Eritropoyetina humana recombinante versus placebo o ningún tratamiento para la anemia de la enfermedad renal crónica en pacientes que no requieren diálisis
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Co‐interventions (both groups)
Duration of study: 8 to 12 weeks until goal HCT of 40% (males) or 37% (females) | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear, reported to be randomised however method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear, states "double‐blind placebo‐controlled..." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All patients completed the first phase of the study |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | "Financial support and erythropoietin was provided by Ortho Pharmaceutical Corporation, Raritan, N.J., USA" |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Duration of study: 1 year | |
| Outcomes |
| |
| Notes |
| |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Iron supplementation
Duration of treatment: 12 weeks | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | "Open randomised parallel‐group study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Attrition was 1/12 (8%) in the epoetin beta arm and 2/10 (20%) in the control arm. As this was > 10% overall this was judged to be high risk |
| Selective reporting (reporting bias) | High risk | Major cardiovascular outcomes were not available |
| Other bias | Unclear risk | Unable to determine |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Patients and physicians blinded were blinded to the identify of the study medication but not the dose |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All patients completed the short phase study |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | High risk | Portions of this study were funded by research grants from National Institutes of Health and Ortho Pharmaceutical Corporation. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
Duration of study: 6 months | |
| Outcomes |
| |
| Notes |
| |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Study said to continue for further 21 months but unclear whether all patients received EPO after 3 months | |
| Outcomes |
| |
| Notes |
| |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Iron administered
Duration of study: 12 weeks or until HCT of 38% or 40% | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 patient withdrew from EPO group |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | High risk | Ortho Pharmaceutical Corporation was an author on the paper |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
All patients received oral maintenance iron supplementation, calcium bicarbonate, statins, beta‐blockers, and calcium channel blockers | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | High risk | Patients allocated in order according to visit to clinic (information from authors) |
| Blinding of participants and personnel (performance bias) | High risk | No blinding; open label |
| Blinding of outcome assessment (detection bias) | Low risk | Relevant outcomes were laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 12/40 (30%) did not complete study (3/20 in treatment group and 9/20 in control group) |
| Selective reporting (reporting bias) | High risk | No report of adverse effects |
| Other bias | Unclear risk | Unable to determine |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Iron: at the investigators discretion | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Control group received no treatment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Unclear risk | Unable to determine |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Iron administered: ferrous sulphate 300 mg orally 3 x day | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Third party |
| Allocation concealment (selection bias) | Low risk | Adequate, third party |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Unable to determine |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Unclear risk | Unable to determine |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Iron administered: investigators discretion | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | Nor reported |
| Incomplete outcome data (attrition bias) | High risk | 23/43 in the EPO group and 25/40 in the control group did not complete the study |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | High risk | Funded by Ortho Biotech |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Iron administered: ferrous sulphate 300 mg 3 times/day Folate administered: 1 mg daily Duration of study: 8 weeks | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind, placebo controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Unable to determine |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | High risk | Funded by Ortho Pharmaceutical |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Data for cardiovascular outcomes not available |
| Other bias | Unclear risk | Unable to determine |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Folate administered: yes | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 106/117 completed the study |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | High risk | Part funded by Ortho Pharmaceuticals |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
Duration of study: 3 years | |
| Outcomes |
| |
| Notes |
| |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
Duration of study: 36 months | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Adequate; sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Unclear risk | Only biochemical parameters reported |
| Other bias | Unclear risk | Unable to determine |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Duration of study: 12 weeks or until target HCT of 38% is achieved | |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed by a third party |
| Allocation concealment (selection bias) | Low risk | Performed by a third party |
| Blinding of participants and personnel (performance bias) | Low risk | Stated double blind; placebo used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | High risk | Funded by Ortho Pharmaceuticals |
BP ‐ blood pressure; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; EPO ‐ erythropoietin; GFR ‐ glomerular filtration rate; Hb ‐ haemoglobin; HCT ‐ haematocrit; HDL ‐ high‐density lipoprotein; IV ‐ intravenous; LDL ‐ low‐density lipoprotein; M/F ‐ male/female; PCV ‐ packed cell volume; QoL ‐ quality of life; RCT ‐ randomised control trial; RRT ‐ renal replacement therapy; SC ‐ subcutaneous; SCr ‐ serum creatinine; SD ‐ standard deviation; SE ‐ standard error
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| All patients received EPO | |
| Comparing the same ESA derivative in different treatment arms | |
| Ineligible population | |
| All patients received EPO | |
| All patients received EPO | |
| All patients received EPO | |
| Not randomised | |
| All patients received EPO | |
| Compares early with late commencement of EPO | |
| All patients received EPO | |
| Unclear how patients allocated to groups. Written to authors for clarification | |
| All patients received EPO | |
| Haemodialysis patients | |
| All patients received EPO | |
| Ineligible population. Cardiac failure patients | |
| Compares EPO delta with EPO alpha | |
| Data only from EPO treated patients | |
| Randomised study. Not all participants were predialysis some had commenced dialysis | |
| Unclear if randomised. Wrote to authors all patients received EPO | |
| All patients received EPO | |
| All patients received EPO |
EPO ‐ erythropoietin
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number starting RRT Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 1 Number starting RRT. | ||||
| 1.1 Starting RRT during the study period | 5 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.43, 1.14] |
| 1.2 Starting RRT in the follow‐up to the study | 1 | 8 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.50, 17.95] |
| 2 GFR Show forest plot | 7 | 283 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐3.08, ‐1.15] |
| Analysis 1.2 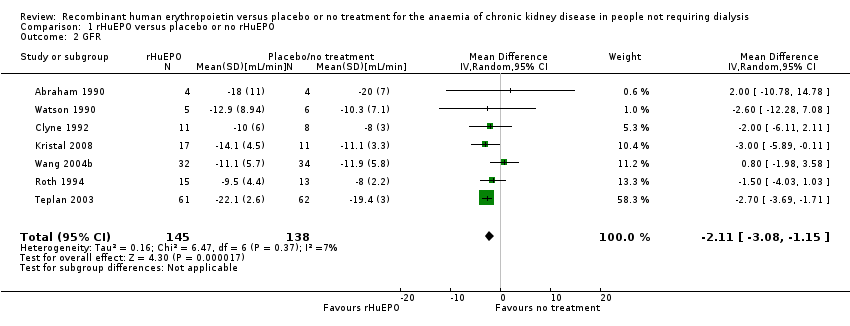 Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 2 GFR. | ||||
| 3 Reduction in GFR Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 3 Reduction in GFR. | ||||
| 4 Serum creatinine Show forest plot | 8 | 327 | Mean Difference (IV, Random, 95% CI) | 27.86 [‐32.04, 87.76] |
| Analysis 1.4 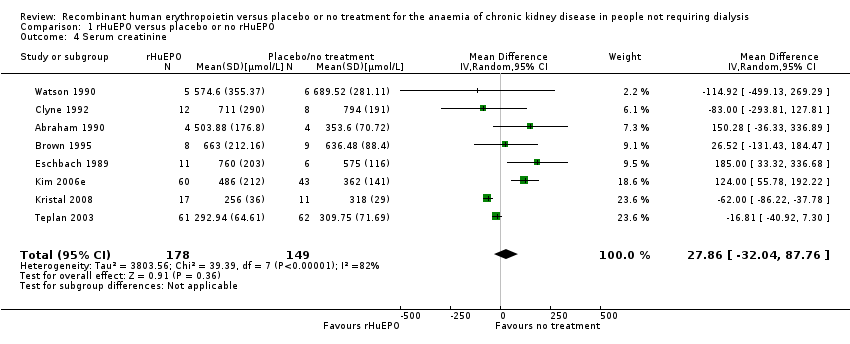 Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 4 Serum creatinine. | ||||
| 5 Increase in serum creatinine Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 5 Increase in serum creatinine. | ||||
| 6 Haemoglobin Show forest plot | 4 | 237 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.34, ‐1.47] |
| Analysis 1.6  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 6 Haemoglobin. | ||||
| 7 Haematocrit Show forest plot | 7 | 145 | Mean Difference (IV, Random, 95% CI) | ‐9.85 [‐11.34, ‐8.35] |
| Analysis 1.7  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 7 Haematocrit. | ||||
| 8 Number of patients transfused Show forest plot | 3 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.83] |
| Analysis 1.8  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 8 Number of patients transfused. | ||||
| 9 Quality of life measures Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.9 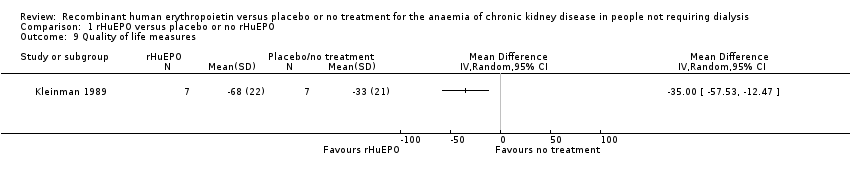 Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 9 Quality of life measures. | ||||
| 10 Change in exercise capacity Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.10 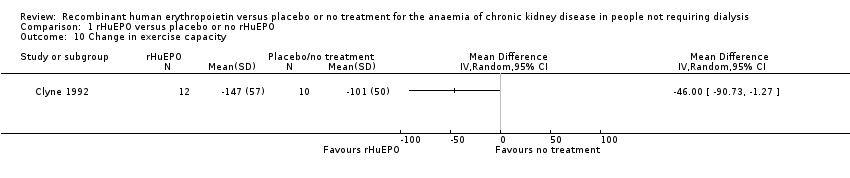 Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 10 Change in exercise capacity. | ||||
| 11 Systolic blood pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.11  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 11 Systolic blood pressure. | ||||
| 12 Diastolic blood pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 12 Diastolic blood pressure. | ||||
| 13 Number with an increase or introduction of antihypertensive treatment Show forest plot | 4 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.76, 2.11] |
| Analysis 1.13  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 13 Number with an increase or introduction of antihypertensive treatment. | ||||
| 14 Number discontinued due to adverse events Show forest plot | 4 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.28, 2.59] |
| Analysis 1.14  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 14 Number discontinued due to adverse events. | ||||
| 15 Seizures Show forest plot | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.02, 1.94] |
| Analysis 1.15  Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 15 Seizures. | ||||
| 16 Mortality Show forest plot | 4 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.13, 2.88] |
| Analysis 1.16 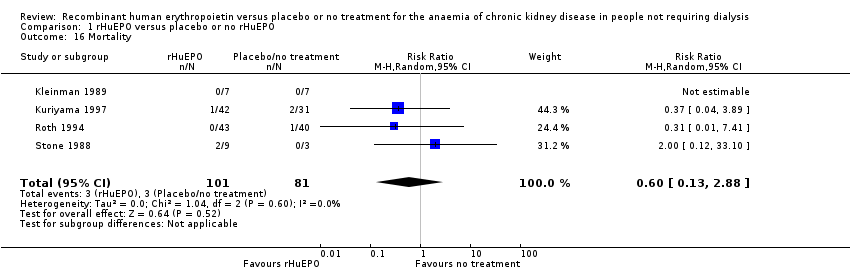 Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 16 Mortality. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The empty rows relate to abstract‐only publications ‐ risk of bias could not be assessed

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 1 Number starting RRT.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 2 GFR.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 3 Reduction in GFR.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 4 Serum creatinine.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 5 Increase in serum creatinine.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 6 Haemoglobin.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 7 Haematocrit.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 8 Number of patients transfused.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 9 Quality of life measures.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 10 Change in exercise capacity.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 11 Systolic blood pressure.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 12 Diastolic blood pressure.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 13 Number with an increase or introduction of antihypertensive treatment.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 14 Number discontinued due to adverse events.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 15 Seizures.

Comparison 1 rHuEPO versus placebo or no rHuEPO, Outcome 16 Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number starting RRT Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Starting RRT during the study period | 5 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.43, 1.14] |
| 1.2 Starting RRT in the follow‐up to the study | 1 | 8 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.50, 17.95] |
| 2 GFR Show forest plot | 7 | 283 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐3.08, ‐1.15] |
| 3 Reduction in GFR Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Serum creatinine Show forest plot | 8 | 327 | Mean Difference (IV, Random, 95% CI) | 27.86 [‐32.04, 87.76] |
| 5 Increase in serum creatinine Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Haemoglobin Show forest plot | 4 | 237 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.34, ‐1.47] |
| 7 Haematocrit Show forest plot | 7 | 145 | Mean Difference (IV, Random, 95% CI) | ‐9.85 [‐11.34, ‐8.35] |
| 8 Number of patients transfused Show forest plot | 3 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.83] |
| 9 Quality of life measures Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Change in exercise capacity Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Systolic blood pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Diastolic blood pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Number with an increase or introduction of antihypertensive treatment Show forest plot | 4 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.76, 2.11] |
| 14 Number discontinued due to adverse events Show forest plot | 4 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.28, 2.59] |
| 15 Seizures Show forest plot | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.02, 1.94] |
| 16 Mortality Show forest plot | 4 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.13, 2.88] |


