La supplémentation en vitamine A pour réduire la transmission materno‐foetale du VIH
Résumé scientifique
Contexte
Les stratégies visant à réduire le risque de transmission materno‐foetale du virus de l'immunodéficience humaine (VIH) incluent un traitement antirétroviral (TAR) permanent pour les femmes séropositives, l'allaitement exclusif pendant les six premières semaines de vie avec de la névirapine ou une alimentation de substitution dès la naissance pendant quatre à six semaines avec de la névirapine, l'accouchement par césarienne programmée et l'administration aux enfants d'aliments intacts (non mastiqués). Dans certains contextes, ces interventions peuvent ne pas être praticables, réalisables, ou accessibles. Des interventions simples, peu onéreuses et efficaces (qui pourraient être mises en œuvre, même en l'absence de programmes de dépistage du VIH prénatal) seraient précieuses. La vitamine A, qui joue un rôle dans la fonction immunitaire, est une intervention peu onéreuse qui a été suggérée dans ce type de contexte.
Objectifs
Résumer les effets de l'administration de suppléments en vitamine A pour les femmes séropositives pendant la grossesse et après l'accouchement
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre Cochrane des essais contrôlés (CENTRAL), PubMed, Embase, et le système d’enregistrement international des essais cliniques de l’Organisation Mondiale de la Santé (ICTRP de l'OMS), jusqu'au 25 août 2017, et nous avons vérifié les références bibliographiques des articles pertinents pour identifier les études éligibles.
Critères de sélection
Nous avons inclus les essais contrôlés randomisés conduits dans tout contexte comparant la supplémentation en vitamine A à un placebo ou à l'absence d'intervention chez les femmes séropositives pendant la grossesse ou après l'accouchement, ou les deux.
Recueil et analyse des données
Au moins deux auteurs de revue ont évalué l’éligibilité des études et extrait les données indépendamment. Nous avons exprimé les résultats des études sous la forme de risques relatifs (RR) ou de différences moyennes (DM) selon le cas, avec un intervalle de confiance (IC) à 95 % et nous avons effectué des méta‐analyses à effets aléatoires. Ceci est une mise à jour d'une revue publiée pour la dernière fois en 2011.
Résultats principaux
Cinq essais remplissaient les critères d'inclusion. Ces essais ont été conduits au Malawi, en Afrique du Sud, en Tanzanie et au Zimbabwe entre 1995 et 2005, et aucun des participants n’a reçu de traitement TAR. Les femmes assignées au bras d'intervention ont reçu de la supplémentation en vitamine A à différentes doses (quotidienne pendant la grossesse ; une dose unique immédiatement après l'accouchement, ou des doses quotidiennes pendant la grossesse avec une dose unique après l'accouchement). Les femmes assignées au bras de comparaison ont reçu un placebo identique (6601 femmes, 4 essais) ou n’ont pas reçu d’intervention (1 essai, 697 femmes). Quatre essais (portant sur 6995 femmes) avaient un faible risque de biais et un essai (portant sur 303 femmes) avait un risque élevé de biais d'attrition.
Les essais ont montré que la supplémentation en vitamine A chez les femmes séropositives pendant la grossesse, la période de post‐partum immédiate ou les deux, a probablement peu ou n’a pas d’effet sur la transmission mère‐enfant du VIH (RR = 1,07, IC à 95 % : 0,91 à 1,26 ; 4428 femmes, 5 essais, preuves de certitude modérée) et peut avoir peu ou n’a pas d’effet sur la mort de l’enfant à deux ans (RR 1,06, IC à 95 % : 0,92 à 1,22 ; 3883 femmes, 3 essais, preuves de certitude faible). Cependant, l'administration de suppléments en vitamine A pendant la grossesse peut augmenter le poids de naissance moyen (DM 34,12 g, IC à 95 % −12,79 à 81,02; 2181 femmes, 3 essais, preuves de faible certitude) et réduit probablement l'incidence de faible poids de naissance (RR 0,78, IC à 95 % 0,63 à 0,97 ; 1819 femmes ; 3 essais, preuves de certitude modérée) ; mais nous ne savons pas si la supplémentation en vitamine A affecte le risque d'accouchement prématuré (1577 femmes, 2 essais), de mortinaissances (2335 femmes, 3 essais), ou la mortalité maternelle (2 essais, 1267 femmes).
Conclusions des auteurs
La supplémentation en vitamine A postpartum ou antepartum, ou les deux,n'a probablement que peu ou pas d'effet sur la transmission mère‐enfant du VIH chez les femmes séropositives ne prenant pas de médicaments antirétroviraux. L'intervention a été désormais supplantée par le TAR, qui est largement disponible et efficace dans la prévention de la transmission materno‐foetale du VIH.
PICO
Résumé simplifié
La supplémentation en vitamine A pour réduire la transmission materno‐foetale de l'infection par le VIH
Quel est l'objectif de cette revue ?
Le principal objectif de cet article de Cochrane était d'évaluer les effets de l'administration de suppléments en vitamine A pour les femmes séropositives, pendant la grossesse ou après l'accouchement, ou les deux, sur le risque de transmission mère‐enfant du VIH. Les chercheurs de Cochrane ont rassemblé et examiné toutes les études pertinentes afin de répondre à cette question et ont pris en compte cinq essais. Ceci est une mise à jour d'une revue publiée pour la dernière fois en 2011.
Quel est le message clé de cette revue ?
L'administration de suppléments en vitamine A pour les femmes séropositives, pendant la grossesse ou après l'accouchement, ou les deux, n'a probablement que peu ou pas de différence au niveau du risque de transmission mère‐enfant du VIH (preuves de certitude modérée).
Quels sont les principaux résultats de la revue ?
Cinq essais remplissaient les critères d'inclusion de la revue. Deux essais venaient d’Afrique du Sud et trois essais venaient chacun du Malawi, la Tanzanie et le Zimbabwe. Les essais ont comparé des femmes recevant des suppléments de vitamine A à des femmes ne recevant pas ces suppléments. Aucun des participants n’a reçu de traitement antirétroviral (ART).
La revue montre que chez les femmes séropositives pour le VIH et ne recevant pas de traitement antirétroviral:
‐l'administration de suppléments en vitamine A pour les femmes séropositives pendant la grossesse, immédiatement après l'accouchement, ou à ces deux périodes, n'a probablement que peu ou pas d'effet sur le risque de transmission materno‐foetale du VIH (preuves de certitude modérée) et pourrait n'avoir que peu ou pas d'effet sur le décès à deux ans des enfants dont la mère était séropositive pour le VIH. (preuves de faible certitude) ;
‐l'administration de suppléments en vitamine A pour les femmes séropositives pendant la grossesse peut augmenter le poids de naissance moyen (preuves de faible certitude) et réduit probablement le nombre de bébés à faible poids de naissance (preuves de certitude modérée), mais on ignore si l'intervention a un effet sur le nombre de naissances prématurées, de mortinaissances, ou de décès parmi les femmes (preuves de très faible certitude).
L'intervention a été désormais supplantée par le TAR, qui est largement disponible et efficace dans la prévention de la transmission materno‐foetale du VIH.
Cette revue est‐elle à jour ?
Les auteurs de la revue ont recherché des études allant jusqu'au 25 août 2017.
Authors' conclusions
Summary of findings
| Population: HIV‐positive women during pregnancy and immediate postpartum period Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Assumed risk with no vitamin A | Corresponding risk with vitamin A supplements | |||||
| HIV infection status of the child | 27 per 100 | 29 per 100 | RR 1.07 (0.91 to 1.26) | 4428 | ⊕⊕⊕⊝ due to imprecision | Vitamin A supplements probably have little or no effect on mother‐to‐child transmission of HIV. |
| Mean birthweight | 2964 g | 34 g higher | MD 34.12 (−12.79 to 81.02) | 2181 | ⊕⊕⊝⊝ due to imprecision | Vitamin A supplements may increase the mean birthweight |
| Low birthweight | 17 per 100 | 13 per 100 | RR 0.78 | 1819 | ⊕⊕⊕⊝ due to imprecision | Vitamin A supplements probably reduce the incidence of low birthweight babies. |
| Child death by two years of age | 14 per 100 | 15 per 100 | RR 1.06 | 3883 | ⊕⊕⊝⊝ due to imprecision | Vitamin A supplements may have little or no effect on child death by two years of age. |
| Preterm delivery | 20 per 100 | 17 per 100 | RR 0.84 | 1577 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on preterm deliveries. |
| Stillbirth | 3 per 100 | 3 per 100 | RR 1.13 | 2335 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on stillbirths. |
| Maternal death | 3 per 100 | 2 per 100 | RR 0.71 | 1267 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on maternal deaths. |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded by 1 for imprecision, as the CI ranges from small benefits to a clinically important increase in harm. | ||||||
Background
Description of the condition
The Global Burden of Disease Study estimates that there were 38.8 million people living with the human immunodeficiency virus (HIV) worldwide in 2015, half of whom were women of childbearing age (Wang 2016). In addition, there are more than 600 new paediatric infections each day worldwide; most of which occur in sub‐Saharan Africa (UNAIDS 2015). Children mostly acquire these infections from their mothers during pregnancy, delivery, or breastfeeding.
The high number of women who are of childbearing age and who are living with HIV makes the prevention of mother‐to‐child transmission of HIV a global health priority (UNAIDS 2015). Current strategies to reduce the risk of transmission include lifelong antiretroviral therapy (ART) to HIV‐positive women, exclusive breastfeeding from birth for six weeks plus nevirapine, or replacement feeding plus nevirapine from birth for four to six weeks (WHO 2015), elective Caesarean section delivery (Read 2005), and avoiding giving children chewed food. Despite their benefits, these interventions are impractical in many resource‐limited countries because they require the determination of the HIV status of pregnant women and are costly, complex, and require skilled personnel. Simple, inexpensive, and effective interventions that could potentially be implemented in the absence of prenatal HIV testing programmes would be valuable. Vitamin A supplementation during pregnancy is one low‐cost intervention that has been suggested (Newell 2000).
Description of the intervention
Vitamin A refers to a group of unsaturated organic compounds that include preformed vitamin A and provitamin A carotenoids (Damodaran 2017). The vitamin exists as preformed retinol in animal food sources, retinyl esters in fortified foods, and provitamin A carotenoids in plant sources. Both preformed vitamin A and provitamin A are metabolized in cells to retinal and retinoic acid, the active forms of vitamin A, to upkeep the vitamin’s multiple biological functions (Thorne‐Lyman 2012).
The biological functions of vitamin A include the regulation and promotion of growth and differentiation of many cells, and maintenance of the integrity of the epithelial cells of the respiratory and digestive tracts. Vitamin A is necessary for formation of the photosensitive visual pigment in the retina, and reproductive functions (Wolf 2001; Tanumihardjo 2011). In the 1920s the vitamin was considered to be an anti‐infective agent (Green 1928), and there is increasing evidence that it is essential for normal immune function (Ross 1996; Semba 1998).
Vitamin A deficiency is most prevalent in areas where diets lack preformed vitamin A, such as in South and Southeast Asia, and Sahel and sub‐Saharan regions of Africa (West 2001). It has been estimated that about 19 million pregnant women in low‐income countries are affected with vitamin A deficiency each year (West 2002; WHO 2009). Vitamin A deficiency in pregnant women is associated with night blindness, severe anaemia, wasting, malnutrition, reproductive and infectious morbidity (Christian 1998a), and increased risk of mortality one to two years following delivery (Christian 2000). About 10 million women suffer from night blindness during pregnancy as a result of Vitamin A deficiency, and this is associated with a constellation of adverse health and nutritional conditions among mothers and their infants (Christian 1998b; Christian 1998c; Christian 2001; WHO 2009).
How the intervention might work
In areas where poor diet and infection coexist, Vitamin A deficiency can increase the severity of infection, which in turn can reduce intake and accelerate body losses of vitamin A to exacerbate deficiency. Vitamin A deficiency and infection in vulnerable groups (notably young children and pregnant or lactating mothers) represent the most compelling consequences of vitamin A deficiency and underlie its significance as a public health problem around the world (WHO 2009).
Observational studies in sub‐Saharan Africa have shown low serum vitamin A levels in HIV‐positive women to be associated with significantly increased rates of mother‐to‐child transmission of HIV (Semba 1994) and infant mortality (Semba 1995). However, three observational studies in the USA provided conflicting results: low serum vitamin A was associated with a higher risk of mother‐to‐child transmission of HIV in one study (Greenberg 1997), but not the other two (Burger 1997; Burns 1999). These studies used different definitions for vitamin A deficiency: two studies used serum retinol levels of less than 30 mg/dL (Greenberg 1997; Burns 1999), and the other study used less than 20 mg/dL (Burger 1997). The studies also had small sample sizes; for example, in Burger 1997, only 4/95 (4.2%) of women had serum retinol levels of less than 20 mg/dL.
Vitamin A was hypothesized to decrease mother‐to‐child transmission of HIV by acting through several maternal, foetal, child risk factors for transmission, or all three. The proposed risk factors were the clinical, immunological, or viral stage of HIV disease among women; the integrity of the epithelial lining of the placenta, maternal lower genital tract, or breast; the occurrence of prematurity and low birthweight; and the status of the systemic and digestive mucosal immune systems of the foetus and the child (Fawzi 1998; Fawzi 2000).
Why it is important to do this review
Even though there have been dramatic reductions in the number of new HIV infections among children (UNAIDS 2015), the magnitude of the paediatric HIV epidemic in resource‐limited countries is still important. The simplicity and low cost of vitamin A supplementation makes the clarification of its role in mother‐to‐child transmission of HIV of considerable importance. We aimed to combine all high‐quality randomized controlled trials (RCTs) conducted to date to estimate the effect of vitamin A supplementation on mother‐to‐child transmission of HIV. This is an update of a Cochrane Review published in 2011 (Wiysonge 2011).
Objectives
To summarize the effects of giving vitamin A supplements to HIV‐positive women during pregnancy and after delivery.
Methods
Criteria for considering studies for this review
Types of studies
RCTs.
Types of participants
Pregnant or breastfeeding women, confirmed HIV‐positive by a validated laboratory test. The women could be of any age, at any clinical stage of HIV disease, and could be living in any setting.
Types of interventions
Intervention
Vitamin A supplementation, irrespective of formulation (retinol with or without beta‐carotene), timing of supplementation (antepartum, postpartum, or both), dosing, or duration of supplementation. We conducted sensitivity analyses to investigate the robustness of the results to the inclusion of trials that used beta‐carotene.
Control
Eligible comparison interventions included placebo or no intervention.
Types of outcome measures
Primary outcomes
-
HIV infection status of the child.
Secondary outcomes
Child
-
Mean birthweight.
-
Low birthweight, defined as birthweight less than 2500 g.
-
Child death by two years of age.
-
Preterm delivery, defined as birth at less than 37 weeks of gestation.
-
Stillbirth.
Mother
-
Maternal death.
-
Postpartum CD4 count.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published or unpublished) up to 25 August 2017 (Table 1).
| Search set | CENTRAL | PubMed | Embase | WHO ICTRP | ClinicalTrials.gov |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | Search ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR 'human immunodeficiency virus:ab,ti' OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'human immunodeficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune‐deficiency virus':ab,ti OR 'human immuno‐deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno‐deficiency syndrome':ab,ti OR 'acquired immune‐deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti | hiv AND vitamin A OR hiv AND retinol OR hiv AND retinoic OR hiv AND micronutrients OR hiv AND carotene | HIV and "VITAMIN A" | Interventional Studies | Studies received from 05/20/2016 to 08/25/2017 |
| #2 | MeSH descriptor: [HIV] explode all trees | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR "clinical trials as topic"[mesh: noexp] OR randomly [tiab] OR trial [tiab]) NOT (animals [mh] NOT humans [mh]) | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR ((doubl* NEAR/3 blind*):ab,ti) OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR ((cross NEXT/1 over*):ab,ti) | — | — |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | Search (infectious disease transmission, vertical[mh] OR vertical transmission[tiab] OR vertical infect*[tiab] OR infection transmission[tiab] OR mother‐to‐child transmission[tiab] OR MTCT[tiab]) | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | — | — |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | Search (vitamin A[mh] OR vitamin*[tiab] OR caroten*[tiab] OR retinol[tiab] OR retinoic[tiab] OR micronutrient*[tiab]) | 'human'/de OR 'normal human'/de OR 'human cell'/de | — | — |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | Search (#1 AND #2 AND #3 AND #4) | #3 AND #4 | — | — |
| #6 | #1 or #2 or #3 or #4 or #5 | Search (((#1 AND #2 AND #3 AND #4))) AND ("2016/05/20"[Date ‐ Publication] : "2017/08/25"[Date ‐ Publication]) | #3 NOT #5 | — | — |
| #7 | [mh "infectious disease transmission, vertical"] or "vertical transmission":ti,ab,kw or vertical next infect*:ti,ab,kw or "infection transmission":ti,ab,kw or "mother‐to‐child transmission":ti,ab,kw or MTCT:ti,ab,kw (Word variations have been searched) | — | #2 NOT #6 | — | — |
| #8 | [mh "vitamin A"] or vitamin*:ti,ab,kw or caroten*:ti,ab,kw or retinol:ti,ab,kw or retinoic:ti,ab,kw or micronutrient*:ti,ab,kw (Word variations have been searched) | — | 'vertical transmission'/de OR 'vertical transmission':ab,ti OR 'infectious disease transmission':ab,ti OR 'mother+to+child transmission':ab,ti OR mtct:ab,ti | — | — |
| #9 | #6 and #7 and #8 | — | caroten*:ab,ti OR retinoic:ab,ti OR 'retinol'/de OR retinol:ab,ti OR vitamin*:ab,ti OR 'vitamin a'/de OR micronutrient*:ab,ti | — | — |
| #10 | #6 and #7 and #8 Publication Year from 2016 to 2017 | — | #1 AND #7 AND #8 AND #9 | — | — |
| #11 | — | — | #1 AND #7 AND #8 AND #9 AND [20‐5‐2016]/sd NOT [25‐8‐2017]/sd | — | — |
The HIV Information Specialist, Joy Oliver, searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, Clinicaltrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) using the search strategies shown in Table 1 and Table 2. We have also provided the search strategy for previous editions of the review in Table 3 and Table 4.
| Search set | CENTRAL | PubMed | Embase |
| #1 | HIV Infections | HIV Infections OR HIV OR hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR hiv infect* OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR (human immun* AND deficiency virus) OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR (acquired immun* AND deficiency syndrome) OR "sexually transmitted diseases, Viral" | human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR hiv OR hiv‐1 OR hiv‐2 OR human immunodeficiency virus OR human immunedeficiency virus OR human immune‐deficiency virus OR human immuno‐deficiency virus OR acquired immunodeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR acquired immunedeficiency syndrome |
| #2 | HIV | randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR "clinical trials as topic" OR randomly OR trial) NOT (animals NOT humans) | randomized controlled trial OR randomized controlled trial OR random* OR trial OR allocat* OR factorial* OR placebo* OR assign* OR volunteer* OR crossover procedure OR crossover procedure OR double‐blind procedure OR double‐blind procedure OR single‐blind procedure OR single‐blind procedure OR doubl* NEAR/3 blind* OR singl* AND blind* OR crossover* OR cross+over* OR cross NEXT/1 over* |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or hiv near infect* or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immuno‐deficiency virus or human immune deficiency virus or human immuno deficiency virus or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun* deficiency syndrome | infectious disease transmission, vertical OR vertical transmission OR vertical infect* OR infection transmission OR mother‐to‐child transmission OR MTCT | animal OR animal experiment OR invertebrate OR animal tissue OR animal cell OR nonhuman |
| #4 | Lymphoma, AIDS‐Related | vitamin A OR vitamin* OR caroten* OR retinol OR retinoic OR micronutrient* | human OR normal human OR human cell |
| #5 | Sexually Transmitted Diseases, Viral | 1‐4/AND | #3 AND #4 |
| #6 | 1‐5/OR | 5 AND (2010/06/01 NOT 2016/05/20) | #3 NOT #5 |
| #7 | infectious disease transmission, vertical or vertical transmission or vertical next infect* or infection transmission or mother‐to‐child transmission or MTCT | — | #2 NOT #6 |
| #8 | vitamin A or vitamin* or caroten* or retinol or retinoic or micronutrient* | — | vertical transmission OR vertical transmission OR infectious disease transmission OR mother+to+child transmission OR mtct |
| #9 | 6‐8/AND | — | caroten* OR retinoic OR retinol OR retinol OR vitamin* OR vitamin a OR micronutrient* |
| #10 | Limit 9 to publication date 2010‐2016 | — | #1 AND #7 AND #8 AND #9 |
| #11 | — | — | #10 AND (2010/06/01 NOT 2016/05/20) |
| Search set | CENTRAL | PubMed | Embase |
| #1 | MeSH descriptor HIV Infections explode all trees | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus'OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab |
| #2 | MeSH descriptor HIV explode all trees | Search (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR allocat*:ti OR allocat*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/exp OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/exp OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'randomised controlled trial'/exp OR 'randomised controlled trial'/de OR 'randomised controlled trial' |
| #3 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME | Search mother‐to‐child‐transmission OR MTCT OR infectious disease transmission, vertical | 'mother‐to‐child transmission' OR 'mother to child transmission' OR mtct OR 'vertical transmission'/de OR 'vertical transmission' |
| #4 | MeSH descriptor Lymphoma, AIDS‐Related, this term only | Search caroten* OR retinoic OR retinol OR vitamin* OR vitamin A OR micronutrient* | caroten* OR retinoicOR 'retinol'/de OR retinolOR vitamin*OR 'vitamin a'/de OR 'vitamin a'OR micronutrient* |
| #5 | MeSH descriptor Sexually Transmitted Diseases, Viral, this term only | Search #1 AND #2 AND #3 AND #4 Limits: Publication Date from 2007/01/01 to 2010/06/08 | #1 AND #2AND #3AND #4 |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) | — | #1 AND #2AND #3AND #4AND [humans]/lim AND [embase]/lim AND [1‐1‐2007]/sd NOT [8‐6‐2010]/sd |
| #7 | mother‐to‐child‐transmission OR MTCT | — | — |
| #8 | MeSH descriptor Infectious Disease Transmission, Vertical, this term only | — | — |
| #9 | (#7 OR #8) | — | — |
| #10 | caroten* OR retinoic OR vitamin* OR vitamin A OR micronutrient* | — | — |
| #11 | (#6 AND #9 AND #10) | — | — |
| #12 | (#6 AND #9 AND #10), from 2007 to 2010 | — | — |
| Search set | PubMed | Embase | AIDSearch | GATEWAY |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] | (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection')) OR ((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'))) OR ((('b cell lymphoma'/exp OR 'b cell lymphoma') OR ('b cell lymphoma'/exp OR 'b cell lymphoma'))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) | Search: (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw]) AND (acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH]) |
| #2 | Search randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | (random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR (((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab))) OR (((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab))) OR (assign*:ti OR assign*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (((('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')) OR (('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')))) OR (((('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')) OR (('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')))) OR (((('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')) OR (('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')))) OR (((('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')) OR (('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')))) OR (allocat*:ti OR allocat*:ab) AND [2003‐2008]/py | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR ("CLINICAL TRIAL") OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND* )) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN ) | Search: (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw]))) OR (( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh])) |
| #3 | Search (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) | 'mother‐to‐child transmission' OR mtct OR 'vertical disease transmission' AND [2003‐2008]/py | (MOTHER‐TO‐CHILD TRANSMISSION) OR MTCT OR (VERTICAL DISEASE TRANSMISSION) | Search: (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) |
| #4 | Search CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | caroten* OR retinoic OR ('retinol'/exp OR 'retinol') OR vitamin* OR micronutrient* AND [2003‐2008]/py | CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | Search: CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* |
| #5 | Search PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* | pregnant OR ('pregnancy'/exp OR 'pregnancy') OR antepartum OR ('ante partum') OR antenatal OR ('ante natal') OR prenatal OR ('pre natal') AND [2003‐2008]/py | PREGNANT OR PREGNANCY OR ANTEPARTUM OR (ANTE‐PARTUM) OR ANTENATAL OR (ANTE‐NATAL) OR PRENATAL OR (PRE‐NATAL) | Search: PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* |
| #6 | Search #1 AND #2 AND #3 AND #4 AND #5 Limits: Publication Date from 2003 to 2008 | #1 AND #2 AND #3 AND #4 AND #5 | #1 AND #2 AND #3 AND #4 AND #5 | #1 and #2 and #3 and #4 and #5 Limit: 2003:2008 |
Data collection and analysis
Two review authors evaluated study eligibility, assessed risk of bias, and extracted data in duplicate. The two authors resolved any disagreements by discussion and consensus. The review authors involved in evaluating study eligibility, assessing risk of bias, and extracting data were not blinded to the names of the trial authors, their institutions, or journals of publication. We reported the data collection and analysis procedures of previous editions of this Cochrane Review in the previous published versions of this review (Wiysonge 2002; Wiysonge 2005; Kongnyuy 2009; Wiysonge 2011).
Selection of studies
Two review authors (either CSW and VNN or CSW and EJK) screened the literature search results by title and abstract for potentially relevant trials and retrieved the full‐text articles as required. The two review authors then independently assessed trial eligibility and resolved differences by discussion and consensus. We considered a trial with multiple publications as one trial. We contacted the corresponding authors of two potentially eligible trials to obtain unpublished data (Chikobvu 2000; Friis 2004). We listed all studies that we excluded after full‐text assessment and the reasons for exclusion in the ʽCharacteristics of excluded studies' table. We constructed a PRISMA flow diagram to illustrate the study selection process.
Data extraction and management
Two review authors (CSW and VNN, CSW and EJK, or CSW and MSS) extracted information on study methods, participants, interventions, and outcomes from each included trial, and reported this information in the ʽCharacteristics of included studies' table.
Assessment of risk of bias in included studies
For each included trial, two review authors (CSW and VNN, CSW and EJK, or CSW and MSS) independently assessed the risk of bias by addressing seven prespecified domains (Higgins 2011): generation of the randomization sequence, concealment of the allocation of interventions, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, completeness of outcome reporting, and any other concerns.
We described what the trial authors reported that they did for each domain and then decided the risk of bias for that domain by assigning a judgement of ʽlow', ʽhigh', or ʽunclear' risk of bias. We summarized the risk of bias for each trial as either low or high. We classified any trial that had a high risk of bias linked to allocation concealment, blinding of outcome assessment, or completeness of outcome data as having a high risk of bias. We considered all other trials to have low risk of bias.
Measures of treatment effect
We expressed each study result as the risk ratio (RR) for dichotomous data or the mean difference (MD) for continuous data, with 95% confidence intervals (CIs).
Unit of analysis issues
There were no unit of analysis issues in this Cochrane Review, as all eligible trials were individually randomized.
Dealing with missing data
We conducted a complete‐case analysis such that we included all participants with a recorded outcome in the analyses. We have reported all missing data and trial dropouts (see the ʽCharacteristics of included studies' table). One trial reported results as means with their standard errors (SE) instead of standard deviations (SD) (Kumwenda 2002). We calculated the SD using the following formula: SD = (square root of N) x (SE).
Assessment of heterogeneity
We assessed the heterogeneity of effects across included trials by visually inspecting the graphical display of data in forest plots and using the Chi² test of homogeneity. In the presence of significant statistical heterogeneity, defined as P < 0.1, we first checked data accuracy to exclude data capturing errors. We quantified observed heterogeneity using the Higgins I² statistic.
Assessment of reporting biases
If we had 10 or more trials included in a meta‐analysis, we would have used funnel plots to assess the risk of publication bias. In such circumstances, we would have assessed the funnel plot visually, followed by formal statistical tests to assess any observed funnel plot asymmetry (Egger 1997; Harbord 2006). Apart from reporting biases, potential causes of funnel plot asymmetry may include high risk of bias, true heterogeneity of effects, and chance (Higgins 2011).
Data synthesis
We used both unpublished (Chikobvu 2000), and published data (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006), to analyse trial participants in groups to which they were randomized, regardless of which or how much treatment they actually received.
Two trials used two‐by‐two factorial designs (Fawzi 2002; Humphrey 2006).
In the first trial, women and their newborns were recruited within four days of delivery and randomly assigned to four treatment groups: Aa, Ap, Pa, and Pp. “A” was maternal vitamin A supplementation, “P” was maternal placebo, “a” was infant vitamin A supplementation, and “p” was infant placebo (Humphrey 2006). In this Cochrane Review, we only used data from Ap (intervention) versus Pp (placebo).
In the second trial with a two‐by‐two factorial design, pregnant women were randomly assigned to receive either "vitamin A alone", "vitamin A plus multivitamins", "multivitamins without vitamin A", or "placebo" (Fawzi 2002). Although the trial authors stated that there was no evidence of clinical interaction between vitamin A and multivitamins, our plan was to consider only the "vitamin A alone" arm as the intervention. However, in the multiple publications from this trial, separate data for "vitamin A alone" were only available for low birthweight and maternal deaths. Thus, for the other outcomes we used data as reported by the trial authors, that is, vitamin A (a combination of "vitamin A alone" and"vitamin A plus multivitamins" arms) versus no vitamin A (consisting of "multivitamins without vitamin A" and "placebo" arms).
We conducted random‐effects meta‐analyses in RevMan 5 because of the diversity of study designs, type of intervention (that is, preformed vitamin A with or without beta‐carotene), timing of intervention (that is, antepartum supplementation, postpartum supplementation, or both), dosing of the supplements, and comparison groups (that is, placebo or no intervention) (RevMan 2014).
We also calculated the optimal information size for the outcomes and presented this information in Table 5. In addition, we used the GRADE approach to assess the certainty of the evidence for the effect of vitamin A supplementation on each key outcome (Guyatt 2008). We constructed a ʽSummary of findings' table using GRADEpro software (GRADEpro GDT 2015).
| Outcome | Assumed risk | Source | Clinically important relative improvement | Sample size required |
| HIV infection in child | 27/100 | Analysis 1.1 | 25% | 1236 |
| Mean birthweight | 2964 | Analysis 1.2 | 25% | 6178 |
| Low birthweight | 17/100 | Analysis 1.3 | 25% | 2194 |
| Still birth | 3/100 | Analysis 1.4 | 25% | 14,264 |
| Preterm birth | 20/100 | Analysis 1.5 | 25% | 1806 |
| Child death | 14/100 | Analysis 1.6 | 25% | 2748 |
| Maternal death | 3/100 | Analysis 1.7 | 25% | 14,264 |
We based the sample size calculations: 2‐sided tests, with ratio of 1:1, power of 0.8 and confidence level of 0.05.
We performed the sample size calculations using http://www.sealedenvelope.com/power/binary‐superiority/
Subgroup analysis and investigation of heterogeneity
For the primary outcome, we conducted a subgroup analysis based on the timing of vitamin A supplementation, that is antepartum supplementation (Chikobvu 2000; Kumwenda 2002), postpartum supplementation (Humphrey 2006), or both (Coutsoudis 1999; Fawzi 2002).
Sensitivity analysis
We included trials that provided supplements containing only preformed vitamin A (Chikobvu 2000; Kumwenda 2002; Humphrey 2006), and trials that used both preformed vitamin A (retinol) and a provitamin A carotenoid (beta‐carotene) (Coutsoudis 1999; Fawzi 2002).
Beta‐carotene is easily converted to retinol, but also has antioxidative properties (Thorne‐Lyman 2012). We therefore conducted sensitivity analyses to investigate the robustness of the results on the primary outcome to the type of intervention (that is, we omitted trials that used beta‐carotene).
Results
Description of studies
Results of the search
Figure 1 shows the search and study selection process for this Cochrane Review, in line with the PRISMA statement (Moher 2009).
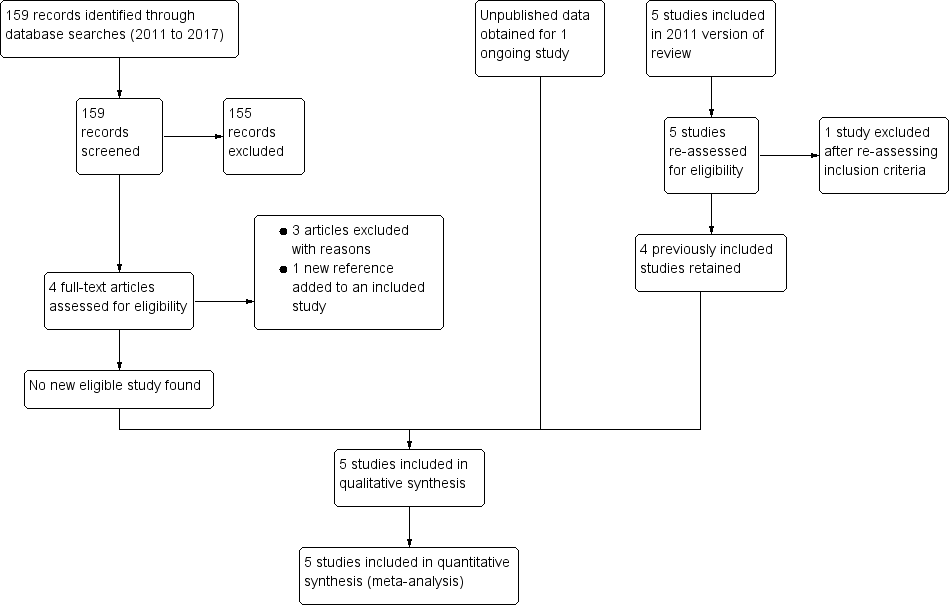
PRISMA flow diagram
Of the five trials included in the previous version of this review, Wiysonge 2011, we retained four of the previously included trials (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006), and excluded one trial because further assessment revealed that it did not meet our inclusion criteria (Friis 2004). We obtained unpublished data for one ongoing trial (Chikobvu 2000). Of the 159 records identified from the updated literature search, we excluded 158 and identified one new article to add to an already included study.
Included studies
Five trials met the inclusion criteria (see Figure 1 and the ʽCharacteristics of included studies' table). These include a trial that we classified as ongoing in previous published versions of this review (Chikobvu 2000). The trial was conducted from September 1997 to December 2000 in South Africa, and the principal investigator of the trial has provided us with outcome data on mother‐to‐child transmission of HIV (Chikobvu 2000). We briefly describe below the designs, participants, interventions, comparisons, and outcome measures of the five included trials.
Period of study
These five included trials were conducted between 1995 and 2005, at the height of the HIV epidemic in sub‐Saharan Africa.
Design
Three of the included trials were randomized trials (Fawzi 2002; Kumwenda 2002; Humphrey 2006). Two trials were described as randomized trials, but the trial authors did not describe the methods used to generate the randomization sequence (Coutsoudis 1999; Chikobvu 2000). Two trials had two‐by‐two factorial designs (Fawzi 2002; Humphrey 2006). All trials used participants as units of randomization. The proportion of participants lost to follow‐up ranged from 3.2% (Humphrey 2006), to more than 48% (Chikobvu 2000). All trial authors excluded mother‐infant pairs lost to follow‐up from their analyses.
Location
The five trials were conducted in four countries in sub‐Saharan Africa: Malawi (one trial; Kumwenda 2002), South Africa (two trials; Coutsoudis 1999; Chikobvu 2000), Tanzania (one trial; Fawzi 2002), and Zimbabwe (one trial; Humphrey 2006).
Population
In trials with antepartum vitamin A supplementation, participants consisted of HIV‐positive pregnant women recruited during their first antenatal visit (Chikobvu 2000), 12 to 27 weeks' gestation (Fawzi 2002), 18 to 28 weeks' gestation (Kumwenda 2002), and 17 to 39 weeks' gestation (Coutsoudis 1999). For the single trial of postpartum vitamin A supplementation, HIV‐positive women were recruited within 96 hours of delivery (Humphrey 2006).The prevalence of vitamin A deficiency among the women at baseline was 30.6% in Coutsoudis 1999, 34% in Fawzi 2002, and 51% in Kumwenda 2002; but was not reported in two trials (Chikobvu 2000; Humphrey 2006).
Interventions
Vitamin A supplements
Three trials used supplements that contained retinol alone (Chikobvu 2000; Kumwenda 2002; Humphrey 2006), and two trials used both retinol and beta‐carotene (Coutsoudis 1999; Fawzi 2002).
Two trials provided vitamin A supplements to women only during pregnancy (Chikobvu 2000; Kumwenda 2002). One trial provided vitamin A during pregnancy, but did not report information on the type or dose of vitamin A used (Chikobvu 2000). The second trial provided pregnant women in the intervention arm with 10,000 IU daily oral doses of vitamin A (Kumwenda 2002).
One trial provided vitamin A supplements only after delivery (Humphrey 2006). This trial had a factorial design and the interventions consisted of a single oral dose of 400,000 IU vitamin A to the mother only; 50,000 IU single oral dose to the newborn only; or 400,000 IU to the mother and 50,000 IU to the newborn (Humphrey 2006). In our analyses, we considered only the group in which the mother alone received vitamin A supplements as the intervention arm.
In two trials, women received vitamin A supplements both during pregnancy and immediately after delivery (Coutsoudis 1999; Fawzi 2002). In the first trial, women in the intervention arm received 5000 IU retinyl palmitate and 30 mg beta‐carotene daily during pregnancy. In addition, at delivery, women in the intervention arm received 200,000 IU of retinyl palmitate (Coutsoudis 1999). The second trial employed a two‐by‐two factorial design (Fawzi 2002). Women in the intervention arms received daily doses of vitamin A (30 mg beta carotene plus 5000 IU retinyl palmitate) alone; vitamin A plus multivitamins (20 mg vitamin B1, 20 mg vitamin B2, 25 mg vitamin B6, 100 mg niacin, 50 µg vitamin B12, 500 mg vitamin C, 30 mg vitamin E, and 0.8 mg folic); or multivitamins alone. At delivery, women receiving any vitamin A were given an additional 200,000 IU oral dose of vitamin A (Fawzi 2002).
Concomitant interventions
Two trials did not provide information on any concomitant interventions (Coutsoudis 1999; Chikobvu 2000). In the remaining three trials, the pregnant women received daily oral doses of iron and folic acid (Fawzi 2002; Kumwenda 2002; Humphrey 2006). One trial also reported providing weekly doses of chloroquine (Fawzi 2002), and another trial provided all women with oral vitamin A (100,000 IU) at six weeks postpartum, according to national policy (Kumwenda 2002). In one trial all children, regardless of whether they were in the intervention or comparison arm, received 100,000 IU vitamin A at six months of age and 200,000 IU of vitamin A every six months afterwards (Fawzi 2002).
Antiretroviral therapy (ART)
None of the five included trials reported giving ART to participants.
Comparison interventions
In four trials, the comparison intervention was a placebo (Coutsoudis 1999; Chikobvu 2000; Fawzi 2002; Humphrey 2006). The fifth study used a "no intervention" comparison group (Kumwenda 2002). All women in this trial received iron and folic acid, and half of them were randomly assigned to receive vitamin A. Supplements that contained vitamin A, iron, and folic acid were indistinguishable from the ones that contained only iron and folic acid (Kumwenda 2002).
Outcome measures
Primary outcomes
HIV infection status of the child
We obtained data on children's HIV infection status at three months (Coutsoudis 1999; Chikobvu 2000), and at 24 months (Fawzi 2002; Kumwenda 2002; Humphrey 2006). A child was determined to be HIV‐positive when a blood specimen tested positive using polymerase chain reaction (PCR) at any point or a plasma specimen obtained at 18 months of age or older tested positive using enzyme‐linked immunosorbent assay (ELISA).
Secondary outcomes
Birthweight
Three trials reported data on mean birthweight (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002).
Child death by two years of age
Three trials reported data on child death by 24 months of age (Fawzi 2002; Kumwenda 2002; Humphrey 2006).
Low birthweight
Three trials reported data on the occurrence of low birthweight, that is, children born with birthweight less than 2500g (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002).
Preterm delivery
Two trials reported data on preterm deliveries, that is, births at less than 37 weeks of gestation (Coutsoudis 1999; Fawzi 2002).
Stillbirth
Three trials reported data on stillbirths (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002).
Maternal death
One trial reported data on maternal deaths from any cause by three months after delivery (Coutsoudis 1999). The second trial reported data on maternal deaths from AIDS‐related causes at two and four years (Fawzi 2002). We have used the two‐year data in this review.
Postpartum CD4 count
One trial reported postpartum CD4 cell count at two and four years (Fawzi 2002). We have used the two‐year data in this review.
Excluded studies
We included Friis 2004 in the previous version of this review (Wiysonge 2011), but excluded the study from this review update because the intervention consisted of multivitamins (including vitamin A) rather than vitamin A alone. We identified 159 records from literature searches. We excluded 155 records after screening by title/abstract. Of the four remaining studies, we excluded three studies after full‐text assessment (Duggan 2012; Olofin 2016; Locks 2017), and identified one new reference to an already included study. We excluded the remaining three studies because they assessed the effects of multivitamins (and not vitamin A) and had as participants, children born to HIV‐positive women (rather than the women themselves) (Duggan 2012; Olofin 2016; Locks 2017).
Risk of bias in included studies
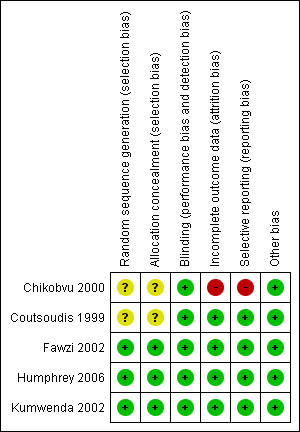
We have provided detailed ʽRisk of bias' assessments of the included trials in the ʽCharacteristics of included studies' table, and provided a summary in Figure 2 and Figure 3.

ʽRisk of bias' graph: review authors' judgements about each ʽRisk of bias' item presented as percentages across all included trials
ʽRisk of bias' summary: review authors' judgements about each ʽRisk of bias' item for each included trial
Allocation
Sequence generation
Regarding sequence generation, three trials were at low risk of bias (Fawzi 2002; Kumwenda 2002; Humphrey 2006), and the risk of bias in two trials was unclear (Coutsoudis 1999; Chikobvu 2000). The authors of the latter trials did not clearly describe the methods used to generate the randomization sequence (Coutsoudis 1999; Chikobvu 2000).
Allocation concealment
Three trials were at low risk of bias as per allocation concealment (Fawzi 2002; Humphrey 2006; Kumwenda 2002). We judged two trials to have an unclear risk of bias (Coutsoudis 1999; Chikobvu 2000), as the trial authors did not describe the methods used to conceal allocation to intervention and comparison groups.
Blinding
All five trials were at low risk of performance bias because participants and care providers were blinded to treatment allocation.
The five included trials performed blinding of outcome assessors, thus we judged them to be at low risk of detection bias.
Incomplete outcome data
Four trials had low risk of bias in relation to completeness of outcome data (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006), but we judged one trial to be at high risk of bias (Chikobvu 2000). The proportion of participants lost to follow‐up was 3.2% in Humphrey 2006, 5.0% in Fawzi 2002, 7.8% in Coutsoudis 1999, 9.0% in Kumwenda 2002, and more than 48% in Chikobvu 2000. Therefore, the proportion of randomized participants with evaluable data ranged from less than 52% (Chikobvu 2000), to 96.8% (Humphrey 2006).
Selective reporting
We judged the risk of reporting bias to be low in four trials (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006). However, we observed evidence of selective reporting in one trial, which we judged to be at high risk of reporting bias (Chikobvu 2000). The trial did not report data on mother‐to‐child transmission of HIV because of high loss to follow‐up (Chikobvu 2000).
Other potential sources of bias
We observed no other potential sources of bias in included studies.
Summary of 'Risk of bias' assessment
Overall, four trials (with 6995 women) were at low risk of bias (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006), and one trial (with 303 women) was at high risk of bias (Chikobvu 2000).
Effects of interventions
We have summarized the effects of vitamin A supplementation of HIV‐positive women during pregnancy, immediately after delivery, or both, on key outcomes in the ʽSummary of findings' table (summary of findings Table for the main comparison).
Primary outcome
HIV infection status of the child
The risk ratio for the effect of vitamin A supplementation during pregnancy on mother‐to‐child transmission of HIV was 0.85 (95% CI 0.67 to 1.09; 650 women, 2 trials) and for vitamin A supplementation immediately after delivery was 1.11 (95% CI 0.98 to 1.09; 2248 women, 1 trial). In the two trials that provided vitamin A supplementation to women during pregnancy and immediately after delivery, the RR was 1.17 (95% CI 0.86 to 1.59; 1530 women, 2 trials).
Overall, the trials show that vitamin A supplementation of HIV‐positive women during pregnancy or the immediate postpartum period, or both, probably has little or no effect on the risk of mother‐to‐child transmission of HIV (RR 1.07, 95% CI 0.91 to 1.26; 4428 women, 5 trials, moderate certainty evidence). There were no substantial differences in effects across the three subgroups (heterogeneity P = 0.15, I² statistic = 48.1%; Analysis 1.1).
The effects were similar between studies that provided supplements containing only retinol (RR 1.00, 95% CI 0.81 to 1.22; 2898 women, 3 trials) and those that provided both retinol and beta‐carotene (RR 1.17, 95% CI 0.86 to 1.59; 1530 women, 2 trials).
Secondary outcomes
Child
Birthweight
Vitamin A supplementation of HIV‐positive women during pregnancy may increase the mean birth weight of babies (MD 34.12 g, 95% CI −12.79g to 81.02; 2181 women, 3 trials, low certainty evidence). The effect was fairly consistent across the three trials (heterogeneity P = 0.34, I² statistic 8%; Analysis 1.2).
Low birthweight
Vitamin A supplementation of HIV‐positive pregnant women probably reduces the incidence of low birthweight (RR 0.78, 95% CI 0.63 to 0.97; 1819 women, 3 trials, moderate certainty evidence). The effect was homogenous across the three included trials (heterogeneity P = 0.56; I² statistic = 0%; Analysis 1.3).
Child death by two years of age
Vitamin A supplementation of HIV‐positive women during pregnancy or the immediate postpartum period, or both, may have little or no effect on child death by two years of age (RR 1.06, 95% CI 0.92 to 1.22; 3883 women, 3 trials, low certainty evidence). This finding was consistent across the three studies (heterogeneity P = 0.49, I² statistic = 0%; Analysis 1.4).
Preterm delivery
It is uncertain whether vitamin A supplementation of HIV‐positive pregnant women has an effect on the risk of preterm deliveries (RR 0.84, 95% CI 0.52 to 1.37; 1577 women, 2 trials, very low certainty evidence). There was unexplained heterogeneity of effects between the two studies (heterogeneity P = 0.03, I² statistic = 79%; Analysis 1.5).
Stillbirth
It is uncertain whether vitamin A supplementation of HIV‐positive pregnant women has an effect on the incidence of stillbirths (RR 1.13, 95% CI 0.72 to 1.77; 2335 women, 3 trials, very low certainty evidence). This uncertainty was consistent across the three trials (heterogeneity P = 0.80, I² statistic = 0%; Analysis 1.6).
Mother
Maternal death
It is uncertain whether vitamin A supplementation of HIV‐positive women during pregnancy and immediate postpartum period has an effect on maternal deaths, as the certainty of the evidence was very low (RR 0.71, 95% CI 0.35 to 1.43; 1267 women, 2 trials). This finding was consistent between the two trials (heterogeneity P = 0.75, I² statistic = 0%; Analysis 1.7).
Postpartum CD4 count
It is uncertain whether vitamin A supplementation of HIV‐positive women during pregnancy and immediate postpartum period has an effect on postpartum maternal CD4 levels, as the certainty of the evidence was very low (mean difference −13.00, 95% CI −60.46 to 34.46; 511 women, 1 trial; Analysis 1.8).
Discussion
Summary of main results
Five randomized trials, which were conducted between 1995 and 2005 and included 7298 HIV‐positive women in sub‐Saharan Africa, met the inclusion criteria of this Cochrane Review. The included trials assessed the effects of vitamin A supplementation during pregnancy, immediately after delivery, or both. A synthesis of the results of the trials shows that vitamin A supplementation probably has little or no effect on mother‐to‐child transmission of HIV (moderate certainty evidence) and may have little or no effect on child death by two years of age (low certainty evidence). However, vitamin A supplements may increase the mean birthweight (low certainty evidence) and probably reduce the incidence of low birthweight (moderate certainty evidence). Due to very low certainty evidence it is uncertain whether vitamin A supplements have an effect on preterm delivery, stillbirth, or maternal death.
Overall completeness and applicability of evidence
By the end of the 20th century, HIV had produced a global epidemic that was far more extensive than was predicted when the infection emerged less than two decades earlier. Antenatal seroprevalence of HIV was more than 10% in many countries in sub‐Saharan Africa, the risk of mother‐to‐child transmission of HIV was about 30% to 45% in the region, and the region was contributing more than 90% of the nearly 2000 new HIV infections in children each day worldwide (De Cock 2000; UNAIDS 2001).
In this context, it was estimated that even with a 3% reduction in transmission, vitamin A supplementation of HIV‐positive women would be a cost‐effective method of preventing mother‐to‐child transmission of HIV. Vitamin A supplements are easily provided through existing health services (Wiysonge 2006). It was thus necessary to clarify the effect of the vitamin on mother‐to‐child transmission of HIV. Such clarification was judged to be important to decision‐makers considering affordable options for reducing mother‐to‐child transmission of HIV in resource‐limited settings (Wiysonge 2002).
Despite our comprehensive search, we found only six potentially eligible trials on the topic, of which five trials met our inclusion criteria. Our review shows that vitamin A supplementation of HIV‐positive women during pregnancy, after delivery, or both, probably has little or no effect on mother‐to‐child transmission of HIV.
Our data suggest that the association between low serum vitamin A levels and increased risk of mother‐to‐child transmission of HIV seen in observational studies (Semba 1994; Greenberg 1997), could have other explanations. Given the observational design of such studies, residual confounding by advanced HIV infection or other factors may explain the seemingly protective association (Fawzi 1998).
In high‐income countries, the combination of (1) antiretroviral prophylaxis, (2) elective Caesarean section delivery, and (3) formula feeding in clinical practice, coupled with increased prenatal HIV counselling and testing, has essentially eliminated mother‐to‐child transmission of HIV in those settings (Mofenson 2000; Navér 2006; UNAIDS 2015). Due to cost and other constraints, many countries in sub‐Saharan Africa had difficulties implementing these interventions (McIntyre 2002). However, in the last decade, there have been dramatic improvements. Antiretroviral drugs are now widely available in sub‐Saharan Africa and other resource‐constrained settings (UNAIDS 2015; WHO 2015; Wang 2016).
Vitamin A is teratogenic when consumed at high doses during early pregnancy, but none of the trials included in this review reported information on adverse events. However, the doses of vitamin A used in the trials were within the recommended safe low doses (WHO 1998).
Quality of the evidence
Due to the inconsistency of the effect of vitamin A supplementation on mother‐to‐child of HIV across included trials, we downgraded the certainty of this evidence to moderate. The GRADE Working Group classifies research evidence as being of moderate certainty if the true effect of the intervention is likely to be close to what was found in the research but there is a possibility that it is substantially different (Guyatt 2008). Therefore, this review does not completely exclude the possibility of a small beneficial or harmful effect of vitamin A supplementation on the risk of mother‐to‐child of HIV.
For most of the other outcomes assessed, we judged the certainty of the evidence to be very low (summary of findings Table for the main comparison), implying that we are uncertain about the true effect of vitamin A supplementation on these outcomes. Our main concerns with the evidence were imprecision (as the CIs ranged from large benefits to clinically important increases in harm) and the possibility of publication bias (because two or more eligible trials did not report the outcomes).
Potential biases in the review process
We minimized bias in the process of conducting and reporting the review by adhering to the Methodological Expectations of Cochrane Intervention Reviews (MECIR) (Higgins 2016).
The ultimate goal of this review was to determine whether vitamin A supplementation of HIV‐positive women could be recommended as a public health policy to reduce mother‐to‐child of HIV. We therefore considered overall mother‐to‐child of HIV, whether occurring during pregnancy, during delivery, or after birth. As such we pooled data from all studies, subgrouped by the timing of supplementation. We do not think that pooling data from these trials has introduced bias to the review.
Agreements and disagreements with other studies or reviews
We found that vitamin A supplements probably make little or no difference to the risk of mother‐to‐child transmission of HIV. This finding is consistent with the findings of previous reviews of maternal vitamin A supplementation by our team (Wiysonge 2002; Wiysonge 2005; Kongnyuy 2009; Wiysonge 2011), and others (Thorne‐Lyman 2012).
Our review also shows that giving vitamin A supplements during pregnancy or the immediate postpartum period, or both, may have little or no effect on child death by two years of age. This finding is consistent with that of other authors (Gorgia 2010; Thorne‐Lyman 2012). In a previous systematic review, Gorgia 2010 pooled data from six randomized trials and found little or no effect on infant mortality of giving synthetic vitamin A supplements to seemingly healthy mothers after delivery.
In another review, Thorne‐Lyman 2012 pooled data from 17 trials among women of any HIV status and found little or no effect of antepartum retinol and beta‐carotene supplements on infant mortality. As in this Cochrane Review, Thorne‐Lyman 2012 found that antepartum supplementation was protective against low birthweight among HIV‐positive women.
Vitamin A is important for embryogenesis; playing a vital role in the development of the foetal heart, embryonal circulatory system, and the regulation of heart asymmetry (Zile 1998). This role could explain the substantial reduction in low birthweight in the vitamin A group compared to placebo or no intervention.

PRISMA flow diagram

ʽRisk of bias' graph: review authors' judgements about each ʽRisk of bias' item presented as percentages across all included trials

ʽRisk of bias' summary: review authors' judgements about each ʽRisk of bias' item for each included trial

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 1 HIV infection status of the child.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 2 Mean birthweight.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 3 Low birthweight.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 4 Child death by two years of age.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 5 Preterm delivery.
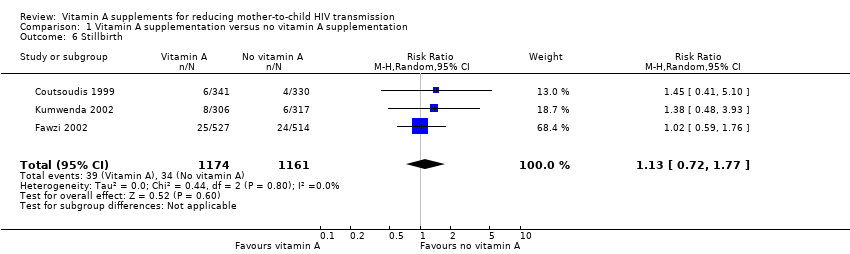
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 6 Stillbirth.
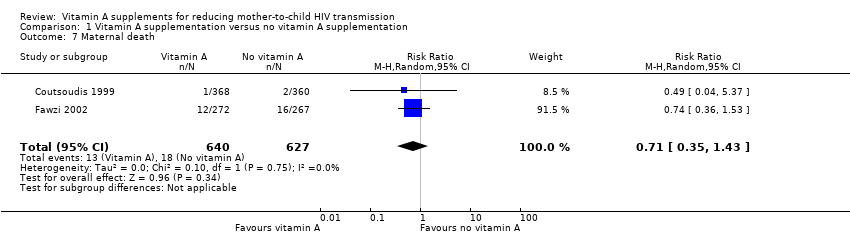
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 7 Maternal death.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 8 Postpartum CD4 count.
| Population: HIV‐positive women during pregnancy and immediate postpartum period Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Assumed risk with no vitamin A | Corresponding risk with vitamin A supplements | |||||
| HIV infection status of the child | 27 per 100 | 29 per 100 | RR 1.07 (0.91 to 1.26) | 4428 | ⊕⊕⊕⊝ due to imprecision | Vitamin A supplements probably have little or no effect on mother‐to‐child transmission of HIV. |
| Mean birthweight | 2964 g | 34 g higher | MD 34.12 (−12.79 to 81.02) | 2181 | ⊕⊕⊝⊝ due to imprecision | Vitamin A supplements may increase the mean birthweight |
| Low birthweight | 17 per 100 | 13 per 100 | RR 0.78 | 1819 | ⊕⊕⊕⊝ due to imprecision | Vitamin A supplements probably reduce the incidence of low birthweight babies. |
| Child death by two years of age | 14 per 100 | 15 per 100 | RR 1.06 | 3883 | ⊕⊕⊝⊝ due to imprecision | Vitamin A supplements may have little or no effect on child death by two years of age. |
| Preterm delivery | 20 per 100 | 17 per 100 | RR 0.84 | 1577 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on preterm deliveries. |
| Stillbirth | 3 per 100 | 3 per 100 | RR 1.13 | 2335 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on stillbirths. |
| Maternal death | 3 per 100 | 2 per 100 | RR 0.71 | 1267 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on maternal deaths. |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded by 1 for imprecision, as the CI ranges from small benefits to a clinically important increase in harm. | ||||||
| Search set | CENTRAL | PubMed | Embase | WHO ICTRP | ClinicalTrials.gov |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | Search ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR 'human immunodeficiency virus:ab,ti' OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'human immunodeficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune‐deficiency virus':ab,ti OR 'human immuno‐deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno‐deficiency syndrome':ab,ti OR 'acquired immune‐deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti | hiv AND vitamin A OR hiv AND retinol OR hiv AND retinoic OR hiv AND micronutrients OR hiv AND carotene | HIV and "VITAMIN A" | Interventional Studies | Studies received from 05/20/2016 to 08/25/2017 |
| #2 | MeSH descriptor: [HIV] explode all trees | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR "clinical trials as topic"[mesh: noexp] OR randomly [tiab] OR trial [tiab]) NOT (animals [mh] NOT humans [mh]) | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR ((doubl* NEAR/3 blind*):ab,ti) OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR ((cross NEXT/1 over*):ab,ti) | — | — |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | Search (infectious disease transmission, vertical[mh] OR vertical transmission[tiab] OR vertical infect*[tiab] OR infection transmission[tiab] OR mother‐to‐child transmission[tiab] OR MTCT[tiab]) | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | — | — |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | Search (vitamin A[mh] OR vitamin*[tiab] OR caroten*[tiab] OR retinol[tiab] OR retinoic[tiab] OR micronutrient*[tiab]) | 'human'/de OR 'normal human'/de OR 'human cell'/de | — | — |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | Search (#1 AND #2 AND #3 AND #4) | #3 AND #4 | — | — |
| #6 | #1 or #2 or #3 or #4 or #5 | Search (((#1 AND #2 AND #3 AND #4))) AND ("2016/05/20"[Date ‐ Publication] : "2017/08/25"[Date ‐ Publication]) | #3 NOT #5 | — | — |
| #7 | [mh "infectious disease transmission, vertical"] or "vertical transmission":ti,ab,kw or vertical next infect*:ti,ab,kw or "infection transmission":ti,ab,kw or "mother‐to‐child transmission":ti,ab,kw or MTCT:ti,ab,kw (Word variations have been searched) | — | #2 NOT #6 | — | — |
| #8 | [mh "vitamin A"] or vitamin*:ti,ab,kw or caroten*:ti,ab,kw or retinol:ti,ab,kw or retinoic:ti,ab,kw or micronutrient*:ti,ab,kw (Word variations have been searched) | — | 'vertical transmission'/de OR 'vertical transmission':ab,ti OR 'infectious disease transmission':ab,ti OR 'mother+to+child transmission':ab,ti OR mtct:ab,ti | — | — |
| #9 | #6 and #7 and #8 | — | caroten*:ab,ti OR retinoic:ab,ti OR 'retinol'/de OR retinol:ab,ti OR vitamin*:ab,ti OR 'vitamin a'/de OR micronutrient*:ab,ti | — | — |
| #10 | #6 and #7 and #8 Publication Year from 2016 to 2017 | — | #1 AND #7 AND #8 AND #9 | — | — |
| #11 | — | — | #1 AND #7 AND #8 AND #9 AND [20‐5‐2016]/sd NOT [25‐8‐2017]/sd | — | — |
| Search set | CENTRAL | PubMed | Embase |
| #1 | HIV Infections | HIV Infections OR HIV OR hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR hiv infect* OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR (human immun* AND deficiency virus) OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR (acquired immun* AND deficiency syndrome) OR "sexually transmitted diseases, Viral" | human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR hiv OR hiv‐1 OR hiv‐2 OR human immunodeficiency virus OR human immunedeficiency virus OR human immune‐deficiency virus OR human immuno‐deficiency virus OR acquired immunodeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR acquired immunedeficiency syndrome |
| #2 | HIV | randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR "clinical trials as topic" OR randomly OR trial) NOT (animals NOT humans) | randomized controlled trial OR randomized controlled trial OR random* OR trial OR allocat* OR factorial* OR placebo* OR assign* OR volunteer* OR crossover procedure OR crossover procedure OR double‐blind procedure OR double‐blind procedure OR single‐blind procedure OR single‐blind procedure OR doubl* NEAR/3 blind* OR singl* AND blind* OR crossover* OR cross+over* OR cross NEXT/1 over* |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or hiv near infect* or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immuno‐deficiency virus or human immune deficiency virus or human immuno deficiency virus or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun* deficiency syndrome | infectious disease transmission, vertical OR vertical transmission OR vertical infect* OR infection transmission OR mother‐to‐child transmission OR MTCT | animal OR animal experiment OR invertebrate OR animal tissue OR animal cell OR nonhuman |
| #4 | Lymphoma, AIDS‐Related | vitamin A OR vitamin* OR caroten* OR retinol OR retinoic OR micronutrient* | human OR normal human OR human cell |
| #5 | Sexually Transmitted Diseases, Viral | 1‐4/AND | #3 AND #4 |
| #6 | 1‐5/OR | 5 AND (2010/06/01 NOT 2016/05/20) | #3 NOT #5 |
| #7 | infectious disease transmission, vertical or vertical transmission or vertical next infect* or infection transmission or mother‐to‐child transmission or MTCT | — | #2 NOT #6 |
| #8 | vitamin A or vitamin* or caroten* or retinol or retinoic or micronutrient* | — | vertical transmission OR vertical transmission OR infectious disease transmission OR mother+to+child transmission OR mtct |
| #9 | 6‐8/AND | — | caroten* OR retinoic OR retinol OR retinol OR vitamin* OR vitamin a OR micronutrient* |
| #10 | Limit 9 to publication date 2010‐2016 | — | #1 AND #7 AND #8 AND #9 |
| #11 | — | — | #10 AND (2010/06/01 NOT 2016/05/20) |
| Search set | CENTRAL | PubMed | Embase |
| #1 | MeSH descriptor HIV Infections explode all trees | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus'OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab |
| #2 | MeSH descriptor HIV explode all trees | Search (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR allocat*:ti OR allocat*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/exp OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/exp OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'randomised controlled trial'/exp OR 'randomised controlled trial'/de OR 'randomised controlled trial' |
| #3 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME | Search mother‐to‐child‐transmission OR MTCT OR infectious disease transmission, vertical | 'mother‐to‐child transmission' OR 'mother to child transmission' OR mtct OR 'vertical transmission'/de OR 'vertical transmission' |
| #4 | MeSH descriptor Lymphoma, AIDS‐Related, this term only | Search caroten* OR retinoic OR retinol OR vitamin* OR vitamin A OR micronutrient* | caroten* OR retinoicOR 'retinol'/de OR retinolOR vitamin*OR 'vitamin a'/de OR 'vitamin a'OR micronutrient* |
| #5 | MeSH descriptor Sexually Transmitted Diseases, Viral, this term only | Search #1 AND #2 AND #3 AND #4 Limits: Publication Date from 2007/01/01 to 2010/06/08 | #1 AND #2AND #3AND #4 |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) | — | #1 AND #2AND #3AND #4AND [humans]/lim AND [embase]/lim AND [1‐1‐2007]/sd NOT [8‐6‐2010]/sd |
| #7 | mother‐to‐child‐transmission OR MTCT | — | — |
| #8 | MeSH descriptor Infectious Disease Transmission, Vertical, this term only | — | — |
| #9 | (#7 OR #8) | — | — |
| #10 | caroten* OR retinoic OR vitamin* OR vitamin A OR micronutrient* | — | — |
| #11 | (#6 AND #9 AND #10) | — | — |
| #12 | (#6 AND #9 AND #10), from 2007 to 2010 | — | — |
| Search set | PubMed | Embase | AIDSearch | GATEWAY |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] | (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection')) OR ((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'))) OR ((('b cell lymphoma'/exp OR 'b cell lymphoma') OR ('b cell lymphoma'/exp OR 'b cell lymphoma'))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) | Search: (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw]) AND (acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH]) |
| #2 | Search randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | (random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR (((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab))) OR (((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab))) OR (assign*:ti OR assign*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (((('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')) OR (('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')))) OR (((('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')) OR (('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')))) OR (((('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')) OR (('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')))) OR (((('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')) OR (('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')))) OR (allocat*:ti OR allocat*:ab) AND [2003‐2008]/py | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR ("CLINICAL TRIAL") OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND* )) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN ) | Search: (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw]))) OR (( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh])) |
| #3 | Search (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) | 'mother‐to‐child transmission' OR mtct OR 'vertical disease transmission' AND [2003‐2008]/py | (MOTHER‐TO‐CHILD TRANSMISSION) OR MTCT OR (VERTICAL DISEASE TRANSMISSION) | Search: (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) |
| #4 | Search CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | caroten* OR retinoic OR ('retinol'/exp OR 'retinol') OR vitamin* OR micronutrient* AND [2003‐2008]/py | CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | Search: CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* |
| #5 | Search PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* | pregnant OR ('pregnancy'/exp OR 'pregnancy') OR antepartum OR ('ante partum') OR antenatal OR ('ante natal') OR prenatal OR ('pre natal') AND [2003‐2008]/py | PREGNANT OR PREGNANCY OR ANTEPARTUM OR (ANTE‐PARTUM) OR ANTENATAL OR (ANTE‐NATAL) OR PRENATAL OR (PRE‐NATAL) | Search: PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* |
| #6 | Search #1 AND #2 AND #3 AND #4 AND #5 Limits: Publication Date from 2003 to 2008 | #1 AND #2 AND #3 AND #4 AND #5 | #1 AND #2 AND #3 AND #4 AND #5 | #1 and #2 and #3 and #4 and #5 Limit: 2003:2008 |
| Outcome | Assumed risk | Source | Clinically important relative improvement | Sample size required |
| HIV infection in child | 27/100 | Analysis 1.1 | 25% | 1236 |
| Mean birthweight | 2964 | Analysis 1.2 | 25% | 6178 |
| Low birthweight | 17/100 | Analysis 1.3 | 25% | 2194 |
| Still birth | 3/100 | Analysis 1.4 | 25% | 14,264 |
| Preterm birth | 20/100 | Analysis 1.5 | 25% | 1806 |
| Child death | 14/100 | Analysis 1.6 | 25% | 2748 |
| Maternal death | 3/100 | Analysis 1.7 | 25% | 14,264 |
| We based the sample size calculations: 2‐sided tests, with ratio of 1:1, power of 0.8 and confidence level of 0.05. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HIV infection status of the child Show forest plot | 5 | 4428 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 1.1 Antepartum supplementation | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| 1.2 Postpartum supplementation | 1 | 2248 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.98, 1.27] |
| 1.3 Antepartum and postpartum supplementation | 2 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.59] |
| 2 Mean birthweight Show forest plot | 3 | 2181 | Mean Difference (IV, Random, 95% CI) | 34.12 [‐12.79, 81.02] |
| 3 Low birthweight Show forest plot | 3 | 1819 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.97] |
| 4 Child death by two years of age Show forest plot | 3 | 3883 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.22] |
| 5 Preterm delivery Show forest plot | 2 | 1577 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.37] |
| 6 Stillbirth Show forest plot | 3 | 2335 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.72, 1.77] |
| 7 Maternal death Show forest plot | 2 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.43] |
| 8 Postpartum CD4 count Show forest plot | 1 | 511 | Mean Difference (IV, Random, 95% CI) | ‐13.0 [‐60.46, 34.46] |

