Mantenimiento con heroína para las personas dependientes crónicas de la heroína
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised controlled trial; Duration of treatment within the study: 12 months | |
| Participants | Diagnosis: heroin addicts (intravenous use) registered in the local methadone maintenance programs, who had failed several methadone programs Age=38.5 years (5.7 SD) Sex= male 82.2% History=a history of heroin dependency (DSM‐IV) of at least five years; a minimum dose level of 50 mg (inhaling) or 60 mg (injecting) of methadone per day for an uninterrupted period of at least four week in the previous five years; in the previous year registered in a methadone program, and during the previous six months in regular contact with the methadone program; chronic heroin addiction and unsuccessfully treated in methadone maintenance treatment; daily or nearly daily use of illicit heroin; poor physical, and/or mental, and/or social functioning; | |
| Interventions | Group A (no. = 98) 12 months methadone; Group B (no. = 76) 12 months methadone+heroin injectable Psychosocial interventions: Psychosocial treatment was offered throughout | |
| Outcomes | Dichotomous, multidomain response index, including validated indicators of physical health, mental status, and social functioning. Information on Substance use; Retention in treatment, Death, Adverse events | |
| Notes | Country: The Netherlands 1998‐2001 website: www.ccbh.nl/ENG/publications.htm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There are no information about procedure of sequence generationand in agreement with the CDAG rule, the judgment has to be "unclear". |
| Allocation concealment (selection bias) | Low risk | "the randomization was organized centrally by an independent monitoring organization, and conducted separately for the trials on injectable heroin and inhalable heroin." Authors were contacted for further details and correspondence is available by the reviewer's author. |
| Incomplete outcome data (attrition bias) | Low risk | "In the present study, it could not be ruled out that the probability of response would systematically and considerably differ between patients with and without endpoint‐assessments. However, since the primary analysis of effectiveness concerned the total treatment‐offer, regardless of possible deviations from the protocol, statistical methods to correct for bias in the findings caused by missing endpoint‐assessments, like multiple imputation or propensity score estimation, were only |
| Selective reporting (reporting bias) | Low risk | prespecified outcomes available from the website of the study, details available on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | In order to reduce the risk of information bias, outcome assessments were conducted by |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Low risk | The validity of the self‐report data was checked through the application of urinalysis, with regard to the concurrent use of illicit drugs, and collection of registered data from the police and justice system, with regard to committed offenses and periods of detention. These latter types of data are insensitive to information bias. |
| Methods | Allocation: randomised controlled trial; Duration of treatment within the study: 12 months | |
| Participants | Diagnosis: heroin addicts (inhaling use) registered in the local methadone maintenance programs, who had failed several methadone programs. Age=39.6 (5.7 SD) History=a history of heroin dependency (DSM‐IV) of at least five years; a minimum dose level of 50 mg (inhaling) or 60 mg (injecting) of methadone per day for an uninterrupted period of at least four week in the previous five years; in the previous year registered in a methadone program, and during the previous six months in regular contact with the methadone program; chronic heroin addiction and unsuccessfully treated in methadone maintenance treatment; daily or nearly daily use of illicit heroin; poor physical, and/or mental, and/or social functioning; | |
| Interventions | Group A (no. = 139) 12 months methadone; Group B (no. = 117) 12 months heroin (inhalable) + methadone; Group C (n=119) 6months methadone+ 6 months Psychosocial interventions: Psychosocial treatment was offered throughout | |
| Outcomes | Dichotomous, multidomain response index, including validated indicators of physical health, mental status, and social functioning. Information on Substance use; Retention in treatment, Death, Adverse events | |
| Notes | Country: The Netherlands 1998‐2001 Website: www.ccbh.nl/ENG/publications.htm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description of sequence generation could be traced in the articles and the report of the study and in agreement with the CDAG rule, the judgment has to be "unclear". |
| Allocation concealment (selection bias) | Low risk | "the randomization was organized centrally by an independent monitoring organization, and conducted separately for the trials on injectable heroin and inhalable heroin." Authors were contacted for further details and correspondence is available by the reviewer's author. |
| Incomplete outcome data (attrition bias) | Low risk | "In the present study, it could not be ruled out that the probability of response would systematically and considerably differ between patients with and without endpoint‐assessments. However, since the primary analysis of effectiveness concerned the total treatment‐offer, regardless of possible deviations from the protocol, statistical methods to correct for bias in the findings caused by missing endpoint‐assessments, like multiple imputation or propensity score estimation, were only |
| Selective reporting (reporting bias) | Low risk | Yes prespecified outcomes available on the website of the study, details on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | In order to reduce the risk of information bias, outcome assessments were conducted by |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Low risk | The validity of the self‐report data was checked through the application of urinalysis, with regard to the concurrent use of illicit drugs, and collection of registered data from the police and justice system, with regard to committed offenses and periods of detention. These latter types of data are insensitive to information bias. |
| Methods | Allocation: Randomized controlled trial, multicenter Randomization: 4x2 stratified randomization Blindness of patients and or care providers in respect to treatment: not specified Duration of threatment within the study: 12 months | |
| Participants | Diagnosis: ICD10 for opiate dependence for at least 5 years (World Health Organization, 1993). (a) people with heroin dependence who were insufficiently responding to treatment owing to continuous intravenous heroin use (n=492); and (b) people with heroin dependence who were not in treatment in the previous 6 months (n=540). N=1032 Age=35.9 ( SD 6.8) | |
| Interventions | Arms 1,2 of each stratum: Heroin+education or Heroin + Case management Maximum single dose of 400mg and a maximum daily dose of 1000mg (none to take home) individually adjusted dose of injectable heroin self‐administered under direct supervision of medical staff Maximally three times a day, 7 days a week out‐patient setting; Up to 60mg of methadone could also be given for take‐home nighttime use to suppress withdrawal Arms 3,4 of each stratum: Methadone + education or Methadone + case‐management minimum daily dose of 60mg oral individually adjusted according to clinical judgement | |
| Outcomes | Two prespecified dichotomous, multidomain primary outcome measures: Primary outcome measure on health. Second primary outcome measure, people were considered responders if they showed a reduction in the use of street heroin and no increase in cocaine use (hair analysis). | |
| Notes | Country: Germany, 2002‐2004 Website: http://www.heroinstudie.de/english.html | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated list of numbers |
| Allocation concealment (selection bias) | Low risk | "Randomisation took place separately for each target group (methadone treatment failure and not in treatment), and treatment allocation was performed using sealed and consecutively numbered envelopes at each study site". |
| Incomplete outcome data (attrition bias) | Low risk | In the intent‐to‐treat analysis, all those randomised were assessed regardless of |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes available from the website of the study, details on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Unclear risk | No mention of blinding of assessors.The assessment by independent research assistants included administration of the European version of the Addiction Severity Index (EuropASI; Kokkevi & Hartgers, 1995), and gathering data on criminal behaviour and on subjective aspects of treatment. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Unclear risk | see above |
| Methods | Allocation: computer generated list of random number; Randomization: unspecified Blindness of patients and or care providers in respect of treatment: patients were aware of the treatment provided but they were not aware of being part of a trial; blindness over interventions by patients and treatment providers and outcomes measurers not mentioned; Duration of treatment within the study: 12 months | |
| Participants | Diagnosis: regular opiate use including daily heroin injection in the last three months sufficient to convince clinical staff to be entered in a substitution program | |
| Interventions | Heroin Maintenance (N= 44); dosages 30‐120 mg/day; Psychosocial interventions: Interviews by a clinic psychiatrist at 3,9 and 12 months; | |
| Outcomes | Health; Use of substances:Total Opiate Consumption (prescribed+illicit); Frequency of Injection during 12 months; Proportion of days spent with other users; Crime activity: Crime as source of outcome during 12 moths; Arrests during 12 months Employment. Retention in treatment, relapse to street heroin use, death | |
| Notes | Country: UK 1972‐75 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated list of random numbers was consulted (without patients being aware of this) and the patients prescribed either HM or refused it and offered Oral Methadone instead." |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) | Low risk | Information where obtained by almost all of the patients some of them where interviewed in the United States and for others family members were interviewed an parallel sources of informations were compared. |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the study not available, due to the older date of the study. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | Data collected by independent research but no blinding of assessors mentioned nevertheless any limitations to validity of objective results are described and taken into consideration in the study results. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Unclear risk | Data collected by independent research but no blinding of assessors mentioned nevertheless any limitations to validity of subjective results are described and taken into consideration in the study results. |
| Methods | Allocation: "A computer‐generated randomisation list of permuted blocks of two, four, and six was used. Patients were assigned to receive diacetylmorphine, methadone, or hydromorphone in a 45:45:10 ratio. | |
| Participants | Opiate‐dependent using injected heroin on regular basis, Diagnosis: opioid dependence (meeting three or more of seven criteria listed in the Diagnostic and Statistical N= 251 | |
| Interventions | Injected heroin + oral methadone (N = 115) oral methadone (N = 111) injected Dilaudid + oral methadone (N = 25) Psychosocial interventions: All patients were offered a comprehensive range of psychosocial and primary care services in keeping with Health Canada best practices. | |
| Outcomes | retention in addiction treatment reduction in illicit‐drug use or other illegal activity | |
| Notes | Country: Canada 2005‐2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors describe "a computer‐generated randomisation list of permuted blocks of two, four, and six". "Randomization was stratified according to center and according to the number of previous methadone treatments (two or fewer vs. three or more)." |
| Allocation concealment (selection bias) | Low risk | central allocation |
| Incomplete outcome data (attrition bias) | Low risk | Almost no missing outcome data: "We obtained 12‐month retention data on 245 of 251participants (97.6%) and response data on 240 of 251 participants (95.6%). The baseline characteristics of the groups were similar" |
| Selective reporting (reporting bias) | Low risk | Details from the published protocol on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | No blinding but the objective outcome measurement were not likely to be influenced by lack of blinding: "Retention was assessed with the use of detailed data on daily prescription‐drug use and, when possible, with the use of administrative data and pharmacy and physician records." |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Low risk | Self‐reported nonuse of illicit heroin was confirmed by means of urine testing at 100% of 46 visits in the group of patients randomly assigned to receive hydromorphone (the double‐blind portion of the study). (information obtained by the authors on request) |
| Methods | Allocation: Permuted blocks of two, four, and six were used (not generated by a list, but ‘manually’ (see risk of bias documents). Patients were assigned to receive diacetylmorphine or methadone in a 50:50 ratio. Blindnessof patients and or care providers in respect to treatment: given the administration routes and different treatment schedules in each group, it was impossible to blind health care personnel to the treatment condition. Nevertheless, the professional who made the assessments and the statistical analysis was blind to the treatment condition. Duration: 12 months | |
| Participants | Diagnosis: opiate dependency for more than 2 years in line with International Statistical Classification of Diseases, 10th Revision criteria; ongoing intravenous opioid habit; have been in MT in the past at least twice according to official certificates issued by authorized centers N= 62 Mental State:mental health problems, and social maladjustment (according to scores of the assessment of severity by the interviewerQ in the social/family situation and legal Addiction Severity Index [ASI] subscales) | |
| Interventions | Experimental group: injected DAM, twice a day, plus oral methadone, once a day. The average DAM dosage was 274.5 mg/day (range: 15–600 mg), and average methadone 42.6 mg/day (range: 18–124 mg). Control group: daily methadone (once a day) 105 mg/day (range:40–180 mg). Comprehensive clinical, psychological, social, and legal support was given to both groups. Psychosocial interventions: see above | |
| Outcomes | General state of health, Quality of life, Severity of the addiction. Consumption of illegal opiate. Consumption of cocaine. Consumption of other psychoactive substances, illegal or legal, not prescribed. Behavior that puts the patient at risk of contracting HIV and hepatitis C Psychological adjustment Symptoms of depression Symptoms of anxiety Family situation Social support Rate of retention. Level of utilization of the psychosocial services of the trial | |
| Notes | Country: Spain 2001‐2004 Website: http://www.easp.es/pepsa/inicio/ensayo_english.htm#Design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information |
| Allocation concealment (selection bias) | Low risk | Treatment name ‘Metadona’ or ‘Heroína’ were introduced in identical opaque envelopes at a 50/50 ratio (i.e. six patients, 3 envelopes will say methadone, 3 will say heroin). There were shuffled in the presence of the participants and each participant will pick an envelope. Our guys had ‘trust’ issues. We early realized that a physician calling the center and tell the participant the treatment he or she randomly got, would generate problems. We knew this from our contact with them, some of them where convinced we were going to ‘cheat’ in the randomisation. Therefore, we had to show them that they had a fifty fifty chance to enter the heroin or methadone arm. (information provided by the authors) |
| Incomplete outcome data (attrition bias) | Low risk | Intention to treat and per protocol analysis were performed (completers in the experimental group: 23/27( 85.1%), and in the control group: 21/23 (91.3%). |
| Selective reporting (reporting bias) | Low risk | Protocol available and study registered, details on table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Unclear risk | "Independent interviewers of the research and clinical teams were responsible for applying the assessment instruments". |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Unclear risk | Our outcomes were based on the ASI, administered by independent interviewers. This instrument is based on self‐report. We tested the use of street heroin with the acetylcodeine test. It did not work; 95% of the test came back ‘negative’ regardless of the allocation group and self‐reported use of street heroin. |
| Methods | Allocation: randomised controlled trial; Randomization Blindness of patients in respect to treatment: non described. Duration of treatment within the study: 6 months | |
| Participants | Diagnosis: Heroin addicts; | |
| Interventions | Heroin injected by the patients themselves + oral methadone if the patient travels or want to reduce the attendance of the clinic; mean daily dosages of intravenous heroin was 509 mg/day in one to three injections; in addition to heroin all the patients occasionally received oral opiates and 16 patients received clorazepate substitution therapy (median dose 60 mg/day) control: waiting list (control patients were encouraged to select any drug treatment program available in Geneva and were enrolled immediately whenever possible). Psychosocial treatment: all patients received psychological counselling, HIV prevention counselling, social and legal support services, and somatic primary care. | |
| Outcomes | Consumption of street heroin; frequency of overdoses; risk behaviour for HIV; number of days ill in the past months; use of health services, health status, work status, living arrangements, quality of social relationships, monthly living and drug related expenditures, sources of income, and criminal behaviour, retention in treatment. | |
| Notes | Country: Switzerland 1995‐1996 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated list of numbers placed in sealed envelopes". |
| Allocation concealment (selection bias) | Low risk | Patients are allocated by the psychiatrist during the first visit through the sealed envelopes. |
| Incomplete outcome data (attrition bias) | Low risk | All experimental group patients and 22 in the control group were reassessed 196 days on |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified probably due to the date of the study. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | High risk | no objective measures adopted: all outcome measures were self reported, which raises the issue of information bias. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | High risk | no actions to reduce the risks reported |
| Methods | Allocation: Randomisation was undertaken independently by the Clinical Trials Unit (IoP). Blindness: Researchers unblinded to treatment allocation, informed both clinicians and patients together of treatment allocation prior to treatment commencing. Duration of treatment within the study: 6 months | |
| Participants | Diagnosis:regular heroin injecting no active significant medical condition N=127 Age= Aged between 18 and 65 years at recruitment to study History= >3 years injecting, in treatment >6 months Mental state= no active significant psychiatric conditions | |
| Interventions | experimental group:supervised injectable heroin (SIH) attendance at clinic twice daily for prescribed split daily dose of injectable heroin; self‐administered under supervision; supplementary oral methadone available (as take‐home dose at clinician's discretion). experimental group :supervised injectable methadone (SIM) attendance at clinic once daily for prescribed single daily dose of injectable methadone; self‐administered under supervision; supplementary oral methadone available (as take‐home dose at clinician's discretion); control group:optimised oral methadone (OOM) enhanced oral methadone treatment, daily doses of >80mg actively encouraged, consumed under supervision on >5 days per week for 3 months; thereafter frequency of supervision reduced if clinically appropriate; | |
| Outcomes |
1.a. objective: (operationally defined as urinalysis negative for street‐heroin for 50% or more of weekly random urines between weeks 14‐26); 1.b. subjective: self‐reported street‐heroin use (over 30 days prior to interview) was elicited through face‐to‐face research interviews with patients and 1.undertaken by independent researchers at baseline, three‐months and six months. | |
| Notes | Country: UK 2005‐2008 Website:http://www.iop.kcl.ac.uk/projects/?id=10114 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible consenting patients were randomised by minimisation in the ratio (1:1:1) to one of three treatment options (OOM, SIM, SIH), with stratification for a) regular cocaine/crack use (>50% days in previous 4 weeks), b) previous optimised oral methadone treatment (doses of >80mg/day; supervised >5 days/week) and c) clinic site (London, Darlington, Brighton). Randomisation was undertaken independently by the Clinical Trials Unit (IoP). |
| Allocation concealment (selection bias) | Unclear risk | Researchers unblinded to treatment allocation, informed both clinicians and patients together of treatment allocation prior to treatment commencing |
| Incomplete outcome data (attrition bias) | Low risk | For the primary outcome measure, missing data were handled using multiple imputation in cases where missing urines occurred due to hospitalisation, imprisonment, pre‐agreed absence (holiday), safety reasons or clinical omission/error. Missing urines from a patient who attempted abstinence were similarly managed. Urine samples not provided due to non‐compliance (refusal to provide or unplanned non‐attendance) were presumed positive. Data were analysed on an Intention‐To‐Treat basis for the primary analysis (all patients randomised included in analyses). Per‐Protocol analyses (only patients who received trial interventions according to protocol) were also conducted: any substantial differences in findings are reported alongside the primary ITT analyses. All the data for each of the groups to which participants were randomised were presented so that the risk of bias of multiple‐intervention study are minimised. |
| Selective reporting (reporting bias) | Low risk | Protocol published and study registered see table 3. |
| Blinding (objective outcomes: drop out, use of substances measured by urine analysis) | Low risk | Urinalysis (primary outcome measure) was preformed by laboratory personnel blinded to treatment allocation and the statistician was blinded to injectable group. |
| Blinding (subjective outcomes: use of substances as measured by self report, side effects) | Low risk | Interviews with patients and undertaken by independent researchers at baseline, three‐months and six months. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study design: retrospective study (audit of 40 patients treated in substitution therapy centres) excluded as the design not in the scope of the review. | |
| Study design: Open randomised clinical trial | |
| Study design: Controlled Clinical Trial | |
| Study design: Randomised Controlled Trial | |
| Study design: Controlled Clinical Trial | |
| Study design: Controlled Clinical Trial | |
| Study design: review | |
| Study design: cross sectional study | |
| Study design: double blinded, randomised study | |
| Study design: prospective observational study | |
| Study design: open‐label crossover design | |
| Study design: Controlled Clinical Trial Excluded as the design and the intervention not in the scope of the review. | |
| Study design: follow‐up study | |
| Study design: Cohort study | |
| Study design: randomised controlled trial | |
| Study design: cohort study |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Projet pilote de traitement assisté par diacétylmorphine: comparaison entre un traitement par diacétylmorphine et un traitement par méthadone. |
| Methods | Muticenter study, allocation performed by a neutral person with the help of informatic procedure (unclear whether randomisation will occur or not) |
| Participants | 2 groups 100 patients each· Beglian citizens or legal residence in Belgium ; |
| Interventions | Intervention: diacetylmorphine (inhaled, injected or in tablets) supervised by a nurse+ psychosocial interventions; Control: Oral methadone + psychosocial interventions. |
| Outcomes | retention in treatment, use of substances and social integration related outcomes |
| Starting date | 2007 expected results: 2010 |
| Contact information | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 4 | 1388 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.19, 1.75] |
| Analysis 1.1  Comparison 1 Supervised Injected Heroin + methadone vs oral methadone, Outcome 1 Retention in treatment. | ||||
| 2 Mortality Show forest plot | 4 | 1477 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.25, 1.69] |
| Analysis 1.2  Comparison 1 Supervised Injected Heroin + methadone vs oral methadone, Outcome 2 Mortality. | ||||
| 3 Adverse events related to intervention medications Show forest plot | 3 | 373 | Risk Ratio (IV, Random, 95% CI) | 13.50 [2.55, 71.53] |
| Analysis 1.3  Comparison 1 Supervised Injected Heroin + methadone vs oral methadone, Outcome 3 Adverse events related to intervention medications. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 6 | 1535 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.16, 1.79] |
| Analysis 2.1  Comparison 2 Heroin Provision (various modality and route of administration) vs any other treatment, Outcome 1 Retention in treatment. | ||||
| 2 Mortality Show forest plot | 5 | 1573 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.32, 1.89] |
| Analysis 2.2  Comparison 2 Heroin Provision (various modality and route of administration) vs any other treatment, Outcome 2 Mortality. | ||||

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
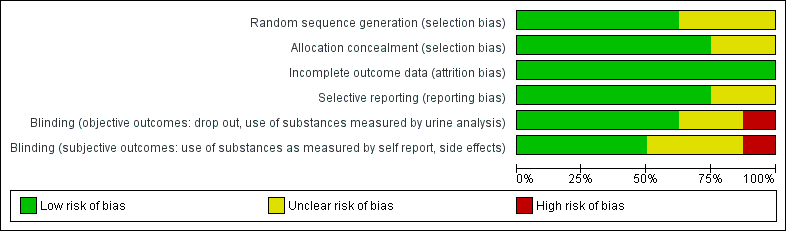
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Supervised injected heroin versus oral methadone: retention in treatment

Heroin provision (any route of administration) versus any other treatment: retention in treatment

Supervised Injected Heroin + methadone vs oral methadone, outcome: Mortality.
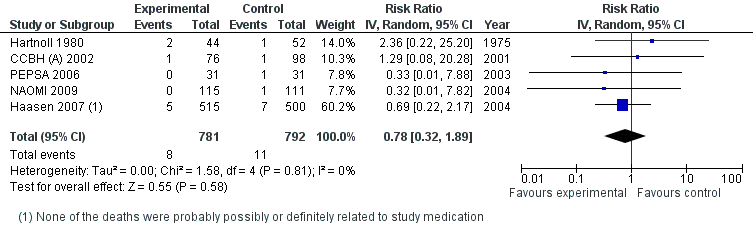
Heroin Provision (various modality and route of administration) vs methadone different modalities, outcome: Mortality.

Comparison 1 Supervised Injected Heroin + methadone vs oral methadone, Outcome 1 Retention in treatment.

Comparison 1 Supervised Injected Heroin + methadone vs oral methadone, Outcome 2 Mortality.

Comparison 1 Supervised Injected Heroin + methadone vs oral methadone, Outcome 3 Adverse events related to intervention medications.

Comparison 2 Heroin Provision (various modality and route of administration) vs any other treatment, Outcome 1 Retention in treatment.

Comparison 2 Heroin Provision (various modality and route of administration) vs any other treatment, Outcome 2 Mortality.
| Study | N Participants | Intervention Heroin mean dosage/day | Intervention Methadone mean dosage/day | Control Methadone mean dosage/day |
| Haasen 2007 | 1015 | 442 mg | 39 mg | 99 mg |
| CCBHA 2002 | 174 | mean heroin dosage 254 mg maximum daily dose 1000 mg, and the maximum single dosage 400 mg | Decided with the help of the treating physician with a minimum daily dose of 30‐50 mg and a maximum of 150 mg. | |
| CCBHB 2002 | 256 | '' | '' | |
| Perneger 1998 | 51 | 509 mg | unspecified | unspecified |
| Hartnoll 1980 | 96 | 30‐120 mg | unspecified | 10‐120 mg |
| RIOTT 2010 | 127 | Injected | Injected methadone doses calculated with the formula: injected methadone dose=0·8×oral dose; dose reassessed continually, Maximum dose of injectable methadone: up to 200 mg/day | Once daily doses of ≥80 mg actively encouraged; optimum doses individually titrated |
| NAOMI 2009 | 226 (+26 INJECTED HYDROMORPHONE) | 392.3 mg | (patients receiving diacetylmorphine plus methadone) mean daily dose of diacetylmorphine was 365.5 | 96.0 mg. |
| PEPSA 2003 | 62 | DAM dosage was 274.5 mg/day (range: 15–600 mg), | methadone dosage was 42.6 mg/day (range: 18–124 mg). | The daily methadone dosage in the control group was 105 mg/day (range:40–180 mg) |
| Study | Definition | Outcome measures |
| Hartnoll 1980 | Total opiate consumption, frequency of injection, involvment with drug subculture. | Interviews (questionnaires not specified), direct observations |
| Perneger 1998 | Self reported drug use, health status , and social functioning | Unpublished questionnaire based on ASI and SF36. |
| CCBHA/B 2002 | Prespecified dichotomous, multidomain outcome index including physical, mental, social dimensions and also completion of | ASI / MAP‐HSS, Case Report Forms (CRF), Composite International |
| PEPSA 2003 | Dichotomous multidimension outcome (MDO) including general health, quality of life, drug‐addiction‐related problems, nonmedical use of heroin, risk behavior for HIV and HCV, and psychological, family, and social status | The ASI, Opiate Treatment Index, Symptom Checklist‐90, and the 12‐item shortform (SF‐12). |
| Haasen 2007 | Two prespecified dichotomous, multidomain primary outcome measures about health and reduction in illicit drug use, were considered. | EuropASI OTI Health Scale (physical health) GSI (mental health) |
| NAOMI 2009 | Retention in addiction treatment at 12 months (defined as receipt of the study medication on at least 10 of the 14 days before the 12‐month assessment, or confirmation of retention in any other treatment program or abstinence from opioids during this interval). Reduction in illicit drug‐use or other illegal activities | Retention in treatment: Data on daily prescription‐drug use and, when possible, with the use of administrative data and pharmacy and physician records Illicit drug use or other illegal activities: Composite scores on the European Addiction Severity Index17 (see the Supplementary Appendix, available with the full text of this article at NEJM.org), |
| RIOTT 2010 | Reduction of regular use of street heroin defined as 50% or more of negative specimens on urinalysis during weeks 14‐26 (responders). Reduction of regular use of street heroin defined as two, one, or zero positive specimens during weeks 14‐26, and a test of zero positive specimens during weeks 23‐26. Self‐reported abstinence from street heroin (zero use) in the past 30 days. | Urine specimens were obtained at random once a week for 26 weeks; Independent researchers in face‐to‐face interviews with patients at baseline (0 weeks), 13 weeks, and 26 weeks. |
| study name | definition of responder | Measure of effect as reported in the published studies (ARR calculated for NNT) | NNT |
| CCBH (A) 2002 and | Responders: at least 40% improvement in at least one of the 3 domains of inclusion (physical, mental, social) at the end of the treatment compared with baseline; if this improvement was not at the expense of a serious ( ≥ 40%) deterioration in functioning in any of the other outcome domains; and if the improvement was not accompanied by a substantial ( ≥ 20%) increase in use of cocaine or amphetamines. | risk difference difference = 22.8%, 95% CI 11.0%‐ 34.6%; ARR= 0.24 | NNT=4.2 (95%CI 2.6‐11.1) |
| see above | risk difference 24.3%, 95% CI 9.6% to 39.0%; ARR= 0.23 | NNT=4.3 (95%CI 2.85‐9.09) | |
| Health Responders: at least a 20% improvement and at least 4 points on the OTI Health Scale (physical health) and/or at least a 20% improvement in the GSI Reduction in Illicit drug use Responders: reduction in the use of street heroin with at least 3 of 5 urine samples negative for the drug in the month prior to the 12‐month assessment and no increase in cocaine use (hair analysis). If less than 3 urine samples or no hair was available at 12 months, data from urine or hair testing at 6 months were used (LOCF). | Health Improvement Adjusted OR=1.54, 95% CI 1.02–2.34, P=0.042. ARR= 0.06 ‘illicit drug use’ Adjusted OR=1.91, 95% CI 1.30–2.79, P=0.001. ARR=0.14 | NNT=16.7 (95% CI 9.09‐100) NNT=7.2 (95%CI 5‐12.5) | |
| Responders: improvement of at least 20% from the baseline score for illicit‐drug use or legal status (or both). In addition, to rule out deterioration in other variables, a patient with a response could have a decrease of 10% or more on at most one of the remaining composite scores. | Reduction in illicit‐drug use or other illegal activities : 67.0% diacetylmorphine group 47.7% methadone group (rate ratio, 1.40; 95% confidence interval [CI], 1.11 to 1.77; P = 0.004) ARR=0.20 Retention in treatment : 87.8% in the diacetylmorphine group 54.1% in the methadone group (rate ratio, 1.62; 95% CI, 1.35 to 1.95; P<0.001). ARR=0.34 | NNT=5.3 (95% CI 3.1‐14.3) NNT=3 (95% CI 2.22‐4.34) | |
| Responders: patients showed at least 20% improvement at 9 months, compared with the baseline values, in general health or psychological or family adjustment, without a deterioration superior to 20% in any of these dimensions evaluated with the respective ASI composite scores. | MDO index 70.4% experimental group; 60.9% control group, difference not statistically significant. ARR=0.10 | NNT=10 (95% CI ‐6.6‐3 *not significant) | |
| Responders: Reduction of regular use of street heroin defined as 50% or more of negative specimens on urinalysis during weeks 14‐26 | ITT weeks 14–26 responders: (72% [n=31]) injectable heroin; oral methadone (27% [n=11], OR 7·42, 95% CI 2·69–20·46, p<0·0001 ARR=0.46 | NNT=2·17 (95% CI 1·60 to 3·97) |
| Study | protocol outcomes | published outcomes | source of protocol information |
| Hartnoll | not available | Health; Use of substances:Total Opiate Consumption (prescribed+illicit); Frequency of Injection during 12 months;Proportion of days spent with other users; Crime activity:Crime as source of outcome during 12 moths;Arrests during 12 months, Employment, Retention in treatment, relapse to street heroin use, death. | info not available |
| Perneger | not available | Consumption of street heroin; frequency of overdoses; risk behaviour for HIV; number of days ill in the past months; use of health services, health status, work status, living arrangements, quality of social relationships, monthly living and drug related expenditures, sources of income, and criminal behaviour, retention in treatment. | info not available |
| CCBH | Physical health, Mental status, Social functioning, Substance use | Dichotomous, multidomain response index, including validated indicators of physical health, mental status, and social functioning. | http://www.ccbh.nl/ |
| PEPSA | General state of health Quality of life Severity of the addiction. Consumption of illegal opiate Consumption of cocaine. Consumption of other psychoactive substances, illegal or legal, not prescribed. Behavior that puts the patient at risk of contracting HIV and hepatitis C Psychological adjustment Symptoms of depression Symptoms of anxiety | General health status | http://www.easp.es/pepsa/inicio/ensayo_english.htm#Protocol http://www.controlled‐trials.com/ISRCTN52023186 |
| NAOMI | A participant was defined as “retained at 12 months” if he or she met any of the following 4 criteria: was compliant with study medication (DAM, HMO and/or MMT) on at least 10 of 14 days prior to the 12‐month date; or was confirmed to be enrolled in detoxification program at the 12‐month date; or was confirmed to be enrolled in a drug‐free program at the 12‐month date; or was confirmed to be abstinent at the 12‐month date. | The first primary outcome was retention in addiction treatment at 12 months (defined as receipt of the study medication The second primary outcome was reduction in illicit‐drug use or other illegal activities. On the basis of composite scores on | Scientific and political challenges in North America's first randomized controlled trial of heroin‐assisted treatment for severe heroin addiction: rationale and design of the NAOMI study. Oviedo‐Joekes E, Nosyk B, Marsh DC, Guh D, Brissette S, Gartry C, Krausz M, Anis A, Schechter MT. Clin Trials. 2009 Jun;6(3):261‐71. |
| RIOTT | Reduction in illicit heroin, measured by urine drug screens taken on a weekly basis over 6 months. Self‐reported to researcher at baseline, 3 months and 6 months (in or out of treatment): | Retention; | Lintzeris et al 2006 (Nicholas Lintzeris, John Strang, Nicola Metrebian, Sarah Byford,Christopher Hallam, Sally Lee, Deborah Zador and RIOTT Group. Methodology for the Randomised Injecting Opioid Treatment Trial (RIOTT): evaluating injectable methadone and injectable heroin treatment versus optimised oral methadone treatment in the UK. Harm Reduction Journal 2006;3:28.) http://www.controlled‐trials.com/ISRCTN01338071 |
| Haasen | improvement of health, | ‘health’ ‘illicit drug use’ | http://www.heroinestudie.de/english.html |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 4 | 1388 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.19, 1.75] |
| 2 Mortality Show forest plot | 4 | 1477 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.25, 1.69] |
| 3 Adverse events related to intervention medications Show forest plot | 3 | 373 | Risk Ratio (IV, Random, 95% CI) | 13.50 [2.55, 71.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention in treatment Show forest plot | 6 | 1535 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.16, 1.79] |
| 2 Mortality Show forest plot | 5 | 1573 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.32, 1.89] |

