Strategije za otklanjanje seksualne disfunkcije inducirane antidepresivima
Abstract
Background
Sexual dysfunction (including altered sexual desire, orgasmic and ejaculatory dysfunction, erectile and other problems) is a relatively common side effect of antidepressant medication. These sexual side effects may compromise a person's lifestyle and result in a lack of compliance with the prescribed antidepressant to the detriment of the person's mental health. A wide range of management strategies are possible to address this problem, including behavioural, psychological and pharmacological approaches.
Objectives
1. To determine the effectiveness of management strategies for sexual dysfunction caused by antidepressants.
2. To determine the adverse effects and acceptability of the different management strategies.
Search methods
We searched the Cochrane Depression, Anxiety and Neurosis Group's Specialized Register (CCDANCTR, to 1 January 2013), which includes relevant randomised controlled trials from the following bibliographic databases: The Cochrane Library (all years), EMBASE (1974 to date), MEDLINE (1950 to date) and PsycINFO (1967 to date). Additional searches were carried out by the author team on the same biomedical databases (using terms for 'sexual dysfunction' only) together with CINAHL (1982 to Jan 2012). The reference lists of reports of all included studies were screened.
Selection criteria
We included randomised controlled trials that compared management strategies for antidepressant‐induced sexual dysfunction versus placebo or any alternative strategy.
Data collection and analysis
Two authors independently extracted data and assessed trial quality. Study authors were contacted for additional information.
Main results
We included 23 trials involving 1886 people in this updated review. Twenty‐two of these trials investigated the addition of medication to treat the identified dysfunction, with most agents studied in only single studies. One study investigated switching to an alternative antidepressant.
In men, data for the phosphodiesterase inhibitors sildenafil (three studies, 255 participants) and tadalafil (one study, 54 participants) indicated they led to a greater improvement in erectile function than placebo. Combined data from three sildenafil studies found benefit over placebo on International Index of Erectile Function ratings of ability to achieve (MD 1.04, 95% CI 0.65 to 1.44), and maintain erections (MD 1.18, 95% CI 0.78 to 1.59). A single point improvement on these ratings is equivalent to an improvement in frequency from 'sometimes' to 'most times'. Men receiving tadalafil were more likely to report improved erectile function (RR 11.50, 95% CI 3.03 to 43.67). For women it remains uncertain whether sildenafil is more effective than placebo. Unpublished data could reduce this uncertainty.
Data from three studies in men and women of bupropion 150 mg twice daily indicate a benefit over placebo on rating scale scores (SMD 1.60, 95% CI 1.40 to 1.81), but response rates in two studies of bupropion 150 mg once daily demonstrated no statistically significant difference in effect (RR 0.62, 95% CI 0.09 to 4.41).
Other augmentation strategies failed to demonstrate significant improvements in sexual dysfunction compared with placebo.
One trial involving 75 people with sexual dysfunction due to sertraline assessed the effect of changing antidepressant. Switching to nefazodone was significantly less likely to result in the re‐emergence of sexual dysfunction than restarting sertraline (RR 0.34, 95% CI 0.19 to 0.60), however, nefazodone is no longer available for clinical use.
There is an absence of randomised trials assessing the effects of switching to currently‐available antidepressant agents with lower rates of adverse sexual effects, the role of psychological or mechanical interventions, or of techniques such as drug holidays.
We identified no data for any of the strategies included in the trials assessed that indicated that they led to a worsening of psychiatric symptoms. However, the relatively small numbers assessed for many of the interventions studied means that the possibility of such an effect cannot confidently be excluded in all cases.
Given the small numbers of studies assessing most of the strategies assessed, the presence of any unpublished trials could have substantial effects on estimates of effect. In some cases, only results from particular items or subscales within ratings scales are available. It is likely that this could act to bias estimates of effect obtained, increasing apparent effectiveness.
Authors' conclusions
The evidence currently available is rather limited. For men with antidepressant‐induced erectile dysfunction, the addition of sildenafil or tadalafil appears to be an effective strategy. For women with antidepressant‐induced sexual dysfunction the addition of bupropion at higher doses appears to be the most promising approach studied so far.
Laički sažetak
Postupci za otklanjanje poremećaja seksualne funkcije uzrokovanog antidepresivima
Lijekovi antidepresivi mogu imati brojne učinke koji negativno utječu na seksualnu funkciju, uključujući promijenjenu seksualnu želju, probleme s erekcijom i orgazmom. Ovaj Cochrane sustavni pregled istražuje različite načine na koje se mogu ukloniti poremećaji seksualne funkcije (seksualne disfunkcije). Uključene su 23 randomizirane studije s ukupno 1886 sudionika koji su razvili seksualne poteškoće tijekom terapije antidepresivima. Dvadeset i dvije od tih studija promatrale su dodatak lijekova dotadašnjoj terapiji antidepresivima. Za muškarce s erektilnom disfunkcijom koja je izazvana antidepresivima, dostupni podatci pokazuju da dodatak sildenafila (Viagra; tri studije, 255 sudionika) ili taladafila (Cialis; jedna studija, 54 sudionika) popravlja stanje. Za žene kod kojih su antidepresivi izazvali seksualnu disfunkciju dodatak bupropiona (Wellbutrin; tri studije, 482 sudionice) u višim dozama se čini kao pristup koji najviše obećava, ali potrebno je još podataka iz randomiziranih studija kako bi se moglo pouzdano propisivati. Nisu pronađeni dokazi da je bilo koja intervencija dovela do pogoršanja psihijatrijskih simptoma, međutim ne možemo biti sigurni da je tako za mnoge istraživane lijekove s obzirom da su studije provedene na malom broju sudionika.
Authors' conclusions
Summary of findings
| Sildenafil compared with placebo for antidepressant‐induced sexual dysfunction | ||||||
| Patient or population: people with antidepressant‐induced sexual dysfunction Settings: outpatient Intervention: sildenafil Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sildenafil | |||||
| Endpoint International Index of Erectile Function (IIEF) total scores (The IIEF is a self‐report measure with 15 questions examining erectile function, orgasmic function, sexual desire, and intercourse satisfaction. Maximum possible score 75) | The mean IIEF score ranged across control groups from | The mean IIEF score in the intervention groups was | 112 men | ⊕⊕⊕⊕ | ||
| Endpoint International Index of Erectile Function (IIEF) scores ‐ question 3: ability to achieve erection (Maximum score 5) | The mean score in control groups was 3.1 | The mean score in the intervention groups was | 231 men | ⊕⊕⊕⊕ | ||
| Endpoint International Index of Erectile Function (IIEF) scores ‐ intercourse satisfaction (questions 6, 7, 8) (Maximum score 15) | The mean score in the control group was 7.2 | The mean score in the intervention group was | 89 men | ⊕⊕⊕⊝ | ||
| Clinical Global Impression ‐Sexual Function not "much/very much improved" by endpoint | Male population | RR 0.44 (0.33 to 0.58) | 187 | ⊕⊕⊕⊝ | ||
| 956 per 1000 | 459 per 1000 | |||||
| Female population | ||||||
| 735 per 1000 | 287 per 1000 | |||||
| Dropouts (People leaving the trial early) | Low risk population | RR 0.68 (0.41 to 1.14) | 353 | ⊕⊕⊕⊕ | ||
| 90 per 1000 | 61 per 1000 | |||||
| Medium risk population | ||||||
| 250 per 1000 | 170 per 1000 | |||||
| High risk population | ||||||
| 360 per 1000 | 245 per 1000 | |||||
| Global Efficacy Questionnaire (questions 1 & 2) (Questions assessing improvement attributed to medication compared to having no treatment at all) | Improvement in erections | RR 2.50 (1.67 to 3.73) and RR 2.55 (1.71 to 3.80) | 284 men | ⊕⊕⊕⊝ | ||
| 282 per 1000 | 705 per 1000 | |||||
| Improvement in ability to have sexual intercourse | ||||||
| 282 per 1000 | 719 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The evidence for effects based on single trials is rated as moderate quality since further trial data may well change the estimate. | ||||||
| Bupropion compared with placebo for antidepressant‐induced sexual dysfunction | ||||||
| Patient or population: people with antidepressant‐induced sexual dysfunction Settings: outpatients Intervention: bupropion (doses of 150 mg daily and 150 mg twice daily) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Bupropion | |||||
| Endpoint scale total scores (150 mg twice daily dose) (Scales of sexual functioning. As studies used different scales to assess sexual functioning, differences are expressed as standardised mean differences (SMD)) | The mean value with this analysis is in effect zero | The mean score in the intervention groups was 1.60 higher (1.40 to 1.81) | 482 | ⊕⊕⊕⊝ | ||
| 50% reduction in score on the Arizona Sexual Experiences Scale (ASEX) (150 mg once daily dose) (The ASEX is a 5‐item self‐report inventory of sexual function) | Lower risk population | RR 0.62 (0.09 to 4.41) | 71 | ⊕⊕⊕⊝ | ||
| 47 per 1000 | 29 per 1000 | |||||
| Higher risk population | ||||||
| 67 per 1000 | 41 per 1000 | |||||
| Dropouts (People leaving the trial early) | Lower risk population | RR 1.08 (0.67 to 1.72) | 579 | ⊕⊕⊕⊕ | ||
| 90 per 1000 | 97 per 1000 | |||||
| Higher risk population | ||||||
| 150 per 1000 | 162 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unexplained inconsistency in effects between studies reduces confidence in this effect | ||||||
Background
Description of the condition
Antidepressant medications are widely prescribed (Donoghue 1996; Jick 1995; Moore 2009). Sexual dysfunction is a common, well‐known adverse effect of all antidepressants (Balon 1993; Baldwin 1997; Williams 2006; Williams 2010). These sexual adverse effects can affect a person's lifestyle considerably, and, where this results in reduced compliance with medication, lead to less effective treatment of the primary psychiatric disorder.
Several different types of sexual dysfunction may be related to antidepressants, including altered sexual desire ‐ such as loss or lack of desire; orgasmic and ejaculatory dysfunction, including anorgasmia (inability to achieve orgasm), hyperorgasmia (significantly more orgasms in a short time period than normal), painful orgasm and inhibited ejaculation; erectile problems, including erectile dysfunction (impotence), priapism (significantly prolonged erection) and painful erection ‐ and other issues, including problems of sexual arousal, reduced sexual satisfaction, lubrication, dyspareunia (painful intercourse) and vaginismus (tensing of vaginal muscles that make intercourse painful or impossible).
Identifying antidepressant‐induced sexual dysfunction can be complicated by the association of sexual dysfunction with some disorders that antidepressants are used to treat. For example, depression is associated with increased rates of reported sexual dysfunction even when no treatment is received (Angst 1998).
Sexual dysfunction has been reported with all classes of antidepressant medication. Reported rates of sexual dysfunction are typically underestimates, as sexual adverse effects are often not specifically asked about in treatment trials, while direct questioning can reveal higher rates than are reported spontaneously (Montejo‐Gonzalez 1997). Studies of the prevalence of antidepressant‐induced sexual dysfunction have exhibited a number of methodological problems (Montgomery 2002). These include the frequent absence of comparison groups or baseline assessments, and inconsistent definitions of sexual dysfunction between studies.
The majority of studies directly comparing rates of sexual dysfunction between different antidepressants have involved selective serotonin reuptake inhibitors (SSRIs). Generally, trials have reported no significant differences between these drugs in rates of sexual dysfunction, with sexual dysfunction reported in population surveys by over one third of participants (Williams 2006; Williams 2010). In randomised trials, nefazodone (a serotonin antagonist and reuptake inhibitor) and bupropion (a norepinephrine‐dopamine reuptake inhibitor) have been associated with less sexual dysfunction than the SSRI, sertraline (Croft 1999; Feiger 1996), and reboxetine (a norepinephrine reuptake inhibitor) with greater sexual satisfaction than the SSRI fluoxetine (Clayton 2003). The monoamine oxidase inhibitor, moclobemide, was more commonly associated with increased sexual desire than the tricyclic antidepressant, doxepin (Philipp 1993). Further information on rates of sexual dysfunction with antidepressants can be found elsewhere (Gregorian 2002; Montgomery 2002; Serretti 2009).
Description of the intervention
Management strategies described for the treatment of antidepressant‐induced sexual dysfunction include waiting for the problem to resolve; behavioural strategies modifying sexual technique; individual and couple psychotherapy; alterations of antidepressant usage, including reducing dose; delaying use until after sexual activity; 'drug holidays'; switching to a different antidepressant; and the use of additional agents (Baldwin 2004). A wide range of additional agents have been employed clinically to try to reverse this problem, for example erectile dysfunction might be treated with a phosphodiesterase inhibitor such as sildenafil or tadalafil. However, additional treatments may themselves have adverse effects and tolerability problems, and could, in theory, affect the primary psychiatric condition for which the antidepressants were prescribed.
How the intervention might work
The mechanisms by which antidepressants cause sexual dysfunction involve complex multi‐system interactions, which are not entirely understood. Psychological factors such as anxiety may also play a role in maintaining dysfunction. The main neurotransmitters involved are serotonin (5HT), acetylcholine, noradrenaline, and dopamine. The adverse sexual effects may be caused centrally or peripherally and may result from the change in function of one or more neurotransmitter. Given this complex system, additional treatments employed have had a wide variety of putative mechanisms. Such treatments have included sildenafil (a phosphodiesterase inhibitor), amantadine (a dopamine agonist), cyproheptadine (an antihistamine and 5HT blocker), yohimbine (an alpha‐2 blocker), buspirone (a 5HT1A receptor agonist), bethanechol (an acetylcholine agonist) and Ginkgo biloba (a herbal medication).
Why it is important to do this review
This is an update of a Cochrane review first published in 2004. That review noted that the available evidence was rather limited, with small numbers of trials assessing each strategy. This review aims to summarise the current evidence regarding potential strategies for managing antidepressant‐induced sexual dysfunction, noting how well the sexual dysfunction responds, as well as risks, such as adverse effects or worsening of the condition for which the antidepressant was initially prescribed. This should assist patients and their clinicians when deciding how best to manage these common problems.
Attention is drawn to the related Cochrane review on the management of sexual dysfunction due to antipsychotic drug therapy (Berner 2007; Schmidt 2012).
Objectives
1. To determine the effectiveness of management strategies for sexual dysfunction caused by antidepressants.
2. To determine the adverse effects and acceptability of the different management strategies.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials in the review. Cluster randomised trials and trials of both parallel group and cross‐over design were considered suitable for inclusion.Trials using non‐randomised allocation were not included, in order to reduce risks of selection and publication bias (Alderson 2004).
Types of participants
Patients aged 16 years and over with sexual dysfunction (including, but not restricted to altered sexual desire, orgasmic and ejaculatory dysfunction, erectile problems, problems of sexual arousal, reduced sexual satisfaction, lubrication, dyspareunia and vaginismus).as a result of being treated with an antidepressant (except mood stabilisers) on any dose regime.
Participants from inpatient or community settings were eligible for inclusion.
Inclusion was not restricted on the basis of the disorder for which the antidepressant had been prescribed.
Types of interventions
Experimental intervention
Any management strategy ‐ pharmacological, psychological or otherwise ‐ for antidepressant‐induced sexual dysfunction.
Comparator intervention
Placebo or any alternative strategy.
Types of outcome measures
Primary outcomes
1. Changes, or post‐treatment differences, in the severity of the identified sexual dysfunction (assessed by self (self‐rated measures) or interviewer (interviewer‐rated measures), or both.
Secondary outcomes
2. Changes, or post‐treatment differences, in sexual satisfaction and functioning (based on self‐ or interviewer‐rated measures, or both).
3. Dropout rates as a measure of the acceptability of specific therapies.
4. Change, or post‐treatment differences, in the primary psychiatric condition for which the antidepressant was being prescribed (based on symptom ratings).
Outcomes at trial endpoint were employed, where available. No decision was made to favour self‐reported or interviewer‐rated measures.
Search methods for identification of studies
CCDAN's Specialized Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintains two clinical trials registers at the editorial base (Bristol, UK), a references register and a studies‐based register. The CCDANCTR‐References Register contains over 29,500 reports of randomised controlled trials in depression, anxiety and neurosis. Approximately 65% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual. Please contact the CCDAN Trials Search Co‐ordinator for further details. Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950 to date), EMBASE (1974 to date) and PsycINFO (1967 to date); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers c/o the World Health Organization's trials portal (ICTRP), drug companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCDAN's generic search strategies can be found on the Group's website.
Electronic searches
Electronic searches included:
1. The CCDANCTR‐Studies Register (all years to 1 August 2012, with a further update search for 1 January 2013), using the following coding terms:
Condition = ( “sexual dysfunction*” and “drug induced”) or Comorbidity = (“sexual dysfunction*”).
2. The CCDANCTR‐References Register, using a more sensitive set of free‐text terms to find additional untagged/uncoded references (Appendix 1).
3. Independent searches by the author team on the Cochrane Register of Controlled Trials (CENTRAL) (2012, Issue 1), CINAHL (1982 to 12 January 2012), EMBASE (1980 to 12 January 2012), MEDLINE (1966 to 12 January 2012) and PsycINFO (1984 to 12 January 2012) (Appendix 2).
4. International trials registries (ClinicalTrials.gov and ICTRP, 1 January 2013), to identify additional ongoing or unpublished studies .
Searching other resources
Reference checking
The reference lists of all trials identified for inclusion were examined, together with other articles on adverse sexual effects of antidepressants, and relevant conference proceedings.
Personal communications
The following experts in the field of sexology were contacted for the original review: J Bancroft, R Basson, J Heiman and R Rosen.
Pharmaceutical companies
For the original review, the authors contacted pharmaceutical companies manufacturing antidepressant medication to find out if they knew of any published or unpublished studies relevant to this review. This search was not repeated for the update.
Data collection and analysis
Selection of studies
Trial inclusion or exclusion was determined independently by two authors. Full reports of studies were used for this assessment, except where a trial could be excluded on the basis of title and abstract alone. Any disagreements were resolved by consensus discussion with a third member of the review team.
Data extraction and management
Data were extracted from the included studies about participants' characteristics, intervention details (including whether or not the study was of a discontinuation design) and outcome measures. The data were extracted independently by two authors. Where inadequate trial data were provided, the authors were contacted in order to obtain further information. Where standard deviations were not reported but could be calculated from standard errors this was done in conventional fashion (Cochrane Handbook chapter 7.7.3.3 Higgins 2009). Any disagreements were resolved by consensus discussion with a third member of the review team.
Main planned comparisons
We planned to compare each experimental intervention with placebo or alternative comparator intervention, and, where possible, to group comparisons of interventions with similar mechanisms.
Assessment of risk of bias in included studies
Two authors independently assessed the methodological quality of the included studies using the Cochrane Collaboration tool for risk of bias (Higgins 2011). Any disagreements were resolved by consensus discussion with a third member of the review team. The risk of bias tool assesses seven domains namely: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and ‘other issues’. Each domain is assigned one of the following judgements: ‘low risk’ of bias, ‘high risk’ of bias, or ‘unclear risk’ of bias, and a supporting statement is provided to back up the judgement.
Measures of treatment effect
Data were analysed using Review Manager software (version 5.2). For binary efficacy outcomes, a pooled risk ratio (with 95% confidence intervals) was calculated using a fixed‐effect model. Risk ratios are reported since this measure can be more readily applied in clinical practice (Sackett 1996; Sinclair 1994). For continuously distributed outcomes, the mean difference (MD) was calculated when the same scale was used by all studies. Standardised mean difference was employed for comparisons across different scales.
We extracted outcome data collected from any time point, but our reported analyses use trial endpoint data, except where specified. Differences between trials in the times of assessment are reported.
Unit of analysis issues
Cluster‐randomised trials
The review protocol did not address how we would include data from cluster‐randomised trials. Although none have been encountered in this review, the appropriate methods, should they need to be included, will be included in a future update of this review.
Cross‐over trials
We intended to use data from the first period of treatment only for cross‐over trials, but we did use pooled data from both periods where these were the only data available.
Studies with multiple treatment groups
Where studies included multiple treatment groups, pair‐wise comparisons are reported. This may lead to increased correlation between estimated intervention effects across such comparisons, as described in the Cochrane Handbook. Where possible, groups were combined to create a single pair‐wise comparison or alternatively the ‘shared’ control group data were split into two or more groups with smaller sample size(s).
Dealing with missing data
We used trial data from intention‐to‐treat analyses when available. Where this was not possible, we used endpoint data for the participants who completed the trial. Where necessary, we wrote to study authors to request missing data.
Assessment of heterogeneity
Statistical heterogeneity between studies was assessed using the I2 statistic. The Cochrane Handbook suggests interpretation of an I2 of 0% to 40% as being potentially unimportant heterogeneity, 30% to 60% as representing moderate heterogeneity, 50% to 90% indicating substantial heterogeneity, and 75% to 100% indicating considerable heterogeneity (Higgins 2003).
Assessment of reporting biases
We considered methods to look for evidence of small study bias, but the number of trials identified for each comparison was too limited for this to be appropriate. Should the number of trials available increase in future updates of this review, the use of methods such as funnel plots and statistical tests to identify possible publication bias will be considered.
Data synthesis
Fixed‐effect analyses are presented unless otherwise stated. Random‐effects models were used routinely to investigate the sensitivity of results to the choice of statistical method, but we observed no cases where this altered estimates of effect qualitatively.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned by:
1. Gender of those experiencing sexual dysfunction (men only, women only, mixed group).
2. Dose of intervention.
Sensitivity analysis
Possible sensitivity analyses were considered to assess the impact of differences in study methodology on outcomes, but no such analyses were performed in view of the small numbers of trials identified for each intervention.
'Summary of findings' table
As this review addresses more than one major treatment comparison, separate 'Summary of findings' tables were prepared for interventions where data were available from three or more trials. Outcome data were summarised separately by gender, where possible. Assumed baseline risks were taken from median control group risks across included studies. Quality of evidence used was assessed using the specific evidence grading system developed by the GRADE working group (GRADE working group 2004).
Results
Description of studies
Results of the search
Over 6000 citations were identified by the search strategy of this review (Figure 1). Of these, 70 citations appeared potentially relevant and 23 were included, 22 of which provided data for quantitative analysis.

Study flow diagram
Further information was provided by the authors of several studies (Clayton 2004; Ferguson 2001; Ginsberg 2001; Jacobsen 1996; Michelson 2002; Nurnberg 2003).
Included studies
Design
We identified 23 studies that met the inclusion criteria for this review (see Characteristics of included studies). Some additional studies were identified too late for inclusion in this version of the review, and so are awaiting classification (see Characteristics of studies awaiting classification). If suitable, they will be included in a future update of the review.
Nineteen studies were of parallel‐group design, and three used a cross‐over design (Bernik 2004; Meston 2004; Nelson 2001). Length of included trials ranged from 14 days to 26 weeks. Where trials described a period of randomised treatment followed by a non‐randomised period, we have reported only the period of randomised treatment.
Sample sizes
Numbers of people randomised within individual studies varied greatly from 12 participants (Bernik 2004; Jespersen 2004), to 288 (Baldwin 2008). This update adds new data from several studies with over 100 participants (Baldwin 2008; Fava 2006; Safarinejad 2010; Safarinejad 2011).
Setting
Participants were recruited to studies taking place in a range of countries. The greatest number of studies took place in the USA and Europe, but studies were identified from South Korea (Kang 2002), and South Africa (Jespersen 2004), with two large new studies from Iran (Safarinejad 2010, Safarinejad 2011).
Participants
The total number of participants randomised in the 23 studies was 1886.
Participants in 12 of the studies had developed adverse sexual effects from the use of a selective serotonin reuptake inhibitor (SSRI). Two participants in one study had received nortriptyline, a tricyclic antidepressant (Kang 2002). One study did not specify the type of antidepressant involved (Jespersen 2004).
Seven studies included only women (Jespersen 2004; Meston 2004; Michelson 2000; Michelson 2002; Nurnberg 2002; Nurnberg 2008; Safarinejad 2011), and four studies included only men ( Fava 2006; Ginsberg 2001; Nurnberg 2003; Safarinejad 2010). One study did not describe the gender of those participating (Masand 2001), and the remaining studies recruited both men and women.
The participants in studies had wholly, or partially, recovered from the disorder for which antidepressants had been prescribed ‐ most commonly depression (see Characteristics of included studies). Two studies reported inclusion of mood and anxiety disorders (Ginsberg 2001; Kang 2002), and one study included participants treated for depression, bipolar disorder and obsessive compulsive disorder (Jacobsen 1996). One study was restricted to people treated for panic disorder (Bernik 2004).
While most studies did not restrict inclusion to particular types of sexual dysfunction, one study employing sildenafil restricted inclusion to antidepressant‐induced erectile dysfunction (Fava 2006), and another investigating bethanecol limited inclusion to ejaculatory delay or anorgasmia (Bernik 2004).
Interventions
The range of intervention types assessed was limited and is outlined below.
Addition of further medication
The majority of studies assessed the addition of further medication to ongoing antidepressant treatment using a placebo control. Two studies had more than one active treatment arm in addition to the placebo arm of the trial (Michelson 2000; Michelson 2002).
The interventions for which the greatest number of studies were identified were bupropion and sildenafil.
Sildenafil, a phosphodiesterase inhibitor, was investigated in five trials with a total of 503 participants (Fava 2006; Ginsberg 2001; Nurnberg 2002; Nurnberg 2003; Nurnberg 2008). The range of doses taken once daily varied between 25 mg and 100 mg in one study (Fava 2006), and a dose between 50 mg and 100mg was employed in the remaining four studies (Ginsberg 2001; Nurnberg 2002; Nurnberg 2003; Nurnberg 2008).
Bupropion, which is thought to act by dual noradrenaline and dopamine reuptake inhibition (Stahl 2004), was investigated in five trials with a total of 579 participants (Clayton 2004; DeBattista 2005; Masand 2001; Safarinejad 2010; Safarinejad 2011). Bupropion (sustained release) was prescribed at a dose of 150 mg daily in two studies (DeBattista 2005; Masand 2001), and at 150 mg twice daily in the remaining studies (Clayton 2004; Safarinejad 2010; Safarinejad 2011).
A wide range of other agents were investigated in fewer studies including:
-
A second phosphodiesterase inhibitor, tadalafil (in doses of 10 mg or 20 mg), tested in one study with 54 participants (Evliyaoğlu 2011).
-
Two 5HT1A receptor agonists: VML‐670, a 5‐HT1A receptor agonist (300 μg once daily) tested in a study with 288 participants (Baldwin 2008), and buspirone, another 5‐HT1A receptor agonist (30 mg daily), tested in a study with 61 participants (Michelson 2000); the Michelson 2000 study also included an active treatment as the comparison treatment, amantadine, a dopaminergic agent (50 mg twice daily).
-
A herbal extract of Ginkgo biloba: two studies with 61 participants tested a herbal extract of Ginkgo biloba (240 mg daily) (Kang 2002; Wheatley 2004).
-
Granisetron, a 5‐HT3 antagonist, was investigated in two studies with 32 participants (Jespersen 2004; Nelson 2001). Nelson 2001 used a dose of 1 mg to 2 mg, but the dosage was not specified in the other study.
-
Bethanecol (20 mg), an agent with mixed cholinergic and adrenergic effects, investigated in one study with 12 participants (Bernik 2004).
-
Two 5HT2 receptor antagonists, olanzapine (2.5 mg daily) and mirtazapine (15 mg daily) were investigated in the same four‐arm study (Michelson 2002). This study also included an active treatment comparison with yohimbine, an alpha‐2 adrenoceptor antagonist (5.4 mg daily). Yohimbine was also studied at a dose of 5.4 mg three times daily in a another trial with 33 participants (Jacobsen 1996).
-
Ephedrine was investigated in one study (Meston 2004).
-
Two different doses of maca root extract were compared in one study (Dording 2008).
Change in antidepressant prescription
One study assessed changing from an SSRI to an antidepressant, nefazodone, with a different mode of action (Ferguson 2001).
Other approaches
No studies were identified that assessed the use of drug holidays, psychological interventions, or mechanical devices to treat sexual dysfunction.
Outcomes
1) Measures of sexual function, dysfunction and satisfaction
The trials used a variety of outcome measures to assess initial sexual function and response to treatment. These included both self‐assessment and externally‐rated measures. The scales used included:
-
International Index of Erectile Function (IIEF; Rosen 1997).
-
Arizona Sexual Experiences Scale (ASEX; McGahuey 2000).
-
Changes in Sexual Functioning Questionnaire (CSFQ; Clayton 1997).
-
Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS; Althof 1999).
-
General Assessment Questions (GAQ; Evliyaoğlu 2011).
-
Sexual Side Effects Scale (SSES; Nelson 2001).
-
Brief Index of Sexual Functioning for Women (BISF‐W; Taylor 1994).
-
Feiger Sexual Function and Satisfaction Questionnaire (FSFSQ; Feiger 1996).
-
Udvalg fur Kliniske Undersogelser side effect rating scale (UKU; Lingjaerde 1987).
-
Massachussetts General Hospital‐Sexual Functioning Questionnaire (MGH‐SFQ; Labbate 2001).
-
Clinical Global Impression Scale adapted for Sexual Function (CGI‐SF; Guy 1976).
-
The Physician's Rating of Sexual Dysfunction Symptoms (PRSDS; Ferguson 2001).
-
A modified version of the Rush‐Presbyterian Sexual Function Inventory (R‐SFI; Ferguson 2001).
-
Interviewer Rating of Sexual Dysfunction, a semi‐structured interview (Michelson 2000).
-
Visual analogue scales (Bernik 2004; Michelson 2000).
-
Investigator‐devised measures (Kang 2002; Wheatley 2004).
2) Measures of psychiatric symptoms
Depressive symptoms were measured using the Hamilton Rating Scale for Depression (HAM‐D; Hamilton 1960), Clinical Global Impression, and Beck Depression Inventory (Beck 1961).
Anxiety symptoms were measured using the Hamilton Rating Scale for Anxiety (HAM‐A; Hamilton 1959), and the State‐Trait Anxiety Inventory (Spielberger 1983).
Excluded studies
A number of studies were excluded from the review (see Characteristics of excluded studies). The most common reason for exclusion was that randomised allocation was not employed, or it was not established that the sexual dysfunction was attributable to use of antidepressants.
Ongoing studies
Six studies were identified that are ongoing, or yet to report (see Characteristics of ongoing studies). It appears, from published trial registers, that one ongoing study is investigating change of antidepressant (Takeda 2011). The remaining studies are investigating trazodone (Chiang 2010), ropinirole (Hellerstein 2008), maca root (Dording 2010), combinations of testosterone with sildenafil or buspirone (Van Rooiji 2010), and Ginkgo biloba, sex therapy, and a combination of the two (Meston 2008).
Studies awaiting classification
Four studies are awaiting classification (see Characteristics of studies awaiting classification), and, if suitable, will be included in future updates of this review.
New studies found at this update
The original version of this review included 15 studies with around 900 participants. The current version includes 23 studies with 1886 participants, so there has been a substantial increase in the availability of data from randomised trials to address this area of interest.
Risk of bias in included studies
Risk of bias judgements are presented graphically in Figure 2 and Figure 3 with further details tabulated in the Characteristics of included studies table.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
There was generally little information provided on methods used for randomisation or used to maintain concealment of allocation.
Blinding
While most studies reported use of blinding, commonly a 'double‐blind' design (Figure 2), the extent of blinding was well described in a minority of studies. Meston 2004 described testing participant blinding for effectiveness, and finding that nine out of 11 women correctly identified the order of treatment received.
Incomplete outcome data
The majority of included studies did not include, or did not report, inclusion of withdrawals or dropouts in analyses. Four of the studies specified that they included withdrawals and dropouts in analyses by carrying forward prior observations (Ferguson 2001; Ginsberg 2001; Michelson 2002; Nurnberg 2003). None followed up withdrawals to obtain further outcome data. The data currently available on dropout rates for Ginsberg 2001 refer to the total over both the initial placebo‐controlled period, and the second stage of the trial in which all participants received sildenafil.
Selective reporting
It was unclear in the majority of studies whether there had been selective reporting of data, since original study protocols were not available. In some cases, only data from particular subscales or questions within a larger scale were reported.
Effects of interventions
See: Summary of findings for the main comparison Sildenafil versus placebo; Summary of findings 2 Bupropion versus placebo
Comparison one: Addition of phosphodiesterase inhibitor
1.1 Sildenafil versus placebo
Six trials were identified that compared the effect of augmenting antidepressant treatment with sildenafil or placebo. A newer trial in men with erectile dysfunction, Fava 2006, was added to the two trials included in the original version of this review (Ginsberg 2001; Nurnberg 2003). Two trials in women were identified (Nurnberg 2002; Nurnberg 2008), but outcome data were only available from one (Nurnberg 2008). A 'Summary of findings' table is available (summary of findings Table for the main comparison).
1.1.1 Effect on severity of the identified sexual dysfunction
For men, ratings of erectile dysfunction showed a benefit of sildenafil over placebo (Figure 4). Data from two studies found those receiving sildenafil scored better than those receiving placebo on questions from the IIEF regarding ability to achieve erection (MD 1.04, 95% CI 0.65 to 1.44; I2 = 34%) and maintain erection (MD 1.18, 95% CI 0.78 to 1.59; I2 = 53%) (Analysis 1.1) (Fava 2006; Nurnberg 2003). The remaining study in men found total EDITS scores also favoured sildenafil (MD 21.60, 95% CI 4.30 to 38.90) (Analysis 1.2) (Ginsberg 2001). The I2values reported are consistent with moderate statistical heterogeneity between studies on the IIEF ratings.
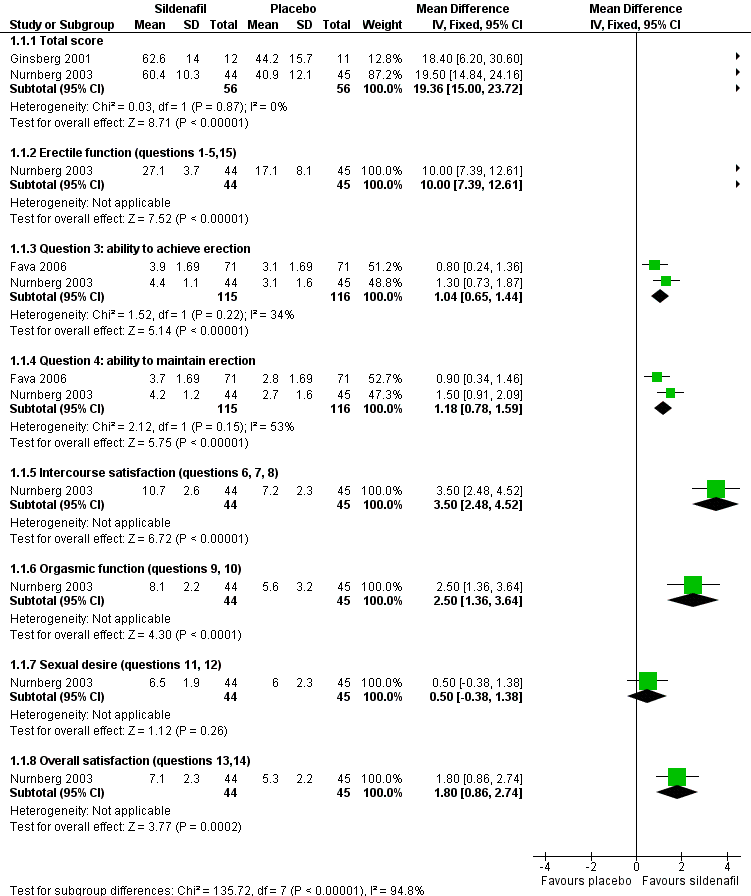
Forest plot of comparison 1: sildenafil vs placebo, outcome: 1.1 endpoint International Index of Erectile Function (IIEF) scores
For women, ratings of overall sexual dysfunction with the clinician‐rated CGI‐SF favoured sildenafil (MD ‐0.80, 95% CI ‐1.20 to ‐0.40) (Analysis 1.3), but results with the participant‐rated ASEX were consistent with benefits from either sildenafil or placebo (MD ‐0.50, 95% CI ‐2.24 to 1.24) (Analysis 1.5).
1.1.2 Effect on sexual satisfaction and functioning
Similar effects of sildenafil were observed for men (Nurnberg 2003), and women (Nurnberg 2008), on the CGI‐SF (RR 0.44, 95% CI 0.33 to 0.58) (Analysis 1.4). ASEX scores from two studies in men favoured sildenafil (MD ‐4.62, 95% CI ‐6.29 to ‐2.95; Figure 5) (Analysis 1.5) (Ginsberg 2001; Nurnberg 2003). This appears to differ from the uncertain effect found for women as noted above. In the study in women from which data are available (Nurnberg 2008), no difference was observed between sildenafil and placebo in endpoint total scores and subscales of SFQ (Analysis 1.14), and UNM‐SFI (Analysis 1.15).

Forest plot of comparison 1: sildenafil vs placebo, outcome: 1.5 endpoint Arizona Sexual Experience Scale (ASEX) total scores
In one study sildenafil was associated with improved scores on the MGH‐SFQ for total score and all domains (Analysis 1.7) (Nurnberg 2003). The IIEF, total score, and several subdomains showed benefit from sildenafil (Analysis 1.1), although increased sexual desire was not demonstrated (MD 0.50, 95% CI ‐0.38 to 1.38). In another study (Fava 2006), GEQ responses indicated improvement in erections and ability to have satisfactory sexual intercourse (Analysis 1.12; Analysis 1.13).
1.1.3 Dropout rates
Reported dropout rates were consistent with effects favouring either sildenafil or placebo (RR 0.68, 95% CI 0.41 to 1.14) (Analysis 1.9).
1.1.4 Change in the primary psychiatric condition
From the two trials that provided data, endpoint HAM‐D scores tended to favour sildenafil but could not exclude a benefit of placebo (MD ‐0.94, 95% CI ‐1.94 to 0.07) (Analysis 1.10); a similar pattern was seen when analysed dichotomously for loss of remission from depression (RR 0.33, 95% CI 0.04 to 3.09) (Analysis 1.11).
1.2 Tadalafil versus placebo
One study was identified that compared the effect of treatment with tadalafil or placebo alongside antidepressant medication (Evliyaoğlu 2011).
1.2.1 Effect on severity of the identified sexual dysfunction
Those receiving tadalafil were more likely than those on placebo to report improved erectile function on the GAQ (RR 11.50, 95% CI 3.03 to 43.67) (Analysis 2.1). IIEF data from Evliyaoğlu 2011 are not currently available in a suitable form for meta‐analysis, but qualitatively they suggest an effect on this measure for tadalafil compared to placebo.
1.2.2 Effect on sexual satisfaction and functioning
Results from use of the Sexual Encounter Profile (SEP) diary mean that a benefit of placebo could not be excluded (Analysis 2.2), including rates of overall satisfaction (RR 6.00, 95% CI 0.78 to 46.29).
1.2.3 Dropout rates
Overall rates of early discontinuation were consistent with benefits for tadalafil or placebo (RR 0.36, 95% CI 0.04 to 3.24) (Analysis 2.3), as were rates of discontinuation for apparent lack of efficacy (RR 0.36, 95% CI 0.04 to 3.24). No dropouts were attributed to adverse effects in either group.
1.2.4 Change in the primary psychiatric condition
No data were available on changes in the primary psychiatric condition.
Comparison two: Addition of bupropion
2.1 Bupropion versus placebo
There are now data available from five trials, with 579 participants, comparing the effect of augmenting antidepressant treatment with bupropion or placebo (Clayton 2004; DeBattista 2005; Masand 2001; Safarinejad 2010; Safarinejad 2011). A 'Summary of findings' table is available (summary of findings Table 2).
2.1.1 Effect on severity of the identified sexual dysfunction
Endpoint data for the total scores on rating scales used to identify sexual dysfunction were available from three studies (Clayton 2004; Safarinejad 2010; Safarinejad 2011), which gave a standardised mean difference (SMD) of 1.60 (95% CI 1.40 to 1.81) favouring bupropion over placebo (Figure 6) (Analysis 3.1). There was a high level of statistical inconsistency identified in this analysis (I2 = 86%), which appears to be driven by the contrast between the striking effect sizes reported in two studies (SMD 1.76, 95% CI 1.45 to 2.07; Safarinejad 2010: and SMD 1.73, 95% CI 1.41 to 2.05; Safarinejad 2011), and a more modest trend in the third (SMD 0.50, 95% CI ‐0.11 to 1.12; Clayton 2004). The reasons for this inconsistency are unclear.
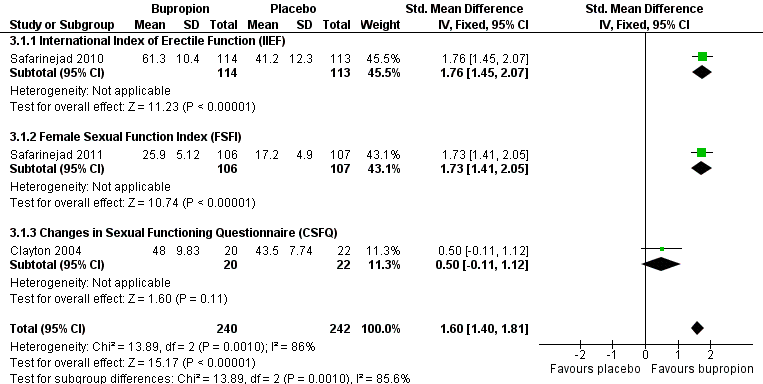
Forest plot of comparison 3: bupropion vs placebo, outcome: 3.1 endpoint scale total scores
Response rates from two studies (DeBattista 2005; Masand 2001), defined as 50% reduction in ASEX score, were consistent with greater effects of either bupropion or placebo (RR 0.62, 95% CI 0.09 to 4.41; Figure 7) (Analysis 3.2), while one study found that women taking bupropion were more likely to achieve a two‐point reduction on CGI‐SF scores than those taking placebo (RR 186.73, 95% CI 11.74 to 2969.23) (Analysis 3.2) (Safarinejad 2011).

Forest plot of comparison 3: bupropion vs placebo, outcome: 3.2 response (as defined by study)
2.1.2 Effect on sexual satisfaction and functioning
Endpoint CGI‐SF scores from two studies favoured bupropion (MD ‐1.74, 95% CI ‐1.87 to ‐1.61) (Analysis 3.8) (Safarinejad 2010; Safarinejad 2011). For men taking part in Safarinejad 2010, total scores and all subscales from IIEF, ASEX, and EDITS scales favoured bupropion over placebo (IIEF Analysis 3.3; ASEX Analysis 3.9; EDITS Analysis 3.10; Analysis 3.11). For women taking part in Safarinejad 2011, FSFI total score and subscales other than pain favoured bupropion (Analysis 3.4).
In Clayton 2004 CSFQ total scores were consistent with a greater effect of either bupropion or placebo (MD 4.50, 95% CI ‐0.89 to 9.89) (Analysis 3.5), as were subscales other than desire/frequency, where a benefit of bupropion was observed (MD 0.88, 95% CI 0.21 to 1.55).
2.1.3 Dropout rates
There was no statistically significant difference in dropout rates between bupropion and placebo (RR 1.08, 95% CI 0.67 to 1.72) (Analysis 3.6).
2.1.4 Change in the primary psychiatric condition
One trial reported endpoint HAM‐D scores (Clayton 2004), and found no significant difference between the groups (MD ‐0.60, 95% CI ‐2.62 to 1.42) (Analysis 3.7).
Comparison three: Change of antidepressant
3.1 Nefazodone versus sertraline
One trial compared the effect of changing antidepressant to nefazodone with effect of restarting sertraline after a two‐week washout period in which sertraline‐induced sexual dysfunction had resolved (Ferguson 2001).
3.1.2 Effect on severity of the identified sexual dysfunction
On a physician‐rated measure (PRSDS), sexual dysfunction was significantly less likely to re‐emerge on treatment with nefazodone compared with restarting sertraline (RR 0.34, 95% CI 0.19 to 0.60) (Analysis 4.1). This means the number needed to treat for an additional beneficial outcome (NNTB) with nefazodone, that is for one additional person to avoid re‐emergence of sexual dysfunction, was two (95% CI 2 to 4). This benefit of using nefazodone was seen by the end of the first week of treatment (Analysis 4.1).
3.1.2 Effect on sexual satisfaction and functioning
Differences in participant‐rated overall sexual satisfaction did not achieve statistical significance (MD 17.22, 95% CI ‐4.57 to 39.01) (Analysis 4.2).
3.1.3 Dropout rates
No significant difference was noted between groups in overall dropout rates, RR 0.83 (95% CI 0.43 to 1.60) nor in dropouts attributed to adverse effects, RR 0.46 (95% CI 0.17 to 1.25).
3.1.4 Change in the primary psychiatric condition
There was no significant difference in HAM‐D between the two groups at the end of the trial (MD ‐1.57, 95% CI ‐4.51 to 1.37).
Comparison four: Addition of Ginkgo biloba
4.1 Ginkgo biloba versus placebo
Two studies compared the effect of augmenting antidepressant treatment with Ginkgo biloba or placebo (Kang 2002; Wheatley 2004).
4.1.1 Effect on severity of the identified sexual dysfunction
There was no significant difference in endpoint sexual dysfunction between the groups on most questions of a nine‐item questionnaire in one study (Kang 2002) . On the 'satisfaction to orgasm' question, scores were better in the placebo arm (MD ‐1.12, 95% CI ‐2.00 to ‐0.24). Only data from the five items assessed in both genders was provided in sufficient detail for analysis.
In the second study (Wheatley 2004), total scores on an investigator‐developed scale of sexual dysfunction were compatible with a benefit of either placebo or Ginkgo biloba (MD 3.80, 95% CI ‐1.94 to 9.54) (Analysis 5.2).
4.1.2 Effect on sexual satisfaction and functioning
No other data on sexual satisfaction or functioning were reported.
4.1.3 Dropout rates
There was no significant difference in the rate of dropouts between the two arms of Kang 2002 (RR 1.33, 95% CI 0.51 to 3.43) (Analysis 5.3). There was an overall dropout rate of 22% in Wheatley 2004, but the distribution of dropouts between treatment arms was unclear.
4.1.4 Change in the primary psychiatric condition
No data were available on changes in the primary psychiatric condition from Kang 2002. Ratings of depression and anxiety symptoms were obtained by Wheatley 2004, but sufficient details are not available for analysis here.
Comparison five: Addition of granisetron
5.1 Granisetron versus placebo
Two trials compared augmentation of antidepressant treatment with granisetron to the addition of placebo (Jespersen 2004; Nelson 2001). Data from Nelson 2001 were derived from both cross‐over periods of the trial.
5.1.1 Effect on severity of the identified sexual dysfunction
In the Nelson 2001 trial both groups had similar change from baseline on SSES scores (MD 0.10, 95% CI ‐2.22 to 2.42). In the Jespersen 2004 trial, total scores on both ASEX and FSFSQ were consistent with greater benefit from either granisetron or placebo (ASEX MD 7.90, 95% CI ‐1.87 to 17.67: FSFSQ MD 1.60, 95% CI ‐5.46 to 8.66).
5.1.2 Effect on sexual satisfaction and functioning
In Jespersen 2004, most item scores on both ASEX and FSFSQ were consistent with greater benefit from either granisetron or placebo (Analysis 6.2; Analysis 6.3). Where items did favour one agent, placebo performed better than granisetron (FSFSQ items 1 and 2; and ASEX orgasm satisfaction).
5.1.3 Dropout rates
There was no statistically significant difference in dropout rates between the two groups in the Jespersen 2004 trial (RR 6.67, 95% CI 0.39 to 114.78) (Analysis 6.4). There was an overall dropout rate of 35% in Nelson 2001, but the distribution of the dropouts between treatments was not clear.
5.1.4 Change in the primary psychiatric condition
Rates of recurrence of mood symptoms were consistent with benefit of either agent in the Nelson 2001 study (RR 2.87, 95%CI 0.12 to 66.75) (Analysis 6.5). In the Jespersen 2004 study it was reported that CGI for depressive symptoms did not differ between groups, but insufficient details were available for analysis.
Comparison six: Addition of a 5HT1A receptor agonist
6.1 VML‐670 versus placebo
Only the Baldwin 2008 study compared augmentation of antidepressant treatment with VML‐670 to the addition of placebo.
6.1.1 Effect on severity of the identified sexual dysfunction
By the end of the trial, rates of absence of sexual dysfunction, defined by ASEX, were consistent with benefits of either VML‐607 or placebo (RR 1.24, 95% CI 0.86 to 1.77) (Analysis 7.1), as were rates of improvement defined by CGI (RR 1.24, 95% CI 0.71 to 2.17) (Analysis 7.2).
6.1.2 Effect on sexual satisfaction and functioning
ASEX total scores were not presented with sufficient detail for analysis in this review. Items of the ASEX were mostly consistent with benefits of either VML‐607 or placebo (Analysis 7.3), with the exception that men randomised to VML‐670 reported a greater improvement in erectile function (MD ‐0.40, 95% CI ‐0.80 to 0.00).
6.1.3 Dropout rates
No significant difference was noted between groups in overall dropout rates (RR 0.97, 95% CI 0.56 to 1.68), or in dropouts attributed to adverse effects (RR 2.32, 95% CI 0.75 to 7.21) (Analysis 7.4).
6.1.4 Change in the primary psychiatric condition
Insufficient details on HAM‐D scores were available for analysis in this review.
6.2 Buspirone versus placebo
One trial compared the effect in women of augmenting antidepressant treatment with buspirone or placebo (Michelson 2000).
6.2.1 Effect on severity of the identified sexual dysfunction
Sexual dysfunction was identified for study inclusion by clinician‐rated global impression, but insufficient details of the results were presented to allow for analysis in this review.
6.2.2 Effect on sexual satisfaction and functioning
Changes in ratings on visual analogue scales of aspects of sexual functioning were consistent with benefits for either buspirone or placebo (Analysis 8.1), as was change in a summary measure of overall functioning (MD 3.10, 95% CI ‐38.33 to 44.53).
6.2.3 Dropout rates
No significant difference was noted between groups in overall dropout rates (RR 2.00, 95% CI 0.20 to 20.41) (Analysis 8.2)
6.2.4 Change in the primary psychiatric condition
Visual analogue scale ratings of mood (MD 0.80, 95% CI ‐7.61 to 9.21) and energy (MD 5.30, 95% CI ‐3.88 to 14.48) (Analysis 8.1) were consistent with benefits for either buspirone or placebo. Insufficient details on specific depression and anxiety rating scale scores were presented to permit analysis in this review.
Comparison seven: Addition of bethanecol
7.1 Bethanecol versus placebo
One study compared augmentation of antidepressant treatment with bethanecol against augmentation with placebo (Bernik 2004).
7.1.1 Effect on severity of the identified sexual dysfunction
Those receiving bethanecol reported higher scores than those receiving placebo on a six‐point visual analogue scale of orgasmic function (MD 3.40, 95% CI 0.99 to 5.81) (Analysis 9.1).
7.1.2 Effect on sexual satisfaction and functioning
Data on erectile function were obtained, but not reported.
7.1.3 Dropout rates
Two of the 12 participants dropped out during the study, but it was unclear from which treatment group(s).
7.1.4 Change in the primary psychiatric condition
One participant (1/12) developed a depressive episode during the study, but it was unclear which treatment s/he was receiving at the time.
Comparison eight: Addition of a 5HT2 receptor antagonist
One trial compared augmentation of antidepressant treatment in women with agents including placebo and two 5HT2 receptor antagonists, olanzapine and mirtazapine (Michelson 2002). These comparisons against placebo are considered separately below:
8.1 Olanzapine versus placebo
8.1.1 Effect on severity of the identified sexual dysfunction
The group receiving olanzapine reported a greater improvement on a scale of overall sexual satisfaction completed at interview (MD ‐0.70, 95% CI ‐1.17 to ‐0.23). Other measures of sexual function completed at the same time were consistent with benefits for either olanzapine or placebo (Analysis 10.1).
8.1.2 Effect on sexual satisfaction and functioning
Diary ratings were all consistent with benefits for either olanzapine or placebo (Analysis 10.2).
8.1.3 Dropout rates
There was an overall dropout rate of 27%, but the distribution of dropouts between treatment group(s) was unclear. Dropouts attributed to adverse effects were consistent with benefits for either olanzapine or placebo (RR 3.59, 95% CI 0.80 to 16.21) (Analysis 10.3).
8.1.4. Change in the primary psychiatric condition
Change in mood did not differ between groups on diary ratings (MD 0.10, 95% CI ‐0.41 to 0.61) (Analysis 10.2). HAM‐D scores were collected, but insufficient details were provided to allow for analysis.
8.2 Mirtazapine versus placebo
8.2.1 Effect on severity of the identified sexual dysfunction
Participant ratings of sexual function completed at interview were consistent with benefits for either mirtazapine or placebo (Analysis 11.1), including overall sexual satisfaction (MD 0.10, 95% CI ‐0.29 to 0.49).
8.2.2 Effect on sexual satisfaction and functioning
Diary ratings of overall sexual function were consistent with benefits for either mirtazapine or placebo (MD ‐1.30, ‐5.71 to 3.11), as were other diary ratings of sexual function (Analysis 11.2).
Kinsey Structured Interview ratings of sexual satisfaction were better in those receiving placebo than mirtazapine (MD 0.60, 95% CI 0.19 to 1.01) (Analysis 11.3).
8.2.3 Dropout rates
There was an overall dropout rate of 27%, but the distribution of the dropouts between treatments was unclear. More participants dropped out of the mirtazapine group than the placebo group because of adverse effects (RR 6.50, 95% CI 1.56 to 27.07) (Analysis 11.4).
8.2.4 Change in the primary psychiatric condition
Change in mood did not differ between groups on diary ratings (MD ‐0.40, 95% CI ‐0.93 to 0.13). Changes in ratings of energy were better in those receiving mirtazapine (MD ‐0.70, 95% CI ‐1.38 to ‐0.02) (Analysis 11.2). HAM‐D scores were collected, but insufficient details were provided to allow for analysis.
Comparison nine: Yohimbine versus placebo
One trial compared augmentation of antidepressant treatment in women with yohimbine or placebo (Michelson 2002), and provided outcome data for this review. A second study has also been performed (Jacobsen 1996), but data were not available in a form suitable for analysis in this review.
9.1. Effect on severity of the identified sexual dysfunction
Participant ratings of sexual function completed at interview were consistent with benefits for either yohimbine or placebo (Analysis 12.1), including overall sexual satisfaction (MD ‐0.30, 95% CI ‐0.79 to 0.19).
9.2. Effect on sexual satisfaction and functioning
Diary ratings of overall sexual function were consistent with benefits for either yohimbine or placebo (MD 1.20, 95% CI ‐3.24 to 5.64), as were other diary ratings of sexual function (Analysis 12.2).
9.3. Dropout rates
There was an overall dropout rate of 27%, but the distribution of dropouts between treatment group(s) was unclear. Dropouts attributed to adverse effects were consistent with benefits for either yohimbine or placebo (RR 2.23, 95% CI 0.43 to 11.43) (Analysis 12.3).
9.4. Change in the primary psychiatric condition
Change in mood did not differ between groups on diary ratings (MD 0.10, 95% CI ‐0.41 to 0.61) (Analysis 12.2). HAM‐D scores were collected, but insufficient details were provided to allow for analysis.
Comparison ten: Amantadine versus placebo
One trial compared augmentation of antidepressant treatment in women with amantadine or placebo (Michelson 2000).
10.1. Effect on severity of the identified sexual dysfunction
Sexual dysfunction was identified for study inclusion by clinician‐rated global impression, but insufficient details of the results were presented to allow for analysis here.
10.2. Effect on sexual satisfaction and functioning
Changes in ratings on visual analogue scales of aspects of sexual functioning were consistent with benefits for either amantadine and placebo (Analysis 13.1).
10.3. Dropout rates
No significant difference was noted between groups in overall dropout rates (RR 1.11, 95% CI 0.07 to 16.47) (Analysis 13.2).
10.4. Change in the primary psychiatric condition
Participants randomised to amantadine reported a greater increase in ratings of both mood (MD 8.10, 95% CI 1.23 to 14.97) and energy (MD 12.70, 95% CI 5.30 to 20.10) (Analysis 13.1). Insufficient details on specific depression and anxiety rating scale scores were presented to allow for analysis here.
Comparison eleven: Ephedrine versus placebo
One trial compared the effect of augmenting antidepressant treatment with ephedrine or placebo (Meston 2004). Data were derived from both cross‐over periods of the trial.
11.1. Effect on severity of the identified sexual dysfunction
Ratings at the end of treatment on the BISF‐W were consistent with benefits from either ephedrine or placebo (Analysis 14.1).
11.2. Effect on sexual satisfaction and functioning
No measures to assess sexual satisfaction and functioning were employed in this trial.
11.3. Dropout rates
There was an overall dropout rate of 34%, but the distribution of dropouts between treatment group(s) was unclear.
11.4. Change in the primary psychiatric condition
Ratings of depression were made, but insufficient details were presented to allow for analysis here.
Comparison twelve: Maca root low dose (1.5 g) versus maca root high dose (3 g)
One study was identified that assessed the effects of two different doses of maca root on antidepressant‐induced sexual dysfunction (Dording 2008).
12.1. Effect on severity of the identified sexual dysfunction
Participants randomised to either dose of maca root reported similar endpoint ratings by ASEX total score (MD ‐0.80, 95% CI ‐6.49 to 4.89) (Analysis 15.1) and MGH‐SFQ total score (MD ‐2.00, 95% CI ‐7.69 to 3.69) (Analysis 15.2).
12.2. Effect on sexual satisfaction and functioning
Similarly, ratings of sexual desire items from both ASEX (MD 0.30, 95% CI ‐1.38 to 1.98) (Analysis 15.1) and MGH‐SFQ (MD ‐0.10, 95% CI ‐1.62 to 1.42) (Analysis 15.2) were similar between groups.
12.3. Dropout rates
Rates of overall dropout did not differ between groups (RR 1.50, 95% CI 0.60 to 3.74) (Analysis 15.3).
12.4. Change in the primary psychiatric condition
The HAM‐D total endpoint score was not significantly different between the groups (MD ‐2.50, 95% CI ‐7.98 to 2.98) nor was the HAM‐A total endpoint score (MD ‐0.40, 95% CI ‐3.15 to 2.35) (Analysis 15.4).
Subgroup and sensitivity analyses
Due to the small number of studies in each comparison we were unable to conduct the planned subgroup and sensitivity analyses.
Discussion
Summary of main results
Twenty‐three trials involving 1886 people met the inclusion criteria for the updated review. The original version of this review included 15 studies with around 900 participants randomised, so there has been a substantial increase in the data available from randomised trials to address this area of interest. This update has included both increased data for previously identified strategies (addition of sildenafil or bupropion) and trials of new agents for this indication. All except one trial (Ferguson 2001) assessed the addition of further medication to treat the sexual dysfunction.
We were unable to do the intended subgroup analyses by gender, but these are summarised descriptively here to facilitate interpretation.
Men
In men, data for the phosphodiesterase inhibitors sildenafil (three studies, 255 participants; see summary of findings Table for the main comparison) and tadalafil (one study, 54 participants) indicated that they led to a greater improvement in erectile function than placebo. The estimates of treatment effect observed are similar to those reported for their use in erectile dysfunction due to other causes (Fink 2002; Carson 2004).
In men, addition of higher dose bupropion has also shown benefit (see summary of findings Table 2), but this is primarily shown in a single study (234 participants; Safarinejad 2010). Replication of this effect is likely to increase confidence in this finding. The magnitude of effect seen on total IIEF score (MD 20.10, 95% CI 17.14 to 23.06) (Analysis 3.3) was similar to that seen in studies of sildenafil (MD 19.36, 95% CI 15.00 to 23.72) (Analysis 1.1).
There are also some promising data from a small cross‐over trial of bethanecol suggesting benefit for men with antidepressant‐induced ejaculatory delay or anorgasmia rather than erectile dysfunction (Bernik 2004). Replication in further studies is likely to be necessary for clinicians and patients to have confidence in this effect.
Women
For women it remains uncertain whether sildenafil is more effective than placebo, but outcome data are only available from one study (Nurnberg 2008). We have identified a larger unpublished study (Nurnberg 2002) ‐ if results from this second study were to become available, it would be expected to reduce this uncertainty substantially.
Data from studies of bupropion 150 mg twice daily indicate a benefit over placebo. These included a study that predominantly included women (Clayton 2004), and one solely in women (Safarinejad 2011). However, response rates in two studies of bupropion 150 mg once daily were consistent with superiority of either bupropion or placebo. The two‐point improvement in CGI taken as a measure of response in Safarinejad 2011 is equivalent to an improvement in rating from 'markedly' to 'mildly' impaired function, which may be a clinically significant change.
Both sexes
Other augmentation strategies studied failed to show significant improvements in sexual dysfunction compared with placebo
One trial involving 75 participants of both genders with sexual dysfunction due to sertraline assessed the effect of changing the antidepressant. Switching to nefazodone was significantly less likely to result in the re‐emergence of sexual dysfunction than restarting sertraline, and was not associated with any worsening of depression. However, since nefazodone is no longer available for use, this strategy is now of limited usefulness.
Adverse effects
We hypothesised that management strategies for antidepressant‐induced sexual dysfunction might differ in acceptability or be associated with a worsening of the condition for which antidepressants were originally being taken. We have identified no data for any of the strategies assessed here that indicated they led to a worsening of psychiatric symptoms. However, the relatively small numbers assessed for many of the interventions studied means that the possibility of such an effect cannot confidently be excluded in all cases.
Rates of dropout from studies with different treatments were not clearly presented in all cases. From the available data, only one intervention was associated with an increase in people dropping out of the study, which was augmentation with mirtazapine (Michelson 2002). However, the analysis of this four‐arm study does not correct for the multiple statistical comparisons that result, and therefore the 95% confidence intervals presented may overestimate the confidence with which this effect has been shown. Further randomised trial data may act to reduce this uncertainty.
Overall completeness and applicability of evidence
The available data mostly investigate the strategy of adding additional medication to counteract antidepressant‐induced sexual dysfunction. There is an absence of randomised data that assesses switching to currently‐available antidepressant agents with lower rates of sexual adverse effects, the role of psychological or mechanical interventions (Hawton 1995), or of techniques such as drug holidays. There is a particular need for more evidence to guide treatment of antidepressant‐induced sexual dysfunction in women. The ongoing studies identified suggest that this limitation of the evidence‐base will be only partially be addressed in the foreseeable future. Of the six studies identified, only one is investigating change of antidepressant (Takeda 2011), and only one other is investigating Ginkgo biloba, sex therapy, and a combination of the two (Meston 2008).
Quality of the evidence
Twenty‐three trials involving 1886 people were included in this review. Many studies were unclear in their reporting of methods, which leaves uncertainty regarding the extent to which their results are subject to bias (see Figure 2). Use of a double‐blind design was commonly reported, but there was generally little information provided on methods used for randomisation or to maintain concealment of allocation. Studies may have been vulnerable to reporting bias, since original study protocols were not available, and, in some cases, only data from particular subscales or questions within a larger scale were reported.
There is now a strong and internally consistent evidence base for the use of phosphodiesterase inhibitors for antidepressant‐induced erectile dysfunction in men. For women with antidepressant‐induced sexual dysfunction, the addition of bupropion at higher doses appears to be the most promising approach studied so far, however, unexplained inconsistencies in effects between studies remain, reducing the confidence with which this strategy can be advocated.
Potential biases in the review process
While we have made efforts to be comprehensive in our methods to identify all published studies, given the small numbers of studies that investigated most of the strategies assessed, the presence of any unpublished trials could have substantial effects on estimates of effect. In some cases, only results from particular items or subscales within ratings scales are available. It is likely that this could act to bias estimates of effect obtained, increasing apparent effectiveness.
The investigation of multiple strategies and often multiple outcomes for each strategy within this review will have increased the risk of reporting false positive findings of effectiveness.
Agreements and disagreements with other studies or reviews
The Cochrane Review on the management of sexual dysfunction due to antipsychotic drug therapy similarly found some support for the addition of sildenafil in men (Schmidt 2012), but the evidence available came from only one small study (32 participants). We are not aware of any non‐Cochrane systematic reviews on this topic.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison 1: sildenafil vs placebo, outcome: 1.1 endpoint International Index of Erectile Function (IIEF) scores

Forest plot of comparison 1: sildenafil vs placebo, outcome: 1.5 endpoint Arizona Sexual Experience Scale (ASEX) total scores

Forest plot of comparison 3: bupropion vs placebo, outcome: 3.1 endpoint scale total scores

Forest plot of comparison 3: bupropion vs placebo, outcome: 3.2 response (as defined by study)

Comparison 1 Sildenafil vs placebo, Outcome 1 Endpoint International Index of Erectile Function (IIEF) scores.

Comparison 1 Sildenafil vs placebo, Outcome 2 Endpoint Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) scores.

Comparison 1 Sildenafil vs placebo, Outcome 3 Endpoint Clinical Global Impression ‐ Sexual Function.

Comparison 1 Sildenafil vs placebo, Outcome 4 Clinical Global Impression ‐Sexual Function not "much/very much improved" by endpoint.

Comparison 1 Sildenafil vs placebo, Outcome 5 Endpoint Arizona Sexual Experience Scale (ASEX) total scores.

Comparison 1 Sildenafil vs placebo, Outcome 6 Males: endpoint Arizona Sexual Experience Scale scores.

Comparison 1 Sildenafil vs placebo, Outcome 7 Endpoint MGH‐Sexual Functioning Questionnaire scores.

Comparison 1 Sildenafil vs placebo, Outcome 8 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint.

Comparison 1 Sildenafil vs placebo, Outcome 9 Dropouts.

Comparison 1 Sildenafil vs placebo, Outcome 10 Endpoint Hamilton Rating Scale for Depression score.

Comparison 1 Sildenafil vs placebo, Outcome 11 Loss of remission: Hamilton Rating Scale for Depression score > 9.

Comparison 1 Sildenafil vs placebo, Outcome 12 Global Efficacy Questionnaire (questions 1 & 2).

Comparison 1 Sildenafil vs placebo, Outcome 13 Global efficacy questionnaire (question 3).

Comparison 1 Sildenafil vs placebo, Outcome 14 Endpoint Sexual Function Questionnaire (SFQ).

Comparison 1 Sildenafil vs placebo, Outcome 15 UNM Sexual Function Inventory.

Comparison 1 Sildenafil vs placebo, Outcome 16 Females: endpoint Arizona Sexual Experience Scale scores.

Comparison 2 Tadalafil vs placebo, Outcome 1 Global Assessment Questions.

Comparison 2 Tadalafil vs placebo, Outcome 2 Endpoint Sexual Encounter Profile (SEP).

Comparison 2 Tadalafil vs placebo, Outcome 3 Dropouts.

Comparison 3 Bupropion vs placebo, Outcome 1 Endpoint scale total scores.

Comparison 3 Bupropion vs placebo, Outcome 2 Response (as defined by study).

Comparison 3 Bupropion vs placebo, Outcome 3 Endpoint International Index of Erectile Function (IIEF).

Comparison 3 Bupropion vs placebo, Outcome 4 Endpoint Female Sexual Function Index score.

Comparison 3 Bupropion vs placebo, Outcome 5 Endpoint Changes in Sexual Functioning Questionnaire score.

Comparison 3 Bupropion vs placebo, Outcome 6 Dropouts.

Comparison 3 Bupropion vs placebo, Outcome 7 Endpoint Hamilton Rating Scale for Depression score.

Comparison 3 Bupropion vs placebo, Outcome 8 Endpoint Clinical Global Impression (CGI ‐ SF).

Comparison 3 Bupropion vs placebo, Outcome 9 Endpoint ASEX.

Comparison 3 Bupropion vs placebo, Outcome 10 Endpoint EDITS (participant).

Comparison 3 Bupropion vs placebo, Outcome 11 Endpoint EDITS (partner).

Comparison 4 Nefazodone vs sertraline, Outcome 1 Re‐emergence of antidepressant‐induced sexual dysfunction (physician rated).

Comparison 4 Nefazodone vs sertraline, Outcome 2 Overall degree of sexual satisfaction (participant rated).

Comparison 4 Nefazodone vs sertraline, Outcome 3 Dropouts.

Comparison 4 Nefazodone vs sertraline, Outcome 4 Hamilton Rating Scale for Depression score.

Comparison 5 Ginkgo biloba vs placebo, Outcome 1 Endpoint sexual function ratings (investigator questionnaire).

Comparison 5 Ginkgo biloba vs placebo, Outcome 2 Sexual Dysfunction Scale (investigator developed).

Comparison 5 Ginkgo biloba vs placebo, Outcome 3 Dropouts.

Comparison 6 Granisetron vs placebo, Outcome 1 Change from baseline on Sexual Side Effects Scale (SSES) total score.

Comparison 6 Granisetron vs placebo, Outcome 2 Endpoint Feiger Sexual Function and Satisfaction Questionnaire score.

Comparison 6 Granisetron vs placebo, Outcome 3 Endpoint Arizona Sexual Experience Scale (ASEX) score.

Comparison 6 Granisetron vs placebo, Outcome 4 Dropouts.

Comparison 6 Granisetron vs placebo, Outcome 5 Recurrence of mood symptoms.

Comparison 7 VML‐670 vs placebo, Outcome 1 Absence of sexual dysfunction at end point.

Comparison 7 VML‐670 vs placebo, Outcome 2 'Improved' or 'much improved' on Clinical Global Impression.

Comparison 7 VML‐670 vs placebo, Outcome 3 Change in Arizona Sexual Experiences Scale (ASEX) item scores.

Comparison 7 VML‐670 vs placebo, Outcome 4 Dropouts.

Comparison 8 Buspirone vs placebo, Outcome 1 Change in patient‐rated visual analogue scales.

Comparison 8 Buspirone vs placebo, Outcome 2 Dropouts.

Comparison 9 Bethanecol vs placebo, Outcome 1 Visual analogue scale of orgasmic function ‐ best score achieved.

Comparison 10 Olanzapine vs placebo, Outcome 1 Change in patient rated assessment of sexual function.

Comparison 10 Olanzapine vs placebo, Outcome 2 Change in diary ratings (visual analogue scales).

Comparison 10 Olanzapine vs placebo, Outcome 3 Dropouts due to adverse effects.

Comparison 11 Mirtazapine vs placebo, Outcome 1 Change in patient rated assessment of sexual function.

Comparison 11 Mirtazapine vs placebo, Outcome 2 Change in diary ratings (visual analogue scales).

Comparison 11 Mirtazapine vs placebo, Outcome 3 Endpoint modified Kinsey Structured Interview.

Comparison 11 Mirtazapine vs placebo, Outcome 4 Dropouts.

Comparison 12 Yohimbine vs placebo, Outcome 1 Change in patient rated assessment of sexual function.

Comparison 12 Yohimbine vs placebo, Outcome 2 Change in diary ratings (visual analogue scales).

Comparison 12 Yohimbine vs placebo, Outcome 3 Dropouts.

Comparison 13 Amantadine vs placebo, Outcome 1 Change in patient‐rated visual analogue scales.

Comparison 13 Amantadine vs placebo, Outcome 2 Dropouts.

Comparison 14 ephedrine vs placebo, Outcome 1 Endpoint Brief Index of Sexual Functioning for Women (BISF‐W).

Comparison 15 Maca root: high vs low dose, Outcome 1 Endpoint Arizona Sexual Experiences Scale (ASEX) score.

Comparison 15 Maca root: high vs low dose, Outcome 2 Endpoint MGH‐SFQ.

Comparison 15 Maca root: high vs low dose, Outcome 3 Dropouts.

Comparison 15 Maca root: high vs low dose, Outcome 4 Endpoint ratings of psychiatric symptoms.
| Sildenafil compared with placebo for antidepressant‐induced sexual dysfunction | ||||||
| Patient or population: people with antidepressant‐induced sexual dysfunction Settings: outpatient Intervention: sildenafil Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sildenafil | |||||
| Endpoint International Index of Erectile Function (IIEF) total scores (The IIEF is a self‐report measure with 15 questions examining erectile function, orgasmic function, sexual desire, and intercourse satisfaction. Maximum possible score 75) | The mean IIEF score ranged across control groups from | The mean IIEF score in the intervention groups was | 112 men | ⊕⊕⊕⊕ | ||
| Endpoint International Index of Erectile Function (IIEF) scores ‐ question 3: ability to achieve erection (Maximum score 5) | The mean score in control groups was 3.1 | The mean score in the intervention groups was | 231 men | ⊕⊕⊕⊕ | ||
| Endpoint International Index of Erectile Function (IIEF) scores ‐ intercourse satisfaction (questions 6, 7, 8) (Maximum score 15) | The mean score in the control group was 7.2 | The mean score in the intervention group was | 89 men | ⊕⊕⊕⊝ | ||
| Clinical Global Impression ‐Sexual Function not "much/very much improved" by endpoint | Male population | RR 0.44 (0.33 to 0.58) | 187 | ⊕⊕⊕⊝ | ||
| 956 per 1000 | 459 per 1000 | |||||
| Female population | ||||||
| 735 per 1000 | 287 per 1000 | |||||
| Dropouts (People leaving the trial early) | Low risk population | RR 0.68 (0.41 to 1.14) | 353 | ⊕⊕⊕⊕ | ||
| 90 per 1000 | 61 per 1000 | |||||
| Medium risk population | ||||||
| 250 per 1000 | 170 per 1000 | |||||
| High risk population | ||||||
| 360 per 1000 | 245 per 1000 | |||||
| Global Efficacy Questionnaire (questions 1 & 2) (Questions assessing improvement attributed to medication compared to having no treatment at all) | Improvement in erections | RR 2.50 (1.67 to 3.73) and RR 2.55 (1.71 to 3.80) | 284 men | ⊕⊕⊕⊝ | ||
| 282 per 1000 | 705 per 1000 | |||||
| Improvement in ability to have sexual intercourse | ||||||
| 282 per 1000 | 719 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The evidence for effects based on single trials is rated as moderate quality since further trial data may well change the estimate. | ||||||
| Bupropion compared with placebo for antidepressant‐induced sexual dysfunction | ||||||
| Patient or population: people with antidepressant‐induced sexual dysfunction Settings: outpatients Intervention: bupropion (doses of 150 mg daily and 150 mg twice daily) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Bupropion | |||||
| Endpoint scale total scores (150 mg twice daily dose) (Scales of sexual functioning. As studies used different scales to assess sexual functioning, differences are expressed as standardised mean differences (SMD)) | The mean value with this analysis is in effect zero | The mean score in the intervention groups was 1.60 higher (1.40 to 1.81) | 482 | ⊕⊕⊕⊝ | ||
| 50% reduction in score on the Arizona Sexual Experiences Scale (ASEX) (150 mg once daily dose) (The ASEX is a 5‐item self‐report inventory of sexual function) | Lower risk population | RR 0.62 (0.09 to 4.41) | 71 | ⊕⊕⊕⊝ | ||
| 47 per 1000 | 29 per 1000 | |||||
| Higher risk population | ||||||
| 67 per 1000 | 41 per 1000 | |||||
| Dropouts (People leaving the trial early) | Lower risk population | RR 1.08 (0.67 to 1.72) | 579 | ⊕⊕⊕⊕ | ||
| 90 per 1000 | 97 per 1000 | |||||
| Higher risk population | ||||||
| 150 per 1000 | 162 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unexplained inconsistency in effects between studies reduces confidence in this effect | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint International Index of Erectile Function (IIEF) scores Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 19.36 [15.00, 23.72] |
| 1.2 Erectile function (questions 1‐5,15) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [7.39, 12.61] |
| 1.3 Question 3: ability to achieve erection | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [0.65, 1.44] |
| 1.4 Question 4: ability to maintain erection | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [0.78, 1.59] |
| 1.5 Intercourse satisfaction (questions 6, 7, 8) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [2.48, 4.52] |
| 1.6 Orgasmic function (questions 9, 10) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.36, 3.64] |
| 1.7 Sexual desire (questions 11, 12) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.38, 1.38] |
| 1.8 Overall satisfaction (questions 13,14) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.86, 2.74] |
| 2 Endpoint Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endpoint Clinical Global Impression ‐ Sexual Function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Clinical Global Impression ‐Sexual Function not "much/very much improved" by endpoint Show forest plot | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.33, 0.58] |
| 4.1 Males | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.66] |
| 4.2 Females | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.24, 0.62] |
| 5 Endpoint Arizona Sexual Experience Scale (ASEX) total scores Show forest plot | 3 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐2.65 [‐3.86, ‐1.44] |
| 5.1 Males | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐6.29, ‐2.95] |
| 5.2 Females | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.24, 1.24] |
| 6 Males: endpoint Arizona Sexual Experience Scale scores Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐6.29, ‐2.95] |
| 6.2 Sexual desire | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.08, ‐0.12] |
| 6.3 Arousal | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.06, ‐0.14] |
| 6.4 Erectile function | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.66, ‐0.74] |
| 6.5 Orgasm (ability) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐1.90, ‐0.90] |
| 6.6 Orgasm (satisfaction) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.58, ‐0.42] |
| 7 Endpoint MGH‐Sexual Functioning Questionnaire scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Dropouts Show forest plot | 4 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.41, 1.14] |
| 10 Endpoint Hamilton Rating Scale for Depression score Show forest plot | 2 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.94, 0.07] |
| 11 Loss of remission: Hamilton Rating Scale for Depression score > 9 Show forest plot | 3 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.09] |
| 12 Global Efficacy Questionnaire (questions 1 & 2) Show forest plot | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.90, 3.35] |
| 12.1 Improvement in erections | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [1.67, 3.73] |
| 12.2 Improvement in ability to have sexual intercourse | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.71, 3.80] |
| 13 Global efficacy questionnaire (question 3) Show forest plot | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.65, 1.75] |
| 13.1 Question 3: Frequency of erection that allowed satisfactory sexual intercourse | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.65, 1.75] |
| 14 Endpoint Sexual Function Questionnaire (SFQ) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Arousal‐sensation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Arousal‐lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.4 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.5 Enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.6 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.7 Partner | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 UNM Sexual Function Inventory Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Sexual arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.5 Ability to reach orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.6 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Females: endpoint Arizona Sexual Experience Scale scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.4 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.5 Orgasm (satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.6 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global Assessment Questions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Has the treatment you have been taking improved your erectile function? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.5 [3.03, 43.67] |
| 1.2 Has the treatment improved your ability to engage in sexual activity? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.5 [3.03, 43.67] |
| 2 Endpoint Sexual Encounter Profile (SEP) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Question 2: Were you able to insert your penis into your partner's vagina? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.68, 8.01] |
| 2.2 Question 3: Did your erection last long enough for you to have successful intercourse? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.78, 46.29] |
| 2.3 Question 4: Were you satisfied with the hardness of your erection? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.78, 46.29] |
| 2.4 Question 5: Were you satisfied overall with this sexual experience? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.78, 46.29] |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Overall | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 3.2 Lack of efficacy | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 3.3 Adverse effects | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint scale total scores Show forest plot | 3 | 482 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.60 [1.40, 1.81] |
| 1.1 International Index of Erectile Function (IIEF) | 1 | 227 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.76 [1.45, 2.07] |
| 1.2 Female Sexual Function Index (FSFI) | 1 | 213 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.73 [1.41, 2.05] |
| 1.3 Changes in Sexual Functioning Questionnaire (CSFQ) | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.11, 1.12] |
| 2 Response (as defined by study) Show forest plot | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 31.77 [10.10, 99.89] |
| 2.1 50% reduction Arizona Sexual Experiences Scale (ASEX) | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.09, 4.41] |
| 2.2 Clinical Global Impression (CGI‐SF) 2‐point improvement | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 186.73 [11.74, 2969.23] |
| 3 Endpoint International Index of Erectile Function (IIEF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Total score | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 20.10 [17.14, 23.06] |
| 3.2 Erectile function | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 9.30 [8.18, 10.42] |
| 3.3 Orgasmic function | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [2.15, 3.25] |
| 3.4 Sexual desire | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.76, 2.44] |
| 3.5 Intercourse satisfaction | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 3.6 [3.00, 4.20] |
| 3.6 Overall satisfaction | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [1.99, 3.21] |
| 4 Endpoint Female Sexual Function Index score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Endpoint Changes in Sexual Functioning Questionnaire score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Desire/interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Desire/frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Arousal/excitement | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Completion/orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Dropouts Show forest plot | 5 | 579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.72] |
| 7 Endpoint Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Endpoint Clinical Global Impression (CGI ‐ SF) Show forest plot | 2 | 440 | Mean Difference (IV, Fixed, 95% CI) | ‐1.74 [‐1.87, ‐1.61] |
| 8.1 Male | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐1.80, ‐1.20] |
| 8.2 Female | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐1.95, ‐1.65] |
| 9 Endpoint ASEX Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 Ability to reach orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 Satisfaction with orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Endpoint EDITS (participant) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Expectations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Likelihood of continuing | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 Confidence | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.6 Partner satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.7 Partner desire to continue treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.8 Naturalness of achieving erection | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.9 Naturalness of erection hardness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.10 Quickness of achieving erection | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.11 Duration that erection lasts | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.12 Ease of use | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Endpoint EDITS (partner) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Expectations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Sexual desirability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Participant's feelings about continuing treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Duration that erection lasts | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Re‐emergence of antidepressant‐induced sexual dysfunction (physician rated) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Week 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Endpoint | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall degree of sexual satisfaction (participant rated) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Last rating recorded | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Overall | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Attributed to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint sexual function ratings (investigator questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Erection maintenance time | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Orgasm frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Satisfaction to orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Sexual Dysfunction Scale (investigator developed) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change from baseline on Sexual Side Effects Scale (SSES) total score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Endpoint Feiger Sexual Function and Satisfaction Questionnaire score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Item 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Item 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Item 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Item 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Item 5 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Item 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endpoint Arizona Sexual Experience Scale (ASEX) score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Orgasm (satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Recurrence of mood symptoms Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 66.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Absence of sexual dysfunction at end point Show forest plot | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.86, 1.77] |
| 2 'Improved' or 'much improved' on Clinical Global Impression Show forest plot | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.71, 2.17] |
| 3 Change in Arizona Sexual Experiences Scale (ASEX) item scores Show forest plot | 1 | 1264 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.19, 0.05] |
| 3.1 How strong is your sexual drive? | 1 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 3.2 How easily are you sexually aroused? | 1 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.47, 0.07] |
| 3.3 Females: how easily does your vagina become moist or wet? | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.22, 0.42] |
| 3.4 Males: can you easily get and keep an erection? | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐0.80, 0.00] |
| 3.5 How easily can you reach orgasm? | 1 | 252 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 3.6 Are your orgasms satisfying? | 1 | 252 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.22, 0.42] |
| 4 Dropouts Show forest plot | 1 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.93] |
| 4.1 Total | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.56, 1.68] |
| 4.2 Attributed to adverse effects | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.75, 7.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient‐rated visual analogue scales Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall function (total of interest, lubrication, orgasm, pleasure) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐38.33, 44.53] |
| 1.2 Mood | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐7.61, 9.21] |
| 1.3 Energy | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [‐3.88, 14.48] |
| 1.4 Interest/desire | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐7.99, 13.39] |
| 1.5 Lubrication | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [‐3.65, 23.45] |
| 1.6 Orgasm | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐6.3 [‐19.98, 7.38] |
| 1.7 Pleasure | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐15.53, 9.13] |
| 1.8 Discomfort | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [‐3.15, 17.15] |
| 2 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visual analogue scale of orgasmic function ‐ best score achieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient rated assessment of sexual function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in diary ratings (visual analogue scales) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mood | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Energy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Physical arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Pleasure/enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient rated assessment of sexual function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in diary ratings (visual analogue scales) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mood | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Energy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Physical arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Pleasure/enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endpoint modified Kinsey Structured Interview Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.19, 1.01] |
| 3.1 Sexual satisfaction | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.19, 1.01] |
| 4 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Attributed to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient rated assessment of sexual function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in diary ratings (visual analogue scales) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mood | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Energy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Physical arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Pleasure/enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Attributed to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient‐rated visual analogue scales Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall function (total of interest, lubrication, orgasm, pleasure) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐29.02, 55.02] |
| 1.2 Mood | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 8.1 [1.23, 14.97] |
| 1.3 Energy | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 12.7 [5.30, 20.10] |
| 1.4 Interest/desire | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐7.96, 9.76] |
| 1.5 Lubrication | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 7.90 [‐5.74, 21.54] |
| 1.6 Orgasm | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [‐11.91, 16.91] |
| 1.7 Pleasure | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐11.28, 14.68] |
| 1.8 Discomfort | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐11.25, 13.85] |
| 2 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint Brief Index of Sexual Functioning for Women (BISF‐W) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual arousability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Lack of vaginal lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Orgasm ability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm intensity/pleasure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Sexual dissatisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint Arizona Sexual Experiences Scale (ASEX) score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Question 1: Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Endpoint MGH‐SFQ Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Item a: Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.60, 3.74] |
| 4 Endpoint ratings of psychiatric symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Hamilton Depression Rating Scale | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐7.98, 2.98] |
| 4.2 Hamilton Anxiety Rating Scale | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐3.15, 2.35] |

