Medicina herbaria china para la esquizofrenia
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2). | |
| Interventions | 1. Xingshen + antipsychotic: dose Xingshen 30ml/bid + antipsychotics (chlorpromazine n=16, clozapine n=38, sulpiride n=6) dosage ˜300‐700 mg/day. N=60. 2. Antipsychotics: dose chlorpromazine n=17, clozapine n=36, sulpiride n=7, dosage ˜250‐800 mg/day (no further details). N=60. Herbal formula ‐ Xingshen: Grams not specified. | |
| Outcomes | Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia (ICD 10 and CCMD‐2‐R). | |
| Interventions | 1. Ginkgo biloba (EGb761) + maintenance antispychotics: dose EGb761, 120 mg/tds, antipsychotics. N=315. 2. Placebo + Antipsychotics: dose maintenance antipsychotics. N=230. Maintenance antipsychotics included clozapine, chlorpromazine, sulpiride, perphenazine and haloperidol. Doses and frequency not reported. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2). | |
| Interventions | 1. Ginkgo biloba (EGb761) + antipsychotics: dose range EGb761, 120 mg/day first week, then 240 mg/day + continuation of antipsychotics (dosage not reported). N=21. 2. Placebo + Antipsychotics: dose (no further details). N=19. | |
| Outcomes | Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: 'divided into groups randomly'. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Dang gui cheng qi tang: dose ˜ mean 50 ml/bid, max. 200 ml/day. N=45. 2. Chlorpromazine: any dose as required. N=45. Herbal formula ‐ Dang gui cheng qi tang: Additional herbs added as required for blood stagnation: Additional herbs added as required for hallucinations, restlessness and insomnia: grams not specified. | |
| Outcomes | Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2) . | |
| Interventions | 1. Herbal medicine: Dang gui cheng qi tang (those with yang syndrome), 100 ml 2‐3/day + antipsychotics (unclear which type or dosage, n=32); Xiao yao san (those with yin syndrome) 100ml 2‐3/day + antipsychotics (unclear which type or dosage, n=34). N=66. 2. Antipsychotics: dose and type not reported. N=57. Herbal formula ‐Dang gui cheng qi tang: Radix Angelica sinensis (Dang gui) 15g Herbal formula ‐ Xiao Yao san: Radix Angelica sinensis (Dang gui) 15g | |
| Outcomes | Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia (DSM III R). | |
| Interventions | 1. Ginkgo biloba (EGb761) + antipsychotics: dose EGb761, 360 mg/day, + haloperidol 0.25 mg/kg/day. N=56. 2. Haloperidol: dose 0.25 mg/kg/day + placebo. N=53. | |
| Outcomes | Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2). | |
| Interventions | 1. Hirudo seu Whitmania (Shui zhi) and Rhizoma Rheum palmatum (Da huang) (doses not specified) + chlorpromazine =/<300 mg/day). N=32. 2. Chlorpromazine: dose =/<400 mg/day. N=35. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
DSM ‐ Diagnostic and Statistical Manual
ICD 10 ‐ International Classification of Diseases
CCMD ‐2‐R ‐ Chinese Classification of Mental Disorders Second Edition Revised
Global state:
CGI ‐ Clinical Global Impression
Mental state:
BPRS ‐ Brief Psychiatric Rating Scale
SANS ‐ Scale for the Assessment of Negative Symptoms
SAPS ‐ Scale for the Assessment of Positive Symptoms
Adverse effects:
TESS ‐Treatment Emergent Symptom Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not reported, double blind (wrote to authors, no reply). | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Not improved/worse ‐ short term Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.24, 2.86] |
| Analysis 1.1  Comparison 1 HERBAL MEDICINE versus ANTIPSYCHOTICS, Outcome 1 Global state: Not improved/worse ‐ short term. | ||||
| 2 Behaviour: Leaving the study early ‐ short term Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.2  Comparison 1 HERBAL MEDICINE versus ANTIPSYCHOTICS, Outcome 2 Behaviour: Leaving the study early ‐ short term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Global state: 1. Not improved/worse ‐ medium term Show forest plot | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.61] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 1 Global state: 1. Not improved/worse ‐ medium term. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Global state: 2. Average score ‐ short term (CGI endpoint , high score=worse) Show forest plot | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.86, ‐0.06] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 2 Global state: 2. Average score ‐ short term (CGI endpoint , high score=worse). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Mental state: 1. Not improved ‐ various measures ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.3  Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 3 Mental state: 1. Not improved ‐ various measures ‐ medium term. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 overall ‐ BPRS | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.16] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 negative symptoms ‐ SANS | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.66, 1.15] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 positive symptoms ‐ SAPS | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 0.99] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Mental state: 2. Average score (BPRS endpoint, high score=worse) Show forest plot | 4 | 724 | Mean Difference (IV, Fixed, 95% CI) | ‐3.33 [‐4.32, ‐2.34] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.4  Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 4 Mental state: 2. Average score (BPRS endpoint, high score=worse). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 short term | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐2.41 [‐3.85, ‐0.97] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 medium term | 2 | 621 | Mean Difference (IV, Fixed, 95% CI) | ‐4.17 [‐5.54, ‐2.79] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Mental state: 3. Average scores ‐ various scales (endpoint, high score=worse, skewed data) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.5
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 5 Mental state: 3. Average scores ‐ various scales (endpoint, high score=worse, skewed data). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.1 overall ‐ medium term ‐ BPRS | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.2 negative symptoms ‐ short term ‐ SANS | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.3 positive symptoms ‐ medium term ‐ SAPS | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Mental state: 4. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse) Show forest plot | 3 | 741 | Mean Difference (IV, Fixed, 95% CI) | ‐9.15 [‐12.10, ‐6.20] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.6  Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 6 Mental state: 4. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 Behaviour: Leaving the study early Show forest plot | 6 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.58] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.7  Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 7 Behaviour: Leaving the study early. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1 short term | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.76] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.2 medium term | 4 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.65] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Adverse effects: 1. Various symptoms ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.8  Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 8 Adverse effects: 1. Various symptoms ‐ short term. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.1 extrapyramidal | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.18, 1.64] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.2 gastrointestinal ‐ constipation | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.45] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Adverse effects: 2. Average score ‐ short term (TESS endpoint, high score=worse, skewed data) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.9
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 9 Adverse effects: 2. Average score ‐ short term (TESS endpoint, high score=worse, skewed data). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average score ‐ short term (CGI endpoint , high score=worse) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 1 Global state: Average score ‐ short term (CGI endpoint , high score=worse). | ||||
| 1.1 ginkgo biloba alone | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.29, ‐0.31] |
| 1.2 other herbs combined | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.86, ‐0.06] |
| 2 Mental state: 1. Average score (BPRS endpoint, high score=worse) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 2 Mental state: 1. Average score (BPRS endpoint, high score=worse). | ||||
| 2.1 ginkgo biloba alone | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐5.97, ‐2.03] |
| 2.2 other herbs combined | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐2.41 [‐3.85, ‐0.97] |
| 3 Mental state: 2. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 3 Mental state: 2. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse). | ||||
| 3.1 ginkgo biloba alone | 2 | 621 | Mean Difference (IV, Fixed, 95% CI) | ‐9.16 [‐12.52, ‐5.79] |
| 3.2 other herbs combined | 3 | 741 | Mean Difference (IV, Fixed, 95% CI) | ‐9.15 [‐12.10, ‐6.20] |
| 4 Behaviour: Leaving the study early Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 4 Behaviour: Leaving the study early. | ||||
| 4.1 short term ‐ ginkgo biloba alone | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.76] |
| 4.2 short term ‐ other herbs combined | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.76] |
| 4.3 medium term ‐ ginkgo biloba alone | 2 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.65] |
| 4.4 medium term ‐ other herbs combined | 4 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.65] |

Comparison 1 HERBAL MEDICINE versus ANTIPSYCHOTICS, Outcome 1 Global state: Not improved/worse ‐ short term.

Comparison 1 HERBAL MEDICINE versus ANTIPSYCHOTICS, Outcome 2 Behaviour: Leaving the study early ‐ short term.

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 1 Global state: 1. Not improved/worse ‐ medium term.
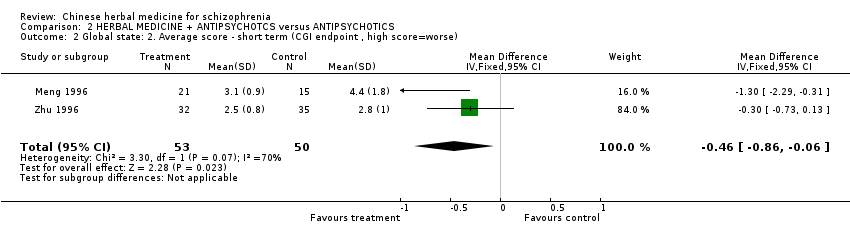
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 2 Global state: 2. Average score ‐ short term (CGI endpoint , high score=worse).

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 3 Mental state: 1. Not improved ‐ various measures ‐ medium term.
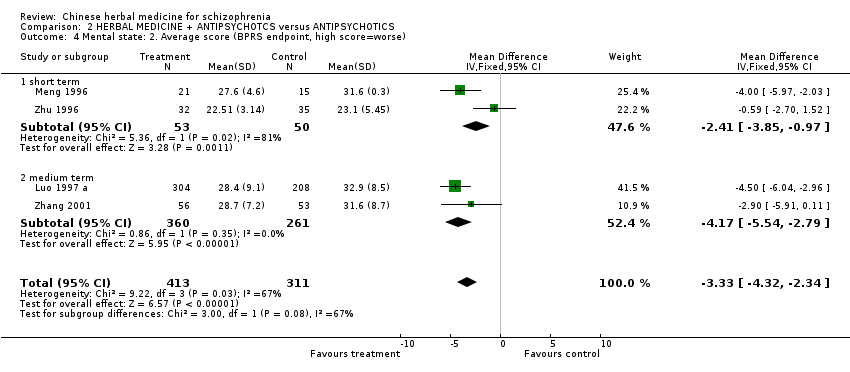
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 4 Mental state: 2. Average score (BPRS endpoint, high score=worse).
| Study | Intervention | mean | SD | N |
| overall ‐ medium term ‐ BPRS | ||||
| Zhang 1997 | Herbal medicine + antipsychotics | 13.4 | 6.7 | 66 |
| Zhang 1997 | Antipsychotics | 26.6 | 15.2 | 57 |
| negative symptoms ‐ short term ‐ SANS | ||||
| Meng 1996 | Ginkgo (EGb61) | 37.1 | 16.0 | 21 |
| Meng 1996 | Antipsychotics | 46.6 | 30.0 | 15 |
| positive symptoms ‐ medium term ‐ SAPS | ||||
| Zhang 2001 | Ginkgo biloba (EGb) + haloperidol | 7.1 | 8.4 | 56 |
| Zhang 2001 | Haloperidol | 11 | 10.5 | 53 |
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 5 Mental state: 3. Average scores ‐ various scales (endpoint, high score=worse, skewed data).
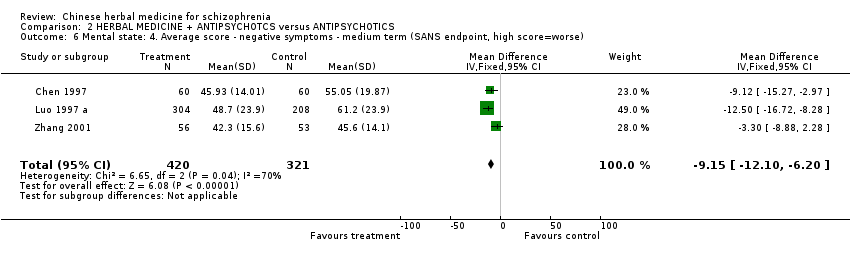
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 6 Mental state: 4. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse).

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 7 Behaviour: Leaving the study early.

Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 8 Adverse effects: 1. Various symptoms ‐ short term.
| Study | Intervention | mean | SD | N |
| Zhang 2001 | Ginkgo biloba (EGb) + haloperidol | 1.8 | 2.8 | 56 |
| Zhang 2001 | Haloperidol | 2.7 | 4.6 | 53 |
Comparison 2 HERBAL MEDICINE + ANTIPSYCHOTCS versus ANTIPSYCHOTICS, Outcome 9 Adverse effects: 2. Average score ‐ short term (TESS endpoint, high score=worse, skewed data).

Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 1 Global state: Average score ‐ short term (CGI endpoint , high score=worse).

Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 2 Mental state: 1. Average score (BPRS endpoint, high score=worse).
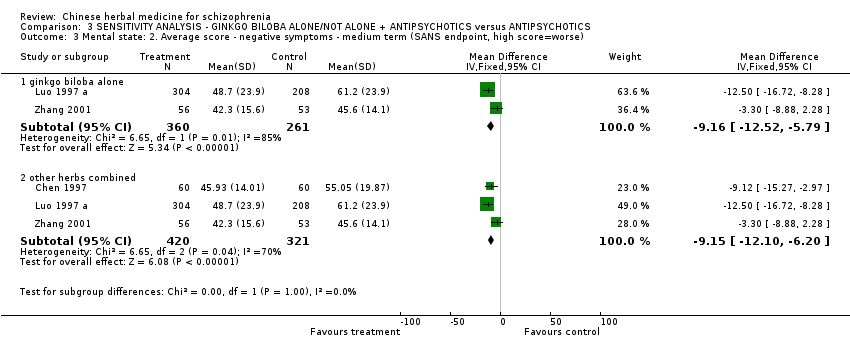
Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 3 Mental state: 2. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse).

Comparison 3 SENSITIVITY ANALYSIS ‐ GINKGO BILOBA ALONE/NOT ALONE + ANTIPSYCHOTICS versus ANTIPSYCHOTICS, Outcome 4 Behaviour: Leaving the study early.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Not improved/worse ‐ short term Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.24, 2.86] |
| 2 Behaviour: Leaving the study early ‐ short term Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Not improved/worse ‐ medium term Show forest plot | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.61] |
| 2 Global state: 2. Average score ‐ short term (CGI endpoint , high score=worse) Show forest plot | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.86, ‐0.06] |
| 3 Mental state: 1. Not improved ‐ various measures ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 overall ‐ BPRS | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.16] |
| 3.2 negative symptoms ‐ SANS | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.66, 1.15] |
| 3.3 positive symptoms ‐ SAPS | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 0.99] |
| 4 Mental state: 2. Average score (BPRS endpoint, high score=worse) Show forest plot | 4 | 724 | Mean Difference (IV, Fixed, 95% CI) | ‐3.33 [‐4.32, ‐2.34] |
| 4.1 short term | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐2.41 [‐3.85, ‐0.97] |
| 4.2 medium term | 2 | 621 | Mean Difference (IV, Fixed, 95% CI) | ‐4.17 [‐5.54, ‐2.79] |
| 5 Mental state: 3. Average scores ‐ various scales (endpoint, high score=worse, skewed data) Show forest plot | Other data | No numeric data | ||
| 5.1 overall ‐ medium term ‐ BPRS | Other data | No numeric data | ||
| 5.2 negative symptoms ‐ short term ‐ SANS | Other data | No numeric data | ||
| 5.3 positive symptoms ‐ medium term ‐ SAPS | Other data | No numeric data | ||
| 6 Mental state: 4. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse) Show forest plot | 3 | 741 | Mean Difference (IV, Fixed, 95% CI) | ‐9.15 [‐12.10, ‐6.20] |
| 7 Behaviour: Leaving the study early Show forest plot | 6 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.58] |
| 7.1 short term | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.76] |
| 7.2 medium term | 4 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.65] |
| 8 Adverse effects: 1. Various symptoms ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 extrapyramidal | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.18, 1.64] |
| 8.2 gastrointestinal ‐ constipation | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.45] |
| 9 Adverse effects: 2. Average score ‐ short term (TESS endpoint, high score=worse, skewed data) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average score ‐ short term (CGI endpoint , high score=worse) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 ginkgo biloba alone | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.29, ‐0.31] |
| 1.2 other herbs combined | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.86, ‐0.06] |
| 2 Mental state: 1. Average score (BPRS endpoint, high score=worse) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 ginkgo biloba alone | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐5.97, ‐2.03] |
| 2.2 other herbs combined | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐2.41 [‐3.85, ‐0.97] |
| 3 Mental state: 2. Average score ‐ negative symptoms ‐ medium term (SANS endpoint, high score=worse) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 ginkgo biloba alone | 2 | 621 | Mean Difference (IV, Fixed, 95% CI) | ‐9.16 [‐12.52, ‐5.79] |
| 3.2 other herbs combined | 3 | 741 | Mean Difference (IV, Fixed, 95% CI) | ‐9.15 [‐12.10, ‐6.20] |
| 4 Behaviour: Leaving the study early Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 short term ‐ ginkgo biloba alone | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.76] |
| 4.2 short term ‐ other herbs combined | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.76] |
| 4.3 medium term ‐ ginkgo biloba alone | 2 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.65] |
| 4.4 medium term ‐ other herbs combined | 4 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.65] |


