Perioperativna optimizacija volumena tekućine nakon prijeloma proksimalnog dijela bedrene kosti
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial Parallel group | |
| Participants | Baseline characteristics Advanced invasive haemodynamic monitoring goal directed
Protocol using standard measures
Included criteria: patients aged > 70 years weighing > 40 kg. Undergoing PFF surgery during regular operating hours Excluded criteria: patients who could be harmed by the treatment (ongoing myocardial infarction, chronic dialysis), concomitant medication with lithium, known allergy to lithium or medical device components, weight ≤ 40 kg, life expectancy < 6 months, pathological fractures and conditions, inability to give informed consent, anticipated difficulties obtaining data during the first postoperative year (as judged by a research team member), operations scheduled during hours when research team was unavailable. (Patients with pathological fractures had presumed survival < 12 months. Given the planned follow‐up of 12 months, these patients were not included.) Sponsorship source: Stockholm county grant Country: Sweden Setting: hospital | |
| Interventions | Intervention characteristics Advanced invasive haemodynamic monitoring goal directed
Protocol using standard measures
| |
| Outcomes | Continuous
Dichotomous
Adverse events
| |
| Notes | Number of participants: Tables showed discrepancy in the number of participants. Review authors have taken number of participants from the text, not from the tables Other: elderly patients. Poor recruitment to study led to early stop at 150 participants. Originally powered to include 460 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation obtained via telephone. Sequence not available to research team |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants, caregivers, anaesthetist not blinded. Unclear whether this could have affected performance of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Personnel collecting data were not blinded, but assigned treatment was coded in the data set for primary and secondary outcome analyses. Therefore, attempts were made to blind data analysts |
| Blinding of outcome assessment (detection bias) | Low risk | Personnel collecting data were not blinded, but assigned treatment was coded in the data set for primary and secondary outcome analyses. Therefore, attempts were made to blind data analysts |
| Incomplete outcome data (attrition bias) | Low risk | Loss of 7 participants after randomization. Reasons for loss given; analyses completed by study authors included ITT analyses |
| Selective reporting (reporting bias) | Low risk | ClinicalTrials.gov; ref NCT01141894. Protocol includes outcomes related to health‐related quality of life and cost analysis, with plans for each participant to have 4 postoperative visits. This is not reported at all in full study publication but is reported in interim report |
| Other bias | Unclear risk | Baseline characteristics well documented. Comparable, except for diabetes mellitus in twice as many participants in the GDHT group ‐ unclear how this could affect results |
| Methods | Study design: randomized controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics Advanced invasive haemodynamic monitoring goal directed
Protocol using standard measures
Included criteria: patients admitted through the emergency department with primary fragility hip fracture, over 60 years of age and listed for surgical repair under spinal anaesthesia. Included patients unable to give consent on their own Excluded criteria: planned general anaesthetic for surgery repair, severe valvular heart disease (as this could affect the accuracy of the LiDCO device), taking therapeutic lithium (as this can affect the calibration of the LiDCO device), multiple injuries, revision hip surgery or requirement for total hip arthroplasty Sponsorship source: NIHR funding Country: UK Setting: hospital | |
| Interventions | Intervention characteristics Advanced invasive haemodynamic monitoring goal directed
Usual care
| |
| Outcomes | Continuous
Dichotomous
Adverse events
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated concealed tables with blocks of unequal size stratified according to gender and mortality risk |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized...via a secure website" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Attending anaesthetist was aware of treatment allocation but was blinded from LiDCO monitor in control group. Unclear whether this could have affected performance of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Data extracted from notes by staff unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Data extracted from notes by staff unaware of treatment allocation |
| Incomplete outcome data (attrition bias) | High risk | Loss of 12% of data |
| Selective reporting (reporting bias) | Low risk | Registered trial; ISRCTN88284896. Had not reported on cost of care or effects on heart and blood vessels, but not relevant to this review |
| Other bias | Unclear risk | Baseline characteristics comparable, although participants in intervention group transferred to theatre more quickly ‐ unclear how this affected results |
| Methods | Study design: randomized controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics Advanced invasive haemodynamic monitoring goal directed
Protocol using standard measures
Included criteria: intracapsular and extracapsular hip fractures; specifics not described Excluded criteria: not described Sponsorship source: no details Country:: Westchester County Medical Centre, New York, USA Setting: single centre, hospital | |
| Interventions | Intervention characteristics Advanced invasive haemodynamic monitoring goal directed
Protocol using standard measures
| |
| Outcomes | Dichotomous
Adverse events
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Paper states that participants were assigned on a random basis on admission to hospital. No details given. Large differences in groups at baseline |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information about whether clinical staff in operating theatre or on ward were aware of participant allocation. Unclear whether knowledge of group allocation would have affected performance. Intervention not clearly defined |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about how outcomes were assessed; no definitions |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome not reported |
| Incomplete outcome data (attrition bias) | Low risk | All randomly assigned participants included in analyses. No information about exclusions due to deviations from protocol |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not fully described |
| Other bias | High risk | Serious baseline imbalances between monitored and non‐monitored group raise questions about the randomization procedure; methods not fully clear; outcomes not fully defined |
| Methods | Study design: randomized controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics Advanced invasive haemodynamic monitoring goal directed
Usual care
Country: London, UK Setting: single centre, teaching hospital Included criteria: adult patients with fractures of the femoral neck Excluded criteria: age < 55 years, fracture secondary to neoplasm, fractures occurring during hospitalization for acute illness, fracture through the site of a previous surgical correction or associated with instability of a previous prosthesis, planned regional anaesthesia (this would preclude the planned intervention) | |
| Interventions | Intervention characteristics Advanced invasive haemodynamic monitoring goal directed
Usual care
| |
| Outcomes | Continuous
Dichotomous
Adverse events
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomization process given |
| Allocation concealment (selection bias) | Unclear risk | Not clear whether sequentially numbered, opaque, sealed envelopes were used |
| Blinding of participants and personnel (performance bias) | Unclear risk | Anaesthetist blinded to Doppler measurements but aware of fluid challenges and therefore likely to know the allocation ‐ probably the surgeon as well. Unclear whether this could have affected performance of anaesthetist. Other medical and nursing staff unaware of randomization of participants |
| Blinding of outcome assessment (detection bias) | Low risk | Medical and nursing staff unaware of randomization of participants |
| Blinding of outcome assessment (detection bias) | Low risk | No discharge criteria given, but staff were blinded; therefore unlikely to bias results |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers in each group included in Results unclear. No details about losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported, but length of stay reported in chart as median and IQR |
| Other bias | Low risk | Study groups similar at baseline |
| Methods | Study design: randomized controlled trial Study grouping: parallel group | |
| Participants | Baseline characteristics Advanced invasive haemodynamic monitoring goal directed
Protocol using standard measures
Usual care
Included criteria: adult patients undergoing repair of PFF under general anaesthesia Excluded criteria: age < 65 years, fracture secondary to neoplasm, oesophageal pathology, patients with central venous cannula in situ, planned regional anaesthesia (this would preclude 1 of the planned interventions) Sponsorship source: no details Country: London, UK Setting: single centre, teaching hospital | |
| Interventions | Intervention characteristics Advanced invasive haemodynamic monitoring goal directed
Protocol using standard measures
Usual care
| |
| Outcomes | Continuous
Dichotomous
Adverse events
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not clear whether sequentially numbered, opaque, sealed envelopes were used |
| Blinding of participants and personnel (performance bias) | Unclear risk | Anaesthetist (and surgeon) aware of fluid challenges and allocation of participants. Unclear whether this could have affected performance of personnel "Postoperative management was performed by orthopaedic medical team and nursing staff who were unaware of patient's randomization" |
| Blinding of outcome assessment (detection bias) | Low risk | Medical and nursing staff unaware of randomization of participants |
| Blinding of outcome assessment (detection bias) | Low risk | No discharge criteria given, but staff were blinded; therefore, unlikely to bias results |
| Incomplete outcome data (attrition bias) | Low risk | 1 participant in CVP group underwent intramedullary nailing but was included in ITT analyses. No losses to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | Study groups similar at baseline |
AM: Austin Moore uncemented hemiarthroplasty; AO: Association for the Study of Internal Fixation; ASA: American Society of Anesthesiologists (physical status classification system); BMI: body mass index; CVP: central venous pressure; DHS: dynamic hip screw; ETS: Exeter trauma stem‐cemented hemiarthroplasty; F: female; GA: general anaesthesia; GDHT: goal‐directed haemodynamic treatment; ITT: intention‐to‐treat; IQR: interquartile range; kg: kilograms; M: male; NIHR: National Institute for Health Research; PA: pulmonary artery; PFF: proximal femoral fracture; RA: right atrial; RIFLE: Risk/Injury/Failure/Loss/End‐stage (classification system for acute kidney injury); RV: right ventricle; SD: standard deviation; TFN: trochanteric femoral nail
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong intervention ‐ Hb targeting, not fluid optimization | |
| Wrong intervention. Study concentrates on titration of Hb levels rather than on optimization of fluid status | |
| Wrong intervention | |
| Wrong intervention. Both groups had identical fluid regimen intraoperatively | |
| Wrong intervention ‐ designed to compare the effects of Ringer`s lactate and hydroxyethyl starch 6% on haemodynamic parameters after spinal anaesthesia | |
| Wrong participants ‐ elective surgery; no orthopaedic participants | |
| Only 1 participant in study may have undergone PFF surgery (unclear) and was assigned to the control group. Would not be appropriate to include such a small sample | |
| Wrong intervention ‐ comparison of spinal and general anaesthesia for participants with femoral fracture | |
| Wrong intervention ‐ effect of iron infusions on postoperative transfusion requirements for participants with femoral fracture | |
| Wrong intervention | |
| No participants with PFF (all general/vascular/urological surgery) |
Hb: haemoglobin; PFF: proximal femoral fracture
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Publication type: full article Allocation random: by computer‐generated sequence Allocation concealment: sequentially numbered opaque, sealed envelopes Baseline comparison: yes Baseline similarity: yes Blinding of caregivers: not considered feasible by investigators Additional features to blind fluid administered: not considered feasible Control of co‐interventions: not described Completeness of follow up: yes ‐ to hospital discharge Intention‐to‐treat analysis: yes |
| Participants | Location: Canada Centre: 19 centres Language: English Inclusion criteria: adults undergoing high‐risk, urgent or elective major thoracic/abdominal/vascular/orthopaedic surgery, then ICU stay Exclusion criteria: nil specified Age: 60 years or older ASA grade: III to IV Surgery type: not specified |
| Interventions | PAC group: goal‐directed fluid therapy, using PAC according to protocol to optimize oxygen delivery Control group: standard fluid therapy |
| Outcomes | In‐hospital all‐cause mortality 6‐Month mortality 12‐Month mortality Length of stay Iatrogenic complications: wound infections; problems due to line insertion Cardiopulmonary complications: myocardial infarction, left ventricular failure, arrhythmia, pneumonia, pulmonary embolism Other complications: renal/liver insufficiency, sepsis |
| Notes | To date, unable to contact study authors to obtain outcome data regarding hip fracture subgroup. If these data become available, this study will be included |
| Methods | Randomized single‐blind controlled trial Parallel design |
| Participants | Location: Greece Inclusion criteria: patients undergoing femoral fracture repair under spinal anaesthesia Number of participants: 20 |
| Interventions | Advanced haemodynamic monitoring: goal‐directed therapy, participants connected to Flo Trac/Vigileo haemodynamic monitoring system to measure cardiac output Control: standard monitoring |
| Outcomes | Duration of hospital stay |
| Notes | Abstract only. Unable to contact study authors to obtain outcome data |
ASA: American Society of Anesthesiologists (physical status classification system); PAC: pulmonary artery catheter
.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Non‐invasive cardiac output monitoring to guide goal‐directed fluid therapy in high‐risk patients undergoing urgent surgical repair of proximal femoral fractures (ClearNOF) |
| Methods | Randomized single‐blind controlled trial |
| Participants | Adults (50 years or older) due to undergo urgent or emergency repair of proximal femoral fracture |
| Interventions | Non‐invasive cardiac monitoring device (Clearsight) vs usual care |
| Outcomes | Incidence of major and minor complications Morbidity at day 3, 5 and 10 Length of stay Time to drinking/eating/mobilization Change in perioperative haemodynamic variables (heart rate, blood pressure, stroke volume) Hypotension Total dose of vasopressor |
| Starting date | January 2015 |
| Contact information | Simon Davies; York Teaching Hospitals NHS Foundation Trust |
| Notes | Estimated enrolment: 250 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.20] |
| Analysis 1.1  Comparison 1 Advanced haemodynamic monitoring versus protocol using standard measures, Outcome 1 All‐cause mortality. | ||||
| 2 Adverse outcomes Show forest plot | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| Analysis 1.2  Comparison 1 Advanced haemodynamic monitoring versus protocol using standard measures, Outcome 2 Adverse outcomes. | ||||
| 2.1 Any complications, including minor | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.07, 2.95] |
| Analysis 2.1 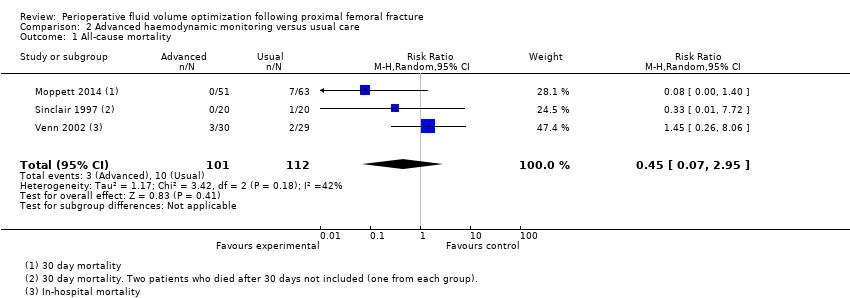 Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 1 All‐cause mortality. | ||||
| 2 Total length of hospital stay Show forest plot | 2 | 173 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐1.70, 2.96] |
| Analysis 2.2  Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 2 Total length of hospital stay. | ||||
| 3 Days until medically fit for discharge Show forest plot | 2 | 173 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐1.74, 1.71] |
| Analysis 2.3  Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 3 Days until medically fit for discharge. | ||||
| 4 Adverse outcomes Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 4 Adverse outcomes. | ||||
| 4.1 Neurological | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.56, 2.18] |
| 4.2 Any complications, including minor | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.31] |

Study flow diagram. Updated search October 2012 to January 2015.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Advanced haemodynamic monitoring versus protocol using standard measures, Outcome 1 All‐cause mortality.
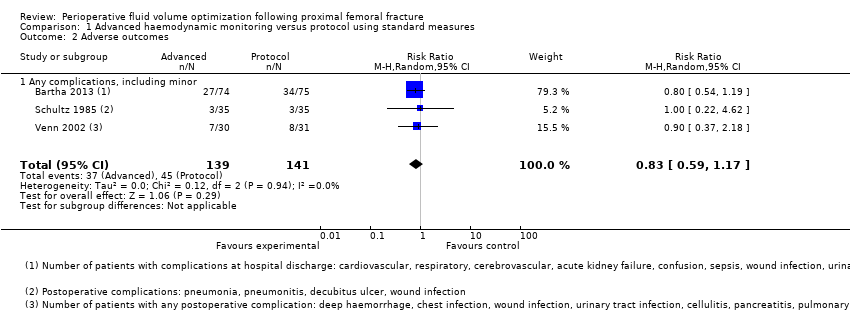
Comparison 1 Advanced haemodynamic monitoring versus protocol using standard measures, Outcome 2 Adverse outcomes.

Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 1 All‐cause mortality.

Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 2 Total length of hospital stay.

Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 3 Days until medically fit for discharge.
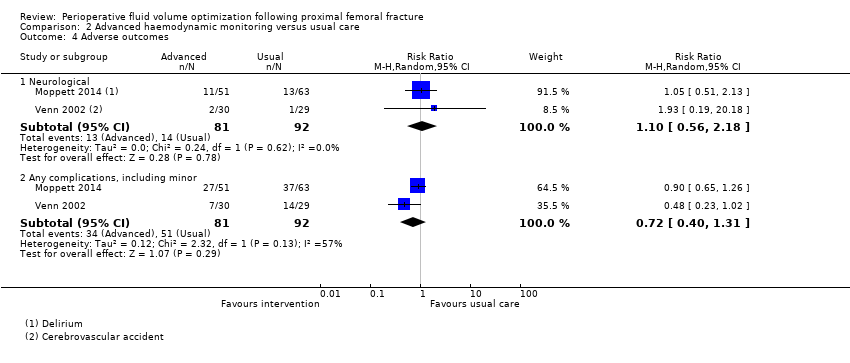
Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 4 Adverse outcomes.
| Advanced haemodynamic monitoring compared with protocol using standard measures such as CVP for proximal femoral fracture | ||||||
| Patient or population: patients with proximal femoral fracture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Protocol using standard measures such as CVP | Advanced haemodynamic monitoring | |||||
| All‐cause mortality | Study population | RR 0.41 | 280 | ⊕⊝⊝⊝ | ||
| 142 per 1000 | 58 per 1000 | |||||
| Moderate | ||||||
| Total length of hospital stay | Not estimabled | 203 | ⊕⊕⊝⊝ | Data reported as median (range) in Bartha 2013 and as mean (95% confidence interval) in Venn 2002 | ||
| Medically fit for discharge | Mean medically fit for discharge in the intervention groups was | 90 | ⊕⊕⊝⊝ | |||
| Return to pre‐fracture accommodation/return to pre‐fracture mobility | Not estimable | ‐ | Not reported | |||
| Adverse outcomes ‐ cardiopulmonary | Study population | Not estimable | 0 | |||
| Moderate | ||||||
| Adverse outcomes ‐ neurological | Not estimable | 0 | ||||
| Adverse outcomes ‐ all | Study population | RR 0.90 | 280 | ⊕⊝⊝⊝ | ||
| 319 per 1000 | 287 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aConcerns about randomization process in Schultz 1985; high risk of selection bias eData from 1 study only. Downgraded 2 levels | ||||||
| Advanced haemodynamic monitoring compared with usual care for perioperative fluid optimization | ||||||
| Patient or population: patients with perioperative fluid optimization | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Advanced haemodynamic monitoring | |||||
| All‐cause mortality | Study population | RR 0.45 | 213 | ⊕⊕⊝⊝ | ||
| 89 per 1000 | 40 per 1000 | |||||
| Moderate | ||||||
| Total length of hospital stay | Mean total length of hospital stay in the control groups was | Mean total length of hospital stay in the intervention groups was | 175 | ⊕⊕⊝⊝ | ||
| Medically fit for discharge | Mean medically fit for discharge in the control groups was | Mean medically fit for discharge in the intervention groups was | 175 | ⊕⊕⊝⊝ | ||
| Return to pre‐fracture accommodation/return to pre‐fracture mobility | Study population | Not estimable | 114 | ⊕⊕⊝⊝ | ||
| 397 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| Adverse outcomes ‐ cardiopulmonary | Study population | Not estimable | 0 | |||
| Moderate | ||||||
| Adverse outcomes ‐ neurological | Study population | RR 1.10 | 173 | ⊕⊕⊝⊝ | ||
| 152 per 1000 | 170 per 1000 | |||||
| Moderate | ||||||
| Adverse outcomes ‐ all | Study population | RR 0.78 | 173 | ⊕⊕⊝⊝ | ||
| 554 per 1000 | 432 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aConfidence intervals cross no effect and are consistent with increased as well as decreased risk. Downgraded 1 level | ||||||
| Protocol using standard measures such as CVP compared with usual care for perioperative fluid optimization | ||||||
| Patient or population: patients with perioperative fluid optimization | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Protocol using standard measures such as CVP | |||||
| All‐cause mortality | Study population | RR 2.81 | 60 | ⊕⊕⊝⊝ | ||
| 69 per 1000 | 194 per 1000 | |||||
| Moderate | ||||||
| Total length of hospital stay | Mean total length of hospital stay in the control groups was | Mean total length of hospital stay in the intervention groups was | 60 | ⊕⊕⊝⊝ | ||
| Medically fit for discharge | Mean medically fit for discharge in the intervention groups was | 60 | ⊕⊕⊕⊝ | |||
| Return to pre‐fracture accommodation/return to pre‐fracture mobility | Not estimable | ‐ | Not reported | |||
| Adverse outcomes ‐ cardiopulmonary | Study population | Not estimable | 0 | Not reported | ||
| Moderate | ||||||
| Adverse outcomes ‐ neurological | Study population | RR 0.94 | 60 | ⊕⊕⊝⊝ | ||
| 34 per 1000 | 32 per 1000 | |||||
| Moderate | ||||||
| Adverse outcomes ‐ all | Study population | RR 0.53 | 60 | ⊕⊕⊝⊝ | ||
| 483 per 1000 | 256 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aBased on 1 study with a small number of events. Wide confidence intervals consistent with increased as well as decreased risk. Downgraded by 1 level | ||||||
| Study ID | Adverse events | Advanced haemodynamic monitoring | Protocol using standard measures |
| n = 74 | n = 75 | ||
| Cardiopulmonary | Cardiovascular 5 Respiratory 5 | Cardiovascular 6 Respiratory 7 | |
| Neurological | Cerebrovascular 0 Confusion 3 | Cerebrovascular 2 Confusion 6 | |
| Other | Acute kidney failure 1 Gastrointestinal bleeding 0 Sepsis 2 Deep vein thrombosis 0 Wound infection 2 Delayed healing 0 Urinary tract infection 16 Decubitus 6 Wound haematoma 0 Other 4 | Acute kidney failure 1 Gastrointestinal bleeding 0 Sepsis 0 Deep vein thrombosis 0 Wound infection 1 Delayed healing 0 Urinary tract infection 12 Decubitus 1 Wound haematoma 1 Other 6 | |
| n = 35 | n = 35 | ||
| Other | Pneumonia 1 Wound infection 1 Pneumonitis 1 | Pneumonia 2 Decubitis ulcer 1 | |
| n = 30 | n = 31 | ||
| Cardiopulmonary | Chest infection 2 Pulmonary embolus 1 Myocardial infarction 0 Cardiac failure 0 Rapid atrial fibrillation 3 Hypotension 0 | Chest infection 3 Pulmonary embolus 0 Myocardial infarction 1 Cardiac failure 1 Rapid atrial fibrillation 1 Hypotension 0 | |
| Neurological | Cerebrovascular accident 2 | Cerebrovascular accident 1 | |
| Other | Deep haemorrhage 1 Haematemesis 0 Wound infection 0 Urinary tract infection 2 Cellulitis 0 Pancreatitis 0 Hypotension 0 Impaired renal function 0 Pseudo‐obstruction 0 | Deep haemorrhage 0 Haematemesis 0 Wound infection 0 Urinary tract infection 1 Cellulitis 1 Pancreatitis 0 Hypotension 0 Impaired renal function 0 Pseudo‐obstruction 1 |
| Study ID | Adverse events | Advanced haemodynamic monitoring | Usual care |
| n = 51 | n = 63 | ||
| Cardiopulmonary | Cardiovascular 8 Respiratory 0 | Cardiovascular 6 Respiratory 0 | |
| Neurological | Acute delirium 11 | Acute delirium 13 | |
| Other | Infectious 21 Abdominal 2 Bleeding 0 Skin 0 Renal (RIFLE) 18* Other 3 | Infectious 34 Abdominal 1 Bleeding 0 Skin 0 Renal (RIFLE) 32* Other 3 | |
| n = 30 | n = 29 | ||
| Cardiopulmonary | Chest infection 2 Pulmonary embolus 1 Myocardial infarction 0 Cardiac failure 0 Rapid atrial fibrillation 3 Hypotension 0 | Chest infection 5 Pulmonary embolus 0 Myocardial infarction 0 Cardiac failure 0 Rapid atrial fibrillation 2 Hypotension 3 | |
| Neurological | Cerebrovascular accident 2 | Cerebrovascular accident 1 | |
| Other | Deep haemorrhage 1 Haematemesis 0 Wound infection 0 Urinary tract infection 2 Cellulitis 0 Pancreatitis 0 Hypotension 0 Impaired renal function 0 Pseudo‐obstruction 0 | Deep haemorrhage 1 Haematemesis 1 Wound infection 2 Urinary tract infection 3 Cellulitis 0 Pancreatitis 1 Hypotension 3 Impaired renal function 2 Pseudo‐obstruction 0 | |
| *RIFLE scores sum of patients at risk, injury or failure | |||
| Outcomes reported in Venn 2002: comparison 3 | Protocol ‐ CVP N = 31 | Standard care
N = 29 | Effect estimate (95% CI) | ||
|
| Mean | SD | Mean | SD | Mean difference |
| Length of hospital stay (days) | 13.3 | 12.1 | 17.5 | 13.8 | ‐4.20 (‐11.0 to 2.60) |
| Time to fitness to discharge | 10 | 5.3 | 13.9 | 6.6 | ‐3.90 (‐7.05 to ‐0.75) |
|
|
|
|
|
|
|
|
| Events |
| Events |
| MH relative risk |
| Mortality | 6 |
| 2 |
| 2.81 (0.61 to 12.81) |
| Adverse events |
|
|
|
|
|
| · Cardiopulmonary ‐ episodes | 6 |
| 7 |
| N/A |
| · Neurological ‐ participants | 1 |
| 1 |
| 0.94 (0.06 to 14.27) |
| · Any, including minor ‐ participants | 8 |
| 14 |
| 0.53 (0.26 to 1.08) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.20] |
| 2 Adverse outcomes Show forest plot | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| 2.1 Any complications, including minor | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.07, 2.95] |
| 2 Total length of hospital stay Show forest plot | 2 | 173 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐1.70, 2.96] |
| 3 Days until medically fit for discharge Show forest plot | 2 | 173 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐1.74, 1.71] |
| 4 Adverse outcomes Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Neurological | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.56, 2.18] |
| 4.2 Any complications, including minor | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.31] |

