Optimisation du volume de fluides périopératoire après fracture du fémur proximal
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003004.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 marzo 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Andrew F Smith (AFS) identified the need for the review update.
Sharon R Lewis (SRL) and Andrew R Butler (ARB) performed the initial searches, applied inclusion criteria and extracted study data.
SRL and ARB compiled the results.
SRL drafted the review.
All review authors reviewed and refined the final manuscript.
Sources of support
Internal sources
-
Oxford Radcliffe Hospitals NHS Trust, UK.
External sources
-
NIHR Cochrane Collaboration Programme Grant, UK.
-
NIHR Cochrane Collaboration Programme Grant. Enhancing the safety, quality and productivity of perioperative care. Project Ref: 10/4001/04, UK. This grant funds the work of AN and AFS on this review.
-
Declarations of interest
Andrew Brammar: none known.
Andrew F Smith: none known.
Sharon R Lewis: none known
Andrew R Butler: none known.
Amanda Nicholson (AN): From June 2015, AN has worked for Q Medical Technology Limited, a firm that markets and distributes a range of medical devices. AN made no substantial contribution to the review while working at Q Medical Technologies Limited. None of the company's products are directly relevant to the subject of this review. AN's husband has small direct holdings in several drug and biotech companies as part of a wider balanced share portfolio.
Acknowledgements
We would like to thank Stephan Kettner (Content Editor), Cathal Walsh (Statistical Editor) and Sheila Page (Consumer Reviewer) for help and editorial advice provided during preparation of this updated systematic review.
We also would like to thank Dr Craig Goldsack, Dr Dominik Krzanicki and Dr Jonathan Pimm for their contributions in reshaping the original review to the new review format before it was updated (Brammar 2013), and we would like to thank previous Consumer Editor Robert Wylie.
We would like to acknowledge the work of Dr James Price, Dr John Sear and Dr Richard Venn, who authored the first review (Price 2004).
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Mar 14 | Perioperative fluid volume optimization following proximal femoral fracture | Review | Sharon R Lewis, Andrew R Butler, Andrew Brammar, Amanda Nicholson, Andrew F Smith | |
| 2013 Sep 11 | Perioperative fluid volume optimization following proximal femoral fracture | Review | Andrew Brammar, Amanda Nicholson, Marialena Trivella, Andrew F Smith | |
| 2004 Jan 26 | Perioperative fluid volume optimization following proximal femoral fracture | Review | James D Price, John JW Sear, Richard RM Venn | |
| 2000 Dec 28 | Perioperative fluid volume optimization following proximal femoral fracture | Review | James D Price, John JW Sear, Richard RM Venn | |
Differences between protocol and review
Alterations made in earlier review (Brammar 2013)
We altered and re‐ran the search strategy using updated key terms from inception of the databases to October 2012. In addition to CENTRAL, MEDLINE and EMBASE, we searched the International Clinical Trials Registry Platform and ClinicalTrials.gov websites for ongoing and unpublished studies (see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5). We carried out backward and forward citation searching for key review articles identified during the initial searches (see Appendix 6). We repeated title selection and full‐text review in full.
We moved one study from excluded to included studies (Schultz 1985). We added one study as awaiting classification pending contact with study authors (Sandham 2003). We added two ongoing studies (GDHT study; NOTTS study).
We used the Cochrane 'Risk of bias' tool to assess the quality of studies. We did not exclude studies on the basis of low quality.
We altered comparison groups so that protocol measures and advanced haemodynamic methods were compared with each other and were not combined.
We redefined outcomes to separate length of stay into time to medical fitness and total stay. The all‐cause mortality time frame was changed to include in‐hospital, 30 days and undefined. We changed reduced return of function outcomes to time to the pre‐fracture category of accommodation and mobility. We re‐classified complications into major iatrogenic, cardiopulmonary, neurological and combined, including minor.
We included 'Summary of findings' tables for all comparisons, using the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram. Updated search October 2012 to January 2015.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Advanced haemodynamic monitoring versus protocol using standard measures, Outcome 1 All‐cause mortality.
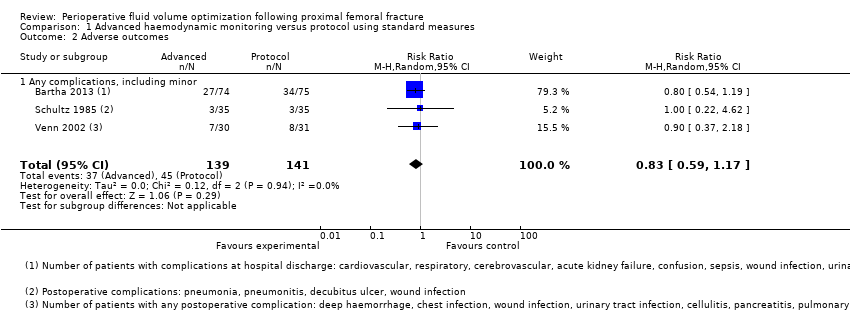
Comparison 1 Advanced haemodynamic monitoring versus protocol using standard measures, Outcome 2 Adverse outcomes.

Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 1 All‐cause mortality.

Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 2 Total length of hospital stay.

Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 3 Days until medically fit for discharge.
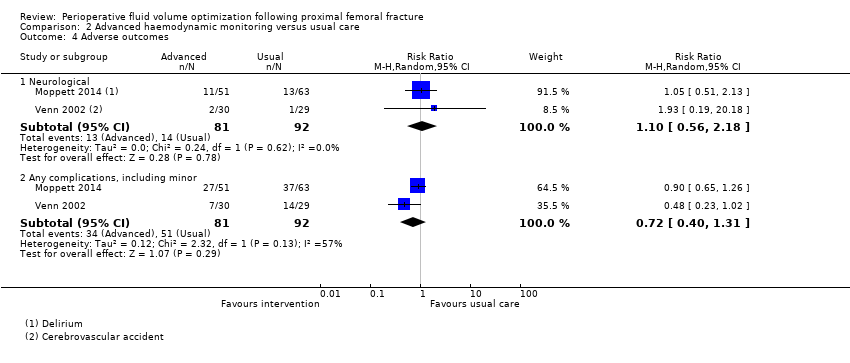
Comparison 2 Advanced haemodynamic monitoring versus usual care, Outcome 4 Adverse outcomes.
| Advanced haemodynamic monitoring compared with protocol using standard measures such as CVP for proximal femoral fracture | ||||||
| Patient or population: patients with proximal femoral fracture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Protocol using standard measures such as CVP | Advanced haemodynamic monitoring | |||||
| All‐cause mortality | Study population | RR 0.41 | 280 | ⊕⊝⊝⊝ | ||
| 142 per 1000 | 58 per 1000 | |||||
| Moderate | ||||||
| Total length of hospital stay | Not estimabled | 203 | ⊕⊕⊝⊝ | Data reported as median (range) in Bartha 2013 and as mean (95% confidence interval) in Venn 2002 | ||
| Medically fit for discharge | Mean medically fit for discharge in the intervention groups was | 90 | ⊕⊕⊝⊝ | |||
| Return to pre‐fracture accommodation/return to pre‐fracture mobility | Not estimable | ‐ | Not reported | |||
| Adverse outcomes ‐ cardiopulmonary | Study population | Not estimable | 0 | |||
| Moderate | ||||||
| Adverse outcomes ‐ neurological | Not estimable | 0 | ||||
| Adverse outcomes ‐ all | Study population | RR 0.90 | 280 | ⊕⊝⊝⊝ | ||
| 319 per 1000 | 287 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aConcerns about randomization process in Schultz 1985; high risk of selection bias eData from 1 study only. Downgraded 2 levels | ||||||
| Advanced haemodynamic monitoring compared with usual care for perioperative fluid optimization | ||||||
| Patient or population: patients with perioperative fluid optimization | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Advanced haemodynamic monitoring | |||||
| All‐cause mortality | Study population | RR 0.45 | 213 | ⊕⊕⊝⊝ | ||
| 89 per 1000 | 40 per 1000 | |||||
| Moderate | ||||||
| Total length of hospital stay | Mean total length of hospital stay in the control groups was | Mean total length of hospital stay in the intervention groups was | 175 | ⊕⊕⊝⊝ | ||
| Medically fit for discharge | Mean medically fit for discharge in the control groups was | Mean medically fit for discharge in the intervention groups was | 175 | ⊕⊕⊝⊝ | ||
| Return to pre‐fracture accommodation/return to pre‐fracture mobility | Study population | Not estimable | 114 | ⊕⊕⊝⊝ | ||
| 397 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| Adverse outcomes ‐ cardiopulmonary | Study population | Not estimable | 0 | |||
| Moderate | ||||||
| Adverse outcomes ‐ neurological | Study population | RR 1.10 | 173 | ⊕⊕⊝⊝ | ||
| 152 per 1000 | 170 per 1000 | |||||
| Moderate | ||||||
| Adverse outcomes ‐ all | Study population | RR 0.78 | 173 | ⊕⊕⊝⊝ | ||
| 554 per 1000 | 432 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aConfidence intervals cross no effect and are consistent with increased as well as decreased risk. Downgraded 1 level | ||||||
| Protocol using standard measures such as CVP compared with usual care for perioperative fluid optimization | ||||||
| Patient or population: patients with perioperative fluid optimization | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Protocol using standard measures such as CVP | |||||
| All‐cause mortality | Study population | RR 2.81 | 60 | ⊕⊕⊝⊝ | ||
| 69 per 1000 | 194 per 1000 | |||||
| Moderate | ||||||
| Total length of hospital stay | Mean total length of hospital stay in the control groups was | Mean total length of hospital stay in the intervention groups was | 60 | ⊕⊕⊝⊝ | ||
| Medically fit for discharge | Mean medically fit for discharge in the intervention groups was | 60 | ⊕⊕⊕⊝ | |||
| Return to pre‐fracture accommodation/return to pre‐fracture mobility | Not estimable | ‐ | Not reported | |||
| Adverse outcomes ‐ cardiopulmonary | Study population | Not estimable | 0 | Not reported | ||
| Moderate | ||||||
| Adverse outcomes ‐ neurological | Study population | RR 0.94 | 60 | ⊕⊕⊝⊝ | ||
| 34 per 1000 | 32 per 1000 | |||||
| Moderate | ||||||
| Adverse outcomes ‐ all | Study population | RR 0.53 | 60 | ⊕⊕⊝⊝ | ||
| 483 per 1000 | 256 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aBased on 1 study with a small number of events. Wide confidence intervals consistent with increased as well as decreased risk. Downgraded by 1 level | ||||||
| Study ID | Adverse events | Advanced haemodynamic monitoring | Protocol using standard measures |
| n = 74 | n = 75 | ||
| Cardiopulmonary | Cardiovascular 5 Respiratory 5 | Cardiovascular 6 Respiratory 7 | |
| Neurological | Cerebrovascular 0 Confusion 3 | Cerebrovascular 2 Confusion 6 | |
| Other | Acute kidney failure 1 Gastrointestinal bleeding 0 Sepsis 2 Deep vein thrombosis 0 Wound infection 2 Delayed healing 0 Urinary tract infection 16 Decubitus 6 Wound haematoma 0 Other 4 | Acute kidney failure 1 Gastrointestinal bleeding 0 Sepsis 0 Deep vein thrombosis 0 Wound infection 1 Delayed healing 0 Urinary tract infection 12 Decubitus 1 Wound haematoma 1 Other 6 | |
| n = 35 | n = 35 | ||
| Other | Pneumonia 1 Wound infection 1 Pneumonitis 1 | Pneumonia 2 Decubitis ulcer 1 | |
| n = 30 | n = 31 | ||
| Cardiopulmonary | Chest infection 2 Pulmonary embolus 1 Myocardial infarction 0 Cardiac failure 0 Rapid atrial fibrillation 3 Hypotension 0 | Chest infection 3 Pulmonary embolus 0 Myocardial infarction 1 Cardiac failure 1 Rapid atrial fibrillation 1 Hypotension 0 | |
| Neurological | Cerebrovascular accident 2 | Cerebrovascular accident 1 | |
| Other | Deep haemorrhage 1 Haematemesis 0 Wound infection 0 Urinary tract infection 2 Cellulitis 0 Pancreatitis 0 Hypotension 0 Impaired renal function 0 Pseudo‐obstruction 0 | Deep haemorrhage 0 Haematemesis 0 Wound infection 0 Urinary tract infection 1 Cellulitis 1 Pancreatitis 0 Hypotension 0 Impaired renal function 0 Pseudo‐obstruction 1 |
| Study ID | Adverse events | Advanced haemodynamic monitoring | Usual care |
| n = 51 | n = 63 | ||
| Cardiopulmonary | Cardiovascular 8 Respiratory 0 | Cardiovascular 6 Respiratory 0 | |
| Neurological | Acute delirium 11 | Acute delirium 13 | |
| Other | Infectious 21 Abdominal 2 Bleeding 0 Skin 0 Renal (RIFLE) 18* Other 3 | Infectious 34 Abdominal 1 Bleeding 0 Skin 0 Renal (RIFLE) 32* Other 3 | |
| n = 30 | n = 29 | ||
| Cardiopulmonary | Chest infection 2 Pulmonary embolus 1 Myocardial infarction 0 Cardiac failure 0 Rapid atrial fibrillation 3 Hypotension 0 | Chest infection 5 Pulmonary embolus 0 Myocardial infarction 0 Cardiac failure 0 Rapid atrial fibrillation 2 Hypotension 3 | |
| Neurological | Cerebrovascular accident 2 | Cerebrovascular accident 1 | |
| Other | Deep haemorrhage 1 Haematemesis 0 Wound infection 0 Urinary tract infection 2 Cellulitis 0 Pancreatitis 0 Hypotension 0 Impaired renal function 0 Pseudo‐obstruction 0 | Deep haemorrhage 1 Haematemesis 1 Wound infection 2 Urinary tract infection 3 Cellulitis 0 Pancreatitis 1 Hypotension 3 Impaired renal function 2 Pseudo‐obstruction 0 | |
| *RIFLE scores sum of patients at risk, injury or failure | |||
| Outcomes reported in Venn 2002: comparison 3 | Protocol ‐ CVP N = 31 | Standard care
N = 29 | Effect estimate (95% CI) | ||
|
| Mean | SD | Mean | SD | Mean difference |
| Length of hospital stay (days) | 13.3 | 12.1 | 17.5 | 13.8 | ‐4.20 (‐11.0 to 2.60) |
| Time to fitness to discharge | 10 | 5.3 | 13.9 | 6.6 | ‐3.90 (‐7.05 to ‐0.75) |
|
|
|
|
|
|
|
|
| Events |
| Events |
| MH relative risk |
| Mortality | 6 |
| 2 |
| 2.81 (0.61 to 12.81) |
| Adverse events |
|
|
|
|
|
| · Cardiopulmonary ‐ episodes | 6 |
| 7 |
| N/A |
| · Neurological ‐ participants | 1 |
| 1 |
| 0.94 (0.06 to 14.27) |
| · Any, including minor ‐ participants | 8 |
| 14 |
| 0.53 (0.26 to 1.08) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.20] |
| 2 Adverse outcomes Show forest plot | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| 2.1 Any complications, including minor | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.07, 2.95] |
| 2 Total length of hospital stay Show forest plot | 2 | 173 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐1.70, 2.96] |
| 3 Days until medically fit for discharge Show forest plot | 2 | 173 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐1.74, 1.71] |
| 4 Adverse outcomes Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Neurological | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.56, 2.18] |
| 4.2 Any complications, including minor | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.31] |

