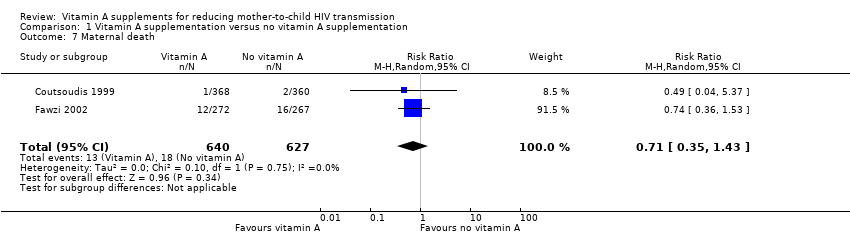
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 7 Maternal death.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 8 Postpartum CD4 count.
| Population: HIV‐positive women during pregnancy and immediate postpartum period Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Assumed risk with no vitamin A | Corresponding risk with vitamin A supplements | |||||
| HIV infection status of the child | 27 per 100 | 29 per 100 | RR 1.07 (0.91 to 1.26) | 4428 | ⊕⊕⊕⊝ due to imprecision | Vitamin A supplements probably have little or no effect on mother‐to‐child transmission of HIV. |
| Mean birthweight | 2964 g | 34 g higher | MD 34.12 (−12.79 to 81.02) | 2181 | ⊕⊕⊝⊝ due to imprecision | Vitamin A supplements may increase the mean birthweight |
| Low birthweight | 17 per 100 | 13 per 100 | RR 0.78 | 1819 | ⊕⊕⊕⊝ due to imprecision | Vitamin A supplements probably reduce the incidence of low birthweight babies. |
| Child death by two years of age | 14 per 100 | 15 per 100 | RR 1.06 | 3883 | ⊕⊕⊝⊝ due to imprecision | Vitamin A supplements may have little or no effect on child death by two years of age. |
| Preterm delivery | 20 per 100 | 17 per 100 | RR 0.84 | 1577 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on preterm deliveries. |
| Stillbirth | 3 per 100 | 3 per 100 | RR 1.13 | 2335 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on stillbirths. |
| Maternal death | 3 per 100 | 2 per 100 | RR 0.71 | 1267 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on maternal deaths. |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded by 1 for imprecision, as the CI ranges from small benefits to a clinically important increase in harm. | ||||||
| Search set | CENTRAL | PubMed | Embase | WHO ICTRP | ClinicalTrials.gov |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | Search ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR 'human immunodeficiency virus:ab,ti' OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'human immunodeficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune‐deficiency virus':ab,ti OR 'human immuno‐deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno‐deficiency syndrome':ab,ti OR 'acquired immune‐deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti | hiv AND vitamin A OR hiv AND retinol OR hiv AND retinoic OR hiv AND micronutrients OR hiv AND carotene | HIV and "VITAMIN A" | Interventional Studies | Studies received from 05/20/2016 to 08/25/2017 |
| #2 | MeSH descriptor: [HIV] explode all trees | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR "clinical trials as topic"[mesh: noexp] OR randomly [tiab] OR trial [tiab]) NOT (animals [mh] NOT humans [mh]) | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR ((doubl* NEAR/3 blind*):ab,ti) OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR ((cross NEXT/1 over*):ab,ti) | — | — |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | Search (infectious disease transmission, vertical[mh] OR vertical transmission[tiab] OR vertical infect*[tiab] OR infection transmission[tiab] OR mother‐to‐child transmission[tiab] OR MTCT[tiab]) | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | — | — |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | Search (vitamin A[mh] OR vitamin*[tiab] OR caroten*[tiab] OR retinol[tiab] OR retinoic[tiab] OR micronutrient*[tiab]) | 'human'/de OR 'normal human'/de OR 'human cell'/de | — | — |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | Search (#1 AND #2 AND #3 AND #4) | #3 AND #4 | — | — |
| #6 | #1 or #2 or #3 or #4 or #5 | Search (((#1 AND #2 AND #3 AND #4))) AND ("2016/05/20"[Date ‐ Publication] : "2017/08/25"[Date ‐ Publication]) | #3 NOT #5 | — | — |
| #7 | [mh "infectious disease transmission, vertical"] or "vertical transmission":ti,ab,kw or vertical next infect*:ti,ab,kw or "infection transmission":ti,ab,kw or "mother‐to‐child transmission":ti,ab,kw or MTCT:ti,ab,kw (Word variations have been searched) | — | #2 NOT #6 | — | — |
| #8 | [mh "vitamin A"] or vitamin*:ti,ab,kw or caroten*:ti,ab,kw or retinol:ti,ab,kw or retinoic:ti,ab,kw or micronutrient*:ti,ab,kw (Word variations have been searched) | — | 'vertical transmission'/de OR 'vertical transmission':ab,ti OR 'infectious disease transmission':ab,ti OR 'mother+to+child transmission':ab,ti OR mtct:ab,ti | — | — |
| #9 | #6 and #7 and #8 | — | caroten*:ab,ti OR retinoic:ab,ti OR 'retinol'/de OR retinol:ab,ti OR vitamin*:ab,ti OR 'vitamin a'/de OR micronutrient*:ab,ti | — | — |
| #10 | #6 and #7 and #8 Publication Year from 2016 to 2017 | — | #1 AND #7 AND #8 AND #9 | — | — |
| #11 | — | — | #1 AND #7 AND #8 AND #9 AND [20‐5‐2016]/sd NOT [25‐8‐2017]/sd | — | — |
| Search set | CENTRAL | PubMed | Embase |
| #1 | HIV Infections | HIV Infections OR HIV OR hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR hiv infect* OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR (human immun* AND deficiency virus) OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR (acquired immun* AND deficiency syndrome) OR "sexually transmitted diseases, Viral" | human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR hiv OR hiv‐1 OR hiv‐2 OR human immunodeficiency virus OR human immunedeficiency virus OR human immune‐deficiency virus OR human immuno‐deficiency virus OR acquired immunodeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR acquired immunedeficiency syndrome |
| #2 | HIV | randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR "clinical trials as topic" OR randomly OR trial) NOT (animals NOT humans) | randomized controlled trial OR randomized controlled trial OR random* OR trial OR allocat* OR factorial* OR placebo* OR assign* OR volunteer* OR crossover procedure OR crossover procedure OR double‐blind procedure OR double‐blind procedure OR single‐blind procedure OR single‐blind procedure OR doubl* NEAR/3 blind* OR singl* AND blind* OR crossover* OR cross+over* OR cross NEXT/1 over* |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or hiv near infect* or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immuno‐deficiency virus or human immune deficiency virus or human immuno deficiency virus or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun* deficiency syndrome | infectious disease transmission, vertical OR vertical transmission OR vertical infect* OR infection transmission OR mother‐to‐child transmission OR MTCT | animal OR animal experiment OR invertebrate OR animal tissue OR animal cell OR nonhuman |
| #4 | Lymphoma, AIDS‐Related | vitamin A OR vitamin* OR caroten* OR retinol OR retinoic OR micronutrient* | human OR normal human OR human cell |
| #5 | Sexually Transmitted Diseases, Viral | 1‐4/AND | #3 AND #4 |
| #6 | 1‐5/OR | 5 AND (2010/06/01 NOT 2016/05/20) | #3 NOT #5 |
| #7 | infectious disease transmission, vertical or vertical transmission or vertical next infect* or infection transmission or mother‐to‐child transmission or MTCT | — | #2 NOT #6 |
| #8 | vitamin A or vitamin* or caroten* or retinol or retinoic or micronutrient* | — | vertical transmission OR vertical transmission OR infectious disease transmission OR mother+to+child transmission OR mtct |
| #9 | 6‐8/AND | — | caroten* OR retinoic OR retinol OR retinol OR vitamin* OR vitamin a OR micronutrient* |
| #10 | Limit 9 to publication date 2010‐2016 | — | #1 AND #7 AND #8 AND #9 |
| #11 | — | — | #10 AND (2010/06/01 NOT 2016/05/20) |
| Search set | CENTRAL | PubMed | Embase |
| #1 | MeSH descriptor HIV Infections explode all trees | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus'OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab |
| #2 | MeSH descriptor HIV explode all trees | Search (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR allocat*:ti OR allocat*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/exp OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/exp OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'randomised controlled trial'/exp OR 'randomised controlled trial'/de OR 'randomised controlled trial' |
| #3 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME | Search mother‐to‐child‐transmission OR MTCT OR infectious disease transmission, vertical | 'mother‐to‐child transmission' OR 'mother to child transmission' OR mtct OR 'vertical transmission'/de OR 'vertical transmission' |
| #4 | MeSH descriptor Lymphoma, AIDS‐Related, this term only | Search caroten* OR retinoic OR retinol OR vitamin* OR vitamin A OR micronutrient* | caroten* OR retinoicOR 'retinol'/de OR retinolOR vitamin*OR 'vitamin a'/de OR 'vitamin a'OR micronutrient* |
| #5 | MeSH descriptor Sexually Transmitted Diseases, Viral, this term only | Search #1 AND #2 AND #3 AND #4 Limits: Publication Date from 2007/01/01 to 2010/06/08 | #1 AND #2AND #3AND #4 |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) | — | #1 AND #2AND #3AND #4AND [humans]/lim AND [embase]/lim AND [1‐1‐2007]/sd NOT [8‐6‐2010]/sd |
| #7 | mother‐to‐child‐transmission OR MTCT | — | — |
| #8 | MeSH descriptor Infectious Disease Transmission, Vertical, this term only | — | — |
| #9 | (#7 OR #8) | — | — |
| #10 | caroten* OR retinoic OR vitamin* OR vitamin A OR micronutrient* | — | — |
| #11 | (#6 AND #9 AND #10) | — | — |
| #12 | (#6 AND #9 AND #10), from 2007 to 2010 | — | — |
| Search set | PubMed | Embase | AIDSearch | GATEWAY |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] | (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection')) OR ((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'))) OR ((('b cell lymphoma'/exp OR 'b cell lymphoma') OR ('b cell lymphoma'/exp OR 'b cell lymphoma'))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) | Search: (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw]) AND (acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH]) |
| #2 | Search randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | (random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR (((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab))) OR (((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab))) OR (assign*:ti OR assign*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (((('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')) OR (('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')))) OR (((('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')) OR (('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')))) OR (((('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')) OR (('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')))) OR (((('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')) OR (('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')))) OR (allocat*:ti OR allocat*:ab) AND [2003‐2008]/py | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR ("CLINICAL TRIAL") OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND* )) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN ) | Search: (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw]))) OR (( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh])) |
| #3 | Search (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) | 'mother‐to‐child transmission' OR mtct OR 'vertical disease transmission' AND [2003‐2008]/py | (MOTHER‐TO‐CHILD TRANSMISSION) OR MTCT OR (VERTICAL DISEASE TRANSMISSION) | Search: (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) |
| #4 | Search CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | caroten* OR retinoic OR ('retinol'/exp OR 'retinol') OR vitamin* OR micronutrient* AND [2003‐2008]/py | CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | Search: CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* |
| #5 | Search PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* | pregnant OR ('pregnancy'/exp OR 'pregnancy') OR antepartum OR ('ante partum') OR antenatal OR ('ante natal') OR prenatal OR ('pre natal') AND [2003‐2008]/py | PREGNANT OR PREGNANCY OR ANTEPARTUM OR (ANTE‐PARTUM) OR ANTENATAL OR (ANTE‐NATAL) OR PRENATAL OR (PRE‐NATAL) | Search: PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* |
| #6 | Search #1 AND #2 AND #3 AND #4 AND #5 Limits: Publication Date from 2003 to 2008 | #1 AND #2 AND #3 AND #4 AND #5 | #1 AND #2 AND #3 AND #4 AND #5 | #1 and #2 and #3 and #4 and #5 Limit: 2003:2008 |
| Outcome | Assumed risk | Source | Clinically important relative improvement | Sample size required |
| HIV infection in child | 27/100 | Analysis 1.1 | 25% | 1236 |
| Mean birthweight | 2964 | Analysis 1.2 | 25% | 6178 |
| Low birthweight | 17/100 | Analysis 1.3 | 25% | 2194 |
| Still birth | 3/100 | Analysis 1.4 | 25% | 14,264 |
| Preterm birth | 20/100 | Analysis 1.5 | 25% | 1806 |
| Child death | 14/100 | Analysis 1.6 | 25% | 2748 |
| Maternal death | 3/100 | Analysis 1.7 | 25% | 14,264 |
| We based the sample size calculations: 2‐sided tests, with ratio of 1:1, power of 0.8 and confidence level of 0.05. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HIV infection status of the child Show forest plot | 5 | 4428 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 1.1 Antepartum supplementation | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| 1.2 Postpartum supplementation | 1 | 2248 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.98, 1.27] |
| 1.3 Antepartum and postpartum supplementation | 2 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.59] |
| 2 Mean birthweight Show forest plot | 3 | 2181 | Mean Difference (IV, Random, 95% CI) | 34.12 [‐12.79, 81.02] |
| 3 Low birthweight Show forest plot | 3 | 1819 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.97] |
| 4 Child death by two years of age Show forest plot | 3 | 3883 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.22] |
| 5 Preterm delivery Show forest plot | 2 | 1577 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.37] |
| 6 Stillbirth Show forest plot | 3 | 2335 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.72, 1.77] |
| 7 Maternal death Show forest plot | 2 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.43] |
| 8 Postpartum CD4 count Show forest plot | 1 | 511 | Mean Difference (IV, Random, 95% CI) | ‐13.0 [‐60.46, 34.46] |

