Elektromagnetska polja za liječenje osteoartritisa
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Inclusion criteria: diagnosis of osteoarthritis of the knee was in accordance with the American College of Rheumatology modified clinical classification system. Plain radiographs available for 64 participants confirmed the diagnosis. Persistent and stable pain (defined as not getting worse or better overall despite short‐term fluctuations) for a minimum of 3 months prior to study entry was confirmed in all participants by telephone interview. Intervention group number: 34 Control/sham group number: 36 | |
| Interventions | Active group: a commercially available TENS stimulator (Metron Digi‐10s) was modified by a biomedical engineer to deliver pulsed electrical stimulation current parameters as follows: pulsed, asymmetrically biphasic, exponentially decreasing waveform with a frequency of 100 Hz and pulse width of 4 ms The placebo device was identical in appearance and method of use; however, the current flow was programmed to turn off after 3 minutes. Since this was a sub‐sensory treatment, this change was not detectable by participants | |
| Outcomes | Primary outcomes: Secondary outcomes: | |
| Notes | Supported by an Arthritis Australia and State & Territory Affiliate Grant and a Physiotherapy Research Foundation Research Seeding grant, and by a Curtin University School of Physiotherapy Early Career Researcher grant to Dr. Fary. Dr. Fary was the recipient of an Australian Government Postgraduate PhD scholarship and a Curtin University School of Physiotherapy Movement Through Life Top‐Up scholarship | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using computer‐generated randomization in blocks of 6." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation, stratified by sex, age (60 years, 60–75 years, and 75 years), and baseline VAS pain scores (25–40 mm, 41–60 mm, and 61–100 mm), was performed independently by an administrator, not otherwise involved in the study" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "This process ensured that all study investigators and participants remained blinded to allocation until analysis was complete." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "This process ensured that all study investigators and participants remained blinded to allocation until analysis was complete." |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | 4 weeks: 1/34 missing from active group (failed to return week 4 data) 16 weeks: 1/34 missing from active group (device uncomfortable to wear) but participant who completed week 4 data returned; 1/36 missing from placebo group (protocol too onerous) 26 weeks: 1/ 34 missing from active group (failed to attend final appointment) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Inclusion criteria: age 18 years or greater, the intellectual ability to understand and sign an informed consent and complete the study questionnaire, and willingness to maintain stable doses of analgesics and NSAIDs for 1 month prior to study entry and during the 3‐month double‐blind period Intervention group number: 39 Control/sham group number: 19 | |
| Interventions | Active group: pulsed electrical stimulation (BioniCare Medical Technologies, Inc., Sparks, Maryland) | |
| Outcomes | Primary outcomes: | |
| Notes | Supported by a grant from BioniCare Medical Technologies, Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each placebo and active device was assigned a unique number." |
| Allocation concealment (selection bias) | Low risk | Quote: "A master random number chart was generated and maintained by an independent observer who had no interaction with the sponsors pendent observer who had no interaction with the sponsors"; "The number coincided with active or placebo units according to the dictates of the random number table." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "A master random number chart was generated and maintained by an independent observer who had no interaction with the sponsors pendent observer who had no interaction with the sponsors." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "A master random number chart was generated and maintained by an independent observer who had no interaction with the sponsors pendent observer who had no interaction with the sponsors"; "The clinical investigators had no influence on the assignment of any specific patient to an active or placebo device. The blind has never been revealed to any of the authors except the statistician." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "A master random number chart was generated and maintained by an independent observer who had no interaction with the sponsors pendent observer who had no interaction with the sponsors"; "The clinical investigators had no influence on the assignment of any specific patient to an active or placebo device. The blind has never been revealed to any of the authors except the statistician." |
| Incomplete outcome data (attrition bias) | Low risk | 1/39 missing from active group (discontinued study participation before the second month follow‐up visit); 1/19 missing from placebo group (discontinued study participation before the second month follow‐up visit) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Methods | Double‐blind, placebo‐controlled, randomised pilot study | |
| Participants | Inclusion criteria: patient selection required that participants had knee pain for at least 3 months with an imaging study that confirmed articular cartilage loss, an initial VAS score ≥ 4, and at least 2 hours of daily standing activity in a physical occupation Exclusion criteria: patients with rheumatoid arthritis, gout and pregnancy; cortisone injections, surgery or an effective visco supplementation series within the past 6 months; implanted electronic devices. Patients on disability or with third party claims were excluded Male/female: 5/10, 5/14 Intervention group number: 15 Control/sham group number: 19 | |
| Interventions | Active group: a pulsed electromagnetic field signal consisting of a 7 ms burst of 6.8 MHz sinusoidal waves repeating at 1 burst/s delivering a peak induced electrical field of 34 ± 8 V/m in the knee from the portable battery operated device (Palermo, Ivivi Health Sciences, LLC, San Francisco, CA) Sham group: sham devices were activated with a switch, same as active devices, and both sham and active units had blinking indicator lights. The pulsed electromagnetic field signal from these devices did not produce heat or cause any other sensation in tissue Once manually activated, treatment was automatically applied for 15 minutes. Device was used for 15 minutes twice daily for 42 days | |
| Outcomes | Primary outcomes: pain: self report maximum daily VAS pain scores on an unmarked horizontal 10 cm line (0 is no pain and 10 is worst possible pain) | |
| Notes | Partially supported by the Department of Orthopaedic Surgery, Henry Ford Hospital, Detroit Michigan, and Ivivi Health Sciences, LLC, San Francisco, CA, who manufactured the pulsed electromagnetic field devices utilised in this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by the blinded assignment of devices according to their serial numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "Device randomization was performed by the manufacturer (Ivivi Health Sciences, LLC) and all devices with the randomization code were sent to the Epidemiology Dept at Henry Ford Hospital, from which assignment to patients was controlled. General unblinding occurred after all data were collected." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "PEMF signal employed in this study is at least 1,000‐fold below the ambient magnetic field and cannot be detected using standard Gauss meters. Therefore, only measurements with specialized laboratory equipment, not readily available to the patient or health care practitioner, could determine whether a device was active. General unblinding occurred after all data were collected." Comment: probably done. |
| Blinding (performance bias and detection bias) | Low risk | Quote: ""PEMF signal employed in this study is at least 1,000‐fold below the ambient magnetic field and cannot be detected using standard Gauss meters. Therefore, only measurements with specialized laboratory equipment, not readily available to the patient or health care practitioner, could determine whether a device was active. General unblinding occurred after all data were collected." Comment: probably done. |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Day 42: 3/15 missing from active group (lack of perceived benefit as the reason, confirmed by VAS scores); 7/19 missing from sham group (lack of perceived benefit as the reason, confirmed by VAS scores) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Methods | Randomised, double‐blind, controlled trial | |
| Participants | Inclusion criteria: patients with symptomatic osteoarthritis of the knee. All patients had radiographic signs of osteoarthritis according to the classification of Kellgren, grade 2 or 3 Intervention group number: 15 Control/sham group number: 17 | |
| Interventions | Treatment group: PMF | |
| Outcomes | 1) Primary outcomes: change between baseline and the end of treatment in the 240‐point WOMAC Osteoarthritis Index: pain score (maximum score, 50), stiffness score (maximum score, 20) and physical function score (maximum score, 170) | |
| Notes | The study was supported by a grant from the "Institut zurwissenschaftlichen Evaluierung alternativer Heilmethoden" (Institute for Research in Complementary Medicine), Rokitan‐skygasse 40/8, 1170 Vienna, Austria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study coordinator assigned the devices on a random basis to patient according to a list created by randomization function (Matlab, Mathworks Incor‐poration, Natick, Massachusetts)." |
| Allocation concealment (selection bias) | Low risk | Quote: "All devices were numbered; only the study coordinator was aware of the code." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The persons involved in patient administration, the evaluator, as well as the person instructing the patients in the use of the device and giving technical support if required, were blinded. At the end of the study the code was broken." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The persons involved in patient administration, the evaluator, as well as the person instructing the patients in the use of the device and giving technical support if required, were blinded. At the end of the study the code was broken." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The persons involved in patient administration, the evaluator, as well as the person instructing the patients in the use of the device and giving technical support if required, were blinded. At the end of the study the code was broken." |
| Incomplete outcome data (attrition bias) | Low risk | 3/18 missing from treatment group (1 did not complete treatment, 2 further patients had to be excluded from the statistical analysis as they did not use the device properly); 1/18 missing from the sham group (withdrew because of excessive pain) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Inclusion criteria: patients had radiographic signs and symptoms of osteoarthritis as judged by the criteria of the ACR Intervention group number: 39 Control/sham group number: 36 (6 patients failed to attend after the screening visit and were excluded from the analysis) | |
| Interventions | Active group: pulsed electromagnetic fields (Snowden Healthcare Ltd., Nottingham, UK) | |
| Outcomes | 1) Primary outcomes: reduction in overall pain assessed on a 4‐point Likert scale ranging from none to severe | |
| Notes | This study was supported by an educational grant from Snowden Healthcare | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients fulfilling the study criteria were randomised on study entry to receive active pulsed electromagnetic fields treatment with the Medicur magnetic devices or placebo using a 12 * 12 randomisation table." |
| Allocation concealment (selection bias) | Low risk | Quote: "Neither the patients nor the medical assessor were aware of the treatment group". "The code numbers were not broken until all patients had complete the study." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the patients nor the medical assessor were aware of the treatment group". "The code numbers were not broken until all patients had complete the study." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the patients nor the medical assessor were aware of the treatment group". "The code numbers were not broken until all patients had complete the study." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the patients nor the medical assessor were aware of the treatment group". "The code numbers were not broken until all patients had complete the study." |
| Incomplete outcome data (attrition bias) | High risk | 10/39 missing from active group (5 attended only for screening and were excluded from the analysis, 5 withdrew before completing treatment); 6/36 missing from sham group (1 attended only for screening and was excluded from the analysis, 5 withdrew before completing treatment) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Inclusion criteria: patients older than 45 years with painful knee osteoarthritis of the femorotibial compartment fulfilling the combined clinical and radiological criteria of the American College of Rheumatology were included Intervention group number: 45 Control/sham group number: 45 | |
| Interventions | Active group: pulsed electromagnetic fields (Biofields Aps, Copenhagen, Denmark) | |
| Outcomes | 1) Primary outcomes: WOMAC osteoarthritis index: a questionnaire addressing the severity of joint pain (5 questions), stiffness (2 questions) and limitation of physical function (17 questions) | |
| Notes | Economic support from IMK Almene Fond and from Københavns Amts Erhvervskontor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a 1:1 randomized, controlled, double‐blind add‐on study." No further information provided |
| Allocation concealment (selection bias) | Low risk | Quote: "This was a 1:1 randomized, controlled, double‐blind add‐on study." "Blinding was maintained until the final database was cleaned and locked." Comment: probably done |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "This was a 1:1 randomized, controlled, double‐blind add‐on study." No further information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "This was a 1:1 randomized, controlled, double‐blind add‐on study." No further information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "This was a 1:1 randomized, controlled, double‐blind add‐on study." No further information provided |
| Incomplete outcome data (attrition bias) | Low risk | 3/45 missing from active group; 4/45 missing from sham group |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Methods | Randomised, placebo‐controlled trial | |
| Participants | Inclusion criteria: all patients met the criteria for the diagnosis of osteoarthritis published by Altman. Patients with osteoarthritis, primarily of the knee with symptoms for greater than 1 year, who had not started any new treatment within 1 month of study Intervention group number: 15 Control/sham group number: 12 | |
| Interventions | Active group: pulsed electromagnetic fields (MFG) | |
| Outcomes | Severity of pain, difficulty performing activities of daily living (ADL), pain performing ADL, worst discomfort in previous week, pain on joint motion by MD exam, joint tenderness by MD exam, assessment of improvement by MDs | |
| Notes | Funded by Bio‐Magnetic Therapy Systems, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized to receive active pulsed electromagnetic fields or placebo using a table of 1000 random digits." |
| Allocation concealment (selection bias) | Low risk | Quote: "A code master record sheet was kept by an office administrator." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Both the patients and the examining physician remained blinded as to whether active pulsed electromagnetic fields or placebo was given." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Both the patients and the examining physician remained blinded as to whether active pulsed electromagnetic fields or placebo was given." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "A code master record sheet was kept by an office administrator." |
| Incomplete outcome data (attrition bias) | High risk | 5/15 missing from active group; 2/12 missing from sham group |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Methods | Randomised, double‐blind, multicentre controlled trial | |
| Participants | Inclusion criteria: patients with osteoarthritis of the knee who met the criteria published by Altman. 11 patients with cervical spine pain were admitted to the study if radiographs showed evidence of disk space narrowing with osteocyte formation and/or subchondral sclerosis in 1 or more locations; osteophyte formation and subchondral sclerosis of facet joints were also accepted as evidence of osteoarthritis, with local symptoms such as pain and stiffness of at least 1 year duration. Patients were instructed not to change their basic therapeutic regimen, including drugs and physical therapy, during the period of treatment and observation Number of patients who finished this study: 86 knee osteoarthritis and 81 cervical osteoarthritis Intervention group number: 86 Control/sham group number: 86 | |
| Interventions | Active group: pulsed electromagnetic fields (M‐T System) | |
| Outcomes | 1) The physician recorded the degree of pain on motion (none, slight, moderate or severe) and tenderness using the Ritchie Scale as described for patients with osteoarthritis by Doyle. For both of these observations the score for 'none' was assigned as 0, 'slight' as 1, 'moderate' as 2 and 'severe' as 3 | |
| Notes | Supported by Bio‐Magnetic Therapy Systems, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One research associate kept the list of 1000 random numbers, divided into pairs, the higher of which was to receive treatment and the lower placebo." |
| Allocation concealment (selection bias) | Low risk | Quote: "Separate lists of random numbers were kept for the patients." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither patient in the trial nor any patient in the center, nor any other staff could tell which patients were receiving active treatment." |
| Blinding (performance bias and detection bias) | High risk | Quote: "Only the therapy technician at the treatment center was informed, by means of a code." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither patient in the trial nor any patient in the center, nor any other staff could tell which patients were receiving active treatment." "Only the therapy technician at the treatment center was informed, by means of a code." |
| Incomplete outcome data (attrition bias) | Low risk | Double‐blind trial of knee osteoarthritis: 5/42 missing from active group; 5/44 missing from sham group |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Methods | Randomised, multicentre,double‐blind, placebo‐controlled trial | |
| Participants | Patients older than 20 years with painful knee osteoarthritis of the femorotibial compartment fulfilling the combined clinical Intervention group number: 41 Control/sham group number: 37 | |
| Interventions | Active group: pulsed electrical stimulation | |
| Outcomes | 1) Primary efficacy outcomes: physician's global evaluation, patient's evaluation of pain, patient's evaluation of function of the treated knee | |
| Notes | This study was supported by Murray Electronics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to receive an active device or an identical appearing placebo device." Comment: no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized to receive an active device or an identical appearing placebo device." Comment: no further information provided |
| Blinding (performance bias and detection bias) | Low risk | Quote: "...in a multicenter, prospective, randomized, placebo controlled, double blind study" Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "A nurse coordinator instructed patients in the proper use of the device." |
| Blinding (performance bias and detection bias) | Unclear risk | No further information provided |
| Incomplete outcome data (attrition bias) | Low risk | 3/41 missing from active group; 4/37 missing from sham group |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
ACR: American College of Rheumatology
DC: direct current
MD: medical doctor
NSAID: non‐steroidal anti‐inflammatory drug
PMF: pulsed magnetic field
TENS: transcutaneous electrical nerve stimulation
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The duration of treatment was only 5 days (once daily). Efficacy requires at least 4 weeks' treatment duration (suggestion of several physiotherapists) | |
| All patients participated in the therapy programmes for 3 weeks (15 sessions). Pulsed electromagnetic field therapy for 30 minutes per day and combined hot‐pack and transcutaneous electrical nerve stimulation (TENS) | |
| Treatments were given for 30 minutes for 15 days. The treatment duration did not match the inclusion criteria of this review | |
| Primary report, not a RCT | |
| Randomisation was not mentioned. Li SS sent an email to Dr. Pelka, the corresponding author of the paper, on 10 December 2007, with 2 questions as follows: 1. Was the randomisation of the allocation procedure adequate? 2. Was the allocation concealment properly done? The author did not reply, so, we considered this a cohort study rather than a RCT | |
| The second version of Fischer 2005 | |
| The aim of this article was to assess the effect of static magnetic fields for chronic knee pain but not osteoarthritis | |
| Prospective, cohort study and not a RCT | |
| Treatment duration: 48 minutes per treatment session 8 times in 2 weeks. This time frame may be too short to assess safety and efficacy based on biological plausibility | |
| A therapy session lasted for 35 minutes and 15 sessions were performed during 3 weeks (5 sessions/week) | |
| Randomisation was mentioned but not described in detail. Li SS telephone interviewed the original author, Wencai Liu, on 3 December 2007. The author could not remember how the randomisation was designed. Therefore we considered the study not to be a RCT | |
| Both the pulsed electromagnetic fields and sham PEMF treatments being evaluated were 55 minutes/session, 5 sessions per week for 2 weeks. The treatment duration did not match the inclusion criteria of this review | |
| The treatment period was only 10 days. The treatment duration did not match the inclusion criteria of this review | |
| The study included patients with cervical spondylosis and shoulder periarthritis without separately reported results and we could not extract data on cervical osteoarthritis | |
| Treatment duration: twice a day for 30 minutes of 3 weeks duration. The duration did not match the inclusion criteria of this review | |
| Both the pulsed electromagnetic field group (n = 16) and the control group (n = 16) participated in therapy, 30 minutes per session twice a day for 3 weeks. The treatment duration did not match the inclusion criteria of this review |
PEMF: pulsed electromagnetic field therapy
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 6 | 434 | Mean Difference (IV, Random, 95% CI) | 15.10 [9.08, 21.13] |
| Analysis 1.1 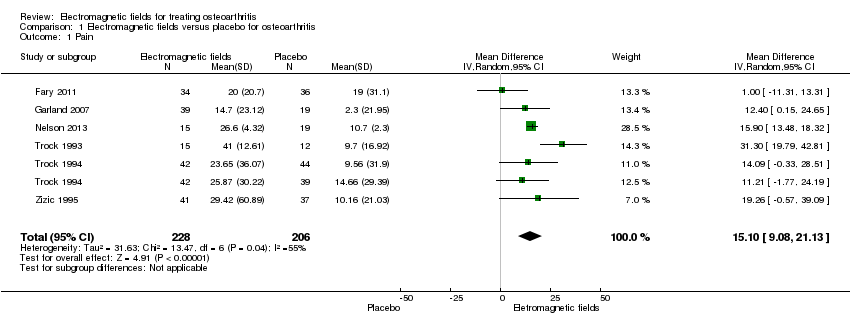 Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 1 Pain. | ||||
| 2 Physical function Show forest plot | 3 | 197 | Mean Difference (IV, Random, 95% CI) | 4.55 [‐2.23, 11.32] |
| Analysis 1.2 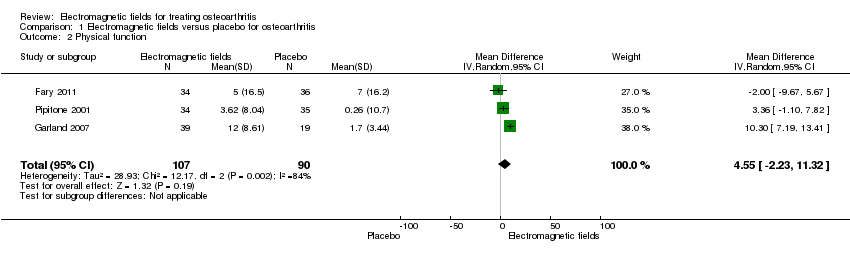 Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 2 Physical function. | ||||
| 3 Quality of life Show forest plot | 2 | 139 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.36, 0.54] |
| Analysis 1.3  Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 3 Quality of life. | ||||
| 4 Number of patients experiencing any adverse event Show forest plot | 4 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.72, 1.92] |
| Analysis 1.4  Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 4 Number of patients experiencing any adverse event. | ||||
| 5 Number of patients who withdrew because of adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 5 Number of patients who withdrew because of adverse events. | ||||
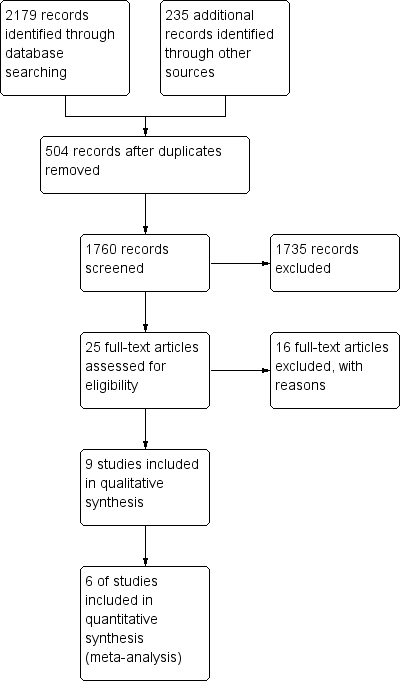
Study flow diagram.
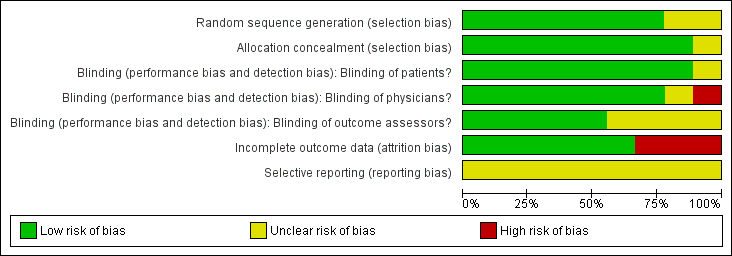
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
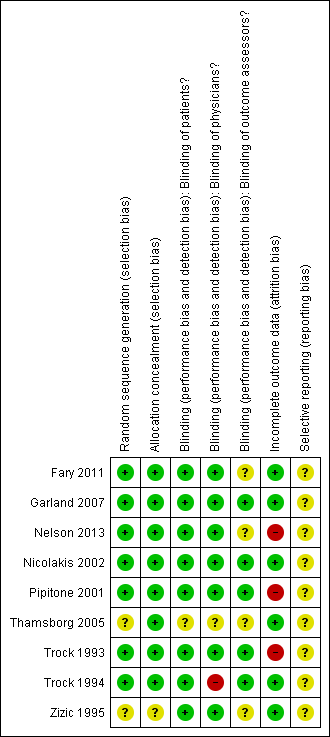
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
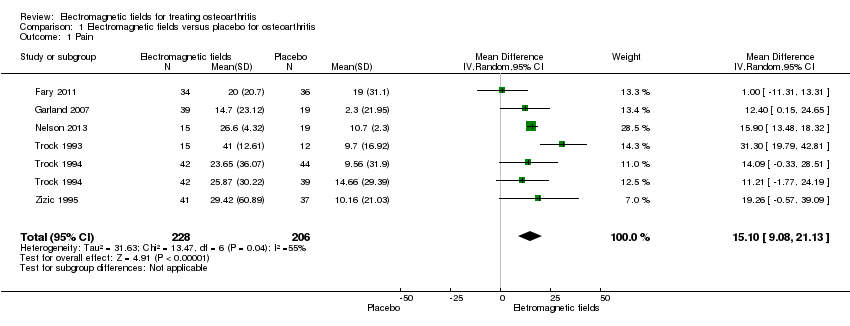
Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 1 Pain.

Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 2 Physical function.
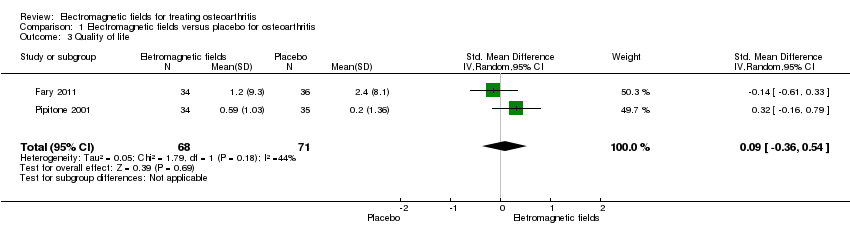
Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 3 Quality of life.

Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 4 Number of patients experiencing any adverse event.
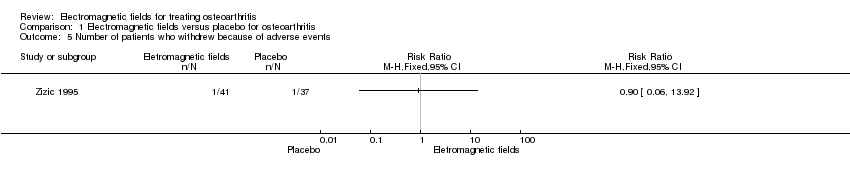
Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 5 Number of patients who withdrew because of adverse events.
| Electromagnetic field treatment compared to placebo for the treatment of osteoarthritis | ||||||
| Patient or population: patients with osteoarthritis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Electromagnetic field treatment | |||||
| Pain Scale from: 0 to 100 (Higher scores mean worse pain) | The mean change in pain in the control groups was 10.7 | The mean change in pain in the intervention groups was | 434 | ⊕⊕⊕⊝ | MD 15.10 (95% CI 9.08 to 21.13) Absolute risk difference: 15% (95% CI 9.08% to 21.13%) Relative per cent change: 21.03% (95% CI 12.65% to 29.43%) NNT: 2 (95% CI 1 to 6) | |
| Physical function WOMAC function Scale from: 0 to 100 (Higher scores mean more severe limitation) | The mean change in physical function in the control groups was | The mean change in physical function in the intervention groups was | 197 | ⊕⊕⊝⊝ | MD 4.55 (95% CI ‐2.23 to 11.32) Absolute risk difference: 4.55% (95% CI ‐2.23% to 11.32%) Relative per cent change: 268% (95% CI ‐131% to 666%) NNT: not statistically significant | |
| Quality of life SF‐36 item Scale from: 0 to 100 (Lower scores mean worse quality) Follow‐up: mean 16 weeks | The mean change in quality of life in the control groups was | The mean change in quality of life in the intervention groups was | 145 | ⊕⊕⊕⊝ | SMD 0.09 (95% CI ‐0.36 to 0.54) Absolute risk difference: 1% (95% CI ‐2.92% to 4.37%) Relative per cent change: 30.38% (95% CI ‐121.5% to 182.25%) NNT: not statistically significant | |
| Radiographic progression Bone scintigraphic examinations Follow‐up: mean 2.5 months | See comment | See comment | Not estimable | 78 | See comment | No related data were available |
| Number of patients experiencing any adverse event Follow‐up: mean 1 month | 167 per 1000 | 195 per 1000 | RR 1.17 | 288 | ⊕⊕⊕⊝ | Absolute risk difference: 3% (95% CI ‐6% to 12%) Relative per cent change: 17% (95% CI ‐28% to 92%) NNT: not statistically significant |
| Number of patients who withdrew because of adverse events Follow‐up: mean 6 months | 27 per 1000 | 24 per 1000 (2 to 376) | RR 0.90 (0.06 to 13.92) | 78 | ⊕⊕⊝⊝ | Only 1 study: 1 participant withdrew from each group because of adverse skin reactions unrelated to the therapy |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded for moderate heterogeneity (I2 = 55%); unclear risk for random sequence generation (Zizic 1995), allocation concealment (Zizic 1995), blinding of outcome assessors (Fary 2011; Nelson 2013; Zizic 1995), selective reporting (all six studies) and high risk for incomplete outcome data (Zizic 1995). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 6 | 434 | Mean Difference (IV, Random, 95% CI) | 15.10 [9.08, 21.13] |
| 2 Physical function Show forest plot | 3 | 197 | Mean Difference (IV, Random, 95% CI) | 4.55 [‐2.23, 11.32] |
| 3 Quality of life Show forest plot | 2 | 139 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.36, 0.54] |
| 4 Number of patients experiencing any adverse event Show forest plot | 4 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.72, 1.92] |
| 5 Number of patients who withdrew because of adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

