Izravno nadgledanje osoba koje se liječe od tuberkuloze da bi im se pomoglo dovršiti liječenje
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis |
| 2 | DOT* | PATIENT COMPLIANCE | PATIENT COMPLIANCE | PATIENT COMPLIANCE | DOT* |
| 3 | directly observed therapy | PATIENT PARTICIPATION | PATIENT PARTICIPATION | PATIENT MONITORING | supervision |
| 4 | 2 or 3 | patient monitoring | MOTIVATION | DOT$ | 2 or 3 |
| 5 | 1 and 4 | MOTIVATION | DECISION SUPPORT TECHNIQUES | directly observed therapy | 1 and 4 |
| 6 | — | DECISION SUPPORT TECHNIQUES | DOT* | compliance | — |
| 7 | — | DOT* | directly observed therapy | motivation | — |
| 8 | — | directly observed therapy | compliance | patient$ | — |
| 9 | — | compliance | patient* | defaulter$ | — |
| 10 | — | defaulter* | defaulter* | adheren$ | — |
| 11 | — | adheren* | adheren* | supervis$ | — |
| 12 | — | supervision* | supervis* | 2‐11/or | — |
| 13 | — | 2‐12/or | 2‐12/or | 1 and 12 | — |
| 14 | — | 1 and 13 | 1 and 13 | Limit 13 to human | — |
| 15 | — | — | Limit 14 to human | — | — |
aCIDG Specialized Register.
bSearch terms used in combination with the search strategy for retrieving trials developed by Cochrane (Higgins 2011); upper case: MeSH or EMTREE heading; lower case: free text term.
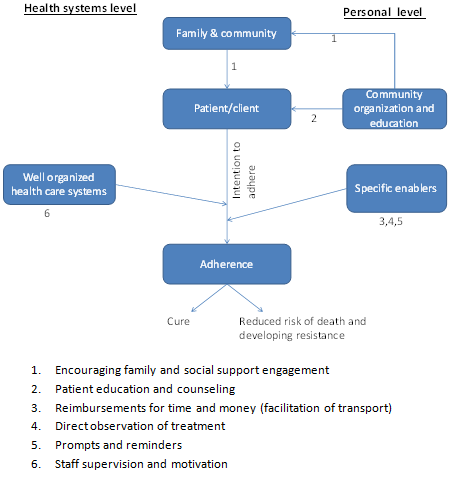
Factors influencing adherence and possible intervention points.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
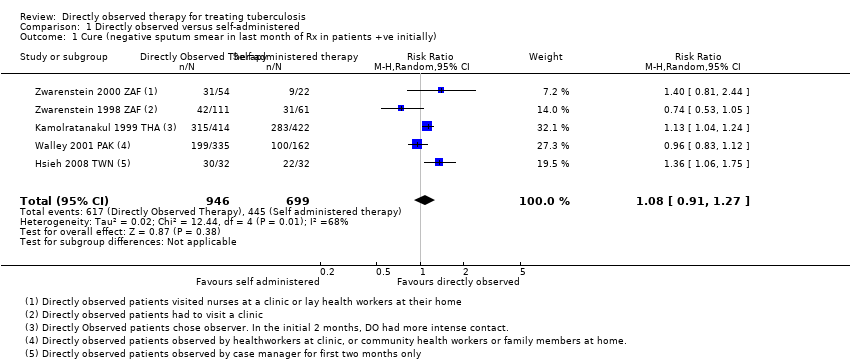
Comparison 1 Directly observed versus self‐administered, Outcome 1 Cure (negative sputum smear in last month of Rx in patients +ve initially).

Comparison 1 Directly observed versus self‐administered, Outcome 2 Cure (by intensity of monitoring in control group).
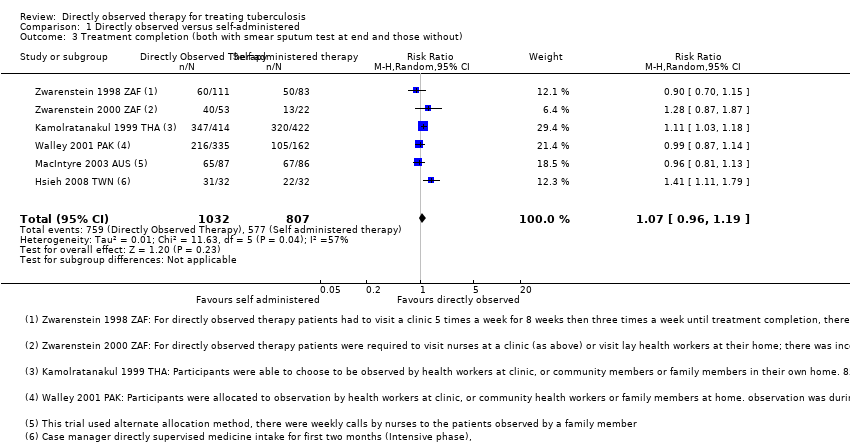
Comparison 1 Directly observed versus self‐administered, Outcome 3 Treatment completion (both with smear sputum test at end and those without).

Comparison 1 Directly observed versus self‐administered, Outcome 4 Treatment completion (grouped by frequency of monitoring in the self‐administered therapy group).
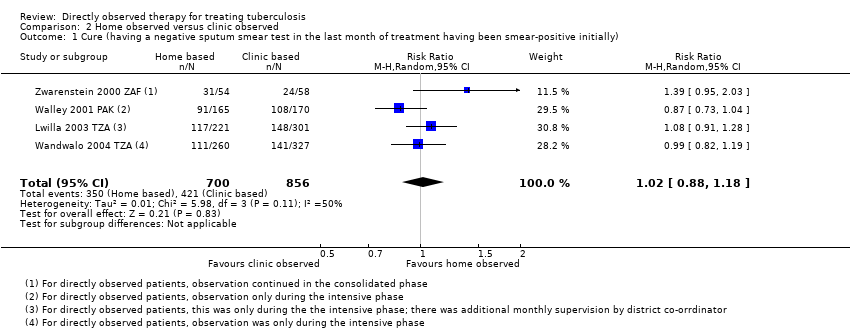
Comparison 2 Home observed versus clinic observed, Outcome 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially).

Comparison 2 Home observed versus clinic observed, Outcome 2 Treatment completion (both with smear sputum test at end and those without).
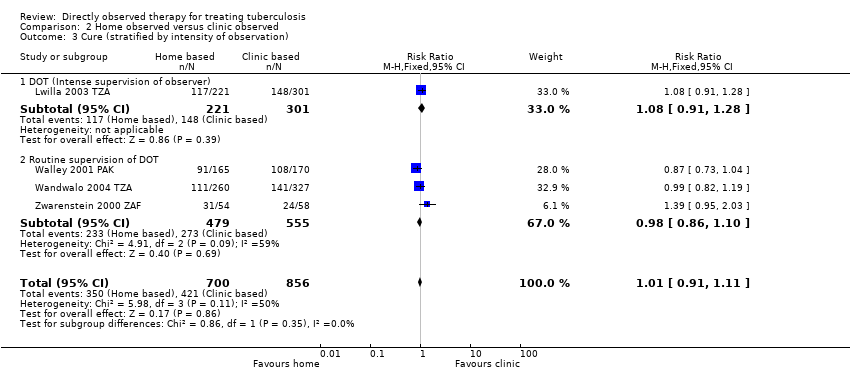
Comparison 2 Home observed versus clinic observed, Outcome 3 Cure (stratified by intensity of observation).

Comparison 3 Community observed vs family observed, Outcome 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially).

Comparison 3 Community observed vs family observed, Outcome 2 Treatment completion (both with smear sputum test at end and those without).

Comparison 4 Injecting drug users, Outcome 1 Treatment completion.
| Directly observed therapy (DOT) versus self‐administered TB treatment | |||||
| Patient or population: Patients on TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Self‐administered therapy | DOT | ||||
| Cure | 617 per 1000 | 666 per 1000 | RR 1.08 | 1645 | ⊕⊕⊕⊝ |
| Treatment completion Follow‐up: 2 to 8 months5 | 709 per 1000 | 751 per 1000 | RR 1.07 | 1839 | ⊕⊕⊕⊝ |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1No serious risk of bias: three trials adequately described allocation concealment. Exclusion of trials at unclear or high risk of bias did not substantially change the result. | |||||
| Home DOT versus clinic DOT | |||||
| Patient or population: Patients with TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Clinic observation | Home observation | ||||
| Cure | 492 per 1000 | 502 per 1000 | RR 1.02 | 1556 | ⊕⊕⊕⊝ |
| Treatment completion4 | 751 per 1000 | 781 per 1000 | RR 1.04 | 1029 | ⊕⊕⊕⊝ |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for risk of bias: selection bias is probable in one trial, Wandwalo 2004 TZA, as there was no blinding and no allocation concealment. In Lwilla 2003 TZA, sequence generation and allocation concealment were unclear and there was no blinding. This could bias the measurement of treatment completion. | |||||
| Community DOT versus family DOT | |||||
| Patient or population: Patients on TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Family DOT | Community DOT | ||||
| Cure Follow‐up: up to 6 months | 766 per 1000 | 781 per 1000 | RR 1.02 (0.86 to 1.21) | 1493 (2 trials) | ⊕⊕⊕⊝ |
| Treatment completion Follow‐up: 2 to 6 months | 827 per 1000 | 869 per 1000 | RR 1.05 (0.90 to 1.22) | 1493 (2 trials) | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for risk of bias. Both trials had unclear random sequence generation and recruitment bias could not be ruled out for Newell 2006 NPL. | |||||
| DOT versus self‐administered therapy for intravenous drug users | |||||
| Patient or population: Patients on TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Self‐administered therapy | DOT | ||||
| Treatment completion Follow‐up for 6 months | 79 per 100 | 79 per 1000 | RR 1.00 (0.88 to 1.13) | 300 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for risk of bias. There was no blinding of outcome assessment and allocation concealment was unclear and treatment completion can be a bit subjective hence the results might be biased. The level of completeness to follow‐up was 88%. | |||||
| Trial ID | DOT | Self administered therapy | |||||||
| Who observed? | Where? | How often? | Adherence recorded at each contact | Cure | Frequency of contact with health service | Adherence recorded at each contact | Cure | ||
| Intensive phase | Consolidation phase | ||||||||
| Nurses | Clinic | 5 times per week | 3 times per week | Yes | 38% (42/111) | Weekly | Yes | 51% (31/61 | |
| Nurse | Clinic | 5 times per week | 3 times per week | Yes | 57% (31/54) | Weekly | Yes | 41% (9/22) | |
| Lay health worker | Lay health workers home | ||||||||
| Healthcare worker | Clinic | Daily | Daily | Yes | 76% | Monthly | Unclear | 67% (283/422) | |
| Community health worker | Home | Daily | Daily | ||||||
| Family member | Home | Daily | Daily | ||||||
| Healthcare worker | Clinic | 6 times per week | 2 times per month | Yes | 59% (199/335) | Every two weeks | Unclear | 62% (100/162) | |
| Community health worker | Home | ||||||||
| Family member | Home | Daily | Daily | ||||||
| Family member | Home | Daily | Daily | Yes | Not reported | Monthly | Yes | Not reported | |
| Case manager or Hospital care | Hospital | Daily | Once per week | Yes | 94% (30/32) | Monthly unscheduled visit | Yes | 69% (22/32) | |
| 1In Kamolratanakul 1999 THA patients could choose which observer they preferred and there a more intense supervision of observers in the intensive phase. | |||||||||
| Trial ID | DOT at patient's home | DOT at clinic | |||||||
| Who observed? | How often? | Supervision of observer | Cure | Who observed? | How often? | Cure | |||
| Intensive phase | Consolidation phase | Intensive phase | Consolidation phase | ||||||
| Family member | Daily | Not described | Observers collected drugs from the clinic every 2 weeks | 55% (91/165) | Health worker | 6 times per week | Self‐supervised | 64% (108/170) | |
| Family member or former TB patient | Daily | Self‐supervised | Observers collected drugs from clinic weekly and spot checks were conducted by health worker | 43% (111/260) | Health worker | Daily | Self‐supervised | 43% (141/327) | |
| Lay health worker2 | 'Several times a week' | Not described | Observer collected drugs monthly | 57% (31/54) | Health worker | 5 times a week | 3 times a week | 41% (24/58) | |
| Community volunteer | Daily | Self‐supervised | Observer was visited every two weeks by the health worker and every month by the district co‐ordinator3 | 53% (117/221) | Health worker | Daily | Self‐supervised | 49% (148/301) | |
| 1In Lwilla 2003 TZA, Walley 2001 PAK and Wandwalo 2004 TZA observation was during the intensive phase, while in the clinic observation arm of Zwarenstein 2000 ZAF it continued in the consolidated phase. | |||||||||
| Trial ID | Who observed? | Where? | How often? | Additional intervention | Who observed? | Where? | How often? | ||
| Intensive phase | Consolidation phase | Intensive phase | Consolidation phase | ||||||
| Family member | Patient's home | Daily | Daily | Drugs supplied to supervisor every week | Community health worker | Patient's home1 | Daily | Daily | |
| Family member | Patient's home | Daily | Daily | Patient reviewed at the diagnostic centre once per month Recorded in a patient adherence card | Community health worker | Community health worker's home | Daily | Daily | |
| 1In Newell 2006 NPL the community health worker mainly visited the patients at their homes but occasionally the patients came to the health worker's home. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (negative sputum smear in last month of Rx in patients +ve initially) Show forest plot | 5 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.91, 1.27] |
| 2 Cure (by intensity of monitoring in control group) Show forest plot | 5 | 1645 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [1.00, 1.15] |
| 2.1 Monthly monitoring of patients in self administered group | 2 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.06, 1.25] |
| 2.2 Once every two weeks monitoring of patients in self‐administered group | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 2.3 Weekly monitoring of patients in self‐administered group | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.21] |
| 3 Treatment completion (both with smear sputum test at end and those without) Show forest plot | 6 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.19] |
| 4 Treatment completion (grouped by frequency of monitoring in the self‐administered therapy group) Show forest plot | 6 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.19] |
| 4.1 Monthly monitoring of self‐administered treatment | 3 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.31] |
| 4.2 Once every two weeks monitoring of self‐administered treatment | 1 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.14] |
| 4.3 Weekly monitoring of self‐administered treatment | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.74, 1.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially) Show forest plot | 4 | 1556 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.18] |
| 2 Treatment completion (both with smear sputum test at end and those without) Show forest plot | 3 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.17] |
| 3 Cure (stratified by intensity of observation) Show forest plot | 4 | 1556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.11] |
| 3.1 DOT (Intense supervision of observer) | 1 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 3.2 Routine supervision of DOT | 3 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (having a negative sputum smear test in the last month of treatment having been smear‐positive initially) Show forest plot | 2 | 1493 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.21] |
| 2 Treatment completion (both with smear sputum test at end and those without) Show forest plot | 2 | 1493 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.90, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment completion Show forest plot | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.88, 1.13] |

