Eritropoyetina humana recombinante versus placebo o ningún tratamiento para la anemia de la enfermedad renal crónica en pacientes que no requieren diálisis
Resumen
Antecedentes
El tratamiento con eritropoyetina humana recombinante (rHuEPO) en pacientes en diálisis ha demostrado ser altamente efectivo en cuanto a la corrección de la anemia y la mejoría de la calidad de vida. Existe polémica sobre los efectos beneficiosos del uso de la rHuEPO en pacientes en prediálisis, ya que puede acelerar el deterioro de la función renal. Sin embargo, el punto de vista opuesto es que si la rHuEPO es igual de efectiva en los pacientes en prediálisis y mejora su sensación de bienestar, puede retrasar el inicio de la diálisis. Ésta es una actualización de una revisión publicada por primera vez en 2001 y actualizada por última vez en 2005.
Objetivos
El objetivo de esta revisión fue determinar los efectos del tratamiento con rHuEPO en pacientes en prediálisis, principalmente en cuanto al momento de inicio de la diálisis; pero también si la rHuEPO en prediálisis: 1) corrige la hemoglobina/hematocrito (marcadores de anemia); 2) mejora la calidad de vida; y 3) no se asocia con una mayor incidencia de eventos adversos como aceleración del inicio de la diálisis, aumento de la hipertensión, coagulación de las fístulas arteriovenosas o convulsiones.
Métodos de búsqueda
Se realizaron búsquedas en el registro especializado del Grupo Cochrane de Riñón y Trasplante (Cochrane Kidney and Transplant Group) hasta el 29 julio 2015, mediante contacto con el coordinador de búsqueda de ensayos, utilizando términos de búsqueda relevantes para esta revisión.
Criterios de selección
Ensayos controlados aleatorizados (ECA) o ensayos controlados cuasialeatorizados que compararon el uso de la rHuEPO con ningún tratamiento o placebo en pacientes en prediálisis.
Obtención y análisis de los datos
Sólo se utilizaron datos publicados. Dos evaluadores realizaron la evaluación de la calidad de forma independiente. Un autor extrajo los datos en un formulario estándar y una muestra del mismo fue verificada por otro autor. Los resultados se presentaron como riesgos relativos (RR) o diferencias de medias (DM), con intervalos de confianza (IC) del 95%.
Resultados principales
Se incluyeron 19 estudios con 993 participantes. Debido a la antigüedad de los estudios incluidos (la mayoría realizados antes de 2000), se consideró que el riesgo de sesgo fue incierto en la mayoría de los estudios en la mayoría de los dominios. Hubo una mejoría significativa en la hemoglobina (DM 1,90 g/dl; IC del 95%: 2,34 a 1,47) y el hematocrito (DM 9,85%; IC del 95%: 8,35 a 11,34) con el tratamiento y una disminución en el número de pacientes que necesitaron transfusiones de sangre (CR 0,32; IC del 95%: 0,12 a 0,83). Los datos de los estudios que informaron la calidad de vida o la capacidad para realizar ejercicios demostraron una mejoría en el grupo de tratamiento. La mayoría de las medidas de progresión de la enfermedad renal no mostraron diferencias estadísticamente significativas. No hubo un aumento significativo en los efectos adversos.
Conclusiones de los autores
El tratamiento con rHuEPO en pacientes en prediálisis corrige la anemia, evita la necesidad de transfusiones de sangre y también mejora la calidad de vida y la capacidad para realizar ejercicios. No fue posible evaluar los efectos de la rHuEPO en la progresión de la enfermedad renal, el retraso del inicio de la diálisis o los eventos adversos. Sobre la base de la evidencia actual, los pronunciamientos sobre si los supuestos efectos beneficiosos en cuanto a la calidad de vida compensan los costes adicionales de la rHuEPO en prediálisis necesitan una evaluación cuidadosa.
PICOs
Resumen en términos sencillos
La eritropoyetina puede ayudar a los pacientes con insuficiencia renal y síntomas de anemia que aún no se encuentran en diálisis
La anemia (bajo nivel de los glóbulos rojos) es una complicación frecuente de la insuficiencia renal. La anemia provoca cansancio y algunos problemas asociados con insuficiencia renal. La eritropoyetina elaborada (una hormona que aumenta la producción de glóbulos rojos) mejora estos problemas y se utiliza en los pacientes en diálisis (tratamiento con un riñón artificial). Esta revisión encontró que la misma también puede reducir la anemia en los pacientes con insuficiencia renal que aún no se encuentran en diálisis. No se sabe si el uso de eritropoyetina puede retrasar la necesidad de diálisis.
Authors' conclusions
Background
Anaemia is often an associated condition in patients with chronic kidney disease (CKD). There is a direct relationship between the severity of the anaemia and the decline in kidney function (Koch 1991). This anaemia is a source of significant morbidity causing symptoms such as lack of energy, breathlessness, dizziness, angina, poor appetite and decreased exercise tolerance (Canadian EPO Study Group 1990; Lundin 1989). The main cause of this anaemia is a decreased production of erythropoietin, a naturally occurring hormone mainly produced by the kidney (Jensen 1994). Much of the impaired quality of life and morbidity suffered by patients with CKD may be a consequence of this anaemia and it may have a major impact on their sense of well‐being as well as impairing their ability to work and affecting their social and sexual lives. In the past, iron and folate were the main treatments for this condition and blood transfusions, with their associated risks of transmission of infection and induction of cytotoxic antibodies, which could jeopardise a future kidney transplant (Ward 1990), were used sparingly. In 1983 the cloning of the human gene for erythropoietin was achieved (Lin 1985), production of recombinant human erythropoietin (rHuEPO) followed and by 1986 the efficacy of rHuEPO treatment in dialysis patients was first demonstrated (Winearls 1986).
Although treatment with rHuEPO in dialysis patients has been shown to be highly effective in terms of correcting anaemia and improving quality of life (Canadian EPO Study Group 1990), there is debate concerning the benefits of rHuEPO use in predialysis patients (patients with CKD anticipated to start renal replacement therapy (RRT) in the near future). Nonetheless there is increasing use of erythropoietin in predialysis patients and professional guidelines are increasingly recommending its use. Anaemia may be a cause of significant morbidity in such patients and substantial numbers of the predialysis population may benefit from treatment with rHuEPO. Its use may enable patients to carry on working longer and, importantly, by improving the patients' sense of well‐being could delay the onset of dialysis. In a survey of 20 investigators in rHuEPO clinical trials and 250 randomly selected nephrologists it was estimated that by improving symptoms of anaemia, rHuEPO therapy could delay the initiation of dialysis for an average of 3.7 months (Sheingold 1990). This could have important cost implications which need to be taken into account in an economic evaluation should such a delay exist. The converse opinion, based on results from animal studies, is that rHuEPO may cause or worsen hypertension (Garcia 1985) and potentially cause an accelerated deterioration in kidney function hence bringing forward initiation of dialysis. Based on work with partially nephrectomised rats, Garcia 1985 suggested that anaemia may have a protective effect on the development of progressive kidney failure and could be an adaptive mechanism. One small clinical study suggested a similar detrimental effect of rHuEPO on kidney function (Muirhead 1994). Other adverse effects which have also been attributed to the use of rHuEPO are clotting of the arterio‐venous fistula which provides access to the circulation for haemodialysis, elevation of blood pressure and seizures (Koch 1991).
The debate about the effectiveness of treating predialysis patients with rHuEPO combined with the perceived increased cost of using this expensive drug may have led to a reluctance to use rHuEPO in this group of patients.
This review aimed to determine the effects of treating the anaemia of CKD with rHuEPO in predialysis patients. Studies evaluating newer erythropoietic stimulating agents (darbepoetin, CERA) have not been included as they are the subject of other reviews (Palmer 2012; Palmer 2014).
Objectives
The objective of this review was to ascertain the effects of rHuEPO treatment in predialysis patients primarily in terms of the timing of the onset of dialysis; but also that predialysis rHuEPO:
-
corrects haemoglobin/haematocrit (markers of anaemia);
-
improves quality of life;
-
is not associated with an increased incidence of adverse events such as hastening of the onset of dialysis, increased hypertension, clotting of arterio‐venous fistulae or seizures.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCT) or quasi‐RCTs (in which concealment of allocation of patients to treatment groups is less secure e.g. alternation where patients are consecutively allocated to different treatments) comparing rHuEPO treatment (experimental group) with either placebo or no rHuEPO (control group) in predialysis patients with renal anaemia were included in this review.
Types of participants
Patients with the anaemia of CKD who have not yet commenced dialysis were included. The definitions of anaemia and CKD used by each individual study were accepted. There were no age exclusions.
Types of interventions
Treatment with rHuEPO irrespective of dose or mode of delivery versus placebo or no rHuEPO were included.
Types of outcome measures
-
Measures of progression of kidney failure: time from start of rHuEPO to start of dialysis; numbers starting RRT in each group; glomerular filtration rate (GFR) at the end of the study; change in GFR; serum creatinine at the end of the study and change in creatinine in each group. Accepted methods for measurement of GFR were inulin clearance, any isotopic measure and formula based estimated GFR (e.g. MDRD and Cockcroft Gault).
-
Measures of correction of anaemia: haemoglobin/haematocrit values; numbers of blood transfusions.
-
Quality of life measures, including changes in exercise capacity.
-
Measures of hypertension: systolic blood pressure; diastolic blood pressure; numbers with an increase or introduction of antihypertensive treatment.
-
Other adverse events: numbers discontinued due to adverse events; access problems for patients commenced on haemodialysis; seizures.
-
Mortality.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register (to 29 June 2015) through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Specialised Register contains studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
-
Reference lists of review articles, relevant studies and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
All electronically‐derived citations and abstracts were read by two assessors. All studies, relevant to the use of erythropoietin in kidney failure which might possibly be an RCT or a quasi‐RCT, were identified. Assessment and identification was designed to be sensitive and not precise (specific). This was to avoid missing possible RCTs/quasi‐RCTs. The full published copies of all identified relevant studies were retrieved.
Data extraction and management
The full published hard copies of studies identified through electronic searching and by our other methods of study ascertainment were assessed for subject relevance, eligibility for inclusion in our review and methodological quality. A standard form, which recorded details of quality of randomisation (particularly security of concealment of randomisation), blinding, description of withdrawals/dropouts and numbers lost to follow‐up and whether intention‐to‐treat analysis was possible on the available data, was used for this quality assessment. If it was unclear from the published study whether it met the methodological inclusion criteria the authors were contacted. These studies remained excluded unless the authors confirmed that our eligibility criteria had been met. Assessment was undertaken by two assessors independently, one of whom was a clinician (nephrologist). If disagreement could not be resolved by discussion a third assessor made the final decision. The assessors were not blind to author, institution or journal. Where appropriate, studies were translated prior to methodological assessment.
Studies which met the criteria for methodological quality and subject relevance for this review passed to the stage of data abstraction. Data relevant to the pre‐stated outcome measures, the characteristics of the study, interventions and participants were abstracted by a single assessor on to a data abstraction form generated for this review. Data relevant to study methodology had already been abstracted onto the quality assessment form. These data were then, where appropriate, entered by a single researcher into Review Manager. No raw data were sought from the authors. Apart from translation by the authors and ascertainment of study methodology all data were obtained from the published paper or abstract.
Assessment of risk of bias in included studies
For the 2016 update all studies were reassessed using the risk of bias assessment tool (Appendix 2) (Higgins 2011).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Where appropriate, data were quantitatively combined using meta‐analysis to determine the typical effect of the intervention. We calculated a relative risk (RR) for dichotomous data and a mean difference (MD) or a standardised mean difference (SMD) for continuous data. A random effects model was used for analyses of both dichotomous and continuous data. Following the convention of the Cochrane Collaboration, all comparisons were framed in terms of unfavourable events such as adverse symptoms or death. RRs of less than one therefore favour the experimental treatment while RRs greater than one favour the control treatment. Ninety‐five per cent confidence intervals (95% CI) were derived for all comparisons.
Assessment of heterogeneity
Evidence of statistical heterogeneity across studies was explored using the Chi‐squared test for heterogeneity and the I2 test (Higgins 2003).
Data synthesis
Data which could not be combined quantitatively were presented in a narrative form.
Certain outcomes were expressed as negative values so that the estimated effect size would lie on the left side of the line of no effect. These outcomes were;
-
GFR: a higher GFR (mL/min) indicates better kidney function.
-
Haemoglobin and haematocrit levels: a higher haemoglobin (g/dL) or haematocrit level (%) is considered a beneficial outcome.
-
Quality of life measures: a higher quality of life measure is a beneficial outcome.
-
Change in exercise capacity: a higher score in change in exercise capacity (watts (W)) is a beneficial outcome.
Subgroup analysis and investigation of heterogeneity
Studies were also examined for methodological and clinical heterogeneity particularly if significant statistical heterogeneity was identified (Thompson 1994).
Results
Description of studies
Results of the search
2001 review
Twelve studies (Abraham 1990; Brown 1995; Clyne 1992; Eschbach 1989; Kleinman 1989; Kuriyama 1997; Lim 1989; Roth 1994; Stone 1988; Teehan 1989; Teehan 1991; Watson 1990) with a total of 232 participants, reported in published papers, one in abstract form (Brown 1995), were included in the review after methodological assessment. Four studies (Abraham 1990; Eschbach 1989; Lim 1989; Stone 1988) formed part of a larger multicentre study (Teehan 1991). If an outcome measure was common to both a small study and the multicentre study, only the results from the multicentre study were included in the meta‐analysis (see Characteristics of included studies); the smaller studies were analyses of individual centres’ data.
Ten studies were conducted in the USA, one in Sweden (Clyne 1992), and one in Japan (Kuriyama 1997). The majority of studies had few participants and were of short duration (8 to 12 weeks) not long enough to assess the effects on the progression of kidney disease. Only three studies (Brown 1995; Kuriyama 1997; Roth 1994) were of longer duration between 36 weeks and one year, however Brown 1995 only included 17 participants.
2005 review update
For the 2005 update of the review, three studies (Ganguli 2003; Teplan 2001b; Teplan 2003) were identified for inclusion, and 10 were excluded either because they did not fit the inclusion criteria of the review or it was unclear how patients had been allocated to groups. This brought the total number of included studies to 15. For the update some authors (Mignon 2001; Teplan 2003) were contacted to seek clarification on how patients were allocated to groups and for data which could be included in the review. Teplan confirmed patients were randomised and that Teplan 2001b was a separate sample of participants from Teplan 2003. No response was received for Mignon and this study was excluded as it appeared both groups received erythropoietin. Unfortunately no additional data were received from Ganguli 2003 and Teplan 2001b.
These new studies were conducted in India (Ganguli 2003) and the Czech Republic (Teplan 2001b; Teplan 2003). The number of participants ranged from 36 to 186 and the duration of the studies was six months to three years.
2016 review update
This update found an additional four studies (four reports) (Akizawa 1993; Kim 2006e; Kristal 2008; Wang 2004b). Three studies were only available as abstracts (Akizawa 1993; Kim 2006e; Wang 2004b). Therefore a total of 19 studies have been included in this review (Figure 1).

Study flow diagram.
These new studies were conducted in Japan (Akizawa 1993), Korea (Kim 2006e), Israel (Kristal 2008), and Hong Kong (Wang 2004b). The number of participants ranged from 40 to 107 and the duration of the studies was 12 weeks to 21 months.
Because the benefit of EPO therapy is now established for patients with CKD not yet on dialysis, further updates of this review with the addition of studies comparing EPO with placebo or no treatment are unnecessary and therefore this review will no longer be updated.
Risk of bias in included studies
The risk of bias was reassessed for 13 studies; for the six studies only reported as a conference abstract (Akizawa 1993; Brown 1995; Ganguli 2003; Kim 2006e; Teplan 2001b; Wang 2004b) the risk of bias was not assessed resulting in blank rows in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The empty rows relate to abstract‐only publications ‐ risk of bias could not be assessed
Allocation
Random sequence generation was at low risk of bias in two studies (Lim 1989; Watson 1990) and unclear in 11 studies
Allocation concealment was at low risk of bias in three studies (Lim 1989; Teplan 2003; Watson 1990), high in Kristal 2008 (patients allocated according to visit to clinic), and unclear in nine studies.
Blinding
Performance bias was assessed as high in five studies; two explicitly stated there was no blinding of patients or health care providers (Clyne 1992; Roth 1994), and three studies randomised the control group to no treatment (Kristal 2008; Kuriyama 1997; Teplan 2003). Six studies were assessed as being at low risk of performance bias (Kleinman 1989; Lim 1989; Stone 1988; Teehan 1989; Teehan 1991; Watson 1990) and performance bias was unclear in two studies (Abraham 1990; Eschbach 1989).
Detection bias was low in two studies (Kristal 2008; Teplan 2003) and unclear in the remaining 11 studies.
Incomplete outcome data
Five studies (Clyne 1992; Kleinman 1989; Kuriyama 1997; Lim 1989; Roth 1994) mentioned the numbers and reasons for withdrawals or dropouts. Attrition bias was low in five studies (Abraham 1990; Eschbach 1989; Kuriyama 1997; Teplan 2003; Watson 1990), high in three studies (Clyne 1992; Kristal 2008; Roth 1994) and unclear in five studies.
Selective reporting
Reporting bias was low in two studies (Abraham 1990; Eschbach 1989), high in three studies (Clyne 1992; Kristal 2008; Teehan 1989), and unclear in eight studies.
Other potential sources of bias
Other potential biases were high (industry funding) in seven studies (Abraham 1990; Eschbach 1989; Kleinman 1989; Roth 1994; Stone 1988; Teehan 1991; Watson 1990) and unclear in six studies.
Effects of interventions
Measures of progression of kidney failure
Number of patients starting renal replacement therapy during study period
Five studies (Brown 1995; Clyne 1992; Kuriyama 1997; Roth 1994; Stone 1988) with a total of 207 patients recorded data on the number of patients starting RRT during the study period. Overall the numbers starting dialysis (Analysis 1.1.1 (5 studies, 207 participants): RR 0.70, 95% CI 0.43 to 1.14; I2 = 24%) showed no evidence of a difference between the two groups. The confidence interval is sufficiently wide for a clinically and economically important difference to exist. There was no evidence of heterogeneity. The different lengths of study duration should be considered when interpreting these data. The study periods ranged from 8 to 10 weeks for three studies (Brown 1995; Clyne 1992; Stone 1988), 48 weeks for Kuriyama 1997 and Roth 1994, and 36 months for Teplan 2003. In the study by Teplan 2003 there was no mention of anyone progressing to dialysis presumably because their progression of kidney failure was less advanced (serum creatinine 2.79 ± 0.97 mg/dL).
Akizawa 1993 reported that the proportions of patients with deteriorating kidney function did not differ between groups.
Time to commencement of dialysis
Only Roth 1994 reported data on time from start of rHuEPO therapy to onset of dialysis. The time to start of dialysis for patients receiving rHuEPO (43) compared with those not receiving rHuEPO (40) was not statistically significant at conventional levels, Kaplan ‐ Meier survival curves (Log‐rank test P = 0.99).
GFR at the end of study
Seven studies (Abraham 1990; Clyne 1992; Kristal 2008; Roth 1994; Teplan 2003; Wang 2004b; Watson 1990) compared GFR in a manner that allowed the data to be combined (i.e. reported both means and standard deviations). The overall estimate of effect favoured treatment and while this was statistically significant it was not clinically significant (Analysis 1.2 (7 studies, 283 participants): (MD ‐2.11 mL/min, 95% CI ‐3.08 to ‐1.15; participants = 283; studies = 7; I2 = 7%).
Reduction in GFR
Roth 1994 recorded change in GFR. Of the original 83 participants reduction in GFR was only available for 28 participants at the end of the study. The result was not statistically significant (Analysis 1.3: MD of ‐0.70 mL/min, 95% CI ‐3.20 to 1.80).
Serum creatinine at end of study
Eight studies (Abraham 1990; Brown 1995; Clyne 1992; Eschbach 1989; Kristal 2008; Kim 2006e; Teplan 2003; Watson 1990) measured serum creatinine at the end of the study period in a way that could be incorporated into the meta‐analysis. There was no statistically significant difference between the groups (Analysis 1.4 (8 studies, 327 participants): MD 27.86 µmol/L, 95% CI ‐32.04 to 87.76; participants = 327; studies = 8; I2 = 82%). There was significant heterogeneity between studies; differences in baseline serum creatinine were not taken into account in this analysis which may have accounted for this.
Change in serum creatinine from baseline
Only Roth 1994 reported change in serum creatinine in a form which could be entered for analysis. Although this showed a significantly greater rise in creatinine at the end of the study with participants receiving rHuEPO (Analysis 1.5 (1 study, 83 participants): MD 114.92 μmol/L, 95% CI 37.83 to 192.01), other included studies reported data which were not consistent with this finding. Teehan 1991 reported mean change in serum creatinine without standard deviations and found no significant difference between participants treated with rHuEPO and those receiving placebo (P > 0.05). Kleinman 1989 recorded the change in kidney function by measuring the change in the slope of the inverse of serum creatinine over time and concluded that there was no statistical difference between those receiving rHuEPO and the controls (P = 0.83). Eschbach 1989 reported the inverse of serum creatinine level multiplied by 100 as the measure of change in kidney function. The slopes were determined for each patient and the pre‐ and post‐treatment slopes compared. There was no significant change (P = 0.78) in the rate of decline of kidney function after a median of 12 months of rHuEPO therapy. Teehan 1991 measured the inverse of serum creatinine as a function of time for 83 participants in whom serum creatinine values were available for at least two months before and after the study; the slopes did not increase after initiation of rHuEPO therapy.
Measures of correction of anaemia
Haemoglobin at the end of the study
Four studies (Clyne 1992; Kristal 2008; Teplan 2003; Wang 2004b) reported haemoglobin at the end of the study. rHuEPO significantly increased Hb compared to placebo or no treatment (Analysis 1.6 (4 studies, 237 participants): MD 1.90 g/dL, 95% CI 1.47 to 2.34; I2 = 30%).
Haematocrit at the end of the study
Seven studies (Abraham 1990; Eschbach 1989; Kuriyama 1997; Lim 1989; Stone 1988; Teehan 1989; Watson 1990) reported haematocrit levels at the end of the study period in a manner that could be combined in a meta‐analysis. rHuEPO significantly improved haematocrit compared to placebo or no treatment (Analysis 1.7 (7 studies, 145 participants): MD 9.85%, 95% CI 8.35 to 11.34; I2 = 20%). Three additional studies measured haematocrit levels results of which could not be used in the meta‐analysis. Kleinman 1989 presented data without standard deviations and showed at the end of the study that a haematocrit of 35.8% was achieved for those treated with rHuEPO compared with 28.3% for the placebo group (P = 0.004). Roth 1994 noted mean haematocrit (again no standard deviations) were available. He reported, however, that correction of anaemia to at least 36% was achieved in 34/43 participants receiving rHuEPO compared with none in the control group. Teehan 1991 demonstrated that 90% of participants treated with 150 units rHuEPO/kg of body weight, 79% who received 100 units and 57% receiving 50 units responded to therapy (defined as an increase of six percentage points in haematocrit) compared with a response rate of 10% in the placebo group. This response rate of 10% was based on an increase in haematocrit, on a single occasion in three placebo participants.
Change in haemoglobin or haematocrit levels
No study recorded change in haemoglobin or haematocrit in a form that could be used in a meta‐analysis and no study recorded data on haemoglobin values one to three months after commencement of RRT.
Number of participants requiring blood transfusions
Three studies (Kleinman 1989; Lim 1989; Roth 1994) reported the number of participants who required blood transfusions. the number requiring blood transfusions in the rHuEPO group was significantly less than those in the placebo or no treatment group (Analysis 1.8 (3 studies, 111 participants): RR 0.32, 95% CI 0.12 to 0.83; I2 = 0%).
Quality of life measures at the end of the study period
Only Kleinman 1989 recorded data on quality of life in a manner suitable for analysis (reported means and standard deviations). Quality of life was assessed by asking participants to:‐
-
"Rate your energy level during the past week"
-
"Judge your ability to do work during the previous week"
-
"Rate your overall quality of life during the past week"
Participants treated with rHuEPO had a better quality of life after 12 weeks. There was evidence of a statistically significant difference between the two groups favouring treatment with rHuEPO (Analysis 1.9: MD 35.00, 95% CI 12.47 to 57.53).
Roth 1994 used a health‐related quality of life (HRQL) assessment carried out at 16, 32 and 48 weeks. Scales from the Sickness Impact Profile (SIP) were used. Four scales taken from the Medical Outcomes Study Short Form and other Medical Outcome Study measures, which have demonstrated acceptable validity and reliability, were also included. During the 48 weeks of follow up the control group showed a significant decrease in physical function (P = 0.03); those receiving rHuEPO showed significant increases in energy (P = 0.045) and physical function (P = 0.015). The results from Kleinman 1989 and Roth 1994 could not be combined as different measures were used. Lim 1989 reported that all 11 participants who received rHuEPO experienced an increased sense of well‐being, felt more energetic and were more able to perform their work. Ganguli 2003 reported a significant improvement in quality of life and work capacity in those receiving EPO.
Change in exercise capacity
Only data from Clyne 1992 could be entered in the analysis. A standardised exercise test was performed using a bicycle ergometer. Clyne 1992 demonstrated a marginally significant result favouring treatment with rHuEPO (Analysis 1.10: MD 46.00 W, 95% CI 1.27 to 90.73). Participants in Teehan 1991 completed questionnaires before and after the study, rating their energy levels and ability to do work during the previous week. Teehan 1991 concluded that correction of anaemia was associated with significant improvements in participants' energy levels.
Measures of blood pressure control
Blood pressure measurements
Clyne 1992 measured systolic blood pressure at the end of the study. The point‐estimate favours treatment, however the result was not statistically significant (Analysis 1.11: MD ‐11.00 mm Hg, 95% CI ‐25.95 to 3.95). Abraham 1990 recorded mean arterial blood pressure and showed no evidence of a significant difference between the groups. Kleinman 1989 measured changes in systolic blood pressure but no standard deviations were reported and hence the data could not be used in the analysis; the investigators however reported no evidence of a significant increase in blood pressure for several months after the study although several participants had begun dialysis at that time. An analysis by Teehan 1991 showed no statistically significant difference either in systolic or diastolic blood pressure at the end of the study. He also reported that no medically significant change occurred in any treatment group. Watson 1990 reported that there were no major problems with blood pressure. No study reported change in systolic blood pressure in a form that could be entered for analysis. Roth 1994 however, reported that the change in systolic blood pressure during the study was not significantly different between the group receiving rHuEPO and the control group (P = 0.67). Diastolic blood pressure at the end of the study was reported by Clyne 1992 and showed a lower diastolic blood pressure at the end of the study in those receiving rHuEPO but the difference was not significant (Analysis 1.12: MD ‐5.00, 95% CI ‐12.39 to 2.39).
Antihypertensive treatment
Seven studies reported data on numbers of participants in whom there was an increase in or introduction of antihypertensive treatment. Data in Abraham 1990, Lim 1989 and Stone 1988 formed a subset of those in the multicentre study of Teehan 1991, and hence only Teehan 1991 data were used in the meta‐analysis. Of the four remaining studies (Clyne 1992; Kleinman 1989; Roth 1994; Teehan 1991) three showed that there was a more frequent need to increase or introduce antihypertensive treatment in participants receiving rHuEPO. The overall estimate of effect was not statistically significant (Analysis 1.13 (4 studies, 232 participants): RR 1.26, 95% CI 0.76 to 2.11; I2 = 50%), however the CIs were sufficiently wide enough to include a clinically important difference. There was some evidence of heterogeneity between the studies however this was not significantly different (P = 0.11). Eschbach 1989 recorded that 11 participants required an increase in or initiation of antihypertensives but the study did not report to which groups the participants belonged. Clyne 1992 reported that in 4/12 participants receiving rHuEPO therapy, rHuEPO treatment was stopped until blood pressure was controlled. It is worth noting that in the earlier studies higher doses of rHuEPO were used than is used in current practice.
Other adverse events
Discontinued treatment due to adverse events
Four studies (Kleinman 1989; Roth 1994; Teehan 1991; Watson 1990) recorded numbers of participants discontinuing treatment due to adverse events including sepsis, myocardial infarction, nausea and vomiting, and suspicion of acceleration of kidney failure. There was no statistically significant difference between the treatment and control groups (Analysis 1.14 (4 studies, 223 participants): RR 0.86, 95% CI 0.28 to 2.59; I2 = 17%). Eschbach 1989 and Roth 1994 both reported "no adverse events attributable to rHuEPO therapy".
Access problems for patient commenced on haemodialysis
Kleinman 1989 was the only study to report on venous access (arterio‐venous fistula/synthetic graft) problems. Two of seven participants in each group had either an arterio‐venous fistula or bovine graft in preparation for haemodialysis and there were no clotting episodes.
Seizures
Three studies reported seizures (Kleinman 1989; Teehan 1991; Watson 1990). Teehan 1991 and Watson 1990 reported one seizure in each of their studies in the control group and Kleinman 1989 reported there were no seizures during the course of their study (Analysis 1.15 (3 studies, 140 participants): RR 0.22, 95% CI 0.02 to 1.94; I2 = 0%).
Mortality
Mortality was reported in four studies (Kleinman 1989; Kuriyama 1997; Roth 1994; Stone 1988). Kuriyama 1997, Roth 1994 and Stone 1988 recorded data on mortality within their studies, however, the wide confidence interval reflects the small number of events (six deaths in total). Kleinman 1989 reported there were no deaths during the course of the study (Analysis 1.16 (4 studies, 182 participants): RR 0.60, 95% CI 0.13 to 2.88; I2 = 0%).
Discussion
This review has generated no clear evidence that rHuEPO treatment has either a beneficial or adverse effect on the progression of CKD or on the timing of initiation of dialysis. Though a formal meta‐analysis, of quality of life and exercise capacity measures were not possible, all the studies reporting these outcomes demonstrated statistically significant differences favouring treatment with rHuEPO. There was no statistically significant result as regards deterioration in blood pressure control or seizure necessitating withdrawal of treatment, however study duration was short and the confidence intervals wide. The combined results from two studies (Teehan 1991; Watson 1990) provide no evidence that rHuEPO treatment was associated with seizures although the short duration of most of the studies resulted in very few events. The meta‐analysis of four studies did not indicate a significant increase in the number of participants in whom antihypertensives were introduced, although as the confidence interval is wide this could not be ruled out. The increase in the use of antihypertensives in some of the studies could have been as a result of the higher doses of rHuEPO used in some of the earlier studies, lower doses are now used. The review also generated convincing evidence that predialysis rHuEPO does correct renal anaemia and reduces blood transfusion requirements.
Left ventricular hypertrophy (LVH) was not one of the predetermined outcomes of this review. Though it may be an independent predictor of mortality in participants with CKD (Harnett 1995; Levin 1996) it is clearly a surrogate marker. There is no convincing evidence that reversing or delaying the progression of LVH has an impact on mortality, morbidity or quality of life. If such evidence becomes available it would support LVH becoming an outcome measure in future RCTs and systematic reviews in this area.
GFR was statistically significantly higher at the end of the study period in the rHuEPO treated group. This result was driven mainly by Teplan 2003 included in this first update. The difference of 2.5 mL/min may however not be clinically significant. In addition the numbers are still small and the GFR was higher at the beginning of Teplan 2003 in the rHuEPO group and when we calculated the relative risk this difference was statistically significant. This imbalance was not incorporated into the meta‐analysis.
It is regrettable that none of the included RCTs continued follow‐up beyond commencement of dialysis. It is possible that participants whose haemoglobin/haematocrit has been partially corrected by rHuEPO may have less morbidity, less hospitalisation, less initial rHuEPO requirements and consume less health care resources around the period of dialysis commencement.
A number of characteristics of the included studies make it difficult to demonstrate either clear evidence of benefit or lack of benefit of predialysis rHuEPO. Many of the studies were of short duration not long enough to assess the effects on the progression of kidney disease and included small numbers of randomised participants. Participants were not equally distributed between treatment and placebo groups in 7/15 studies (Clyne 1992; Eschbach 1989; Kuriyama 1997; Lim 1989; Roth 1994; Stone 1988; Teehan 1991). The way data were reported in many of the studies included in this review (without means or measures of dispersion) made incorporation into a meta‐analysis impossible and thus the statistical power of combined patient numbers could not always be utilised. Teplan 2003 was one of the largest studies and longest duration of 36 months, and contributed to the statistically significant result in the GFR favouring EPO, although clinical significance is questionable. There was no mention of any of the participants progressing to dialysis, presumably because their kidney failure was less advanced, a follow‐up study of these participants to start of dialysis would be interesting.
With the exception of two studies (Brown 1995; Kuriyama 1997) which included participants with diabetes, the studies generally excluded participants with significant comorbidity. The results of this review may not be generalisable to the present predialysis patient population.
This review also demonstrated the potential pitfall of double‐counting randomised participants in systematic reviews when some individual centres in a multi‐centre RCT publish their data separately in addition to their inclusion in the full report of the complete RCT. All the reports from multi‐centre RCTs should clearly describe the involvement of all centres and highlight any previous or potential future publications derived from the RCT.
In summary there was a marked improvement in measures of anaemia with the treatment and a decrease in the number of participants requiring blood transfusion. Though difficult to summarise, the data from all studies which reported quality of life or exercise capacity demonstrated an improvement in the rHuEPO group. Only one of the measures of progression of kidney disease (GFR) (when a summary statistic was calculated) demonstrated a statistically significantly difference but with questionable clinical significance. Hence there is no evidence from this review of the hypothesised delay in requirement for RRT or of a further deterioration in kidney function attributable to rHuEPO treatment. The requirement for antihypertensive treatment appears not to be increased by rHuEPO therapy and there was no other statistically significant increase in adverse events.
The addition of four studies in 2016 did not alter the conclusions of the review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The empty rows relate to abstract‐only publications ‐ risk of bias could not be assessed

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 1 Number starting RRT.
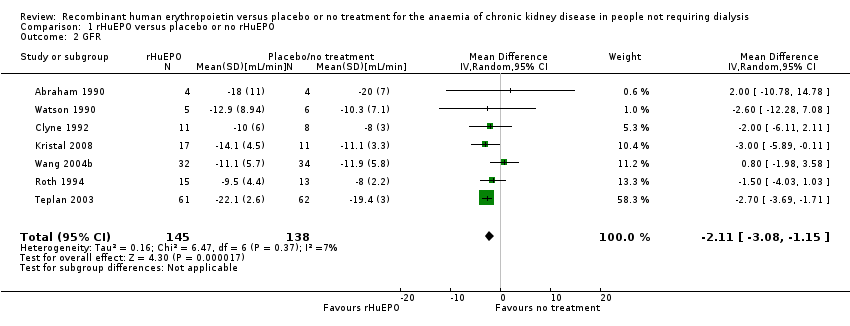
Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 2 GFR.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 3 Reduction in GFR.
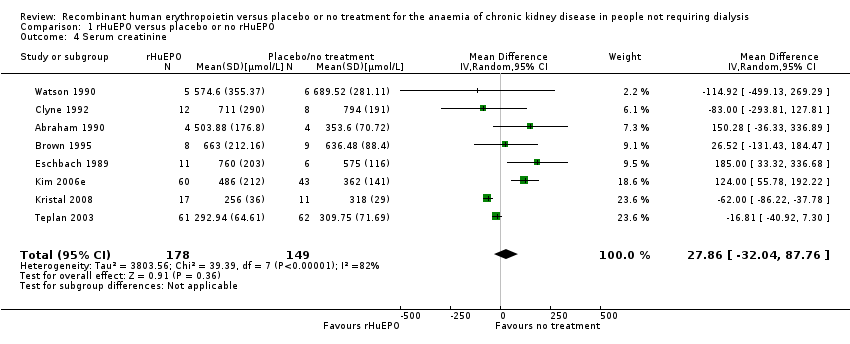
Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 4 Serum creatinine.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 5 Increase in serum creatinine.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 6 Haemoglobin.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 7 Haematocrit.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 8 Number of patients transfused.
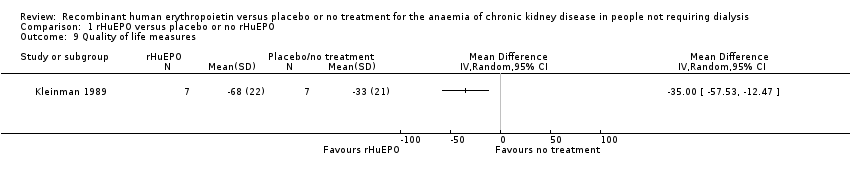
Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 9 Quality of life measures.
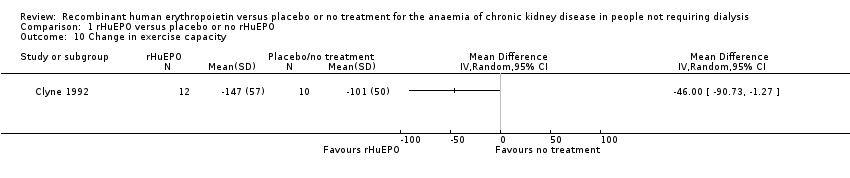
Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 10 Change in exercise capacity.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 11 Systolic blood pressure.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 12 Diastolic blood pressure.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 13 Number with an increase or introduction of antihypertensive treatment.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 14 Number discontinued due to adverse events.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 15 Seizures.
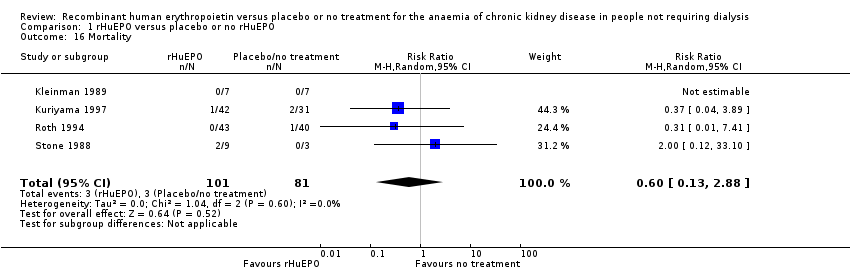
Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 16 Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number starting RRT Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Starting RRT during the study period | 5 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.43, 1.14] |
| 1.2 Starting RRT in the follow‐up to the study | 1 | 8 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.50, 17.95] |
| 2 GFR Show forest plot | 7 | 283 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐3.08, ‐1.15] |
| 3 Reduction in GFR Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Serum creatinine Show forest plot | 8 | 327 | Mean Difference (IV, Random, 95% CI) | 27.86 [‐32.04, 87.76] |
| 5 Increase in serum creatinine Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Haemoglobin Show forest plot | 4 | 237 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.34, ‐1.47] |
| 7 Haematocrit Show forest plot | 7 | 145 | Mean Difference (IV, Random, 95% CI) | ‐9.85 [‐11.34, ‐8.35] |
| 8 Number of patients transfused Show forest plot | 3 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.83] |
| 9 Quality of life measures Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Change in exercise capacity Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Systolic blood pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Diastolic blood pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Number with an increase or introduction of antihypertensive treatment Show forest plot | 4 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.76, 2.11] |
| 14 Number discontinued due to adverse events Show forest plot | 4 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.28, 2.59] |
| 15 Seizures Show forest plot | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.02, 1.94] |
| 16 Mortality Show forest plot | 4 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.13, 2.88] |

