Pengubahsuaian tekanan atau pelembapan untuk meningkatkan pengunaan mesin CPAP (continuous positive airway pressure) bagi orang dewasa yang mengalami OSA (obstructive sleep apnea)
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind, parallel group study | |
| Participants | N = 76 participants (53 M/23 F). Age not reported. BMI 35.6 kg/m2; AHI 60.2; ESS 13.6 Inclusion criteria: CPAP naive Exclusion criteria: significant cardiac, respiratory, psychiatric, or sleep comorbidities | |
| Interventions | CPAP versus C‐Flex (identical devices) Study duration: 3 months | |
| Outcomes |
| |
| Funding & conflicts of interest statements | 'This study was funded by Philips‐Respironics. All authors received research support from Philips‐Respironics. Philips‐Respironics manufactures and markets CPAP and C‐Flex devices.' | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to treatment was performed prior to manual titration using a (1, 2) urn randomization procedure" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization to treatment was performed prior to manual titration using a (1, 2) urn randomization procedure" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was a double‐blinded, parallel‐arm RCT of C‐Flex versus CPAP" Quote: "Patients were not able to access the C‐Flex menu option, and all references to "C‐Flex" on the device were covered" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was a double‐blinded, parallel‐arm RCT of C‐Flex versus CPAP" Quote: "Patients were not able to access the C‐Flex menu option, and all references to "C‐Flex" on the device were covered" |
| Blinding of outcome assessment (detection bias) | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The data analyst remained blinded by random 3‐digit codes being assigned to all patients" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Two patients were withdrawn".........2 patients dropped out of the CPAP group............they were replaced with additional recruitment, and therefore analysis was conducted on a per‐protocol basis" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, double‐blind, parallel group trial | |
| Participants | N = 104 participants (67 M/37 F); Age 52 years; BMI 33 kg/m2; AHI 42; ESS not reported Inclusion criteria: non‐adherent with CPAP based on 14‐day run‐in (< 4 hours/day); previous diagnosis of OSA Exclusion criteria: compliant with CPAP during run‐in | |
| Interventions | Bi‐PAP with flexible pressure setting versus fixed CPAP setting (identical machine) Run‐in: phase 1 of study identified non‐adherent CPAP users (those using CPAP < 4 hours/day) Study duration: 12 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This study was supported by a grant from Respironics, Inc. Respironics reimbursed National Jewish Medical and Research Center for part of Dr. Ballard’s time. Dr. Gay has received research support from ResMed. Dr. Strollo has indicated no financial conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were then provided a modified positive airway pressure device (BiPAP Pro, Respironics Inc.) randomly set to either CPAP or BiFlex mode at appropriate pressure(s)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were then provided a modified positive airway pressure device (BiPAP Pro, Respironics Inc.) randomly set to either CPAP or BiFlex mode at appropriate pressure(s)" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both patients and the investigators were blinded as to the specific mode assigned to each patient." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both patients and the investigators were blinded as to the specific mode assigned to each patient." |
| Blinding of outcome assessment (detection bias) | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Blinding of outcome assessment (detection bias) | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Study reported to have been analysed on ITT principles. Unlikley to bias machine usage data but quality of life collected from completers. There was differential dropout and this may have influenced the results. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, open‐label, parallel group, singe‐centre trial | |
| Participants | N = 156 participants (145 M/11 F). Age: 59 years; BMI: 36 kg/m2; AHI: 28.5 ESS: 14.8 Inclusion criteria: AHI ≥ 10/hour; ESS ≥ 8; living within 200 miles of treatment centre; age > 18 years Exclusion criteria: previous CPAP therapy; shift work; unstable depression/psychosis; non‐adherence with medication; COPD; uncontrolled hypertension or restless legs syndrome; narcolepsy; supplemental oxygen use; congestive heart failure; nightly narcotic use; hypoventilation; neuromuscular weakness; regular sleep of < 4 hours per night; low baseline SaO2; central apnoea index > 5/hour | |
| Interventions | Auto‐CPAP versus home PSG CPAP titration followed by fixed pressure CPAP treatment Study duration: 6 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This study was supported by a research grant from the Res Med Foundation and an unrestricted research grant from Philips Respironics. Both grants were made to the North Florida Foundation for Research and Education. The CPAP and APAP equipment were purchased by the VA as part of the routine clinical care of the patients. The PAP setups were performed by clinical PAP respiratory therapists as part of the patient’s usual care. A registered polysomnographic technologist (CPAP titrations) and study coordinator were paid by research funding. The principal investigators received no salary support from research funding. This work was also supported by resources provided by the North Florida/South Georgia Veterans Health System, Gainesville, FL. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The authors have indicated no other financial conflicts of interest. The study was performed at the Malcom Randall VA Medical Center, Gainesville, FL." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details available to determine process |
| Allocation concealment (selection bias) | Low risk | Quote: "The method of randomization was by opening sequential envelopes prepared by the research service." |
| Blinding of participants and personnel (performance bias) | High risk | Study had open‐label design which likely affects usage, symptoms, quality of life attrition outcomes. |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by open‐label design. |
| Blinding of outcome assessment (detection bias) | High risk | Impact of study design on outcome assessment related to nature of outcome. Usage, AHI and treatment pressure measured from technical readings. Symptoms, quality of life more likely affected by open‐label design. |
| Blinding of outcome assessment (detection bias) | Low risk | Treatment pressure and AHI unlikely to be affected by open‐label design. |
| Incomplete outcome data (attrition bias) | Unclear risk | Balanced but potentially meaningful dropout which was slightly higher in fixed CPAP group (19/78 versus 12/78). Participants not using machines at clinic visit assumed to be non‐users and assigned values of 0 for usage outcome. Likely to be greater impact on symptoms and quality of life outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes presented according to details provided on trials registry record. |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, double‐blind, parallel group trial | |
| Participants | N = 35 participants (34 M/1 F); Age 54.2 years; BMI 30.9 kg/m2; AHI ≥ 39; ESS: 10.2 Inclusion criteria: 18 to 75 years; AHI ≥ 15; BMI < 45 kg/m²; ability to follow study specific instructions Exclusion criteria: other sleep, cardiac, pulmonary, psychiatric or neurological disorder; previous abuse of alcohol, hypnotics or drugs; previous treatment for OSA (including CPAP); inability to wear a mask | |
| Interventions | ABRP‐PAP versus fixed CPAP Study duration: 12 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This study was supported by an unrestricted grant from Philips Respironics, Inc. 1001 Murry Ridge Lane, Murrysville, PA, 15668, USA" Conflict of interest quote: "'Dr. Fietze, Prof. Penzel, Dr. Peter and Dr. Blau have received travel grants and honorariums for lecturing from Philips Respironics. The other authors have no significant conflicts of interest with any companies/organizations whose products or services may be discussed in this article." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We generated a list of N = 32 uniformly distributed pseudo‐random numbers of either 0 (CPAP) or 1 (Auto bi‐level) by using the Mersenne Twister algorithm" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation of the patient was told directly via telephone in case of randomisation from an not otherwise involved team member, situated in another campus of our university clinic." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Devices were set by the study coordinators who deactivated the LCD display so that the patient and investigators did not become aware of device allocation" Quote: "There were no concrete information about characteristics of these different PAP types in the informed consent" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Devices were set by the study coordinators who deactivated the LCD display so that the patient and investigators did not become aware of device allocation" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The investigator making and analysing the PSG recordings on therapy and other outcome measures did not have access to information from the therapy device within their PSG montage" |
| Blinding of outcome assessment (detection bias) | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | 3 withdrawals after allocation (1 from CPAP group and 2 from ABRP‐PAP group); these patients were not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, double‐blind, parallel group trial | |
| Participants | N = 208 participants (177 M/31 F). Age 55.5; BMI 32.7 kg/m2; AHI 48.4; ESS 13 Inclusion criteria: ESS ≥ 8; AHI ≥ 10/hour; age 18‐75 Exclusion criteria: psychophysiological incapacity to perform questionnaires, other sleep disorders, psychiatric disease, previous CPAP therapy, previous uvulopalatopharyngoplasty, chronic nasal obstruction, cancer, COPD, with FEV1 < 50% predicted, symptomatic cardiovascular disease, previous stroke, cheyne‐Stokes respiration, chronic pain syndromes, fibromyalgia, drug or alcohol addiction | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 years | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "The study was supported by the Swiss National Science Foundation, the lung leagues of Zurich, St. Gallen and Thurgau and by unconditional grants from the respironics Foundation and resMed Switzerland. This was an investigator initiated trial, and the commercial companies were not involved in study design, data acquisition and analysis or writing the manuscript. competing interests KEB reports grants to his institution from Swiss National Science Foundation, Zurich lung league, respironics Foundation, resMed Switzerland, during the conduct of the study. The other authors report no competing interests." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation of participants was performed according to a 1:1 balanced block design by the study centre.......envelopes containing codes for the treatment mode and CPAP brand for 12‐24 participants were sent to participating centres as needed. The local co‐ordinator drew a paper (from an opaque envelope) with the codes for each participant." |
| Allocation concealment (selection bias) | Low risk | Quote: "...envelopes containing codes for the treatment mode and CPAP brand for 12‐24 participants were sent to participating centres as needed. The local co‐ordinator drew a paper (from an opaque envelope) with the codes for each participant." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "True blinding of participants and clinical care‐givers was not feasible since all participants had an initial phase of autoCPAP therapy." This is likely to impact on usage data, quality of life and symptom scores. |
| Blinding of participants and personnel (performance bias) | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "True blinding of participants and clinical care‐givers was not feasible since all participants had an initial phase of autoCPAP therapy." This is likely to impact on usage data, quality of life and symptom scores |
| Blinding of outcome assessment (detection bias) | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | 15 out of 95 randomised to fixed CPAP group withdrew from treatment by 24 months. 21 out of 113 randomised to auto‐CPAP group withdrew from treatment by 24 months. Data were presented for ITT analysis and per protocol. Multiple imputation method used to address attrition. In view of large attrition rates there is some uncertainty over the reliability of data for usage, symptoms and quality of life. Treatment pressure and AHI unlikely to have been affected since short term measurement is informative in determining intervention effects. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised cross‐over study. Data analysed with paired t tests. | |
| Participants | N = 70 participants (48 M/22 F); age: 50.78; BMI: 35.93; AHI: not reported. ESS: 10.7 Inclusion criteria: 18 to 75 years of age; AHI ≥ 10 per hour; successful in‐lab polysomnography; seven hours’ sleep on most nights; bedtime midnight or earlier; fluent English speakers Exclusion criteria: use of CPAP in last 2 years; CPAP therapy contraindicated; factor or disease that might interfere with study participation (e.g. psychiatric disease, non‐adherence to medical regimens); significant sleep disorder(s) that make use of CPAP challenging; use of hypnotics and/or sedating medications; surgery of the mouth, nose, sinuses, or airways in previous 12 months; patients who are required by the nature of their employment to not comply with therapy (e.g. truck drivers, airline pilots) | |
| Interventions | Fixed CPAPexp designed to detect transition from sleep to wakefulness versus fixed pressure CPAP Duration: 4 weeks per treatment arm | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "The authors declare that there are no conflicts of interest regarding the publication of this paper. Dr Chris Frampton is an independent, statistical consultant hired to analyse the results for this study. Medical writing assistance was provided by Anita Fitzgerald, on behalf of Fisher & Paykel Healthcare. Irene Cheung is an employed staff from Fisher & Paykel Healthcare who helped in inputting data." Study sponsored by Fisher & Paykel, manufacturers' of SensAwake expiratory relief | |
| Notes | The type of expiratory pressure relief mechanism used in this study works differently to those evaluated by other studies in this review. We present the findings of this study separately to analyses of CPAP expiratory pressure relief devices. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random permuted blocks were used to randomise patients into the two treatment sequence groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization records were kept in a patient master log. The study coordinator set the device to the appropriate treatment arm according to the patient master log during the device setup visit." The process described is consistent with an approach that conceals the assignment from both the study personnel and the participants and so is probably adequate. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both the physician and the patient were blinded to the treatment. To ensure adequate blinding, SensAwake was turned ON in all devices and this setting displayed to the user." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both the physician and the patient were blinded to the treatment. To ensure adequate blinding, SensAwake was turned ON in all devices and this setting displayed to the user." |
| Blinding of outcome assessment (detection bias) | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Blinding of outcome assessment (detection bias) | Low risk | Identical machine appearance unlikely to affect measurement of these outcomes. |
| Incomplete outcome data (attrition bias) | High risk | 5 participants withdrew before completion of both arms. Data on machine usage available for participants who completed both arms. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in accordance with trial protocol. |
| Other bias | Low risk | No significant concerns identified. |
| Methods | Randomised, cross‐over study. Statistical analysis approach not described | |
| Participants | N = 50 participants. 40 completed and analysed. Age: 53 years. No other baseline details reported. Inclusion criteria: severe OSA (RDI > 30) | |
| Interventions | Auto‐CPAP versus fixed CPAP (RemStar machines set in 2 different modes) Study duration: 2 x 4 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Details not available (conference abstract) | |
| Notes | TJL wrote for confirmation of data and methods on 3 September 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Global cognitive functioning, attention, vigilance, language, memory.......were blindly assessed." No further information available to judge this |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | High risk | 20% attrition. Non‐completers not analysed |
| Selective reporting (reporting bias) | Unclear risk | Results reported in abstract form. Not all outcomes reported |
| Other bias | Unclear risk | Information not available |
| Methods | Prospective, randomised, cross‐over study. Statistical analysis methods unclear | |
| Participants | N = 19 participants (18 M/1 F). Age 46.2; BMI 30.2; AHI 59.7; ESS 9.6 Inclusion criteria: age > 20, AHI > 15, consent to wear CPAP Exclusion criteria: not consenting to positive pressure device, treatment for mood disorders such as anxiety and depression | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 12 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Funding not declared. Conflict of interest: none | |
| Notes | Unadjusted values used in the analysis as the P values were high and errors were very small. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We used single sequence of random assignments for randomisation." Not clear how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "There was no selection bias if the patient wanted to enrol in our study." Not clear how allocation was concealed from study personnel or participants |
| Blinding of participants and personnel (performance bias) | High risk | Study was not blinded |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Study was not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Six of the 25 enrolled OSA patients (24%; 3 received APAP first and three received CPAP first) withdrew during the first month due to intolerance to CPAP/APAP." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: random sampling number tables (correspondence with trialist). All participants accounted for Data analysis: paired t test where ANOVA significant. T test used for treatment pressure. Analysis on usage not paired t test. | |
| Participants | N = 25 participants (22 M/3 F). Mean age 57; mean AHI 57.8 Inclusion criteria: OSA confirmed by PSG; AHI > 10/hr; ATS recommended indication for CPAP treatment | |
| Interventions | Auto‐CPAP versus fixed CPAP. No washout period Study duration: 2 x 4 week treatment arms | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Funded by Institut National de la sante et de la Recherche Medicale & by Nellcor‐Puritan Bennett. No declaration of interests provided. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling number tables |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Patients were not aware of the order of administration (i.e. they were blinded to the setting mode until the first night of use after which they could easily guess which mode they were using.).........The questionnaire was completed with the help of sleep laboratory technicians who were unaware of CPAP mode." |
| Blinding of participants and personnel (performance bias) | Low risk | Awareness of treatment group assignment unlikely to affect objective outcome data from the study |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Patients were not aware of the order of administration (i.e. they were blinded to the setting mode until the first night of use after which they could easily guess which mode they were using.).........The questionnaire was completed with the help of sleep laboratory technicians who were unaware of CPAP mode." |
| Blinding of outcome assessment (detection bias) | Low risk | Awareness of treatment group assignment unlikely to affect objective outcome data from the study |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised controlled, parallel group trial | |
| Participants | N = 100 participants (78 M/22 F); mean age 57; BMI 31 kg/m2 Inclusion criteria: AHI > 15, with or without corresponding daytime symptoms Exclusion criteria: 1. global respiratory failure; 2. central sleep apnoea syndrome; 3. severe mental or psychological impairment | |
| Interventions | 4 groups. Autoadjusting CPAP with or without intensive support versus fixed CPAP with or without intensive support Study duration: 9 months | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Information not available (link to declarations of interest no longer live) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Pulling mixed and sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "Pulling mixed and sealed envelopes... At time of recruitment, recruiters were also unaware of allocation." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Patients were not told about their pressure mode, however, full blinding of pressure delivery was not possible. At time of recruitment, recruiters were also unaware of allocation." |
| Blinding of participants and personnel (performance bias) | Low risk | Awareness of treatment group assignment unlikely to affect objective outcome data from the study |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Outcome assessors were not blinded" |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | No information on withdrawals provided |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, parallel group trial. Method of randomisation not reported | |
| Participants | N = 184 participants (138 M/46 F); age: 48 years; ESS: 15 Inclusion criteria: newly diagnosed OSA; study participants who used PAP for 4 hours night or more in the first week of treatment were followed up; AHI > 10; ESS ≥ 10 Exclusion criteria: prior surgical procedure for OSA; significant hypoventilation; CPAP exposure; significant comorbidities | |
| Interventions | CPAP with expiratory pressure relief (C‐Flex) versus fixed pressure CPAP Study duration: 24 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | This study was funded by Respironics. No author declarations provided. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was centralized by the sponsor. Randomization lists were generated in block sizes of 4 and 6 randomly chosen with respect to order. The actual block size of 10 comprised a block of 4 (or 6) followed by a block of 6 (or 4). Randomization cards were provided to the clinical sites at the time of study initiation." |
| Allocation concealment (selection bias) | Low risk | Quote: "Research assistants received sealed random condition assignment cards from Respironics to determine standard CPAP or C‐Flex therapy." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Participants were not made aware of the treatment they were assigned. This study was therefore a.......single‐blinded, controlled study". Only participants were blinded. |
| Blinding of participants and personnel (performance bias) | Low risk | Study design unlikely to affect these outcomes. |
| Blinding of outcome assessment (detection bias) | High risk | Study personnel were aware of treatment group assignment as the study was single‐blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Study design unlikely to affect these outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Data were analyzed with multivariate mixed models procedures that allowed for analysis including participants with missing observations." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group study. Methods of randomisation not reported. Devices were identical in appearance. | |
| Participants | N = 100 participants. Mean age: 54.3; BMI: 31.8; AHI: 47.9; ESS: 12.6 Inclusion criteria: diurnal somnolence (≥ 8 on ESS); AHI > 10; written consent Exclusion criteria: prior CPAP therapy; central sleep apnoea or Cheyne‐Stokes respiration; severe nasal obstruction or other conditions contraindicating CPAP treatment; COPD (FEV1 < 70% predicted); congestive heart failure (NYHA III or IV) | |
| Interventions | Auto‐CPAP (forced oscillation technique) versus fixed CPAP Conference abstract reported 8 weeks duration (published paper reported 2 nights data from laboratory studies) | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Randomised, double‐blind, parallel group study. Participants randomised for 2 night cross‐over and retained device assigned on second night for subsequent 6‐week period. | |
| Participants | N = 21 (20 M/1 F) participants. Mean age 54.2; BMI: 30.9 kg/m2. AHI: 41.8. ESS: 12.9 Inclusion criteria: AHI > 10 or excessive sleepiness (if AHI < 10). Participants who did not have excessive sleepiness at baseline also eligible if AHI > 20 Exclusion criteria: other sleep disorders (e.g. restless leg syndrome or periodic leg movement syndrome; cardiac, pulmonary or other medical disorders;psychiatric/neurological disorders; abuse of sleep‐inducing agents or other drugs; suspected or confirmed central sleep apnoea syndrome; prior OSA treatment (e.g. CPAP, oral devices or surgery) | |
| Interventions | Auto‐CPAP versus fixed pressure CPAP (established by manual titration after 2 night cross‐over study) Study duration: 6 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Funding quote: "This study was supported by an unrestricted grant from Respironics Inc.". No declarations reported from authors. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to allow judgement. |
| Blinding of participants and personnel (performance bias) | Low risk | Same device used with different pressure settings (REMstar Auto CPAP). |
| Blinding of participants and personnel (performance bias) | Low risk | Same device used with different pressure settings (REMstar Auto CPAP). |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate blinding procedure in place and unlikely that this has impacted on subjective outcomes. |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate blinding procedure in place and unlikely that this has impacted on objective outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | All 21 participants who started completed the study. |
| Selective reporting (reporting bias) | High risk | Symptoms and quality of life data measured but reported as aggregate of two treatment groups and described as not significantly different. |
| Other bias | Low risk | No concerns identified. |
| Methods | Randomised, single‐blind, cross‐over study (participants not informed of order/setting) Statistical test: Wilcoxon test | |
| Participants | N = 20 participants (16 M/4 F) completed and analysed. Mean age: 56 years. AHI: 33; ESS: 10.3 Inclusion criteria: new diagnosis of OSA (diagnosis established through polysomnography, AHI > 10) Exclusion criteria: COPD, congestive heart failure and other serious medical disorders | |
| Interventions | Auto‐CPAP versus fixed pressure CPAP Same machine delivered the different treatment pressure settings Study duration: 2 x 8 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "a randomized, single‐blinded, cross‐over study." Only participants were blinded. |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by open‐label design. |
| Blinding of outcome assessment (detection bias) | High risk | Only participants were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by open‐label design. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, double‐blind, parallel group trial. Machines had settings: 'Set CPAP'; 'Set NBL' and 'A'. Assessors could alter the settings for safety reasons. Randomisation not reported | |
| Participants | N = 27 participants (22 M/5 F). Age: 44 years; BMI: 35 kg/m2; AHI: 43; ESS: 13.8 Inclusion criteria: > 18 years; AHI > 10 and < 100; ability to follow instructions and provide informed consent; willingness to return for follow‐up visit 30 days after random allocation to CPAP/BiPAP; residence within 200 miles of clinic Exclusion criteria: inability to wear a mask; prior surgical treatment for OSA; prior CPAP usage; other significant comorbidities | |
| Interventions | Bi‐level PAP versus CPAP. Participants also given instruction via educational video on CPAP and OSA. Study duration: 30 days | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "Dr. Peter Gay received grant support for this study by Respironics Inc. (noted in manuscript)." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Low risk | Quote: "the technician selected the 'A' mode which in a double‐blinded and selective way, locked in the optimal settings for either CPAP or NBL" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "the technician selected the 'A' mode which in a double‐blinded and selective way, locked in the optimal settings for either CPAP or NBL.............true identification of the 'A' mode was not revealed until the end of the trial" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by study design. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "the technician selected the 'A' mode which in a double‐blinded and selective way, locked in the optimal settings for either CPAP or NBL.............true identification of the 'A' mode was not revealed until the end of the trial" |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by study design. |
| Incomplete outcome data (attrition bias) | Low risk | Randomisation was performed prior to baseline PSG, after which a number of participants became ineligible. All participants recruited to the 2nd phase completed the study. There were no withdrawals from this study, although the sample reflects a selected population. |
| Selective reporting (reporting bias) | High risk | Data on quality of life (FOSQ) were not reported but described as 'equivalent' between two treatment arms. |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, cross‐over study. Statistical analysis not clear | |
| Participants | N = 18 (15 M/3 F); mean age: 56.8 years; AHI: 41.4; BMI: 36 Inclusion criteria: non‐sleepy OSA patients | |
| Interventions | Pressure relief CPAP versus fixed CPAP Study duration: 2 x 4 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | TJL emailed for confirmation of methods and data on 4 September 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available; requested |
| Allocation concealment (selection bias) | Unclear risk | Information not available; requested |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Randomised parallel group study. Randomisation and blinding not described | |
| Participants | N = 20; ESS: 12. No other baseline details provided Inclusion criteria: OSA and obstructive hyperventilation syndrome Exclusion criteria: not reported | |
| Interventions | Bi‐PAP versus fixed pressure CPAP Study duration: 12 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | Unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Prospective, randomised, cross‐over study in patients who were suboptimally compliant with CPAP despite appropriate interventions. Data analysed as paired t test | |
| Participants | N = 28 participants (24 M/4 F). Mean Age 56.7 years; BMI 35 kg/m2; ESS 13.2; AHI 35 Inclusion criteria: OSA with AHI > 5, CPAP compliance < 4 hours per night for 6 weeks after CPAP prescription despite technical and educational interventions, symptoms of pressure intolerance Exclusion criteria: significant airflow obstruction (FEV1/FVC < 60%), pretreatment study showing central sleep apnoea, clinical evidence of congestive heart failure, daytime hypercapnia (PaCO2 > 6.5kPa) or previous prescription of Bi‐PAP | |
| Interventions | Bi‐PAP versus new CPAP (brand of fixed CPAP different from the one used prior to study entry) Study duration: 2 x 4 weeks with 2 weeks washout in‐between | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Funding source: not declared; conflict of interest: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomisation technique was used to allocate patients into different groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "(Allocation sequence concealment) was done independently by the research and development officer, so the patients and researchers were not aware of the allocation sequence." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Neither (participants or research personnel) were blinded as the machines used were different in each arm." |
| Blinding of participants and personnel (performance bias) | Low risk | Outcome assessors were not blinded to treatment allocation, but the outcomes unlikely to be affected by open‐label design. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were not blinded to treatment allocation, but the outcomes unlikely to be affected by open‐label design. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "31 gave consent and were recruited. One developed a stroke during the treatment period (on Bi‐level PAP arm) and 2 others did not complete the study (one of them dropped out after first using Bi‐level PAP and the other after using the new CPAP). These subjects were excluded from the analyses." |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group study | |
| Participants | N = 74 participants (M/F 60/14). Mean age 58 years; BMI 31; AHI 35; ESS 9 Inclusion criteria: newly diagnosed OSA patients (AHI > 15 on polysomnography) | |
| Interventions | CPAP with warm air humidifier versus CPAP without warm air humidifier Study duration: 12 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Funding source: study was funded by manufacturers. Quote: "Diese Studie wurde finanziell unterstützt durch die Firmen Fisher & Paykel Healthcare und Air Products Medical GmbH" Author declaration of interests are identical to funding source | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study participants assigned according to a randomised schedule but no more detail provided about sequence of treatment group assignments |
| Allocation concealment (selection bias) | Unclear risk | No detail described. Insufficent information available to judge |
| Blinding of participants and personnel (performance bias) | Low risk | Identical devices provided |
| Blinding of participants and personnel (performance bias) | Low risk | Identical devices provided |
| Blinding of outcome assessment (detection bias) | Low risk | Identical devices provided |
| Blinding of outcome assessment (detection bias) | Low risk | Identical devices provided |
| Incomplete outcome data (attrition bias) | High risk | Non‐users excluded from the analysis |
| Selective reporting (reporting bias) | High risk | Outcome data not available for symptoms. Objective of the study differed from that of the review question, but symptoms likely to have been collected. |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: hospital number (odd versus even last digit) Paired t test used for continuous data | |
| Participants | N = 60 (53 with OSA and 7 with UARS). 21 withdrawals 2 stopped due to medical complications (not stated) and the rest did not complete the study. Further 6 did not have machine usage data. (21 M/18 F). Total number of OSA patients completing trial is 29. Data analysed for 33 patients which included 4 patients with UARS Mean age: 46 years; AHI 30; BMI: 42 kg/m2 Inclusion criteria: diagnosed OSA or UARS (confirmation by polysomnography) Exclusion criteria: prior CPAP treatment, facial/pharyngeal abnormalities requiring surgery, chronic airways disease necessitating bronchodilator usage, obesity hypoventilation syndrome, shift workers, congestive heart failure, seizure disorder, mental retardation, sedative/antidepressant/hypnotic treatment | |
| Interventions | Auto‐CPAP versus fixed CPAP. No washout Study duration: 2 x 12 week treatment periods | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Hospital number (odd versus even last digit) |
| Allocation concealment (selection bias) | High risk | Study investigators likely to be aware of treatment group assignment |
| Blinding of participants and personnel (performance bias) | High risk | Study investigators likely to be aware of treatment group assignment |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by study design |
| Blinding of outcome assessment (detection bias) | High risk | Study investigators likely to be aware of treatment group assignment |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by open‐label design |
| Incomplete outcome data (attrition bias) | High risk | High withdrawal rate and non‐completers not included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: sealed envelopes (off‐site) Statistical analysis: paired t test | |
| Participants | N = 55 adults (48 M/7 F) randomised (46 completed). Age: 50 years; BMI: 35; AHI: 54; ESS: 12.5 Inclusion criteria: AHI ≥ 5; optimal treatment PSG determined optimal treatment pressure; no previous home use of CPAP Exclusion criteria: significant comorbidity; complication (e.g. hypercapnic respiratory failure); non‐OSA; patients unable to use masks with Autoset T machines | |
| Interventions | Auto‐CPAP (Autoset T) versus fixed pressure CPAP Study duration: 2 x 8‐week treatment periods | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This was an industry supported study by ResMed Australia. Dr. Hukins received research equipment from ResMed Australia." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by a laboratory scientist not involved with the study using the technique of shuffled sealed envelopes containing equal numbers of each treatment arm" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed by a laboratory scientist not involved with the study using the technique of shuffled sealed envelopes containing equal numbers of each treatment arm" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "....this single‐blinded, randomised cross‐over study.......The Autoset T was used for both treatment modes in an attempt to blind the patient to the mode...Investigators were not blinded..." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by single‐blind study design. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Investigators were not blinded..." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by open‐label design. |
| Incomplete outcome data (attrition bias) | High risk | Non‐completers not included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation not described. Statistical test: paired t tests | |
| Participants | N = 10 (9 M/1 F). Mean age: 44.98; AHI: 47.2; BMI: 35.9; ESS: 11.1 Inclusion criteria: CPAP‐naive at baseline; symptomatic OSA (AHI > 15/h) Exclusion criteria: not described | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 x 4‐week treatment periods (washout 2 weeks) | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This study was funded by Respironics Inc., Murrysville, PA." Author conflicts of interest: not declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "...this randomized, prospective, single‐blind cross‐over trial.....Patients were unaware of the treatment mode..." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by single‐blind study design. |
| Blinding of outcome assessment (detection bias) | High risk | Study personnel would have been aware of treatment group assignment. |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by single‐blind study design. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, cross‐over study. Statistical analysis methods unclear but paired data obtained via correspondence | |
| Participants | N = 20 participants Inclusion criteria: diagnosed with OSA; established on CPAP therapy | |
| Interventions | Modified APAP (bi‐level pressure mode) versus fixed CPAP Study duration: 2 x 2 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Resmed sponsored the study but no other details were available. | |
| Notes | TJL emailed for confirmation of data and methods 5 September 2008. Reply from Resmed October 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study was randomised by pulling the order of treatment received 'out of a hat'" |
| Allocation concealment (selection bias) | Low risk | Quote: "The person who pulled the order was a ResMed employee independent of the study and with no knowledge of the study." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | All completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, double‐blind, cross‐over study | |
| Participants | N = 41 (38M/3F). 27 completed the study. Mean age: 52.4 years; BMI: 32.3kg/m2; ESS 13.9 Eligibility criteria not provided | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 x 2‐week treatment periods | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Randomised, single‐blind, parallel group study. Method of randomisation not reported | |
| Participants | N = 50 participants (44 M/6F); Age 53.5. No other baseline details available | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 3 to 6 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | Sleep study following treatment done between 3 and 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive either automatically adjusting or conventional nCPAP." |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "the design of the study was single‐blinded" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "the design of the study was single‐blinded" |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Non‐completers did not contribute to the analysis (4%) |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
| Methods | A prospective, randomised, double‐blinded, three‐arm, multicenter trial | |
| Participants | N = 168 participants (128 M/40 F). Age: 49 years; BMI: 34 kg/m2; AHI: 39; ESS: 11 Inclusion criteria: age 21‐75 years, AHI > 15/hour, able to consent, agreeable to commence CPAP as initial therapy, adequate titration within 2 weeks of enrolment Exclusion criteria: previous study participation < 30 days, > 1 titration, sedatives, medical/psychiatric illness potentially interfering with CPAP adherence, CPAP exposure < 1 year, chronic respiratory disease, upper airway surgery < 90 days, previous surgery for OSA, non‐OSA sleep disorder, excess alcohol use, shift workers | |
| Interventions | Comparing effects of autoadjusting PAP EXPssure relief with autoadjusting PAP and fixed CPAP Study duration: 6 months (see notes) | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "Philips Respironics provided funding for this study; Drs. Kushida, Berry, Blau, Fietze, Kryger, Kuna, Pegram, and Penzel received research support for the conduct of this study through contracts between Philips Respironics and their respective institutions. Ms. Crabtree received consulting fees for statistical data analysis from Philips Respironics. Dr. Kushida has received research support from Philips Respironics, ResMed, Ventus Medical, and Pacific Medico. Dr. Berry has received research support from Philips Respironics, ResMed, and Ventus Medical. Dr. Blau has received research support from Philips Respironics, Breas, and Hoffrichter) Dr. Fietze has received research support from Philips Respironics, ResMed, Advanced Sleep Research, Breas, Hoffrichter, and Weinmann. Dr. Kryger has received research support from Ventus, and ResMed. Dr. Penzel has received research support from Philips Respironics, ResMed, Advanced Sleep Research, Breas, Hoffrichter, Somnomedics, and Weinmann." | |
| Notes | Patients in APAP group ‐ initially APAP for two weeks followed by fixed CPAP for six months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Urn randomization was used to control for the potentially confounding variables (age, gender, education, AHI, subjective sleepiness)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The Principal Investigator (PI) and research staff administering questionnaires or interacting with the participant were blinded to randomization and the results of all participant evaluations...participants were blinded to treatment" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The Principal Investigator (PI) and research staff administering questionnaires or interacting with the participant were blinded to randomization and the results of all participant evaluations" |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Fourteen participants did not receive the therapy to which they were randomized, but were included in the intention‐to‐treat analysis." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, double‐blind, cross‐over trial | |
| Participants | N = 30 participants (22 M/8 F). Age: 55.4 years, BMI 32 Inclusion criteria: clinical suspicion of OSAS with AHI > 5 on polysomnography Exclusion criteria: severe comorbidity, such as acute or chronic heart failure (NYHA grade 3 or 4), severe COPD, dementia, alcoholism, drug abuse, and age under 18 | |
| Interventions | CPAP versus C‐Flex Study duration: 2 x 6 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Funding quote: "This study was supported by Air Products Medical GmbH." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The physicians and technicians performing the titration and scoring the polysomnographic data were blinded to the treatment mode which was chosen...The patients were only informed that they would receive two different modes of therapy, but they did not know if they got CPAP or C‐Flex" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The physicians and technicians performing the titration and scoring the polysomnographic data were blinded to the treatment mode which was chosen" |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Twelve patients dropped out of the study (7 after C‐Flex, 5 after CPAP); 4 of them gave up the therapy completely (2 after CPAP, 2 after C‐Flex)" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised study. Design and method of randomisation not reported. Single‐blind trial | |
| Participants | N = 16 participants. Distribution and baseline details not reported Inclusion criteria: participants with newly diagnosed CPAP; AHI > 15; uncomplicated CPAP lab PSG Exclusion criteria: REM‐related/supine positional OSA | |
| Interventions | C‐Flex PAP versus fixed pressure CPAP Study duration: 4 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | Unpublished conference abstract. Details requested by email | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available; requested |
| Allocation concealment (selection bias) | Unclear risk | Information not available; requested |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available; requested |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: not described. Statistical analysis: unpaired t test | |
| Participants | N = 22 participants (21 M/1 F). Mean age 53.45; BMI: 32.9; ESS: 16.3 Inclusion criteria: newly diagnosed OSA; AHI ≥ 30 Exclusion criteria: not described | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 x 4 weeks. No washout described | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Funding quote: "This study was supported by Air Products Medical GmbH." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Machines were assigned in a single blind, random fashion." |
| Allocation concealment (selection bias) | High risk | Given that the study was single‐blind, the pressure setting of the machines may have been known by investigators |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind study |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No information available |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, parallel group trial | |
| Participants | N = 19 participants (15 M/4 F). Mean age: 47; AHI: 78; ESS: 14 Inclusion criteria: severe OSA (AHI > 30; or symptomatic and AHI > 20) Exclusion criteria: other significant medical disorders | |
| Interventions | C‐Flex versus fixed CPAP Study duration: 4 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "None of the authors have had any financial relationships with Respironics Inc., who are the manufacturers of the device tested. Respironics International Inc., through their New Zealand suppliers Care Medical, provided six C‐Flex machines for the purposes of this trial." | |
| Notes | Information on randomisation available from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sequence of allocation to treatment was determined by sleep technicians randomly picking one of a set of pre‐prepared opaque envelopes containing the treatment allocation." |
| Allocation concealment (selection bias) | Low risk | Quote: "An urn with 2 differently coloured paper clips was prepared. One clip was blindly withdrawn, and its corresponding treatment noted on a folded piece of paper in an opaque envelope which was then sealed. The clip was then replaced in the urn along with another coloured clip, which represented the other treatment. This was repeated until 20 envelopes had been produced. Because a number of patients who did not meet the entry requirements, were randomised and then withdrawn from the study, a further 5 envelopes were subsequently prepared using the same method. This method leads toward roughly equal group sizes whilst maintaining unpredictability of sequence and sample sizes." |
| Blinding of participants and personnel (performance bias) | High risk | Study personnel would have been aware of treatment group allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Measurements of sleepiness and reaction times were undertaken by an investigator blinded to treatment allocation; patients were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Whilst all 19 patients were included in the primary analysis under the intention‐to‐treat principle due to missed appointments only 17 patients are included in the Epworth analysis." This is a low rate of attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, three‐arm, parallel group | |
| Participants | N = 151 participants entered into treatment groups relevant to this review question (66 M/85 F); age: 60 years; BMI: 44; AHI: 69; ESS: 11 Inclusion criteria: 15‐80 years; AHI: > 30; no other significant sleep disorders (e.g. narcolepsy or restless leg syndrome); correctly executed 30‐minute CPAP/NIV test Exclusion criteria: significant comorbidity | |
| Interventions | Fixed CPAP versus Non‐invasive ventilation treatment set at bilevel pressure with assured volume. Study assigned to bi‐level PAP comparison. Supplemental oxygen offered if participants met additional criteria (daytime PaO2 < 55 mmHg, with the necessary flow to maintain waking arterial oxygen saturation between 88% and 92% or PaO2 greater than or equal to 55 mmHg for at least 17 h/d). Third treatment arm consisting of a usual care control was not of interest to this review. Study duration: 3 years (for hospitalisation and withdrawal outcomes). Other outcome data reported at 8 weeks unless stated. | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "Supported by the Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo) grant PI050402, the Spanish Respiratory Foundation 2005 (FEPAR), and Air Liquide Spain." Funders did not participate in the design or conduct of the study, analysis or interpretation of data, or manuscript preparation. The authors all declared that they had no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: participants "randomized by an electronic database (simple randomization)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to judge. |
| Blinding of participants and personnel (performance bias) | High risk | For most outcomes of interest to the review open‐label nature of the study places the study at high risk of performance bias. |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | For most outcomes of interest to the review open‐label nature of the study places the study at high risk of detection bias. |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Unclear risk | 10% of participants missing data at 2 months. ITT was undertaken with missing data imputed for secondary outcomes (quote): "following a multiple imputation method with iterative multivariable regression, because the missing data had characteristics compatible with a missing at random pattern." |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest reported in accordance with trial registry record. |
| Other bias | Low risk | No other sources of bias identified. |
| Methods | Randomised, single‐blind, cross‐over study. Methods of randomisation not reported. Comparisons between treatment using 2‐way analysis of variance ‐ treatment order as between‐subject factor, and treatment type within‐subject factor | |
| Participants | N = 46 participants (36 M/10 F) 1 dropout and 1 data unavailable from machine. Mean age: 49; BMI: 32kg/m2 Inclusion criteria: 18 to 65 years; symptomatic OSA; AHI > 15; > 10 cmH2O to correct AHI Exclusion criteria: pre‐existing lung disease; awake resting SaO2 < 90%; 10 or more central apnoeas/hr; patients taking medication considered to interfere with sleep respiration | |
| Interventions | Auto‐CPAP versus fixed CPAP. No washout period Study duration: 2 x 6‐week treatment periods | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Supported by a grant from ResMed Corporation. One of the authors (Neil Douglas) declared a role as medical advisor to ResMed | |
| Notes | Participants aware that machine usage was monitored; stipend offered for completion of study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: ".....randomised, single‐blinded, cross‐over study....(patients) were not informed of the type of therapy they were receiving" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Single blind study |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Low rate of non‐completers excluded. One excluded from study and additional participant excluded from analysis of machine usage due to unreadable data. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group study. Statistical analysis based on t tests but unadjusted data presented for all outcomes. | |
| Participants | N = 16 participants (16 M). Mean age: 54; BMI: 34.2 kg/m2; AHI: 43.6; ESS: 14.8 Inclusion criteria: diagnosis of OSA (confirmed by polysomnography; untreated OSA) Exclusion criteria: not reported | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 3 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Low risk | Participants unaware as to group they were randomised to (CPAP machines were identical). |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants unaware as to group they were randomised to (CPAP machines were identical). |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, multicentre, parallel group trial | |
| Participants | N = 83. Mean age: 56 years; AHI: 52; ESS: 11.5 Inclusion criteria: new diagnosis of OSA; CPAP‐naive; AHI > 30 | |
| Interventions | Four auto‐CPAP machines assessed:
All 4 compared against fixed pressure CPAP Study duration: 24 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | Data aggregated from 4 auto‐CPAP groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation was carried out centrally..." |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly coded envelopes opened by a coordinator from envelopes batched for each centre in order to have similar proportions of patients in each group from each centre." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Participants who withdrew were not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, cross‐over trial. Statistical methods unclear | |
| Participants | N = 20. Age 57 years | |
| Interventions | Automatic‐CPAP device combined with pressure reduction during exhalation (auto C‐Flex) versus fixed CPAP Study duration: 2 x 1 month | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blinded study |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) | High risk | Single‐blinded study |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) | Unclear risk | No information available |
| Selective reporting (reporting bias) | Unclear risk | No information available. Information only available as a conference abstract. No further details about the study are available. |
| Other bias | Unclear risk | No information available |
| Methods | Randomised, cross‐over study. Statistical analysis unclear | |
| Participants | N = 26 participants. Baseline details not available Inclusion criteria: diagnosis of OSA; CPAP‐naive | |
| Interventions | CPAP + expiratory pressure relief versus fixed CPAP Study duration: 2 x 2 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "A randomized two‐arm prospective crossover unblinded....." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "A randomized two‐arm prospective crossover unblinded....." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Randomised, double‐blind, cross‐over study. Method of randomisation not reported. Statisitical analysis unclear | |
| Participants | N = 16 participants. Mean age: 59 years; BMI: 31 kg/m2; AHI: 69 Inclusion criteria: previously documented OSA and poor compliance with CPAP (< 3 hours/night) | |
| Interventions | Bi‐level PAP versus fixed CPAP Study duration: 2 x 8‐week treatment periods Pressure levels for inspiratory pressure were: 12.3 cmH2O (SD 1.8), and expiratory pressure: 7.6 cmH2O (SD 2.2) for bilevel PAP treatment, and for fixed CPAP: 9.4 cmH2O (SD 2.3) (no P value reported) | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | Study published as conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Double‐blind, crossover study". No further information available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | We have judged the risk of bias on objective outcomes to be unclear in the absence of more detail beyond the study being double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Double‐blind, crossover study". No further information available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | We have judged the risk of bias on objective outcomes to be unclear in the absence of more detail beyond the study being double‐blind. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Randomised, double‐blind, cross‐over study. Method of randomisation not reported. Statisical test: not reported although exact P values presented for between group tests and confidence interval reported for machine usage. | |
| Participants | N = 42 randomised (37 completed study protocol and were analysed). Mean age: 49 years. BMI: 35 kg/m2; RDI: 50; ESS: 12.1 Inclusion criteria: newly diagnosed OSA requiring treatment with CPAP Exclusion criteria: significant nasal obstruction; requirement for supplemental oxygen | |
| Interventions | Humidification in addition to nasal CPAP versus sham humidifier in addition to nasal CPAP Study duration: 2 x 3‐week treatment periods (3 day washout) | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This study was funded by an Otago University Research Grant." Author declarations not provided (conference abstract) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The authors attempted to create a placebo humidification arm in which the HC100 was used without the heating unit turned on.........An investigator blinded to research treatment interviewed the patients at the end of each treatment arm..." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "An investigator blinded to research treatment interviewed the patients at the end of each treatment arm..." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Non‐completers excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | Not possible to determine based on details provided |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group trial | |
| Participants | N = 51 participants; mean age: 57 years; AHI: 53.3 Inclusion criteria: AHI > 20 Exclusion criteria: Inability to follow instructions, failure to give informed consent, acute infection, acute cardiac disease such as acute coronary artery syndrome, NYHA grade 3 or 4 heart failure, and acute pulmonary thromboembolism | |
| Interventions | CPAP with expiratory pressure relief versus fixed CPAP Study duration: 7 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This study was financed by a gift from Heinen U. Lowenstein. Dr. Ruhle has received research funding from Fisher A. Paykel, Heinen U. Lowenstein, ResMed, and Weinmann, but this funding has gone into department funds. Dr. Nilius, Andreas Happel, and Ulrike Domanski have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available; requested |
| Allocation concealment (selection bias) | Low risk | Third party responsible for randomisation and setting machines to relevant treatment mode |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patients were informed that they would receive two different forms of treatment .....but were given no further details...........The technician had no information from the investigators about the modality that the patients were receiving" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The physician and technicians reviewing the polysomnographic data and performing the manual CPAP titration were blinded to the treatment mode employed" |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, cross‐over study. Statistical test: Wilcoxon matched pair test | |
| Participants | N = 34 participants (completed: 29 (26 M/3 F)). Mean age: 53 years; BMI: 29.9 kg/m2; AHI: 14.7; ESS: 12.3 Inclusion criteria: mild to moderate OSA (AHI 5‐30) Exclusion criteria: not reported | |
| Interventions | Auto‐CPAP versus fixed pressure CPAP Study duration: 2 x 8‐week treatment periods | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This was not an industry supported study. Drs. Nolan, Doherty, and Mc Nicholas have indicated no financial conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent person not involved in the study design, protocol, or analysis assigned the devices to the patients in random order." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "....patients were not informed about the different technologies used in the devices.....the trial was fully blinded to the investigator performing the analysis." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...the trial was fully blinded to the investigator performing the analysis." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | High rate of participants not crossing over on to subsequent treatment arm |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, cross‐over study. Method of randomisation: random numbers table. Statistical analysis: student's t test (paired data) | |
| Participants | N = 27 participants (23 M/4 F). Withdrawals: 3. Total completed and analysed N = 24. Mean age: 49 years; BMI: 32.3 kg/m2; AHI: 50.9; ESS 10.7 Inclusion criteria: AHI > 20/hour; MAI: > 30/hour; high variability of within night pressure to correct AHI Exclusion criteria: prior treatment with CPAP; central OSA/Cheyne Stokes; major facial abnormality; night/shift work; severe chronic heart failure/COPD; seizure disorder; mental retardation; sedative, hypnotic or antidepressant therapy; previous UPPP; prolonged hypoventilation during REM | |
| Interventions | Auto‐CPAP versus fixed CPAP. Need for pressure assessed over a 14‐night run‐in period with auto‐CPAP. No washout period described Study duration: 2 x 8‐week treatment periods | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation tables |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: ".....single‐blind, randomized, crossover trial........subjects were told that they would sleep with the machine functioning in two distinct modes.....no further explanation was given." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Single‐blind nature of the study means that these outcomes are at risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Rate of participants not crossing over could be high enough to introduce bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, cross‐over study. Double‐blinding: investigators and participants unaware as to treatment. Same machine used to deliver both treatment pressure modalities. Identical chip card given to patient which contains algorithm for either fixed or automatic titration mode. Statistical test: paired t test | |
| Participants | N = 38 participants (30 completed the study and contributed to the analysis) (27 M/3 F). Mean age: 49 years; BMI: 31 kg/m2; ESS: 12.7; AHI: 41.1 Inclusion criteria: AHI > 10 events/hour Exclusion criteria: CHF; chronic rhinitis; other sleep disorders | |
| Interventions | Auto‐CPAP versus fixed CPAP No washout period described Study duration: 2 x 4‐week treatment periods | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "Study supported by MADELA AG, distributors of Respironics products in Switzerland". No author conflicts provided. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatments given in "random order" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients and attending physicians were blinded to treatment modes and order of application." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "attending physicians were blinded to treatment modes and order of application." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Non‐completers excluded from the analysis (N = 8) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group trial | |
| Participants | N = 31 participants (25 M/ 6 F). Mean age: 48 years; BMI: 36.5kg/m2; AHI: 47; ESS: 15 Inclusion criteria: AHI > 20; ESS > 12 Exclusion criteria: not specified | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 12 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This work was supported by a University of Milan Fondo Interuniversitario per la Ricerca Scienfifica e Technologia Grant and a Minister for Instruction, University and Research Progetto di Ricerca di Interesse Nazionale 2003 grant to Dr. Montano. The authors have no financial or other potential conflicts of interest to disclose." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After CPAP titration, all subjects were randomised to receive either fixed‐level CPAP for 3 months." |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | 40 participants recruited; 9 withdrew (no information on which groups they were assigned to); 3 of these participants withdrew due to poor compliance |
| Selective reporting (reporting bias) | High risk | Data on ESS not presented: requested from authors along with details of randomisation and withdrawals |
| Other bias | Low risk | No concerns identified |
| Methods | Parallel group, randomized, double‐blind, controlled study | |
| Participants | N = 48 participants (37 M/9 F). Age: 55 years; BMI: 33 kg/m2; ESS: 9 Inclusion criteria: 21 to 75 years; AHI > 15; suboptimal CPAP titration after at least 3 hours of attempted titration Exclusion criteria: major medical or psychiatric illness; chronic respiratory failure; upper airway/ENT surgery in last 90 days; shift workers; alcohol/drug abuse within last three years; hypnotic use < 3 months; PLMI > 10 per hour; complex/central sleep apnoea; CPAP contraindication | |
| Interventions | Auto bi‐PAP (nasal) versus fixed CPAP Study duration: 90 days | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This study was supported by a grant from Phillips Respironics. Dr. Powell has received research support from Philips Respironics, Inc, Fisher – Paykel Healthcare, and Takeda Pharmaceuticals Dr. Malhotra has received consulting and/or research support from NIH, AHA, Philips, Medtronic, Apnex, SHC, SGS, Apnicure, Galleon, Pfizer, Merck, Cephalon, and Sepracor. Dr. Litinski has received research support from the American Sleep Medicine Foundation. Dr. Gay has received research support from Philips Respironics, Inc. Dr. Ojile has indicated no financial conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Urn randomization was used for treatment group placement using the variables of gender, age, diagnostic AHI, and education" |
| Allocation concealment (selection bias) | Low risk | Quote: "Urn randomization was used for treatment group placement using the variables of gender, age, diagnostic AHI, and education" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The participant, investigator, respiratory therapist, and research staff were all blinded to the therapy group" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The participant, investigator, respiratory therapist, and research staff were all blinded to the therapy group" |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A total of 48 participants were randomized into the trial......A total of 48 participants were analyzed for the primary endpoint on an intent‐to‐treat basis" |
| Selective reporting (reporting bias) | High risk | Study protocol indicates that AHI was measured but this was not reported in main trial publication. |
| Other bias | Low risk | No concerns identified |
| Methods | Multicentre, randomised, controlled, double‐blind trial | |
| Participants | N = 218 (172 M/46 F). Age: 55 years; BMI: 31 kg/m2; AHI: 44; ESS: 11.5 Inclusion criteria: newly diagnosed sleep apnoea patients over 18 years of age who were referred for CPAP Exclusion criteria: pregnancy, medically unstable, predominantly central sleep apnoea | |
| Interventions | Comparing effect of C‐Flex versus fixed CPAP Study duration: 3 months | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This work was supported by grants from 'Comite ́ National des Maladies Respiratoires' and Respironics. Author conflicts not reported." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization was performed by a computer‐generated schedule in random blocks of six and was stratified according to study centers' |
| Allocation concealment (selection bias) | Low risk | 'Randomization was performed by a computer‐generated schedule in random blocks of six and was stratified according to study centers' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Patients were not aware of whether they were receiving CPAP with or without C‐Flex activated'.........'The investigators who assessed outcome were unaware of the randomization status of the patients' |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | 'The investigators who assessed outcome were unaware of the randomization status of the patients and did not set up or maintain the machines' |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | 'The data for all outcomes were analyzed on an intention‐to treat basis'. Balanced but high rates of withdrawal. Reasons for withdrawal include refusal to continue with treatment |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Single‐centre, randomised controlled, double‐blind, parallel group trial | |
| Participants | N = 322 participants (225 M/97 F). Age: 58; BMI: 30 kg/m2 AHI: 38.8 Inclusion criteria: age: 18 to 80 years, capable of providing written informed consent, patients claiming social insurance and patients with OSA needing CPAP treatment Exclusion criteria: cardiac failure known and treated, central apnoea syndrome, patients who stopped CPAP treatment in the previous year, pregnancy, patients under guardianship, imprisoned patients, patients in hospital, patients included in another clinical study | |
| Interventions | Fixed versus auto‐CPAP Study duration: 4 months | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "The study was funded by the Fondation Agir pour les maladies chroniques. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication." Author conflicts of interest: none declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was conducted by...a computer‐generated random numbers list (six patients per block)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was conducted by telephone call to a clinical trials statistician, independent of the study..." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All patients, investigators and outcome assessment technicians were blinded to the arms to which the patients were allocated." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All patients, investigators and outcome assessment technicians were blinded to the arms to which the patients were allocated." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | High and imbalanced rate of withdrawal between intervention groups. Multiplie imputation method used to replace missing values from ITT analysis. |
| Selective reporting (reporting bias) | High risk | Quality of life, as measured by SF‐36 was described as not different between the two arms. The trial registry record indicated that symptoms was also measured but this was not reported in the trial publication. |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, cross‐over study. ANOVA test with Bonferroni correction used (for differences between baseline and treatment mode, and for differences between treatment mode) | |
| Participants | N = 52 participants (45 M/7 F). Mean age: 54.7 years; BMI: 32.4 kg/m2; AHI 35.1 Inclusion criteria: confirmed OSA by polysomnography Exclusion criteria: prior treatment with CPAP | |
| Interventions | Auto‐CPAP versus fixed CPAP. No washout Study duration: 2 x 6‐week treatment periods 24‐hour telephone helpline was at the disposal of the participants | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "The devices were supplied by the Weinmann Company, Hamburg, Germany." No author conflicts provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the person who managed the randomisation knew about the order of the treatment modes in the individual patients. This person adjusted the devices to the different treatment modes." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Those who did the questioning and cared for the patients in the hospital and sleep laboratory and those who evaluated results of PSG were blinded to the mode of treatment applied.........The patients were not informed about the sequence in which the two treatment modes were applied." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Those who did the questioning and cared for the patients in the hospital and sleep laboratory and those who evaluated results of PSG were blinded to the mode of treatment applied." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | 5/52 participants withdrew; their data were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group trial | |
| Participants | N = 83 (gender available for 62 completers: 45 M/17 F). Mean age: 47; BMI: 40 kg/m2; AHI: 51 Inclusion criteria: OSA diagnosed according to American Sleep Disorders Association AHI > 10; "heavy snoring"; excessive daytime sleepiness Exclusion criteria: concomitant illness requiring hospitalisation 6 months previously; psychiatric illness; pregnancy | |
| Interventions | Bi‐PAP versus CPAP administered at home Study duration: 52 weeks Prescribed inspiratory pressure was 11 mmHg ± 0.3 and expiratory pressure was 7 mmHg ± 0.3 in the BiPAP group versus 10 mmHg ± 0.2 in the fixed CPAP group at baseline | |
| Outcomes |
| |
| Funding & conflicts of interest statements | 'Supported in part by Respironics'. Author conflicts not provided. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Non‐completers did not contribute to analysis |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group trial. Single‐blinded study | |
| Participants | N = 20 participants (18 M/2 F). Mean age: 47 years; BMI: 37; ESS: 14 Inclusion criteria: untreated OSA; PSG‐confirmed diagnosis of OSA (ASDA criteria) Exclusion criteria: not reported | |
| Interventions | Auto‐CPAP versus fixed pressure CPAP. CPAP titration undertaken manually in sleep laboratory Study duration: 4 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "...random, single‐blind fashion..." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, cross‐over study. Statistical analysis: information not available | |
| Participants | N = 13 participants (10 M/3 F). Mean age: 48.2 years; AHI: 22.5; ESS: 11.2 Inclusion criteria: newly diagnosed OSA patients Exclusion criteria: not reported | |
| Interventions | Auto‐CPAP (Autoset Spirit, ResMed) versus fixed CPAP Auto‐CPAP (APAP, Compumedics) versus fixed CPAP Study duration: 3 x 4‐week duration. 2‐week washout | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | Study available as conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Single‐blind, randomised, cross‐over trial | |
| Participants | N = 33 participants. Mean age: 52; BMI: 30.6 kg/m2; AHI: 35; ESS: 7.5 Inclusion criteria: age > 18 years, CPAP‐naive with diagnosis of OSA, understand Dutch language, AHI > 15 events per hour with mild sleepiness or AHI > 5 events/hour with moderate/severe sleepiness Exclusion criteria: central sleep apnoea, Cheyne‐Stoke Respiration, severe nasal obstruction, facial/pharyngeal abnormalities, shift work, psychiatric disorder, heart failure, COPD, seizure disorder, pregnancy, learning disability | |
| Interventions | Pressure restricted auto‐adapting CPAP versus fixed CPAP Study duration 2 x 12 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By block randomisation of a statistical program (Randomisation program BSR, Windows version 5.0) with a block size of 4‐4‐8" |
| Allocation concealment (selection bias) | Low risk | Quote: "Both the patient and the person who recruited the participants were unaware of the allocation sequence." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The trial was fully blinded to the patients. It was not possible to fully blind the investigator performing the analysis." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "It was not possible to fully blind the investigator performing the analysis...The registered technologists were not aware of the group allocation of the patients. However they could deduct therapy as the PAP‐pressure was recorded during the sleep study." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | In both groups 3 drop‐outs occurred. 17 subjects completed 6 weeks of CPAP with subsequently 6 weeks of RAPAP and 16 subjects completed 6 weeks of RAPAP with subsequently 6 weeks of CPAP. |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, cross‐over study. Statistical analysis methods not reported. | |
| Participants | N = 30. No baseline details provided. Participants were on long‐term CPAP for OSA, but were using it for less than 4 hours per night. | |
| Interventions | Auto‐CPAP (AutoSet T) versus fixed pressure CPAP Study duration: 2 x 4‐week treatment periods | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not available (conference abstract) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Randomised, cross‐over study | |
| Participants | N = 51 participants (gender breakdown available for 44 completers: 39 M/5 F). Age 51.5; BMI: 30.9 kg/m2; AHI: 43; ESS 10.3 Inclusion criteria: all patients referred with OSA, aged between 30 and 80 and without nasal or throat complaints Exclusion criteria: > 5 central apnoeas per hour of sleep, acute infection, NYHA III or IV heart failure, acute pulmonary embolism or acute coronary syndrome. Previous use of CPAP | |
| Interventions | CPAP with heated humidification versus CPAP without heated humidification Study duration: 2 x 4 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "K‐H. Ruhle and G. Nilius received research funding from Fisher & Paykel Healthcare, Heinen und Löwenstein, ResMed and Weinmann. This funding has gone into department funds. The author's study was supported by a grant from Fisher & Paykel Healthcare Germany GmbH & Co. KG, 73636 Welzheim, Germany.Karl‐Josef Franke and Ulrike Domanski have no financial or other potential conflict of interest associated with this investigation." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study had a randomised, cross‐over design and used a previously prepared randomisation list. |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were told about the group allocation only after consent. The recruiting doctor was blinded to the randomisation." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "However, it must be kept in mind that patients knew when cHH was used and this may impact on the reported symptoms" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The outcome assessor who calculated the study results was not blinded to the allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Fifty one patients were recruited, 7 dropped out during the course of the study, of which 5 were allocated to the humidification group and 2 in the CPAP group.....44 patients completed the study." |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group trial | |
| Participants | N = 125 participants (116 M/9 F). Age: 48; BMI: 35 kg/m2; AHI: 36; ESS: 12.5 Inclusion criteria: AHI > 10, CPAP‐naive, successful nasal CPAP titration study, adequate nasal breathing Exclusion criteria: Bi‐PAP or supplemental oxygen; malignant disease; psychiatric disease; regular use of narcotics; sedatives or psychoactive substances | |
| Interventions | Standard (dry) CPAP versus CPAP with heated humidification versus CPAP with nasal steroid spray Study duration: 4 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This was not an industry supported study. The authors have indicated no financial conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used to randomise patients to different treatment groups |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred centrally |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "However, patients were not blinded, since blinding would be difficult to achieve and requiring the use of placebo humidification, which has also been a limitation of previous studies." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Although the investigators administering questionnaires and downloading data from the devices were blinded to the treatment arm, participants rating symptoms and other subjective outcomes would have been aware of the treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "All analyses were undertaken using the intention‐to‐treat principle" Quote: "Of the 123 patients participating in the study, 112 subjects (91%) completed the trial... Of the 10 patients wishing to discontinue For dichotomous outcomes the difference in drop out was low and unlikely to affect the size or direction of effect. For machine usage and symptom scores dropouts may have influenced the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, cross‐over study. Method of randomisation not reported. Statistical methods: ANOVA. Unclear how within subject design accounted for in analysis. | |
| Participants | N = 31 participants. Withdrawals: N = 2. (23 M/6 F). Mean age: 53 years; BMI: 33.3 kg/m2; AHI: 45.8; ESS: 14.2 Inclusion criteria: AHI > 10 per hour; CPAP‐naive | |
| Interventions | Auto‐CPAP (DeVilbiss ‐ response to apnoeas and snoring) and AutoSet T ‐ response to apnoea and snoring + flow limitation) versus fixed CPAP Study duration: 2‐week run‐in with either auto‐CPAP device. 3 x 4 week treatment periods. | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "Supported by the Lung League of Zurich, Lung League of Schaffhausen, Lamprecht AG & Labhardt AG". Author conflicts not provided. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind, cross‐over study. Quote: "Patients...... were blinded to exact study purpose and treatment modes." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition rate (2 participants/31); reasons for missing data given as lack of time (N = 1), and moving away from area (N = 1) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Prospective, single‐blinded, randomised, cross‐over study in climate with a high humidity level. Data analysed with paired t test | |
| Participants | N = 20 participants. (M/F 14/6). Mean age 48.9 years; BMI 28.1 kg/m2; AHI 53.7; ESS 11.5 Inclusion criteria: age > 18 years; AHI > 15 on split‐night polysomnogarphy; nasopharyngeal symptoms according to modified XERO questionnaire Exclusion criteria: > 5 central apnoeas per hour; acute infection; heart failure with NYHA class 3 or 4; acute pulmonary embolus; acute coronary syndrome; travel outside of Thailand within 2 months of study baseline pattern of split‐night PSG < 2 hours, less than optimal CPAP titration, use of humidification during split‐night study | |
| Interventions | CPAP with heated humidification versus conventional CPAP alone Study duration: 2 x 4 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This work was supported by the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University. All CPAP machines and related equipment were sponsored by Fisher and Paykel Healthcare Limited. However, the company had no impact on study design or interpretation of the results of the study.SR has disclosed relationships with Sanofi,Medtronic, and Merck. The other authors have disclosed no conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned by bloc sizes of 4 to receive CPAP with or without heated humidification". Insufficient information available. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation sequence was based on the random order which was in the sealed envelope prepared by our research statistician and the investigators who conducted the study and the patients were not aware of." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Single blind randomized cross‐over study......the subjects were blinded to the treatment arm." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Single‐blind study design |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Unclear risk | Twenty‐two were subjects enrolled.....Quote: "2 more subjects dropped out from the study in the first week after CPAP use, and the reasons for dropout were CPAP refusal." Rate of withdrawal could potentially have influenced some outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (quality of life and AHI reported as medians so could not be used in meta‐analysis) |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, parallel group study | |
| Participants | N = 36 No dropouts. Age range 36 to 65; AHI: 43.6; ESS: 15.5 Inclusion criteria: OSA confirmed by polysomnography and by clinical features; participants chosen to be treated by CPAP Exclusion criteria: life‐threatening OSA (severe hypersomnolence); OSA associated with non‐obstructive breathing disorders (narcolepsy); estimated pressure < 15 cmH2O. All participants were recruited from the Hôpital Laval sleep clinic. | |
| Interventions | Auto‐CPAP 1 (measured effective pressure based upon polysomnography) versus Auto‐CPAP 2 (effective pressure estimated by prespecified formula) versus fixed CPAP. Data entered from Auto‐CPAP 1. Study duration: 3 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Funded in part by Pierre Medical France. Author declarations not provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table (block of 3) |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind study |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group trial | |
| Participants | N = 48. 40 had previously participated in other trials of auto and fixed CPAP. Mean age: 48; BMI: 39.5 kg/m2 Inclusion criteria: PSG‐diagnosed OSA Exclusion criteria: corrective surgery for OSA | |
| Interventions | Auto‐CPAP (Morphée) versus fixed CPAP Study duration: 3 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | TJL emailed for details of randomisation and outcome data 8 September 2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "This was done using a binary randomisation list provided by our statistician who was not involved in the study." |
| Blinding of participants and personnel (performance bias) | High risk | Some participants had previously participated in a study and could have become aware of different devices irrespective of masking treatment group assignment |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Some participants had previously participated in a study and could have become aware of different devices irrespective of masking treatment group assignment |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Most outcomes in this review unaffected by the following exclusions: Quote: "The sleep stage‐ and body position‐dependence could not be characterized in 15 patients who did not change body position or whose sleep architecture did not include REM sleep during baseline sleep recording. These subjects were therefore excluded from the comparison between sleep stages/body position‐dependent and independent patients." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, double‐blind, cross‐over study Two‐factor repeated measures analysis of variance (AVOVA). Order of testing (fixed CPAP versus auto‐CPAP first) taken as one fixed factor, mode of treatment (fixed CPAP versus auto‐CPAP as second factor) | |
| Participants | N = 10 participants (10 M). Mean age 52 years; AHI 52.9 Inclusion criteria: > 20 AHI, residence < 50 km from clinic and newly diagnosed with OSA Exclusion criteria: co‐existing airways disease (asthma/COPD), rhinitis or cardiac failure | |
| Interventions | Auto‐CPAP versus fixed CPAP. No washout period Study duration: 2 x 8‐week treatment periods | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The staff were blinded as to whether the machine was in auto or conventional mode. Patients were not told in which mode the machine was operating." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Study had double‐blind design. |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, cross‐over study. Statisitical analysis based on paired t test. | |
| Participants | N = 43 participants (2 lost to follow‐up). BMI: 28.7 kg/m2; AHI: 54.3; ESS: 13.4 Inclusion criteria: 18 to 65 years; newly diagnosed OSA (AHI > 30) Exclusion criteria: prior treatment for OSA | |
| Interventions | Auto‐CPAP versus fixed CPAP Study duration: 2 x 8 weeks (washout: 1 week) | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "The authors declared no conflict of interest between ResMed Company and the participating institutions, which received no external funding support for this study." | |
| Notes | TJL emailed for clarification of data from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned by random table allocation into one of the two arms of the study." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by an investigator external to the trial. The sequence of treatment group assignment was concealed from investigators conducting the screening and ongoing assessments. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Low rate of exclusions from the analysis (N = 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, blinded, cross‐over trial. Statistical analysis based on paired tests. | |
| Participants | N = 200 participants (154 M/46 F). Mean age 50; BMI 34.5 kg/m2; AHI: 33; ESS 14 Inclusion criteria: ESS > 10 or sleepiness while driving; AHI > 15 on PSG or > 25 apnoeas/hypopneas per hour on limited sleep study; age 18 to 75; CPAP naive Exclusion criteria: neurological deficit compromising CPAP use; significant comorbidity; co‐existing narcolepsy/periodic limb movements; contraindication to CPAP | |
| Interventions | Fixed pressure versus variable pressure CPAP Study duration: 2 x 6 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "This study was supported by a grant from ResMed, Poway, CA. Dr. Douglas is a shareholder in ResMed. The other authors have indicated no additional conflicts of interest. The study was proposed, designed and all analysis performed solely by the authors." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized...using a randomization schedule of balanced blocks..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized...by a worker otherwise uninvolved in the trial." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients were informed that 2 different methods of giving CPAP were to be assessed, but were not told which was the new method" Quote: "None of the staff involved in data acquisition or analysis were aware of the mode of treatment the patient was receiving" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The blinded staff member conducted the follow‐up assessments unaware of the type of CPAP the patient was using at the time of assessment." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Nineteen patients did not complete the study; at the time of dropout 9 of these were receiving fixed and 10 variable pressure CPAP" Balanced drop out which could impact on machine usage and symptoms |
| Selective reporting (reporting bias) | High risk | Side effects were rated by the Edinburgh questionnaire but not reported. |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, single‐blind, cross‐over study (participants not informed of order/setting). Statistical analysis based on Wicoxon methods (not specified if paired). | |
| Participants | N = 20 participants completed and analysed (16 M/4 F). AHI: 45; ESS: 10.9 Inclusion criteria: new diagnosis of OSA (diagnosis established through polysomnography) Exclusion criteria: mixed or central apnoea | |
| Interventions | CPAPexp (C‐Flex) versus fixed pressure CPAP Same machine delivered the different treatment pressure settings Study duration: 2 x 6 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Not provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "....randomised, single‐blinded cross‐over study" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "...single‐blinded cross‐over study" |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group trial. Identical machines used. Withdrawals described | |
| Participants | N = 98 participants (N considered for this review: 65 (55 M/10 F). Mean age: 47; ESS: 16. Inclusion criteria: 18 to 75 years of age; ESS > 9; proven OSA (PSG); 10 dips/hr in arterial O2 saturation; CPAP‐naive Exclusion criteria: respiratory failure requiring urgent treatment; unable to give written consent Participants were not excluded on the basis of comorbidities | |
| Interventions | Auto‐CPAP versus algorithm established fixed CPAP Additional treatment group not considered for this review: 1 week auto‐titration followed by fixed pressure at the level of 95th centile pressure from the auto‐CPAP week data. Study duration: 24 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "ResMed UK provided part financial support for the purchase of CPAP machines for the study but was not involved in its design or analysis. D Jones was supported in part by a Helen Bearpark Scholarship from the Australasian Sleep Association and by the Sleep Apnoea Trust Association (UK). None of the authors has any conflict of interest." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated schedule (MINIM) |
| Allocation concealment (selection bias) | Low risk | Quote: "...investigators carrying out the assessment studies were blind to their group allocation." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patients and the investigators carrying out the assessment studies were blind to their group allocation." |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...the investigators carrying out the assessment studies were blind to their group allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | High risk | Nine participants lost to follow‐up or excluded from the analysis: Quote: "No data were entered for those who did not attend." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
| Methods | Randomised, parallel group study | |
| Participants | N = 54 participants (43 M/11 F). Mean age 55 years; AHI 46. ESS 14 Consecutive OSA patients referred for CPAP, under a program paid for by the Victorian State government, were enrolled. | |
| Interventions | Fixed pressure CPAP + humidification versus fixed pressure CPAP alone Study duration: 12 weeks | |
| Outcomes |
| |
| Funding & conflicts of interest statements | Quote: "Fisher and Paykel Healthcare, Auckland, New Zealand funded this study. They were not involved in the design of this study, in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication. Author conflict of interest: None." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was concealed using a statistical randomisation package so that each subject was randomised to receive either treatment independently of the other subjects." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was concealed using a statistical randomisation package..." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "It was not possible to blind patients to the type of treatment they were getting" |
| Blinding of participants and personnel (performance bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The one CPAP therapist saw each patient at each visit. It was not possible to blind her to the treatment that the patients were getting, but she was instructed not to make any attempts to determine what type of pump each patient was using, and to try to treat each patient in a similar manner" |
| Blinding of outcome assessment (detection bias) | Low risk | These outcomes unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All of the fifty‐four subjects completed the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No concerns identified |
ABRP‐PAP: automatic bi‐level therapy with pressure relief; AHI: Apnoea Hypopnoea Index; ATS: American Thoracic Society; auto‐CPAP: auto‐titrating CPAP; Bi‐PAP: bilevel positive airway pressure; BMI: body mass index; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure; CPAPexp ‐ CPAP with expiratory pressure relief; DI: desaturation index; ESS: Epworth Sleepiness Scale; FEV1: forced expiratory volume in one second; FOSQ: Functional Outcomes of Sleep Questionnaire; FOT: forced oscillation technique; FVC: forced vital capacity; ITT: intention‐to treat‐analysis; MWT: Maintenance of Wakefulness Test; nCPAP: nasal CPAP; NIV: non‐invasive ventilation; NYHA: New York Heart Association; OSA: obstructive sleep apnoea; PAP: positive airway pressure; PSG: polysomnography; RDI: respiratory disturbance index; REM: rapid eye movement; SaO2: oxygen saturation; SAQLI: Sleep Apnoea Quality of Life Index; SF‐36: Short‐Form 36 quality of life questionnaire; TST: total sleep time; UARS: upper airway resistance syndrome; UPPP: WASO: wake after sleep onset
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No fixed CPAP arm | |
| Study of different humidifying units plus CPAP | |
| CBT | |
| Review article | |
| CPAP or C‐Flex given in a sequential, non‐randomised order | |
| Not randomised | |
| Study assessing oral versus nasal interface of CPAP | |
| Study assessing chinstrap over a 2‐night laboratory titration study | |
| No fixed CPAP arm; study duration 2 days | |
| Placebo‐controlled trial | |
| Non‐randomised study of treatment failure in central sleep apnoea | |
| Review article | |
| Review article | |
| Non‐randomised study of APAP for OSA treatment | |
| Comparison of effects of auto‐titrating CPAP (APAP) versus auto‐titrating CPAP with expiratory pressure relief (A‐Flex) on AHI. No fixed CPAP arm, study duration 2 nights | |
| Comparison of auto‐CPAP with A‐Flex (auto‐CPAP with pressure relief during expiration) | |
| Non‐randomised study of CPAP treatment | |
| Effect of nose drops | |
| Not randomised | |
| Different algorithms of auto‐CPAP compared | |
| Inadequate duration | |
| Study assessing interface chamber of CPAP | |
| Educational/psychosocial intervention | |
| No fixed CPAP arm | |
| Inadequate duration | |
| Non‐randomised study of nasal mucosal tissue changes with CPAP treatment | |
| CPAP versus subtherapeutic pressure of CPAP | |
| Study assessing efficacy of CPAP | |
| Overnight study only | |
| Educational/psychosocial support | |
| Comparison between different auto‐CPAP devices | |
| Overnight study only; no fixed CPAP arm | |
| One‐night study | |
| One‐night study | |
| No fixed CPAP arm | |
| Non‐randomised study of objective compliance measure of CPAP use | |
| Educational/psychosocial intervention | |
| Assessment of nose drops on CPAP machine usage | |
| Laboratory‐based study | |
| Laboratory‐based study | |
| Laboratory‐based study | |
| Educational/psychosocial intervention | |
| Non‐randomised study of CPAP compliance | |
| Non‐randomised study on effectiveness of AutoSet to determine treatment pressure | |
| Manual versus auto‐titrating study | |
| No fixed CPAP arm | |
| Control group received humidification in addition to fixed pressure CPAP | |
| Inadequate duration | |
| Placebo control | |
| Non‐randomised study on CPAP compliance | |
| Not a comparative trial of pressure modification devices in OSA | |
| Participants randomised to receive auto‐CPAP as a titration strategy | |
| Laboratory‐based titration study | |
| Split‐night titration study | |
| Different levels of Bi‐PAP compared | |
| Review article | |
| Laboratory‐based study | |
| No fixed CPAP arm | |
| Educational/psychosocial intervention | |
| Non‐randomised study | |
| Educational/psychosocial intervention | |
| Non‐randomised study of CPAP effectiveness | |
| Different pressure levels of CPAP compared (therapeutic and subtherapeutic) | |
| Different titration strategies compared | |
| No fixed CPAP control group | |
| Two‐night laboratory titration study | |
| Comparison outside the focus of the review: oral versus nasal interface | |
| Participants with significant cardiac comorbidity | |
| Observational study | |
| Non‐randomised study on CPAP compliance following simplified diagnostic procedure for OSA | |
| Review article | |
| Non‐randomised study of CPAP compliance | |
| Inadequate duration | |
| Study assessing the effects of daytime CPAP titration | |
| Not assessment of pressure modification | |
| Laboratory based titration study | |
| Randomisation between immediate provision of humidification and delayed provision of humidification | |
| Participants randomised to CPAP or inactive control | |
| Not assessment of pressure modification | |
| Different titration strategies compared | |
| No control group receiving only fixed pressure CPAP | |
| Comparison of effects of manual titration versus laboratory APAP titration versus home APAP titration on CPAP compliance. Participants switched to fixed CPAP after titration study | |
| Editorial | |
| Non‐randomised study of CPAP compliance | |
| Randomised comparison of 2 types of auto‐CPAP | |
| Study of educational interventions | |
| Inadequate duration | |
| Journal correspondence | |
| Randomised trial comparing nose and face mask CPAP therapy | |
| Different diagnostic strategies compared | |
| Inadequate duration | |
| No fixed CPAP arm | |
| Responder analysis | |
| Randomised trial comparing 6 autoadapting CPAP devices in patients previously treated with fixed CPAP. Fixed CPAP arm not run concurrently with auto‐CPAP arms | |
| Randomisation between different auto‐titrating CPAP machines; data from fixed CPAP machines captured from start of trial | |
| Randomised study conducted when participants were awake | |
| Educational/psychosocial intervention | |
| Laboratory‐based study | |
| No fixed CPAP control | |
| Different APAP therapies compared | |
| Laboratory‐based study | |
| Participants recruited with obesity hypoventilation syndrome | |
| Randomised trial comparing auto with fixed pressure CPAP. This trial was excluded as an educational intervention administered at baseline was not standardised between the two treatment groups. Titration was also performed in different settings for auto and fixed pressure CPAP. | |
| No fixed CPAP arm | |
| Study in people with positional OSA | |
| Non‐randomised trial on side effects of nasal CPAP therapy | |
| Non‐randomised study assessing educational interventions in 4 children with OSA (PsycINFO) | |
| No fixed CPAP arm | |
| This study compared different media for informing patients about CPAP. Overnight study | |
| Laboratory‐based study | |
| No fixed CPAP arm | |
| Study of CBT | |
| This study was excluded as participants were prescribed CPAP machines set at different hours of use (< 6.5 hours and > 7.5 hours) | |
| No fixed CPAP arm | |
| Inadequate duration | |
| Humidification added to APAP. No fixed pressure comparator | |
| No attempt to measure compliance | |
| Overnight study | |
| Assessment of different strategies to diagnose and manage OSA | |
| Non‐randomised cohort study on the effects of a complex intervention on patient compliance with CPAP therapy | |
| No fixed CPAP arm | |
| Laboratory‐based study | |
| Participants randomised to auto‐CPAP or no treatment as a means of titration prior to fixed pressure CPAP | |
| Assessment of telemedicine intervention | |
| Inadequate duration (2 x 1 week treatment arms) | |
| Different titration strategies | |
| No fixed CPAP arm | |
| Educational/behavioural intervention | |
| No fixed CPAP arm | |
| 2‐night titration study | |
| Comparison of S9 (humidification with autoadjusting CPAP) versus CPAP. Not a randomised trial |
AHI: Apnoea Hypopnoea Index; APAP: CBT: cognitive behavioural therapy; CPAP: continuous positive airway pressure; OSA: obstructive sleep apnoea
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Multicentre cross‐over study |
| Participants | N = 40 participants (23 M/17 F); Age: 62.4 years; BMI: 30.7 kg/m2; AHI: 46.7; ESS 8.6; FOSQ 10 29. Inclusion criteria: diagnosis of OSA (AHI >30 (or less than 30 if respiratory arousal index >10 events/hour); no prior treatment with CPAP; using medicine known to induce nasal dryness; previous nasal symptoms or nasal surgery. Exclusion criteria: heart failure (New York Heart Association level 3 or 4), lung disease; pregnancy; prior treatment for OSA; >5 central oxygen saturation/hour. |
| Interventions |
Study duration: 4 weeks per treatment period |
| Outcomes |
|
| Notes |
| Methods | Open‐label, randomised controlled trial |
| Participants | 800 adult participants not previously treated with CPAP. AHI in excess of 30 events per hour |
| Interventions | Auto CPAP and fixed CPAP for a period of 3 months |
| Outcomes |
The study objective is to relate effective treatment pressure with clinical outcomes and polysomnography measurements. |
| Notes | NCT02749812 record states that study completed in September 2015. Date registered as April 2016. Status update (November 2018): manuscript undergoing revision by author team. Likely publication date 2nd quarter 2019. |
| Methods | Part of large adherence trial |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Conference abstract |
AHI: Apnoea Hypopnoea Index; BMI: body mass index; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; OSA: obstructive sleep apnoea
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Comparing the usage of continuous positive airway pressure (PAP) against the RACer airway device in the treatment of obstructive sleep apnoea (OSA) in naive PAP users |
| Methods | Randomised cross‐over study |
| Participants | Planned sample size: 30. Recruitment from tertiary sleep clinic in New Zealand. Inclusion criteria: age 18 to 70 years, no prior treatment with CPAP, ability to tolerate a nasal pillow mask, AHI or RDI of > 20 events per hour |
| Interventions | Standard CPAP device or Rest‐Activity‐Cycle (RACer) system used with positive airway pressure device (RACer CPAP). The RACer device delivers pressurised air into the upper airway into one nostril at a time. Both modalities are combined in a single device, although RACer mode necessitates the use of nasal pillow. Planned duration: 4 weeks of treatment on each arm either side of a 3‐day washout period between arms. |
| Outcomes |
|
| Starting date | 20/09/2017 |
| Contact information | Dr Angela Campbell WellSleep University of Otago, Wellington 98 Churchill Drive Crofton Downs, 6035 Wellington |
| Notes |
| Trial name or title | Auto‐titrating versus fixed continuous positive airway pressure in obesity hypoventilation syndrome |
| Methods | Randomised parallel group trial |
| Participants | Planned sample size: 40 Inclusion criteria: age 18 to 80 years; BMI > 30; PaCO2 45 mmHg to 60 mmHg; blood pH 7.35 to 7.45; no use of CPAP in the past 12 months; AHI ≥ 30 |
| Interventions | Fixed CPAP versus auto‐CPAP Study duration: 12 weeks |
| Outcomes |
|
| Starting date | May 2018 |
| Contact information | Dr Yizhong Zheng Department of Respiratory and Sleep Medicine Australia |
| Notes |
| Trial name or title | The effects of humidification of nasal CPAP on adherence, compliance, patient satisfaction and symptoms: a preliminary report |
| Methods | NA |
| Participants | 80 recruited. 40 completed protocol. Mean age 52.2 (10.3). 32 M: 8 F. 5 dropped out. |
| Interventions | CPAP with humidifier versus CPAP without humidifier for 3/12 |
| Outcomes |
|
| Starting date | Not stated |
| Contact information | Sharon Morton, Flinders Medical Centre Bedford Pk. |
| Notes |
| Trial name or title | None given |
| Methods | Double‐blind cross‐over randomised trial. Randomisation sequence generated by computer |
| Participants | 400 planned as per published protocol (440 planned as per CT.gov record). Participants recruited from responders exposed to dust from collapse of World Trade Centre in 2001. Inclusion criteria: nasal resistance and sleep apnoea diagnosed by home‐based study |
| Interventions | Fixed pressure CPAP and flexible CPAP (CPAPFlex) Treatment arms scheduled to last for 4 weeks in published protocol (9 weeks according to CT.gov record) |
| Outcomes |
|
| Starting date | December 2012 |
| Contact information | |
| Notes |
| Trial name or title | Decrease in sympathetic tone in OSA patients: Is CPAP more effective than APAP? |
| Methods | Randomised parallel group |
| Participants | Planned sample size: 68
Inclusion criteria: AHI ≥ 20; daytime sleepiness; no prior exposure to CPAP Exclusion criteria: serious heart failure; central sleep apnoea index above 20% of AHI; serious comorbidity |
| Interventions | Fixed CPAP versus auto‐CPAP Study duration: 4 weeks |
| Outcomes |
|
| Starting date | 1 March 2018 |
| Contact information | Renaud Tamisier, MD, PhD University Hospital Grenoble France |
| Notes |
| Trial name or title | Not available |
| Methods | Randomised, cross‐over study |
| Participants | 5 recruited |
| Interventions | Auto‐CPAP versus CPAP for 2/52 |
| Outcomes | Heart rate variability |
| Starting date | Not stated |
| Contact information | — |
| Notes | TJL emailed for data 14 November 2008 |
AHI: Apnoea Hypopnoea Index; BMI: body mass index; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; OSA: obstructive sleep apnoea; PaCO2: partial pressure of arterial carbon dioxide; RDI: Respiratory Disturbance Index; SF‐36: Short‐Form 36; SAQLI: Sleep Apnoea Quality of Life Index
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 31 | 1452 | Mean Difference (Fixed, 95% CI) | 0.21 [0.11, 0.31] |
| Analysis 1.1  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 Machine usage (hours/night) (Pepin imputed) Show forest plot | 32 | 1774 | Mean Difference (Fixed, 95% CI) | 0.19 [0.10, 0.29] |
| Analysis 1.2  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 2 Machine usage (hours/night) (Pepin imputed). | ||||
| 3 Number of participants who used CPAP therapy > 4 hours per night Show forest plot | 2 | 346 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.75, 1.81] |
| Analysis 1.3  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 3 Number of participants who used CPAP therapy > 4 hours per night. | ||||
| 4 Machine usage (on nights when CPAP used 'effectively') Show forest plot | 3 | Mean Difference (Fixed, 95% CI) | 0.42 [0.05, 0.78] | |
| Analysis 1.4  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 4 Machine usage (on nights when CPAP used 'effectively'). | ||||
| 5 Machine usage (frequency of usage as % of days) Show forest plot | 9 | Mean Difference (Fixed, 95% CI) | 1.60 [‐0.83, 4.03] | |
| Analysis 1.5  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 5 Machine usage (frequency of usage as % of days). | ||||
| 6 Machine usage (% of nights of > 4 hours of use) ‐ cross‐over studies Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | 6.25 [‐0.05, 12.54] | |
| Analysis 1.6  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 6 Machine usage (% of nights of > 4 hours of use) ‐ cross‐over studies. | ||||
| 7 Symptoms (Epworth Sleepiness Scale) Show forest plot | 25 | 1285 | Mean Difference (Fixed, 95% CI) | ‐0.44 [‐0.72, ‐0.16] |
| Analysis 1.7  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 7 Symptoms (Epworth Sleepiness Scale). | ||||
| 8 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 13 | 1275 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.64, 1.27] |
| Analysis 1.8  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 8 Withdrawals (parallel group trials/first arm cross‐over trials). | ||||
| 9 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 3 | 352 | Mean Difference (Fixed, 95% CI) | 0.12 [‐0.21, 0.46] |
| Analysis 1.9  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 9 Quality of life (Functional Outcomes of Sleep Questionnaire). | ||||
| 10 Quality of life (Sleep Apnoea Quality of Life Index) Show forest plot | 2 | 97 | Mean Difference (Fixed, 95% CI) | ‐0.14 [‐0.54, 0.27] |
| Analysis 1.10  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 10 Quality of life (Sleep Apnoea Quality of Life Index). | ||||
| 11 Quality of life (SF‐36 questionnaire) Show forest plot | 8 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 11 Quality of life (SF‐36 questionnaire). | ||||
| 11.1 Physical functioning | 3 | Mean Difference (Fixed, 95% CI) | 0.76 [‐3.50, 5.01] | |
| 11.2 Role physical | 2 | Mean Difference (Fixed, 95% CI) | ‐3.73 [‐13.46, 6.01] | |
| 11.3 Bodily pain | 2 | Mean Difference (Fixed, 95% CI) | 4.21 [‐4.23, 12.64] | |
| 11.4 General health | 2 | Mean Difference (Fixed, 95% CI) | 2.49 [‐4.99, 9.97] | |
| 11.5 Vitality | 6 | Mean Difference (Fixed, 95% CI) | 1.32 [‐1.25, 3.88] | |
| 11.6 Social functioning | 2 | Mean Difference (Fixed, 95% CI) | 3.31 [‐4.29, 10.92] | |
| 11.7 Role emotional | 3 | Mean Difference (Fixed, 95% CI) | 0.70 [‐4.19, 5.59] | |
| 11.8 Mental health | 3 | Mean Difference (Fixed, 95% CI) | 0.20 [‐1.88, 2.27] | |
| 12 Apnoea Hypopnoea Index (events/hr) Show forest plot | 26 | 1256 | Mean Difference (Fixed, 95% CI) | 0.48 [0.16, 0.80] |
| Analysis 1.12  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 12 Apnoea Hypopnoea Index (events/hr). | ||||
| 13 Arousals (events/hr) Show forest plot | 4 | 136 | Mean Difference (Fixed, 95% CI) | ‐0.66 [‐2.90, 1.58] |
| Analysis 1.13  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 13 Arousals (events/hr). | ||||
| 14 Pressure of CPAP treatment (cmH2O) Show forest plot | 24 | 1171 | Mean Difference (Fixed, 95% CI) | ‐1.01 [‐1.17, ‐0.84] |
| Analysis 1.14  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 14 Pressure of CPAP treatment (cmH2O). | ||||
| 15 Systolic blood pressure Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | 1.87 [‐1.08, 4.82] |
| Analysis 1.15  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 15 Systolic blood pressure. | ||||
| 16 Diastolic blood pressure Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | 2.92 [1.06, 4.77] |
| Analysis 1.16  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 16 Diastolic blood pressure. | ||||
| 17 24‐hour mean BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [‐1.05, 2.22] |
| Analysis 1.17  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 17 24‐hour mean BP. | ||||
| 18 24‐hour systolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐2.21, 1.91] |
| Analysis 1.18  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 18 24‐hour systolic BP. | ||||
| 19 24‐hour diastolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.65, 2.44] |
| Analysis 1.19  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 19 24‐hour diastolic BP. | ||||
| 20 Diurnal mean BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐1.05, 2.32] |
| Analysis 1.20  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 20 Diurnal mean BP. | ||||
| 21 Diurnal systolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐2.44, 1.74] |
| Analysis 1.21  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 21 Diurnal systolic BP. | ||||
| 22 Diurnal diastolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [‐0.74, 2.55] |
| Analysis 1.22  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 22 Diurnal diastolic BP. | ||||
| 23 Nocturnal mean BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐1.29, 2.15] |
| Analysis 1.23  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 23 Nocturnal mean BP. | ||||
| 24 Nocturnal systolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [‐1.81, 3.57] |
| Analysis 1.24  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 24 Nocturnal systolic BP. | ||||
| 25 Nocturnal diastolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.81, 2.40] |
| Analysis 1.25  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 25 Nocturnal diastolic BP. | ||||
| 26 Tolerability outcomes Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.26  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 26 Tolerability outcomes. | ||||
| 26.1 Intolerable treatment pressure | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Mask leak | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.3 Dry mouth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.4 Stuffy nose | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 Patient preference (auto‐CPAP/not auto‐CPAP) Show forest plot | 14 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.27  Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 27 Patient preference (auto‐CPAP/not auto‐CPAP). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 4 | 268 | Mean Difference (Fixed, 95% CI) | 0.14 [‐0.17, 0.45] |
| Analysis 2.1  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 4 | 226 | Mean Difference (Fixed, 95% CI) | ‐0.49 [‐1.46, 0.48] |
| Analysis 2.2  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale). | ||||
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 3 | 261 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.17] |
| Analysis 2.3  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials). | ||||
| 4 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 4 Quality of life (Functional Outcomes of Sleep Questionnaire). | ||||
| 5 Quality of life (Sleep Apnoea Quality of Life Index) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 5 Quality of life (Sleep Apnoea Quality of Life Index). | ||||
| 6 Quality of life (SF‐36 questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 6 Quality of life (SF‐36 questionnaire). | ||||
| 6.1 Physical health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Mental heath | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Apnoea Hypopnoea Index (events/hr) Show forest plot | 2 | 179 | Mean Difference (Fixed, 95% CI) | 1.36 [‐6.92, 9.63] |
| Analysis 2.7  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 7 Apnoea Hypopnoea Index (events/hr). | ||||
| 8 Patient preference ‐ BiPAP/no preference or CPAP Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.8  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 8 Patient preference ‐ BiPAP/no preference or CPAP. | ||||
| 9 Tolerability outcomes Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.9  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 9 Tolerability outcomes. | ||||
| 9.1 Dry mouth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Mask intolerance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Treatment comfort score Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.10  Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 10 Treatment comfort score. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 9 | 609 | Mean Difference (Fixed, 95% CI) | 0.14 [‐0.07, 0.35] |
| Analysis 3.1  Comparison 3 CPAPexp versus fixed CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 6 | 515 | Mean Difference (Fixed, 95% CI) | 0.17 [‐0.26, 0.60] |
| Analysis 3.2  Comparison 3 CPAPexp versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale). | ||||
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 2 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.55] |
| Analysis 3.3  Comparison 3 CPAPexp versus fixed CPAP, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials). | ||||
| 4 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 CPAPexp versus fixed CPAP, Outcome 4 Quality of life (Functional Outcomes of Sleep Questionnaire). | ||||
| 5 Quality of life (SF‐36 questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 CPAPexp versus fixed CPAP, Outcome 5 Quality of life (SF‐36 questionnaire). | ||||
| 5.1 Physical functioning | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [‐3.05, 15.45] |
| 5.2 Role physical score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐9.5 [‐25.38, 6.38] |
| 5.3 Bodily pain score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 7.20 [‐3.69, 18.09] |
| 5.4 General health score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐8.05, 10.05] |
| 5.5 Vitality | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐11.38, 6.38] |
| 5.6 Social functioning score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐8.30 [‐17.87, 1.27] |
| 5.7 Role emotional score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐7.30 [‐21.83, 7.23] |
| 5.8 Mental health score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐4.86, 8.86] |
| 6 Apnoea Hypopnoea Index (events/hr) Show forest plot | 5 | 342 | Mean Difference (Fixed, 95% CI) | 0.24 [‐0.49, 0.96] |
| Analysis 3.6  Comparison 3 CPAPexp versus fixed CPAP, Outcome 6 Apnoea Hypopnoea Index (events/hr). | ||||
| 7 Pressure of CPAP treatment (cmH2O) Show forest plot | 2 | 241 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.63, 0.52] |
| Analysis 3.7  Comparison 3 CPAPexp versus fixed CPAP, Outcome 7 Pressure of CPAP treatment (cmH2O). | ||||
| 8 Treatment satisfaction score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.8  Comparison 3 CPAPexp versus fixed CPAP, Outcome 8 Treatment satisfaction score. | ||||
| 9 Treatment comfort score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.9  Comparison 3 CPAPexp versus fixed CPAP, Outcome 9 Treatment comfort score. | ||||
| 10 Treatment interface score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.10  Comparison 3 CPAPexp versus fixed CPAP, Outcome 10 Treatment interface score. | ||||
| 11 Preference Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.11  Comparison 3 CPAPexp versus fixed CPAP, Outcome 11 Preference. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 6 | 277 | Mean Difference (Fixed, 95% CI) | 0.37 [0.10, 0.64] |
| Analysis 4.1  Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 1 Machine usage (hours/night). | ||||
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 4 | 184 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.93, 0.26] |
| Analysis 4.2  Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 2 Symptoms (Epworth Sleepiness Scale). | ||||
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 3 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.45, 2.24] |
| Analysis 4.3  Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials). | ||||
| 4 Apnoea Hypopnoea Index (events/hr) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 4 Apnoea Hypopnoea Index (events/hr). | ||||
| 5 Quality of life (SF‐36 questionnaire) Show forest plot | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐6.97, 7.18] |
| Analysis 4.5  Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 5 Quality of life (SF‐36 questionnaire). | ||||
| 6 Nasal symptoms (parallel group trials) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.6  Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 6 Nasal symptoms (parallel group trials). | ||||
| 6.1 Runny nose | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.09, 1.15] |
| 6.2 Congested or blocked nose | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.51] |
| 7 Nasal symptoms (parallel group trials) Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.7  Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 7 Nasal symptoms (parallel group trials). | ||||
| 7.1 Dry nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.78, 0.01] |
| 7.2 Runny nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.69, 0.09] |
| 7.3 Blocked nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.78, 0.01] |
| 7.4 Bleeding nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.99, 0.10] |
| 8 Preference Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.8  Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 8 Preference. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 2 | 113 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.60, 0.67] |
| Analysis 5.1  Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale). | ||||
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials). | ||||
| 4 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 4 Quality of life (Functional Outcomes of Sleep Questionnaire). | ||||
| 5 Apnoea Hypopnoea Index (events/hr) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.5  Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 5 Apnoea Hypopnoea Index (events/hr). | ||||
| 6 Pressure of CPAP treatment (cmH2O) Show forest plot | 2 | 113 | Mean Difference (Fixed, 95% CI) | ‐0.92 [‐1.77, ‐0.07] |
| Analysis 5.6  Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 6 Pressure of CPAP treatment (cmH2O). | ||||
| 7 Systolic blood pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.7  Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 7 Systolic blood pressure. | ||||
| 8 Diastolic blood pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.8  Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 8 Diastolic blood pressure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Bi‐PAPexp versus fixed CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 Number of participants who used CPAP therapy > 4 hours per night Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Bi‐PAPexp versus fixed CPAP, Outcome 2 Number of participants who used CPAP therapy > 4 hours per night. | ||||
| 3 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Bi‐PAPexp versus fixed CPAP, Outcome 3 Quality of life (Functional Outcomes of Sleep Questionnaire). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | ‐0.00 [‐0.70, 0.70] | |
| Analysis 7.1  Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 Number of participants who used CPAP therapy > trialist defined threshold Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 2 Number of participants who used CPAP therapy > trialist defined threshold. | ||||
| 3 Symptoms (Epworth Sleepiness Scale) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | 0.97 [‐0.56, 2.51] | |
| Analysis 7.3  Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 3 Symptoms (Epworth Sleepiness Scale). | ||||
| 4 Withdrawals Show forest plot | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.33, 17.43] |
| Analysis 7.4  Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 4 Withdrawals. | ||||
| 5 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.5  Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 5 Quality of life (Functional Outcomes of Sleep Questionnaire). | ||||
| 6 Apnoea Hypopnoea Index (events/hr) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 7.6  Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 6 Apnoea Hypopnoea Index (events/hr). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale). | ||||
| 3 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 8.3  Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 3 Quality of life (Functional Outcomes of Sleep Questionnaire). | ||||
| 4 Apnoea Hypopnoea Index (events/hr) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 8.4  Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 4 Apnoea Hypopnoea Index (events/hr). | ||||
| 5 Mask leak Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 8.5  Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 5 Mask leak. | ||||

Study flow diagram: review update
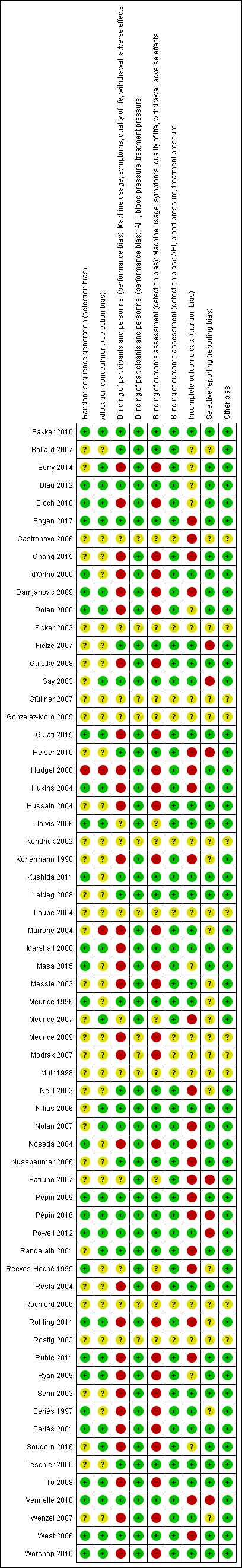
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Auto‐CPAP versus fixed CPAP, outcome: 1.1 Machine usage (hours/night).

Forest plot of comparison: 2 Bi‐PAP versus fixed CPAP, outcome: 2.1 Machine usage (hours/night).

Forest plot of comparison: 3 CPAP with expiratory pressure relief versus fixed CPAP, outcome: 3.1 Machine usage (hours/night).

Forest plot of comparison: 4 Heated humidification + fixed pressure CPAP versus fixed pressure CPAP alone, outcome: 4.1 Machine usage (hours/night).
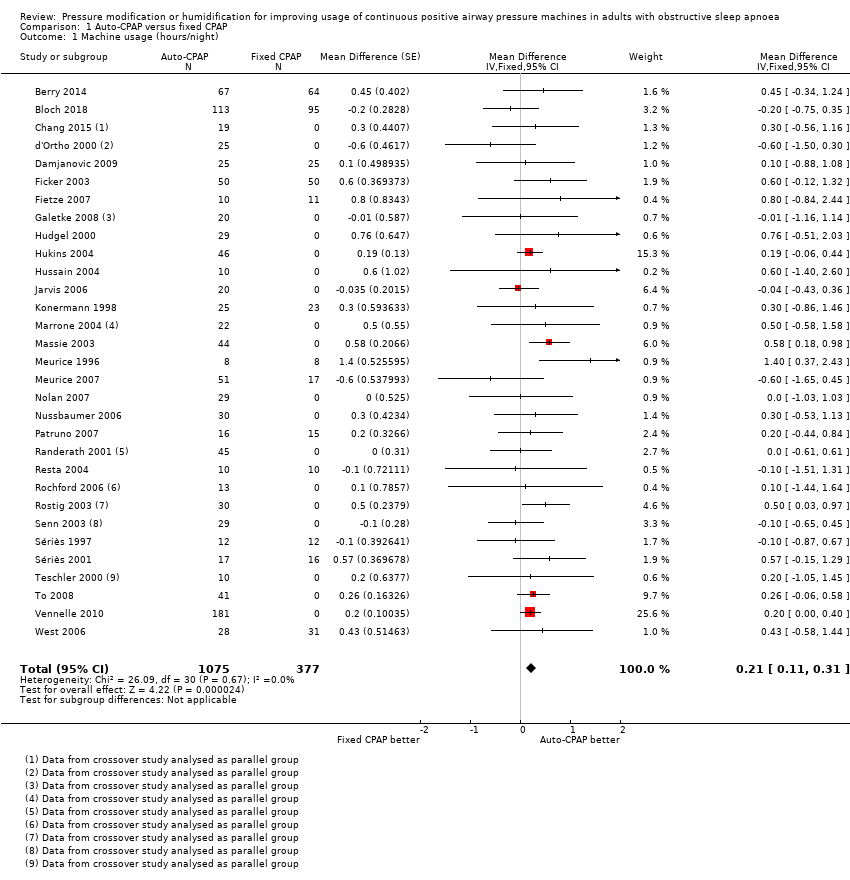
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 1 Machine usage (hours/night).

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 2 Machine usage (hours/night) (Pepin imputed).
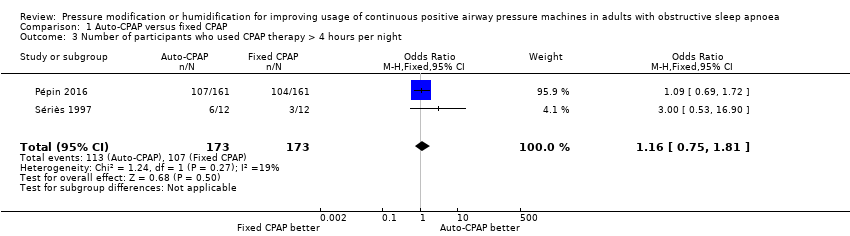
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 3 Number of participants who used CPAP therapy > 4 hours per night.
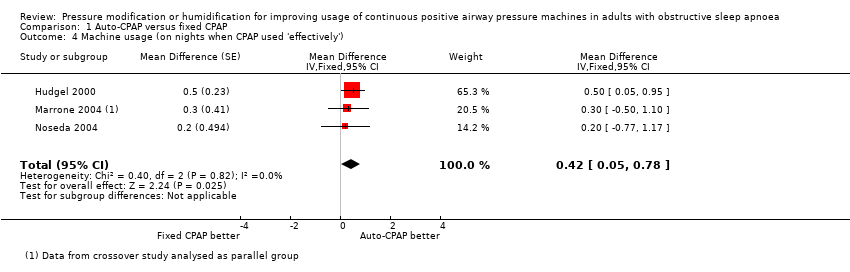
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 4 Machine usage (on nights when CPAP used 'effectively').

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 5 Machine usage (frequency of usage as % of days).
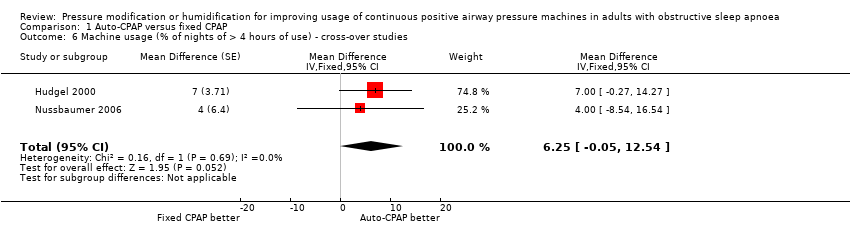
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 6 Machine usage (% of nights of > 4 hours of use) ‐ cross‐over studies.
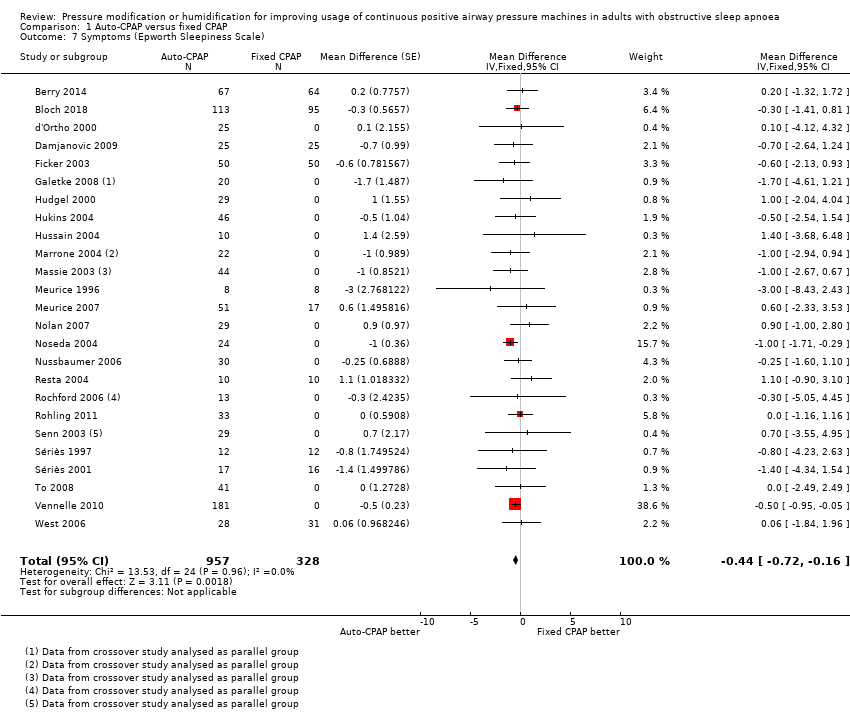
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 7 Symptoms (Epworth Sleepiness Scale).

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 8 Withdrawals (parallel group trials/first arm cross‐over trials).
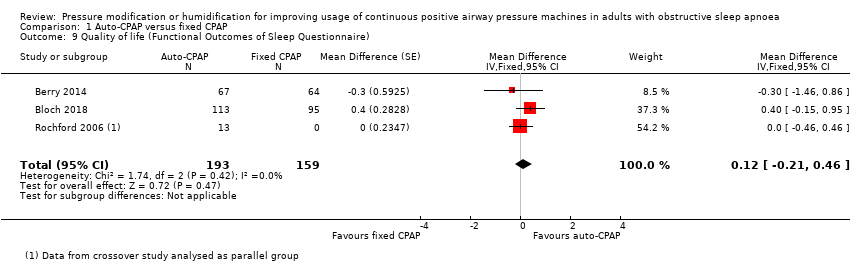
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 9 Quality of life (Functional Outcomes of Sleep Questionnaire).

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 10 Quality of life (Sleep Apnoea Quality of Life Index).

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 11 Quality of life (SF‐36 questionnaire).

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 12 Apnoea Hypopnoea Index (events/hr).

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 13 Arousals (events/hr).
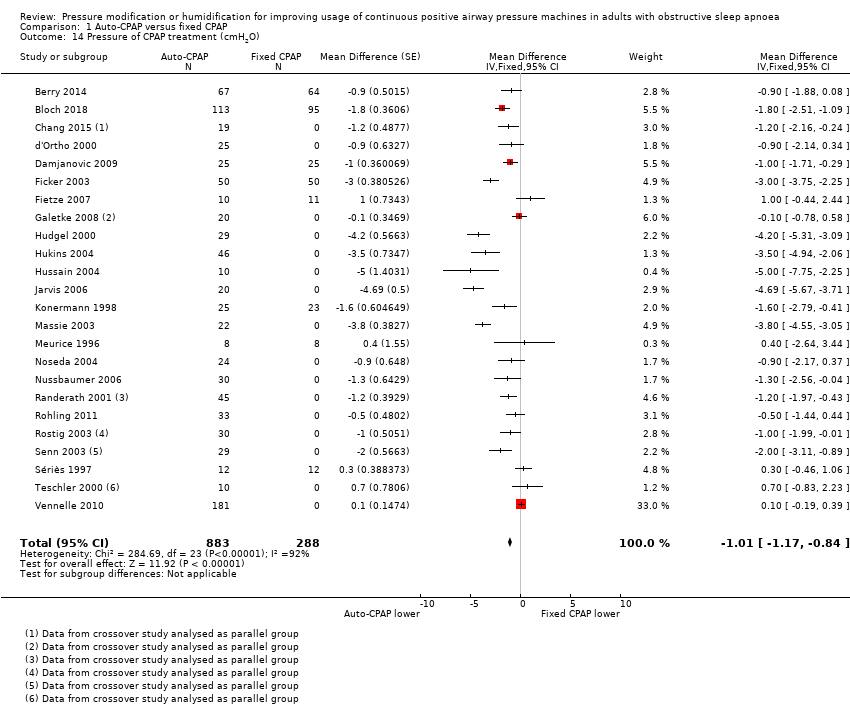
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 14 Pressure of CPAP treatment (cmH2O).
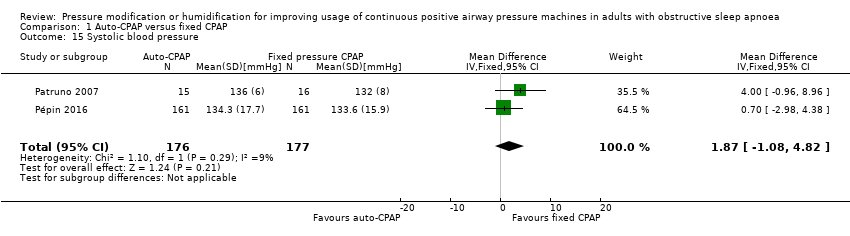
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 15 Systolic blood pressure.
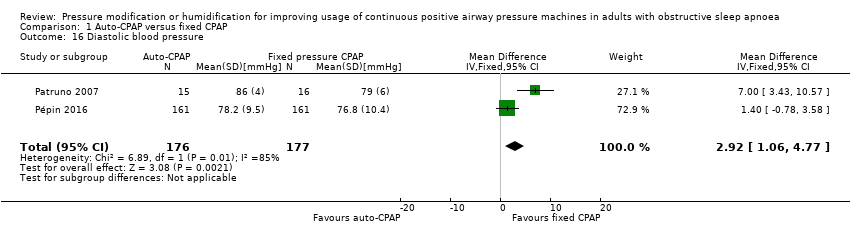
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 16 Diastolic blood pressure.
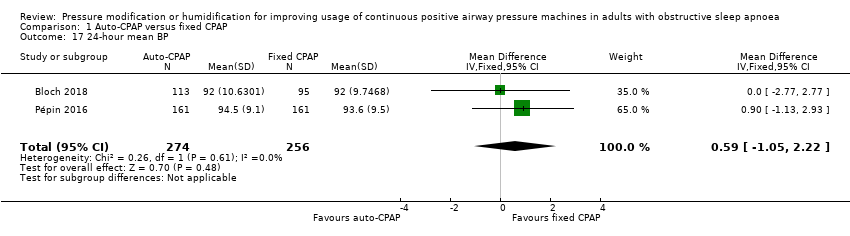
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 17 24‐hour mean BP.

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 18 24‐hour systolic BP.

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 19 24‐hour diastolic BP.

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 20 Diurnal mean BP.
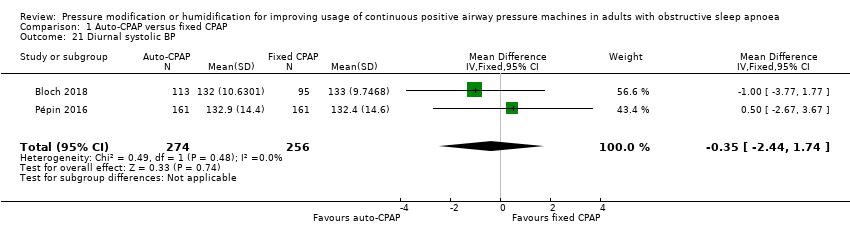
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 21 Diurnal systolic BP.

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 22 Diurnal diastolic BP.

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 23 Nocturnal mean BP.

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 24 Nocturnal systolic BP.
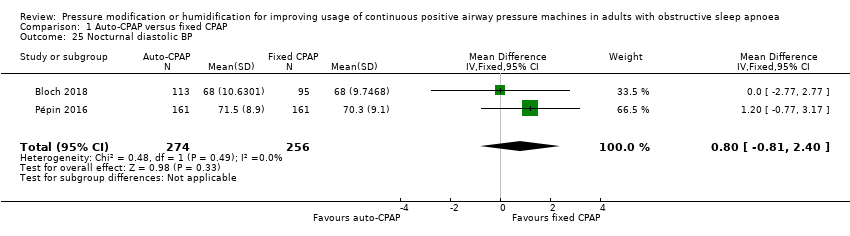
Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 25 Nocturnal diastolic BP.

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 26 Tolerability outcomes.

Comparison 1 Auto‐CPAP versus fixed CPAP, Outcome 27 Patient preference (auto‐CPAP/not auto‐CPAP).
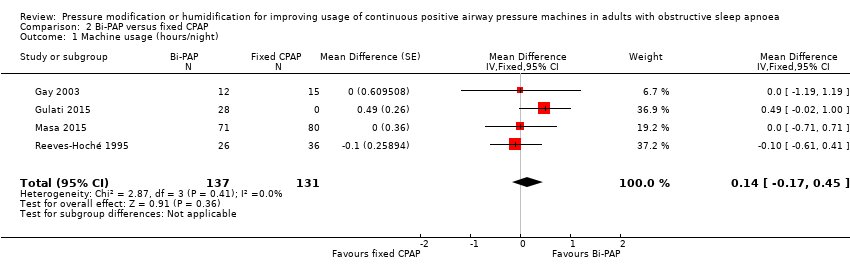
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 1 Machine usage (hours/night).
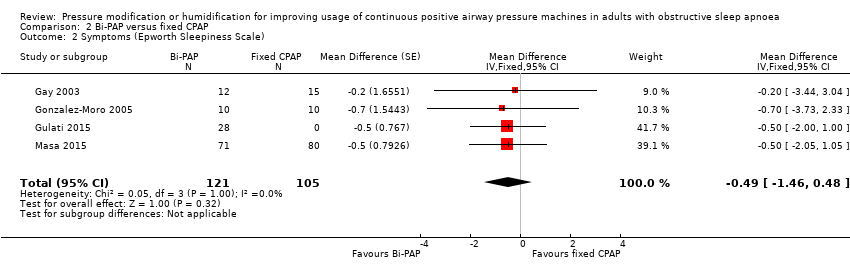
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale).

Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials).
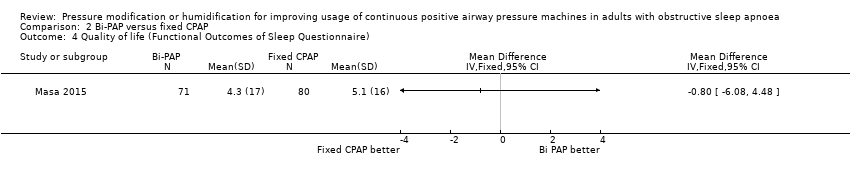
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 4 Quality of life (Functional Outcomes of Sleep Questionnaire).
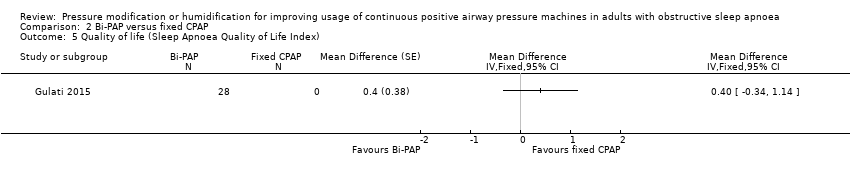
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 5 Quality of life (Sleep Apnoea Quality of Life Index).

Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 6 Quality of life (SF‐36 questionnaire).
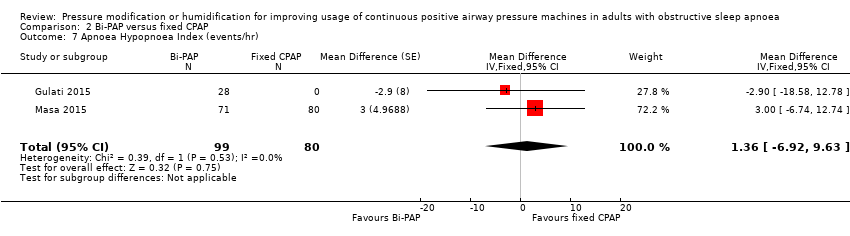
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 7 Apnoea Hypopnoea Index (events/hr).
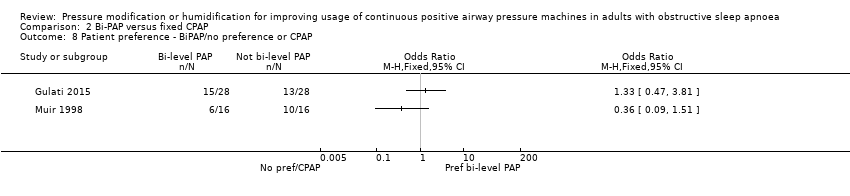
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 8 Patient preference ‐ BiPAP/no preference or CPAP.
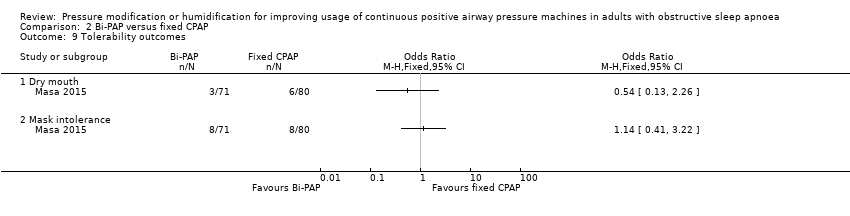
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 9 Tolerability outcomes.
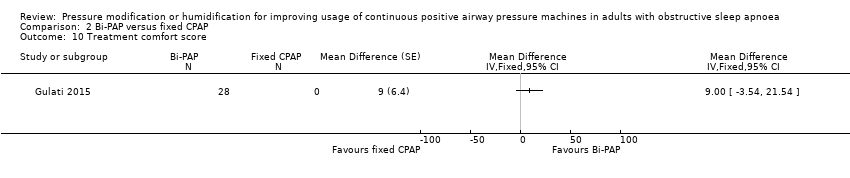
Comparison 2 Bi‐PAP versus fixed CPAP, Outcome 10 Treatment comfort score.

Comparison 3 CPAPexp versus fixed CPAP, Outcome 1 Machine usage (hours/night).

Comparison 3 CPAPexp versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale).
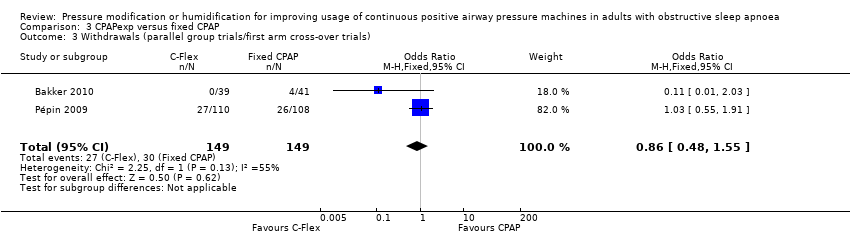
Comparison 3 CPAPexp versus fixed CPAP, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials).

Comparison 3 CPAPexp versus fixed CPAP, Outcome 4 Quality of life (Functional Outcomes of Sleep Questionnaire).
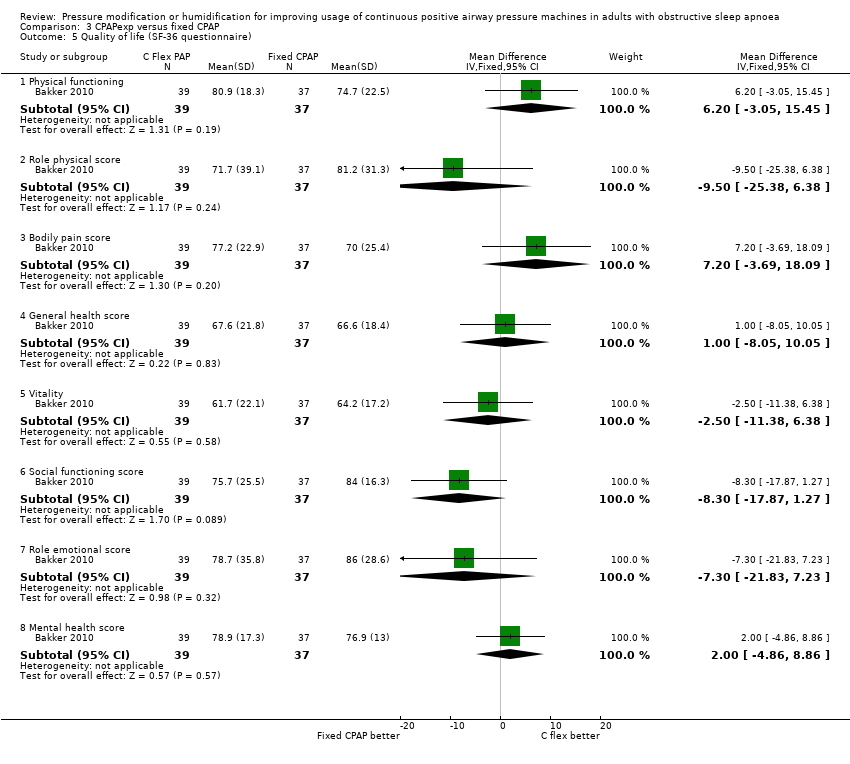
Comparison 3 CPAPexp versus fixed CPAP, Outcome 5 Quality of life (SF‐36 questionnaire).
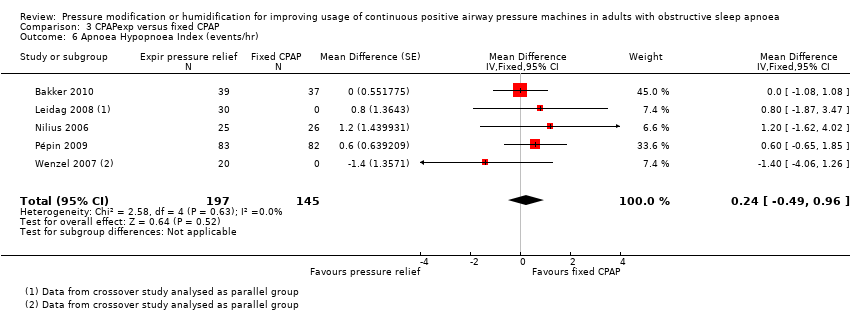
Comparison 3 CPAPexp versus fixed CPAP, Outcome 6 Apnoea Hypopnoea Index (events/hr).

Comparison 3 CPAPexp versus fixed CPAP, Outcome 7 Pressure of CPAP treatment (cmH2O).
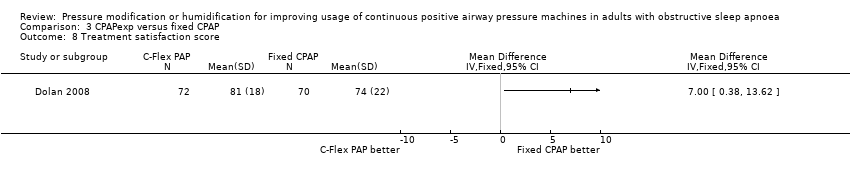
Comparison 3 CPAPexp versus fixed CPAP, Outcome 8 Treatment satisfaction score.

Comparison 3 CPAPexp versus fixed CPAP, Outcome 9 Treatment comfort score.

Comparison 3 CPAPexp versus fixed CPAP, Outcome 10 Treatment interface score.

Comparison 3 CPAPexp versus fixed CPAP, Outcome 11 Preference.

Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 1 Machine usage (hours/night).

Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 2 Symptoms (Epworth Sleepiness Scale).

Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials).
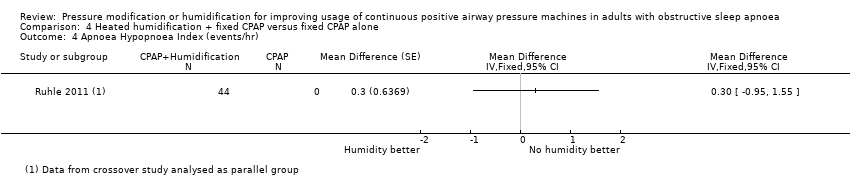
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 4 Apnoea Hypopnoea Index (events/hr).
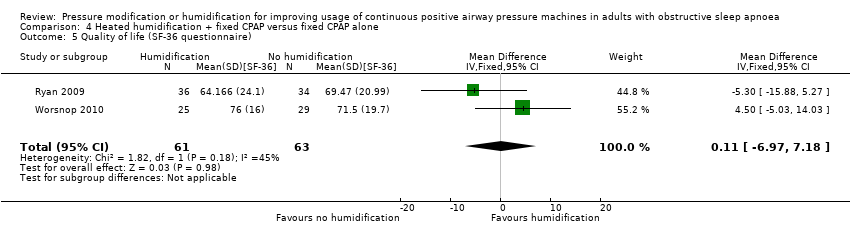
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 5 Quality of life (SF‐36 questionnaire).
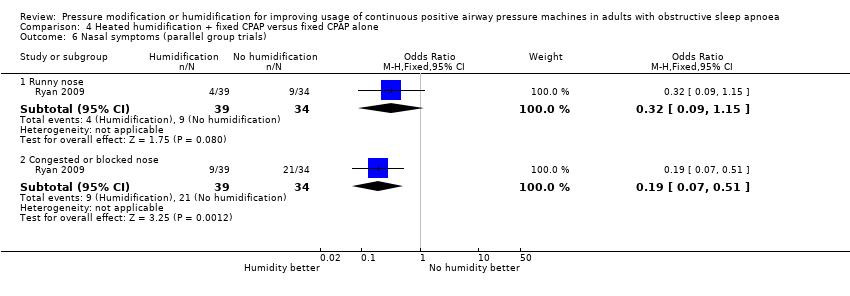
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 6 Nasal symptoms (parallel group trials).

Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 7 Nasal symptoms (parallel group trials).
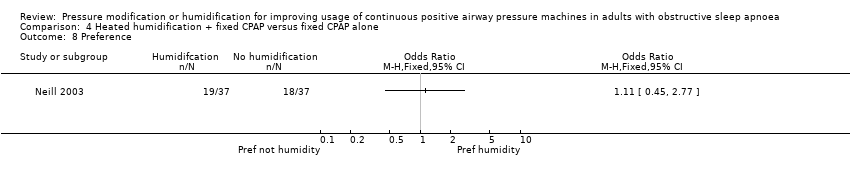
Comparison 4 Heated humidification + fixed CPAP versus fixed CPAP alone, Outcome 8 Preference.
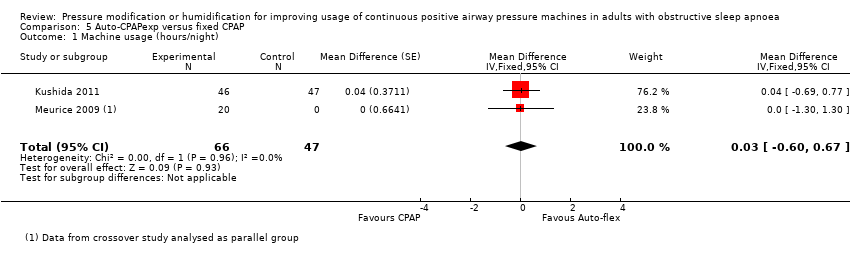
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 1 Machine usage (hours/night).
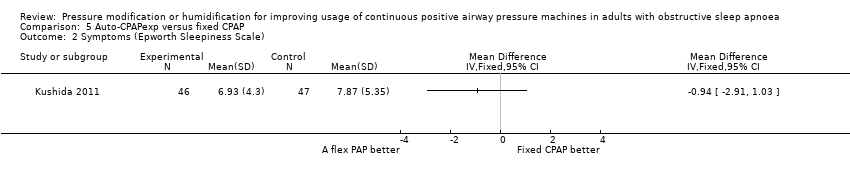
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale).
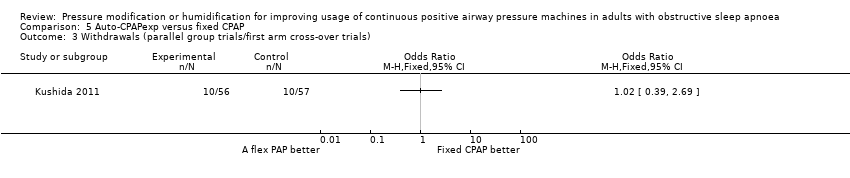
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 3 Withdrawals (parallel group trials/first arm cross‐over trials).

Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 4 Quality of life (Functional Outcomes of Sleep Questionnaire).

Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 5 Apnoea Hypopnoea Index (events/hr).
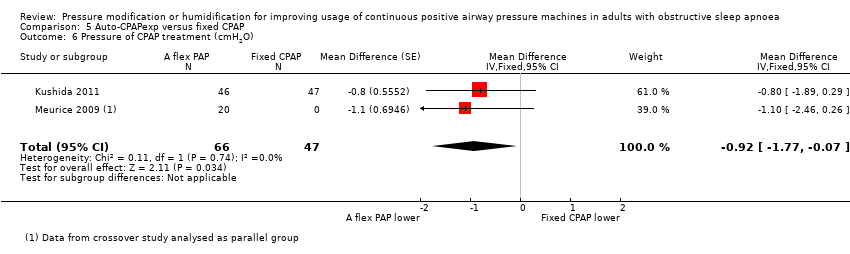
Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 6 Pressure of CPAP treatment (cmH2O).

Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 7 Systolic blood pressure.

Comparison 5 Auto‐CPAPexp versus fixed CPAP, Outcome 8 Diastolic blood pressure.
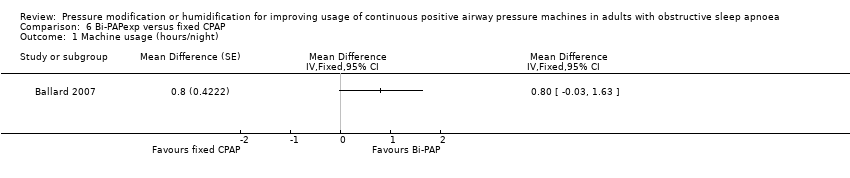
Comparison 6 Bi‐PAPexp versus fixed CPAP, Outcome 1 Machine usage (hours/night).
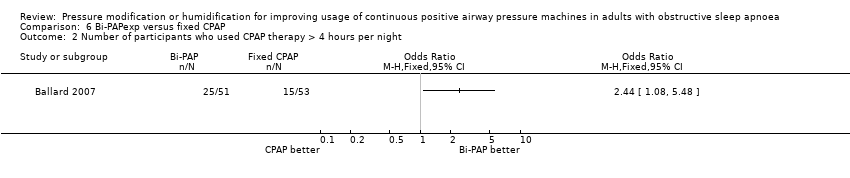
Comparison 6 Bi‐PAPexp versus fixed CPAP, Outcome 2 Number of participants who used CPAP therapy > 4 hours per night.
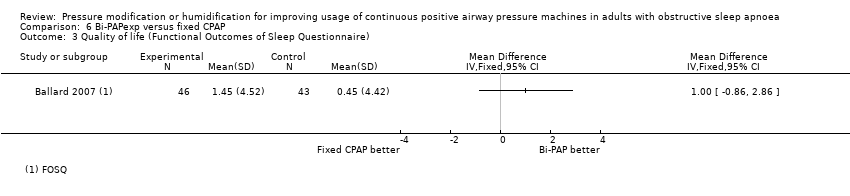
Comparison 6 Bi‐PAPexp versus fixed CPAP, Outcome 3 Quality of life (Functional Outcomes of Sleep Questionnaire).

Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 1 Machine usage (hours/night).

Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 2 Number of participants who used CPAP therapy > trialist defined threshold.
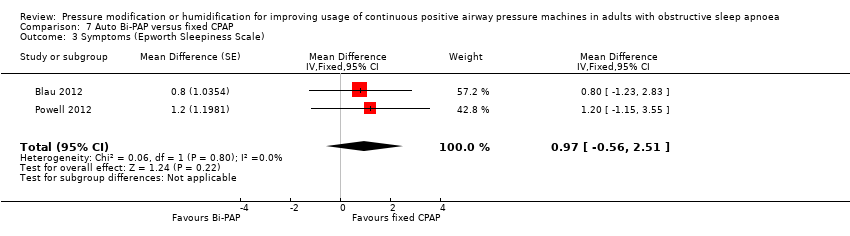
Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 3 Symptoms (Epworth Sleepiness Scale).

Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 4 Withdrawals.
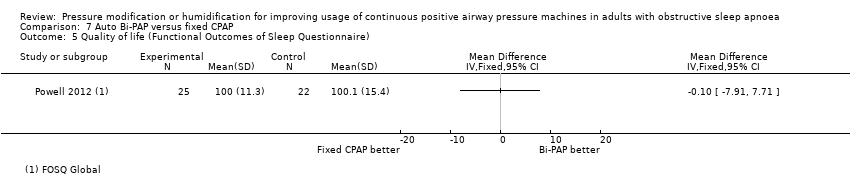
Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 5 Quality of life (Functional Outcomes of Sleep Questionnaire).

Comparison 7 Auto Bi‐PAP versus fixed CPAP, Outcome 6 Apnoea Hypopnoea Index (events/hr).
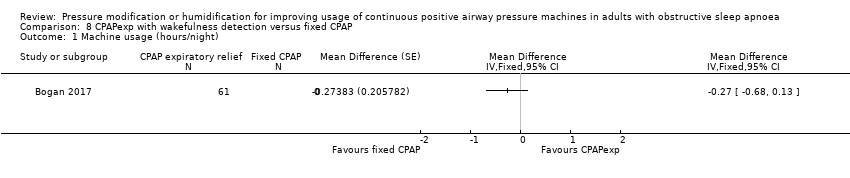
Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 1 Machine usage (hours/night).
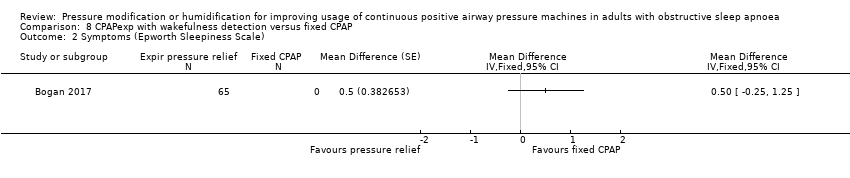
Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 2 Symptoms (Epworth Sleepiness Scale).

Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 3 Quality of life (Functional Outcomes of Sleep Questionnaire).

Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 4 Apnoea Hypopnoea Index (events/hr).
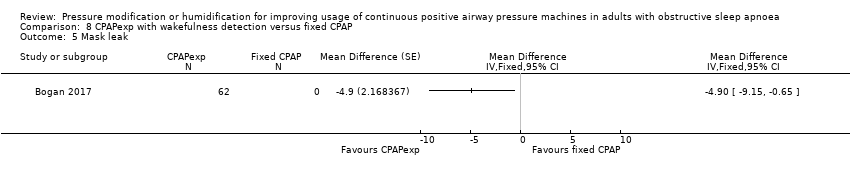
Comparison 8 CPAPexp with wakefulness detection versus fixed CPAP, Outcome 5 Mask leak.
| Auto‐CPAP compared to fixed CPAP for adults with a diagnosis of OSA | ||||||
| Patient or population: adults with a diagnosis of OSA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Average effect or risk with fixed CPAP | Average effect or risk with auto‐CPAP | |||||
| Average machine usage (hours per night) Median follow‐up: 6 weeks | Average nightly machine usage 5 hours per night across all CPAP arms | 0.21 hours per person per night longer The average effect is about 13 minutes longer per person per night (6 minutes to 20 minutes) | ‐ | 1452 | ⊕⊕⊕⊝ | |
| Number of participants using machine for 4 or more hours per night Follow‐up: range 3 to 16 weeks | Study population | OR 1.16 (0.75 to 1.81) | 346 (2 RCTs) | ⊕⊕⊝⊝ | ||
| 601 per 1000 | 636 per 1000 (530 to 732) | |||||
| Symptoms assessed with ESS from 0 to 24 Median follow‐up: 6 weeks | Average symptom scores ranged from 4.1 to 8.6 on the ESS | 0.44 ESS units lower | ‐ | 1285 | ⊕⊕⊕⊝ | MCID of between 2 to 3 has been proposed by Patel 2018. |
| Withdrawals (parallel group trials/first arm cross‐over trials) Median follow‐up: 6 weeks | Study population | OR 0.90 | 1275 | ⊕⊕⊕⊝ | ||
| 110 per 1000 | 100 per 1000 | |||||
| Quality of life assessed with FOSQ scale from 5 to 20 Follow‐up: range 4 to 104 weeks | The mean quality of life score was 5.58 on the FOSQ | 0.12 FOSQ units higher | ‐ | 352 | ⊕⊕⊝⊝ | MCID for FOSQ has not been confirmed; a change of 1 unit has been proposed as representing a possible meaningful change (Billings 2014). |
| AHI measured by number of events/hr Median follow‐up: 6 weeks | Average AHI ranged from 2 to 9 events per hour | 0.48 events per hour higher | ‐ | 1256 | ⊕⊕⊕⊕ | |
| Blood pressure (mmHg) Follow‐up 12 and 16 weeks | Systolic blood pressure | ‐ | 353 | ⊕⊕⊕⊝ | ||
| The mean systolic blood pressure was 133 mmHg | 1.87 mmHg higher | |||||
| Diastolic blood pressure | ‐ | 353 | ⊕⊕⊝⊝ | |||
| The mean diastolic blood pressure was 78 mmHg | 2.92 mmHg higher | |||||
| Adverse events (machine tolerability outcomes) Follow‐up: 4 to 36 weeks | Nine studies provided information on tolerability outcomes, but data could not be combined because they were measured and analysed inconsistently across the studies. Studies used different scales and data to report on four main outcome types: nasal blockage, dry mouth, tolerance of treatment pressure and mask leak. The direction and size of effect varied between the studies across the outcomes. | ‐ | 574 | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: Apnoea Hypopnoea Index; CI: confidence interval; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; MCID: minimal clinically important difference; mmHg: millimetres of mercury (used to measure pressure); OR: odds ratio; OSA: obstructive sleep apnoea; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias. All studies contributing data to machine usage were judged to be at unclear or high risk of bias for performance or attrition bias. | ||||||
| Bi‐PAP compared to fixed CPAP for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with a diagnosis of sleep apnoea | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with fixed CPAP | Risk with Bi‐PAP | |||||
| Machine usage (hours/night) Follow‐up: 4 to 52 weeks | Average nightly machine usage 5.5 hours per night across CPAP | 0.14 hours/night higher | ‐ | 268 | ⊕⊕⊝⊝ | |
| Machine usage assessed by number of participants using machine for 4 or more hours per night ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Symptoms assessed with ESS from 0 to 24 Follow‐up: 4 to 12 weeks | The mean symptoms ranged from 8 to 11 ESS units | 0.49 ESS units lower (1.46 lower to 0.48 higher) | ‐ | 226 | ⊕⊕⊝⊝ | MCID of between 2 to 3 has been proposed by Patel 2018. |
| Withdrawals (parallel group trials/first arm cross‐over trials) Follow‐up: 4 to 52 weeks | Study population | OR 0.55 | 261 | ⊕⊕⊝⊝ | ||
| 188 per 1000 | 113 per 1000 (57 to 213) | |||||
| Quality of life assessed with FOSQ: scale from 5 to 20 Follow‐up: 8 weeks | Mean change from baseline 5.1 units | 0.8 FOSQ units lower (6.08 lower to 4.48 units higher) | ‐ | 151 (1 RCT) | ⊕⊕⊝⊝ | MCID for FOSQ has not been confirmed; a change of 1 unit has been proposed as representing a possible meaningful change (Billings 2014). |
| AHI measured with number of events/hr Follow‐up: 4 to 8 weeks | The mean AHI was 6.6 events/hour | 1.36 events/hour lower | ‐ | 179 | ⊕⊕⊝⊝ | |
| Blood pressure ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events (machine tolerability outcomes) Follow‐up: 4 to 52 weeks | Five studies provided information on tolerability outcomes but data could not be combined because they were measured and analysed inconsistently across the studies. One study (N = 62) reported 5 withdrawals in CPAP group due to mask discomfort or device intolerance. 20 participants across both arms complained of nasal dryness. 1 trial (N = 151) reported similar rates of dry mouth (4.2% and 7.5%) and mask intolerance (11% and 10%) in Bi‐PAP and CPAP groups, respectively. Two small studies (N = 46) reported non‐specific adverse events. One reported that telephone contact did not identify the need for further interventions, and the other that there were similar rates of non‐specific adverse events. One study (N = 28) used a global treatment comfort score on a 0 to 00 VAS. There was insufficient evidence to determine whether Bi‐PAP improved comfort scores (69 versus fixed CPAP 60, P = 0.16). | ‐ | 239 | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias. Open‐label design from one study and insufficient detail available to assess blinding and allocation process in other studies. 2 Downgraded one level due to serious imprecision. Wide confidence interval including higher average usage with either device. 3 Downgraded one level due to serious imprecision. Wide confidence interval. 4 Downgraded one level due to serious risk of bias. Lack of detailed description of randomisation process and lack of blinding in two studies contributing data to the analysis. 5 Downgraded one level due to risk of bias. Single study at high risk of bias from lack of blinding. 6 Downgraded two levels due to very serious imprecision. Wide confidence interval and small sample size. 7 Downgraded due to very serious inconsistency. Variation in the measurement of tolerability across the studies meant that no data could be combined, | ||||||
| CPAP with expiratory pressure relief compared to fixed CPAP for improving usage of CPAP machines in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with a diagnosis of sleep apnoea | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with fixed CPAP | Risk with CPAP with expiratory pressure relief | |||||
| Machine usage Follow‐up: range 2 to 24 weeks | The mean machine usage was 5.1 hours/night | MD 0.14 hours/night higher | ‐ | 609 | ⊕⊕⊝⊝ | |
| Machine usage assessed by number of participants using machine for 4 or more hours per night ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Symptoms Follow‐up: range 4 to 24 weeks | The mean symptoms was 7 ESS | 0.17 ESS units higher | ‐ | 515 | ⊕⊕⊕⊝ | MCID of between 2 to 3 has been proposed by Patel 2018. |
| Withdrawals (parallel group trials/first arm cross‐over trials) Follow‐up: 12 weeks | Study population | OR 0.86 | 298 | ⊕⊕⊝⊝ | ||
| 201 per 1000 | 178 per 1000 | |||||
| Quality of life assessed with FOSQ: scale from 5 to 20 Follow‐up: 12 weeks | The mean quality of life was 18.7 FOSQ units | 0.4 FOSQ units lower | ‐ | 74 | ⊕⊕⊝⊝ | MCID for FOSQ has not been confirmed; a change of 1 unit has been proposed as representing a possible meaningful change (Billings 2014). |
| AHI measured by number of events/hr Follow‐up: range 6 to 12 weeks | The mean AHI was 5.3 events/hour | 0.24 events/hour higher | ‐ | 342 | ⊕⊕⊕⊕ | |
| Blood pressure ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events (machine tolerability outcomes) Follow‐up: range 4 to 24 weeks | No specific measures of nasal or oral symptoms were carried out in six studies providing information on tolerability outcomes. Four studies assessed treatment comfort scores but data could not be combined. None of the studies reported that there were differences between the different treatment modes. Different measures of mask leak were made by two studies with no differences reported in either 90th percentile leak or average leak. | ‐ | 577 (6 RCTs) | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias. Uncertainty over study design from trials published as abstracts and open‐label design in other studies. 2 Downgraded one level due to serious imprecision. Confidence interval includes no difference and increase of up to 20 minutes per person per night. 3 Downgraded two levels due to very serious imprecision. Wide confidence intervals and small sample size. 4 Downgraded two levels due to very serious imprecision. Single study with small sample size. 5 Downgraded one level due to serious imprecision. Small sample size. 6 Downgraded two levels due to very serious inconsistency. Different study designs and approaches taken to capturing tolerability outcomes. | ||||||
| Heated humidification + fixed CPAP compared to fixed CPAP alone for improving usage of CPAP machines in adults with obstructive sleep apnoea | ||||||
| Patient or population: adults with a diagnosis of sleep apnoea | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with fixed CPAP alone | Risk with heated humidification + fixed CPAP | |||||
| Machine usage Follow‐up: range 3 weeks to 12 weeks | The mean machine usage was 5 hours | MD 0.37 hours higher | ‐ | 277 | ⊕⊕⊝⊝ | |
| Machine usage assessed by number of participants using machine for 4 or more hours per night ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Symptoms Follow‐up: range 3 weeks to 12 weeks | The mean symptoms ranged from 4 to 9 ESS | MD 0.34 ESS lower | ‐ | 184 | ⊕⊕⊝⊝ | |
| Withdrawals (parallel group trials/first arm cross‐over trials) Follow‐up: median 12 weeks | Study population | OR 1.00 | 316 | ⊕⊝⊝⊝ | ||
| 159 per 1000 | 159 per 1000 | |||||
| Quality of life assessed with FOSQ: scale from 5 to 20 ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| AHI measured by number of events/hr Follow‐up: 4 weeks | The mean AHI (events/hr) was 4.2 events/hr | MD 0.3 events/hr higher | ‐ | 44 | ⊕⊕⊝⊝ | |
| Blood pressure ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events (machine tolerability outcomes) Follow‐up: mean 4 weeks | Study population | OR 0.32 | 147 | ⊕⊕⊝⊝ | This outcome was selected from different measures of nasal symptoms. | |
| 648 per 1000 | 371 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: Apnoea Hypopnoea Index; CI: confidence interval; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias. Open‐label study design in a number of studies and lack of detail regarding methods of allocation. 2 Downgraded one level due to serious imprecision. The sample size of 277 may be sufficient for effect size observed, but confirmatory studies would help to establish this more reliably. 3 Downgraded one level due to serious risk of bias, lack of blinding in one trial 4 Downgraded one level due to serious imprecision. Given baseline values and the change in both groups from using CPAP, the confidence interval includes a potentially meaningful difference of 1 unit on the ESS. 5 Downgraded two levels due to imprecision: small sample size and wide confidence intervals 6 Downgraded two levels due to very serious imprecision. Sample size across the studies is small. There were a number of different measures of nasal symptoms and the effect observed here may reflect multiplicity. | ||||||
| Device | Mechanism |
| Fixed CPAP | A single pressure is set. The device attempts to maintain this during inspiration and expiration and throughout the period of use. |
| Automatically adjusting‐CPAP (auto‐CPAP) | High and low pressure limits are set. The device adjusts its pressure within these limits to try to maintain a patent (clear and open) airway. The pressure does not vary between inspiration and expiration but will vary across the period of use. |
| Bilevel positive airway pressure (Bi‐PAP) | Two pressure levels are set. The device aims to co‐ordinate with patient breaths to deliver the higher pressure throughout inspiration and the lower pressure throughout expiration. If the patient has no respiratory effort some machines will produce timed or back up breaths. |
| CPAP with expiratory pressure relief (CPAPexp) | A basic pressure is set for the period of use. The device tracks patient effort and drops from the basic pressure by a preset amount at the start of expiration, increasing back to the basic pressure at the end of expiration. The pressure drops by 1 of 3 amounts selected according to patient comfort. |
| Heated humidification | The addition of heated humidification to the CPAP circuit increases the humidity and temperature of inspired air; this aims to reduce dryness of the upper respiratory tract and improve comfort. |
| Auto‐CPAPexp | This device combines the modalities of automatically adjusting continuous positive airway pressure and expiratory pressure relief. |
| Bi‐PAPexp | This device combines the modalities of bilevel positive airway pressure and expiratory pressure relief. |
| Auto bi‐PAP with pressure relief | This device reduces the pressure delivered at the end of inspiration and pressure during the early part of expiration, combined with an automatic titration modality. |
| CPAPexp with wakefulness detection | This CPAP device with expiratory pressure relief incorporates a sensor to detect when the user is rousing from sleep. |
| Intervention arm | No. of studies (participants) | Average study duration (weeks) | Average Age | % Male participants | Average BMI (kg/m2) | Average AHI (events/hr) | Average ESS |
| Auto‐CPAP | 36 (2135) | 11 | 52 | 71 | 34 | 42 | 13 |
| Bi‐PAP | 6 (325) | 16 | 55 | 76 | 35 | 50 | 13 |
| CPAPexp | 10 (658) | 8 | 53 | 60 | 34 | 54 | 13 |
| Humidification + fixed CPAP | 6 (359) | 7 | 52 | 74 | 32 | 42 | 12 |
| Auto‐CPAPexp | 2 (188) | 14 | 49 | 77 | 34 | 39 | 11 |
| Bi‐PAP (multimodality) | 3 (187) | 12 | 54 | 86 | 32 | 41 | 10 |
| CPAPexp with wakefulness detection | 1 (70) | 4 | 51 | 69 | 36 | ‐ | 11 |
| All interventions are compared with fixed CPAP. AHI: Apnoea Hypopnoea Index; BMI: body mass index; ESS: Epworth Sleepiness Scale. Averages calculated as mean of baseline means from studies contributing to each comparison. For cross‐over studies duration reflects amount of time treatment groups are exposed to one of the treatment arms, rather than the entire length of the study (conventionally double the duration of a single treatment arm exposure). | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 31 | 1452 | Mean Difference (Fixed, 95% CI) | 0.21 [0.11, 0.31] |
| 2 Machine usage (hours/night) (Pepin imputed) Show forest plot | 32 | 1774 | Mean Difference (Fixed, 95% CI) | 0.19 [0.10, 0.29] |
| 3 Number of participants who used CPAP therapy > 4 hours per night Show forest plot | 2 | 346 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.75, 1.81] |
| 4 Machine usage (on nights when CPAP used 'effectively') Show forest plot | 3 | Mean Difference (Fixed, 95% CI) | 0.42 [0.05, 0.78] | |
| 5 Machine usage (frequency of usage as % of days) Show forest plot | 9 | Mean Difference (Fixed, 95% CI) | 1.60 [‐0.83, 4.03] | |
| 6 Machine usage (% of nights of > 4 hours of use) ‐ cross‐over studies Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | 6.25 [‐0.05, 12.54] | |
| 7 Symptoms (Epworth Sleepiness Scale) Show forest plot | 25 | 1285 | Mean Difference (Fixed, 95% CI) | ‐0.44 [‐0.72, ‐0.16] |
| 8 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 13 | 1275 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.64, 1.27] |
| 9 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 3 | 352 | Mean Difference (Fixed, 95% CI) | 0.12 [‐0.21, 0.46] |
| 10 Quality of life (Sleep Apnoea Quality of Life Index) Show forest plot | 2 | 97 | Mean Difference (Fixed, 95% CI) | ‐0.14 [‐0.54, 0.27] |
| 11 Quality of life (SF‐36 questionnaire) Show forest plot | 8 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 11.1 Physical functioning | 3 | Mean Difference (Fixed, 95% CI) | 0.76 [‐3.50, 5.01] | |
| 11.2 Role physical | 2 | Mean Difference (Fixed, 95% CI) | ‐3.73 [‐13.46, 6.01] | |
| 11.3 Bodily pain | 2 | Mean Difference (Fixed, 95% CI) | 4.21 [‐4.23, 12.64] | |
| 11.4 General health | 2 | Mean Difference (Fixed, 95% CI) | 2.49 [‐4.99, 9.97] | |
| 11.5 Vitality | 6 | Mean Difference (Fixed, 95% CI) | 1.32 [‐1.25, 3.88] | |
| 11.6 Social functioning | 2 | Mean Difference (Fixed, 95% CI) | 3.31 [‐4.29, 10.92] | |
| 11.7 Role emotional | 3 | Mean Difference (Fixed, 95% CI) | 0.70 [‐4.19, 5.59] | |
| 11.8 Mental health | 3 | Mean Difference (Fixed, 95% CI) | 0.20 [‐1.88, 2.27] | |
| 12 Apnoea Hypopnoea Index (events/hr) Show forest plot | 26 | 1256 | Mean Difference (Fixed, 95% CI) | 0.48 [0.16, 0.80] |
| 13 Arousals (events/hr) Show forest plot | 4 | 136 | Mean Difference (Fixed, 95% CI) | ‐0.66 [‐2.90, 1.58] |
| 14 Pressure of CPAP treatment (cmH2O) Show forest plot | 24 | 1171 | Mean Difference (Fixed, 95% CI) | ‐1.01 [‐1.17, ‐0.84] |
| 15 Systolic blood pressure Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | 1.87 [‐1.08, 4.82] |
| 16 Diastolic blood pressure Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | 2.92 [1.06, 4.77] |
| 17 24‐hour mean BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [‐1.05, 2.22] |
| 18 24‐hour systolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐2.21, 1.91] |
| 19 24‐hour diastolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.65, 2.44] |
| 20 Diurnal mean BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐1.05, 2.32] |
| 21 Diurnal systolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐2.44, 1.74] |
| 22 Diurnal diastolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [‐0.74, 2.55] |
| 23 Nocturnal mean BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐1.29, 2.15] |
| 24 Nocturnal systolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [‐1.81, 3.57] |
| 25 Nocturnal diastolic BP Show forest plot | 2 | 530 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.81, 2.40] |
| 26 Tolerability outcomes Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 26.1 Intolerable treatment pressure | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Mask leak | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.3 Dry mouth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.4 Stuffy nose | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 Patient preference (auto‐CPAP/not auto‐CPAP) Show forest plot | 14 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 4 | 268 | Mean Difference (Fixed, 95% CI) | 0.14 [‐0.17, 0.45] |
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 4 | 226 | Mean Difference (Fixed, 95% CI) | ‐0.49 [‐1.46, 0.48] |
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 3 | 261 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.17] |
| 4 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life (Sleep Apnoea Quality of Life Index) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Quality of life (SF‐36 questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Physical health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Mental heath | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Apnoea Hypopnoea Index (events/hr) Show forest plot | 2 | 179 | Mean Difference (Fixed, 95% CI) | 1.36 [‐6.92, 9.63] |
| 8 Patient preference ‐ BiPAP/no preference or CPAP Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Tolerability outcomes Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Dry mouth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Mask intolerance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Treatment comfort score Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 9 | 609 | Mean Difference (Fixed, 95% CI) | 0.14 [‐0.07, 0.35] |
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 6 | 515 | Mean Difference (Fixed, 95% CI) | 0.17 [‐0.26, 0.60] |
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 2 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.55] |
| 4 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life (SF‐36 questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Physical functioning | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [‐3.05, 15.45] |
| 5.2 Role physical score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐9.5 [‐25.38, 6.38] |
| 5.3 Bodily pain score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 7.20 [‐3.69, 18.09] |
| 5.4 General health score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐8.05, 10.05] |
| 5.5 Vitality | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐11.38, 6.38] |
| 5.6 Social functioning score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐8.30 [‐17.87, 1.27] |
| 5.7 Role emotional score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐7.30 [‐21.83, 7.23] |
| 5.8 Mental health score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐4.86, 8.86] |
| 6 Apnoea Hypopnoea Index (events/hr) Show forest plot | 5 | 342 | Mean Difference (Fixed, 95% CI) | 0.24 [‐0.49, 0.96] |
| 7 Pressure of CPAP treatment (cmH2O) Show forest plot | 2 | 241 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.63, 0.52] |
| 8 Treatment satisfaction score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Treatment comfort score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Treatment interface score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Preference Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 6 | 277 | Mean Difference (Fixed, 95% CI) | 0.37 [0.10, 0.64] |
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 4 | 184 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.93, 0.26] |
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 3 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.45, 2.24] |
| 4 Apnoea Hypopnoea Index (events/hr) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life (SF‐36 questionnaire) Show forest plot | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐6.97, 7.18] |
| 6 Nasal symptoms (parallel group trials) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Runny nose | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.09, 1.15] |
| 6.2 Congested or blocked nose | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.51] |
| 7 Nasal symptoms (parallel group trials) Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Dry nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.78, 0.01] |
| 7.2 Runny nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.69, 0.09] |
| 7.3 Blocked nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.78, 0.01] |
| 7.4 Bleeding nose | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.99, 0.10] |
| 8 Preference Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 2 | 113 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.60, 0.67] |
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Withdrawals (parallel group trials/first arm cross‐over trials) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Apnoea Hypopnoea Index (events/hr) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Pressure of CPAP treatment (cmH2O) Show forest plot | 2 | 113 | Mean Difference (Fixed, 95% CI) | ‐0.92 [‐1.77, ‐0.07] |
| 7 Systolic blood pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Diastolic blood pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Number of participants who used CPAP therapy > 4 hours per night Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | ‐0.00 [‐0.70, 0.70] | |
| 2 Number of participants who used CPAP therapy > trialist defined threshold Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptoms (Epworth Sleepiness Scale) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | 0.97 [‐0.56, 2.51] | |
| 4 Withdrawals Show forest plot | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.33, 17.43] |
| 5 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Apnoea Hypopnoea Index (events/hr) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Symptoms (Epworth Sleepiness Scale) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Quality of life (Functional Outcomes of Sleep Questionnaire) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Apnoea Hypopnoea Index (events/hr) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Mask leak Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |

