| 1 Cognition Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 1.1 Dose 30mg/day, SKT (change from baseline at 6 weeks) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐3.04 [‐5.68, ‐0.40] |
| 2 Activities of daily living Show forest plot | 3 | | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 2.1 Dose 30mg/day, (change from baseline at 4‐6 weeks) | 2 | 119 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.36 [‐1.77, ‐0.96] |
| 2.2 Dose 10mg/day, BGP subscore care dependence (change from baseline at 12 weeks) | 1 | 166 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.47, 0.14] |
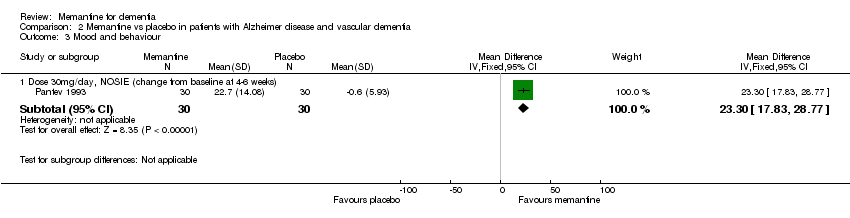
| 3 Mood and behaviour Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 3.1 Dose 30mg/day, NOSIE (change from baseline at 4‐6 weeks) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 23.3 [17.83, 28.77] |
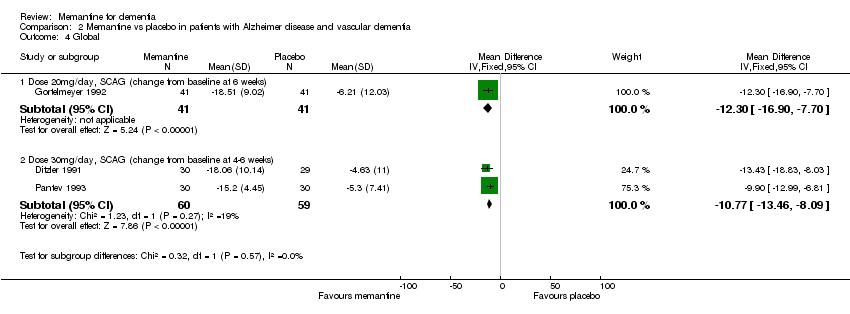
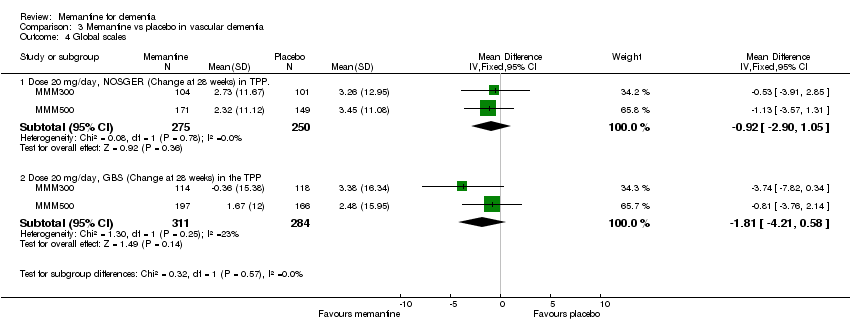
| 4 Global Show forest plot | 3 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 4.1 Dose 20mg/day, SCAG (change from baseline at 6 weeks) | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐12.3 [‐16.90, ‐7.70] |
| 4.2 Dose 30mg/day, SCAG (change from baseline at 4‐6 weeks) | 2 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐10.77 [‐13.46, ‐8.09] |
| 5 CGIC (numbers improved) Show forest plot | 3 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 5.1 Dose 20mg/day, time 6 weeks | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.85 [1.52, 9.75] |
| 5.2 Dose 30mg/day, time 6 weeks | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.25 [1.72, 15.98] |
| 5.3 Dose 10mg/day, time 12 weeks | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.72, 6.33] |
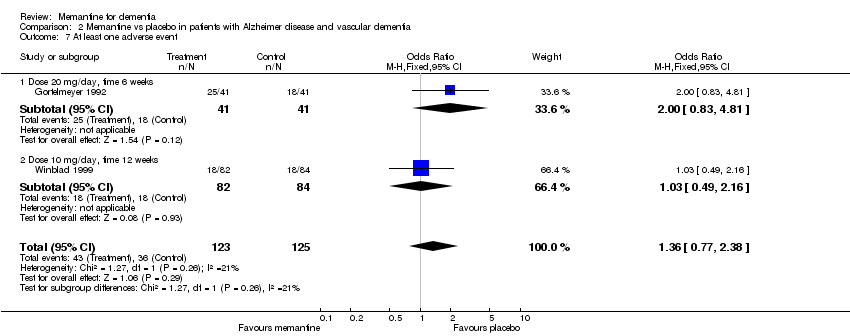
| 6 Dropouts by the end of the treatment Show forest plot | 3 | 305 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.40, 1.89] |
|
| 6.1 Dose 20 mg/day, time 6 weeks | 1 | 88 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.35, 11.53] |
| 6.2 Dose 30 mg/day, time 6 weeks | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.07, 2.15] |
| 6.3 Dose 10 mg/day, time 12 weeks | 1 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.30, 2.55] |
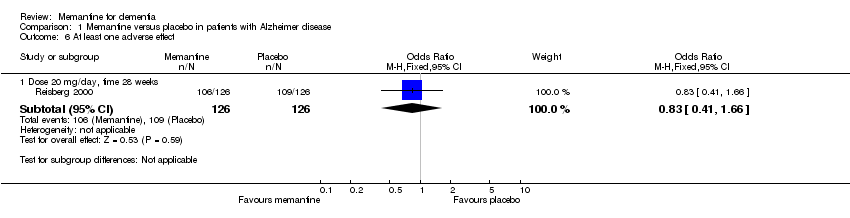
| 7 At least one adverse event Show forest plot | 2 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.77, 2.38] |
|
| 7.1 Dose 20 mg/day, time 6 weeks | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.83, 4.81] |
| 7.2 Dose 10 mg/day, time 12 weeks | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.16] |
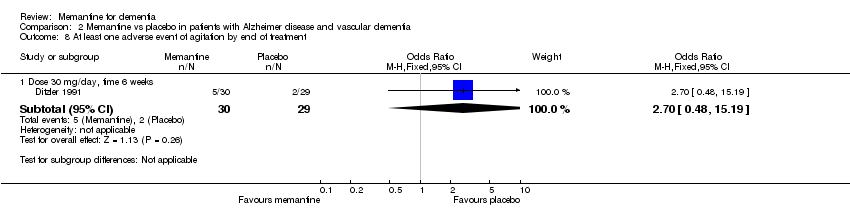
| 8 At least one adverse event of agitation by end of treatment Show forest plot | 1 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 8.1 Dose 30 mg/day, time 6 weeks | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.48, 15.19] |
| 9 At least one advese event of restlessness by the end of treatment Show forest plot | 1 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 9.1 Dose 30 mg/day, time 6 weeks | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.5 [2.71, 67.18] |