Terapi tingkah laku kognitif (CBT), CBT gelombang ketiga dan intervensi berasaskan terapi interpersonal (IPT) untuk mencegah kemurungan pada kanak‐kanak dan remaja
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: BDI‐II: 13.5 (mild)
Mean age: 14.5 Age range: not specified Percentage male: 55.5% Setting: school
Psychiatric diagnoses excluded: unclear Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: unclear
Country: Chile | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: I Think, Feel, and Act Number of sessions: 11 sessions plus 2 booster sessions Length of sessions: 1 hour Intensity (total number of hours): 13 hours Duration of treatment period: unclear. Booster sessions were conducted at 2 and 7 months. Group size: unclear Delivered by: trained mental health research workers, including: psychologists, teachers, social workers and others Fidelity: not assessed Type of comparison: TAU comprising normal teaching activities and assessments which, according to school curriculum, were described as ‘counselling’. Teachers advised to place more emphasis on emotional problems, provide better information to students, to allow students to exchange experiences, and provide mutual support to one another. | |
| Outcomes | Diagnosis: established from cut‐points on the BDI of 17.0 for the overall sample; 14.0 for boys and 20.0 for girls (however, we were unable to obtain these data from the authors) Name of self‐report depression measure: BDI‐II Name of clinician report depression measure: N/A Name of anxiety measure: RCADS (omitting the depression and separation anxiety subscales) Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...a computer‐generated list of random numbers..." (p.1005) |
| Allocation concealment (selection bias) | Unclear risk | "The trial statistician..." (p.1005) Unclear whether the sequence was concealed from the other researchers involved in the trial, however |
| Blinding (performance bias and detection bias) | High risk | The nature of the intervention suggests that it is likely participants were aware to which group they had been allocated. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 17.7% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: sensitivity analyses were conducted by imputing missing data using multiple imputations. However, the authors state that results did not differ from those using observed cases and therefore chose to present outcomes based on observed cases only. |
| Selective reporting (reporting bias) | High risk | Trial protocol (i.e. Araya 2011) would suggest that scores on the "Self‐Harm Questionnaire" and that clinically significant depression (as established from cut scores on the BDI‐II) were also assessed |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: 75th to 90th percentile on the CDI What risk was basis of inclusion for selected studies: 75th percentile or higher on the negative attribution style composite of the CSAQ Diagnostic interview to exclude those with current or previous depression: those with current depression excluded as well as those scoring above the 90th percentile of the CDI, and those with a past episode of a depressive disorder Baseline severity of depression: CDI: 14.9 (mild‐moderate)
Mean age: not specified Age range: 14 to 15 Percentage male: 49.4% Setting: school
State what psychiatric diagnoses were excluded: dysthymia, cyclothymia, anorexia, bulimia, any psychotic disorder, bipolar disorder (types I or II), comorbid substance use/disorder, conduct disorder, oppositional defiance disorder and attention deficit hyperactivity disorder Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: unclear
Country: Iceland | |
| Interventions | Broad category: CBT and IPT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: not specified Number of sessions: 14 sessions Length of sessions: unclear. As sessions were delivered during usual class time, assumption is 1 hour. Intensity (total number of hours): 14 hours (on assumption each session has a duration of 1 hour) Duration of treatment period: 11 weeks Group size: 6 to 8 Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising the ability to seek any school‐based or other services as necessary except those associated with systematic interventions | |
| Outcomes | Diagnosis: Hodges' Child Assessment Scale, the A‐Life (for follow‐up interviews between 2003‐2005), or the K‐SADS (between 2004‐2005) Name of self‐report depression measure: CDI (data not reported in a usable format) Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) for depression diagnosis only | |
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no Coding for depression severity at baseline: where baseline severity was mild to moderate as in this trial, it was rated as mild. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants...were randomly assigned..." (p.581) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to an assessment only control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Low risk | "All interviewers were uninformed as to the intervention condition of participants at all interviews" (p.580) |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 12.87% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken. |
| Selective reporting (reporting bias) | High risk | Scores on the CDI at follow‐up not reported |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: BDI‐II ≥ 18.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current episodes of depression included. Additionally, as no student self‐disclosed past psychiatric treatment for any mental illness, it is likely that those with past episodes of depression were also included. Baseline severity of depression: BDI‐II: 24.7 (moderate) Mean age: 15.7 Age range: 14 to 17 Percentage male: 30.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified. However, no student self‐disclosed past psychiatric treatment for any mental illness. Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Nigeria | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: not specified Number of sessions: 5 sessions Length of sessions: 45 to 60 minutes Intensity (total number of hours): up to 5 hours Duration of treatment period: 5 weeks Group size: 20 Delivered by: Child and Adolescent Psychiatrist Fidelity: assessed as adequate Type of comparison: WL | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐II Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and approx. 4 months (short‐term) | |
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes Author contacted for outcome data: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "...randomly designated...by ballot" (manuscript p.6) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.5% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: LOCF |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes (only 2 of the 5 sessions, however) Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past episodes of depression were not excluded, however. Baseline severity of depression: CES‐D: 11.8 (subthreshold)
Mean age: 14.3. Age range: 12 to 17 Percentage male: 44.1% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: N/A as MoodGYM freely available to access Online: yes Name of programme: MoodGYM Number of sessions: 5 sessions Length of sessions: 20 to 40 minutes Intensity (total number of hours): up to 3.3 hours Duration of treatment period: 5 weeks Group size: N/A as MoodGYM individual‐based programme Delivered by: N/A Fidelity: online, therefore standardised Type of comparison: WL | |
| Outcomes | Diagnosis: established from cut‐points on the CES‐D of ≥ 24 Name of self‐report depression measure: CES‐D Name of clinician report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: N/A as MoodGYM freely available to access Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...a computerized random number generator..." (p.1023) |
| Allocation concealment (selection bias) | Low risk | "An independent statistician randomly allocated schools... The identity of the schools was concealed from the statistician during this process." (p.1023) |
| Blinding (performance bias and detection bias) | High risk | "Information and consent forms outlining the details of the trial and the school's assignment to either intervention or control were distributed to all participating students and their parents" (p.1023) |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 13.3% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past episodes of depression not excluded, however. Baseline severity of depression: CDI: 9.5 (subthreshold)
Mean age: 11.1 Age range: 10 to 12 Percentage male: 47.4% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes. Penn Resiliency Program manual is freely available on request. Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 10 Delivered by: masters‐level graduate students (clinical psychology, educational psychology, counselling) Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: established from cut‐points on the CDI of ≥ 30 Name of self‐report depression measure: CDI Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 3 months (short‐term), 12 months (medium‐term) and 24 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no. Penn Resiliency Program manual is freely available on request. Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 13.5% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of Latino versus African children and high symptomatic versus low symptomatic children. |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: scoring one standard deviation above the school mean on the negative thinking subscale of the Substance Use Risk Profile Scale (SURPS; Conrod 2002) Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past depression not excluded, however. Baseline severity of depression: BSI: 16.0 (unclear)
Mean age: 14.0 Age range: 13 to 16 Percentage male: 35.7% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: UK | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: not specified Number of sessions: 2 sessions Length of sessions: 90 minutes Intensity (total number of hours): 3 hours Duration of treatment period: unclear Group size: 2 to 9 Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BSI Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 36.89% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: authors state they use LOCF method, however, the number of participants included in this analysis is unclear |
| Selective reporting (reporting bias) | High risk | Protocol not available. Numbers of participants included in analyses are unclear, however, and yet there is a high proportion of treatment drop‐outs. |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past depression not excluded, however. Baseline severity of depression: CDI: approximately 8.0 (subthreshold)
Mean age: 12.2 Age range: 11 to 14 Percentage male: 50.5% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes. Penn Resiliency Program manual is freely available on request. Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 9 to 14 Delivered by: both non‐mental health and mental health experts Fidelity: assessed but only for the purposes of supervision Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no. Penn Resiliency Program manual is freely available on request. Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... randomly assigned... using a computer‐generated random numbers table" (p.114) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 23.80% Means and SDs used in meta‐analysis based on what data: observed cases (girls only) Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. Data on 12‐month outcomes not presented as too few participants had been assessed by this time point. |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | High risk | Implementation integrity assessed: yes (only for purposes of supervision, however) Implementation integrity adequate: no Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: scoring above the average on the negative reactivity and negative intensity subscales of the Affect Intensity Measure (AIM; Larsen 1986). Diagnostic interview to exclude those with current or previous depression: not undertaken. Unclear whether those with current and/or past episodes of depression were excluded. Baseline severity of depression: CES‐D: 19.9 (mild)
Mean age: 18.0 Age range: 17 to 19 Percentage male: 0.0% Setting: university
Psychiatric diagnoses excluded: unclear Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: unclear
Country: USA | |
| Interventions | Broad category: third wave (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Women and Relaxation, Openness Contemplation and Kindness (ROCK) Number of sessions: 8 sessions Length of sessions: 1 hour Intensity (total number of hours): 8 hours Duration of treatment period: 8 weeks Group size: 10 to 12 Delivered by: masters‐level graduate student (clinical psychology) Fidelity: not assessed. However, researcher who developed the intervention also delivered all 8 intervention sessions. Type of comparison: unclear. Described as a “control group”. | |
| Outcomes | Diagnosis: SCID‐I Name of self‐report depression measure: CES‐D Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: SACQ Assessment point: post‐intervention, short‐term and medium‐term | |
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | Content of the control condition was not adequately described, therefore difficult to determine whether participants would have been able to determine to which group they had been allocated or not. |
| Blinding (performance bias and detection bias) | Low risk | "Interviewers were blinded to participant condition" (p.31) |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.00% dropped out whilst a further 12.50% did not complete the post‐intervention assessment. Means and SDs used in meta‐analysis based on what data: for outcomes measured on a continuous scale (e.g. self‐reported depression scores), trial authors imputed missing item scores for those participants with fewer than 3 items missing. However, scores for those participants who missed more than 3 items on a scale, or who missed the scale entirely, were not imputed. Data for continuous outcomes therefore may contain some imputed values. Data for categorical outcomes (e.g. depression diagnosis) are based on observed cases (defined as those who attended at least 1 session). Intention‐to‐treat analyses: hierarchical linear modelling |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial and therapy sessions conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past depression were not excluded, however. Baseline severity of depression: CES‐D: 16.3 (mild)
Mean age: 15.1. Age range: 14 to 16 Percentage male: 53.9% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: BT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: not specified Number of sessions: 5 sessions Length of sessions: 50 minutes Intensity (total number of hours): 4.2 hours Duration of treatment period: 5 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising the usual health class curriculum delivered on the same days as the intervention, however, assessment of the control class curriculum revealed no overlap in content with regard to depressive disorders and/or related mental health issues. | |
| Outcomes | Diagnosis: established from cut‐points on the CES‐D of ≥ 24 Name of self‐report depression measure: CES‐D Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 weeks (short‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes. Correspondence with authors revealed manual was no longer available. Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | The nature of the intervention suggests it may have been possible to blind participants to allocation. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 21.05% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of males versus females. |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Low risk | Implementation integrity assessed: research assistants observed classes and rated fidelity Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 24 and K‐SADS What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression, however, were not excluded. Baseline severity of depression: CES‐D: 22.9 (mild)
Mean age: 15.3 Age range: 14 to 16 Percentage male: 30.0% Setting: school
State what psychiatric diagnoses were excluded: dysthymia, bipolar disorder Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Coping with Stress Number of sessions: 15 sessions Length of sessions: 45 minutes Intensity (total number of hours): 11.25 hours Duration of treatment period: 5 weeks Group size: unclear Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising freedom to continue with any pre‐existing treatment or to seek new assistance during the study period | |
| Outcomes | Diagnosis: K‐SADS and LIFE Name of self‐report depression measure: CES‐D Name of clinician report depression measure: modified 14‐item version of the HAM‐D Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 5 weeks (short‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes. Correspondence with authors revealed no manual was available. Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 16.67% Means and SDs used in meta‐analysis based on what data: observed cases (defined as those who completed at least one of the follow‐up assessments) Intention‐to‐treat analyses: unclear whether intention‐to‐treat analyses were undertaken |
| Selective reporting (reporting bias) | High risk | Data on General Assessment of Functioning scores presented, which is not indicated as an outcome measure within the methods section |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 24 What risk was basis of inclusion for selected studies: parental depression Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression, however, were not excluded. Baseline severity of depression: CES‐D: 24.4 (mild)
Mean age: 14.6 Age range: 13 to 18 Percentage male: 35.6% Setting: HMO
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Coping with Stress Number of sessions: 15 sessions Length of sessions: 60 minutes Intensity (total number of hours): 15 hours Duration of treatment period: unclear Group size: 6 to 10 Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising freedom to continue with any pre‐existing treatment or to seek new assistance during the study period provided by the HMO and/or by outside healthcare providers | |
| Outcomes | Diagnosis: K‐SADS Name of self‐report depression measure: CES‐D Name of clinical report depression measure: modified 14‐item version of the HAM‐D Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... assignment was preprinted using a computer program..." (p.1129) |
| Allocation concealment (selection bias) | Low risk | "... sealed in sequentially numbered envelopes, which were opened in sequential order by the project coordinator..." (p.1129) |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Low risk | "Assessors were unaware of the experimental condition of interviewed subjects" (p.1128) |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 4.30% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using random‐effects regression |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: parental depression Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression were not excluded (13% of intervention group and 23% of control group). Baseline severity of depression: YSR depression/anxiety subscale: 55.9 (moderately elevated)
Mean age: 11.5 Age range: 9 to 15 Percentage male: 54.8% Setting: mental health clinics/practices, family and general medical practices
State what psychiatric diagnoses were excluded: autism spectrum disorders, mental retardation, bipolar I, schizophrenia, conduct disorder, comorbid substance use/disorder, Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: those with parents diagnosed with bipolar I, schizophrenia, schizoaffective disorder, substance use/abuse excluded as were those whose parents were currently suicidal Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: not specified Number of sessions: 8 sessions plus 4 booster sessions Length of sessions: unclear. As sessions delivered during visits, assumption is 1 hour. Intensity (total number of hours): 12 hours (on assumption each session has a duration of 1 hour) Duration of treatment period: 8 weeks plus 1 booster session per month for an additional 4 months Group size: 4 families per group Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: other | |
| Outcomes | Diagnosis: K‐SADS‐PL Name of self‐report depression measure: CES‐D Name of clinician report depression measure: N/A Name of anxiety measure: YSR anxiety subscale Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of randomization was determined by a random number generator..." (p.1012) |
| Allocation concealment (selection bias) | Low risk | "...the assignment order was kept in a series of sealed envelopes that were opened by research assistants who were blind to assignment until the envelopes were opened..." (p.1012) |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Low risk | "Doctoral candidates in clinical psychology, who were blind to condition, conducted the structured diagnostic interviews..." (p.1011) |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 29.68% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: using multivariate mixed‐effects models with maximum likelihood estimation |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: BDI‐II ≥ 7.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression could participate in the intervention, but they were excluded from all subsequent analyses. Unclear if those with past episodes of depression were excluded, however. Baseline severity of depression: BDI‐II: 17.85 (intervention group) and 16.80 (control group) (mild) Mean age: not specified Age range: 14 to 15 Percentage male: 0% Setting: schools State what psychiatric diagnoses were excluded: exclusion criteria not stated Suicide risk excluded: exclusion criteria not stated Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not stated Country: Chile | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: unclear Online: no Name of programme: not specified Number of sessions: approx. 11 sessions. The intervention programme was, however, adapted to fit with students' timetables so some students may have received fewer sessions. Length of sessions: approx. 1.5 hours. The intervention programme was, however, adapted to fit with students' timetables so some students may have received shorter sessions. Intensity (total number of hours): approx. 16.5 hours (on assumption each participants received 11 sessions of 1.5 hours duration) Duration of treatment period: unclear as frequency of sessions not stated Group size: 15 to 23 Delivered by: mental health experts (graduate‐level psychologists) Fidelity: unclear if assessed Type of comparison: correspondence with study authors suggests NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐II Name of clinician report depression measure: N/A Name of anxiety measure: BAI Name of general functioning measure: N/A Assessment points: 7 months (medium‐term) | |
| Notes | Author contacted for methodological detail: yes Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation "by chance" Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 14.8% Means and SDs used in meta‐analysis based on what data: observed cases (girls only) Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: being the child of a Mexican immigrant woman Diagnostic interview to exclude those with current or previous depression: those with current depression were excluded. Unclear whether those with past episodes of depression were also excluded, however. Baseline severity of depression: CDI: 9.2 (sub‐threshold) Mean age: 10.4 Age range: not specified Percentage male: not specified Setting: school State what psychiatric diagnoses were excluded: depression. Children attending special education classes were also excluded. Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: mothers with current depression were excluded. Unclear whether those with a past history of any mental illness were also excluded, however. Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Mexican American Problem Solving Program (Stop, Think, and Act) Number of sessions: 10 sessions Length of sessions: unclear. As sessions delivered during visits, assumption is 1 hour. Intensity (total number of hours): 10 hours (on assumption each session has a duration of 1 hour) Duration of treatment period: unclear Group size: 4 to 5 Delivered by: non‐mental health experts (nurses) Fidelity: assessed but unclear if assessed as adequate Type of comparison: NT | |
| Outcomes | Diagnosis: established from cut‐points on the CDI of ≥ 12 (numbers not reported in manuscript, however) Name of self‐report depression measure: CDI Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 10 weeks (short‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Schools were randomised to intervention and control groups" (p.179) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | The clustered nature of allocation suggests it is possible participants could have been blind to treatment allocation. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.3% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: using "Ruben's Hot deck imputation (1987)..." (p.187) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 24 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current and/or past episodes of depression were excluded Baseline severity of depression: CES‐D: 32.1 (moderate)
Mean age: 15.3 Age range: 13 to 18 Percentage male: 30.4% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Canada | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Coping with Stress Number of sessions: 15 sessions Length of sessions: 45 minutes Intensity (total number of hours): 11.25 hours Duration of treatment period: unclear. Typically the Coping with Stress programme is delivered as 2 sessions per week. Assumption, therefore, is that the duration of the treatment period was 8 weeks. Group size: unclear Delivered by: doctoral students in clinical psychology Fidelity: assessed as adequate Type of comparison: AP entitled “Let’s Talk” | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CES‐D and CDI Name of clinician report depression measure: N/A Name of anxiety measure: BAI and MASQ. Name of general functioning measure: N/A Assessment points: post‐intervention, 3 months (short‐term) and 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no. Coping with Stress manual is freely available on request. Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomly generated list of the two conditions was generated by a computer program..." (p.296) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: sensitivity analyses were conducted by imputing missing data using the expectation‐maximisation algorithm. However, the authors state that results did not differ from those using observed cases and therefore chose to present outcomes based on observed cases only. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: those with "low to moderate levels of psychological distress" on the K‐10. No cut‐point is specified, however. What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. However, those with K‐10 ≥ 30 were excluded. Those with past episodes of depression were not excluded, however. Baseline severity of depression: DASS depression subscale: 15.0 (moderate)
Mean age: 19.7 Age range: not specified Percentage male: 23.0% Setting: university
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: N/A as MoodGYM freely available to access Online: yes Name of programme: MoodGYM Number of sessions: 3 sessions Length of sessions: 60 minutes Intensity (total number of hours): 3 hours Duration of treatment period: 3 weeks Group size: N/A as MoodGYM is an individual‐based programme Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS depression subscale (DASS‐21‐d) Name of clinician report depression measure: N/A Name of anxiety measure: DASS anxiety subscale (DASS‐21‐a) Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: N/A as MoodGYM freely available to access Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly allocated..." (p.462) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: unclear. Abstract suggests 39 participants were included, and outcome data are available for 39 participants. However, gender breakdown in the methods section seems to indicate 40 participants were included. Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: students excluded, or at risk of being excluded, from mainstream education due to behavioural problems Diagnostic interview to exclude those with current or previous depression: not undertaken, although those with “extreme depression” were excluded (p.531). Unclear whether those with past episodes of depression were also excluded Baseline severity of depression: CDRS‐R: 39.6 (moderate)
Mean age: 14.9 Age range: 13 to 16 Percentage male: 56.0% Setting: schools (alternative education)
State what psychiatric diagnoses were excluded: "Only those judged not to be safe using the computerized program were excluded" (p.531) Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no
Country: New Zealand | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: N/A Online: yes Name of programme: SPARX Number of sessions: 7 modules Length of sessions: 30 minutes Intensity (total number of hours): 3.5 hours Duration of treatment period: 5 weeks Group size: unclear Delivered by: online Fidelity: online, therefore standardised Type of comparison: WL | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: RADS‐2 Name of clinician report depression measure: CDRS‐R Name of anxiety measure: SAS Name of general functioning measure: PQ‐LES‐Q Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out in a 1:1 ratio using a computer generated randomization sequence. Allocation was stratified by study site and arranged in permuted blocks" (p.533) |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was ensured by allocating each participant a unique study number..." (p.533) |
| Blinding (performance bias and detection bias) | High risk | "It was not possible to blind participants to their treatment allocation" (p.533) |
| Blinding (performance bias and detection bias) | High risk | "The researcher was unblinded after the baseline assessment" (p.533) |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 6.30%; 0% (for depression diagnosis) Means and SDs used in meta‐analysis based on what data: observed cases (defined as those who completed at least one SPARX module and completed the 5 week post‐treatment assessment Intention‐to‐treat analyses: ITT analyses were undertaken, however, the sample was not large enough to form the primary outcome |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: scoring in the top percentile on the Expanded Attributional Style Questionnaire (Pessimism) Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: BDI‐I: 9.4 (sub‐threshold)
Mean age: 19.2 Age range: not specified Percentage male: 22.0% Setting: university
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Self Administered Optimism Training (SOT) Number of sessions: 1 session plus daily monitoring Length of sessions: 10 minute session plus daily monitoring of unclear duration Intensity (total number of hours): unclear Duration of treatment period: 28 days Group size: N/A as monitoring was individual‐based intervention Delivered by: unclear Fidelity: unclear if assessed Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐I Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... participants were...randomly assigned..." (p.354) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 12.5% (unbalanced) Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: expectation‐maximisation imputation algorithm |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 9.4 (sub‐threshold)
Mean age: 9.9 Age range: 9 to 11 Percentage male: 47.4% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Mexico | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: AMISTAD (Mexican version of the FRIENDS for Life program) Number of sessions: 10 sessions plus 2 booster sessions Length of sessions: 60 to 75 minutes Intensity (total number of hours): up to 12.5 hours (including booster sessions) Duration of treatment period: 10 weeks (booster sessions at 1 month and 3 months post‐intervention) Group size: unclear Delivered by: non‐mental health experts Fidelity: assessed as adequate Type of comparison: NT | |
| Outcomes | Diagnosis: established from CDI of ≥ 19.0 Name of self‐report depression measure: CDI (Spanish version) Name of clinician report depression measure: N/A Name of anxiety measure: SAS (Spanish version) Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Schools... were randomly assigned..." (p.62) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.8% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Low risk | As this trial was reported in a thesis, it is unlikely selective outcome reporting was present |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes (for 17.00% of cases) Implementation integrity adequate: unclear Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 20.0 What risk was basis of inclusion for selected studies: parental depression Diagnostic interview to exclude those with current or previous depression: those with current episodes of depression excluded. However, inclusion criteria allowed for those who had experienced a previous episode, but were currently in remission for at least 2 months. 55.3% of intervention group and 55.4% of control group had experienced a previous episode. Baseline severity of depression: CES‐D: 18.6 (mild)
Mean age: 14.8 Age range: 13 to 17 Percentage male: 58.5% Setting: HMO, university medical centres, schools
State what psychiatric diagnoses were excluded: bipolar I disorder, schizophrenia Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: yes
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Coping with Stress Number of sessions: 14 sessions Length of sessions: 90 minutes Intensity (total number of hours): 21.0 hours Duration of treatment period: overall 8 months (first 8 weeks acute) Group size: 3 to 10 Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising freedom to initiate or continue any non‐intervention mental health care | |
| Outcomes | Diagnosis: established from K‐SADS‐PL and LIFE ≥ 4.0 Name of self‐report depression measure: CES‐D Name of clinician report depression measure: CDRS‐R Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 33 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... randomized using the Begg and Iglewicz modification of the Efron biased coin toss... by a computer program" (p.2217) |
| Allocation concealment (selection bias) | Low risk | "Participants were randomized centrally at the Pittsburgh site..." (p.2217) |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Low risk | "Independent evaluators were blinded to experimental condition throughout the study..." (p.2116) |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.20% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: mixed models including LOCF |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Reported that 5.1% of participants had experienced a previous mental health condition. Baseline severity of depression: DASS‐d: 10.9 (mild)
Mean age: 14.8 Age range: 14 to 16 Percentage male: 0.0% Setting: school
State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no
Country: Mexico | |
| Interventions | Broad category: third wave (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Project Wings Number of sessions: 16 sessions Length of sessions: 3 hours Intensity (total number of hours): 48 hours Duration of treatment period: 16 weeks plus booster sessions at 3 and 7 months Group size: unclear Delivered by: mental health workers (youth workers) Fidelity: not assessed Type of comparison: AP | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐d Name of clinical report depression measure: N/A Name of anxiety measure: DASS‐a Name of general functioning measure: N/A Assessment points: post‐intervention, 3 months (short‐term), 9 months (medium‐term) | |
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerised permuted block randomisation schedule was created..." (p.439) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported and there is no report of blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 14.30% Means and SDs used in meta‐analysis based on what data: observed cases (those with at least 2 post‐intervention assessments) Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: not specified What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 7.7 (sub‐threshold) Mean age: not specified Age range: 10 to 12 Percentage male: 53.4% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Depression Prevention Program plus a parental component (now referred to as Penn Resiliency Program) Number of sessions: 12 sessions Length of sessions: 2 hours Intensity (total number of hours): 24 hours Duration of treatment period: 12 weeks Group size: 8 to 12 Delivered by: Doctoral students in clinical psychology Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: established from CDI of ≥ 15.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 2 months (short‐term), 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no We have assumed that there were 4 clusters ‐ 2 intervention and 2 in control ‐ for ICC and sample size adjustment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correspondence with study authors indicated that randomisation was conducted using a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 9.59% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, longer‐term follow‐up data have not been reported and follow‐up data have not been reported for those children recruited in year 2. |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Unclear whether those with past depression were also excluded, however. Baseline severity of depression: CDI: 8.4 (subthreshold)
Mean age: 12.1 Age range: 11 to 14 Percentage male: 54.1% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 6 to 14 Delivered by: all Fidelity: assessed as satisfactory to good Type of comparison: AP | |
| Outcomes | Diagnosis: established from CDI of ≥ 13.0 or from clinically significant symptoms on the CDRS‐R of ≥ 65.0 Name of self‐report depression measure: CDI Name of clinician report depression measure: CDRS‐R Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) and 36 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no (manual freely available on request) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... computer‐generated random numbers sequence" (p.10) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Low risk | "Interviewers and coders were not informed of participants'... assignments" (p.13) |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.86% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: undertaken, but based on only those who completed baseline and at least one post‐intervention assessment |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, CDRS‐R raw scores are not presented nor are the proportion of children with elevated, high, and clinically significant levels of depression. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: described as "adequate to good" Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: not specified What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: although a diagnostic interview was undertaken, those with current and/or past episodes of depression were not excluded Baseline severity of depression: CDI: 10.6 (sub‐threshold)
Mean age: not stated Age range: 10 to 15 Percentage male: 52.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 10 sessions plus 6 booster sessions offered once every 6 months post‐intervention Length of sessions: 90 minutes Intensity (total number of hours): 15 hours (length of booster sessions unclear) Duration of treatment period: 10 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: fidelity of first 2 to 3 sessions assessed and descried as satisfactory. However, the authors note that fidelity for this trial was lower than that observed for other trials of PRP. Type of comparison: described as “control group”; probably TAU | |
| Outcomes | Diagnosis: computer‐assisted DISC‐IV Name of self‐report depression measure: CDI and RADS‐2 Name of clinician report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no (manual freely available on request) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... computer‐generated random number sequence..." (p.625) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Low risk | "Interviewers were not informed of students' condition assignment" (p.627) |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 7.90% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: described as "satisfactory" but less than that achieved in other trials of PRP Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDI ≥ 7.0 for girls and ≥ 9.0 for boys What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded Baseline severity of depression: CDI: 12.9 (subthreshold) Mean age: not specified Age range: 11 to 12 Percentage male: 46.9% Setting: HMO State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: mental health experts Fidelity: assessed but unclear if adequate fidelity (therapist compliance scores ranged between 88.1% and 95.8% according to a measure of fidelity developed by the study authors) Type of comparison: TAU comprising usual care in an HMO setting | |
| Outcomes | Diagnosis: established from computerised HMO databases across the 2‐year follow‐up period Name of self‐report depression measure: CDI Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term), 24 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no (manual freely available on request) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer generated random number sequence..." (p.207) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | High risk | No information specified with respect to blinding of those extracting diagnostic information from the HMO database |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 20.30% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: undertaken but unclear what method used |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: unclear (64% to ‐95%) Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 10.8 (subthreshold) Mean age: not specified Age range: 11 to 13 Percentage male: 70.5% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar excluded: exclusion criteria not specified Country: USA | |
| Interventions | Broad category: CBT‐parent adaption (for further information on intervention components, see Table 1) Manualised: yes (manual freely available on request) Online: no Name of programme: Penn Resiliency Program‐Parent Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 10 to 12 Delivered by: research associates with at least an undergraduate degree in psychology (1 had a doctorate in psychology) Fidelity: not assessed Type of comparison: NT. Families were free to pursue counselling and/or other psychological therapies. | |
| Outcomes | Diagnosis: CDI ≥ 19.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no (manual freely available on request) Author contacted for outcome data: no There were 44 clusters and we assume 22 for each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned to one of two study conditions" (p.330) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 9.10% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: undertaken but unclear what method used |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. However, data on parental outcomes are not reported due to a high level of non‐response from parents and therefore large amounts of missing data. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 9.7 (sub‐threshold)
Mean age: 14.4 Age range: 14 to 15 Percentage male: 46.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: CB programme (based on Coping with Stress programme) Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 8 to 15 (median 11) Delivered by: students Fidelity: not assessed Type of comparison: TAU comprising normal health classes in which students were taught the standard wellness curriculum | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A random number list was used...to assign participants..." (p.695) "Within class periods, participants were randomly assigned to condition unless there were fewer than 15 students participating. This occurred for only two classes...for those two classes, randomization was done at the class level rather than at the individual level" (p.695). Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | "Participants and group leaders were aware of group assignment..." (p.695) |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 1.32% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: the authors undertook sensitivity analyses using an unspecified method. However, the authors state that results did not differ from those using observed cases. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | See Horowitz a2007 | |
| Participants | See Horowitz a2007 | |
| Interventions | Broad category: IPT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: IPT‐AST Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 8 to 15 (median 11) Delivered by: students Fidelity: not assessed Type of comparison: TAU comprising normal health classes in which students were taught the standard wellness curriculum | |
| Outcomes | See Horowitz a2007 | |
| Notes | See Horowitz a2007 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Horowitz a2007 |
| Allocation concealment (selection bias) | High risk | See Horowitz a2007 |
| Blinding (performance bias and detection bias) | High risk | See Horowitz a2007 |
| Blinding (performance bias and detection bias) | Low risk | See Horowitz a2007 |
| Incomplete outcome data (attrition bias) | Low risk | See Horowitz a2007 |
| Selective reporting (reporting bias) | Unclear risk | See Horowitz a2007 |
| Other bias | Unclear risk | See Horowitz a2007 |
| Implementation integrity | Unclear risk | See Horowitz a2007 |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: residing in a shelter for homeless and runaway youth Diagnostic interview to exclude those with current or previous depression: unclear whether a diagnostic interview was undertaken. Those with elevated depression scores and/or past depression were not excluded. Baseline severity of depression: BDI: 15.3 (mild)
Mean age: 15.5 Age range: not specified Percentage male: 100% Setting: homeless shelter
State what psychiatric diagnoses were excluded: those diagnosed with any psychiatric disorder were excluded Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: no
Country: South Korea | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: unclear. Session by session information on intervention components available in the publication. Online: no Name of programme: not specified Number of sessions: 8 sessions Length of sessions: 50 minutes Intensity (total number of hours): 6.7 hours Duration of treatment period: 8 weeks Group size: 6 to 8 Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐I Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Research participants were randomly assigned..." (p.162) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.63% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: ≥ 0.50 on a composite score based on the z score of the CDI and the Child’s Perception Questionnaire of parental conflict. Those scoring below this composite score, however, were included in the trial subject to availability and space in the groups. What risk was basis of inclusion for selected studies: child’s perception of parental conflict Diagnostic interview to exclude those with current or previous depression: not undertaken. Unclear whether those with current and/or past episodes of depression were excluded. Baseline severity of depression: CDI: 9.5 (sub‐threshold)
Mean age: 11.4 Age range: 10 to 13 Percentage male: 53.8% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Penn Prevention Program (Penn Resiliency Program) Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 10 to 12 Delivered by: students Fidelity: not assessed Type of comparison: WL | |
| Outcomes | Diagnosis: established from CDI of ≥ 15 Name of self‐report depression measure: CDI, RADS and a composite measure Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 weeks (short‐term) | |
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no There were 8 clusters and we assumed 4 in each | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correspondence with study authors revealed that the randomisation sequence was generated by pulling envelopes were picked out of a hat |
| Allocation concealment (selection bias) | Low risk | Correspondence with study authors revealed that the person generating the allocation sequence could not see what was written in each envelope |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.38% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: unclear if undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the trial commenced with 3 separate intervention groups that were subsequently combined in a post‐hoc manner as there were no differences between them in terms of main outcomes at post‐test. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: unclear | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: those children that were reported to be "depressed". No cut‐point specified, however. What risk was basis of inclusion for selected studies: parental divorce Diagnostic interview to exclude those with current or previous depression: not undertaken. Unclear whether those with current and/or past episodes of depression were excluded. Baseline severity of depression: unclear as means and SDs on CDI at baseline not specified Mean age: 12.0 Age range: 10 to 13 Percentage male: not specified Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Islamic Republic of Iran | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: unclear Online: no Name of programme: not specified Number of sessions: 8 sessions Length of sessions: 60 minutes Intensity (total number of hours): 8 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: not specified Fidelity: not assessed Type of comparison: unclear; presumably TAU comprising usual care from the welfare centre | |
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A | |
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.78) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: unclear as numbers of participants included in final analyses not specified Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: unclear if undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear if trial undertaken by those that developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: K10 ≥ 16.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear whether a diagnostic interview was undertaken and whether those with current and/or past episodes of depression were excluded Baseline severity of depression: DASS‐21 depression subscale: 20.0 (moderate)
Mean age: 18.1 Age range: 14 to 24 Percentage male: 28.0% Setting: GP clinics
State what psychiatric diagnoses were excluded: those diagnosed with any severe psychiatric or medical condition (e.g. current psychosis) and those requiring imminent hospitalisation Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: no
Country: Australia | |
| Interventions | Broad category: BT (for further information on intervention components, see Table 1) Manualised: N/A Online: telephone Name of programme: MOBILETYPE Number of sessions: recommended 2 entries per day Length of sessions: 1 to 3 minutes Intensity (total number of hours): 2.5 hours (based on reported average number of messages sent per day being 3, for an average of 17 days, assuming 3 minutes per message) Duration of treatment period: 2 to 4 weeks Group size: N/A (individual) Delivered by: N/A (self‐monitoring) Fidelity: not assessed Type of comparison: AP | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐d Name of clinical report depression measure: N/A Name of anxiety measure: DASS‐a Name of general functioning measure: N/A Assessment points: post‐intervention, between 2 to 4 weeks (short‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...a random seed generators to allocate each program to the 200 identification numbers in at the individual level..." (no pagination specified) |
| Allocation concealment (selection bias) | Low risk | "A research assistant downloaded each program by selecting the next consecutive link for the next study mobile and was blinded to allocation..." (no pagination specified) |
| Blinding (performance bias and detection bias) | High risk | "Participants...became aware of the group allocation at the post‐test..." (no pagination specified) |
| Blinding (performance bias and detection bias) | High risk | "...GPs because aware of the group allocation at the post‐test..." (no pagination specified) |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 26.3% (numbers from Reid 2011, Figure 1) Means and SDs used in meta‐analysis based on what data: unclear, assume observed cases Intention‐to‐treat analyses: maximum likelihood estimation (based on 114 rather than 118 participants, however) |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, information on the SF‐12 Health Survey, the AUDIT, the Adolescent Coping Scale, and a range of other outcome measures no reported in this paper or in a related publication (i.e. Reid 2011). In addition, 6‐month follow‐up data are also not reported. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: not specified Mean age: 16.8. Age range: 15 to 19 Percentage male: 57.9% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA | |
| Interventions | Broad category: third wave (for further information on intervention components, see Table 1) Manualised: unclear Online: no Name of programme: Yoga Ed Number of sessions: 23 to 32 sessions Length of sessions: 30 to 40 minutes Intensity (total number of hours): up to 21.3 hours Duration of treatment period: 11 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: TAU comprising normal physical education classes | |
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A | |
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly assigned by class..." (p.82) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | "...lack of blinding of subjects..." (p.88) |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 17.30% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: low income. To be included schools had to have at least 30% of their pupils living in low‐income areas, however, all young people attending these schools were then eligible for inclusion. Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past episodes of depression were not excluded, however. Baseline severity of depression: CDI: 8.5 (sub‐threshold)
Mean age: 13.4 Age range: 11 to 16 Percentage male: unclear Setting: school
State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no
Country: The Netherlands | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Op Volle Kracht ("At Full Strength") Number of sessions: 16 sessions Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 5 months Group size: 25 Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: TAU comprising usual school curriculum which, in some schools, did include social skills training | |
| Outcomes | Diagnosis: Established from CDI of ≥ 19.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was conducted within schools at the class level…with an allocation ratio of 1:1…[using] a computerized random number generator with a blocked randomization scheme (block size 2)…" (p.5277) |
| Allocation concealment (selection bias) | Unclear risk | "An independent researcher from the research institute…" (p.5277) generated the randomization sequence. However, the "list of classes that were allocated to control or intervention condition…was communicated to the school by the first author." (p.5277), suggesting that allocation may not have been adequately concealed from study authors. |
| Blinding (performance bias and detection bias) | High risk | "...the study was not blind...adolescents knew whether they received the program or not" (p.5288) |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 13.00% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: multiple imputations |
| Selective reporting (reporting bias) | High risk | Trial protocol (i.e. Kindt 2012) would suggest that scores on the Children's Negative Cognitive Errors Questionnaire‐Revised, the Children's Response Styles Questionnaire, the Adolescent Cognitive Style Questionnaire, and the substance use and happiness sub‐scales of the Adolescent Life Event Schedule will also be assessed |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | High risk | Implementation integrity assessed: "We decided not to check... program integrity and adherence to the program..." (p.5289) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDI ≥ 18.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past episodes of depression not excluded, however. Baseline severity of depression: CDI: 21.6 (severe)
Mean age: 14.6 Age range: 13 to 16 Percentage male: 0.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Adolescents Coping with Emotions (ACE) Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 8 to 10 Delivered by: mental health experts Fidelity: not assessed Type of comparison: WL | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Correspondence with study authors indicates that 16 of the 17 schools included in this trial were allocated randomly. One "non‐compliant" school changed its allocation from that determined by the randomisation sequence. Method of randomisation also not clear. |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.83% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: LOCF |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, although a complete dataset is available for 126 participants, only 9 of the participants with complete data are male. Therefore, a post hoc decision was made to present data for females only. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: those form an ethnic minority group in the USA recruited during a summer camp Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: SMFQ: 8.8 (sub‐threshold)
Mean age: 9.5 Age range: not specified Percentage male: 71.0% Setting: summer camps
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: third wave (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Mindful Schools (mindfulschools.org) Number of sessions: 10 sessions Length of sessions: 15 minutes Intensity (total number of hours): 2.5 hours Duration of treatment period: 2 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: TAU comprising lessons prepared by a health educator on the importance of activity, healthy eating and stress management | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: SMFQ Name of clinician‐report depression measure: N/A Name of anxiety measure: SAI‐C Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.70) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 5.55% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Brief report. |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the intervention was translated into Norwegian by the research team. | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CES‐D: 11.2 (sub‐threshold) Mean age: 16.8 Age range: 15 to 20 Percentage male: 43.2% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Norway | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: N/A as MoodGYM freely available to access Online: yes Name of programme: MoodGYM Number of sessions: 5 sessions Length of sessions: 45 minutes Intensity (total number of hours): 3.75 hours Duration of treatment period: 6 weeks Group size: N/A as MoodGYM individual‐based programme Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: NT | |
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A | |
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: no (MoodGYM freely available) Author contacted for outcome data: yes (not provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…randomization was undertaken using the SPSS program to generate the random numbers, which then were ordered in ascending order and allocated numbers from 1‐4." (p.4) |
| Allocation concealment (selection bias) | High risk | "…randomization…was undertaken by the first author." (p.4) |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Correspondence with study authors confirmed all outcomes were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 30.00%. Note that only 8.54% of those randomised actually registered with the MoodGYM programme. Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: mean substitution |
| Selective reporting (reporting bias) | Low risk | Trial protocol indicates that all proposed outcome measures were reported |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: those who, according to school counsellors, were experiencing mild to moderate depression symptoms. Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear whether those with current and/or past episodes of depression were excluded. Baseline severity of depression: RADS‐2: 65.21 (subthreshold) Mean age: 14.6 Age range: 12.5 ‐to 17.75 Percentage male: 0% Setting: mixed State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia | |
| Interventions | Broad category: third wave (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Acceptance and Commitment Therapy Number of sessions: 8 sessions Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 8 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: RADS‐2 Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using a random number table..." (no pagination specified) |
| Allocation concealment (selection bias) | High risk | Correspondence with study authors revealed that although students' names were concealed, the allocation sequence itself was not concealed. Instead, the number table included the condition next to the number sequence. |
| Blinding (performance bias and detection bias) | High risk | Correspondence with study authors revealed that participants were not blind to allocation |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 12.1% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: mixed model repeated measures |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D > 13. Symptoms were also required to be persistent as eligible participants were also required to have a score on the CES‐D of > 16.0 at first screen. What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression were not excluded, however. Baseline severity of depression: CES‐D: 27.0 (moderate)
Mean age: 12.7 Age range: not specified Percentage male: 44.0% Setting: mixed
State what psychiatric diagnoses were excluded: dysthymia and mania Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: N/A Online: yes Name of programme: Blues Blaster Number of sessions: 6 modules Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 6 weeks Group size: N/A (individual‐based intervention) Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: AP | |
| Outcomes | Diagnosis: established from K‐SADS and LIFE Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.33) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported but no detail of blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 14.3% (unbalanced) Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: estimation maximisation algorithm |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the data on the proportion of participants diagnosed with a depressive disorder at the 6‐month follow‐up period not reported. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: DASS‐21: 8.9 (sub‐threshold) Mean age: 15.4 Age range: 15 to 18 Percentage male: 32.5% Setting: schools and youth centres State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia | |
| Interventions | Broad category: third wave (for further information on intervention components, see Table 1) Manualised: N/A as website freely available Online: yes Name of programme: Bite Back Number of sessions: 6 sessions Length of sessions: 60 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 6 weeks Group size: N/A (individual‐based intervention) Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: AP | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐21 Name of clinician report depression measure: N/A Name of anxiety measure: DASS‐21 Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly allocated...through a block randomization method…[using] a random number generator in Excel to allocate blocks of 10 participants to one of [the] two conditions" (no pagination specified) |
| Allocation concealment (selection bias) | Low risk | "An independent researcher not associated with this study... " (no pagination specified) |
| Blinding (performance bias and detection bias) | Low risk | "It was important to conceal...participants' allocated condition..." (no pagination specified) |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 34.5% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken. Instead non‐compliant participants and non‐completers were excluded from subsequent analyses. |
| Selective reporting (reporting bias) | High risk | Trial protocol would suggest that scores on the Student Life Satisfaction Scale (SLSS), the Scale of Positive and Negative Experience (SPANE), modified General Self‐Efficacy Scale (GSE), the modified Rosenberg's Self‐Esteem scale and a number of Wellbeing indicators, as measured by the modified Life Orientation Test ‐ Revised (LOT‐R), were also assessed |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention. Although correspondence with study authors revealed that the research team provided no involvement to participants during the trial. Participants were instead instructed to navigate the website on their own. |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: MFQ ≥ 14.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear whether diagnostic interview undertaken and whether those with current and/or past episodes of depression were excluded. Those with high scores on the PHQ were, however, excluded. Baseline severity of depression: MFQ: 14.6 (sub‐threshold)
Mean age: 13.0 Age range: 12 to 13 Percentage male: 49.2% Setting: school
State what psychiatric diagnoses were excluded: none Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Positive Thoughts and Actions Program Number of sessions: 12 sessions Length of sessions: 50 minutes Intensity (total number of hours): 10 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: unclear Fidelity: correspondence with authors confirmed fidelity was assessed as adequate Type of comparison: TAU comprising freedom to seek school‐based or other services but not any systematic interventions | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: MFQ. However, data on this outcome could not be included in meta‐analyses because scores were adjusted for baseline depression symptoms as measured by the CDRS‐R. Name of clinician report depression measure: CDRS‐R. However, data on this outcome could not be included in meta‐analyses because scores were adjusted for baseline depression symptoms as measured by the CDRS‐R. Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 18 months (long‐term) | |
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Low risk | Correspondence with study authors indicated that parents were given 2 opaque manilla envelopes and were instructed to open or the other at the end of the baseline interview. Each set of envelopes included one with a card indicating "PTA Group" and one indicating "Control". RAs had no way of knowing which card was in which envelope. They were also instructed to return the sealed envelope at the end of the interview for verification. |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Low risk | Correspondence with study authors indicated that the assessors who conducted interviews, including the administration of the CDRS‐R, were blinded to group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.5% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: undertaken but method unclear |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, mean and SD scores on the CDRS‐R were not reported |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes (via correspondence) Implementation integrity adequate: yes (via correspondence) Implementation integrity reported: yes (via correspondence) |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: MFQ ≥ 14.0 (top 25%) What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear whether diagnostic interview undertaken and whether those with current and/or past episodes of depression were excluded. Those with high scores on the PHQ‐9, indicative of current probable MDD, however, were excluded. Baseline severity of depression: MFQ: 14.7 (sub‐threshold) Mean age: 12.7 Age range: 11 to 15 Percentage male: 39.2% Setting: school State what psychiatric diagnoses were excluded: intellectual disability. Unclear whether other psychiatric diagnoses were excluded, however. Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Positive Thoughts and Actions Program Number of sessions: 12 sessions Length of sessions: 50 minutes Intensity (total number of hours): 10 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: other | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: MFQ Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...random number sequences..." (p.556) |
| Allocation concealment (selection bias) | Low risk | "A statistician applied [the] random number sequences..." (p.556) |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to the control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.3% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: general linear model repeated measures analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes (via correspondence) Implementation integrity adequate: yes. Described as excellent for 92.00% of classes. Implementation integrity reported: yes (via correspondence) |
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the team were involved in adapting the intervention for this setting. | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: "Clinically significant" scores on either the BDI‐II or CES‐D. No cut‐point specified, however. What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear whether diagnostic interview undertaken and whether those with current and/or past depression were excluded. Those with elevated depression scores were, however, excluded. Baseline severity of depression: CES‐D: 19.4 (mild)
Mean age: 11.8 Age range: 10 to 15 Percentage male: 59.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: modified version of the Adolescent Coping with Depression programme Number of sessions: 10 sessions Length of sessions: 50 minutes Intensity (total number of hours): 8.2 hours Duration of treatment period: 10 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising the Vernon 1998 curriculum (a school‐based coping skills and problem‐solving curriculum) | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐Y Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...random number generator..." (p.70) |
| Allocation concealment (selection bias) | High risk | "...school psychologist and school psychology intern...The first halves of numbers generated were assigned to the experimental group and the last half of numbers generated were assigned to the treatment as usual group" (p.70) |
| Blinding (performance bias and detection bias) | Unclear risk | The nature of the intervention suggests it may have been possible to blind participants to allocation. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | High risk | "The school psychologist, school psychology intern, and a school counsellor were...the data collectors...The school psychology intern is also the author...the experimenter had knowledge of the hypotheses for the study..." (p.77) |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 4.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Low risk | As this trial was reported in a thesis, it is unlikely selective outcome reporting was present |
| Other bias | Unclear risk | Trial not conducted by those who developed the intervention. However, intervention was adapted (reduced to 10 sessions) by the author for this setting. |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: those living in disadvantaged and/or underserved urban communities Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: SMFQ: score not specified
Mean age: 10.1 Age range: 9 to 11 Percentage male: 39.2% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: third wave (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: none specified Number of sessions: 48 sessions Length of sessions: 45 minutes Intensity (total number of hours): 36 hours Duration of treatment period: 12 weeks Group size: 25 Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: WL | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: SMFQ Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.4% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes. The team were involved in adapting the intervention for this setting. | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression, as established from: i) total BDI‐II scores of ≥ 23 and scores of 2 or 3 on items 2 or 9 of the BDI‐II; ii) total BDI‐II scores of ≥ 30; iii) a score of 3 on item 9 of the BDI‐II; iv) total RADS scores of ≥ 77; v) a positive score on any of the "critical items" on the RADS were excluded from all analyses (although they were still eligible to participate in the programme). Unclear whether those with past episodes of depression were also excluded. Baseline severity of depression: BDI‐II: 8.9 (sub‐threshold) Mean age: 14.2 Age range: 13 to 15 Percentage male: 48.4% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: excluded from all analyses (although they were still eligible to participate in the programme) Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: New Zealand | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: RAP‐Kiwi Number of sessions: 11 sessions Length of sessions: 60 minutes Intensity (total number of hours): 11 hours Duration of treatment period: in one school sessions were conducted twice a week for 6 weeks, whilst in the second sessions were conducted once a week for 11 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: AP | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐II, RADS Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term), 18 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomization tables..." (p.539) |
| Allocation concealment (selection bias) | Low risk | "Participating students were given a study number. A research assistant who did not know the pupils used these numbers and randomization tables to assign students..." (p.539) |
| Blinding (performance bias and detection bias) | Unclear risk | Participants were tested to determine whether they were aware to which group they had been allocated. Some were able to correctly determine to which group they had been allocated (11% guess correctly; 14% in the intervention group). |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.6% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: although ITT analyses were also undertaken, data presented are based on observed cases |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDS between 96 and 140 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDS: 109.4 (unclear) Mean age: 16.0 Age range: not specified Percentage male: not specified Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Islamic Republic of Iran | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: not specified Number of sessions: not specified Length of sessions: not specified Intensity (total number of hours): not specified Duration of treatment period: not specified Group size: not specified Delivered by: not specified Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDS Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly..." assigned Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 6.1%. Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear if trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the team were involved in adapting the intervention for this setting. | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: ≥ 10.0 on CES‐D What risk was basis of inclusion for selected studies: living in a rural community Diagnostic interview to exclude those with current or previous depression: those with current depression not excluded. Unclear whether those with past episodes of depression were excluded Baseline severity of depression: CES‐D: 14.9 (sub‐threshold) Mean age: 13.8 Age range: 13 to 15 Percentage male: 0.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Talk 'n' Time Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 8 Delivered by: non‐mental health experts Fidelity: assessed, but unclear if assessed as adequate Type of comparison: WL | |
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A | |
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…a random number table [was used]..." (p.11) |
| Allocation concealment (selection bias) | Low risk | "…a research assistant who did not do any of the assessments..." (p.11) |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "...a trained interviewer [undertook assessments]..." (p.11) Unclear whether this interviewer was blind to treatment allocation, however |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: the authors state that "[a]pproximately 8 percent of participants dropped out before providing complete data..." (p.1) Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: possibly LOCF |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, follow‐up data are not reported. |
| Other bias | Unclear risk | Trial not conducted by those who developed the intervention. However, intervention was adapted by the author for this setting. |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: no reported Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: those scoring 1 SD above the school average on the hopelessness subscale of the SURPS What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: BSI‐depression: 17.4 (unclear) Mean age: unclear for this sub‐sample Age range: unclear for this sub‐sample Percentage male: unclear for this sub‐sample Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: UK | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: not specified Number of sessions: 2 sessions Length of sessions: 90 minutes Intensity (total number of hours): 3 hours Duration of treatment period: unclear Group size: 6 Delivered by: non‐mental health experts Fidelity: assessed, but not unclear if assessed as adequate Type of comparison: NT | |
| Outcomes | Diagnosis: established from BSI. Cut‐point, however, unclear. Name of self‐report depression measure: BSI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: 12 months (medium‐term), 24 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…a computerized randomization procedure." (p.912) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: data at post‐intervention not available Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: full information maximum likelihood estimation |
| Selective reporting (reporting bias) | High risk | Protocol implies that data on binge drinking frequency, drinking frequency, drinking quality, drinking problems, as assessed by an abbreviated version of Rutger's Alcohol Problem Index, illicit drug use frequency, emotional and behavioural problems, school attendance, grade attainment, coping skills, motives for drinking, and antisocial behaviours assessed using the BSI were also assessed |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: no reported Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 7.9 (sub‐threshold)
Mean age: 10.4 Age range: 9 to 12 Percentage male: 48.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 10 sessions Length of sessions: 120 minutes Intensity (total number of hours): 20 hours Duration of treatment period: 11 weeks Group size: 16 Delivered by: unclear Fidelity: not assessed Type of comparison: AP | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: trait anxiety subscale of the STAIC Name of general functioning measure: N/A Assessment points: post‐intervention, 8 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported but there is no detail about blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 4.2% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: not specified
Mean age: not specified Age range: 11 to 13 Percentage male: not specified Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: unclear Online: no Name of programme: not specified Number of sessions: 16 sessions Length of sessions: 40 minutes Intensity (total number of hours): 10.7 hours Duration of treatment period: 3 months Group size: unclear Delivered by: mental health experts and students Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.7% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, diagnostic data not presented. |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: RADS ≥ 60.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: RADS: 70.3 (mild)
Mean age: 16.0 Age range: 14.1 to 18.3 Percentage male: 18.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Teaching Kids to Cope Number of sessions: 10 sessions Length of sessions: 45 minutes Intensity (total number of hours): 7.5 hours Duration of treatment period: 10 weeks Group size: unclear Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: TAU. No further description provided. | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: RADS Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... equal allocation using permuted block randomization within school sites..." (p.74) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 7.9% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: repeated measures analysis using mixed modelling methods |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CES‐D: 8.6 (sub‐threshold).
Mean age: 14.0 Age range: not specified Percentage male: 52.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Germany | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: LISA‐T Number of sessions: 10 sessions Length of sessions: 90 minutes Intensity (total number of hours): 15 hours Duration of treatment period: 10 weeks Group size: 8 to 24 Delivered by: Mental health experts and students Fidelity: assessed but unclear if assessed as adequate Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 3 months (short‐term), 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...classes were to be randomly assigned...We tried to recruit both training and control groups in each school; however, there was one school with only one class, which we assigned to the training group. In another school with three classes, we randomly assigned two classes to the training group" (p.1005) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 27.4% Means and SDs used in meta‐analysis based on what data: observed cases (based on those who did not miss more than 2 assessments) Intention‐to‐treat analyses: unclear if undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: no Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: SBB‐DES: 0.6 (sub‐threshold)
Mean age: 13.7 Age range: not specified Percentage male: 53.5% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Germany | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: LARS and LISA‐T Number of sessions: 10 sessions Length of sessions: 90 minutes Intensity (total number of hours): 15 hours Duration of treatment period: 10 weeks Group size: 8 to 18 (median 14) Delivered by: mental health experts and students Fidelity: assessed but unclear if assessed as adequate Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: SBB‐DES Name of clinical report depression measure: N/A Name of anxiety measure: SBB‐ANG Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...classes were randomly assigned to the intervention and control groups..." (p.108) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | "Adolescents, parents, and teachers of the intervention and control groups were informed about the program’s objectives...It was explained that having a control group is essential in order to study the program’s effects" (p.109) |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.0% Means and SDs used in meta‐analysis based on what data: observed cases (based on 163 and 138 rather than 163 and 136) Intention‐to‐treat analyses: hierarchical linear model analyses |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: N/A Implementation integrity reported: no |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: not specified Mean age: 15.1 Age range: not specified Percentage male: 37.3% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: LARS and LISA Number of sessions: 10 sessions Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 10 weeks Group size: unclear Delivered by: masters' level clinical psychology students Fidelity: assessed but unclear if assessed as adequate Type of comparison: TAU comprising usual wellness classes | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: SBB‐ANG Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly assigned…" (p.433) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | "Both interventions were described to students... as probably efficacious" (p.434) |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 12.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Brief report. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | High risk | Implementation integrity assessed: no. Only assessed via self‐ratings of the material covered within each session. Implementation integrity adequate: unclear Implementation integrity reported: no |
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the team were involved in adapting the intervention for this setting. | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 7.4 (sub‐threshold)
Mean age: not specified Age range: 11 to 12 Percentage male: 0.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: The Optimism and Lifeskills Program (adapted from the Penn Resiliency Program) Number of sessions: 8 sessions Length of sessions: 80 minutes Intensity (total number of hours): 10.7 hours Duration of treatment period: 8 weeks Group size: 12 Delivered by: students Fidelity: not assessed Type of comparison: WL | |
| Outcomes | Diagnosis: established from CDI ≥ 13.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 29.8% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. Analyses of those scoring above the clinical cut‐point for depression appear post hoc. |
| Other bias | High risk | Trial not conducted by those who developed the intervention. However, intervention was adapted by the author for this setting. |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: DASS‐d: 5.0 (subthreshold)
Mean age: 17.9 Age range: not specified Percentage male: 45.7% Setting: college
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: BT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Brief Behavioral Activation Treatment for Depression (BATD) Number of sessions: 15 sessions Length of sessions: 120 minutes Intensity (total number of hours): 30 hours Duration of treatment period: 15 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising classes to facilitate student adjustment, including: academic skills, career exploration, library resources, campus safety, sexuality, diversity and responsible decision making. Students were encouraged to make contact with a faculty advisor and to keep diaries reflecting on the process of adjusting to college life. | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐d Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "Research assistants were not affiliated in any way with the courses...Research assistants were blind to the class condition as well as the study hypotheses" (p.557). However, primary outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 9.6% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: generalised estimating equations |
| Selective reporting (reporting bias) | High risk | Protocol not available. Outcomes assessed with the DASS, which includes an anxiety subscale. Data on this outcome not reported, however. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: RADS: 15.2 (sub‐threshold)
Mean age: 14.0 Age range: 12 to 16 Percentage male: 50.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Mauritius | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: RAP Number of sessions: 11 sessions Length of sessions: 60 minutes Intensity (total number of hours): 11 hours Duration of treatment period: 11 weeks Group size: 8 to 12 Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: WL | |
| Outcomes | Diagnosis: unclear how this was established Name of self‐report depression measure: RADS‐2 Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.70) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | High risk | "Teachers running the RAP‐A program randomly assigned the students..." (p.70) |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "All forms were scored by the primary researcher (not blinded to group allocation" (p.88) However, primary outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: N/A as 0% dropouts |
| Selective reporting (reporting bias) | Low risk | As this trial was reported in a thesis, it is unlikely selective outcome reporting was present |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: children in each class were rank ordered on the basis of CDI scores. The 13 children with the highest score from each class were invited to participate. In classes with fewer than 13 students, all were eligible to participate. Diagnostic interview to exclude those with current or previous depression: those with current and/or past depression not excluded Baseline severity of depression: CDI: 11.1 (sub‐threshold)
Mean age: 11.9 Age range: 11 to 13 Percentage male: 50.3% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 120 minutes Intensity (total number of hours): 24 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: mental health experts and school nurses Fidelity: assessed as adequate
Type of comparison: TAU comprising monitoring of symptoms and regular health curriculum | |
| Outcomes | Diagnosis: CDI ≈ 15.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 30 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.623) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | "Parents were informed of their child's school group status..." (p.623). Parents therefore could have communicated allocation to their children. |
| Blinding (performance bias and detection bias) | High risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 5.3% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: "With only one exception, facilitators achieved a high level of program integrity..." (p.623) Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Reported that between 5% to 7% of participants had experienced a mental health condition at some point in their life. Baseline severity of depression: CDI: 7.8 (sub‐threshold)
Mean age: 12.0 Age range: 11 to 13 Percentage male: 45.6% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Aussie Optimism Program Number of sessions: 20 sessions Length of sessions: 60 minutes Intensity (total number of hours): 20 hours Duration of treatment period: 20 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising 20 regular health education classes relating to self‐improvement and interpersonal skills. Lessons had similar learning outcomes to the intervention. | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 18 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "...trained research assistants [who were] blind to group allocation" (p.70) However, primary outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 13.9% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: assessed as high fidelity Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: endorsed 2 or more symptoms on the CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear if those with current and/or past episodes of depression were excluded Baseline severity of depression: CES‐D: 1.40 (subthreshold)
Mean age: 15.5 Age range: 13 to 19 Percentage male: 32.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: none specified Number of sessions: 6 sessions Length of sessions: 60 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 6 weeks Group size: 4 to 8 Delivered by: female masters‐level graduate students Fidelity: assessed as adequate Type of comparison: TAU comprising an NIMH educational brochure describing symptoms of MDD and treatment options | |
| Outcomes | Diagnosis: established from the K‐SADS Name of self‐report depression measure: N/A Name of clinician report depression measure: K‐SADS Name of anxiety measure: N/A Name of general functioning measure: SAS‐SR‐Y Assessment points: post‐intervention, 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated random numbers" (p.67) |
| Allocation concealment (selection bias) | High risk | Correspondence with study authors indicated the project co‐ordinator who derived the random sequence was not independent of the research team |
| Blinding (performance bias and detection bias) | High risk | Correspondence with study authors revealed that participants were not blind to treatment allocation |
| Blinding (performance bias and detection bias) | Unclear risk | "Assessors...were blind to condition..." (p.67) However, social functioning outcomes are self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 4.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using multiple imputation |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: all sessions assessed as delivered with competence Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: endorsed 2 or more symptoms on the CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear if those with current and/or past episodes of depression were excluded Baseline severity of depression: CES‐D: 1.47 (subthreshold) Mean age: 19.0 Age range: 17‐22 Percentage male: 30.5% Setting: college State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: none specified Number of sessions: 6 sessions Length of sessions: 60 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 6 weeks Group size: 4 to 8 Delivered by: female masters‐level graduate students Fidelity: assessed as adequate Type of comparison: TAU comprising an NIMH educational brochure describing symptoms of MDD and treatment options | |
| Outcomes | Diagnosis: established from the K‐SADS Name of self‐report depression measure: N/A Name of clinician report depression measure: K‐SADS. Please note that mean and SDs for this outcome variable obtained through correspondence differ modestly from the published values. There is no material difference in terms of direction or magnitude, however. Name of anxiety measure: N/A Name of general functioning measure: adapted from 17 items from the SAS‐SR‐Y Assessment points: post‐intervention, 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated random numbers…" (p.49) |
| Allocation concealment (selection bias) | Unclear risk | "...assigned by the project coordinator..." (p.49) Unclear if the project co‐ordinator was independent from the research team, however |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "Assessors were blind to condition..." (p.49) However, social functioning outcomes are self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using imputed data in 20 data sets |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: described as "...good or very good fidelity..." (p.51) Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear whether those with current depression excluded. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 13.9 (mild)
Mean age: 9.1 Age range: 8 to 9 Percentage male: 56.7% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Positive Thinking Program Number of sessions: 8 sessions Length of sessions: 60 minutes Intensity (total number of hours): 8 hours Duration of treatment period: 8 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising regular health education curriculum | |
| Outcomes | Diagnosis: established from the DICA‐IV Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 18 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly allocated..." (p.79) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.8% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear whether those with current and/or past depression excluded Baseline severity of depression: CDI: 12.0 (subthreshold) Mean age: 8.8 Age range: 9 to 10 Percentage male: 51.4% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar excluded: exclusion criteria not specified Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Aussie Optimism: Positive Thinking Program Number of sessions: 10 sessions Length of sessions: 60 minutes Intensity (total number of hours): 10 hours Duration of treatment period: 10 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising regular health education curriculum | |
| Outcomes | Diagnosis: established from the DICA‐IV Name of self‐report depression measure: CDI (minus item 9) Name of clinical report depression measure: Name of anxiety measure: SCAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 18 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly allocated..." (p.847) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "...clinicians were blind to the school conditions and they were not aware of the intervention effects on the students" (p.852) However, primary outcomes are self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.4% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using GLMM (i.e. LOCF) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes (specifically, PIR component) | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 8.2 (sub‐threshold) Mean age: 12.2 Age range: 9‐14 Percentage male: 56.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: RAP plus Peer Interpersonal Relatedness (PIR) Number of sessions: 20 sessions Length of sessions: 50 minutes Intensity (total number of hours): 16.7 hours Duration of treatment period: 20 weeks Group size: 6 to 12 Delivered by: students Fidelity: assessed as adequate Type of comparison: AP | |
| Outcomes | Diagnosis: established from scores on the major depression subscale of the DISCAP Name of self‐report depression measure: CDI and RADS‐2. Scores on the CDI will be extracted in preference in the present review. Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and approx. 9 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.512) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Diagnostic interviews were "...administered by a senior clinical psychologist who was unaware of the experimental conditions" (p.513). However, primary outcomes are self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 1.4% (for RAP‐PIR and control groups) Means and SDs used in meta‐analysis based on what data: hierarchical linear modelling which the authors explain "...can accommodate missing data..." (p.514) Intention‐to‐treat analyses: N/A |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the PIR intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 14.4 (sub‐threshold) Mean age: 13.1 Age range: not specified Percentage male: 47.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: beyondblue Secondary Schools Research Initiative Number of sessions: 10 sessions over 3 years Length of sessions: 45 minutes Intensity (total number of hours): 22.5 hours Duration of treatment period: 3 years Group size: unclear Delivered by: non‐mental health experts Fidelity: assessed Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term), 24 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly allocated..." (p.202) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Low risk | "...by a research assistant who was blind to the groups to which schools were being allocated" (p.202) |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: unclear Means and SDs used in meta‐analysis based on what data: adjusted conditional model using a dummy variable coded as 1 if the assessment was incomplete and as 0 if compete Intention‐to‐treat analyses: N/A |
| Selective reporting (reporting bias) | Low risk | Protocol not available. However, supplementary files on the beyondblue website would indicate that all intended outcomes were reported. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: families in which a parent had recently died Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but those with current and/or past episodes of depression not excluded Baseline severity of depression: CDI: 9.8 (subthreshold)
Mean age: 11.4 Age range: not specified Percentage male: 53.0% Setting: mail solicitation, media outlets, agencies in contact with recently bereaved families (e.g. churches, schools, hospitals)
State what psychiatric diagnoses were excluded: conduct disorder, oppositional defiance disorder, ADHD (unmedicated), aggressive and/or delinquent children Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: The Family Bereavement Program Number of sessions: 12 sessions Length of sessions: 120 minutes Intensity (total number of hours): 24 hours Duration of treatment period: 12 weeks (3 months) Group size: 5 to 9 Delivered by: mental health experts Fidelity: not assessed Type of comparison: AP | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention and 11 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...computer program..." (p.589, Sandler 2003) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, attention placebo condition was not credible. Without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 3.7% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: modelling approaches undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. However, many outcomes are also reported in Sandler 2003. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: scoring in the bottom quartile on the ASQ Diagnostic interview to exclude those with current or previous depression: those with current depression (indicated by a score of ≥ 19.0 on the BDI) were excluded. Those with past episodes of depression not excluded (7.5%). Baseline severity of depression: BDI: 7.3 (subthreshold)
Mean age: not stated (19.0) Age range: All participants were 19 Percentage male: 48.0% Setting: university
State what psychiatric diagnoses were excluded: those with current anxiety disorders, substance use/disorder, mania, cyclothymia, psychosis, somatisation disorder, hypochondriasis, undifferentiated somatoform disorder, anorexia, and bulimia Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Prevention Program (APEX) Number of sessions: 8 sessions Length of sessions: 120 minutes Intensity (total number of hours): 16 hours Duration of treatment period: 8 weeks Group size: 10 to 12 Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: LIFE Name of self‐report depression measure: BDI Name of clinical report depression measure: HAM‐D Name of anxiety measure: BAI Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term), and 36 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "To determine if diagnostic interviewers were blind as to which condition participants were in, following each interview, we had them guess...At all the evaluations but one...interviewers were unable to accurately guess which condition participants were in" (no pagination specified). Some outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 3.5% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using survival analysis |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of those with moderate versus severe depression symptomatology. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: BDI score between 9 and 24 Diagnostic interview to exclude those with current and/or past episodes of depression: those with current and/or past episodes of depression not excluded Baseline severity of depression: BDI: 10.1 (subthreshold)
Mean age: not stated (19.0) Age range: All participants were 19 Percentage male: 35.0% Setting: university
State what psychiatric diagnoses were excluded: exclusion criteria not stated Suicide risk excluded: exclusion criteria not stated Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not stated
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: partly. Included ongoing web‐based materials such as email coaching. Name of programme: APEX Number of sessions: 8 sessions Length of sessions: 120 minutes Intensity (total number of hours): 16 hours Duration of treatment period: 8 weeks Group size: 10 to 12. Email coaching individual. Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: LIFE Name of self‐report depression measure: BDI Name of clinical report depression measure: HAM‐D Name of anxiety measure: BAI Name of general functioning measure: N/A Assessment points: post‐intervention and between 4 to 6 months (medium term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "...research assistants asked participants not to tell the interviewer which condition they were in" (p.1118) Primary outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 5.4% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using survival analysis |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of those with moderate versus severe depression symptomatology. Additionally, follow‐up data were not reported. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: not specified What risk was basis of inclusion for selected studies: mild to moderate depression according to DASS‐21 scores Diagnostic interview to exclude those with current and/or past episodes of depression: those with past episodes of depression not excluded, however, those with current depression, as indicated by extremely high scores on the DASS‐21, were excluded Baseline severity of depression: DASS‐21‐d: 18.20 (moderate) Mean age: 19.5 Age range: 18 to 23 Percentage male: 21% Setting: university State what psychiatric diagnoses were excluded: exclusion criteria not stated Suicide risk excluded: exclusion criteria not stated Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not stated Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: N/A as MoodGYM freely available to access Online: yes Name of programme: MoodGYM Number of sessions: 3 sessions plus 2 assessment‐only sessions Length of sessions: between 20 to 40 minutes Intensity (total number of hours): up to 3.33 hours Duration of treatment period: 3 weeks Group size: N/A (individual‐based intervention) Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐21‐d Name of clinical report depression measure: N/A Name of anxiety measure: DASS‐21‐a Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: N/A as MoodGYM freely available to access Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: N/A as 0% dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current and/or past episodes of depression not excluded Baseline severity of depression: CDI: 12.3 (mild)
Mean age: 12.7 Age range: 12 to 14 Percentage male: 53.3% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 120 minutes Intensity (total number of hours): 24 hours Duration of treatment period: 12 weeks Group size: 9 Delivered by: non‐mental health experts and students Fidelity: assessed as adequate Type of comparison: AP | |
| Outcomes | Diagnosis: CDI ≥ 12.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | Each programme "...was presented to parents and teachers as based on established psychological theory..." (p.17) |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported and no detail of blinding of participants. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 6.6% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: undertaken, but method unclear |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of those completing 3 of the initial 4 sessions versus those who completed 8 of the 12 sessions. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: scoring in the top 20% on the combined CDI and CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interviews not undertaken to exclude those with current depression. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 22.0 (severe)
Mean age: 14.3 Age range: 13 to 15 Percentage male: 31.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Problem‐Solving for Life Number of sessions: 8 sessions Length of sessions: 45 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 2 school terms Group size: unclear Delivered by: non‐mental health experts and students Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: SCAS Name of general functioning measure: CASAFS Assessment points: post‐intervention and 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly allocated [...]using a number drawn by the researchers at random from a container..." (p.67) |
| Allocation concealment (selection bias) | Low risk | "...the sequence [was] concealed until assignment..." (p.67) |
| Blinding (performance bias and detection bias) | High risk | "...participants...were not blind to experimental condition" (p.70) |
| Blinding (performance bias and detection bias) | High risk | "...participants...and assessors were not blind to experimental condition" (p.70) |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.2% (universal intervention) and 7.3% (targeted intervention) Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using hierarchical linear modelling |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: scoring in the top 20% on the combined CDI and CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interviews not undertaken to exclude those with current depression. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 22.0 (severe)
Mean age: 14.3 Age range: 13 to 15 Percentage male: 31.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Problem‐Solving for Life Number of sessions: 16 sessions Length of sessions: 8 sessions of 45 minutes plus 8 sessions of 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 2 school terms Group size: 8 to 10 Delivered by: non‐mental health experts and students Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: ADIS‐C MDD, DYS and LIFE Name of self‐report depression measure: CDI and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: SCAS Name of general functioning measure: CASAFS Assessment points: post‐intervention and 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Sheffield a2006 |
| Allocation concealment (selection bias) | Low risk | See Sheffield a2006 |
| Blinding (performance bias and detection bias) | High risk | See Sheffield a2006 |
| Blinding (performance bias and detection bias) | Unclear risk | See Sheffield a2006 |
| Incomplete outcome data (attrition bias) | Unclear risk | See Sheffield a2006 |
| Selective reporting (reporting bias) | Unclear risk | See Sheffield a2006 |
| Other bias | Unclear risk | See Sheffield a2006 |
| Implementation integrity | Unclear risk | See Sheffield a2006 |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interviews not undertaken to exclude those with current depression. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 11.1 (subthreshold)
Mean age: 14.3 Age range: 13 to 15 Percentage male: 46.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Problem‐Solving for Life Number of sessions: 8 sessions Length of sessions: 45 minutes Intensity (total number of hours): 6 hours Duration of treatment period: one school term Group size: unclear Delivered by: non‐mental health professionals Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: SCAS Name of general functioning measure: CASAFS Assessment points: post‐intervention and 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes(not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Sheffield a2006 |
| Allocation concealment (selection bias) | Low risk | See Sheffield a2006 |
| Blinding (performance bias and detection bias) | High risk | See Sheffield a2006 |
| Blinding (performance bias and detection bias) | Unclear risk | See Sheffield a2006 |
| Incomplete outcome data (attrition bias) | Unclear risk | See Sheffield a2006 |
| Selective reporting (reporting bias) | Unclear risk | See Sheffield a2006 |
| Other bias | Unclear risk | See Sheffield a2006 |
| Implementation integrity | Unclear risk | See Sheffield a2006 |
| Methods | Design: RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview not undertaken to exclude those with current depression, those with past episodes of depression not excluded Baseline severity of depression: CES‐D: 8.4 (subthreshold)
Mean age: not specified Age range: 13 to 14 Percentage male: 40.0% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: USA | |
| Interventions | Broad category: third wave (positive psychology) (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Positive Psychoeducation Number of sessions: 7 sessions Length of sessions: 40 minutes Intensity (total number of hours): 4.7 hours Duration of treatment period: 7 weeks Group size: 5 Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: AP | |
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.30) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | "To keep parents and participants blind to the hypotheses, limited detail about the content of the groups was provided" (p.30) |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported but unclear detail about blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 7.1% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Low risk | As this trial was reported in a thesis, it is unlikely selective outcome reporting was present |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression not excluded. Baseline severity of depression: BDI: 7.8 (subthreshold)
Mean age: 12.8 Age range: 12 to 14 Percentage male: 48.5% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Problem Solving for Life Number of sessions: 8 sessions Length of sessions: 45 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 8 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: ADIS‐C and LIFE Name of self‐report depression measure: BDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: CASAFS Assessment points: post‐intervention, 12 months (medium‐term) and 36 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.6% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: unclear if undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: MFQ ≥ 2.0 over 2 assessments approx. 2 weeks apart What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview not used to exclude those with current depression. Those with past episodes of depression not excluded. Baseline severity of depression: SMFQ: 10.6 (subthreshold)
Mean age: not specified Age range: 8 to 11 Percentage male: 34.9% Setting: school
State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no
Country: UK | |
| Interventions | Broad category: CBT with elements of IPT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: RAP Number of sessions: 9 sessions and 2 booster sessions Length of sessions: 50 to 60 minutes Intensity (total number of hours): up to 9 hours (not including booster sessions) Duration of treatment period: unclear Group size: unclear Delivered by: students with at least an undergraduate degree in a relevant discipline Fidelity: assessed as adequate Type of comparison: TAU comprising usual personal health and social education classes provided by the school | |
| Outcomes | Diagnosis: SMFQ ≥ 5.0 Name of self‐report depression measure: SMFQ Name of clinical report depression measure: N/A Name of anxiety measure: RCADS Name of general functioning measure: N/A Assessment points: 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...we allocated year groups on a 1:1:1 ratio. We balanced the trial arms for key characteristics by calculating an imbalance statistic for a large random sample of possible allocation sequences" (no pagination specified). |
| Allocation concealment (selection bias) | Low risk | "A statistician with no other involvement in the study randomly selected one sequence from a subset..." (no pagination specified) |
| Blinding (performance bias and detection bias) | Unclear risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported but there is no detail about blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 21.1% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: multiple imputation |
| Selective reporting (reporting bias) | Low risk | Trial protocol (i.e. Stallard 2010) would suggest that all intended outcomes were assessed |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes (5% of sessions) Implementation integrity adequate: 89.00% of sessions covered core tasks, with at least 75% of the core tasks covered in the remaining 11% of sessions Implementation integrity reported: yes |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 20.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken and those with current depression indicated by BDI ≥ 30.0 were excluded. Those with previous episodes of depression not excluded. Baseline severity of depression: BDI: 19.9 (mild‐moderate)
Mean age: 18.4 Age range: 15 to 22 Percentage male: 30.0% Setting: school
State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: The Blues Group Number of sessions: 4 sessions Length of sessions: 60 minutes Intensity (total number of hours): 4 hours Duration of treatment period: 4 weeks Group size: 6 to 10 Delivered by: students Fidelity: not assessed Type of comparison: WL | |
| Outcomes | Diagnosis: BDI ≥ 30.0 Name of self‐report depression measure: BDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (short‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no Coding for depression severity at baseline: where baseline severity was mild to moderate as in this trial, it was rated as mild. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 18.0% by 6 months Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: full information maximum likelihood ratio based on expectation‐maximisation algorithm |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 20.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken and those with current and/or previous episodes of depression excluded Baseline severity of depression: BDI: 19.8 (mild‐moderate)
Mean age: 15.6 Age range: 14 to 19 Percentage male: 44.0% Setting: school
State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: not specified Number of sessions: 6 sessions Length of sessions: 60 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 6 weeks Group size: 6 to 10 Delivered by: students Fidelity: assessed as adequate Type of comparison: NT | |
| Outcomes | Diagnosis: 16 item version of the K‐SADS Name of self‐report depression measure: BDI and a continuous score created by summing items from the K‐SADS Name of clinical report depression measure: 16 item version of the K‐SADS Name of anxiety measure: N/A Name of general functioning measure: SAS‐SR‐Y Assessment points: post‐intervention and 6 months (short‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no Coding for depression severity at baseline: where baseline severity was mild to moderate as in this trial, it was rated as mild. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated random numbers..." (p.597) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "...assessors...were blind to condition..." (p.597) Primary outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 1.2% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: full information maximum likelihood ratio based on expectation‐maximisation algorithm |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. However, depression outcomes at 2 years not reported. Additionally, the authors did undertake post‐hoc analyses of clinically significant change in depression symptomatology. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: a score of 50 to 70 on the CDI What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current and/or past episodes of depression not excluded Baseline severity of depression: CDI: 10.6 (subthreshold)
Mean age: 15.0 Age range: not specified Percentage male: 41.0% Setting: school
State what psychiatric diagnoses were excluded: those with any “current psychiatric diagnosis” excluded from analyses Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: no
Country: USA | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Coping with Depression Number of sessions: 10 sessions Length of sessions: 50 minutes Intensity (total number of hours): 8.3 hours Duration of treatment period: 10 weeks Group size: 20 Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising didactic lectures about general topics in psychology | |
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A | |
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: no Author contacted for outcome data: yes (not provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.41) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | The clustered nature of allocation suggests it is possible participants could have been unable to determine to which group they had been allocated. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.9% Means and SDs used in meta‐analysis based on what data: observed cases (based on those who completed 8 or more sessions) Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview was not undertaken, however, those with RADS scores ≥ 76.0 or those with current depression according to the CRDS‐R were excluded. Unclear whether those with past episodes of depression were also excluded. Baseline severity of depression: RADS‐II: 53.5 (subthreshold)
Mean age: 14.3 Age range: 13 to 17 Percentage male: 31.7% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: New Zealand | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: N/A Online: telephone Name of programme: MEMO Number of sessions: 2 mobile telephone messages per day (mixture of SMS messages and links to videos and/or external websites) Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 9 weeks Group size: telephone messages (individual) Delivered by: N/A (self‐monitoring) Fidelity: N/A, although only three‐quarters of participants viewed at least half of the messages sent Type of comparison: AP | |
| Outcomes | Diagnosis: K‐SADS Name of self‐report depression measure: RADS‐2 and MFQ Name of clinical report depression measure: CDRS‐R Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐based randomisation..." (no pagination specified) |
| Allocation concealment (selection bias) | Low risk | "...allocation concealment was maintained by computer‐based randomisation so that researchers were unaware of possible allocation" (no pagination specified) |
| Blinding (performance bias and detection bias) | Low risk | "Participants were not aware of which program was the intervention and which was the control..." (no pagination specified) |
| Blinding (performance bias and detection bias) | Low risk | "The interviews were conducted by research assistants blinded to allocation..." |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.3% Means and SDs used in meta‐analysis based on what data: observed cases (via correspondence) Intention‐to‐treat analyses: LOCF (via correspondence) |
| Selective reporting (reporting bias) | Low risk | Trial protocol would suggest that all intended outcomes were assessed |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDI ≥ 16.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: although diagnostic interviews were not undertaken, those with CDI score of ≥ 19.0 were excluded. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 20.9 (severe) Mean age: 13.3 Age range: 11 to 15 Percentage male: 0% Setting: school
State what psychiatric diagnoses were excluded: those currently receiving mental health care were excluded Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no
Country: The Netherlands | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Op Volle Kracht ("At Full Strength") Number of sessions: 8 sessions Length of sessions: 50 minutes Intensity (total number of hours): 6.7 hours Duration of treatment period: 8 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDU and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using a computerized random number generator..." (p.3) |
| Allocation concealment (selection bias) | Low risk | "An independent researcher performed the randomization..."(p.3) |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.3% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: full information maximum likelihood ratio based on expectation‐maximisation algorithm |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: PHQ‐5: 2.9 (unclear) Mean age: not specified Age range: 14 to 15 Percentage male: 30.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not reported Suicide risk excluded: exclusion criteria not reported Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not reported Country: Australia | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: N/A (online) Online: yes Name of programme: Thiswayup Schools Number of sessions: 7 sessions Length of sessions: 40 minutes Intensity (total number of hours): 4.7 hours Duration of treatment period: 7 weeks Group size: individual‐based therapy Delivered by: N/A (self‐monitoring) Fidelity: N/A. Online, therefore standardised. Type of comparison: TAU comprising regular health and personal development classes | |
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A | |
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly allocated..." (p.91) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 72.8% Means and SDs used in meta‐analysis based on what data: N/A Intention‐to‐treat analyses: linear mixed‐model repeated measures analysis |
| Selective reporting (reporting bias) | High risk | Protocol indicates knowledge gained with respect to causes and symptoms of depression will also be assessed |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the team were involved in adapting the intervention for this setting. | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDI ≥ 63.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 24.5 (moderate) Mean age: 14.0 Age range: not specified Percentage male: not specified Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: New Zealand | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: ACE‐Kiwi Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 8 to 12 Delivered by: mental health experts Fidelity: unclear if assessed Type of comparison: TAU comprising ongoing counselling with the school counsellor and/or referral to mental health services as required | |
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 2 months (short‐term), 12 months (medium‐term) | |
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated random assignment..." (p.43) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 57.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Trial not conducted by those who developed the intervention. However, intervention was adapted by the author for this setting. |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 16.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken and those with current depression and/or those with CES‐D scores ≥ 40.0 were excluded. Those with past episodes of depression were not excluded. Baseline severity of depression: CES‐D: 25.2 (mild)
Mean age: 13.4 Age range: 11 to 16 Percentage male: 14.6% Setting: school
State what psychiatric diagnoses were excluded: panic disorder, obsessive‐compulsive disorder, post‐traumatic stress disorder, conduct disorder, oppositional defiance disorder, bipolar disorder, psychosis and ADHD (untreated) Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not reported
Country: USA | |
| Interventions | Broad category: IPT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Interpersonal Psychotherapy‐Adolescent Skills Training Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 3 to 7 Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising referral to school counsellors and/or social worker as required. Additional psychotherapy and/or medication was also available as required. | |
| Outcomes | Diagnosis: K‐SADS‐PL Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: CGAS Assessment points: post‐intervention and 6 months (short‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using a table of random numbers..." (p.1257) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "...interviews were performed by a clinical evaluator...who was blind to treatment condition" (p.1257) Primary outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.4% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: LOCF |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: unclear | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 16.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those who met diagnostic criteria for depression were excluded. Unclear whether those with past episodes of depression were also excluded. Baseline severity of depression: CES‐D: 15.2 (subthreshold)
Mean age: 14.5 Age range: 11 to 17 Percentage male: 40.3% Setting: school
State what psychiatric diagnoses were excluded: panic disorder, obsessive‐compulsive disorder, post‐traumatic stress disorder, conduct disorder, oppositional defiance disorder, bipolar disorder, psychosis and ADHD (untreated) Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no
Country: USA | |
| Interventions | Broad category: IPT (for further information on intervention components, see Table 1) Manualised: unclear Online: no Name of programme: Interpersonal Psychotherapy‐Adolescent Skills Training Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: unclear Group size: 4 to 6 Delivered by: mental health experts Fidelity: assessed but unclear if assessed as adequate Type of comparison: TAU comprising referral to school counsellors and/or social worker as required | |
| Outcomes | Diagnosis: K‐SADS Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: CGAS Assessment points: post‐intervention, 12 months (medium‐term) and 18 months (long‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: yes (provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...table of random numbers..." (p.428) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | "The evaluations were conducted by independent evaluators..." (p.429) Primary outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.8% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using hierarchical linear modelling and LOCF |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: N/A Implementation integrity reported: N/A |
| Methods | Design: RCT Conducted by the team who developed the intervention: yes | |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: those with scores in the top 25% for their age bracket on combined z scores on the CDI and on the perception of family relationships item of the Cohesion and Conflict subscale of the FES What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview not undertaken, however, those with current and/or past episodes of depression not excluded Baseline severity of depression: CDI: 17.1 (moderate)
Mean age: 11.8 Age range: 8 to 15 Percentage male: 55.5% Setting: school
State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified
Country: China | |
| Interventions | Broad category: CBT (for further information on intervention components, see Table 1) Manualised: yes Online: no Name of programme: Penn Resiliency Program, Chinese adaption Number of sessions: 10 sessions Length of sessions: 120 minutes Intensity (total number of hours): 20 hours Duration of treatment period: 10 weeks Group size: 10 to 14 Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: NT | |
| Outcomes | Diagnosis: CDI ≥ 15.0 (moderate depression) and CDI ≥ 20.0 (severe depression) Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (short‐term) | |
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.3% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: N/A |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
ADHD: attention deficit hyperactivity disorder
ADIS‐C: Anxiety Disorders Interview Schedule for Children
ASQ: Attribution Style Questionniare
AP: attention placebo
BAI: Beck Anxiety Inventory
BDI: Beck Depression Inventory
BDI‐II: Beck Depression Inventory‐second revision
BSI: Brief Symptom Inventory
BT: behavioural therapy
CASAFS: Child and Adolescent Social and Adaptive Functioning Scale
CATCH‐IT: Competent Adulthood Transition with Cognitive‐behavioral and Interpersonal Training
CBT: cognitive behavioural therapy
CDI: Children's Depression Inventory
CDRS‐R: Children's Depression Rating Scale‐Revised
CDS: Children's Depression Scale
CES‐D: Center for Epidemiologic Studies Depression Scale
CGAS: Children's Global Assessment Scale
CSAQ: Cognitive Somatic Anxiety Questionniare
CURB:
DASS: Depression Anxiety Stress Scale
DICA‐IV: Diagnostic Interviewfor Children and Adolescents, version four
DISC‐IV: Diagnostic Interview Schedule for Children, version four
DISCAP: Diagnostic Interview Schedule for Children, Adolescents, and Parents
FES: Family Environment Scale
GLMM: Generalised Linear Mixed Model
GP: general practitioner
HAM‐D: Hamilton Depression Rating Scale
HMO: health maintenance organisation
IPT: interpersonal therapy
IPT‐AST: interpersonal psychotherapy‐adolescent skills training
ITT: intention‐to‐treat
K‐SADS: Kiddie‐Schedule for Affective Disorders and Schizophrenia for School‐Age Children
K‐SADS‐E: Kiddie‐Schedule for Affective Disorders and Schizophrenia‐Epidemiological version
K‐SADS‐PL: Kiddie‐Schedule for Affective Disorders and Schizophrenia‐Present and Lifetime version
LARS&LISA‐T: Ease of Handling Social Aspects in Everyday Life‐Training (English Translation)
LIFE: Longitudinal Interval Follow‐up Evaluation
LOCF: last observation carried forward
MASQ: Mood and Anxiety Symptom Questionnaire
MDD: major depressive disorder
MFQ: Mood and Feeling Questionnaire
N/A: not available
NIMH: National Institute of Mental Health
NT: no treatment
PIR: Peer Interpersonal Relatedness
PHQ‐9: Patient Health Questionniare‐9 item version.
PLS: plain language statement
PQ‐LES‐Q: Paediatric Quality of Life Enjoyment and Satisfaction Questionnaire
PRP: Penn Resilience Program
RAP‐PIR: Resourceful Adolescent Program‐Peer Interpersonal Relatedness
RADS: Reynold's Adolescent Depression Scale
RADS‐2: Reynold's Adolescent Depression Scale, version two
RCADS: Revised Child Anxiety and Depression Scale
RCMAS: Revised Children's Manifest Anxiety Scale
RCT: randomised controlled trial
SACQ: Student Adaption to College Questionnaire
SAI‐C: State Anxiety Inventory for Children
SAS‐SR‐Y: Social Adjustment Scale‐Self‐Report for Youth
SAS: Social Adjustment Scale
SBB‐DES: Selbstbeurteilungsbogen‐Depressive Störungen (Self‐Report Questionnaire‐Depression)
SCAS: Spence Children's Anxiety Scale
SCID‐I: Structured Clinical Interview for DSM‐IV
SD: standard deviation
SMFQ: Short Mood Feeling Questionnaire
SWEMWBS: Short Warwick‐Edinburgh Mental Well‐Being Scale
TAU: treatment as usual
WL: wait‐list
YSR: Youth Self‐Report
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Participants recruited on the basis of depressive or anxious symptoms | |
| Not a RCT | |
| Not primarily a depression prevention programme ‐ the focus is on anxiety prevention | |
| Not primarily a depression prevention programme ‐ th focus is instead on broad mental health or well‐being, or both | |
| Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) | |
| Focus is on anxiety prevention | |
| Not primarily a depression prevention programme ‐ the focus is on trauma | |
| Not primarily a depression prevention programme ‐ the focus is on anxiety prevention | |
| Not primarily a depression prevention programme ‐ the focus is on broad mental health or well‐being, or both | |
| Treatment study | |
| Not primarily a depression prevention programme ‐ the focus is instead on improving relationships, parenting and coping in children with divorced parents | |
| No suitable validated depression outcome measure | |
| No suitable validated depression outcome measure | |
| Not primarily a depression prevention programme ‐ the focus is instead on broad mental health or well‐being, or both | |
| Intervention not primarily delivered to the individual (e.g. family therapy or parenting skills) | |
| Not primarily a depression prevention programme ‐ the focus is on treating disruptive behaviours | |
| Focus is on broad mental health or well‐being, or both | |
| Focus is on trauma or PTSD | |
| Participants not within the age bracket specified for this review | |
| Study 1: use of alternate allocation. Study 2: participants not within the age bracket specified for this review | |
| Not primarily a depression prevention programme ‐ the focus is on anxiety, stress and anger | |
| Not primarily a depression prevention programme ‐ the focus is on anxiety, stress and anger | |
| Not primarily a depression prevention programme ‐ the focus is instead on anxiety, stress and anger | |
| Intervention not primarily delivered to the individual (e.g. family therapy or parenting skills) | |
| Not primarily a depression prevention programme ‐ the focus is instead on depression or anxiety, or both | |
| Not primarily a depression prevention programme ‐ the focus is instead on broad mental health or well‐being, or both. | |
| Not primarily a depression prevention programme ‐ the focus is instead on improving social skills | |
| Participants not within the age bracket specified for this review | |
| Not primarily a depression prevention programme ‐ the focus is on treating disruptive behaviours | |
| Participants recruited on the basis of depressive or anxious symptoms | |
| Not primarily a depression prevention programme ‐ the focus is on broad mental health or well‐being, or both | |
| Not primarily a depression prevention programme ‐ the focus is on broad mental health or well‐being, or both | |
| Treatment study | |
| Not primarily a depression prevention programme ‐ the focus is on trauma | |
| Not primarily a depression prevention programme ‐ the focus is on anxiety prevention | |
| Not primarily a depression prevention programme ‐ the focus is on anxiety prevention | |
| Not primarily a depression prevention programme ‐ the focus is instead on depression and/or anxiety | |
| Participants recruited on the basis of depressive or anxious symptoms | |
| Does not contain a suitable psychological intervention | |
| Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) | |
| Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) | |
| Not a RCT | |
| Intervention not focused on addressing participants' own cognitions to reduce depressive symptomatology. Focus is instead on generic psychoeducation to increase awareness of the link between depressive symptoms and perceptions. | |
| Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) | |
| No suitable psychological intervention | |
| Not primarily a depression prevention programme ‐ the focus is on chronic pain | |
| Treatment study | |
| No suitable comparison or control group | |
| Not primarily a depression prevention programme ‐ the focus is on trauma | |
| Not primarily a depression prevention programme ‐ the focus is instead on broad mental health or well‐being, or both | |
| No suitable depression outcome measure | |
| Not primarily a depression prevention programme ‐ the focus is on trauma | |
| Not primarily a depression prevention programme ‐ the focus is instead on broad mental health or well‐being, or both | |
| Not primarily a depression prevention programme ‐ participants recruited on the basis of depressive or anxious symptoms | |
| Not a RCT | |
| Focus is on anxiety prevention | |
| Focus is on trauma or PTSD | |
| Treatment study | |
| Not primarily a depression prevention programme ‐ the focus is on trauma | |
| Not a RCT | |
| No suitable validated depression outcome measure | |
| Not a suitable control/comparison condition ‐ head to head trial of 2 active interventions instead | |
| Not primarily a depression prevention programme ‐ the focus is on vocational preparedness | |
| No suitable comparison or control group | |
| Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) | |
| Not primarily a depression prevention programme ‐ the focus is on trauma |
PTSD: post‐traumatic stress disorder
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: no information available Description: no information available |
| Participants | Age range: no information available Country: no information available |
| Interventions | Broad category: no information available Name of programme: no information available Comparison group: no information available |
| Outcomes | Diagnosis: no information available Name of self‐report depression measure: no information available |
| Notes | — |
| Methods | Design: no information available Description: no information available |
| Participants | Age range: no information available Country: no information available |
| Interventions | Broad category: no information available Name of programme: none Comparison group: no information available |
| Outcomes | Diagnosis: no information available Name of self‐report depression measure: no information available |
| Notes | — |
| Methods | Design: RCT Description: targeted |
| Participants | Age range: 15 to 22 years Country: The Netherlands |
| Interventions | Broad category: CBT Name of programme: rumination focused CBT Comparison group: no treatment |
| Outcomes | Diagnosis: no Name of self‐report depression measure: Beck Depression Inventory II |
| Notes | — |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: question if this is included: participants must report symptoms of social anxiety and/or depression that exceed clinical cut‐offs on the Social Anxiety Scale for Adolescents (SAS‐A > or = to 50) or the Center for Epidemiologic Studies‐Depression Scale (CES‐D > or = to 16) What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: 13 to 18 years Percentage male: N/A Setting: school Psychiatric diagnoses excluded: social anxiety, depression, PTSD, bipolar disorder, psychosis, eating disorder, substance use disorder, conduct disorder Suicide risk excluded: yes. Must not endorse active suicidal items on the Columbia Suicide Severity Rating Scale (C‐SSRS). Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: USA |
| Interventions | Broad category: IPT Manualised: not reported Online: no Name of programme: PEERS/UTalk Comparison: education/support (ES) |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: Centre of Epidemiological Studies Depression Scale (CES‐D; Radloff 1977). This is a secondary outcome measure. Name of clinician report depression measure: not reported Name of anxiety measure: Anxiety Disorder Interview Schedule ‐ Children (ADIS‐C) Name of general functioning measure: Clinicians Global Impression Scale Assessment points: baseline, 12 weeks, 6 months |
| Notes | — |
| Methods | Design: RCT Description: universal |
| Participants | Age range: 18 to 20 years Country: USA |
| Interventions | Broad category: 3rd wave CBT Name of programme: ACT on college life (ACT‐CL) Comparison group: wait‐list |
| Outcomes | Diagnosis: no Name of self‐report depression measure: DASS |
| Notes | — |
| Methods | Design: RCT Description: targeted |
| Participants | Age range: 12 to 15 years Country: USA |
| Interventions | Broad category: CBT Name of programme: Coping and Support Training for the Transition (CAST‐T) Comparison group: TAU |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported |
| Notes | — |
| Methods | Design: RCT |
| Participants | Description: indicated and selective Cut‐point for inclusion for indicated studies: Children’s Depression Inventory 2 (CDI 2; Kovacs 1992) and Spence Children Anxiety Scale (SCAS; Spence 2003) What risk was basis of inclusion for selected studies: a parent with elevated levels of depression or anxiety as determined by the Brief Symptom Inventory (BSI; De Beurs 2005) Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: 11 to 15 years Percentage male: N/A Setting: school Psychiatric diagnoses excluded: not reported Suicide risk excluded: prominence of suicidal ideation excluded (score 2 on CDI item: a desire to kill oneself, if given the chance) Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: The Netherlands |
| Interventions | Broad category: CBT Manualised: not reported Online: no Name of programme: ‘Een Sprong Vooruit’ (A Jump Forward) Comparison: no intervention |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: (CDI 2; Kovacs 1992) Name of clinician report depression measure: not reported Name of anxiety measure: (SCAS; Spence 2003) Name of general functioning measure: not reported Assessment points: baseline, T2 (after session 2), T3 (after session 4), post‐intervention, 6 months follow‐up, 12 months follow‐up |
| Notes | — |
| Methods | Design: no information available Description: no information available |
| Participants | Age range: no information available Country: no information available |
| Interventions | Broad category: no information available Name of programme: no information available Comparison group: no information available |
| Outcomes | Diagnosis: no information available Name of self‐report depression measure: no information available |
| Notes | — |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: not reported What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: not reported Percentage male: N/A Setting: primary care clinic, school clinic, hospital Psychiatric diagnoses excluded: not reported Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: USA |
| Interventions | Broad category: CBT, BT, IPT plus additional motivational component Manualised: yes Online: yes Name of programme: CURB (modification of CATCH‐IT) Comparison: wait‐list |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: not reported |
| Notes | — |
| Methods | Design: cluster‐RCT |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: 12 to 14 years Percentage male: N/A Setting: school Psychiatric diagnoses excluded: not reported Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: The Netherlands |
| Interventions | Broad category: CBT and social problem‐solving Manualised: yes Online: no Name of programme: Op Volle Kracht Comparison: usual school curriculum, which in some schools does include social skills |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: CDI Name of clinician report depression measure: none Name of anxiety measure: Revised Children’s Manifest Anxiety Scale (RCMAS) Name of general functioning measure: none Assessment points: baseline, post‐intervention |
| Notes | — |
| Methods | Design: RCT Description: targeted |
| Participants | Age range: not reported Country: Taiwan |
| Interventions | Broad category: IPT Name of programme: none Comparison group: TAU |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported |
| Notes | — |
| Methods | Design: RCT Description: targeted |
| Participants | Age range: 13 to 17 years Country: USA |
| Interventions | Broad category: CBT and IPT Name of programme: CATCH‐IT Comparison group: Attention Monitoring Psycho‐education (AMPE) Arm |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported |
| Notes | — |
CATCH‐IT: Competent Adulthood Transition with Cognitive Behavioral Humanistic and Interpersonal Training
CBT: cognitive behavioural therapy
CDI: Children's Depression Inventory
CES‐D: Center for Epidemiologic Studies Depression Scale
DASS: Depression Anxiety Stress Scale
IPT: interpersonal therapy
N/A: not available
RCT: randomised controlled trial
SAS: Social Adjustment Scale
SCAS: Spence Children's Anxiety Scale
TAU: treatment as usual
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Adapted and Translated, Adolescent Depression, Internet Intervention |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: 16 on the CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear Baseline severity of depression: N/A Mean age: N/A Age range: 13 to 21 years Percentage male: N/A Setting: community Psychiatric diagnoses excluded: Depression, Schizophrenia and Bipolar Affective Disorder Suicide risk excluded: imminent suicide risk excluded Parents with history of schizophrenia/bipolar disorder excluded: yes Country: Hong Kong |
| Interventions | Broad category: CBT and IPT Manualised: yes Online: yes Name of programme: AT‐CATCH (adaption of CATCH‐IT) Comparison: interactive anti‐smoking website |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: Centre of Epidemiological Studies Depression Scale (CES‐D; Radloff 1977) and Depression Anxiety Stress Scale (DASS; Lovibond 1995) Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: baseline, 3 months, 6 months, 12 months |
| Starting date | 31 January 2013 |
| Contact information | Dr David Chim, The University of Hong Kong |
| Notes | — |
| Trial name or title | A Family Depression Prevention Program (FDP) |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: parent with a current or history of a depressive disorder within child's life Diagnostic interview to exclude those with current or previous depression: yes Baseline severity of depression: N/A Mean age: N/A Age range: 9 to 15.6 years Percentage male: N/A Setting: community Psychiatric diagnoses excluded: schizophrenia, bipolar I disorder, current depressive disorder, developmental disability, substance use disorder Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: yes Country: USA |
| Interventions | Broad category: CBT Manualised: not reported Online: no Name of programme: Family Depression Prevention (FDP) program Comparison: written information. Families receive written materials about depression and the effects of parental depression on children. |
| Outcomes | Diagnosis: Longitudinal Interval Follow‐up Evaluation (LIFE) Name of self‐report depression measure: Youth Self‐report (YSR) depression subscale Name of clinician report depression measure: not reported Name of anxiety measure: Youth Self‐report (YSR) anxiety subscale Name of general functioning measure: not reported Assessment points: baseline, post‐intervention, 6 months, 12 months, 18 months and 24 months |
| Starting date | December 2014 |
| Contact information | Robin Weersing ([email protected]); San Diego University Judy Garber ([email protected]); Vanderbilt University Bruce Compas ([email protected]); Vanderbilt University |
| Notes | — |
| Trial name or title | Web based personalized intervention for risky drinking college students with depressed mood: examining the moderating effect of drinking motives |
| Methods | Design: RCT |
| Participants | Description: Targeted Cut‐point for inclusion for indicated studies: BDI score of 14 or more What risk was basis of inclusion for selected studies: elevated depression and risk drinking Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: not reported Mean age: not reported Age range: university students Percentage male: 35% Setting: community Psychiatric diagnoses excluded: not reported Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: not reported |
| Interventions | Broad category: CBT Manualised: yes Online: yes Name of programme: unnamed Comparison: assessment only |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: not reported |
| Starting date | not reported |
| Contact information | not reported |
| Notes |
| Trial name or title | Screening and Training: Enhancing Resilience in Kids |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: 80th percentile of either the subscale for depression or the cluster of subscales for anxiety What risk was basis of inclusion for selected studies: at least one parent has a current or in the last 5 years has had a unipolar mood or anxiety disorder; meet 2 of the 3 High Risk Index criteria i.e. being female, have 2 affected parents, having a parent with a history of past suicidal behaviour Diagnostic interview to exclude those with current or previous depression: yes Baseline severity of depression: N/A Mean age: N/A Age range: 8 to 17 years Percentage male: N/A Setting: mental health services, GP, media (including digital) Psychiatric diagnoses excluded: mental retardation; current diagnosis of a mental disorder that warrants regular treatment but included those with e.g. a diagnosis like ADHD that was sufficiently treated (stable) Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: yes Country: The Netherlands |
| Interventions | Broad category: CBT Manualised: not reported Online: no Name of programme: none Comparison: attention placebo (minimal written information) |
| Outcomes | Diagnosis: unclear Name of self‐report depression measure: Revised Child Anxiety and Depression Scale (RCADS; Chorpita 2005) Name of clinician report depression measure: not reported Name of anxiety measure: Revised Child Anxiety and Depression Scale (RCADS; Chorpita 2005) Name of general functioning measure: not reported Assessment points: baseline, 4 months, 12 months, 24 months |
| Starting date | 1 October 2010 |
| Contact information | Maaike Nauta ([email protected]); University of Groningen |
| Notes | — |
| Trial name or title | The PRODO trial |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: at least one parent who meets diagnostic criteria for a current (or past, during the child's lifetime) diagnosis of depression Diagnostic interview to exclude those with current or previous depression: yes Baseline severity of depression: N/A Mean age: N/A Age range: 8 to 17 years Percentage male: N/A Setting: recruitment from adult psychiatric clinics and advertisements Psychiatric diagnoses excluded: any current or previous psychiatric disorder, or has undergone treatment or is receiving treatment for depression Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: N/A Country: Germany |
| Interventions | Broad category: CBT Manualised: yes Online: no Name of programme: Raising Healthy Children Comparison: no intervention |
| Outcomes | Diagnosis: Diagnostic Interview for Psychiatric Disorders for Children and Adolescents (K‐DIPS; Unnewehr 2008) Name of self‐report depression measure: Depression Inventory for Children and Adolescents (DIKJ; children aged 8 to 12; Stiensmeier‐Pelster 2000) and the German‐version of the revised Beck Depression Inventory (BDI‐II; children aged 13 and over; Hautzinger 1994) Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: baseline, 6 months, 9 months, 15 months |
| Starting date | 7 April 2014 |
| Contact information | Belinda Platt ([email protected]‐muenchen.de); Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, Munich |
| Notes | — |
| Trial name or title | Interpersonal Psychotherapy for Adolescent Girls (IPT‐A) |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: child maltreatment Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: 13 to 15 years Percentage male: 0% Setting: community Psychiatric diagnoses excluded: not reported Suicide risk excluded: yes ‐ actively suicidal excluded Parents with history of schizophrenia/bipolar disorder excluded: N/A Country: USA |
| Interventions | Broad category: IPT Manualised: not reported Online: no Name of programme: none Comparison: enhanced care that they would typically receive in a community‐based setting |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: baseline, mid‐intervention (6 weeks), post‐intervention (12 weeks), 12 months and 18 months |
| Starting date | July 2011 |
| Contact information | Sheree Toth ([email protected]). |
| Notes | — |
| Trial name or title | Competent Adulthood Transition with Cognitive Behavioral Humanistic and Interpersonal Training (CATCH‐IT) |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: must score between 8 to 17 on the CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: past depression and dysthymia included Baseline severity of depression: N/A Mean age: 14.87 years (based on those currently enrolled at 2015) Age range: 13 to 18 years Percentage male: N/A Setting: primary care clinic Psychiatric diagnoses excluded: current MDD, schizophrenia, bipolar disorder Suicide risk excluded: yes ‐ if at serious imminent risk of suicide Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: USA |
| Interventions | Broad category: CBT and IPT Manualised: yes Online: yes Name of programme: CATCH‐IT Comparison: Health Education‐attention control |
| Outcomes | Diagnosis: K‐SADS Name of self‐report depression measure: CES‐D Name of clinician report depression measure: not reported Name of anxiety measure: the Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher 1997) Name of general functioning measure: Pediatric Quality of Life and Enjoyment and Satisfaction Questionnaire ‐ parent and child versions (PQ‐LES‐Q; Endicott 1981) Assessment points:baseline and at 2, 6, 12, 18 and 24 months post‐intake |
| Starting date | 2012 |
| Contact information | Benjamin Van Voorhees ([email protected]) |
| Notes | — |
ADHD: attention deficit hyperactivity disorder
BT: Behavioural therapy
CATCH‐IT: Competent Adulthood Transition with Cognitive Behavioral Humanistic and Interpersonal Training
CURB: Adaptation of CATCH‐IT
CBT: cognitive behavioural therapy
CES‐D: Center for Epidemiologic Studies Depression Scale
GP: general practitioner
IPT: interpersonal therapy
K‐SADS: Kiddie‐Schedule for Affective Disorders and Schizophrenia for School‐Age Children
MDD: major depressive disorder
N/A: not available
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive diagnosis (by population) post‐intervention Show forest plot | 20 | 3232 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.08, ‐0.02] |
| Analysis 1.1  Comparison 1 Psychological intervention versus any comparison, Outcome 1 Depressive diagnosis (by population) post‐intervention. | ||||
| 1.1 Targeted | 13 | 2022 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.02] |
| 1.2 Universal | 7 | 1210 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| 2 Depressive diagnosis short‐term follow‐up Show forest plot | 6 | 724 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| Analysis 1.2  Comparison 1 Psychological intervention versus any comparison, Outcome 2 Depressive diagnosis short‐term follow‐up. | ||||
| 2.1 Targeted | 4 | 360 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.19, ‐0.02] |
| 2.2 Universal | 2 | 364 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 3 Depressive diagnosis medium‐term follow‐up Show forest plot | 32 | 5965 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.05, ‐0.01] |
| Analysis 1.3  Comparison 1 Psychological intervention versus any comparison, Outcome 3 Depressive diagnosis medium‐term follow‐up. | ||||
| 3.1 Targeted | 22 | 3915 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 3.2 Universal | 10 | 2050 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 4 Depressive diagnosis long‐term follow‐up Show forest plot | 10 | 1769 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.05, 0.02] |
| Analysis 1.4  Comparison 1 Psychological intervention versus any comparison, Outcome 4 Depressive diagnosis long‐term follow‐up. | ||||
| 4.1 Targeted | 6 | 1043 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.03] |
| 4.2 Universal | 4 | 726 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 5 Depression symptoms (by population) post‐intervention Show forest plot | 73 | 13829 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.27, ‐0.15] |
| Analysis 1.5  Comparison 1 Psychological intervention versus any comparison, Outcome 5 Depression symptoms (by population) post‐intervention. | ||||
| 5.1 Targeted | 42 | 4816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| 5.2 Universal | 31 | 9013 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.05] |
| 6 Depression symptoms short‐term follow‐up Show forest plot | 16 | 1558 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.45, ‐0.17] |
| Analysis 1.6  Comparison 1 Psychological intervention versus any comparison, Outcome 6 Depression symptoms short‐term follow‐up. | ||||
| 6.1 Targeted | 11 | 999 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.54, ‐0.20] |
| 6.2 Universal | 5 | 559 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.37, 0.01] |
| 7 Depression symptoms medium‐term follow‐up Show forest plot | 53 | 11913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.18, ‐0.05] |
| Analysis 1.7  Comparison 1 Psychological intervention versus any comparison, Outcome 7 Depression symptoms medium‐term follow‐up. | ||||
| 7.1 Targeted | 29 | 4448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.33, ‐0.12] |
| 7.2 Universal | 24 | 7465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 8 Depression symptoms long‐term follow‐up Show forest plot | 15 | 3836 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| Analysis 1.8  Comparison 1 Psychological intervention versus any comparison, Outcome 8 Depression symptoms long‐term follow‐up. | ||||
| 8.1 Targeted | 7 | 847 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.21, 0.11] |
| 8.2 Universal | 8 | 2989 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.06, 0.09] |
| 9 Depression symptoms clinician‐rated (by population) post‐intervention Show forest plot | 11 | 2175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.41, ‐0.05] |
| Analysis 1.9  Comparison 1 Psychological intervention versus any comparison, Outcome 9 Depression symptoms clinician‐rated (by population) post‐intervention. | ||||
| 9.1 Targeted | 10 | 1340 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.44, ‐0.11] |
| 9.2 Universal | 1 | 835 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.06, 0.21] |
| 10 Depression symptoms clinician‐rated medium‐term follow‐up Show forest plot | 9 | 1754 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.24, 0.07] |
| Analysis 1.10  Comparison 1 Psychological intervention versus any comparison, Outcome 10 Depression symptoms clinician‐rated medium‐term follow‐up. | ||||
| 10.1 Targeted | 8 | 968 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.09] |
| 10.2 Universal | 1 | 786 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.14, 0.14] |
| 11 Depression symptoms clinician‐rated long‐term follow‐up Show forest plot | 6 | 894 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.01] |
| Analysis 1.11  Comparison 1 Psychological intervention versus any comparison, Outcome 11 Depression symptoms clinician‐rated long‐term follow‐up. | ||||
| 11.1 Targeted | 6 | 894 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.01] |
| 12 Anxiety symptoms (by population) post‐intervention Show forest plot | 23 | 5017 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.16, 0.02] |
| Analysis 1.12  Comparison 1 Psychological intervention versus any comparison, Outcome 12 Anxiety symptoms (by population) post‐intervention. | ||||
| 12.1 Targeted | 13 | 1666 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.31, 0.04] |
| 12.2 Universal | 10 | 3351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.13, 0.05] |
| 13 Anxiety symptoms (by population) short‐term follow‐up Show forest plot | 3 | 334 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| Analysis 1.13  Comparison 1 Psychological intervention versus any comparison, Outcome 13 Anxiety symptoms (by population) short‐term follow‐up. | ||||
| 13.1 Targeted | 3 | 334 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| 14 Anxiety symptoms (by population) medium‐term follow‐up Show forest plot | 18 | 4957 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.14, ‐0.01] |
| Analysis 1.14  Comparison 1 Psychological intervention versus any comparison, Outcome 14 Anxiety symptoms (by population) medium‐term follow‐up. | ||||
| 14.1 Targeted | 10 | 1827 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.18, 0.04] |
| 14.2 Universal | 8 | 3130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.17, ‐0.01] |
| 15 Anxiety symptoms (by population) long‐term follow‐up Show forest plot | 5 | 971 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.44, 0.14] |
| Analysis 1.15  Comparison 1 Psychological intervention versus any comparison, Outcome 15 Anxiety symptoms (by population) long‐term follow‐up. | ||||
| 15.1 Targeted | 2 | 293 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.43, 0.03] |
| 15.2 Universal | 3 | 678 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 16 Social and general functioning (by population) post‐intervention Show forest plot | 10 | 2067 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.06, 0.41] |
| Analysis 1.16  Comparison 1 Psychological intervention versus any comparison, Outcome 16 Social and general functioning (by population) post‐intervention. | ||||
| 16.1 Targeted | 9 | 1021 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.04, 0.50] |
| 16.2 Universal | 1 | 1046 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [0.04, 0.28] |
| 17 Social and general functioning (by population) short‐term follow‐up Show forest plot | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.81 [0.12, 1.49] |
| Analysis 1.17  Comparison 1 Psychological intervention versus any comparison, Outcome 17 Social and general functioning (by population) short‐term follow‐up. | ||||
| 17.1 Targeted | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.81 [0.12, 1.49] |
| 17.2 Universal | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Social and general functioning (by population) medium‐term follow‐up Show forest plot | 11 | 2449 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [0.02, 0.28] |
| Analysis 1.18  Comparison 1 Psychological intervention versus any comparison, Outcome 18 Social and general functioning (by population) medium‐term follow‐up. | ||||
| 18.1 Targeted | 9 | 1058 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.00, 0.38] |
| 18.2 Universal | 2 | 1391 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.01, 0.20] |
| 19 Social and general functioning (by population) long‐term follow‐up Show forest plot | 4 | 744 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.16, 0.14] |
| Analysis 1.19  Comparison 1 Psychological intervention versus any comparison, Outcome 19 Social and general functioning (by population) long‐term follow‐up. | ||||
| 19.1 Targeted | 3 | 342 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.22, 0.21] |
| 19.2 Universal | 1 | 402 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive diagnosis medium‐term follow‐up Show forest plot | 22 | 3915 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| Analysis 2.1  Comparison 2 Psychological intervention versus any comparison for targeted interventions, Outcome 1 Depressive diagnosis medium‐term follow‐up. | ||||
| 1.1 Treatment as usual | 12 | 2464 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 1.2 No treatment | 8 | 1286 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 1.3 Wait‐list | 1 | 95 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.21, 0.05] |
| 1.4 Other | 1 | 70 | Risk Difference (M‐H, Random, 95% CI) | ‐0.12 [‐0.29, 0.04] |
| 2 Depression symptoms post‐intervention Show forest plot | 42 | 4816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| Analysis 2.2  Comparison 2 Psychological intervention versus any comparison for targeted interventions, Outcome 2 Depression symptoms post‐intervention. | ||||
| 2.1 Treatment as usual | 16 | 2514 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.45, ‐0.15] |
| 2.2 No treatment | 14 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.57, ‐0.21] |
| 2.3 Attention placebo | 4 | 466 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.32, 0.13] |
| 2.4 Wait‐list | 6 | 361 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.72, ‐0.26] |
| 2.5 Other | 2 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.51, 0.04] |
| 3 Depression symptoms medium‐term follow‐up Show forest plot | 29 | 4448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.33, ‐0.12] |
| Analysis 2.3  Comparison 2 Psychological intervention versus any comparison for targeted interventions, Outcome 3 Depression symptoms medium‐term follow‐up. | ||||
| 3.1 Treatment as usual | 15 | 2315 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.42, ‐0.13] |
| 3.2 No treatment | 9 | 1207 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.09] |
| 3.3 Attention placebo | 3 | 761 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.26, 0.03] |
| 3.4 Wait‐list | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.55, 0.28] |
| 3.5 Other | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.64, ‐0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive diagnosis medium‐term follow‐up Show forest plot | 10 | 2050 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| Analysis 3.1  Comparison 3 Psychological intervention versus any comparison for universal interventions, Outcome 1 Depressive diagnosis medium‐term follow‐up. | ||||
| 1.1 Treatment as usual | 3 | 656 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 1.2 No treatment | 2 | 316 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.05, 0.07] |
| 1.3 Attention placebo | 2 | 861 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 1.4 Wait‐list | 3 | 217 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.24, 0.09] |
| 2 Depression symptoms post‐intervention Show forest plot | 31 | 9013 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.05] |
| Analysis 3.2  Comparison 3 Psychological intervention versus any comparison for universal interventions, Outcome 2 Depression symptoms post‐intervention. | ||||
| 2.1 Treatment as usual | 9 | 1791 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.31, 0.00] |
| 2.2 No treatment | 9 | 4231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.25, ‐0.05] |
| 2.3 Attention placebo | 9 | 2180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.09, 0.08] |
| 2.4 Wait‐list | 4 | 811 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.28, 0.04] |
| 3 Depression symptoms medium‐term follow‐up Show forest plot | 24 | 7465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| Analysis 3.3  Comparison 3 Psychological intervention versus any comparison for universal interventions, Outcome 3 Depression symptoms medium‐term follow‐up. | ||||
| 3.1 No treatment | 7 | 3367 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.10, 0.16] |
| 3.2 Treatment as usual | 6 | 1505 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.16, 0.05] |
| 3.3 Attention placebo | 7 | 1813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 3.4 Wait‐list | 4 | 780 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.34, 0.07] |
| 3.5 Other | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive diagnosis medium‐term follow‐up Show forest plot | 22 | 3915 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| Analysis 4.1  Comparison 4 Psychological intervention versus any comparison for selected and indicated interventions, Outcome 1 Depressive diagnosis medium‐term follow‐up. | ||||
| 1.1 Selective | 3 | 963 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.07, 0.12] |
| 1.2 Indicated | 16 | 2374 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.06, ‐0.01] |
| 1.3 Combined | 3 | 578 | Risk Difference (M‐H, Random, 95% CI) | ‐0.14 [‐0.21, ‐0.07] |
| 2 Depression symptoms (by population) post‐intervention Show forest plot | 42 | 4816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| Analysis 4.2  Comparison 4 Psychological intervention versus any comparison for selected and indicated interventions, Outcome 2 Depression symptoms (by population) post‐intervention. | ||||
| 2.1 Selective | 9 | 1394 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.30, ‐0.02] |
| 2.2 Indicated | 29 | 2740 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.50, ‐0.24] |
| 2.3 Combined | 4 | 682 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.45, ‐0.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression scores (by assessor) post‐intervention Show forest plot | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Self‐reported depression symptoms versus clinician‐rated depression symptoms, Outcome 1 Depression scores (by assessor) post‐intervention. | ||||
| 1.1 Self‐reported | 9 | 1877 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.53, ‐0.12] |
| 1.2 Clinician‐rated | 9 | 1884 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.46, ‐0.04] |
| 2 Depression scores medium‐term follow‐up Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Self‐reported depression symptoms versus clinician‐rated depression symptoms, Outcome 2 Depression scores medium‐term follow‐up. | ||||
| 2.1 Self‐reported | 7 | 1465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.41, ‐0.02] |
| 2.2 Clinician‐rated | 7 | 1468 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.32, 0.06] |
| 3 Depression scores long‐term follow‐up Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Self‐reported depression symptoms versus clinician‐rated depression symptoms, Outcome 3 Depression scores long‐term follow‐up. | ||||
| 3.1 Self‐reported | 4 | 390 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.37, 0.16] |
| 3.2 Clinician‐rated | 4 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.27, 0.14] |

'Risk of bias' graph: Review authors' judgements about each risk of bias item presented as percentages across all included studies.
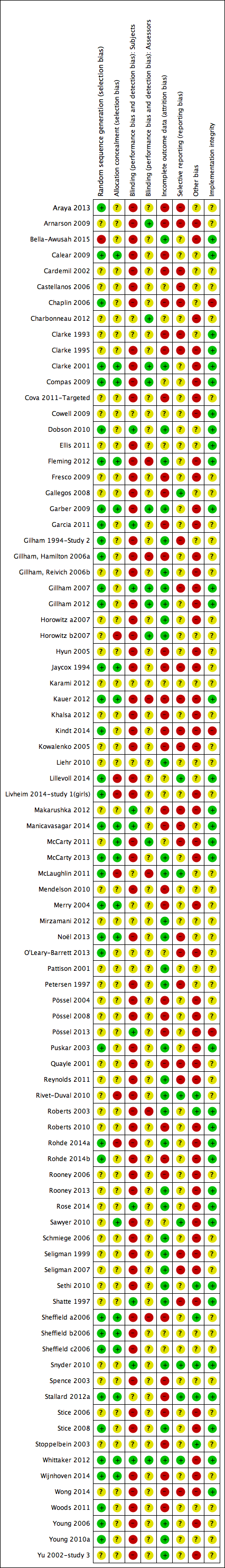
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
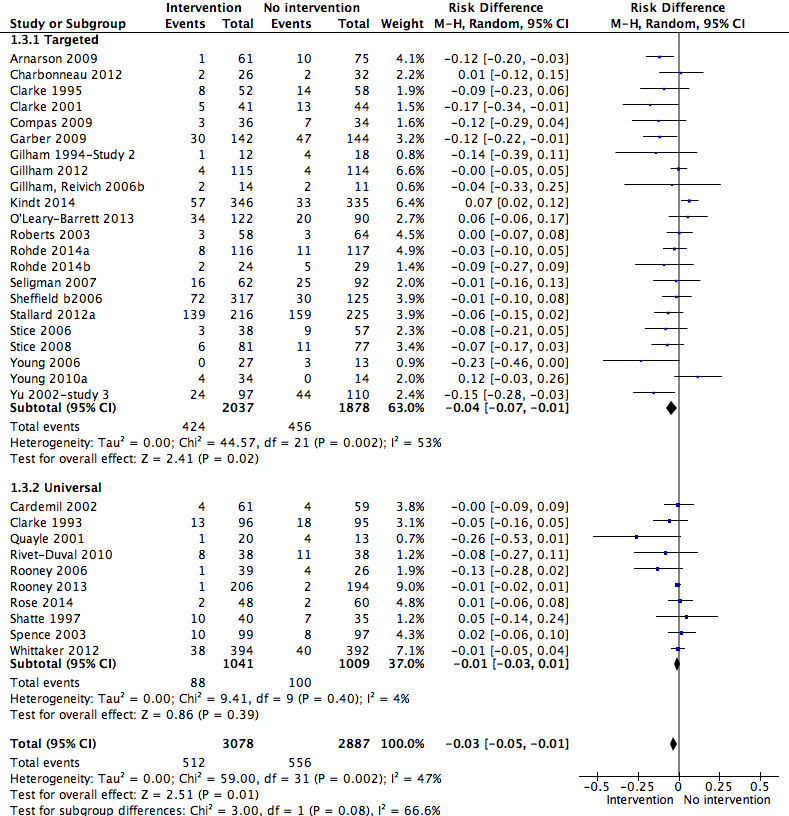
Forest plot of comparison: 1 Psychological intervention versus any comparison post‐intervention, outcome: 1.3 Depressive disorder medium‐term follow‐up (primary outcomes).
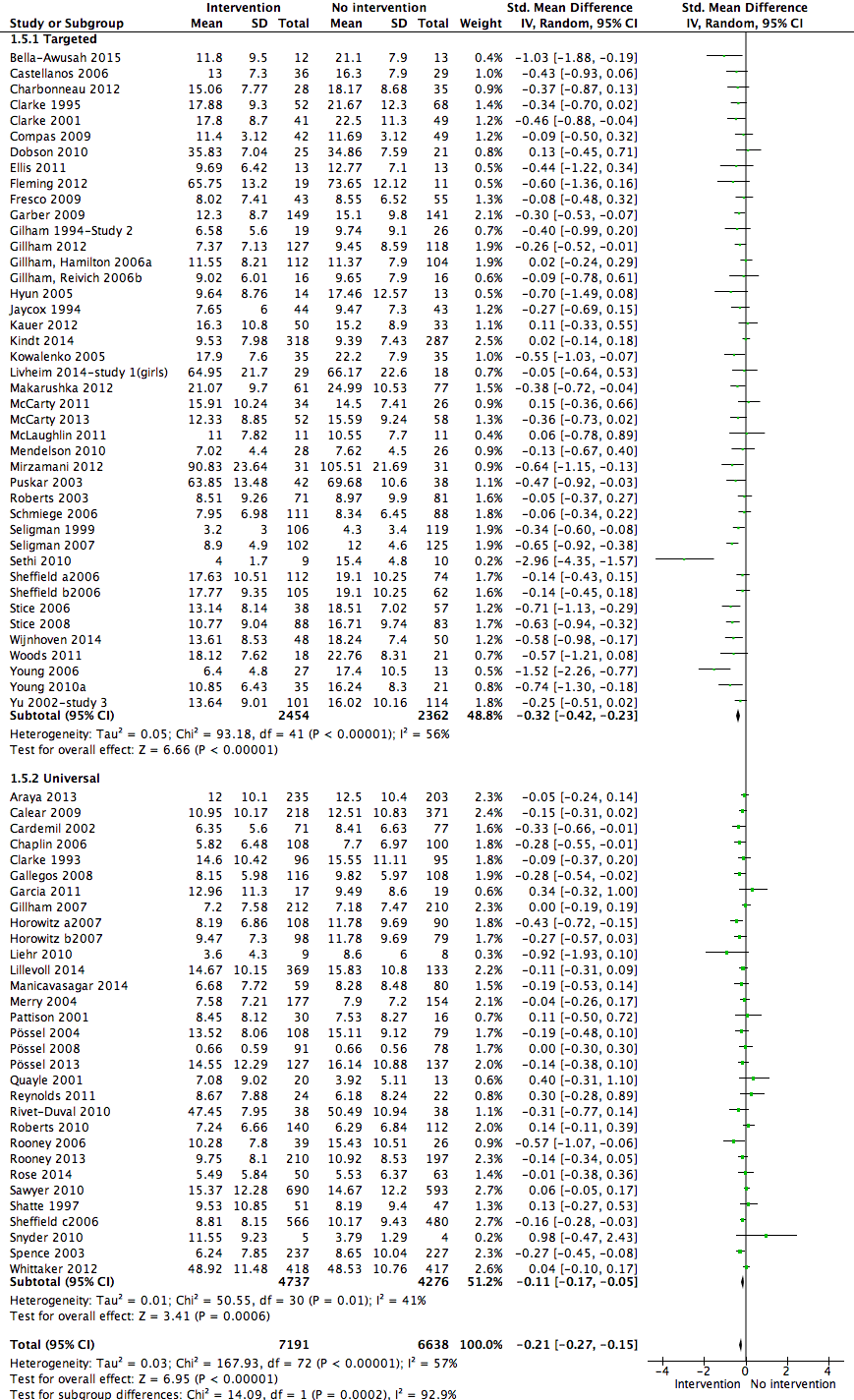
Forest plot of comparison: 1 Psychological intervention versus any comparison post‐intervention, outcome: 1.5 Depression scores (self‐report) post‐intervention follow‐up (primary outcome).
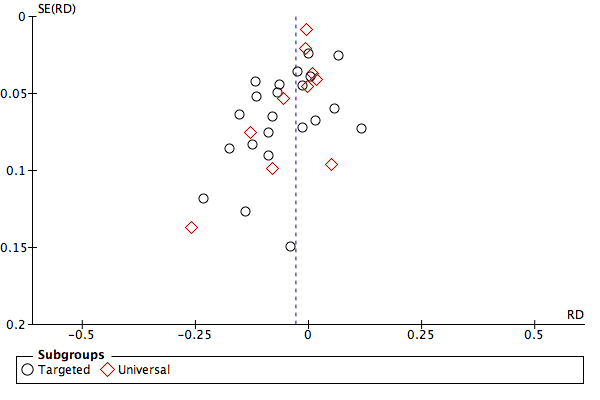
Funnel plot of analysis 1.4: Psychological intervention versus any comparison post‐intervention for depressive disorder at the medium‐term follow‐up.

Funnel plot of analysis 1.6: Psychological intervention versus any comparison post‐intervention for depression scores at the post‐intervention assessment.

Comparison 1 Psychological intervention versus any comparison, Outcome 1 Depressive diagnosis (by population) post‐intervention.

Comparison 1 Psychological intervention versus any comparison, Outcome 2 Depressive diagnosis short‐term follow‐up.
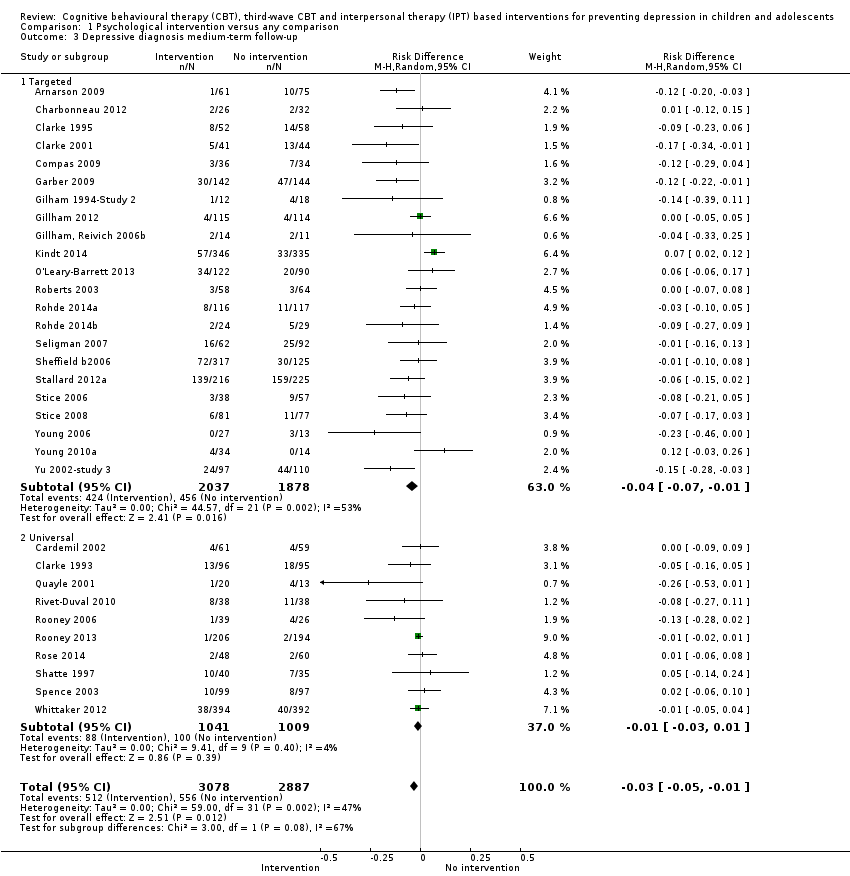
Comparison 1 Psychological intervention versus any comparison, Outcome 3 Depressive diagnosis medium‐term follow‐up.
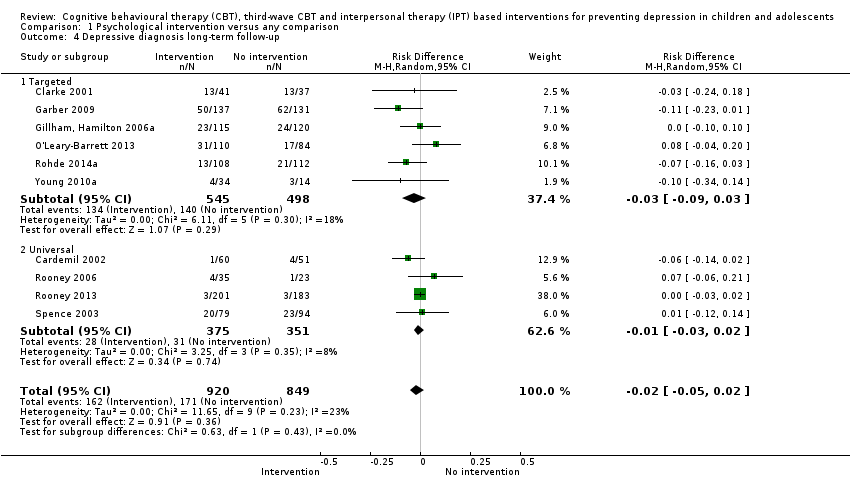
Comparison 1 Psychological intervention versus any comparison, Outcome 4 Depressive diagnosis long‐term follow‐up.
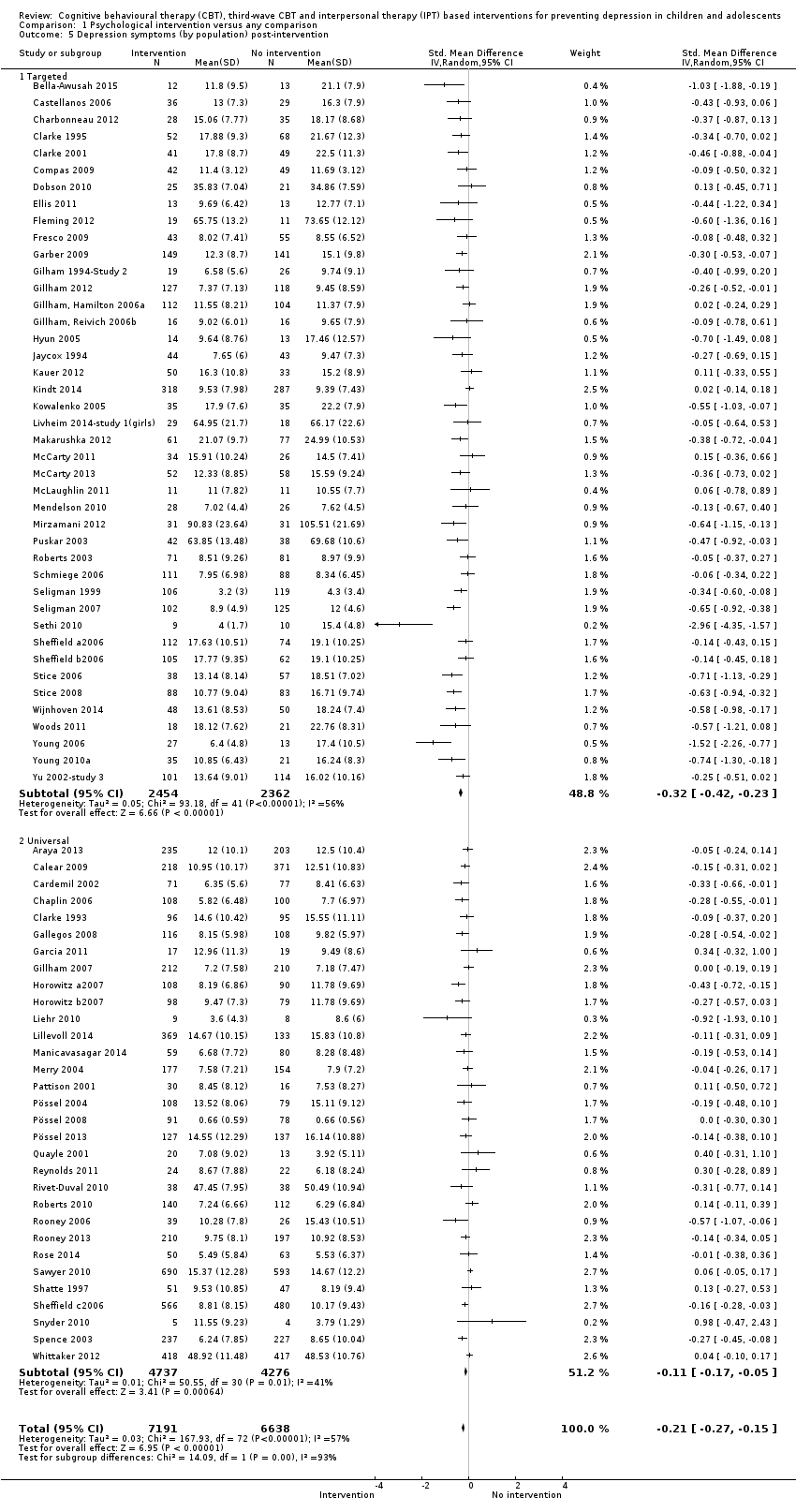
Comparison 1 Psychological intervention versus any comparison, Outcome 5 Depression symptoms (by population) post‐intervention.
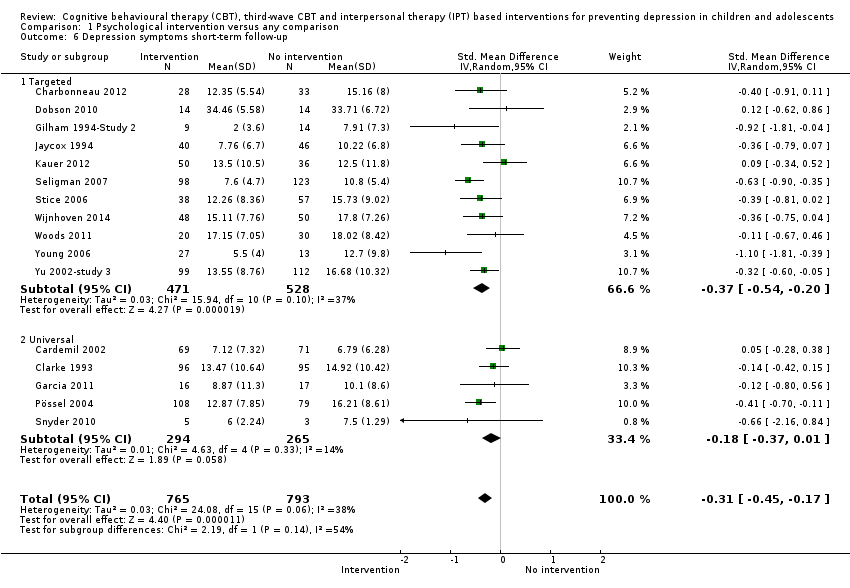
Comparison 1 Psychological intervention versus any comparison, Outcome 6 Depression symptoms short‐term follow‐up.

Comparison 1 Psychological intervention versus any comparison, Outcome 7 Depression symptoms medium‐term follow‐up.

Comparison 1 Psychological intervention versus any comparison, Outcome 8 Depression symptoms long‐term follow‐up.

Comparison 1 Psychological intervention versus any comparison, Outcome 9 Depression symptoms clinician‐rated (by population) post‐intervention.

Comparison 1 Psychological intervention versus any comparison, Outcome 10 Depression symptoms clinician‐rated medium‐term follow‐up.
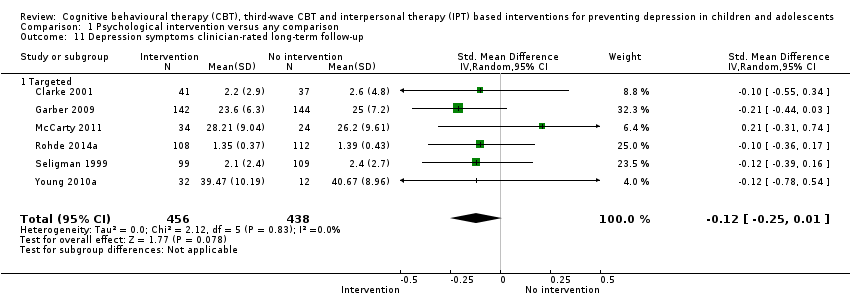
Comparison 1 Psychological intervention versus any comparison, Outcome 11 Depression symptoms clinician‐rated long‐term follow‐up.

Comparison 1 Psychological intervention versus any comparison, Outcome 12 Anxiety symptoms (by population) post‐intervention.

Comparison 1 Psychological intervention versus any comparison, Outcome 13 Anxiety symptoms (by population) short‐term follow‐up.
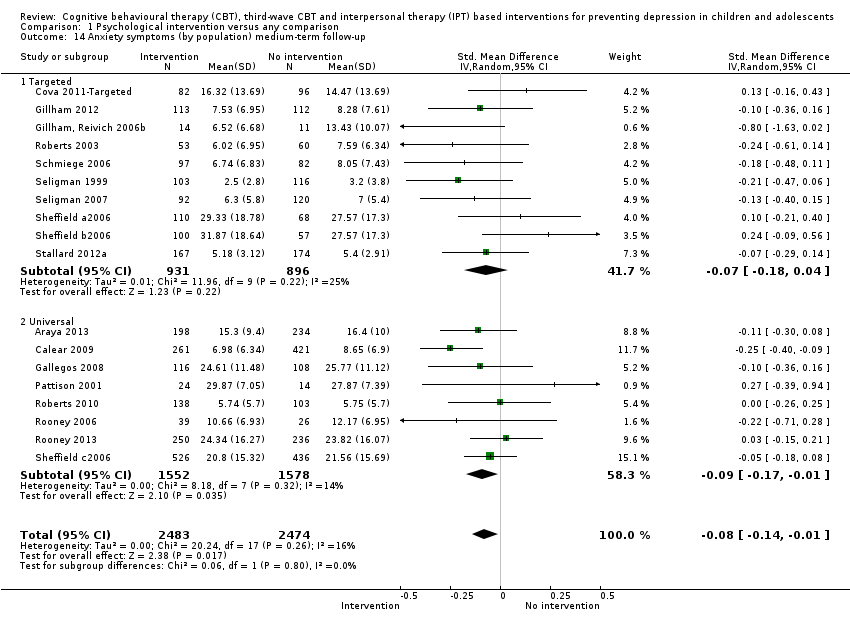
Comparison 1 Psychological intervention versus any comparison, Outcome 14 Anxiety symptoms (by population) medium‐term follow‐up.
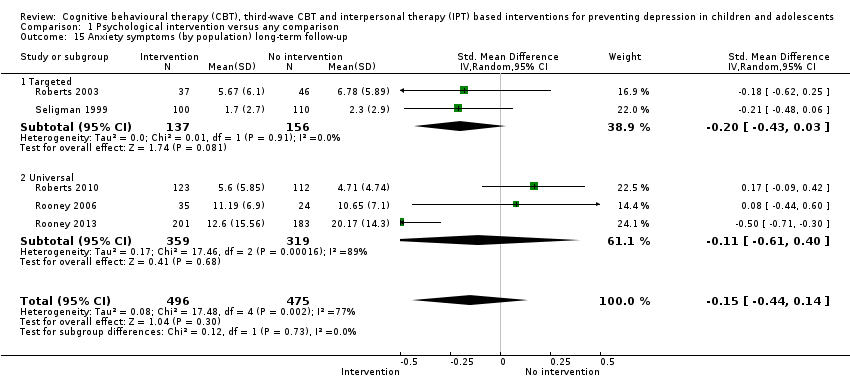
Comparison 1 Psychological intervention versus any comparison, Outcome 15 Anxiety symptoms (by population) long‐term follow‐up.
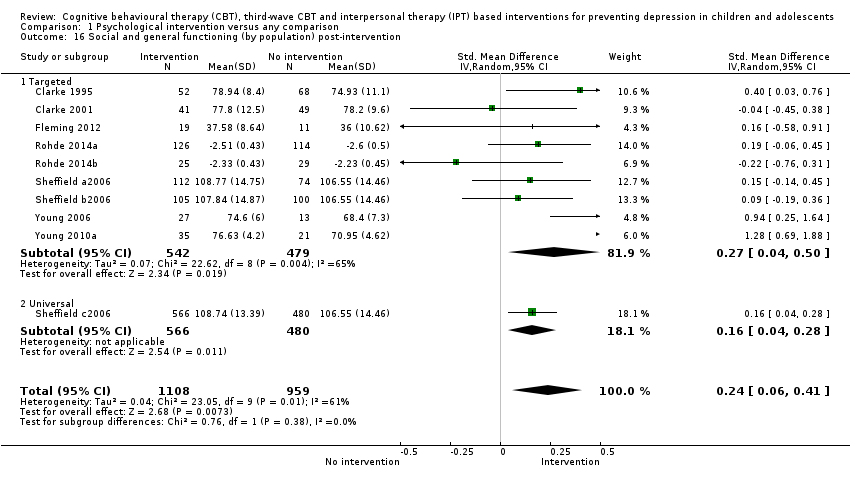
Comparison 1 Psychological intervention versus any comparison, Outcome 16 Social and general functioning (by population) post‐intervention.

Comparison 1 Psychological intervention versus any comparison, Outcome 17 Social and general functioning (by population) short‐term follow‐up.

Comparison 1 Psychological intervention versus any comparison, Outcome 18 Social and general functioning (by population) medium‐term follow‐up.

Comparison 1 Psychological intervention versus any comparison, Outcome 19 Social and general functioning (by population) long‐term follow‐up.

Comparison 2 Psychological intervention versus any comparison for targeted interventions, Outcome 1 Depressive diagnosis medium‐term follow‐up.

Comparison 2 Psychological intervention versus any comparison for targeted interventions, Outcome 2 Depression symptoms post‐intervention.
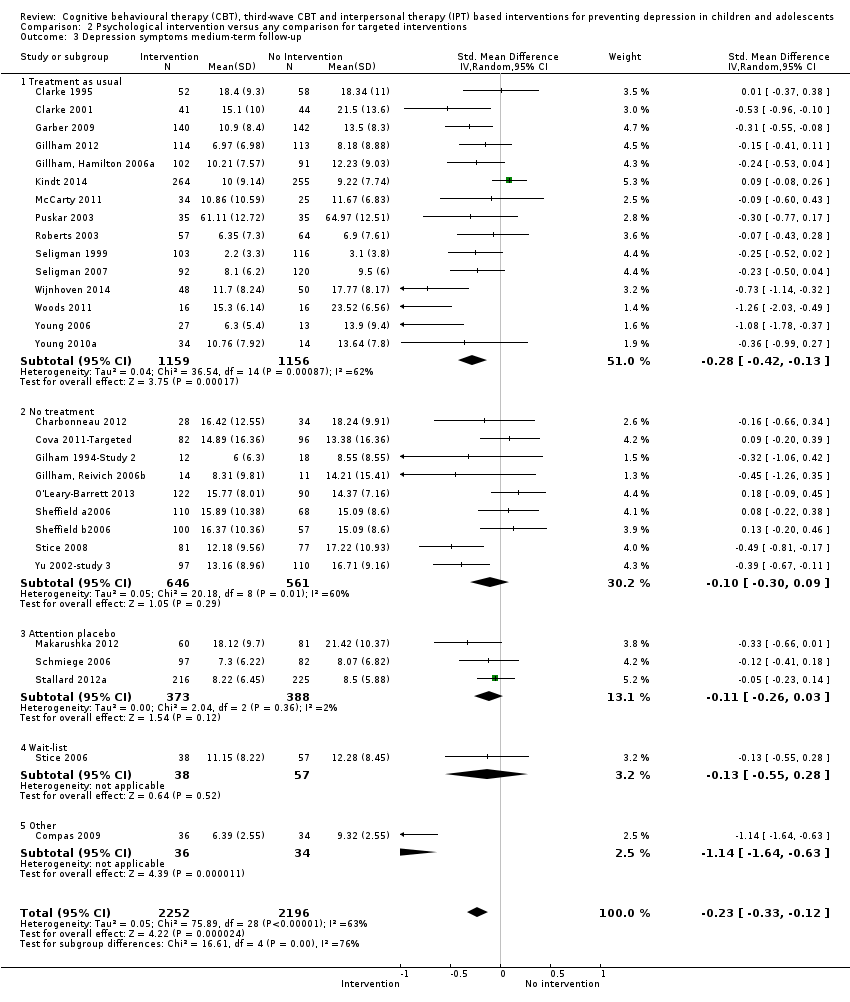
Comparison 2 Psychological intervention versus any comparison for targeted interventions, Outcome 3 Depression symptoms medium‐term follow‐up.

Comparison 3 Psychological intervention versus any comparison for universal interventions, Outcome 1 Depressive diagnosis medium‐term follow‐up.
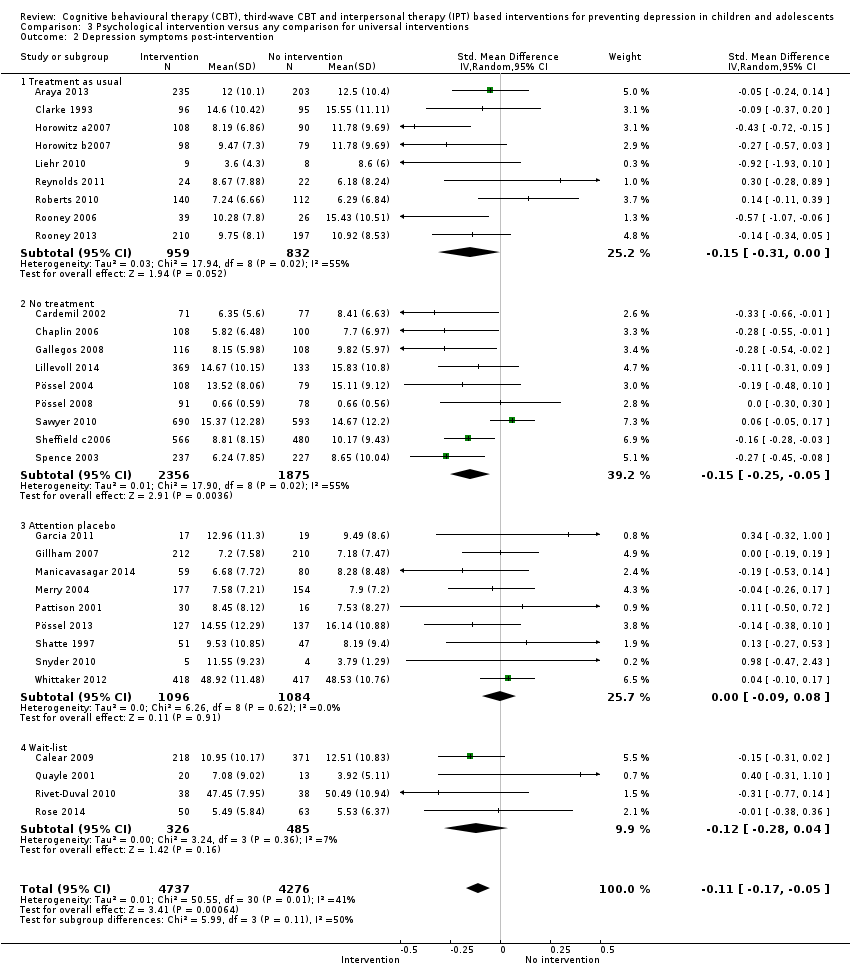
Comparison 3 Psychological intervention versus any comparison for universal interventions, Outcome 2 Depression symptoms post‐intervention.

Comparison 3 Psychological intervention versus any comparison for universal interventions, Outcome 3 Depression symptoms medium‐term follow‐up.
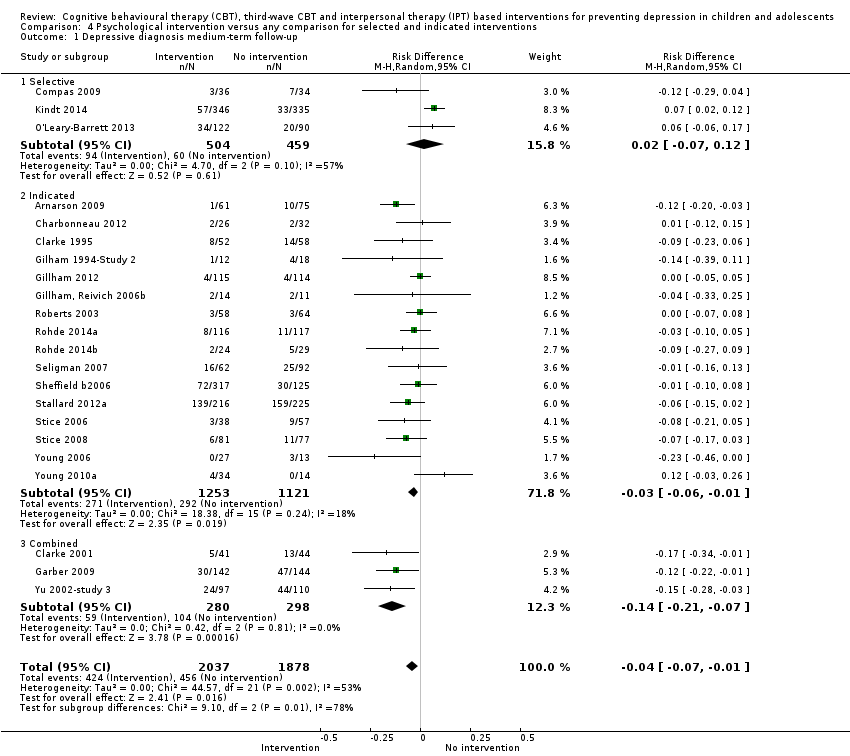
Comparison 4 Psychological intervention versus any comparison for selected and indicated interventions, Outcome 1 Depressive diagnosis medium‐term follow‐up.

Comparison 4 Psychological intervention versus any comparison for selected and indicated interventions, Outcome 2 Depression symptoms (by population) post‐intervention.
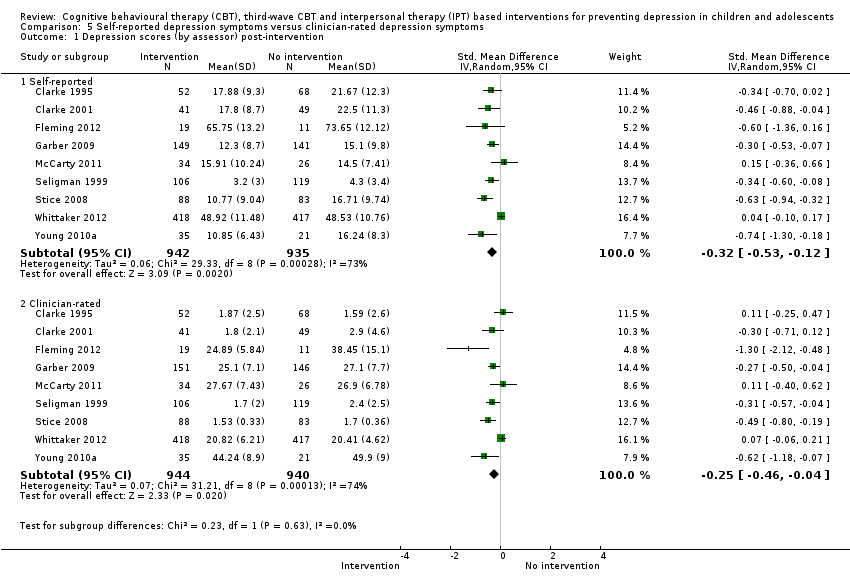
Comparison 5 Self‐reported depression symptoms versus clinician‐rated depression symptoms, Outcome 1 Depression scores (by assessor) post‐intervention.
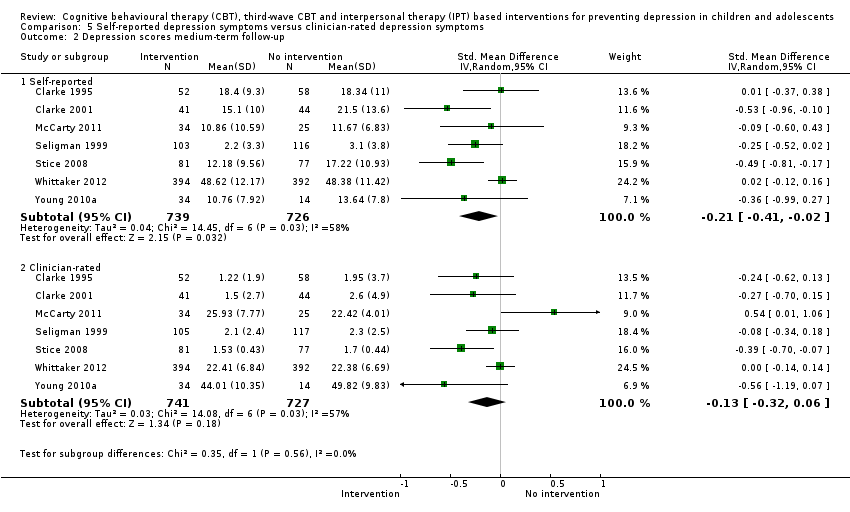
Comparison 5 Self‐reported depression symptoms versus clinician‐rated depression symptoms, Outcome 2 Depression scores medium‐term follow‐up.
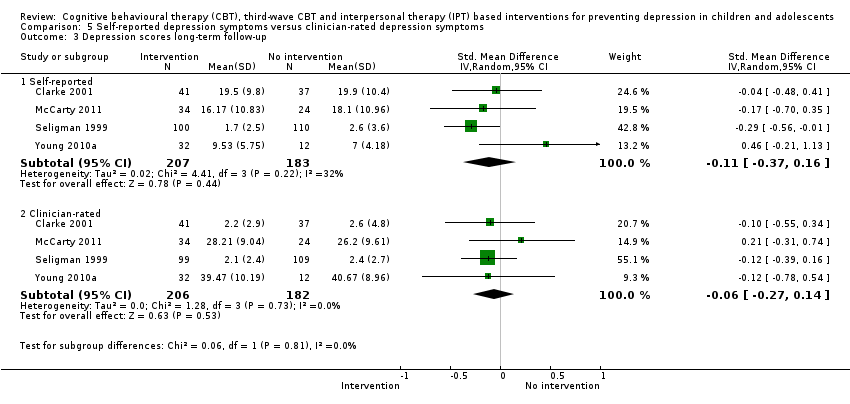
Comparison 5 Self‐reported depression symptoms versus clinician‐rated depression symptoms, Outcome 3 Depression scores long‐term follow‐up.
| Evidence‐based psychological interventions compared to any comparator for depression diagnosis at the medium‐term follow‐up | |||||
| Patient or population: children and adolescents | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Any comparator | Evidence‐based psychological interventions | ||||
| Evidence‐based psychological interventions versus any comparator (Overall) ‐ effect on diagnosis of depression The assumed risk is based on control group rates of depression diagnosis at medium‐term follow‐up (from a rank ordering of control group rates of each included study). | Study population | RR 0.84 (0.72 to 0.97) | ⊕⊕⊕⊝ | — | |
| 193 per 1000 | 162 per 1000 (139 to 187) | ||||
| Low (0%) | |||||
| 0 per 1000 (0 to 0) | |||||
| Moderate (18.5%) | |||||
| 185 per 1000 | 155 per 1000 (133 to 180) | ||||
| High (70.7%) | |||||
| 707 per 1000 | 594 per 1000 (509 to 685) | ||||
| Evidence‐based psychological interventions versus any comparator (Targeted programmes) ‐ effect on diagnosis of depression The assumed risk is based on control group rates of depression diagnosis at medium‐term follow‐up (from a rank ordering of control group rates of each included study). | Study population | RR 0.82 | ⊕⊝⊝⊝ | — | |
| 243 per 1000 | 199 per 1000 | ||||
| Low (0%) | |||||
| 0 per 1000 (0 to 0) | |||||
| Moderate (20.4%) | |||||
| 204 per 1000 | 167 per 1000 (139 to 202) | ||||
| High (76.7%) | |||||
| 767 per 1000 | 629 per 1000 (521 to 759) | ||||
| Evidence‐based psychological interventions versus any comparator (Universal programmes) ‐ effect on diagnosis of depression The assumed risk is based on control group rates of depression diagnosis at medium‐term follow‐up (from a rank ordering of control group rates of each included study). | Study population | RR 0.87 | ⊕⊕⊕⊝ | — | |
| 99 per 1000 | 86 per 1000 | ||||
| Low (1.0%) | |||||
| 10 per 1000 | 9 per 1000 (7 to 12) | ||||
| Moderate (14.5%) | |||||
| 144 per 1000 | 125 per 1000 (95 to 164) | ||||
| High (30.8%) | |||||
| 308 per 1000 | 268 per 1000 (203 to 351) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1We downgraded quality owing to lack of clarity over allocation concealment and presence of other bias. | |||||
| Evidence‐based psychological interventions versus any comparator for self‐rated depression scores at the post‐intervention assessment | ||||||
| Patient or population: children and adolescents Settings: various | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Any comparator | Evidence‐based psychological interventions | |||||
| Evidence‐based psychological interventions versus any comparator (Overall) ‐ self‐rated depression scores (higher score is equivalent to a poorer outcome) | The mean self‐reported depression score ranged across control groups from 0.66 to 105.51 points. | The mean self‐rated depression score in the intervention group was 0.21 standard deviations lower (0.27 to 0.15 lower) | — | 13,829 (73 trials) | ⊕⊕⊝⊝ Low1,2 | — |
| Evidence‐based psychological interventions versus any comparator (Targeted ‐ self‐rated depression scores (higher score is equivalent to a poorer outcome)) | The mean self‐reported depression score ranged across control groups from 4.30 to 105.51 points. | The mean self‐rated depression score in the intervention group was 0.32 standard deviations lower (0.42 to 0.23 lower) | — | 4816 | ⊕⊕⊕⊝ Moderate3 | — |
| Evidence‐based psychological interventions versus any comparator (Universal programmes) ‐ self‐rated depression scores (higher score is equivalent to a poorer outcome) | The mean self‐reported depression score ranged across control groups from 0.66 to 50.49 points. | The mean self‐rated depression score in the intervention group was 0.11 standard deviations lower (0.17 to 0.05 lower) | — | 9013 | ⊕⊕⊕⊝ | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded quality owing to a lack of clarity about random sequence generation and allocation concealment and the presence of other bias. 2Heterogeneity (I2 = 57%). 3We downgraded quality owing to a lack of clarity over allocation concealment and the presence of other bias. | ||||||
| Study | Cognitive restructuring (Y/N) | Behavioural techniques (Y/N) | Problem‐solving (Y/N) | Social skills training (Y/N) | Relaxation techniques (Y/N) | Third wave techniques (Y/N) | Anxiety management techniques (Y/N) | Component/s focusing on management of specific problems (Y/N) | Parental component/s (Y/N) | Predominant therapeutic focus |
| Y | N | Y | N | N | N | N | N | Y | CBT (cognitive) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT plus IPT | |
| Y | Y | N | N | Y | N | N | N | N | CBT (behavioural) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | N | Y | Y | N | N | N | N | N | CBT (cognitive) | |
| Y | N | N | N | N | N | N | N | N | CBT (cognitive) | |
| Y | N | Y | Y | Y | N | N | Y1 | N | CBT (cognitive) | |
| N | N | N | N | Y | Y | N | N | N | Third wave | |
| N | Y | N | N | N | N | N | N | N | Behaviour therapy (third wave) | |
| Y | N | N | N | N | N | N | N | N | CBT (cognitive) | |
| Y | N | N | N | N | N | N | Y2 | Y | CBT (cognitive) | |
| Y | Y | N | N | N | Y | N | Y2 | Y | CBT (cognitive and behavioural) | |
| Y | N | Y | Y | Y | Y | Y | Y3 | N | CBT (cognitive) | |
| N | N | Y | Y | N | N | N | Y4 | Y |
| |
| Y | N | N | N | N | N | N | N | N | CBT (cognitive) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | N | N | N | N | N | N | N | N | CBT (cognitive) | |
| Y | N | Y | N | Y | N | Y | N | Y | CBT (cognitive) | |
| Y | N | N | N | N | N | N | N | Y | CBT (cognitive) | |
| Y | N | Y | Y | Y | Y | Y | Unclear | Unclear | Third wave | |
| Y | N | Y | Y | Y | N | N | Y | N5 | CBT (cognitive) | |
| Y | N | Y | Y | Y | N | N | Unclear | N | CBT (cognitive) | |
| Y | N | Y | Y | Y | N | N | Unclear | Y | CBT (cognitive) | |
| Y | N | Y | Y | Y | N | N | Unclear | N | CBT (cognitive) | |
| Y | N | N | Y | Y | N | N | Y | N | CBT (cognitive) | |
| Y | N | N | N | N | N | N | N | N | CBT (cognitive) | |
| N | N | N | Y | N | N | N | N | N | IPT | |
| Y | Y | N | N | Y | N | N | Y6 | N | CBT (cognitive and behavioural) | |
| Y | N | Y | Y | Y | N | N | Y7 | N | CBT (cognitive) | |
| Y | N | Y | Y | Y | N | N | Y8 | N | CBT (cognitive) | |
| N | Y | N | N | N | N | N | N | N | Behaviour therapy (third wave) | |
| N | N | N | N | Y | Y | N | N | N | Third wave | |
| Y | N | N | Y | Y | N | N | N | N | CBT (cognitive) | |
| Y | Y | Y | Y | N | N | N | N | N | CBT (cognitive and behavioural) | |
| N | N | N | N | N | Y | N | N | N | Third wave | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| N | N | N | N | N | Y | N | N | N | Third wave | |
| Y | Y | N | N | N | N | N | N | N | CBT (cognitive and behavioural) | |
| Unclear | Unclear | N | N | Y | Y | N | N | N | Third wave | |
| Y | Y | Y | Y | Y | N | N | N | Y | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | Y | N | N | N | Y | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| N | N | N | N | N | Y | N | N | N | Third wave | |
| Y | Y | Y | Y | Y | N | Y | N | N | CBT plus IPT | |
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Y | Y | Y | Y | N | N | N | Y9 | N | CBT (cognitive and behavioural) | |
| Y | N | N | N | N | N | N | N | N | CBT (cognitive) | |
| Y | N | Y | Y | Y | N | N | Unclear | N | CBT (cognitive) | |
| Y | N | Y | Y | Y | N | N | N | N | Problem‐solving | |
| Y | N | N | Y | N | N | N | N | N | CBT (cognitive) | |
| Y | Y | N | Y | N | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | N | Y | N | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | N | Y | Y | N | N | N | Y1 | N | CBT (cognitive) | |
| N | Y | N | N | N | N | N | N | N | Behaviour therapy (third wave) | |
| Y | Y | Y | Y | Y | N | Y | N | N | CBT plus IPT | |
| Y | N | Y | Y | N | N | N | Y1 | N | CBT (cognitive) | |
| Y | Y | Y | Y | N | N | Unclear | Unclear | N | CBT (cognitive) | |
| Y | Y | N | N | N | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | N | N | N | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | N | N | N | Y | N | N | N | N | CBT (cognitive) | |
| Y | Y | N | N | Y | N | Y | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | N | N | Unclear | N | N | CBT plus IPT | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | N | N | N | Y10 | Y | CBT (cognitive) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | N | Y | Y | Y | N | N | Y6 | N | CBT (cognitive) | |
| Y | Y | Y | Y | N | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | N | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | N | Y | N | N | N | N | N | N | CBT (cognitive) | |
| N | N | N | N | N | Y | N | N | N | Third wave | |
| Y | N | Y | N | N | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | Y | N | Y | N | N | CBT plus IPT | |
| Y | Y | N | N | N | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | N | N | N | N | Y | N | N | CBT (cognitive and behavioural) | |
| Y | Y | N | N | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | N | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | N | N | N | N | N | N | N | N | CBT (cognitive) | |
| Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) | |
| Y | Y | Y | Y | N | N | N | N | N | CBT (cognitive and behavioural) | |
| N | N | N | Y | N | N | N | N | N | IPT | |
| N | N | N | Y | N | N | N | N | N | IPT | |
| Y | N | Y | Y | Y | N | N | Y1 | N | CBT (cognitive) | |
| 1Penn Resiliency programmes place some emphasis on resolution of family conflict. 2Addresses beliefs related to or coping with a parent diagnosed with depression, or both. 3Addresses resolving conflict with family and friends. 4 Addresses being an immigrant 5Although for some participants there was a parental component, this was not controlled. Instead only the feasibility of offering parental sessions was evaluated. 6Addresses factors involved in the participants' decision to run away from home. 7Addresses coping with parental conflict. 8Addresses coping with parental divorce. 9Addresses coping with rural living. 10Addresses coping with grief after the death of a parent. | ||||||||||
| k | RR | (95% CI) | β | (95% CI) | P value (moderator) | Adjusted R2 (%) | I2 (Res) | P value | |
| Overall effect | 22 | 0.82 | (0.68 to 0.99) | ‐0.20 | (‐0.40 to 0.01) | 0.06 | 0 | 37.0 | 0.04 |
| Continuous | |||||||||
| Intensity of intervention (hours) | 21 | — | — | ‐0.02 | (‐0.04 to 0.01) | 0.08 | 92.0 | 0.9 | 0.08 |
| Binary | |||||||||
| Focus of intervention | |||||||||
| CBT (reference) | 17 | 0.81 | (0.65 to 1.01) | — | — | — | 0 | 44.9 | 0.95 |
| CBT + IPT | 2 | 0.44 | (0.07 to 2.90) | ‐0.03 | (‐0.74 to 0.68) | 0.93 | — | — | — |
| IPT | 2 | 0.53 | (0.01 to 26.34) | ‐0.39 | (‐2.64 to 1.85) | 0.72 | — | — | — |
| Third wave | 1 | 1.23 | (0.19 to 8.15) | 0.44 | (‐1.71 to 2.59) | 0.67 | — | — | — |
| Depression severity at baseline | |||||||||
| Subthreshold (reference) | 10 | 1.01 | (0.81 to 1.27) | — | — | — | 99.0 | 0.5 | 0.02 |
| Mild | 8 | 0.57 | (0.43 to 0.77) | ‐0.52 | (‐0.86 to ‐0.17) | 0.01 | — | — | — |
| Moderate | 2 | 0.59 | (0.40 to 0.88) | ‐0.48 | (‐0.93 to ‐0.03) | 0.04 | — | — | — |
| Severe | 1 | 0.95 | (0.65 to 1.37) | ‐0.01 | (‐0.44 to 0.41) | 0.95 | — | — | — |
| Focus of CBT (for CBT studies only) | |||||||||
| CBT – cognitive and behavioural (reference) | 9 | 0.87 | (0.76 to 1.01) | — | — | — | 0 | 41.8 | 0.62 |
| CBT ‐ cognitive | 10 | 0.83 | (0.59 to 1.18) | 0.10 | (‐0.33 to 0.54) | 0.62 | |||
| CBT ‐ behavioural | 0 | — | — | — | — | — | — | — | — |
| Inclusion of relaxation component (for CBT studies only) | |||||||||
| No mention of relaxation component (reference) | 11 | 0.77 | (0.63 to 0.95) | — | — | — | 0 | 37.2 | 0.28 |
| Relaxation component described as included | 8 | 0.90 | (0.65 to 1.24) | 0.22 | (‐0.20 to 0.63) | 0.28 | — | — | — |
| Inclusion of problem‐solving skills training component (for CBT studies only) | |||||||||
| No mention of problem‐solving component (reference) | 11 | 0.77 | (0.55 to 1.08) | — | — | — | 0 | 41.8 | 0.99 |
| Problem‐solving component described as included | 8 | 0.86 | (0.74 to 1.01) | ‐0.01 | (‐0.43 to 0.43) | 0.99 | — | — | — |
| Inclusion of social skills training (for CBT studies only) | |||||||||
| No mention of social skills component (reference) | 9 | 0.70 | (0.54 to 0.91) | — | — | — | 11.0 | 32.9 | 0.13 |
| Social skills component described as included | 10 | 0.93 | (0.73 to 1.18) | 0.30 | (‐0.09 to 0.70) | 0.13 | — | — | — |
| Type of facilitator | |||||||||
| Mental health expert (reference group) | 9 | 0.64 | (0.45 to 0.90) | — | — | — | 0 | 21.2 | 0.12 |
| Students | 8 | 0.89 | (0.78 to 1.01) | 0.18 | (‐0.34 to 0.70) | 0.48 | — | — | — |
| Non‐mental health expert | 5 | 1.05 | (0.73 to 1.53) | 0.49 | (0.01 to 0.98) | 0.05 | — | — | — |
| Mode of delivery | |||||||||
| Face‐to‐face (group or individual) | 22 | 0.82 | (0.68 to 0.99) | ‐0.20 | (‐0.40 to 0.01) | 0.06 | 0 | 37.0 | 0.04 |
| Online/telephone | 0 | — | — | — | — | — | — | — | — |
| k refers to number of trials. CBT: cognitive behavioural therapy | |||||||||
| k | SMD | (95% CI) | β | (95% CI) | P value (moderator) | Adjusted R2 (%) | I2 (Res) (%) | P value | |
| Overall effect | 42 | ‐0.32 | (‐0.42 to ‐0.23) | ‐0.33 | (‐0.44 to ‐0.22) | > 0.001 | 0 | 56.0 | > 0.001 |
| Continuous | |||||||||
| Intensity of intervention (hours) | 37 | — | — | 0.02 | (‐0.01 to 0.03) | 0.06 | 15.0 | 50.5 | 0.06 |
| Binary | |||||||||
| Focus of intervention | |||||||||
| CBT (reference) | 36 | ‐0.32 | (‐0.42 to ‐0.22) | — | — | — | 17.0 | 54.2 | 0.03 |
| CBT + IPT | 0 | — | — | — | — | — | — | — | — |
| IPT | 2 | ‐1.11 | (‐1.89 to ‐0.33) | ‐0.75 | (‐1.35 to ‐0.15) | 0.02 | — | — | — |
| Third wave | 4 | ‐0.10 | (‐0.35 to 0.15) | 0.21 | (‐0.16 to 0.59) | 0.26 | — | — | — |
| Depression severity at baseline | |||||||||
| Subthreshold (reference) | 15 | ‐0.20 | (‐0.33 to ‐0.07) | — | — | — | 12.0 | 56.0 | 0.20 |
| Mild | 10 | ‐0.51 | (‐0.69 to ‐0.33) | ‐0.31 | (‐0.60 to ‐0.02) | 0.03 | — | — | — |
| Moderate | 10 | ‐0.41 | (‐0.71 to ‐0.11) | ‐0.14 | (‐0.45 to 0.16) | 0.35 | — | — | — |
| Severe | 4 | ‐0.31 | (‐0.54 to ‐0.07) | ‐0.12 | (‐0.49 to 0.25) | 0.52 | — | — | — |
| Focus of CBT (for CBT studies only) | |||||||||
| CBT – cognitive and behavioural (reference) | 18 | ‐0.42 | (‐0.58 to ‐0.27) | — | — | — | 31.0 | 47.4 | 0.06 |
| CBT ‐ cognitive | 17 | ‐0.20 | (‐0.30 to ‐0.10) | 0.20 | (‐0.01 to 0.40) | 0.05 | — | — | — |
| CBT ‐ behavioural | 1 | ‐1.07 | (‐1.91 to ‐0.23) | ‐0.66 | (‐1.68 to 0.37) | 0.20 | — | — | — |
| Inclusion of relaxation component (for CBT studies only) | |||||||||
| No mention of relaxation component (reference) | 17 | ‐0.30 | (‐0.41 to‐0.91) | — | — | — | 0 | 57.2 | 0.93 |
| Relaxation component described as included | 18 | ‐0.33 | (‐0.50 to ‐0.17) | ‐0.01 | (‐0.24 to 0.22) | 0.93 | — | — | — |
| Inclusion of problem‐solving skills training component (for CBT studies only) | |||||||||
| No mention of problem‐solving component (reference) | 15 | ‐0.35 | (‐0.49 to 0.20) | — | — | — | 0 | 57.7 | 0.59 |
| Problem‐solving component described as included | 20 | ‐0.29 | (‐0.43 to ‐0.15) | 0.06 | (‐0.17 to 0.29) | 0.59 | — | — | — |
| Inclusion of social skills training component (for CBT studies only) | |||||||||
| No mention of social skills component (reference) | 13 | ‐0.40 | (‐0.54 to ‐0.27) | — | — | — | 13.0 | 52.5 | 0.19 |
| Social skills component described as included | 22 | ‐0.26 | (‐0.39 to ‐0.13) | 0.15 | (‐0.08 to 0.38) | 0.19 | |||
| Type of facilitator | |||||||||
| Mental health expert (reference group) | 20 | ‐0.39 | (‐0.52 to ‐0.26) | — | — | — | 30.0 | 43.5 | 0.08 |
| Students | 7 | ‐0.40 | (‐0.62 to ‐0.19) | ‐0.02 | (‐0.29 to 0.24) | 0.85 | — | — | — |
| Non‐mental health expert | 7 | ‐0.11 | (‐0.21 to ‐0.01) | 0.24 | (0.02 to 0.46) | 0.03 | — | — | — |
| Mode of delivery | |||||||||
| Face‐to‐face (group or individual) (reference group) | 36 | ‐0.32 | (‐0.42 to ‐0.23) | — | — | — | 0 | 59.2 | 0.87 |
| Online/telephone | 6 | ‐0.45 | (‐0.98 to ‐0.02) | ‐0.03 | (‐0.39 to 0.33) | 0.87 | — | — | — |
| k refers to number of trials. CBT: cognitive behavioural therapy | |||||||||
| k | RR | (95% CI) | β | (95% CI) | P value (moderator) | AdjustedR2 (%) | I2 (Res) (%) | P value | |
| Overall effect | 10 | 0.87 | (0.66 – 1.14) | ‐0.14 | (‐0.45 to 0.17) | 0.33 | 0 | 0 | 0.30 |
| Continuous | |||||||||
| Intensity of intervention (hours) | 9 | — | — | 0.02 | (‐0.04 to 0.08) | 0.38 | 0 | 0 | 0.38 |
| Binary | |||||||||
| Focus of intervention | |||||||||
| CBT (reference) | 7 | 0.92 | (0.64 to 1.31) | — | — | — | 0 | 0 | 0.76 |
| CBT + IPT | 2 | 0.79 | (0.38 to 1.64) | ‐0.16 | (‐1.13 to 0.80) | 0.70 | — | — | — |
| IPT | 0 | ||||||||
| Third wave | 1 | 0.72 | (0.37 to 1.38) | ‐0.26 | (‐1.14 to 0.62) | 0.51 | — | — | — |
| Depression severity at baseline | |||||||||
| Subthreshold (reference) | 7 | 0.90 | (0.65 to 1.23) | — | — | — | 0 | 0 | 0.73 |
| Mild | 3 | 0.77 | (0.37 to 1.58) | ‐0.11 | (‐0.81 to 0.59) | 0.73 | — | — | — |
| Moderate | 0 | — | — | — | — | — | — | — | — |
| Severe | 0 | — | — | — | — | — | — | — | — |
| Focus of CBT (for CBT studies only) | |||||||||
| CBT – cognitive and behavioural (reference) | 5 | 0.93 | (0.67 to 1.30) | — | — | — | 0 | 0 | 0.70 |
| CBT ‐ cognitive | 4 | 0.61 | (0.23 to 1.64) | ‐0.15 | (‐1.03 to 0.73) | 0.70 | — | — | — |
| CBT ‐ behavioural | 0 | — | — | — | — | — | — | — | — |
| Inclusion of relaxation component (for CBT studies only) | |||||||||
| No mention of relaxation component (reference) | 4 | 0.93 | (0.47 to 1.86) | — | — | — | 0 | 0 | 0.87 |
| Relaxation component described as included | 5 | 0.89 | (0.64 to 1.24) | ‐0.06 | (‐0.95 to 0.82) | 0.87 | — | — | — |
| Inclusion of problem‐solving skills training component (for CBT studies only) | |||||||||
| No mention of problem‐solving component (reference) | 2 | 0.26 | (0.05 to 1.30) | — | — | — | 0 | 0 | 0.17 |
| Problem‐solving component described as included | 7 | 0.94 | (0.70 to 1.28) | 1.27 | (‐0.68 to 3.23) | 0.17 | — | — | — |
| Inclusion of social skills training component (for CBT studies only) | |||||||||
| No mention of social skills component (reference) | 4 | 0.91 | (0.60 to 1.39) | — | — | — | 0 | 0 | 0.85 |
| Social skills component described as included | 5 | 0.87 | (0.53 to 1.43) | ‐0.06 | (‐0.81 to 0.68) | 0.85 | — | — | — |
| Type of facilitator | |||||||||
| Mental health expert (reference group) | 2 | 0.26 | (0.05 to 1.30) | — | — | — | 0 | 0 | 0.39 |
| Students | 3 | 0.68 | (0.22 to 2.04) | 0.97 | (‐1.36 to 3.30) | 0.35 | — | — | — |
| Non‐mental health expert | 4 | 0.90 | (0.61 to 1.32) | 1.22 | (‐0.83 to 3.27) | 0.19 | — | — | — |
| Mode of delivery | |||||||||
| Face‐to‐face (group or individual) | 9 | 0.82 | (0.57 to 1.16) | — | — | — | 0 | 0 | 0.62 |
| Online/telephone | 1 | 0.94 | (0.62 to 1.44) | 0.15 | (‐0.50 to 0.79) | 0.62 | — | — | — |
| k refers to number of trials. CBT: cognitive behavioural therapy | |||||||||
| k | SMD | (95% CI) | β | (95% CI) | P value (moderator) | AdjustedR2 (%) | I2 (Res) (%) | P value (overall) | |
| Overall effect | 31 | ‐0.11 | (‐0.17 to ‐0.05) | ‐0.11 | (‐0.17 to ‐0.04) | >0.001 | 0 | 41.0 | > 0.001 |
| Continuous | |||||||||
| Intensity of intervention (hours) | 29 | — | — | 0.01 | (0.00 to 0.02) | > 0.001 | 68.0 | 18.0 | > 0.001 |
| Binary | |||||||||
| Focus of intervention | |||||||||
| CBT (reference) | 21 | ‐0.11 | (‐0.18 to ‐0.04) | — | — | — | 0 | 46.5 | 0.79 |
| CBT + IPT | 3 | ‐0.08 | (‐0.25 to 0.10) | 0.02 | (‐0.24 to 0.28) | 0.87 | — | — | — |
| IPT | 1 | ‐0.27 | (‐0.57 to 0.02) | ‐0.16 | (‐0.58 to 0.25) | 0.43 | — | — | — |
| Third wave | 6 | ‐0.01 | (‐0.31 to 0.30) | 0.07 | (‐0.19 to 0.33) | 0.57 | — | — | — |
| Depression severity at baseline | |||||||||
| Subthreshold (reference) | 25 | ‐0.11 | (‐0.18 to ‐0.04) | — | — | — | 0 | 45.9 | 0.62 |
| Mild | 5 | ‐0.06 | (‐0.26 to 0.14) | 0.05 | (‐0.16 to 0.27) | 0.62 | |||
| Moderate | 0 | — | — | — | — | — | — | — | — |
| Severe | 0 | — | — | — | — | — | — | — | — |
| Focus of CBT (for CBT studies only) | |||||||||
| CBT – cognitive and behavioural (reference) | 11 | ‐0.08 | (‐0.15 to ‐0.01) | — | — | — | 2.0 | 42.7 | 0.42 |
| CBT ‐ cognitive | 13 | ‐0.14 | (‐0.24 to ‐0.03) | ‐0.05 | (‐0.19 to 0.08) | 0.42 | |||
| CBT ‐ behavioural | 0 | — | — | — | — | — | — | — | — |
| Inclusion of relaxation component (for CBT studies only) | |||||||||
| No mention of relaxation component (reference) | 11 | ‐0.13 | (‐0.23 to ‐0.04) | — | — | — | 9.0 | 40.8 | 0.45 |
| Relaxation component described as included | 13 | ‐0.08 | (‐0.16 to ‐0.01) | 0.05 | (‐0.09 to 0.19) | 0.45 | — | — | — |
| Inclusion of problem‐solving skills training component (for CBT studies only) | |||||||||
| No mention of problem‐solving component (reference) | 6 | ‐0.20 | (‐0.34 to ‐0.07) | — | — | — | 14.0 | 40.4 | 0.13 |
| Problem‐solving component described as included | 18 | ‐0.08 | (‐0.15 to ‐0.01) | 0.12 | (‐0.04 to 0.28) | 0.13 | — | — | — |
| Inclusion of social skills training component (for CBT studies only) | |||||||||
| No mention of social skills component (reference) | 8 | ‐0.18 | (‐0.29 to ‐0.07) | — | — | — | 13.0 | 39.5 | 0.11 |
| Social skills component described as included | 16 | ‐0.06 | (‐0.13 to 0.01) | 0.11 | (‐0.03 to 0.24) | 0.11 | |||
| Type of facilitator | |||||||||
| Mental health expert (reference group) | 11 | ‐0.11 | (‐0.23 to 0.02) | — | — | — | 0 | 48.0 | 0.57 |
| Students | 6 | ‐0.21 | (‐0.38 to ‐0.05) | ‐0.10 | (‐0.35 to 0.14) | 0.38 | — | — | — |
| Non‐mental health expert | 8 | ‐0.09 | (‐0.22 to 0.03) | 0.01 | (‐0.19 to 0.22) | 0.88 | — | — | — |
| Mode of delivery | |||||||||
| Face‐to‐face (group or individual) | 27 | ‐0.11 | (‐0.19 to ‐0.04) | — | — | — | 0 | 43.9 | 0.76 |
| Online/telephone | 4 | ‐0.07 | (‐0.18 to 0.03) | 0.03 | (‐0.15 to 0.20) | 0.76 | — | — | — |
| k refers to number of trials. CBT: cognitive behavioural therapy | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive diagnosis (by population) post‐intervention Show forest plot | 20 | 3232 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.08, ‐0.02] |
| 1.1 Targeted | 13 | 2022 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.02] |
| 1.2 Universal | 7 | 1210 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| 2 Depressive diagnosis short‐term follow‐up Show forest plot | 6 | 724 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 2.1 Targeted | 4 | 360 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.19, ‐0.02] |
| 2.2 Universal | 2 | 364 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 3 Depressive diagnosis medium‐term follow‐up Show forest plot | 32 | 5965 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.05, ‐0.01] |
| 3.1 Targeted | 22 | 3915 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 3.2 Universal | 10 | 2050 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 4 Depressive diagnosis long‐term follow‐up Show forest plot | 10 | 1769 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.05, 0.02] |
| 4.1 Targeted | 6 | 1043 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.03] |
| 4.2 Universal | 4 | 726 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 5 Depression symptoms (by population) post‐intervention Show forest plot | 73 | 13829 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.27, ‐0.15] |
| 5.1 Targeted | 42 | 4816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| 5.2 Universal | 31 | 9013 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.05] |
| 6 Depression symptoms short‐term follow‐up Show forest plot | 16 | 1558 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.45, ‐0.17] |
| 6.1 Targeted | 11 | 999 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.54, ‐0.20] |
| 6.2 Universal | 5 | 559 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.37, 0.01] |
| 7 Depression symptoms medium‐term follow‐up Show forest plot | 53 | 11913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.18, ‐0.05] |
| 7.1 Targeted | 29 | 4448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.33, ‐0.12] |
| 7.2 Universal | 24 | 7465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 8 Depression symptoms long‐term follow‐up Show forest plot | 15 | 3836 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 8.1 Targeted | 7 | 847 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.21, 0.11] |
| 8.2 Universal | 8 | 2989 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.06, 0.09] |
| 9 Depression symptoms clinician‐rated (by population) post‐intervention Show forest plot | 11 | 2175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.41, ‐0.05] |
| 9.1 Targeted | 10 | 1340 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.44, ‐0.11] |
| 9.2 Universal | 1 | 835 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.06, 0.21] |
| 10 Depression symptoms clinician‐rated medium‐term follow‐up Show forest plot | 9 | 1754 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.24, 0.07] |
| 10.1 Targeted | 8 | 968 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.09] |
| 10.2 Universal | 1 | 786 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.14, 0.14] |
| 11 Depression symptoms clinician‐rated long‐term follow‐up Show forest plot | 6 | 894 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.01] |
| 11.1 Targeted | 6 | 894 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.01] |
| 12 Anxiety symptoms (by population) post‐intervention Show forest plot | 23 | 5017 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.16, 0.02] |
| 12.1 Targeted | 13 | 1666 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.31, 0.04] |
| 12.2 Universal | 10 | 3351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.13, 0.05] |
| 13 Anxiety symptoms (by population) short‐term follow‐up Show forest plot | 3 | 334 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| 13.1 Targeted | 3 | 334 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| 14 Anxiety symptoms (by population) medium‐term follow‐up Show forest plot | 18 | 4957 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.14, ‐0.01] |
| 14.1 Targeted | 10 | 1827 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.18, 0.04] |
| 14.2 Universal | 8 | 3130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.17, ‐0.01] |
| 15 Anxiety symptoms (by population) long‐term follow‐up Show forest plot | 5 | 971 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.44, 0.14] |
| 15.1 Targeted | 2 | 293 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.43, 0.03] |
| 15.2 Universal | 3 | 678 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 16 Social and general functioning (by population) post‐intervention Show forest plot | 10 | 2067 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.06, 0.41] |
| 16.1 Targeted | 9 | 1021 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.04, 0.50] |
| 16.2 Universal | 1 | 1046 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [0.04, 0.28] |
| 17 Social and general functioning (by population) short‐term follow‐up Show forest plot | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.81 [0.12, 1.49] |
| 17.1 Targeted | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.81 [0.12, 1.49] |
| 17.2 Universal | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Social and general functioning (by population) medium‐term follow‐up Show forest plot | 11 | 2449 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [0.02, 0.28] |
| 18.1 Targeted | 9 | 1058 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.00, 0.38] |
| 18.2 Universal | 2 | 1391 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.01, 0.20] |
| 19 Social and general functioning (by population) long‐term follow‐up Show forest plot | 4 | 744 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.16, 0.14] |
| 19.1 Targeted | 3 | 342 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.22, 0.21] |
| 19.2 Universal | 1 | 402 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive diagnosis medium‐term follow‐up Show forest plot | 22 | 3915 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 1.1 Treatment as usual | 12 | 2464 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 1.2 No treatment | 8 | 1286 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 1.3 Wait‐list | 1 | 95 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.21, 0.05] |
| 1.4 Other | 1 | 70 | Risk Difference (M‐H, Random, 95% CI) | ‐0.12 [‐0.29, 0.04] |
| 2 Depression symptoms post‐intervention Show forest plot | 42 | 4816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| 2.1 Treatment as usual | 16 | 2514 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.45, ‐0.15] |
| 2.2 No treatment | 14 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.57, ‐0.21] |
| 2.3 Attention placebo | 4 | 466 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.32, 0.13] |
| 2.4 Wait‐list | 6 | 361 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.72, ‐0.26] |
| 2.5 Other | 2 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.51, 0.04] |
| 3 Depression symptoms medium‐term follow‐up Show forest plot | 29 | 4448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.33, ‐0.12] |
| 3.1 Treatment as usual | 15 | 2315 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.42, ‐0.13] |
| 3.2 No treatment | 9 | 1207 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.09] |
| 3.3 Attention placebo | 3 | 761 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.26, 0.03] |
| 3.4 Wait‐list | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.55, 0.28] |
| 3.5 Other | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.64, ‐0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive diagnosis medium‐term follow‐up Show forest plot | 10 | 2050 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 1.1 Treatment as usual | 3 | 656 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 1.2 No treatment | 2 | 316 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.05, 0.07] |
| 1.3 Attention placebo | 2 | 861 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 1.4 Wait‐list | 3 | 217 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.24, 0.09] |
| 2 Depression symptoms post‐intervention Show forest plot | 31 | 9013 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.05] |
| 2.1 Treatment as usual | 9 | 1791 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.31, 0.00] |
| 2.2 No treatment | 9 | 4231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.25, ‐0.05] |
| 2.3 Attention placebo | 9 | 2180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.09, 0.08] |
| 2.4 Wait‐list | 4 | 811 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.28, 0.04] |
| 3 Depression symptoms medium‐term follow‐up Show forest plot | 24 | 7465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 3.1 No treatment | 7 | 3367 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.10, 0.16] |
| 3.2 Treatment as usual | 6 | 1505 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.16, 0.05] |
| 3.3 Attention placebo | 7 | 1813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 3.4 Wait‐list | 4 | 780 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.34, 0.07] |
| 3.5 Other | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depressive diagnosis medium‐term follow‐up Show forest plot | 22 | 3915 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 1.1 Selective | 3 | 963 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.07, 0.12] |
| 1.2 Indicated | 16 | 2374 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.06, ‐0.01] |
| 1.3 Combined | 3 | 578 | Risk Difference (M‐H, Random, 95% CI) | ‐0.14 [‐0.21, ‐0.07] |
| 2 Depression symptoms (by population) post‐intervention Show forest plot | 42 | 4816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| 2.1 Selective | 9 | 1394 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.30, ‐0.02] |
| 2.2 Indicated | 29 | 2740 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.50, ‐0.24] |
| 2.3 Combined | 4 | 682 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.45, ‐0.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression scores (by assessor) post‐intervention Show forest plot | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Self‐reported | 9 | 1877 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.53, ‐0.12] |
| 1.2 Clinician‐rated | 9 | 1884 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.46, ‐0.04] |
| 2 Depression scores medium‐term follow‐up Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Self‐reported | 7 | 1465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.41, ‐0.02] |
| 2.2 Clinician‐rated | 7 | 1468 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.32, 0.06] |
| 3 Depression scores long‐term follow‐up Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Self‐reported | 4 | 390 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.37, 0.16] |
| 3.2 Clinician‐rated | 4 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.27, 0.14] |