Rehabilitación cognitiva y entrenamiento cognitivo para la enfermedad de Alzheimer y la demencia vascular de leve a moderada
Appendices
Appendix 1. Pre‐publication search: November 2012
| Source
| Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Keyword search: "cognitive rehabilitation" OR "cognitive stimulation" OR "cognitive training" | 113 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. exp Dementia/ 2. Delirium, Dementia, Amnestic, Cognitive Disorders/ 3. dement*.mp. 4. alzheimer*.mp. 5. (lewy* adj2 bod*).mp. 6. (chronic adj2 cerebrovascular).mp. 7. ("organic brain disease" or "organic brain syndrome").mp. 8. (cerebr* adj2 deteriorat*).mp. 9. (cerebral* adj2 insufficient*).mp. 10. (pick* adj2 disease).mp. 11. or/1‐10 12. *Cognitive Therapy/ 13. (cognit* adj2 stimulation).ti,ab. 14. (cognit* adj2 rehabilitation).ti,ab. 15. (cognit* adj2 training).ti,ab. 16. (cognit* adj2 retrain*).ti,ab. 17. "cognitive support".ti,ab. 18. "memory function*".ti,ab. 19. (memory adj2 rehabilitation).ti,ab. 20. (memory adj2 therap*).ti,ab. 21. "memory aid*".ti,ab. 22. "memory group*".ti,ab. 23. "memory training".ti,ab. 24. ("memory retraining" or "memory re‐training").ti,ab. 25. "memory support".ti,ab. 26. "memory stimulation".ti,ab. 27. "memory strateg*".ti,ab. 28. "memory management".ti,ab. 29. or/12‐28 30. 11 and 29 31. randomized controlled trial.pt. 32. controlled clinical trial.pt. 33. randomized.ab. 34. placebo.ab. 35. randomly.ab. 36. trial.ab. 37. groups.ab. 38. or/31‐37 39. (animals not (humans and animals)).sh. 40. 38 not 39 41. 30 and 40 42. (201111* or 201112*).ed. 43. 2012*.ed. 44. 42 or 43 45. 41 and 44
| 53 |
| 3. EMBASE 1980‐2011 week 39 (Ovid SP) | 1. exp dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (lewy* adj2 bod*).mp. 5. (chronic adj2 cerebrovascular).mp. 6. ("organic brain disease" or "organic brain syndrome").mp. 7. (cerebr* adj2 deteriorat*).mp. 8. (cerebral* adj2 insufficient*).mp. 9. CADASIL.mp. 10. or/1‐9 11. (cognit* adj2 stimulation).ti,ab. 12. (cognit* adj2 rehabilitation).ti,ab. 13. (cognit* adj2 training).ti,ab. 14. (cognit* adj2 retrain*).ti,ab. 15. "cognitive support".ti,ab. 16. (memory adj2 rehabilitation).ti,ab. 17. (memory adj2 therap*).ti,ab. 18. "memory aid*".ti,ab. 19. "memory group*".ti,ab. 20. "memory training".ti,ab. 21. ("memory retraining" or "memory re‐training").ti,ab. 22. "memory support".ti,ab. 23. "memory stimulation".ti,ab. 24. "memory strateg*".ti,ab. 25. "memory management".ti,ab. 26. or/11‐25 27. 10 and 26 28. randomly.ab. 29. placebo*.ti,ab. 30. "double‐blind*".ti,ab. 31. randomized controlled trial/ 32. trial.ti,ab. 33. or/28‐32 34. 27 and 33 35. (2011* or 2012*).em. 36. 34 and 35
| 52 |
| 4. PSYCINFO 1806‐October week 5 2011 (Ovid SP) | 1. exp Dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (chronic adj2 cerebrovascular).mp. 5. ("organic brain disease" or "organic brain syndrome").mp. 6. (cerebr* adj2 deteriorat*).mp. 7. (cerebral* adj2 insufficient*).mp. 8. or/1‐7 9. (cognit* adj2 stimulation).ti,ab. 10. (cognit* adj2 rehabilitation).ti,ab. 11. (cognit* adj2 training).ti,ab. 12. (cognit* adj2 retrain*).ti,ab. 13. "cognitive support".ti,ab. 14. (memory adj2 rehabilitation).ti,ab. 15. (memory adj2 therap*).ti,ab. 16. "memory aid*".ti,ab. 17. "memory group*".ti,ab. 18. "memory training".ti,ab. 19. ("memory retraining" or "memory re‐training").ti,ab. 20. "memory support".ti,ab. 21. "memory stimulation".ti,ab. 22. "memory strateg*".ti,ab. 23. "memory management".ti,ab. 24. or/9‐23 25. 8 and 24 26. randomly.ab. 27. randomi?ed.ab. 28. placebo*.ti,ab. 29. trial.ti,ab. 30. RCT.ti,ab. 31. groups.ab. 32. or/26‐31 33. 25 and 32 34. (2011* or 2012*).up. 35. 33 and 34
| 41 |
| 5. CINAHL (EBSCOhost) | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 (MH "Rehabilitation, Cognitive") S21 TX (cognit* rehab*) S22 TX (cognit* train*) S23 TX (memory train*) S24 TX (memory support*) S25 TX (memory stimul*) S26 S20 or S21 or S22 or S23 or S24 or S25 S27 S19 and S26 S28 EM 2011 S29 EM 2012 S30 S28 or S29 S31 S27 and S30 | 67 |
| 6. Web of Science (1945‐present) and conference proceedings via Web of Knowledge | Topic=(dement* OR VCI OR "vascular cognitive impairment*" OR VaD OR alzheimer*) AND Topic=("cognit* train*" OR "cognit* rehab*" OR "memory aid*" OR "memory train*" OR "memory support*" OR "memory stimul*") AND Topic=(randomly OR placebo OR groups OR trial OR RCT OR randomized OR randomised) AND Year Published=(2011‐2012) Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH. Lemmatization=On
| 86 |
| 7. LILACS (BIREME) | demenc$ OR dement$ OR alzheimer$ [Words] and memory [Words] and randomly OR randomised OR randomized OR trial OR ensaio clínico [Words] | 10 |
| 8. CENTRAL (The Cochrane Library) (Issue 2 of 4, 2011) | #1 MeSH descriptor Dementia explode all trees #2 dement* #3 alzheimer* #4 "chronic cerebrovascular" #5 "organic brain disease" or "organic brain syndrome" #6 "benign senescent forgetfulness" #7 "cerebr* deteriorat*" #8 "cerebral* insufficient*" #9 "pick* disease" #10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 "cognit* rehab*" #12 "cognit* train*" #13 "cognit* stimul*" #14 "memory train*" #15 "memory support*" OR "memory aid*" #16 "memory therap*" #17 "memory group*" #18 "memory stimul*" OR "memory strateg*" #19 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 (#10 AND #19) #21 #20 AND (2011 OR 2012) | 9 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Interventional Studies | dementia OR alzheimer OR alzheimers OR VCI OR vascular dementia OR VaD OR vascular cognitive impairment OR cadasil OR multi‐infarct OR binswanger | cognitive rehabilitaion OR cognitive training OR memory | Senior
| 183 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry-India; Clinical Research Information Service-Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] | Interventional Studies | dementia OR Alzheimer OR vascular impairment OR VCI OR Alzheimers | cognitive rehabilitaion OR cognitive training OR memory | received from 01/11/2011 to 02/11/2012 | 19 |
| TOTAL before de‐duplication | 633 | |
| TOTAL after de‐dupe and first‐assess | 123 | |
Appendix 2. Update search: December 2011
| Source
| Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | ("cognitive training" OR "cognitive rehabilitation" OR "memory training") AND (dementia OR alzheimer) AND (2009 OR 2010 OR 2011) | 129 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. exp Dementia/ 2. Delirium, Dementia, Amnestic, Cognitive Disorders/ 3. dement*.mp. 4. alzheimer*.mp. 5. (lewy* adj2 bod*).mp. 6. (chronic adj2 cerebrovascular).mp. 7. ("organic brain disease" or "organic brain syndrome").mp. 8. (cerebr* adj2 deteriorat*).mp. 9. (cerebral* adj2 insufficient*).mp. 10. (pick* adj2 disease).mp. 11. or/1‐10 12. *Cognitive Therapy/ 13. (cognit* adj2 stimulation).ti,ab. 14. (cognit* adj2 rehabilitation).ti,ab. 15. (cognit* adj2 training).ti,ab. 16. (cognit* adj2 retrain*).ti,ab. 17. "cognitive support".ti,ab. 18. "memory function*".ti,ab. 19. (memory adj2 rehabilitation).ti,ab. 20. (memory adj2 therap*).ti,ab. 21. "memory aid*".ti,ab. 22. "memory group*".ti,ab. 23. "memory training".ti,ab. 24. ("memory retraining" or "memory re‐training").ti,ab. 25. "memory support".ti,ab. 26. "memory stimulation".ti,ab. 27. "memory strateg*".ti,ab. 28. "memory management".ti,ab. 29. or/12‐28 30. 11 and 29 31. randomized controlled trial.pt. 32. controlled clinical trial.pt. 33. randomized.ab. 34. placebo.ab. 35. randomly.ab. 36. trial.ab. 37. groups.ab. 38. or/31‐37 39. (animals not (humans and animals)).sh. 40. 38 not 39 41. 30 and 40 42. (2009* or 2010* or 2011*).ed. 43. 41 and 42
| 110 |
| 3. EMBASE 1980‐2011 week 49 (Ovid SP) | 1. exp dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (lewy* adj2 bod*).mp. 5. (chronic adj2 cerebrovascular).mp. 6. ("organic brain disease" or "organic brain syndrome").mp. 7. (cerebr* adj2 deteriorat*).mp. 8. (cerebral* adj2 insufficient*).mp. 9. CADASIL.mp. 10. or/1‐9 11. (cognit* adj2 stimulation).ti,ab. 12. (cognit* adj2 rehabilitation).ti,ab. 13. (cognit* adj2 training).ti,ab. 14. (cognit* adj2 retrain*).ti,ab. 15. "cognitive support".ti,ab. 16. (memory adj2 rehabilitation).ti,ab. 17. (memory adj2 therap*).ti,ab. 18. "memory aid*".ti,ab. 19. "memory group*".ti,ab. 20. "memory training".ti,ab. 21. ("memory retraining" or "memory re‐training").ti,ab. 22. "memory support".ti,ab. 23. "memory stimulation".ti,ab. 24. "memory strateg*".ti,ab. 25. "memory management".ti,ab. 26. or/11‐25 27. 10 and 26 28. randomly.ab. 29. placebo*.ti,ab. 30. "double‐blind*".ti,ab. 31. randomized controlled trial/ 32. trial.ti,ab. 33. or/28‐32 34. 27 and 33 35. (2009* or 2010* or 2011*).em. 36. 34 and 35
| 63 |
| 4. PsycINFO 1806‐December week 2 2011 (Ovid SP) | 1. exp Dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (chronic adj2 cerebrovascular).mp. 5. ("organic brain disease" or "organic brain syndrome").mp. 6. (cerebr* adj2 deteriorat*).mp. 7. (cerebral* adj2 insufficient*).mp. 8. or/1‐7 9. (cognit* adj2 stimulation).ti,ab. 10. (cognit* adj2 rehabilitation).ti,ab. 11. (cognit* adj2 training).ti,ab. 12. (cognit* adj2 retrain*).ti,ab. 13. "cognitive support".ti,ab. 14. (memory adj2 rehabilitation).ti,ab. 15. (memory adj2 therap*).ti,ab. 16. "memory aid*".ti,ab. 17. "memory group*".ti,ab. 18. "memory training".ti,ab. 19. ("memory retraining" or "memory re‐training").ti,ab. 20. "memory support".ti,ab. 21. "memory stimulation".ti,ab. 22. "memory strateg*".ti,ab. 23. "memory management".ti,ab. 24. or/9‐23 25. 8 and 24 26. randomly.ab. 27. randomi?ed.ab. 28. placebo*.ti,ab. 29. trial.ti,ab. 30. RCT.ti,ab. 31. groups.ab. 32. or/26‐31 33. 25 and 32 34. (2009* or 2010* or 2011*).up. 35. 33 and 34
| 48 |
| 5. CINAHL (EBSCOhost) |
|
|
| 6. ISI Web of Knowledge-all databases [includes Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] | Topic=(dement* OR VCI OR "vascular cognitive impairment*" OR VaD OR alzheimer*) AND Topic=("cognit* train*" OR "cognit* rehab*" OR "memory aid*" OR "memory train*" OR "memory support*" OR "memory stimul*") AND Topic=(randomly OR placebo OR groups OR trial OR RCT OR randomized OR randomised) AND Year Published=(2009‐2011) Timespan=2009‐2011.
| 88 |
| 7. LILACS (BIREME) | memory [Words] and demenc$ OR dement$ OR alzheimer$ [Words] and randomly OR randomised OR randomized OR trial OR ensaio clínico [Words] |
|
| 8. CENTRAL (The Cochrane Library) (Issue 4 of 4, Oct 2010) | #1 MeSH descriptor Dementia explode all trees #2 dement* #3 alzheimer* #4 "chronic cerebrovascular" #5 "organic brain disease" or "organic brain syndrome" #6 "benign senescent forgetfulness" #7 "cerebr* deteriorat*" #8 "cerebral* insufficient*" #9 "pick* disease" #10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 "cognit* rehab*" #12 "cognit* train*" #13 "cognit* stimul*" #14 "memory train*" #15 "memory support*" OR "memory aid*" #16 "memory therap*" #17 "memory group*" #18 "memory stimul*" OR "memory strateg*" #19 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 (#10 AND #19) |
|
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Interventional Studies | dementia | cognitive rehabilitaion OR cognitive training | Senior | received from 01/01/2009 to 12/14/2011 | 23 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry-India; Clinical Research Information Service-Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] | Interventional Studies | dementia | cognitive rehabilitaion OR cognitive training | Senior | received from 01/01/2009 to 14/12/2011 | 18 |
| TOTAL before de‐duplication | 489 | |
| TOTAL after de‐dupe | 259 | |
Appendix 3. Update search: January 2006 to January 2009
| Source | Date Searched | Hits Retrieved |
| MEDLINE (PubMed) | January 7 | 27 |
| EMBASE (Ovid SP) | January 8 | 32 |
| PsycINFO (Ovid SP) | January 8 | 8 |
| CINAHL (Ovid SP) | January 8 | 7 |
| LILACS (bireme) | January 8 | 0 |
| CDCIG SR* | January 7 | 42 |
| CENTRAL (The Cochrane Library) | Issue 4 2008 | 48 |
| ISTP Conference Proceedings http://portal.isiknowledge.com/portal.cgi | January 8 | 32 |
| Australian Digital Theses Programme | January 12 | 0 |
| Canadian Theses and Dissertations | January 12 | 0 |
| WHO trials register | January 12 | 8 |
| Current Controlled trials: Meta Register of Controlled Trials (mRCT) | January 11 | 9 |
| ISRCTN Register
| January 11 | // |
| Nederlands Trial Register http://www.trialregister.nl/trialreg/index.asp | January 12 | 0 |
| ClinicalTrials.gov | Included in WHO portal | // |
| IPFMA Clinical Trials Register | January 12 | 0 |
| UMIN Japan Trial Register | January 12 | 2 |
| OPENsigle | January 12 | 2 |

Study flow diagram.
RCT = randomised controlled trial.
CT = cognitive training.
CR = cognitive rehabilitation.
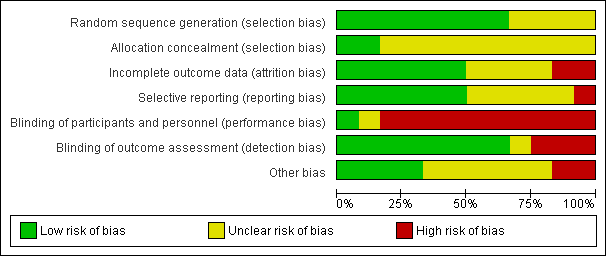
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
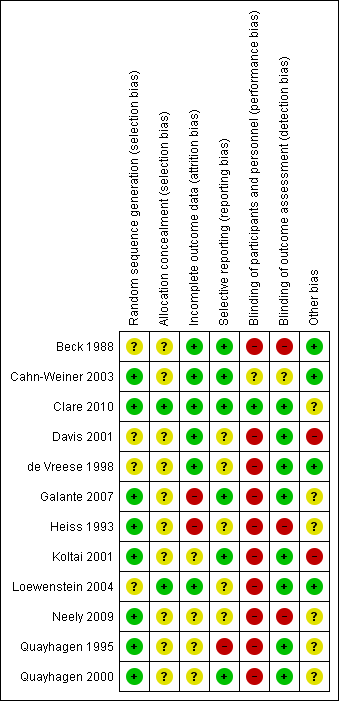
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.1 A1.1 Change in a global measure of cognition.

Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.13 B1.2 Change in participant's mood (self‐reported).
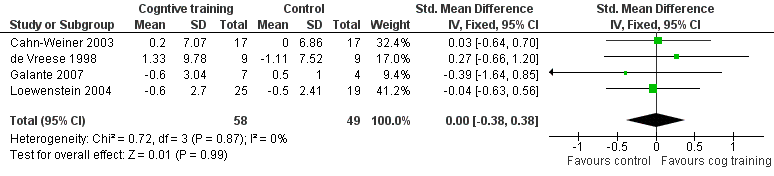
Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.15 B1.4 Change in participant's capacity for activities of daily living (Carer reported).

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 1 A1.1 Change in a global measure of cognition.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 2 A1.2 Change in orientation.
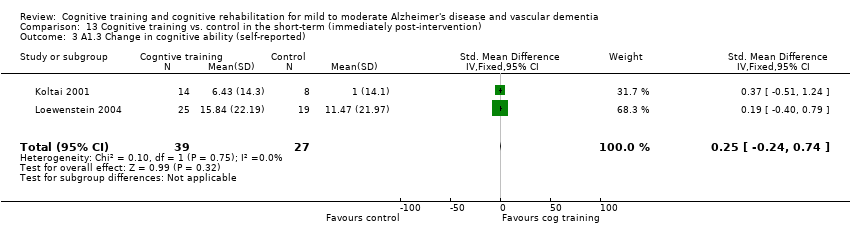
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 3 A1.3 Change in cognitive ability (self‐reported).

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 4 A1.4 Change in cognitive ability (carer reported).
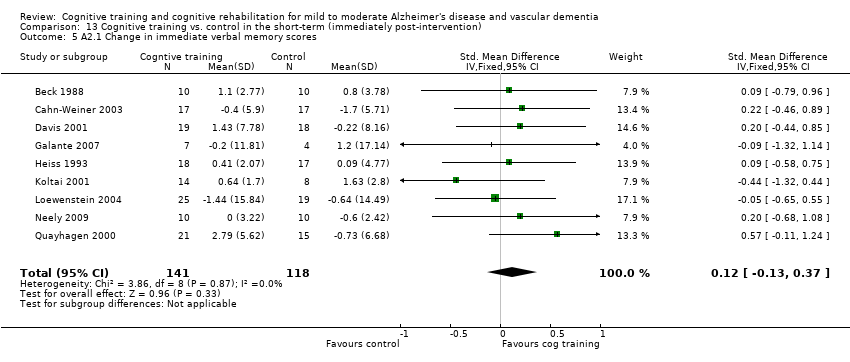
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 5 A2.1 Change in immediate verbal memory scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 6 A2.2 Change in delayed verbal memory scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 7 A2.3 Change in verbal memory recognition scores.
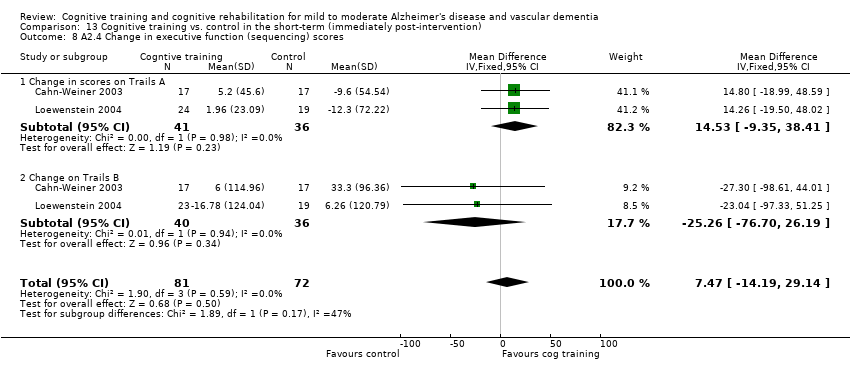
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 8 A2.4 Change in executive function (sequencing) scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 9 A2.5 Change in verbal letter fluency scores.
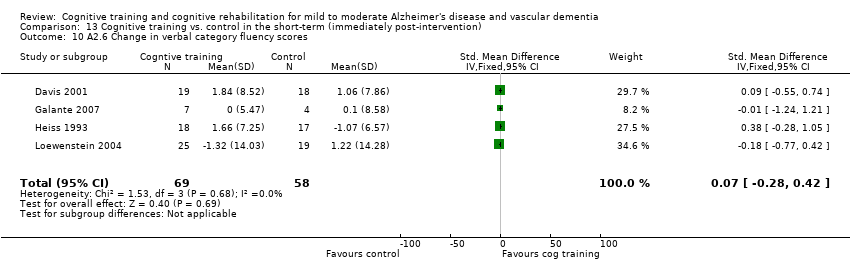
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 10 A2.6 Change in verbal category fluency scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 11 A2.7 Change in attention and working memory scores.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 13 B1.2 Change in participant's mood (self‐reported).

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 15 B1.4 Change in participant's capacity for activities of daily living (Carer reported).

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 16 B1.5 Change in participant's mood (carer reported).
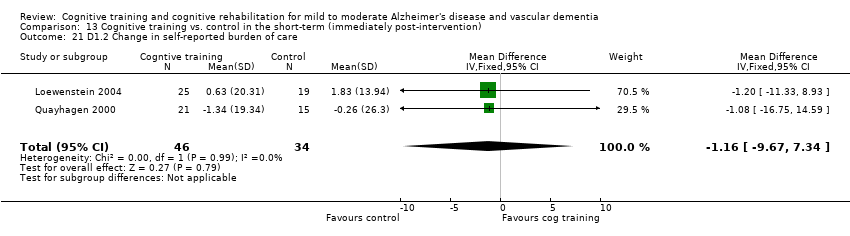
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 21 D1.2 Change in self‐reported burden of care.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 23 E1.1 Effect of cognitive training on biomarker evidence of brain function.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 1 A2.1.1 Change in a global measure of cognition.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 3 A2.1.3 Change in cognitive ability (carer reported).

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 4 A2.2.1 Change in immediate verbal memory scores.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 6 A2.2.3 Change in executive function (sequencing) scores.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 7 A2.2.4 Change in verbal letter fluency scores.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 8 A2..2.5Change in verbal category fluency scores.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 11 B2.2 Change in participant's mood (self‐reported).
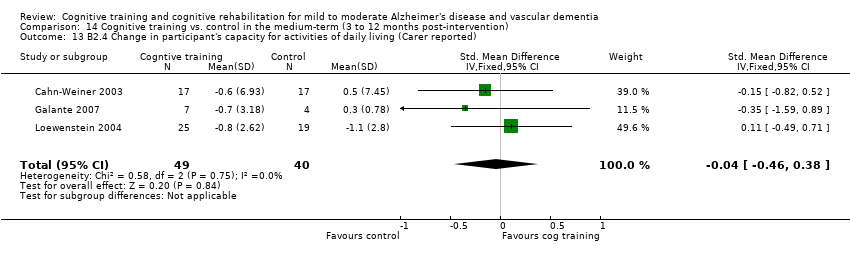
Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 13 B2.4 Change in participant's capacity for activities of daily living (Carer reported).
| Cognitive training compared to control in the short‐term (i.e. post‐intervention) for early‐stage Alzheimer's disease and vascular dementia | ||||||
| Patient or population: participants with early‐stage Alzheimer's disease and vascular dementia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control in the short‐term (i.e. post‐intervention) | Cognitive training | |||||
| Change in a global measure of cognition | The mean change in a global measure of cognition in the intervention groups was | 173 | ⊕⊕⊝⊝ | |||
| Change in participant's capacity for activities of daily living (Caregiver reported) | The mean change in participant's capacity for activities of daily living (caregiver reported) in the intervention groups was | 107 | ⊕⊕⊝⊝ | SMD 0 (‐0.38 to 0.38) | ||
| Change in participant's mood (self‐reported) | The mean change in participant's mood (self‐reported) in the intervention groups was | 114 | ⊕⊕⊕⊝ | SMD 0.03 (‐0.34 to 0.41) | ||
| Change in rates of admission to residential care-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in measures of dementia severity-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in immediate verbal memory scores | The mean change in immediate verbal memory scores in the intervention groups was | 201 | ⊕⊕⊝⊝ | SMD 0.1 (‐0.18 to 0.38) | ||
| Change in self‐reported burden of care | The mean change in self‐reported burden of care in the intervention groups was | 80 | ⊕⊕⊕⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence: | ||||||
| 1 The confidence interval of the effect included a zero effect. Therefore, imprecision is likely. | ||||||
| Cognitive rehabilitation compared to control in the short‐term (i.e. post‐intervention) for early‐stage Alzheimer's disease and vascular dementia | ||||||
| Patient or population: participants with early‐stage Alzheimer's disease and vascular dementia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control in the short term (i.e. post‐intervention) | Cognitive rehabilitation | |||||
| Change in a global measure of cognition-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in participant's self‐reported performance in relation to individual goals (COPM Performance, self‐reported) | The mean change in participant's capacity for activities of daily living (COPM Performance, self‐reported) in the intervention groups was | 39 | ⊕⊕⊕⊕ | |||
| Change in participant's mood (Depression, self‐reported) | The mean change in participant's mood (depression, self‐reported) in the intervention groups was | 41 | ⊕⊕⊕⊕ | SMD ‐0.24 (‐0.86 to 0.37) | ||
| Change in rates of admission to residential care-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in measures of dementia severity-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in self‐reported mood (Depression-caregiver) | The mean change in self‐reported mood (depression, caregiver) in the intervention groups was | 18 | ⊕⊕⊕⊕ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence: | ||||||
| Study | Condition | n (completed baseline assessment) | Age mean (SD), range | Gender balance (m:f) | Years of education | Number taking AChE‐I | Baseline MMSE score | Discontinue rates |
| Cognitive training | 10 | 74 (range 68‐75) | 5:5 | Attended college = 2 | none | not reported | 0 | |
| Control | 10 | 76 (range 70‐93) | 3:7 | Attended college = 1 | none | not reported | 0 | |
| Cognitive training | not reported (18 completed the study) | 65.9 (6.28) | 9:9 | not reported | none | 20.55 (4.42) | not reported | |
| Control | not reported (17 completed the study) | 66.6 (10.17) | 10:7 | not reported | none | 20.23 (4.10) | not reported | |
| Cognitive training | 25 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Control | 25 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Cognitive training | 9 | not reported | not reported | not reported | all | 17.33 (3.39) | 0 | |
| Control | 9 | not reported | not reported | not reported | all | 17 (3.2) | 0 | |
| Cognitive training | 21 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Control | 15 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Cognitive training | 19 | 68.67 (3.86) | 10:9 | 15.06 (3.86) | 5 | 21.84(4.03) | 0 | |
| Control | 18 | 72.56 (7.62) | 6:12 | 12.97 (2.56) | 4 | 22.78 (4.45) | 0 | |
| Cognitive training | 16 | 72.9 (6.7) | not reported | 15.0 (4.0) | not reported | 22.9 (3.6) | 2 | |
| Control | 8 | 73.9 (7.2) | not reported | 15.0 (4.0) | not reported | 26.6 (2.5) | 0 | |
| Cognitive training | 19 | 77. 8 (6.9) | 9:8 | 12.7 (2.1) | all | 24.3 (2.2) | 2 | |
| Control | 20 | 76.0 (7.7) | 5:12 | 13.1 (3.5) | all | 25.1 (1.7) | 3 | |
| Cognitive training | 28 | 78.12 (4.3) | 15:10 | 13.08 (4.1) | all | 23.4 (2.9) | 3 | |
| Control | 21 | 74.74 (7.5) | 11:8 | 14.37 (3.0) | all | 24.53 (4.5) | 2 | |
| Cognitive training | 7 | not reported | not reported | not reported | all | 22.9 (3.1) | 0 | |
| Control | 4 | not reported | not reported | not reported | all | 23.1 (1.8) | 1 | |
| Cognitive training | 10 | 74.8 (6.7) | 6:4 | not reported | not reported | 22.9 (4.15) | 0 | |
| Control | 10 | 77.0 (6.6) | 6:4 | not reported | not reported | 18.6 (5.7) | 1 | |
| Cognitive rehabilitation | 22 | 76.3 (6.39), 64‐89 | 9:13 | 11.41 (2.81), 9‐19 | all | 23.14 (3.12), 18‐27 | 2 | |
| Control | 22 | 78.1 (6.61), 56‐87 | 9:13 | 11.43 (2.99), 9‐19 | all | 22.32 (3.05), 18‐30 | 1 | |
| Date in the table are generally reported only for those participants who completed the interventions. | ||||||||
| Study | Intervention length | Initial assessment | Interim assessment | Post‐interv assessment | Follow‐up assessments | Details of sessions | Format of sessions |
| 6 weeks | week 0 | n/a | week 6 | n/a | 18 × 30‐ to 40‐minute sessions | Individual | |
| 24 weeks | week 0 | weeks 8 and 16 (plus monthly physician appointments) | week 25 | n/a | 48 × 1‐hour sessions | Individual | |
| 12 weeks | week 0 | n/a | week 13 | week 38 | 72 × 1‐hour caregiver‐facilitated sessions | Individual | |
| 12 weeks (after 12 weeks on drug) | weeks 0 and 13 | n/a | week 26 | n/a | 24 × 45‐minute sessions | Individual | |
| 8 weeks | week 0 | n/a | week 12 | n/a | 40 × 1‐hour caregiver‐facilitated sessions | Individual | |
| 5 to 6 weeks | weeks 0 to 2 | n/a | weeks 6 to 8 | n/a | 5 × 1‐hour sessions (group) or mean of 6 × 1‐hour sessions (group) | Group or individual | |
| 5 weeks | week 0 | n/a | week 6 | week 12 (cross‐over) | 5 × 1‐hour sessions | Individual | |
| 6 weeks | week 0 | n/a | weeks 8 to 9 (mean 59 days post‐baseline) | week 16 (mean 114.5 days post‐baseline) | 6 × 45‐minute sessions | Group | |
| 12 to 16 weeks | week 0 | n/a | weeks 13 to 18 | weeks 25 to 31 | 24 × 45‐minute sessions | Individual | |
| 4 weeks | week 0 | n/a | week 5 | 3, 6 & 9 months (MMSE only) post‐interventions | 12 × 60‐minute sessions 3 times per week | Individual | |
| 8 weeks | week 0 | n/a | week 9 | n/a | 8 × 60‐minute sessions | Dyads or Individual | |
| 8 weeks | week 0 | n/a | week 9 | 6 months | 8 × 60‐minute sessions | Individual |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 A1.1 Change in a global measure of cognition Show forest plot | 6 | 173 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.21, 0.40] |
| 2 A1.2 Change in orientation Show forest plot | 2 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.13, 0.76] |
| 3 A1.3 Change in cognitive ability (self‐reported) Show forest plot | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.24, 0.74] |
| 4 A1.4 Change in cognitive ability (carer reported) Show forest plot | 3 | 100 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.17, 0.63] |
| 5 A2.1 Change in immediate verbal memory scores Show forest plot | 9 | 259 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.13, 0.37] |
| 6 A2.2 Change in delayed verbal memory scores Show forest plot | 3 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.27, 0.51] |
| 7 A2.3 Change in verbal memory recognition scores Show forest plot | 2 | 69 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.22, 0.73] |
| 8 A2.4 Change in executive function (sequencing) scores Show forest plot | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | 7.47 [‐14.19, 29.14] |
| 8.1 Change in scores on Trails A | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 14.53 [‐9.35, 38.41] |
| 8.2 Change on Trails B | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐25.26 [‐76.70, 26.19] |
| 9 A2.5 Change in verbal letter fluency scores Show forest plot | 3 | 82 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.46, 0.42] |
| 10 A2.6 Change in verbal category fluency scores Show forest plot | 4 | 127 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.28, 0.42] |
| 11 A2.7 Change in attention and working memory scores Show forest plot | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.64, 0.72] |
| 12 B1.1 Change in participant's capacity for activities of daily living (self‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 B1.2 Change in participant's mood (self‐reported) Show forest plot | 4 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.34, 0.41] |
| 14 B1.3 Change in participant's general quality of life (self‐report) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 B1.4 Change in participant's capacity for activities of daily living (Carer reported) Show forest plot | 4 | 107 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.38, 0.38] |
| 16 B1.5 Change in participant's mood (carer reported) Show forest plot | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.38, 0.61] |
| 17 B1.6 Change in participant's general quality of life (carer‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 C1.1 Change in rates of admission to residential care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 C1.2 Change in measures of dementia severity | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 D1.1 Change in self‐reported mood (carer) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 D1.2 Change in self‐reported burden of care Show forest plot | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐9.67, 7.34] |
| 22 D1.3 Change in self‐reported overall wellbeing and quality of life | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 E1.1 Effect of cognitive training on biomarker evidence of brain function Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐3.67, 1.79] |
| 23.1 Change in glucose metabolism at rest (FDG PET) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐3.67, 1.79] |
| 23.2 Effects on glucose metabolism at activation (FDG PET task) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 E1.2 Effect of cognitive training on biomarker measures of neuropathology | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 A2.1.1 Change in a global measure of cognition Show forest plot | 2 | 61 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.01, 1.02] |
| 2 A2.1.2 Change in cognitive ability (self‐reported) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 A2.1.3 Change in cognitive ability (carer reported) Show forest plot | 2 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.44, 0.45] |
| 4 A2.2.1 Change in immediate verbal memory scores Show forest plot | 3 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.48, 0.37] |
| 5 A2.2.2 Change in delayed verbal memory scores | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 A2.2.3 Change in executive function (sequencing) scores Show forest plot | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | 9.38 [‐9.88, 28.65] |
| 6.1 Change in scores on Trails A | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 12.62 [‐7.98, 33.23] |
| 6.2 Change on Trails B | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.13 [‐67.45, 41.19] |
| 7 A2.2.4 Change in verbal letter fluency scores Show forest plot | 1 | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.18, 1.28] |
| 8 A2..2.5Change in verbal category fluency scores Show forest plot | 1 | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐1.28, 1.18] |
| 9 A2.2.6 Change in attention and working memory scores | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 B2.1 Change in participant's capacity for activities of daily living (self‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 B2.2 Change in participant's mood (self‐reported) Show forest plot | 1 | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐1.34, 1.12] |
| 12 B2.3 Change in participant's general quality of life (self‐report) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 B2.4 Change in participant's capacity for activities of daily living (Carer reported) Show forest plot | 3 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.46, 0.38] |
| 14 B2.5 Change in participant's mood (carer reported) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 B2.6 Change in participant's general quality of life (carer‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 C2.1 Change in rates of admission to residential care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 C2.2 Change in measures of dementia severity | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 D2.1 Change in self‐reported mood | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 D2.2 Change in self‐reported burden of care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 D2.3 Change in self‐reported overall wellbeing and quality of life | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 E2.1 Effect of cognitive training on biomarker evidence of brain function | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Change in glucose metabolism at rest (FDG PET) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Effects on glucose metabolism at activation (FDG PET task) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 E2.2 Effect of cognitive training on biomarker measures of neuropathology | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

