Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term
Appendices
Appendix 1. Methods used to assess trials included in the initial version of this review
Kelly 2003
The trials included in the primary reviews were extracted from an initial set of trials covering all interventions used in induction of labour (see above for details of search strategy). The data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with The Pregnancy and Childbirth Group of The Cochrane Collaboration. This process allowed the data extraction process to be standardised across all the reviews.
The trials were initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, data were extracted to a standardised data extraction form which was piloted for consistency and completeness. The pilot process involved the researchers at the CESU and previous reviewers in the area of induction of labour.
Information was extracted regarding the methodological quality of trials on a number of levels. This process was completed without consideration of trial results. Assessment of selection bias examined the process involved in the generation of the random sequence and the method of allocation concealment separately. These were then judged as adequate or inadequate using the criteria described in Table 1 for the purpose of the reviews.
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer‐generated sequence, random number tables, lot drawing, coin tossing, shuffling cards, throwing dice. | Case number, date of birth, date of admission, alternation. |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially sealed opaque envelopes. | Open allocation sequence, any procedure based on inadequate generation. |
Performance bias was examined with regards to whom was blinded in the trials, i.e. patient, caregiver, outcome assessor or analyst. In many trials the caregiver, assessor and analyst were the same party. Details of the feasibility and appropriateness of blinding at all levels was sought.
Predefined subgroup analyses are: previous caesarean section or not; nulliparity or multiparity; membranes intact or ruptured, and cervix unfavourable, favourable or undefined. Only those outcomes with data appear in the analysis tables.
Individual outcome data were included in the analysis if they meet the prestated criteria in Types of outcome measures. Included trial data were processed as described in the Cochrane Reviewers' Handbook (Clarke 2002). Data extracted from the trials were analysed on an intention to treat basis (when this was not done in the original report, re‐analysis is performed if possible). Where data were missing, clarification was sought from the original authors. If the attrition was such that it might significantly affect the results, these data were excluded from the analysis. This decision rested with the reviewers of primary reviews and is clearly documented. If missing data become available, they will be included in the analyses.
Data were extracted from all eligible trials to examine how issues of quality influence effect size in a sensitivity analysis. In trials where reporting was poor, methodological issues were reported as unclear or clarification sought.
Due to the large number of trials, double data extraction was not feasible and agreement between the three data extractors was therefore assessed on a random sample of trials.
Once the data had been extracted, they were distributed to individual reviewers for entry onto the Review Manager computer software (RevMan 2003), checked for accuracy, and analysed as above using the RevMan software. For dichotomous data, risk ratios and 95% confidence intervals were calculated, and in the absence of heterogeneity, results were pooled using a fixed‐effect model.
The predefined criteria for sensitivity analysis included all aspects of quality assessment as mentioned above, including aspects of selection, performance and attrition bias.
Primary analysis was limited to the prespecified outcomes and subgroup analyses. In the event of differences in unspecified outcomes or subgroups being found, these were analysed post hoc, but clearly identified as such to avoid drawing unjustified conclusions.
Appendix 2. Methods used to assess trials included in previous version of this review
Kelly 2009
The following methods were used to assess: Al Malt 1995; Al‐Sebai 1993; Buchanan 1984; Campbell 1984; Cardozo 1986; Chaterjee 1990; Chua 1995; Chung 1992; Curet 1989; Doany 1997; Dommisse 1980; Duhl 1997; Dunston‐Boone 1991; Egarter 1989; El‐Mardi 1991; El Shawarby 2006; Glanville 2002; Graves 1985; Green 1998; Greer 1990; Hage 1993; Hannah 1996; Hayashi 1983; Kalkat 2008; Liggins 1979; MacKenzie 1979; MacKenzie 1981; MacKenzie 1997a; MacLennan 1980; Mahmood 1989; Mahmood 1992; Mahmood 1995; McCaul 1997; McLaren 1987; Miller 1991; Mukhopadhyay 2002; Murphy 1980; Murray 1995; Neilson 1983; Newman 1997; Nuutila 1996; O'Brien 1995; Ohel 1996; Payne 1993; Perryman 1992; Prasad 1989; Prins 1983; Rabl 2002; Rath 1999; Rayburn 1988; Rayburn 1992; Roach 1997; Sawai 1991; Sawai 1994; Shoaib 1994; Smith 1990; Smith 1994; Stampe Sorensen 1992; Thiery 1984; Tomlinson 2001; Ulmsten 1985; Witter 1992; Witter 1996.
In 2008, the methods and software for carrying out reviews were updated, as a result of which new reviews and updates, where appropriate, will use these new methods (Higgins 2008; RevMan 2008), which will be described in the Methods section of all the individual new and updated reviews.For this update, we used the following methods when assessing the trials identified by the updated search.
Assessment of methodological quality of included studies
We assessed the validity of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We described methods used for generation of the randomisation sequence for each trial.
(1) Selection bias (randomisation and allocation concealment)
We assigned a quality score for each trial, using the following criteria:
-
adequate concealment of allocation: such as telephone randomisation, consecutively‐numbered, sealed opaque envelopes;
-
unclear whether adequate concealment of allocation: such as list or table used, sealed envelopes, or study does not report any concealment approach;
-
inadequate concealment of allocation: such as open list of random‐number tables, use of case record numbers, dates of birth or days of the week.
(2) Attrition bias (loss of participants, for example, withdrawals, dropouts, protocol deviations)
We assessed completeness to follow up using the following criteria:
-
(A) less than 5% loss of participants;
-
(B) 5% to 9.9% loss of participants;
-
(C) 10% to 19.9% loss of participants;
-
(D) more than 20% loss of participants.
(3) Performance bias (blinding of participants, researchers and outcome assessment)
We assessed blinding using the following criteria:
-
blinding of participants (yes/no/unclear);
-
blinding of caregiver (yes/no/unclear);
-
blinding of outcome assessment (yes/no/unclear).
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. If heterogeneity was found, we explored this by sensitivity analysis, followed by random‐effects if required.
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but use different methods. Where there was evidence of skewness, this has been reported.
Dealing with missing data
We analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If, in the original reports, participants were not analysed in the group to which they were randomised and there is sufficient information in the trial report, we attempted to restore them to the correct group.
Assessment of heterogeneity
We applied tests of heterogeneity between trials, if appropriate, using the I² statistic. If we identified high levels of heterogeneity among the trials (exceeding 50%), we explored it by prespecified subgroup analysis and performed sensitivity analysis. We used a random‐effects meta‐analysis as an overall summary if this was considered appropriate.
Subgroup analyses
We conducted planned subgroup analyses as performed for the other reviews in this series (seeData collection and analysis). When assessing differences between subgroups, e.g. within the comparison of vaginal PGE2 versus placebo, we explored this using an inverse variance method of meta‐ analysis and presenting the statistics for subgroup differences using chi² and I² statistics.
Sensitivity analyses
We carried out sensitivity analysis to explore the effect of trial quality assessed by concealment of allocation, by excluding studies with clearly inadequate allocation of concealment.
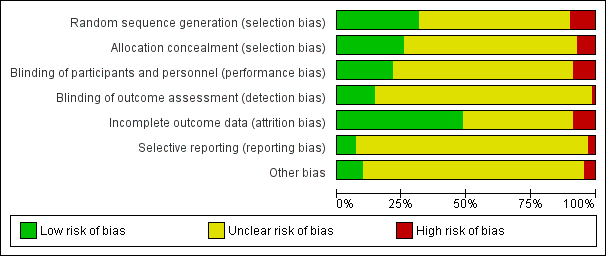
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
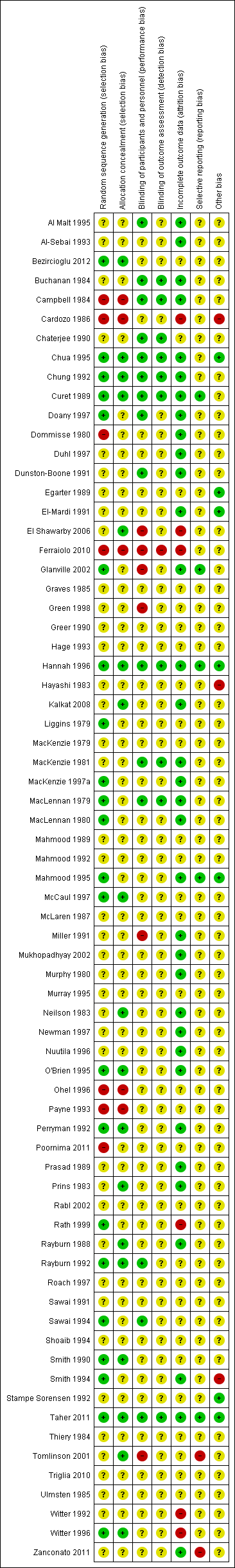
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 (1.1) PGE2 (all regimens) vs placebo/no treatment (all women), outcome: 1.13 Apgar score < 7 at 5 minutes.
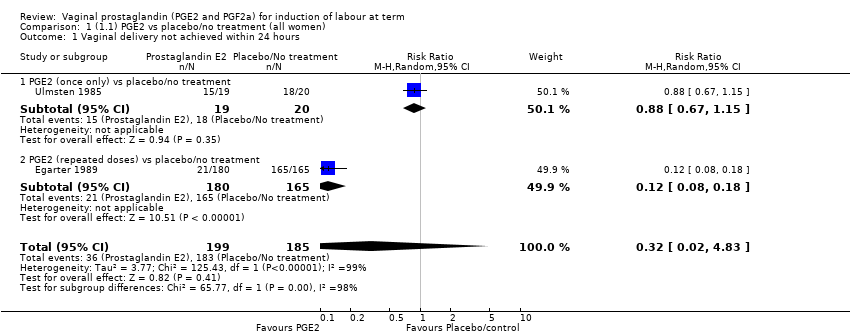
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 2 Uterine hyperstimulation with FHR changes.
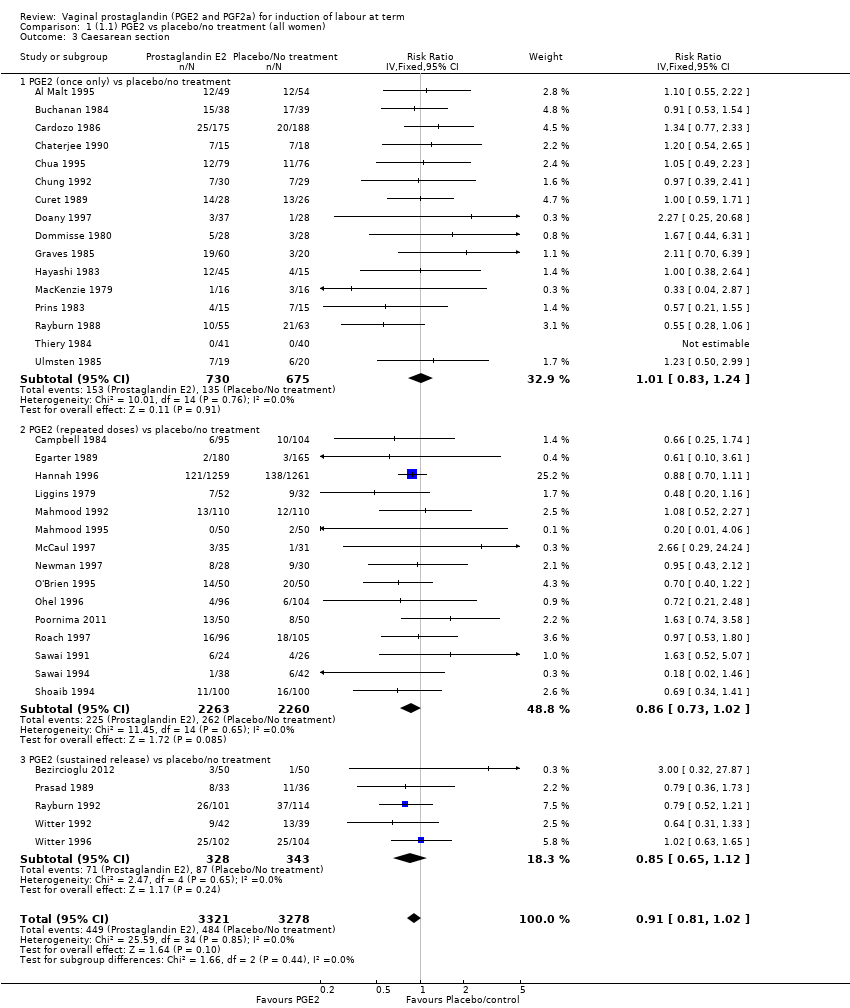
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 3 Caesarean section.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 4 Serious neonatal morbidity or perinatal death.
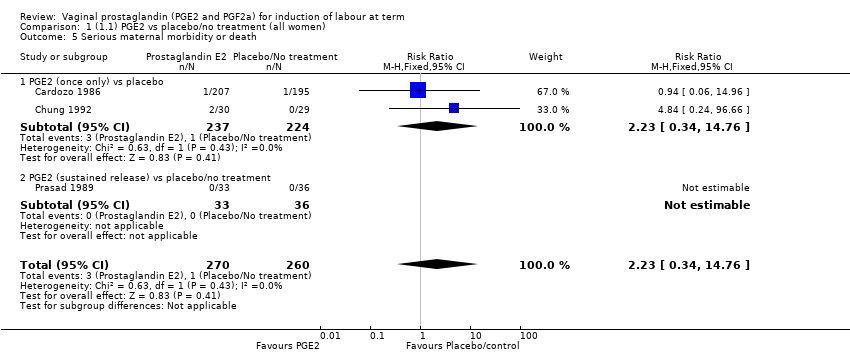
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 5 Serious maternal morbidity or death.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 7 Oxytocin augmentation.
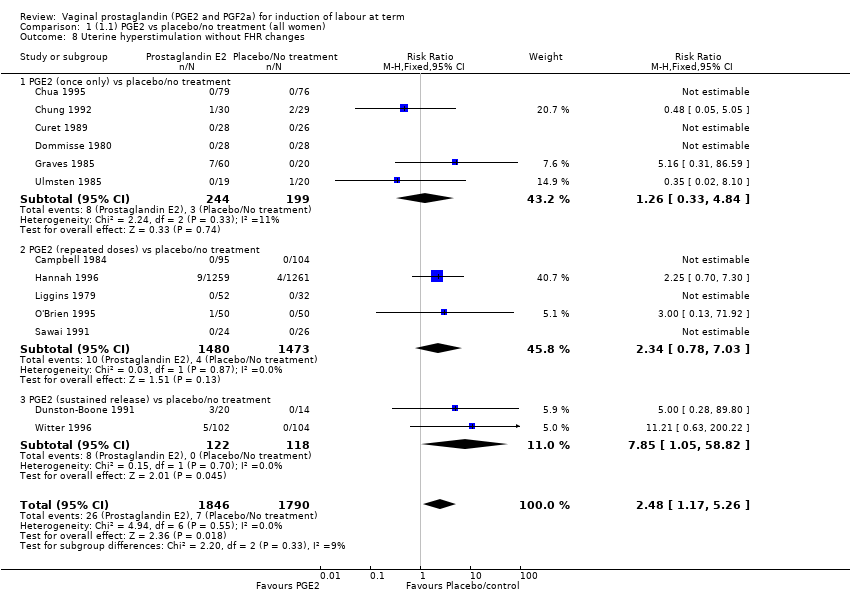
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 8 Uterine hyperstimulation without FHR changes.
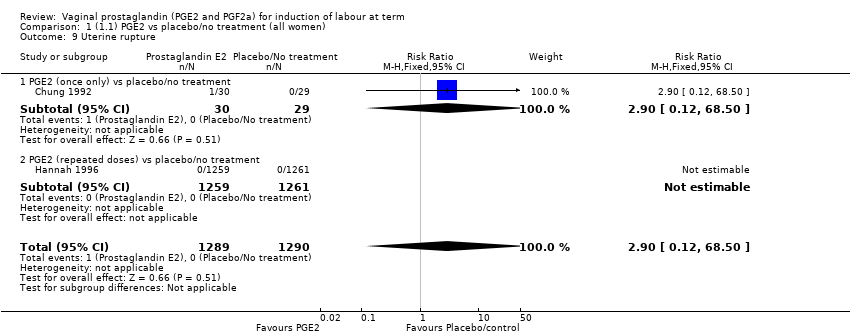
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 9 Uterine rupture.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 10 Epidural analgesia.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 11 Instrumental vaginal delivery.
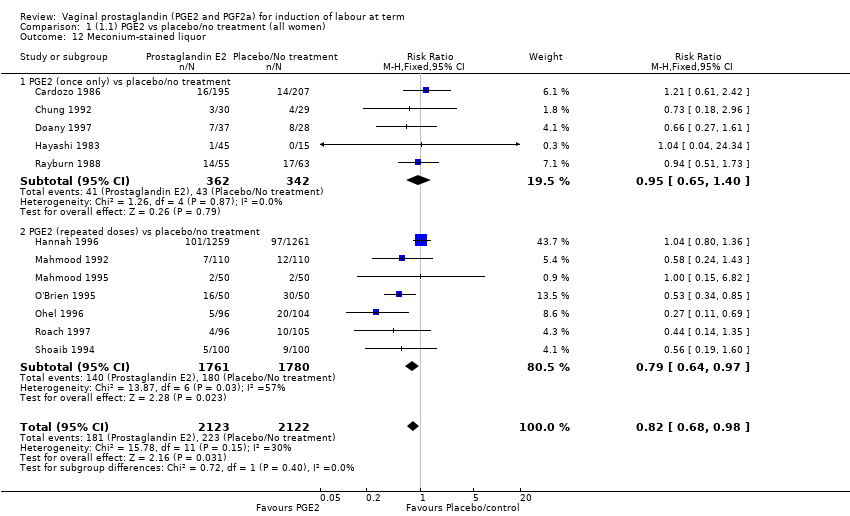
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 12 Meconium‐stained liquor.
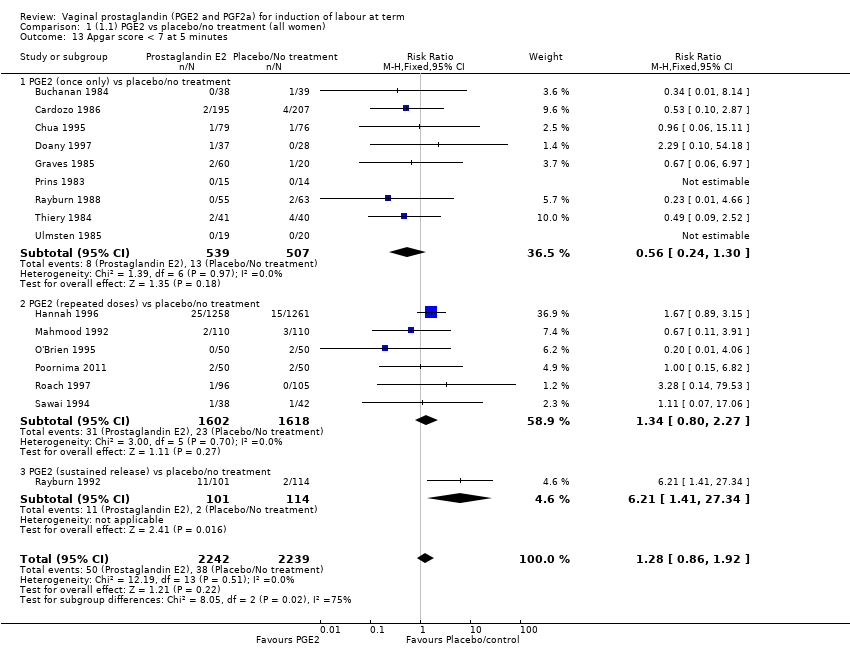
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 13 Apgar score < 7 at 5 minutes.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 14 Neonatal intensive care unit admission.
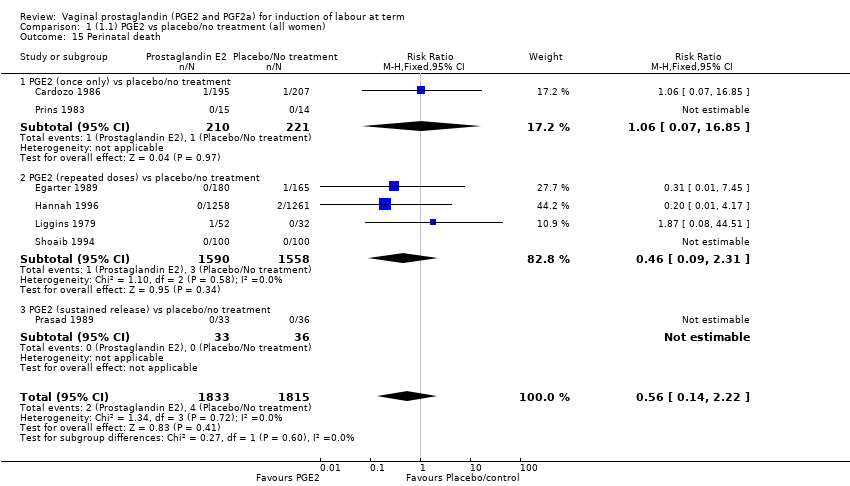
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 15 Perinatal death.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 16 Maternal side‐effects (all).
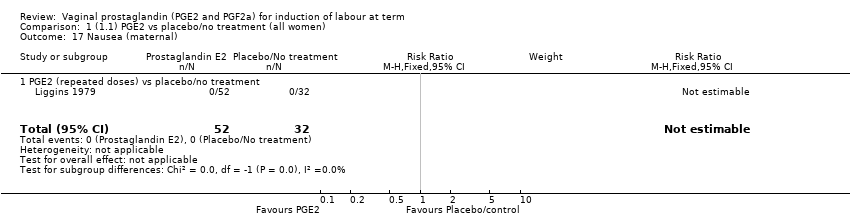
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 17 Nausea (maternal).
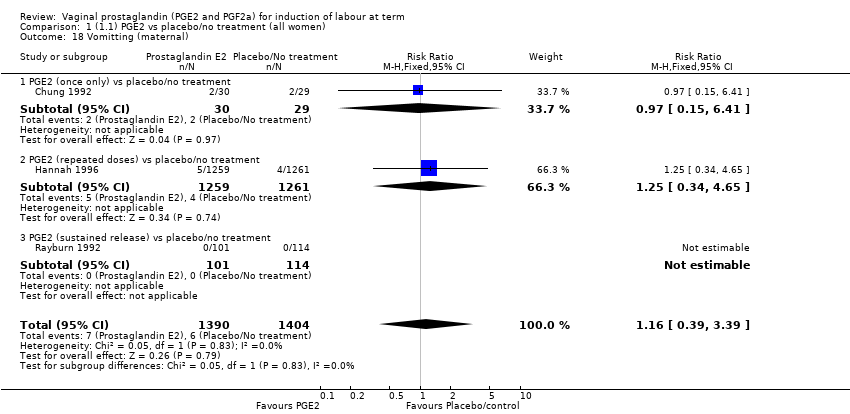
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 18 Vomitting (maternal).
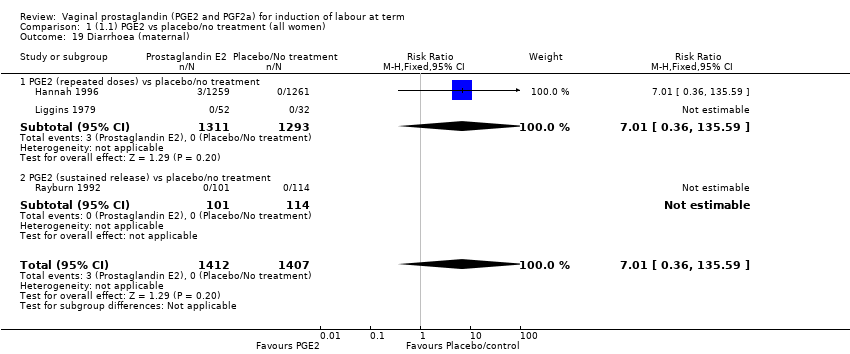
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 19 Diarrhoea (maternal).
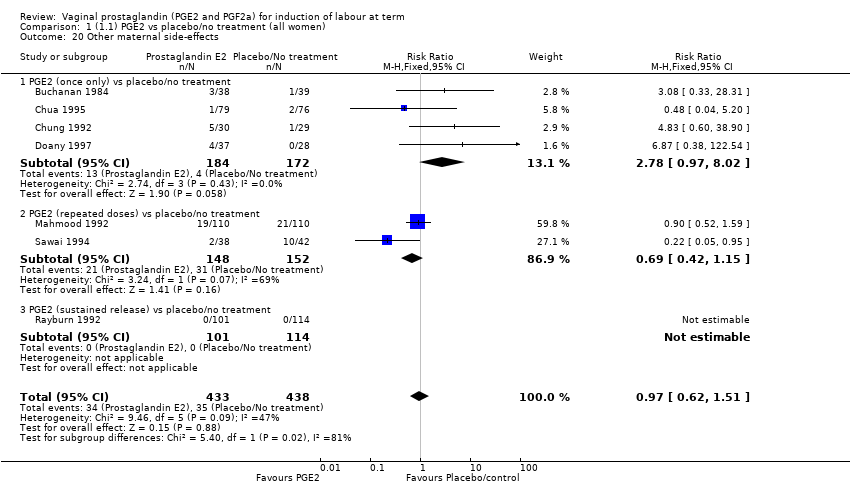
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 20 Other maternal side‐effects.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 21 Postpartum haemorrhage.
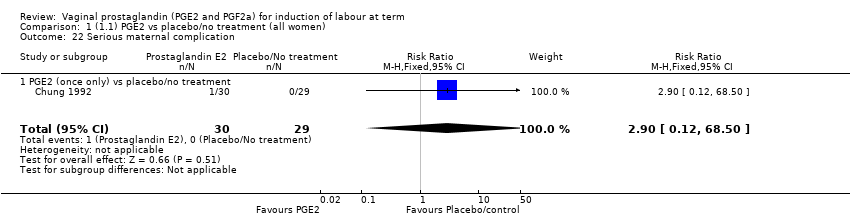
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 22 Serious maternal complication.
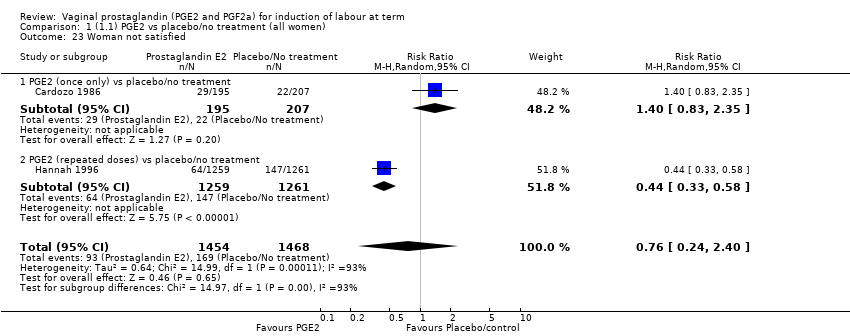
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 23 Woman not satisfied.
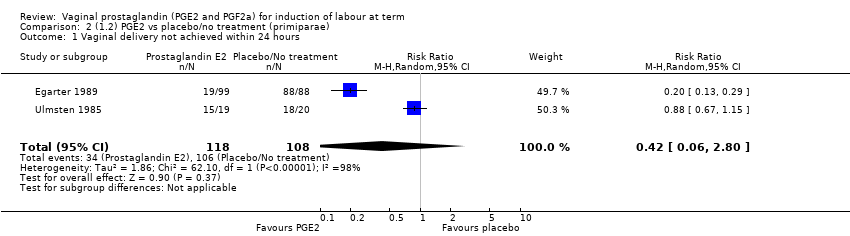
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
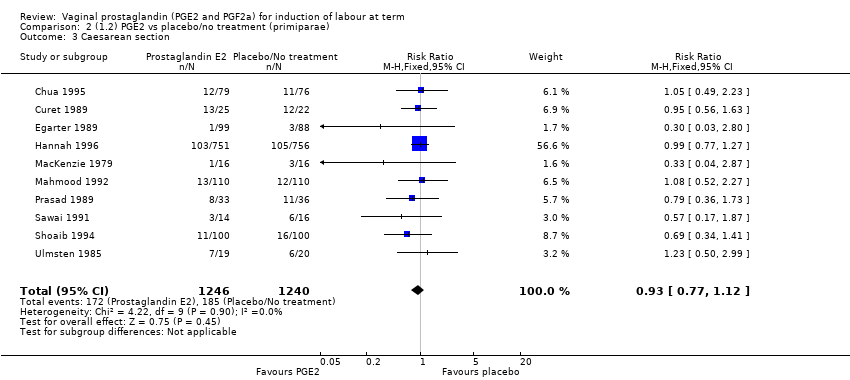
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 3 Caesarean section.

Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 4 Serious neonatal morbidity or perinatal death.

Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 5 Serious maternal morbidity or death.
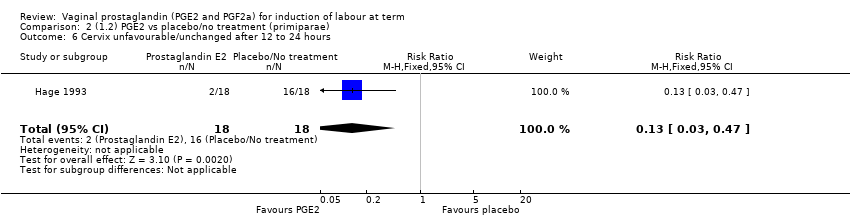
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.
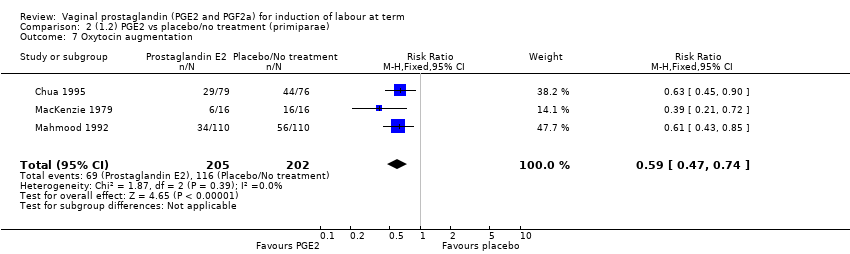
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 7 Oxytocin augmentation.
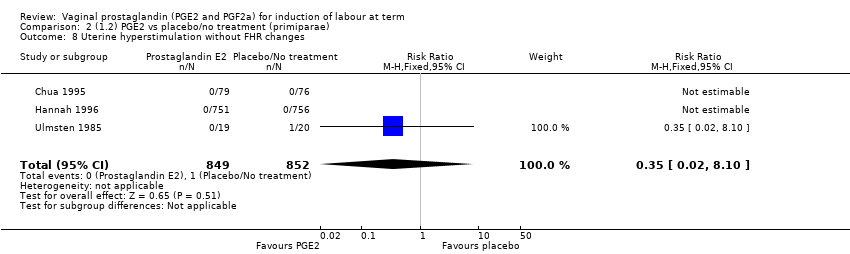
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 8 Uterine hyperstimulation without FHR changes.
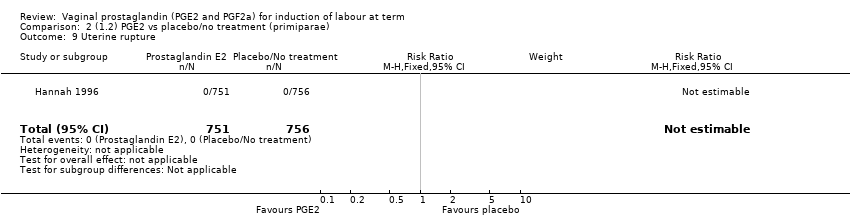
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 9 Uterine rupture.

Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 10 Epidural analgesia.
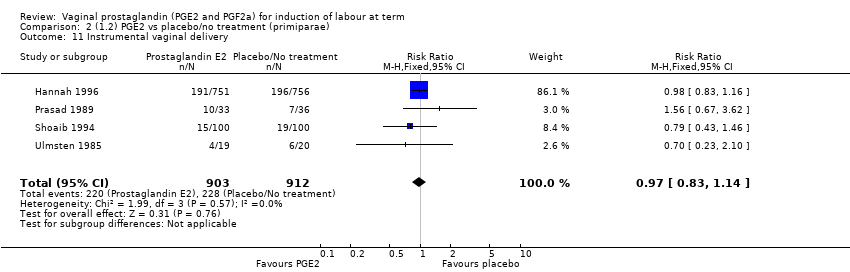
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 11 Instrumental vaginal delivery.
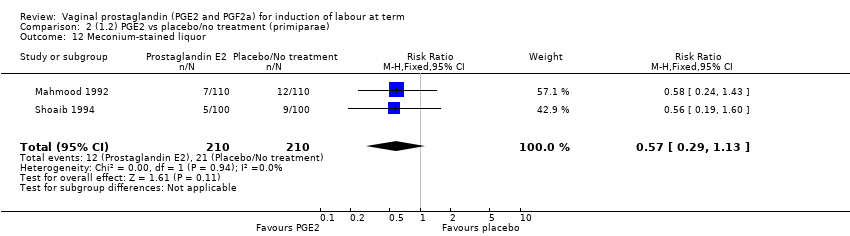
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 12 Meconium‐stained liquor.
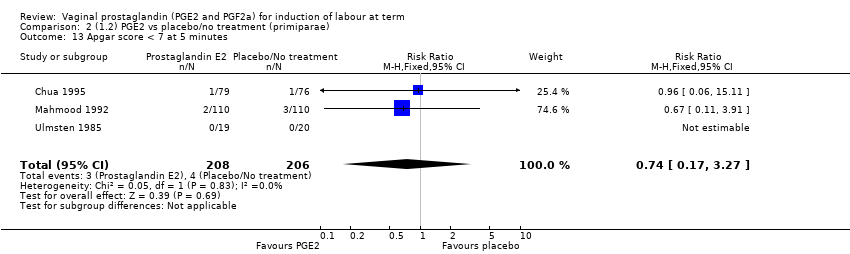
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 13 Apgar score < 7 at 5 minutes.

Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 14 Neonatal intensive care unit admission.

Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 15 Perinatal death.

Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 16 Maternal side‐effects (all).
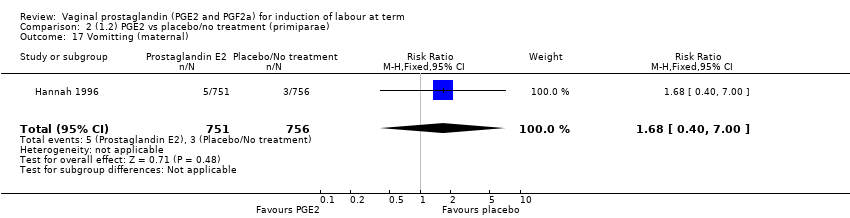
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 17 Vomitting (maternal).
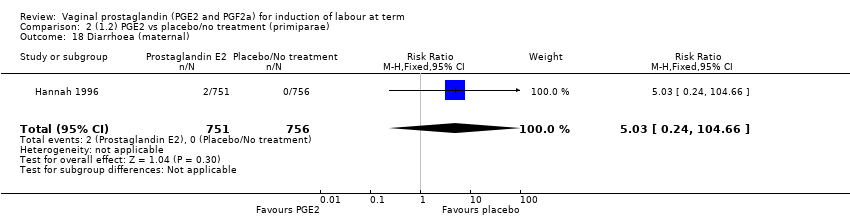
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 18 Diarrhoea (maternal).
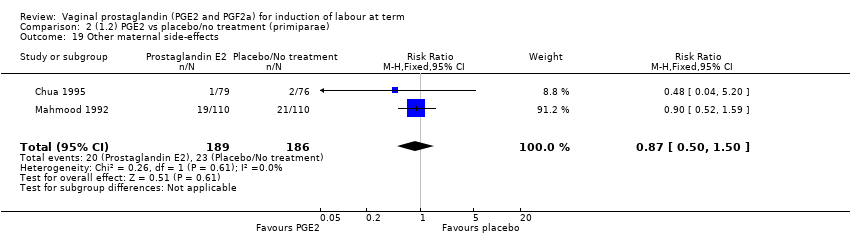
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 19 Other maternal side‐effects.

Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 20 Postpartum haemorrhage.

Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 3 Caesarean section.

Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 4 Serious neonatal morbidity or perinatal death.
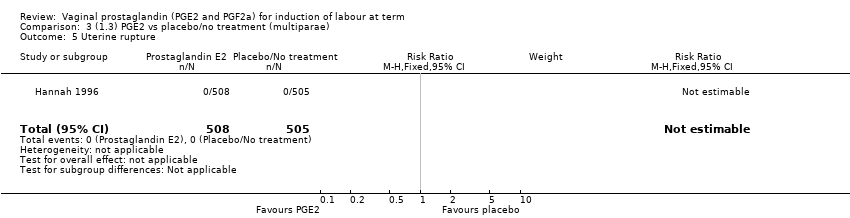
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 5 Uterine rupture.
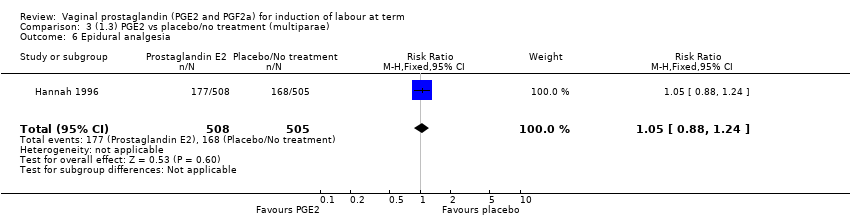
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 6 Epidural analgesia.
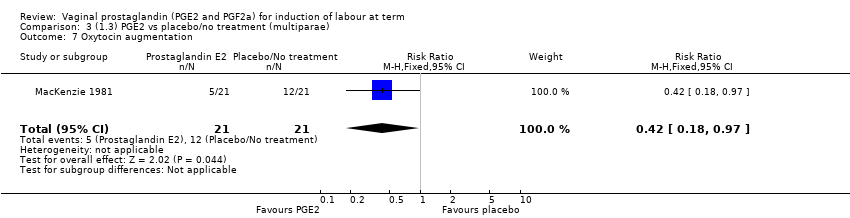
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 7 Oxytocin augmentation.

Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 8 Uterine hyperstimulation without FHR changes.

Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 9 Instrumental vaginal delivery.

Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 10 Meconium‐stained liquor.

Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 11 Perinatal death.
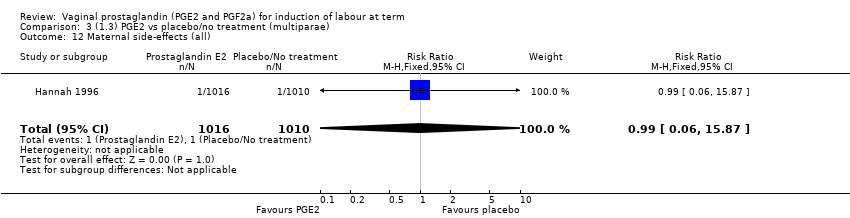
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 12 Maternal side‐effects (all).

Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 13 Vomitting (maternal).
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 14 Diarrhoea (maternal).
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 15 Postpartum haemorrhage.

Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 1 Vaginal delivery not achieved within 24 hours.
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 3 Caesarean section.

Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 4 Serious neonatal morbidity or perinatal death.
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.
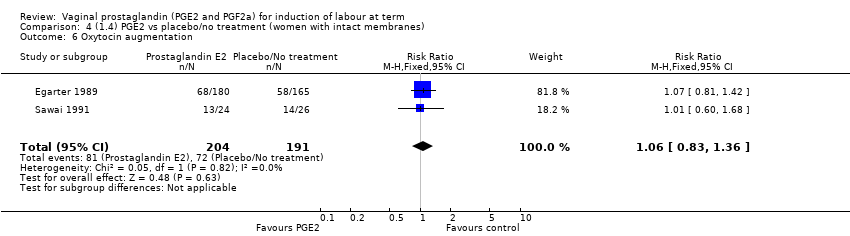
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 6 Oxytocin augmentation.
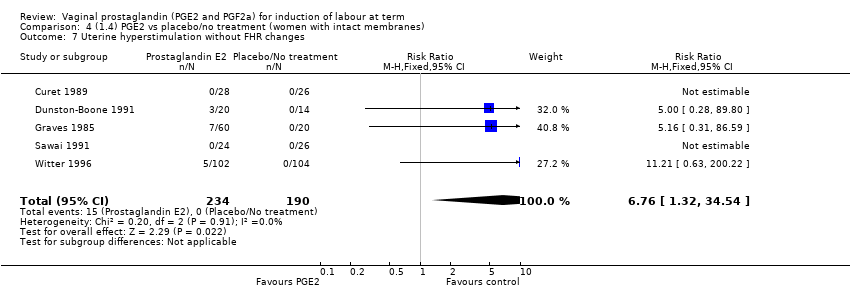
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 7 Uterine hyperstimulation without FHR changes.
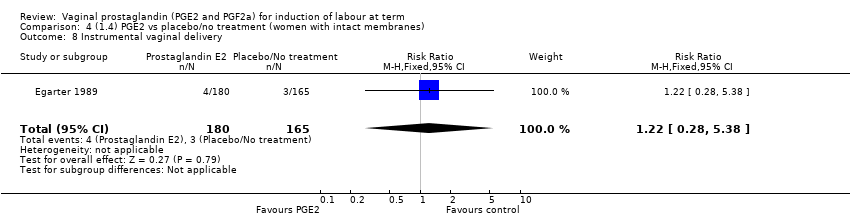
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 8 Instrumental vaginal delivery.

Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 9 Apgar score < 7 at 5 minutes.

Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 10 Neonatal intensive care unit admission.

Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 11 Maternal side‐effects (all).
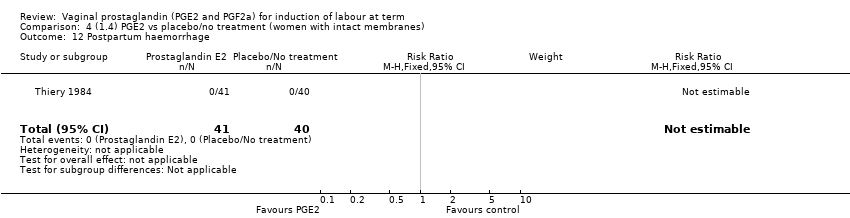
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 12 Postpartum haemorrhage.

Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 13 Perinatal death.
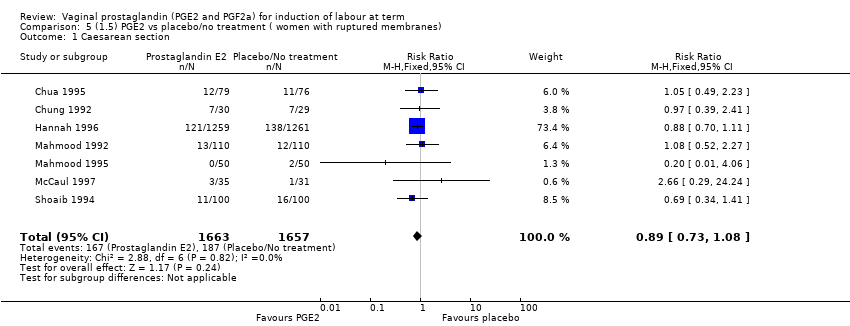
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 1 Caesarean section.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 2 Serious neonatal morbidity or perinatal death.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 3 Uterine hyperstimulation without FHR changes.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 4 Uterine rupture.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 5 Epidural analgesia.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 6 Instrumental vaginal delivery.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 7 Meconium‐stained liquor.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 8 Apgar score < 7 at 5 minutes.
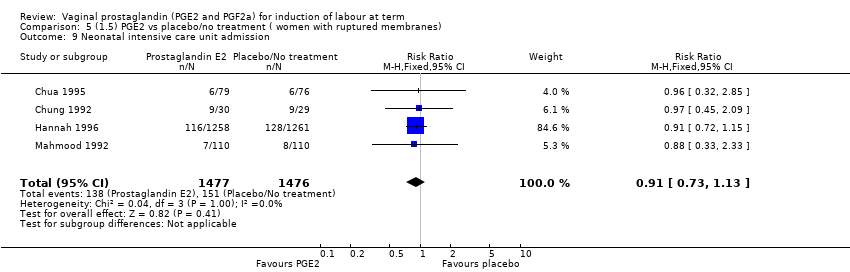
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 9 Neonatal intensive care unit admission.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 10 Perinatal death.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 11 Maternal side‐effects (all).

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 12 Vomitting (maternal).

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 13 Diarrhoea (maternal).

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 14 Postpartum haemorrhage.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 15 Woman not satisfied.
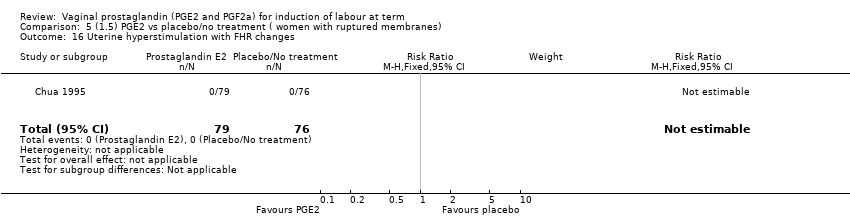
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 16 Uterine hyperstimulation with FHR changes.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 17 Serious maternal morbidity or death.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 18 Oxytocin augmentation.

Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 19 Other maternal side‐effects.
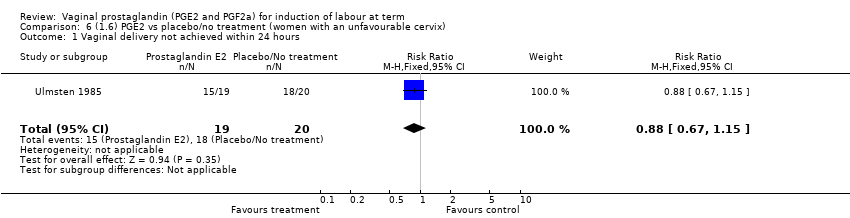
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
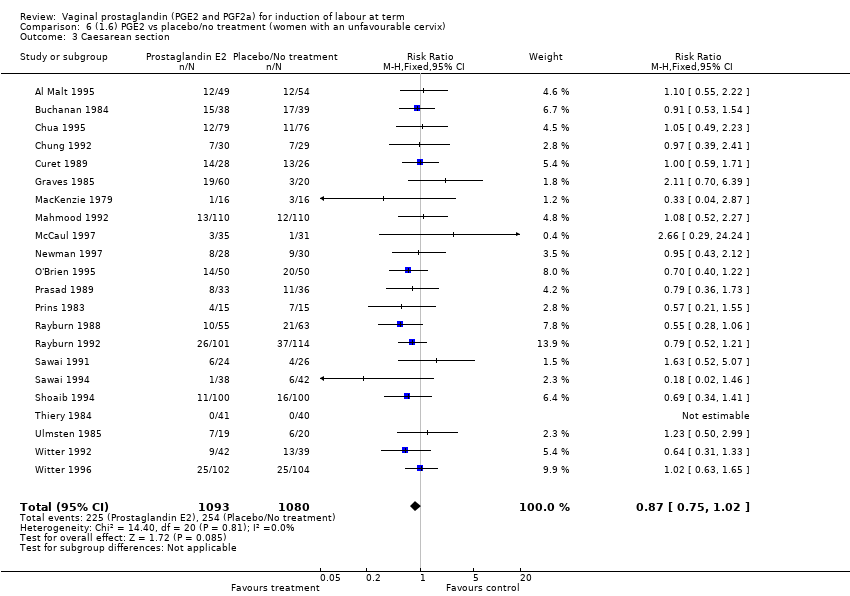
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 3 Caesarean section.
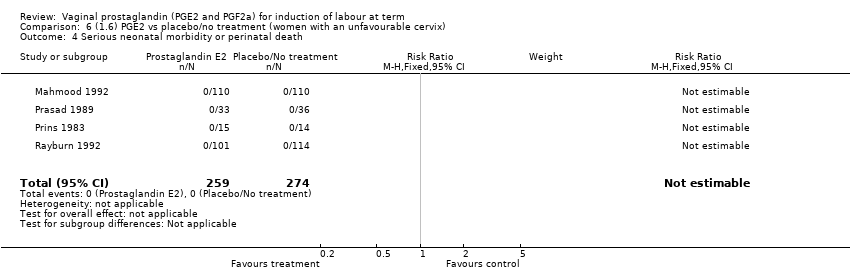
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 4 Serious neonatal morbidity or perinatal death.
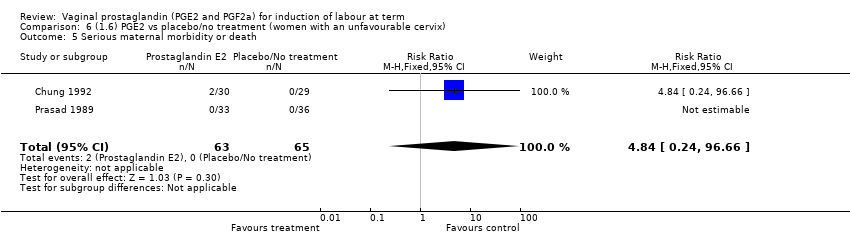
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 5 Serious maternal morbidity or death.

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 7 Oxytocin augmentation.
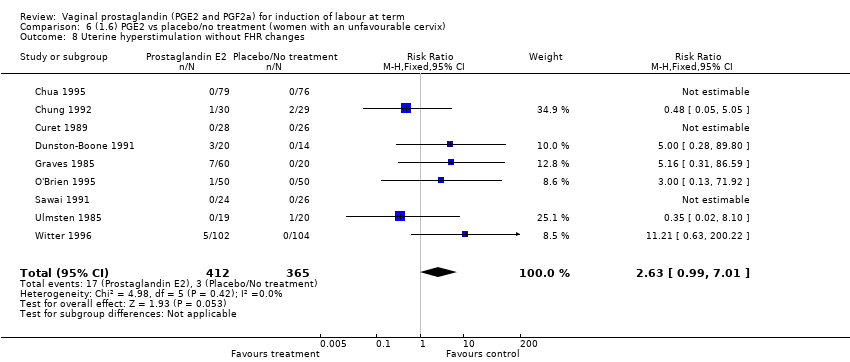
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 8 Uterine hyperstimulation without FHR changes.

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 9 Uterine rupture.
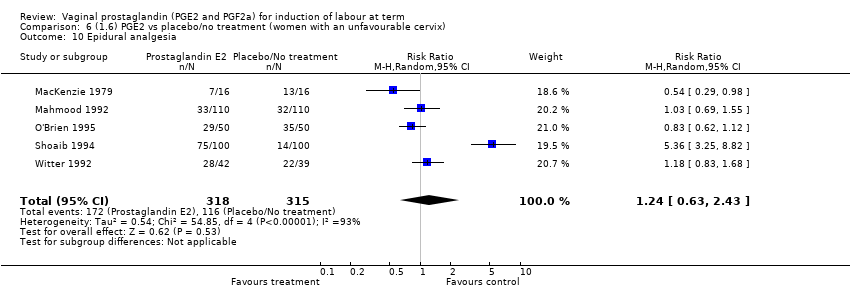
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 10 Epidural analgesia.
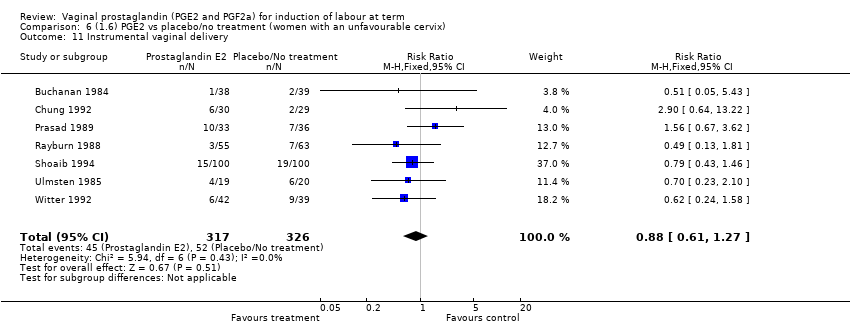
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 11 Instrumental vaginal delivery.

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 12 Meconium‐stained liquor.
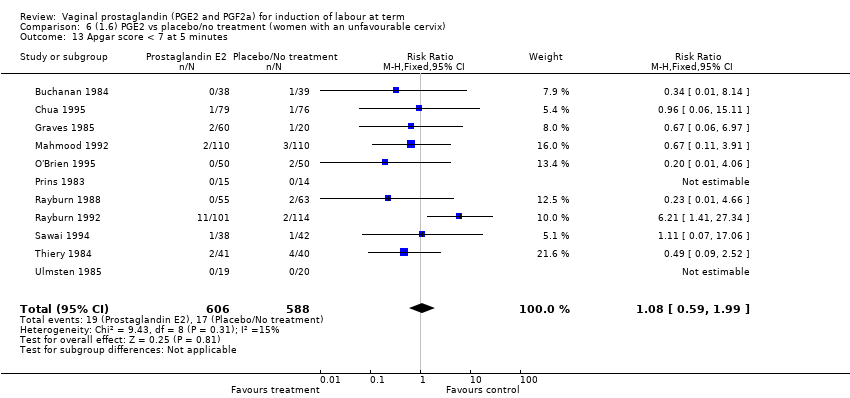
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 13 Apgar score < 7 at 5 minutes.

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 14 Neonatal intensive care unit admission.

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 15 Perinatal death.
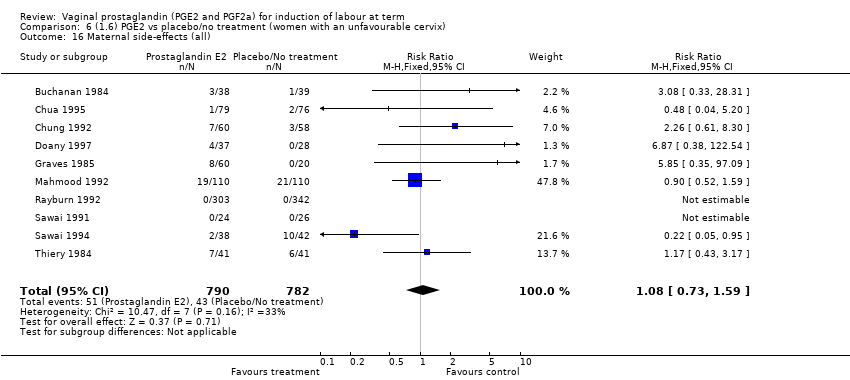
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 16 Maternal side‐effects (all).
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 17 Nausea (maternal).
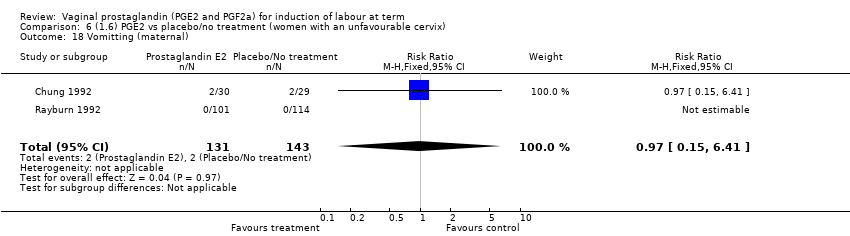
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 18 Vomitting (maternal).

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 19 Diarrhoea (maternal).
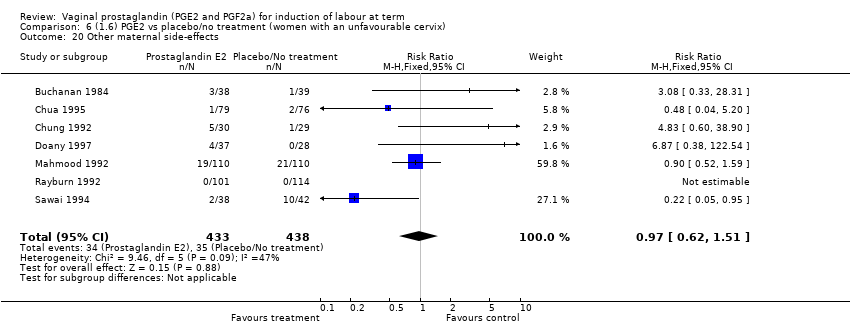
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 20 Other maternal side‐effects.
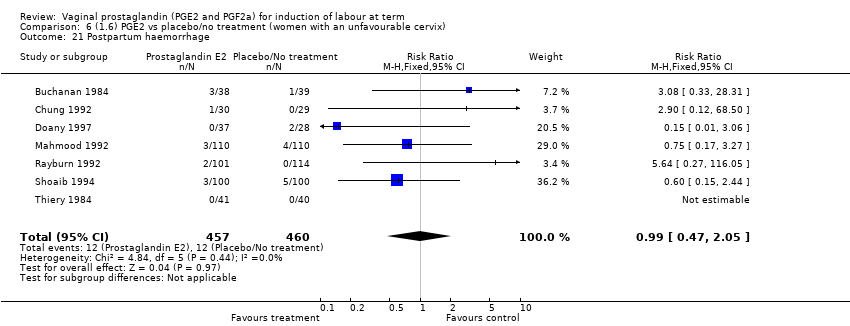
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 21 Postpartum haemorrhage.

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 22 Serious maternal complication.
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 1 Uterine hyperstimulation without FHR changes.
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 3 Caesarean section.

Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 4 Serious neonatal morbidity or perinatal death.

Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 5 Oxytocin augmentation.

Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 6 Vaginal delivery not achieved within 24 hours.
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 7 Instrumental vaginal delivery.
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 8 Perinatal death.

Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 1 Uterine hyperstimulation with FHR changes.
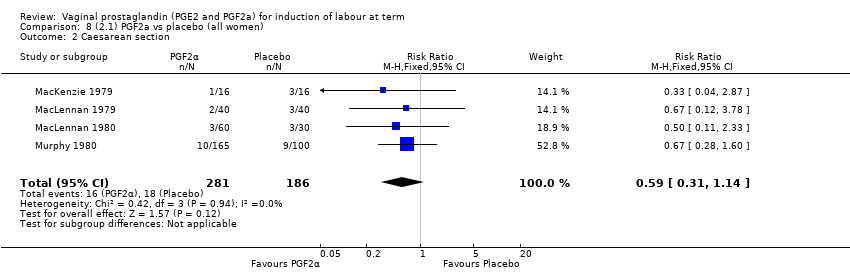
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 2 Caesarean section.
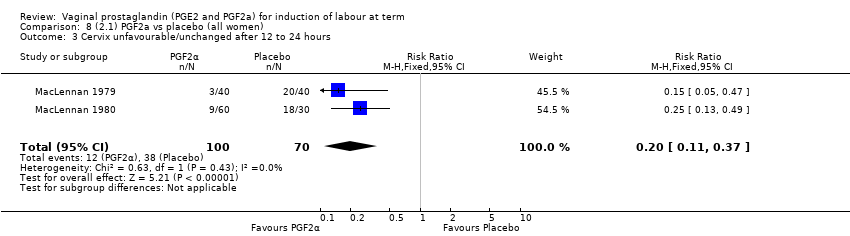
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 3 Cervix unfavourable/unchanged after 12 to 24 hours.
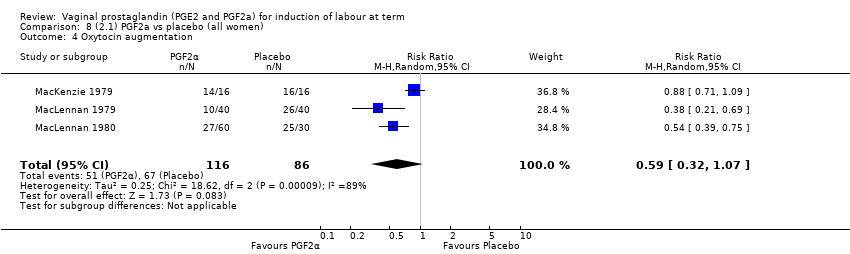
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 4 Oxytocin augmentation.

Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 5 Epidural analgesia.
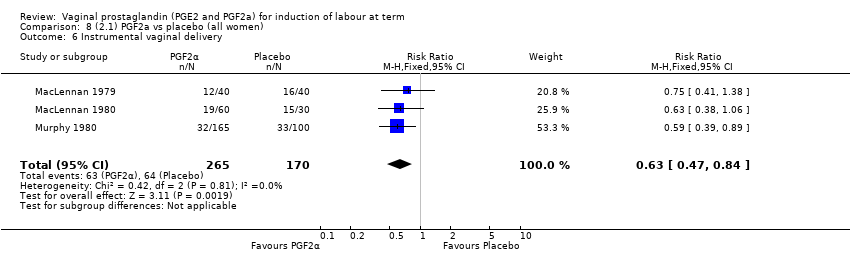
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 6 Instrumental vaginal delivery.
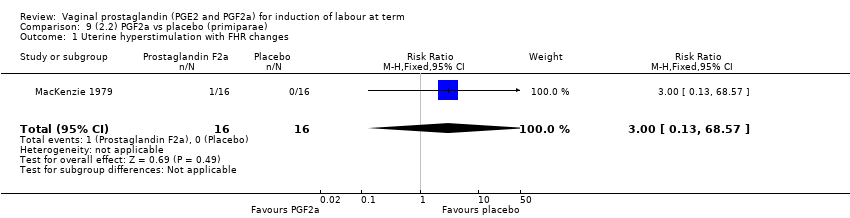
Comparison 9 (2.2) PGF2a vs placebo (primiparae), Outcome 1 Uterine hyperstimulation with FHR changes.
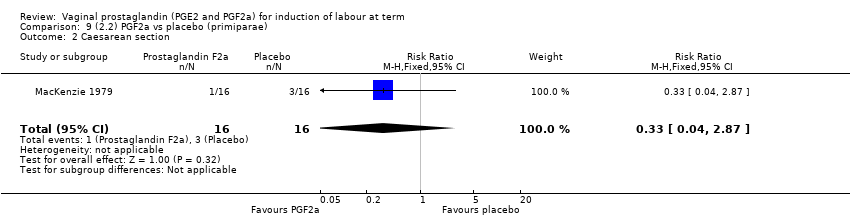
Comparison 9 (2.2) PGF2a vs placebo (primiparae), Outcome 2 Caesarean section.
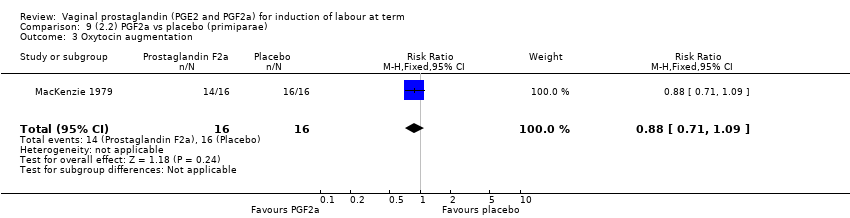
Comparison 9 (2.2) PGF2a vs placebo (primiparae), Outcome 3 Oxytocin augmentation.

Comparison 9 (2.2) PGF2a vs placebo (primiparae), Outcome 4 Epidural analgesia.
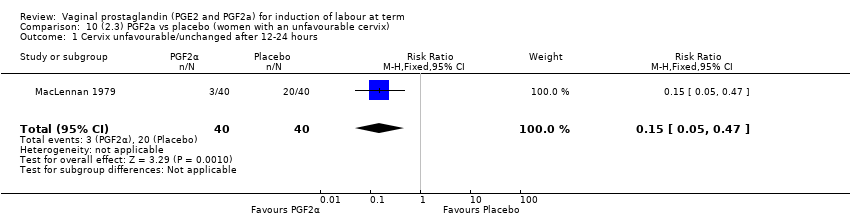
Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 1 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 3 Caesarean section.
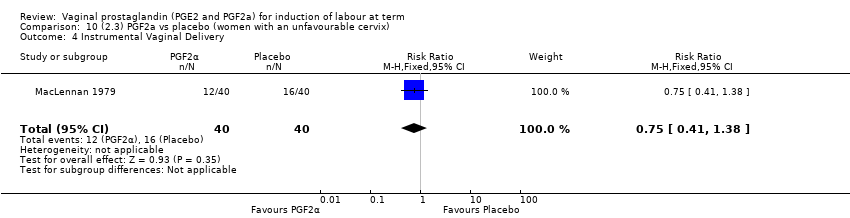
Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 4 Instrumental Vaginal Delivery.

Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 5 Oxytocin augmentation.
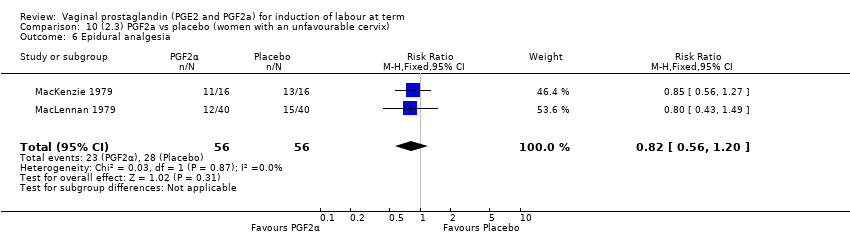
Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 6 Epidural analgesia.

Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 3 Caesarean section.
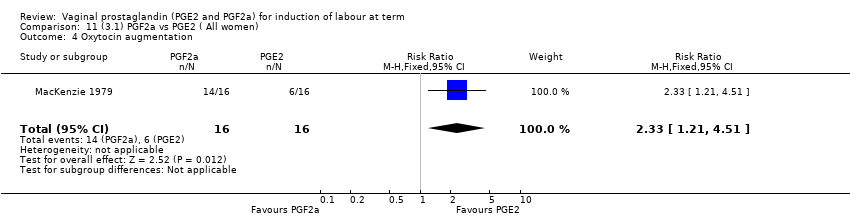
Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 4 Oxytocin augmentation.
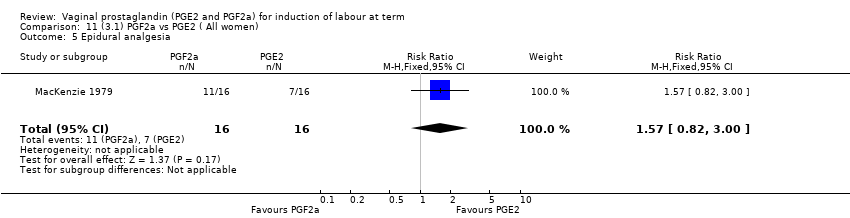
Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 5 Epidural analgesia.

Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 6 Apgar score < 7 at 5 minutes.

Comparison 12 (3.2) PGF2a vs PGE2 (primiparae), Outcome 1 Uterine hyperstimulation with FHR changes.
Comparison 12 (3.2) PGF2a vs PGE2 (primiparae), Outcome 2 Caesarean section.

Comparison 12 (3.2) PGF2a vs PGE2 (primiparae), Outcome 3 Oxytocin augmentation.
Comparison 12 (3.2) PGF2a vs PGE2 (primiparae), Outcome 4 Epidural analgesia.
Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 3 Caesarean section.
Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 4 Oxytocin augmentation.

Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 5 Epidural analgesia.

Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 6 Apgar score < 7 at 5 minutes.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 3 Caesarean section.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 4 Serious maternal morbidity or death.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 6 Oxytocin augmentation.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 7 Epidural analgesia.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 8 Instrumental vaginal delivery.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 9 Meconium Stained Liquor.
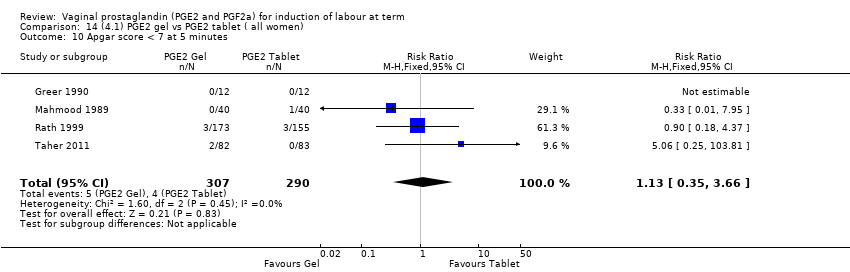
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 10 Apgar score < 7 at 5 minutes.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 11 Neonatal Intensive Care Unit Admission.

Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 12 Postpartum haemorrhage.

Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 3 Caesarean section.

Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 4 Serious maternal morbidity or death.
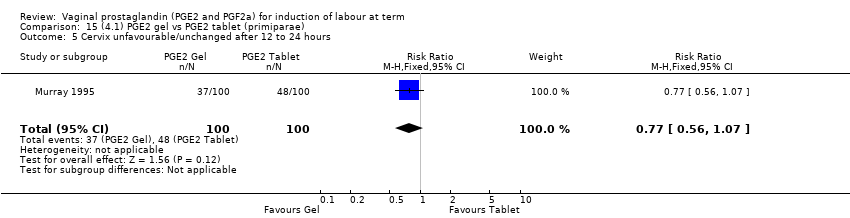
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.
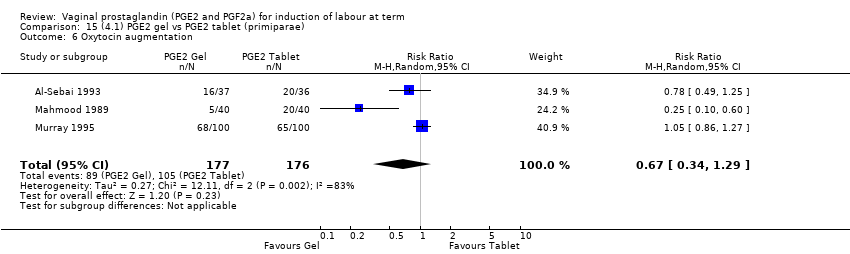
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 6 Oxytocin augmentation.

Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 7 Epidural analgesia.
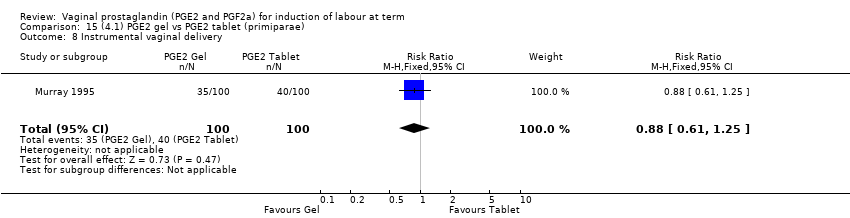
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 8 Instrumental vaginal delivery.

Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 9 Apgar score < 7 at 5 minutes.

Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 10 Postpartum haemorrhage.
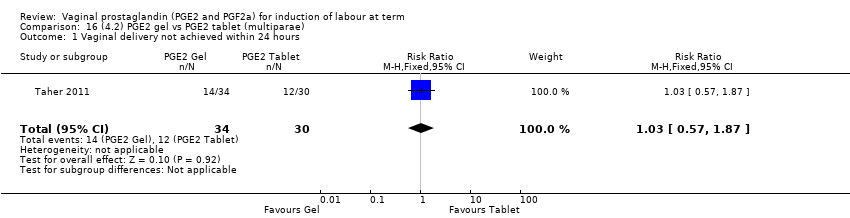
Comparison 16 (4.2) PGE2 gel vs PGE2 tablet (multiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 16 (4.2) PGE2 gel vs PGE2 tablet (multiparae), Outcome 2 Caesarean section.

Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 1 Vaginal delivery not achieved within 24 hours.
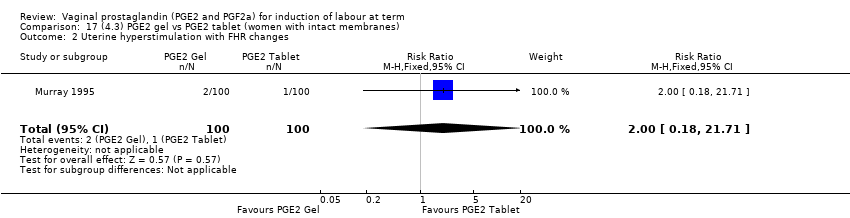
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 2 Uterine hyperstimulation with FHR changes.
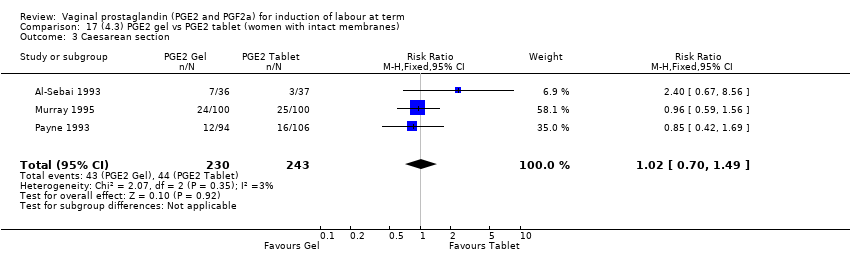
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 3 Caesarean section.
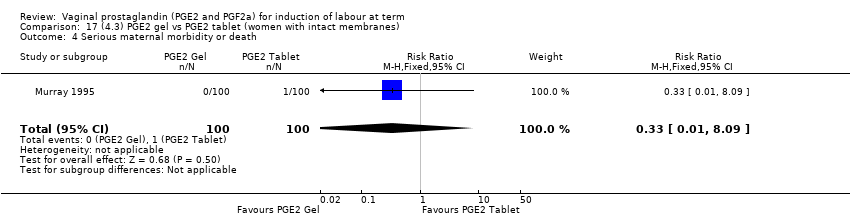
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 4 Serious maternal morbidity or death.

Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.

Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 6 Oxytocin augmentation.

Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 7 Epidural analgesia.
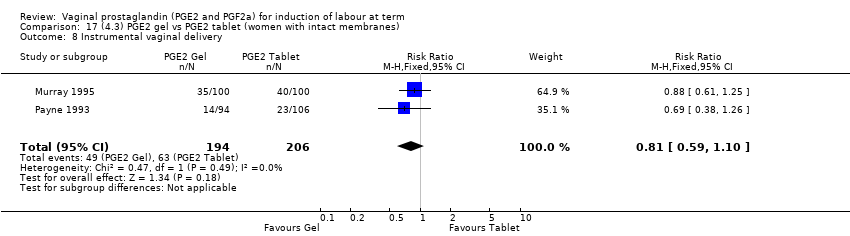
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 8 Instrumental vaginal delivery.

Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 9 Postpartum haemorrhage.

Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 3 Caesarean section.
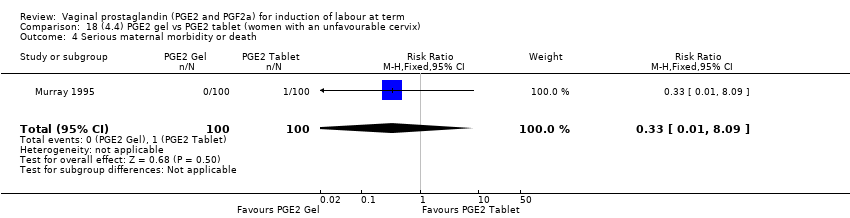
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 4 Serious maternal morbidity or death.
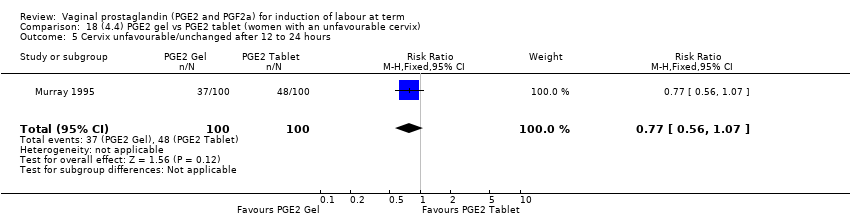
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.

Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 6 Oxytocin augmentation.
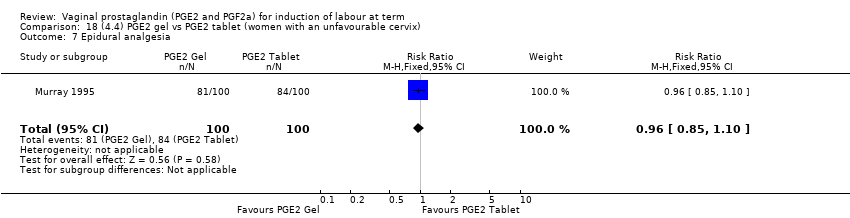
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 7 Epidural analgesia.
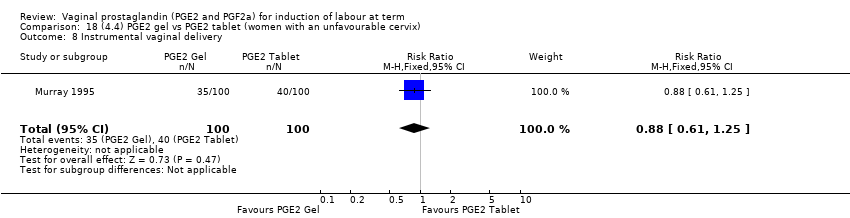
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 8 Instrumental vaginal delivery.

Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 9 Apgar score < 7 at 5 minutes.
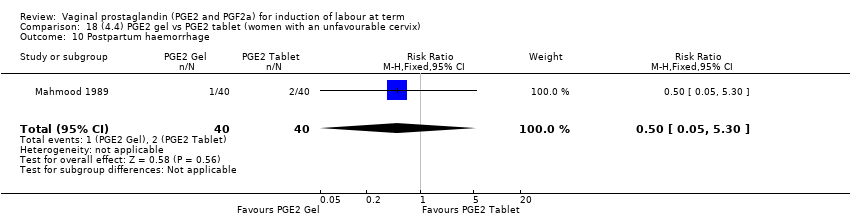
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 10 Postpartum haemorrhage.
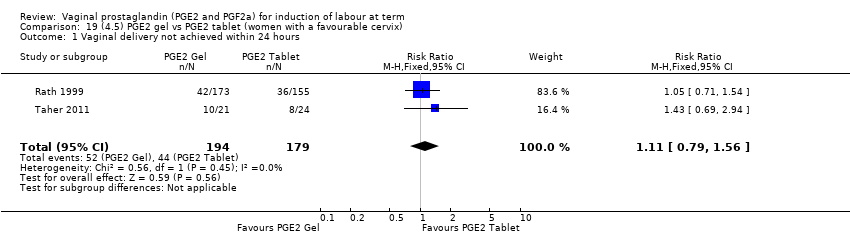
Comparison 19 (4.5) PGE2 gel vs PGE2 tablet (women with a favourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.
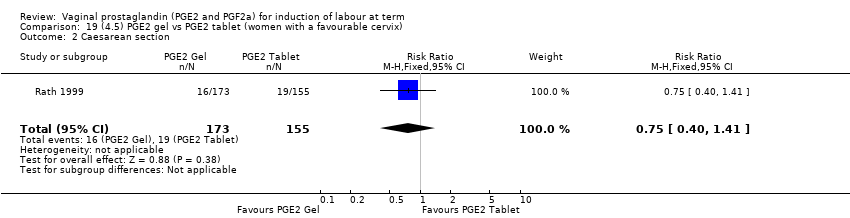
Comparison 19 (4.5) PGE2 gel vs PGE2 tablet (women with a favourable cervix), Outcome 2 Caesarean section.

Comparison 19 (4.5) PGE2 gel vs PGE2 tablet (women with a favourable cervix), Outcome 3 Oxytocin augmentation.

Comparison 19 (4.5) PGE2 gel vs PGE2 tablet (women with a favourable cervix), Outcome 4 Apgar score < 7 at 5 minutes.
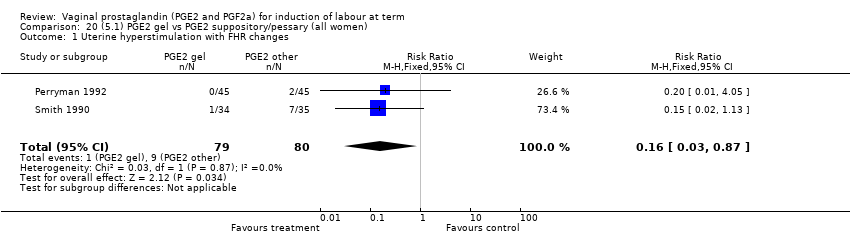
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 2 Caesarean section.
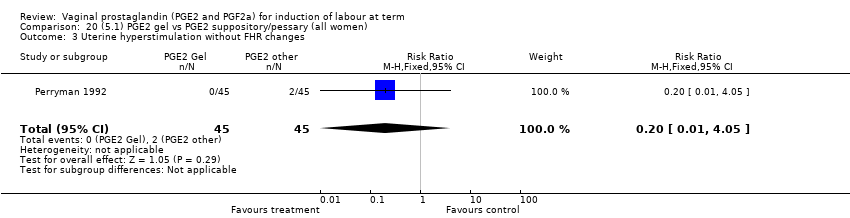
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 3 Uterine hyperstimulation without FHR changes.

Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 4 Apgar score < 7 at 5 minutes.

Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 5 Maternal side‐effects (all).
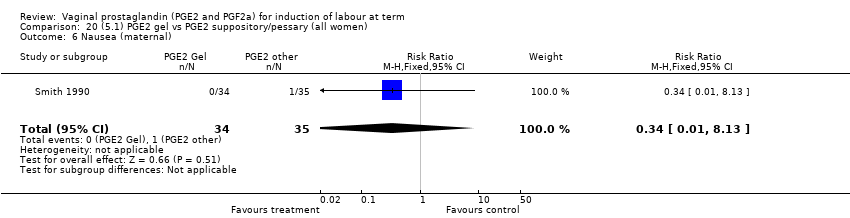
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 6 Nausea (maternal).

Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 7 Vomitting (maternal).

Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 8 Diarrhoea (maternal).

Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 9 Other maternal side‐effects.

Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.
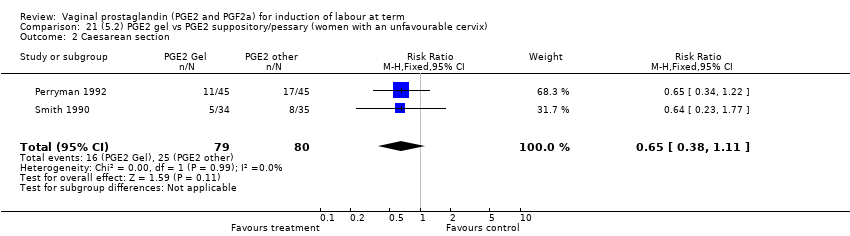
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 2 Caesarean section.
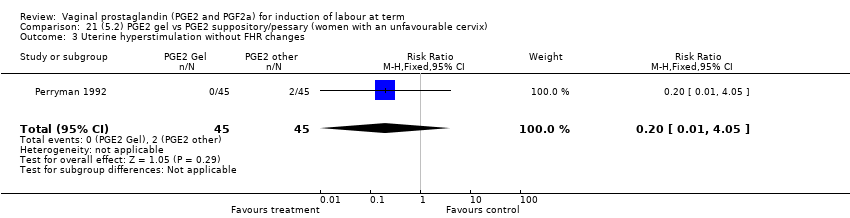
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 3 Uterine hyperstimulation without FHR changes.
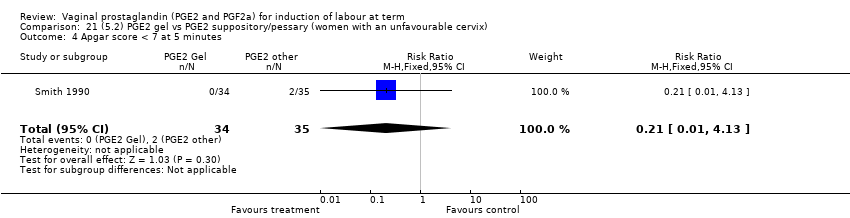
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 4 Apgar score < 7 at 5 minutes.

Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 5 Maternal side‐effects (all).

Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 6 Nausea (maternal).
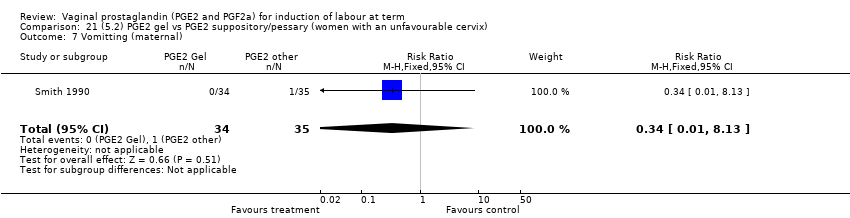
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 7 Vomitting (maternal).
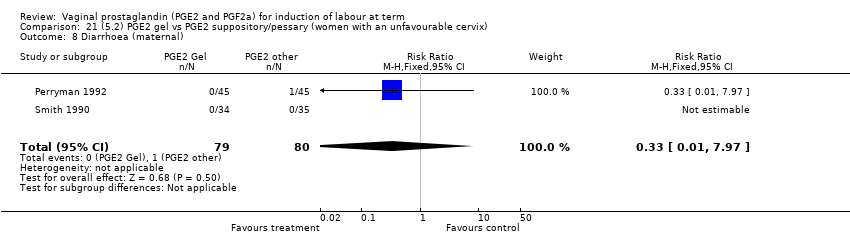
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 8 Diarrhoea (maternal).
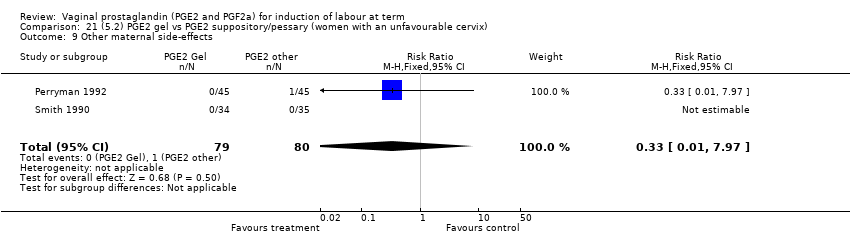
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 9 Other maternal side‐effects.
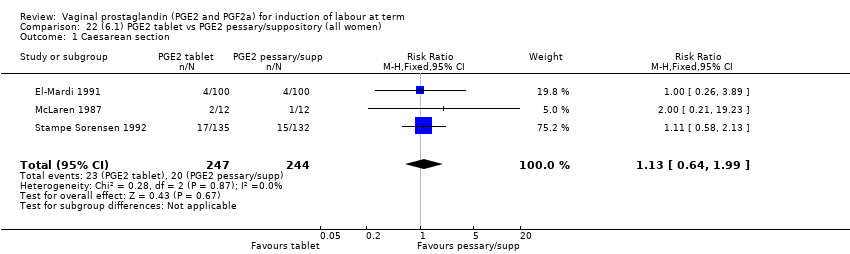
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 1 Caesarean section.

Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 2 Oxytocin augmentation.

Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 3 Uterine hyperstimulation without FHR changes.

Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 4 Epidural analgesia.

Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 5 Instrumental vaginal delivery.

Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 6 Apgar score < 7 at 5 minutes.
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 7 Maternal side‐effects (all).

Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 8 Vomitting (maternal).

Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 9 Diarrhoea (maternal).
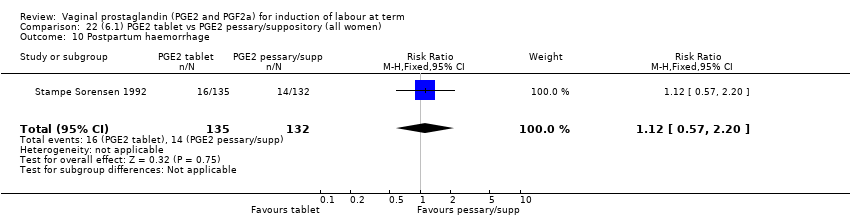
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 10 Postpartum haemorrhage.
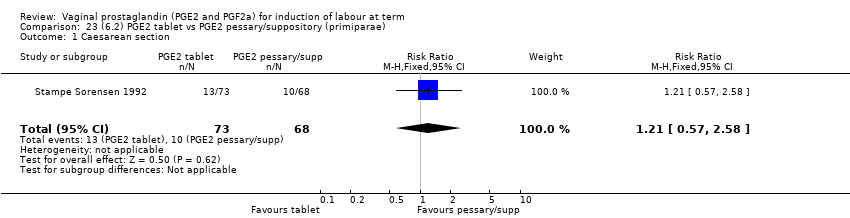
Comparison 23 (6.2) PGE2 tablet vs PGE2 pessary/suppository (primiparae), Outcome 1 Caesarean section.

Comparison 23 (6.2) PGE2 tablet vs PGE2 pessary/suppository (primiparae), Outcome 2 Oxytocin augmentation.

Comparison 24 (6.3) PGE2 tablet vs PGE2 pessary/suppository (multiparae), Outcome 1 Caesarean section.

Comparison 24 (6.3) PGE2 tablet vs PGE2 pessary/suppository (multiparae), Outcome 2 Oxytocin augmentation.

Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 1 Caesarean section.

Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 2 Oxytocin augmentation.

Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 3 Uterine hyperstimulation without FHR changes.
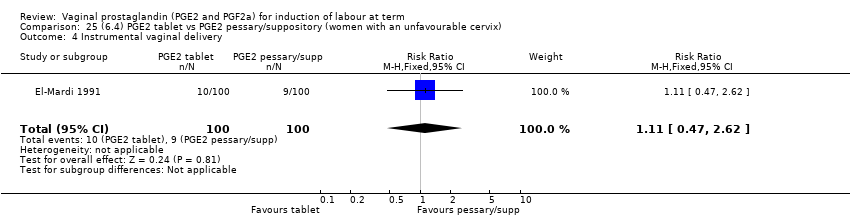
Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 4 Instrumental vaginal delivery.

Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.

Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 6 Maternal side‐effects (all).

Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 7 Vomitting (maternal).
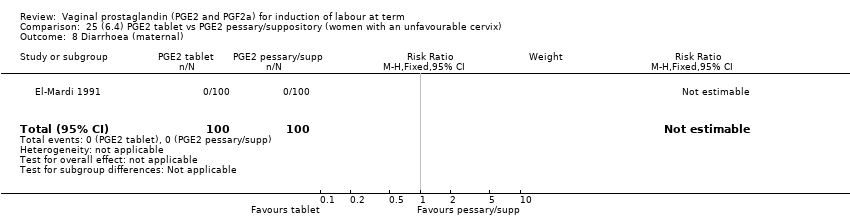
Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 8 Diarrhoea (maternal).

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 1 Vaginal delivery not achieved within 24 hours.
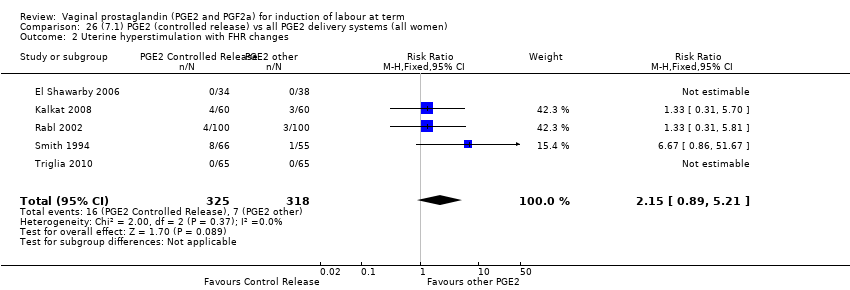
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 2 Uterine hyperstimulation with FHR changes.

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 3 Caesarean section.
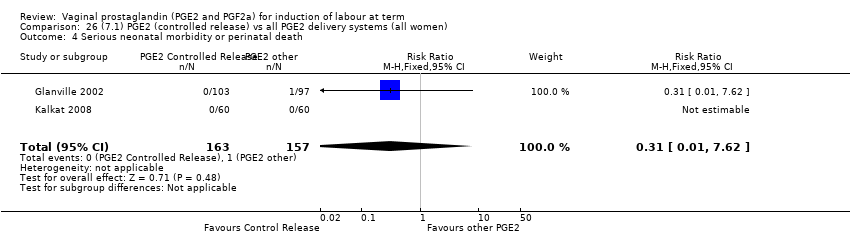
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 4 Serious neonatal morbidity or perinatal death.
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 5 Serious maternal morbidity or death.

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 6 Cervix unfavourable/unchanged after 12 ‐24 hours (BS < 3).

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 7 Oxytocin augmentation.
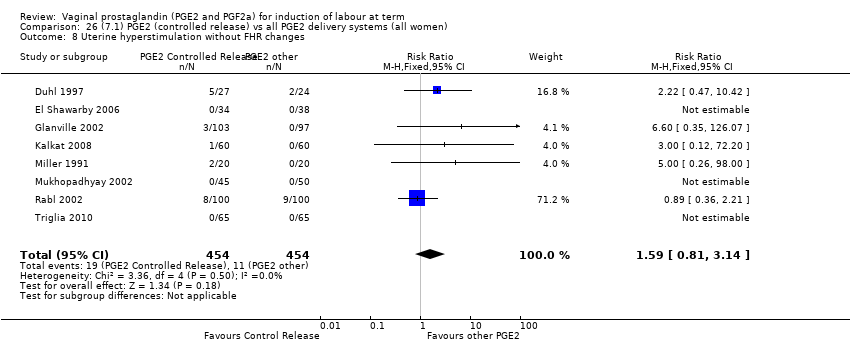
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 8 Uterine hyperstimulation without FHR changes.

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 9 Uterine rupture.

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 10 Epidural analgesia.
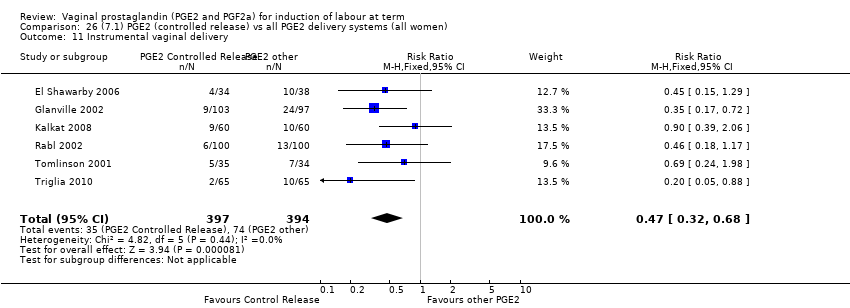
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 11 Instrumental vaginal delivery.

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 12 Postpartum haemorrhage.

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 13 Apgar score < 7 at 5 minutes.
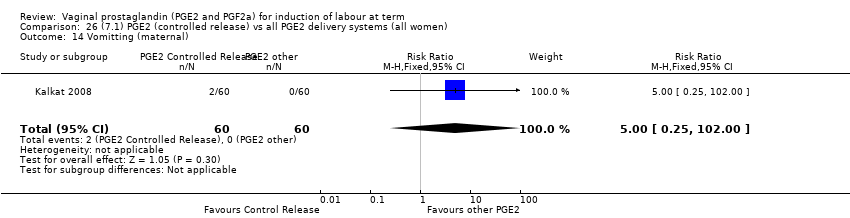
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 14 Vomitting (maternal).

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 15 Diarrhoea (maternal).

Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.
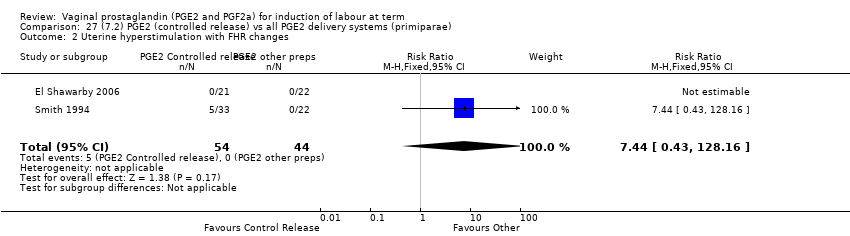
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
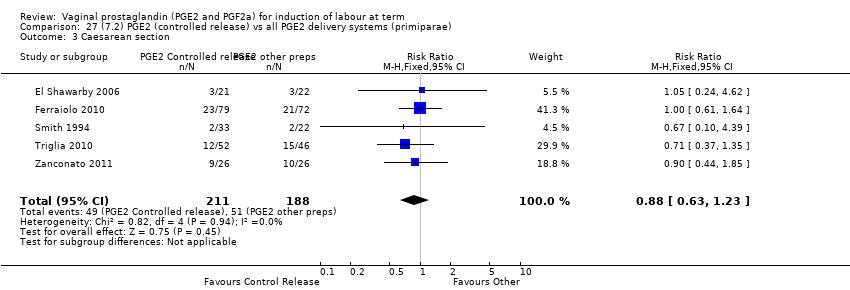
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 3 Caesarean section.

Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
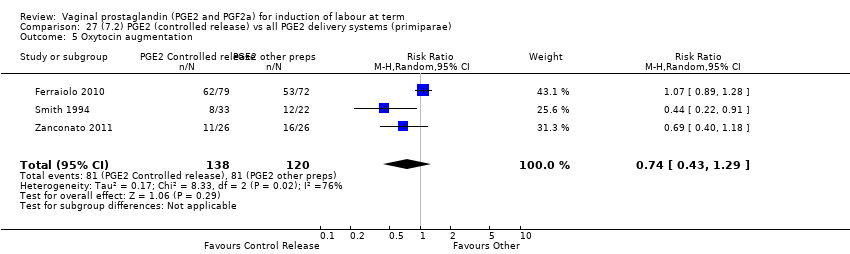
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 5 Oxytocin augmentation.
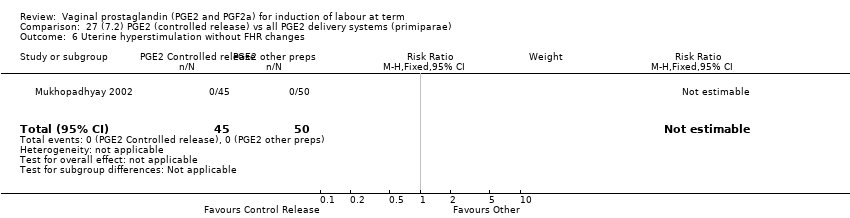
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 6 Uterine hyperstimulation without FHR changes.
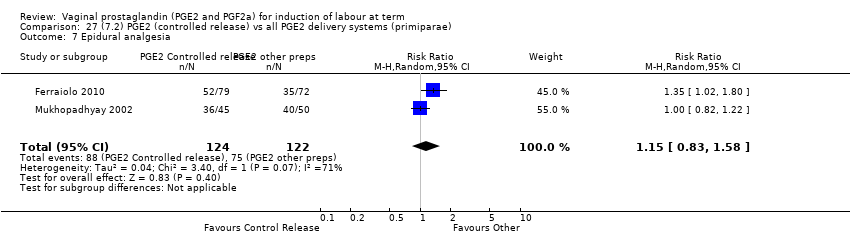
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 7 Epidural analgesia.

Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 8 Instrumental vaginal delivery.

Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
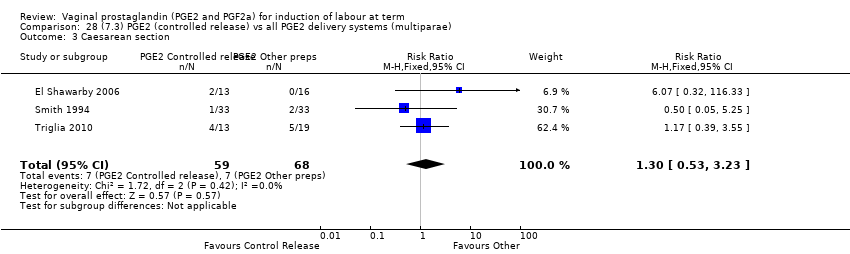
Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 3 Caesarean section.

Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 4 Oxytocin augmentation.

Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 5 Instrumental vaginal delivery.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
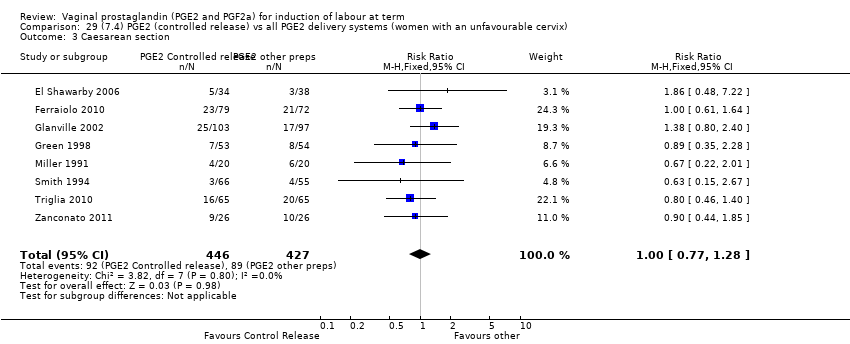
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 3 Caesarean section.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 4 Serious neonatal morbidity or perinatal death.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 5 Serious maternal morbidity or death.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 7 Oxytocin augmentation.
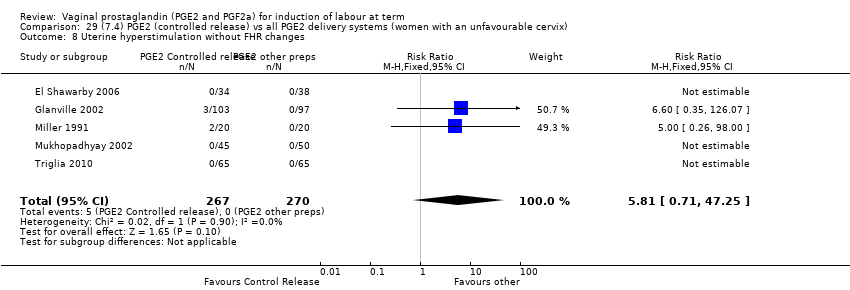
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 8 Uterine hyperstimulation without FHR changes.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 9 Uterine rupture.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 10 Epidural analgesia.
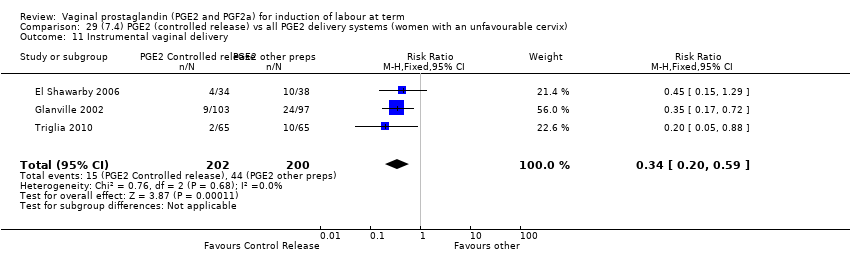
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 11 Instrumental vaginal delivery.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 12 Apgar score < 7 at 5 minutes.

Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 13 Postpartum haemorrhage.
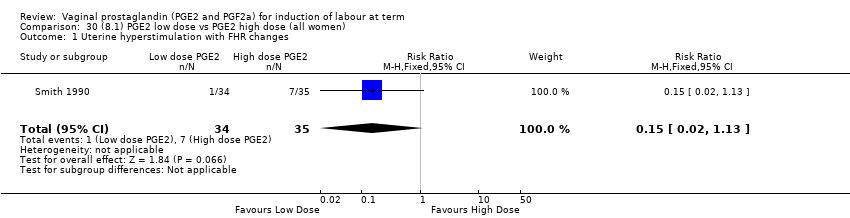
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 1 Uterine hyperstimulation with FHR changes.

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 2 Caesarean section.

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 3 Serious neonatal morbidity or perinatal death.

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 4 Cervix unfavourable/unchanged after 12‐24hrs .

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 5 Oxytocin augmentation.

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 6 Uterine hyperstimulation without FHR changes.

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 7 Epidural analgesia.

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 8 Instrumental vaginal delivery.
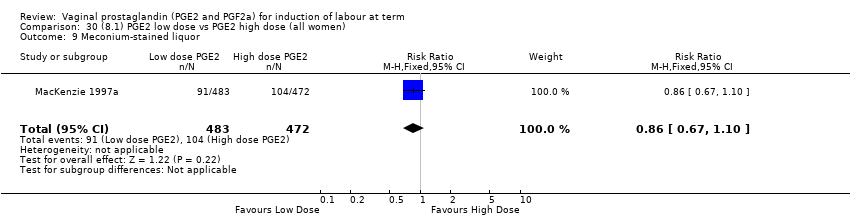
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 9 Meconium‐stained liquor.
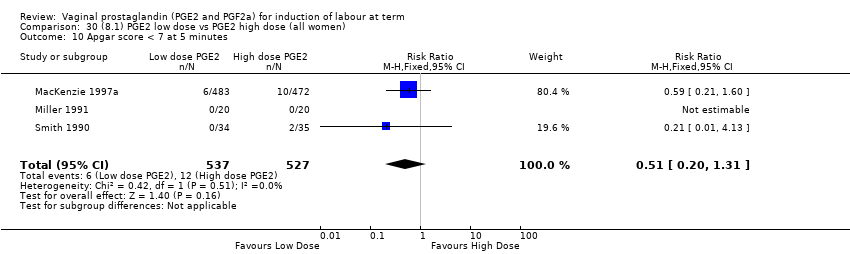
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 10 Apgar score < 7 at 5 minutes.
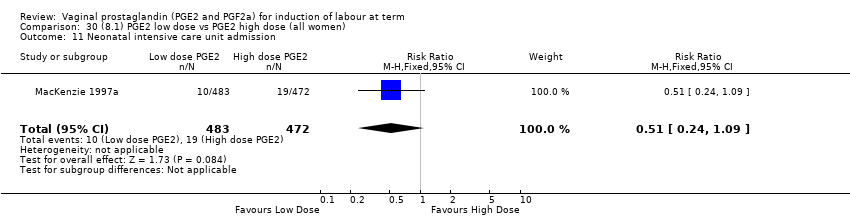
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 11 Neonatal intensive care unit admission.

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 12 Perinatal death.
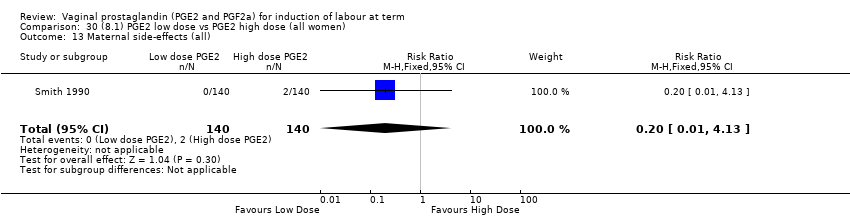
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 13 Maternal side‐effects (all).
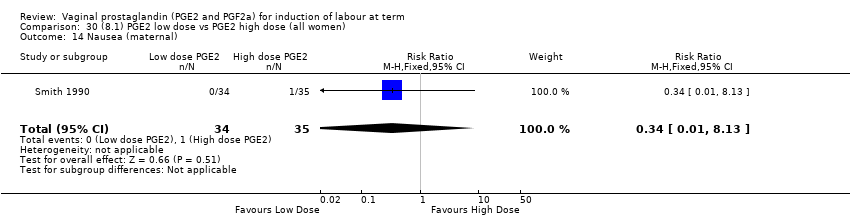
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 14 Nausea (maternal).
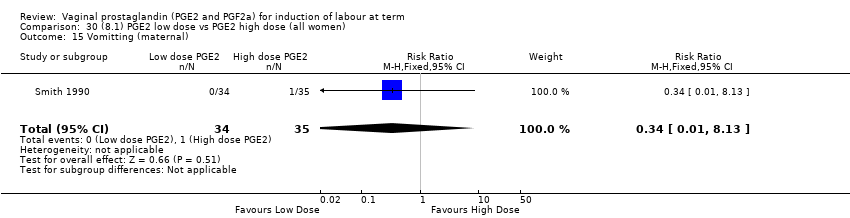
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 15 Vomitting (maternal).

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 16 Diarrhoea (maternal).

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 17 Other maternal side‐effects.

Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 18 Postpartum haemorrhage.
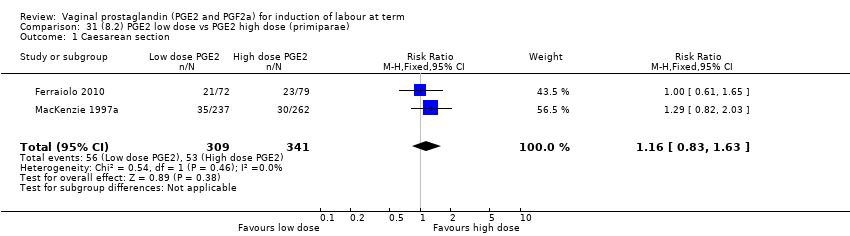
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 1 Caesarean section.

Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 2 Serious neonatal morbidity or perinatal death.
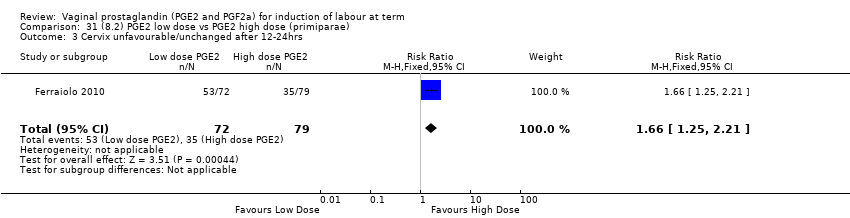
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 3 Cervix unfavourable/unchanged after 12‐24hrs .

Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 4 Oxytocin augmentation.

Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 5 Epidural analgesia.

Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 6 Instrumental vaginal delivery.
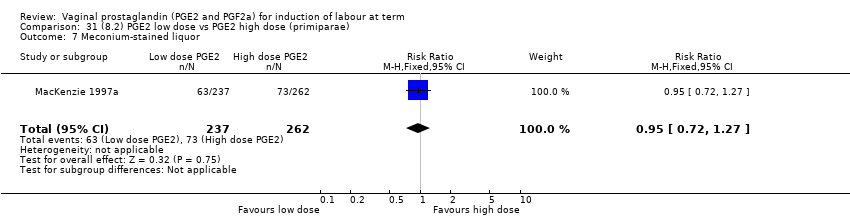
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 7 Meconium‐stained liquor.

Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 8 Apgar score < 7 at 5 minutes.

Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 9 Neonatal intensive care unit admission.
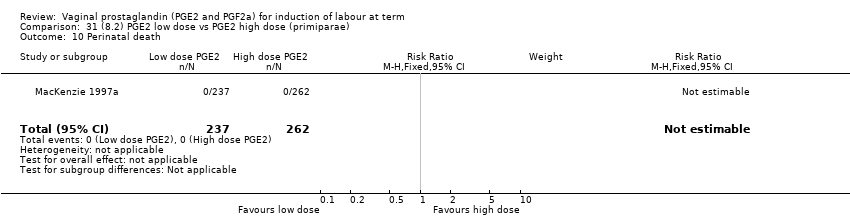
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 10 Perinatal death.

Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 11 Postpartum haemorrhage.
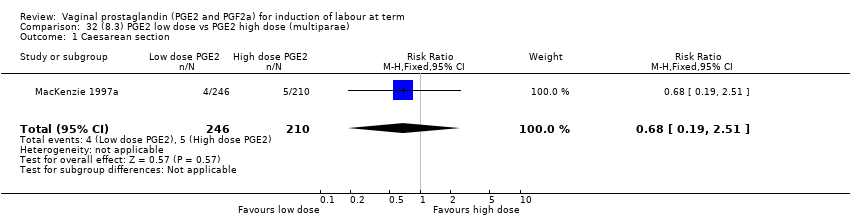
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 1 Caesarean section.

Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 2 Serious neonatal morbidity or perinatal death.
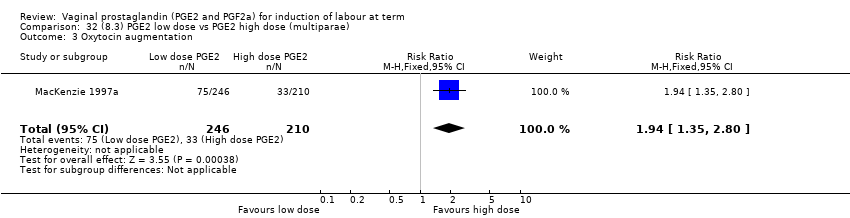
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 3 Oxytocin augmentation.

Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 4 Epidural analgesia.

Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 5 Instrumental vaginal delivery.
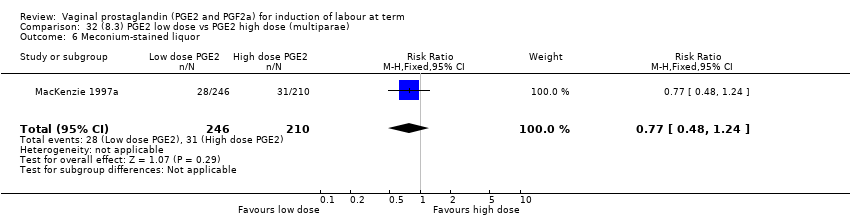
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 6 Meconium‐stained liquor.

Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 7 Apgar score < 7 at 5 minutes.
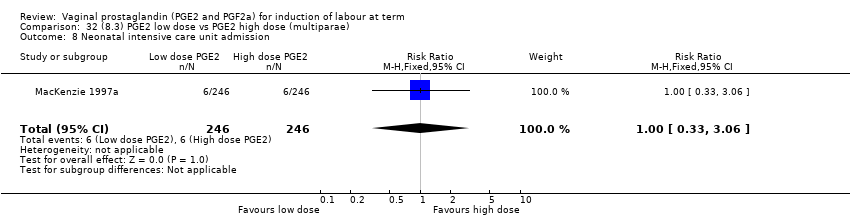
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 8 Neonatal intensive care unit admission.

Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 9 Perinatal death.
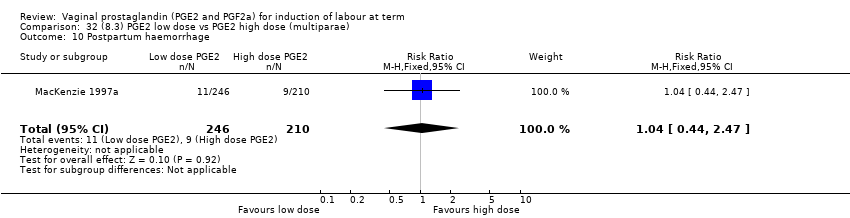
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 10 Postpartum haemorrhage.
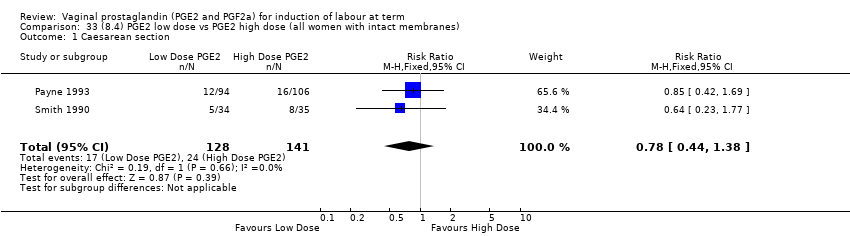
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 1 Caesarean section.
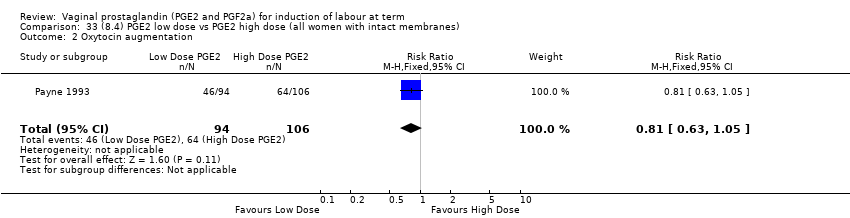
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 2 Oxytocin augmentation.
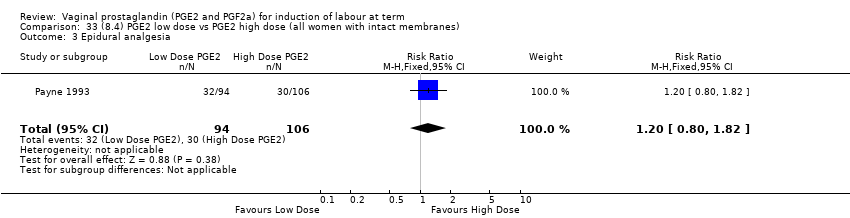
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 3 Epidural analgesia.
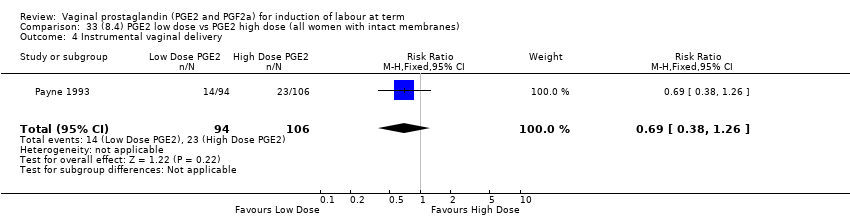
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 4 Instrumental vaginal delivery.
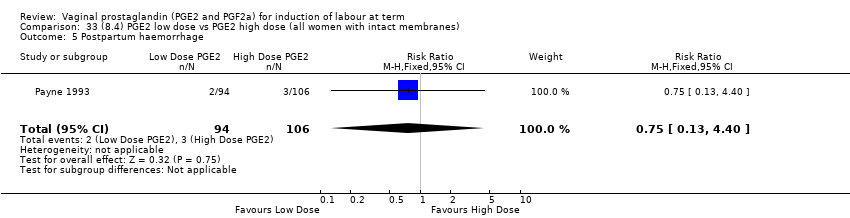
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 5 Postpartum haemorrhage.

Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 6 Uterine hyperstimulation with FHR changes.

Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 8 Maternal side‐effects (all).
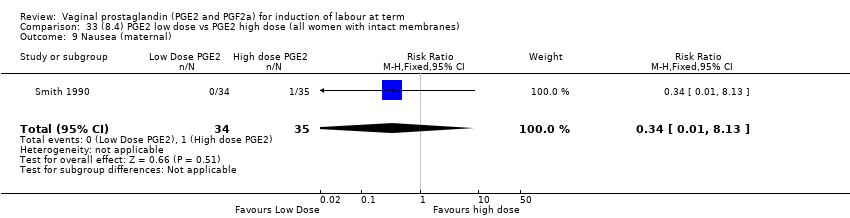
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 9 Nausea (maternal).

Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 10 Vomitting (maternal).

Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 11 Diarrhoea (maternal).
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 12 Other maternal side‐effects.

Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.
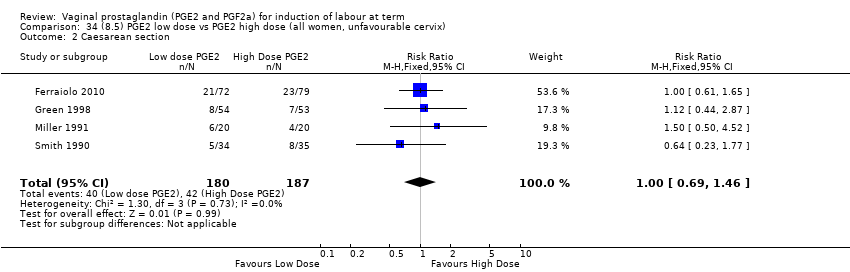
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 2 Caesarean section.
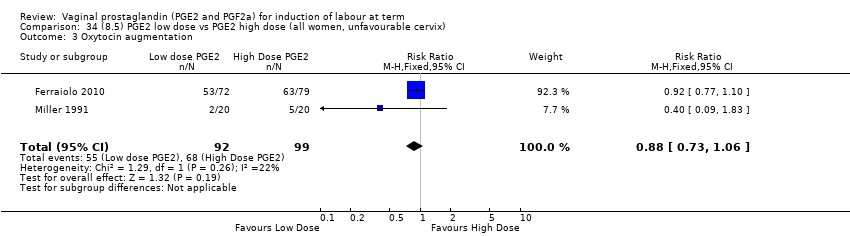
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 3 Oxytocin augmentation.
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 4 Uterine hyperstimulation without FHR changes.

Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.

Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 6 Maternal side‐effects (all).
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 7 Nausea (maternal).
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 8 Vomitting (maternal).
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 9 Diarrhoea (maternal).

Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 10 Other maternal side‐effects.
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 1 Uterine hyperstimulation with FHR changes.
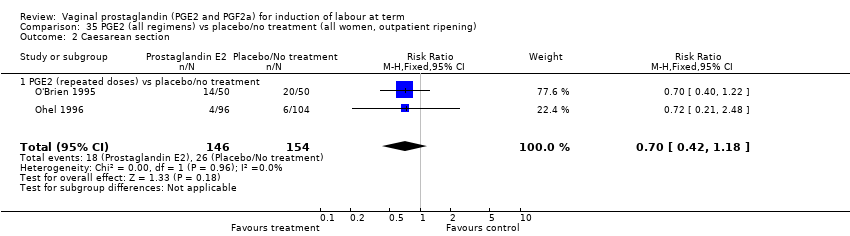
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 2 Caesarean section.

Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 3 Cervix unfavourable/unchanged after 12 to 24 hours.
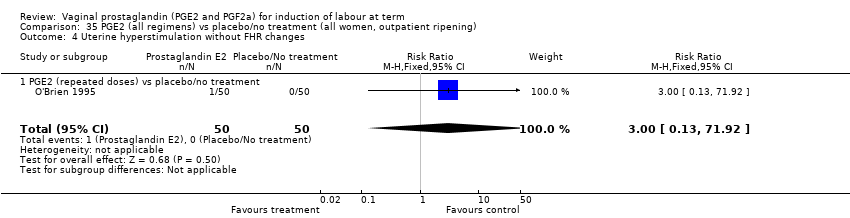
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 4 Uterine hyperstimulation without FHR changes.

Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 5 Epidural analgesia.

Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 6 Meconium‐stained liquor.
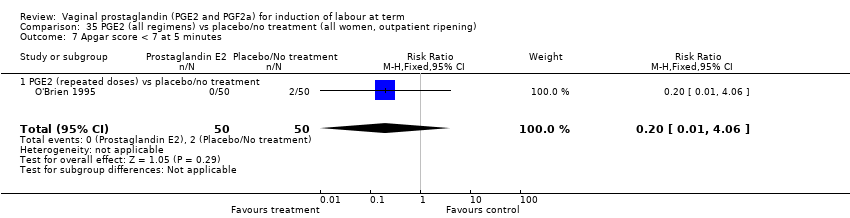
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 7 Apgar score < 7 at 5 minutes.

Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 8 Neonatal intensive care unit admission.
| PGE2 compared with placebo or no treatment for induction of labour at term (all women) | ||||||
| Patient or population: patients with induction of labour at term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment (all women) | PGE2 (all regimens) | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 0.32 | 384 | ⊕⊝⊝⊝ | Probable reduction in time to delivery using PGE2. Useable data only available in 2 of 15 studies reporting time as an outcome. 39 studies in this comparison. | |
| 989 per 1000 | 317 per 1000 | |||||
| Moderate | ||||||
| 950 per 1000 | 304 per 1000 | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 3.16 | 1359 | ⊕⊕⊕⊝ | The risk of bias is "unclear" for most quality domain of the 15 RCT's and this may be a serious limitation. | |
| 10 per 1000 | 33 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Caesarean section | Study population | RR 0.91 | 6599 | ⊕⊕⊕⊕ | The risk of bias is unclear for most of the studies, but the largest study with a quarter of the participants) has a low risk of bias. | |
| 148 per 1000 | 134 per 1000 | |||||
| Moderate | ||||||
| 166 per 1000 | 151 per 1000 | |||||
| Serious neonatal morbidity or perinatal death | Study population | RR 0.46 | 3638 | ⊕⊕⊝⊝ | Neonatal morbidity or mortality is rare, several studies have no events. Underpowered to detect a difference even if one exists. | |
| 2 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Serious maternal morbidity or death | Study population | RR 2.23 | 530 | ⊕⊝⊝⊝ | A very rare outcome, so underpowered to detect a difference if one exists. | |
| 4 per 1000 | 9 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| (4.1) PGE2 gel compared with PGE2 tablet (all women) for induction of labour at term | ||||||
| Patient or population: patients with induction of labour at term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PGE2 tablet (all women) | (4.1) PGE2 gel | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 1.03 | 566 | ⊕⊕⊕⊝ | Most quality domains unclear or low risk but loss to follow up and reporting bias high in 1 trial. | |
| 369 per 1000 | 380 per 1000 | |||||
| Moderate | ||||||
| 528 per 1000 | 544 per 1000 | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 2 | 200 | ⊕⊝⊝⊝ | Only 1 small trial with an unclear risk of bias reports this outcome. | |
| 10 per 1000 | 20 per 1000 | |||||
| Moderate | ||||||
| 10 per 1000 | 20 per 1000 | |||||
| Caesarean section | Study population | RR 0.91 | 1046 | ⊕⊕⊕⊕ | The risk of bias is unclear for most studies, but the largest study has a low risk of bias. | |
| 198 per 1000 | 180 per 1000 | |||||
| Moderate | ||||||
| 201 per 1000 | 183 per 1000 | |||||
| Serious maternal morbidity or death | Study population | RR 0.33 | 200 | See comment | Study far too small to detect a difference. | |
| 10 per 1000 | 3 per 1000 | |||||
| Moderate | ||||||
| 10 per 1000 | 3 per 1000 | |||||
| Instrumental vaginal delivery | Study population | RR 0.77 | 565 | ⊕⊕⊕⊝ | The largest study has a high risk of bias. This is a secondary outcome in this review. | |
| 287 per 1000 | 221 per 1000 | |||||
| Moderate | ||||||
| 241 per 1000 | 186 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| (7.1) PGE2 (controlled release) compared with all PGE2 delivery systems (all women) for induction of labour at term | ||||||
| Patient or population: patients with induction of labour at term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| All PGE2 delivery systems (all women) | (7.1) PGE2 (controlled release) | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 1.15 | 450 | ⊕⊕⊕⊝ | Although all published after 2002, the risk of bias for most quality domains unclear. | |
| 373 per 1000 | 429 per 1000 | |||||
| Moderate | ||||||
| 333 per 1000 | 383 per 1000 | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 2.15 | 643 | ⊕⊕⊕⊝ | 4 of the studies are recent but risk of bias unclear. | |
| 22 per 1000 | 47 per 1000 | |||||
| Moderate | ||||||
| 18 per 1000 | 39 per 1000 | |||||
| Caesarean section | Study population | RR 1.02 | 1262 | ⊕⊕⊕⊝ | Risk of bias unclear, recent studies poorly reported. | |
| 201 per 1000 | 205 per 1000 | |||||
| Moderate | ||||||
| 177 per 1000 | 181 per 1000 | |||||
| Serious neonatal morbidity or perinatal death | Study population | RR 0.31 | 320 | ⊕⊕⊝⊝ | Underpowered to detect effect even if exists. | |
| 6 per 1000 | 2 per 1000 | |||||
| Moderate | ||||||
| 5 per 1000 | 2 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer‐generated sequence, random number tables, lot drawing, coin tossing, shuffling cards, throwing dice. | Case number, date of birth, date of admission, alternation. |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially sealed opaque envelopes. | Open allocation sequence, any procedure based on inadequate generation. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 2 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.02, 4.83] |
| 1.1 PGE2 (once only) vs placebo/no treatment | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.15] |
| 1.2 PGE2 (repeated doses) vs placebo/no treatment | 1 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.08, 0.18] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 15 | 1359 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.67, 5.98] |
| 2.1 PGE2 (once only) vs placebo/no treatment | 7 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.46, 4.15] |
| 2.2 PGE2 (repeated doses) vs placebo/no treatment | 3 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.34 [0.27, 106.70] |
| 2.3 PGE2 (sustained release) vs placebo/no treatment | 5 | 636 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.53 [1.92, 10.65] |
| 3 Caesarean section Show forest plot | 36 | 6599 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.81, 1.02] |
| 3.1 PGE2 (once only) vs placebo/no treatment | 16 | 1405 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.83, 1.24] |
| 3.2 PGE2 (repeated doses) vs placebo/no treatment | 15 | 4523 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.73, 1.02] |
| 3.3 PGE2 (sustained release) vs placebo/no treatment | 5 | 671 | Risk Ratio (IV, Fixed, 95% CI) | 0.85 [0.65, 1.12] |
| 4 Serious neonatal morbidity or perinatal death Show forest plot | 9 | 3638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.31] |
| 4.1 PGE2 (once only) vs placebo/no treatment | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 3269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.31] |
| 4.3 PGE2 (sustained release) vs placebo/no treatment | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death Show forest plot | 3 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.34, 14.76] |
| 5.1 PGE2 (once only) vs placebo | 2 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.34, 14.76] |
| 5.2 PGE2 (sustained release) vs placebo/no treatment | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 6 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.27, 0.65] |
| 6.1 PGE2 (once only) vs placebo/no treatment | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.39, 0.73] |
| 6.2 PGE2 (repeated doses) vs placebo/no treatment | 2 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.07, 1.08] |
| 6.3 1.6.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.05, 0.45] |
| 7 Oxytocin augmentation Show forest plot | 13 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.05] |
| 7.1 PGE2 (once only) vs placebo/no treatment | 7 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.59, 1.47] |
| 7.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 795 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.01] |
| 7.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.64] |
| 8 Uterine hyperstimulation without FHR changes Show forest plot | 13 | 3636 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.17, 5.26] |
| 8.1 PGE2 (once only) vs placebo/no treatment | 6 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.33, 4.84] |
| 8.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 2953 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.78, 7.03] |
| 8.3 PGE2 (sustained release) vs placebo/no treatment | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.85 [1.05, 58.82] |
| 9 Uterine rupture Show forest plot | 2 | 2579 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 9.1 PGE2 (once only) vs placebo/no treatment | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 9.2 PGE2 (repeated doses) vs placebo/no treatment | 1 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia Show forest plot | 7 | 3555 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.85, 1.60] |
| 10.1 PGE2 (once only) vs placebo/no treatment | 2 | 434 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.41, 1.55] |
| 10.2 PGE2 (repeated doses) vs placebo/no treatment | 4 | 3040 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.81, 2.44] |
| 10.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.83, 1.68] |
| 11 Instrumental vaginal delivery Show forest plot | 13 | 4219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 11.1 PGE2 (once only) vs placebo/no treatment | 6 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.28] |
| 11.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 3348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.13] |
| 11.3 PGE2 (sustained release) vs placebo/no treatment | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.55, 1.86] |
| 12 Meconium‐stained liquor Show forest plot | 12 | 4245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.98] |
| 12.1 PGE2 (once only) vs placebo/no treatment | 5 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.40] |
| 12.2 PGE2 (repeated doses) vs placebo/no treatment | 7 | 3541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.97] |
| 13 Apgar score < 7 at 5 minutes Show forest plot | 16 | 4481 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.86, 1.92] |
| 13.1 PGE2 (once only) vs placebo/no treatment | 9 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.30] |
| 13.2 PGE2 (repeated doses) vs placebo/no treatment | 6 | 3220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.80, 2.27] |
| 13.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.21 [1.41, 27.34] |
| 14 Neonatal intensive care unit admission Show forest plot | 12 | 4022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.14] |
| 14.1 PGE2 (once only) vs placebo/no treatment | 4 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.70, 2.15] |
| 14.2 PGE2 (repeated doses) vs placebo/no treatment | 7 | 3272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.10] |
| 14.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.36, 29.93] |
| 15 Perinatal death Show forest plot | 7 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.14, 2.22] |
| 15.1 PGE2 (once only) vs placebo/no treatment | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.85] |
| 15.2 PGE2 (repeated doses) vs placebo/no treatment | 4 | 3148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.31] |
| 15.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal side‐effects (all) Show forest plot | 12 | 6780 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.67] |
| 16.1 PGE2 (once only) vs placebo/no treatment | 6 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.02, 3.74] |
| 16.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 5558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.34] |
| 16.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Nausea (maternal) Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Vomitting (maternal) Show forest plot | 3 | 2794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.39] |
| 18.1 PGE2 (once only) vs placebo/no treatment | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.15, 6.41] |
| 18.2 PGE2 (repeated doses) vs placebo/no treatment | 1 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.65] |
| 18.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Diarrhoea (maternal) Show forest plot | 3 | 2819 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.01 [0.36, 135.59] |
| 19.1 PGE2 (repeated doses) vs placebo/no treatment | 2 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.01 [0.36, 135.59] |
| 19.2 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Other maternal side‐effects Show forest plot | 7 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.51] |
| 20.1 PGE2 (once only) vs placebo/no treatment | 4 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.97, 8.02] |
| 20.2 PGE2 (repeated doses) vs placebo/no treatment | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.42, 1.15] |
| 20.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postpartum haemorrhage Show forest plot | 9 | 3537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.04, 2.09] |
| 21.1 PGE2 (once only) vs placebo/no treatment | 4 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.33, 3.97] |
| 21.2 PGE2 (repeated doses) vs placebo/no treatment | 4 | 3040 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.01, 2.11] |
| 21.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.64 [0.27, 116.05] |
| 22 Serious maternal complication Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 22.1 PGE2 (once only) vs placebo/no treatment | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 23 Woman not satisfied Show forest plot | 2 | 2922 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.24, 2.40] |
| 23.1 PGE2 (once only) vs placebo/no treatment | 1 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.83, 2.35] |
| 23.2 PGE2 (repeated doses) vs placebo/no treatment | 1 | 2520 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.33, 0.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 2 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.06, 2.80] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 3 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 3 Caesarean section Show forest plot | 10 | 2486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.77, 1.12] |
| 4 Serious neonatal morbidity or perinatal death Show forest plot | 3 | 1796 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.22] |
| 5 Serious maternal morbidity or death Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.47] |
| 7 Oxytocin augmentation Show forest plot | 3 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.47, 0.74] |
| 8 Uterine hyperstimulation without FHR changes Show forest plot | 3 | 1701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.10] |
| 9 Uterine rupture Show forest plot | 1 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia Show forest plot | 4 | 1959 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.64, 2.73] |
| 11 Instrumental vaginal delivery Show forest plot | 4 | 1815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.14] |
| 12 Meconium‐stained liquor Show forest plot | 2 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.13] |
| 13 Apgar score < 7 at 5 minutes Show forest plot | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.17, 3.27] |
| 14 Neonatal intensive care unit admission Show forest plot | 3 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.54, 2.09] |
| 15 Perinatal death Show forest plot | 3 | 1776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.22] |
| 16 Maternal side‐effects (all) Show forest plot | 3 | 1882 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.63, 1.71] |
| 17 Vomitting (maternal) Show forest plot | 1 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.40, 7.00] |
| 18 Diarrhoea (maternal) Show forest plot | 1 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [0.24, 104.66] |
| 19 Other maternal side‐effects Show forest plot | 2 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.50] |
| 20 Postpartum haemorrhage Show forest plot | 3 | 1927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.97, 2.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.12] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 5 | 1298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.48, 1.42] |
| 4 Serious neonatal morbidity or perinatal death Show forest plot | 2 | 1113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 5 Uterine rupture Show forest plot | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Epidural analgesia Show forest plot | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.24] |
| 7 Oxytocin augmentation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.18, 0.97] |
| 8 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.94 [0.61, 197.24] |
| 9 Instrumental vaginal delivery Show forest plot | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.77, 1.95] |
| 10 Meconium‐stained liquor Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.82] |
| 11 Perinatal death Show forest plot | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 12 Maternal side‐effects (all) Show forest plot | 1 | 2026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.87] |
| 13 Vomitting (maternal) Show forest plot | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 14 Diarrhoea (maternal) Show forest plot | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.12, 73.04] |
| 15 Postpartum haemorrhage Show forest plot | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.59, 2.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.08, 0.18] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 5 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.57, 8.21] |
| 3 Caesarean section Show forest plot | 6 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.82, 1.57] |
| 4 Serious neonatal morbidity or perinatal death Show forest plot | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.45] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.68] |
| 6 Oxytocin augmentation Show forest plot | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.83, 1.36] |
| 7 Uterine hyperstimulation without FHR changes Show forest plot | 5 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.76 [1.32, 34.54] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.28, 5.38] |
| 9 Apgar score < 7 at 5 minutes Show forest plot | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.14, 2.05] |
| 10 Neonatal intensive care unit admission Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.28] |
| 11 Maternal side‐effects (all) Show forest plot | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.66, 4.31] |
| 12 Postpartum haemorrhage Show forest plot | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Perinatal death Show forest plot | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 7 | 3320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.08] |
| 2 Serious neonatal morbidity or perinatal death Show forest plot | 3 | 2840 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.17] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 3 | 2734 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.61, 4.52] |
| 4 Uterine rupture Show forest plot | 2 | 2579 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 5 Epidural analgesia Show forest plot | 3 | 2940 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.73, 4.14] |
| 6 Instrumental vaginal delivery Show forest plot | 3 | 2779 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.13] |
| 7 Meconium‐stained liquor Show forest plot | 5 | 3099 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.21] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 3 | 2894 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.83, 2.63] |
| 9 Neonatal intensive care unit admission Show forest plot | 4 | 2953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.13] |
| 10 Perinatal death Show forest plot | 2 | 2719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.17] |
| 11 Maternal side‐effects (all) Show forest plot | 4 | 5533 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.73, 1.83] |
| 12 Vomitting (maternal) Show forest plot | 2 | 2579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.39] |
| 13 Diarrhoea (maternal) Show forest plot | 1 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.01 [0.36, 135.59] |
| 14 Postpartum haemorrhage Show forest plot | 5 | 3099 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.02, 2.13] |
| 15 Woman not satisfied Show forest plot | 1 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.33, 0.58] |
| 16 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Serious maternal morbidity or death Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.84 [0.24, 96.66] |
| 18 Oxytocin augmentation Show forest plot | 2 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.79] |
| 19 Other maternal side‐effects Show forest plot | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 12 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.47 [2.01, 9.93] |
| 3 Caesarean section Show forest plot | 22 | 2173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.02] |
| 4 Serious neonatal morbidity or perinatal death Show forest plot | 4 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death Show forest plot | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.84 [0.24, 96.66] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.35, 0.79] |
| 7 Oxytocin augmentation Show forest plot | 8 | 813 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.53, 1.10] |
| 8 Uterine hyperstimulation without FHR changes Show forest plot | 9 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.99, 7.01] |
| 9 Uterine rupture Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 10 Epidural analgesia Show forest plot | 5 | 633 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.63, 2.43] |
| 11 Instrumental vaginal delivery Show forest plot | 7 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.27] |
| 12 Meconium‐stained liquor Show forest plot | 5 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.47, 0.89] |
| 13 Apgar score < 7 at 5 minutes Show forest plot | 11 | 1194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.59, 1.99] |
| 14 Neonatal intensive care unit admission Show forest plot | 7 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.27] |
| 15 Perinatal death Show forest plot | 3 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal side‐effects (all) Show forest plot | 10 | 1572 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.73, 1.59] |
| 17 Nausea (maternal) Show forest plot | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Vomitting (maternal) Show forest plot | 2 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.15, 6.41] |
| 19 Diarrhoea (maternal) Show forest plot | 2 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Other maternal side‐effects Show forest plot | 7 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.51] |
| 21 Postpartum haemorrhage Show forest plot | 7 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.47, 2.05] |
| 22 Serious maternal complication Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 2 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.40, 3.18] |
| 4 Serious neonatal morbidity or perinatal death Show forest plot | 2 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.45] |
| 5 Oxytocin augmentation Show forest plot | 3 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.51] |
| 6 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.08, 0.18] |
| 7 Instrumental vaginal delivery Show forest plot | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.28, 5.38] |
| 8 Perinatal death Show forest plot | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 2 Caesarean section Show forest plot | 4 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.31, 1.14] |
| 3 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.11, 0.37] |
| 4 Oxytocin augmentation Show forest plot | 3 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.32, 1.07] |
| 5 Epidural analgesia Show forest plot | 4 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.56, 0.97] |
| 6 Instrumental vaginal delivery Show forest plot | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 2 Caesarean section Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.87] |
| 3 Oxytocin augmentation Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.09] |
| 4 Epidural analgesia Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.47] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 3 Caesarean section Show forest plot | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 1.90] |
| 4 Instrumental Vaginal Delivery Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.41, 1.38] |
| 5 Oxytocin augmentation Show forest plot | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.17, 2.11] |
| 6 Epidural analgesia Show forest plot | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.56, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.42] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 3 Caesarean section Show forest plot | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.47, 2.22] |
| 4 Oxytocin augmentation Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.21, 4.51] |
| 5 Epidural analgesia Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.82, 3.00] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 2 Caesarean section Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 3 Oxytocin augmentation Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.21, 4.51] |
| 4 Epidural analgesia Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.82, 3.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.42] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 3 Caesarean section Show forest plot | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.47, 2.22] |
| 4 Oxytocin augmentation Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.21, 4.51] |
| 5 Epidural analgesia Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.82, 3.00] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 3 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.84, 1.26] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 3 Caesarean section Show forest plot | 6 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.17] |
| 4 Serious maternal morbidity or death Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 2 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.70, 1.07] |
| 6 Oxytocin augmentation Show forest plot | 6 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.08] |
| 7 Epidural analgesia Show forest plot | 3 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 8 Instrumental vaginal delivery Show forest plot | 3 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.02] |
| 9 Meconium Stained Liquor Show forest plot | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.39, 2.13] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 4 | 597 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.35, 3.66] |
| 11 Neonatal Intensive Care Unit Admission Show forest plot | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.47] |
| 12 Postpartum haemorrhage Show forest plot | 3 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.72, 1.51] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 3 Caesarean section Show forest plot | 4 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 4 Serious maternal morbidity or death Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.07] |
| 6 Oxytocin augmentation Show forest plot | 3 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.29] |
| 7 Epidural analgesia Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.10] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| 9 Apgar score < 7 at 5 minutes Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 10 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.57, 1.87] |
| 2 Caesarean section Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.24, 3.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.87, 1.87] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 3 Caesarean section Show forest plot | 3 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.70, 1.49] |
| 4 Serious maternal morbidity or death Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.07] |
| 6 Oxytocin augmentation Show forest plot | 3 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 7 Epidural analgesia Show forest plot | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.19] |
| 8 Instrumental vaginal delivery Show forest plot | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.10] |
| 9 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.13, 4.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.62, 1.59] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 3 Caesarean section Show forest plot | 3 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.48, 1.86] |
| 4 Serious maternal morbidity or death Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.07] |
| 6 Oxytocin augmentation Show forest plot | 4 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.43, 1.25] |
| 7 Epidural analgesia Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.10] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| 9 Apgar score < 7 at 5 minutes Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 10 Postpartum haemorrhage Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.56] |
| 2 Caesarean section Show forest plot | 1 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.40, 1.41] |
| 3 Oxytocin augmentation Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.58] |
| 4 Apgar score < 7 at 5 minutes Show forest plot | 2 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.18, 4.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.87] |
| 2 Caesarean section Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.38, 1.11] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.05] |
| 4 Apgar score < 7 at 5 minutes Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
| 5 Maternal side‐effects (all) Show forest plot | 2 | 460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.70] |
| 6 Nausea (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 7 Vomitting (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 8 Diarrhoea (maternal) Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
| 9 Other maternal side‐effects Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.87] |
| 2 Caesarean section Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.38, 1.11] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.05] |
| 4 Apgar score < 7 at 5 minutes Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
| 5 Maternal side‐effects (all) Show forest plot | 2 | 460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.70] |
| 6 Nausea (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 7 Vomitting (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 8 Diarrhoea (maternal) Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
| 9 Other maternal side‐effects Show forest plot | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 3 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.64, 1.99] |
| 2 Oxytocin augmentation Show forest plot | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.31, 1.40] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.78] |
| 5 Instrumental vaginal delivery Show forest plot | 3 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.09, 2.70] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.58, 3.05] |
| 7 Maternal side‐effects (all) Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Vomitting (maternal) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Diarrhoea (maternal) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Postpartum haemorrhage Show forest plot | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.57, 2.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.58] |
| 2 Oxytocin augmentation Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.85, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.23, 2.93] |
| 2 Oxytocin augmentation Show forest plot | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.37, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.89] |
| 2 Oxytocin augmentation Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.19, 0.64] |
| 3 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.47, 2.62] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.51, 3.04] |
| 6 Maternal side‐effects (all) Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Vomitting (maternal) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Diarrhoea (maternal) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 3 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.45] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 5 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.89, 5.21] |
| 3 Caesarean section Show forest plot | 11 | 1262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.26] |
| 4 Serious neonatal morbidity or perinatal death Show forest plot | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.62] |
| 5 Serious maternal morbidity or death Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 ‐24 hours (BS < 3) Show forest plot | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.80] |
| 7 Oxytocin augmentation Show forest plot | 7 | 884 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.13] |
| 8 Uterine hyperstimulation without FHR changes Show forest plot | 8 | 908 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.81, 3.14] |
| 9 Uterine rupture Show forest plot | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 10 Epidural analgesia Show forest plot | 3 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.95, 1.36] |
| 11 Instrumental vaginal delivery Show forest plot | 6 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 12 Postpartum haemorrhage Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.72] |
| 13 Apgar score < 7 at 5 minutes Show forest plot | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 14 Vomitting (maternal) Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 102.00] |
| 15 Diarrhoea (maternal) Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.77, 2.10] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.44 [0.43, 128.16] |
| 3 Caesarean section Show forest plot | 5 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.23] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.80] |
| 5 Oxytocin augmentation Show forest plot | 3 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.43, 1.29] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Epidural analgesia Show forest plot | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.83, 1.58] |
| 8 Instrumental vaginal delivery Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.23, 2.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.35, 6.15] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.38] |
| 3 Caesarean section Show forest plot | 3 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.53, 3.23] |
| 4 Oxytocin augmentation Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.20, 0.86] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.13, 2.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vaginal delivery not achieved within 24 hours Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.85, 2.21] |
| 2 Uterine hyperstimulation with FHR changes Show forest plot | 3 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [0.86, 51.67] |
| 3 Caesarean section Show forest plot | 8 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.28] |
| 4 Serious neonatal morbidity or perinatal death Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.62] |
| 5 Serious maternal morbidity or death Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours Show forest plot | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.80] |
| 7 Oxytocin augmentation Show forest plot | 5 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.54, 1.21] |
| 8 Uterine hyperstimulation without FHR changes Show forest plot | 5 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.81 [0.71, 47.25] |
| 9 Uterine rupture Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia Show forest plot | 3 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.84, 1.63] |
| 11 Instrumental vaginal delivery Show forest plot | 3 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.20, 0.59] |
| 12 Apgar score < 7 at 5 minutes Show forest plot | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Postpartum haemorrhage Show forest plot | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.13] |
| 2 Caesarean section Show forest plot | 7 | 1546 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.33] |
| 3 Serious neonatal morbidity or perinatal death Show forest plot | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24hrs Show forest plot | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.25, 2.21] |
| 5 Oxytocin augmentation Show forest plot | 5 | 1370 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.77, 1.20] |
| 6 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 7 Epidural analgesia Show forest plot | 4 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.26] |
| 8 Instrumental vaginal delivery Show forest plot | 3 | 1179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.13] |
| 9 Meconium‐stained liquor Show forest plot | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 3 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.20, 1.31] |
| 11 Neonatal intensive care unit admission Show forest plot | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.09] |
| 12 Perinatal death Show forest plot | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal side‐effects (all) Show forest plot | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.13] |
| 14 Nausea (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 15 Vomitting (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 16 Diarrhoea (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Other maternal side‐effects Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Postpartum haemorrhage Show forest plot | 2 | 1155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.79, 2.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 2 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.63] |
| 2 Serious neonatal morbidity or perinatal death Show forest plot | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cervix unfavourable/unchanged after 12‐24hrs Show forest plot | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.25, 2.21] |
| 4 Oxytocin augmentation Show forest plot | 2 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.18] |
| 5 Epidural analgesia Show forest plot | 2 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.22] |
| 6 Instrumental vaginal delivery Show forest plot | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.18] |
| 7 Meconium‐stained liquor Show forest plot | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.27] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.27] |
| 9 Neonatal intensive care unit admission Show forest plot | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.11, 1.03] |
| 10 Perinatal death Show forest plot | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Postpartum haemorrhage Show forest plot | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.86, 3.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.19, 2.51] |
| 2 Serious neonatal morbidity or perinatal death Show forest plot | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Oxytocin augmentation Show forest plot | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.35, 2.80] |
| 4 Epidural analgesia Show forest plot | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.77, 1.70] |
| 5 Instrumental vaginal delivery Show forest plot | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.98 [1.37, 25.99] |
| 6 Meconium‐stained liquor Show forest plot | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.48, 1.24] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.34, 5.88] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.33, 3.06] |
| 9 Perinatal death Show forest plot | 1 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Postpartum haemorrhage Show forest plot | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.44, 2.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 2 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.44, 1.38] |
| 2 Oxytocin augmentation Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.63, 1.05] |
| 3 Epidural analgesia Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.80, 1.82] |
| 4 Instrumental vaginal delivery Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.38, 1.26] |
| 5 Postpartum haemorrhage Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.13, 4.40] |
| 6 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.13] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
| 8 Maternal side‐effects (all) Show forest plot | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.13] |
| 9 Nausea (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 10 Vomitting (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 11 Diarrhoea (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Other maternal side‐effects Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.13] |
| 2 Caesarean section Show forest plot | 4 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.46] |
| 3 Oxytocin augmentation Show forest plot | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.06] |
| 4 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
| 6 Maternal side‐effects (all) Show forest plot | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.13] |
| 7 Nausea (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 8 Vomitting (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 9 Diarrhoea (maternal) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Other maternal side‐effects Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Uterine hyperstimulation with FHR changes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.18] |
| 2.1 PGE2 (repeated doses) vs placebo/no treatment | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.18] |
| 3 Cervix unfavourable/unchanged after 12 to 24 hours Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.47] |
| 3.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.47] |
| 4 Uterine hyperstimulation without FHR changes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 4.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 5 Epidural analgesia Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.12] |
| 5.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.12] |
| 6 Meconium‐stained liquor Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
| 6.1 PGE2 (repeated doses) vs placebo/no treatment | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.06] |
| 7.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.06] |
| 8 Neonatal intensive care unit admission Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.65] |
| 8.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.65] |

