Высокодозная химиотерапия и аутологичная трансплантация костного мозга или стволовых клеток по сравнению с общепринятой химиотерапией у женщин с ранним плохим прогнозом рака молочной железы
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | INCLUDED: | |
| Interventions | After randomisation all women received 4 standard cycles of doxorubicin (75 mg/m²) then HDC or CDC. HDC group received PBPC mobilisation (cyclophosphamide 4.0 gm/m² + filgrastim) followed by a single cycle of PBPC‐supported HDC (cyclophosphamide 6.0 gm/m², thiotepa 800 mg/m² + filgrastim). CDC group received conventional course of CMF (cyclophosphamide, methotrexate and fluorouracil) | |
| Outcomes | Overall survival | |
| Notes | Power calculation: 300 participants in total would give power to detect a 12% survival difference at 5 years, assuming a survival with conventional chemotherapy (A‐CMF) of 50% at 10 years. Rapid accrual enabled inclusion of 600 women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation programme used |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned to their treatment by telephone from the trial management office." |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | 603/605 (over 99%) of randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
| Methods | Randomised controlled trial | |
| Participants | INCLUDED: | |
| Interventions | 2 ‐ 8 weeks after primary surgery all women received 3 cycles of standard dose CAF chemotherapy (cyclophosphamide 600 mg/m²; doxorubicin 60 mg/m²; fluorouracil 1200 mg/m²). Women were then re‐evaluated and if disease‐free were randomised to HDC or CDC. HDC group had bone marrow harvest before or after a 4th cycle of standard‐dose CAF and GCSF‐primed PBPC harvest after the 4th cycle. They then received a course of HDC (cyclophosphamide 5625 mg/m², cisplatin 165 mg/m², carmustine 600 mg/m²) with both bone marrow and PBPC support, plus GCSF. The CDC group completed the 4th cycle of CAF then received an intermediate level dose of cyclophosphamide (900 mg/m²), cisplatin (90 m/m²) and carmustine (90 mg/m²), plus GCSF | |
| Outcomes | Disease‐free survival | |
| Notes | Power calculation: 380 participants per arm give 90% power to detect 15% absolute difference in disease‐free survival at 5 years (P = 0.05) Participants relapsing on CDC eligible for HDC, but post‐relapse transplant not part of protocol Data are immature. 3‐year survival data in our tables of comparison have been calculated from percentages reported by trialists at median of 37 months' follow‐up, and 5‐year data have been calculated from 5‐year percentage survivals quoted by trialists at median of 7.3 years' follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | 112 women initially declared ineligible (with minor protocol violations) but subsequently randomised |
| Methods | Randomised controlled trial | |
| Participants | INCLUDED: | |
| Interventions | Women randomised to the HDC group received 4 cycles of FEC (fluorouracil 500 mg/m², epirubicin 90 mg/m², cyclophosphamide 500 mg/m²) and 1 cycle of CTC (cyclophosphamide 6 g/m², thiotepa 480 mg/m², carboplatin 1600 mg/m²) with PBPC support | |
| Outcomes | Relapse‐free survival | |
| Notes | Power calculation: 880 participants give 90% power to detect a reduction in hazard of 24% after 571 events (progression‐free survival 30% to 40%) 3‐year data are mature: 3‐year survival results in our tables estimated from graphs published at median 57 month follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Treatment allocation by phone call to centralised trial office |
| Blinding (performance bias and detection bias) | Unclear risk | A centralised review of pathological specimens was carried out in a blinded fashion. Otherwise blinding was not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | Quote: "In two patients data were lacking on infectious complications and in three patients data were lacking on bacterial cultures. In 99% of patients (437 patients) sufficient data could be retrieved from the medical records and case record forms. Ultimately, 392 patients actually received high‐dose chemotherapy. Reasons not to proceed with the high‐dose regimen were an infected central venous catheter prior to high‐dose chemotherapy in three patients, venous access problems in one patient and catheter‐unrelated in the remaining patients." |
| Other bias | Low risk | No other potential bias identified |
| Methods | Randomised controlled trial Country: The Netherlands | |
| Participants | INCLUDED: | |
| Interventions | Women were assessed for appropriate breast surgery. | |
| Outcomes | Disease‐free survival | |
| Notes | Power calculation: designed to provide 80% power to predict 30% increase in progression‐free survival at 4 years (30% ‐ 60%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Treatment allocation by phone call to centralised trial office |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | Seems to be free of selective reporting |
| Other bias | Low risk | No other potential bias identified |
| Methods | Randomised controlled trial Country: USA | |
| Participants | INCLUDED: Women aged 15 ‐ 60 years with stage II or III epithelial breast cancer, within 12 weeks of breast surgery, with histologically free surgical margins and at least 10 positive ipsilateral lymph nodes EXCLUDED: | |
| Interventions | All women had 6 cycles of cyclophosphamide 100 mg/m² orally for 14 days, and doxorubicin 30 mg/m² I/V and fluorouracil 500 mg/m² I/V on days 1 and 8 in 28‐day cycles. Women randomised to the HDC group then received 1 cycle of high‐dose CTM (cyclophosphamide 6 gm/m² and thiotepa 800 mg/m², continuously for 4 days with autologous stem cell support. | |
| Outcomes | Event‐free survival, overall survival, time to recurrence, toxicity | |
| Notes | Data immature: 6‐year results in our tables based on percentage survival figures at median follow‐up 6.1 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | 511/540 (95%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | "The primary analysis was originally planned to include the subgroup of eligible patients. However, owing to the high rates of ineligibility, this policy was reviewed in July 1999, whereupon we decided to divide protocol violations into major and minor categories and to include patients with minor violations in the primary analysis." |
| Methods | Randomised controlled trial Country: Germany | |
| Participants | INCLUDED: | |
| Interventions | All women had 4 cycles of EC (epirubicin 90 mg/m², cyclophosphamide 600 mg/m²). Women randomised to the HDC group then received 1 cycle of high‐dose CTM (cyclophosphamide 6 gm/m² , thiotepa 600 mg/m², mitoxanthrone 40 mg/m²) with PBPC support Application of radiotherapy not specified in protocol until 1998: from then on, radiotherapy recommended after both mastectomy and breast ‐conserving surgery, to start within 3 ‐ 6 weeks postoperatively | |
| Outcomes | Event‐free survival Toxicity | |
| Notes | Power calculation: 320 participants would give 80% power to detect an improvement in the 5‐year event‐free survival rate from 25% to 40% (P = 0.05) Data are immature: 4‐year data in our tables based on results at median 3.8 year follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization code was produced by the statistical center using a computerized random‐number generator. The clinical center was used as a stratification criterion, and within each center block, randomization with varying block size was performed." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomised treatment was communicated centrally by phone after registration of the patient, guaranteeing concealment of the randomised treatment." |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "Obviously, blinding was not possible, and the statistician was also aware of the treatment." This appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | 302/307 (98%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | More women in the high‐dose arm than in the control arm had in excess of 16 positive lymph nodes (52% compared to 38% in the control arm) |
| Methods | Randomised controlled trial Countries: Australia, New Zealand, Italy, Switzerland, Hong Kong, Slovenia | |
| Participants | INCLUDED: | |
| Interventions | HDC arm had 3 cycles of epirubicin 200 mg/m² and cyclophosphamide 4 gm/m² with PBPC support | |
| Outcomes | Event‐free survival | |
| Notes | Data immature: 8‐year data in our tables based on 8‐year estimates reported by trialists at median follow‐up 8.3 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted centrally (at the coordinating centres in Bern, Switzerland, and Sydney, Australia). A permuted blocks randomization schedule was produced by use of pseudorandom numbers generated by a congruence method." |
| Allocation concealment (selection bias) | Low risk | Carried out by central data centre |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
| Methods | Randomised controlled trial Countries: UK, Italy, Spain, Australia | |
| Participants | INCLUDED: Women with primary breast cancer, T1 ‐ T4, aged 60 or less, with at least 4 positive axillary nodes after complete surgical resection and no metastatic disease on bone scan | |
| Interventions | All women had 1 3‐week cycle of FEC (cyclophosphamide 600 mg/m², epirubicin 50 mg/m², fluorouracil 600 mg/m²) followed by 2 4‐week cycles of FEC (as above but 2 doses per cycle) | |
| Outcomes | Disease‐free survival | |
| Notes | Power calculation: 300 participants would show an improvement from 30% ‐ 45% in 5‐year survival with 80% power (α = 0.05) Accrual failed following early reports from other trials Data immature: 5‐year data in our tables based on 5‐year estimates by trialists at median 50 months follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Centres randomised their patients by telephoning the ICCG Data Centre. The randomisation method used was adapted minimisation, where the weighted probabilities ensure a random component to the allocation. Stratification factors were centre, menopausal status and number of axillary nodes involved (4–9, 10+)". |
| Allocation concealment (selection bias) | Low risk | By telephone to central data centre |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | 279/281 (99%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
| Methods | Randomised controlled trial Country: Japan | |
| Participants | INCLUDED: | |
| Interventions | After randomisation, all women had 6 cycles of CAF (cyclophosphamide 500 mg/m², adriamycin 40 mg/m², fluorouracil 500 mg/m²) | |
| Outcomes | Relapse‐free survival | |
| Notes | 1. Power calculation: 90% power to detect 30% increase in relapse‐free survival at 5 years of a 60% power to detect a 20% increase (P = 0.05) (1‐sided logrank) Data immature ‐ median follow‐up 5.25 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible patients were randomly assigned to the STD or HDC arm at the time of enrolment by |
| Allocation concealment (selection bias) | Low risk | Allocation by phone call to centralised trial office |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | 95/97 (98%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
| Methods | Randomised controlled trial Country: Italy | |
| Participants | INCLUDED: | |
| Interventions | Women randomised to the high‐dose arm received 1 cycle of cyclophosphamide 7 gm/m², then 1 cycle of methotrexate 8 gm/m², then 2 cycles of epirubicin 120 mg/m², then 1 cycle of thiotepa 600 mg/m² plus melphalan 160 ‐ 180 mg/m² plus PBPC transplant | |
| Outcomes | Progression‐free survival Toxicity | |
| Notes | Power calculation: 80% power to detect a 15% increase in progression‐free survival at 5 years Progression‐free survival not defined; this outcome is reported as event‐free survival in this review 12‐year data in our tables based on percentages for survival reported by trialists at median follow‐up 136 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Low risk | Allocation by fax to centralised trial office |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | 382/398 (96%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
| Methods | Randomised controlled trial Country: USA | |
| Participants | INCLUDED: | |
| Interventions | Women entered the trial in 2 ways: | |
| Outcomes | Time to relapse | |
| Notes | Power calculation: Needed 40 participants in each arm to 80% power to detect 30% improvement in 3‐year relapse‐free survival (P = 0.05) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomization was performed by remote computer access; blocks of four patients (1122, 1221, 1212, 2121, etc.) were used in random order to ensure balance between the two treatment arms." |
| Allocation concealment (selection bias) | Low risk | Remote allocation. "Access to the computerized randomization program was restricted to research nurses and was not available to treating physicians." |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | 10/39 women randomised to high‐dose arm did not receive high‐dose chemo |
| Methods | Randomised control trial Number of dropouts pre‐randomisation: Not stated Country: USA | |
| Participants | Women with breast cancer who had completed modified radical mastectomy or breast‐conserving surgery with axillary dissection within 12 weeks of registration. Initially, the study included patients with four to nine involved lymph nodes. In March 2000, the study was amended to include patients with 10 or more involved lymph | |
| Interventions | High‐dose arm: doxorubicin and cyclophosphamide X 4 followed by high‐dose STAMP I or STAMP V (depending on centre) with autograft STAMP I consisted of cyclophosphamide 1.85 g/m²/d and cisplatin 55 mg/m²/d, each for 3 days (days 6, 5, and 4), followed by carmustine 600 mg/m² (day 3). STAMP V consisted of cyclophosphamide 1.5 g/m²/d, carboplatin 200 mg/m²/d, and thiotepa 125 mg/m²/d for 4 days (days 7 through 4). Control arm: doxorubicin, paclitaxel and cyclophosphamide X 3 of each in intensive sequential doses supported by GCSF | |
| Outcomes | Disease‐free survival Overall survival Toxicity | |
| Notes | Initial plan was to enrol 1000 women as approximately 350 events were required to achieve 90% power to be able to detect 45% improvement in the transplantation arm. The process of enrolment began in July 1996 and was stopped in February 2001 as the data from transplantation trials in breast cancer were not very encouraging 5‐year data immature; median follow‐up 70 months (max 102 months) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
| Methods | Randomised controlled trial Country: France | |
| Participants | INCLUDED: | |
| Interventions | All women received 4 cycles of FEC (fluorouracil 500 mg/m²; cyclophosphamide 500 mg/m²; epirubicin 100 mg/m²) | |
| Outcomes | Event‐free survival | |
| Notes | Power calculation: 90% power to detect a 20% increase in disease‐free survival at 3 years (P = 0.05) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
| Methods | Randomised controlled trial Allocation concealment: Done centrally by telephone or fax Country: Germany | |
| Participants | INCLUDED: * One man was randomised: it is unclear whether this was a breach of protocol | |
| Interventions | All women received 2 cycles of EC (cyclophosphamide 600 mg/m²; epirubicin 90 mg/m²) 2 weeks apart with GCSF priming | |
| Outcomes | Event‐free survival Quality of life: European Organisation for Research and Treatment of Cancer quality‐of‐life C30 questionnaire (only administered to "about" the first 200 participants) | |
| Notes | Power calculation: 80% power to detect a 10% absolute reduction in event‐free survival after 3 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remotely generated, with a random permuted‐block design and stratification by tumour size (<4 cm or >/=4 cm) and by centre." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was done centrally by telephone or fax in the WSG study office" |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
CAF = cyclophosphamide, doxorubicin & fluorouracil
CDC = Conventional adjuvant chemotherapy
CMF = Cyclophosphamide, Methotrexate and 5‐Fluorouracil
CXR = chest X‐ray
EC = epirubicin & cyclophosphamide
EORTC = European Organisation for Research and Treatment of Cancer
FEC = fluorouracil, epirubicin & cyclophosphamide
GCSF = Granulocyte colony‐stimulating factor
HDC = High‐dose chemotherapy
LVE = Left ventricular ejection fraction (cardiac function test)
PBPC = Peripheral blood progenitor cells
PPC = Peripheral blood progenitor cells
STAMP I = cyclophosphamide, cisplatin and carmustine
STAMP V = cyclophosphamide, carboplatin and thiotepa
US = ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This study did not have a control group receiving conventional‐dose chemotherapy: both arms of the study were treated with experimental therapies. Participants with bony micro‐metastases were not excluded from the study. | |
| This study was formally withdrawn from the scientific domain at the request of the University of Witwatersrand in South Africa in February 2000 after an investigation into possible serious breaches of scientific honesty and integrity. The data presented at ASCO 1999 are incorrect. | |
| Not an RCT |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Isaacs |
| Methods | |
| Participants | 4 ‐ 9 positive lymph nodes |
| Interventions | High‐dose arm: adriamycin X 4 then high‐dose cyclophosphamide, then 1 cycle of STAMP with autograft |
| Outcomes | Disease‐free survival |
| Starting date | Accrued 1996 ‐ 98 |
| Contact information | |
| Notes | Some preliminary data presented ASCO 1999 but not available for review. |
| Trial name or title | BCIRG 002 |
| Methods | |
| Participants | 4+ positive lymph nodes |
| Interventions | High‐dose arm: TAC X 4, then high‐dose mitoxantrone, cyclophosphamide and vinorelbine |
| Outcomes | Disease‐free survival |
| Starting date | |
| Contact information | Chuck Vogel; Miguel Martin |
| Notes | Enrolled: 476 |
| Trial name or title | Pegase 06 |
| Methods | |
| Participants | 8+ positive lymph nodes |
| Interventions | High‐dose arm: High‐dose EC X 4 |
| Outcomes | |
| Starting date | December 2000 |
| Contact information | Prof. P. Pouillart, Institute Curie Paris |
| Notes | Target population: 400 |
| Trial name or title | |
| Methods | |
| Participants | ? 10+ positive lymph nodes |
| Interventions | High‐dose arm: EX X 3 then high‐dose cyclophosphamide, carboplatin, thiotepa and mitoxantrone |
| Outcomes | |
| Starting date | |
| Contact information | Dr Seeber, West German Cancer Center of Essen |
| Notes | For possible submission for publication 2003 |
HDC = High‐dose chemotherapy
AC = doxorubicin
CAF = cyclophosphamide, doxorubicin and fluorouracil
CMF = cyclophosphamide, methotrexate and fluorouracil
GCSF = Granulocyte colony‐stimulating factor
EC = epirubicin and cyclophosphamide
FEC = fluorouracil, epirubicin and cyclophosphamide
CET = cyclophosphamide, epirubicin and thiotepa
STAMP I = cyclophosphamide, cisplatin and carmustine
STAMP V = cyclophosphamide, carboplatin and thiotepa
TAC = docetaxel, doxorubicin & cyclophosphamide
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 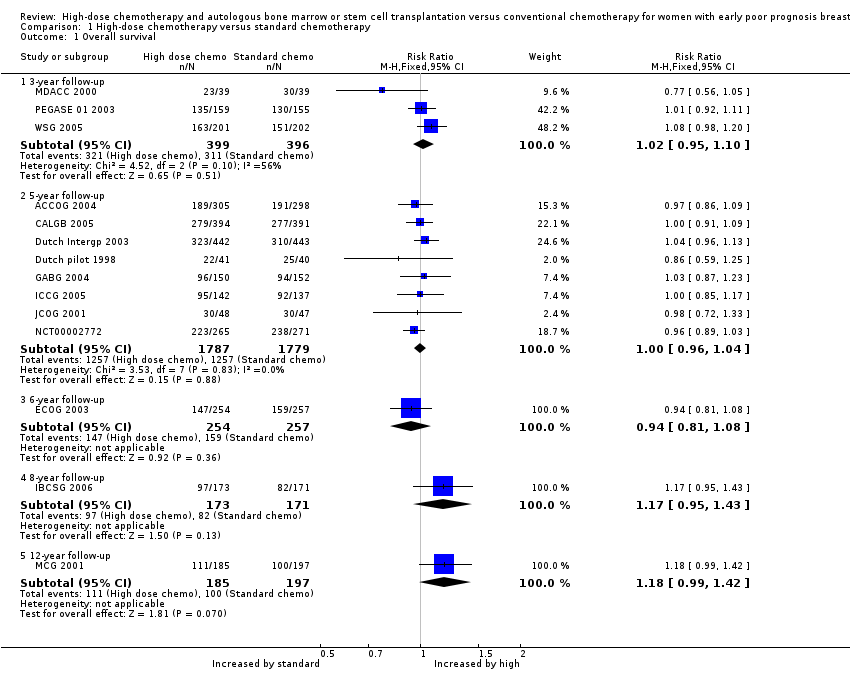 Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 1 Overall survival. | ||||
| 1.1 3‐year follow‐up | 3 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.10] |
| 1.2 5‐year follow‐up | 8 | 3566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.04] |
| 1.3 6‐year follow‐up | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.08] |
| 1.4 8‐year follow‐up | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.95, 1.43] |
| 1.5 12‐year follow‐up | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.42] |
| 2 Event‐free survival Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 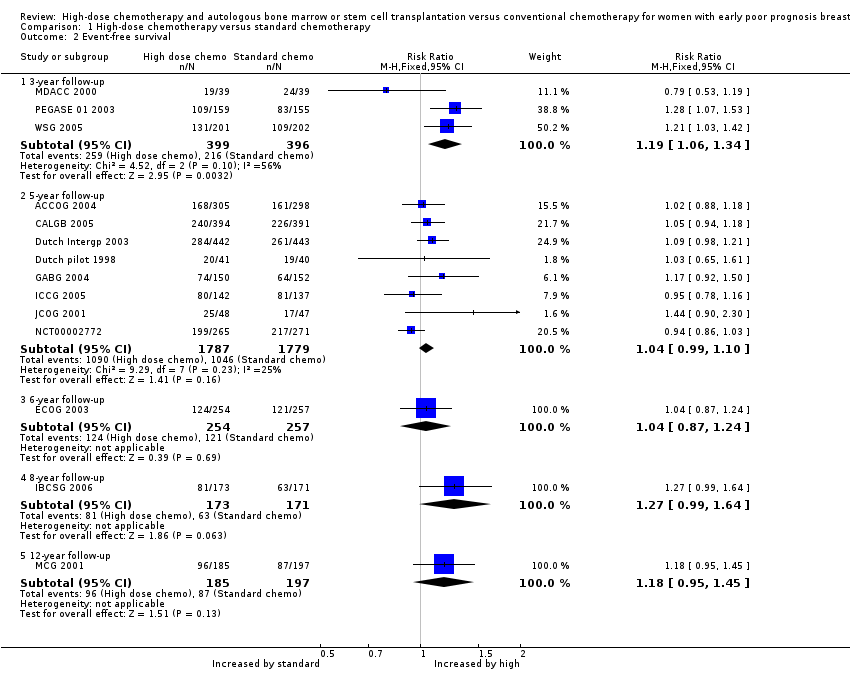 Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 2 Event‐free survival. | ||||
| 2.1 3‐year follow‐up | 3 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.06, 1.34] |
| 2.2 5‐year follow‐up | 8 | 3566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.99, 1.10] |
| 2.3 6‐year follow‐up | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.24] |
| 2.4 8‐year follow‐up | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.99, 1.64] |
| 2.5 12‐year follow‐up | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.45] |
| 3 Treatment‐related mortality Show forest plot | 14 | 5600 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.97 [3.99, 15.92] |
| Analysis 1.3  Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 3 Treatment‐related mortality. | ||||
| 4 Second cancers Show forest plot | 7 | 3423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.90, 1.73] |
| Analysis 1.4  Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 4 Second cancers. | ||||
| 4.1 By median 4‐ to 5‐year follow‐up | 2 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.61, 8.99] |
| 4.2 By median 6‐year follow‐up | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.75, 3.78] |
| 4.3 By median 7‐year follow‐up | 3 | 1751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.51] |
| 4.4 By median 8‐ to 9‐year follow‐up | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.61, 14.49] |
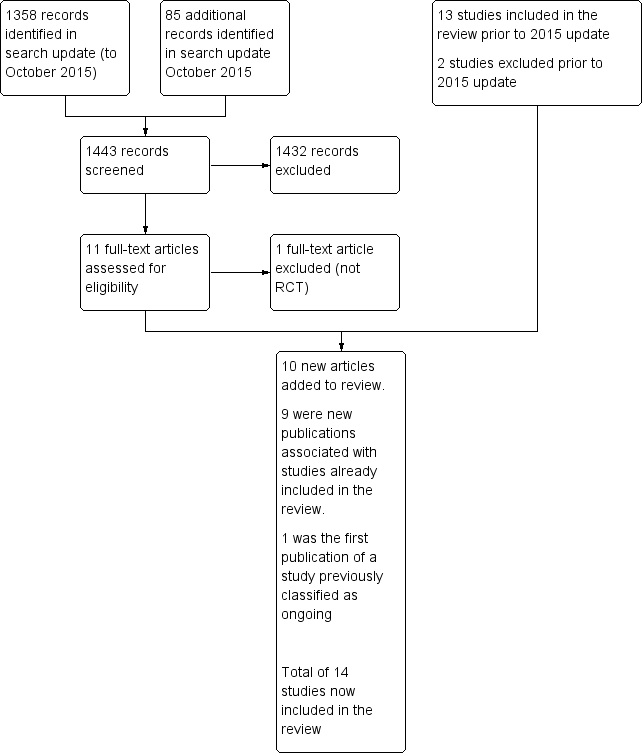
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
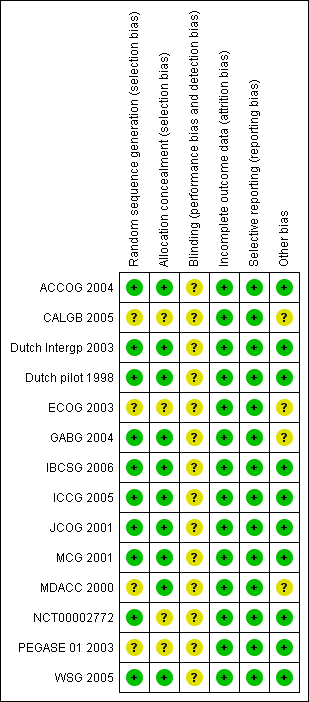
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 High‐dose chemotherapy versus standard chemotherapy, outcome: 1.1 Overall survival.

Forest plot of comparison: 1 High‐dose chemotherapy versus standard chemotherapy, outcome: 1.2 Event‐free survival.

Funnel plot of comparison: 1 High‐dose chemotherapy versus standard chemotherapy, outcome: 1.3 Treatment‐related mortality.

Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 1 Overall survival.
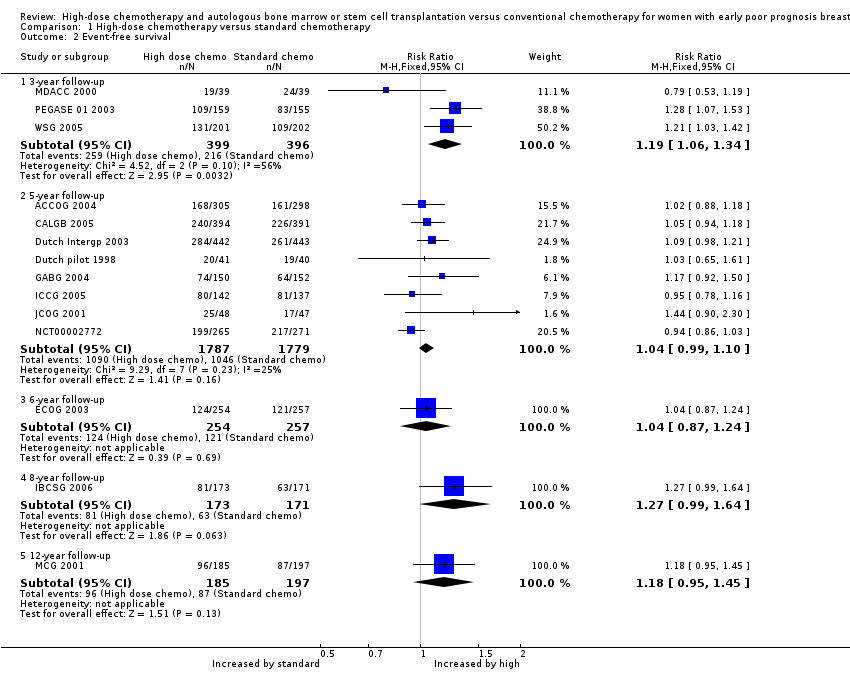
Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 2 Event‐free survival.

Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 3 Treatment‐related mortality.

Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 4 Second cancers.
| High‐dose chemotherapy versus chemotherapy without bone marrow transplant or stem cell rescue | ||||||
| Population: women with early poor prognosis breast cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard chemotherapy | Risk with high dose chemotherapy | |||||
| Overall survival at 5‐year follow‐up | 672 per 1000 | 672 per 1000 | RR 1.00 | 3566 (8 RCTs) | ⨁⨁⨁⨁ | |
| Event‐free survival at 5‐year follow‐up | 578 per 1000 | 601 per 1000 | RR 1.04 | 3566 | ⨁⨁⨁⨁ | |
| Treatment‐related mortality | 2 per 1000 | 14 per 1000 | RR 7.97 | 5600 | ⨁⨁⨁⨁ | Most deaths occurred within the first year of treatment |
| Second cancers at 4 ‐ 9‐year median follow‐up | 25 per 1000 | 31 per 1000 | RR 1.25 | 3423 | ⨁⨁⨁⨁ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the median risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| Study ID | Median Age | Tumour | Median nodes positive | Minimum nodes positive | > 9 nodes | Oestro positive | Progest. positive | Other | Premenop'sal |
| 45 | 3 cm max. | 9 | 4 | 45% | 31% (ER or PR +ve) | 31% (ER or PR +ve) | 43% receptor unknown | ‐ | |
| 45 | 3 cm median | 14 (range 10 ‐ 52) | 10 | 100% | 69% | ‐ | ‐ | ‐ | |
| 45 | T1 5%; T2 30%; T3 45%; T4 10%; Tx 10% | ‐ | N/A: Had pre‐op chemo | N/A | 20% | 25% | 54% receptor unknown | 83% | |
| 45.7 | T1 22%; T2 60%; T3 16% | ‐ | 4 | 35.8% | 65% | 53% | 28% oestrogen receptor negative | ‐ | |
| 44 | ‐ | ‐ | 10 | ‐ | 60% | 59% | 46% > 14 +ve nodes | 72% | |
| ‐ | ‐ | ‐ | 10 | 100% | 60% | 40% | ‐ | 58% | |
| 46 | T1 26%; T2 51%; T3 20% | 13 | 5 ‐ 10 depending on other prognostic factors | 73% | ‐ | ‐ | 40% oestrogen & progesterone receptor ‐ve | 67% | |
| 47 (range 24 ‐ 60) | T1 28%; T2 54%; T3 14%; unknown 4% | 9 (range 4 ‐ 36) | 4 | 45% | 43% | 25% | 38% receptor status not known | 70% | |
| 46 | ‐ | 16 (range 10 ‐ 49) | 10 | 100% | ‐ | ‐ | ‐ | 74% | |
| 45 | ‐ | ‐ | 10 at diagnosis or 4 after initial chemo | > 60% | 50% | 45% | 5% receptor unknown | 68% | |
| ‐ | ‐ | ‐ | 4 | 62% | ‐ | ‐ | ‐ | ‐ | |
| 46 (mean) | ‐ | 13 | 8 | ? | 31% | ‐ | ‐ | 68% | |
| Not stated. 45% were aged 40 ‐ 49 yrs | 20% had T3 tumour | 8% were N2 | ‐ | ‐ | ‐ | ‐ | 66% ER/PgR +ve; 8% receptor unknown | ‐ | |
| 47 | Mean size 3.3 ‐ 3.5 cm | 17 ‐ 18 | 10 | 100% | 63% | ‐ | ‐ | 53% | |
| +ve = positive | |||||||||
| Stage | What stage means |
| I | Breast tumour 2 cm or less in diameter and does not appear to have spread beyond the breast |
| IIA | Breast tumour over 2 cm in diameter OR has spread to the axillary (underarm) lymph nodes on the same side as the breast cancer. The nodes are not stuck to one another or to the surrounding tissues |
| IIB | Breast tumour over 2 cm in diameter AND has spread to the axillary nodes on the same side as the breast cancer. The nodes are not stuck together or to the surrounding tissues. OR the tumour is larger than 5 cm in diameter (and nodes are clear) |
| IIIA | Breast tumour over 5 cms in diameter AND has spread to the axillary lymph nodes on the same side OR tumour has spread to the lymph nodes on the same side as the breast cancer and the nodes are stuck to each other or to the surrounding tissues |
| IIIB | Breast tumour has spread to chest wall or skin OR tumour has spread to internal mammary lymph nodes on the same side as breast tumour |
| IV | Tumour has spread from breast to distant sites or to supraclavicular (above collarbone) lymph nodes |
| Study | Phase 1 | Phase 2 |
| doxorubicin 75 mg | cyclophosphamide | |
| cyclophosphamide 600 mg | cyclophosphamide 900 mg | |
| cyclophosphamide 500 mg | ‐ | |
| cyclophosphamide 500 mg | ‐ | |
| cyclophosphamide 1400 mg (po) | ‐ | |
| cyclophosphamide 600 mg | cyclophosphamide 1 gm | |
| doxorubicin 60mg or epirubicin 90 mg | cyclophosphamide 1400 mg (po) | |
| cyclophosphamide 600 mg | cyclophosphamide 1200 mg | |
| cyclophosphamide 500 mg | ‐ | |
| epirubicin 120 mg 3 cycles | cyclophosphamide 600 mg | |
| cyclophosphamide 500 mg | ‐ | |
| cyclophosphamide 500 mg | ‐ | |
| sequential administration of 3 cycles each of doxorubicin 80 mg/m², paclitaxel 200 mg/m², and cyclophosphamide | ‐ | |
| cyclophosphamide 600 mg | cyclophosphamide 600 mg |
| Study | Initial phase | High‐dose cycle 1 | High‐dose cycle 2 | High‐dose cycle 3 | High‐dose cycle 4 | Regimen |
| 4 cycles of doxorubicin (as control arm) followed by: | cyclophosphamide 4 gm | cyclophosphamide 6 gm | ‐ | ‐ | Divided doses over 4 days | |
| 4 cycles of cyclophosphamide, doxorubicin and fluorouracil (as control arm) followed by: | cyclophosphamide 5.625 gm | ‐ | ‐ | ‐ | Divided doses over 3 days | |
| 4 cycles of cyclophosphamide, epirubicin and fluorouracil (doses as control arm) followed by: | cyclophosphamide 6 gm | ‐ | ‐ | ‐ | Divided doses over 4 days | |
| 4 cycles of cyclophosphamide, epirubicin and fluorouracil (as control arm) followed by: | cyclophosphamide 6 gm | ‐ | ‐ | ‐ | Divided doses over 4 days | |
| 6 cycles of cyclophosphamide, doxorubicin and 5FU (as control arm) followed by: | cyclophosphamide 6 gm | ‐ | ‐ | ‐ | Continuous infusion over 4 days | |
| 4 cycles of cyclophosphamide and epirubicin (as control arm) followed by: | cyclophosphamide 6 gm | ‐ | ‐ | ‐ | Divided doses over 4 days | |
| No common path with control group protocol | epirubicin 200 mg | As cycle 1 | As cycle 1 | 3 X 21‐day cycles | ||
| 2 cycles of cyclophosphamide, epirubicin and fluorouracil (as control arm cycles 1 and 2) | cyclophosphamide 6 gm | ‐ | ‐ | ‐ | Continuous infusion over 4 days | |
| 6 cycles of cyclophosphamide, doxorubicin and fluorouracil (as control arm), followed by: | cyclophosphamide 6 gm | ‐ | ‐ | ‐ | ‐ | |
| No common path with control group protocol | cyclophosphamide 7 gm | methotrexate 8gm | epirubicin 120 mg X 2 | thiotepa 600 mg melphalan 160 ‐ 180 mg | 4 high‐dose treatments in sequence | |
| 8 cycles of cyclophosphamide, doxorubicin and fluorouracil (as control arm), followed by: | cyclophosphamide 5.25 gm | As cycle 1 | ‐ | ‐ | Divided doses over 3 days. 2nd cycle given when haematologically safe | |
| 4 cycles of cyclophosphamide, epirubicin and fluorouracil (as control arm), followed by: | cyclophosphamide 120 mg | ‐ | ‐ | ‐ | ‐ | |
| 4 cycles of doxorubicin 80 mg/m² and cyclophosphamide 600 mg/m² (AC) every 3 weeks | STAMP I or STAMP V HDC regimen. STAMP I consisted of cyclophosphamide 1.85 g/m²/d and cisplatin 55 mg/m²/d, followed by carmustine 600 mg/m²; STAMP V consisted of cyclophosphamide 1.5 g/m²/d, carboplatin 200 mg/m²/d, and thiotepa 125 mg/m²/d | ‐ | ‐ | ‐ | ‐ | |
| 2 cycles of cyclophosphamide and epirubicin (as control arm) | cyclophosphamide 3 gm | As cycle 1 | ‐ | ‐ | High‐dose cycles over 28 days |
| Study ID | Data maturity | Median follow‐up |
| No | 4 years | |
| No | 7.3 years | |
| 5 years | 6.9 years | |
| 3 years | 7 years | |
| No | 6.1 years | |
| No | 6.1 years | |
| No | 8.3 years | |
| No | 4.2 years | |
| No | 63 months | |
| 3 years | 11.9 years | |
| No | 11.33 years | |
| 3 years | 3.25 years | |
| No | 5.8 years | |
| 3 years | 4 years |
| Study ID | Haemopoietic | Gastrointestinal | Pulmonary | Cardiac events | Neurological | Other toxicity | Late/ long term | Second cancers | Trialist's summary |
| Standard chemo: Grade 4 neutropenia 15% | Haemorrhage ≥ grade 2: | Nausea ≥ grade 3: | Rhythm toxicity ≥ grade 2: | Cortical neurotoxicity ≥ 1 | Both trial arms: Menopausal symptoms common. | ‐ | ‐ | ‐ | |
| Leukopenia and thrombocytopenia common in both groups but more severe and persistent in HDC arm | ‐ | Toxicity ≥ grade 3: | ‐ | Toxicity ≥ grade 3: | Hepatic toxicity ≥ grade 3: | ‐ | By median 7.5 yrs High‐dose arm: 16 second cancers (4%) (including acute myeloid leukaemia or myelodysplatic syndrome 7; breast cancer 5) | ‐ | |
| High‐dose chemo: all hospitalised for 13 ‐ 30 days for haemopoietic recovery. Median neutropenic fever 5 days Standard chemo: neutropenic fever after 4% of cycles | High‐dose: mucositis 85% (severe in 22%), diarrhoea common. Standard chemo: Mild nausea and vomiting, mucositis (28% of cycles), diarrhoea (4% of cycles) | ‐ | See long‐term events | ‐ | Both arms: alopecia 100%, fatigue common, lymphoedema of arm in 20% High‐dose: ovarian failure 100%, radiation pneumonitis 10%, Standard dose: radiation pneumonitis 2% | High‐dose arm: 1 case hypothyroidism, 1 case auto‐antibody production | At median follow‐up of 7 years: | High‐dose: "Moderately well tolerated but substantial though reversible toxic effects". Standard dose: "Mild toxicity" | |
| High‐dose: transfusion‐dependent 100% | High‐dose: nausea and vomiting 100% | ‐ | High‐dose: cardiac arrhythmia 1/442, possible heart failure 1/442 | ‐ | High‐dose: high fever (necessitating early termination of treatment): 4 women (1%) | ‐ | By median follow‐up 7 years: | High‐dose: "Well tolerated" | |
| High‐dose: leukopenia 98%, granulocytopenia 94%, thrombocytopenia 97%, anaemia 62%, | High‐dose: nausea 32%, vomiting 16%, diarrhoea 22%, stomatitis 37% Standard chemo: nausea 11%, vomiting 8%, stomatitis 4% (all grade 3 or 4) | Standard chemo: 1% (grade 3 or 4) | ‐ | Standard chemo: 6% (grade 3 or 4) | High‐dose: infection 21%, liver effects 13%, skin effects 11%, diabetes 14% Standard dose: hyperglycaemia 2%, phlebitis 1%, hepatotoxicity 1% (all grade 3 or 4) | ‐ | By median 6.1 years: | ‐ | |
| ‐ | High‐dose: Grade 3 or 4 gastrointestinal toxicity < 1%; | Grade 3 or 4 toxicity < 1% | ‐ | High‐dose: Grade 3 or 4 toxicity nil | High‐dose: Grade 3 or 4 toxicity: Bladder < 1%; kidney nil; liver nil | ‐ | ‐ | ‐ | |
| High‐dose: myelosuppression | High‐dose: nausea and vomiting; mucositis | ‐ | ‐ | ‐ | ‐ | Permanent amenorrhoea: High‐dose arm 77/95 (81% overall, age < 40 years 61%; age > 40 years 96%); Standard‐dose arm 61/98 (63% overall age < 40 years 24%; age > 40 years 84%) | By median 8.3 years: | High‐dose: Overall toxicities Grade 3 1%; Grade 4 98%; | |
| High‐dose: leucopenia and thrombocytopenia presumed 100% but nadir count not always available (grade 3 or 4) | High‐dose: nausea and vomiting 46%, mucositis 22% (grade 3 or 4) | High‐dose: Pulmonary embolus 1/143; respiratory failure requiring ventilator 1/143 | High‐dose: severe cardiac arrhythmia 2% (3/143) | ‐ | High‐dose: hair loss 100%, fever (no infection) 17%, infection 24%, "other" 28% (grade 3 or 4), deep vein thrombosis 1/143 | After chemotherapy: 227 toxic events occurred (127 in high‐dose arm, 110 in control arm), of which 30% related to tamoxifen. Of the others, 7 events deemed life‐threatening (5 in high‐dose group, 2 in control arm) | High‐dose: 2/143 (breast 1, ovarian 1) | ||
| High‐dose: All 34 women receiving HDC actually developed grade 4 leukopenia and grade 4 neutropenia; 27 (79%) developed grade 4 and the other 7 grade 3 thrombocytopenia. Standard dose: 7 women (8%) developed grade 4 neutropenia | High‐dose: vomiting 62%, diarrhoea 29%, mucositis 15%, (grade 3 or 4) | ‐ | High‐dose: grade 3 arrhythmia 3%, | ‐ | High‐dose: Grade 3 or 4 infection: 6% | ‐ | ‐ | ‐ | |
| High‐dose: Length of hospital stay not stated. Standard dose: 22% admitted with infection or fever | High‐dose: mild/moderate vomiting 80%, mild/moderate diarrhoea 58%, mild/moderate mucositis 83%. Standard dose: Nausea and vomiting moderate 75%, severe 16%. Diarrhoea moderate 19%, severe 8%. Mucousitis moderate 36%, severe 10% | High‐dose: 1 case (severe) | High‐dose: moderate/severe 8%. Standard dose: 1 woman (1%) had myocardial infarction | High‐dose: hearing loss 2 cases (6%) ‐ 1 permanent, mild/moderate peripheral neuropathy 11% | High‐dose: Renal: 25% (22% mild, < 3% severe), hepatic (mild/moderate) 31%, bladder (moderate) 25%, skin (mild) 8% | High‐dose: 1 case of avascular necrosis | High‐dose: 1 case of acute myeloid leukaemia | "Overall there was greater and more frequent morbidity associated with high dose chemotherapy" | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| High‐dose: 62% had haematologic toxicity during induction and 92% had it during transplantation. 3 women had myelodysplastic syndrome Controls: 59% had haematologic toxicity 2 women had myelodysplastic syndrome | ‐ | ‐ | ‐ | ‐ | ‐ | High‐dose: 44% of women experienced grade 3 or 4 nonhaematologic toxicity during induction while 80% experienced grade 3 or 4 nonhematologic toxicity during transplantation. Control arm: Approximately 63% experienced grade 3 or 4 nonhaematologic toxicity, most commonly fatigue, nausea and vomiting, infection, febrile neutropenia, mucositis, and sensory neuropathy | ‐ | High‐dose: 44% had | |
| ‐ | High‐dose arm: nausea 25%, mucositis 18%, diarrhoea 5% | High‐dose arm: 1% | High‐dose arm: 3% | ‐ | High‐dose arm: grade 3 or 4 skin toxicity 3%, amenorrhoea 100% | ‐ | ‐ | Both high‐dose chemotherapy and dose‐dense conventional chemotherapy are feasible with tolerable toxicity in a multicentre setting |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 3‐year follow‐up | 3 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.10] |
| 1.2 5‐year follow‐up | 8 | 3566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.04] |
| 1.3 6‐year follow‐up | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.08] |
| 1.4 8‐year follow‐up | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.95, 1.43] |
| 1.5 12‐year follow‐up | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.42] |
| 2 Event‐free survival Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 3‐year follow‐up | 3 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.06, 1.34] |
| 2.2 5‐year follow‐up | 8 | 3566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.99, 1.10] |
| 2.3 6‐year follow‐up | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.24] |
| 2.4 8‐year follow‐up | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.99, 1.64] |
| 2.5 12‐year follow‐up | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.45] |
| 3 Treatment‐related mortality Show forest plot | 14 | 5600 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.97 [3.99, 15.92] |
| 4 Second cancers Show forest plot | 7 | 3423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.90, 1.73] |
| 4.1 By median 4‐ to 5‐year follow‐up | 2 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.61, 8.99] |
| 4.2 By median 6‐year follow‐up | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.75, 3.78] |
| 4.3 By median 7‐year follow‐up | 3 | 1751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.51] |
| 4.4 By median 8‐ to 9‐year follow‐up | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.61, 14.49] |

