| 1 Global impression (SCAG) Show forest plot | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 2.7 [0.07, 5.33] |
|
| 2 Global impression (CGI) Show forest plot | 1 | 339 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.16, 2.91] |
|
| 3 Adverse events Show forest plot | 6 | 615 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.60] |
|
| 4 Functional performance and behaviour Show forest plot | 2 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.73, ‐0.35] |
|
| 4.1 CBRS | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.29, ‐0.65] |
| 4.2 SGRS | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.25, ‐0.25] |
| 5 Mood Show forest plot | 2 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.26, ‐0.34] |
|
| 5.1 HRSD | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐1.80, ‐0.26] |
| 5.2 Depressive Scale | 1 | 51 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.24, ‐0.10] |
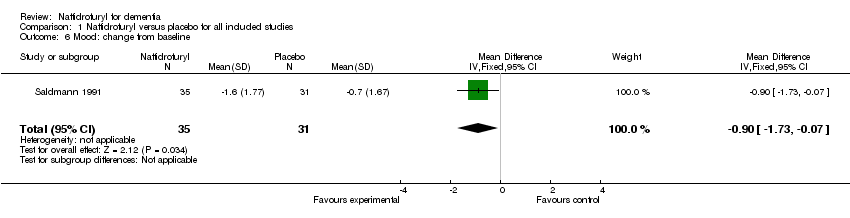
| 6 Mood: change from baseline Show forest plot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.73, ‐0.07] |
|
| 7 Cognition Show forest plot | 2 | 135 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.71, ‐0.02] |
|
| 7.1 ADAS‐Cog | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.92, ‐0.05] |
| 7.2 EST | 1 | 51 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.72, 0.38] |
| 8 Cognition: EACG, change from baseline Show forest plot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐2.15, ‐0.75] |
|
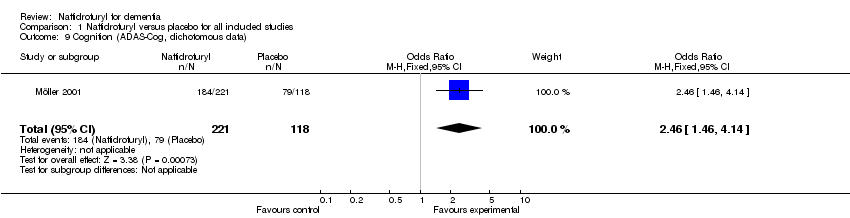
| 9 Cognition (ADAS‐Cog, dichotomous data) Show forest plot | 1 | 339 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.46, 4.14] |
|
| 10 Combination scales: SCAG, change from baseline Show forest plot | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.03, 1.97] |
|
| 11 Combination scales: SCAG, dichotomous data Show forest plot | 1 | 339 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [1.68, 4.78] |
|
| 12 Combination scales (GBS) Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐9.29, 12.09] |
|
| 13 Combination scales (BDRS) Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐1.47, 3.27] |
|
| 14 Combination scales: NOSGER, dichotomous data Show forest plot | 1 | 339 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.98, 2.42] |
|
| 15 Death Show forest plot | 3 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.34, 3.72] |
|