Rehabilitación cognitiva y entrenamiento cognitivo para la enfermedad de Alzheimer y la demencia vascular de leve a moderada
Resumen
Antecedentes
El deterioro cognitivo, en particular los problemas de memoria, son una característica definitoria de las primeras etapas de la enfermedad de Alzheimer (EA) y la demencia vascular. La rehabilitación cognitiva y el entrenamiento cognitivo son enfoques de intervención específicos diseñados para abordar las dificultades de la memoria y otros aspectos del funcionamiento cognitivo. La presente revisión es una actualización de las versiones anteriores de esta revisión.
Objetivos
El objetivo principal de esta revisión fue evaluar la eficacia y la repercusión de la rehabilitación cognitiva y el entrenamiento cognitivo de los pacientes con enfermedad de Alzheimer o demencia vascular leve en relación con resultados cognitivos y no cognitivos importantes para el paciente con demencia y el cuidador primario a corto, medio y largo plazo.
Métodos de búsqueda
Se buscó en el Registro Especializado del Grupo Cochrane de Demencia y Trastornos Cognitivos (Cochrane Dementia and Cognitive Improvement Group), ALOIS, que contiene registros de MEDLINE, EMBASE, CINAHL, PsycINFO, LILACS y en muchas otras bases de datos de ensayos clínicos y fuentes de literatura gris y la búsqueda más recientemente fue el 2 de noviembre de 2012.
Criterios de selección
Se consideraron para inclusión los ensayos controlados aleatorizados (ECA), publicados en inglés, que compararon la rehabilitación cognitiva o las intervenciones de entrenamiento cognitivo con condiciones control, y que informaron sobre resultados relevantes para la persona con demencia y el cuidador familiar.
Obtención y análisis de los datos
En la revisión se incluyeron 11 ECA que informaron sobre intervenciones de entrenamiento cognitivo. En los diferentes estudios se utilizaron un gran número de medidas y fue posible realizar un metanálisis de 11 de los resultados primarios y secundarios de interés. Algunos resultados no se midieron en alguno de los estudios. La unidad de análisis en el metanálisis fue el cambio a partir de la puntuación inicial. Las estimaciones generales del efecto del tratamiento se calcularon mediante un modelo de efectos fijos y la heterogeneidad estadística se midió con la estadística Ji2 estándar. Se identificó un ECA sobre rehabilitación cognitiva que permitió examinar los tamaños de los efectos, pero no fue posible realizar un metanálisis.
Resultados principales
El entrenamiento cognitivo no se asoció con efectos positivos o negativos en relación con los resultados informados. La calidad general de los ensayos fue baja a moderada. El único ECA sobre rehabilitación cognitiva encontró resultados prometedores en relación con varios resultados de los participantes y los cuidadores, y en general fue de calidad alta.
Conclusiones de los autores
La evidencia disponible sobre el entrenamiento cognitivo es limitada y su calidad debe mejorar. Sin embargo, todavía no hay evidencia de efectos beneficiosos significativos derivados del entrenamiento cognitivo. Los informes de los ensayos indican que algunos de los efectos beneficiosos resultantes de la intervención pueden no ser detectadas adecuadamente por las medidas de resultados estandarizadas disponibles. Los resultados del único ECA de rehabilitación cognitiva son prometedores, pero son de naturaleza preliminar. Además, se necesitan estudios bien diseñados de la rehabilitación cognitiva y el entrenamiento cognitivo para obtener evidencia más definitiva. Los investigadores deben describir y clasificar las intervenciones de manera apropiada con el uso de la terminología disponible.
PICO
Resumen en términos sencillos
Rehabilitación cognitiva y entrenamiento cognitivo para la enfermedad de Alzheimer y la demencia vascular de leve a moderada
La demencia debido al Alzheimer y a la enfermedad vascular es un enorme problema de salud pública. Más de 36 000 000 de personas en el mundo viven actualmente con demencia y se espera que este número aumente a más de 115 000 000 en el año 2050. Se necesitan con urgencia intervenciones efectivas para reducir la carga de la demencia. La rehabilitación cognitiva y el entrenamiento cognitivo son métodos no farmacológicos que tienen como objetivo ayudar a los pacientes con demencia en estadio precoz a aprovechar al máximo su memoria y su funcionalidad cognitiva a pesar de las dificultades que experimentan. El entrenamiento cognitivo se centra en la práctica guiada de una serie de tareas que reflejan funciones cognitivas particulares, como la memoria, la atención o la resolución de problemas. La rehabilitación cognitiva se centra en la identificación y el tratamiento de las necesidades y objetivos individuales, lo que puede requerir estrategias para la asimilación de nueva información o métodos compensatorios, como el uso de ayudas a la memoria.
Esta revisión incluyó 11 ensayos de entrenamiento cognitivo y un único ensayo de rehabilitación cognitiva. No se encontró evidencia de la eficacia del entrenamiento cognitivo para mejorar el funcionamiento cognitivo, el estado de ánimo o las actividades cotidianas en pacientes con enfermedad de Alzheimer o demencia vascular de leve a moderada; sin embargo, la calidad de los estudios generalmente no fue alta. El único ensayo de rehabilitación cognitiva proporcionó indicaciones preliminares de los posibles efectos beneficiosos de la rehabilitación cognitiva individual para mejorar las actividades cotidianas en pacientes con enfermedad de Alzheimer leve. Se necesitan más ensayos de alta calidad de rehabilitación cognitiva y de entrenamiento cognitivo para establecer su eficacia en pacientes con demencia en estadio precoz.
Authors' conclusions
Summary of findings
| Cognitive training compared to control in the short‐term (i.e. post‐intervention) for early‐stage Alzheimer's disease and vascular dementia | ||||||
| Patient or population: participants with early‐stage Alzheimer's disease and vascular dementia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control in the short‐term (i.e. post‐intervention) | Cognitive training | |||||
| Change in a global measure of cognition | The mean change in a global measure of cognition in the intervention groups was | 173 | ⊕⊕⊝⊝ | |||
| Change in participant's capacity for activities of daily living (Caregiver reported) | The mean change in participant's capacity for activities of daily living (caregiver reported) in the intervention groups was | 107 | ⊕⊕⊝⊝ | SMD 0 (‐0.38 to 0.38) | ||
| Change in participant's mood (self‐reported) | The mean change in participant's mood (self‐reported) in the intervention groups was | 114 | ⊕⊕⊕⊝ | SMD 0.03 (‐0.34 to 0.41) | ||
| Change in rates of admission to residential care-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in measures of dementia severity-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in immediate verbal memory scores | The mean change in immediate verbal memory scores in the intervention groups was | 201 | ⊕⊕⊝⊝ | SMD 0.1 (‐0.18 to 0.38) | ||
| Change in self‐reported burden of care | The mean change in self‐reported burden of care in the intervention groups was | 80 | ⊕⊕⊕⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence: | ||||||
| 1 The confidence interval of the effect included a zero effect. Therefore, imprecision is likely. | ||||||
| Cognitive rehabilitation compared to control in the short‐term (i.e. post‐intervention) for early‐stage Alzheimer's disease and vascular dementia | ||||||
| Patient or population: participants with early‐stage Alzheimer's disease and vascular dementia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control in the short term (i.e. post‐intervention) | Cognitive rehabilitation | |||||
| Change in a global measure of cognition-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in participant's self‐reported performance in relation to individual goals (COPM Performance, self‐reported) | The mean change in participant's capacity for activities of daily living (COPM Performance, self‐reported) in the intervention groups was | 39 | ⊕⊕⊕⊕ | |||
| Change in participant's mood (Depression, self‐reported) | The mean change in participant's mood (depression, self‐reported) in the intervention groups was | 41 | ⊕⊕⊕⊕ | SMD ‐0.24 (‐0.86 to 0.37) | ||
| Change in rates of admission to residential care-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in measures of dementia severity-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in self‐reported mood (Depression-caregiver) | The mean change in self‐reported mood (depression, caregiver) in the intervention groups was | 18 | ⊕⊕⊕⊕ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence: | ||||||
Background
Cognitive impairment is a defining feature of dementia caused by neurodegenerative conditions such as Alzheimer's disease (AD) and cerebrovascular disease. In the milder stages of dementia, cognitive impairments are often the most disabling and distressing features for the individual and for the family. For the person with dementia, memory and other cognitive difficulties can have a major impact on self‐confidence and can lead to anxiety, depression and withdrawal from activities, which in turn can make the difficulties seem worse. This is an example of what has been termed 'excess disability' (Reifler 1990). Family caregivers are also affected by the practical impact of cognitive problems on everyday life and by the strain and frustration that can result. Interventions designed to assist with aspects of cognitive functioning such as memory problems are therefore important in the milder stages of dementia, as they may allow the person greater independence and can potentially minimise the risk of excess disability. The current review is an update of previous versions of this review (Clare 2003; Clare 2008).
Description of the condition
AD and cerebrovascular disease are the most common aetiologies underlying dementia among older individuals (Alzheimer's Disease International 2009). Dementia due to AD is generally characterised by an insidious onset; vascular dementia is often associated with a more rapid onset. However, both disorders have a progressive course that eventually culminates in global cognitive impairment and compromised functional independence. During milder stages, clinical signs typically include forgetfulness for recent events and other cognitive impairments such as word‐finding difficulties or increased confusion in navigating unfamiliar environments. These signs often precede the formal diagnosis by several years, but they can be difficult to distinguish from the common forgetfulness associated with normal ageing—a factor that often leads to delays in bringing the situation to medical attention. During this pre‐dementia phase, there is often no, or minimal, impairment in the ability of the individual to carry out most activities of daily living. With disease progression, difficulties develop in most other cognitive domains, such as semantics, praxis and executive functioning. Functional impairment also becomes increasingly evident. In more advanced dementia, most cognitive and functional abilities are profoundly impaired, and behavioural changes such as apathy, depression, aggression and agitation are frequently observed (Lyketsos 2002; Mortby 2011).
On neuropsychological examination, the earliest signs are almost invariably related to episodic memory function, particularly in the person with AD. Deficits in new learning and delayed recall of information precede the diagnosis by several years (Arnaiz 2003; Collie 2000). Studies have established that associative memory functions, particularly the ability to form arbitrary inter‐modal and intra‐modal associations, show a striking deficit very early in AD (Fowler 2002; Lowndes & Savage 2007). Although deficits noted on measures of episodic memory are central to vascular dementia, people with vascular dementia display a more striking deficit on executive and attention tasks, as well as on measures of semantic knowledge and visuospatial function (Graham 2004)
Pathologically, AD is characterised by the build‐up of extra‐cellular Aβ plaques and intra‐cellular neurofibrillary tangles, which spread in a predictable and well‐described manner through cortical and subcortical regions (Braak & Braak 2012). In the case of both Alzheimer's and vascular pathology, the pathological cascade commences years or even decades before the onset of obvious clinical symptoms, at which stage individuals are increasingly brought to clinical attention.
Description of the intervention
Cognition‐focused interventions as a group fall under the broader umbrella of non‐pharmacological interventions. Cognition‐focused interventions can be broadly defined as interventions that directly or indirectly target cognitive functioning as opposed to interventions that focus primarily on behavioural (e.g. wandering), emotional (e.g. anxiety) or physical (e.g. sedentary lifestyle) function. Several types of cognition‐based interventions have been described. The potential benefits of non‐specific stimulation of cognitive functioning for people with dementia have long been recognised. These interventions typically involve engaging the person with dementia in a range of general activities and discussions, are commonly conducted in groups and are aimed at general enhancement of cognitive and social functioning. A separate recent Cochrane Review, which focuses on interventions that fall under this category (collectively termed 'cognitive stimulation'), has concluded that general cognitive stimulation and reality orientation approaches consistently produce improvements in general cognition and, in some cases, in self‐reported quality of life and wellbeing, primarily for people with mild to moderate dementia (Woods 2012).
Progress in understanding the operation of memory and related cognitive functions and of the mechanisms underpinning learning has facilitated the development of more specific approaches designed to help maintain or enhance cognitive functioning and wellbeing for people with AD or vascular dementia-most commonly those in the milder stages. These more recent approaches to cognition‐based interventions are most commonly classified as either cognitive training (or 'retraining' or 'remediation' or ‘brain training’) or cognitive rehabilitation. These terms have been and continue to be applied somewhat interchangeably in the literature (e.g. Fernandez‐Prado 2012; Giordano 2010); therefore in previous versions of this review (Clare 2003; Clare 2008), we have offered the following broad definitions and descriptions with the aim of clarifying the nature of these two related but distinct forms of intervention.
Cognitive training
Cognitive training typically involves guided practice on a set of standardised tasks designed to reflect particular cognitive functions such as memory, attention or problem‐solving. Tasks may be presented in paper‐and‐pencil (Davis 2001; de Vreese 1998; Quayhagen 1995; Quayhagen 2000) or computerised (Heiss 1993; Hofmann 1996) form, or may involve analogues of activities of daily living (Farina 2002; Zanetti 1994; Zanetti 1997; Zanetti 2001; Loewenstein 2004; Neely 2009). Tailoring of task difficulty based on individual performance level and adaptive training (i.e. adjustment of task difficulty in response to changes in performance level) are becoming more available through computerised packages (e.g. Peretz 2011). One assumption underlying cognitive training is that practice has the potential to improve or at least maintain functioning in the given domain. An additional assumption is that any effects of practice will generalise beyond the immediate training context. Although this last assumption has not often been supported by the evidence (Owen 2010; Papp 2009), some have argued that failure to produce transferable benefits is related in part to problems with task design (Jaeggi 2010). Some authors have recently broadened the definition of cognitive training to include strategy training, which involves instruction in and practice of strategies designed to minimise cognitive impairment while enhancing performance (e.g. method of loci, visual imagery) and cognitive exercise (Gates 2011). Cognitive training may be offered through individual (Davis 2001; de Vreese 1998; Koltai 2001; Loewenstein 2004; Farina 2002) or group (Cahn‐Weiner 2003; Koltai 2001; Ermini Fuenfsch 1995; Kesslak 1997; Moore 2001) sessions or may be facilitated by family members (Quayhagen 1995; Quayhagen 2000; Neely 2009) with therapist support. In accordance with the suggestion that cognitive training may enhance the effects of pharmacological therapy (Newhouse 1997), some studies have evaluated the efficacy of cognitive training in combination with the use of acetylcholinesterase‐inhibiting (Cahn‐Weiner 2003; de Vreese 1998; Loewenstein 2004) or other (Yesavage 1981; Heiss 1993) medications. In addition, cognitive training for the person with dementia has sometimes been included as a component of supportive interventions for caregivers (Brodaty 1989; Brodaty 1997).
Cognitive rehabilitation
Historically, rehabilitation has been viewed as a process aimed at helping people achieve or maintain an 'optimal level of physical, psychological and social functioning' in the context of specific impairments arising from illness or injury (McLellan 1991), thus facilitating participation in preferred activities and valued social roles (WHO 2001). More recent views of rehabilitation include a deeper appreciation of the complex interplay between disease and ability to function: A disability may endure even once the disease that triggered it has been eliminated, and equally, disability can be reduced in the face of permanent injury or even chronic disease (Institute of Medicine 2011). Cognitive rehabilitation, originally developed mainly through work with younger brain‐injured people but equally applicable to progressive conditions, refers to the rehabilitation of people with cognitive impairments. Although the concept continues to evolve, cognitive rehabilitation generally refers to an individualised approach to helping people with cognitive impairments, by which those affected, and their families, work together with healthcare professionals to identify personally relevant goals and to devise strategies for addressing these (Wilson 2002). The emphasis is not on enhancing performance on cognitive tasks as such, but rather on improving functioning in the everyday context. Cognitive rehabilitation interventions aim to tackle directly those difficulties considered most relevant by the person with dementia and by his or her family members or supporters and to target everyday situations in the real‐life context. Cognitive rehabilitation approaches tend to be implemented in real‐world settings because there is no implicit assumption that changes instituted in one setting would necessarily generalise to another. Goals for intervention are selected collaboratively, and interventions are usually provided on an individual basis.
Both cognitive training and cognitive rehabilitation might be accompanied by (1) psychoeducational activities aimed at facilitating an understanding of cognitive strengths and difficulties, and (2) supportive discussion related to individual emotional reactions or other needs; where appropriate, links may be made to other possible sources of support. Table 1 summarizes the main differences between the attributes of cognitive training and cognitive rehabilitation.
| Table 1. Selected differences between cognitive training and cognitive rehabilitation | ||
| Cognitive training | Cognitive rehabilitation | |
| Target | Impairment | Participation restriction |
| Context | Structured tasks and environments | Real‐world setting |
| Focus of intervention | Isolated cognitive abilities and processes | Groups of cognitive abilities and processes required to perform everyday tasks |
| Format | Individualised or group | Individualised |
| Proposed mechanism of action | Mainly restorative; sometimes combined with psychoeducation and strategy training | A combination of restorative and compensatory approaches combined with psychoeducation and strategy training |
| Goals | Improved or maintained ability in specific cognitive domains | Performance and functioning in relation to collaboratively set goals |
How the intervention might work
Cognition‐based interventions for persons with acquired disorders of the central nervous system (including traumatic brain injury, stroke and neurodegenerative conditions) are driven by knowledge of brain‐behaviour relationships and mechanisms of injury, disease and recovery. Historically, such interventions have reflected two broad conceptual frameworks for the recovery of function after brain illness or injury: a traditional or restorative approach, and a contextualised or compensatory approach (Ylvisaker 2002). Techniques usually associated with cognitive training such as the repeated exercise of standardised cognitive tests of increasing difficulty, targeting specific cognitive domains, tend to reflect restorative principles and “thrive on the lure of neuroplasticity” (Rabipour & Raz 2012, p. 2). Evidence in support of this comes from a recent functional magnetic resonance imaging (fMRI) study that reported increased memory‐related brain activation following cognitive training in several brain regions of individuals at high risk of dementia due to mild cognitive impairment (Belleville 2011). Such increased brain activation may be the result of processes of synaptic growth and repair triggered by repeated practice on standardised tests. Techniques usually associated with cognitive rehabilitation, on the other hand, such as optimising residual cognitive abilities in impaired domains and making the most of unimpaired cognitive abilities, lend themselves more to compensatory approaches. For example, in relation to memory and learning, it is well established that the processes of memory encoding and consolidation, as well as the sub‐system of declarative memory, tend to be profoundly impaired even in the milder stages of AD (Christensen 1998). Nevertheless, research has shown that given appropriate conditions and support, and sufficient time, people with dementia can retain the ability to learn and can hold onto some information and skills despite their memory difficulties (Bäckman 1992; Bäckman 1996; Kopelman 1985; Little 1986). A cognitive rehabilitation approach may focus on helping people with dementia and their families make the most of residual memory ability, for example, by identifying the best ways of taking in important information (Bäckman 1991; Camp 1989; Camp 2000; Clare 1999; Clare 2000; Clare 2001; Hill 1987; Clare 2002; Anderson 2001) or by carrying out important real‐life practical skills (Josephsson 1993). Indeed, several learning principles and techniques (e.g. errorless learning, spaced retrieval) have been found to lead to improved rates of learning and memory among patients with mild dementia (Boudreaux 2011Clare, Wilson et al 2000; Dunn 2007). It is well documented that despite the severity of memory difficulties, certain memory systems and processes such as implicit memory (e.g. priming, procedural memory) are relatively preserved in the milder stages of AD and vascular dementia (Brandt 1995; Morris 1996). This profile suggests that interventions may aim to build on areas of relative strength reflected in preserved aspects of memory by helping patients develop strategies for learning information via less impaired components of the memory system. Finally, cognitive rehabilitation interventions also attempt to assist patients in developing ways to compensate for impairments in those aspects of memory that are significantly affected (e.g. using external memory aids, making environmental changes), so as to minimise the cognitive demands of various activities (Bird 2001; Bourgeois 1990; Clare 2000; Kurlychek 1983). Cognitive rehabilitation interventions use these and other techniques to enhance or maintain everyday functioning and wellbeing and to reduce excess disability for the person with dementia, while reducing strain for family caregivers.
Why it is important to do this review
Both pharmacological treatments with cholinesterase inhibitors and cognition‐based interventions can be defined as symptomatic treatments in that they do not target hypothesised disease mechanisms. Extensive efforts to develop disease‐modifying treatments continue; however, consistently disappointing results from drug trials of various agents have resulted in considerable doubt that disease‐modifying treatments can show a positive effect by the time dementia is fully developed (Salomone 2012), and efforts in this direction are increasingly being shifted to the pre‐dementia or even the pre‐symptomatic stage. In contrast, non‐pharmacological interventions, particularly cognition‐based interventions, are increasingly recognised as an important adjunct and in some cases as an alternative to pharmacological treatments for individuals with dementia and those at risk of dementia. Nevertheless, earlier studies suggested that cognition‐based interventions are not appropriate, as they are ineffective and result in frustration and depression among participants and caregivers (Small 1997). With growing emphasis on early detection and intervention in dementia care, the need for a clear evidence base for cognition‐focused interventions is becoming apparent (Woods & Clare 2006). As was already mentioned, a recent systematic review concluded that general cognitive stimulation and reality orientation provide benefit in terms of the overall cognitive status of patients and aspects of their wellbeing (Woods 2012). Whether or not more targeted approaches such as cognitive training and cognitive rehabilitation can produce similarly encouraging outcomes has not yet been determined.
The present review is an update of the original review and updated versions of this review (Clare 2003; Clare 2008). The latest update of this review included 9 randomised controlled trials (RCTs) of cognitive training and found no evidence for efficacy of cognitive training in relation to cognitive outcomes for the person with dementia. No RCTs of cognitive rehabilitation were found in searches for previous versions of this review; therefore no conclusions could be drawn regarding the efficacy of this type of intervention.
In selecting studies for this review, we have classified interventions on the basis of the ways in which they are described in relation to the definitions previously provided. In some cases, this led to classification of an intervention as ‘cognitive training’ even when the term ‘cognitive rehabilitation’ was used by the study authors. In other cases, an intervention described as ‘cognitive training’ might be deemed to fit more closely with the principles of ‘cognitive stimulation’, thus leading to exclusion from the current review. We acknowledge that the identified categories represent broad definitions and that some cases may reflect an overlap between techniques found in cognitive 'training' and those classified as cognitive 'rehabilitation', which in turn may have some commonalities with cognitive 'stimulation'. Therefore, although the current classification of cognition‐based interventions is gradually gaining some consensus among researchers, this classification should remain open to additional refinement in the future.
Objectives
-
To evaluate the effects of cognitive training and cognitive rehabilitation for people with mild AD or vascular dementia in relation to cognitive and non‐cognitive outcomes for individuals affected and for their caregivers.
-
To update previous versions of this review (Clare 2003; Clare 2008).
-
To consider the nature and quality of available evidence on this topic as derived from RCTs.
-
To assist in establishing the appropriateness of cognitive training and cognitive rehabilitation interventions offered to people with early‐stage dementia and, where relevant, to identify the factors associated with efficacy.
Methods
Criteria for considering studies for this review
Types of studies
-
Randomised controlled trials for which adequate information was provided or could be obtained from the researchers were considered for inclusion. For consistency with previous versions of the review, we decided to include only studies that were published in the English language. Although in some cases this may lead to an increased language bias, evidence suggests that the effects of language bias have diminished as a result of the continuous shift towards publishing of trial results in English (Sterne 2011). No study was excluded solely on the basis of language other than English, as whole non‐English studies that were screened beyond the title (n = 3) were found to fail other inclusion criteria (non‐randomised trials or trials of cognitive stimulation).
Types of participants
-
Participants with a medical diagnosis of dementia, possibly further specified as Alzheimer's disease, vascular dementia or mixed Alzheimer's and vascular dementia according to the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV), the International Classification of Diseases, Tenth Revision (ICD‐10), criteria of the National Institute of Neurological and Communicative Disorders-Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) or research diagnostic criteria of the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NINDS‐AIREN) (APA 1995; WHO 1992; McKhann 1984; Roman 1993). Given common limitations of available data regarding specific diagnoses, we decided to consider these diagnostic categories together. We excluded data from participants for whom dementia was known to have an aetiology other than AD or cerebrovascular pathology (e.g. frontotemporal dementia, Lewy body dementia), as the format of cognitive training and rehabilitation in other dementia types is likely to differ substantially from that applied in AD or vascular dementia.
-
We included only studies that reported the severity of dementia through group mean scores, ranges of scores or individual scores on a standardized scale such as the Mini‐Mental State Examination (MMSE; Folstein 1975) or the Clinical Dementia Rating (CDR; Hughes 1982).
-
Studies targeting primarily people with minimal, mild or moderate dementia (MMSE score > 12 or CDR score < or = 2), although studies with a small proportion of participants in the more severe ranges (< 20%) were considered acceptable.
-
Qualifying participants were expected in the main to be residing at home, but interventions might be offered in a range of settings, and data from home, outpatient, day‐care and residential settings were considered acceptable for inclusion. However, it was considered appropriate to exclude data from long‐term residents of psychiatric hospitals, where pre‐existing psychiatric conditions were likely to occur.
-
No specific restrictions were set regarding age. Although it was previously planned to examine the potential moderation of age on observed outcomes, given the limited data available, it was decided not to examine the role of age at this point.
-
No restrictions were placed on current pharmacological treatment. Where available, we noted information about participants' use of cholinesterase inhibitors.
-
It was decided that data from family caregivers would be included where available, and where the relationship between the caregiver and the person with dementia was specified, including whether the two were co‐residents.
-
In previous versions, it was proposed that information regarding the use of coping strategies used by participants or caregivers to maintain or enhance cognitive function would be noted. However, no study provided this information.
Types of interventions
Experimental interventions
-
Interventions meeting our definition of cognitive training or cognitive rehabilitation were acceptable for inclusion. These might also be described as memory 'therapy', 'groups', 'retraining', 'support' or 'stimulation', or as cognitive 'training', 'retraining', 'remediation', 'support' or 'stimulation'.
-
Interventions were required to specifically address one or more target areas relevant to cognitive functioning, either singly or in combination with interventions directed at other targets (e.g. relieving anxiety or depression) or other cognitive functions (e.g. attention or problem‐solving).
-
When more than one experimental group was included in the study, the group that provided the treatment most similar to that described in other included studies was selected for analysis (e.g. individual interventions were selected over interventions delivered to dyads, and stand‐alone cognitive training or rehabilitation interventions were selected over interventions that combined pharmacological and non‐pharmacological components).
Comparator interventions
-
No treatment/standard treatment. Unless otherwise specified, whenever groups were described as 'no treatment' in individual studies, it was assumed that this referred to the usual/standard treatment, and not to withholding of treatment. 'Usual or standard treatment' refers to what would normally be provided in the study locality to participants with early‐stage Alzheimer's or vascular dementia, and might include provision of medication, clinic consultations, contact with a community mental health team, day care or support from voluntary organisations, but not cognitive training or rehabilitation interventions.
-
Wait‐list control. In studies of this kind, the experimental intervention was offered to the control group after the study had ended.
-
Active control condition. For example, active control conditions consisted of an equivalent number of sessions or visits in which general social support was provided, but during which no structured cognitive training or rehabilitation intervention was offered.
-
When more than one comparator intervention was included in the study, the group that was most similar to that included in other studies was selected for analyses. This was usually a 'no treatment' group.
All interventions
-
Interventions conducted in individual or group modalities, with or without involvement of family caregivers, were acceptable for inclusion.
-
Interventions included at least a baseline assessment and an immediate post‐intervention assessment, with or without follow‐up assessment.
-
No restrictions were imposed regarding duration of intervention or number of treatment sessions. It was decided to consider differences in these parameters when making comparisons between studies.
Types of outcome measures
Primary and secondary outcomes were examined in three categories:
-
Cognitive and non‐cognitive outcomes of the intervention for the person with dementia
-
Outcomes for the primary caregiver
-
The impact of the intervention on the course of the disorder
Outcomes for the person with dementia and for the primary caregiver were considered for inclusion in the meta‐analysis when they were assessed using scores on at least one standardised test or questionnaire measure. When more than one measure was used to assess a particular outcome (e.g. immediate memory), we included in the comparison the measure on which group differences were observed at post‐intervention or follow‐up assessments (if relevant), or the measure that ,most resembled the measures contributed by other studies. Behavioural observations and ad hoc measures were considered as additional information.
Rates of attrition and reasons for attrition were noted where available. Drop‐out rates in the context of progressive conditions may in part reflect changes in the needs of the individual that prompted a needed change in therapeutic approach. With a progressive condition, individual needs may change during the course of an intervention and follow‐up period, requiring implementation of a different approach, but this should not be interpreted as evidence that the approach itself is ineffective.
Outcome measures for the person with dementia seek to identify whether changes are observed after the intervention, and to determine the extent to which these can be attributed to the intervention itself. Given the progressive nature of dementia, improved performance may not necessarily be a goal. Instead, preserved performance on a trained task in the context of a decline in untrained tasks could be interpreted as evidence of efficacy. Differences in the trajectory of change between scores on intervention targets and standardised measures are as important as the overall level of change; for example, maintenance of functioning on a target task in the context of a decline in scores on standardised assessments might indicate that the intervention was effective in relation to the targeted area of functioning.
Primary outcomes
For each of the outcomes described previously, we intended to conduct separate comparisons for those measured short term (immediately post‐intervention), medium term (3 to 12 months post‐intervention) and long term (> 12 months post‐intervention). However, no study reported relevant outcomes beyond the medium term.
(A) Cognitive outcomes for the person with dementia
-
(A1) Change in scores on global cognitive screening measures (e.g. MMSE) and in orientation and self‐reported and caregiver‐reported cognitive abilities in the short term (i.e. immediately post‐intervention-A1.1), in the medium term (i.e. 3 months up to one year-A1.2) and in the long term (i.e. longer than a year-A1.3).
-
(A2) Change in performance on neuropsychological measures (immediate and delayed memory, working memory and attention, language, executive function) in the short term (i.e. immediately post‐intervention-A2.1), in the medium term (i.e. 3 months up to one year-A2.2) and in the long‐term (i.e. longer than a year-A2.3).
(B) Non‐cognitive outcomes for the person with dementia
-
Self‐reported or caregiver‐reported changes in mood, capacity for activities of daily living, behaviour, adjustment to disability, general health and quality of life in the short term (B1), in the medium term (B2) and in the long‐term (B3).
Secondary outcomes
(C) Outcomes regarding the course of dementia
-
Change in scores on measures of dementia severity (e.g. CDR) or rates of admission to residential care in the short term (C1), in the medium term (C2) and in the long term (C3).
(D) Outcomes for the family caregiver
-
Self‐reported changes in mood, wellbeing, burden of care and quality of life in the short term (D1), in the medium term (D2) and in the long term (D3).
(E) Outcomes for disease biomarkers of the person with dementia
-
(E1) Changes in in vivo measures of neuropathology (e.g. amyloid or tau pathology, brain atrophy) in the short term (E1.1), in the medium term (E1.2) and in the long term (E1.3).
-
(E2) Changes in measures of brain function (e.g. fluorodeoxyglucose positron emission tomography (FDG PET), fMRI) in the short term (E2.1), in the medium term (E2.2) and in the long term (E2.3).
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois)-the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register-on 2 November 2012.
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy individuals. Studies are identified from the following:
-
Monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO and LILACS.
-
Monthly searches of a number of trial registers, including ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others).
-
Quarterly search of The Cochrane Central Register of Controlled Trials (CENTRAL).
-
Six‐monthly searches of a number of grey literature sources, including ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS Website.
Details of the search strategies used for retrieval of reports of trials from healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed using many of the sources previously listed to cover the time frame from the last searches performed for ALOIS, to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
Searches carried out in previous versions of the review can be viewed in Appendix 2 and Appendix 3.
Data collection and analysis
Selection of studies
The search results (covering the period April 2006-November 2012) were reviewed by one review author (AB‐F), who identified all relevant RCTs of cognition‐based interventions in mild AD or vascular dementia and retrieved the full texts. Two review authors (AB‐F and LC) then independently reviewed each article to determine whether inclusion criteria were met. There were no disagreements regarding the inclusion of studies.
Data extraction and management
All relevant data were extracted from the studies selected for inclusion, recorded on a data entry form and entered into Review Manager (RevMan). Additional information was sought from study authors as appropriate. Data extracted from each trial included characteristics of the experimental and control groups used in each study, as well as characteristics of the interventions provided. Mean scores and standard deviations from baseline, post‐intervention and, where available, follow‐up assessments on all relevant outcome measures for treatment and comparison groups were also extracted. Two studies (Koltai 2001; Beck 1988) directly reported the data in terms of change from baseline. In the remaining studies, changes from baseline statistics were calculated from group means and standard deviations at baseline, post‐intervention and follow‐up. Baseline was defined as the latest assessment available before randomisation, but no more than two months before.
Assessment of risk of bias in included studies
Assessment of risk of bias was conducted by AB‐F using The Cochrane Collaboration's Risk of Bias tool (Higgins 2011) and was subsequently reviewed by LC. Consistent with the risk of bias tool, study quality was assessed in the following domains: sequence generation, allocation concealment, blinding of participants and investigators, incomplete outcome data and selective reporting of outcomes. Studies were rated as 'low risk', 'high risk' or 'unclear risk' in each of these domains. There were no disagreements between review authors in ratings of risk of bias.
Measures of treatment effect
The meta‐analysis was conducted on change‐from‐baseline scores. A zero correlation between measurements at baseline and those at subsequent time points was assumed. This method overestimates the standard deviation of the change from baseline but provides a conservative approach considered to be preferable in a meta‐analysis. Outcome measures were treated as continuous measures. In some cases, outcomes were derived from ordinal rating scales; provided these contained a reasonably large number of categories (> 10), the data were treated as continuous variables arising from a normal distribution. There were no examples of binary outcome measures, which would have required an odds ratio calculation.
The mean difference (MD) with 95% confidence interval (CI) was used whenever studies used the same outcome measure, and the standardised mean difference (SMD), which is the absolute mean difference divided by the pooled standard deviation, was used when the same outcome was assessed with the use of different measures.
Unit of analysis issues
Three types of unit of analysis issues were encountered: cross‐over trial designs, multiple treatment groups and repeated assessments. For cross‐over trials, only data from the first treatment period were used. In the case of studies that compared more than two treatment groups, the analysis focused on the two groups providing the most pertinent data that most resembled conditions included in other studies. Wherever possible, a condition in which individual cognitive training or rehabilitation was delivered was compared with a condition that included no cognitive intervention. To address the issue of repeated assessments (more than one post‐intervention assessment), we intended to conduct separate comparisons to assess outcomes immediately post‐intervention (the first post‐intervention assessment), short‐term outcomes (up to 12 months post‐intervention) and longer‐term outcomes (more than 12 months post intervention).
Dealing with missing data
Numbers of participants who commenced and who completed the intervention in each group were noted where available, and these numbers contributed to the assessment of risk due to incomplete outcomes data. Studies generally provided minimal detail on the causes and impact of missing data. In general, it was assumed that data were missing at random, and analyses in individual studies were generally performed per protocol rather than on an intention‐to‐treat basis.
Assessment of heterogeneity
Statistical heterogeneity was assessed using a standard Chi2 statistic and an associated l2 statistic. Consistent with recommendations, heterogeneity was deemed to be present when the Chi2 statistic was significant at the P = 0.1 level, or when the l2 suggested that more than 40% of the variability in effect estimate was due to heterogeneity (Higgins 2011).
Data synthesis
As no evidence of statistical heterogeneity was found, all analyses were conducted using a fixed‐effect model and the inverse variance method.
Subgroup analysis and investigation of heterogeneity
As no heterogeneity was detected, no subgroup analyses were conducted.
Sensitivity analysis
Inflated estimates of the standard deviation of change scores, associated with the assumption of zero correlation between pre‐intervention and post‐intervention scores on outcome measures, can potentially obscure real effects of the interventions. To address this possibility, we re‐ran the meta‐analysis for some of the central outcome measures using post‐intervention scores only, thus avoiding the need to estimate the standard deviation of change scores. This sensitivity analysis did not lead to a change in any of the results reported here.
Results
Description of studies
Results of the search
Electronic searches conducted in November 2012, December 2011 and September 2009 retrieved a combined total of 1339 results. Following preliminary screening and removal of duplicate studies by Anna Noel‐Storr, Trial Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group, 495 records were forwarded to the review authors for further evaluation. After title and abstract review by one review author (AB‐F), 49 records were selected for closer assessment, and full records were retrieved and reviewed independently by two review authors (AB‐F, LC). Upon review and discussion, three trials were identified that met the inclusion criteria-two trials describing a cognitive training intervention (Galante 2007; Neely 2009) and one trial describing a cognitive rehabilitation intervention (Clare 2010). The two cognitive training studies were added to the nine studies that were included in the previous meta‐analysis, bringing the total number of studies in the meta‐analysis to 11. Because no previous trials on individualised cognitive rehabilitation had been undertaken, no meta‐analysis of cognitive rehabilitation could be performed. The flow of studies through the review process is shown in Figure 1.

Study flow diagram.
RCT = randomised controlled trial.
CT = cognitive training.
CR = cognitive rehabilitation.
Additional information
Additional information was sought from study authors where necessary. With regard to de Vreese 1998, another abstract was published in 1999 (see under de Vreese 1998), additional data were reported in a later review article (see under de Vreese 1998) and further information including mean scores was kindly supplied by the author. Additional data related to Loewenstein 2004 were also kindly supplied by the author. Queries related to Koltai 2001, Beck 1988, Quayhagen 1995, Quayhagen 2000, Galante 2007 and Heiss 1993 were answered by the investigators. No responses were received to queries related to the studies by Davis 2001, Cahn‐Weiner 2003 and Neely 2009.
Included studies reported that a total of 117 measures (100 measuring patient outcomes, 17 measuring caregiver outcomes) were used to examine the 22 primary and secondary outcomes selected for examination in this review. For cognitive training interventions, data for meta‐analysis were available for 8 of the 14 primary outcomes and for 6 of the 8 secondary outcomes over the short term. Meta‐analysis could be performed on 2 of 14 primary outcome measures and on 2 of 8 secondary outcome measures over the medium term. No cognitive training studies reported an outcome measure over the long term. As only one study of cognitive rehabilitation met inclusion criteria for this review, no meta‐analysis of cognitive rehabilitation could be conducted.
Included studies
Significant diversity was noted among the 12 studies on a range of parameters. Seven studies included only participants diagnosed with AD, but the other four included participants diagnosed with AD, vascular dementia or mixed dementia. In one study (Quayhagen 2000), participants were included if they were diagnosed with dementia due to Parkinson's disease (PD) in addition to AD and vascular and mixed dementia, but it was not possible to ascertain how many of the included participants had PD, because data for all aetiologies were reported together. Severity of dementia varied in the included studies from very mild to moderate; this was generally determined on the basis of scores on a measure of dementia severity or global cognition (e.g. Clinical Dementia Rating, MMSE). Although not stated explicitly in most studies, it appears that in most cases, patients were recruited from the community; in a small number of studies, patients who resided in residential care homes were also included. The duration of interventions provided in the included studies varied considerably, ranging from 4 to 24 weeks. Four studies reported follow‐up assessments over the medium term; these occurred at 8 weeks, as well as at 3, 6 and 9 months, after the end of treatment. The content of the interventions also varied considerably, ranging from training in the use of compensatory strategies to practice on computerised tasks to working toward achieving collaboratively derived goals. Selected features of the included studies are further described here and are summarised in the Characteristics of included studies table.
Objectives of the studies
Beck 1988: Compared 'cognitive skills remediation training', delivered on a one‐to‐one basis, with a usual‐treatment control condition.
Heiss 1993: Compared computerised cognitive training alone with two conditions in which computerised cognitive training was combined with drug treatment (cognitive training plus pyritinol and cognitive training plus phosphatidylserine) and an active control condition (social support). The relevant comparison for this review is that between cognitive training alone and social support.
Quayhagen 1995: Compared cognitive training with active and wait‐list control conditions. The relevant comparison for this review is that between cognitive training and the wait‐list control condition.
de Vreese 1998: The study initially set out to compare cognitive training alone, acetylcholinesterase‐inhibiting medication (AChEI) alone, cognitive training plus AChEI and placebo. Raw data for the group receiving cognitive training alone were not reported in the 1998 paper and are no longer available. The design was subsequently amended as caregivers were dissatisfied with the possibility of receiving cognitive training alone, so the comparisons reported in 2001 and augmented with further information from the author involve three groups: AChEI alone, cognitive training plus AChEI and active control. For the purposes of this review, the comparison of interest lies in the difference between AChEI alone and cognitive training plus AChEI.
Quayhagen 2000: Compared four intervention approaches-cognitive training, dyadic counselling, dual supportive seminar groups and early‐stage day care with caregiver support-with a wait‐list control condition. For the purposes of this review, the comparison of interest is that between cognitive training and the wait‐list control condition.
Davis 2001: Compared cognitive training with a 'mock' (active control) intervention in a cross‐over design. The comparison of interest is that between training and active control groups following the initial intervention stage; cross‐over data are not considered here.
Koltai 2001: Compared a memory and coping programme, delivered in individual or group session format, with a wait‐list control condition. The results for individual and group training were analysed together in the trial report as no differences were observed between them.
Cahn‐Weiner 2003: Compared a memory training programme delivered in small‐group format with a control condition involving didactic presentation.
Loewenstein 2004: Compared 'cognitive rehabilitation training' with 'mental stimulation', delivered in one‐to‐one sessions.
Galante 2007: Compared individual computerised cognitive training with an active control condition.
Neely 2009: Compared collaborative cognitive training (dyadic), individual cognitive training and a no treatment control condition. The relevant comparison for this review is that between individual cognitive training and no treatment groups.
Clare 2010: Compared individual, goal‐oriented cognitive rehabilitation with relaxation therapy, and with a no treatment control condition. The relevant comparison for this review is that between the cognitive rehabilitation and no treatment groups.
Participant numbers and characteristics in the overall samples
Beck 1988: Participants included 20 individuals over 55 years of age with moderately impaired cognitive functioning (MMSE score of 15 to 20) and findings compatible with a diagnosis of Alzheimer's disease or mixed dementia, living in one of four nursing homes or in the geriatric unit of a Veterans Administration hospital.
Heiss 1993: Of 80 people who entered the study, data were available for 70. Included in this group were 37 men and 33 women with a diagnosis of possible or probable AD according to NINCDS‐ADRDA criteria and a modified Hachinski score of 3 or less, ranging in age from 48 to 79 years (average age 66.63 years), and with MMSE scores ranging from 13 to 26. On entry to the study, none were taking any medications known to affect the central nervous system. This study was carried out in Germany.
Quayhagen 1995: Of 135 care recipient/caregiver dyads initially assessed, 95 were eligible for inclusion, 79 completed the study and data were available for 78. These were families in which one person had a diagnosis of possible/probable AD and was in the mild or moderate stage with a Mattis Dementia Rating Scale (DRS) score of 90 or above. People with dementia included 51 men and 27 women, with an average age of 73.6 years (standard deviation (SD) 8.0) and an average education level of 12.6 years (SD 4.1). They were not participating in any clinical trials of anti‐dementia medication. Caregivers consisted of 18 men and 60 women, with an average age of 66.7 years (SD 10.8) and an average education level of 14.1 years (SD 2.7). Twenty‐nine percent of caregivers attended support groups periodically, and 14% had previously sought psychological help. This study was conducted in California, USA, and ethnicity within the whole sample was described as 85% white, 3% African American and 11% Hispanic.
de Vreese 1998: The 1998 paper reports the inclusion of 24 participants with a diagnosis of AD according to NINCDS‐ADRDA orDSM‐IV criteria and a CDR rating of 1 to 2. Average age was 72.6 years (range 61 to 83 years). The 2001 review paper reports the inclusion of 27 participants with early‐stage AD and MMSE scores ranging from 20 to 26, representing the removal of the 6 people in the original cognitive training alone condition and the addition of 3 more participants to each of the other groups. Participants were taking no concurrent medication known to affect the central nervous system. This study was undertaken in Italy.
Quayhagen 2000: Participants included 103 dyads consisting of a person with dementia and a caregiving spouse. The people with dementia had a diagnosis of possible or probable AD (more than 70% were in this category), vascular dementia or Parkinson's dementia, and were in the mild or moderate stages, scoring above 100 on the DRS. They included 65 men and 38 women, with an average age of 74.51 years (SD 7.11) and an average education level of 14.57 years (SD 3.05). The caregivers were 38 men and 65 women, with an average age of 71.83 years (SD 8.12) and an average education level of 14.42 years (SD 3.05). The study took place in California, USA, and the ethnic mix within the whole sample was described as 93% white, 2% African American, 1% Asian and 4% Hispanic.
Davis 2001: The participants were 37 individuals (16 men and 21 women) with a diagnosis of probable AD according to NINCDS‐ADRDA criteria. MMSE score range was 15 to 29 (average score 22.31). Average age in the sample was 70.62 years, and average level of education was 14.02 years. Mean score on the 30‐item Geriatric Depression Scale (5.02) was within the normal range. This study was carried out in Texas, USA.
Koltai 2001: The participants were 24 older people aged 60 to 84 with a diagnosis of AD and a CDR score of 0.5 or 1.0. Of the 25 people initially identified as eligible, one found the group treatment modality unacceptable and declined to take part. The study was carried out in North Carolina, USA.
Cahn‐Weiner 2003: The participants were 34 individuals (20 women and 14 men) with a diagnosis of probable AD according to NINCDS‐ADRDA criteria. The study was conducted in Rhode Island, USA.
Loewenstein 2004: The participants were 44 individuals (26 men and 18 women) with a diagnosis of probable or possible AD according to NINCDS‐ADRDA criteria. The authors note that those with a diagnosis of probable AD met DSM‐IV criteria for dementia, and those with possible AD did not show sufficient functional impairment to merit a DSM‐IV diagnosis of dementia. All participants had been on a stable dose of an acetylcholinesterase inhibitor for 8 weeks at the start of the study; 41 of these were taking donepezil (doses ranged from 5 to 15 mg). Approximately two‐thirds of participants were English speakers, and the remaining 14 were Spanish speakers, mostly of Cuban origin, for whom all components of the programme were conducted in Spanish. The study took place in Florida, USA.
Galante 2007: Participants were 12 individuals who met NINCDS‐ADRDA criteria for mild AD and scored 19 to 26 on the MMSE or 70 to 90 on the Milan Overall Dementia Assessment (MODA). All were treated with AChEI for at least 3 months. The mean age of the sample was 76 years (SD 6), and the mean educational level was 6.3 years (SD 2.2). One control participant was excluded from analysis "due to poor compliance". The study was conducted in Italy.
Neely 2009: Forty‐seven individuals who met DSM‐IV criteria for mild to moderate AD or vascular dementia and their spouses were approached for the study, and 30 patients (15 males, 15 females) consented to participate. All participants were diagnosed with dementia within the 8 months immediately before the study, were living at home with their spouses and were free from significant psychiatric disorders. The mean age of patients was 75.4 years (SD 6.4). The study was conducted in the Stockholm area of Sweden.
Clare 2010: Participants were 69 individuals (41 women, 28 men) with a mean age of 77.78 years (SD 6.32), and a mean education level of 10.64 years (SD 1.67). They were diagnosed with AD (n = 56) or mixed AD and vascular dementia (n = 13) according to NINCDS‐ADRDA criteria. The mean MMSE score was 23 (SD = 3.02), and all participants were on a stable dose of AChEIs. Forty‐four participants had family members involved, and in all but 4 cases, these individuals were living with the person with dementia. The study was conducted in the North Wales area of the UK.
Characteristics of participants in the treatment and comparison groups
Beck 1988: Characteristics of participants in the treatment and control groups are summarised in Table 1. No significant differences were found between the two groups. Each group comprised 7 white and 3 black participants. In the treatment group 2 had completed grade school, 6 high school and 2 college, and in the control group, 2 had completed grade school, 7 high school and 1 college. In the treatment group 6 people resided in nursing homes and 4 in hospital, and in the control group 9 people resided in nursing homes and 1 in hospital.
| Study | Condition | n (completed baseline assessment) | Age mean (SD), range | Gender balance (m:f) | Years of education | Number taking AChE‐I | Baseline MMSE score | Discontinue rates |
| Cognitive training | 10 | 74 (range 68‐75) | 5:5 | Attended college = 2 | none | not reported | 0 | |
| Control | 10 | 76 (range 70‐93) | 3:7 | Attended college = 1 | none | not reported | 0 | |
| Cognitive training | not reported (18 completed the study) | 65.9 (6.28) | 9:9 | not reported | none | 20.55 (4.42) | not reported | |
| Control | not reported (17 completed the study) | 66.6 (10.17) | 10:7 | not reported | none | 20.23 (4.10) | not reported | |
| Cognitive training | 25 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Control | 25 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Cognitive training | 9 | not reported | not reported | not reported | all | 17.33 (3.39) | 0 | |
| Control | 9 | not reported | not reported | not reported | all | 17 (3.2) | 0 | |
| Cognitive training | 21 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Control | 15 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Cognitive training | 19 | 68.67 (3.86) | 10:9 | 15.06 (3.86) | 5 | 21.84(4.03) | 0 | |
| Control | 18 | 72.56 (7.62) | 6:12 | 12.97 (2.56) | 4 | 22.78 (4.45) | 0 | |
| Cognitive training | 16 | 72.9 (6.7) | not reported | 15.0 (4.0) | not reported | 22.9 (3.6) | 2 | |
| Control | 8 | 73.9 (7.2) | not reported | 15.0 (4.0) | not reported | 26.6 (2.5) | 0 | |
| Cognitive training | 19 | 77. 8 (6.9) | 9:8 | 12.7 (2.1) | all | 24.3 (2.2) | 2 | |
| Control | 20 | 76.0 (7.7) | 5:12 | 13.1 (3.5) | all | 25.1 (1.7) | 3 | |
| Cognitive training | 28 | 78.12 (4.3) | 15:10 | 13.08 (4.1) | all | 23.4 (2.9) | 3 | |
| Control | 21 | 74.74 (7.5) | 11:8 | 14.37 (3.0) | all | 24.53 (4.5) | 2 | |
| Cognitive training | 7 | not reported | not reported | not reported | all | 22.9 (3.1) | 0 | |
| Control | 4 | not reported | not reported | not reported | all | 23.1 (1.8) | 1 | |
| Cognitive training | 10 | 74.8 (6.7) | 6:4 | not reported | not reported | 22.9 (4.15) | 0 | |
| Control | 10 | 77.0 (6.6) | 6:4 | not reported | not reported | 18.6 (5.7) | 1 | |
| Cognitive rehabilitation | 22 | 76.3 (6.39), 64‐89 | 9:13 | 11.41 (2.81), 9‐19 | all | 23.14 (3.12), 18‐27 | 2 | |
| Control | 22 | 78.1 (6.61), 56‐87 | 9:13 | 11.43 (2.99), 9‐19 | all | 22.32 (3.05), 18‐30 | 1 |
Date in the table are generally reported only for those participants who completed the interventions.
Heiss 1993: Mean ages and gender distributions for cognitive training and social support conditions are summarised in Table 1. No significant differences were noted between groups.
Quayhagen 1995: Details are not reported separately for the cognitive training and comparison groups, but the authors comment that no significant differences were observed between groups.
de Vreese 1998: In the 1998 paper, groups were reportedly matched on educational level and illness severity, although the cognitive training plus AChEI group had a significantly longer duration of illness. The 2001 paper reports that groups were matched on MMSE scores; mean MMSE scores are reported in Table 1.
Quayhagen 2000: Details are not reported separately for the cognitive training and comparison groups, but the authors comment that no significant differences were noted between groups.
Davis 2001: Characteristics of participants in each of the two groups are summarised in Table 1. No statistically significant differences were reported, but some trends were apparent; participants in the cognitive training group were on average younger and better educated and were more likely to be male and to be receiving anti‐depressant medication.
Koltai 2001: Characteristics of participants in training and control groups are summarised in Table 1. Participants in the control group had significantly higher MMSE scores at baseline (26.6 vs 22.9) and significantly lower relative rated levels of depression on the Geriatric Depression Scale (GDS) (8.3 vs 14.7).
Cahn‐Weiner 2003: Characteristics of participants in each of the two groups are summarised in Table 1. No statistically significant differences were noted between the groups on these parameters.
Loewenstein 2004: Characteristics of participants in each of the two groups are summarised in Table 1. No statistically significant differences were noted between the groups on these parameters, except that three‐month follow‐up was significantly later for the cognitive training group (13.67 weeks from post‐intervention, compared with 12.79 for the mental stimulation group).
Galante 2007: The authors provided the mean age and education level for the full sample, but no information regarding these patient characteristics was provided at the group level. In addition, the authors report in a table the means and SDs for the two groups on the cognitive measures at all time points, but significance levels are provided only for the Time × Group interaction. Therefore, it is not possible to ascertain whether the groups were equivalent at baseline. Visual inspection shows clear trends for group differences on a number of cognitive (e.g. prose memory), functional (e.g. instrumental activities of daily living (IADL)) and mood (e.g. Neuropsychiatric Inventory (NPI)) measures at baseline. Available characteristics of participants in the training and control groups are summarised in Table 1.
Neely 2009: Relevant characteristics of the treatment and control groups are summarised in Table 1. The groups did not differ in age, levels of depression, MMSE scores or subjective health, or on any of the cognitive measures included at baseline. Data on participants' education level was not reported.
Clare 2010: Characterisitcs of the intervention and control groups are summarised in Table 1. No group differences were found at baseline in any of the demographic, cognitive or functional measures or in the presence of comorbid medical conditions.
Description of the interventions
Beck 1988: Cognitive skills remediation training included exercises on attention and reading, concentration on detail and remembering. Exercises were graded for difficulty level, and participants were given assistance when they had problems with the tasks.
Heiss 1993: Computerised cognitive training for one hour, twice a week, with commercially available software designed for use in neurological rehabilitation (produced by Rigling Reha‐Service), running on a Commodore C64 computer. Participants had to solve memory, perceptual or motor tasks, selected according to the profile of cognitive impairment, of varying difficulty levels. Duration of training was 24 weeks.
Quayhagen 1995: One hour per day of active cognitive stimulation, six days per week, facilitated by the family caregiver in the home setting, using ecologically valid exercises addressing memory, problem‐solving and conversational fluency. A workbook provided for family caregivers contained exercises of varying difficulty levels from which they could select appropriate tasks. The exercises were continued for 12 weeks.
de Vreese 1998: Twice‐weekly, 45‐minute individual sessions with caregivers present, aimed at '(re)training memory (in particular autobiographical and implicit), language and executive abilities associated with reality orientation therapy, to be repeated at home by the caregiver'. The 2001 paper describes the sessions as 30 to 40 minutes in length and involving individually tailored memory training exercises that provided support for encoding (use of real‐life material, involvement of motor activity, self‐generation of cues) and for retrieval (provision of supplementary cues, use of forced‐choice recognition). The sessions were introduced after a 3‐month run‐in period on the drug treatment and were continued for 12 weeks.
Quayhagen 2000: As for Quayhagen 1995, but given 5 days per week for 8 weeks. Post‐treatment assessment was carried out at 12 weeks.
Davis 2001: Weekly individual one‐hour sessions at the clinic, covering (1) spaced retrieval training for personal information (although half recalled the information without training, so there was a ceiling effect); (2) the 'peg' task mnemonic strategy (for those who required little or no spaced retrieval training) and (3) face‐name association using mnemonics. Home attention exercises were carried out for 30 minutes per day, 6 days a week, and were directed by the caregiver. Duration of treatment was five weeks.
Koltai 2001: The memory and coping programme was delivered in individual or group modality. The group format consisted of five weekly, one‐hour sessions conducted in groups of four. The individual format consisted of a mean of six individual sessions. Caregivers joined the last 10 to 15 minutes of each session, where available. The programme involved training and practice in the following techniques: spaced retrieval, face‐name recall, verbal elaboration, concentration/overt repetition, external memory aids and coping strategies.
Cahn‐Weiner 2003: The memory training provided was a modified version of a manualised protocol, involving practice with memory strategies such as categorisation and visualisation and word list learning.
Loewenstein 2004: The cognitive rehabilitation training covered time and place orientation, face‐name association learning, object manipulation, attention and visuomotor training with a computer, making change for a purchase from a $20 bill and balancing a cheque book. Participants were encouraged to use a memory notebook and to practice what they had learned at home between sessions.
Galante 2007: The computerised cognitive training group was trained on a set of computerised exercises selected from a software package covering the domains of memory, language, perception, attention, spatial cognition and intelligence. Exercises were administered in a fixed sequence to all participants, and most exercises lasted 3 minutes.
Neely 2009: The cognitive training was conducted with the support of a research assistant. Participants were trained on a name‐face learning task and on a table‐setting activity. Spaced retrieval and the provision of letter cues were used to support training on the face‐name learning task, whereas a hierarchical cueing technique was used to support training on the table‐setting activity.
Clare 2010: The focus of the intervention was addressing personally meaningful goals; goals were collaboratively identified, and individualised interventions were developed. This was supported by the provision of practical aids and strategies, techniques for learning new information, practice in maintaining attention and concentration and techniques for stress management. Participants were encouraged to work on goals and practise strategies between intervention sessions, and caregivers were invited to participate in the final 15 minutes of each session to assist with between‐session implementation.
Length and duration of the interventions
A summary of the duration of interventions and the timing of assessments is shown in Table 2.
| Study | Intervention length | Initial assessment | Interim assessment | Post‐interv assessment | Follow‐up assessments | Details of sessions | Format of sessions |
| 6 weeks | week 0 | n/a | week 6 | n/a | 18 × 30‐ to 40‐minute sessions | Individual | |
| 24 weeks | week 0 | weeks 8 and 16 (plus monthly physician appointments) | week 25 | n/a | 48 × 1‐hour sessions | Individual | |
| 12 weeks | week 0 | n/a | week 13 | week 38 | 72 × 1‐hour caregiver‐facilitated sessions | Individual | |
| 12 weeks (after 12 weeks on drug) | weeks 0 and 13 | n/a | week 26 | n/a | 24 × 45‐minute sessions | Individual | |
| 8 weeks | week 0 | n/a | week 12 | n/a | 40 × 1‐hour caregiver‐facilitated sessions | Individual | |
| 5 to 6 weeks | weeks 0 to 2 | n/a | weeks 6 to 8 | n/a | 5 × 1‐hour sessions (group) or mean of 6 × 1‐hour sessions (group) | Group or individual | |
| 5 weeks | week 0 | n/a | week 6 | week 12 (cross‐over) | 5 × 1‐hour sessions | Individual | |
| 6 weeks | week 0 | n/a | weeks 8 to 9 (mean 59 days post‐baseline) | week 16 (mean 114.5 days post‐baseline) | 6 × 45‐minute sessions | Group | |
| 12 to 16 weeks | week 0 | n/a | weeks 13 to 18 | weeks 25 to 31 | 24 × 45‐minute sessions | Individual | |
| 4 weeks | week 0 | n/a | week 5 | 3, 6 & 9 months (MMSE only) post‐interventions | 12 × 60‐minute sessions 3 times per week | Individual | |
| 8 weeks | week 0 | n/a | week 9 | n/a | 8 × 60‐minute sessions | Dyads or Individual | |
| 8 weeks | week 0 | n/a | week 9 | 6 months | 8 × 60‐minute sessions | Individual |
Beck 1988: The intervention was delivered in one‐to‐one sessions lasting 30 to 40 minutes, held three times a week for six weeks.
Heiss 1993: Twice‐weekly, one‐hour individual sessions for 24 weeks.
Quayhagen 1995: One hour per day, 6 days per week, for 12 weeks, facilitated by caregiver, plus weekly session with member of research team.
de Vreese 1998: Twice‐weekly 45‐minute individual sessions for 12 weeks, supplemented by home practice.
Quayhagen 2000: One hour per day, 5 days per week, for 8 weeks, facilitated by caregiver, plus modelling of the intervention by member of the research team to assist the caregiver.
Davis 2001: Weekly individual one‐hour sessions for 5 weeks, supplemented by home practice, 30 minutes per day, 6 days per week.
Koltai 2001: The group format consisted of five weekly, one‐hour sessions conducted in groups of four. The individual format consisted of a mean of six individual sessions. Caregivers joined the last 10 to 15 minutes of each session where available. As no differences were observed in results for group and individual training, the data were analysed together.
Cahn‐Weiner 2003: Weekly small‐group sessions lasting 45 minutes each, over a 6‐week period.
Loewenstein 2004: The interventions were delivered in 24 individual sessions, each lasting 45 minutes over a 12‐ to 16‐week period.
Galante 2007: The intervention was delivered individually, with each participant receiving twelve 60‐minute sessions, three times a week, over 4 weeks,
Neely 2009: Participants were offered a one‐hour session of home‐based training each week for a period of 8 weeks.
Clare 2010: Cognitive rehabilitation was delivered in eight weekly, 1‐hour individual sessions conducted in participants' homes.
Description of the comparison conditions
Beck 1988: Participants in the control condition received treatment as usual with no additional intervention.
Heiss 1993: The social support condition consisted of weekly, one‐hour individual sessions that included conversation about personal problems in managing daily life, as well as past experiences, sometimes assisted by games. It is not clear whether the sessions were carried out individually or in groups.
Quayhagen 1995: Placebo intervention consisted of similar types of caregiver‐facilitated exercises, offered for an equivalent length of time but designed to elicit only passive responses rather than active processing and engagement.
de Vreese 1998: Participants received AChEI medication alone for a 6‐month period.
Quayhagen 2000: Wait‐list control condition.
Davis 2001: 'Mock' intervention involved five weekly, one‐hour individual sessions comprising unstructured conversation, recitation of 'overlearned material' and watching of health‐related videos.
Koltai 2001: Wait‐list control condition.
Cahn‐Weiner 2003: The comparison condition was an educational group intervention in which didactic information about ageing and dementia was provided.
Loewenstein 2004: The mental stimulation intervention included individual sessions comprising computer games, exercises like 'hangman', word‐finding tasks and discussion of the 'topic of the day'.
Galante 2007: Participants in the nonspecific treatment condition participated in a semi‐structured interview on current affairs and relevant events of their own life history. The neuropsychologist who conducted the sessions made use of audiovisual material and information received from participants and their relatives on the participant's life history, hobbies and favourite activities. The control condition was matched with the intervention group in terms of number, duration and frequency of sessions.
Neely 2009: Participant dyads in the control condition did not receive any intervention between the pretest and the post‐test.
Clare 2010: Participants in the 'no treatment' group had no contact with the research team between baseline and post‐intervention assessments.
Excluded studies
Characterisitcs of excluded studies are summarised in the Characteristics of excluded studies table.
Risk of bias in included studies
Risk of bias for individual studies, along with a justification for our ratings, is summarised in tables under the Characteristics of included studies section. Risk of bias for specific outcomes across studies is summarised in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most studies had a low risk of bias in relation to random sequence generation procedures, although lack of detail in four studies led to difficulty in evaluating whether random sequence generation was adequately performed. In addition, in all but two studies, insufficient detail was provided to ascertain whether concealment of the randomisation sequence was attempted.
Blinding
In most of the included studies (8/12), outcome assessments were conducted by individuals described as blind to participants' group allocation. However, most studies were classified as high risk in relation to blinding of participants and personnel. Although it is recognised that blinding of participants and personnel is difficult or impossible in trials of cognition‐based interventions, researchers can take steps to reduce the risk of bias due to lack of blinding of participants and research personnel (Higgins 2011).
Incomplete outcome data
Six studies (50%) were classified as low risk as the result of incomplete outcome data, two studies were classified as high risk and the remaining four were classified as unclear risk.
Selective reporting
Again, 6 of the 12 studies (50%) were classified as having low risk of bias as the result of selective reporting, one study was classified as having a high risk of bias and the remaining five as having unclear risk.
Effects of interventions
See: Summary of findings for the main comparison Cognitive training compared to control in the short‐term (i.e. immediately post‐intervention) for early‐stage Alzheimer's disease and vascular dementia; Summary of findings 2 Cognitive rehabilitation compared to control in the short‐term (i.e. immediately post‐intervention) for early‐stage Alzheimer's disease and vascular dementia
Cognitive training
The meta‐analysis revealed no differences between cognitive training and control conditions on any of the primary or secondary outcomes included in the analyses (Data and analyses). summary of findings Table for the main comparison shows the results for a selected number of central outcomes, and details of selected analyses of interest are shown in Figure 4, Figure 5 and Figure 6. As can be seen in the summary of findings Table for the main comparison, longer‐term outcomes related to the trajectory of dementia (i.e. severity of dementia, rates of admission to residential care) have not been assessed in any of the included studies. Furthermore, evidence from cognitive training interventions to date has been found to be of low to moderate quality.

Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.1 A1.1 Change in a global measure of cognition.

Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.13 B1.2 Change in participant's mood (self‐reported).

Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.15 B1.4 Change in participant's capacity for activities of daily living (Carer reported).
Cognitive rehabilitation
Because only a single trial of cognitive rehabilitation (Clare 2010) met criteria for inclusion in this review, no meta‐analysis could be conducted. summary of findings Table 2 shows the results for a selected number of important outcomes in Clare 2010. As can be seen, cognitive rehabilitation was found to be superior to the control condition in the primary outcome of patient‐reported improvement in goal performance over the short term as measured by the Canadian Occupational Performance Measure (effect size Z = 2.11, P = 0.04). Cognitive rehabilitation was also found to be superior to the control condition in relation to outcomes not shown in summary of findings Table 2, Specifically, participants in the cognitive rehabilitation group rated themselves as more satisfied with their ability to carry out meaningful activities of daily living compared with the control condition immediately following the intervention, and they were more satisfied with their memory performance six months after the intervention compared with the control group. A trend that approached significance suggested that six months after the intervention, participants in the cognitive rehabilitation group rated their overall quality of life as higher than that of participants in the control condition (Clare 2010). Evidence also indicated that caregivers of participants in the cognitive rehabilitation group had improved social relationships after the intervention relative to the control condition. Finally, in a subset of participants who underwent functional neuroimaging with fMRI while performing a learning task, cognitive rehabilitation was associated with an increase in brain activation relative to the control condition in the right fusiform face area-the right medial prefrontal cortex. In addition, although participants in the control condition showed lower brain activation after the intervention in the right parahippocampal cortex and in the right temporal parietal junction, no reduction in brain activity in these regions was noted for the group that underwent cognitive rehabilitation (Van Paasschen 2013). As can be seen in the Characteristics of included studies and summary of findings Table 2, the evidence from Clare 2010 was generally regarded as of high quality. However, because the evidence comes from a single study carried out in one setting with a limited sample, the overall quality of evidence in relation to cognitive rehabilitation is best described as moderate.
Discussion
Summary of main results
The aim of this updated review was to evaluate current evidence regarding the efficacy of cognitive training and cognitive rehabilitation interventions for people with mild AD or vascular dementia. Eleven studies of cognitive training were identified for inclusion in the review (nine of which were included in the previous version of this review), and meta‐analysis could be conducted on 8 primary and 6 secondary outcomes in the short term, and on 2 primary and 2 secondary outcome measures in the medium term. No positive or adverse effects of cognitive training were detected in the meta‐analysis. The finding of no adverse effects of cognitive training is relevant in light of proposals from previous commentators (e.g. Small 1997) that cognitive training may have a negative impact, particularly on mood.
Only one RCT of individualised cognitive rehabilitation was identified (Clare 2010). Hence, no meta‐analysis could be conducted. Howevever, the results of this single, high‐quality trial are positive, indicating that cognitive rehabilitation is likely to provide some benefit for patients in the short term and in the medium term related to self‐rated competence and satisfaction in performing meaningful personal goals, memory capacity and general quality of life.
Overall completeness and applicability of evidence
Number of publications meeting inclusion criteria
Since the publication of the previous version of this review (Clare 2008), only two additional RCTs of cognitive training in participants with AD or vascular dementia were published that met the review criteria (Galante 2007; Neely 2009). In addition, only a single study met our inclusion criteria for individual cognitive rehabilitation (Clare 2010). Several factors appear to account for the small number of new studies that met criteria for the present review. First, insufficient methodological quality, namely, the absence of randomisation, led to several published trials (e.g. Bentwich 2011; Hwang 2012) not being included in the review. Second, several RCTs of cognition‐based interventions did not meet our definitions of cognitive training and cognitive rehabilitation, or they described multi‐component interventions (e.g. Graff 2006; Kurz 2012). Issues related to the inclusion criteria used in the current review are further discussed later. A third factor that may have contributed to inclusion of a smaller number of relevant studies in the literature is likely to be associated with the widely held belief that interventions, pharmacological and non‐pharmacological alike, have the greatest chance of success when applied in the earliest possible stage of AD or vascular dementia. Hence, in recent years studies have increasingly targeted individuals who do not meet criteria for dementia, but who nevertheless show significant cognitive decline-such as persons with amnestic mild cognitive impairment (MCI) (Albert 2011; Gauthier 2006). Indeed, many of the records that were retrieved in the updated literature search now exclusively focus on individuals with MCI, and separate reviews focusing on individuals with MCI are now available (Jean 2010Martin 2011).
Issues related to the inclusion of RCTs only
The original protocol for the current review (Clare, Woods, Moniz‐Cook et al 2001) stated that only RCTs would be included in the review. RCTs have been long regarded as the highest forms of evidence in medical research because of the lower risk of bias associated with them. However, most of the studies of cognitive training included in the present review have been rated as having substantial risk of bias in several domains, and the quality of evidence has been found to be low to moderate. Low‐quality RCTs can in principle be associated with a greater threat to internal validity of the study than high‐quality non‐randomised trials and even well conducted single‐case studies. Hence, although more recent studies are generally of a higher methodological quality, this trend is likely to continue, it might be justifiable to include, under strict conditions, high‐quality non‐randomised trials and single‐case studies in future versions of this review to increase the evidence base from which conclusions can be drawn. Several possible advantages are derived by including high‐quality non‐randomised trials in a systematic review, and pooled estimates of effect sizes from randomised and non‐randomised trials can be analysed separately (Reeves 2011).
Issues related to definitions of interventions and multi‐component interventions
Despite progress in the application of a clearer and more consistent terminology in referring to various cognition‐based interventions in mild dementia, interventions often continue to be inaccurately labelled. Specifically, studies continue to be published in which interventions are described as cognitive training or as cognitive rehabilitation, when in fact they appear to more closely reflect cognitive stimulation or reality orientation (e.g. Giordano 2010). This state of affairs means that, in reviewing the available literature and in choosing studies to include in the review, it was generally insufficient to examine the title used in the publication, and in many cases, the Methods section of a published trial had to be closely scrutinised to clarify whether the intervention actually provided was consistent with the one suggested by the title.
In addition, the present review excluded trials in which an intervention was described as a combination of elements from various approaches-such as cognitive behaviour therapy combined with elements of cognition‐focused intervention (e.g. Kurz 2012). This decision is related to the fact that different techniques are likely to have different mechanisms of action, and that it is generally not possible with such interventions to isolate the contributions of different components to the measured outcomes. The definitions of cognition‐based interventions provided in this review essentially reflect groups of intervention techniques that tend to go together, but some overlap has been noted in the techniques used in cognitive stimulation, training and rehabilitation (e.g. psychoeducation may be a component of each of these approaches). Because each of these broad approaches to intervention is likely to involve the use of more than one intervention technique with different mechanisms of action (e.g. setting goals, discovering effective ways to learn new information, using repeated practice), these approaches can also be regarded as essentially ‘multi‐component’ interventions. Additional work is required to better characterise the essential or core components of each of the broad approaches to intervention. It is possible that inclusion of studies based on their use of discrete intervention techniques (e.g. goal‐setting, practice of structured tasks, use of specific learning strategies such as errorless learning) rather than on whether they neatly fit into the definitions offered here might prove more informative.
Outcomes measured in included studies
A further issue influencing the completeness and applicability of the evidence is the range of outcomes reported in the included studies. Trials, particularly studies of cognitive training, have traditionally measured mainly cognitive outcomes in the form of performance on standardised cognitive measures. Very few studies have measured non‐cognitive outcomes for the person with dementia or for the primary caregiver (e.g. mood, quality of life, general health and wellbeing) or longer‐term outcomes that are likely to be of critical importance to policy‐makers, such as those related to the course of dementia, for example, dementia severity and rates of admission to residential care. Although obvious methodological constraints are applied to the measurement of longer‐term outcomes, such as admission to residential care, it is nonetheless important that future trials of cognition‐based interventions, particularly those found to be effective, routinely measure and report outcomes other than direct cognitive ones, and that attempts are made to capture outcomes related to the future trajectory of dementia. Given the nature and aims of individualised cognitive rehabilitation interventions, these approaches tend to emphasize individualised goals and activities of daily living over performance on standardised cognitive tests. Indeed, the single trial of cognitive rehabilitation included in the present review measured and reported several important outcomes other than cognitive outcomes that are of direct clinical relevance.
Methodological limitations of included studies
The lack of significant effects achieved in cognitive training studies must be interpreted in the context of methodological limitations that may have constrained the possibility of demonstrating significant gains, including issues related to power, choice of control condition and choice of outcome measures along with the impact of individual characteristics that may moderate treatment response.
Power to detect effects: Many of the included trials are likely to have suffered from limited statistical power to detect effects. Lack of power of individual studies to detect effects is commonly associated with small sample sizes, which is a frequent limitation in cognition‐based interventions for people with mild AD and vascular dementia. However, this explanation is unlikely to account for the lack of significant findings, as a meta‐analysis is designed to overcome limitations derived from individual studies associated with such factors as sample size. Indeed, not only was the size of the effects in individual studies small, but, and this is possibly of greater relevance, the direction of effects associated with some outcomes did not consistently favour cognitive training over the control condition. For example, in three out of five studies that reported the impact of cognitive training on a global measure of cognition in the short term, the direction of the effect favoured the control group, whereas in only one of the trials did the effect clearly favour the cognitive training condition (Figure 4). Indeed, such inconsistency in the direction of effects was found to be the case for a substantial number of outcomes reported by the studies, even when the same measures were used by different studies to evaluate a given outcome. Other factors that might contribute to the difficulty involved in detecting significant effects are difficulties in determining the right ‘dose’ of an intervention (i.e. frequency, intensity and duration of interventions), the presence of 'ceiling' or 'floor' effects, rendering it impossible to demonstrate improvements in a given domain, and baseline differences between treatment and control groups.
Choice of control condition: The difficulty of defining what constitutes an appropriate comparison condition is particularly important because in some studies (e.g. Cahn‐Weiner 2003; Loewenstein 2004) cognitive training may have been compared with other active treatments, thus masking potentially beneficial effects. Clinical practice requires the ability to distinguish which of a range of possible psychosocial interventions is most likely to be useful for a given individual, and the study designs used here do not allow this question to be addressed.
Use of neuropsychological tests as cognitive outcomes: The use of neuropsychological tests to measure cognitive outcomes effectively means that what is actually being assessed is transfer of benefit from trained to untrained tasks, rather than the effects of training on trained tasks. However, as was discussed in the introduction, very limited evidence has been found to support such transfer effects from trained to untrained tasks. When the trained tasks are analogous in some way to daily activities, however, improvement in such tasks may have direct relevance to daily functioning, but this would be missed if these benefits were not transferred to performance on standardised neuropsychological tests. For example, Davis 2001 noted improvement on tasks during training, such as recall of personal information and face‐name associations, but this was not captured by the neuropsychological measures selected to assess cognitive outcomes. A further problem with the use of standardised neuropsychological tests before and after the intervention to measure cognitive outcomes involves the potential for practice effects that may obscure possible effects of specific treatments. Finally, in some studies, more than one neuropsychological test or self‐report scale is used to measure the same outcome (e.g. executive function, general wellbeing). This leads to difficulties in choosing which is the most appropriate or relevant measure of the outcome under consideration for inclusion in the meta‐analysis.
Moderating role of patient characteristics in intervention outcomes: Investigators have learned that various patient characteristics have the potential to moderate response to the intervention, and as more evidence becomes available regarding important moderators, cognition‐focused interventions might be better able to control for the effects of such moderators. For example, Koltai 2001 retrospectively classified participants' level of awareness of their own impairments and found that a higher level of awareness was a predictor of a more successful outcome-a finding that has also been demonstrated in a prospective study of cognitive rehabilitation outcomes for a small group of people with mild AD (Clare 2004). The moderating impact of factors such as awareness of deficits or the presence of neuropsychiatric symptoms (e.g. apathy) may strengthen the rationale for inclusion of high‐quality single‐case studies in future versions of this review.
Study context: Non‐pharmacological studies are more likely than drug trials to be affected by the study context, including the healthcare setting, as well as by cultural and linguistic factors. Given that the studies reviewed have taken place in a variety of contexts, one cannot exclude the possibility that cognition‐based interventions are better suited for some contexts than others.
Quality of the evidence
As has been discussed, the generally low methodological quality of trials continues to limit the ability of researchers to evaluate the evidence base. The quality of most of the studies of cognitive training interventions included in the review was often compromised by significant risks of bias-particularly as a result of insufficient detail regarding the method used to generate a random group allocation sequence, concealment of this sequence from relevant members of the research team and attempts to blind participants, researchers or both to group allocation. Hence, the finding of no significant benefit (or harm) from cognitive training interventions needs to be interpreted with caution, and the estimate of effect sizes may vary in the future as the evidence comes from studies of greater quality. However, the methodological quality of trials is gradually improving, and this trend is expected to continue in coming years. Indeed, although only a single study of individualised cognitive rehabilitation was identified, this study was rated to be of high methodological quality and hence to have lower risk of bias-permitting the drawing of positive conclusions regarding the efficacy of this approach. Although effect estimates that are based on high‐quality evidence are generally regarded as estimates that are unlikely to change significantly with the publication of further studies, confidence in the positive outcomes of cognitive rehabilitation will nevertheless increase as evidence accumulates from further high‐quality, preferably multi‐site, trials of cognitive rehabilitation with larger sample sizes.
Agreements and disagreements with other studies or reviews
In recent years, two main systematic reviews that included an examination of the efficacy of cognitive training for people with mild dementia have been published. In reviewing the literature to 2004, Sitzer 2006 concluded that “cognitive training evidenced promise in the treatment of AD, with primarily medium effect sizes for learning, memory, executive functions, activities of daily living, general cognitive problems, depression, and self‐rated general functioning”. Closer examination of the methodology described in Sitzer 2006 reveals important differences that explain the inconsistency in the results of the current review. First, Sitzer 2006 applied inclusion criteria that were much less strict and included both randomised and non‐randomised trials (total 19), as well as studies that included participants with moderate to severe AD. Second, although Sitzer 2006 described their review as a review of cognitive training, of the 14 RCTs that met their inclusion criteria, 6 were in fact studies of other cognition‐based interventions (primarily reality orientation/cognitive stimulation) or multi‐component interventions. Indeed, in separate analyses, performed only on the five “high‐quality trials” (all of which were included in the current review)-the observed effects were very small and non‐significant. It is quite plausible that if studies of cognitive stimulation, training and rehabilitation for people with mild AD or vascular dementia were assessed together, some benefits would have been detected. However, given the important differences among the various cognition‐focused approaches to intervention, these should be treated separately. Indeed, although the current review did not identify any benefits associated with cognitive training, the results of a single, high‐quality trial of cognitive rehabilitation tentatively suggest that this approach may be associated with important benefits for the person with dementia and the primary caregiver. In addition, a separate Cochrane review of cognitive stimulation for mild AD has recently confirmed that this approach was associated with several positive outcomes for the person with dementia (Woods 2012).
More recently, Olazaran 2010 reviewed the general literature on the efficacy of 26 categories of non‐pharmacological interventions for people with dementia. In relation to cognitive training, these authors concluded that a Grade B recommendation (recommendation associated with low‐quality RCTs) can be given for the efficacy of individual and group cognitive training in improving cognitive functions. No effects of cognitive training on other outcomes were found. This conclusion seems to be different from that of the current review, and important differences are evident between this review and that of Olazaran 2010. Specifically, Olazaran 2010 included in their review participants with any kind of dementia, and in fact allowed for inclusion of a small proportion of participants with cognitive decline but without confirmed dementia. In addition, like Sitzer 2006, Olazaran 2010 used less strict inclusion criteria, leading to inclusion of several low‐quality studies. Finally, rather than examining different cognitive domains separately, Olazaran 2010 analysed cognition broadly, and studies contributed diverse measures of cognition to the evaluation of this outcome, whereas cognitive outcomes in the current study were evaluated separately against widely agreed cognitive domains. These methodological differences are most likely to account for the differences between the current review and the review by Olazaran 2010.

Study flow diagram.
RCT = randomised controlled trial.
CT = cognitive training.
CR = cognitive rehabilitation.
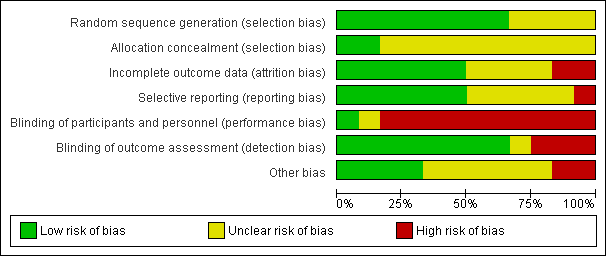
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
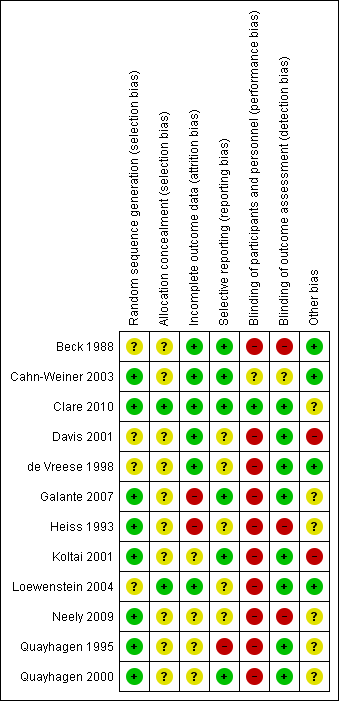
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.1 A1.1 Change in a global measure of cognition.

Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.13 B1.2 Change in participant's mood (self‐reported).
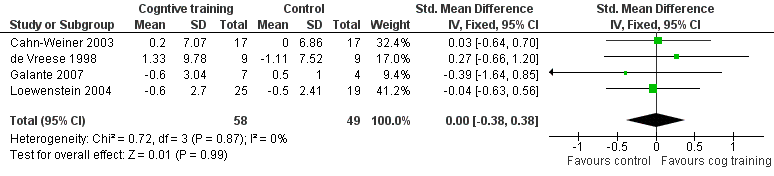
Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.15 B1.4 Change in participant's capacity for activities of daily living (Carer reported).

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 1 A1.1 Change in a global measure of cognition.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 2 A1.2 Change in orientation.
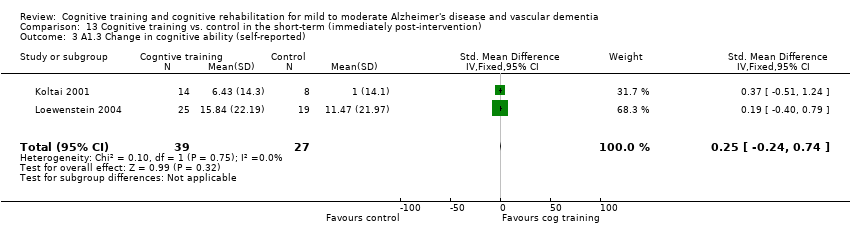
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 3 A1.3 Change in cognitive ability (self‐reported).

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 4 A1.4 Change in cognitive ability (carer reported).
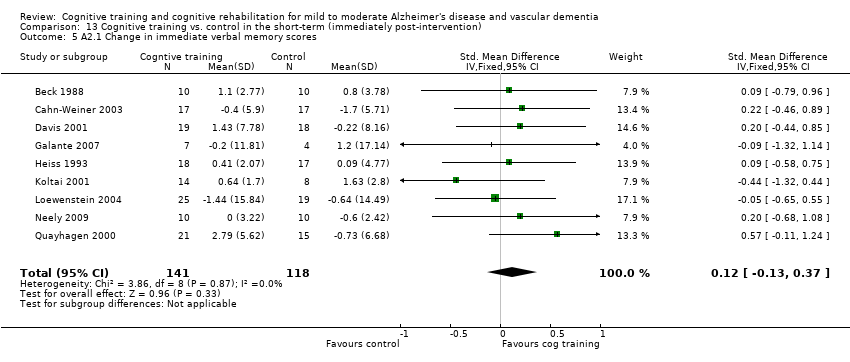
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 5 A2.1 Change in immediate verbal memory scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 6 A2.2 Change in delayed verbal memory scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 7 A2.3 Change in verbal memory recognition scores.
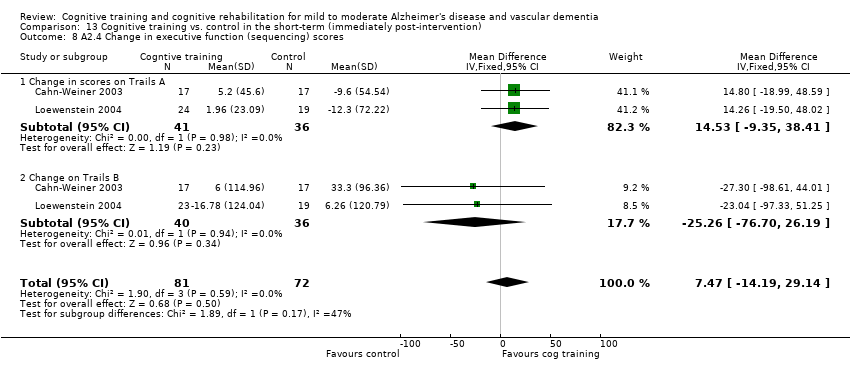
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 8 A2.4 Change in executive function (sequencing) scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 9 A2.5 Change in verbal letter fluency scores.
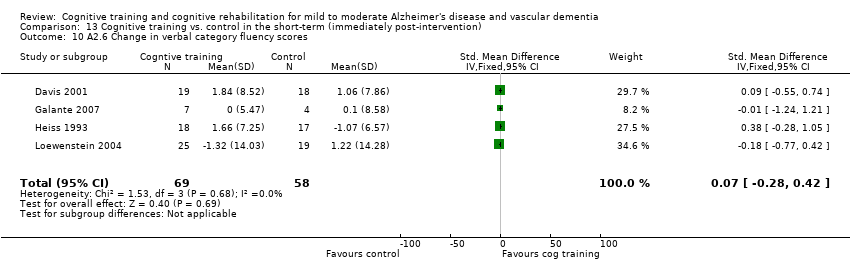
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 10 A2.6 Change in verbal category fluency scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 11 A2.7 Change in attention and working memory scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 13 B1.2 Change in participant's mood (self‐reported).

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 15 B1.4 Change in participant's capacity for activities of daily living (Carer reported).

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 16 B1.5 Change in participant's mood (carer reported).
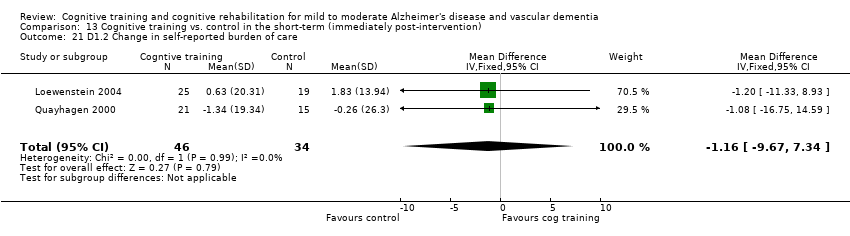
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 21 D1.2 Change in self‐reported burden of care.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 23 E1.1 Effect of cognitive training on biomarker evidence of brain function.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 1 A2.1.1 Change in a global measure of cognition.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 3 A2.1.3 Change in cognitive ability (carer reported).

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 4 A2.2.1 Change in immediate verbal memory scores.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 6 A2.2.3 Change in executive function (sequencing) scores.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 7 A2.2.4 Change in verbal letter fluency scores.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 8 A2..2.5Change in verbal category fluency scores.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 11 B2.2 Change in participant's mood (self‐reported).
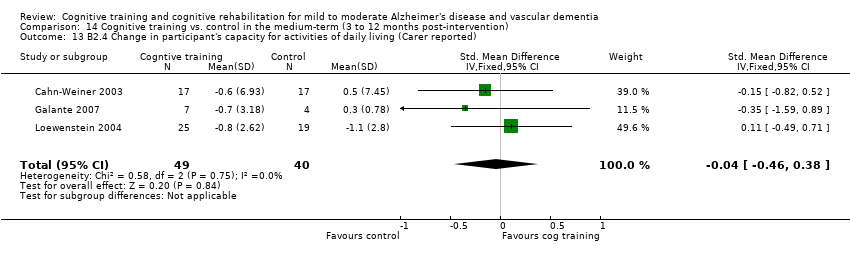
Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 13 B2.4 Change in participant's capacity for activities of daily living (Carer reported).
| Cognitive training compared to control in the short‐term (i.e. post‐intervention) for early‐stage Alzheimer's disease and vascular dementia | ||||||
| Patient or population: participants with early‐stage Alzheimer's disease and vascular dementia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control in the short‐term (i.e. post‐intervention) | Cognitive training | |||||
| Change in a global measure of cognition | The mean change in a global measure of cognition in the intervention groups was | 173 | ⊕⊕⊝⊝ | |||
| Change in participant's capacity for activities of daily living (Caregiver reported) | The mean change in participant's capacity for activities of daily living (caregiver reported) in the intervention groups was | 107 | ⊕⊕⊝⊝ | SMD 0 (‐0.38 to 0.38) | ||
| Change in participant's mood (self‐reported) | The mean change in participant's mood (self‐reported) in the intervention groups was | 114 | ⊕⊕⊕⊝ | SMD 0.03 (‐0.34 to 0.41) | ||
| Change in rates of admission to residential care-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in measures of dementia severity-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in immediate verbal memory scores | The mean change in immediate verbal memory scores in the intervention groups was | 201 | ⊕⊕⊝⊝ | SMD 0.1 (‐0.18 to 0.38) | ||
| Change in self‐reported burden of care | The mean change in self‐reported burden of care in the intervention groups was | 80 | ⊕⊕⊕⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence: | ||||||
| 1 The confidence interval of the effect included a zero effect. Therefore, imprecision is likely. | ||||||
| Cognitive rehabilitation compared to control in the short‐term (i.e. post‐intervention) for early‐stage Alzheimer's disease and vascular dementia | ||||||
| Patient or population: participants with early‐stage Alzheimer's disease and vascular dementia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control in the short term (i.e. post‐intervention) | Cognitive rehabilitation | |||||
| Change in a global measure of cognition-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in participant's self‐reported performance in relation to individual goals (COPM Performance, self‐reported) | The mean change in participant's capacity for activities of daily living (COPM Performance, self‐reported) in the intervention groups was | 39 | ⊕⊕⊕⊕ | |||
| Change in participant's mood (Depression, self‐reported) | The mean change in participant's mood (depression, self‐reported) in the intervention groups was | 41 | ⊕⊕⊕⊕ | SMD ‐0.24 (‐0.86 to 0.37) | ||
| Change in rates of admission to residential care-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in measures of dementia severity-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in self‐reported mood (Depression-caregiver) | The mean change in self‐reported mood (depression, caregiver) in the intervention groups was | 18 | ⊕⊕⊕⊕ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence: | ||||||
| Study | Condition | n (completed baseline assessment) | Age mean (SD), range | Gender balance (m:f) | Years of education | Number taking AChE‐I | Baseline MMSE score | Discontinue rates |
| Cognitive training | 10 | 74 (range 68‐75) | 5:5 | Attended college = 2 | none | not reported | 0 | |
| Control | 10 | 76 (range 70‐93) | 3:7 | Attended college = 1 | none | not reported | 0 | |
| Cognitive training | not reported (18 completed the study) | 65.9 (6.28) | 9:9 | not reported | none | 20.55 (4.42) | not reported | |
| Control | not reported (17 completed the study) | 66.6 (10.17) | 10:7 | not reported | none | 20.23 (4.10) | not reported | |
| Cognitive training | 25 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Control | 25 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Cognitive training | 9 | not reported | not reported | not reported | all | 17.33 (3.39) | 0 | |
| Control | 9 | not reported | not reported | not reported | all | 17 (3.2) | 0 | |
| Cognitive training | 21 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Control | 15 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Cognitive training | 19 | 68.67 (3.86) | 10:9 | 15.06 (3.86) | 5 | 21.84(4.03) | 0 | |
| Control | 18 | 72.56 (7.62) | 6:12 | 12.97 (2.56) | 4 | 22.78 (4.45) | 0 | |
| Cognitive training | 16 | 72.9 (6.7) | not reported | 15.0 (4.0) | not reported | 22.9 (3.6) | 2 | |
| Control | 8 | 73.9 (7.2) | not reported | 15.0 (4.0) | not reported | 26.6 (2.5) | 0 | |
| Cognitive training | 19 | 77. 8 (6.9) | 9:8 | 12.7 (2.1) | all | 24.3 (2.2) | 2 | |
| Control | 20 | 76.0 (7.7) | 5:12 | 13.1 (3.5) | all | 25.1 (1.7) | 3 | |
| Cognitive training | 28 | 78.12 (4.3) | 15:10 | 13.08 (4.1) | all | 23.4 (2.9) | 3 | |
| Control | 21 | 74.74 (7.5) | 11:8 | 14.37 (3.0) | all | 24.53 (4.5) | 2 | |
| Cognitive training | 7 | not reported | not reported | not reported | all | 22.9 (3.1) | 0 | |
| Control | 4 | not reported | not reported | not reported | all | 23.1 (1.8) | 1 | |
| Cognitive training | 10 | 74.8 (6.7) | 6:4 | not reported | not reported | 22.9 (4.15) | 0 | |
| Control | 10 | 77.0 (6.6) | 6:4 | not reported | not reported | 18.6 (5.7) | 1 | |
| Cognitive rehabilitation | 22 | 76.3 (6.39), 64‐89 | 9:13 | 11.41 (2.81), 9‐19 | all | 23.14 (3.12), 18‐27 | 2 | |
| Control | 22 | 78.1 (6.61), 56‐87 | 9:13 | 11.43 (2.99), 9‐19 | all | 22.32 (3.05), 18‐30 | 1 | |
| Date in the table are generally reported only for those participants who completed the interventions. | ||||||||
| Study | Intervention length | Initial assessment | Interim assessment | Post‐interv assessment | Follow‐up assessments | Details of sessions | Format of sessions |
| 6 weeks | week 0 | n/a | week 6 | n/a | 18 × 30‐ to 40‐minute sessions | Individual | |
| 24 weeks | week 0 | weeks 8 and 16 (plus monthly physician appointments) | week 25 | n/a | 48 × 1‐hour sessions | Individual | |
| 12 weeks | week 0 | n/a | week 13 | week 38 | 72 × 1‐hour caregiver‐facilitated sessions | Individual | |
| 12 weeks (after 12 weeks on drug) | weeks 0 and 13 | n/a | week 26 | n/a | 24 × 45‐minute sessions | Individual | |
| 8 weeks | week 0 | n/a | week 12 | n/a | 40 × 1‐hour caregiver‐facilitated sessions | Individual | |
| 5 to 6 weeks | weeks 0 to 2 | n/a | weeks 6 to 8 | n/a | 5 × 1‐hour sessions (group) or mean of 6 × 1‐hour sessions (group) | Group or individual | |
| 5 weeks | week 0 | n/a | week 6 | week 12 (cross‐over) | 5 × 1‐hour sessions | Individual | |
| 6 weeks | week 0 | n/a | weeks 8 to 9 (mean 59 days post‐baseline) | week 16 (mean 114.5 days post‐baseline) | 6 × 45‐minute sessions | Group | |
| 12 to 16 weeks | week 0 | n/a | weeks 13 to 18 | weeks 25 to 31 | 24 × 45‐minute sessions | Individual | |
| 4 weeks | week 0 | n/a | week 5 | 3, 6 & 9 months (MMSE only) post‐interventions | 12 × 60‐minute sessions 3 times per week | Individual | |
| 8 weeks | week 0 | n/a | week 9 | n/a | 8 × 60‐minute sessions | Dyads or Individual | |
| 8 weeks | week 0 | n/a | week 9 | 6 months | 8 × 60‐minute sessions | Individual |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 A1.1 Change in a global measure of cognition Show forest plot | 6 | 173 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.21, 0.40] |
| 2 A1.2 Change in orientation Show forest plot | 2 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.13, 0.76] |
| 3 A1.3 Change in cognitive ability (self‐reported) Show forest plot | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.24, 0.74] |
| 4 A1.4 Change in cognitive ability (carer reported) Show forest plot | 3 | 100 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.17, 0.63] |
| 5 A2.1 Change in immediate verbal memory scores Show forest plot | 9 | 259 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.13, 0.37] |
| 6 A2.2 Change in delayed verbal memory scores Show forest plot | 3 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.27, 0.51] |
| 7 A2.3 Change in verbal memory recognition scores Show forest plot | 2 | 69 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.22, 0.73] |
| 8 A2.4 Change in executive function (sequencing) scores Show forest plot | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | 7.47 [‐14.19, 29.14] |
| 8.1 Change in scores on Trails A | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 14.53 [‐9.35, 38.41] |
| 8.2 Change on Trails B | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐25.26 [‐76.70, 26.19] |
| 9 A2.5 Change in verbal letter fluency scores Show forest plot | 3 | 82 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.46, 0.42] |
| 10 A2.6 Change in verbal category fluency scores Show forest plot | 4 | 127 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.28, 0.42] |
| 11 A2.7 Change in attention and working memory scores Show forest plot | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.64, 0.72] |
| 12 B1.1 Change in participant's capacity for activities of daily living (self‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 B1.2 Change in participant's mood (self‐reported) Show forest plot | 4 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.34, 0.41] |
| 14 B1.3 Change in participant's general quality of life (self‐report) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 B1.4 Change in participant's capacity for activities of daily living (Carer reported) Show forest plot | 4 | 107 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.38, 0.38] |
| 16 B1.5 Change in participant's mood (carer reported) Show forest plot | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.38, 0.61] |
| 17 B1.6 Change in participant's general quality of life (carer‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 C1.1 Change in rates of admission to residential care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 C1.2 Change in measures of dementia severity | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 D1.1 Change in self‐reported mood (carer) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 D1.2 Change in self‐reported burden of care Show forest plot | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐9.67, 7.34] |
| 22 D1.3 Change in self‐reported overall wellbeing and quality of life | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 E1.1 Effect of cognitive training on biomarker evidence of brain function Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐3.67, 1.79] |
| 23.1 Change in glucose metabolism at rest (FDG PET) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐3.67, 1.79] |
| 23.2 Effects on glucose metabolism at activation (FDG PET task) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 E1.2 Effect of cognitive training on biomarker measures of neuropathology | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 A2.1.1 Change in a global measure of cognition Show forest plot | 2 | 61 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.01, 1.02] |
| 2 A2.1.2 Change in cognitive ability (self‐reported) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 A2.1.3 Change in cognitive ability (carer reported) Show forest plot | 2 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.44, 0.45] |
| 4 A2.2.1 Change in immediate verbal memory scores Show forest plot | 3 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.48, 0.37] |
| 5 A2.2.2 Change in delayed verbal memory scores | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 A2.2.3 Change in executive function (sequencing) scores Show forest plot | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | 9.38 [‐9.88, 28.65] |
| 6.1 Change in scores on Trails A | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 12.62 [‐7.98, 33.23] |
| 6.2 Change on Trails B | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.13 [‐67.45, 41.19] |
| 7 A2.2.4 Change in verbal letter fluency scores Show forest plot | 1 | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.18, 1.28] |
| 8 A2..2.5Change in verbal category fluency scores Show forest plot | 1 | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐1.28, 1.18] |
| 9 A2.2.6 Change in attention and working memory scores | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 B2.1 Change in participant's capacity for activities of daily living (self‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 B2.2 Change in participant's mood (self‐reported) Show forest plot | 1 | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐1.34, 1.12] |
| 12 B2.3 Change in participant's general quality of life (self‐report) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 B2.4 Change in participant's capacity for activities of daily living (Carer reported) Show forest plot | 3 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.46, 0.38] |
| 14 B2.5 Change in participant's mood (carer reported) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 B2.6 Change in participant's general quality of life (carer‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 C2.1 Change in rates of admission to residential care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 C2.2 Change in measures of dementia severity | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 D2.1 Change in self‐reported mood | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 D2.2 Change in self‐reported burden of care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 D2.3 Change in self‐reported overall wellbeing and quality of life | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 E2.1 Effect of cognitive training on biomarker evidence of brain function | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Change in glucose metabolism at rest (FDG PET) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Effects on glucose metabolism at activation (FDG PET task) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 E2.2 Effect of cognitive training on biomarker measures of neuropathology | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

