Conduta ativa versus expectante para pré‐eclâmpsia grave entre 24 e 34 semanas de gestação
Resumo
Introdução
A pré‐eclâmpsia grave é uma causa importante de mortalidade e morbidade para a mãe e a criança, especialmente quando se instala entre 24 e 34 semanas, ou seja, muito tempo antes do término da gestação. A única cura conhecida para a doença é o parto. Alguns obstetras defendem o parto imediato para evitar o surgimento de complicações maternas graves, como a eclâmpsia (convulsões) e a insuficiência renal. Outros preferem uma conduta expectante, adiando o parto na tentativa de reduzir a morbidade e a mortalidade perinatal decorrentes da prematuridade.
Objetivos
O objetivo da revisão foi comparar os efeitos de uma conduta ativa e antecipação do parto com uma conduta expectante e postergação do parto, em gestantes com pré‐eclâmpsia grave de início precoce.
Métodos de busca
Procuramos no Cochrane Pregnancy and Childbirth Group's Trials Register (até 28 de fevereiro de 2013).
Critério de seleção
Ensaios clínicos randomizados comparando as duas condutas para gestantes com pré‐eclâmpsia grave de início precoce.
Coleta dos dados e análises
Dois autores da revisão selecionaram independentemente os estudos para inclusão, extraíram os dados e avaliaram o risco de viés. A acurácia dos dados dos estudos foi verificada.
Principais resultados
Foram incluídos nesta revisão quatro estudos, com um total de 425 mulheres. Os estudos tinham um baixo risco de viés quanto aos métodos de randomização e de ocultação da alocação, mas tinham alto risco de viés de cegamento; o risco de viés foi considerado como incerto para dados de desfechos incompletos e outros tipos de viés; e o risco de viés foi considerado baixo para relatos seletivos. Para a maioria dos desfechos maternos, os dados são insuficientes para conclusões confiáveis sobre os efeitos das duas condutas. Para os desfechos relativos à criança, não há evidências suficientes para conclusões confiáveis sobre os efeitos das diferentes condutas sobre a natimortalidade ou morte neonatal (risco relativo, RR, de 1,08, intervalo de confiança de 95%, 95% CI, de 0,69 a 1,71; quatro estudos, 425 mulheres). Os bebês cujas mães tinham sido alocadas para o grupo intervencionista tiveram mais hemorragia intraventricular (RR 1,82, 95% CI de 1,06 a 3,14; um estudo; 262 mulheres), mais membrana hialina (RR 2,30, 95% CI 1,39 a 3,81; dois estudos; 133 mulheres), precisaram de mais ventilação (RR 1,50, 95% CI de 1,11 a 2,02; dois estudos; 300 mulheres) e tiveram maior probabilidade de ter uma gestação mais curta (diferença média, MD, ‐9,91 dias, 95% CI ‐16,37 a ‐3,45 dias; quatro estudos; 425 mulheres), de precisarem de internação em unidade de terapia intensiva (UTI) neonatal (RR 1,35, 95% CI 1,16 a 1,58) e de terem uma estadia mais longa na UTI neonatal (MD 11,14 dias, 95% CI 1,57 a 20,72 dias; dois estudos; 125 mulheres) do que aqueles que foram alocados ao grupo de conduta expectante. No entanto, os bebês alocados no grupo da conduta ativa tiveram menor probabilidade de serem pequenos para a idade gestacional (RR 0,30, 95% CI 0,14 a 0,65; dois estudos; 125 mulheres). As gestantes alocadas no grupo de conduta ativa tiveram maior probabilidade de ter uma cesariana (RR 1,09, 95% CI 1,01 a 1,18; quatro estudos; 425 mulheres) do que aquelas alocadas ao grupo de conduta expectante. Não houve nenhuma diferença estatisticamente significativa entre as duas estratégias para quaisquer outros desfechos.
Conclusão dos autores
Esta revisão sugere que a conduta expectante para gestantes com pré‐eclâmpsia grave de início precoce pode estar associada com diminuição da morbidade para o bebê. No entanto, essa evidência é baseada em dados de apenas quatro estudos. Mais estudos grandes são necessários para confirmar ou refutar esses achados e estabelecer se essa abordagem é segura para a mãe.
PICOs
Resumo para leigos
Conduta ativa versus expectante para gestantes com pré‐eclâmpsia grave precoce
As gestantes que desenvolvem pré‐eclâmpsia (pressão arterial alta e proteína na urina) antes de 34 semanas de gravidez (início precoce) estão em risco de ter complicações graves e até mesmo morrer. As complicações incluem problemas nos rins, no fígado e no sistema de coagulação e causam distúrbios neurológicos, tais como dor de cabeça, alterações visuais e exacerbação dos reflexos musculares. Nos casos em que a placenta é afetada, pode haver restrição do crescimento do feto ou redução do líquido amniótico, colocando a criança em risco. A única cura conhecida para a pré‐eclâmpsia é o nascimento do bebê. Porém, nascer cedo demais pode trazer problemas para o bebê, mesmo com a administração de corticosteroides 24 a 48 horas antes do parto para ajudar a amadurecer os pulmões dos fetos. Alguns hospitais seguem uma conduta ativa que consiste na realização do parto antecipado dentro de 24 a 48 horas após a internação de gestantes com pré‐eclâmpsia grave e precoce.
Esta revisão incluiu quatro estudos que distribuíram gestantes com menos de 34 semanas com pré‐eclâmpsia grave em dois grupos, de forma aleatória: um grupo era tratado de forma ativa (parto imediato) e o outro recebia o tratamento expectante (adiar o parto). Um total de 425 mulheres foi incluído nesses quatro estudos. Os bebês nascidos de mulheres tratadas de forma ativa tiveram maior probabilidade de ter eventos adversos, tais como hemorragia intraventricular (sangramento cerebral) e síndrome de angústia respiratória neonatal (problema de respiração). Eles também tiveram maior probabilidade de precisar ficar internados em unidade de terapia intensiva (UTI) neonatal e de ventilação mecânica (respirar com ajuda de aparelhos), ficar mais tempo na UTI neonatal e pesar menos no nascimento do que os bebês nascidos de mulheres recebendo conduta expectante. As mulheres no grupo ativo também eram mais propensas a necessitar de cesariana para o parto. Portanto, a conduta expectante (que consiste em adiar o parto) pode ser mais benéfica para o bebê. No entanto, existem dados insuficientes para conclusões confiáveis sobre os efeitos das duas condutas para a maioria dos desfechos maternos. Consequentemente, não se sabe qual é a segurança de uma abordagem expectante para a mãe.
A evidência dessa revisão sistemática é baseada em dados de apenas quatro pequenos estudos. São necessários mais estudos grandes, com acompanhamento a longo prazo das crianças, para confirmar ou refutar se a conduta expectante seria melhor do que parto imediato para as gestantes com pré‐eclâmpsia grave antes de 34 semanas de gravidez.
Authors' conclusions
Background
Pre‐eclampsia is a multisystem disorder that is usually associated with raised blood pressure and proteinuria, but can also involve the woman's liver, kidneys, clotting system, or brain. If the placenta is involved this may lead to growth restriction or premature birth. Pre‐eclampsia is a relatively common complication of pregnancy, and can occur at any time during the second half of pregnancy or in the first few weeks after delivery. Prediction models for adverse maternal outcome have been developed and validated in recent times (von Dadelszen 2011), but there is still a paucity of data to guide the clinician on the timing of delivery to ensure safety of both the mother and the baby in the long term. Pre‐eclampsia is described in more detail in the generic protocol on interventions for treatment of pre‐eclampsia and its consequences (Duley 2009).
Description of the condition
Hypertension in pregnancy is defined as a systolic blood pressure of 140 mmHg or more, and/or a diastolic pressure of 90 mmHg or more. To be diagnosed with pre‐eclampsia the hypertension has to arise de novo after 20 weeks of pregnancy in combination with proteinuria defined as greater than 300 mg of total protein in a 24‐hour urine collection (Davey 1988). Recently, proteinuria has been assessed using a spot urine measuring the protein to creatinine ratio. A protein: creatinine ratio of 30 mg/mmol correlates with a 24‐hour protein excretion of greater than 300 mg in 24 hours (Morris 2012). This method of estimating the amount of protein being excreted has several advantages over the 24‐hour urine collection and has been endorsed in a Royal College of Obstetricians and Gynaecologists (RCOG) Pre‐eclampsia study group consensus statement (RCOG 2003). However, pre‐eclampsia is a multi‐system disorder and the diagnosis of hypertension and proteinuria is considered to be too restrictive for clinical practice. Clinicians are all too aware that the disease can present in several ways and it is necessary to be vigilant when assessing women with symptoms and signs that are strongly associated with the disease. This has led to a widening of the definition for clinical purposes, to include the following: de novo hypertension after 20 weeks' gestation and new onset of one of the following: a) proteinuria as defined above; b) renal insufficiency (creatinine > 0.09 mmol/L, or oliguria; c) liver disease (raised transaminases and/or severe right upper quadrant or epigastric pain); d) neurological problems, convulsions (eclampsia), hyper‐reflexia with clonus (involuntary muscular contractions), severe headaches, persistent visual disturbances (scotoma); e) haematological disturbances: thrombocytopenia (reduced numbers of platelets), disseminated intravascular coagulation, haemolysis; or f) fetal growth restriction (Brown 2001).
There is no widely accepted definition of severe pre‐eclampsia (Duley 2009). Nevertheless, the features described above in combination with the early onset of the disease between 24 and 34 weeks' gestation, would be considered by most clinicians to represent severe pre‐eclampsia. We therefore did not further define nor categorise "severity".
Description of the intervention
Within clinical practice, some units advocate early delivery, which has been referred to as 'aggressive management' (Sibai 1984), but in this review the term 'interventionist' is preferred. This means delivery by either induction of labour or caesarean section after corticosteroids have been given to improve fetal lung maturation, which in practice is after 24 to 48 hours (Crowley 1996). Others prefer to give corticosteroids, stabilise the woman's condition and then, if possible, aim to delay delivery. This is usually known as 'expectant management' (Derham 1989). The greatest dilemma in when to deliver is balancing the risks to mother and baby when the pregnancy is somewhere between 24 to 34 weeks. Early delivery resulting in a very premature baby could lead to more neonatal complications such as respiratory distress syndrome (difficulty in breathing and oxygenation), intraventricular haemorrhage (bleeding into the cavities of the brain) and necrotising enterocolitis (bleeding into the wall of the bowel due to a lack of oxygen). Conversely, delaying delivery in an attempt to allow fetal maturation could place the mother in jeopardy and at risk of multisystem organ failure as outlined above. It also prolongs the time that a fetus is in a potentially hostile in utero environment. This in turn will continue to adversely affect the growth of the fetus and may result in an intrauterine death, from severe hypoxia or an acute event such as an abruption. Although the precise cut offs for gestational age will vary with different settings, before 24 weeks the child has little chance of survival. After 34 weeks the prognosis improves with nearly 100 per cent survival. Between 24 and 34 weeks mortality decreases with increasing gestational age, but especially below 28 weeks there is also considerable risk of survival with severe disability. A structured review of observational studies found that expectant care for severe pre‐eclampsia was associated with a prolongation of the pregnancy by between one and two weeks with better outcomes for babies and low risks for the mother. There were fewer neonatal deaths and complications of prematurity (Magee 2009).
Why it is important to do this review
This difficult clinical dilemma occurs relatively frequently in large units, and currently decisions are based mainly upon personal experience rather than good evidence. There is a great need for reliable data to help inform this decision‐making.
Other aspects of care for women with severe pre‐eclampsia are dealt with in other reviews. These include drugs for lowering very high blood pressure (Duley 2006), prophylactic anticonvulsants (Duley 2010) and plasma volume expansion (Duley 1999b). Prevention of pre‐eclampsia is covered by reviews of calcium supplementation (Hofmeyr 2010), antiplatelets (Duley 2007), salt intake (Duley 1999a; Duley 2005) and magnesium supplementation (Makrides 2001).
Objectives
To evaluate the comparative benefits and risks of a policy of early delivery by induction of labour or by caesarean section after sufficient time has elapsed to administer corticosteroids, and allow them to take effect; with a policy of delaying delivery (expectant care) for women with severe pre‐eclampsia between 24 and 34 weeks.
Methods
Criteria for considering studies for this review
Types of studies
All adequately randomised trials comparing interventionist (aggressive) with expectant care (delayed delivery) for women with severe early onset pre‐eclampsia. Quasi‐random designs, such as alternate numbers or allocation by the day of the week, were excluded.
Types of participants
Women with severe pre‐eclampsia before or equal to 34 weeks' gestation. Severe pre‐eclampsia was defined as high blood pressure, > 140/90 mmHg on two consecutive occasions four or more hours apart and proteinuria greater than 300 mg/24 hours. Alternatively as:
-
severe hypertension (blood pressure at least 160 mmHg systolic, or 110 mmHg diastolic) alone;
or hypertension as defined above plus one or more of the following criteria:
-
severe proteinuria (usually at least 3 g (range 2 g to 5 g) protein in 24 hours, or 3+ on dipstick);
-
reduced urinary volume (less than 500 mL in 24 hours), upper abdominal pain, pulmonary oedema;
-
neurological disturbances (such as headache, visual disturbances, and exaggerated tendon reflexes);
-
impaired liver function tests, high serum creatinine, low platelets;
-
suspected intrauterine growth restriction or reduced liquor volume.
This latter set of criteria reflect the natural history of the disease and clinical practice when diagnosing severe pre‐eclampsia.
Types of interventions
Any comparison of a policy of early elective delivery by induction of labour or by caesarean section (interventionist management) with a policy of delayed delivery (expectant management). If corticosteroids were used within the trial, they should have been used for both types of care. As the beneficial effects of a course of corticosteroids are so important, any study where corticosteroids were only administered to one group but not the other was excluded.
Types of outcome measures
Primary outcomes
For the woman
-
Death
-
Eclampsia (fitting)
-
Stroke (brain damage)
-
HELLP (haemolysis, elevated liver enzymes and low platelets) syndrome
-
Pulmonary oedema (fluid in the lungs)
For the baby
-
Stillbirth
-
Neonatal death
-
Intraventricular haemorrhage (bleeding in the brain) and/or hypoxic ischaemic encephalopathy
Secondary outcomes
For the woman
-
Renal failure (kidney failure)
-
Liver failure
-
Cardiac arrest
-
The need for invasive monitoring, such as central venous catheterisation (intravenous lines into the great veins around the heart)
-
Caesarean section
-
Placental abruption
For the baby
-
Low Apgar score at five minutes
-
Neonatal seizures
-
Hyaline membrane disease (stiff lungs)
-
Pneumothorax (air leaks from the lungs)
-
Necrotising enterocolitis (bleeding into the bowel wall)
-
Ventilation (any ventilation, duration of ventilation)
-
Measures of long‐term growth and development, such as important impairment and cerebral palsy
-
Small‐for‐gestational age
-
Gestation at birth
Use of health service resources
-
Need for intensive care for the woman
-
Need for high‐dependency care or observation, or both, for the woman
-
Length of stay in neonatal intensive care
-
Admission to neonatal intensive care unit
-
Surfactant for the baby
-
Ventilation for the baby
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (28 February 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For details of additional searching carried out in the previous version of the review, please see Appendix 1.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 2.
For this update we used the following methods when assessing the reports identified by the updated search. The two already included studies (Odendaal 1990; Sibai 1994) were also re‐assessed using the following methods for 'Risk of bias' assessment.
Selection of studies
Two review authors (D Churchill (DC); L Jones (LJ)) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (DC; LJ) independently extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third author. We entered data into Review Manager software (RevMan 2012) and checked for accuracy. When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreement was resolved by discussion or by involving a third author.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We planned to assess blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We planned to assess blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other sources of bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods, if required.
Unit of analysis issues
Cluster‐randomised trials
If we identify cluster‐randomised trials in future updates, we will include them in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not a valid study design for this review.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T2, I² and Chi² statistics. We regarded heterogeneity as substantial if the T2 is greater than zero and either the I2 was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an averaged treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with its 95% confidence interval, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses based on:
-
gestation at trial entry: 24 to 28 weeks' gestation; 29 to 34 weeks' gestation; gestation mixed or unknown;
-
whether suspected intrauterine growth restriction at trial entry: suspected intrauterine growth restriction; no suspected intrauterine growth restriction; mixed or unknown.
The following primary outcomes will be used in subgroup analysis.
For the woman
-
Death
-
Eclampsia (fitting)
-
Stroke (brain damage)
-
HELLP syndrome
-
Pulmonary oedema
For the baby
-
Stillbirth
-
Neonatal death
-
Intraventricular haemorrhage
We will assess differences between subgroups using interaction tests available within (RevMan 2012).
Sensitivity analysis
In future updates, if more studies are identified and included in analyses, we plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
A total of seven trials were identified and four trials met our inclusion criteria (GRIT 2003; Mesbah 2003; Odendaal 1990; Sibai 1994) ‐ see table of Characteristics of included studies.
Two trials were excluded (Gruppo di Studio1998; Langenveld 2011) ‐ see table of Characteristics of excluded studies.
One trial is ongoing (Duvekot 2011) ‐ see table of Ongoing studies.
Included studies
Four trials with a total of 425 women are included in this review.
Setting
One trial, was a UK based multi‐centre trial involving 69 hospitals in 13 European countries (GRIT 2003). The other three trials were single‐centre trials, based in Egypt (Mesbah 2003), South Africa (Odendaal 1990), and the USA (Sibai 1994).
Participants
In one trial, (GRIT 2003), 548 pregnant women with fetal growth restriction between 24 and 36 weeks' gestation, an umbilical artery Doppler waveform recorded and clinical uncertainty whether immediate delivery was indicated were examined. A subset of women from this trial (GRIT 2003) at less than or equal to 34 weeks' gestation (n = 262), who had severe pre‐eclampsia were included in this review. Mesbah 2003 included 30 women with severe pre eclampsia between 28 and 33 weeks' gestation; Odendaal 1990 included 38 women with severe pre‐eclampsia between 28 and 34 weeks' gestation; and Sibai 1994 included 95 women with severe pre‐eclampsia at 28 to 32 weeks' gestation.
Interventions
In all trials, women had a 24‐ to 48‐hour period of stabilisation during which they were given steroids to accelerate fetal lung maturity and if necessary magnesium sulphate to prevent seizures and antihypertensives to lower blood pressure. If they continued to meet the eligibility criteria at the end of this period they were then randomised. They were either randomised to the interventionist group, which involved immediate delivery by caesarean section or induction, or to the expectant management group, who were managed with hospitalisation and intensive maternal and fetal monitoring. Earlier delivery in this expectant group was implemented if either the maternal or fetal condition deteriorated, as determined by prespecified criteria.
Outcomes
The main outcomes in all studies included maternal, perinatal and neonatal morbidity and mortality outcomes. Only one trial included long‐term outcomes (GRIT 2003): measures of long‐term growth and development at two years.
For further details seeCharacteristics of included studies.
Excluded studies
Two trials were excluded as they did not meet the inclusion criteria of the review (Gruppo di Studio1998; Langenveld 2011). In both trials, the women did not have severe pre‐eclampsia.
See table of Characteristics of excluded studies.
Risk of bias in included studies
The four trials were relatively small. Overall, two trials were judged to have a low risk of bias (GRIT 2003; Sibai 1994), one was unclear (Odendaal 1990) and one a high risk of bias (Mesbah 2003).
See Figure 1 and Figure 2 for summaries of 'Risk of bias' assessment.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
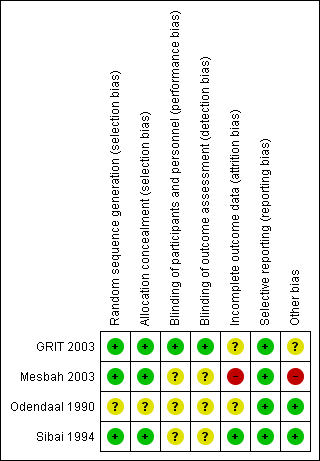
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For one study, the method used for randomisation and concealment of allocation was not described (Odendaal 1990); in the other, three trials both methods of randomisation and concealment were adequate (GRIT 2003; Mesbah 2003; Sibai 1994).
Blinding
Blinding of participants, personnel or outcome assessors was not described in three of the trials (Mesbah 2003; Odendaal 1990; Sibai 1994). Blinding of outcome assessment for long‐term outcomes such as Griffiths assessment was reported in one trial (GRIT 2003).
Incomplete outcome data
In one trial (Sibai 1994), all women appear to have been accounted for in the results. In one trial individual patient data for a subset of women with severe pre‐eclampsia were provided by the authors of the original trial and it is not possible to tell how complete this data set is (GRIT 2003). In another trial, it is not clear from results tables how many were included in the analyses (Odendaal 1990). In one trial 41 women were recruited, but 11 (27%) were judged too compromised for expectant management and were delivered by caesarean section and after randomisation, five patients appear to be missing from the results table 2 (Mesbah 2003).
Selective reporting
All expected outcomes appear to have been reported upon in all four trials (GRIT 2003; Mesbah 2003; Odendaal 1990; Sibai 1994).
Other potential sources of bias
In two studies, baseline characteristics were similar between groups and no other sources of bias were apparent (Odendaal 1990; Sibai 1994). In one study, other bias may have been introduced as only a subset of the original randomised sample provided data for analysis, but this was not clear (GRIT 2003). In one study, the severe group were excluded from the study and no baseline characteristics described for this group of patients (Mesbah 2003).
Effects of interventions
1. Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia
Primary outcomes
Only one study (95 women) reported on primary outcomes of relevance to the woman. In this study there were no reports of eclampsia or pulmonary oedema in either group. There is insufficient evidence about the effects on HELLP (haemolysis, elevated liver enzymes and low platelets) syndrome (one trial; 95 women; risk ratio (RR) 0.53; 95% confidence interval (CI) 0.05 to 5.68), Analysis 1.2. Death, stroke and pulmonary oedema were not reported in any of the trials.
For the baby, there is insufficient evidence for any reliable conclusions about the effects on stillbirth (four trials; 425 women; RR 0.20; 95% CI 0.03 to 1.16) or death after delivery (four trials; 425 women; RR 1.33; 95% CI 0.80 to 2.23), Analysis 1.5. Babies whose mothers had been allocated to the interventionist group had more intraventricular haemorrhage (one trial; 262 women; RR 1.82, 95% CI 1.06 to 3.14), Analysis 1.6.
Secondary outcomes
Women allocated to the interventionist group were more likely to have a caesarean section (four trials; 425 women; RR 1.09, 95% CI 1.01 to 1.18), Analysis 1.18, than those allocated an expectant policy. There were no statistically significant differences between the two management strategies for renal failure (two trials; 133 women; RR 0.30, 95% CI 0.01 to 6.97), Analysis 1.7, or placental abruption (two trials; 133 women; RR 0.80, 95% CI 0.26 to 2.40), Analysis 1.9. Liver failure, cardiac arrest and the need for invasive monitoring for the woman were not reported in any of the trials.
This review suggests that an interventionist policy of care may be associated with increased morbidity for the baby. For example, those babies whose mothers had been allocated to the interventionist group had more hyaline membrane disease (two trials; 133 women; RR 2.30, 95% CI 1.39 to 3.81), Analysis 1.12, were more likely to require ventilation (two trials; 300 women; RR 1.50, 95% CI 1.11 to 2.02), Analysis 1.14, and were more likely to have a lower gestation at birth (days) (four trials; 425 women; random‐effects analysis; average mean difference (MD) ‐9.91, 95% CI ‐16.37 to ‐3.45; Heterogeneity: Tau² = 31.74; I² = 76%), Analysis 1.19. Also, babies whose mothers had been allocated to the interventionist group were at increased risk of developing necrotising enterocolitis, (three trials; 395 women; RR 2.10, 95% CI 0.93 to 4.79), Analysis 1.13, although the results were not statistically significant, the confidence interval only just overlaps the line of no effect. This effect could be clarified by more data in future updates. Nevertheless, babies allocated to the interventionist policy were less likely to be small‐for‐gestational age (two trials; 125 women; RR 0.30, 95% CI 0.14 to 0.65), Analysis 1.18. There were no statistically significant differences between the two management strategies for low Apgar score at five minutes, neonatal seizures, and measures of long‐term growth and development, seeAnalysis 1.10; Analysis 1.11; Analysis 1.15; Analysis 1.16; Analysis 1.17.
Babies whose mothers had been allocated to the interventionist group were more likely to be admitted to neonatal intensive care (two trials; 125 women; RR 1.35, 95% CI 1.16 to 1.58), Analysis 1.21, and have a longer stay in the neonatal intensive care unit (two trials; 125 women; random‐effects analysis; average MD 11.14 days, 95% CI 1.57 to 20.72 days; Heterogeneity: Tau² = 40.93; I² = 85%), Analysis 1.20, than those allocated an expectant policy. Other outcomes on use of health service resources were not reported in any of the trials (need for intensive care for the woman; need for high‐dependency care or observation, or both, for the woman; surfactant for the baby).
Discussion
Timing the delivery of a very premature infant in the presence of severe pre‐eclampsia is a difficult clinical decision. When the mother's life is in danger there is no doubt that delivery is the only correct course of action. This situation is rare. More usually, the risks of maternal morbidity or intrauterine fetal demise, if the pregnancy is continued, have to be constantly balanced against the hazards of prematurity to the fetus if delivered. Most obstetricians would probably be cautious and expedite delivery in favour of the outcome for the mother and being able to guarantee a live baby at delivery. What is not clear is to what level this (if at all), adversely affects the baby.
Only the GRIT study pre‐specified fetal assessment parameters as entry criteria into the study. The other studies used fetal assessment to trigger delivery if there was evidence of significant compromise. It is therefore not possible to compare the trials for the condition of the fetuses on trial entry. However it is unlikely that there would have been any clinical differences where this was not formally assessed at trial entry. If there were signs of imminent fetal demise then the women would not have been randomised into the trials. But there is the potential for unseen bias and future trials must include a formal assessment of fetal wellbeing on trial entry.
Currently there are insufficient data to justify any of our prespecified subgroup analyses. These will be included in future updates of this review, when larger trials become available.
It is not possible to draw firm conclusions from this review. However, the evidence is promising that short‐term morbidity for the baby may be reduced by a policy of expectant care. This is perhaps surprising given that expectant management increases the length of time a fetus is within the hostile environment, with the potential to adversely affect fetal growth. In fact this is often stated as a reason for intervention. The results of this review suggest otherwise. While the babies in the expectant management group were smaller, their short‐term outcomes were better. Before this policy can be recommended in clinical practice, further evidence is required to demonstrate that there is truly a short‐term benefit for the baby and that it continues in the longer term. Reassurance is also needed to demonstrate that there is no increase in mortality for the child, or in morbidity for the mother.
Summary of main results
There is insufficient evidence for reliable conclusions about the effects of either management approach on stillbirth or death after delivery. However, there is some evidence from this review to suggest that a policy of delaying delivery reduces the morbidity experienced in the neonatal period of life. Fewer babies had intraventricular haemorrhages, hyaline membrane disease and reduced levels of ventilation in those allocated to expectant management. Babies in this group were also less likely to be admitted to the neonatal intensive care unit and when admitted stayed there for shorter periods of time. There were insufficient data to draw any conclusions about the effect a policy of expectant care had on the mothers health. None of the studies included had sufficient sample size to demonstrate differences in maternal outcome.
Overall completeness and applicability of evidence
There is insufficient evidence from this review to recommend a particular management policy for this area of obstetric care. The numbers of participants in the trials is too small to be able to demonstrate differences in most significant (primary) outcomes and where differences are found there is a considerable level heterogeneity or the contribution is from only one trial. The same is true for the analysis of the secondary outcome measures.
Quality of the evidence
Three of the trials included in the review were judged to be at unclear risk of both performance and detection bias. It is not possible to blind personnel and participants to interventions and most outcomes are objective outcomes, and are unlikely to be affected by blinding e.g. death, eclampsia. One study (Mesbah 2003), was in addition judged to be at risk of attrition bias. The GRIT 2003 trial was not originally designed to examine severe pre‐eclampsia. It looked at interventionist versus expectant management for babies with growth restriction. A by‐product of this study, was that a subset of women also had severe pre‐eclampsia and it is these women who have been included in the review.
Potential biases in the review process
J Thornton was the Principle Investigator for the GRIT 2003 trial. To remove the potential for bias, the GRIT trial data were supplied directly to two other review authors (D Churchill, L Jones) from the trial statistician. J Thornton had no dealings with the acquisition, preparation or analysis of the GRIT trial data in this review.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 1 Eclampsia.

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 2 HELLP syndrome.
Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 3 Pulmonary oedema.

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 4 Death of the baby (all stillbirths, neonatal and infant deaths).
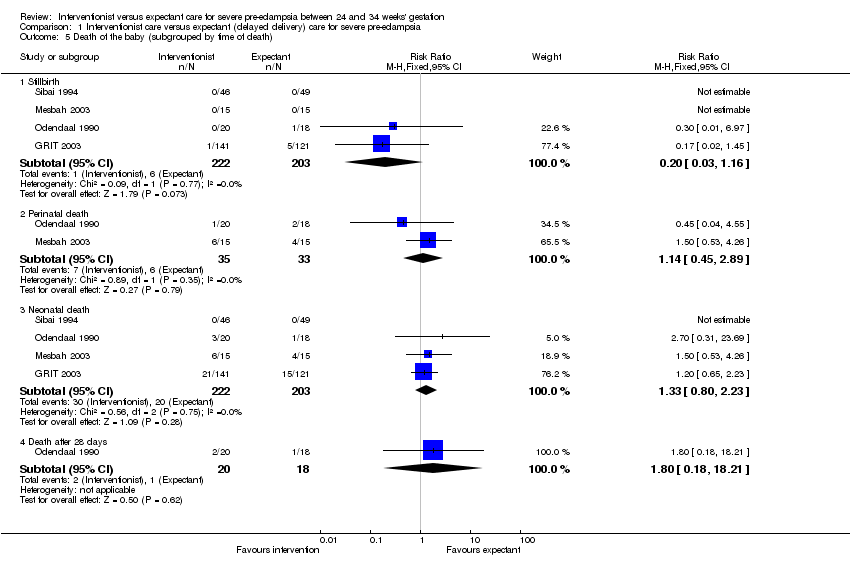
Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 5 Death of the baby (subgrouped by time of death).
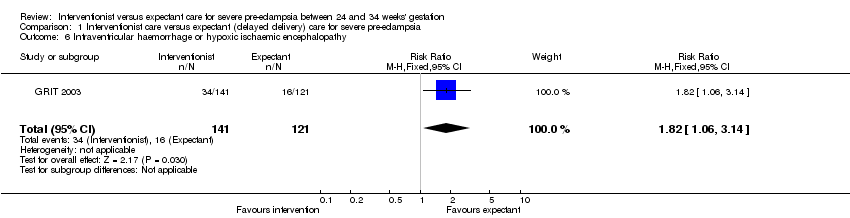
Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 6 Intraventricular haemorrhage or hypoxic ischaemic encephalopathy.
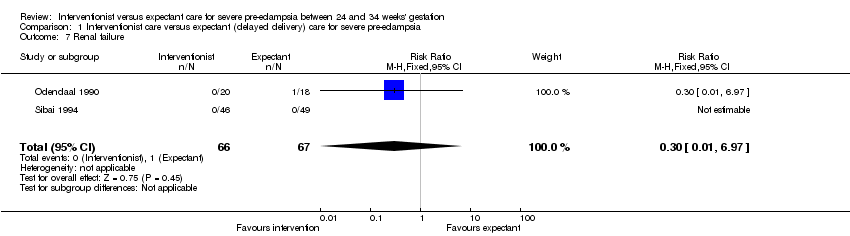
Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 7 Renal failure.

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 8 Caesarean section.
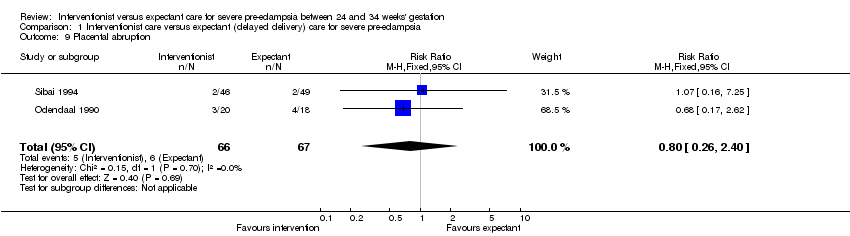
Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 9 Placental abruption.

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 10 Low Apgar score at five minutes (< 7 at five minutes).

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 11 Neonatal seizures.

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 12 Hyaline membrane disease.

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 13 Necrotising enterocolitis.
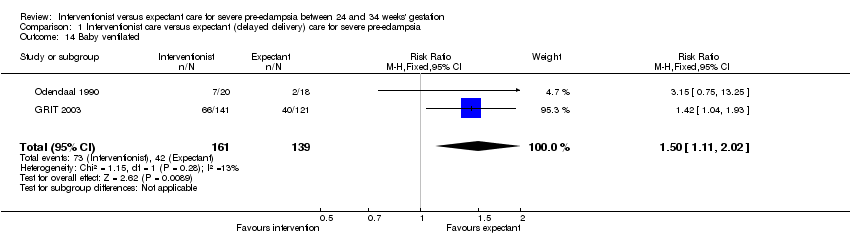
Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 14 Baby ventilated.

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 15 Measures of long‐term growth & development (cerebral palsy).

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 16 Measures of long‐term growth & development (poor hearing/hearing aid).
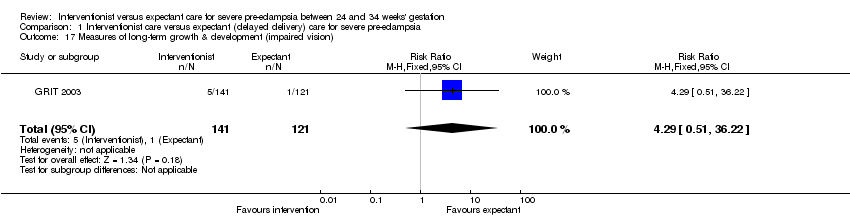
Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 17 Measures of long‐term growth & development (impaired vision).

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 18 Small‐for‐gestational age.

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 19 Gestation at birth (days).
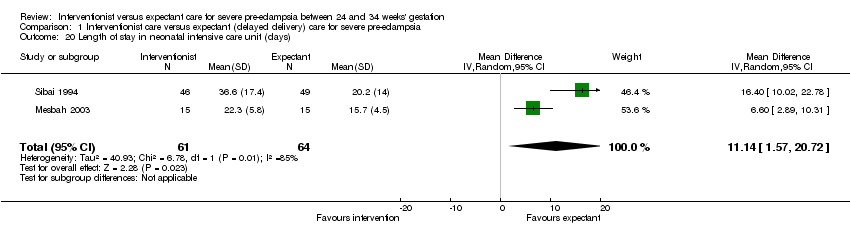
Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 20 Length of stay in neonatal intensive care unit (days).

Comparison 1 Interventionist care versus expectant (delayed delivery) care for severe pre‐eclampsia, Outcome 21 Admission to neonatal intensive care unit.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eclampsia Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 HELLP syndrome Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.68] |
| 3 Pulmonary oedema Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Death of the baby (all stillbirths, neonatal and infant deaths) Show forest plot | 4 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.69, 1.71] |
| 5 Death of the baby (subgrouped by time of death) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Stillbirth | 4 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.03, 1.16] |
| 5.2 Perinatal death | 2 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.45, 2.89] |
| 5.3 Neonatal death | 4 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.80, 2.23] |
| 5.4 Death after 28 days | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.18, 18.21] |
| 6 Intraventricular haemorrhage or hypoxic ischaemic encephalopathy Show forest plot | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.06, 3.14] |
| 7 Renal failure Show forest plot | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.97] |
| 8 Caesarean section Show forest plot | 4 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.01, 1.18] |
| 9 Placental abruption Show forest plot | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.26, 2.40] |
| 10 Low Apgar score at five minutes (< 7 at five minutes) Show forest plot | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.87, 2.50] |
| 11 Neonatal seizures Show forest plot | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.27, 24.43] |
| 12 Hyaline membrane disease Show forest plot | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.39, 3.81] |
| 13 Necrotising enterocolitis Show forest plot | 3 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.93, 4.79] |
| 14 Baby ventilated Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.11, 2.02] |
| 15 Measures of long‐term growth & development (cerebral palsy) Show forest plot | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.01 [0.75, 48.14] |
| 16 Measures of long‐term growth & development (poor hearing/hearing aid) Show forest plot | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.74] |
| 17 Measures of long‐term growth & development (impaired vision) Show forest plot | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [0.51, 36.22] |
| 18 Small‐for‐gestational age Show forest plot | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.65] |
| 19 Gestation at birth (days) Show forest plot | 4 | 425 | Mean Difference (IV, Random, 95% CI) | ‐9.91 [‐16.37, ‐3.45] |
| 20 Length of stay in neonatal intensive care unit (days) Show forest plot | 2 | 125 | Mean Difference (IV, Random, 95% CI) | 11.14 [1.57, 20.72] |
| 21 Admission to neonatal intensive care unit Show forest plot | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.16, 1.58] |

