Tratamento supervisionado no controle da tuberculose
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Generation of allocation sequence: randomized, with factorial overlay; computer‐generated random numbers. Allocation concealment: not stated. Blinding: none. Completeness of follow‐up: 88%. | |
| Participants | Number: 300 randomized; 73% men; 85% unemployed; 27% with documented human immunodeficiency virus (HIV) infection. Included: adult, intravenous drug users with positive tuberculin skin test (at least 10 mm induration or 5 mm if HIV positive); given isoniazid preventive therapy for 6 months. Excluded: people with active TB. | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Location: Baltimore City Health Department TB Clinic, USA. Date: 1995 to 1997. Duration of DOT duration not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "generated using computer algorithm". |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of outcome assessment (detection bias) | High risk | None. "Blinding of the study was not possible." |
| Incomplete outcome data (attrition bias) | Unclear risk | There were losses to follow‐up in each arm though not differential there are no reports on them. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methodology are reported. |
| Other bias | Unclear risk | Not applicable. |
| Methods | Generation of allocation sequence: not stated. Randomization: stratified. Allocation concealment: not stated. Blinding: not stated. Completeness of follow‐up: no losses (18/114) dropped to enable matching. | |
| Participants | Number; 96 randomized into three groups; Matched by age and gender; confirmed TB diagnosis and over 18 yrs. | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Location: Taiwan. Trial period: May 2002 to July 2003. Duration of observation was 6 months. The patients were not given a choice of DOT observer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "There were 114 subjects meeting the sampling criteria who were then matched by age and gender and randomized into one of three groups". |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | 18/114 dropped to enable matching. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methodology are reported. |
| Other bias | Unclear risk | Not applicable. |
| Methods | Generation of allocation sequence: central block random allocation scheme prepared for each of 15 trial sites; random‐number table used. Allocation concealment: none. Blinding: no blinding of assessors. Completeness of follow‐up: 100% (no losses). | |
| Participants | Number: 837 randomized; 73% male. Included: new smear positive adults (aged 15+). | |
| Interventions |
All participants received the same drug regimen: isoniazid‐rifampicin‐pyrazinamide‐ethambutol for 2 months and isoniazid‐rifampicin for 4 months. | |
| Outcomes |
| |
| Notes | Location: Thailand. Date: 1996 to 1997. Duration of DOT not stated. Informed consent not obtained as participants were not told that they were participating in a study. Choice of supervisor for DOT participants: 352 chose a family member; 34 chose a community member; and 24 chose health centre staff. One participant in daily supervision arm excluded due to protocol violation so not strictly intention‐to‐treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated using random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information. |
| Blinding of outcome assessment (detection bias) | High risk | Investigators not blinded though the patients were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | There were no exclusions. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methodology are reported. |
| Other bias | Unclear risk | Not applicable. |
| Methods | Cluster‐RCT: 9 pairs of centres matched by type and size. Generation of allocation sequence: unclear. Allocation concealment: unclear. Blinding: none. Completeness of follow‐up: 87% at 2 months and 69% at 7 months. | |
| Participants | Number: 18 clusters randomized; 522 participants; mean age 35; 60% male. Included: new smear positive adults. | |
| Interventions |
Continuation phase of 6 months: both groups managed the same and expected to self‐administer treatment daily. | |
| Outcomes |
| |
| Notes | Location: Tanzania. Date: 1999 to 2000. Duration of DOT not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of outcome assessment (detection bias) | High risk | None. "This study was an unmasked cluster randomized trial". |
| Incomplete outcome data (attrition bias) | High risk | Only 68% (311/437 participants) were evaluated at 7 months. (This could affect the cure outcome). |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methodology are reported. |
| Recruitment bias | Unclear risk | No details of any shifting, though the cluster sizes varied from as low as 2 persons to 232 persons. |
| Baseline imbalance | Low risk | Clusters were similar though the size varied and one cluster had possibly a more sicker patient profile due to its highly specialized nature. |
| Loss of clusters | Unclear risk | One cluster in the community based intervention did not have patients hence was dropped in the analysis. |
| Incorrect analysis | Low risk | Cluster adjusted hence comparable to other RCTs randomizing individuals. |
| Methods | Quasi‐RCT Generation of allocation sequence: alternate allocation Concealment of allocation: none Blinding: assessment of urinary isoniazid blinded Completeness of follow‐up: not stated | |
| Participants | Number: 173 recruited, mostly foreign nationals; male 51%; mean age 41 (range 14 to 83). Included: new TB participants. Excluded: multiple‐drug resistant TB; relapsed TB; human immunodeficiency virus (HIV)‐positive cases; and nontuberculous mycobacterial infections. | |
| Interventions |
Both groups had monthly visits to health facilities and standardized recording charts. | |
| Outcomes | Treatment completion measured by:
| |
| Notes | Location: Australia. Date: 1998 to December 2000. Duration of DOT not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly. "Patients were systematically allocated to receive FDOT or ST". |
| Allocation concealment (selection bias) | High risk | Systematic allocation "The first patient was randomly allocated to the ST arm, every second patient was allocated to FDOT, and the remainder to ST". |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information as to what happened to those who refused family DOT. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methodology are reported. |
| Other bias | Unclear risk | Not applicable. |
| Methods | Cluster‐RCT. Generation of allocation sequence: 5 randomly selected districts allocated to each arm; the name of each district was written on an individual paper and randomly drawn from a basket. Allocation concealment: method not stated. Blinding: laboratory technicians assessing the primary outcomes were blinded. Completeness of follow‐up: 100% (no clusters or individuals lost). | |
| Participants | Number: 10 districts with 907 people randomized; all smear positive; 67% male. Included: people with TB (aged 15+); new smear‐positive cases, diagnosed at health facilities in the trial area; human immunodeficiency virus (HIV) status not known. | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Location: hill and mountain districts of Nepal. Date: 2002 to 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on selection of 10 districts out of 17. |
| Allocation concealment (selection bias) | Low risk | Randomly picked papers from an opaque bag. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No cluster was lost to follow‐up or excluded. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methodology are reported. |
| Recruitment bias | Unclear risk | Not reported if there were patients who shifted to the different intervention arms, though they were separated by a mountainous region. |
| Baseline imbalance | Low risk | Characteristics similar. |
| Loss of clusters | Low risk | No loss reported. |
| Incorrect analysis | Low risk | Cluster adjustment done. |
| Methods | Generation of allocation sequence: computer‐generated random numbers. Allocation concealment: opaque, sealed envelopes. Blinding: assessors blinded. Completeness of follow‐up: not stated. | |
| Participants | Number: 497 randomized; 51.3% male. Included: adults (aged 15+); new smear‐positive cases. | |
| Interventions |
All participants received isoniazid‐rifampicin‐pyrazinamide‐ethambutol for 2 months and isoniazid‐ethambutol for 6 months. | |
| Outcomes |
| |
| Notes | Location: Pakistan Date: 1996 to 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were used and third party calls. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | There were no exclusions after randomization. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methodology are reported. |
| Other bias | Unclear risk | Not applicable. |
| Methods | Generation of allocation sequence: coin tossing in each of 5 clinics. Allocation concealment: none. Blinding: none. Completeness of follow‐up: 100% (no losses). | |
| Participants | Number: 587 randomized; 322 smear positive, 182 smear negative, and 83 extrapulmonary TB; 57% male. Included: people with TB (aged 5+); new smear positive, smear negative, and extrapulmonary cases; human immunodeficiency virus (HIV) status not known. Excluded: previously treated for TB; severe illness; transferred from another clinic; previously enrolled in the study. | |
| Interventions |
Apart from the observation option participants received the same standardized management including drug therapy. | |
| Outcomes |
| |
| Notes | Location: Dar es Salaam, Tanzania. Date: 2001 to 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly by coin toss". |
| Allocation concealment (selection bias) | High risk | None. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methodology are reported. |
| Other bias | Unclear risk | Not applicable. |
| Methods | Generation of allocation sequence: unclear; stratified into adults and children; then, within each group, randomized by type of TB (sputum positive, sputum negative, extrapulmonary, relapse). Allocation concealment: unclear; sealed, sequentially numbered envelopes not stated if opaque. Blinding: assessors of sputum results blinded. Completeness of follow‐up: 98%. | |
| Participants | Number: 1353 randomized; 55% male; most 15+ years. Included: adults and children with smear positive or negative, extrapulmonary TB, or relapse of previously treated TB. Excluded: died before discharge; or too ill to receive outpatient treatment; lived in area without treatment supporter; or referred in after treatment commenced. | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Location: Swaziland. Date: 2000 to 2002. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes not clear whether opaque. " sealed, sequentially numbered, stratum specific envelopes containing treatment assignments". |
| Blinding of outcome assessment (detection bias) | Low risk | Laboratory assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Non differential loss to follow‐up (4/664 and 5/662). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methodology are reported. |
| Other bias | Unclear risk | Not applicable. |
| Methods | Generation of allocation sequence: computer‐generated random numbers. Allocation concealment: consecutively numbered, opaque, sealed envelopes in each of 5 clinics. Blinding: none. Completeness of follow‐up: 114/120 (95%) in 1 trial and 102/120 (85%) in other trial excluded from analysis. | |
| Participants | Number: 216 included in analysis; 62% male; 57% < 35 years. Included: adults (aged 15+) with pulmonary TB; both new and re‐treatment cases. Excluded: severe disease or multiple drug resistance; treatment at a non‐study clinic for more than 2 weeks; need to be supervised at school or at the workplace; and leaving the area within a month. | |
| Interventions |
New cases received Rifater (combined rifampicin‐isoniazid‐pyrazinamide) for 8 weeks followed by Rifinah 4 (combined rifampicin‐isoniazid) plus additional isoniazid for 18 weeks. Retreatment participants received Rifater plus ethambutol for 12 weeks and Rifinah plus rifampicin‐ethambutol for 22 weeks. | |
| Outcomes |
| |
| Notes | Location: 1 trial in each of 2 low‐income communities near Cape Town, South Africa. Date: 1994 to 1995. Results combined. 54 participants in 1 trial allocated to community supervision not reported in this paper. Exclusions from analysis: trial 1 (6 cases of multiple drug resistance) and trial 2 (12 cases of multiple drug resistance and 6 not TB). Number of exclusions per arm of the 2 trials not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random sequence generated by a computer algorithm". |
| Allocation concealment (selection bias) | Low risk | "Consecutively numbered opaque sealed envelops were used". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on whether there was any blinding or not. |
| Incomplete outcome data (attrition bias) | High risk | There was differential exclusions between the intervention and control arms. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methodology are reported. |
| Other bias | Unclear risk | Not applicable. |
| Methods | Generation of allocation sequence: computer‐generated random numbers. Allocation concealment: consecutively numbered, opaque, sealed envelopes. Blinding: none. Completeness of follow‐up: not stated. | |
| Participants | Number: 174 randomized. Included: new or re‐treatment participants aged 15+ who were sputum or culture positive. | |
| Interventions |
| |
| Outcomes | As for Zwarenstein 1998 ZAF. | |
| Notes | Location: 4 clinics in a township near Cape Town, South Africa. Date: 1994 to 1995. 18 participants excluded from analysis: 12 with multiple‐drug resistant TB and 6 not TB. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by a computer algorithm. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered opaque sealed envelopes. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information on whether there was any blinding or not. |
| Incomplete outcome data (attrition bias) | Low risk | There were exclusions though not differentiated between intervention arms. "After exclusion of 12 MDR and six non‐TB patients". |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methodology are reported. |
| Other bias | Unclear risk | Not applicable. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Compared direct observation plus with methadone treatment for injecting drug users with routine TB treatment without methadone. | |
| Before‐and‐after study; no control group. | |
| Not randomized. | |
| Different criteria for allocation to self‐administration or direct observation. | |
| An educational intervention was evaluated. | |
| Evaluates incentives for IV drug users within the context of a direct observation programme. | |
| Cohort study. | |
| Trial evaluating devices that monitor treatment using uranium along a strip of photographic film. | |
| Not randomly allocated; A publication reporting same data as Pungrassami 2002b. | |
| Not randomly allocated; A publication reporting same data as Pungrassami 2002a. | |
| Cohort study. | |
| Described as a RCT, but the randomization led to very different numbers in the 2 groups; subsequently over 50 participants (out of a total of 379) crossed over from self‐treatment to direct observation and were excluded from the analysis; little detail for the rest of the study provided. | |
| Multifaceted intervention including DOT. | |
| Patients in hospital. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (negative sputum smear in last month of Rx in patients +ve initially) Show forest plot | 5 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.91, 1.27] |
| Analysis 1.1 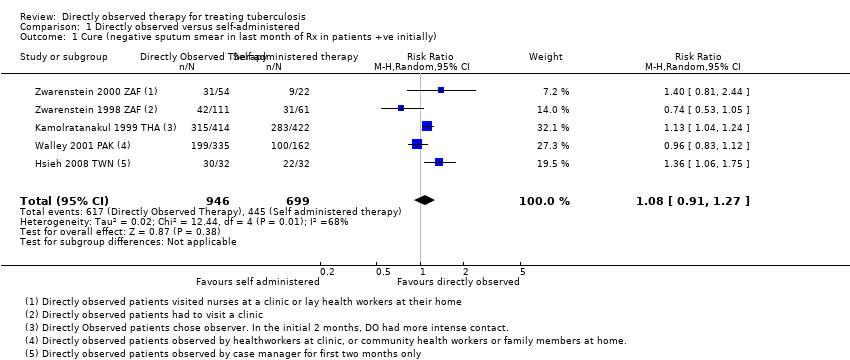 Comparison 1 Directly observed versus self‐administered, Outcome 1 Cure (negative sputum smear in last month of Rx in patients +ve initially). | ||||
| 2 Cure (by intensity of monitoring in control group) Show forest plot | 5 | 1645 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [1.00, 1.15] |
| Analysis 1.2  Comparison 1 Directly observed versus self‐administered, Outcome 2 Cure (by intensity of monitoring in control group). | ||||
| 2.1 Monthly monitoring of patients in self administered group | 2 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.06, 1.25] |
| 2.2 Once every two weeks monitoring of patients in self‐administered group | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 2.3 Weekly monitoring of patients in self‐administered group | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.21] |
| 3 Treatment completion (both with smear sputum test at end and those without) Show forest plot | 6 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.19] |
| Analysis 1.3 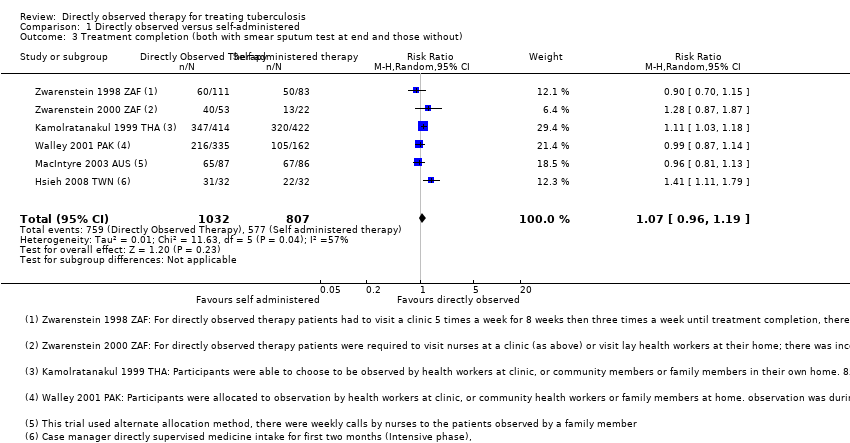 Comparison 1 Directly observed versus self‐administered, Outcome 3 Treatment completion (both with smear sputum test at end and those without). | ||||
| 4 Treatment completion (grouped by frequency of monitoring in the self‐administered therapy group) Show forest plot | 6 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.19] |
| Analysis 1.4  Comparison 1 Directly observed versus self‐administered, Outcome 4 Treatment completion (grouped by frequency of monitoring in the self‐administered therapy group). | ||||
| 4.1 Monthly monitoring of self‐administered treatment | 3 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.31] |
| 4.2 Once every two weeks monitoring of self‐administered treatment | 1 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.14] |
| 4.3 Weekly monitoring of self‐administered treatment | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.74, 1.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially) Show forest plot | 4 | 1556 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.18] |
| Analysis 2.1 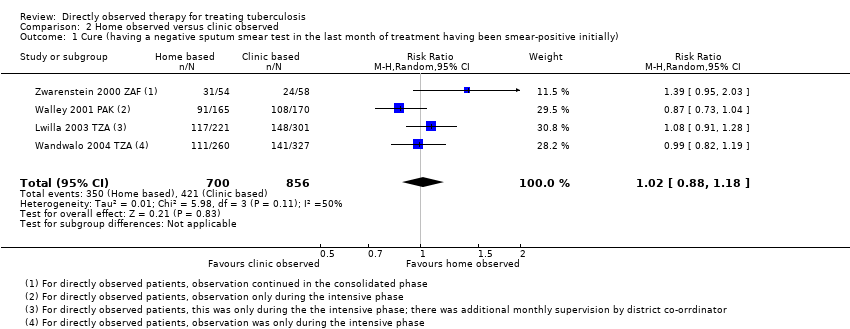 Comparison 2 Home observed versus clinic observed, Outcome 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially). | ||||
| 2 Treatment completion (both with smear sputum test at end and those without) Show forest plot | 3 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.17] |
| Analysis 2.2  Comparison 2 Home observed versus clinic observed, Outcome 2 Treatment completion (both with smear sputum test at end and those without). | ||||
| 3 Cure (stratified by intensity of observation) Show forest plot | 4 | 1556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.11] |
| Analysis 2.3 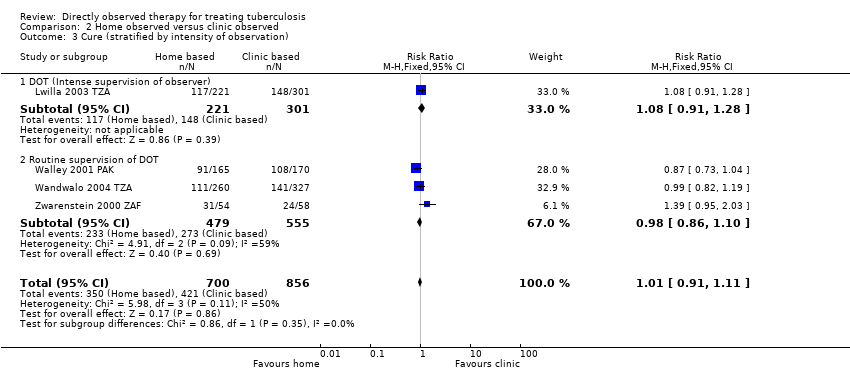 Comparison 2 Home observed versus clinic observed, Outcome 3 Cure (stratified by intensity of observation). | ||||
| 3.1 DOT (Intense supervision of observer) | 1 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 3.2 Routine supervision of DOT | 3 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially) Show forest plot | 2 | 1493 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.21] |
| Analysis 3.1 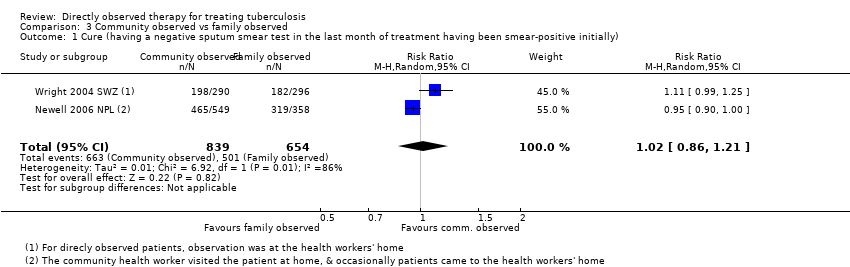 Comparison 3 Community observed vs family observed, Outcome 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially). | ||||
| 2 Treatment completion (both with smear sputum test at end and those without) Show forest plot | 2 | 1493 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.90, 1.22] |
| Analysis 3.2 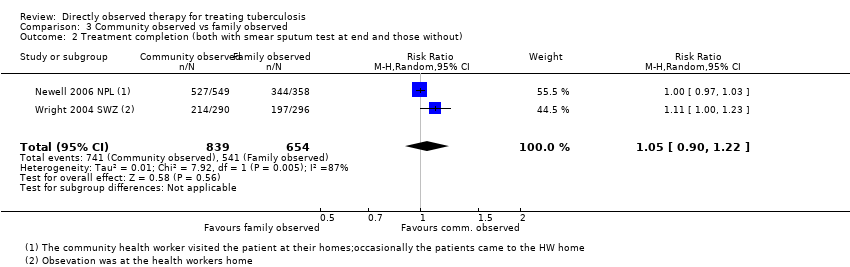 Comparison 3 Community observed vs family observed, Outcome 2 Treatment completion (both with smear sputum test at end and those without). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment completion Show forest plot | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.88, 1.13] |
| Analysis 4.1  Comparison 4 Injecting drug users, Outcome 1 Treatment completion. | ||||
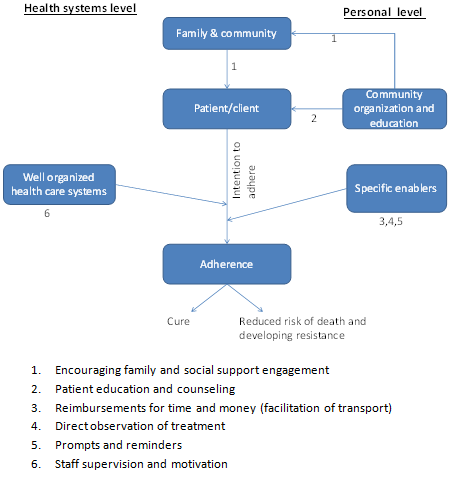
Factors influencing adherence and possible intervention points.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
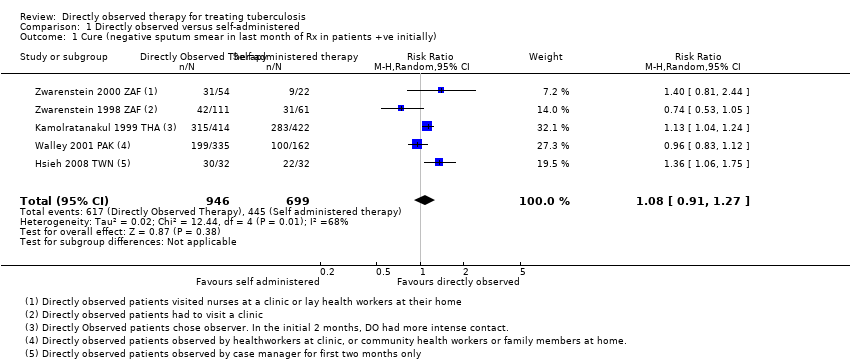
Comparison 1 Directly observed versus self‐administered, Outcome 1 Cure (negative sputum smear in last month of Rx in patients +ve initially).

Comparison 1 Directly observed versus self‐administered, Outcome 2 Cure (by intensity of monitoring in control group).
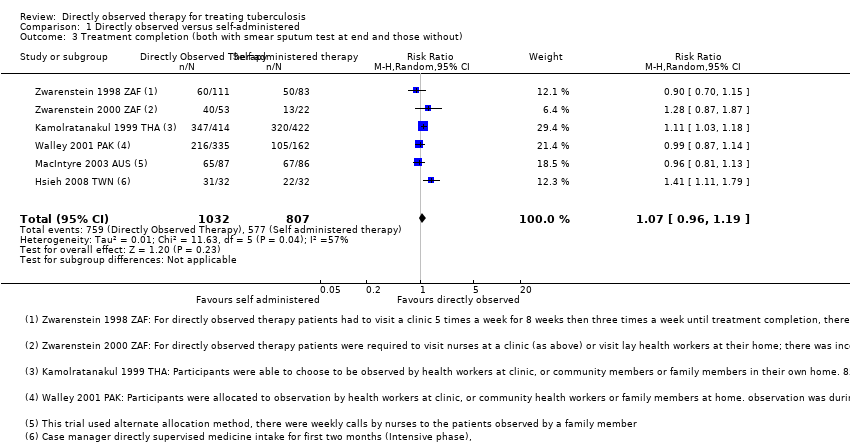
Comparison 1 Directly observed versus self‐administered, Outcome 3 Treatment completion (both with smear sputum test at end and those without).

Comparison 1 Directly observed versus self‐administered, Outcome 4 Treatment completion (grouped by frequency of monitoring in the self‐administered therapy group).
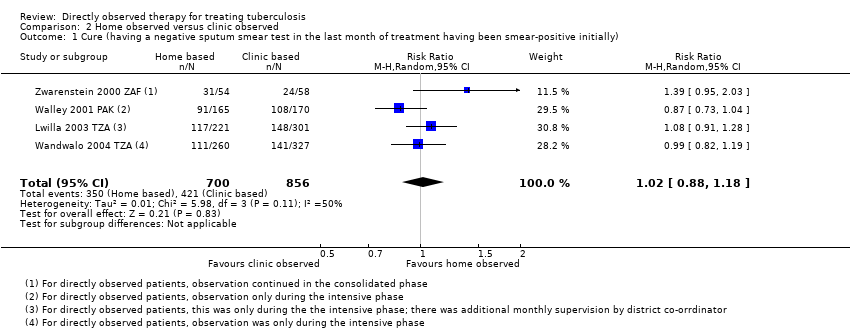
Comparison 2 Home observed versus clinic observed, Outcome 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially).

Comparison 2 Home observed versus clinic observed, Outcome 2 Treatment completion (both with smear sputum test at end and those without).
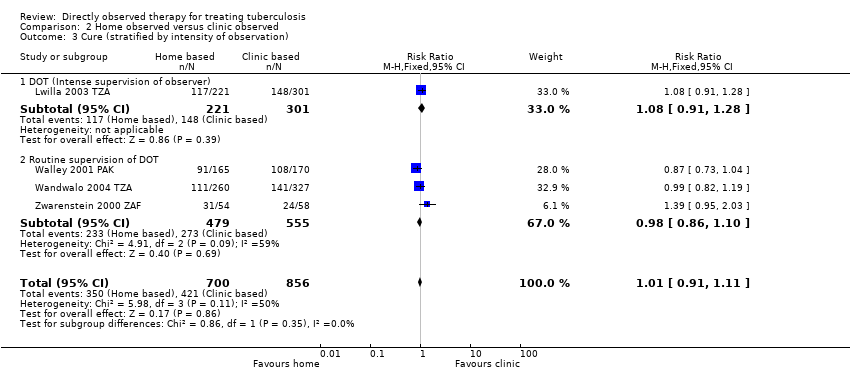
Comparison 2 Home observed versus clinic observed, Outcome 3 Cure (stratified by intensity of observation).

Comparison 3 Community observed vs family observed, Outcome 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially).

Comparison 3 Community observed vs family observed, Outcome 2 Treatment completion (both with smear sputum test at end and those without).

Comparison 4 Injecting drug users, Outcome 1 Treatment completion.
| Directly observed therapy (DOT) versus self‐administered TB treatment | |||||
| Patient or population: Patients on TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Self‐administered therapy | DOT | ||||
| Cure | 617 per 1000 | 666 per 1000 | RR 1.08 | 1645 | ⊕⊕⊕⊝ |
| Treatment completion Follow‐up: 2 to 8 months5 | 709 per 1000 | 751 per 1000 | RR 1.07 | 1839 | ⊕⊕⊕⊝ |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1No serious risk of bias: three trials adequately described allocation concealment. Exclusion of trials at unclear or high risk of bias did not substantially change the result. | |||||
| Home DOT versus clinic DOT | |||||
| Patient or population: Patients with TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Clinic observation | Home observation | ||||
| Cure | 492 per 1000 | 502 per 1000 | RR 1.02 | 1556 | ⊕⊕⊕⊝ |
| Treatment completion4 | 751 per 1000 | 781 per 1000 | RR 1.04 | 1029 | ⊕⊕⊕⊝ |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for risk of bias: selection bias is probable in one trial, Wandwalo 2004 TZA, as there was no blinding and no allocation concealment. In Lwilla 2003 TZA, sequence generation and allocation concealment were unclear and there was no blinding. This could bias the measurement of treatment completion. | |||||
| Community DOT versus family DOT | |||||
| Patient or population: Patients on TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Family DOT | Community DOT | ||||
| Cure Follow‐up: up to 6 months | 766 per 1000 | 781 per 1000 | RR 1.02 (0.86 to 1.21) | 1493 (2 trials) | ⊕⊕⊕⊝ |
| Treatment completion Follow‐up: 2 to 6 months | 827 per 1000 | 869 per 1000 | RR 1.05 (0.90 to 1.22) | 1493 (2 trials) | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for risk of bias. Both trials had unclear random sequence generation and recruitment bias could not be ruled out for Newell 2006 NPL. | |||||
| DOT versus self‐administered therapy for intravenous drug users | |||||
| Patient or population: Patients on TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Self‐administered therapy | DOT | ||||
| Treatment completion Follow‐up for 6 months | 79 per 100 | 79 per 1000 | RR 1.00 (0.88 to 1.13) | 300 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for risk of bias. There was no blinding of outcome assessment and allocation concealment was unclear and treatment completion can be a bit subjective hence the results might be biased. The level of completeness to follow‐up was 88%. | |||||
| Trial ID | DOT | Self administered therapy | |||||||
| Who observed? | Where? | How often? | Adherence recorded at each contact | Cure | Frequency of contact with health service | Adherence recorded at each contact | Cure | ||
| Intensive phase | Consolidation phase | ||||||||
| Nurses | Clinic | 5 times per week | 3 times per week | Yes | 38% (42/111) | Weekly | Yes | 51% (31/61 | |
| Nurse | Clinic | 5 times per week | 3 times per week | Yes | 57% (31/54) | Weekly | Yes | 41% (9/22) | |
| Lay health worker | Lay health workers home | ||||||||
| Healthcare worker | Clinic | Daily | Daily | Yes | 76% | Monthly | Unclear | 67% (283/422) | |
| Community health worker | Home | Daily | Daily | ||||||
| Family member | Home | Daily | Daily | ||||||
| Healthcare worker | Clinic | 6 times per week | 2 times per month | Yes | 59% (199/335) | Every two weeks | Unclear | 62% (100/162) | |
| Community health worker | Home | ||||||||
| Family member | Home | Daily | Daily | ||||||
| Family member | Home | Daily | Daily | Yes | Not reported | Monthly | Yes | Not reported | |
| Case manager or Hospital care | Hospital | Daily | Once per week | Yes | 94% (30/32) | Monthly unscheduled visit | Yes | 69% (22/32) | |
| 1In Kamolratanakul 1999 THA patients could choose which observer they preferred and there a more intense supervision of observers in the intensive phase. | |||||||||
| Trial ID | DOT at patient's home | DOT at clinic | |||||||
| Who observed? | How often? | Supervision of observer | Cure | Who observed? | How often? | Cure | |||
| Intensive phase | Consolidation phase | Intensive phase | Consolidation phase | ||||||
| Family member | Daily | Not described | Observers collected drugs from the clinic every 2 weeks | 55% (91/165) | Health worker | 6 times per week | Self‐supervised | 64% (108/170) | |
| Family member or former TB patient | Daily | Self‐supervised | Observers collected drugs from clinic weekly and spot checks were conducted by health worker | 43% (111/260) | Health worker | Daily | Self‐supervised | 43% (141/327) | |
| Lay health worker2 | 'Several times a week' | Not described | Observer collected drugs monthly | 57% (31/54) | Health worker | 5 times a week | 3 times a week | 41% (24/58) | |
| Community volunteer | Daily | Self‐supervised | Observer was visited every two weeks by the health worker and every month by the district co‐ordinator3 | 53% (117/221) | Health worker | Daily | Self‐supervised | 49% (148/301) | |
| 1In Lwilla 2003 TZA, Walley 2001 PAK and Wandwalo 2004 TZA observation was during the intensive phase, while in the clinic observation arm of Zwarenstein 2000 ZAF it continued in the consolidated phase. | |||||||||
| Trial ID | Who observed? | Where? | How often? | Additional intervention | Who observed? | Where? | How often? | ||
| Intensive phase | Consolidation phase | Intensive phase | Consolidation phase | ||||||
| Family member | Patient's home | Daily | Daily | Drugs supplied to supervisor every week | Community health worker | Patient's home1 | Daily | Daily | |
| Family member | Patient's home | Daily | Daily | Patient reviewed at the diagnostic centre once per month Recorded in a patient adherence card | Community health worker | Community health worker's home | Daily | Daily | |
| 1In Newell 2006 NPL the community health worker mainly visited the patients at their homes but occasionally the patients came to the health worker's home. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (negative sputum smear in last month of Rx in patients +ve initially) Show forest plot | 5 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.91, 1.27] |
| 2 Cure (by intensity of monitoring in control group) Show forest plot | 5 | 1645 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [1.00, 1.15] |
| 2.1 Monthly monitoring of patients in self administered group | 2 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.06, 1.25] |
| 2.2 Once every two weeks monitoring of patients in self‐administered group | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 2.3 Weekly monitoring of patients in self‐administered group | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.21] |
| 3 Treatment completion (both with smear sputum test at end and those without) Show forest plot | 6 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.19] |
| 4 Treatment completion (grouped by frequency of monitoring in the self‐administered therapy group) Show forest plot | 6 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.19] |
| 4.1 Monthly monitoring of self‐administered treatment | 3 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.31] |
| 4.2 Once every two weeks monitoring of self‐administered treatment | 1 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.14] |
| 4.3 Weekly monitoring of self‐administered treatment | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.74, 1.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially) Show forest plot | 4 | 1556 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.18] |
| 2 Treatment completion (both with smear sputum test at end and those without) Show forest plot | 3 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.17] |
| 3 Cure (stratified by intensity of observation) Show forest plot | 4 | 1556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.11] |
| 3.1 DOT (Intense supervision of observer) | 1 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 3.2 Routine supervision of DOT | 3 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially) Show forest plot | 2 | 1493 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.21] |
| 2 Treatment completion (both with smear sputum test at end and those without) Show forest plot | 2 | 1493 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.90, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment completion Show forest plot | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.88, 1.13] |

