针对红斑痤疮的干预措施
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, prospective, active‐controlled, open‐label Date of study Unreported Setting | |
| Participants | Randomised: 67 participants (mean age 47.93 years (SD 14.18), 37 male, 30 female) Inclusion criteria
Exclusion criteria
Neither ocular involvement nor phymas Dropouts and withdrawals
Baseline data mean (SD) Lesion counts; azithromycin group 19.24 (9.67), doxycycline group 18.86 (8.95) | |
| Interventions | Three months Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, month 1, 2, 3, and 5 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 288): "The authors wish to acknowledge Pakhshe Razi Co. (Tehran, Iran) for providing azithromycin (azithromycin, 250 mg capsule, Chemiedaru)." | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) Skewed data for lesion counts See comparison 45 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 285): "Patients were allocated to the trial using a randomized numbers table in a one‐to‐one fashion" Comment: Probably done |
| Allocation concealment (selection bias) | High risk | Following extensive e‐mail contact with the investigators we were informed that the providers of care had access to the computer‐generated list Comment: We judged this as at high risk of bias |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 284): "....an open clinical trial." The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Quote (page 284): "....an open clinical trial." Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 9/67 (13.4%); 5 in azithromycin group, 4 in doxycycline group. Analysis followed ITT principle, withdrawals were balanced across groups, reasons were reported, all participants were accounted for and included in the analysis Comment: We considered this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration and wash‐out period adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, active‐controlled, double‐blind, within‐patient comparison Date of study January to July 2012 Setting | |
| Participants | Randomised: 16 participants (mean age 42 years (range 24 to 52), 8 male, 8 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Nothing reported | |
| Interventions | Six months Intervention
Comparator
| |
| Outcomes | Assessments (2): baseline, month 7 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 438): "Funded by the Northwestern University Department of Dermatology" | |
| Declaration of interest | Quote (page 438): "None declared" | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 63 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 439): "This was a randomized controlled split‐face study with allocation ratio 1:1, using random block size of 2" and "A random number generator was used to generate 0s and 1s, which were designated as left or right" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 440): "Each random assignment was sealed individually in an opaque, sequentially numbered envelope (M.A.). Assignments were made consecutively, with subjects receiving PDL to the left or right side of the face, and Nd:YAG laser to the contralateral side" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 440): "Subjects were blinded as to which facial side received which laser treatment. They were laser naive before the study, and both laser treatments were performed (N.V.) in the same room after subjects donned occlusive eye‐protective goggles. The investigator obtaining spectroscopy measurements (M.W.) was not present during treatments and blinded regarding allocation" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 2/16 (12.5%) dropped out reporting post‐treatment swelling. Per‐protocol analysis Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was available on clinicaltrials.gov (NCT01529996) and the pre‐specified primary outcome "rating on global improvement scale" has not been assessed, nor mentioned anymore in the methods section of present publication Comment: We judged this as at unclear risk of bias |
| Other bias | Low risk | Study duration adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Setting Dermatology Section, St Mary's ‐ Duluth Clinic Health System, Duluth, Minnesota, US | |
| Participants | Randomised: 44 participants (mean age 56.9 years (SD 12.9) in treatment group, 58.9 years (SD 11.9) in control group, gender unreported) Inclusion criteria:
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data mean (SD) Duluth Rosacea score; clarithromycin group 10.8 (3.5), placebo group 11.1 (4.2) | |
| Interventions | Two weeks Intervention
Comparator
| |
| Outcomes | Assessments (2): baseline, day 60 Outcomes of the trial (as reported) Primary outcomes
Method: Duluth Rosacea Scoring Instrument Secondary outcomes None ✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 663): "Astra Merck, Wayne, PA, provided the major funding for the study as well as omeprazole (Prilosec) and matching placebos. Abbott laboratories, North Chicago, Ill, supplied the clarithromycin. Cortecs Diagnostics Ltd, London, England, donated the Helisal Rapid Whole Blood Test. Meretek Diagnostics, Inc, Houston, Tex, donated the 13C urea breath tests." | |
| Declaration of interest | None reported | |
| Notes | None of our primary outcomes were addressed. Follow‐up 2 months; 25% in the treatment group tested positive still for Helicobacter pylori after treatment. For the N of pustules the data are quite skewed and for the total score very skewed See comparison 1 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 660): "Patients were randomly assigned to groups receiving active treatment or placebo. Dispensing of study medications according to a randomised registry list provided by the project programmer." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (660): "Treatment status was not disclosed to investigators, coordinators or patients throughout study." The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 660): "Double‐blind, placebo medication resembled active treatment." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator‐assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 2/44 (4.5%); 2 withdrawals in clarithromycin group, reasons reported Comment: Low number of dropouts at follow‐up, and although per‐protocol analysis considered to be at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, no wash‐out period described, groups treated equally Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship and support represented any additional bias |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study August 2006 to July 2008 Setting | |
| Participants | Randomised: 53 participants (mean age 47.3 years, 14 male, 39 female) Inclusion criteria
Exclusion criteria
Ocular involvement: Unclear Dropouts and withdrawals
Baseline data mean Rosacea severity; zinc group 6.30 (95% CI 5.83 to 6.76), placebo group 6.77 (95% CI 6.22 to 7.32) | |
| Interventions | Three months Intervention
Comparator
Subjects were required to refrain from using oral or topical treatments for rosacea while participating in the trial | |
| Outcomes | Assessments (2): baseline, month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 462): "thank the Duluth Clinic Foundation for grant support that made this study possible" | |
| Declaration of interest | Quote (page 459): "None declared" | |
| Notes | Two of our primary outcomes were addressed (quality of life and adverse events) See comparison 57 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 460): "Randomization was carried out following a sequence of random numbers using random block size created by a biostatistician and maintained at the research pharmacy of the healthcare organization" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 460): "Randomization was carried out following a sequence of random numbers using random block size created by a biostatistician and maintained at the research pharmacy of the healthcare organization" Comment: Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 459‐60): "double‐blind" and "Treatment was masked from participants, investigators, and study staff". Capsules probably of identical appearance Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 9/53 (17%); zinc group (5), placebo group (4), reasons reported. Per‐protocol analysis Comment: We judged this as at an unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT00395226). Only the primary outcome was listed in the protocol. The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, investigator‐blinded, within‐patient comparison Date of study Unreported Setting Department of Ophthalmology, Cleveland Clinic Foundation, Cleveland, US | |
| Participants | Randomised: 13 participants (mean age 72.8 years (range 40 to 90), 7 male, 6 female) Inclusion criteria
Exclusion criteria
Dropouts and withdrawal
Baseline data mean (SD) Eye and eyelid grading: metronidazole site 4.5 (1.1), control site 4.5 (1.0) | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline, week 6 and 12 Outcomes of the trial (as reported) Primary outcomes
Method: grading sheet (1 to 5) (higher score is worse) Pre‐treatment scores were compared with post‐treatment scores with respect to ocular surface, eyelid margin, and combined eyelid plus ocular surface Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Withdrawals were not included in the analysis by the review authors. Because it is a within‐patient study, patients can make errors with which eye to treat or treat both eyes. One of our primary outcomes was addressed (adverse events) See comparison 1 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1881): "One eye was assigned randomly to receive lid hygiene and warm compresses twice daily, while the other eye received lid hygiene and compresses twice daily." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 1881): "An observer who was masked to the treated and control eye completed a physician data sheet." Participants were not blinded Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes were investigator as well as participant‐assessed Quote (page 1881): "An observer who was masked to the treated and control eye completed a physician data sheet." Comment: We judged this at unclear risk of bias |
| Incomplete outcome data (attrition bias) | High risk | 3/13 (23%), reasons reported Comment: We considered this as at high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate Comment: The study appeared to be free of other forms of bias |
| Methods | Randomised, prospective, within‐patient comparison Date of study Unreported | |
| Participants | Randomised: 102 participants (mean age 41.6 years, 40 male, 62 female) Inclusion criteria
Ocular involvement: Unclear, probably not Exclusion criteria
Dropouts and withdrawals
Baseline data (number) CEA score 4 (severe); 0.07% group 5, 0.18% QD group 3, 0.18% BID group 5, 0.5% group 0 | |
| Interventions | Four weeks
Comparator 1
Comparator 2
Comparator 3
Each subject received one drop of brimonidine tartrate 0.2% ophthalmic solution in each eye every 8 hours over a 24 hour period, as proposed in the US prescribing information. After a 2 day wash‐out period they received the dermal applications as described above | |
| Outcomes | Assessments (47): day 1 (10x), after 2 days wash‐out day 4 (10x), 5, 10, 18 (10x), 19, 24 and 32 (13x) Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 162): "Funding for this study was provided by Galderma R&D, SNC. Funding for writing assistance was provided by Galderma Laboratories, L.P." | |
| Declaration of interest | Quote (page 162): "K. Benkali, F. Rony, R. Bouer, and N. Wagner are employees of Galderma R&D, Sophia Antipolis, France. M. Leoni, A. Fernando, and M. Graeber are employees of Galderma R&D, Princeton, NJ, USA" | |
| Notes | None of our primary nor secondary outcomes were addressed (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 163): "One hundred and two (102) subjects were randomly assigned to 1 of the 4 brimonidine gel regimens" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups After e‐mail communication: "Regarding the allocation sequence generated for the 4 subsequent groups consisting of different doses or regimen for topical applications, the randomization list was created before the study started, with a 1:1:1:1 ratio and block size of 4. This randomization list was generated by a designated biostatistician and was distributed to the clinical supply team in a sealed envelope" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: "This randomization list was generated by a designated biostatistician and was distributed to the clinical supply team in a sealed envelope" Comment: Adequate, probably done |
| Blinding of participants and personnel (performance bias) | High risk | No blinding reported |
| Blinding of outcome assessment (detection bias) | High risk | No blinding reported Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | 14/102 (13.7%); 6/102 during ophthalmic dosing, and an additional 8/102 during dermal dosing, unclear from which group, reasons unreported. Per‐protocol analysis Comment: We judged this as at an unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate Comment: The study appeared to be free of other forms of bias |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Between April and June 2009 Setting | |
| Participants | Randomised: 42 participants (mean age 39.8 years (range 20 to 60), 11 male, 31 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: None Baseline data mean Nothing reported | |
| Interventions | Four weeks
Comparator
| |
| Outcomes | Assessments (5): baseline, day 7, 14, 28 and 42 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed See comparison 41 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 38): " were randomized" After e‐mail communication: "according to a computer generated randomization list".. "with a 2:1 ratio using blocks of 3" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: Form of central allocation, "the randomization list was generated by the statistician and kept under lock and key until the data base lock, as usual" and "Patients were sequentially assigned to the next available randomization number, starting from the lowest number provided to each investigational site" Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 37): "double‐blind" After e‐mail communication: "placebo cream units, which were identical to the active product in terms of size, shape, volume, color. The tubes (P‐3075 and placebo) were identically labeled for clinical use as it is in a double‐blind procedure." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 37): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study After e‐mail communication: "placebo cream units, which were identical to the active product in terms of size, shape, volume, color. The tubes (P‐3075 and placebo) were identically labeled for clinical use as it is in a double‐blind procedure." Outcomes were investigator‐assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow up Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | No exact data were provided regarding assessment of sign and symptoms of rosacea, only generic comment Comment: We judged this as at an unclear risk of bias |
| Other bias | Low risk | Study duration adequate, no wash‐out period described, groups treated equally Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study March 2003 to January 2004 Setting Multicentre study in the US | |
| Participants | Randomised: 1299 participants (557 in metronidazole gel group, 553 in metronidazole cream group, and 189 in vehicle gel group) (mean age 48.4 ± 13.02 years, range 18 to 92 for metronidazole gel group; 48.3 ± 13.04 years, range 18 to 88 for metronidazole cream group; 47.8 ± 12.05 years, range 22 to 81 for vehicle gel group; sex 149 male, 408 female for metronidazole gel group; 143 male, 410 female in metronidazole cream group; and 48 male, 141 female in vehicle gel group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data (mean) Lesion count: metronidazole gel group (18.3), metronidazole cream group (18.1) vehicle group (18.4) | |
| Interventions | 10 weeks Intervention
Comparator 1
Comparator 2
| |
| Outcomes | Assessments (5): baseline, week 2, 4, 7 and 10 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 10): "Supported by Galderma R&D Inc." | |
| Declaration of interest | Page 10; Dr Beutner and Mr Calvarese are employees of Dow Pharmaceutical Sciences. Dr Graeber is an employee of Galderma R&D Inc | |
| Notes | One of our primary outcomes is addressed (adverse events) Poster presentation, after e‐mail contact extensive information has been provided by authors See comparison 1 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 10): "This was a multicenter, randomized, investigator‐blind, active and vehicle‐controlled, parallel comparison." After e‐mail contact with investigators we received additional information which enabled us to change the grading for this criterion from 'Unclear' to 'Yes' Quote: "Prior to the start of the study, a randomization list was supplied by the Sponsor. Drug supplies for the entire trial were numbered sequentially. The drug supplies for Metronidazole Gel 1%, Noritate Cream 1%, and Vehicle Gel were packaged according to the randomization list in blocks of 7 using a ratio of 3:3:1. Study drug supplies were distributed to each of the investigational sites in complete blocks in order to maintain the randomization ratio within an investigational site. A unique drug kit number was associated with each drug supply kit, and this corresponded to the subject number. These numbers were assigned sequentially as subjects entering the study at each investigational site." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence was not described in sufficient detail in the report E‐mail contact with the investigator confirmed "the randomization schedule remained blinded from those involved in the clinical conduct of the study until the database lock memo was issued" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. This was probably done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 10): "...investigator blind." E‐mail contact with the investigator confirmed "the study drugs were different in appearance. To protect the blinding, a study staff designee, other than the Investigator making evaluations, dispensed and collected study drug from subjects. Additionally, both the person in charge of study drug dispensation and the subject were instructed not to discuss the study treatment with the Investigator or other evaluator(s)". Participants were not blinded Comment: We judged this as at unclear risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 10): "...investigator blind." Comment: As the investigators were the outcome assessors the report was unclear how they were blinded Blinding of the outcomes assessors, key personnel, was ensured, and it was unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Number of participants unclear, dropouts not reported E‐mail contact with the investigator confirmed "57 (10.2%) discontinued in metronidazole gel group, 72 (13.0%) in metronidazole cream and 27 (14.3%) in vehicle gel group". Reasons for dropouts stated and ITT analysis LOCF Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, but unclear if there was a 'wash‐out' period, unclear if groups were treated equally E‐mail contact with the investigator confirmed "no financial arrangements have been made with any of the investigators. Each listed investigator was required to disclose to the sponsor whether the investigator had a proprietary interest in this product or a significant equity in the sponsor and none disclosed any such interests" Comment: We judged this as at a low risk of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Department of Dermatology, Hotel‐Dieu Hospital; University of Montreal, Montréal, Québec, Canada | |
| Participants | Randomised: 100 participants (mean age 50.3 years (SD 1.6) in treatment group, 50.8 years (1.9) in control group, 41 male, 59 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Number of papules; metronidazole group 8.1 (0.7), control group 8.9 (0.7) Number of pustules; metronidazole group 3.2 (0.4), control group 4.3 (0.6) | |
| Interventions | Two months Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline, month 1 and 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 248): "This study was supported by Rhône‐Poulenc Pharma Inc, Montréal, Canada" | |
| Declaration of interest | None declared | |
| Notes | We only included first 4 weeks (quality of the study declined after 4 weeks) One of our primary outcomes was addressed (adverse events) See comparison 1 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 243): "50 patients were randomly assigned to treatment with metronidazole 1% cream, while the other 50 patients received placebo cream." "Metronidazole 1% cream and placebo cream were randomly distributed." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 242): "...double‐blind." Quote (page 243): "Tubes were identical in appearance and creams were of same colour and consistency." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 242‐3): "...double‐blind." "Tubes were identical in appearance and creams were of same colour and consistency." Outcomes were investigator‐ and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 18/100 (18%); metronidazole group (8), control group (10) in second month, similar reasons reported and balanced across both groups. ITT analysis only first month Comment: No dropouts in first month and we only included data for the first month, therefore considered as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration was adequate, and participants on antibiotics or vasodilators were excluded. Compliance was assessed Quote (page 243): Concomitant medications which were "considered to be vital to the general health of the patients were permitted and noted", i.e. nonsteroidal anti‐inflammatory and antihypertensive agents. The dropout rate was high in the second month in both groups, and in the absence of an ITT analysis only data from the first month was entered into the RevMan analysis Comment: We considered this as at low risk of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting | |
| Participants | Randomised: 97 participants (mean age 47 years (range 18 to 77), 44 male, 53 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) No details reported | |
| Interventions | Two months Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline, week 4 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | Page 187, one of the investigators is employed by Dumex, the manufacturer of metronidazole. No conflict of interest declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 1 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 188): "The trial was a randomized...." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 188): "The trial was double‐blind." Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 188): "The trial was double‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Low risk | 4/97 (4.1%), ITT analysis. Reasons for withdrawals reported Comment: We considered this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Wash‐out period before study started unclear, no other local or oral treatment was allowed, study duration adequate, no sponsoring mentioned, however, study details are incomplete Comment: Insufficient information to assess whether important risk of bias exists |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, Dermatology Department, Haukeland Hospital, of Ullevål, and National Hosital (Rikshospitalet), Oslo, Norway | |
| Participants | Randomised: 116 participants (mean age 48.4 years in treatment group, 50.3 years in control group, 57 male, 59 female) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of inflammatory lesions; azelaic acid group 30.8, placebo group 31.7 | |
| Interventions | Three months Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, month 1, 2 and 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | One of the investigators was employed by Schering AG Berlin, Germany, the manufacturer of the azelaic acid cream. However, none declared | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 6 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 456): "The assignment of study medication was random." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 456): "double‐blind, parallel group comparison between azelaic acid 20% cream and its vehicle." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 456): "..double‐blind.." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Low risk | 8/116 (6.9%); azelaic acid group (5), placebo group (1), unclear from which group (2) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate. Unclear if there was a wash‐out period before study, unclear if groups were treated equally, no sponsoring mentioned Comment: Insufficient information to assess whether important risk of bias exists |
| Methods | RCT, prospective, placebo‐controlled, double‐blind, within‐patient comparison Date of study Unreported Setting Two centres, Department of Dermatology, Harvard Medical School; Department of Dermatology, Massachusetts General Hospital, Boston, US | |
| Participants | Randomised: 40 participants (mean age 48.7 years, 16 male, 24 female) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of lesions counts; 30.8 | |
| Interventions | Nine weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 614): "Study was funded, in part, by Curatek Pharmaceuticals, Elk Grove Village, Ill. The metronidazole gel and vehicle placebo used were also provided by Curatek Pharmaceuticals. Statistical analysis was performed by an independent statistical consultant." | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 1 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 610): "Patients were randomly assigned to receive either 0.75% metronidazole in a water based gel or the gel‐base alone to each half of the face." "Randomization by Curatek Pharmaceuticals, Elk grove Village, Ill." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 610): "Randomization by Curatek Pharmaceuticals, Elk grove Village, Ill." Comment: Appears to be a form of central randomisation, probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 609 and 610): "double‐blind" and "Identical appearing tubes, colour coded and labelled right and left containing active treatment or placebo." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 609‐10): "...double‐blind." and "Identical appearing tubes, colour coded and labelled right and left containing active treatment or placebo." Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 611 and 612): "Two patients did not complete the study. One discontinued after 2 days due to a flare‐up in rosacea related to withdrawal from his systemic antibiotic therapy. Data on this patient were not included in the results. A second patient withdrew at five weeks because of a severe unilateral flare‐up on the placebo‐treated side." Comment: Second patient was included in the analysis. We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate. Wash‐out period before study at least 3 weeks. Other treatments that might affect rosacea were required to be discontinued |
| Methods | Randomised, prospective, active‐controlled, double‐blind Date of study Setting Department of Dermatology, regional Hospital Örebro, Sweden | |
| Participants | Randomised: 40 (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean | |
| Interventions | Four weeks Intervention
Comparator
Unreported how many participants were randomised into each group | |
| Outcomes | Assessments (2): baseline and week 4 Outcomes of the trial (as reported) Primary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 359): "This work was supported by Essex Läkemedel AB" | |
| Declaration of interest | None declared | |
| Notes | Participants who failed to respond or got worse were switched to the alternative treatment, unclear who and how many. Lack of usable data and inability to trace the investigators (see Table 6) One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 358): "Patients were allocated to either regimen 1 or 2 according to a randomization code" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 358): "double‐blind study" Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 358): "double‐blind study" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Low risk | 3/40 dropouts, probably in lymecycline group, no reasons mentioned. Per‐protocol analysis Comment: Low number of dropouts and although per protocol analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | High risk | Only number of papules and pustules is addressed and not the other primary efficacy outcome measures Comment: We judged this as at a high risk of bias |
| Other bias | High risk | Participants who failed to respond or got worse were switched to the alternative treatment, unclear who and how many |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, setting not specified other than in Cincinnati, Ohio, US | |
| Participants | Randomised: 156 participants (mean age 48.5 years (SD 12.6) in treatment group and 46.9 years (SD 11.9) in control group, 51 male, 105 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Number of papules; metronidazole group 13, placebo group 15 Number of pustules; metronidazole group 2, placebo group 3 Baseline rosacea severity score; metronidazole group 2.10 (0.24), placebo group 2.16 (0.33) | |
| Interventions | 10 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 2, 4, 7 and 10 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Unclear, reprint requests "Dermik Laboratories Inc,", the manufacturer of metronidazole, but no source of funding reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed See comparison 1 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 44): "This was a double‐blind, randomized, parallel group clinical trial..." "Patients were randomly assigned..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 44): "This was a double‐blind, randomized, parallel group clinical trial comparing the efficacy of metronidazole 1% cream to vehicle." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 44) : "..double‐blind.." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Low risk | 17/156 (10.8%); metronidazole group (15), placebo group (2). Reasons reported. Per‐protocol analysis Comment: Double the number (104) patients were enrolled in the active treatment group compared to 52 in the vehicle group. The percentage of excluded patients in the treatment group was higher than in the vehicle group. Because far more people in this group took prohibited medication that could have influenced in a positive way the outcomes on rosacea, the review authors consider that this does not pose any threat to the validity of the results in this study Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate wash‐out period before the study. Adequate study duration. No medication allowed that might influence outcome Comment: The study appears to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Setting Multicentre, University Dermatology Consultants, Cincinnati; The Savin Centre, New Haven; Department of Dermatology, University of Pennsylvania School of Medicine, US | |
| Participants | Randomised: 53 participants (mean age 43.1 years (SD 11.7) in treatment group and 45.7 years (12.9) in control group, 8 male and 18 female in treatment group, 9 male and 17 female in control group) Inclusion criteria
No ocular rosacea Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Number of papules and pustules; treatment group 17.7 (9.7), vehicle group 19.3 (11.4) | |
| Interventions | Twelve weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
Leyden 2004 ‐ same study, different outcome measures. Overall global improvement as rated by 3 independent investigators using photographs ✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 381): "This study was supported by Dermik Laboratories, a division of Aventis Pharmaceuticals Inc, Berwyn, PA", Dermik Laboratories is the manufacturer of BenzaClin® | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) Some SDs are lacking, and most data are skewed. This also applies to Leyden 2004 See comparison 19 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 382): "Patients were randomly assigned in a 1:1 ratio...... Randomization was performed according to a computer generated random code." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 382): "Treatments were identified by a code number, which was assigned in chronological order at each site." Comment: Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 382): "BP/C gel and vehicle only gel were supplied in identical jars and were indistinguishable in color, texture, and smell. Both were packaged in identical patient kits with indistinguishable labelling." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 17/156 (10.8%); metronidazole group (15), placebo group (2). ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | No information about sponsorship or support was reported. Wash‐out period before study unreported, nor if other medications were recorded or allowed that might influence the outcomes Comment: Insufficient information to assess whether important risk of bias exists |
| Methods | RCT, prospective, placebo‐controlled, single‐blind Date of study Setting | |
| Participants | Randomised: 65 participants (age 25 to 67 years, 32 male, 33 female) Inclusion criteria
Ocular rosacea: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data (N or mean (range)) IGA score minimal; praziquantel (4), vehicle (1) IGA score mild; praziquantel (11), vehicle (9) IGA score moderate; praziquantel (28), vehicle (12) CEAS score mild; praziquantel (5), vehicle (3) CEAS score moderate; praziquantel (12), vehicle (8) CEAS score significant; praziquantel (26), vehicle (11) DLQI; praziquantel 15.8 (4 to 23), vehicle 14.6 (5 to 21) | |
| Interventions | 12 weeks with 4 weeks follow‐up Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 1 Epub): "Funding: None" | |
| Declaration of interest | Quote (page 1 Epub): "Conflicts of interest: None" | |
| Notes | Two of our primary outcomes was addressed (quality of life and adverse events) See comparison 34 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 2 Epub): "were randomly assigned" and "using a computer‐generated randomization schedule" Comment: Probably done |
| Allocation concealment (selection bias) | High risk | Quote (page 2 Epub): "The assignment was performed in a single‐blinded manner (for safety reasons" The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Not sure if allocation concealment and blinding are confused. There was insufficient information to permit a clear judgement After e‐mail communication it became clear that two investigators had access to the list Comment: We judged this as at a high risk of bias |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 2 Epub): "The assignment was performed in a single‐blinded manner (for safety reasons" in which the subjects were blinded to the treatment affectation" Comment: Investigators not blinded. The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Quote (page 2 Epub): "The assignment was performed in a single‐blinded manner (for safety reasons" in which the subjects were blinded to the treatment affectation" |
| Incomplete outcome data (attrition bias) | Low risk | 2/65 (3%), ITT analysis. Reasons for withdrawals reported Comment: We considered this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period prior to study entry adequate, no other treatments allowed, no sponsoring Comment: The study appears to be free of other forms of bias |
| Methods | Randomised, prospective, placebo‐controlled, within‐patient comparison Unreported Setting Instituto de Fotomedicina, Centro Medico Teknon, Barcelona, Spain | |
| Participants | Randomised: 31 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals : Not reported Baseline data (mean) | |
| Interventions | One treatment, follow‐up 30 days Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, day 1, 9, 21 and 30 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Nothing reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed Abstract, few data presented. Unable to contact principal investigator, no exact data are provided (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 43): "applied on a randomized side of the face" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding reported |
| Blinding of outcome assessment (detection bias) | High risk | No blinding reported Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on dropouts and withdrawals Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | RCT, prospective, placebo‐controlled, double‐blind, within‐patient comparison Date of study Unreported Setting Department of Dermatology, University Wales College of Medicine, Cardiff, UK | |
| Participants | Randomised: 33 participants (mean age 56.9 years for males and 52.8 years for females, 15 male, 18 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: None Baseline data mean (SEM) Number of papules; azelaic acid site 13.0 (1.5), vehicle site 13.3 (1.6) Number of pustules; azelaic acid site 1.2 (0.4), vehicle site 1.6 (0.5) | |
| Interventions | 13 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 13 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared. Two investigators were employed by Schering AG, Berlin, Germany, the manufacturer of azelaic acid cream | |
| Notes | One of our primary outcomes was addressed (adverse events) Subjective severity scale and overall rating by physicians is not consistent and data on inflammatory lesions were skewed See comparison 6 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page S19): "Allocation of the preparations to the facial side was randomized." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page S19): "Comparison between 20% azelaic acid and its identical‐appearing vehicle." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | There were no dropouts (page S21). ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out period adequate before start, study duration adequate. No topical or systemic medications that could influence outcomes were allowed Comment: The study appears to be free of other forms of bias |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 83 participants (mean age 52.2 years, 23 male, 57 female and 3 gender unreported) Inclusion criteria
Exclusion criteria
Ocular involvement: Unclear Dropouts and withdrawals
Baseline data mean (SD) Number of inflammatory lesions; clindamycin + tretinoin group 14.3 (9.5), placebo group 18.7 (14.1) | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 2, 6 and 12 Outcomes of the trial (as reported) Primary outcomes:
Secondary outcomes:
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (338): "This study was funded by a grant from Medicis" | |
| Declaration of interest | Quote (338):"The authors have no conflict of interest to disclose" | |
| Notes | Two of our primary outcomes were addressed (quality of life and adverse events) See comparison 28 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 334): "Qualifying subjects were randomized via a computerized random number generator" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 334): "The research staff member who randomized the study population was not involved in any study assessments." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 334): "CT gel and placebo gel were indistinguishable on visual inspection with respect to color, consistency and odor" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator‐ and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 3/83 were not included in the analyses. Per‐protocol analysis Comment: Low number of participants excluded from analysis and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT00823901). In the protocol reduction of transient erythema was the single secondary outcome and was specified in Methods section of the report but embedded in improvement of clinical features of rosacea. The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, groups treated equally The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre (6 centres) in US We only included second phase (first phase was open and not controlled) | |
| Participants | Randomised: 88 participants (mean age 48.6 years in treatment group versus 43.7 years in control group, 32 male, 56 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean (SD) Number of inflammatory lesions; metronidazole group 0.9 (2.2) and vehicle group 0.5 (1.0) | |
| Interventions | Six months Intervention
Comparator
| |
| Outcomes | Assessments (7): baseline, week 4, 8, 12, 16, 20 and 24 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 683): "The study was funded by a grant from Galderma Laboratories Inc, Fort Worth, Tex." | |
| Declaration of interest | Quote (page 683): "Dr Herndon is a paid consultant for Galderma Laboratories Inc. Drs Tuley and Czernielewski and Mr Baker are employees of Galderma laboratories Inc." | |
| Notes | None of our primary outcomes were addressed. We only included the double‐blind randomised second phase See comparison 1 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 680): "...were randomized into 2 treatment groups." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 680): "...double‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 680): "..double‐blind.." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Low risk | 33/88 (37.5%); metronidazole group (14) and vehicle group (19). 9/44 in metronidazole group relapsed, versus 18/44 in vehicle group. No subjects discontinued because of adverse events Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Adequate study duration, sponsorship and declaration of interest stated. No wash‐out period (first phase was active treatment), unclear if groups were treated equally aside from intervention Comment: Insufficient information to assess whether an important risk of bias exists |
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Unreported Setting Department of Dermatology, Mayo Medical School, Scottsdale; Department of Dermatology, Baylor College of Medicine, Houston; Department of Dermatology, University of Missouri, Kansas City School of Medicine, US | |
| Participants | Randomised: 72 participants (mean age 45 years (range 22 to 78) in 0.75% cream group versus 47 years (range 28 to 75) in metronidazole 1% group, 10 male and 26 female in metronidazole 0.75% group versus 11 male and 25 female in metronidazole 1% group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of inflammatory lesions; metronidazole 0.75% group 19, metronidazole 1% group 25 | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 738): "Supported by Galderma Laboratories, Inc." | |
| Declaration of interest | Quote (page 738): "Dr Tuley and Mr Baker are employees of Galderma Laboratories. Drs Dahl, Jarratt, and Kaplan all received financial compensation from Galderma Laboratories, Inc for performing this study" | |
| Notes | None of our primary outcomes were addressed See comparison 3 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 725): "Patients were randomly assigned to receive 0.75% metronidazole cream or 1.0% metronidazole cream." E‐mail contact with the investigator confirmed "subjects were randomised to 1 of the 2 treatment groups at a ratio of 1:1. The randomisation process was done in blocks of 4, stratified by investigators. The randomisation was carried out using SAS PROC PLAN." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 724): "A double‐blind format was not used because the study drugs were label‐blinded commercial products contained in tubes of different sizes and shapes." Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Quote (page 724) : "A double‐blind format was not used because the study drugs were label‐blinded commercial products contained in tubes of different sizes and shapes." Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | 11/72 (15.3%); metronidazole 0.75% group (4), metronidazole 1% group (7). ITT analysis, based on LOCF However, "Intention to treat population ranged from 30 to 35 subjects in 0.75% metronidazole group and from 29 to 34 in 1.0% metronidazole group." Page 725 Comment: ITT population did not appear to include all randomised participants. Unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Wash‐out period adequate, study duration adequate, groups treated equally. Sponsoring by Galderma Laboratories, Inc. 2 authors are employees of Galderma Quote (page 723): "The authors received financial compensation from Galderma Laboratories, Inc for performing this study." Comment: The study was not double‐blind combined with the financial support may pose a potential risk of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study June 2004 to April 2005 Setting Multicentre, 14 sites in US | |
| Participants | Randomised: 251 participants (age 46.8 (SD 13.2) in treatment group and 47.6 (SD 11.5) in placebo group, 91% (SD 71.7) female in treatment group, and 95% (SD 76.6) female in placebo group) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Lesion counts (papules, pustules, nodules); doxycycline group 19.5 (8.8), placebo group 20.3 (10.4) Clinical erythema assessment; doxycycline group 9.7 (3.0), placebo group 9.5 (2.7) | |
| Interventions | 16 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 791): "Supported by CollaGenex Pharmaceuticals, Inc." | |
| Declaration of interest | All authors have received grants from Collagenex or worked as consultants for Collagenex (page 791) | |
| Notes | One of our primary outcomes was addressed (adverse events) Some SD were missing and these were calculated by the review authors See comparison 43 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 794): "For each study site, a master randomisation list in blocks of 4 was prepared by the sponsor for all study sites. With the use of a computer‐generated randomisation scheme, patients were assigned in equal proportions (1:1) to receive drug or placebo." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 794): "Master randomisation list in blocks of 4 was prepared by the sponsor for all study sites." Comment: A form of central randomisation was used. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 794): "Investigators, study site personnel, and patients were blinded with respect to the identity of the study medication being taken. All the employees of the sponsor and its affiliates who were involved in data monitoring, data entry, or data analysis were blinded as well." "Study drug and placebo capsules were identical in size, shape, and colour." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 794): "Investigators, study site personnel, and patients were blinded with respect to the identity of the study medication being taken. All the employees of the sponsor and its affiliates who were involved in data monitoring, data entry, or data analysis were blinded as well." "Study drug and placebo capsules were identical in size, shape, and colour." Blinding of the outcomes assessors, key personnel, was ensured, and it was unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete outcome data were adequately addressed, reasons for withdrawal reported, no differences between the 2 groups. ITT analysis Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate wash‐out period before the study, adequate study duration, clinically significant concomitant drug therapy was forbidden Study supported by Collagenex Pharmaceuticals. All authors have received grants from Collagenex or worked as consultants for Collagenex Comment: As the study appeared to be triple‐blinded and there was no selective reporting we do not consider that the sponsorship and support represented any additional bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study June 2004 to April 2005 Setting Multicentre, 14 sites in US | |
| Participants | Randomised: 286 participants (age 46.3 (SD 12.7) in treatment group and 47.6 in placebo group, 94% (SD 66.2) female in treatment group, and 95% (SD 66.0) female in placebo group) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Lesion count; doxycycline group 20.5 (11.7), placebo group 21.23 (12.5) Clinical erythema assessment; doxycycline group 9.5 (2.9), placebo group 9.1 (2.5) | |
| Interventions | 16 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 791): "Supported by CollaGenex Pharmaceuticals, Inc." | |
| Declaration of interest | All authors have received grants from Collagenex or worked as consultants for Collagenex (page 791) | |
| Notes | One of our primary outcomes was addressed (adverse events) Some SD were missing and these were calculated by the review authors See comparison 43 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 794): "For each study site, a master randomisation list in blocks of 4 was prepared by the sponsor for all study sites. With the use of a computer‐generated randomisation scheme, patients were assigned in equal proportions (1:1) to receive drug or placebo." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 794): "Master randomisation list in blocks of 4 was prepared by the sponsor for all study site." Comment: A form of central randomisation was used. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 794): "Investigators, study site personnel, and patients were blinded with respect to identity of the study medication being taken. All the employees of the sponsor and its affiliates who were involved in data monitoring, data entry, or data analysis were blinded as well." "Study drug and placebo capsules were identical in size, shape, and colour." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 794): "Investigators, study site personnel, and patients were blinded with respect to identity of the study medication being taken. All the employees of the sponsor and its affiliates who were involved in data monitoring, data entry, or data analysis were blinded as well." "Study drug and placebo capsules were identical in size, shape, and colour." Blinding of the outcomes assessors, key personnel, was ensured, and it was unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete outcome data were adequately addressed, reasons for withdrawal reported, no differences between the 2 groups. ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate wash‐out period before the study, adequate study duration, clinically significant concomitant drug therapy was forbidden Study supported by Collagenex Pharmaceuticals. All authors have received grants from Collagenex or worked as consultants for Collagenex Comment: As the study appeared to be triple‐blinded and there was no selective reporting we do not consider that the sponsorship and support represented any additional bias |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Setting Department of Dermatology, Valley Hospital Medical Center, Las Vegas; Department of Dermatology, Advanced Skin Research Center, Omaha, University of Washington, Washington, US | |
| Participants | Randomised: 91 participants (age 44.3 years in 40 mg group and 45.2 in 100 mg group, 29 females and 15 males in 40 mg group and 35 females and 12 males in 100 mg group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and wWithdrawals
Baseline data mean (SD) Nothing reported | |
| Interventions | 16 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 576): "The study was supported through educational grants from Collagenex Corporation" | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) All SD are missing and these were calculated by the review authors See comparison 50 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 574): "Subjects were randomized to receive daily administration of drugs." Comment: Insufficient information about the method used to generate the allocation sequence to allow an assessment of whether it should produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 574): "Both the doxycycline 100 mg capsules and the 40 mg capsules were over encapsulated to ensure the capsules were indistinguishable during administration and to maintain a double‐blind study." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 574): "Both the doxycycline 100 mg capsules and the 40 mg capsules were over encapsulated to ensure the capsules were indistinguishable during administration and to maintain a double‐blind study." Blinding of the outcomes assessors, key personnel, and participants was ensured, and it was unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Incomplete outcome data were adequately addressed, reasons for withdrawal reported, no differences between the 2 groups. ITT analysis Comment: High but balanced dropout rate and although combined with ITT analysis (LOCF) judged as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate wash‐out period before study started, adequate study duration, clinically significant concomitant drug therapy was not permitted Comment: The study appears to be free of other forms of bias |
| Methods | Randomised, prospective, active‐control, investigator‐blind Date of study February to July 2009 Setting Multicentre US | |
| Participants | Randomised: 207 participants (mean age 49 years, 71 male, 136 female) Inclusion criteria
No ocular involvement
Dropouts and withdrawals
Baseline data mean) Number of inflammatory lesions; azelaic acid group 20.6, metronidazole group 21.9 | |
| Interventions | 12 weeks Intervention
Comparator
Patients were instructed how to clean their face and what to use to clean their face and what moisturizer to use. No other soaps, cleansers and moisturizers were allowed | |
| Outcomes | Assessments (6): baseline week 2, 4, 6, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 612): "This study was supported by Intendis" | |
| Declaration of interest | Quote (page 612): "Dr Del Rosso is a consultant to and serves as a speaker for ...Galderma...Intendis...Dr Bruce has served as an investigator (grants) for Actavis......Dr Jaratt has served as consultant for Stiefel...He has received honoraria from ...Galderma, ...He has been principal investigator for...Galderma..Intendis...Dr Menter is a consultant, speaker, and is on the advisory board for Abbott....He is a consultant and speaker for Eli Lilly and Stiefel. He is an investigator for .....He has received grants and honoraria from ....etc "He received honoraria from Galderma...." | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 51 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 608‐9): "were randomized at a ratio of 1:1.." and "randomly assigned" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 608): "investigator‐blinded" Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement Comment: Blinding of investigators effective, however participants were not blinded but unlikely to represent a threat to performance bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 608): "investigator‐blinded". Outcomes were investigator as well participant‐assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Low risk | 13/207 (6.3%); azelaic acid group (6), metronidazole group (7), reasons in part reported. Per‐protocol analysis Comment: Low number of dropouts and although per‐protocol analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available at clinicaltrials.gov NCT00855595, and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, clinically significant concomitant drug therapy was not permitted, groups treated equally Comment: The study appears to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Department of Dermatology, Wake Forest University School of Medicine, Winston‐Salem, North Carolina, US | |
| Participants | Randomised: 30 participants (ages between 20 and 65, gender unreported, both sexes) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean (SD) Nothing reported | |
| Interventions | Four weeks
Comparator
| |
| Outcomes | Assessments (2): baseline and week 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 881): "This study was funded by an educational grant from Cutanix Corporation" | |
| Declaration of interest | Quote (page 881): "Zoe Draelos, MD, has indicated no significant interest with commercial supporters, Bryan Fuller, PhD, is the inventor of the active, which was licensed through the Oklahoma Health Sciences Center to Cutanix" | |
| Notes | One of our primary outcomes was addressed (adverse events) SDs are missing from the report See comparison 32 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 882): "The 30 subjects were randomized at a 2:1 ratio." Comment: Unclear E‐mail contact with the investigator confirmed a random number generator was used Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 882): "All products were dispensed in identical bottles with identical labelling. Neither the dermatologist investigator nor the subjects knew the contents of the bottle." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 882): "All products were dispensed in identical bottles with identical labelling. Neither the dermatologist investigator nor the subjects knew the contents of the bottle." Blinding of the outcomes assessors, key personnel, and participants was ensured, and it was unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for the 2 withdrawals were reported, but unclear in which group. After clarification with the author this was confirmed as 1 in each group. Per‐protocol analysis Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | High risk | Percentage improvement in dermatitis was not addressed, no exact data were reported for the self‐assessments carried out by the participants Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | One of the investigators is the inventor of the formula, which may represent a potential conflict of interests. No baseline balance descriptives. Treatment duration adequate, no wash‐out prior to study described Comment: We judged this as at unclear risk of bias |
| Methods | RCT, prospective, "placebo"‐controlled, investigator‐blinded Date of study Setting Department of Dermatology; Wake Forest University School of Medicine, Winston‐Salem, North Carolina, US | |
| Participants | Randomised: 67 participants (age between 19 to 66, gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not specified Dropouts and withdrawals
Baseline data mean (SD) Lesion counts; group non‐standardised care 10, group PHA skin care 7 (estimated from a graph) | |
| Interventions | 12 weeks Intervention
Comparator
Unclear to which groups the other five participants were allocated | |
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes None ✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | Two investigators were employed by NeoStrata Company, Inc., Princeton, NJ, however, no conflict of interest declared | |
| Notes | None of our primary outcomes were addressed The combination of incomplete and selective reporting of outcome data did not permit entry of any data into a meta‐analysis. It was unclear how many participants were randomised to each intervention and because very limited outcomes data were reported no reliable conclusions could be drawn (Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 23): "The investigation was designed as a 12‐week investigator blinded, randomized study of parallel groups." Comment: Unclear E‐mail contact with the investigator confirmed "a randomisation schedule with a random number generator was developed" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 23): "...investigator‐blinded." Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 23): "...investigator‐blinded." Comment: Both the participant and the investigator were outcomes assessors and the report was unclear what measures were used, if any, to blind study personnel from knowledge of which intervention a participant received |
| Incomplete outcome data (attrition bias) | High risk | Five participants were lost to follow‐up for "personal reasons", and it was unclear how many occurred in each group, at which stage of the study, and whether data were available for any of the other assessment time points. Per‐protocol analysis. After e‐mail contact with the author: "the dropouts were for personal reasons, not related to product. They were random between the groups" Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | High risk | Not all predefined outcomes were addressed or reported clearly, i.e. Investigator's Global Assessment of rosacea, observations of tingling and tightness by participants. No precise data were reported, data had to be estimated from figures Comment: We judged this as at a high risk of bias |
| Other bias | High risk | Wash‐out period adequate, study duration adequate. No baseline descriptives Study sponsorship was not reported, but 2 authors were from Neostrata Company the manufacturer of the PHA cleanser and moisturizer. Unclear how many participants started in each group. Possible imbalance in the baseline scores of lesion count in the 2 groups. The actual comparison was non‐standardised skin care versus PHA moisturizer Comment: We judged this as at a high risk of bias |
| Methods | Randomised, prospective, active‐controlled, double‐blind Unreported Setting | |
| Participants | Randomised: 146 women, age not reported Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data mean | |
| Interventions | Six weeks (first 2 weeks wash‐out period) Intervention
Comparator
Unclear how many were randomised to each group | |
| Outcomes | Assessments (2): baseline, week 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared but four investigators are employed by The Proctor and Gamble Company, Cincinnati, OH, US | |
| Notes | Poster abstract, limited data None of our primary outcomes was addressed, no response from PI to fill in gaps (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page AB82): "subjects were randomized to" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page AB82): "double‐blind" Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page AB82): "double‐blind". Outcomes were investigator as well participant‐assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on drop‐outs and withdrawals Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 401 participants (mean age 48.5 years (range 19 to 83 years), 103 male, 298 female) Inclusion criteria
No ocular involvement
Dropouts and withdrawals
Baseline data N (%) Moderate rosacea; azelaic acid group 172 (86.9), vehicle group 189 (93.1) Severe rosacea; azelaic acid group 26 (13.1), vehicle group 14 (6.9) | |
| Interventions | 12 weeks
Comparator
| |
| Outcomes | Assessments (5); baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None declared. Quote (page 315): "Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals" | |
| Declaration of interest | Quote (page 306): "Dr. Draelos is a researcher for Bayer HealthCare Pharmaceuticals. Dr. Elewski has conducted clinical research for Bayer HealthCare Pharmaceuticals and Galderma Laboratories, LP. Mr. Staedtler and Dr. Havlickova are employees of Bayer HealthCare Pharmaceuticals" | |
| Notes | All our primary outcomes are addressed See comparison 6 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 308): "The computer‐generated randomization procedure used blocks. Whole randomization blocks were allocated to the study centers, ensuring that the comparison groups maintained the planned allocation ratio for the treatment groups overall and within each center" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Form of central allocation Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 307): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement After e‐mail communication: "The blind was maintained by dispensing the vehicle and the vehicle plus the active in identical containers" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 307): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study After e‐mail communication: "The blind was maintained by dispensing the vehicle and the vehicle plus the active in identical containers" Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 41/401 (10.2%); azelaic acid group (21), vehicle group (20), reasons reported. ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was available on clinicaltrials.gov (NCT01025635). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. However, exact data on QoL scores were missing which is a primary outcome in our review Comment: We judged this as at an unclear risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, groups treated equally The study appeared to be free of other forms of bias |
| Methods | Randomised, prospective, active‐controlled, double‐blind Unreported Setting Dermatology clinic and the routine setting of a woman's home, US | |
| Participants | Randomised: 40 women (age unreported) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals: None Baseline data mean Nothing reported | |
| Interventions | Three weeks Intervention
Comparator
| |
| Outcomes | Assessments (3); baseline, week 1 and 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared. Three investigators are employed by Johnson & Johnson Consumer Companies, Inc, Skillman, NU, US | |
| Notes | None of our primary outcomes were addressed There are no separate data on women with rosacea (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 314‐6): "randomized".."were divided equally into two groups" and "Study participants were stratified and balanced for demographics and presence and severity of acne, eczema, rosacea and atopic dermatitis" After e‐mail communication: "Subjects were randomized in two balanced populations based on a computer generated randomization sequence" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: No further additional information to change our judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 314‐5): "double‐blind" Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 314‐5): "double‐blind". Outcomes were investigator as well participant‐assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study After e‐mail communication: "..identically appearing products packaged identically" Comment: Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses to follow up Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, clinically significant concomitant drug therapy was not permitted, groups treated equally Comment: The study appears to be free of other forms of bias |
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Multicentre, several centres in France | |
| Participants | Randomised: 100 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Unclear Dropouts and withdrawals
Baseline data mean (SD) Nothing reported | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared. One investigator was employed by Galderma, manufacturer of at least one of the investigated drugs | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 4 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (S272): "This multicenter, controlled, randomized, investigator‐masked study..." Comment: Insufficient information about the method used to generate the allocation sequence to allow an assessment of whether it should produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page S272): "...investigator‐masked study." Not clear what measures were used to blind study participants and personnel from knowledge of which intervention a participant received Outcomes assessments: Principally by the investigators Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page S272): "...investigator‐masked study." |
| Incomplete outcome data (attrition bias) | High risk | Quote (S272): "100 patients enrolled and analysed for ITT..." (21 withdrew/12 losses to follow up). Per‐protocol analysis at week 12 ‐ 67/100 Comment: Losses were accounted for but the data analysis as reported appeared to be per‐protocol with exclusion of outcome data for 33/100 participants. We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | High risk | One pre‐specified outcome was inadequately addressed and reported: Investigator's Global Assessment of improvement Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | Wash‐out period not stated, study duration adequate, unclear if groups were treated equally. Poster abstract Comment: Insufficient information to permit a clear judgement |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Multicentre, 13 centres in US | |
| Participants | Randomised: 251 participants (mean age 49 years in treatment group versus 46 years in control group, 32 male and 92 female versus 34 male and 93 female) Inclusion criteria
Ocular involved: Participants with marked involvement were excluded Exclusion criteria
Dropouts and withdrawals
Baseline data mean Lesion counts; azelaic group 18, metronidazole group 19 | |
| Interventions | 15 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 15 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | Quote (page 1444): "The authors received financial compensation from Berlex Laboratories Inc, Montville, NJ, for serving as principal investigators for this study" | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 14 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1145): "Computer‐generated block wise randomisation method was used to ensure balance between the groups..." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 1445): "Assignment occurred by the physician in ascending order with newly accepted patient receiving study medication with the lowest randomisation number available in the center." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 1445): "To preserve blinding, study medication was dispensed and collected only by a study nurse or assistant not involved with selection and assessment of patients." Comment: The report was also unclear what measures were used to blind study participants from knowledge of which intervention they received or any information relating to whether the intended blinding was effective Comment: Insufficient information to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 1445): "To preserve blinding, study medication was dispensed and collected only by a study nurse or assistant not involved with selection and assessment of patients." Comment: Assignment to intervention was by the investigators who were also the outcomes assessors. No satisfactory evidence of blinding. Outcomes were investigator and participant assessed |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis. All participants were accounted for Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Wash‐out period and study duration adequate, not permitted to receive any concurrent therapy. Authors received financial compensation from Berlex Laboratories, Inc, Montville, NJ, for serving as principal investigators for this study Comment: Insufficient information to assess whether important risk of bias exists |
| Methods | Randomised, prospective, placebo‐controlled (both groups have same topical treatment but different systemic treatments), double‐blind, cross‐over Date of study | |
| Participants | Randomised: 22 participants (mean age 59 years, 12 male, 10 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria:
Dropouts and withdrawals
Baseline data mean | |
| Interventions | 16 weeks to cross‐over but oral isotretinoin withheld Intervention
Comparator 1
Comparator 2
| |
| Outcomes | Assessments (2): baseline and week 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | After 16 weeks cross‐over but oral isotretinoin withheld; second phase unbalanced comparison. We only included first phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 320): "three separate treatment groups were randomly assigned" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 320) : "Subjects were given coded bottles containing either isotretinoin or placebo capsules. The creams were dispensed in tubes containing either 0.025% tretinoin cream or the vehicle" |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 2/22 lost to follow‐up; data presented as individual participant data Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | High risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. Data unreliable, its re‐analysis using the individual participant data confirmed its flawed analysis by the investigators Comment: We judged this as at a high risk of bias |
| Other bias | Low risk | Wash‐out phase before study started adequate, study duration adequate, groups treated equally, in first 16 weeks, no sponsoring Comment: The study appeared to be free of other forms of bias |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study April to October 1990 Setting Multicentre (18), France | |
| Participants | Randomised: 51 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | Six weeks Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline week 3 and 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | Two investigators were employees of Schering Plough | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) Allocation to intervention was based on up to 4 participants in each of 18 clinics but not all clinics enrolled 4 participants. The report did not provide any reassurance that the allocation sequence was adequately generated (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote (page 129): "la randomisation a porté sur des groups de 4, chaque médicin constituent un centre et devant inclure 4 malades" Comment: Allocation to intervention was based on up to 4 participants in each of 18 clinics but not all clinics enrolled 4 participants. The report did not provide any reassurance that the allocation sequence was adequately generated and there was lack of evidence that any form of central randomisation had been employed for the 18 clinics involved in this study |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 130): "Les emballages, les tubes, la coloration des gels étaient strictement comparables et indiscernables par les malades ou les expérimateureurs" (packaging, tubes, colour of gels were indistinguishable for participants and investigators). |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 6/51 (11.7%); metronidazole group (2), placebo group (4), ITT (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out phase before study started adequate, study duration adequate, groups treated equally |
| Methods | Randomised, prospective, controlled, within‐patient comparison Date of study Unreported Setting | |
| Participants | Randomised: 20 participants (mean age 46.5 years, 2 male, 9 female, 9 gender unreported) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data mean | |
| Interventions | Six weeks Intervention
Comparator
| |
| Outcomes | Assessments (3); baseline, week 2 and 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Poster abstract, limited data, unable to contact investigators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 969): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding reported |
| Blinding of outcome assessment (detection bias) | High risk | No blinding reported. Outcomes were investigator as well participant‐assessed Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 9/20 (45%); reasons unreported. Per‐protocol analysis Comment: High dropout rate assessed as at a high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | RCT, prospective 'placebo'‐controlled (both treatment arms had same topical treatment; one arm systemic active treatment versus placebo), double‐blind Date of study Unreported Setting Multicentre ‐ unclear which ones but at least Department of Dermatology, University of Louisville, Louisville, US | |
| Participants | Randomised: 72 participants (age unclear, 16 male, 56 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of lesions; doxycycline group 21.3 and placebo group 18.7 Basal erythema score; doxycycline group 8.6 and placebo group 9.2 | |
| Interventions | 16 weeks Intervention
Comparator
After 12 weeks, metronidazole gel stopped, but oral medication or placebo continued until week 16 | |
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed We only included data from the first 12 weeks of the study See comparison 52 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 642): "This was a randomized, multi‐center, outpatient, double‐blind placebo‐controlled trial." E‐mail contact with the investigator confirmed randomisation was carried out using a computer‐generated table provided by the sponsor Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence was not reported E‐mail contact with the investigator confirmed "pharmacy‐controlled central allocation and neither investigators or study staff were involved in the generation of the sequence" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 642): "This was a randomized...double‐blind..." Comment: The report did not describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Insufficient information to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 642): "This was a randomized...double‐blind..." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers) during the study. |
| Incomplete outcome data (attrition bias) | Low risk | 8/72 (11.1%); doxycycline group (6) and placebo group (2). Per‐protocol analysis Comment: Low number of dropouts, and although slightly unbalanced, judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate. Wash‐out phase before study not reported, groups treated equally Comment: We judged this as at low risk of bias |
| Methods | Randomised, prospective, active and placebo‐controlled, double‐blind Setting Multicentre (5) in US | |
| Participants | Randomised: 122 participants (mean age 45.7 years (SD 12.1), 30 male, 92 female) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals: None Baseline data N (%) PSA mild; BT 0.07% 1 (3.6), BT 0.18% 1 (3.2), BT 0.5% 0 (0), vehicle 2 (6.3) PSA moderate; BT 0.07% 12 (42.9), BT 0.18% 24 (77.4), BT 0.5% 26 (83.9), vehicle 26 (81.3) PSA severe; BT 0.07% 15 (53.6), BT 0.18% 6 (19.4), BT 0.5% 5 (16.1), vehicle 4 (12.5) | |
| Interventions | One application, follow‐up 12 hours
Comparator 1
Comparator 2
Comparator 3
| |
| Outcomes | Assessments (14): baseline, 30 min, 1 hour and then each hour until 12 hours Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 633): "The two studies were funded by Galderma R&D" | |
| Declaration of interest | Quote (page 633): "The investigators received grants for conducting the studies. YL and ML are employees of Galderma R&D" | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 10 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 634): "Subjects were randomized in a 1:1:1:1 ratio to receive" and "randomization lists were generated prior to study initiation by an independent statistician using SAS hoc Plan procedure (SAS Institute, Cary, NC, U.S.A.)." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 634): "The randomization lists were then sent to the clinical supply group, and only the personnel directly involved with labelling and packaging had access." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 634): "The integrity of the blinding was ensured by packaging the topical gels in identical tubes and requiring a third party other than the investigator/evaluator to dispense the medication." The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up. ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT00989014). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | This is a phase II study, duration for this design adequate, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
| Methods | Randomised, prospective, active‐ and placebo‐controlled, double‐blind Setting Multicentre (17) in US | |
| Participants | Randomised: 269 participants (mean age 44.3 years, 52 male, 217 female) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data N (%) PSA moderate; BT 0.18% QD 45 (83.3), BT 0.18% BID 45 (83.3), BT 0.5% 44 (83), vehicle QD 46 (83.6), vehicle BID 45 (84.9) PSA severe; BT 0.18% QD 9 (16.7), BT 0.18% BID 9 (16.7), BT 0.5% 9 (17), vehicle QD 9 (16.4), vehicle BID 8 (5.1) | |
| Interventions | Four weeks, and four weeks follow‐up
Comparator 1
Comparator 2
Comparator 3
Comparator 4
| |
| Outcomes | Assessments (23): baseline (5x), day 1 (5x), 15 (5x), 29 (5x), week 5, 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 633): "The two studies were funded by Galderma R&D" | |
| Declaration of interest | Quote (page 633): "The investigators received grants for conducting the studies. YL and ML are employees of Galderma | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 11 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 634): "Subjects were randomized in a 1:1:1:1:1 ratio to the groups" and "randomization lists were generated prior to study initiation by an independent statistician using SAS hoc Plan procedure (SAS Institute, Cary, NC, U.S.A.)." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 634): "The randomization lists were then sent to the clinical supply group, and only the personnel directly involved with labelling and packaging had access." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 634): "The integrity of the blinding was ensured by packaging the topical gels in identical tubes and requiring a third party other than the investigator/evaluator to dispense the medication." The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 9/269 (3.3%); BT 0.18% QD (2), BT 0.18% BID (2), BT 0.5% (2), vehicle QD (2), vehicle BID (1), reasons reported. ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT01174030). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | This is a phase II study, duration for this design adequate, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Setting Multicentre in US and Canada | |
| Participants | Randomised: 260 participants (mean age 48.8 years, 54 male, 206 female) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data N (%) CEA moderate; brimonidine tartrate 0.5% gel group 111 (86), vehicle gel group 113 (86.3) CEA severe; brimonidine tartrate 0.5% gel group 18 (14), vehicle gel group 18 (13.7) PSA mild; brimonidine tartrate 0.5% gel group 0 (0), vehicle gel group 1 (0.8) PSA moderate; brimonidine tartrate 0.5% gel group 107 (82.9), vehicle gel group 114 (87) PSA severe; brimonidine tartrate 0.5% gel group 22 (17.1), vehicle gel group 16 (12.2) | |
| Interventions | Four weeks with four weeks follow up
Comparator
A wash‐out period was mandatory for subjects receiving prescription medications for inflammatory conditions, rosacea, or acne (for most treatments 4 weeks, isotretinoin 6 months) | |
| Outcomes | Assessments (6): baseline, day 1, 15, 29, week 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 656): "The two studies were funded by Galderma R&D" | |
| Declaration of interest | Quote (page 656): "The investigators received grants for conducting the studies. Ms. Rudisill and Dr. Leoni are employees of Galderma R&D." | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 12 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 651): "Subjects were randomized in a 1:1 ratio to the groups of BT gel 0.5% and vehicle gel" and "Randomization lists were generated prior to study initiation by an independent statistician using SAS Proc Plan procedure" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 651): "The randomization lists were then sent to the clinical supply group, and only the personnel directly involved with labeling and packaging had access" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 651): "The integrity of the blinding was ensured by packaging the topical gels in identical tubes and requiring a third party other than the investigator/evaluator to dispense the medication." The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 6/260 (2.3%); brimonidine tartrate 0.5% gel group (2), vehicle gel group (4). ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT01355458). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Setting Multicentre in US and Canada | |
| Participants | Randomised: 293 participants (mean age 47.5 years, 80 male, 213 female) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data N (%) CEA moderate; brimonidine tartrate 0.5% gel group 108 (73), vehicle gel group 115 (79.3) CEA severe; brimonidine tartrate 0.5% gel group 40 (27), vehicle gel group 30 (20.7) PSA mild; brimonidine tartrate 0.5% gel group 0 (0), vehicle gel group 2 (6.3) PSA moderate; brimonidine tartrate 0.5% gel group 129 (87.2), vehicle gel group 122 (84.1) PSA severe; brimonidine tartrate 0.5% gel group 19 (12.8), vehicle gel group 23 (15.9) | |
| Interventions | Four weeks with four weeks follow‐up
Comparator
A wash‐out period was mandatory for subjects receiving prescription medications for inflammatory conditions, rosacea, or acne (for most treatments 4 weeks, isotretinoin 6 months) | |
| Outcomes | Assessments (6): baseline, day 1, 15, 29, week 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 656): "The two studies were funded by Galderma R&D" | |
| Declaration of interest | Quote (page 656): "The investigators received grants for conducting the studies. Ms. Rudisill and Dr. Leoni are employees of Galderma R&D." | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 12 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 651): "Subjects were randomized in a 1:1 ratio to the groups of BT gel 0.5% and vehicle gel" and "Randomization lists were generated prior to study initiation by an independent statistician using SAS Proc Plan procedure" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 651): "The randomization lists were then sent to the clinical supply group, and only the personnel directly involved with labeling and packaging had access" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 651): "The integrity of the blinding was ensured by packaging the topical gels in identical tubes and requiring a third party other than the investigator/evaluator to dispense the medication." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 10/293 (3.4%); brimonidine tartrate 0.5% gel group (7), vehicle gel group (3). Reasons not reported. ITT analysis. Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT01355471). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
| Methods | Randomised, prospective, active‐ and placebo‐control, double‐blind Date of study Multicentre (35) in Germany | |
| Participants | Randomised: 573 participants (mean age 53.3 years (SD 14.0), 259 male, 290 female, 24 gender unreported) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data median Number of inflammatory lesions; isotretinoin 0.1 group 17, isotretinoin 0.3 group 18, isotretinoin 0.5 group 16, doxy 18, placebo 19 Physician's Global Assessment; all groups 5 | |
| Interventions | 12 weeks Intervention
Comparator 1
Comparator 2
Comparator 3
Comparator 4
| |
| Outcomes | Assessments (5): baseline, week 2, 4, 6, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 514): "The study was supported by Almirall Hermal GmbH" | |
| Declaration of interest | Quote (page 514): "Professor Gollnick received lecturer fees for the subject rosacea from various firms: Almirall Hermal GmbH, Galderma, Schering/Intendis" | |
| Notes | Two of our primary outcome were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 58 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 506): "were allocated to 5 different treatment groups in a randomized and blinded manner" and "For random assignment to the different treatment groups patients were stratified according to weight (50–70, 71–90, 91–110 and 111–130 kg). After a request by fax through the treating physician a central stratified randomization and mailing of the medication occurred." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 506): "The study medications were blinded according to § 10 of the German Drug Law (Arzneimittelgesetz, AMG) and provided by Almirall Hermal GmbH, Reinbek, Germany. Isotretinoin was employed as capsules with 10 mg isotretinoin and doxycycline as tablets with 50 mg doxycycline each. Due to the double dummy study design each patients had to take both isotretinoin/placebo capsules or doxycycline/placebo tablets." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 72/573 (12.6%); Isotretinoin 0.1 mg/kg (10/111), Isotretinoin 0.3 mg/kg (18/147), Isotretinoin 0.5 mg/kg (16/116), doxycycline (20/152), placebo (8/47). reasons reported, Per‐protocol analysis Comment: Low and balanced number of dropouts and although per‐protocol analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on https://www.clinicaltrialsregister.eu/ctr‐search/search as EudraCT‐Nr 2006‐002410‐35. The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, groups treated equally. However, cohorts, and flow diagram are rather unclear. Study supported by Almirall Hermal GmbH and the Principal Investigator received fees Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Clinique Dermatologique des Hospiteaux Universitaires de Strasbourg, France | |
| Participants | Randomised: 34 participants (mean age 44 years (SD 13) in treatment group versus 49 years (14) in control group, 6 male, 28 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Nothing reported | |
| Interventions | Four months Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline, week 6 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) Males tend to have more severe rosacea and all the males were in the control group See comparison 60 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 688): "Il' s' aggisait d'un essai randomisé en double insu." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 687): "....en double insu." [translated as 'double‐blind'] Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 687): "....en double insu." [translated as 'double‐blind'] |
| Incomplete outcome data (attrition bias) | Low risk | 5/34 (14.7%); rilmenidine group (2) and placebo group (3). ITT analysis. All participants appear to have been accounted for (pages 688, 689) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out period long enough before the study, no concomitant therapy for rosacea was allowed, additional medication recorded, sponsorship or support not reported Comment: We judged this as at a low risk of bias |
| Methods | RCT, prospective, active‐controlled, investigator‐masked Date of study Unreported Multicentre, 9 centres in Europe (France, Ireland, Spain, and Belgium) | |
| Participants | Randomised: 114 participants (age 22 to 82 years, gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts/Withdrawals: Unclear | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (2): baseline, week 12, and maybe more Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | A poster of an old study, much information is either poorly reported or missing, e.g. number of dropouts None of our primary outcomes were addressed (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page S145): "The randomised, investigator‐blinded study lasted twelve weeks." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page S145): "...investigator masked." The report did not clarify what measures were used to blind study participants and personnel from knowledge of which intervention a participant received Comment: Insufficient information to make a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page S145): "...investigator masked." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers) during the study. Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Inadequate reporting of rates of attrition and exclusions to permit clear judgement of (e.g. number randomised not stated, no reasons for missing data provided) |
| Selective reporting (reporting bias) | Unclear risk | Methods section not specific about which outcomes were being sought Quote (page S145): "To compare the efficacy and safety as well as the cosmetic acceptability?" Comment: Insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Study duration adequate, wash‐out phase before study started adequate, groups treated equally, no information about sponsorship or support. Inadequate detail about the baseline characteristics of the participants, the interventions delivered, and methods of standardisation of outcomes assessment across the 9 international centres Comment: Insufficient information to assess whether important risk of bias exists |
| Methods | Randomised, prospective, active‐controlled Date of study Setting | |
| Participants | Randomised: 60 participants (mean age 31.63 years (SD 9.16), 36 male, 24 female) Inclusion criteria
Ocular involvement: Unclear
Dropouts/Withdrawals: None Baseline data mean | |
| Interventions | Three months
Comparator
| |
| Outcomes | Assessments (3): baseline, week 4 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Translated from Chinese, see Acknowledgements See comparison 66 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 308): "divided randomly into two groups" |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding reported and no sham laser treatment Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding reported and no sham laser treatment. Investigator and participant assessed outcomes Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period before study started too short Comment: Insufficient information to assess whether important risk of bias exists |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Setting | |
| Participants | Randomised: 170 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals: Not reported Baseline data mean | |
| Interventions | 12 weeks
Comparator
Unclear how many were randomised to each group | |
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page AB9): "Funded by Galderma Laboratories LP" | |
| Declaration of interest | None declared. Several investigators are employed by Galderma Laboratories LP | |
| Notes | One of our primary outcomes was addressed (adverse events) Limited data from poster abstract (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page AB9): "randomized" |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page AB9): "double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page AB9): "double‐blind" Comment: Outcomes were investigator and participant assessed. Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Poster abstract, with limited information Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided. Pubished as protocol NCT01308619 in clinicaltrials.gov Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | Randomised, prospective, active‐controlled, double‐blind Setting | |
| Participants | Randomised: 60 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | 12 weeks with four week follow up
Comparator
| |
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 298): "Funding for the study was provided by Medicis" | |
| Declaration of interest | Quote (page 298): "Dr Jackson has served as a speaker, consultant, and investigator for Medicis" | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 54 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 294/295): "Treatment was randomly allocated in blocks of 2. Blocks were centrally assigned to investigators as needed and based on enrollment" "The randomization process assigned equal numbers of patients to each treatment group." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 294): "Treatment was randomly allocated in blocks of 2. Blocks were centrally assigned to investigators as needed and based on enrollment" Comment: Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 295): "Blinded study medication was identified using the patient randomization number" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 295): "Blinded study medication was identified using the patient randomization number" |
| Incomplete outcome data (attrition bias) | Low risk | 5/60 (8.3%); all in minocycline + azelaic acid group, reasons reported Comment: Low number of dropouts and ITT analysis (LOCF) judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out before the study started adequate, no concomitant therapy for rosacea was allowed Comment: We judged this as at low risk of bias |
| Methods | RCT, prospective, placebo‐controlled and active‐controlled (4 treatment arms), double‐blind Date of study Unreported Setting | |
| Participants | Randomised: 277 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Unclear Baseline data mean (SD) | |
| Interventions | 10 weeks Intervention
Comparator 1
Comparator 2
Comparator 3
Unclear how many participants started in each group | |
| Outcomes | Assessments (5): baseline, week 2, 4, 7 and 10 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 502): "Supported by Dermik Laboratories, Inc., 500 Arcola Rd, Collegeville, PA 19426." | |
| Declaration of interest | Quote (page 502): "Dr Tobey formerly was formerly Vice President of Research and Development, Dermik Laboratories, Inc." | |
| Notes | One of our primary outcomes was addressed (adverse events) Unclear how many participants started in each group (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 502) : ".... randomized, double‐blind, multicenter trial." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 502): "Patients were blinded as to treatment, and evaluators were blinded as to treatment and application regimen." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 502): "Patients were blinded as to treatment, and evaluators were blinded as to treatment and application regimen." |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts reported. ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | High risk | Unclear how many participants were in each intervention group. Withdrawals were unreported Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period prior to study entry adequate. Study was supported by Dermik Laboratories, Inc, 500 Arcola Rd, Collegeville, PA 19426. One co‐investigator was formerly vice‐president of research and development, Dermik Laboratories Comment: Realistic and potential risk of bias |
| Methods | RCT, prospective, active‐controlled, double‐blind, within‐patient comparison Date of study Setting | |
| Participants | Randomised: 20 participants (age 62 years ± 12.3, 14 male, 6 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria:
Dropouts and withdrawals: None | |
| Interventions | One treatment Intervention
Comparator 1
Comparator 2
If no effect, treatment was repeated up to 3 times in same session Evaluation after 4 weeks | |
| Outcomes | Assessments (2): baseline and week 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | Quote (page 702): "The authors have indicated no significant interest with commercial supporters" | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 62 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 703): "Patients were randomized to receive one of four treatment regimens." "Twenty patients were studied using the sequence delivery of PDL and NdYAG wavelets combined on one side of their nose This could be right or left side. The other side received either PDL, or NdYAG." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 704): "blinded assessment of photographs taken before and after final evaluation". Investigators were blinded with respect to treatment modality, it is unclear if participants knew what treatment they were receiving on each side of the nose. Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study |
| Incomplete outcome data (attrition bias) | Low risk | There were no dropouts reported. Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias. |
| Other bias | High risk | The investigator used a Chi2 statistic on cell values less than 5, invalidating the analysis Also, reports "possible confounding with ages that was not accounted for" Comment: We judged this as at a high risk of bias |
| Methods | Randomised, prospective, active‐controlled, double‐blind, cross‐over Setting | |
| Participants | Randomised: 70 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 2/70 (2.9%) in brimonidine group; adverse event (1) and protocol deviation (1) Baseline data mean | |
| Interventions | 15 days
Comparator
Wash‐out period (unspecified) and cross‐over | |
| Outcomes | Assessments (2): baseline and day 15 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared, investigators employed by Galderma Laboratories, L.P., Fort Worth, TX | |
| Notes | Poster, limited data One of our primary outcomes was addressed (participants‐assessed changes in rosacea severity (PSA)) See comparison 24 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page A182): "Subjects were randomized 1:1 to.." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page A181): "double‐masked" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page A181): "double‐masked". Investigator and participant assessed outcomes Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | 2/70 (2.9%) in brimonidine group; adverse event (1) and protocol deviation (1). Poster abstract, limited information Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data. Comment: There was insufficient information to permit a clear judgement |
| Methods | Randomised, prospective, active‐controlled, open label, within‐patient comparison Date of study August 2009 to March 2010 Setting Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea | |
| Participants | Randomised: 18 participants (mean age 31.1 years, 5 male, 13 female) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data mean | |
| Interventions | Three treatments at three weekly intervals Intervention
Comparator
| |
| Outcomes | Assessments (5), baseline, week 3, 6, 9 and 15 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 67 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 575): "According to a computer‐generated randomization, each cheek was randomly assigned to.." Comment: Probably done |
| Allocation concealment (selection bias) | High risk | Quote (page 575): "The randomization schedule was not concealed from physicians who carried out the treatment" Comment: We judged this as at a high risk of bias |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 574): "randomized, open, split‐face.." Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 574): randomized, open, split‐face.." and "Photographs were taken by the same blinded physician at baseline".. "on the blinded investigators’ and patients’ own evaluation. Three blinded dermatologists assessed.." Comment: These statements are contradictory. Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | 3/18 (16.6%); due to difficulty in attending follow‐up because of distance. Per‐protocol analysis Comment: The moderate dropout rate with per‐protocol analysis represents a potential risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Pre‐study wash‐out period adequate, study duration adequate |
| Methods | RCT, prospective, active‐controlled, open‐label Date of study Dermatology department, Zonguldak Karaelmas University, Turkey | |
| Participants | Randomised: 49 participants (age 50.7 ± 9.1 years in metronidazole group versus 48.4 ± 9.4 years in pimecrolimus group, 16 male and 8 female in metronidazole group and 13 male and 12 female in pimecrolimus group) Inclusion criteria
No ocular rosacea Exclusion criteria
Dropouts and withdrawals: 1 in pimecrolimus group (deterioration of disease) | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) Conclusions do not reflect data reported, therefore the data could not be included in the meta‐analysis See comparison 23 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 2): "Patients were randomly assigned to receive either pimecrolimus 1% cream or metronidazole 1% cream twice daily for 12 weeks." "Randomization was carried out using random‐number generation from standard tables." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | High risk | Quote (251): "Open‐label" Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Quote (251): "Open‐label" Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis, all participants were accounted for. One lost to follow up in pimecrolimus group (deterioration of disease) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | High risk | Substantial baseline imbalance between groups: mean of inflammatory lesion count at baseline was higher in pimecrolimus group, 26.0 ± 11.7, versus 16.0 ± 4.6 in metronidazole group and disease duration was also longer in pimecrolimus group, 33.7 ± 33.4 months versus 16.8 ± 18.3 months in metronidazole group Study duration adequate, wash‐out period before study adequate, groups treated equally, sponsorship or support and other potential conflicts of interest not reported Comment: Baseline imbalance may be a result of 'failed' randomisation. We judged this as at a high risk of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Dermatological Practice Kassel, Germany | |
| Participants | Randomised: 30 participants (age unclear, 11 male, 19 female) Inclusion criteria
Exclusion criteria: Not stated Dropouts and withdrawals: Not stated | |
| Interventions | Six weeks Intervention
Comparator
Unclear how many in each group | |
| Outcomes | Assessments (3): baseline, week 3 and 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Poster, very limited reporting of trial details and outcomes data One of our primary outcomes was addressed (adverse events) See comparison 61 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 143‐4): "A double‐blind, randomised, placebo controlled clinical study..." and "Patients randomly received either..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 143‐4): "Double‐blind." and "...coated tablets with 200 mg sodium salt of dark sulfonated shale oil, died substance per tablet, or optically identical coated tablets without any active ingredient." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 143‐4): "Double‐blind." and "...coated tablets with 200 mg sodium salt of dark sulfonated shale oil, died substance per tablet, or optically identical coated tablets without any active ingredient." Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Poster with a lot of missing data Comment: Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Quote (page 143): "To evaluate the efficacy and tolerance..." Primary and secondary outcomes unclear, difficult to judge if all outcomes were addressed. Subjective reporting of several outcomes unsupported by data Comment: Insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Study duration adequate, wash‐out period unclear, unclear if groups were treated equally, sponsorship, support unreported Comment: Inadequate trial details to enable a clear judgement |
| Methods | RCT, prospective, active‐ and placebo‐controlled (3‐armed study), double‐blind Date of study Outpatient Clinic of Dermatology at Ankara Education and Research Hospital, Turkey | |
| Participants | Randomised: 63 participants (mean age 51 years (range 20 to 80), 15 male, 48 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 0 Papules; permethrin group 6.04 (7.60), metronidazole group 8.00 (6.70), placebo group 4.85 (4.10) Pustules; permethrin group 2.30 (3.73), metronidazole group 4.90 (4.78), placebo group 2.60 (3.36) Demodex folliculorum; permethrin group 2.20 (1.04), metronidazole group 2.60 (0.74), placebo group 2.70 (0.80) | |
| Interventions | Two months Intervention
Comparator 1
Comparator 2
| |
| Outcomes | Assessments (5): baseline, day 15, 30, 45 and 60 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported. However, investigators thanked Glaxo‐Wellcome, quote (page 269): "The authors thank Glaxo‐Wellcome for their contributions to packaging the two drugs and the placebo in identical boxes." | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) Data on number of papules, pustules and Demodex folliculorum were skewed See comparison 1, 16 and 17 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 266): "They were randomly assigned to three groups to receive permethrin (n = 23), metronidazole (n = 20) and placebo (n = 20)." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 266): "Patients were given permethrin 5% cream, metronidazole 0.75% gel, placebo cream in packages looking identical." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 266): "Patients were given permethrin 5% cream, metronidazole 0.75% gel, placebo cream in packages looking identical." Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals reported. ITT analysis Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Appears to have been in part sponsored by Glaxo Wellcome (page 269). Wash‐out period unreported. Unclear if concomitant therapy that might influence rosacea was allowed Comment: Insufficient information to assess whether important risk of bias exists |
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Unreported Department of Dermatology, Mount Sinai Medical Center, New York and Chicago, Illinois, US | |
| Participants | Randomised: 63 participants (age range 25 to 80, 21 male, 42 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of papules; sulphacetamide and sulphur group 12.1 and metronidazole group 13.5 Number of pustules; sulphacetamide and sulphur group 4.6 and metronidazole group 3.3 | |
| Interventions | Eight weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 2, 4, 6 and 8 Outcomes of the trial (as reported)
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 191): "This study was supported by a grant of Dermik laboratories." | |
| Declaration of interest | Two of the investigators are employed by Dermik Laboratories, however none declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events), however data were not reported, only that there was no statistical difference between the two groups See comparison 21 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (192): "...were randomly assigned to the two treatment groups." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 191): "...investigator blinded." The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 191): "...investigator blinded." |
| Incomplete outcome data (attrition bias) | Unclear risk | Six participants withdrawn, 5 in the sulphacetamide and sulphur group, 1 in metronidazole group Comment: Unclear whether dropouts were included in analysis. Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | High risk | No data were available for "participants evaluation of overall response", "cosmetic acceptability as considered by participant", and "willingness to use again by participant". Only information reported that "there was no statistical difference between the 2 groups" Comment: We judged this as at a high risk of bias. Participant's evaluation is one of the principal outcome measures |
| Other bias | Unclear risk | Study duration adequate, wash‐out period before study adequate, groups were treated equally. The sodium sulphacetamide group tended to have greater overall severity scores and greater number of pustules but this was not statistically significant. Supported by a grant from Dermik Laboratories, 2 investigators were employees of Dermik Laboratories Comment: Insufficient information to assess whether important risk of bias exists |
| Methods | Randomised, prospective, active‐controlled, investigator‐blinded Setting | |
| Participants | Randomised: 30 participants (mean age 45 years, all female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean | |
| Interventions | Four weeks
Comparator 1
Comparator 2
The women were instructed to apply the supplied sunscreen daily and to wear protective clothing when exposed to sun | |
| Outcomes | Assessments (3): baseline, week 2 and 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 1185): "The study was funded by OMP, Inc" | |
| Declaration of interest | Quote (page 1185): "Dr Leyden has been an investigator and consultant for OMP, Inc" | |
| Notes | One of our primary outcomes was addressed (participant assessed changes in rosacea severity) See comparison 29, 30 and 31 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1180): "patients were randomly assigned (in a 1:1:1 ratio)" |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 1179): "...investigator blinded." Comment: The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 1179): "...investigator blinded." Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study. |
| Incomplete outcome data (attrition bias) | Low risk | 1/30 (3.3%); metronidazole plus standard skin care group, reason reported Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Treatment duration adequate, wash‐out period before study started adequate Comment: The study appeared to be free of other forms of bias |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 92 participants (mean age 51.2 years, 25 male, 67 female) Inclusion criteria:
Ocular involvement: Unclear
Dropouts and withdrawals: None reported Baseline data mean | |
| Interventions | 12 weeks
Comparator 1
Comparator 2
| |
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | Quote (page 688): "The author has not disclosed any relevant conflicts" | |
| Notes | None of our primary outcomes was addressed See comparison 18 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 685): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 685): "...double‐blind" Comment: The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 685): "...double‐blind" Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts reported Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available (NCT00940992), and the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate, no wash‐out period before study started described. Limited information Comment: There was insufficient information to permit a clear judgement |
| Methods | Randomised, prospective, placebo‐controlled Date of study Unreported Setting | |
| Participants | Randomised: 61 participants (mean age 51.7 years, 13 male, 48 female) Inclusion criteria
No ocular involvement
Dropouts and withdrawals
Baseline data mean (SD) Total rosacea standard grading system (Wilkin 2004); TDT 068 7.8 (1.66), vehicle 8.1 (1.73) | |
| Interventions | Four weeks Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline, week 2 and 4 (and 2 phone calls, one at week 1 and one at week 5) Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 1): Editorial assistance with the preparation of the manuscript was provided by Bollin Strategies Ltd., UK, and was funded by Pro Bono Bio Entrepreneur Ltd., UK | |
| Declaration of interest | Quote (page 1): "T. Luger and N. Peukert have no conflict of interest to declare. M. Rother is a paid consultant of Pro Bono Bio Entrepreneur Ltd" | |
| Notes | Two of our primary outcomes were addressed (quality of life and adverse events) See comparison 37 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 2): "..were stratified in a 4:1 female/male ratio and randomized according to a random permuted block scheme in a 2:1 ratio .." |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 2): "The investigator and other study team members involved in the evaluation of the safety and efficacy end‐points, the patients, the monitors, the sponsor and clinical research organization staff remained blinded to treatment until database lock." Comment: The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement Comment: Blinding ensured |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 2): "The investigator and other study team members involved in the evaluation of the safety and efficacy end‐points, the patients, the monitors, the sponsor and clinical research organization staff remained blinded to treatment until database lock." |
| Incomplete outcome data (attrition bias) | Low risk | 6/61 (9.8%); TDT 068 (3), vehicle (3), reasons reported. Per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available (NCT01666509), and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
| Methods | Randomised, prospective, active‐controlled, open‐label Date of study Unreported Setting | |
| Participants | Randomised: 12 participants (mean age 49.8 years, gender unreported) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals: None reported Baseline data mean | |
| Interventions | One or two treatments
Comparator
Unclear how many were randomised to each group | |
| Outcomes | Assessments (4): baseline, week 2, 4 and week 12/13 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page AB43): "Supported by Ulthera" | |
| Declaration of interest | None declared | |
| Notes | Two of our outcomes were addressed (participant‐assessed changes in rosacea severity, and adverse events). After 3 attempts failed to contact PI for further details (see Table 3 and Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page AB 43): "were randomized.." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | High risk | No blinding reported |
| Blinding of outcome assessment (detection bias) | High risk | No blinding reported. Investigator and participant assessed outcomes Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided. Protocol available at clinicaltrials.gov NCT01756027 Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | RCT, prospective, active‐controlled, double‐blind, within‐patient comparison Date of study Division of Dermatology Skin Care Centre at University of British Columbia, Canada | |
| Participants | Randomised: 40 participants (mean age 52.2 years for males and 49.6 years for females, 11 male, 29 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 3/40 (7.5%)
Baseline data mean (SEM) | |
| Interventions | 15 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 8 and 9 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 961): "Supported by a grant provided by Allergan, Inc." | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 14 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 962): "A single‐center, randomized, double‐blind, contralateral, split‐face..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 962): "...double‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 962): "...double‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study |
| Incomplete outcome data (attrition bias) | Low risk | The 3 withdrawals were accounted for and reasons for withdrawal reported. ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study adequate, additional medications that might influence outcome were not allowed Comment: We judged this as at a low risk of bias |
| Methods | RCT, prospective, active‐controlled and placebo‐controlled (3‐armed study), double‐blind Date of study Unreported Setting | |
| Participants | Randomised: 64 participants (mean age 47.8 years, 27 male, 29 female and 8 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | Six weeks Intervention
Comparator 1
Comparator 2
Number of participants reported as having completed the trial, but unclear how many were initially randomised to each group | |
| Outcomes | Assessments (8): baseline, week 1, 2, 3, 4, 5, 6 and 7 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 1051): "We are grateful to Pfizer Ltd. for supplying the tetracycline, ampicillin, and placebo packed in identical capsules; and to Miss C. Pullin, of the Wellcome Research Laboratories, for statistical analysis of the results. R. M. is in receipt of a grant from the Medical Research Council." | |
| Declaration of interest | None declared | |
| Notes | Total number randomised not explicitly stated. 56 participants completed the trial, but at least 64 participant numbers were allocated so it is possible that eight or more participants dropped out Dosage of ampicillin not reported Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events). Data on lesions counts were quite skewed See comparison 42, 46 and 47 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1049): "Dispensing of study medications randomly allocated to one of 3 coded treatment groups by hospital dispensary." Comment: Appears to have been done centrally by the dispensary. Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: Central allocation by the dispensary Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 1049): "The placebo, tetracycline, and ampicillin were supplied in identical capsules." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 1049): "The placebo, tetracycline, and ampicillin were supplied in identical capsules." Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Total number randomised not explicitly stated. 56 participants completed the trial, but at least 64 were allocated, so eight or more participants dropped out. Unclear how many participants were initially randomised in each group. Further one withdrawal in the placebo group Comment: Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration short (6 weeks), wash‐out period at start of the study adequate, no concomitant medication that might influence rosacea were allowed Quote (page 1051): "We are grateful to Pfizer Ltd. for supplying the tetracycline, ampicillin, and placebo packed in identical capsules;.... R. M. is in receipt of a grant from the Medical Research Council." Comment: We judged this as at a low risk of bias |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 33 participants (mean age 46.9 years in metronidazole 0.75% gel + placebo capsules group, and 50.7 years in placebo gel + oxytetracycline group, 8 male and 8 female versus 9 male and 8 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Erythema grade; metronidazole group 2.5 and oxytetracycline group 2.4 | |
| Interventions | Nine weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 3, 6 and 9 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 91): "We wish to thank Bioglan Laboratories for kindly providing the materials for this study" | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 56 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 91): "The patients were randomly allocated in a double‐blind fashion of treatment." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 91): "...placebo gel (having the same base as the active preparation)." Comment: Assuming the placebo capsules were similar. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 91): "Double‐blind" "...placebo gel (having the same base as the active preparation)." Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 6/33 (18.2%); metronidazole group (4) and oxytetracycline group (2). Incomplete outcome data were adequately addressed, reasons for withdrawal were reported. Per‐protocol analysis Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out period before start of the study adequate, no concomitant rosacea therapy was allowed. Additional therapy was noted Comment: We judged this as at low risk of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Setting Only data from the first 4 weeks included. Study biased after 4 weeks | |
| Participants | Randomised: 64 participants (age unclear, 19 male, 39 female, 6 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Nothing reported | |
| Interventions | Four weeks, then a further four weeks for participants who showed improvement Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 2, 4, 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed. Only first 4 weeks included. Study biased after 4 weeks as people who did not respond to treatment "were dropped from the study" (page 187) See comparison 18 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 186): "This randomized, double‐blind...". Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 186) : "...matching placebo gel." "double‐blind" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 186) : "...matching placebo gel." "double‐blind" Outcomes were investigator assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | High risk | Participants who showed no improvement at end of the first 4 weeks were dropped from the study. Per‐protocol analysis (page 186‐7). Only data up to week 4 are considered Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate (bit short), sponsorship or support unreported. Other topical or systemic treatment were not allowed Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, active‐controlled, 3‐armed, double‐blind, within‐patient comparison Date of study Unreported Department of Dermatology and Venereology, Faculty of Medicine, Zagazig University, Zagazig, Egypt | |
| Participants | Randomised: 24 participants (mean age 51.08 ± 5.9 years (range 42 to 61), 1 male, 23 female) Inclusion criteria
Ocular Involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Nothing reported Baseline data mean (SD) Inflammatory lesion count; azelaic acid group 4.2 (2.7), metronidazole 5.4 (3.3), permethrin group 4.5 (3.7) | |
| Interventions | 15 weeks Intervention
Comparator 1
Comparator 2
| |
| Outcomes | Assessments (11): baseline, week 3, 6, 9 and 15, and then monthly for another 6 months Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes None ✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | Quote (page 22): "None declared" | |
| Notes | One of our primary outcomes was addressed (adverse events). Participant' assessment was evaluated but not regarding rosacea severity. Investigators conclusions were based on the analysis of skewed and unreliable data analysis See comparison 15 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 23): "The 24 patients were randomly allocated to three groups." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 23): "...double‐blind comparison of azelaic acid 20% cream, metronidazole 0.75% cream and permethrin 20% cream." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 23): "...double‐blind comparison of azelaic acid 20% cream, metronidazole 0.75% cream and permethrin 20% cream." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts unclear Comment: Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | High risk | Not all pre‐specified outcomes are addressed adequately or reported completely, e.g. photographic assessment and Physician's Gobal Assessment Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period unclear, and unclear if groups were treated equally Comment: Insufficient information to assess whether an important risk of bias exists |
| Methods | Randomised, prospective, active and placebo‐controlled, single‐blind Date of study November 2005 to May 2006 | |
| Participants | Randomised: 400 participants (age and gender unreported) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals: One participant randomised in error but did not receive treatment, for rest nothing reported Baseline data mean (SD) Nothing reported | |
| Interventions | 12 weeks Intervention
Comparator 1
Comparator 2
Comparator 3
Comparator 4
Unclear how many were randomised to each group | |
| Outcomes | Assessments (6): baseline, week 2, 4, 8, 12 and 13 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Allergan sponsored the study | |
| Declaration of interest | No information on clinicaltrials.gov | |
| Notes | Study has been completed May 2006. Website accessed 19‐7‐2014 additional information on http://www.allerganclinicaltrials.com/results/medical_aesthetics.htm One of our primary outcomes was addressed (adverse events). Unclear how many were randomised to each group (see Table 6), no reply from Allergan after several email attempts (see Table 3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (published as pdf on Allergan website ): "Randomization: Subjects were assigned in a 1:1:1:1:1 ratio to the five treatment groups" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (clinicaltrials.gov): "...single‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (clinicaltrials.gov): "...single‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropout reported. ITT. Limited data available Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | No exact data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Limited data are provided Comment: There was insufficient information to permit a clear judgement |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Unreported Multicentre (US) | |
| Participants | Randomised: 130 participants (mean age 49.4 years, 44 male, 86 female) Inclusion criteria
Ocular Involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | All participants receive doxycycline 40 mg and metronidazole gel 1% once daily during phase 1 (baseline to week 12) Intervention
Comparator
| |
| Outcomes | Assessments (11): baseline, every 4 weeks up to 40 weeks Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (clinicaltrials.gov) "Sponsor: Galderma Laboratories, L.P." | |
| Declaration of interest | Quote (clinicaltrials.gov) "Principal Investigators are not employed by the organization sponsoring the study" | |
| Notes | Study includes two phases; first phase (12 weeks) all participants (230) received doxycycline 40 mg combined with topical metronidazole gel. We only included the randomised second phase. All our primary outcomes were addressed Information found on clinicaltrial.gov and of a poster provided by Galderma See comparison 44 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (clinicaltrials.gov): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (clinicaltrials.gov): "...double‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (clinicaltrials.gov): "...double‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study |
| Incomplete outcome data (attrition bias) | High risk | 85/130 (65%); 38/65 in doxycycline group, 47/65 in placebo group, reasons reported and 27 were due to relapse (primary endpoint for this study) Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol was available at clinicaltrials.gov. The pre‐specified outcomes appeared to have been reported in addition to RosaQol scores and a patient satisfaction questionnaire Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Treatment duration adequate, groups treated equally. Not all study data are available as study is not published yet Comment: There was insufficient information to permit a clear judgement |
| Methods | Randomised, prospective, active and placebo‐controlled, double‐blind Date of study September 2011 to February 2012 | |
| Participants | Randomised: 36 participants (mean age 47.4 years, 12 male, 24 female) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts withdrawals
Baseline data mean (SD) | |
| Interventions | 12 weeks Intervention
Comparator 1
Comparator 2
Application frequency not reported | |
| Outcomes | Assessments (8): baseline, week 1, 2, 4, 8, 10, 12 and 14 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes prespecified for this review | |
| Funding source | Sponsor: Novartis Pharmaceuticals | |
| Declaration of interest | No information on clinicaltrials.gov | |
| Notes | Study was completed December 2012. Website accessed 19‐7‐2014. Data reported on: http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public See comparison 36 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (on Novartis website): "This was a multicenter, randomized, blinded, comparator‐ and vehicle‐controlled study". Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (clinicaltrials.gov): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (clinicaltrials.gov): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study |
| Incomplete outcome data (attrition bias) | Low risk | 4/36 (11.1%); BFH772 (1), vehicle (1), metronidazole (2), reasons reported. Per‐protocol analysis. Comment: Low and balanced number of dropouts and although per‐protocol analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Treatment duration adequate, no wash‐out period before start of study reported, groups treated equally |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study | |
| Participants | Randomised: 92 participants (mean age 54.1 years (SD 12.8), 36 male, 56 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 4/92 (4.3%), unclear from which group Baseline data N (%) CEA moderate; brimonidine group 20 (41.7), vehicle group 25 (56.8) | |
| Interventions | Eight days
Comparator
| |
| Outcomes | Assessments (3): baseline, day 2, and day 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Sponsor: Galderma | |
| Declaration of interest | No information on clinicaltrials.gov | |
| Notes | This study was completed November 2013. Website accessed 21‐7‐2013. Galderma provided additional data See comparison 13 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (clinicaltrials.gov): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (clinicaltrials.gov): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (clinicaltrials.gov): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | 4/92 (4.3%), unclear from which group, analysis not clear |
| Selective reporting (reporting bias) | Unclear risk | Data incomplete poster/conference abstract (EADV 2014) full study not yet published Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Limited data are provided Comment: There was insufficient information to permit a clear judgement |
| Methods | RCT, prospective, active‐controlled and controlled with "no treatment, investigator‐blinded, within‐patient comparison Date of study Unspecified Dermatologic Surgery and Laser Center, Department of Dermatology, University of California, San Francisco, US | |
| Participants | Randomised: 30 participants (mean age 45.8 ± 10.6 years, 9 male, 20 female and 1 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Nothing reported | |
| Interventions | Three treatment sessions, each month Intervention
Comparator 1
Comparator 2
| |
| Outcomes | Assessments (4): baseline, month 1, 2 and 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 927): "This study was funded by the American Society for Dermatologic Surgery Cutting Edge Research Grant." | |
| Declaration of interest | Quote (page 920): "The authors have indicated no significant interest with commercial supporters" | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 64 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 921): "Treatment randomization was performed using a random number generator." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 921): "Only the patient and the investigator performing the therapies were aware of their treatment allocation." "A blinded investigator gave.." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 921): "Only the patient and the investigator performing the therapies were aware of their treatment allocation." "A blinded investigator gave.." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 922): "One patient in the IPL/control group dropped out after first treatment because of excessive swelling reaction. This patient did not return for follow‐up. Remaining 29 patients completed all three treatment sessions." Comment: Low number of dropouts and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | High risk | No exact data are provided, only P values. All outcome measures are addressed but without exact data Comment: We judged this as at a high risk of bias |
| Other bias | Low risk | No wash‐out period before study, study duration adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study January to February 1982 Setting | |
| Participants | Randomised: 81 participants (mean age 47 years, 32 male, 49 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean Number of papules; metronidazole group 23.8 and placebo group 27.5 Number of pustules; metronidazole group 0.6 and placebo group 1.0 | |
| Interventions | Two months Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline, month 1 and 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 332): "The assays of plasma metronidazole were kindly performed by A/S Dumex Laboratories, | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 1 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 328): "....in accordance with a randomized administration scheme." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 327): "...double‐blind." Comment: Although not explicitly stated it would appear that the active intervention and placebo cream were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 327): "...double‐blind." Outcomes were investigator‐ and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 4/81 (4.9%); metronidazole group (1) and placebo group (3) due to absence of improvement. Withdrawals/dropouts were accounted for but not included in the analysis. Per‐protocol analysis Comment: Low number of drop‐outs and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | A/S Dumex manufactured the test creams. Study duration adequate, no additional rosacea treatment allowed, adequate wash‐out period before study started Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 51 participants (mean age 44 years, 17 male, 34 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | Two months Intervention
Comparator
| |
| Outcomes | Assessments (2): baseline and month 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 65): "Coded tablets and test creams were kindly provided by A/S Dumex, Copenhagen" | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes was addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 56 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 63): "Fifty‐one randomly selected patients etc..." "Patients were assigned at random to one of the two courses of treatment." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Low risk | Quote (page 65): 'Coded tablets and test creams were kindly provided by A/S Dumex." Comment: Form of central allocation. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 63): "...double‐blind." Comment: Although not explicitly stated in the report it would appear that the active interventions were matched with similar and indistinguishable placebos |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 63): "...double‐blind." Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | The dropouts are accounted for and included in ITT analysis Comment: Low number of dropouts combined with ITT analysis, judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate study duration, adequate wash‐out period before study started. No additional rosacea therapy allowed Comment: The study appeared to be free of other forms of bias |
| Methods | Randomised, active‐controlled, investigator‐blind, within‐patient comparison Date of study Unreported Setting Dermatology Department of Bispebjerg Hospital, Copenhagen, Denmark | |
| Participants | Randomised: 40 participants (mean age 54 years, gender unreported) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data mean Nothing reported | |
| Interventions | Three treatments at six week intervals Intervention
Comparator
| |
| Outcomes | Assessments (2): baseline and month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes:
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 143): "Dermatologic Development, Hørsholm, Denmark lent the Ellipse Flex. | |
| Declaration of interest | Quote (page 143): "None declared" | |
| Notes | 31 had telangiectasia related to rosacea, one had telangiectasia due to treatment with corticosteroids, and seven had idiopathic telangiectasia (and one died) Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 65 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 144): "Patients were randomly allocated.." and "Randomization was carried out by patients drawing lots between opaque sealed envelopes, containing cards with subject number and split side treatment code" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 144): "Randomization was carried out by patients drawing lots between opaque sealed envelopes, containing cards with subject number and split side treatment code" |
| Blinding of participants and personnel (performance bias) | High risk | Investigators and participants were not blinded during the treatment phase Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 145): "Clinical efficacy was evaluated by one blinded trained physician" Outcomes were investigator and participant‐assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers) during the study. |
| Incomplete outcome data (attrition bias) | Low risk | 1/40 (2.5%); died not related to treatment. Per‐protocol analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period adequate before study started Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Department of Dermatology, Bristol Royal Infirmary, Bristol, UK | |
| Participants | Randomised: 29 participants (age 24 to 86 years, gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 1 in each group because of headache Baseline data mean (SD) | |
| Interventions | Six weeks Intervention
Comparator
| |
| Outcomes | Assessments (2): baseline and week 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review | |
| Funding source | Nothing reported | |
| Declaration of interest | Nothing declared | |
| Notes | None of our primary outcomes were addressed See comparison 55 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1212): "The treatment was allocated at random..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 1212): "The treatment was allocated at random without the knowledge of the doctor or the patient." The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 1211): "...double‐blind" "...metronidazole 200 mg twice daily or a lactose placebo tablet.." Comment: Although not explicitly stated it would appear that the active intervention and placebo tablets were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 1211): "...double‐blind." "...metronidazole 200 mg twice daily or a lactose placebo tablet.." Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 2 withdrawals in each group were accounted for. Per‐protocol analysis Comment: Low number of dropouts and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration short, unclear if groups were treated equally and if additional rosacea therapy was allowed, adequate wash‐out period before study Comment: Insufficient information to assess whether an important risk of bias exists |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 40 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean | |
| Interventions | 12 weeks Intervention
Comparator
No other skin care products or emollients were allowed for the duration of the study | |
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page AB64): "100% supported by Nu Skin Enterprises" | |
| Declaration of interest | Quote (page AB64): "Salary support through clinical trials sponsored by Nu Skin Enterprises" | |
| Notes | Poster abstract, no results presented, limited data (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page AB64): "Each subject was randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page AB64): "double‐blind." Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page AB64): "double‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study. Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | No data provided Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Outcomes unclear and no data provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Multicentre. Department of Dermatology, University of Athens, Andreas Aygros Hospital, Athens, Greece; IRIS, Institute de Recherches et d'Innovations Scientifiques, Paris, France; 4 Private Practices, Germany | |
| Participants | Randomised: 246 participants (mean age 48.9 years (range 18 to 80), 34 male and 91 female in treatment group, 36 male and 85 female in placebo group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Rosacea overall severity; treatment group 3.21 (0.1) and placebo group 3.3 (0.08) | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 568): "This study was supported by a grant from the research Division of the European Council – 5th plan." | |
| Declaration of interest | Quote (page 568): "None of the authors has any conflict of interest to declare including financial arrangements, interest or share holding options with the company manufacturing the product." | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events). The review authors imputed SDs for mean reduction from baseline in rosacea severity score using 3 correlations between the baseline and final measurements See comparison 33 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 565): "This multicentre, randomized, double‐blind, parallel group, placebo‐controlled, study..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 564‐5): "Double‐blind" and "As the active ingredient resulted in a slightly coloured final product, colour of placebo (vehicle) was adjusted accordingly." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 564‐5): "Double‐blind" and "As the active ingredient resulted in a slightly coloured final product, colour of placebo (vehicle) was adjusted accordingly." Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | High risk | Only 96 participants in the treatment group and 100 in the placebo group appear to be included in analysis. The analysis excluded the remaining participants (20%) because of "missing grade values for any examination" (quote page 566). Per‐protocol analysis Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out period adequate, study duration adequate, groups treated equally. Study was supported by a grant from the research Division of The European Council ‐ fifth plan None of the authors have any conflicts of interest to declare, including financial arrangements Comment: The study appeared to be free of other forms of bias |
| Methods | Randomised, prospective, active‐controlled, double‐blind Setting | |
| Participants | Randomised: 34 participants (mean age unreported, 11 male, 20 female, 3 gender unreported) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data mean | |
| Interventions | 45 days Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 2, 4, 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 38 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 128): "se dividieron al azar en dos grupos" (were divided at random in two groups) |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 126): "estudio doble ciego" (double‐blind) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 126): "estudio doble ciego" (double‐blind) Comment: Outcomes were investigator and participant‐assessed. Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Low risk | 3/34 (8.8%) of benzyl benzoate group, lost to follow‐up (2), pregnancy (1). Per‐protocol analysis Comment: Unbalanced, but low number of follow up, judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, no wash‐out period before study described, groups treated equally Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Department of Dermatology, Bristol Royal Infirmary, Bristol, UK | |
| Participants | Randomised: 40 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not specified Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline, week 6 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review | |
| Funding source | Nothing reported | |
| Declaration of interest | Nothing declared | |
| Notes | Although independent assessments were made by the participant and 2 doctors these were combined and presented as a composite score See comparison 48 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 443): "...treated...on a random double‐blind basis." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Low risk | Quote (page 443): "...coded tablets being issued by the pharmacist." Comment: Pharmacy‐controlled, probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 443): "Double‐blind basis...coded tablets." Comment: Although not explicitly stated it would appear that the active intervention and placebo tablets were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 443): "Double‐blind basis...coded tablets." Outcomes were investigator assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Two dropouts were accounted for but not included in analyses. Per‐protocol analysis Comment: Low number of dropouts and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period before study adequate. No sponsorship or conflict of interest reported Comment: Insufficient information to assess whether an important risk of bias exists |
| Methods | Randomised, prospective, active‐controlled, single‐blind Date of study Setting | |
| Participants | Randomised: 120 participants (mean age 36.1 years (SD 12.4), 56 male, 64 female) Inclusion criteria
Ocular involvement: Participants with ocular manifestations were included
Additionally in patients with anterior blepharitis
Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | Two weeks
Comparator
| |
| Outcomes | Assessments (5): baseline, week 1, 2, 3 and 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | Quote (page e347): "Conflict of Interest: None" | |
| Notes | None of our primary outcomes was addressed See comparison 59 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page e344): "randomly assigned to either combined therapy or ivermectin treatment at a ratio of 1:1 (15 patients for each treatment regimen from each group) using a computer‐generated randomization schedule." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (page e344): "The assignment was done in a single‐blinded manner, in which the subjects were blinded to the treatment assignment." Comment: It looks like allocation concealment is confused with blinding, we did not receive additional information of the principal investigators. The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page e344): "The assignment was done in a single‐blinded manner, in which the subjects were blinded to the treatment assignment." Comment: Investigators were not blinded. The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | High risk | Quote (page e344): "Assessment of the outcome samples was done by two unblinded parasitologists and then reviewed by another independent blinded professor of parasitology to avoid bias" |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up. Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before start of study adequate, groups treated equally |
| Methods | RCT, prospective, "placebo"‐controlled (placebo tablets, but also topical metronidazole), double‐blind Date of study Unreported Unspecified, US | |
| Participants | Randomised: 40 participants (mean age 41.6 years in metronidazole + doxycycline group versus 38.8 years in metronidazole + placebo group, 3 male and 17 female versus 5 male and 15 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline, week 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 791): "Supported by CollaGenex Pharmaceuticals, Inc." | |
| Declaration of interest | Quote (page 791): "Conflicts of interest: None identified". One of the investigators was employed by CollaGenex | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 53 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 792): "Randomization was accomplished by assigning numbers to the sub antimicrobial dose doxycycline and placebo bottles based on the SAS statistical software randomization procedure. Each patient entering the study received the next sequentially numbered bottle." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Unclear if this was done 'centrally'. There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 792): "...double‐blind." and "All study tablets were identical in size, shape, and colour (white)." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 792): "...double‐blind." and "All study tablets were identical in size, shape, and colour (white)." Outcomes were investigator and participant‐assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for withdrawal were reported. ITT analysis (LOCF) was carried out Comment: Low number of dropouts, ITT analysis, judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period adequate. Concomitant medications that could influence rosacea were prohibited Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Multicentre, Dermatology department of different centres in Canada and UK | |
| Participants | Randomised: 103 participants (mean age 50 years, 40 male, 63 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals | |
| Interventions | Eight weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 1, 4 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 85): "This study was funded in part by GenDerm Corporation, Lincolnshire, Illinois" | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events). Unclear how many participants in each group, although it was reported "comparable numbers". Skewed data See comparison 20 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 80): "...dispensed to patients in a randomized, double‐blind fashion." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 80): "...double‐blind fashion..." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 80): "...double‐blind fashion..." Outcomes were investigator‐ and participant assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | 9/103 (8.7%); treatment group (2) and placebo group (1), due to adverse events, for the remaining 6 it is unclear from which group, but were also excluded from the analysis Comment: Low number of dropouts, but unclear how many started in each group and how many dropped out in each group, judged as unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration rather short, wash‐out period before study adequate, no concomitant medication allowed Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Multicentre, Women's College Hospital, Toronto, Ontario; General Practice in Ontario, and Rhône Poulenc Rorer Canada, Montreal, Quebec, Canada | |
| Participants | Randomised: 125 participants (mean age 45.4 ± 1.3 (SEM), 40 male, 61 female, 24 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 24/125 (19.2 %) withdrew for reasons not related to treatment, unclear from which group | |
| Interventions | Two months Intervention
Comparator
Only number of participants that completed the study | |
| Outcomes | Assessments (3): baseline, month 1 and 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes None | |
| Funding source | None reported, but one investigator was employed by Rhone Poulenc Rorer Canada, manufacturer of metronidazole | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were assessed (participant‐assessed changes in rosacea severity and adverse events) See comparison 56 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 221): "Patients were randomly administered either metronidazole cream and placebo capsules or placebo cream and tetracycline capsules." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 221): "Placebo and active cream and capsules were matched as appropriate." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 221): "Placebo and active cream and capsules were matched as appropriate." Outcomes were investigator‐ and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | High risk | Unclear how many participants were actually allocated to each group. After randomisation 24/125 (19%) withdrew from the study for reasons unrelated to treatment. Additional withdrawals occurred during the course of the study (9) Tetracycline group 2/52 discontinued because of adverse events The dropouts and withdrawals were not included in the analysis Comment: The high dropout rate and the per‐protocol analysis represents a potentially high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Data presented in graphs had to be extracted from figures All pre‐specified outcomes appear to have been addressed Comment: Insufficient information to permit a clear judgement |
| Other bias | Low risk | Wash‐out period adequate, study duration adequate, groups treated equally, sponsorship or support not reported Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Florida Eye, Microsurgical Institute, Florida, US | |
| Participants | Randomised: 37 participants (age 75.6 years in cyclosporine group and 69.6 years in artificial tears group, 15 males and 6 females in cyclosporine group and 9 males and 7 females in artificial tears group) Inclusion criteria
Exclusion criteria Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | Three months Intervention
Comparator
| |
| Outcomes | Assessments (2): baseline, month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 658): "This study was funded by an unrestricted educational grant from Allergan, Inc." | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (quality of life) See comparison 27 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 653): "Patients were randomised by computer." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 653): "...double‐masked clinical trial." "The vials for each product were identical, ensuring patient and clinicians masking." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 653): "...double‐masked clinical trial." "The vials for each product were identical, ensuring patient and clinicians masking." Outcomes were investigator‐ and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 3/37 (8.1%) Dropouts and withdrawals reasons were clarified. Per‐protocol analysis Comment: Low number of dropouts and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcomes were addressed, other than one of the secondary outcome measures, the quality of excreta of the Meibomian glands Comment: We judged this as at an unclear risk of bias |
| Other bias | Low risk | Participants were in the older age group and almost exclusively Caucasians. Wash‐out period before study and duration adequate, no additional medication allowed that might influence outcome. The study was funded by an unrestricted educational grant from Allergan, Inc (page 659) Comment: The study appeared to be free of other forms of bias |
| Methods | Randomised, prospective, vehicle‐controlled, double‐blind Date of study | |
| Participants | Randomised: 66 participants (mean age 52 years (SD 11), 19 males, 47 females) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals: None Baseline data mean (SD) | |
| Interventions | Eight weeks Intervention
Comparator
All participants were treated the 8 weeks before with topical metronidazole | |
| Outcomes | Assessments (5): baseline, week 2, 4, 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes ✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 924): "The studies were funded by la Roche‐Posay Pharmaceutica Laboratories France" | |
| Declaration of interest | Quote (page 924): "All the authors except M Skalikova, L. Gibejova and H. Zelenkova, are employees of L'Oréal.' | |
| Notes | The article covers 3 studies, only study 3 is a RCT and is included in this review See comparison 39 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 922): " randomized....." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups After e‐mail communication: "The allocation sequence was generated by a statistician using a specific software" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement No further information after e‐mail communication |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 922): "double blind...." After e‐mail communication: "Both products was in the same packaging (blind white packaging) without any indication about formula reference" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 922): "double blind...." After e‐mail communication: "Both products was in the same packaging (blind white packaging) without any indication about formula reference" Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | The article provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | RCT, prospective, placebo‐controlled, double‐blind, cross‐over Date of study Setting | |
| Participants | Randomised: 25 participants (age 48.2 ± 9.3 years (range 21 to 64), 9 male, 16 female) Inclusion criteria
Ocular involvement: Yes in nine participants Exclusion criteria
Dropouts and withdrawals: 6/25 (24%) for unknown reasons, 5 from placebo group and 1 from treatment group | |
| Interventions | Three months (thereafter cross‐over) Intervention
Comparator
| |
| Outcomes | Assessments (7): baseline, each month up to month 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | We only included data for the first 3 months of the study. One of our primary outcomes was addressed (adverse events) See comparison 57 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 858): "Patients were randomly allocated." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 858): "Zinc sulphate or the identical placebo capsules were given in a double‐blind manner." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 858): "Zinc sulphate or the identical placebo capsules were given in a double‐blind manner." Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | High risk | 6/25 (24%) participants dropped out for unknown reasons and were not included in the analysis Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | For intermediate outcomes only means reported, no SDs. Inadequate reporting of lesion counts Comment: Insufficient information to permit a clear judgement |
| Other bias | Low risk | Wash‐out period adequate, study duration adequate, groups treated equally, sponsorship or support unreported Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 85 participants (mean age 47 years, 26 male, 52 female, 7 gender not reported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | Four weeks Intervention
Comparator
Number of participants that completed the study (78) | |
| Outcomes | Assessments (3): baseline, week 2 and 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed See comparison 42 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 649): "...dispensed by the pharmacist according to a random table." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (649): "...tetracycline 250 mg twice daily or a dummy placebo indistinguishable in appearance." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (649): "...tetracycline 250 mg twice daily or a dummy placebo indistinguishable in appearance." Outcomes were investigator‐assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how many participants were actually randomised to each group. Withdrawals were accounted for for first month. Per‐protocol analysis Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Older study of short duration. Unreported if wash‐out period before study, if concomitant medication was allowed, sponsorship or support Comment: Insufficient information to assess whether an important risk of bias exists |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study December 2011 to July 2013 Setting Multicentre, US and Canada | |
| Participants | Randomised: 683 participants (mean age 50.4 years (SD 12.09) 217 male, 466 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Number of inflammatory lesions; 30.9 (14.33) IGA severe; 123 (18%) participants | |
| Interventions | 12 weeks Intervention
Comparator
Subjects were instructed to avoid rosacea triggers such as sudden exposure to heat, certain foods and excessive sun exposure | |
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 323): "The study was funded by Galderma R&D" | |
| Declaration of interest | Quote (page 323): "The investigators received grants for conducting the studies. Ms Liu and Dr Jacovella are employees of Galderma R&D" | |
| Notes | All our primary outcomes were addressed See comparison 9 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 317): "Randomization lists were generated prior to study initiation by a statistician, and were then sent to a clinical supply group, and only personnel directly involved with labeling and packaging (not site personnel) had access." Comment: Central allocation, probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 317): "Randomization lists were generated prior to study initiation by a statistician, and were then sent to a clinical supply group, and only personnel directly involved with labeling and packaging (not site personnel) had access." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 317): "The integrity of the blinding was ensured by packaging the topical creams in identical tubes with no visible difference between the creams, and requiring a third party other than the investigator to dispense the medication." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator‐assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 59/683 (8.6%); ivermectin group (37), vehicle group (22), reasons reported. Per‐protocol analysis and ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available at clinicaltrials.gov (NCT01493687) and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started not reported, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship/support represented any additional bias |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study December 2011 to August 2013 Setting Multicentre, US and Canada | |
| Participants | Randomised: 688 participants (mean age 50.2 years (SD 12.29) 229 male, 459 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Number of inflammatory lesions; 32.9 (13.70) IGA severe; 166 (24%) participants | |
| Interventions | 12 weeks Intervention
Comparator
Subjects were instructed to avoid rosacea triggers such as sudden exposure to heat, certain foods and excessive sun exposure | |
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 323): "The study was funded by Galderma R&D" | |
| Declaration of interest | Quote (page 323): "The investigators received grants for conducting the studies. Ms Liu and Dr Jacovella are employees of Galderma R&D" | |
| Notes | All our primary outcomes were addressed See comparison 9 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 317): "Randomization lists were generated prior to study initiation by a statistician, and were then sent to a clinical supply group, and only personnel directly involved with labeling and packaging (not site personnel) had access." Comment: Central allocation, probably done. |
| Allocation concealment (selection bias) | Low risk | Quote (page 317): "Randomization lists were generated prior to study initiation by a statistician, and were then sent to a clinical supply group, and only personnel directly involved with labeling and packaging (not site personnel) had access." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 317): "The integrity of the blinding was ensured by packaging the topical creams in identical tubes with no visible difference between the creams, and requiring a third party other than the investigator to dispense the medication." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were investigator‐assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 59/683 (8.6%); ivermectin group (37), vehicle group (22), reasons reported. Per‐protocol analysis and ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available at clinicaltrials.gov (NCT01493687) and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started not reported, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship/support represented any additional bias |
| Methods | Randomised, prospective, active‐controlled, investigator‐blinded Date of study Setting | |
| Participants | Randomised: 962 (mean age 51.5 years (SD 13.3), 335 male, 627 female) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data mean (SD) | |
| Interventions | 16 weeks Intervention
Comparator
| |
| Outcomes | Assessments (6): baseline, week 3, 6, 9, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Epub page 1 "This study was funded by Galderma R&D." | |
| Declaration of interest | Epub page 1 "The investigators received grants for conducting the studies. Mrs. Peirone and Mr. Jacovella are employees of Galderma R&D." | |
| Notes | All our primary outcomes were addressed See comparison 25 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (Epub): "randomized" |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (Epub): "...investigator‐blinded." Comment: The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement Comment: Blinding investigators ensured, low risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (Epub): "...investigator‐blinded." |
| Incomplete outcome data (attrition bias) | Low risk | 50/962 (5.2%); ivermectin group (32), metronidazole group (28), reasons reported. Authors state to have performed an ITT analysis (LOCF) Comment: Low and balanced number of dropouts, combined with ITT analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available at clinicaltrials.gov (NCT01493947) and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Treatment duration adequate, wash‐out period not described, groups treated equally |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Multicentre (6), Canada (Windsor, Ontario; Montreal, Quebec; Alberta, Quebec; Waterloo, Ontario; Sainte‐Foy, Ontario; Winnipeg, Manitoba) | |
| Participants | Randomised: 120 participants (mean age 51 years (treatment group) versus 47.7 years (placebo group), 31 male, 89 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Erythema score; metronidazole group 2.13 (0.04) and placebo group 2.10 (0.04) Telangiectasia score; metronidazole group 1.70 (0.08) and placebo group 1.73 (0.08) | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 529): "Sponsored by Stiefel Canada Inc", one of the investigators was employed by Stiefel | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events). Data are skewed See comparison 2 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 530): "This randomized, double‐blind...study..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment:There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (529): "...double‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (529): "...double‐blind." Outcomes were investigator and participant assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study |
| Incomplete outcome data (attrition bias) | High risk | 31/120 (25.8%); metronidazole group (17) and placebo group (14). All participants were accounted for (including the dropouts and withdrawals). Per‐protocol analysis Comment: High dropout rate combined with a per‐protocol analysis judged as at high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration and wash‐out period before study adequate, no concomitant medication that could influence rosacea permitted. Sponsored by Stiefel Canada Inc, manufacturer of the active intervention. One investigator was employed by Stiefel Comment: Insufficient information to assess whether an important risk of bias exists |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Multicentre, 13 centres in the US | |
| Participants | Randomised: 329 participants (mean age 48 years (treatment group) versus 49 years (placebo group), 39 male and 125 female versus 45 male and 120 female) Inclusion criteria
Ocular involvement: Unclear, no participants with marked ocular involvement were included Exclusion criteria
Dropouts and withdrawals
Baseline data mean Inflammatory lesion count; azelaic group 17.5 and vehicle group 17.6 | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 836): "Supported by Berlex Laboratories" | |
| Declaration of interest | Quote (page 836): "Dr Thieroff‐Ekerdt is an employee of Berlex Laboratories. Dr Graupe is an employee of Schering AG. Dr Thiboutot received financial compensation from Berlex Laboratories for her role as a principal investigator in these studies" | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 6 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 837): "Patients were randomly assigned to treatment with either AzA gel or vehicle gel. The randomization list was prepared by a computer program ensuring equal numbers of patients per treatment group." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 837): "Patients were allocated to treatment in the sequence of entry into the studies, i.e., in each center each newly admitted patient received the study medication with the lowest randomization number available." The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 836): "double‐blind" Comment: Vehicle gel was used. Probably identical appearance. Although not explicitly stated it would appear that the active intervention and placebo tablets were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 836): "double‐blind" Comment: Vehicle gel was used. Probably identical appearance. Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 46/329 (13.9%); azelaic group (31) and vehicle group (15), dropouts and withdrawals were accounted for. ITT analysis (LOCF) Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration and wash‐out period before study adequate, no concomitant medication that could influence rosacea allowed. Other medication recorded Sponsoring: Berlex Laboratories, second investigator was an employee of Berlex laboratories, third author is an employee of Schering, and first author received financial compensation from Berlex laboratories for her role as principal investigator (page 836) Comment: The review authors do not consider that the commercial sponsorship introduced any additional bias the study was double‐blind, and all pre‐specified outcome measures were addressed and analysis of data was according to ITT principle |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Ureported Multicentre, 14 centres in the US | |
| Participants | Randomised: 335 participants (mean age 48 years (treatment group) versus 47 years (placebo group), 47 male and 122 female versus 46 male and 120 female) Inclusion criteria
Ocular involvement: Unclear, no participants with marked ocular involvement were included Exclusion criteria
Dropouts and withdrawals
Baseline data mean Inflammatory lesion count; azelaic group 17.8 and vehicle group 18.5 | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 836): "Supported by Berlex Laboratories" | |
| Declaration of interest | Quote (page 836): "Dr Thieroff‐Ekerdt is an employee of Berlex Laboratories. Dr Graupe is an employee of Schering AG. Dr Thiboutot received financial compensation from Berlex Laboratories for her role as a principal investigator in these studies" | |
| Notes | Same reference as Thiboutot 2003a (report of 2 studies). Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 6 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 837): "Patients were randomly assigned to treatment with either AzA gel or vehicle gel. The randomization was prepared by a computer program ensuring equal numbers of patients per treatment group." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 837): "Patients were allocated to treatment in the sequence of entry into the studies, ie, in each center each newly admitted patient received the study medication with the lowest randomization number available." The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 836): "double‐blind" Comment: Vehicle gel was used. Probably identical appearance. Although not explicitly stated it would appear that the active intervention and placebo tablets were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 836): "double‐blind" Comment: Vehicle gel was used. Probably identical appearance. Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 39/335 (11.6%); azelaic group (19) and vehicle group (20). Dropouts and withdrawals were accounted for and included in the analysis. ITT analysis (LOCF) Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported. Comment: We judged this as at a low risk of bias. |
| Other bias | Low risk | Wash‐out period adequate, study duration adequate, no concomitant medication that could influence rosacea allowed. Other medication recorded Sponsoring: Berlex Laboratories, second investigator was an employee of Berlex laboratories, third investigator was an employee of Schering and first investigator received financial compensation from Berlex laboratories for her role as principal investigator (page 836) Comment: The review authors do not consider that the commercial sponsorship introduced any additional bias the study was double‐blind, and all pre‐specified outcome measures were addressed and analysis of data was according to ITT principle |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 134 participants (mean age 44.5 years in doxycycline group and 48.9 years in placebo group, 40 male, 94 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean | |
| Interventions | 16 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 12 and week 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 17): "100% supported by CollaGenex" | |
| Declaration of interest | Quote (page 17): "Drs. Thiboutot, Beer, and Skidmore have received consulting and speaking fees from CollaGenex. Dr. Berman has received research grant support from CollaGenex. Drs. Leyden and Fowler have received consulting fees from CollaGenex." | |
| Notes | Poster presentation, a lot of information is lacking (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 17): "A multi‐center, double‐blind, randomized, placebo‐controlled trial was undertaken" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 17): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 17): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | 25/134 (18.7%); unclear from which group. Analysis unclear Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported | |
| Participants | Randomised: 92 participants (mean age 48.5 years in QD group versus 49.6 years in BID group, 11 male and 34 female versus 17 male and 30 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean Nothing reported | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | One of the investigators was employed by Intendis | |
| Declaration of interest | Quote (page 545): "Dr Thiboutot has participated in clinical trials and has been a consultant/advisor for Intendis Inc,....Dr Fleischer has participated in clinical trials and has been a consultant/advisor for Intendis Inc,....Dr Del Rosso has received grant/research support/honoraria from and has been a consultant for etc and....Intendis Inc...Dr Graupe is employed by Intendis GmbH, Berlin, Germany" | |
| Notes | 1 centre (20 participants) was excluded as assessments were not in conformity with protocol One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 7 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 542): "Patients were randomized to receive either AzA 15% gel once daily or AzA 15% gel twice daily." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Low risk | As both groups received 2 tubes for each study day it is unlikely that allocation could have been foreseen Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 541): "double‐blind". Both groups received a morning and evening tube for each study day. The subjects in the QD group received 1 application with vehicle gel each day of the study (page 542) Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 541): "double‐blind". Both groups received a morning and evening tube for each study day. The subjects in the QD group received 1 application with vehicle gel each day of the study (page 542). Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 4/92 (4.3%); 2 in each group, 1 centre was excluded (20 participants, as IGA assessments were not in conformity with study protocol). Reasons for withdrawal are reported. ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | High risk | All of the investigators in this study have received sponsorship from pharmaceutical industry. The study was designed as a superiority study, but was inappropriately reported as a successful non‐inferiority study, without any reference to the number of participants recruited, the planned design, or sample size. Study duration adequate, unclear if there was a wash‐out period before study or if other medications were allowed Comment: A potential risk of bias cannot be excluded |
| Methods | RCT (only second phase), prospective, placebo‐controlled (only second phase), double‐blind (only second phase), cross‐over study (we only included second phase) Date of study | |
| Participants | Randomised: 136 participants (mean age 46.4 years in azelaic acid gel group versus 47.5 years in vehicle group, 18 male and 49 female in azelaic acid group versus 17 male and 52 female in vehicle group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Inflammatory lesion count; azelaic acid group 1.39, vehicle group 1.55 | |
| Interventions | 24 weeks Intervention
Comparator
| |
| Outcomes | Assessments (7): baseline, every for weeks up to 24 weeks Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 647): "Research funding was provided by Indendis Inc" | |
| Declaration of interest | Quote (page 647): "Dr Fleischer.... he served as a consultant for...Intendis..He has served as an investigator for...Intendis...He also served on the speaker bureaus for...Intendis..Dr Del Rosso has served as a consultant, speaker and researcher for...Intendis...Dr Thiboutot has served as an investigator and consultant for Intendis Inc..." | |
| Notes | We only included second phase as first phase (up to 12 weeks). All participants received doxycycline 100 mg BID + azelaic acid 15% gel BID in first phase. However, participants who did not respond in first phase were not included in second phase, this means these data cannot be generalised for all participants with papulopustular rosacea. Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 9 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 640): "Subjects eligible for the maintenance phase of the study were randomized to apply either AzA 15% gel or its vehicle twice daily for an additional 24 weeks." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page (640); "double‐blind" Comment. Vehicle gel was used. Probably identical appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page (640); "double‐blind" Comment. Vehicle gel was used. Probably identical appearance. Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | We only included second phase of the study. 14/136 (10.3%), 7 in both groups. ITT analysis carried out (LCOF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | High risk | Not all pre‐specified outcomes were addressed and reported. Data missing in the maintenance phase, self‐assessment by participants Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | No wash‐out phase, only participants were included who had attained at least a 75% reduction in number of inflammatory lesions in the first phase on a combination of doxycycline 100 mg to 200 mg plus azelaic acid 15% gel twice daily. Study duration adequate, not allowed to use other medications that might influence rosacea. Research funding was provided by Intendis, Inc. First author served as an investigator and consultant for Intendis, as did second and third author Comment: Phase 1 of this study is a run‐in period (equivalent to wash‐out). However, insufficient information to assess whether an important risk of bias exists |
| Methods | Randomised, prospective, active‐controlled, double‐blind, within‐patient comparison Date of study Setting | |
| Participants | Randomised: 12 participants (mean age 39.8 years, 5 male, 7 female) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data mean (SEM) Inflammatory lesion count; micro emulsion group 3.75 (0.74), commercial gel group 3.01 (0.59) Erythema score; micro emulsion group 2.50 (0.22), commercial gel group 2.08 (0.26) Telangiectasia; micro emulsion group 1.50 (0.17), commercial gel group 1.08 (0.31) | |
| Interventions | Six weeks
Comparator
To prevent cross‐over, hands were washed between the right and left applications. None of the patients used any other kind of treatment during the study period. No restrictions were placed on diet or the use of cosmetics | |
| Outcomes | Assessments (4); baseline, week 2, 4 and 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 591): "This study was partly supported by a Grant from Gazi University, Turkey (SBE‐11‐2001/10)" | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 5 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 585): "This study was designed as a randomized, double‐blind, bilateral split‐face paired comparison" and "Patients were assigned to receive the commercial gel and microemulsion formulation to each half of the face." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 585): "Each patient received a pair of identical‐appearing vials, labeled right and left, one containing commercial gel and the other microemulsion formulation." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 585): "Each patient received a pair of identical‐appearing vials, labeled right and left, one containing commercial gel and the other microemulsion formulation Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts reported Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Treatment duration adequate, wash‐out period before the study adequate Comment: The study appeared to be free of other forms of bias |
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Unreported Multicentre (6) in US. Trillium Creek Dermatology Center, Medina, Ohio; Department of Dermatology, Thomas Jefferson University, Pennsylvania; Radiant Research, Tucson, Arizona; International Research Services, Inc, Rockland, Maine; Derm Research, Inc, Austin, Texas | |
| Participants | Randomised: 152 participants (mean age 47 years (range 19 to 77), 43 male, 109 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Inflammatory lesion count; sulphacetamide group 18 (1), metronidazole group 17 (1) | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 357): "This study was supported by Stiefel Laboratories, Inc" | |
| Declaration of interest | Quote (page 357): "Dr Torok is a consultant and advisory board member for, is on speaker's bureau and received research grants from Galderma Laboratories, LP, Stiefel Laboratories Inc....Dr Webster is a consultant and speaker for and has received a grant from ..Galderma Laboratories, LP, Stiefel Laboratories Inc..Dr Egan is a consultant for Stiefel Laboratories Inc". Others no conflict of interest | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 21 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 358): "Subjects were randomly assigned to treatment with either sodium sulphacetamide 10% and sulfur 5% with sunscreens or metronidazole 0.75% cream." E‐mail contact with the investigator confirmed computer‐generated and central allocation Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Method of allocation concealment not reported E‐mail contact with the investigator confirmed central allocation Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 357): "...investigator‐blinded." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 357): "...investigator‐blinded." |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts were accounted for and included in analysis, but unclear how missing data was imputed Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | High risk | Participant's assessment of global improvement was not addressed Comment: As this was one of our principal outcomes, this was considered as at a high risk of bias |
| Other bias | Unclear risk | Study duration and wash‐out period adequate, groups treated equally aside from intervention. Study sponsored by Stiefel Laboratories, Inc. The first two investigators have received grants from Stiefel Laboratories Comment: Sponsorship and the fact that one investigator is a consultant for the sponsor raises concerns about the potential for bias |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 15 participants (mean age 60 years, 3 male, 7 female, 5 gender unreported) Inclusion criteria
Ocular involvement: Unclear
Dropouts and withdrawals
Baseline data mean IGA score; SEI003 group 1.8, vehicle group 2.0 | |
| Interventions | 12 weeks Intervention
Comparator
Application frequency unclear | |
| Outcomes | Assessments (5): baseline, week 2, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 1145): "In vitro analysis described in this work was supported in part by the United States National Institutes of Health (NIH) grant R01‐AR052728 to RLG." | |
| Declaration of interest | Quote (page 1145): "Neither Therapeutics nor Skin Epibiotics provided any financial compensation for the study or to any members of the study team with the exception of EH, who left his position at UCSD for employment opportunities at these companies part‐way through the study" | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 40 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1143): " randomized, double‐blind, placebo controlled study".."randomized 2:1...." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: "The allocation sequence was created prior to enrolling any subjects in the study" by a third party Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 1143): "Subjects, study coordinators, and those performing clinical assessments were blinded" After e‐mail communication: "study medication was placed into a bottle labeled with the participant’s unique study identification number that was assigned to the participant at the time of enrolment in the trial" by a third party, and "Both the treatment and the control creams were identical in appearance and viscosity so that the two drugs could not be distinguished by look or feel." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 1143): "Subjects, study coordinators, and those performing clinical assessments were blinded" Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | High risk | 4/15 (26.6%) in the SEI003 group and per‐protocol analysis Comment: High and unbalanced dropout rate combined with per‐protocol analysis judged as at high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for this study NCT01398280 was available at https://www.clinicaltrialsregister.eu/ctr‐search/search and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, no information regarding wash‐out period Comment: The study appeared to be free of other forms of bias |
| Methods | Randomised, prospective, active and placebo‐controlled, double‐blind Date of study Setting Department of Dermatology, Erciyes University Medical School, Kayseri, Turkey | |
| Participants | Randomised: 53 participants (mean age 46.9 years, 7 male, 46 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean | |
| Interventions | Two weeks Intervention
Comparator 1
Comparator 2
Comparator 1
Comparator 1
| |
| Outcomes | Assessments (2): baseline and week 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed Older study, described in letter, a lot of information is lacking (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 69): "Quote: "The patients were randomized into 5 groups" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 69): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 69): "double‐blind". Only investigator assessed outcomes Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported, no exact data were provided Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Outcomes unclear and no data provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Letter provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | Randomised, prospective, active‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 60 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Not reported | |
| Interventions | 30 days Intervention
Comparator
| |
| Outcomes | Assessments (at least 3): baseline, 15 days and 30 days Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | This is a very brief interim report, full study has never been published, data only reported for 30 participants and largely unusable (see Table 6) None of our primary outcomes were addressed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 729): "randomisée" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 729): "double insu." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 729): "double insu" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Interim report on 30 participants Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Multicentre, Department of Dermatology, Marselisborg Hospital, Arhus; Department of Dermatology, Genthofte Hospital, Copenhagen; Odense University Hospital, Odense; and Dermatology Clinic, Aalborg, Denmark | |
| Participants | Randomised: 76 participants (mean age 52.4 years, 36 male/39 female and 1 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts/Withdrawals: 6/76 (7.9%); unclear how many from each group Baseline data mean (SD) Means for lesions or erythema were not reported | |
| Interventions | Eight weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 2, 4 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated | |
| Funding source | Quote (page 210); "The metronidazole cream and tetracycline tablets were supplied by the Danish drug company, Dumex Ltd." | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed See comparison 56 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 209): "The study was performed in 4 centers as a double‐blind, randomized trial." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 209): "double‐blind" "...placebo tablets identical in appearance to the tetracycline tablets." "...placebo cream was cream base of metronidazole cream." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 209): "double‐blind" "...placebo tablets identical in appearance to the tetracycline tablets." "...placebo cream was cream base of metronidazole cream." Outcomes were investigator assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 6/76 (7.9%); unclear how many participants from each group, per‐protocol analysis Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period before study rather short for oral therapy (2 weeks), unclear if other medications were allowed Comment: Insufficient information to assess whether an important risk of bias exists |
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Dermatology department, Juan Canalejo Hospital, La Coruña, Spain | |
| Participants | Randomised: 40 participants (mean age 57.8 (14) years in the erythromycin group and 62.2 (12) years in metronidazole group, 13 male, 27 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data total Number of papules; erythromycin group 571 and metronidazole group 476 Number of pustules; erythromycin group 160 and metronidazole group 63 | |
| Interventions | Three months Intervention
Comparator
| |
| Outcomes | Assessments (2): baseline, month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 26 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 474) (translation): "Patients included in the study were assigned a key number by the pharmacy service that assigned them to one of the two groups through a table of random numbers generated by computer." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 474) (translation): "They were randomised by a computer generated distribution numbered list by the pharmacy." Comment: Pharmacy‐controlled randomisation. Probably done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 474): "...doble ciego." [Translated as double‐blind] Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 474): "...doble ciego." [Translated as double‐blind] Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers and participants) during the study |
| Incomplete outcome data (attrition bias) | High risk | Dropouts and withdrawals (> 20% in erythromycin group) were reported but unclear at which time points and no evidence of ITT analysis (page 475) Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | High risk | Physician's Global Assessment was not addressed or reported Comment: We judged this as at a high risk of bias |
| Other bias | High risk | Wash‐out period unclear, study duration adequate, groups probably treated equally. Baseline imbalance between the groups in number of pustules and papules Comment: The baseline imbalance in the groups puts the study at serious risk of bias |
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Department of Dermatology and Allergy Biederstein, Munich, Germany | |
| Participants | Randomised: 40 participants (mean age 58 years, 25 male, 15 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals: None Baseline data mean | |
| Interventions | Four weeks Intervention
Comparator
| |
| Outcomes | Assessments (4): baseline, week 1, 2 and 3 Outcomes of the trial (as reported) Primary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | Quote (page 728): "M.B. is employed by Novartis Pharma, the manufacturer of Elidel (pimecrolimus)." | |
| Notes | We only included data from the first 4 weeks, second part of study was open‐phase. Two of our primary outcomes were addressed (quality of life and participant‐assessed changes in rosacea severity) See comparison 22 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 729): "Forty patients with papulopustular rosacea were investigated in a single‐centre, randomized, double‐blind vehicle‐controlled study." Comment:Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 728): "double‐blind", only first 4 weeks Comment: Vehicle cream was used. Probably identical appearance The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 728): "double‐blind", only first 4 weeks Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | There were no withdrawals reported Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Unclear if there was a wash‐out period, duration of double‐blinded part too short (4 weeks), unclear if additional medications were allowed and recorded. One of the investigators was employed by the manufacturer of Elidel (Novartis Pharma) Comment: Insufficient information to assess whether an important risk of bias exists |
| Methods | RCT, prospective, active‐controlled, placebo‐controlled, double‐blind, cross‐over Date of study Unreported McGuire Veterans Administration Medical Center, Richmond, US | |
| Participants | Randomised: 15 participants (age range 41 to 60 years, 4 male, 11 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Not stated, unclear Baseline data mean (SD) | |
| Interventions | 53 days Intervention
Comparator 1
Comparator 2
Comparator 3
| |
| Outcomes | Assessments for period A (2): baseline, day 18 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 202): "Supported by a grant from E.R. Squibb & Sons, Inc, New Brunswick, New Jersey" | |
| Declaration of interest | None declared | |
| Notes | We included only period A, first study period, however, no separate data for this period (see Table 6) None of our primary outcomes were addressed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 202): "All persons were randomly assigned to one of four 2‐way cross‐over treatment groups in a double‐blind manner." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear if tablets were comparable/similar in appearance Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 203): "were analyzed in a blinded manner" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers and participants) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts not reported, other than 1 participant who dropped out reasons unclear Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | High risk | Wash‐out period was included in study design, other rosacea treatment did not have to be stopped, study duration too short (period of 17 to 18 days), groups treated equally. Small sample size Comment: We judged this as at a high risk of bias |
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Unreported | |
| Participants | Randomised: 43 participants (age range 25 to 70 years, both male and female, numbers not specified) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Unclear Baseline data mean (SD) | |
| Interventions | 12 weeks Intervention
Comparator
| |
| Outcomes | Assessments (2): baseline, week 12 Outcomes of the trial (as reported) Primary outcomes:✴
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 65): "Supported by a grant of Upjohn Company, Kalamazoo, Michigan" | |
| Declaration of interest | None declared | |
| Notes | Unclear how many participants were assigned to each group, dropouts not mentioned, no exact data provided (see Table 6) One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 66): "Patients were randomly assigned to one of two regimens." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 65): "...investigator‐blinded." "...double‐blinded." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 65): "...investigator‐blinded." "...double‐blinded." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers and participants) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Minimal outcomes data reported, dropouts and withdrawals unreported Comment: Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Unclear what study outcomes were, not stated in Methods section Comment: Insufficient information to permit a clear judgement |
| Other bias | Low risk | Study duration and wash‐out period adequate, groups appear to have been treated equally Comment: The study appears to be free of other forms of bias |
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Setting | |
| Participants | Randomised: 20 (age and gender unreported) Inclusion criteria
Ocular involvement: Yes Exclusion criteria
Dropouts and withdrawals
Baseline data mean | |
| Interventions | Three months Intervention
Comparator
Unclear how many were randomised to each group, application frequency unclear | |
| Outcomes | Assessments (at least 2): baseline and month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (in abstract): "None" | |
| Declaration of interest | Quote (in abstract): "JR Wittpenn, Allergan, B Schechter, Allergan" | |
| Notes | None of our outcomes were addressed. This study was part of NCT00348335 (see Table 3). Poster with very limited data (see Table 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (on poster): "patients were randomized to cyclosporine A or artificial tears for 3 months." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (on poster): "double‐masked." Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (on poster): "double‐masked." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Only limited data were provided, no report on dropouts Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
| Methods | RCT, prospective, active‐controlled, investigator‐blind Date of study Unreported | |
| Participants | Randomised: 160 participants (mean age 51.1 ± 10.7 years (range 32 to 78) in metronidazole group and 51.1 ± 11.3 years (range 31 to 77) in azelaic acid group, 26 male and 56 female in metronidazole group, and 18 male and 60 female in azelaic group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data median Inflammatory lesions; metronidazole group 17 and azelaic acid group 14.5 | |
| Interventions | 15 weeks Intervention
Comparator
| |
| Outcomes | Assessments (6): baseline, week 3, 6, 9, 12 and 15 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | Quote (page 3): "This study was supported by a grant from Galderma Laboratories, LP" | |
| Declaration of interest | Quote (page 3): "Mr Kerrouche and Ms Arsonnaud are from Galderma Laboratories, LP, Sophi‐Antipolis, France. Dr Wolf is an advisory board member, consultant, researcher and speaker for Galderma Laboratories | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 14 in Effects of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 4): "Patients were randomized in a 1:1 fashion to treatment with metronidazole 1% gel once daily or azelaic acid 15% gel twice daily for a period of 15 weeks." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 4): 'investigator‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 4): 'investigator‐blind" |
| Incomplete outcome data (attrition bias) | Low risk | 24/160 (15%); metronidazole group (14) and azelaic acid group (10). Both ITT and per‐protocol analyses reported performed Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration and wash‐out period adequate, groups treated equally. Supported by a grant from Galderma Laboratories. First investigator was an advisory board member, consultant, researcher, and speaker for Galderma Laboratories, two other investigators are from Galderma Comment: We judged this as at unclear risk of bias |
| Methods | Randomised, prospective, active‐controlled, single‐blind, within‐patient comparison Setting | |
| Participants | Randomised: 6 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean | |
| Interventions | 12 weeks (4 sessions of laser with 2 week intervals) Intervention
Comparator
| |
| Outcomes | Assessments (3): baseline, 16 and 20 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review | |
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 918): "and concurrently received PDL treatment to one randomized side" |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 918): "single‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 918): "single‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | 1/6 for personal reasons lost to follow‐up. Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided (protocol available at clinical trials.gov NCT00945373) Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
BID = twice a day, BZP = benzoyl peroxide, ITT = intention‐to‐treat analysis, N = number, n/a = not applicable, ns = not significant, no further data available, QD = once daily, RCT = randomised controlled trial, RWBT = rapid whole blood test, SD = standard deviation, SEM = standard error of the mean, TID = three times a day, UBT = urea breath test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised controlled trial (RCT). No description of rosacea, unclear if additional medication was allowed, no site of evaluation is recorded, no intention‐to‐treat analysis (ITT). Lots of information is lacking | |
| Not a RCT | |
| Quote: "Patients were randomly assigned..." Page 253 Comment: The investigator confirmed by email that this consisted of "60 sealed envelopes including names of treatments, half to half. Patients selected an envelope" This is a form of quasi‐randomisation. CCT | |
| Open allocation, "based on arrival", quasi‐randomised. CCT | |
| Not a RCT | |
| Not a RCT | |
| CCT, no evidence of randomisation | |
| After e‐mail communication with the investigators to clarify aspects of trial conduct the judgement for sequence generation was changed from 'unclear' to 'high risk of bias'. The participants were allocated to the intervention by alternation. CCT | |
| CCT | |
| Not a RCT | |
| Not a RCT. Narrative report about treatment | |
| CCT | |
| Not a RCT. Case report | |
| Study to assess cumulative irritation potential and not treatment effect on rosacea | |
| CCT | |
| Not a RCT. Narrative report about 2 studies | |
| Not a RCT. Open‐label study | |
| Not a RCT of effects of interventions on rosacea. Unit of randomisation = barrier tests on the arms | |
| Not a RCT | |
| Not a RCT | |
| Open‐label, observational study | |
| After e‐mail contact appeared to be quasi‐randomised | |
| Not a RCT | |
| Quote: "Treatment (either doxycycline protocol or tetracycline hydrochloride protocol) was suggested to each patient at random. Those who refused the suggested protocol were offered the treatment with the other protocol." Page 89 Comment: Method used to randomise participants to the interventions was inadequate. Not a RCT | |
| Not a RCT. Open‐label study | |
| Not a RCT. Rosacea patients were given triple therapy consisting of amoxicillin, clarithromycin and lansoprazole | |
| Not a RCT | |
| Not a RCT. Narrative review | |
| Not a RCT, no blinding, all participants were treated with isotretinoin | |
| Not a RCT | |
| Poster, without data. Unsuccessful attempts at contacting authors | |
| Contact with investigators via electronic mail, responses clear that the allocation sequence was inadequately generated | |
| Quote: "...randomly divided into two groups." Comment: Following extensive email communication with the principal investigator we were unable to receive reassurances that the allocation sequence was adequately generated and therefore this study has been classified as a CCT | |
| This study did not match the inclusion criteria for this review | |
| Participants with steroid‐induced rosacea, and rosacea patients were excluded | |
| Systematic review of 5 studies, all included in present review | |
| Not a RCT | |
| After reading full text appears to be CCT | |
| No participants with rosacea | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT. Open‐label study | |
| Not a RCT | |
| Not a RCT. Open, observational study | |
| This study did not match the inclusion criteria for this review | |
| Participants with chronic blepharitis, few had associated rosacea. No separate data available for participants with rosacea. Many criteria were assessed as unclear or inadequate. No ITT | |
| Not a RCT. Case report | |
| Not a RCT. Case report | |
| Open‐label pilot study with 6 participants, of which 1 dropped out Quote: "Patients were selected randomly and consecutively." "Patients were instructed to apply 0.75% metronidazole gel to the right side of the face...and 5% permethrin to the left side." Page 177 Comment: Quasi‐randomised. CCT | |
| Poster, limited data available. Not a RCT | |
| Ten participants with telangiectasia, no mention of rosacea at all | |
| Not a RCT. Case report of 4 treated participants | |
| Not a RCT | |
| Not a RCT, no control. Open‐label | |
| Population did not fit the inclusion criteria. Participants with photodamage without rosacea were also included. Unclear which participants had rosacea and no separate data available for participants with rosacea | |
| Not a clinical trial | |
| Not a RCT | |
| Not a RCT. A narrative review on incyclinide | |
| Not a RCT, open‐label study | |
| Not a RCT |
RCT = randomised controlled trial
CCT = controlled clinical trial (quasi‐randomised)
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, single‐blind, placebo‐controlled |
| Participants | 138 participants with baseline facial Rosacea Severity Score (RSS) of 2 or greater |
| Interventions | Topical medical grade Kanuka honey versus cetomacrogol cream |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Study has been completed. Website accessed 21‐7‐2014. Study is currently being written‐up, will be included when published |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 40 participants with papulopustular rosacea |
| Interventions | Permethrin 5% gel versus placebo gel for 12 weeks |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Website accessed 23‐9‐2014, study is submitted for publication. Will be included when full data is reported |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 72 participants with facial rosacea and blepharitis |
| Interventions | Doxycycline 40 mg versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Completed July 2009, no study results reported. Attempts to contact CollaGenex Pharmaceuticals unsuccessful Website accessed 16‐7‐2014, sent mail to Galderma NL and international. There are some outcomes data reported on clinicaltrials.gov. Will be included when full data is reported |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 84 participants with papulopustular rosacea |
| Interventions | Azelaic acid 15% foam twice daily versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Completed June 2008, no study results reported yet. Website accessed 18‐7‐2014, sent e‐mail, is now changed into Bayer, some outcome data reported on clinicaltrials.gov. Information Bayer that study is not published yet. Will be included when full data is reported |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 156 participants with papulopustular rosacea |
| Interventions | Isotretinoin 0.25 mg/kg, 1 per day, 4 months of treatment versus placebo |
| Outcomes | Primary Outcome measures
Secondary outcome measures
|
| Notes | Study completed, but not yet published Website accessed 18‐7‐2014, sent e‐mail to O Chosidow, they will send submitted abstract (will be later this year). Will be included when published |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 68 participants with erythematotelangiectatic rosacea |
| Interventions | 46 weeks, Atralin gel 0.05% versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Study completed January 2013. Website accessed 19‐7‐2014 (some outcome data on clinicaltrials.gov), sent mail 19‐7‐2014 reply 29‐7‐2014. The study has not been published yet, but they will notify us. Will be included when published |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 60 participants with rosacea |
| Interventions | Laropiprant versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Study completed April 2012. Website accessed 19‐7‐2014, submitted for publication. Will be included when published |
| Methods | Randomised, double‐blind, active and placebo‐controlled |
| Participants | 64 participants with moderate to severe facial erythema associated with rosacea |
| Interventions | AGN‐199201 Dose A versus AGN‐199201 Dose B versus AGN‐199201 Dose C versus vehicle (once and twice daily dosages) |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Study completed June 2013. Website accessed 21‐7‐2014. E‐mail sent 22‐7‐2014 through website. Some data published on ClinicalTrials.gov. Will be included when published |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 10 participants with rosacea |
| Interventions | Incobotulinumtoxin A versus saline injections |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Study was completed August 2014. Website accessed 19‐7‐2014. Paper is written‐up will be included in the review when published |
| Methods | Randomised, single‐blind, active‐controlled, within participant |
| Participants | 10 subjects with mild to moderate rosacea |
| Interventions | Azelaic acid 15% gel + Nd: YAG laser versus azelaic acid 15% gel |
| Outcomes | Primary outcome measures
|
| Notes | Study was completed February 2011. Website accessed 20‐7‐2014. Article written‐up not yet published, unclear if it is truly randomised or CCT, no further reply received |
| Methods | Randomised, double‐blind, active and placebo‐controlled |
| Participants | 357 participants with moderate to severe facial erythema associated with rosacea |
| Interventions | AGN‐199201 Dose A versus AGN‐199201 Dose B versus AGN‐199201 Dose C versus vehicle (once and twice daily dosages) |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | The study has been completed June 2013. Website accessed 21‐7‐2014. Part of data published on ClinicalTrials.gov. Will be included when published |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 117 participants with mild to moderate rosacea |
| Interventions | Anatabloc cream versus vehicle cream |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Study has been completed August 2013. Website accessed 20‐7‐2014. They are writing study down. Will be included when published |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Assessment of the efficacy and safety of three concentration: 1%, 0.3%, 0.1% of CD5024 cream once daily and CD5024 1% cream twice daily, versus its vehicle and versus metronidazole cream (Rozex®) in patients with papulopustular rosacea over 12 weeks |
| Methods | Randomised, single‐blind, active and placebo‐controlled |
| Participants | 270 |
| Interventions | CD5024 1% once daily, CD5024 0.3% once daily, CD5024 0.1% once daily, CD5024 1% twice daily, versus vehicle versus metronidazole 0.75% |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 10‐10‐2006, completed 2‐8‐2007 |
| Contact information | |
| Notes | Dose finding study for ivermectin, website accessed 23‐9‐2014, sent mail to Galderma NL |
| Trial name or title | Activity of twice daily per os administration of CD06713 at 8 mg versus its placebo during 4 weeks treatment, in patients with erythemato‐telangiectatic rosacea |
| Methods | Randomised, single‐blind, placebo‐controlled |
| Participants | 48 participants with erythemato‐telangiectatic rosacea |
| Interventions | Ondansetron 8 mg versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 9‐1‐2007, completed 23‐5‐2007 |
| Contact information | |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
| Trial name or title | Non inferiority study of metronidazole 0.75% cream versus reference therapy in the local treatment of papulopustular rosacea |
| Methods | Randomised, single‐blind, placebo and active‐controlled |
| Participants | 300 participants with papulopustular rosacea |
| Interventions | Metronidazole 0.75% cream versus metronidazole 0.75% gel versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 08‐05‐2007 |
| Contact information | Not provided, but sponsored by Pierre Fabre Dermatologie |
| Notes | Website accessed 23‐9‐2014, still ongoing in France |
| Trial name or title | An investigator blind parallel group vehicle control study comparing the efficacy and safety of CD 5024 1% cream with metronidazole 0.75% cream in subjects with papulopustular rosacea over 16 weeks treatment |
| Methods | Randomised, active and vehicle‐controlled, investigator‐blinded |
| Participants | 600 participants with papulopustular rosacea |
| Interventions | Ivermectin 1% versus placebo versus metronidazole 0.75% |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 15‐01‐2009, still ongoing |
| Contact information | Not provided but sponsored by GALDERMA R&D SNC |
| Notes | Website accessed 23‐9‐2014 |
| Trial name or title | Effect of CD08514 versus placebo, in patients presenting with type 1 rosacea, over an 8‐week treatment |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | Number unclear, participants with moderate to severe erythemato‐telangiectactic rosacea |
| Interventions | Famotidin‐ratiopharm® 40 and 10 mg BID versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 9‐10‐2009, completed 7‐6‐2010 |
| Contact information | Galderma R&D SNC |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
| Trial name or title | A double‐blind, vehicle controlled, parallel group study assessing the activity of CD5024 1% cream in subjects with papulopustular rosacea over 12 weeks treatment |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 317 participants with papulopustular rosacea |
| Interventions | CD5024 1% cream (ivermectin) versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 17‐08‐2010, study completed 02‐05‐2011 |
| Contact information | GALDERMA R&D SNC, France |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
| Trial name or title | Doxycycline versus minocycline in the treatment of rosacea: a randomised controlled trial ‐ DoMino‐study |
| Methods | Randomised, single‐blind, active‐controlled |
| Participants | Number not stated, participants with papulopustular rosacea |
| Interventions | Doxycycline 40 mg versus minocycline 100 mg |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 5‐10‐2010 |
| Contact information | Study is ongoing |
| Notes | Website accessed 23‐9‐2014, study of Mireille MD van der Linden |
| Trial name or title | Multizentrische, randomisierte, doppelblinde, kontrollierte Phase III‐Studie zur Behandlung der papulopustulären Rosazea mit Permethrin Creme 5 % (InfectoScab®) versus Permethrin Creme 2,5 % versus Metronidazol Creme 0,75 % (Rozex®) ‐ Papulopustuläre Rosazea‐Behandlung mit Permethrin Creme versus Metronidazol Creme |
| Methods | Randomised, double‐blind, active‐controlled |
| Participants | Number of participants unclear, participants with papulopustular rosacea |
| Interventions | Permethrin 5% cream versus permethrin 2.5% cream versus metronidazole 0.75% cream |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | Study is completed 27‐02‐2013 |
| Contact information | INFECTOPHARM Arzneimittel GmbH Dr. Bertil Wachall, [email protected] |
| Notes | Website accessed 23‐9‐2014, sent e‐mail |
| Trial name or title | Effect of CD08100/02 3% gel versus placebo in subjects presenting with erythematotelangiectatic rosacea over a 4 week treatment period |
| Methods | Randomised, single‐blind, placebo‐controlled, within‐participant |
| Participants | Number unclear, participants with erythematotelangiectatic rosacea |
| Interventions | Diclofenac sodium 3% gel versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 23‐12‐2013 study completed |
| Contact information | Galderma R&D SNC, France. [email protected] |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
| Trial name or title | Effect of CD08100/02 3% gel versus placebo gel in subjects presenting with papulopustular rosacea over a 6‐week treatment period |
| Methods | Randomised, single‐blind, placebo‐controlled, within‐participant |
| Participants | Number unclear, participants with papulopustular rosacea |
| Interventions | Diclofenac sodium 3% gel versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | Study is completed 23‐03‐2012 |
| Contact information | Galderma R&D SNC, France. [email protected] |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
| Trial name or title | Efficacy and safety of CD5024 1% cream versus metronidazole 0.75% cream in subjects with papulopustular rosacea over 16 weeks treatment, followed by a 36‐week extension period |
| Methods | Randomised, single‐blind, active‐controlled |
| Participants | 960 participants with papulopustular rosacea |
| Interventions | CD5024 1% cream (ivermectin) versus metronidazole 0.75% cream |
| Outcomes | Primary outcome measures
Secondary outcome measures
For second part:
|
| Starting date | 10‐1‐2012, study has been completed 19‐12‐2013 |
| Contact information | Galderma R&D SNC, France [email protected] |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL. Same number of participants as Taieb 2015 (part A), however, that study did not have an extension of 36 weeks (part B) |
| Trial name or title | A multicentre, randomised, double‐blind, vehicle‐controlled, parallel group study to demonstrate the efficacy and assess the safety of CD07805/47 gel 0.5% applied topically once daily in subjects with moderate to severe facial erythema of rosacea |
| Methods | Randomised, double‐blind, vehicle‐controlled |
| Participants | 140 participants with facial rosacea |
| Interventions | Briminodine tartrate 0.5% versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 15‐08‐2012 |
| Contact information | |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL. Looks like design of Fowler 2013a but other number of participants and Jackson 2013 |
| Trial name or title | Effect of CD07805/47 gel in subjects presenting with flushing related to erythematotelangiectatic or papulopustular rosacea ‐ Effect of CD07805/47 gel in rosacea flushing |
| Methods | Randomised, single‐blind, placebo‐controlled |
| Participants | Number unreported, mild to moderate erythematotelangiectatic rosacea (ETR) or mild to moderate papulopustular rosacea (PPR) |
| Interventions | Brimonidine 0.5% gel versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures Period 1
Period 2
Others:
|
| Starting date | 28‐2‐2014, study completed June 2014 |
| Contact information | |
| Notes | Website accessed 23‐9‐2014, email sent 23‐9‐2014 to Galderma NL |
| Trial name or title | Comparison of dapsone 5% topical gel with metronidazole 0.75% efficacy in combination with oral doxycycline In papulopustular rosacea |
| Methods | Randomised, double‐blind, active‐controlled |
| Participants | 56 participants with papulopustular rosacea |
| Interventions | Dapsone 5% gel b.i.d. + doxycycline 100 mg versus metronidazole 0.75% gel + doxycycline 100 mg for 12 weeks |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 10‐4‐2013 study is completed |
| Contact information | Dr. Gita Faghihi [email protected] or Dr. Parastoo Khosravani [email protected] |
| Notes | Website accessed 23‐9‐2014, email sent 23‐9‐2014 |
| Trial name or title | Clinical trial for development of topical rapamycin treatment for rosacea |
| Methods | Randomised, placebo‐controlled, cross‐over |
| Participants | 5 participants with rosacea |
| Interventions | 0.2% rapamycin ointment versus vehicle |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | Still recruiting |
| Contact information | Mari Wataya‐Kaneda, [email protected]‐u.ac.jp |
| Notes | Website accessed 23‐9‐2014, not sent mail as they are still recruiting |
| Trial name or title | A multicentre, randomised, double‐blind, placebo‐controlled, clinical trial to determine the effects of doxycycline hyclate 20 mg tablets (Periostat(R)) administered twice daily for the treatment of acne rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 150 men and women with rosacea, erythema, papules/pustules, and telangiectasia |
| Interventions | Doxycycline hyclate 20 mg tablets (Periostat(R)) administered twice daily versus placebo |
| Outcomes | Not specified |
| Starting date | June 2002 |
| Contact information | Not provided |
| Notes | Study has been completed. Tried to contact CollaGenex Pharmaceuticals without success Website accessed 16‐7‐2014, sent e‐mail to D. Pariser for more information, and asked Galderma NL and international |
| Trial name or title | MetroGel 1% hydration study: a kinetic regression study |
| Methods | Randomised, single blind, no treatment control, within‐participant |
| Participants | 26 participants with rosacea |
| Interventions | Metronidazole gel 1% versus no treatment |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2006 |
| Contact information | Galderma |
| Notes | Study has been completed August 2006. Website accessed 19‐7‐2014, sent e‐mail to Galderma NL and international |
| Trial name or title | Determine the effects of COL‐101 administered once daily with metronidazole topical gel, 1% versus doxycycline hyclate 100 mg administered once daily with metronidazole topical gel, 1% in patients with moderate to severe rosacea |
| Methods | Randomised, double‐blind, active‐controlled |
| Participants | 91 participants with papulopustular rosacea, with erythema and telangiectasia |
| Interventions | Vibramycin plus metronidazole versus Oracea® delayed‐release plus metronidazole |
| Outcomes | Not specified |
| Starting date | March 2007 |
| Contact information | CollaGenex Pharmaceuticals (C Powala, VP, Drug Development & Regulatory Affairs) |
| Notes | Study completed December 2007. Tried to contact CollaGenex Pharmaceuticals without success Website accessed 16‐7‐2014, sent message via LinkedIn, and asked Galderma NL and international |
| Trial name or title | A pilot study to compare tretinoin gel, 0.05% to tretinoin gel vehicle when dosed once or twice daily in female subjects with classical rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 26 with erythrophagocytotic rosacea |
| Interventions | Tretinoin gel 0.05% bid versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | February 2008 |
| Contact information | Coria Laboratories, Ltd (D. Innes Cargill, PhD) |
| Notes | Study completed December 2008, no study results reported yet Website accessed 18‐7‐2014, sent e‐mail, company is acquired by Valeant Pharmaceuticals |
| Trial name or title | A phase 2, multi‐centre, evaluator‐blind, randomised, vehicle‐controlled clinical study to assess the safety and efficacy of IDP‐115 in the treatment of rosacea |
| Methods | Randomised, single‐blind, 3 arms, placebo‐controlled |
| Participants | 140 with facial rosacea and inflammatory lesions |
| Interventions | Drug: IDP‐115 topical application for 12 weeks versus vehicle versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | November 2007 |
| Contact information | Dow Pharmaceutical Sciences, Inc |
| Notes | Study has been completed July 2008, no published data yet, seeking initial approval September 2010 Website accessed 18‐7‐2014, Dow Pharmaceuticals Sciences is acquired by Valeant Pharmaceuticals, sent e‐mail |
| Trial name or title | A phase II, single‐centre, two‐way crossover relative systemic bioavailability study of Col‐118 administered topically as a 0.18 % facial gel and brimonidine ophthalmic solution 0.2% administered to the eye in subjects with moderate to severe erythematous rosacea |
| Methods | Randomised, double‐blind, active‐control |
| Participants | 20 male and female subjects with moderate to severe erythematous rosacea will be randomised into 2 groups of 10 subjects |
| Interventions | 0.18% Col‐118 facial gel (1.8 mg brimonidine) versus 0.2% brimonidine ophthalmic solution (0.1 mg brimonidine tartrate drop) versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2008 |
| Contact information | Galderma (Michael Graeber, MD, Head of US Development) |
| Notes | Study completed June 2008, study results not reported Website assessed 18‐7‐2014, sent e‐mail to Galderma NL and international |
| Trial name or title | Multi‐centre, double‐blind, randomised, vehicle‐controlled, parallel group study of 0444 gel |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 867 participants with rosacea |
| Interventions | 70 days, 0444 gel versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | January 2008 |
| Contact information | Fougera Pharmaceuticals Inc |
| Notes | Study completed 2009, results not reported Website accessed 19‐7‐2014, company acquired by Sandoz in 2012, sent e‐mail through website Sandoz |
| Trial name or title | Pilot, randomised, double blind, placebo controlled, parallel group, dose range finding study, to evaluate the tolerability and safety of FXFM244 antibiotic foam and to monitor its clinical effect in moderate to severe rosacea patients |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 21 moderate to severe rosacea patients |
| Interventions | 12 weeks, 1% FXFM244 versus 4% FXFM244 versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | June 2010 |
| Contact information | Foamix Ltd |
| Notes | Study terminated (difficulties in recruitment). Website accessed 19‐7‐2014, sent e‐mail. Reply 21‐7‐2014, according to Dov Tamarkin, PhD the study is still ongoing |
| Trial name or title | A randomised, double‐blind, vehicle‐controlled, parallel‐group study of the dose‐response profile of V‐101 cream in subjects with erythematous rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 175 participants with erythematous rosacea |
| Interventions | V‐101 versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2010 |
| Contact information | Vicept Therapeutics, Inc. (Chief Operating Officer) |
| Notes | Study has been completed (no data reported), but after e‐mail contact not yet published |
| Trial name or title | Investigator‐blinded, randomised, cross‐over, multiple dose phase I study on safety and pharmacokinetics of topically applied azelaic acid foam, 15% compared to azelaic acid gel, 15% in subjects with papulopustular rosacea |
| Methods | Randomised, investigator‐blind, cross‐over, multiple dose phase I study |
| Participants | 21 participants with papulopustular rosacea |
| Interventions | Azelaic acid foam versus azelaic acid gel |
| Outcomes | Primary outcome measures
|
| Starting date | January 2011 |
| Contact information | Bayer, no further contact details are provided, sent mail through website Bayer, Novum [email protected], e‐mails sent, but not yet published |
| Notes | Study has been completed March 2011. Website accessed 19‐7‐2014 |
| Trial name or title | A multicentre, randomised, double‐blind, placebo‐controlled evaluation of rosacea‐related inflammatory biochemical markers in the skin of adults with papulopustular rosacea treated with daily doxycycline 40 mg (30 mg immediate release / 10 mg delayed release beads) capsules |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 170 participants with papulopustular rosacea |
| Interventions | Doxycycline 40 mg versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | April 2011 |
| Contact information | Galderma Laboratories, no further contact information is provided |
| Notes | Study has been completed August 2012. Website accessed 19‐7‐2014, sent mail to Galderma NL and international |
| Trial name or title | A therapeutic equivalence study of two metronidazole 1% topical gel treatments for patients with rosacea (MTZG). A randomised, double‐blind, placebo controlled, parallel design, multi‐site clinical study to compare the bioequivalence of two metronidazole 1% topical gel formulations in patients with moderate to severe rosacea |
| Methods | Randomised, double‐blind, active and placebo‐controlled |
| Participants | 602 participants with moderate to severe rosacea |
| Interventions | Metronidazole topical gel 1% versus metronidazole topical gel 1% (Metrogel) versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2011 |
| Contact information | Taro Pharmaceuticals USA [email protected]. Study has not been published yet, e‐mail 21‐7‐2014 [email protected], confirmed, not yet submitted |
| Notes | Study has been completed September 2012. Website accessed 19‐7‐2014 |
| Trial name or title | A randomised, double‐blind, vehicle‐controlled, multicentre, parallel‐group clinical trial to assess the safety and efficacy of azelaic acid foam, 15% topically applied twice daily for 12 weeks in subjects with papulopustular rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 961 participants with papulopustular rosacea |
| Interventions | Azelaic acid foam 15% versus vehicle |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | September 2012 |
| Contact information | Bayer, no further contact details provided, e‐mail contact with Bayer not yet submitted for publication |
| Notes | Study has been completed January 2014. Website accessed 20‐7‐2014 |
| Trial name or title | A multicentre, randomised, controlled, double‐masked, crossover design study to compare efficacy and assess safety of CD07805/47 gel 0.5% applied once daily vs azelaic acid gel 15% applied twice daily in subjects with erythema of rosacea |
| Methods | Randomised, double‐blind, cross‐over, active and placebo‐controlled |
| Participants | 70 participants with erythema of rosacea |
| Interventions | CD07805/47 gel 0.5% versus azelaic acid 15% gel versus vehicle |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | September 2012 |
| Contact information | Galderma Laboratories, L.P. No further contact details provided |
| Notes | Study has been completed December 2012. Website accessed 20‐7‐2014, sent e‐mail (outcomes reported on clinicaltrials.gov) not yet published according to Galderma, but I thought already submitted, sent another e‐mail |
| Trial name or title | A phase 2, randomised, vehicle‐controlled, double‐blind, multicentre study to evaluate the safety and efficacy of three once‐daily CLS001 topical gels versus vehicle administered for 12 weeks to subjects with papulopustular rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 240 participants with papulopustular rosacea |
| Interventions | Omiganan versus placebo |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | March 2013 |
| Contact information | Cutanea Life Sciences, Inc. No further contact details [email protected], sent mail 23‐7‐2014 and 10‐8‐2014 |
| Notes | Study has been completed March 2014. Website accessed 20‐7‐2014 |
| Trial name or title | A multicentre randomised evaluator‐blinded vehicle‐controlled parallel group evaluation of twice daily PDI‐320 in comparison to its monads in adults with rosacea |
| Methods | Randomised, single‐blind, active and placebo‐controlled |
| Participants | 200 participants with rosacea |
| Interventions | PDI‐320 versus PDI‐320 monad #1 versus PDI‐320 monad #2 versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | June 2013 |
| Contact information | PreCision Dermatology, Inc.Syd Dromgoole, PhD. No further contact details provided, as study is still ongoing, not sent mail |
| Notes | Study is ongoing. Website accessed 20‐7‐2014 |
| Trial name or title | Efficacy of Pulsed Light Therapy for Meibomian gland dysfunction and dry eye syndrome |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 140 participants with facial rosacea and diagnosis of mild to moderate dry eye syndrome with meibomian gland dysfunction and ocular rosacea |
| Interventions | Pre‐existing dry eye treatment + sham treatment versus pre‐existing dry eye treatment + Pulsed Light Therapy |
| Outcomes | Primary outcome measures
|
| Starting date | June 2013 |
| Contact information | Angela Chang, University of Miami [email protected], Bradford Lee [email protected] |
| Notes | This study is currently recruiting participants. Website accessed 21 July 2014. As study is still recruiting not sent mail |
| Trial name or title | An analysis of the effect of topical cromolyn sodium on rosacea‐associated erythema |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 10 participants with rosacea associated erythema |
| Interventions | Cromolyn sodium versus normal saline |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2013 |
| Contact information | Anna Di Nardo, MD, PhD, University of California, San Diego. As study is still recruiting not sent mail |
| Notes | This study is currently recruiting participants. Website accessed 20‐7‐2014 |
| Trial name or title | A randomised, double‐blind, vehicle‐controlled study of the safety and efficacy of topical DRM02 in subjects with rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 30 participants with rosacea |
| Interventions | DRM02 versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | October 2013 |
| Contact information | Dermira, Inc. Beth Zib no further contact details provided, [email protected] sent mail 23‐7‐2014 and 11‐8‐2014 |
| Notes | Study has been completed March 2014. Website accessed 20‐7‐2014 |
| Trial name or title | A randomised, double blind, placebo‐controlled, half‐face study to evaluate the effect of topical ivermectin cream 0.5% on demodicidosis |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 50 subjects with clinical and laboratory diagnosis of demodicidosis with symmetrical facial eruption (including papulopustular rosacea) |
| Interventions | Ivermectin 0.5% cream versus vehicle cream |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | February 2014 |
| Contact information | Rina Segal, Rabin Medical Center, [email protected], not sent mail as not yet open to recruitment |
| Notes | This study is not yet open for participant recruitment. Website accessed 20‐7‐2014 |
| Trial name or title | An open label pilot study to evaluate the efficacy of PAC‐14028 in the treatment of erythematotelangiectatic rosacea and papulopustular rosacea |
| Methods | Randomised, open‐label, active and placebo‐controlled |
| Participants | 80 participants with erythema‐telangiectatic or papulopustular rosacea |
| Interventions | PAC‐14028 cream 1% versus metronidazole gel 0.75% versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | February 2013 |
| Contact information | Amorepacific Corporation BeomJoon Kim, Professor Department of Dermatology, Chungang University Hospital 23‐7‐2014, sent mail through website |
| Notes | Study has been completed August 2013. Website accessed 20‐7‐2014 |
| Trial name or title | Photodynamic therapy for papulopustular rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 30 participants with papulopustular rosacea |
| Interventions | Aminolevulinic acid topical solution 20% + Blu‐U Light versus vehicle + Blu‐U Light |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | April 2014 |
| Contact information | George Washington University, Jack Short, [email protected], as they are still recruiting, not sent mail |
| Notes | This study is currently recruiting participants. Website accessed 20‐7‐2014 |
| Trial name or title | A multicentre, double‐blind, randomised, parallel‐group, vehicle‐controlled study to evaluate the safety and clinical equivalence of a generic azelaic acid gel, 15% and the reference listed Finacea® (azelaic acid) gel, 15% in patients with moderate facial rosacea |
| Methods | Randomised, double‐blind, active and placebo‐controlled |
| Participants | 1100 participants wild moderate rosacea |
| Interventions | Generic azelaic acid gel, 15% versus Finacea® (azelaic acid) gel, 15% versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | July 2013 |
| Contact information | Actavis Inc. No further contact details provided |
| Notes | Still recruiting. Website accessed 20‐7‐2014. As they are still recruiting, not sent mail |
| Trial name or title | Safety and efficacy of AGN‐199201 in patients with persistent erythema associated with rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 440 participants with persistent erythema associated with rosacea |
| Interventions | AGN‐199201 versus vehicle |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | June 2014 |
| Contact information | Allergan, [email protected] seems the same as NCT02131636 which I did not add, sent mail, see Table 3 |
| Notes | This study is currently recruiting participants. Website accessed 20‐7‐2014 |
| Trial name or title | of the safety and efficacy of the Ulthera® System for the treatment of signs and symptoms of erythematotelangiectatic rosacea |
| Methods | Randomised, single‐blind, active‐controlled |
| Participants | 88 participants with erythematotelangiectatic rosacea |
| Interventions | Two low‐density Ulthera System treatments versus three low‐density Ulthera System treatments versus two high‐density Ulthera System treatments versus three high‐density Ulthera System treatments |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2014 |
| Contact information | Ulthera, Inc. Mark Lupin, MD. No further contact details provided. (is Lupin 2014, now an include, part of this study?). Sent mail 29‐7‐2014 |
| Notes | Still recruiting. Website accessed 20‐7‐2014 |
| Trial name or title | Finacea 15% and brimonidine 0.33% gel in the treatment of rosacea ‐ a pilot study |
| Methods | Randomised, single‐blind, active‐controlled |
| Participants | 20 participants with moderate to severe rosacea |
| Interventions | Azelaic acid 15% gel + brimonidine 0.33% gel versus brimonidine 0.33% gel |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2014 |
| Contact information | Leon Kircik, M.D., Derm Research, PLLC, as they are still recruiting, not sent mail |
| Notes | This study is currently recruiting participants. Website accessed 20‐7‐2014 |
| Trial name or title | Prospective, open label, randomised study comparing bipolar radiofrequency potentiated by infrared light to doxycycline in patient with papulopustular rosacea |
| Methods | Randomised, open label, active‐controlled |
| Participants | 40 participants with papulopustular rosacea |
| Interventions | Bipolar radiofrequency potentiated by infrared light versus doxycycline |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | Recruiting |
| Contact information | Florence le Duff, leduff.f2@chu‐nice.fr |
| Notes | Website accessed 23‐9‐2014, as they are still recruiting, not sent e‐mail |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Adverse events Show forest plot | 6 | 1773 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.94, 1.51] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.1  Comparison 1 Topical metronidazole versus placebo, Outcome 1 Adverse events. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Physician's global evaluation of improvement Show forest plot | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.29, 3.02] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.2  Comparison 1 Topical metronidazole versus placebo, Outcome 2 Physician's global evaluation of improvement. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Incomplete data on which further analysis is not possible Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.3
Comparison 1 Topical metronidazole versus placebo, Outcome 3 Incomplete data on which further analysis is not possible. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||||||||||||
| 1 Participant‐assessed improvement of rosacea Show forest plot | 4 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.30, 1.63] | |||||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 Topical azelaic acid versus placebo, Outcome 1 Participant‐assessed improvement of rosacea. | |||||||||||||||||||||||||||||||||||||||
| 2 Physician's global evaluation of improvement Show forest plot | 4 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.18, 1.47] | |||||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 Topical azelaic acid versus placebo, Outcome 2 Physician's global evaluation of improvement. | |||||||||||||||||||||||||||||||||||||||
| 3 Incomplete data on which further analysis is not possible Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||
| Analysis 2.3
Comparison 2 Topical azelaic acid versus placebo, Outcome 3 Incomplete data on which further analysis is not possible. | |||||||||||||||||||||||||||||||||||||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
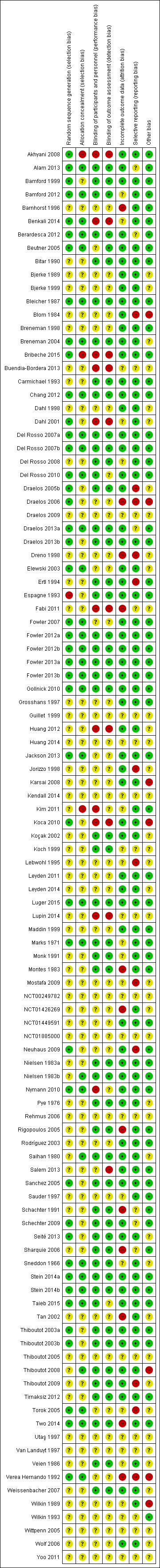
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Topical metronidazole versus placebo, Outcome 1 Adverse events.

Comparison 1 Topical metronidazole versus placebo, Outcome 2 Physician's global evaluation of improvement.
| Study | Interventions | Summary Outcomes | Comment | Notes |
| Barnhorst 1996 | 13 participants were treated with lid hygiene plus warm compresses plus metronidazole 0.75% gel in one eye BID, versus lid hygiene plus warm compresses in the other eye. Within‐patient comparison. | No adverse events reported. Eye and eyelid grading pre‐post mean (SD) ‐1.5 (1.7) versus ‐1.0 (1.7). Authors report significant improvement in treatment group but not in control group, P = 0.022 versus P = 0.10 [inappropriate analysis]. No direct comparison reported. Eye pre‐post mean (SD) ‐0.4 (1.0) versus ‐0.3 (0.9). Eyelid pre‐post mean (SD) ‐1.1 (0.9) versus ‐0.7 (0.8) | Small group (13 participants), Not much data. Participant not blinded. | BID = twice a day SD = standard deviation |
| Beutner 2005 | 557 were treated with metronidazole gel 1% QD versus 553 with metronidazole 1% cream QD versus 189 with metronidazole gel vehicle. | Adverse events 186/557 versus 176/553 versus 51/189. Reduction in lesion count 66.7% versus 58.3% vs. 46.2% | Large vehicle effect. | QD = once daily |
| Bitar 1990 | 50 were treated with metronidazole cream 1% BID versus 50 with placebo cream BID. | Erythema and telangiectasia, no statistical difference. | Data on papules and pustules are skewed. | BID = twice a day |
| Bjerke 1989 | 50 were treated with metronidazole cream 1% BID versus 47 with placebo cream BID. | Erythema: 3 score reduction 2% versus 5%, 2 score reduction 26% versus 5% and 1 score reduction 46% versus 45%, unchanged 26% versus 41%, worse 0% versus 5%. | No SDs were reported. | BID = twice a day N = number SD = standard deviation |
| Bleicher 1987 | 40 were treated with metronidazole 0.75% BID versus 40 with placebo BID. | Adverse events, one complained of tearing when gel came to close to the eyes. Decrease in lesion counts, 65.1% versus 14.9%. | No SDs were reported. Within‐patient comparison. | BID = twice a day SD = standard deviation |
| Breneman 1998 | 104 were treated with metronidazole 1% QD versus 52 with placebo QD. | Mean decrease in erythema score of 0.9 in metronidazole group versus 0.5 in placebo group. Decrease in lesion count 8 versus 3. | No SDs were reported. | SD = standard deviation QD = once daily |
| Dahl 1998 | 44 were treated with metronidazole 0.75% BID versus 44 with placebo BID. | At baseline 35/44 had no or mild erythema versus 32/44. At end of study this was 32/43 versus 24/44. Telangiectasia (no significant difference or effect) | No SDs reported. | BID = twice a day SD = standard deviation |
| Koçak 2002 | 20 patients were treated with metronidazole 0.75% gel BID versus 20 with placebo BID. | No local adverse events in any group Mean change from baseline in papules ‐5.10 (23.36) versus 0.25 (11.25) with a MD of ‐5.35 (95% CI ‐16.71 to 6.01). Mean change from baseline in pustules ‐2.50 (13.65) versus ‐0.20 (9.20) with a MD of ‐2.30 (95% CI ‐9.51 to 4.91). No effects on rhinophyma and telangiectasia. | Most data are skewed. | BID = twice a day SD = standard deviation |
| Nielsen 1983a | 41 were treated with metronidazole 1% QD versus 40 with placebo QD. | Reduction on erythema from 3.8 to 2.5 for metronidazole group and from 3.7 to 3.1 in placebo group. Authors state P < 0.05. There were no effects on telangiectasia. Papules count 8.6 versus 16.6, and pustules count 0.3 versus 0.8. | No SDs were reported. | SD = standard deviation QD = once daily |
Comparison 1 Topical metronidazole versus placebo, Outcome 3 Incomplete data on which further analysis is not possible.
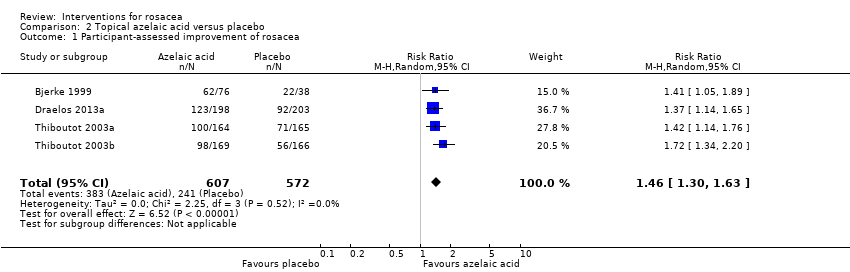
Comparison 2 Topical azelaic acid versus placebo, Outcome 1 Participant‐assessed improvement of rosacea.
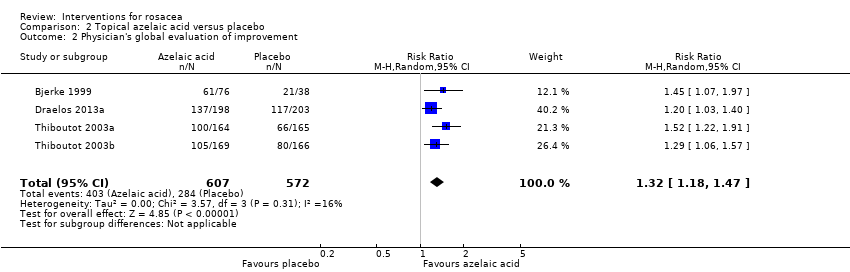
Comparison 2 Topical azelaic acid versus placebo, Outcome 2 Physician's global evaluation of improvement.
| Study | Intervention | Summary Outcomes | Comment | Notes |
| Bjerke 1999 | 76 were treated with azelaic cream 20% BID versus 38 with placebo BID. | Decrease in erythema 47.9% versus 37.9%, in telangiectasia 22.3% versus 23.5%. Decrease in lesions 73.4% versus 50.6%. | No SDs were reported. | BID = twice a day SD = standard deviation |
| Carmichael 1993 | Azelaic cream 20% BID versus placebo BID. Within‐patient comparison in 33 patients. | VAS scale of improvement 6.9 (1.15) to 2.6 (1.72) for azelaic acid treated side versus 7.0 (1.15) to 4.5 (2.30) for placebo treated side Papule count 2.5 (2.87) versus 6.3 (4.6), pustule count 0.0 (0.17) versus 0.4 (0.57). | Data are skewed. | BID = twice a day |
| Draelos 2013a | 198 were treated with azelaic acid 15% foam BID versus 203 vehicle foam BID | There were no statistically significant differences between the 2 groups in end‐of‐treatment or end‐of‐study erythema and telangiectasia | BID = twice a day | |
| Thiboutot 2003a | 164 were treated with azelaic acid 15% BID versus 165 with vehicle BID. | Marked improvement or complete remission according to investigator: 51% versus 27% (investigators reported P < 0.001). | No SDs were reported, can only be estimated from figures | BID = twice a day SD = standard deviation |
| Thiboutot 2003b | 169 were treated with azelaic acid 15% BID versus 166 with vehicle BID Same reference describes 2 studies. | Marked improvement or complete remission according to investigator: 46% versus 31% (investigators reported P < 0.0048). Overall improvement in erythema : 46% versus 28% (investigators reported P = 0.0005). Change in number of inflammatory lesions from 17.8 to 8.9 versus 18.5 to 12.1. | No SDs were reported, can only be estimated from figures | BID = twice a day SD = standard deviation |
Comparison 2 Topical azelaic acid versus placebo, Outcome 3 Incomplete data on which further analysis is not possible.
| Metronidazole compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Metronidazole | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 252 | ⊕⊕⊕⊝ | Bjerke 1989 RR 1.68, 95% CI 1.25 to 2.28; P = 0.0007, Nielsen 1983a RR 3.05, 95% CI 1.57 to 5.94; P = 0.001, Bleicher 1987 (within‐participant study) RR 7. These are clinically important improvements |
| Proportion of participants with adverse event | 161 per 1000 | 191 per 1000 | RR 1.19 | 1773 | ⊕⊕⊕⊕ | Most instances of these adverse events were mild and consisted of pruritus, skin irritation and dry skin |
| Physician‐assessed improvement in rosacea severity | 288 per 1000 | 570 per 1000 | RR 1.98 | 334 | ⊕⊕⊕⊝ | The results are both statistically significant and clinically important |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 602 | ⊕⊕⊕⊝ | In the separate studies (but not in Bitar 1990) there was a greater reduction of erythema in the groups treated with metronidazole, but data were inadequately reported. Except in Koçak 2002 data were adequately reported with a MD of ‐1.40 (95% CI ‐2.47 to ‐0.33; P = 0.01) in favour of metronidazole |
| Lesion count | See comment | See comment | Not estimable | 1964 | ⊕⊕⊕⊝ | No SDs reported, data were skewed but appeared to support data of physician‐assessed improvement |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 514 | ⊕⊕⊕⊕ | Based on interim data improvement started around four weeks |
| Duration of remission | 409 per 1000 | 205 per 1000 | RR 0.50 | 88 | ⊕⊕⊕⊝ | 9/44 in metronidazole group relapsed, versus 18/44 in vehicle group during six months follow‐up |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Bjerke 1989, Nielsen 1983a, Bleicher 1987 11 Although we judged the domains for sequence generation, allocation concealment as unclear and the method of blinding of participants and physicians was not reported, there was no attrition bias nor selective reporting and therefore we concluded there was no serious risk of bias for this outcome assessment 12 Downgraded one level due to serious imprecision (low sample size, optimal sample size is not met) | ||||||
| Azelaic acid compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Azelaic acid | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | 421 per 1000 | 636 per 1000 | RR 1.46 | 1179 | ⊕⊕⊕⊕ | This is a clinically important improvement in favour of azelaic acid |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 1245 | ⊕⊕⊕⊕ | Bjerke 1999 RR 1.00, 95% CI 0.62 to 1.62; P = 0.02, Carmichael 1993 (within‐participant) 24/33 on the azelaic acid side and 19/33 on placebo side, Draelos 2013a RR 2.39, 95% CI 1.12 to 5.09; P = 0.02, Thiboutot 2003a and Thiboutot 2003b 18% and 8% respectively for azelaic acid treated groups and limited to no data for the placebo groups |
| Physician‐assessed improvement in rosacea severity | 497 per 1000 | 655 per 1000 | RR 1.32 | 1179 | ⊕⊕⊕⊕ | Data for these assessments from four studies illustrated that azelaic acid was more effective than placebo |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 1245 | ⊕⊕⊕⊕ | Decrease in erythema in groups treated with azelaic acid ranged from 44% to 47.9% and for placebo from 28% to 37.9%, telangiectasia minimal changes. SDs missing |
| Lesion count | The mean lesion count in the control group was ‐9.5 inflammatory lesions | The mean lesion count in the control group was 3.90 lower (5.87 to 1.93 lower) | 401 | ⊕⊕⊕⊝ | No SDs were reported in (Bjerke 1999; Thiboutot 2003a; Thiboutot 2003b) and data were skewed in Carmichael 1993. All four studies showed a greater reduction in lesions in azelaic acid treated groups (see Analysis 2.3) | |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 1245 | ⊕⊕⊕⊕ | This was not a pre‐specified outcome, but all studies showed clear improvement after three to six weeks |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Bjerke 1999, Draelos 2013a, Thiboutot 2003a, Thiboutot 2003b | ||||||
| Topical ivermectin compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical ivermectin | |||||
| HRQOL | See comment | See comment | Not estimable | 1371 | ⊕⊕⊕⊕ | Although data were statistically significant in favour of ivermectin, the clinical importance is unclear as MID in reduction of DLQI score was not reached and the MID is not yet established for RosaQoL2 |
| Participant‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 1371 | ⊕⊕⊕⊕ | RR 1.78, 95% CI 1.50 to 2.11 (Stein 2014a), RR 1.92, 95% CI 1.59 to 2.32 (Stein 2014b). Both studies showed a statistically significant and clinically important improvement in favour of topical ivermectin |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 1371 | ⊕⊕⊕⊕ | RR 0.54, 95% CI 0.29 to 1.01 (Stein 2014a), RR 1.00, 95% CI 0.55 to 1.82 (Stein 2014b) |
| Physician‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 1371 | ⊕⊕⊕⊕ | RR 3.30, 95% CI 2.27 to 4.79 (Stein 2014a), RR 2.10, 95% CI 1.57 to 2.81 (Stein 2014b). The results of both studies are in concordance with the assessments of the participants |
| Assessment of erythema or telangiectasia ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion count | See comment | See comment | Not estimable | 1371 | ⊕⊕⊕⊕ | MD ‐8.40, 95% CI ‐9.93 to ‐6.87 (Stein 2014a), MD ‐8.90, 95% CI ‐10.45 to ‐7.35 (Stein 2014b). Both of these differences are statistically significant and clinically important |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 1371 | ⊕⊕⊕⊕ | Improvement in both studies was seen after four weeks |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Stein 2014a, Stein 2014b | ||||||
| Topical brimonidine compared to vehicle for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical brimonidine | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 553 | ⊕⊕⊕⊕ | At 3 hours RR 2.21, 95% CI 1.52 to 3.22 (Fowler 2013a) and RR 2.00, 95% CI 1.33 to 3.01 (Fowler 2013b). At each time point in both studies brimonidine was shown to be more effective than vehicle in an improvement which was statistically significant |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 553 | ⊕⊕⊕⊕ | RR 1.17, 95% CI 0.79 to 1.74 (Fowler 2013a), RR 1.40, 95% CI 0.97 to 2.02 (Fowler 2013b). Adverse events were mild and transient |
| Physician‐assessed improvement in rosacea severity ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No reporting of data other than "No aggravations in the severity of IGA were observed" |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 553 | ⊕⊕⊕⊕ | At 3 hours RR 2.82, 95% CI 1.85 to 4.30 (Fowler 2013a), RR 1.78, 95% CI 1.25 to 2.55 (Fowler 2013b) |
| Lesion count ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No reporting of data other than "No aggravations in the severity of lesion counts were observed" |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 553 | ⊕⊕⊕⊕ | Improvement was seen within 30 min |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | There was no rebound or worsening of erythema after treatment cessation in comparison to baseline assessments |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Topical azelaic acid compared to topical metronidazole for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical metronidazole | Topical azelaic acid | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 491 | ⊕⊕⊝⊝ | RR 1.23, CI 95% 1.04 to 1.44; P = 0.01 (Elewski 2003), RR 1.00, 95% CI 0.83 to 1.21 (Wolf 2006), Maddin 1999 (within‐participant) authors report P = 0.02 in favour of azelaic acid |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 491 | ⊕⊕⊝⊝ | RR 3.64, 95% CI 1.81 to 7.31; P = 0.0003 (Elewski 2003), RR 0.74, 95% CI 0.52 to 1.07 (Wolf 2006). In Maddin 1999 1 participant reported stinging on azelaic acid treated site |
| Physician‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 491 | ⊕⊕⊝⊝ | RR 1.26, 95% CI 1.03 to 1.53; P = 0.02 (Elewski 2003), RR 1.05, 95% CI 0.79 to 1.39 (Wolf 2006), Maddin 1999 score 2.7 (SD 1.0) versus 3.1 (SD 1.0) (higher is worse) |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 491 | ⊕⊕⊝⊝ | RR 1.35, 95% CI 1.05 to 1.75; P = 0.02 (Elewski 2003), RR 0.99, 95% CI 0.69 to 1.42 (Wolf 2006), in Maddin 1999 the participants and physicians had contradictory judgements |
| Lesion counts | See comment | See comment | Not estimable | 491 | ⊕⊕⊕⊝ | No SDs were reported, all three studies demonstrated a clinically important reduction in lesion count in both treatment arms |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 491 | See comment | Improvement for both arms was seen after four to six weeks in all three studies |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Elewski 2003, Maddin 1999, Wolf 2006 | ||||||
| Topical ivermectin compared to topical metronidazole for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical metronidazole | Topical ivermectin | |||||
| HRQOL | 640 per 1000 | 711 per 1000 | RR 1.11 | 962 | ⊕⊕⊕⊕ | Reduction in DLQI was 5.18 in ivermectin group and 3.92 in metronidazole group (both meeting minimal important difference) |
| Participant‐assessed improvement in rosacea severity | 748 per 1000 | 853 per 1000 | RR 1.14 | 962 | ⊕⊕⊕⊕ | This is a statistically significant difference and in concordance with the results on number of participants that experienced no deleterious effect on their quality of life |
| Proportion of participants with adverse event | 8 per 1000 | 19 per 1000 | RR 2.28 | 962 | ⊕⊕⊕⊝ | |
| Physician‐assessed improvement in rosacea severity | 754 per 1000 | 852 per 1000 | RR 1.13 | 962 | ⊕⊕⊕⊕ | These assessments are consistent with the assessments of the participants |
| Assessment of erythema or telangiectasia ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion count | The mean lesion count in the control groups was | The mean lesion count in the intervention groups was | 962 | ⊕⊕⊕⊕ | Both treatments showed clinically important reductions in lesion counts | |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 962 | ⊕⊕⊕⊕ | This was not a predefined outcome, but clear improvement could be seen for both treatment arms around six weeks |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Taieb 2015 | ||||||
| Ciclosporin ophthalmic emulsion 0.05% compared to artificial tears for ocular rosacea | ||||||
| Patient or population: Participants with ocular rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Artificial tears | Ciclosporinophthalmic emulsion 0.05% | |||||
| HRQOL | The mean OSDI in the control group was | The mean OSDI in the intervention group was | 37 | ⊕⊕⊝⊝ | The difference between change scores at end of study equates to a moderate improvement in quality of life in favour of ciclosporin ophthalmic emulsion | |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | RR 2.32 | 37 | ⊕⊕⊝⊝ | |||
| Physician‐assessed improvement in rosacea severity | The mean physician‐assessed improvement in rosacea severity in the control group was | The mean physician‐assessed improvement in rosacea severity in the intervention group was | 37 | ⊕⊕⊝⊝ | ||
| Assessment of erythema or telangiectasia ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion count ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Time needed until improvement of the skin lesions ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Schechter 2009 | ||||||
| Clindamycin phosphate 1.2% + tretinoin 0.025% gel compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Clindamycin phosphate 1.2% + tretinoin 0.025% gel | |||||
| HRQOL | See comment | See comment | Not estimable | 83 | ⊕⊕⊕⊝ | No mean scores were provided, only percentages of participants that had improved per item on the 21 survey items, no statistically significant difference for any item |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | 275 per 1000 | 674 per 1000 | RR 2.45 | 83 | ⊕⊕⊕⊝ | Worsening of rosacea, facial scaling, as well as dry skin were reported most often in the active treatment group |
| Physician‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 83 | ⊕⊕⊕⊝ | None of the primary features of the PGA showed statistically significant differences between the treatment groups except for oedema in favour of placebo |
| Assessment of erythema or telangiectasia | 150 per 1000 | 257 per 1000 | RR 1.71 | 83 | ⊕⊕⊕⊝ | RR 1.71 (95% CI 0.70 to 4.18) refers to erythema. Telangiectasia RR 2.42, 95% CI 0.95 to 6.17 |
| Lesion count | The mean lesion count in the control group was | The mean lesion count in the intervention group was | 83 | ⊕⊕⊕⊝ | ||
| Time needed until improvement of the skin lesions ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | There was no improvement |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Chang 2012 | ||||||
| Tetracycline compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Tetracycline | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | 474 per 1000 | 701 per 1000 | RR 1.48 | 39 | ⊕⊕⊕⊝ | |
| Proportion of participants with adverse event | 53 per 1000 | 50 per 1000 | RR 0.95 | 39 | ⊕⊕⊕⊝ | Only one adverse event was reported in each group, diarrhoea in the tetracycline group, maculopapular rash in the placebo group |
| Physician‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 107 | ⊕⊕⊕⊝ | RR 4.04, 95% CI 1.66 to 9.83; P = 0.002 (Marks 1971) and RR 1.72, 95% CI 1.18 to 2.50; P = 0.005 (Sneddon 1966) |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 39 | ⊕⊕⊕⊝ | There were no significant changes in erythema (Marks 1971) |
| Lesion count | The mean lesion count in the control group was | The mean lesion count in the intervention group was | 39 | ⊕⊕⊕⊝ | Crude MD ‐14.64 but skewed data (Marks 1971) | |
| Time needed until improvement of the skin lesions ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Marks 1971 | ||||||
| Doxycycline 40 mg compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Doxycycline 40 mg | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 537 | ⊕⊕⊕⊕ | RR 1.14, 95% CI 0.85 to 1.53 (Del Rosso 2007a) and RR 1.27, 95% CI 1.04 to 1.55 (Del Rosso 2007b) |
| Physician‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 537 | ⊕⊕⊕⊕ | RR 1.77, 95% CI 1.24 to 2.52; P = 0.002 (Del Rosso 2007a) and RR 1.41, 95% CI 0.87 to 2.29 (Del Rosso 2007b) and IGA score of 0 or 1 RR 1.59, 95% CI 1.02 to 2.47; P = 0.04 (Del Rosso 2007a) and RR 2.37, 95% CI 1.12 to 4.99; P = 0.02 (Del Rosso 2007b) |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 537 | ⊕⊕⊕⊕ | Mean change in CEA ‐2.7 (doxycycline group) versus ‐1.8 (placebo group), investigators report P = 0.017 (Del Rosso 2007a); and ‐1.4 and ‐1.2 respectively (Del Rosso 2007b) |
| Lesion counts | See comment | See comment | Not estimable | 537 | ⊕⊕⊕⊝ | MD ‐5.90, 95% CI ‐9.37 to ‐2.43; P = 0.0009 (Del Rosso 2007a) and MD ‐5.20, 95% CI ‐8.27 to ‐2.13; P = 0.0009 (Del Rosso 2007b) |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 537 | ⊕⊕⊕⊕ | The steepest changes in graph plots occurred within three weeks in the doxycycline group |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Del Rosso 2007a and Del Rosso 2007b | ||||||
| Azithromycin compared to doxycycline 100 mg for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline 100 mg | Azithromycin | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | 800 per 1000 | 784 per 1000 | RR 0.98 | 67 | ⊕⊝⊝⊝ | There was no statistically significant difference between the groups, but in both treatment arms the majority of participants considered themselves improved |
| Proportion of participants with adverse event | 67 per 1000 | 108 per 1000 | RR 1.62 | 67 | ⊕⊝⊝⊝ | |
| Physician‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Assessment of erythema or telangiectasia ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion counts | The mean lesions count in the control group was | The mean lesions count in the intervention group was | 67 | ⊕⊝⊝⊝ | Lesion count decreased in azithromycin group from 19.24 (SD 9.67) to 1.90 (SD 3.28) at 3 months and for doxycycline from 18.86 (SD 8.95) to 2.34 (SD 3.47). Skewed data | |
| Time needed until improvement of the skin lesions ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission | See comment | See comment | Not estimable | 67 | ⊕⊝⊝⊝ | No data on duration of remission, but both groups showed no statistically significant change between the third month of treatment and the second month post‐treatment in the mean inflammatory lesion counts |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Akhyani 2008 | ||||||
| Doxycycline 40 mg + metronidazole 1% gel compared to doxycycline 100 mg + metronidazole 1% gel for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline 100 mg + metronidazole 1% gel | Doxycycline 40 mg + metronidazole 1% gel | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | 553 per 1000 | 138 per 1000 | RR 0.25 | 91 | ⊕⊕⊝⊝ | The majority of these adverse events were gastrointestinal complaints |
| Physician‐assessed improvement in rosacea severity | The mean physician‐assessed improvement in rosacea severity in the control group was | The mean physician‐assessed improvement in rosacea severity in the intervention group was | 91 | ⊕⊕⊝⊝ | ||
| Assessment of erythema or telangiectasia | The mean assessment of erythema or telangiectasia in the control group was | The mean assessment of erythema or telangiectasia in the intervention group was | 91 | ⊕⊕⊝⊝ | Reduction in CEA 4.2 in doxycycline 40 mg and 4.0 in doxycycline 100 mg group, investigator's state P = 0.50 | |
| Lesion count | The mean lesion count in the control group was | The mean lesion count in the intervention group was | 91 | ⊕⊕⊝⊝ | ||
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 91 | ⊕⊕⊝⊝ | A clear improvement was seen from week four for both groups. |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Del Rosso 2008 | ||||||
| Doxycycline 40 mg + azelaic acid gel compared to doxycycline 40 mg + metronidazole gel for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline 40 mg + metronidazole gel | Doxycycline 40 mg + azelaic acid gel | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | 465 per 1000 | 489 per 1000 | RR 1.05 | 207 | ⊕⊕⊕⊕ | Excellent improvement was reported in approximately half of each intervention group |
| Proportion of participants with adverse event | 69 per 1000 | 19 per 1000 | RR 0.27 | 207 | ⊕⊕⊕⊕ | |
| Physician‐assessed improvement in rosacea severity | 723 per 1000 | 781 per 1000 | RR 1.08 | 207 | ⊕⊕⊕⊕ | |
| Clinician's Erythema Assessment ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion count | The mean lesion count in the control group was | The mean lesion count in the intervention group was | 207 | ⊕⊕⊕⊝ | ||
| Time needed until improvement | See comment | See comment | 207 | ⊕⊕⊕⊕ | From four weeks on improvement could be seen for both treatment arms | |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Del Rosso 2010 | ||||||
| Minocycline 45 mg compared to minocycline 45 mg + azelaic acid gel for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Minocycline 45 mg + azelaic acid gel | Minocycline 45 mg | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | 533 per 1000 | 368 per 1000 | RR 0.69 | 60 | ⊕⊕⊝⊝ low2,3 | |
| Physician‐assessed improvement in rosacea severity | The mean physician‐assessed improvement in rosacea severity in the control groups was | The mean physician‐assessed improvement in rosacea severity in the intervention groups was | 60 | ⊕⊕⊝⊝ low2,3 | ||
| Assessment of erythema or telangiectasia | The mean assessment of erythema or telangiectasia in the control group was | The mean assessment of erythema or telangiectasia in the intervention group was | 60 | ⊕⊕⊝⊝ low2,3 | ||
| Lesion count | The mean lesion count in the control group was | The mean lesion count in the intervention group was | 60 | ⊕⊕⊝⊝ low2,3 | In both groups there was a clinically important reduction in lesion counts of 11.00 (SD 4.49) in the minocycline group and 12.00 (SD 3.00) in the comparator group | |
| Time needed until improvement | See comment | See comment | Not estimable | 60 | ⊕⊕⊝⊝ low2,3 | Improvement was seen in both arms at four weeks |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Jackson 2013 | ||||||
| Topical metronidazole compared to oral (oxy)tetracycline for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral (oxy) tetracycline | Topical metronidazole | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 182 | ⊕⊕⊕⊝ | RR 0.71, 95% CI 0.40 to 1.26 (Monk 1991), RR 0.96, 95% CI 0.80 to 1.17 (Nielsen 1983b) and in Schachter 1991 no exact data were provided other than that "both groups considered their condition much improved" |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 258 | ⊕⊕⊕⊝ | No adverse event (Nielsen 1983b), RR 1.06, 95% CI 0.32 to 3.55 (Monk 1991), 12 adverse events reported in metronidazole group and 9 in tetracycline group (Schachter 1991), RR 0.70, 95% CI 0.30 to 1.65 (Veien 1986) |
| Physician‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 81 | ⊕⊕⊕⊝ | RR 0.80, 95% CI 0.47 to 1.35 (Monk 1991), RR 1.00, 95% 0.89 to 1.13 (Nielsen 1983b) |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 258 | ⊕⊕⊝⊝ | Erythema score ‐1.4 versus ‐1.3 (Monk 1991), "the reduction of erythema was the same in both groups, and the number and extent of telangiectases were unchanged" (Nielsen 1983b), in Schachter 1991 no differences in erythema nor telangiectasia were seen in either group. In Veien 1986 the percentage of no improvement was 11.1 in the metronidazole group versus 12.5 in the tetracycline group |
| Lesion count | See comment | See comment | 258 | ⊕⊕⊕⊝ | Complete clearance in 75% versus 66% of participants (Monk 1991), "the reduction of papules and pustules was the same in both groups" (Nielsen 1983b), decrease of 68% versus 77% in papule count and of 53% and 61% in pustule count (Schachter 1991). In Veien 1986 only medians were provided with 11.1 lesions in the metronidazole group and 0 in the tetracycline group | |
| Time needed until improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Monk 1991, Nielsen 1983b, Schachter 1991 (number of participants randomised in Schachter 1991 was unclear) | ||||||
| Low dose isotretinoin 0.3 mg/kg compared to doxycycline 100 mg for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline 100 mg | Low dose isotretinoin 0.3 mg/kg | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity1 | 644 per 1000 | 792 per 1000 | RR 1.23 | 261 | ⊕⊕⊕⊕ | Low dose isotretinoin is considered by the participants to be slightly more effective than doxycycline 100 mg |
| Proportion of participants with adverse event | 171 per 1000 | 204 per 1000 | RR 1.19 | 299 | ⊕⊕⊕⊕ | |
| Physician‐assessed improvement in rosacea severity1 | 689 per 1000 | 813 per 1000 | RR 1.18 | 261 | ⊕⊕⊕⊕ | In agreement with the participant‐assessed changes |
| Assessment of erythema or telangiectasia | 783 per 1000 | 736 per 1000 | RR 0.94 | 285 | ⊕⊕⊕⊕ | Telangiectasia were improved or "healed" RR 1.03, 95% CI 0.77 to 1.37 |
| Lesion count1 | The mean lesion count in the control group was | The mean lesion count in the intervention group was | 261 | ⊕⊕⊕⊕ | ||
| Time needed until improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Per‐protocol analysis | ||||||
| Pulsed dye laser compared to Nd:YAG laser for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Nd: YAG laser | Pulsed dye laser | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity1 | The mean participant‐assessed improvement in rosacea severity in the control group was | The mean participant‐assessed improvement in rosacea severity in the intervention group was | 14 | ⊕⊕⊝⊝ low3 | ||
| Proportion of participants with adverse event1 | See comment | See comment | Not estimable | 14 | ⊕⊕⊝⊝ low4 | Pain was assessed on the PDL treated side 3.87 and 3.07 on the Nd:YAG side, the investigators state P = 0.0028 |
| Physician‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Assessment of erythema or telangiectasia1 | The mean assessment of erythema or telangiectasia in the control group was | The mean assessment of erythema or telangiectasia in the intervention group was | 14 | ⊕⊕⊝⊝ low3 | ||
| Lesion count ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Time until improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Within‐participant | ||||||
| Pulsed dye laser compared to intense pulsed light therapy for rosacea | ||||||
| Patient or population: Participants with rosacea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intense Pulsed Light Therapy | Pulsed Dye Laser | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity1 | The mean participant‐assessed improvement in rosacea severity in the control group was | The mean participant‐assessed improvement in rosacea severity in the intervention group was | 40 | ⊕⊕⊝⊝ low3,4 | Median was 8 (range 2 to 10) for PDL group and 7 (range 2 to 10) for IPL group (10% and 90% percentiles) | |
| Proportion of participants with adverse event | Pain assessed on a VAS scale in the control group was | Pain assessed on a VAS scale in the intervention group was | 40 | ⊕⊕⊝⊝ low3,4 | Median was 4 (range 2 to 6) for PDL group and 7 (range 2 to 10) for IPL group (10% and 90% percentiles) | |
| Physician‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Assessment of erythema or telangiectasia | See comment | See comment | 40 | ⊕⊕⊕⊝ moderate4,5 | On the PDL treated side 18 had an excellent (75% to 100% vessel clearance) response and 12 a good response (50% to 74% clearance) and on the IPL treated sides 11 had an excellent response and 19 a good response | |
| Lesion count ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Time until improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Within‐participant design | ||||||
| Term | Definition |
| Acne | A skin condition characterised by the inflammation or infection of sebaceous glands (usually attached to hair follicles) resulting in comedones (whiteheads and blackheads) and inflammatory lesions such as papules (pimples), pustules, and nodules) |
| Bacillus oleronius | A bacteria found in Demodex mites |
| Bacterial resistance | Resistance of a micro‐organism to an antimicrobial drug that was originally effective for treatment of infections caused by this micro‐organism |
| Body dysmorphic disorder | An anxiety disorder surrounding perceived flaws in one's own appearance |
| Cytokines | A small protein released by cells, and having a specific effect on the behavior of other cells, or on the interactions or communications between cells |
| Demodex folliculorum | A species of face mite found in human hair follicles |
| Down‐regulation | Process of reducing or suppressing a response to a stimulus |
| Epidermal barrier | The skin's front line of defence in the upper layer of the skin (the epidermis) against environmental factors such as UV light, chemicals, bacteria and other organisms and limits water loss from the body |
| Innate immune response | The first line generic defence of the immune system against infection and other organisms |
| Keratinocytes | A predominant cell type in the outermost layer of skin (epidermis), and when found in the basal layer, are referred to as 'basal cells' or 'basal keratinocytes'. Their main function is the formation of a barrier against environmental damage |
| Matrix‐Metalloproteinases | Zinc dependent enzymes that promote break down of proteins like collagen. They regulate various inflammatory and repair processes |
| Neurovascular dysregulation | A failure of the vascular response, vasodilation, and neurosensory symptoms to regulate properly Dysfunction of both nerves and vascular elements, controlling the calibre of blood vessels |
| Nodule | Solid, raised area in or under the skin |
| Nodularities | An increased density of tissues |
| Pathophysiology | The functional changes that accompany a particular syndrome or disease (combined terms of ‘patho’ (path, related to disease) and ‘physiology’ (a branch of biology that specialises in the study of the functions of living organisms and their parts) |
| Phototype | A classification of skin type based on a person's sensitivity to sunlight |
| Pustule | A small bump on the skin containing purulent material (pus) in the top layer (epidermis) or beneath it (dermis) |
| Reactive oxygen species (ROS) | Chemically reactive molecules containing oxygen, or oxygen‐derived radicals, having important roles in cell signalling (communication and interaction) and homeostasis (the maintenance of a steady state) |
| Retinoids | Chemical compounds related chemically to Vitamin A |
| Stratum corneum | The outermost layer of the epidermis |
| Stye | A bacterial infection of a gland at the base of any eyelash, causing painful swelling on the inner or outer eyelid |
| Toll‐like receptors | A class of proteins that play a key role in the innate immune system, activating immune cell responses |
| Name | Response | Additional | Comment |
| Bayer | Yes | Yes | Added information on Del Rosso 2010 Christopher Billis <[email protected]> and that ongoing studies were not yet published |
| Roche | Yes | No | ‐ |
| ASTA Medica | Yes | No | ‐ |
| Merck | Yes | No | ‐ |
| Dumex‐Alpharma | Yes | No | ‐ |
| Galderma | Yes | Yes | [email protected], [email protected] August 2014 several times contact with Galderma NL, France and US, provided lots of extra information regarding brimonidine and ivermectin |
| AHP Pharma | No | No | ‐ |
| Yamanouchi | No | No | ‐ |
| Dermik Laboratories | No | No | ‐ |
| CollaGenex | No | No | Taken over by Galderma |
| Name | Response | Additional | Comment |
| Yes | Yes | [email protected]. (sequence generation and allocation concealment) "In efficacy of azithromycin vs. doxycycline in the treatment of rosacea: a randomised open clinical trial" Patients were allocated to the trial using a randomised numbers table. Unfortunately this trial was not blinded" "The randomised number table generated by computer. The list was only in access of physician, and patients could not see that | |
| Yes | Yes | After email contact with the primary investigator and following on from discussion between the review authors, this was judged to be quasi‐randomised, i.e. a CCT | |
| Yes | Yes | [email protected], 1‐8‐2014 (sequence generation and allocation concealment) 2) As explained above, only the 4 arms treated with topical products were to be randomised. The block size of 4 was not known by the sites, so foreseeing the next allocation was possible but unlikely. Since the study had 2 treatment groups for QD regimen and 2 treatment groups for BID regimen, subjects and the personnel who distributed the medication necessarily knew this information Of note, the primary objective of this study was PK assessment (and not efficacy), an objective measure, and the primary comparison was topical versus eye drop which was in no way planned to be randomised or blinded 3) This study was not posted on CT.gov since it was classified as a phase 1 study | |
| Yes | Yes | [email protected]. After email communication with the investigators to clarify aspects of trial conduct, the criterion for sequence generation was changed from UNCLEAR to High risk, i.e. the study was not a RCT and participants appear to have been allocated to the intervention by alternation | |
| Yes | Yes | 18‐8‐2014 [email protected] (sequence generation and allocation concealment and blinding) 29‐9‐2013 replies 1. This was a randomised, double‐blind, parallel‐group, placebo‐controlled study Patients, having signed their informed consent and who satisfied all inclusion and exclusion criteria at inclusion visit were randomly assigned to one of two treatment groups (P‐3075 cream, placebo), according to a computer‐generated randomisation list Patients were sequentially assigned to the next available randomisation number, starting from the lowest number provided to each investigational site Furthermore, for ethical reason, in order to minimise the exposure to placebo, randomisation was unbalanced between the P‐3075 and placebo groups with a 2:1 ratio using blocks of 3 treatments 2/3. The double blind study design was guaranteed by the use of placebo cream units, which were identical to the active product in terms of size, shape, volume, colour. The tubes (P‐3075 and placebo) were identically labelled for clinical use as it is in a double‐blind procedure 4. Thank you for this observation (you are the first). We confirm that the correct value is ‐167.00 and not 167.00, as reported in our database. It was a typing error that was not detected when the manuscript was transformed in draft paper by the editor 5. As described in the paper, a 4‐point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) was used by the investigators at each visit for the clinical evaluation of erythema The results at Day 28 (end of treatment) showed that, in the P‐3075 group, erythema was absent in 27 patients (96.4%) and mild in 1 (3.6%), while in the placebo group, erythema was absent in 9 patients (64.3%) and mild in 5 (35.7%). There were no cases of moderate or severe intensity at Day 28 in both groups. The statistically significance values were reported in the paper as you underlined. For completeness the baseline clinical assessment for erythema was as follows: for P‐3075 absent in 7 patients, mild in 14, moderate in 6 and severe in 1 and for placebo absent in 3 patients, mild in 6 and moderate in 5 | |
| Yes | Yes | [email protected] and [email protected], [email protected]. Useful additional information provided by primary investigator, on randomisation, allocation concealment and characteristics of patients | |
| Yes | Yes | '[email protected]' 30‐8‐2014 Reply 10‐9‐2014: Me and professor Fedotov VP, were responsible for using this programme, both of us had access to the generated list and a doctor from our department (Dr Makurina); who was fully unaware of the aims of the study and overseen the enrolment | |
| Can't find mail address, sent invite on LinkedIn | |||
| Chosidow 2014 | Yes | [email protected] and [email protected],18‐7‐2014 Reply 18‐7‐2014 "Hi Esther, we are still in processing the manuscript and hope submitting the paper before the end of 2014" and "We could send your our submitted manuscript?" | |
| No | No | ‐ | |
| Yes | Yes | [email protected]."Subjects will be randomised to 1 of the 2 treatment groups at a ratio of 1:1. The randomisation process will be done in blocks of 4, stratified by investigators. The randomisation will be carried out using SAS PROC PLAN" | |
| Yes | Yes | [email protected], 1‐8‐2014 (sequence generation, allocation concealment) Chris Billis [[email protected]]; Keith Flanders [[email protected]] | |
| Yes | No | Old study, no further data available | |
| Yes | Yes | [email protected]. On 2006. Sequence generation? "Subjects were randomised based on the order in which they presented to the office". Allocation concealment? "The research coordinator maintained the blind which was not shared with anyone, including the investigator." | |
| No | No | [email protected], 2‐8‐2014 (sequence generation and allocation concealment and blinding, how many randomised to each group, separate data for participants with rosacea? losses to follow‐up?) 9‐8‐2014 sent again. No reaction | |
| Yes | Yes | [email protected], 2‐8‐2014 (blinding and details on RosaQoL data) Reply 2‐8‐2014 This was the pivotal trial for FDA approval. The blind was maintained by dispensing the vehicle and the vehicle plus the active in identical containers. I do not have more detail on the QOL scores | |
| Yes | Yes | [email protected] (sequence generation, stratification and allocation concealment and blinding) Reply 2‐8‐2014 follow‐up mail that allocation concealment is not yet satisfactorily answered | |
| Yes | No | Dr Levine, study 17 years old, no further data available | |
| No | No | [email protected] 2‐8‐2014 (sequence generation and allocation concealment and dropouts?) Follow‐up mail 11‐8‐2014 and 17‐8‐2014, no reply | |
| Yes | Yes | [email protected] and [email protected]. "Randomisation was done by using a computer generated table provided by the sponsor. Neither subjects nor investigators and study staff had any control over this" | |
| Yes | Yes | [email protected] and Jean Jacovella ([email protected]) 22‐8‐2014, 27‐8 Asked for separate exact data of PSA and CEA at different time points, wash‐out period and details on AE Received replies 25‐9‐2014 | |
| Yes | Yes | 20‐7‐2014 asked Galderma if it is published (Patricia van Lith) is this Fowler 2013? 28‐7‐2014 confirmed (NCT01355471 and NCT01789775 are the same studies) Received replies 25‐9‐2014 | |
| Yes | Yes | '[email protected]' 3‐8‐2014 (sequence generation and allocation concealment and blinding) 3) Investigators were not privy to the medication/placebo selection process. No medication tubes were shown to the investigators. No questions were asked about the topical product (i.e. Odor, color or feel). There was no communication between the study coordinator and the investigators regarding the medication/placebo selection follow‐up mail 12‐8‐2014 | |
| No | No | ‐ | |
| Yes | Yes | 3‐8‐2014 [email protected] [email protected] (sequence generation and allocation concealment clarification of N) | |
| No | No | 18‐7‐2014 [email protected] (sequence generation and allocation concealment) 9‐8‐2014 follow‐up mail and 17‐8‐2014, no reply | |
| No | No | ‐ | |
| Yes | Yes | [email protected] 9‐8‐2014 | |
| No | No | ‐ | |
| Yes | No | ‐ | |
| Yes | Yes | [email protected]. After email contact with the primary investigator this study was excluded | |
| No | No | ‐ | |
| Yes | Yes | [email protected] 6‐8‐2014 (sequence generation and allocation concealment and blinding and dropouts) 9‐9‐2014 reply: I did not receive your previous e‐mails as my address is [email protected] 70 patients were enrolled in the study. Two subjects withdrew from the study and they were both in the brimonidine tartrate gel 0.5% treatment group in phase 1 and did not enter phase 2. One for an adverse event and one for a protocol deviation | |
| Yes | No | Appeared to be wrong R Koch | |
| Yes | Yes | After extensive email contact, clarified as a CCT | |
| No | No | ‐ | |
| Yes | No | Old study, no further data available | |
| Yes | No | [email protected] 7‐8‐2014 (sequence generation and allocation concealment) | |
| Yes | Yes | 19‐7‐2014, info@sol‐gel.com Ofer.Toledano@sol‐gel.com My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? Reply 19‐7‐2014 Ofer.Toledano@sol‐gel.com, Gaby.Peleg@sol‐gel.com . The study results were published by James Leyden in the JDD (journal of drugs in dermatology) in June 2014, volume 13, issue 6, p.685 | |
| Yes | Yes | Mderma@uni‐muenster.de (allocation concealment and blinding) Reply 18‐8‐2014 | |
| No | No | Ulthera, Inc. Mark Lupin, M.D 23‐7‐2014 info @cosmedica.ca [email protected], sent several mails no reply (sequence generation and allocation concealment, dropouts) | |
| No | No | S Mokadem no response | |
| National Rosacea Society | No | No | ‐ |
| No | No | ‐ | |
| G Plewig | No | No | ‐ |
| Yes | No | ‐ | |
| A Rebora | No | No | ‐ |
| No | No | ‐ | |
| No | No | ‐ | |
| No | No | [email protected] 10‐08‐2014 Resent 17‐8‐2014 and 3‐9‐2014 (sequence generation and allocation concealment and blinding), no replies | |
| Yes | Yes | [email protected] 16‐7‐2014 (sequence generation and allocation concealment and blinding, dropouts) 1. The allocation sequence was generated by a statistician using a specific software 2. As soon as they have been recruited (because they answered to the inclusion criteria) by the investigating dermatologist (only one = Dr Zelenkova) a number given chronologically, as indicated in the allocation sequence purchase to the investigator, was attributed to the patient (the first was the N°1, the 2nd the N° 2…) 3. After enrolment and at the end of the 1st visit, a nurse (in the absence of the investigating dermatologist) give the products allocated to the patient’s number. Both products was in the same packaging (blind white packaging) without any indication about formula reference (only reference of study and number of patient) and some information about use (topical use only…) 4. None dropped out between the stop of metronidazole treatment (Week 8) and the end of the study (week 16). 67 patients were included before metronidazole treatment, 1 dropped out due to irritative dermatitis at day 53 (before the end of the 8‐week Metronidazole treatment); So 66 patients remained after 8 weeks, 32 received the test formula and 34 the vehicle 5. More detail about the 66 patients included in this study are available (see below (printscreens)) | |
| No | No | ‐ | |
| Yes | Yes | 19‐7‐2014 asked Galderma if NCT01493687 it is published and looks the same as NCT01494467 (Patricia van Lith), confirmed 28‐7 are the same and are Stein Gold 3‐9‐2014, received data | |
| Yes | Yes | 19‐7‐2014 asked Galderma if NCT01493947 is published (Patricia van Lith), confirmation 28‐7‐2014, EADV abstract 2014 alain.taieb@chu‐bordeaux.fr (sequence generation and allocation concealment and blinding, dropouts) The study was a parallel group study of 960 subjects; however 1800 kit numbers are randomised in blocks of 6. The RANUNI routine of the SAS system was used to randomly assign, in balanced blocks, kit to a treatment (Ivermectin 1% cream, Metronidazole 0.75% cream). Prior to the start of the study, a randomisation list was generated by the statistician and was secured with restricted access. Treatment assignment was balanced into consecutive blocks in a 1:1 ratio and kit numbers were assigned sequentially in chronological order. The study design was investigator‐blinded. The integrity of the blinding was ensured by packaging the products in identical tubes, not allowing the investigator and subject to discuss study treatments, and requiring a third party other than the investigator to dispense the medication. Study population and causes for withdrawal are summarised in the figure below | |
| Thiboutot 2003a; Thiboutot 2003b; Thiboutot 2008; Thiboutot 2009 | No | No | ‐ |
| No | No | [email protected] 17‐8‐2014 ((sequence generation and allocation concealment) | |
| Yes | Yes | [email protected]. "The patients were not cognizant nor were they aware of the different formulations Nor their unique characteristics so they were easily utilized and dispensed in unmarked tubes". "Central randomisation that was computer generated" | |
| Yes | Yes | [email protected] 17‐8‐2014 (sequence generation and allocation concealment and blinding) 1. The allocation sequence was generated by an unblinded member of the study team who worked off‐site in a separate laboratory to group in a 2‐to‐1 fashion, so that 8 of those numbers were assigned to the treatment group, and 4 to the control group. As subjects were enrolled in the study, they were sequentially assigned a unique study identification number from 1‐12 by the blinded study coordinator, with the first subject to enrol in the study being assigned the study identification number of 1. The list matching study identification numbers to their corresponding treatment group was only accessible by this unblinded member of the study team 2. The allocation sequence was created prior to enrolling any subjects in the study, therefore ensuring that intervention allocations could not be foreseen in advance of, or during, enrolment 3. This study was conducted in a double‐blind fashion so that both participants and investigators were blinded as to which intervention group participants were assigned. As stated previously, randomisation was completed by an unblinded member of the study team who worked off‐site and had no contact with enrolled subjects. The list of treatment group assignments was stored on a password‐protected computer accessible only to this unblinded study team member. This same unblinded member of the study team was also responsible for preparing all study medication. Once prepared, the study medication was placed into a bottle labelled with the participant’s unique study identification number that was assigned to the participant at the time of enrolment in the trial. The unblinded study team member dispensed the bottles of prepared medication to the study’s clinical coordinator, who was also blinded, for distribution to subjects. Both the treatment and the control creams were identical in appearance and viscosity so that the two drugs could not be distinguished by look or feel | |
| No | No | ‐ | |
| Yes | Yes | Additional information could not be used | |
| No | No | ‐ | |
| No | No | [email protected] 18‐8‐2014 (sequence generation and allocation concealment and blinding) Resent 3‐9 | |
| Yes | No | Medical Research Institute of New Zealand, Anna Hunt [email protected]. sent 23‐7‐2014 My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? email reply 29‐7‐2014 Dear Dr van Zuuren, Thank you for your email and interest in our study. We are currently analysing this 138 participant Phase 3 study. It is not yet published, but we would be happy to send you the publication when that time comes | |
| Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply | |
| Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply | |
| Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply | |
| Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply | |
| Yes | No | Study of Mireille, is still ongoing | |
| Yes | No | 23‐9‐2014 Dr. Bertil Wachall, [email protected] 2‐10‐2014 Thank you for your request concerning our permethrin rosacea trial (permethrin 5% and 2.5% vs metronidazole cream). Unfortunately, the data are not published or submitted up to now. We hope this will be done in the next months, but the principal investigator who is responsible for the publication seems to be very busy. In addition, please be informed that we are currently conducting another permethrin trial (permethrin 5% vs placebo cream) in PPR‐patients. We expect the results of this trial in spring 2015 (we discussed with PI, study does NOT appear in EUCTR (EUDRACT‐Nr. 2013‐000979‐32) | |
| Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply | |
| Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply | |
| Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply | |
| Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply | |
| Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply | |
| No | No | 23‐9‐2014 [email protected], [email protected] My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already completed or submitted for publication. | |
| Yes | No | 23‐9‐2014 [email protected]; [email protected]; [email protected] My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already submitted for publication. Reply: 23‐9‐2014 Dear Dr Zuuren Many thanks for your query, We are in the process of completing and submitting the article. Regards, Mehdi | |
| Mari Wataya‐Kaneda [email protected]‐u.ac.jp not sent mail as they are still recruiting | |||
| Yes | No | [email protected] 16‐7‐2014 Follow up mail to Dr Pariser 11‐8‐2014 | |
| No | No | Allergan, results published on the Internet, as word doc and pdf we made several, but unsuccessful, attempts to contact Allergan | |
| NCT00348335 | Yes | No | [email protected], [email protected] 16‐7‐2014 My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion (NCT00348335 “Efficacy of topical cyclosporin 0.05% for the treatment of ocular rosacea”) Follow‐up mail 11‐8‐2014 John Wittpenn |
| NCT00417937 | Yes | Yes | 19‐7‐2014 Alan Fleischer <[email protected]>My colleagues and I are updating our Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already included in our review? Reply 19‐7‐2014: I do believe that this is the exact same study. Sorry that my name appears in lots of clinical trials settings (Thiboutot 2008) |
| Yes | No | 19‐7‐2014, asked Galderma if it is published (Patricia van Lith), several follow‐up mails also to Maria‐Jose Rueda marie‐[email protected] last 11‐8‐2014 | |
| NCT00483145 | Yes | No | [email protected] 16‐7‐2014 Reply 16‐7‐2014: We were unsuccessful in recruiting rosacea patients and as thus, published a case report, which is attached. Results from treating acne patients were published as a RCT, we have removed this one from ongoing studies |
| Yes | No | Sent 16‐7‐2014 message via LinkedIn, and Galderma (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐[email protected] last 11‐8‐2014 | |
| Yes | No | 16‐7‐2014, asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐[email protected] last 11‐8‐2014 | |
| Yes | No | 18‐7‐2014 clinical‐trials‐[email protected] | |
| No | No | 18‐7‐2014 via website Valeant Pharmaceuticals who took over Coria Laboratories, asked if it is published | |
| No | No | 18‐7‐2014 via website Valeant Pharmaceuticals who took over Dow Pharmaceutical Sciences, Inc, asked if it is published | |
| Yes | 18‐7‐2014 asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐[email protected] last 11‐8‐2014 | ||
| No | No | 19‐7‐2014, mail though website Sandoz. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? | |
| Yes | No | 19‐7‐2014, [email protected]. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? Dear Dr. van Zuuren, The study has not yet been published yet. When it has been accepted for publication, I can notify you. Thank you, Lisa Maier | |
| Yes | No | 19‐7‐2014 [email protected]. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT01134991 “Study to Evaluate the Safety and Efficacy of Topical Minocycline FXFM244 in Rosacea Patients”. Can you tell us if NCT01134991 is published and if so give us a pdf of the publication? Reply 21‐7 Dov Tamarkin, Ph.D. [email protected], study is still ongoing | |
| Yes | No | 19‐7‐2014 [email protected] My colleagues and I are updating our Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? Follow‐up 11‐8‐2014 [email protected] | |
| Yes | No | 22‐7‐2014. Bayer sent though website, Novum, [email protected] My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? The study was supported by Bayer and Novum are listed as "locations" on clinicaltrials.gov NCT01257919 “ Safety and Pharmacokinetics of Azelaic Acid Foam, 15% in Papulopustular Rosacea Can you tell us if the study has been published if so could I request a pdf of the publication? If not could we please access the data? | |
| Yes | 20‐7‐2014 asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐[email protected] last 11‐8‐2014 | ||
| NCT01398280 | Yes | Yes | 22‐7‐2014 Tissa Hata, MD, University of California, San Diego, [email protected] My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT01398280 Effects of Aminocaproic Acid (ACA) on Rosacea‐specific Inflammation If this has been published could I kindly ask for the citation and/or if you have a pdf available? If it has not been published are the data available? 17‐8‐2014: EvZ: This is Two 2014!!! Reply: 18‐8‐2014: Thank you for your interest in our research. Attached is the paper which was published in the JID. Thanks! Tissa |
| Yes | 21‐7‐2014 asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐[email protected] last 11‐8‐2014 5‐9‐2014: [email protected]. 22‐9‐2014, received all we needed | ||
| Yes | Yes | Novartis Pharmaceuticals, no contact details, sent mail through Dutch website U vraagt om informatie over de studie NCT01449591 (CBFH772A2203) met als compound BFH772. De enige informatie die we nu kunnen delen over deze studie zijn gepubliceerd op ‘Novartis clinical trial database’, onder ‘Novartis Institute for Biomedical Research’, Dermatology/Skin, CBFH772 http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public Reply 9‐9‐2014: BFH772 is currently under investigation and has not been approved for use other than for use as part of a clinical trial. | |
| Yes | No | 22‐7‐2014. Merck Sharp & Dohme Corp, [email protected], [email protected], [email protected] | |
| Yes | No | 19‐7‐2014 [email protected]. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT01513863 “A Therapeutic Equivalence Study of Two Metronidazole 1%Topical Gel Treatments for Patients With Rosacea (MTZG)”. Can you tell us if NCT01513863 is published and if so give us a pdf of the publication? 21‐7‐2014 reply Aimee Brown, [email protected]. "Thank you Dr. Zuuren for your inquiry, however this study has not yet been published." | |
| No | No | Bayer, mailed via website 22‐7‐2014 | |
| No | No | Allergan, sent mail 22‐07‐2014 through website no response | |
| Yes | No | 22‐7‐2014 Steven H. Dayan, Medical Director, DeNova Research, [email protected] My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, could you kindly confirm the status? It is reported on clinicaltrials.gov as This study is ongoing, but not recruiting participants When do you expect to complete it or if it is completed ... publish the data? Reply 22‐7‐2014. We appreciate your inquiry, and look forward to speaking with you. We are currently forwarding your message to Annie, our patient coordinator, and she will respond soon. emailed again 29‐7 2014. reply 29‐7‐2014 of [email protected] I appreciate you reaching out to us. This study is in the process of being written up, unfortunately due to a signed confidentiality agreement, we cannot provide you with any of the data at this time | |
| Yes | Yes | Amy McMichael, Wake Forest School of Medicine 23‐7‐2014 [email protected], [email protected] (sequence generation, allocation concealment), several e‐mail exchanges, no replies to this | |
| Yes | No | 20‐7‐2014 asked Galderma if it is published (Patricia van Lith), 28‐7‐2014, not yet published, but I thought already submitted so sent another mail. several follow‐up mails also to Maria‐Jose Rueda marie‐[email protected] last 11‐8‐2014 | |
| No | No | Allergan, results posted on clinicaltrials.gov [email protected], 23‐7‐2014 My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published, we saw results published on clinicaltrials.gov? NCT01735201 “AGN‐199201 for the Treatment of Erythema With Rosacea”. Can you tell us if NCT01735201 is published and if so give us a pdf of the publication? Follow‐up 11‐8‐2014 and 17‐8‐2014 no replies | |
| Yes | No | Rock Creek Pharmaceuticals, Inc. M Varga, 23‐7‐2014 [email protected] | |
| No | No | Cutanea Life Sciences, Inc 23‐7‐2014, [email protected] This study has been completed. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) this study has been identified as potentially eligible for inclusion. Can you please indicate if the study has been published and if so could I request a pdf or the citation? If not are the data available? 10‐8‐2014 Resent e‐mail | |
| PreCision Dermatology, Inc.Syd Dromgoole, as study is still ongoing not sent mail | |||
| Yes | Yes | 21‐7‐2014 asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐[email protected] last 11‐8‐2014 19‐8‐2014 received poster abstracts Layton 2014 | |
| Angela Chang, University of Miami [email protected] or Bradford Lee [email protected] Still recruiting patients at the moment, not sent mail | |||
| Anna Di Nardo, MD, PhD, University of California, San Diego. As study is still recruiting not sent mail | |||
| No | No | Dermira, Inc. Beth Zib, [email protected] 23‐7‐2014 Follow‐up 11‐8‐2014 no replies | |
| Rina Segal, Rabin Medical Center, [email protected] not sent mail as not yet open to recruitment | |||
| No | No | Amorepacific Corporation BeomJoon Kim, Professor Department of Dermatology, Chungang University Hospital, sent mail through website 23‐7‐2014 My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? | |
| George Washington University, Jack Short, [email protected] as they are still recruiting, not sent mail | |||
| Actavis Inc. John Capicchioni Akesis, LLC As they are still recruiting, not sent mail | |||
| No | No | Allergan [email protected] seems the same as NCT02131636 which I did NOT add? NCT02131636 Efficacy and Safety of AGN‐199201 in Patients With Persistent Erythema Associated With Rosacea NCT02132117 Safety and Efficacy of AGN‐199201 in Patients With Persistent Erythema Associated With Rosacea Also NCT02095158 Longterm and efficacy and safety | |
| Evaluation of the Safety and Efficacy of the Ulthera® System for the Treatment of Signs and Symptoms of Erythematotelangiectatic Rosacea | No | No | Ulthera, Inc. Mark Lupin, MD is LUPIN 2014 part of this? As they are still recruiting, not sent mail e‐mail sent 29‐7‐2014 to confirm, [email protected] Dear Colleagues I have received no further response could you please confirm with Dr Lupin?There appears to be a poster in JAAD 2014 vol17 Iss 5 referring to this trial? NCT01756027 Evaluation of the safety and effectiveness of microfocused ultrasound with visualization (MFU‐V) for the treatment of erythematotelangiectatic rosacea Mark Lupin, MD, The Department of Dermatology and Skin Science, University of British Columbia, Vancouver, Canada |
| Leon Kircik, M.D., Derm Research, PLLC [email protected] As they are still recruiting, not sent mail | |||
| Florence Le Duff, leduff.f2@chu‐nice.fr, not sent e‐mail as they are still recruiting | |||
| RCT = randomised controlled trial | |||
| Newly included studies | |
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| Formerly excluded studies | |
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 |
| 1. What were PROs measuring? |
| 2. Omissions |
| 3. If randomised trials and other studies measured PROs, what were the instruments' measurement strategies? c. Who exactly completed the instruments? |
| 4. Did the instruments work in the way they were supposed to work ‐ validity? |
| 5. Did the instruments work in the way they were supposed to work ‐ ability to measure change? |
| 6. Can you make the magnitude of effect (if any) understandable to readers? |
| Table 17.6.a Patrick D, Guyatt GH, Acquadro C. Chapter 17: Patient‐reported outcomes. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. |
| Study ID | Interventions and comparisons | N | Comments |
| Four different concentrations brimonidine tartrate gel | 102 | None of our outcomes were addressed | |
| Sulphur 10% cream versus lymecycline | 40 | Unclear how many were randomised to each group, minimal reporting of outcomes. Participants who failed to respond or got worse were switched to the alternative treatment, unclear who and how many | |
| PDL + post‐laser serum versus PDL + placebo gel | 31 | Poster, very limited data reported, not able to contact PI | |
| Azelaic acid 15% gel + habitual self‐selected skin cleanser and moisturizer versus azelaic acid 15% gel + standardised PHA (polyhydroxy acid) containing cleanser, and anti‐aging moisturizer | 67 | None of our primary outcomes were addressed combined with that it was unclear how many participants were randomised to each intervention. Because very limited outcomes data were reported no reliable conclusions could be drawn | |
| Facial foundation with niacinamide and N‐acetylglucosamine, cleanser and moisturizer versus marketed foundation + cleanser and moisturizer | 146 | Poster, lot of data missing, PI did not reply to e‐mail. Also included patients with sensitive skin, no separate data reported for participants with rosacea | |
| Gentle foaming cleanser containing hydrophobically modified polymers versus commercial gentle liquid non‐foaming facial cleanser | 40 | Participants with other skin diseases (atopic dermatitis, eczema, acne) were included and no separate data reported for participants with rosacea | |
| Isotretinoin + topical tretinoin versus topical tretinoin versus isotretinoin | 22 | Data unreliable, its re‐analysis using the individual participant data confirmed its flawed analysis by the investigators | |
| Metronidazole gel versus placebo gel | 51 | Allocation to intervention was based on up to four participants in each of 18 clinics but not all clinics enrolled four participants. The report did not provide any reassurance that the allocation sequence was adequately generated and no evidence that any form of central randomisation had been employed for the 18 clinics involved in this study | |
| IPL + azelaic acid versus IPL | 20 | Poster, very limited data reported, PI failed to respond to several e‐mails | |
| Metronidazole 75% gel versus metronidazole 0.75% lotion | 114 | Poster, very limited data reported, old study, not able to contact PI | |
| Doxycycline 40 mg versus placebo | 170 | Poster abstract, limited data, unclear how many were randomised to each group, PI failed to respond to several e‐mails | |
| Metronidazole versus placebo | 277 | Unclear how many participants were initially recruited. Unclear how many participants started in each group, no SDs, dropout rate unclear. Data seem very skewed | |
| MFU‐V one treatment versus MFU‐V two treatments | 12 | Poster abstract, limited data, unclear how many were randomised to each group, PI failed to respond to several e‐mails | |
| Dapsone 5% gel QD vs dapsone 5% BID, versus metronidazole gel versus dapsone + metronidazole gel versus vehicle | 400 | Unclear how many were randomised to each group, Allergan failed to respond to several e‐mails requesting further data | |
| Antiinflammatory cream versus placebo | 40 | Poster, no results provided, very limited data reported | |
| Doxycycline versus placebo | 134 | Poster, lot of data are missing, PI did not reply to e‐mail | |
| Ketoconazole oral versus ketoconazole cream versus ketoconazole oral + cream versus placebo cream versus placebo pills | 53 | Letter, limited and no exact data | |
| Clonidine versus placebo | 60 | Interim report only on first 30 participants, incomplete and very limited data | |
| Nadolol versus placebo, four arms, crossover, 3 periods | 15 | Small groups, unclear what dropout rate was. No separate data for period A | |
| Topical clindamycin versus tetracycline | 43 | Unclear how many participants were assigned to each group, dropouts not mentioned, no exact data provided | |
| Topical ciclosporin A versus artificial tears | 20 | Unclear how many randomised to each group, poster with very limited data see also Table 3 | |
| PDL + calcium dobesilate versus PDL | 6 | Poster, with incomplete and missing data |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 6 | 1773 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.94, 1.51] |
| 2 Physician's global evaluation of improvement Show forest plot | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.29, 3.02] |
| 3 Incomplete data on which further analysis is not possible Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐assessed improvement of rosacea Show forest plot | 4 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.30, 1.63] |
| 2 Physician's global evaluation of improvement Show forest plot | 4 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.18, 1.47] |
| 3 Incomplete data on which further analysis is not possible Show forest plot | Other data | No numeric data | ||

