Haloperidol versus placebo para la esquizofrenia
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: random assignment. Consent: written. | |
| Participants | Diagnosis: (DSM‐III‐R) schizophrenia. | |
| Interventions | 1. Haloperidol: fixed dose (FD) 12 mg/day, increased day 1‐14. N = 52 | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind"; "[Blood] samples were shipped to the sponsor and analysed". No further details reported. |
| Incomplete outcome data (attrition bias) | High risk | "Of all patients evaluated, 149 (41%) completed 6 weeks of treatment. Lack of efficacy was the primary reason for withdrawal and was seen most often in the placebo group". No further details reported. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | High risk | Supported by a grant from Zeneca Pharmaceuticals. |
| Methods | Allocation: random assignment. Consent: written. | |
| Participants | Diagnosis: (DSM‐III‐R) schizophrenia. | |
| Interventions | 1. Haloperidol: dose 10, 15 or 20 mg/day; initial dose 15 mg/day, adjusted accordingly thereafter. N = 69. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | Data only taken from the initial 'acute phase' trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information reported. |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up ranged from 51% ‐ 68% in the five treatment arms. |
| Selective reporting (reporting bias) | High risk | Patient Global Impression (PGI) assessed but not reported. |
| Other bias | High risk | Source of funding from industry "From the Psychopharmacology Division, Lilly Research Laboratories, Eli Lilly and Company" |
| Methods | Allocation: random assignment. Consent: not stated. | |
| Participants | Diagnosis: (ICD‐9) schizophrenia. | |
| Interventions | 1. Haloperidol: dose 5 ‐ 20 mg/day. N = 30. | |
| Outcomes | Adverse event: various observed effects. | |
| Notes | If, after entering the trial, participants showed improvement they could be discharged from hospital. If, however, they were readmitted due to relapse they could then re‐enter the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were assigned to 3 groups of 30 each in a random and probabilistic manner, after stratification", no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Low risk | "The assessments were always performed by the same investigator in a double‐blind trial. Only at the end of the study after the data were analysed were the patient group assignments identified". |
| Incomplete outcome data (attrition bias) | Low risk | "Five patients ran away between the 6th and 27th day of the study. Three belonged to the pipotiazine group, 1 belonged to the haloperidol group, and 1 to the placebo group". "These patients were excluded from the analysis". |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes reported. |
| Other bias | Unclear risk | Source of funding not reported. |
| Methods | Allocation: random assignment. Consent: written. | |
| Participants | Diagnosis: (DSM‐III) schizophrenia. | |
| Interventions | 1. Haloperidol: dose 15 ‐ 75 mg/day. N = 8. | |
| Outcomes | Adverse events: various observed effects, use of antiparkinson medication. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...subjects were assigned on a randomized blind schedule to treatment" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | "...subjects were assigned on a randomized blind schedule to treatment" no further details reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐ blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information reported. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up or missing data were balanced across intervention groups, with similar reasons for missing data. "...three placebo‐treated patients were terminated prior to study completion due to lack of efficacy. Two patients receiving haloperidol terminated early due to positive response and the desire to leave the hospital, and one patient in the haloperidol treatment group was terminated for administrative reasons. The only patient who left the study prematurely due to an apparent adverse reaction was one receiving placebo, who developed chest pain and electrocardiographic changes" |
| Selective reporting (reporting bias) | Unclear risk | Study does not state in methods which outcomes will be measured, and/or no protocol available. |
| Other bias | High risk | Researchers currently imprisoned for research fraud. |
| Methods | Allocation: random assignment. Consent: written. | |
| Participants | Diagnosis: (DSM‐III‐R) schizophrenia. | |
| Interventions | 1. Haloperidol: dose 4‐20 mg/day, dose adjusted as required days 1‐18. N = 12. | |
| Outcomes | Adverse events: various observed effects. Unable to use ‐ | |
| Notes | Participants already part of larger multicentre trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind" no further details reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | No details reported. |
| Selective reporting (reporting bias) | High risk | Several outcomes not fully reported (BPRS, CGI, SANS, or ESRS). |
| Other bias | High risk | Source of funding not reported. Richard Borison, MD, former psychiatry chief at the Augusta Veterans Affairs medical center and Medical College of Georgia, was sentenced to 15 years in prison for a $10 million clinical trial fraud. |
| Methods | Allocation: random assignment. Consent: written. | |
| Participants | Diagnosis: (DSM‐III‐R) schizophrenia. | |
| Interventions | 1. Haloperidol: dose 20 mg/day, initial dose 2 mg/day increased in fixed increments day 2‐7. N=21. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | Part of North American Trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind", "Study medication was administered under double‐blind conditions as identical tablets of risperidone, haloperidol and placebo". |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind", "Assessment of symptoms was based on clinical interviews conducted by a psychiatrist". No further details reported. |
| Incomplete outcome data (attrition bias) | High risk | A total of 65 participants (48.1%) left the study early: 16 (72.7%) from the placebo group and 13 (61.9%) from the haloperidol group. "Statistical analyses of efficacy and safety parameters were conducted according to the intent‐to‐treat analysis principle". |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | Source of funding not reported. |
| Methods | Allocation: random assignment. Consent: unknown. | |
| Participants | Diagnosis: schizophrenia (40%), neurosis (60%).* | |
| Interventions | 1. Haloperidol: dose 2‐25 mg/day, mean 6 mg/day. N = 19. | |
| Outcomes | Global effect: improved/not improved. Unable to use ‐ | |
| Notes | * use only data for those suffering from schizophrenia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...all drugs were given at random" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "...'pharmacotherapeutically blind unit,' that is, in a hospital service where all drugs were given at random and used without the service team (made up of one intern, two assistant residents, one resident, one psychologist, two to four nurses, one social worker and one occupational therapist) knowing what drugs were being investigated." |
| Blinding of outcome assessment (detection bias) | Low risk | "The assessment of the patients (and the drugs) was a result of the pooling of the opinions of the different members of the team." |
| Incomplete outcome data (attrition bias) | Low risk | There were no drop‐outs from the study. |
| Selective reporting (reporting bias) | High risk | Details on side effects were not fully reported although "Side effects were relatively numerous and disturbing to the patients." |
| Other bias | Unclear risk | "Haloperidol was generously supplied by G. D. Searle & Co." |
| Methods | Allocation: randomised. Consent: written. | |
| Participants | Diagnosis: schizophrenia DSM‐4. | |
| Interventions | 1. Blonanserin: dose 2.5 mg/day. N = 61. 2. Blonanserin: dose 5 mg/day. N = 58. 3. Blonanserin: dose 10 mg/day. N = 64. 4. Haloperidol: dose 10 mg/day. N = 60. 5. Placebo. N = 64. | |
| Outcomes | Leaving the study early Global state: No overall improvement (< 20% reduction in PANSS‐total score) Mental state: PANSS‐total score, mean change from baseline at 6 weeks Mental state: PANSS‐positive score, mean change from baseline at 6 weeks Mental state: PANSS‐negative score, mean change from baseline at 6 weeks Global state: CGI‐S, mean change from baseline at 6 weeks Adverse effects: Extrapyramidal symptoms (akathisia, dyskinesia, dystonia, parkinsonism, rigidity, tremor) Adverse effects: Cardiovascular (bradycardia) Adverse effects: Other (insomnia, drooling, headache, weight loss, agitation, anxiety, sleepiness) Unusable data (no measurement of variance reported) ‐ Adverse effects: Extrapyramidal symptoms: SAS, BAS and AIMS scales | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of medication was performed using a computer‐generated schedule". |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Low risk | “Double‐blind”, "...blinding was ensured by over‐encapsulating all capsules to ensure the same appearance". |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Double‐blind”, no further details reported. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up or missing data were balanced across intervention groups, with similar reasons for missing data. Missing data have been imputed using appropriate methods (intention‐to‐treat analysis). |
| Selective reporting (reporting bias) | High risk | Not all of the study's pre‐specified primary outcomes have been reported (BPRS, UKU assessments). |
| Other bias | High risk | "Laboratorios Almirall SA provided financial support for performing the study and Dainippon Sumitomo Pharma Co., Ltd funded the preparation of this manuscript. Drs Garcia, Robert and Peris are employees of Laboratorios Aimirall SA. H. Nakamura, Dr Sato and Y. Terazawa are employees of Dainippon Sumitomo Pharma Co., Ltd. Drs Garcia and Peris have received stock options from Laboratorios Almirall SA." |
| Methods | Allocation: random assignment. Consent: not stated. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Haloperidol: dose 0.75 ‐ 4.5 mg/day, increased day 1 ‐ 42. N = 26. | |
| Outcomes | Adverse events: various observed effects* | |
| Notes | *Adverse effects reported for haloperidol group only, reviewers assumed that placebo group had no adverse effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants "were divided into two groups on the basis of a list of random numbers supplied by the drug company" |
| Allocation concealment (selection bias) | Low risk | Central allocation "Our pharmacist allotted the patients from an alphabetical list supplied by us" |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind" no further details reported. |
| Incomplete outcome data (attrition bias) | Low risk | "Two patients were withdrawn from the trial, one had a recurrence of a skin rash (he was eventually found to be on the placebo) and the other was found to have early pulmonary tuberculosis on routine chest X‐ray. We were finally left with 50 patients (25 on drug and 25 on controls) who completed the trial". |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | Messrs. G. D. Searle and Co. Ltd. supplied the haloperidol and control tablets. |
| Methods | Allocation: random assignment. Consent: not stated. | |
| Participants | Diagnosis: schizophrenia (80%). | |
| Interventions | 1. Haloperidol: dose < 200 mg/day. N = 17. | |
| Outcomes | Global effect: improved/not improved. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the patients were randomly assigned" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "The study medications were prepared in identical appearing capsules". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were balanced across intervention groups, with similar reasons for missing data. |
| Selective reporting (reporting bias) | High risk | All outcomes were not fully reported (no SD s were reported for BPRS and NOSIE). |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists (does not report source of funding). |
| Methods | Allocation: random assignment. Consent: written. | |
| Participants | Diagnosis: (DSM‐III‐R) schizophrenia. | |
| Interventions | 1. Haloperidol: dose up to 75 mg/day, dose individually adjusted weekly. N = 18. | |
| Outcomes | Adverse events: various observed effects. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Identical looking capsules" were used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) | High risk | "Only 8 patients in the placebo group completed the 6‐week study". "For the haloperidol group, 12 patients completed the study". |
| Selective reporting (reporting bias) | High risk | All pre‐stated outcomes were not reported (no data reported for the AIMS scale). |
| Other bias | Unclear risk | Source of funding not reported. |
| Methods | Allocation: random. Consent: given. | |
| Participants | Diagnosis: (DSM‐IV) schizophrenia or schizoaffective disorder. | |
| Interventions | 1. Aripiprazole: dose 15 mg/day. N = 102. | |
| Outcomes | Leaving the study early. Unable to use: | |
| Notes | Data taken from haloperidol and placebo groups only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further information reported. |
| Blinding of outcome assessment (detection bias) | Low risk | "The same rater conducted the assessment throughout the study and was blinded to the patient's treatment". |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of losses and reasons for leaving the study similar across groups. "Analysis of efficacy parameters was performed on an intention‐to‐treat basis using data obtained from each patient's last visit (i.e. last observation carried forward analysis at week 4". "Of the 414 randomised patients, 248 competed the 4‐week study period". |
| Selective reporting (reporting bias) | High risk | All pre‐stated outcomes were not fully reported (SDs were not reported for PANSS, BPRS, CGI, AIMS, SAS and BAS scales, weight and serum prolactin levels). |
| Other bias | High risk | Sponsored by Otsuka Pharmaceutical Co. Ltd (Tokyo, Japan) and Bristol‐Meyers Squibb Company (Princeton, NJ). |
| Methods | Allocation: randomised. Consent: written. | |
| Participants | Diagnosis: schizophrenia, DSM‐IV. | |
| Interventions | 1. Asenapine: dose 5 mg/day. N = 114. 2. Asenapine: dose 10 mg/day. N = 106. 3. Placebo. N = 123. 4. Haloperidol: dose 4 mg/day. N = 115. | |
| Outcomes | Leaving the study early Global state: no overall improvement (no CGI‐I score of 1 [very much improved] or 2 [much improved]) (also measured as < 30% reduction in PANSS total score, not used) Mental state: PANSS subscale scores (positive, negative), mean change from baseline at 6 weeks Global state: CGI‐S and CGI‐I, mean change from baseline at 6 weeks Mental state: Calgary Depression Scale for Schizophrenia (CDSS), mean change from baseline at 6 weeks Adverse effects: Extrapyramidal symptoms (SAS, BAS and AIMS scales), mean change from baseline at 6 weeks Adverse effects: Extrapyramidal symptoms (parkinsonism, akathisia, dystonia, rigidity) Adverse effects: Other (insomnia, oral hypoaesthesia, sleepiness, agitation, headache, anxiety, weight loss, weight gain) Adverse effects: Autonomic (sedation) Use of anti‐Parkinson medication Unable to use (measurements of variance not reported) ‐ PANSS total score mean change from baseline to day 42 (primary outcome) Modified International Suicide Prevention Trial (InterSePT) Scale for Suicidal Thinking Readiness to Discharge Questionnaire (RDQ) | |
| Notes | *Patients were hospitalised for at least 2 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized”, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | “Randomized”, no further details provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind", no further details reported. |
| Incomplete outcome data (attrition bias) | Low risk | "Safety assessments were made using data from the treated population (all patients who received >1 dose of study medication); efficacy analyses were based on data from the intent‐to‐treat (ITT) population (treated patients who had >1 postbaseline PANSS assessment)" |
| Selective reporting (reporting bias) | High risk | Not all pre‐specified outcomes were reported fully. |
| Other bias | High risk | "funded by Schering‐Plough Corporation, now Merck & Co, Inc" |
| Methods | Allocation: random assignment. Consent: not stated. | |
| Participants | Diagnosis: schizophrenia (63%), major depressive disorder (37%). | |
| Interventions | 1. Haloperidol: dose 20 mg/day. N = 20*. | |
| Outcomes | Leaving the study early | |
| Notes | *number of people with schizophrenia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind" no further details reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of losses from each treatment group and reasons for loss to follow‐up not reported. "Fourteen patients left the study on day 3, 19 patients left on day 7, and 6 patients left on day 14". |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes were reported. |
| Other bias | Unclear risk | Source of funding not reported. |
| Methods | Allocation: random assignment. Consent: given. | |
| Participants | Diagnosis: (DSM‐III‐R) schizophrenia. | |
| Interventions | 1. Haloperidol: dose 20 mg/day. N = 66. | |
| Outcomes | Leaving the study early Unable to use ‐ | |
| Notes | Part of 'North America 1997'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomization was in blocks of 12" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information reported. |
| Incomplete outcome data (attrition bias) | High risk | "early termination in 53% of the patients...62 % of the placebo patients; and 38% of patients receiving haloperidol...Both observed case and last observation carried forward (or end point) analyses were performed". |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported. |
| Other bias | Unclear risk | Source of funding from a pharmaceutical company "Supported by a grant from the Janssen Research Foundation". |
| Methods | Allocation: randomised. Consent: written. | |
| Participants | Diagnosis: schizophrenia and schizoaffective disorder DSM‐IV. | |
| Interventions | 1. Haloperidol: dose 10 mg/day. N = 98. 2. Placebo. N = 98. 3. 5‐HT2A/2C antagonist: dose 5 mg/day. N = 70. 4. NK3 antagonist: dose 200 mg/day. N = 67. 5. CB1 antagonist: dose 20 mg/day. N = 69. 6. NTS1 antagonist: dose 180 mg/day. N = 63. | |
| Outcomes | Leaving the study early* Unable to use (losses to follow‐up > 50%) ‐ Mental state: PANSS (total, positive, negative, general), mean change from baseline at 6 weeks BPRS (total), mean change from baseline at 6 weeks Global state: CGI‐I, mean endpoint score at 6 weeks Global state: CGI‐S, mean change from baseline at 6 weeks Mental state: Calgary Depression Scale (CDS), mean change from baseline at 6 weeks Adverse effects: Extrapyramidal symptoms: various scales (SAS, BAS, AIMS), mean change from baseline at 6 weeks Adverse effects: Other (headache, insomnia, psychosis, agitation, abdominal pain, dyspepsia, vomiting, extrapyramidal symptoms, hyperkinesia) | |
| Notes | *This study had > 50% losses to follow‐up, only the outcome Leaving the study early could be used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized”, no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | “Double‐blind”, no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Double‐blind”, no further details reported. |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up greater than 50%. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported. |
| Other bias | High risk | Funding sources are pharmaceutical companies, "Dr. Meltzer has received grant support from and is a consultant to Acadia, AstraZeneca, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutica, Lundbeck, Novartis, Pfizer, Sanofi‐Synthelabo, and Solvay. He is a consultant to Psychiatric Genomics, Precision Med, Pharmacia, and Roche. Drs. Arvanitis and Rein and Ms. Bauer are employees of Sanofi‐Synthelabo." |
| Methods | Allocation: randomised. Consent: not stated. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Lurasidone: dose 20mg/day. N = 71. 2. Lurasidone: dose 40mg/day. N = 69. 3. Lurasidone: dose 80mg/day. N = 71. 4. Haloperidol: dose 10mg/day. N = 73. 5. Placebo. N = 72. | |
| Outcomes | Leaving the study early* Unable to use (losses to follow‐up > 50%) ‐ Adverse effects: Extrapyramidal symptoms (akathisia, tremor, dystonia, EPS) Adverse effects: Autonomic (sedation) Adverse effects: Cardiovascular and gastric (dizziness, diarrhoea, constipation, abdominal discomfort) Adverse effects: Other (nausea, vomiting, headache, sleepiness, agitation, anxiety, insomnia) Mental state: BPRS total, mean change from baseline at 6 weeks Mental state: PANSS, mean change from baseline at 6 weeks Global state: CGI‐S, mean change from baseline at 6 weeks Mental state: MADRS, mean change from baseline at 6 weeks | |
| Notes | ClinicalTrials.gov Identifier: NCT00044044. *This study had > 50% losses to follow‐up, only the outcome Leaving the study early could be used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized”, no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | “Randomized”, no further details reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The placebo was an "Oral Capsule matching treatment" indicating blinding of participants. Further, the study was described as “Double blind”, but there were no further details on blinding of personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Double blind”, no further details reported. |
| Incomplete outcome data (attrition bias) | High risk | There was a high rate of drop‐outs (> 50%). |
| Selective reporting (reporting bias) | Low risk | All pre‐stated outcomes were reported. |
| Other bias | High risk | "Sponsored by: Sumitomo Pharmaceuticals America" |
| Methods | Allocation: random assignment. Consent: not stated. | |
| Participants | Diagnosis: schizophrenia | |
| Interventions | 1. Haloperidol: dose 3 mg/day. N = 10. | |
| Outcomes | Relapse: number remaining in remission < 1 year. Unable to use ‐ | |
| Notes | *Initial 'cross‐over' design of trial was disregarded after participant/clinician reluctance to switch neuroleptics. Data taken from first arm only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" no further information reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind", "Drug appearance, with respect to powder colour, taste and volumes, was made identical by adding a kind of stomachics, SMP (Sankyo, Japan)." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind" no further details reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Study does not report which treatment groups the losses to follow‐up were from "Nine patients were dropped from the study for various reasons. The reasons included: failure to report to the hospital for scheduled appointment (N=3); admissions to other hospitals (N=2); strong requests from the patient not to change the previous drug (N=3); and a suicide after admission to the hospital (N=1)". |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes were reported. |
| Other bias | Unclear risk | Source of funding was not reported. |
| Methods | Allocation: random assignment. Consent: not stated. | |
| Participants | Diagnosis: (DSM III) schizophrenia. | |
| Interventions | 1. Haloperidol: dose 1 mg/day. N = 13. | |
| Outcomes | Relapse: number remaining in remission < 1 year. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind", "Drug appearance, with respect to powder colour, taste and volume, was made identical by gastric aid, SMP (Sankyo, Japan)". |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind" no further details reported. |
| Incomplete outcome data (attrition bias) | High risk | "Among the entire group of 87 patients, 19 patients continued to receive the assigned drugs since they were in remission during the entire year of the trial, while other patients discontinued use of the assigned drugs due to overdose (N=16), relapse (N=48) and drop‐out (N=4)". |
| Selective reporting (reporting bias) | High risk | Results not reported for placebo group for number of symptom free days and serum prolactin. |
| Other bias | Unclear risk | Source of funding not reported. |
| Methods | Allocation: randomised. Consent: written. | |
| Participants | Diagnosis: schizophrenia, DSM‐IV. | |
| Interventions | 1. Iloperidone: dose 4 mg/day. N = 121. 2. Iloperidone: dose 8 mg/day. N = 125. 3. Iloperidone: dose 12 mg/day. N = 124. 4. Haloperidol: dose 15 mg/day. N = 124. 5. Placebo. N = 127. | |
| Outcomes | Leaving the study early* Unable to use (losses to follow‐up > 50%, variance not reported) ‐ Mental state: PANSS total, PANSS positive, PANSS negative, PANSS general psychopathology, BPRS, mean change from baseline at 6 weeks Adverse effects | |
| Notes | *This study had > 50% losses to follow‐up, only the outcome Leaving the study early could be used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized”, no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | “Double‐blind”, no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Double‐blind”, no further details reported. |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up greater than 50%. |
| Selective reporting (reporting bias) | High risk | SDs not reported for PANSS or BPRS. |
| Other bias | High risk | Source of funding from pharmaceutical companies. "Dr Potkin has received grant funding from Astra‐ Zeneca, Bioline, Bristol‐Myers Squibb, Dainippon‐Sumitomo, Elan, Forest Laboratories, Fujisawa Healthcare, Janssen Pharmaceutica, Merck, Novartis, Ono, Organon, Otsuka, Pfizer Inc, Solvay Pharmaceuticals, Roche" |
| Methods | Allocation: random assignment. Consent: not stated. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Haloperidol: dose 4‐12 mg/day. N = 29. | |
| Outcomes | Adverse effects: various observed effects. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "A double‐blind process was used in which neither patient nor investigator knew what medication was received until after the study was completed", "All medication was administered in identically appearing capsules". |
| Blinding of outcome assessment (detection bias) | Low risk | "A double‐blind process was used in which neither patient nor investigator knew what medication was received until after the study was completed". |
| Incomplete outcome data (attrition bias) | Unclear risk | "Eight patients were excluded (1 loxapine, 3 haloperidol and 4 placebo", "All eight were dropped for administrative reasons such as unauthorised departure from the hospital or family objections to patients' participation in the study". Last observation carried forward method used "Patients who continued beyond the fourth week but did not complete the full 12 weeks were included in the analysis through their final rating period, at either 4 or 8 weeks. These included 29 patients". |
| Selective reporting (reporting bias) | High risk | All pre‐stated outcomes were not fully reported (SDs were not reported for the BPRS and NOSIE scales). |
| Other bias | High risk | Loxitane (Loxapine succinate) supplied by Lederle Laboratories, Division of America Cyanamid Co, Pearle River, New York, who also supported the study. |
| Methods | Allocation: random assignment. Consent: not stated | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Haloperidol: dose up to 15 mg/day. N = 14. | |
| Outcomes | Adverse events: various observed effects. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind", "All medications were prepared in identically appearing capsules". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information reported. |
| Incomplete outcome data (attrition bias) | Low risk | "Four of the 57 subjects, three on CPZ, and one on PL, failed to complete the 12 weeks of study. The PL subject and one CPZ subject were terminated because of behavioural deterioration after 4 and 8 weeks respectively. The other two CPZ subjects developed intestinal obstruction and were terminated in the 7th week of study". |
| Selective reporting (reporting bias) | High risk | All pre‐stated outcomes were not reported (no data reported for cognitive response, no SDs reported for the BPRS, NOSIE and OBRS scales). |
| Other bias | Low risk | Study supported in part by US Public Health Service Grant MH 11666 and by National Institute of Mental Health Research Scientist Development Award K135278. |
| Methods | Allocation: random assignment. Consent: not stated. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Haloperidol: dose 6 mg/day. N = 8. | |
| Outcomes | Adverse events: use of antiparkinson medication. Unable to use ‐ | |
| Notes | Group numbers not stated in text, reviewers have assumed that they were divided into three sets of 8 (see table 1). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind"; "the staff correctly guessed which patients were on active medication in a high percentage of cases". |
| Blinding of outcome assessment (detection bias) | Unclear risk | The code was broken at the end of the study. |
| Incomplete outcome data (attrition bias) | Unclear risk | No details reported. |
| Selective reporting (reporting bias) | High risk | All pre‐stated outcomes were not fully reported (SDs were not reported for IMPS and PRP). |
| Other bias | Unclear risk | "The haloperidol (HALDOL®) used in this study was supplied by McNeil Laboratories. Inc.," |
| Methods | Allocation: random assignment. Consent: not stated. | |
| Participants | Diagnosis: (DSM‐III‐R) schizophrenia. | |
| Interventions | 1. Haloperidol: dose 4 weeks 0.5‐10 mg/day followed by 4 weeks placebo. N = 12. | |
| Outcomes | Global effect: improved/not improved. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random assignment" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind" For CPRS (one of the outcomes scales), the raters were "all blind to treatment status", no further details reported. |
| Incomplete outcome data (attrition bias) | Low risk | There were no drop‐outs. |
| Selective reporting (reporting bias) | High risk | No SDs reported for CGI, CPRS, BPRS‐C. No data reported for adverse events for placebo group. No data reported for cognitive assessments (WISC‐R, WPPSI, DICA‐R). |
| Other bias | Unclear risk | Study supported by NIMH Child and Adolescent Mental Health Academic Award MH‐00763 and NIMH Institutional Training Grant MH‐18915. Haloperidol and placebo tablets provided by McNeil Pharmaceutical. |
| Methods | Allocation: random assignment. Consent: unknown. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Haloperidol: dose 6 weeks 4.5 mg/day followed by 6 weeks placebo. N = 30. | |
| Outcomes | Global effect: FFS, improved/not improved. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind"; "The code was worked out by the head of Female In‐patient section, which kept it secret until the investigation had ended."; "...it soon became clear that the trial was not, in fact blind. The patients on haloperidol showed extrapyramidal symptoms". |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind"; "The code was worked out by the head of Female In‐patient section, which kept it secret until the investigation had ended." |
| Incomplete outcome data (attrition bias) | Low risk | Only one drop‐out reported. |
| Selective reporting (reporting bias) | Unclear risk | Side‐effects in placebo group not mentioned. |
| Other bias | Unclear risk | Does not report source of funding. |
AIMS ‐ Assessment of Involuntary Movement Scale.
BAS ‐ Barnes Akathisa Scale.
BHGRS ‐ Bunney‐Hamburg Global Rating Scale.
BPRS ‐ Brief Pyschiatric Rating Scale.
BPRS‐C ‐ Brief Psychiatric Rating Scale for Children.
CDS ‐ Calgary Depression Scale.
CGI ‐ Clinical Global Impression.
CGI‐I ‐ Clinical Global Impression ‐ Improvement.
CGI‐S ‐ Clinical Global Impression ‐ Severity.
CPRS ‐ Childrens Psychiatric Rating Scale.
DICA‐R ‐ Diagnostic Interview for Children & Adolescents (Revised).
DSM‐III‐R ‐ Diagnositic and statistical manual of mental disorders: 3rd edition ‐ revised.
EPS ‐ Extrapyramidal Sypmtoms.
ESRS ‐ Extrapyramidal Symptom Rating Scale.
FFS ‐ Fergus Falls Scale.
GPS ‐ General Psychopathology Subscale.
ICD‐9 ‐ 9th International Classification of Diseases.
IMPS ‐ Inpatient Multidimensional Psychiatric Scale.
MADRS ‐ Montgomery Asberg‐Depression Scale.
MSC ‐ Mental Status Checklist.
NOSIE ‐ Nurses' Observation Scale for Inpatient Evaluation.
OBRS ‐ Oklahoma Behaviour Rating Scale.
PANSS ‐ Positive And Negative Syndrome Scale.
PGI ‐ Patient Global Impression.
PRP ‐ Psychotic Reaction Profile.
SANS ‐ Scale for the Assessment of Negative Symptoms.
SAS ‐ Simpson Angus Scale.
SD ‐ standard deviation
UKU ‐ side effect rating scale.
WISC‐R ‐ Wechsler Intelligence Scale for Children (Revised).
WPPSI ‐ Wechsler Preschool & Primary Scale of Intelligence.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: unclear. | |
| Allocation: post‐hoc analysis of two RCTs (no references provided, conference abstract). | |
| Allocation: unclear. | |
| Allocation: unclear. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: unclear. | |
| Allocation: unclear. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: unclear. | |
| Allocation: random. | |
| Allocation: random, cross‐over study . | |
| Allocation: random. | |
| Allocation: unclear, no recording of random allocation. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: not random, ABA design. | |
| Allocation: random. | |
| Allocation: not random, ABA design. | |
| Allocation: unclear. | |
| Allocation: random. | |
| Allocation: not an RCT, only one treatment group. | |
| Allocation: not random, ABA design. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: not random, ABA design. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: not random, ABA design. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: not an RCT, narrative review. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: retrospective study of 3 already excluded or included RCTs. | |
| Allocation: not random, ABA design. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: not random, ABA design. | |
| Allocation: unclear. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: not random, meta‐analysis. | |
| Allocation: random, cross‐over. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: not random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: unclear. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: unclear . | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random but with non‐random additions. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: not random, ABA design. | |
| Allocation: random. | |
| Allocation: random, cross‐over. | |
| Please note: this study was included in the previous versions of this review; as it was clarified that the route of haloperidol administration was not oral, but by intramuscular injection, we decided to exclude this study for this update. Allocation: randomised. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: unclear. | |
| Allocation: random. | |
| Allocation: not random, ABA design. | |
| Allocation: random. | |
| Allocation: unclear if randomised. | |
| Allocation: quasi‐randomised. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. Outcome: all data unusable (reported measures of immune index and P values of correlation of immune index and BPRS/SAPS scores). | |
| Allocation: random. | |
| Allocation: random. | |
| Allocation: random. |
ABA: Before and after trial.
AMPT ‐ alpha‐methyl‐p‐tyrosine.
BPRS ‐ Brief Pyschiatric Rating Scale.
CGI ‐ Clinical Global Impression.
.
HAM‐D: Hamilton Depression Scale.
PET ‐ Positron emission tomography.
RCT ‐ randomised controlled trial.
SAPS ‐ Simplified Acute Physiology Score.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1a. Overall improvement: No marked global improvement Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 1 Global state: 1a. Overall improvement: No marked global improvement. | ||||
| 1.1 up to 6 weeks (clinician rated) | 4 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.80] |
| 1.2 > 6‐24 weeks (clinician rated) | 8 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.58, 0.78] |
| 1.3 > 6‐24 weeks (nurse rated) | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.37, 0.92] |
| 2 Global state: 1b. Overall improvement: Average change in CGI‐S score (up to 6 weeks; high = poor) Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.73, ‐0.25] |
| Analysis 1.2  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 2 Global state: 1b. Overall improvement: Average change in CGI‐S score (up to 6 weeks; high = poor). | ||||
| 3 Global state: 2. Hospital discharge: Not discharged from hospital (> 6‐24 weeks) Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.47, 1.52] |
| Analysis 1.3  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 3 Global state: 2. Hospital discharge: Not discharged from hospital (> 6‐24 weeks). | ||||
| 4 Global state: 3. Relapse (< 52 weeks) Show forest plot | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.55, 0.86] |
| Analysis 1.4  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 4 Global state: 3. Relapse (< 52 weeks). | ||||
| 5 Global state: 4. Leaving the study early Show forest plot | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 5 Global state: 4. Leaving the study early. | ||||
| 5.1 up to 6 weeks | 16 | 1812 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.95] |
| 5.2 > 6‐24 weeks | 8 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.29, 1.00] |
| 5.3 < 52 weeks | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.14, 46.83] |
| 6 Mental state: 1. No clinical improvement (< 20% reduction in BPRS score; up to 6 weeks) Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.08] |
| Analysis 1.6  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 6 Mental state: 1. No clinical improvement (< 20% reduction in BPRS score; up to 6 weeks). | ||||
| 7 Mental state: 2a. General symptoms: Average BPRS total score (up to 6 weeks; high = poor) Show forest plot | 3 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐9.76 [‐14.60, ‐4.93] |
| Analysis 1.7  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 7 Mental state: 2a. General symptoms: Average BPRS total score (up to 6 weeks; high = poor). | ||||
| 8 Mental state: 2b. General symptoms: Average change in PANSS total score (up to 6 weeks; high = poor) Show forest plot | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐15.58 [‐23.92, ‐7.24] |
| Analysis 1.8  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 8 Mental state: 2b. General symptoms: Average change in PANSS total score (up to 6 weeks; high = poor). | ||||
| 9 Mental state: 3. Positive symptoms: Average change in PANSS positive score (up to 6 weeks; high = poor) Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐3.29 [‐4.70, ‐1.89] |
| Analysis 1.9  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 9 Mental state: 3. Positive symptoms: Average change in PANSS positive score (up to 6 weeks; high = poor). | ||||
| 10 Mental state: 4. Negative symptoms: Average change in PANSS negative score (up to 6 weeks; high = poor) Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐2.32, ‐0.04] |
| Analysis 1.10  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 10 Mental state: 4. Negative symptoms: Average change in PANSS negative score (up to 6 weeks; high = poor). | ||||
| 11 Mental state: 5. Mood: Average change in CDS score (up to 6 weeks; high = poor) Show forest plot | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.20, 0.60] |
| Analysis 1.11  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 11 Mental state: 5. Mood: Average change in CDS score (up to 6 weeks; high = poor). | ||||
| 12 Adverse effects: 1a. Movement disorders: Extrapyramidal symptoms Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 12 Adverse effects: 1a. Movement disorders: Extrapyramidal symptoms. | ||||
| 12.1 akathisia | 6 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.66 [2.24, 5.97] |
| 12.2 dystonia | 5 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.49 [3.23, 40.85] |
| 12.3 needing antiparkinson medication | 4 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [2.20, 4.72] |
| 12.4 oculogyric crises | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.57] |
| 12.5 parkinsonism (including EPS) | 5 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [2.68, 11.22] |
| 12.6 rigidity | 5 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.98 [2.74, 9.05] |
| 12.7 teeth grinding | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.11, 57.83] |
| 12.8 'thick' speech | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.89 [0.33, 105.81] |
| 12.9 tremor | 5 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.93 [1.96, 7.91] |
| 13 Adverse effects: 1b. Movement disorders: Tardive dyskinesia Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 13 Adverse effects: 1b. Movement disorders: Tardive dyskinesia. | ||||
| 13.1 dyskinesia and tardive dyskinesia | 2 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.13] |
| 14 Adverse effects: 1c. Movement disorders: Average changes scores (various scales; up to 6 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 14 Adverse effects: 1c. Movement disorders: Average changes scores (various scales; up to 6 weeks). | ||||
| 14.1 AIMS (high = poor) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.71, 0.13] |
| 14.2 BAS (high = poor) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.10, 0.52] |
| 14.3 SAS (high = poor) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.48 [0.76, 2.20] |
| 15 Adverse effects: 2. Other CNS Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 15 Adverse effects: 2. Other CNS. | ||||
| 15.1 blurred vision | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [1.21, 12.93] |
| 15.2 confusion | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.11, 57.83] |
| 15.3 dry mouth | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.62, 4.46] |
| 15.4 sedation | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.24, 3.11] |
| 16 Adverse effects: 3. Cardiovascular effects Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 16 Adverse effects: 3. Cardiovascular effects. | ||||
| 16.1 blood pressure ‐ dizziness/low BP | 3 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.36, 2.79] |
| 16.2 blood pressure ‐ high BP | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 64.26] |
| 16.3 bradycardia | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.27 [0.49, 37.10] |
| 17 Adverse effects: 4. Other adverse effects Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 17 Adverse effects: 4. Other adverse effects. | ||||
| 17.1 agitation | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.54, 2.12] |
| 17.2 anxiety | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.33, 2.16] |
| 17.3 drooling | 3 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.88, 18.21] |
| 17.4 facial oedema | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.12, 64.89] |
| 17.5 headache | 4 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.62, 1.39] |
| 17.6 infection | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.40, 122.44] |
| 17.7 insomnia | 4 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.76, 1.63] |
| 17.8 nausea/vomiting | 2 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.49, 1.65] |
| 17.9 oral hypoaesthesia | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.92] |
| 17.10 perspiration | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.58, 38.81] |
| 17.11 sleepiness | 7 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.51, 6.31] |
| 17.12 weight gain | 2 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [1.41, 16.95] |
| 17.13 weight loss | 3 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.64] |
| 18 SUBGROUP ANALYSIS: 1. MEN vs WOMEN: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.18  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 18 SUBGROUP ANALYSIS: 1. MEN vs WOMEN: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated). | ||||
| 18.1 only men | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.82] |
| 18.2 only women | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.55, 0.87] |
| 19 SUBGROUP ANALYSIS: 2. 18‐65 YEARS vs < 18 YEARS: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.19  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 19 SUBGROUP ANALYSIS: 2. 18‐65 YEARS vs < 18 YEARS: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated). | ||||
| 19.1 18‐65 years | 7 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.61, 0.80] |
| 19.2 < 18 years | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.74] |
| 20 SUBGROUP ANALYSIS: 3. ACUTE vs CHRONIC: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.20  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 20 SUBGROUP ANALYSIS: 3. ACUTE vs CHRONIC: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated). | ||||
| 20.1 acute phase of illness | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.06] |
| 20.2 chronic phase of illness | 7 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.59, 0.78] |
| 21 SUBGROUP ANALYSIS: 4. LOW DOSE vs MEDIUM TO HIGH DOSE Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.21  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 21 SUBGROUP ANALYSIS: 4. LOW DOSE vs MEDIUM TO HIGH DOSE. | ||||
| 21.1 low dose (≤ 5 mg/day) ‐ Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.69, 1.04] |
| 21.2 medium to high dose (> 5 mg/day) ‐ Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) | 3 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.35, 0.66] |
| 21.3 low dose (≤ 5 mg/day) ‐ Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) | 4 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.81] |
| 21.4 medium to high dose (> 5 mg/day) ‐ Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) | 5 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.83] |
| 22 SUBGROUP ANALYSIS: 5. DIAGNOSTIC CRITERIA vs NO DIAGNOSTIC CRITERIA Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.22  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 22 SUBGROUP ANALYSIS: 5. DIAGNOSTIC CRITERIA vs NO DIAGNOSTIC CRITERIA. | ||||
| 22.1 operational criteria used for diagnosis ‐ Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.81] |
| 22.2 no operational criteria used for diagnosis ‐ Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.24] |
| 22.3 operational criteria used for diagnosis ‐ Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.74] |
| 22.4 no operational criteria used for diagnosis ‐ Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) | 7 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.61, 0.80] |
| 22.5 operational criteria used for diagnosis ‐ Global state: Relapse (< 52 weeks) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.91] |
| 22.6 no operational criteria used for diagnosis ‐ Global state: Relapse (< 52 weeks) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.37, 1.03] |
| 23 SENSITIVITY ANALYSIS: 1. ASSUMPTIONS FOR MISSING DATA vs NO ASSUMPTIONS FOR MISSING DATA: Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.23  Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 23 SENSITIVITY ANALYSIS: 1. ASSUMPTIONS FOR MISSING DATA vs NO ASSUMPTIONS FOR MISSING DATA: Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated). | ||||
| 23.1 no assumptions for missing data | 3 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.87] |
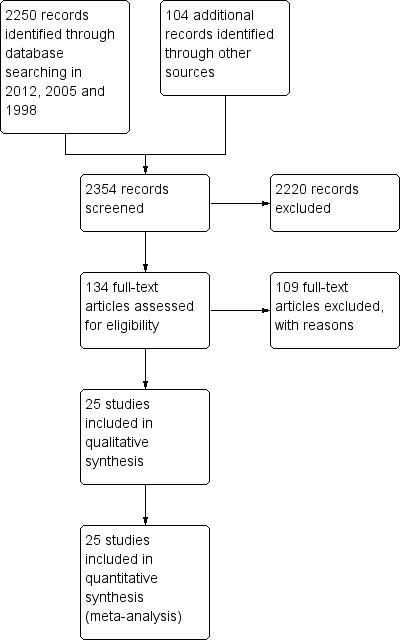
Study flow diagram.
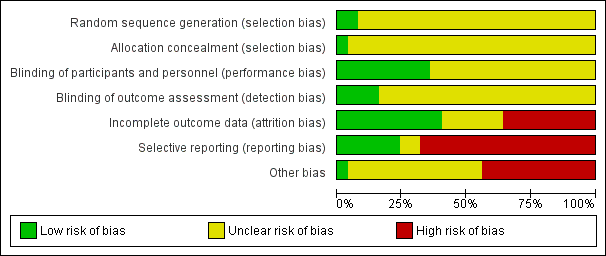
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
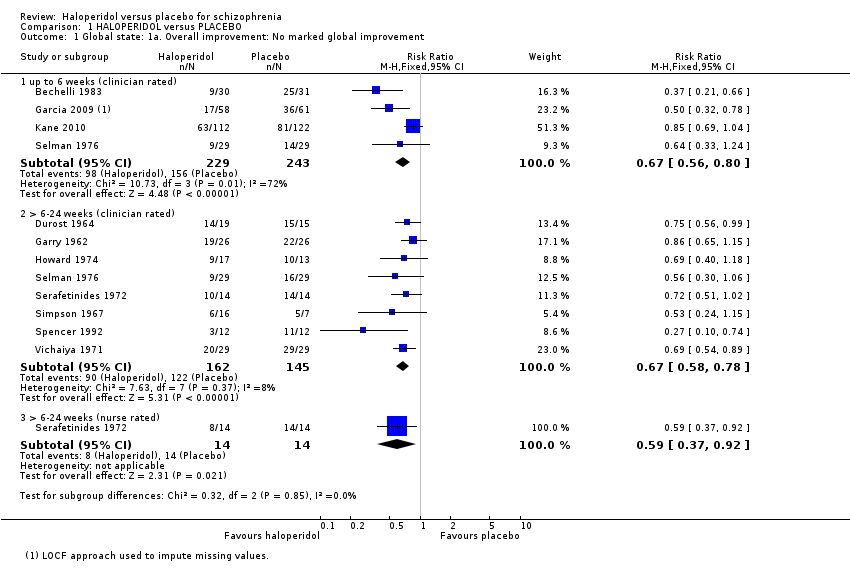
Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 1 Global state: 1a. Overall improvement: No marked global improvement.

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 2 Global state: 1b. Overall improvement: Average change in CGI‐S score (up to 6 weeks; high = poor).
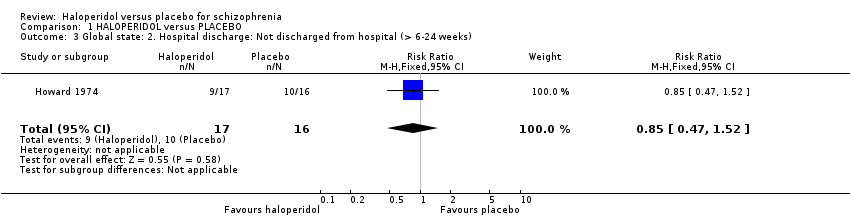
Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 3 Global state: 2. Hospital discharge: Not discharged from hospital (> 6‐24 weeks).

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 4 Global state: 3. Relapse (< 52 weeks).
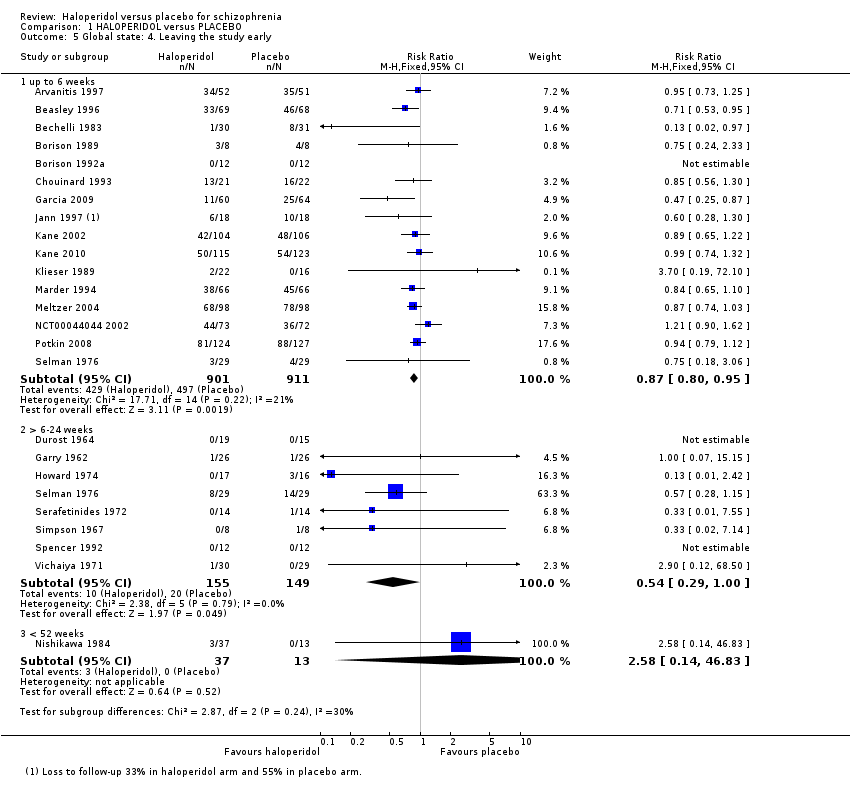
Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 5 Global state: 4. Leaving the study early.

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 6 Mental state: 1. No clinical improvement (< 20% reduction in BPRS score; up to 6 weeks).

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 7 Mental state: 2a. General symptoms: Average BPRS total score (up to 6 weeks; high = poor).
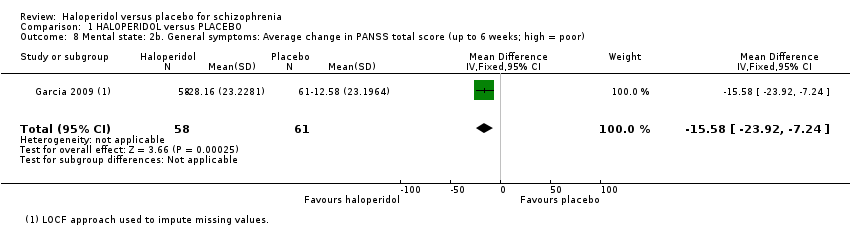
Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 8 Mental state: 2b. General symptoms: Average change in PANSS total score (up to 6 weeks; high = poor).

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 9 Mental state: 3. Positive symptoms: Average change in PANSS positive score (up to 6 weeks; high = poor).

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 10 Mental state: 4. Negative symptoms: Average change in PANSS negative score (up to 6 weeks; high = poor).

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 11 Mental state: 5. Mood: Average change in CDS score (up to 6 weeks; high = poor).

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 12 Adverse effects: 1a. Movement disorders: Extrapyramidal symptoms.
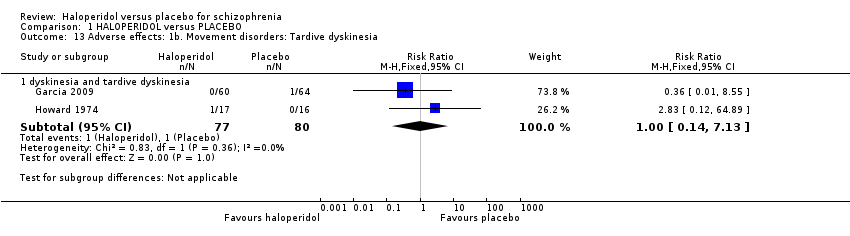
Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 13 Adverse effects: 1b. Movement disorders: Tardive dyskinesia.
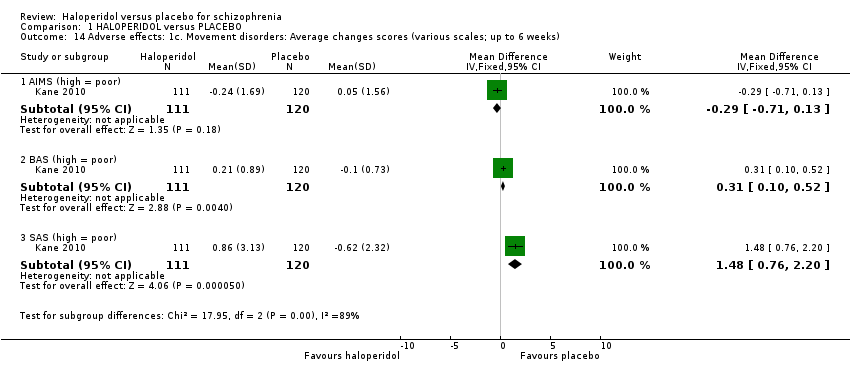
Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 14 Adverse effects: 1c. Movement disorders: Average changes scores (various scales; up to 6 weeks).

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 15 Adverse effects: 2. Other CNS.

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 16 Adverse effects: 3. Cardiovascular effects.

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 17 Adverse effects: 4. Other adverse effects.
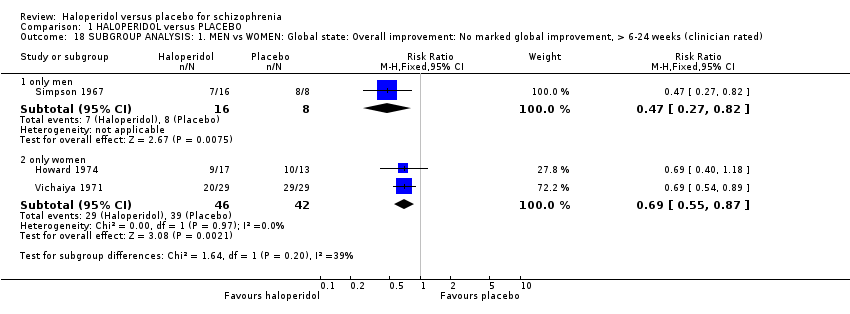
Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 18 SUBGROUP ANALYSIS: 1. MEN vs WOMEN: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated).
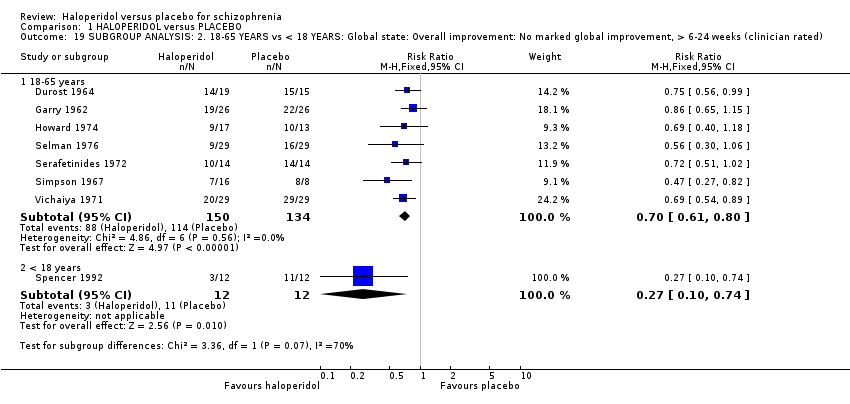
Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 19 SUBGROUP ANALYSIS: 2. 18‐65 YEARS vs < 18 YEARS: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated).

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 20 SUBGROUP ANALYSIS: 3. ACUTE vs CHRONIC: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated).

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 21 SUBGROUP ANALYSIS: 4. LOW DOSE vs MEDIUM TO HIGH DOSE.
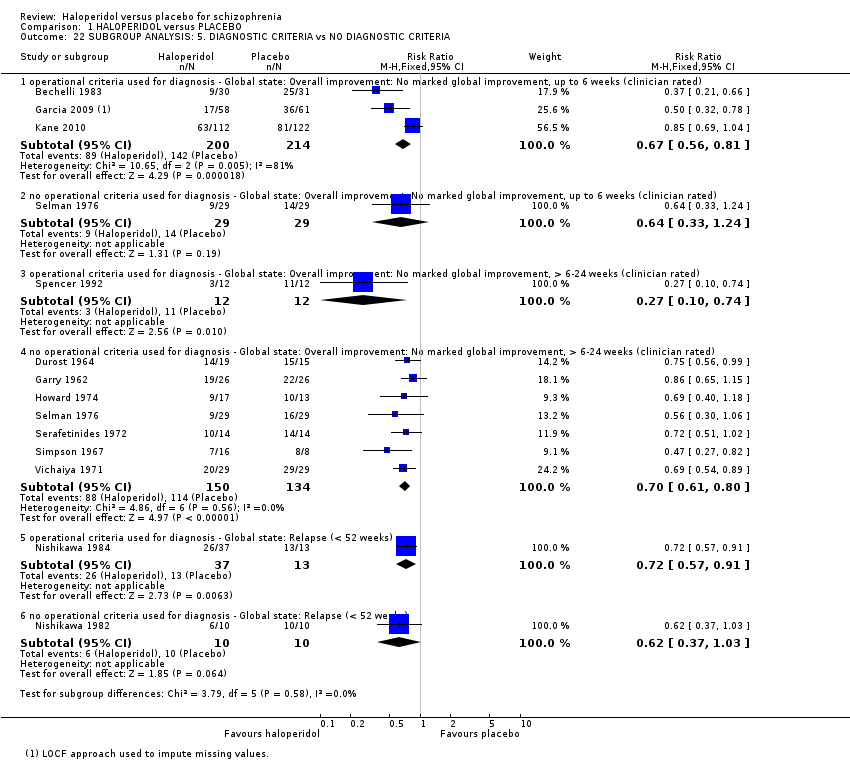
Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 22 SUBGROUP ANALYSIS: 5. DIAGNOSTIC CRITERIA vs NO DIAGNOSTIC CRITERIA.

Comparison 1 HALOPERIDOL versus PLACEBO, Outcome 23 SENSITIVITY ANALYSIS: 1. ASSUMPTIONS FOR MISSING DATA vs NO ASSUMPTIONS FOR MISSING DATA: Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated).
| HALOPERIDOL versus PLACEBO for schizophrenia | ||||||
| Patient or population: patients with schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | HALOPERIDOL versus PLACEBO | |||||
| Death ‐ suicide and natural causes | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| Overall improvement: No marked global improvement | 841 per 1000 | 564 per 1000 | RR 0.67 | 307 | ⊕⊕⊕⊝ | Another four trials reported on this outcome at up to six weeks follow‐up, and one trial at > 6‐24 weeks follow‐up using a nurse‐rated scale, both sub‐analyses showed significant results in favour of haloperidol. |
| Not discharged from hospital | 625 per 1000 | 531 per 1000 | RR 0.85 | 33 | ⊕⊝⊝⊝ | |
| Relapse | 1000 per 1000 | 690 per 1000 | RR 0.69 | 70 | ⊕⊝⊝⊝ | |
| Leaving the study early | 134 per 1000 | 72 per 1000 | RR 0.54 | 304 | ⊕⊕⊕⊝ | Another 16 trials reported on this outcome at up to six weeks follow‐up showing a significant result in favour of haloperidol. One trial at < 52 weeks follow‐up showed no difference between haloperidol and placebo. |
| Satisfaction with treatment | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| Adverse effects: Movement disorders ‐ parkinsonism | 28 per 1000 | 154 per 1000 | RR 5.48 | 485 | ⊕⊕⊕⊝ | Several studies also reported on other, specific movement disorders: there was a significant result favouring placebo for akathisia, dystonia, needing anti‐Parkinson medication, rigidity and tremor; there was no difference between haloperidol and placebo for tardive dyskinesia, oculogyric crises, teeth grinding and 'thick' speech. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Seven out of the eight included studies had an unclear risk of bias for random sequence generation and for allocation concealment. Blinding of participants and personnel was unclear in four studies and blinding of assessors was unclear in six. Two studies had an unclear risk of bias for incomplete outcome data. One study had a high risk of other bias as they were funded by industry and three an unclear risk of bias as the drugs were provided by a pharmaceutical company. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1a. Overall improvement: No marked global improvement Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 up to 6 weeks (clinician rated) | 4 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.80] |
| 1.2 > 6‐24 weeks (clinician rated) | 8 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.58, 0.78] |
| 1.3 > 6‐24 weeks (nurse rated) | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.37, 0.92] |
| 2 Global state: 1b. Overall improvement: Average change in CGI‐S score (up to 6 weeks; high = poor) Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.73, ‐0.25] |
| 3 Global state: 2. Hospital discharge: Not discharged from hospital (> 6‐24 weeks) Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.47, 1.52] |
| 4 Global state: 3. Relapse (< 52 weeks) Show forest plot | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.55, 0.86] |
| 5 Global state: 4. Leaving the study early Show forest plot | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 up to 6 weeks | 16 | 1812 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.95] |
| 5.2 > 6‐24 weeks | 8 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.29, 1.00] |
| 5.3 < 52 weeks | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.14, 46.83] |
| 6 Mental state: 1. No clinical improvement (< 20% reduction in BPRS score; up to 6 weeks) Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.08] |
| 7 Mental state: 2a. General symptoms: Average BPRS total score (up to 6 weeks; high = poor) Show forest plot | 3 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐9.76 [‐14.60, ‐4.93] |
| 8 Mental state: 2b. General symptoms: Average change in PANSS total score (up to 6 weeks; high = poor) Show forest plot | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐15.58 [‐23.92, ‐7.24] |
| 9 Mental state: 3. Positive symptoms: Average change in PANSS positive score (up to 6 weeks; high = poor) Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐3.29 [‐4.70, ‐1.89] |
| 10 Mental state: 4. Negative symptoms: Average change in PANSS negative score (up to 6 weeks; high = poor) Show forest plot | 2 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐2.32, ‐0.04] |
| 11 Mental state: 5. Mood: Average change in CDS score (up to 6 weeks; high = poor) Show forest plot | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.20, 0.60] |
| 12 Adverse effects: 1a. Movement disorders: Extrapyramidal symptoms Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 akathisia | 6 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.66 [2.24, 5.97] |
| 12.2 dystonia | 5 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.49 [3.23, 40.85] |
| 12.3 needing antiparkinson medication | 4 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [2.20, 4.72] |
| 12.4 oculogyric crises | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.57] |
| 12.5 parkinsonism (including EPS) | 5 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.48 [2.68, 11.22] |
| 12.6 rigidity | 5 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.98 [2.74, 9.05] |
| 12.7 teeth grinding | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.11, 57.83] |
| 12.8 'thick' speech | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.89 [0.33, 105.81] |
| 12.9 tremor | 5 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.93 [1.96, 7.91] |
| 13 Adverse effects: 1b. Movement disorders: Tardive dyskinesia Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 dyskinesia and tardive dyskinesia | 2 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.13] |
| 14 Adverse effects: 1c. Movement disorders: Average changes scores (various scales; up to 6 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 AIMS (high = poor) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.71, 0.13] |
| 14.2 BAS (high = poor) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.10, 0.52] |
| 14.3 SAS (high = poor) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.48 [0.76, 2.20] |
| 15 Adverse effects: 2. Other CNS Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 blurred vision | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [1.21, 12.93] |
| 15.2 confusion | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.11, 57.83] |
| 15.3 dry mouth | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.62, 4.46] |
| 15.4 sedation | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.24, 3.11] |
| 16 Adverse effects: 3. Cardiovascular effects Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 blood pressure ‐ dizziness/low BP | 3 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.36, 2.79] |
| 16.2 blood pressure ‐ high BP | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 64.26] |
| 16.3 bradycardia | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.27 [0.49, 37.10] |
| 17 Adverse effects: 4. Other adverse effects Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 agitation | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.54, 2.12] |
| 17.2 anxiety | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.33, 2.16] |
| 17.3 drooling | 3 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.88, 18.21] |
| 17.4 facial oedema | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.12, 64.89] |
| 17.5 headache | 4 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.62, 1.39] |
| 17.6 infection | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.40, 122.44] |
| 17.7 insomnia | 4 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.76, 1.63] |
| 17.8 nausea/vomiting | 2 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.49, 1.65] |
| 17.9 oral hypoaesthesia | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.92] |
| 17.10 perspiration | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.58, 38.81] |
| 17.11 sleepiness | 7 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.51, 6.31] |
| 17.12 weight gain | 2 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [1.41, 16.95] |
| 17.13 weight loss | 3 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.64] |
| 18 SUBGROUP ANALYSIS: 1. MEN vs WOMEN: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 only men | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.82] |
| 18.2 only women | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.55, 0.87] |
| 19 SUBGROUP ANALYSIS: 2. 18‐65 YEARS vs < 18 YEARS: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 18‐65 years | 7 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.61, 0.80] |
| 19.2 < 18 years | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.74] |
| 20 SUBGROUP ANALYSIS: 3. ACUTE vs CHRONIC: Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 acute phase of illness | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.06] |
| 20.2 chronic phase of illness | 7 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.59, 0.78] |
| 21 SUBGROUP ANALYSIS: 4. LOW DOSE vs MEDIUM TO HIGH DOSE Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 low dose (≤ 5 mg/day) ‐ Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.69, 1.04] |
| 21.2 medium to high dose (> 5 mg/day) ‐ Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) | 3 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.35, 0.66] |
| 21.3 low dose (≤ 5 mg/day) ‐ Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) | 4 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.81] |
| 21.4 medium to high dose (> 5 mg/day) ‐ Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) | 5 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.83] |
| 22 SUBGROUP ANALYSIS: 5. DIAGNOSTIC CRITERIA vs NO DIAGNOSTIC CRITERIA Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 operational criteria used for diagnosis ‐ Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.81] |
| 22.2 no operational criteria used for diagnosis ‐ Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.24] |
| 22.3 operational criteria used for diagnosis ‐ Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.74] |
| 22.4 no operational criteria used for diagnosis ‐ Global state: Overall improvement: No marked global improvement, > 6‐24 weeks (clinician rated) | 7 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.61, 0.80] |
| 22.5 operational criteria used for diagnosis ‐ Global state: Relapse (< 52 weeks) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.91] |
| 22.6 no operational criteria used for diagnosis ‐ Global state: Relapse (< 52 weeks) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.37, 1.03] |
| 23 SENSITIVITY ANALYSIS: 1. ASSUMPTIONS FOR MISSING DATA vs NO ASSUMPTIONS FOR MISSING DATA: Global state: Overall improvement: No marked global improvement, up to 6 weeks (clinician rated) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 no assumptions for missing data | 3 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.87] |

