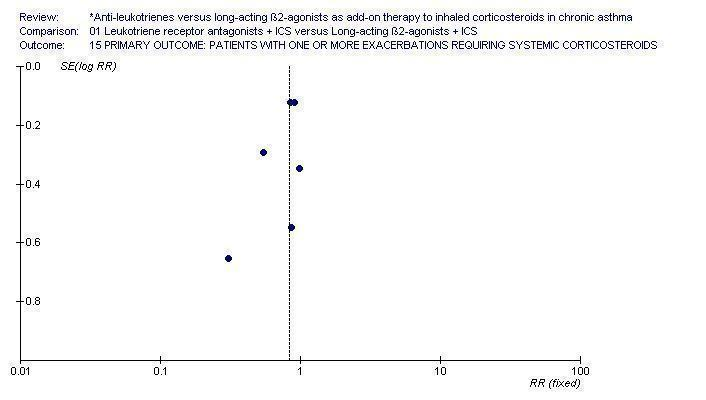
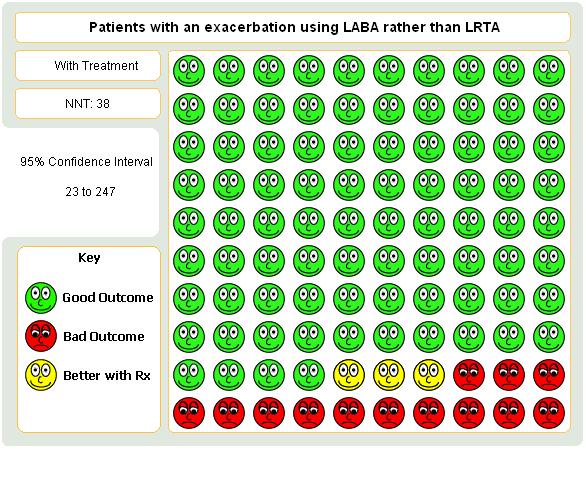
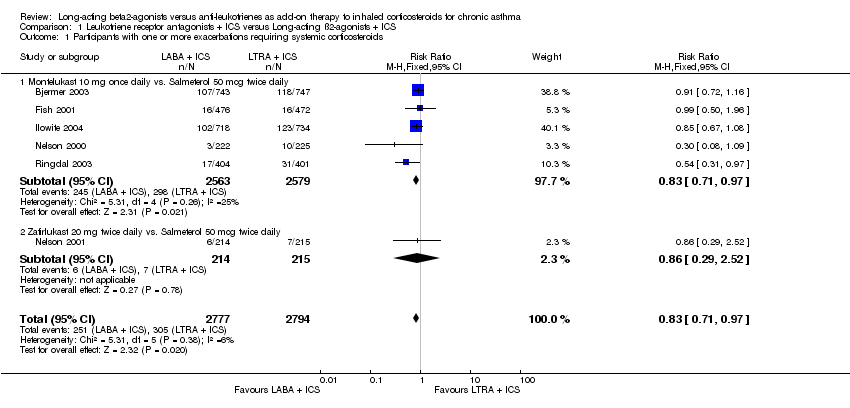
| 1 Participants with one or more exacerbations requiring systemic corticosteroids Show forest plot | 6 | 5571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.97] |
|
| 1.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 5 | 5142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.97] |
| 1.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.29, 2.52] |
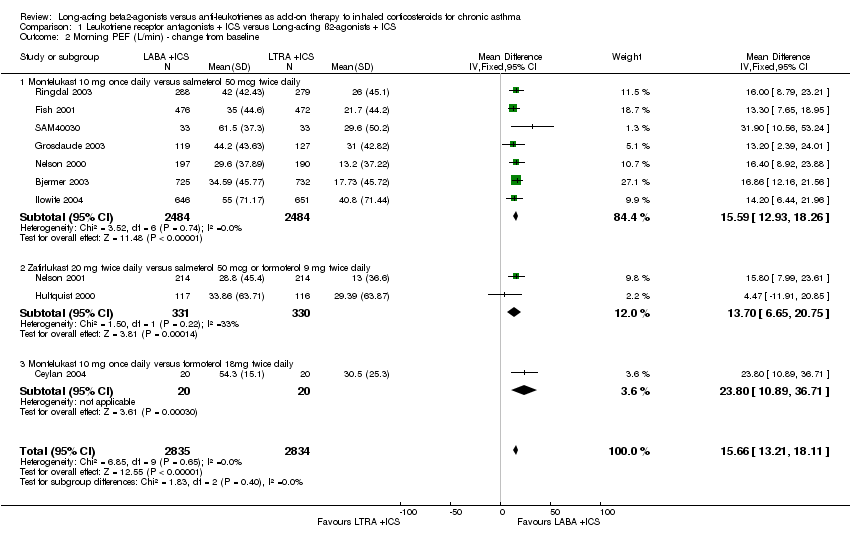
| 2 Morning PEF (L/min) ‐ change from baseline Show forest plot | 10 | 5669 | Mean Difference (IV, Fixed, 95% CI) | 15.66 [13.21, 18.11] |
|
| 2.1 Montelukast 10 mg once daily versus salmeterol 50 mcg twice daily | 7 | 4968 | Mean Difference (IV, Fixed, 95% CI) | 15.59 [12.93, 18.26] |
| 2.2 Zafirlukast 20 mg twice daily versus salmeterol 50 mcg or formoterol 9 mg twice daily | 2 | 661 | Mean Difference (IV, Fixed, 95% CI) | 13.70 [6.65, 20.75] |
| 2.3 Montelukast 10 mg once daily versus formoterol 18mg twice daily | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 23.80 [10.89, 36.71] |
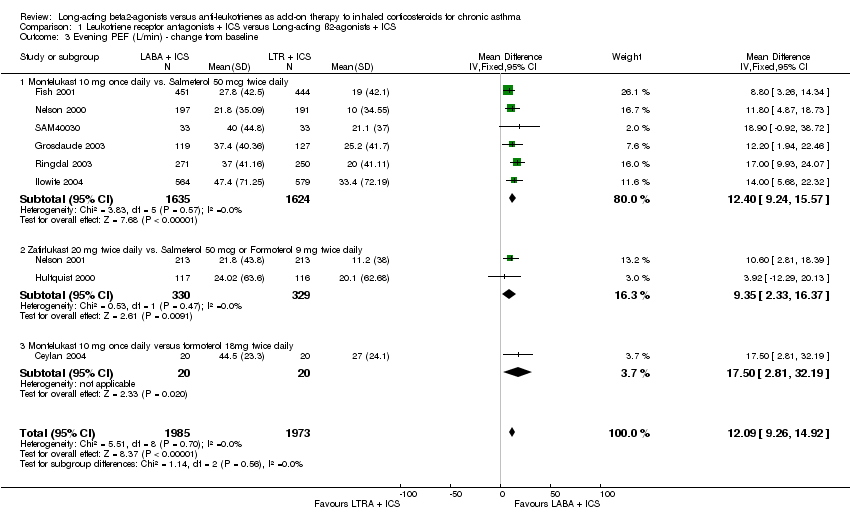
| 3 Evening PEF (L/min) ‐ change from baseline Show forest plot | 9 | 3958 | Mean Difference (IV, Fixed, 95% CI) | 12.09 [9.26, 14.92] |
|
| 3.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 6 | 3259 | Mean Difference (IV, Fixed, 95% CI) | 12.40 [9.24, 15.57] |
| 3.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg or Formoterol 9 mg twice daily | 2 | 659 | Mean Difference (IV, Fixed, 95% CI) | 9.35 [2.33, 16.37] |
| 3.3 Montelukast 10 mg once daily versus formoterol 18mg twice daily | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 17.5 [2.81, 32.19] |
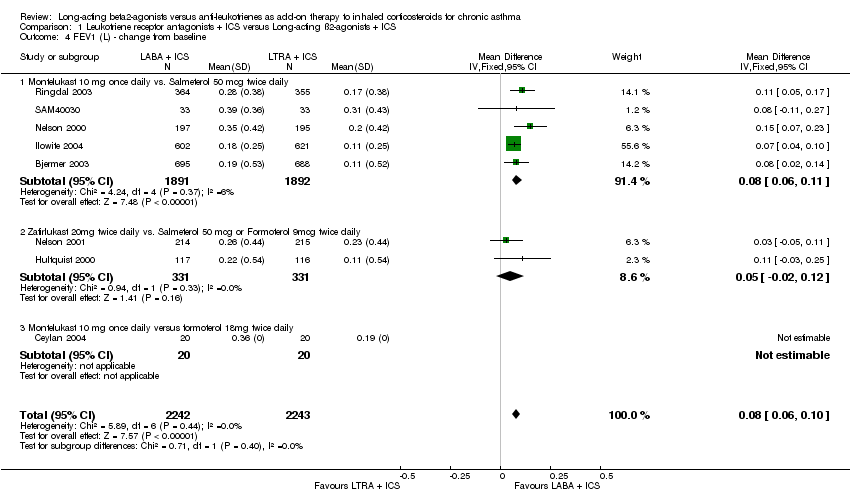
| 4 FEV1 (L) ‐ change from baseline Show forest plot | 8 | 4485 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.06, 0.10] |
|
| 4.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 5 | 3783 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.06, 0.11] |
| 4.2 Zafirlukast 20mg twice daily vs. Salmeterol 50 mcg or Formoterol 9mcg twice daily | 2 | 662 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.02, 0.12] |
| 4.3 Montelukast 10 mg once daily versus formoterol 18mg twice daily | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
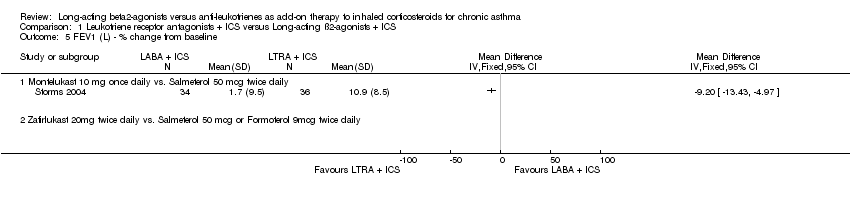
| 5 FEV1 (L) ‐ % change from baseline Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
|
| 5.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 1 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Zafirlukast 20mg twice daily vs. Salmeterol 50 mcg or Formoterol 9mcg twice daily | 0 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
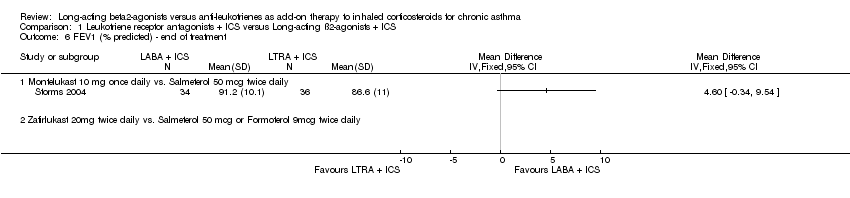
| 6 FEV1 (% predicted) ‐ end of treatment Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
|
| 6.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 1 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Zafirlukast 20mg twice daily vs. Salmeterol 50 mcg or Formoterol 9mcg twice daily | 0 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 % fall in FEV1 POST‐EXERCISE Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
|
| 7.1 Montelukast 10 mg once daily versus salmeterol 50 mcg twice daily | 1 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Zafirlukast 20mg twice daily versus.salmeterol 50 mcg or formoterol 9mcg twice daily | 0 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
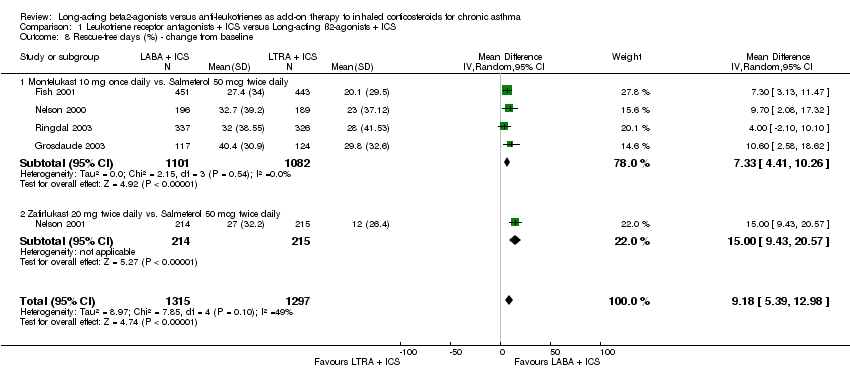
| 8 Rescue‐free days (%) ‐ change from baseline Show forest plot | 5 | 2612 | Mean Difference (IV, Random, 95% CI) | 9.18 [5.39, 12.98] |
|
| 8.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 4 | 2183 | Mean Difference (IV, Random, 95% CI) | 7.33 [4.41, 10.26] |
| 8.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Mean Difference (IV, Random, 95% CI) | 15.0 [9.43, 20.57] |
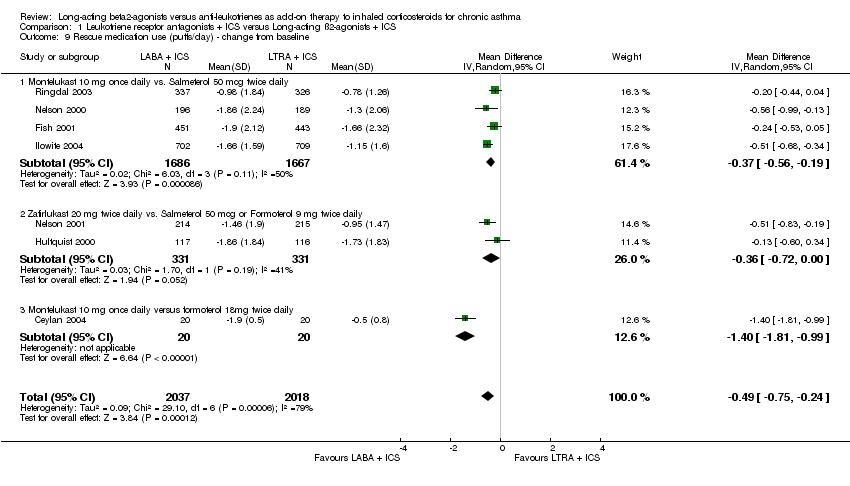
| 9 Rescue medication use (puffs/day) ‐ change from baseline Show forest plot | 7 | 4055 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.75, ‐0.24] |
|
| 9.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 4 | 3353 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.56, ‐0.19] |
| 9.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg or Formoterol 9 mg twice daily | 2 | 662 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.72, 0.00] |
| 9.3 Montelukast 10 mg once daily versus formoterol 18mg twice daily | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.4 [‐1.81, ‐0.99] |
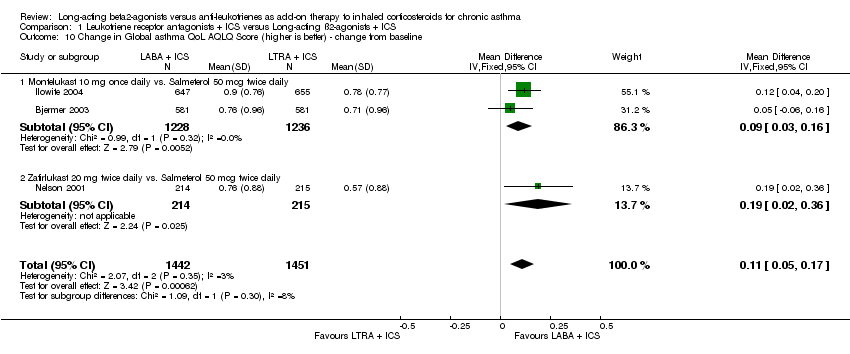
| 10 Change in Global asthma QoL AQLQ Score (higher is better) ‐ change from baseline Show forest plot | 3 | 2893 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.05, 0.17] |
|
| 10.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 2 | 2464 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.03, 0.16] |
| 10.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.02, 0.36] |
| 11 Symptom free days (%) ‐ change from baseline Show forest plot | 5 | 2626 | Mean Difference (IV, Random, 95% CI) | 6.75 [3.11, 10.39] |
|
| 11.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 4 | 2197 | Mean Difference (IV, Random, 95% CI) | 5.42 [1.80, 9.05] |
| 11.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Mean Difference (IV, Random, 95% CI) | 11.0 [6.10, 15.90] |
| 12 Night‐time symptom score (5pt scale, higher score is worse) ‐ change from baseline Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
|
| 12.1 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
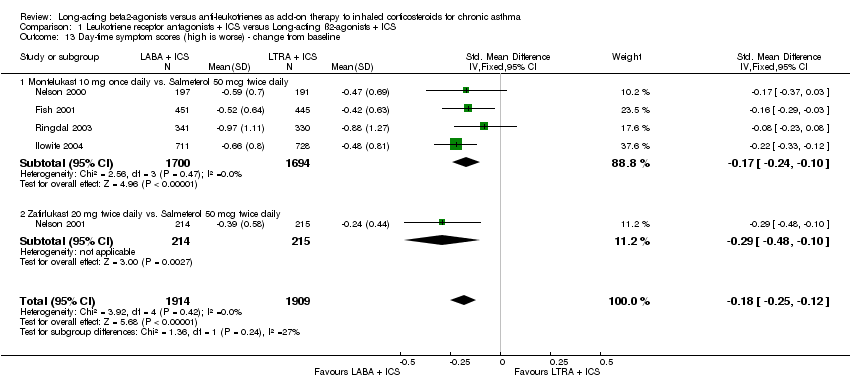
| 13 Day‐time symptom scores (high is worse) ‐ change from baseline Show forest plot | 5 | 3823 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.25, ‐0.12] |
|
| 13.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 4 | 3394 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.24, ‐0.10] |
| 13.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.48, ‐0.10] |
| 14 Morning symptoms ‐ change from baseline Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
|
| 14.1 Montelukast 10 mg once daily versus formoterol 18mg twice daily | 1 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
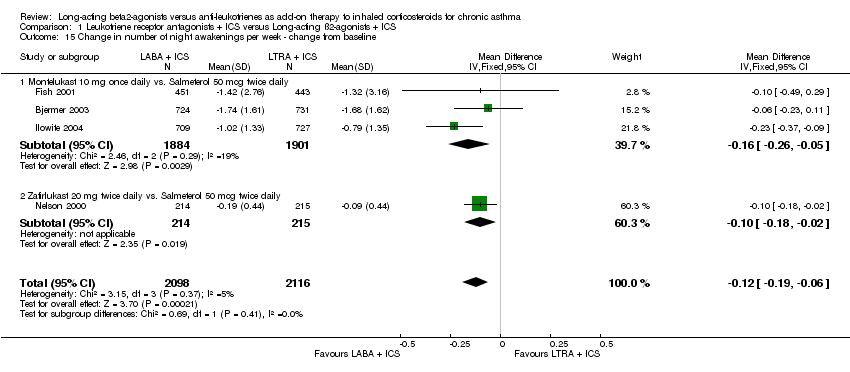
| 15 Change in number of night awakenings per week ‐ change from baseline Show forest plot | 4 | 4214 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.19, ‐0.06] |
|
| 15.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 3 | 3785 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.26, ‐0.05] |
| 15.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Mean Difference (IV, Fixed, 95% CI) | ‐0.1 [‐0.18, ‐0.02] |
| 16 Change in % of nights with no awakenings per week ‐ change from baseline Show forest plot | 2 | 673 | Mean Difference (IV, Fixed, 95% CI) | 6.89 [2.87, 10.91] |
|
| 16.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 1 | 244 | Mean Difference (IV, Fixed, 95% CI) | 6.60 [‐1.06, 14.26] |
| 16.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [2.28, 11.72] |
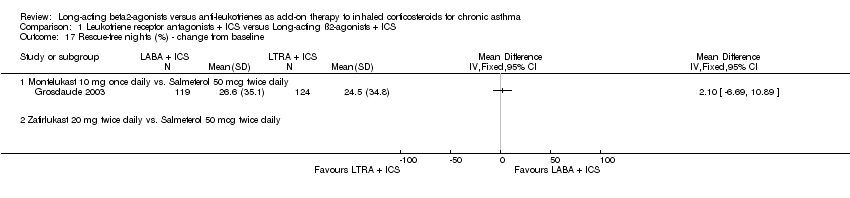
| 17 Rescue‐free nights (%) ‐ change from baseline Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
|
| 17.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 1 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 0 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Withdrawals for any reason Show forest plot | 10 | 6225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.73, 0.95] |
|
| 18.1 Montelukast 10mg/day vs Salmeterol 50ug twice daily | 8 | 5560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.97] |
| 18.2 Zafirlukast 20 mg twice daily vs Salmeterol 50 mcg twice daily | 2 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.40, 1.06] |
| 19 Withdrawals due to adverse events Show forest plot | 10 | 6225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.80, 1.32] |
|
| 19.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 8 | 5560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.79, 1.35] |
| 19.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 2 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.05] |
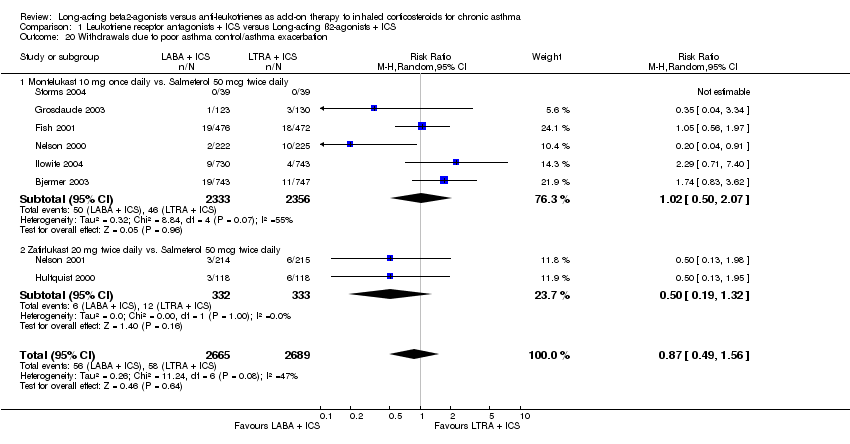
| 20 Withdrawals due to poor asthma control/asthma exacerbation Show forest plot | 8 | 5354 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.49, 1.56] |
|
| 20.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 6 | 4689 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.50, 2.07] |
| 20.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 2 | 665 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.19, 1.32] |
| 21 Patients with one or more exacerbations requiring hospital admission Show forest plot | 4 | 3993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.58, 2.98] |
|
| 21.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 4 | 3993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.58, 2.98] |
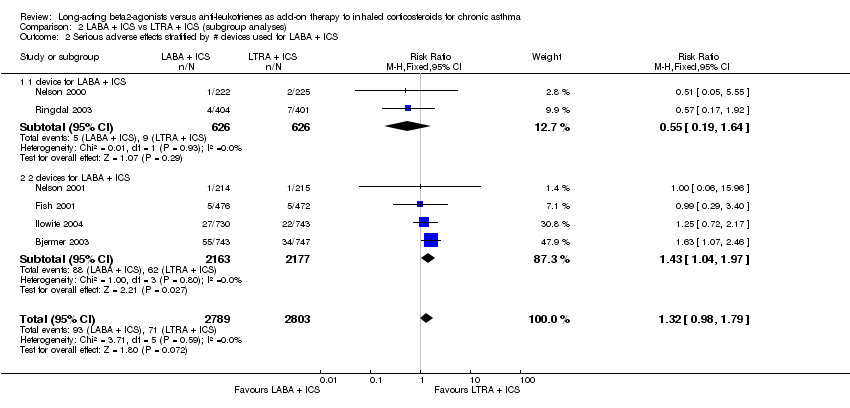
| 22 Serious Adverse events Show forest plot | 6 | 5592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.98, 1.79] |
|
| 22.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 5 | 5163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.98, 1.79] |
| 22.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.96] |
| 23 Death Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 23.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Headache Show forest plot | 10 | 6187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.90, 1.26] |
|
| 24.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 7 | 5482 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 24.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 2 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.57] |
| 24.3 Montelukast 10 mg once daily versus formoterol 18mg twice daily | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.90] |
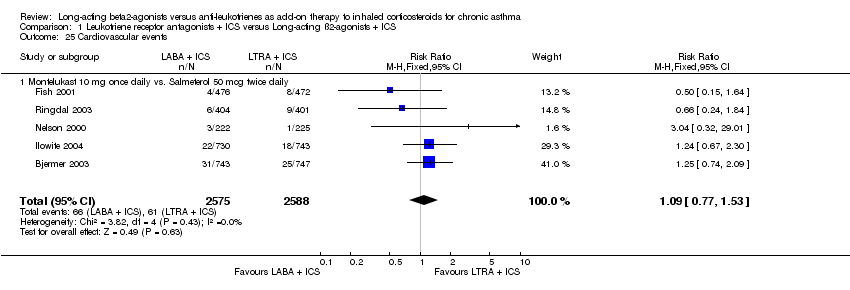
| 25 Cardiovascular events Show forest plot | 5 | 5163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.77, 1.53] |
|
| 25.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 5 | 5163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.77, 1.53] |
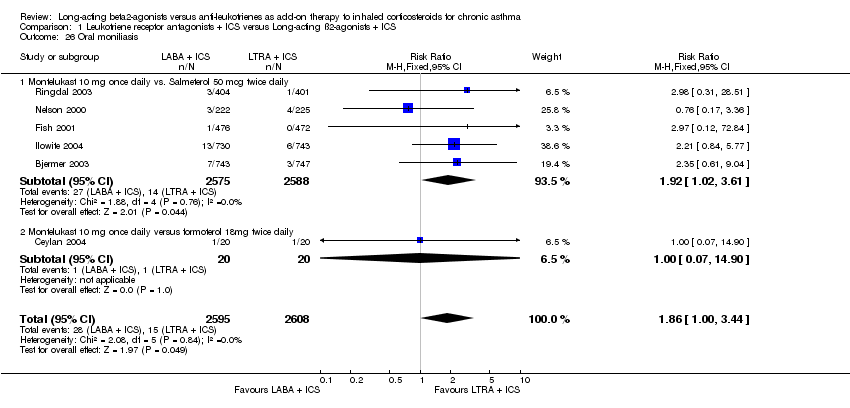
| 26 Oral moniliasis Show forest plot | 6 | 5203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.00, 3.44] |
|
| 26.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 5 | 5163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.02, 3.61] |
| 26.2 Montelukast 10 mg once daily versus formoterol 18mg twice daily | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.90] |
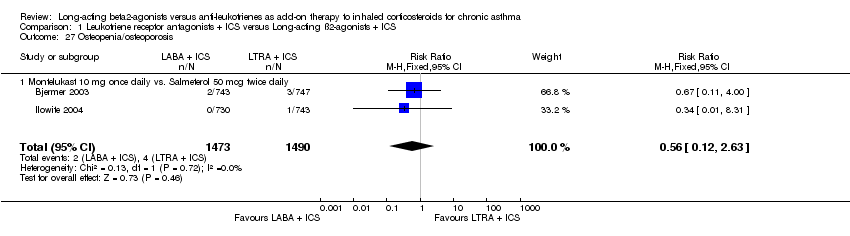
| 27 Osteopenia/osteoporosis Show forest plot | 2 | 2963 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.12, 2.63] |
|
| 27.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 2 | 2963 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.12, 2.63] |
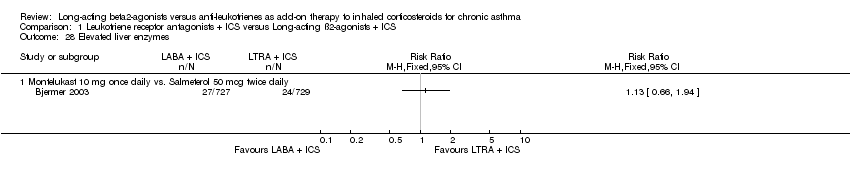
| 28 Elevated liver enzymes Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 28.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Overall adverse events Show forest plot | 8 | 5911 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
|
| 29.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 7 | 5482 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
| 29.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.81, 1.31] |
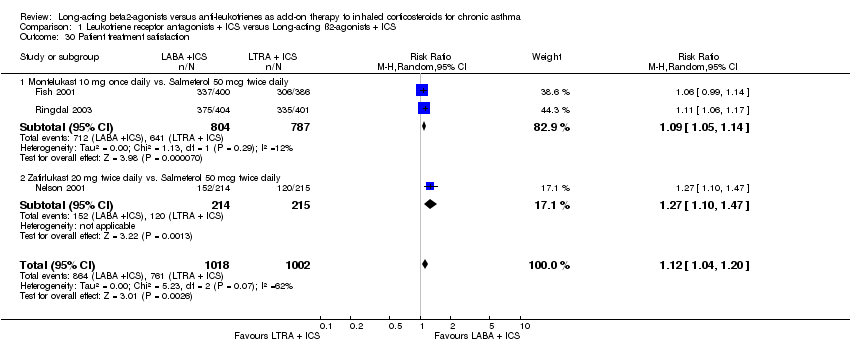
| 30 Patient treatment satisfaction Show forest plot | 3 | 2020 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.04, 1.20] |
|
| 30.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 2 | 1591 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.05, 1.14] |
| 30.2 Zafirlukast 20 mg twice daily vs. Salmeterol 50 mcg twice daily | 1 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.10, 1.47] |
| 31 Change from baseline in serum eosinophils ( x 10e9/L) Show forest plot | 2 | 2787 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.02, 0.05] |
|
| 31.1 Montelukast 10 mg once daily vs. Salmeterol 50 mcg twice daily | 2 | 2787 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.02, 0.05] |