Interwencje, których celem jest poprawa przepisywania antybiotyków dla pacjentów hospitalizowanych
Appendices
Appendix 1. Search strategies
MEDLINE <1946 to Present> and MEDLINE In‐Process & Other Non‐Indexed Citations (Searched 19 January 2015) (OvidSP)
1 (hospital$ and antibiotic?).ti.
2 ((antibiotic? or alamethicin? or amdinocillin? or amdinocillin pivoxil? or amikacin? or amoxicillin? or amoxicillin‐potassium clavulanate combination? or amphotericin? or ampicillin? or anisomycin? or antimycin? or aurodox? or azithromycin? or azlocillin? or aztreonam? or bacitracin? or bacteriocin? or bambermycin? or bongkrekic acid? or brefeldin? or butirosin sulfate? or calcimycin? or candicidin? or capreomycin? or carbenicillin? or carfecillin? or cefaclor? or cefadroxil? or cefamandole? or cefatrizine? or cefazolin? or cefixime? or cefmenoxime? or cefmetazole? or cefonicid? or cefoperazone? or cefotaxime? or cefotetan? or cefotiam? or cefoxitin? or cefsulodin? or ceftazidime? or ceftizoxime? or ceftriaxone? or cefuroxime? or cephacetrile? or cephalexin? or cephaloglycin? or cephaloridine? or cephalosporin? or cephalothin? or cephamycin? or cephapirin? or cephradine? or chloramphenicol? or chlortetracycline? or citrinin? or clarithromycin? or clavulanic acid? or clavulanic acid? or clindamycin? or cloxacillin? or colistin? or cyclacillin? or dactinomycin? or daptomycin? or demeclocycline? or dibekacin? or dicloxacillin? or dihydrostreptomycin sulfate? or diketopiperazine? or distamycin? or doxycycline? or echinomycin? or edeine? or enviomycin? or erythromycin? or erythromycin estolate? or erythromycin ethylsuccinate? or filipin? or floxacillin? or fluoroquinolone? or fosfomycin? or framycetin? or fusidic acid? or gentamicin? or gramicidin? or hygromycin? or imipenem? or josamycin? or kanamycin? or kitasamycin? or lactam? or lasalocid? or leucomycin? or lincomycin? or lincosamide? or lucensomycin? or lymecycline? or mepartricin? or methacycline? or methicillin? or mezlocillin? or mikamycin? or minocycline? or miocamycin? or moxalactam? or mupirocin? or mycobacillin? or nafcillin? or natamycin? or nebramycin? or neomycin? or netilmicin? or netropsin? or nigericin? or nisin? or norfloxacin? or novobiocin? or nystatin? or ofloxacin? or oleandomycin? or oligomycin? or oxacillin? or oxytetracycline? or paromomycin? or penicillanic acid? or penicillic acid? or penicillin?? or piperacillin? or pivampicillin? or polymyxin b? or polymyxin? or pristinamycin? or prodigiosin? or ribostamycin? or rifabutin? or rifamycin? or ristocetin? or rolitetracycline? or roxarsone? or roxithromycin? or rutamycin? or sirolimu? or sisomicin? or spectinomycin? or spiramycin? or streptogramin?? or streptomycin? or streptovaricin? or sulbactam? or sulbenicillin? or sulfamerazine? or sulfamethoxypyridazine? or talampicillin? or teicoplanin? or tetracycline? or thiamphenicol? or thienamycin? or thiostrepton? or ticarcillin? or tobramycin? or troleandomycin? or tunicamycin? or tylosin? or tyrocidine? or tyrothricin? or valinomycin? or vancomycin? or vernamycin? or viomycin? or virginiamycin? or beta‐lactams) adj2 (resistant or resistance)).ti,ab. and (pc.fs. or (preventi$ or best practice? or evidence$ or policy or policies or pathway?).ti,ab,hw. or (guidance or guiding or guide? or guideline? or algorithm? or collaborat$ or computer$ or decision$ or emergency or formulary or guidance or guideline? or icu or impact or initiat$ or intensive care interdisciplin$ or interprofession$ or multidisciplin$ or multi‐disciplin$ or notification? or order entry or pharmacist? or pharmacy or pharmacies or policy or policies or prescrib$ or (quality adj2 (manag$ or improv$ or circle?)) or ((patient? or hospital?) adj2 record?) or reminder? or rotating or rotation or support or team$).ti,ab.)
3 (antibiotic? and (education$ or continuing‐education$ or cme or decision‐making or evidence‐based or ebm or guidance or guideline? or habit? or impact or improper$ or inappropriat$ or influenc$ or intervention? or management or overprescrib$ or overuse or overusing or pattern? or policy or policies or prescribing or prudent$ or stewardship? or rational or unnecessary or "use" or "usage")).ti.
4 (antibiotic? adj4 (education$ or continuing‐education$ or cme or decision‐making or evidence‐based or ebm or guidance or guideline? or habit? or impact or improper$ or inappropriat$ or influenc$ or intervention? or management or overprescrib$ or overuse or overusing or pattern? or policy or policies or prescribing or prudent$ or rational or stewardship or unnecessary or "use" or "usage")).ab.
5 antibiotic?.ti. and evidence‐based.hw.
6 ((antimicrobial? or anti‐microbial? or penicillin?) and (stewardship or guidance or guideline? or policy or policies)).ti.
7 ((antimicrobial? or anti‐microbial? or penicillin?) adj3 (stewardship or guidance or guideline? or policy or policies)).ab.
8 (antibiotic? adj5 (hour? or immediat$ or emergency)).ab. or (antibiotic? and (hour? or immediat$ or emergency)).ti. or (antibiotic? adj3 (rotat$ or timing or time or decision$ or notification or appropriat$)).ab. or (antibiotic? and (rotat$ or timing or time or decision$ or notification or appropriat$)).ti.
9 or/1‐8
10 exp anti‐bacterial agents/
11 antibiotic?.ti,ab.
12 (alamethicin or amdinocillin or amdinocillin pivoxil or amikacin or amoxicillin or amoxicillin‐potassium clavulanate combination or amphotericin or ampicillin or anisomycin or antimycin or aurodox or azithromycin or azlocillin or aztreonam or bacitracin or bacteriocins or bambermycins or bongkrekic acid or brefeldin or butirosin sulfate or calcimycin or candicidin or capreomycin or carbenicillin or carfecillin or cefaclor or cefadroxil or cefamandole or cefatrizine or cefazolin or cefixime or cefmenoxime or cefmetazole or cefonicid or cefoperazone or cefotaxime or cefotetan or cefotiam or cefoxitin or cefsulodin or ceftazidime or ceftizoxime or ceftriaxone or cefuroxime or cephacetrile or cephalexin or cephaloglycin or cephaloridine or cephalosporins or cephalothin or cephamycins or cephapirin or cephradine or chloramphenicol or chlortetracycline or citrinin or clarithromycin or clavulanic acid or clavulanic acids or clindamycin or cloxacillin or colistin or cyclacillin or dactinomycin or daptomycin or demeclocycline or dibekacin or dicloxacillin or dihydrostreptomycin sulfate or diketopiperazines or distamycins or doxycycline or echinomycin or edeine or enviomycin or erythromycin or erythromycin estolate or erythromycin ethylsuccinate or filipin or floxacillin or fluoroquinolones or fosfomycin or framycetin or fusidic acid or gentamicins or gramicidin or hygromycin or imipenem or josamycin or kanamycin or kitasamycin or lactams or lasalocid or leucomycins or lincomycin or lincosamides or lucensomycin or lymecycline or mepartricin or methacycline or methicillin or mezlocillin or mikamycin or minocycline or miocamycin or moxalactam or mupirocin or mycobacillin or nafcillin or natamycin or nebramycin or neomycin or netilmicin or netropsin or nigericin or nisin or norfloxacin or novobiocin or nystatin or ofloxacin or oleandomycin or oligomycins or oxacillin or oxytetracycline or paromomycin or penicillanic acid or penicillic acid or penicillin? or piperacillin or pivampicillin or polymyxin b or polymyxins or pristinamycin or prodigiosin or ribostamycin or rifabutin or rifamycins or ristocetin or rolitetracycline or roxarsone or roxithromycin or rutamycin or sirolimus or sisomicin or spectinomycin or spiramycin or streptogramin? or streptomycin or streptovaricin or sulbactam or sulbenicillin or sulfamerazine or sulfamethoxypyridazine or talampicillin or teicoplanin or tetracycline or thiamphenicol or thienamycins or thiostrepton or ticarcillin or tobramycin or troleandomycin or tunicamycin or tylosin or tyrocidine or tyrothricin or valinomycin or vancomycin or vernamycin or viomycin or virginiamycin or beta‐lactams).ti,ab.
13 (infection control$ or nosocomial$ or cross infection? or hospital acquired infection? or mrsa).ti,ab.
14 methicillin resistan$.ti,ab.
15 aminoglycosides/ or metronidazole/ or anti‐infective agents/ or anti‐infective agents, urinary/
16 or/10‐15
17 (programs or programmes).ti.
18 empiric.ti.
19 (quality adj3 improvement?).ti.
20 (adherence or alert? or benchmark$ or (change adj3 treatment) or computer assist$ or computer support or computeri?ed or clinical decision$ or dosing or education$ or formulary or guidance or guideline? or impact or intervention or justification or methicillan‐resistant or overuse or over‐prescrib$ or overprescrib$ or pathway? or pharmacist? or policy or policies or program or programme or (quality adj3 improv$) or reminder? or resistance or restriction? or rotation? or timing or turnaround or unnecessary).ti.
21 or/17‐20
22 16 and 21
23 22 not 9
24 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.
25 exp animals/ not humans.sh.
26 43 not 45
27 intervention?.ti. or (intervention? adj6 (clinician? or collaborat$ or community or complex or design$ or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or gp or general practice? or hospital? or impact? or improv$ or individuali?e? or individuali?ing or interdisciplin$ or multicomponent or multi‐component or multidisciplin$ or multi‐disciplin$ or multifacet$ or multi‐facet$ or multimodal$ or multi‐modal$ or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib$ or prescription? or primary care or professional$ or provider? or regulatory or regulatory or tailor$ or target$ or team$ or usual care)).ab.
28 (pre‐intervention? or preintervention? or "pre intervention?" or post‐intervention? or postintervention? or "post intervention?").ti,ab.
29 (hospital$ or patient?).hw. and (study or studies or care or health$ or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw.
30 demonstration project?.ti,ab.
31 (pre‐post or "pre test$" or pretest$ or posttest$ or "post test$" or (pre adj5 post)).ti,ab.
32 (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab.
33 trial.ti. or ((study adj3 aim?) or "our study").ab.
34 (before adj10 (after or during)).ti,ab.
35 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab,hw.
36 ("time series" adj2 interrupt$).ti,ab,hw.
37 (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month$ or hour? or day? or "more than")).ab.
38 pilot.ti.
39 pilot projects/ [ml]
40 (clinical trial or controlled clinical trial or multicenter study).pt. [ml]
41 (multicentre or multicenter or multi‐centre or multi‐center).ti.
42 andom$.ti,ab. or controlled.ti.
43 (control adj3 (area or cohort? or compare? or condition or design or group? or intervention? or participant? or study)).ab. not (controlled clinical trial or randomized controlled trial).pt. [ml]
44 "comment on".cm. or review.ti,pt. or randomized controlled trial.pt. [ml]
45 review.ti.
46 (rat or rats or cow or cows or chicken? or horse or horses or mice or mouse or bovine or animal?).ti.
47 exp animals/ not humans.sh.
48 (animal$ not human$).sh,hw.
49 *experimental design/ or *pilot study/ or quasi experimental study/ [em]
50 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab.
51 ("time series" adj2 interrupt$).ti,ab.
52 or/26‐43
53 or/44‐48
54 52 not 53
55 9 or 23
56 54 and 55
EMBASE <1996 to 2015 Week 03> (Searched 22 January 2015) (OvidSP)
1 exp *antibiotic agent/
2 (bundle or bundles or education$ or continuing‐education$ or cme or decision‐making or guidance or (guideline? adj2 (adherence or implement$ or complian$ or comply$)) or improper$ or inappropriat$ or incorrect$ or nurse led or overprescrib$ or overuse or overusing or pharmacist initiated or physician? practice? or policy or policies or practice pattern? or (prescribing adj2 (ebm or evidence‐based or habit? or pattern? or practice or practices)) or prudent$ or rational or stewardship or unnecessary or underprescrib$).ti.
3 ("antibiotic use" or "antibiotic usage").ti.
4 (hospital$ and antibiotic?).ti.
5 ((antibiotic? or alamethicin? or amdinocillin? or amdinocillin pivoxil? or amikacin? or amoxicillin? or amoxicillin‐potassium clavulanate combination? or amphotericin? or ampicillin? or anisomycin? or antimycin? or aurodox? or azithromycin? or azlocillin? or aztreonam? or bacitracin? or bacteriocin? or bambermycin? or bongkrekic acid? or brefeldin? or butirosin sulfate? or calcimycin? or candicidin? or capreomycin? or carbenicillin? or carfecillin? or cefaclor? or cefadroxil? or cefamandole? or cefatrizine? or cefazolin? or cefixime? or cefmenoxime? or cefmetazole? or cefonicid? or cefoperazone? or cefotaxime? or cefotetan? or cefotiam? or cefoxitin? or cefsulodin? or ceftazidime? or ceftizoxime? or ceftriaxone? or cefuroxime? or cephacetrile? or cephalexin? or cephaloglycin? or cephaloridine? or cephalosporin? or cephalothin? or cephamycin? or cephapirin? or cephradine? or chloramphenicol? or chlortetracycline? or citrinin? or clarithromycin? or clavulanic acid? or clavulanic acid? or clindamycin? or cloxacillin? or colistin? or cyclacillin? or dactinomycin? or daptomycin? or demeclocycline? or dibekacin? or dicloxacillin? or dihydrostreptomycin sulfate? or diketopiperazine? or distamycin? or doxycycline? or echinomycin? or edeine? or enviomycin? or erythromycin? or erythromycin estolate? or erythromycin ethylsuccinate? or filipin? or floxacillin? or fluoroquinolone? or fosfomycin? or framycetin? or fusidic acid? or gentamicin? or gramicidin? or hygromycin? or imipenem? or josamycin? or kanamycin? or kitasamycin? or lactam? or lasalocid? or leucomycin? or lincomycin? or lincosamide? or lucensomycin? or lymecycline? or mepartricin? or methacycline? or methicillin? or mezlocillin? or mikamycin? or minocycline? or miocamycin? or moxalactam? or mupirocin? or mycobacillin? or nafcillin? or natamycin? or nebramycin? or neomycin? or netilmicin? or netropsin? or nigericin? or nisin? or norfloxacin? or novobiocin? or nystatin? or ofloxacin? or oleandomycin? or oligomycin? or oxacillin? or oxytetracycline? or paromomycin? or penicillanic acid? or penicillic acid? or penicillin?? or piperacillin? or pivampicillin? or polymyxin b? or polymyxin? or pristinamycin? or prodigiosin? or ribostamycin? or rifabutin? or rifamycin? or ristocetin? or rolitetracycline? or roxarsone? or roxithromycin? or rutamycin? or sirolimu? or sisomicin? or spectinomycin? or spiramycin? or streptogramin?? or streptomycin? or streptovaricin? or sulbactam? or sulbenicillin? or sulfamerazine? or sulfamethoxypyridazine? or talampicillin? or teicoplanin? or tetracycline? or thiamphenicol? or thienamycin? or thiostrepton? or ticarcillin? or tobramycin? or troleandomycin? or tunicamycin? or tylosin? or tyrocidine? or tyrothricin? or valinomycin? or vancomycin? or vernamycin? or viomycin? or virginiamycin? or beta‐lactams) adj2 (resistant or resistance) adj10 (best practice? or (chang$ adj (practice or clinical practice)) or evidence‐base? or policy or policies or pathway? or ((treatment or care) adj (algorithm? or pathway? or protocol)) or collaborat$ or computeri?ed or computer‐supported or decision‐mak$ or (support adj decision?) or formulary or guidance or (guideline? adj (adher$ or implement$ or concord$ or comply or complian$)) or interdisciplin$ or interprofession$ or multidisciplin$ or multi‐disciplin$ or notification? or order entry or (pharmacist? adj2 (led or initiat$ or intervention? or participat$)) or policy or policies or (prescrib$ adj (practice? or method? or algorithm? or protocol? or habit?)) or (quality adj (manag$ or improv$ or circle?)) or ((patient? or medical or electronic) adj2 record?) or reminder? or rotating or rotation or team$)).ti,ab.
6 (antibiotic? and (bundle or bundles or education$ or continuing‐education$ or cme or decision‐making or guidance or (guideline? adj2 (adherence or implement$ or complian$ or comply$)) or improper$ or inappropriat$ or incorrect$ or nurse led or overprescrib$ or overuse or overusing or pharmacist initiated or physician? practice? or policy or policies or practice pattern? or (prescribing adj2 (ebm or evidence‐based or habit? or pattern? or practice or practices)) or prudent$ or rational or stewardship or unnecessary or underprescrib$)).ti.
7 (antibiotic? adj3 (bundle or bundles or education$ or continuing‐education$ or cme or decision‐making or guidance or (guideline? adj2 (adherence or implement$ or complian$ or comply$)) or improper$ or inappropriat$ or incorrect$ or nurse led or overprescrib$ or overuse or overusing or pharmacist initiated or physician? practice? or policy or policies or practice pattern? or (prescribing adj2 (ebm or evidence‐based or habit? or pattern? or practice or practices)) or prudent$ or rational or stewardship or unnecessary or underprescrib$)).ab.
8 ((antimicrobial? or anti‐microbial? or penicillin?) and (bundle or bundles or education$ or continuing‐education$ or cme or decision‐making or guidance or (guideline? adj2 (adherence or implement$ or complian$ or comply$)) or improper$ or inappropriat$ or incorrect$ or nurse led or overprescrib$ or overuse or overusing or pharmacist initiated or physician? practice? or policy or policies or practice pattern? or (prescribing adj2 (ebm or evidence‐based or habit? or pattern? or practice or practices)) or prudent$ or rational or stewardship or unnecessary or underprescrib$)).ab. or ((antimicrobial? or anti‐microbial? or penicillin?) and (bundle or bundles or education$ or continuing‐education$ or cme or decision‐making or guidance or (guideline? adj2 (adherence or implement$ or complian$ or comply$)) or improper$ or inappropriat$ or incorrect$ or nurse led or overprescrib$ or overuse or overusing or pharmacist initiated or physician? practice? or policy or policies or practice pattern? or (prescribing adj2 (ebm or evidence‐based or habit? or pattern? or practice or practices)) or prudent$ or rational or stewardship or unnecessary or underprescrib$)).ti.
9 1 and 2
10 or/3‐8
11 9 or 10
12 intervention?.ti. or (intervention? adj6 (clinician? or collaborat$ or community or complex or design$ or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or gp or general practice? or hospital? or impact? or improv$ or individuali?e? or individuali?ing or interdisciplin$ or multicomponent or multi‐component or multidisciplin$ or multi‐disciplin$ or multifacet$ or multi‐facet$ or multimodal$ or multi‐modal$ or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib$ or prescription? or primary care or professional$ or provider? or regulatory or regulatory or tailor$ or target$ or team$ or usual care)).ab.
13 (pre‐intervention? or preintervention? or "pre intervention?" or post‐intervention? or postintervention? or "post intervention?").ti,ab.
14 (hospital$ or patient?).hw. and (study or studies or care or health$ or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw.
15 demonstration project?.ti,ab.
16 (pre‐post or "pre test$" or pretest$ or posttest$ or "post test$" or (pre adj5 post)).ti,ab.
17 (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab.
18 trial.ti. or ((study adj3 aim?) or "our study").ab.
19 (before adj10 (after or during)).ti,ab.
20 (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month$ or hour? or day? or "more than")).ab.
21 pilot.ti.
22 (multicentre or multicenter or multi‐centre or multi‐center).ti.
23 random$.ti,ab. or controlled.ti.
24 review.ti.
25 or/12‐23
26 25 not 24
27 11 and 26
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library Issue 1 2015 (Searched 22 January 2015)
#1 antibiotic?:ti,ab,kw
#2 ((antibacterial or anti‐bacterial or antiinfective or anti‐infective) and (agent? or drug?)):ti,ab,kw
#3 ((alamethicin? or amdinocillin? or amdinocillin pivoxil? or amikacin? or amoxicillin? or amoxicillin‐potassium clavulanate combination? or amphotericin? or ampicillin? or anisomycin? or antimycin? or aurodox? or azithromycin? or azlocillin? or aztreonam? or bacitracin? or bacteriocin? or bambermycin? or bongkrekic acid? or brefeldin? or butirosin sulfate? or calcimycin? or candicidin? or capreomycin? or carbenicillin? or carfecillin? or cefaclor? or cefadroxil? or cefamandole? or cefatrizine? or cefazolin? or cefixime? or cefmenoxime? or cefmetazole? or cefonicid? or cefoperazone? or cefotaxime? or cefotetan? or cefotiam? or cefoxitin? or cefsulodin? or ceftazidime? or ceftizoxime? or ceftriaxone? or cefuroxime? or cephacetrile? or cephalexin? or cephaloglycin? or cephaloridine? or cephalosporin? or cephalothin? or cephamycin? or cephapirin? or cephradine? or chloramphenicol? or chlortetracycline? or citrinin? or clarithromycin? or clavulanic acid? or clavulanic acid? or clindamycin? or cloxacillin? or colistin? or cyclacillin? or dactinomycin? or daptomycin? or demeclocycline? or dibekacin? or dicloxacillin? or dihydrostreptomycin sulfate? or diketopiperazine? or distamycin? or doxycycline? or echinomycin? or edeine? or enviomycin? or erythromycin? or erythromycin estolate? or erythromycin ethylsuccinate? or filipin? or floxacillin? or fluoroquinolone? or fosfomycin? or framycetin? or fusidic acid? or gentamicin? or gramicidin? or hygromycin? or imipenem? or josamycin? or kanamycin? or kitasamycin? or lactam? or lasalocid? or leucomycin? or lincomycin? or lincosamide? or lucensomycin? or lymecycline? or mepartricin? or methacycline? or methicillin? or mezlocillin? or mikamycin? or minocycline? or miocamycin? or moxalactam? or mupirocin? or mycobacillin? or nafcillin? or natamycin? or nebramycin? or neomycin? or netilmicin? or netropsin? or nigericin? or nisin? or norfloxacin? or novobiocin? or nystatin? or ofloxacin? or oleandomycin? or oligomycin? or oxacillin? or oxytetracycline? or paromomycin? or penicillanic acid? or penicillic acid? or penicillin?? or piperacillin? or pivampicillin? or polymyxin b? or polymyxin? or pristinamycin? or prodigiosin? or ribostamycin? or rifabutin? or rifamycin? or ristocetin? or rolitetracycline? or roxarsone? or roxithromycin? or rutamycin? or sirolimu? or sisomicin? or spectinomycin? or spiramycin? or streptogramin?? or streptomycin? or streptovaricin? or sulbactam? or sulbenicillin? or sulfamerazine? or sulfamethoxypyridazine? or talampicillin? or teicoplanin? or tetracycline? or thiamphenicol? or thienamycin? or thiostrepton? or ticarcillin? or tobramycin? or troleandomycin? or tunicamycin? or tylosin? or tyrocidine? or tyrothricin? or valinomycin? or vancomycin? or vernamycin? or viomycin? or virginiamycin? or beta‐lactams) and (prescrib$ or resistance or "use" or "usage" or utlii?ation)):ti,ab,kw
#4 ((antibacterial agent? or anti‐bacterial agent?) and (prescrib$ or resistance or "use" or "usage" or utili?ation)):ti,ab,kw
#5 "stewardship":ti,ab,kw
#6 (antibiotic* or antimicrobial*) and (prescrib* or prescrip*):ti,ab,kw
#7 #1 or #2 or #3 or #4 or #5 or #6
Appendix 2. Decisions based on 5 GRADE criteria about quality of evidence from RCTs in 'Summary of findings' table
Outcome prescribing, % compliance with guideline
| Criterion | Evidence | Decision |
| Risk of bias | Effect estimate lower for 15 studies with low/medium risk of bias. | Not serious, 95% confidence interval for effect estimate 10% to 12% in studies at low or medium risk of bias. |
| Imprecision1 | 23,394 patients and 3660 events | Not serious |
| Inconsistency | Chi2 = 367.98, df = 28 (P < 0.00001); I2 = 92% | Not serious, effect size rather than direction (Figure 3). Variation partially explained by prespecified subgroup analysis by intervention function (Figure 7). Direction of effect consistent despite high levels of statistical heterogeneity. |
| Indirectness | Only 2 RCTs of restrictive interventions (Singh 2000; Strom 2010) | Not serious because this is a concern for safety rather than effectiveness. |
| Publication bias | Large trials, few commercially sponsored | Not serious |
1Imprecision, optimal information size threshold 862 patients for Δ 10%, control compliance 43%, α 0.05, β 0.2, dropout 10%.
Outcome prescribing, reduction in duration of all antibiotic treatment
| Criterion | Evidence | Decision |
| Risk of bias | Effect estimate greater for 3 studies with low/medium risk of bias (Analysis 1.6). | Not serious |
| Imprecision1 | 3318 patients | Not serious, number of patients is > OIS to detect Δ 1 day (3018 patients). |
| Inconsistency2 | All trials: Chi2 = 119.95, df = 13 (P < 0.00001); I2 = 89% | Not serious, most variation is effect size rather than direction (Figure 4). |
| Indirectness | Not serious for effectiveness | Not serious |
| Publication bias | Large trials, few commercially sponsored | Not serious |
1Imprecision, OIS is 754 patients for Δ 2 days, standard deviation 9.3 days (highest of the 3 studies contributing > 10% of weight), α 0.05, β 0.8, dropout 10%, and 3018 patients for Δ 1 day.
OIS: optimal information size
Outcome mortality
| Criterion | Evidence | Decision |
| Risk of bias | Effect estimate and confidence interval similar for 8 studies with low/medium risk of bias | Not serious |
| Imprecision1 | 17,697 patients and 1587 events | Not serious |
| Inconsistency | Heterogeneity: Chi2 = 16.55, df = 28 (P = 0.96); I2 = 0% | Not serious (Figure 5) |
| Indirectness | No trials of restrictive interventions. Mortality lower in trials at low/medium risk of bias. | Serious |
| Publication bias | Large trials, few commercially sponsored | Not serious |
1Imprecision, OIS threshold for patients for non‐inferiority is 6726 patients for a 2% difference in mortality.
OIS: optimal information size
All trials:
Mortality, control 11%, power 80%, dropout 10%
| Non‐inferiority criteria | Total number of patients to be recruited |
| 1% | 26,900 |
| 2% | 6726 |
| 3% | 2988 |
| 4% | 1682 |
Outcome length of hospital stay
| Criterion | Evidence | Decision |
| Risk of bias | Effect size only slightly smaller for 6 RCTs at low or medium risk of bias, and the 95% CI did not include increase in length of stay. | Not serious |
| Imprecision1 | 3834 patients (> OIS for Δ 1 day but not 0.5 day). The lower bound of CI is reduction by 0.7 days for all RCTs and 0.3 days for RCTs at low or medium risk of bias. | Not serious |
| Inconsistency | Heterogeneity: Chi2 = 17.32, df = 14 (P = 0.24); I2 = 19% | Not serious, effect size rather than direction (Figure 6) |
| Indirectness | No trials of restrictive interventions | Serious |
| Publication bias | Large trials, few commercially sponsored | Not serious |
1Imprecision, OIS is 2014 patients for Δ 1 day and 7640 patients for Δ 0.5 day, standard deviation 7.6 (highest of the 3 studies contributing > 20% of weight), α 0.05, β 0.2, dropout 10%.
CI: confidence interval
OIS: optimal information size
RCT: randomised controlled trial
Appendix 3. Details of power calculations for RCTs
Based on a previous study, the authors estimated that on day 5, ∼85% of control patients would be on antibiotics. They thus calculated that 57 patients in each arm would be needed to detect in a two‐sided test with an 80% probability and a 0.05 type I error, a 25% absolute reduction in the proportion of antibiotic‐treated patients on day 5. They also estimated that 20% of patients would eventually be withdrawn from the study after showing indisputable infection. One hundred and forty patients in total (70 in each arm) would thus be needed.
Power: Assuming a mean of 12 days without antibiotics for the control group, 133 patients per study group would provide 90% power to detect a 3‐day increase in number of days without antibiotics.
The sample calculation was based on the difference in mortality of 6.5% as detected by Doern 1994. With 296 patients in each study group in each study period, the study would have power of 80.1% to yield a statistically significant result (α = 0.05, two‐tailed, specific proportions 0.120 vs 0.055).
We designed the trial to enrol 105 patients with completed follow‐up in each group. This number gave the study 95% power to detect a 30% reduction in antibiotic exposure. Assumptions included use of a two‐tailed test, a 5% level of significance, and a standard deviation (SD) of 6 days in both groups.
A study sample of 150 patients in each group gave the study a power of 95% to detect a 30% reduction in antibiotic exposure from 10 to 7 days per patient assuming a two‐tailed test, a 1% level of significance, and a SD of 6 days in both groups. This sample size gave the study a power of 74% to detect a 10% increase in the combined treatment failure and complication rate (from 10% to 20%), using the procalcitonin algorithm with a one‐sided value of 0.05.
This study was designed to compare the two cefotaxime groups with the hypothesis that a higher proportion of cefotaxime orders would be within hospital guidelines in the intervention group. Inappropriate antibiotic prescribing has been shown to be as high as 40% (17). By assuming an alpha of 5% (two‐tailed), power of 80%, probability of appropriate prescribing with and without the intervention at 75% and 60% (absolute difference = 15%), respectively, the case sample size for the uncorrected Chi2 test in this randomised study was 300, which was then increased by 10% to account for patient dropouts.
Pre‐study power calculations (with 90% power) showed that 76 patients in each group were necessary to detect a 15% lower antibiotic use, considering that 100% of children hospitalised for community‐acquired pneumonia were treated with antibiotics and assuming a two‐tailed test and a 5% level of significance. Since we planned to analyse the data in subgroups of mild and severe community‐acquired pneumonia, we doubled the number of patients per group (n = 152). We thus decided to enrol 160 patients in each group to allow for a 5% dropout participant.
This study was designed with 80% power to detect a 1‐day decrease in length of stay from an assumed baseline of 7.2 days. The sample size was adjusted for the clustering on physician group assuming an average of 3.5 patients per group and an intraclass correlation coefficient of 0.1.
The sample size calculation was based on the following assumptions: a significance level .05, a power .80, a proportion of initially missed infections of 4% in the interleukin‐8 group and 9% in the standard group, and an equivalence limit of 3%. On the basis of these assumptions, a sample size of 207 patients with infection in each group was required to demonstrate 1‐sided equivalence of the proportions of initially missed infections. Assuming a rate of bacterial infection of 18% in the study population, a total of 1150 patients needed to be enrolled into the study.
We calculated the power using standard formulae for comparison of proportions in a completely randomised design and estimated that with 40 hospitals, we would have 90% power to detect a decrease or an increase in a practice equal to the SD between hospitals, in a one‐sided significance test at 5% level of significance. For example, if the SD of use of episiotomy is 20%, we would be able to detect a decrease in the end‐of‐study rate of use of episiotomy from 70% to 50%. We used a one‐sided significance test because we believed the intervention could only improve the use of evidence‐based practices.
The final (adjusted) sample size of 1200 patients was based on an estimated mortality in the standard‐of‐care‐only group of 31.0% and a proposed absolute risk reduction of 7.5%. Detailed sample size considerations are available in the supplemental data (see Supplemental Digital Content 2, links.lww.com/CCM/A257).
It was calculated that 1500 patients were needed to demonstrate a 6% absolute reduction in mortality (power of 80% and a two‐sided alpha of 0.05) from 25% in the control group to 18% in the rapid group (Sample Power, SPSS, Chicago, USA).
Pre‐study power calculations (with 90% power) showed that 107 patients in each group were necessary to detect a 20% reduction in antibiotic use (from 10 to 8 days), assuming a two‐tailed test and a 5% level of significance.
A priori power calculations determined that 40 hospitals sampling 100 cases per measurement period would give 80% power to detect a 15% difference in the pre–post change between groups in the timing of prophylaxis based on an intraclass correlation coefficient of 0.15, estimated from an earlier study of intensive care unit process improvement (0.05, 2‐tailed test).
Power calculation suggested that 97 patients should be enrolled in each group to give 80% power at the 5% level of significance to detect a 20% difference in antibiotic prescription rate. Taking into account the possibility for lost to follow‐up patients or missing or incomplete results, we considered including 140 patients in each group.
Assuming a mean stay of 7 days with 50% antibiotic exposure, a study sample of at least 250 patients in each group was deemed necessary to detect a 20% reduction in antibiotic consumption with 95% power at the 5% significance level.
We hypothesized that the intervention might result in a 20% reduction of the duration of hospitalisation. The sample size was estimated based on the results of previous observations performed in our hospital showing that the mean length of hospital stay for patients treated with one of the targeted antibiotics was 15 ± 7 days. To detect a 20% reduction in the length of hospital stay in the intervention group with a type I error of 5% and a type II error of 80%, it was necessary to enrol a total of 506 patients (253 patients in each group).
"Assuming 90% of the patients in the control group would use antibiotics, and anticipating a 15% decrease in antibiotic usage in the procalcitonin (PCT) group, a sample size of 158 patients (79 patients per group) was necessary to detect a significant difference in antibiotic prescription rate between the groups with 80% power and an α error of 0.05. To account for possible loss of patients to follow‐up, we planned to enrol 180 patients." One hundred and eighty eligible patients were randomised to intervention (n = 90) or control (n = 90).
We hypothesised a difference of at least 15% in defined daily doses of the targeted antibiotics between intervention and control groups based on the results of previous reports. One hundred and forty‐four patients were required in each group to reach 80% power, alpha 0.05, and, within awaited group, standard deviation of 5 days.
The trial was designed to enrol at least 66 patients to obtain a power of 90% to detect a 33% (4‐day) difference in the duration of antibiotic therapy for the initial infection between the two groups based on an estimated baseline duration of 12 days.
Sample size calculation was based on data from a previous study, in which the mean duration of antibiotic therapy for the index infection was 8.6 ± 5.0 days among patients treated according to a PCT‐guided protocol, as compared with 10.7 (± 4.0) days in the control group (V. Nobre, unpublished observation, 2008). We thus hypothesised that the duration of the antibiotic therapy in patients treated with a PCT‐guided protocol would be at least 25% shorter than the duration observed in patients treated according to a protocol based on the serum C‐reactive protein levels. We found that 58 patients per group (a total of 116 individuals) would be necessary to demonstrate this difference, with a power of 80% and an alpha error of 5%.
In the control group, all patients were expected to receive a complete course of antibiotic treatment. On the basis of an expected detection rate of 20% for atypical and viral pathogens in the intervention group and an estimate of the number of possible dropouts, 100 patients would be required to demonstrate a reduction in the use of antibiotic treatment from 100% to 80%.
The primary outcome measure was % inappropriate treatment, which could only be assessed in patients with microbiologically documented infections. The planned sample of 1500 patients in 15 wards had a power of greater than 99% to detect a 15% reduction in inappropriate antibiotic treatment (from 35% to 20%), for a two‐tailed test, assuming cluster randomisation of wards stratified within three hospitals by a two‐way analysis of variance and a between‐ward variance of 0.0005. We chose a sample size that would allow us to detect a difference even if two wards defaulted. The authors say that "Owing to the grant time limits the trial was stopped before attaining the planned sample size"; they recruited 570 patients for the primary outcome measure instead of the planned 1500.
To define non‐inferiority with regard to the primary combined endpoint, the planning committee agreed on a 7.5% absolute difference as the clinically tolerable upper limit (i.e. at worst the risk of an overall adverse outcome in the PCT group was increased by 7.5%). Based on this non‐inferiority boundary, a minimal sample size of 1002 patients was determined, allowing for an overall adverse outcome rate in the control group of at most 20% and aiming for a power of 90%. Instead of a fixed sample size, we predefined a fixed recruitment period of 18 months with the goal to randomise all eligible patients from the 6 participating hospitals during that period and an extension if fewer than 1002 patients had been recruited. This prospective rule allows for the possibility of a higher number of patients and thus better power for subgroup analyses, while maintaining the integrity of the trial.
The sample size was estimated according to the Freedman method of sample size estimation under the proportional‐hazards model, on the basis of pre‐study observation. One hundred and thirty‐five patients were required in each group to reach 80% power of demonstrating a 40% increase in the hazard ratio (a difference that would correspond approximately to a 25% reduction in the expected number of antibiotic‐days until modification). For practical reasons, study duration was determined before the beginning of prospective data collection: we chose a five‐month period, which was the estimated time necessary to achieve the calculated sample size. However, the observed effect (14% reduction) was lower than predicted, so the trial was underpowered.
Sample size calculations were derived from the findings of Schuetz in which patients with lower respiratory tract infections treated with a PCT‐based algorithm showed a 35% (29% to 40%) reduction in antibiotic exposure. Assuming a median baseline exposure level of 9 days and a standard deviation of 6 days, with 165 patients per group this study had greater than 90% power to detect a clinically relevant reduction in duration of antibiotic usage of 25% (9.0 versus 6.7 days). As duration of antibiotic usage is unlikely to follow a normal distribution, in accordance with Lehmann this figure was inflated by 15%. To further account for potential dropout or loss to follow‐up (anticipated to be less than 5%), a total of 400 participants were recruited.
Assuming that the patients in the experimental therapy group would have 10% worse outcome than patients in the standard therapy arm, a sample size of 200 patients (100 in each arm) would detect a difference at 0.05 and power 0.5. Assuming a 20% incidence of development of resistance in the standard therapy group and 5% in the experimental therapy group, a sample size of 176 patients (88 in each group) would be needed for significance at 0.05 and power 0.8.
NB: The study was terminated prematurely because providers caring for patients in the control group were influenced by the favourable results in the intervention group.
The trial was designed to demonstrate the persistent superiority of procalcitonin guidance in decreasing antibiotic use up to six months after the index exacerbation. The sample size was calculated from the following assumptions: a 75% use of antibiotics to treat the index exacerbation and an expected absolute reduction of this frequency from 75% to 45% with procalcitonin guidance. Considering an exacerbation rate of 70% within 6 months and 75% antibiotic use in the following exacerbations, a sample size of 186 patients (93 patients per group) was necessary to detect a significant difference in antibiotic use between both groups with a power of 85% and an error of 0.05. Considering a 20% dropout rate after assignment to the study, 223 inclusions were planned.
Considering 13 antibiotic‐free days in the control group and 18 antibiotic‐free days in the procalcitonin group, a sample size of 84 patients (42 per group) was necessary to detect a significant difference in antibiotic‐free days alive between both groups with a power of 90% and an error of 0.05 using a two‐tailed test. Assuming 8% lost to follow‐up, we planned the inclusion of 100 participants.
The primary outcome was site of treatment rather than the antibiotic process measures. "We estimated that we would need 96 eligible patients per hospital (3072 in total) to achieve 80% power to detect a 12% difference across the intervention groups for the site‐of‐treatment decision among low‐risk patients."
"For the site‐of‐treatment decision, this study achieved greater than 80% power to detect differences of 10% between high‐intensity and moderate‐intensity groups and differences of 12% between high‐intensity and low‐intensity groups according to separate 1‐tailed tests in which the level was 0.025."
Appendix 4. Contribution of 49 RCTs to meta‐analyses and to meta‐regression
| Study | MA | MR | |||||
| Annane 2013 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Bailey 1997 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Bouza 2004 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Bouza 2007 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Burton 1991 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| Camins 2009 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Christ‐Crain 2004 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Christ‐Crain 2006 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Danaher 2009 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ding 2013 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Dranitsaris 2001 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Esposito 2011 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| Fine 2003 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Franz 2004 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Fraser 1997 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Gulmezoglu 2007 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Gums 1999 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Hochreiter 2009 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Kerremans 2008 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Kristofferson 2009 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Kritchevsky 2008 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Lacroix 2014 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Layios 2012 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Liu 2013 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Long 2014 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Maravic‐Stojkovic 2011 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| Masia 2008 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Micek 2004 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Oliveira 2013 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Paul 2006 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| Poehling 2006 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Qu 2012 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Schnoor 2010 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| Schouten 2007 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Schroeder 2009 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Schuetz 2009 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Senn 2004 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| Shen 2011 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Shojania 1998 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Singh 2000 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Solomon 2001 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Stocker 2010 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Stolz 2007 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Stolz 2009 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Strom 2010 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Trehnholme 1989 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Walker 1998 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Wyatt 1998 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Yealy 2005 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Totals | 49 | 29 | 29 | 14 | 4 | 28 | 15 |
Appendix 5. Contribution of 109 ITS studies to meta‐regression of prescribing outcomes for intervention effect (n = 107) or removal (n = 5, 2 studies only had data about intervention removal)
| Intervention effect | Intervention removal Table 7 | ||||
| TOTALS | 107 | 5 | 91 | 29 | 43 |
| Study | |||||
| Abramowitz 1982 | 1 | 0 | 1 | 0 | 1 |
| Adachi 1997 | 1 | 0 | 1 | 0 | 1 |
| Akenroye 2014 | 1 | 0 | 1 | 0 | 1 |
| Aldeyab 2012 | 1 | 0 | 1 | 1 | 0 |
| Ananda Rajah 2010 | 1 | 0 | 0 | 0 | 0 |
| Ansari 2003 | 1 | 0 | 1 | 0 | 1 |
| Avorn 1988 | 1 | 0 | 1 | 0 | 1 |
| Bantar 2006 | 1 | 0 | 1 | 1 | 0 |
| Barlow 2007 | 1 | 0 | 1 | 0 | 1 |
| Bassetti 2009 | 1 | 0 | 1 | 1 | 0 |
| Belliveau 1996 | 1 | 0 | 1 | 1 | 0 |
| Benson 2014 | 1 | 0 | 1 | 0 | 1 |
| Berild 2002 | 1 | 0 | 0 | 0 | 0 |
| Borde 2014a | 1 | 0 | 1 | 0 | 1 |
| Borde 2015a | 1 | 0 | 1 | 0 | 1 |
| Borde 2015b | 1 | 0 | 1 | 0 | 1 |
| Bradley 1999 | 1 | 0 | 1 | 1 | 0 |
| Buising 2008a | 1 | 0 | 1 | 1 | 0 |
| Buising 2008b | 1 | 0 | 1 | 0 | 1 |
| Bunz 1990 | 1 | 0 | 1 | 1 | 0 |
| Buyle 2010 | 1 | 0 | 1 | 0 | 1 |
| Chan 2011 | 1 | 0 | 1 | 1 | 0 |
| Chan 2014 | 1 | 0 | 1 | 1 | 0 |
| Chandy 2014 | 1 | 0 | 1 | 0 | 0 |
| Cheng 2009 | 1 | 0 | 1 | 0 | 1 |
| Cook 2011 | 1 | 0 | 1 | 0 | 1 |
| Cook 2011a | 1 | 0 | 1 | 1 | 0 |
| Cortoos 2011 | 1 | 0 | 1 | 0 | 0 |
| Dancer 2013 | 1 | 0 | 1 | 1 | 0 |
| Dull 2008 | 1 | 0 | 1 | 0 | 1 |
| Elligsen 2012a | 1 | 0 | 1 | 0 | 1 |
| Everitt 1990 | 1 | 0 | 1 | 1 | 0 |
| Fitzpatrick 2008 | 1 | 0 | 0 | 0 | 0 |
| Fowler 2007 | 1 | 0 | 1 | 0 | 1 |
| Fukuda 2014 | 1 | 0 | 1 | 0 | 1 |
| Grohs 2014 | 1 | 0 | 0 | 0 | 0 |
| Gupta 1989 | 1 | 0 | 1 | 1 | 0 |
| Hadi 2008 | 1 | 0 | 1 | 0 | 0 |
| Halm 2004 | 1 | 0 | 0 | 0 | 0 |
| Hess 1990 | 1 | 0 | 1 | 0 | 1 |
| Hitti 2012 | 1 | 0 | 1 | 0 | 0 |
| Huber 1982 | 1 | 0 | 0 | 0 | 0 |
| Hulgan 2004 | 1 | 0 | 1 | 0 | 1 |
| Inaraja 1986 | 1 | 0 | 0 | 0 | 0 |
| Jobson 2015 | 1 | 0 | 1 | 0 | 1 |
| Jump 2012 | 1 | 0 | 1 | 0 | 1 |
| Kallen 2009 | 1 | 1 | 0 | 0 | 0 |
| Kim 2008 | 1 | 1 | 1 | 1 | 0 |
| Knudsen 2014 | 1 | 0 | 1 | 0 | 1 |
| Kumana 2001 | 1 | 0 | 1 | 0 | 1 |
| Lafuarie 2012 | 1 | 0 | 1 | 0 | 1 |
| Lautenbach 2003 | 1 | 0 | 0 | 0 | 0 |
| Lee 1995 | 1 | 0 | 1 | 0 | 1 |
| Lee 2007 | 1 | 0 | 0 | 0 | 0 |
| Lee 2014 | 1 | 0 | 1 | 0 | 1 |
| Liebowitz 2008 | 1 | 0 | 1 | 0 | 0 |
| Magedanz 2012 | 1 | 0 | 1 | 0 | 1 |
| Marwick 2013 | 1 | 0 | 1 | 0 | 1 |
| May 2000 | 1 | 0 | 1 | 0 | 0 |
| McElnay 1995 | 1 | 0 | 1 | 1 | 0 |
| McGowan 1976 | 1 | 0 | 0 | 0 | 0 |
| McNulty 1997 | 1 | 0 | 1 | 1 | 0 |
| Mercer 1999 | 1 | 0 | 1 | 1 | 0 |
| Meyer 2007 | 1 | 0 | 1 | 0 | 0 |
| Meyer 2009 | 1 | 0 | 1 | 0 | 0 |
| Meyer 2010 | 1 | 0 | 1 | 0 | 0 |
| Mittal 2014 | 1 | 0 | 1 | 0 | 1 |
| Mol 2005 | 1 | 0 | 0 | 0 | 0 |
| Newland 2012 | 1 | 0 | 1 | 0 | 1 |
| Parikh 2014 | 1 | 0 | 1 | 0 | 0 |
| Patel 1989 | 1 | 0 | 0 | 0 | 0 |
| Perez 2003, Intervention 2 | 1 | 0 | 1 | 0 | 1 |
| Peto 2008 | 1 | 0 | 1 | 1 | 0 |
| Petrikkos 2007 | 1 | 0 | 1 | 0 | 0 |
| Po 2012, Intervention 1 | 1 | 0 | 1 | 0 | 0 |
| Popovski 2014 | 1 | 0 | 1 | 0 | 0 |
| Price 2010 | 1 | 0 | 1 | 1 | 0 |
| Richards 2003 | 1 | 0 | 1 | 1 | 0 |
| Ross 2014 | 1 | 0 | 1 | 0 | 0 |
| Saizy‐Callaert 2003 | 1 | 0 | 0 | 0 | 0 |
| Salama 1996 | 1 | 0 | 1 | 1 | 0 |
| Schwann 2011 | 1 | 0 | 1 | 0 | 1 |
| Schwartz 2007 | 1 | 0 | 1 | 0 | 0 |
| Sirinavin 1998 | 1 | 0 | 0 | 0 | 0 |
| Skaer 1993 | 1 | 0 | 1 | 0 | 1 |
| Standiford 2012 | 1 | 1 | 1 | 0 | 1 |
| Stevenson 1988 | 1 | 0 | 1 | 0 | 0 |
| Sun 2011 | 1 | 0 | 1 | 0 | 1 |
| Suwangool 1991 | 1 | 0 | 1 | 1 | 0 |
| Talpaert 2011 | 1 | 0 | 1 | 1 | 0 |
| Tangden 2011 | 1 | 0 | 1 | 0 | 0 |
| Toltzis 1998 | 1 | 0 | 1 | 1 | 0 |
| Valiquette 2009 | 1 | 0 | 1 | 0 | 1 |
| van Kasteren 2005 | 1 | 0 | 1 | 0 | 1 |
| Volpe 2012 | 1 | 0 | 1 | 0 | 1 |
| Wang 2014 | 1 | 0 | 1 | 1 | 0 |
| Wax 2007 | 1 | 0 | 1 | 0 | 0 |
| Weinberg 2001 | 1 | 0 | 0 | 0 | 0 |
| Weiner 2009 | 1 | 0 | 1 | 0 | 1 |
| Wenisch 2014 | 1 | 0 | 1 | 1 | 0 |
| Willemsen 2010 | 1 | 0 | 1 | 0 | 1 |
| Wilson 1991 | 1 | 0 | 1 | 0 | 0 |
| Woodward 1987 | 1 | 0 | 1 | 1 | 0 |
| Yeo 2012 | 1 | 0 | 1 | 0 | 1 |
| Yong 2010 | 1 | 0 | 1 | 0 | 1 |
| Yoon 2014 | 1 | 0 | 1 | 1 | 0 |
| Young 1985 | 1 | 0 | 1 | 1 | 0 |
Appendix 6. RCTs and ITS studies not included in any evidence synthesis
Reasons for exclusion of 9 RCTs from prescribing meta‐analysis. Note that these studies had no valid clinical outcome data and so were not included in any meta‐analysis:
| Reason | Number | Studies |
| Prescribing outcome continuous variable with no standard deviation | 5 | Lesprit 2013; Nobre 2008; Oosterheert 2005; Palmay 2014; Shehabi 2014 |
| Insufficient detail to quantify impact on prescribing outcomes used in the meta‐analyses | 4 |
Reasons for exclusion of 28 ITS studies from meta‐regression:
16 ITS studies did not include time series data about prescribing outcomes: Aldeyab 2014; Calil 2001; Carling 2003; Charbonneau 2006; Climo 1998; de Champs 1994; Dempsey 1995; Dua 2014; Gerding 1985; Khan 2003; Landman 1999; Lawes 2012; Leverstein‐van Hall 2001; Nuila 2008; Pear 1994; Toltzis 2014. Note that Bell 2014 did not include data about prescribing outcomes but did include valid clinical outcome data (Table 4).
13 ITS studies included time series data about prescribing outcomes but were excluded from meta‐regression for the following reasons:
| Study | Reason |
| Only 3 postintervention points and compound outcome (choice and dose) not comparable with other studies | |
| Intervention was substitution of ertapenem for ampicillin‐sulbactam, but there are no ampicillin‐sulbactam data. | |
| Effect size reported for segmented regression analysis but no variance. | |
| Large, unjustified gap between pre‐ and postintervention data | |
| Restriction of cephalosporins was in place throughout the study period. The paper reports an outbreak of cephalosporin‐resistant Klebsiella pneumoniae. Following the outbreak "approvals were reduced by 80%", but unclear whether this was because of change in restriction or reduction in requests. | |
| Removal of restriction of fluoroquinolone and effect on MRSA, BUT only one data point prior to removal so cannot be re‐analysed. | |
| Non‐standardised intervention and prescribing outcomes across multiple hospitals | |
| "Intervention" was introduction of ertapenem into the formulary with no instruction to use less of anything else. | |
| 4 months' pre‐ and postintervention data in 2 weekly time points. Data format not compatible with other studies. | |
| Removal of restriction only, and there is not enough unnecessary use before de‐restriction to detect change. | |
| Not truly 3 pre‐intervention time points, and time intervals irregular. | |
| Comparison is between the deliverer of the same intervention (infectious disease physicians with and without infection control training). No pre‐intervention data | |
| Large, unjustified gap between pre‐ and postintervention data |
Appendix 7. Details of disagreements with other reviews
A systematic review on current evidence about antimicrobial stewardship objectives reported that "guideline‐adherent empirical therapy was associated with a relative risk reduction for mortality of 35% (odds ratio 0.65, 95% CI 0.54‐0.80)" (Schuts 2016). This analysis was based on 39 studies, of which 19 were identified by our literature search. We have reviewed the 20 studies that were not identified by our literature search. Only two of the 39 studies in this review reported an intervention, and both were identified by our literature review: one was invalid because it was an uncontrolled before‐after study (Garcia 2007), and one controlled before‐after study (CBA) is in our 'Characteristics of included studies' table (Dean 2006). The remaining 27 studies used case control or cohort designs to compare the outcomes of patients with and without guideline‐adherent antibiotic treatment, and did not include an intervention to change professional practice. The results of this review are in marked contrast to our analysis of mortality in 11 randomised controlled trials targeting antibiotic choice (Analysis 3.1). The aim of these interventions was to increase adherence with antibiotic guidelines for the antibiotic or route of administration. We have presented results as risk differences (Figure 8), but the odds ratio for mortality in these 11 randomised controlled trials is 0.96 (95% confidence interval (CI) 0.82 to 1.13). The most likely explanation for the discrepancy between our results and those of Schuts 2016 is confounding by indication. It is likely that patients with less complex or severe illness were more likely to receive guideline‐adherent antibiotic treatment and that there was residual confounding after adjustment for available clinical information. The only valid intervention study in the analysis by Schuts 2016 was a CBA. This study compared outcomes for community‐acquired pneumonia (CAP) for patients in 16 hospitals that had implemented a policy based on national guidelines with 19 control hospitals from the same state (Dean 2006). The CAP policy included several important elements in addition to antibiotic choice, such as antibiotic administration in the outpatient or emergency department before admission to hospital; administration of enoxaparin; and early ambulation of hospital inpatients. This study did not include any measures of process compliance, so it is unclear whether there is any relationship between mortality and adherence with the antibiotics recommended in the CAP policy.
A systematic review on the effect of antibiotic stewardship programmes on Clostridium difficile infection (CDI) reported that interventions were associated with a consistent, significant protective effect (pooled risk ratio for CDI 0.48, 95% CI 0.38 to 0.62) (Feazel 2014). This analysis was based on 16 studies, of which 10 were identified by our literature search. We have reviewed the six studies that were not identified by our literature search. Of the 16 studies included in this systematic review, four were interrupted time series (ITS) studies that we have included in our review (Elligsen 2012; Fowler 2007; Price 2010; Talpaert 2011); the remaining 12 studies were either uncontrolled before‐after or inadequate ITS studies. Elligsen 2012 only has reliable data about prescribing outcomes; CDI data are in the form of an inadequate CBA with aggregated before and after data from one intervention and one control site. The statistical analysis in this review, Feazel 2014, was not appropriate for the three ITS studies included in our review (Fowler 2007; Price 2010; Talpaert 2011). Calculation of risk ratios for the post‐ versus pre‐intervention periods is an uncontrolled before‐after analysis, which does not provide a reliable estimate of intervention effect. This is most clearly demonstrated by the results of one study (Price 2010), in which CDIs were declining pre‐intervention by ‐0.04 cases per 1000 occupied bed days per month (95% CI ‐0.08 to ‐0.01; P = 0.03). Postintervention CDI continued to decline at a slightly greater rate, but our estimate of the intervention effect was only a 10% reduction at 12 months (95% CI 85% reduction to 65% increase). In the systematic review (Feazel 2014), the reported risk ratio in the post‐ versus pre‐intervention phase was 0.52 (95% CI 0.44 to 0.61), but this result is mainly attributable to a steady decline in CDI over the entire study period rather than to any intervention effect.

Figure 1 Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Blank sections in this graph are due to use of different ROB criteria for CBA, NRT and RCT versus ITS studies

Forest plot of comparison: 1 Prescribing: RCTs of all interventions to reduce unnecessary prescribing, outcome: 1.1 Dichotomous outcomes, increase in desired practice.

Forest plot of comparison: 1 Effectiveness: Prescribing outcomes from RCTs of interventions to reduce unnecessary antibiotic use, outcome: 1.4 Continuous outcomes, duration of all antibiotic treatment (days).
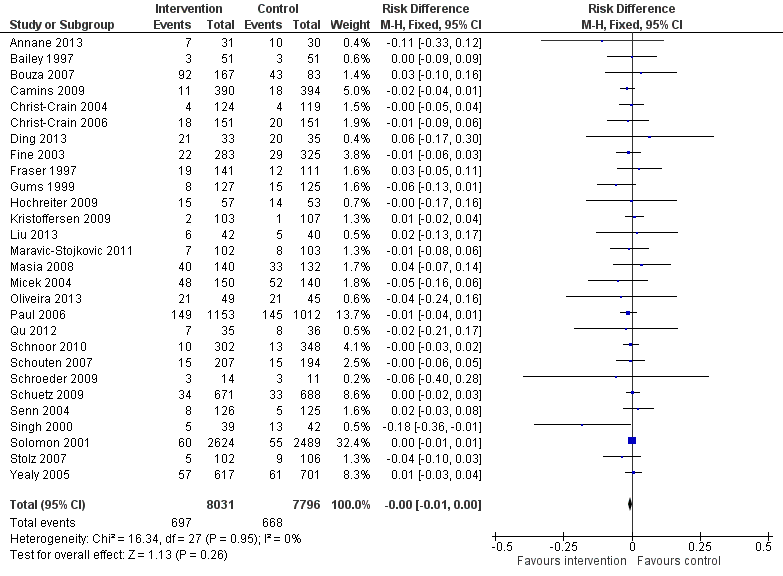
Forest plot of comparison: 2 Adverse effects: Clinical outcomes from RCTs of interventions to reduce unnecessary antibiotic use, outcome: 2.1 Mortality, all RCTs.
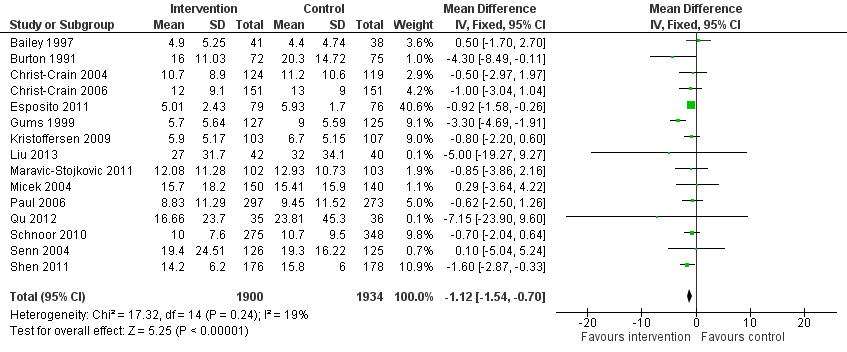
Forest plot of comparison: 2 Adverse effects: Clinical outcomes from RCTs of interventions to reduce unnecessary antibiotic use, outcome: 2.4 Length of stay, all RCTs.
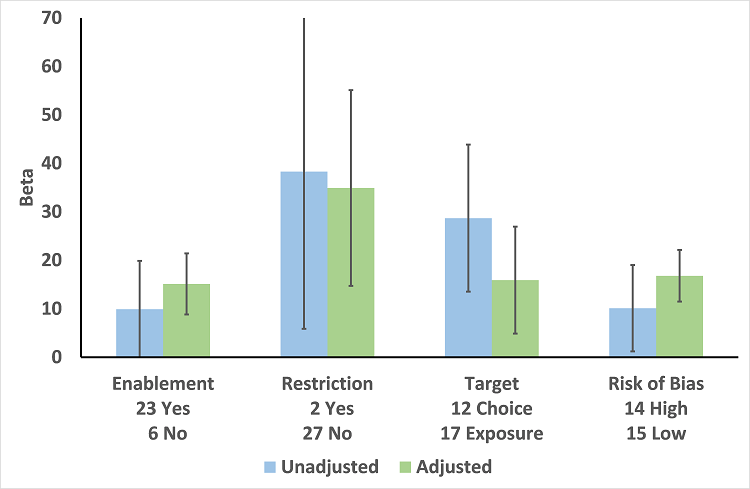
Meta‐regression by effect modifier for 29 RCTs. A positive value for Beta indicates enhanced intervention effect. One RCT had both enabling and restrictive components in the intervention (Strom 2010).

Forest plot of comparison 5: RCTs of enablement with and without feedback, outcome: 5.1 Enablement plus feedback.
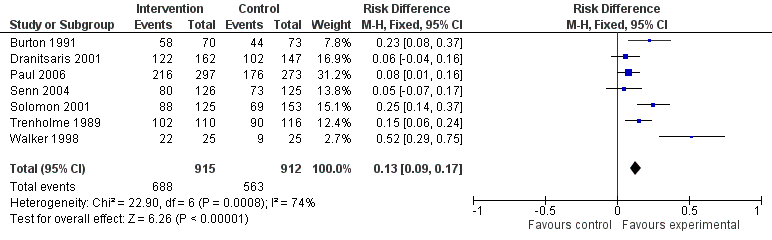
Forest plot of comparison 5: RCTs of enablement with and without feedback, outcome: 5.2 Enablement without feedback.
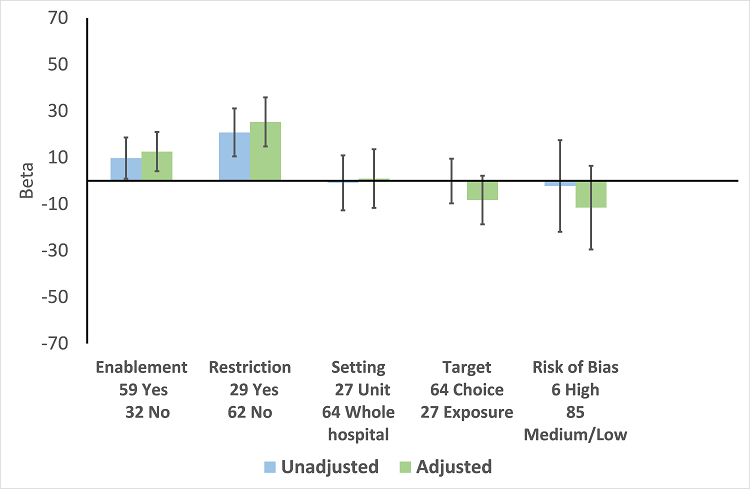
Meta‐regression by effect modifiers of intervention for 91 ITS studies. Outcome is effect on prescribing six months' postintervention. There are 16 studies with both enabling and restricting intervention components (Figure 11).

Meta‐regression of prescribing outcome by effect modifiers for 29 ITS studies of interventions that included restriction.
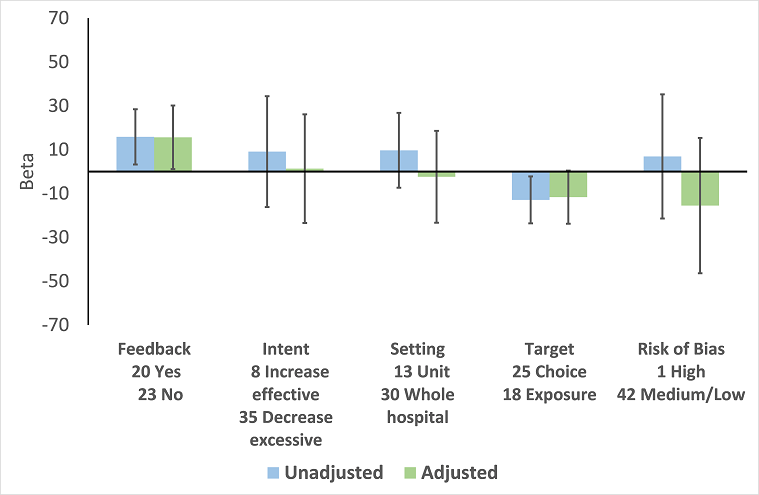
Meta‐regression by effect modifier for 43 ITS studies of interventions that included enablement but not restriction. Outcome is effect on prescribing six months' postintervention. Note that four studies with feedback were not included in this analysis because they also included restriction.
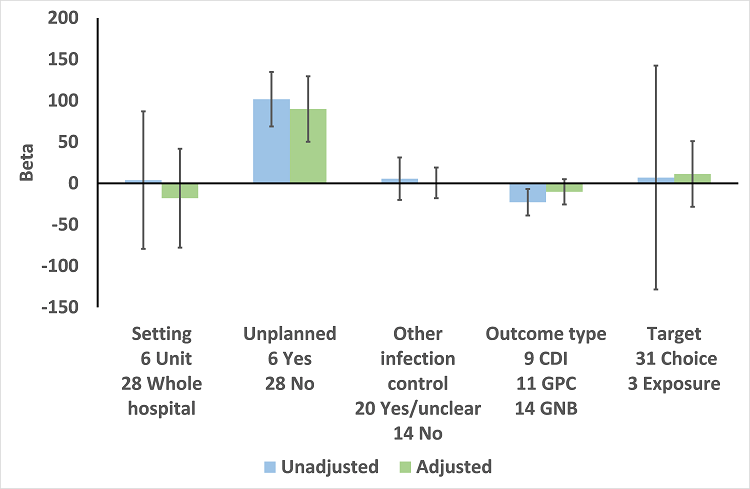
Meta‐regression by effect modifiers for 34 microbial outcomes 12 months' postintervention from 26 ITS studies. The bars show the results for unadjusted versus adjusted analyses, the comparison for unplanned interventions is with planned interventions in both the unadjusted and adjusted analysis.
CDI: Clostridium difficile infection
GPC: infection with antibiotic‐resistant gram‐positive cocci
GNB: infection with antibiotic‐resistant gram‐negative bacteria
Other infection control: 'Yes' means there were changes to infection control processes during the study period.
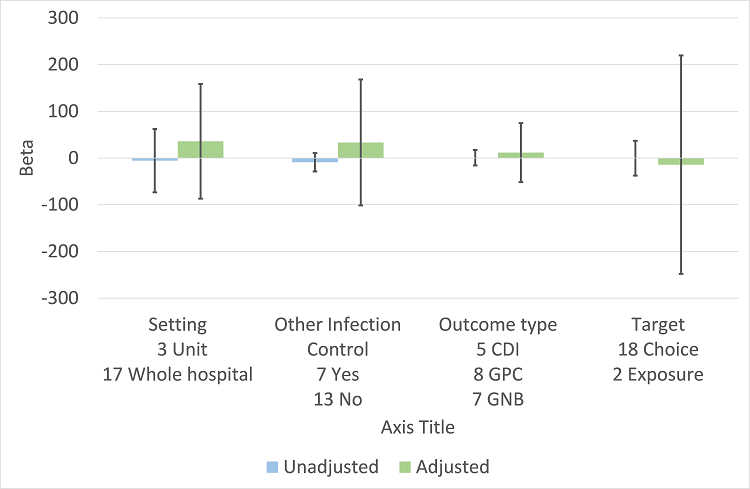
Meta‐regression by effect modifiers for 20 microbial outcomes 12 months' postintervention from 14 ITS studies of planned interventions that provided details about other infection control changes or interventions.
CDI: Clostridium difficile infection
GPC: infection with antibiotic‐resistant gram‐positive cocci
GNB: infection with antibiotic‐resistant gram‐negative bacteria
Other infection control: 'Yes' means there were changes to infection control processes during the study period.

Comparison 1 Effectiveness: Prescribing outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 1 Dichotomous outcomes, increase in desired practice.
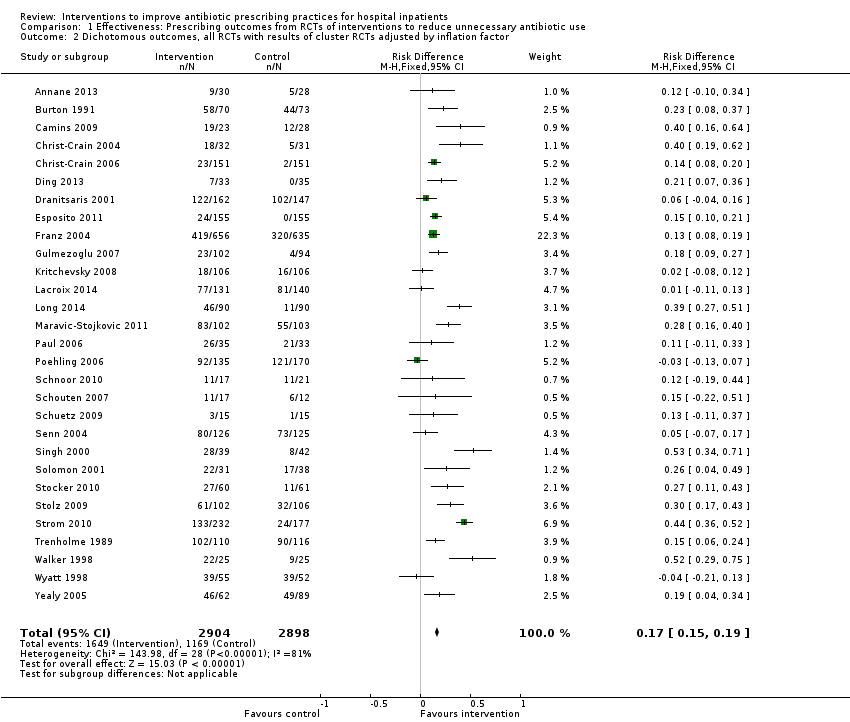
Comparison 1 Effectiveness: Prescribing outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 2 Dichotomous outcomes, all RCTs with results of cluster RCTs adjusted by inflation factor.

Comparison 1 Effectiveness: Prescribing outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 3 Dichotomous outcomes, low or medium 'Risk of bias' studies only.
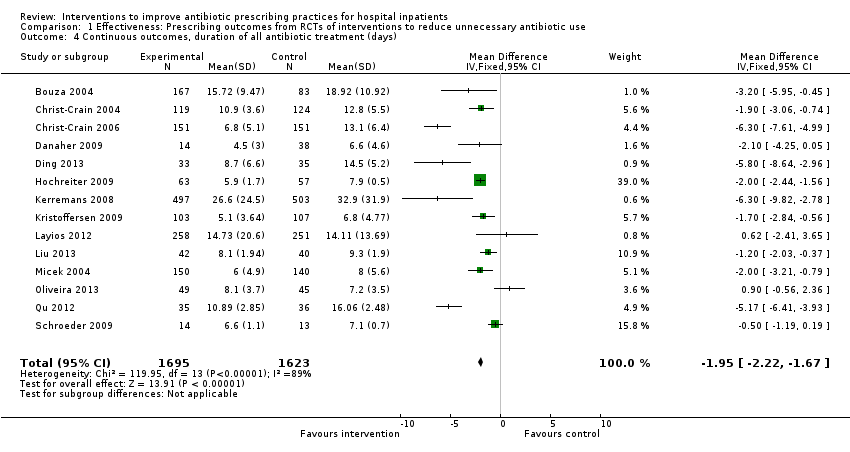
Comparison 1 Effectiveness: Prescribing outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 4 Continuous outcomes, duration of all antibiotic treatment (days).

Comparison 1 Effectiveness: Prescribing outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 5 Continuous outcomes, duration of all antibiotic treatment with results of cluster RCTs adjusted by inflation factor.

Comparison 1 Effectiveness: Prescribing outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 6 Continuous outcomes, low or medium 'Risk of bias' studies only.

Comparison 1 Effectiveness: Prescribing outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 7 Continuous outcome, consumption of targeted antibiotic only, standardised mean reduction (original outcome cost, days or DDD).
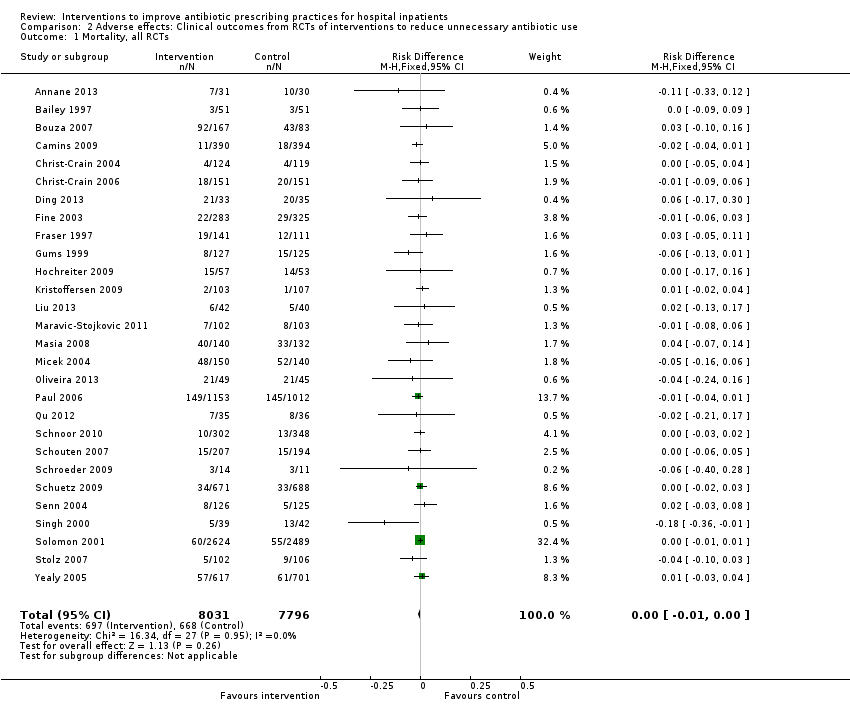
Comparison 2 Adverse effects: Clinical outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 1 Mortality, all RCTs.

Comparison 2 Adverse effects: Clinical outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 2 Mortality, all RCTs with results of cluster RCTs adjusted by inflation factor.
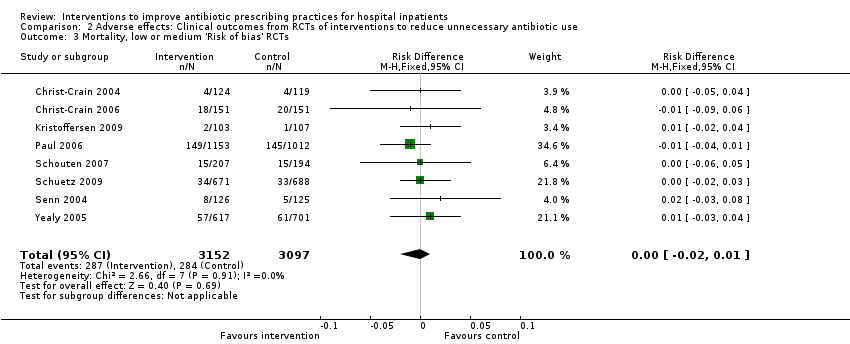
Comparison 2 Adverse effects: Clinical outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 3 Mortality, low or medium 'Risk of bias' RCTs.

Comparison 2 Adverse effects: Clinical outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 4 Length of stay, all RCTs.
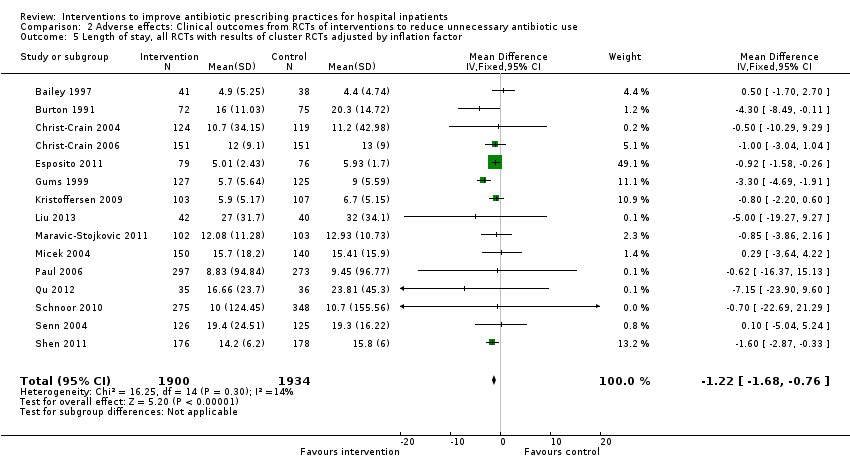
Comparison 2 Adverse effects: Clinical outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 5 Length of stay, all RCTs with results of cluster RCTs adjusted by inflation factor.
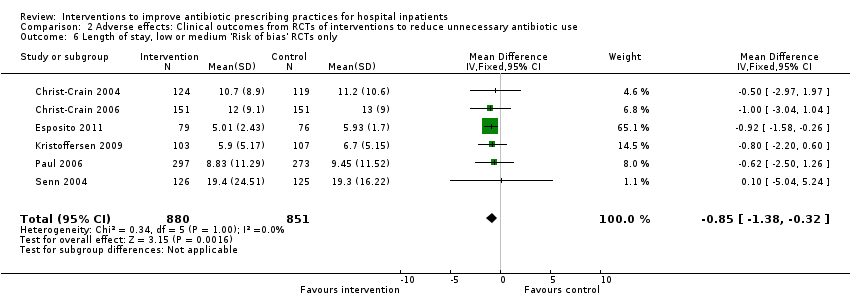
Comparison 2 Adverse effects: Clinical outcomes from RCTs of interventions to reduce unnecessary antibiotic use, Outcome 6 Length of stay, low or medium 'Risk of bias' RCTs only.
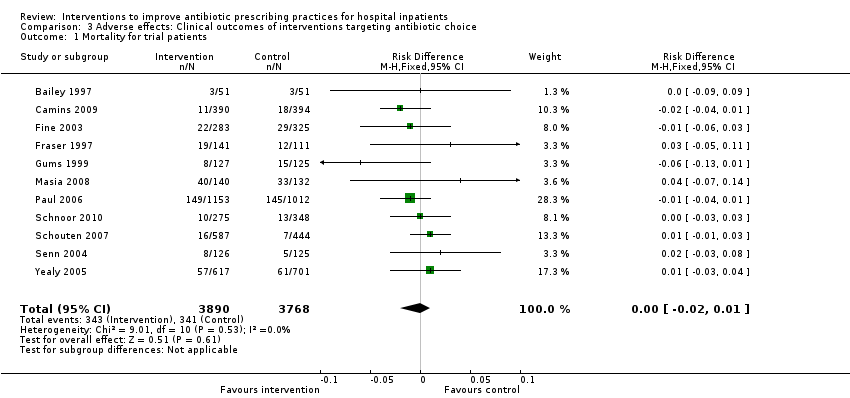
Comparison 3 Adverse effects: Clinical outcomes of interventions targeting antibiotic choice, Outcome 1 Mortality for trial patients.

Comparison 3 Adverse effects: Clinical outcomes of interventions targeting antibiotic choice, Outcome 2 Length of stay for trial patients.

Comparison 4 Adverse effects: Clinical outcomes of interventions targeting antibiotic exposure, Outcome 1 Mortality for trial patients.

Comparison 4 Adverse effects: Clinical outcomes of interventions targeting antibiotic exposure, Outcome 2 Length of stay for trial patients.

Comparison 5 Modifiers of intended effect: Comparison of enabling interventions with and without feedback, Outcome 1 Enablement with feedback.

Comparison 5 Modifiers of intended effect: Comparison of enabling interventions with and without feedback, Outcome 2 Enablement without feedback.
| Patient or population: adults or children undergoing inpatient antibiotic prophylaxis or treatment Settings: mainly high‐income countries (North America or Western Europe) Intervention: any intervention targeting healthcare professionals that aimed to improve antibiotic prescribing to hospital inpatients Comparison: usual care (varied across studies) | |||||
| Effectiveness: prescribing outcomes from RCTs | |||||
| Outcomes | Absolute effect* | No of participants (No of studies) | Certainty of the evidence (GRADE) | Comments | |
| Without intervention | With intervention | ||||
| Proportion of participants who were treated according to antibiotic prescribing guidelines Follow‐up to end of study | 43 per 100 | 58 per 100 | 23,394 participants (29 RCTs) | ⊕⊕⊕⊕ | We have graded the certainty of evidence as high because heterogeneity was explained by prespecified effect modifiers (see below). The intervention effect varied between the studies, but the direction of effect was consistent. Restricting the analysis to studies at low risk of bias gave a similar result (RD 11%, 95% CI 10% to 12%). |
| Difference: 15 more participants per 100 (95% CI 15 to 23) received appropriate treatment following intervention. | |||||
| Duration of all antibiotic treatment | 11.0 days | 9.1 days | 3318 participants (14 RCTs) | ⊕⊕⊕⊕ | |
| Difference: 1.95 fewer days per participant (95% CI 2.22 to 1.67) | |||||
| Mortality Follow‐up to end of study | 11 per 100 | 11 per 100 | 15,827 participants 28 (RCTs) | ⊕⊕⊕⊝1 | Mortality and length of stay were measured to determine the impact of reduced antibiotic use on clinical outcomes. The results were similar for studies that targeted antibiotic choice or exposure. Only 1 of the interventions in the RCTs with mortality or length‐of‐stay outcomes had a restrictive component (Singh 2000). This evidence is therefore at high risk of indirectness because 7 studies in the next section of the table (see below) raise concerns about the safety of restrictive interventions. Moreover, the ITS studies showed that restrictive components were included in 42 (34%) of 123 hospital interventions. |
| Difference: 0 more deaths per 100 participants (95% CI 1 to 0 fewer) | |||||
| Mean length of hospital stay per participant | 12.9 days | 11.8 days | 3834 participants 15 (RCTs) | ⊕⊕⊕⊝1 | |
| Difference: 1.1 fewer days per participant (95% CI 1.5 to 0.7 fewer) | |||||
| Delay in treatment | Restrictive interventions increased the risk of delay in all 3 studies. The risk to patients resulted in termination of the RCT by the Trial Monitoring Committee. | 1 RCT, 2 cohort | ⊕⊕⊝⊝2 | The evidence from these 7 studies of unintended consequences raises concerns about the directness of the evidence of safety from the 29 RCTs in the previous section of the table (see above). | |
| Negative professional culture | Loss of trust in infection specialists because of failure to record approvals for restricted drugs or provide warning about stopping treatment Misleading or inaccurate information from prescribers in order to meet criteria for restricted drugs. In 1 hospital, misdiagnosis of hospital‐acquired infection was large enough to trigger an outbreak investigation. | 1 case control, 2 cohort, 1 qualitative | ⊕⊕⊖⊖3 | ||
| Effect modifiers (heterogeneity) for immediate effect of intervention on prescribing outcomes: | |||||
| Effect modifier | Adjusted effect in meta‐regression | Number of studies | Certainty of the evidence (GRADE) | Comments | |
| Enablement | 15.12 (8.45 to 21.8) | 29 RCTs | ⊕⊕⊕⊕ | The effect of enablement and restriction is similar in the RCTs and ITS studies. Of the 29 RCTs, only 8 (31%) of interventions were hospital‐wide, the majority being in single units. In contrast, 64 (70%) of the interventions in ITS studies were hospital‐wide. | |
| 12.86 (4.11 to 21.6) | 91 ITS | ||||
| Restriction | 34.91 (13.52 to 56.29) | 29 RCTs | ⊕⊕⊕⊕ | ||
| 24.69 (13.74 to 35.64) | 91 ITS | ||||
| Addition of feedback to enablement | 10.88 (7.16 to 19.32) | 23 RCTs | ⊕⊕⊕⊝2 | Feedback was included in 4 (17%) of 23 RCTs and 20 (47%) of 43 ITS studies with interventions that included enablement. There were not enough interventions with goal setting and action planning to analyse as effect modifiers. | |
| 15.63 (0.56 to 30.70) | 43 ITS | ||||
| Addition of enablement to restriction | 38.36 (18.94 to 57.78) | 29 ITS | ⊕⊕⊖⊖3 | Enablement was included in 13 (45%) of 29 ITS studies with restrictive interventions. | |
| *The risk WITHOUT the intervention is based on the median control group risk across studies. The corresponding risk WITH the intervention (and the 95% confidence interval for the difference) is based on the overall relative effect (and its 95% confidence interval). | |||||
| GRADE Working Group grades of evidence | |||||
| Details of five GRADE criteria for all outcomes from RCTs are in Appendix 2. 1We downgraded the evidence to moderate because of indirectness. | |||||
| Intervention Function | Definition | Intervention components |
| Education | Increasing knowledge or understanding | Educational meetings; Dissemination of educational materials; Educational outreach |
| Persuasion | Using communication to induce positive or negative feelings or to stimulate action | Educational outreach by academic detailing or review and recommend change |
| Restriction | Using rules to reduce the opportunity to engage in the target behaviour (or increase the target behaviour by reducing the opportunity to engage in competing behaviours) | Restrictive |
| Environmental restructuring | Changing the physical context | Reminders (physical) such as posters, pocket‐size or credit card‐size summaries or on laboratory test reports; Structural (e.g. new laboratory tests or rapid reporting of results) |
| Enablement | Increasing means/reducing barriers to increase capability or opportunity | Audit and feedback; Decision support through computerised systems or through circumstantial reminders that were triggered by actions or events related to the targeted behaviour; Educational outreach by review and recommend change |
| Study | Prescribing target | Restriction | Design of analysis | Effect estimate | 95% CI |
| Choice of drug | No | Cohort | Incidence rate ratio 1.1 | 0.9 to 1.5 | |
| Choice of drug | No | Cohort | Increase by 1.4% | ‐1.2% to 4.1% | |
| Choice of drug | Yes | ITS, segmented regression | Change in slope ‐0.0172 | No data | |
| Choice of drug | Yes | Cohort | +0.43 per 1000 OBD | No data | |
| *Mortality was measured in all patients in the hospital rather than just those patients who were the targets of the interventions. CI: confidence interval | |||||
| Study | Prescribing target | Restrictive | Design of analysis | Effect estimate | 95% CI |
| Exposure, % treated | No | Cohort | ‐0.5 days | No data | |
| Choice of drug | No | Cohort | ‐0.1 days | ‐0.49 to +0.29 | |
| *Length of stay was measured in all patients in the hospital rather than just those patients who were the targets of the interventions. CI: confidence interval | |||||
| Study | Prescribing target | Design of analysis | Effect measure | Effect estimate | 95% CI |
| Antibiotic choice | ITS, segmented regression | Risk of postoperative acute kidney injury | Increase 98% | 93.8% to 94.2% | |
| Exposure, duration | Cohort | Surgical‐site infection | Decrease 0.8% | ‐2.2% to 0.6% | |
| Time to first antibiotic dose | Cohort | Left without being seen rate | Decrease 0.4% | No data | |
| CI: confidence interval | |||||
| Study | Design | Patients | Intended target | Unintended consequence | Effect estimate | 95% CI |
| Interventions with a restrictive component | ||||||
| Qualitative | 36 physicians | Reduce unnecessary use of restricted antibiotics | Inaccurate feedback | Not quantified; qualitative study | ||
| Case control | Not clear | Increase in physician‐based diagnosis of nosocomial infection | No denominator data | |||
| Cohort | 120 | Failure to warn prescribers about discontinuation | — | — | ||
| Cohort | 222 | Reduce unnecessary laboratory tests | Delay in TFAD (HR > 1 shows delay less likely in intervention period) | Multivariate HR 1.56 | 1.17 to 2.07 | |
| Cross‐sectional | 15,440 | Reduce unnecessary use of restricted antibiotics | Orders for restricted antibiotics (% all orders) from 10 to 11 pm vs all other hours | — | — | |
| Cohort | 360 | % appropriate orders 10 to 11 pm vs 9 to 10 pm | ‐23.7% | ‐31.8% to ‐15.5% | ||
| Cohort | 200 | Risk of inaccurate information in orders judged inappropriate vs appropriate | OR 2.2 | 1.0 to 4.4 | ||
| Cohort | 3251 | Risk of 1‐hour delay in TFAD | OR 1.5 | 1.2 to 1.8 | ||
| Risk of 2‐hour delay in TFAD | OR 1.8 | 1.4 to 2.2 | ||||
| Interventions with no restrictive component | ||||||
| Cohort | 13,042 | Reduce time to first antibiotic dose for patients with community‐acquired pneumonia | % CAP diagnoses | 1% increase | No denominator data | |
| Cohort | 518 | % correct CAP diagnoses | ‐7.9% decrease | ‐15.4% to ‐0.4% | ||
| Cohort | 548 | % correct CAP diagnoses | ‐16.0% decrease | ‐7.6% to ‐24.4% | ||
| CAP: community‐acquired pneumonia | ||||||
| Intervention function and components | RCT | ITS |
| Enablement | 24 studies | 59 studies |
| Number of enabling or restrictive intervention components | 27 | 76 |
| Studies with > 1 Enabling intervention component | 2 8%* | 19 32%* |
| Audit and feedback | 4 17% | 24 41% |
| Computerised decision support | 1 4% | 3 5% |
| Circumstantial reminders | 16 67% | 18 31% |
| Review and recommend change | 6 25% | 31 53% |
| Restriction | 2 studies | 29 studies |
| Number of Restrictive intervention components | 3 | 41 |
| Studies with > 1 Restrictive intervention component | 1 50% | 10 34% |
| Expert approval | 1 50% | 18 62% |
| Compulsory order form | 1 50% | 7 24% |
| Removal | 0 | 10 34% |
| Review and make change | 1 50% | 6 21% |
| No Enablement or Restriction | 4 studies | 18 studies |
| Number of intervention components | 6 | 25 |
| Studies with > 1 intervention component | 2 50% | 6 33% |
| Educational materials or meetings | 3 75% | 16 89% |
| Educational outreach (academic detailing) | 1 25% | 6 33% |
| Physical reminders | 1 25% | 2 11% |
| Structural intervention | 1 25% | 1 6% |
| *The denominator for all percentages is the number of studies for each intervention function. One RCT, Strom 2010, and 16 ITS studies (Figure 11) included both enabling and restrictive intervention components. ITS: interrupted time series | ||
| Study | Intervention function | Intervention effect (95% CI) | Time intervention was in place | Effect of removal (95% CI) |
| Restriction | ‐87.5% ‐115.4 to ‐59.7 | 6 months | 398.9% 238.2 to 559.5 | |
| Restriction | ‐23.1% ‐53.7 to +7.4 | 9 months | 6.0% ‐23.4 to 35.4 | |
| Enablement | ‐28.6% ‐46.5 to ‐10.6 | 7 years | 31.0% 6.8 to 55.3 | |
| Restriction | No data | “long‐standing” | 301.2% 230.9 to 371.5 | |
| Restriction | 2 years | 255.8% 194.7 to 316.9 | ||
| CI: confidence interval | ||||
| Study | Design | Microbial outcome | Reason not in meta‐analysis |
| RCT | Colonisation with MRSA (nasal swab) and GNRB (rectal swabs) | Not comparable with any other RCT | |
| RCT | Number of cases of Clostridium difficile | Not in prescribing meta‐analysis | |
| RCT | Secondary infection and/or colonisation with multidrug‐resistant bacteria in the 6 months following randomisation | Not in prescribing meta‐analysis. It is impossible to assess the impact of the intervention on colonisation or infection with bacteria resistant to specific antibiotics. | |
| RCT | CDI and infection with antibiotic resistant organisms cases/1000 OBD | Not in prescribing meta‐analysis | |
| RCT | Number of participants with "antimicrobial resistance and/or superinfections" from randomisation until discharge from hospital | It is impossible to assess the impact of the intervention on colonisation or infection with bacteria resistant to specific antibiotics. | |
| CDI: Clostridium difficile infection | |||
| Prescribing target | Microbial outcome | N | Study ID |
| Cephalosporins | GNRB | 8 | Grohs 2014; Kim 2008; Knudsen 2014; Lee 2007; McNulty 1997; Meyer 2009; Petrikkos 2007; Tangdén 2011 |
| MRSA | 1 | ||
| Carbapenems | GNRB | 1 | |
| Fluoroquinolones | GNRB | 3 | |
| MRSA | 1 | ||
| High‐risk antibiotics | CDI | 6 | Aldeyab 2012; Chan 2011; Dancer 2013; Fowler 2007; Talpaert 2011; Valiquette 2007 |
| GNRB | 4 | ||
| MRSA | 6 | Aldeyab 2014; Ananda‐Rajah 2010; Chan 2011; Dancer 2013; Fowler 2007; Liebowitz 2008 | |
| Total antibiotic use | CDI | 2 | |
| MRSA | 1 | ||
| Vancomycin | VRE | 1 | |
| Total microbial | 34* | ||
| *Some studies had more than one microbial outcome, so the total is 34 microbial outcomes from 26 studies. CDI: Clostridium difficile infection | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dichotomous outcomes, increase in desired practice Show forest plot | 29 | 23394 | Risk Difference (M‐H, Fixed, 95% CI) | 0.15 [0.14, 0.16] |
| 2 Dichotomous outcomes, all RCTs with results of cluster RCTs adjusted by inflation factor Show forest plot | 29 | 5802 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [0.15, 0.19] |
| 3 Dichotomous outcomes, low or medium 'Risk of bias' studies only Show forest plot | 15 | 13086 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [0.10, 0.12] |
| 4 Continuous outcomes, duration of all antibiotic treatment (days) Show forest plot | 14 | 3318 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐2.22, ‐1.67] |
| 5 Continuous outcomes, duration of all antibiotic treatment with results of cluster RCTs adjusted by inflation factor Show forest plot | 14 | 3318 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐2.23, ‐1.67] |
| 6 Continuous outcomes, low or medium 'Risk of bias' studies only Show forest plot | 3 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐3.06 [‐3.76, ‐2.37] |
| 7 Continuous outcome, consumption of targeted antibiotic only, standardised mean reduction (original outcome cost, days or DDD) Show forest plot | 4 | 1053 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.37, ‐0.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality, all RCTs Show forest plot | 28 | 15827 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 2 Mortality, all RCTs with results of cluster RCTs adjusted by inflation factor Show forest plot | 28 | 8332 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 3 Mortality, low or medium 'Risk of bias' RCTs Show forest plot | 8 | 6249 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 4 Length of stay, all RCTs Show forest plot | 15 | 3834 | Mean Difference (IV, Fixed, 95% CI) | ‐1.12 [‐1.54, ‐0.70] |
| 5 Length of stay, all RCTs with results of cluster RCTs adjusted by inflation factor Show forest plot | 15 | 3834 | Mean Difference (IV, Fixed, 95% CI) | ‐1.22 [‐1.68, ‐0.76] |
| 6 Length of stay, low or medium 'Risk of bias' RCTs only Show forest plot | 6 | 1731 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.38, ‐0.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality for trial patients Show forest plot | 11 | 7658 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 2 Length of stay for trial patients Show forest plot | 7 | 2276 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐2.16, ‐0.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality for trial patients Show forest plot | 18 | 9173 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 2 Length of stay for trial patients Show forest plot | 8 | 1558 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.42, ‐0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Enablement with feedback Show forest plot | 4 | 3747 | Risk Difference (M‐H, Fixed, 95% CI) | 0.19 [0.16, 0.22] |
| 2 Enablement without feedback Show forest plot | 7 | 1827 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [0.09, 0.17] |

