Fármacos para el lupus eritematoso discoide
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, 8 weeks of treatment, with further 8‐week follow‐up. | |
| Participants | 10 adults with facial DLE; diagnosis clinical and histological. | |
| Interventions | Pimecrolimus 1% vs betamethasone 17‐valerate 0.1% cream, twice daily. | |
| Outcomes | Digital photographs; clinical assessment by 3 blinded dermatologists, using a clinical score* | |
| Notes | * Reported erythema and adverse events. Did not report complete resolution or participant satisfaction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "all patients with odd numbers were allocated to group A (pimecrolimus 1%) and all patients with even numbers to group B (betamethasone valerate 0.1%". |
| Allocation concealment (selection bias) | Low risk | All patients referred were randomised into the study. As referrals were from multiple sources it would not have been possible for allocations to be known when patients were referred. |
| Blinding of participants and personnel (performance bias) | Low risk | Test products in identical jars. |
| Blinding of outcome assessment (detection bias) | Low risk | Clearly stated ‐ outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data was reported for all participants, using a combined score, including erythema. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
| Other bias | Unclear risk | None apparent. |
| Comparability of the two arms | Low risk | Activity scores were similar at baseline: '4.2 +/‐ 0.9' pimecrolimus vs '4.4 +/‐ 2.6' betamethasone |
| Methods | RCT, 8 weeks. | |
| Participants | 37 adults with DLE, clinically and histologically. | |
| Interventions | R‐salbutamol 0.5% topical cream vs placebo, applied twice daily. | |
| Outcomes | CLASI score; clinician global assessment; participant global assessment, adverse events*. | |
| Notes | * Reported complete resolution, but not relapse rate or 50% clearing of erythema Two authors receive support from Astion Pharma A ⁄S. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT, using random number chart. |
| Allocation concealment (selection bias) | Unclear risk | "The randomisation list consisted of randomisation numbers 1–64 and uniquely assigned each patient to one of the two treatments". It was not clear whether this list was kept hidden from investigators who were responsible for recruitment. |
| Blinding of participants and personnel (performance bias) | Low risk | Assessors and participants were blinded; creams appeared identical. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors and participants were blinded; creams appeared identical. |
| Incomplete outcome data (attrition bias) | Low risk | Intent‐to‐treat analysis was carried out on all patients randomised, with last observation carried forward for any missing values. |
| Selective reporting (reporting bias) | High risk | Impact on erythema: no detail supplied. |
| Other bias | Unclear risk | Short duration (6 weeks). |
| Comparability of the two arms | Low risk | At baseline, the groups were similar according to score. All participants had an active lesion. |
| Methods | Within‐patient RCT, 12 weeks; diagnosis clinical and histological. | |
| Participants | 30 adults with cutaneous LE; 14 of them with DLE (subgroup analysis). | |
| Interventions | Tacrolimus 0.1% cream vs placebo (vehicle). | |
| Outcomes | Digital photography; clinical score; participant satisfaction, adverse events* | |
| Notes | * Did not report complete resolution, relapse rate. Astellas Pharma GmbII assisted with protocol design. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Allocation by pharmacy independent of the investigators. Quote "patients were randomly assigned to either 0.1% tacrolimus ointment or the placebo (vehicle) by the pharmacy at each center". |
| Blinding of participants and personnel (performance bias) | Low risk | Containers identical. |
| Blinding of outcome assessment (detection bias) | Low risk | "Patients and physicians were blinded during the trial". |
| Incomplete outcome data (attrition bias) | Low risk | 2 of the 14 participants with DLE did not complete the trial. Intention to treat analysis was used in a secondary analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | None apparent. |
| Comparability of the two arms | Low risk | Matched skin lesions used in individual participants. |
| Methods | RCT, cross‐over at 6 weeks, duration 12 weeks. Data was analysed at 6 weeks as a parallel trial; data after the cross‐over component was excluded. | |
| Participants | 78 adults, clinical diagnosis DLE well‐matched. | |
| Interventions | Fluocinonide cream 0.05% vs hydrocortisone cream 1% given 3 times daily without occlusion. | |
| Outcomes | Skin cleared or much improved ‐ the lowest score of 1 if the lesion was worse, the highest score 5 if the lesion was excellent or clear*. | |
| Notes | *Did report resolution and adverse events, not % change in erythema, relapse rate or participant satisfaction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned... " "Statistical tests of equality ..showed successful randomisation". |
| Allocation concealment (selection bias) | Unclear risk | No details about how the allocation sequence was concealed from the participants and clinicians. Creams were provided in identical base. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as a "double‐blind" trial. "Both medications were supplied in a specifically formulated cream base...in identical tubes". Participants had one active lesion which was monitored every 3 weeks ‐ it is not clear if they were aware which lesion this was. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated, but "double‐blind". |
| Incomplete outcome data (attrition bias) | High risk | 78 of 93 participants were assessed at 6 weeks, 15 of the 93 patients who did not complete the first phase of the study did so for reasons which may relate to outcome. |
| Selective reporting (reporting bias) | Low risk | Not apparent. |
| Other bias | Unclear risk | Short duration (6 weeks). |
| Comparability of the two arms | Low risk | The fluocinonide and hydrocortisone groups were similar at baseline with respect to sex, age, race, duration of disease and duration of monitored lesion. |
| Methods | RCT, duration 8 weeks (included DLE and subacute LE). | |
| Participants | 58 adults, clinical diagnosis DLE or SCLE, not matched for diagnosis. | |
| Interventions | Acitretin 50 mg vs hydroxychloroquine 400 mg daily. | |
| Outcomes | An ordinal scale used to assess the skin initially and at follow‐up visits: Complete clearing = 0, improvement = 1, no change or deterioration = 2. Clearing of skin lesions and erythema were assessed* | |
| Notes | Reported complete resolution and adverse events, not participant satisfaction or relapse rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..was conducted in a randomised fashion" and "patients were randomly assigned" but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details about how the allocation sequence was concealed from the participants and clinicians. |
| Blinding of participants and personnel (performance bias) | Low risk | Study described as "double‐blind" ‐ but no further details were given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Stated as double‐blind but no details given as to how outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 2 out of 60 patients randomised did not contribute to the analysis. ITT not described. |
| Selective reporting (reporting bias) | Unclear risk | None apparent. |
| Other bias | High risk | Clinical heterogeneity. |
| Comparability of the two arms | High risk | More participants with DLE in hydroxychloroquine group. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Results for DLE subset not reported; not an RCT. | |
| Trial results included other disorders, outcome in DLE could not be established. | |
| Not randomised trial. | |
| Participants with SLE, results for DLE subset not reported. | |
| Participants with SLE, results for DLE subset not reported. | |
| Not an RCT. | |
| Participants with SLE, results for DLE subset not reported. | |
| Participants with SLE, results for DLE subset not reported. | |
| Not RCT. | |
| Photoprotection trial, not drug treatment. | |
| Not RCT. | |
| Not RCT. Results for DLE subset not reported separately. | |
| Study of people with SLE. Results for DLE not reported. | |
| Study of people with SLE. Results for DLE not reported. | |
| Study of people with SLE. Results for DLE not reported | |
| Study of people with SLE. Results for DLE not reported. | |
| Not RCT. | |
| Study of people with SLE. Results for DLE not reported. | |
| Not RCT. | |
| Most participants had malar rash of acute SLE (13 of 18). Results for DLE subset (4 people) could not be established. | |
| Uncertain diagnosis. | |
| Included participants with all forms of lupus, results for DLE subset not reported. | |
| Participants with SLE, results for DLE subset not reported. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT, two matched lesions in same participant. |
| Participants | 17 adults with DLE. |
| Interventions | Thalidomide 20% ointment under occlusion. |
| Outcomes | Completed. |
| Notes | ‐ |
| Methods | RCT. |
| Participants | Peope with DLE, all ages. |
| Interventions | Pimecrolimus cream vs betamethasone valerate 0.1% cream. |
| Outcomes | Clinical score, adverse events. |
| Notes | ‐ |
| Methods | Randomised, double‐blind trial. |
| Participants | Adults with DLE. |
| Interventions | ASF cream 0.5% (R‐salbutamol) vs placebo. |
| Outcomes | GA, CLASI, QoL. |
| Notes | Completed, results not posted. |
| Methods | RCT, multicentre. |
| Participants | 32 adults with DLE. |
| Interventions | ASF cream vs placebo. |
| Outcomes | Safety, CLASI, Global assessment by participant and investigator. |
| Notes | Completed 2007. |
| Methods | RCT, cross‐over study. |
| Participants | 16 adults with DLE. |
| Interventions | AMG811 (interferon gamma blocker) injection vs placebo. |
| Outcomes | Safety; secondary outcome is CLASI score changes. |
| Notes | Passed completion date. |
| Methods | Randomised, double‐blind study. |
| Participants | People with DLE and SCLE. |
| Interventions | CC 11050 (Celgene). |
| Outcomes | Adverse effects, pharmacokinetics, CLASI. |
| Notes | ‐ |
| Methods | Randomised, double‐blind. |
| Participants | 7 adults with cutaneous lupus. |
| Interventions | Alitretinoin (Toctino®) vs placebo. |
| Outcomes | CLASI, percentage with improvement by global assessment, adverse events. |
| Notes | ‐ |
| Methods | Randomised, double‐blind trial. |
| Participants | 54 adults with DLE. |
| Interventions | R932333 6% cream vs placebo. |
| Outcomes | Erythema and scaling score. |
| Notes | Trial discontinued. Results not published. |
| Methods | Randomised, to right or left side of the body, for 6 weeks. |
| Participants | 21 Thai adults with DLE. |
| Interventions | Tacrolimus 0.1% cream vs clobetasol propionate 0.05%. |
| Outcomes | CLASI score. |
| Notes | Information from abstract only. We were unable to obtain full text. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A Double‐blind (Sponsor Unblinded) Study to Investigate Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Clinical Effect of Repeat Dosing of GSK2646264 in Cutaneous Lupus Erythematosus Patients |
| Methods | Randomised, double‐blind trial. |
| Participants | 40 people with cutaneous LE. |
| Interventions | GSK2646264 1% cream vs placebo. |
| Outcomes | Adverse effects, CLASI, pharmacokinetics. |
| Starting date | January 2017. |
| Contact information | US GSK Clinical Trials Call Center 877‐379‐3718 |
| Notes | ‐ |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of skin lesions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Fluocinonide versus hydrocortisone cream, Outcome 1 Resolution of skin lesions. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of skin lesions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Acitretin versus hydroxychloroquine, Outcome 1 Resolution of skin lesions. | ||||
| 2 Clearing of erythema Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Acitretin versus hydroxychloroquine, Outcome 2 Clearing of erythema. | ||||
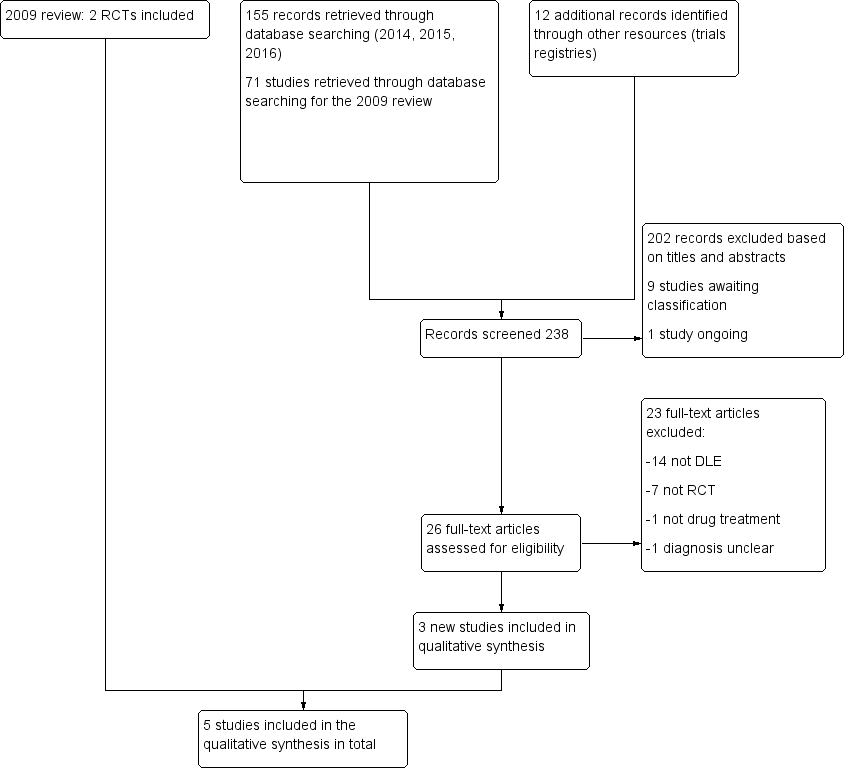
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Fluocinonide versus hydrocortisone cream, Outcome 1 Resolution of skin lesions.

Comparison 2 Acitretin versus hydroxychloroquine, Outcome 1 Resolution of skin lesions.
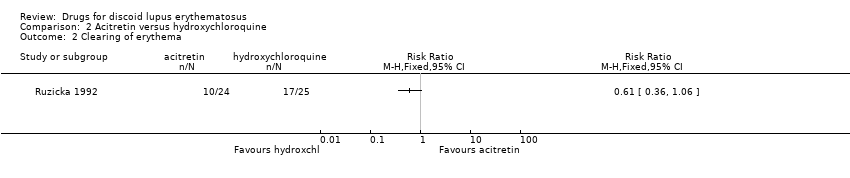
Comparison 2 Acitretin versus hydroxychloroquine, Outcome 2 Clearing of erythema.
| Fluocinonide 0.05% compared with hydrocortisone 1% for discoid lupus erythematosus | ||||||
| Patient or population: people with discoid lupus erythematosus Settings: not stated Intervention: fluocinonide Comparison: hydrocortisone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| hydrocortisone | fluocinonide | |||||
| Clearing or excellent improvement (after 6 weeks of treatment) | 10 per 100 | 27 per 100 (9 to 79) | 2.77 (0.95 to 8.08) | 78 (1 study) | Low1 | ‐ |
| At least 50% reduction in erythema | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Quality of life measure | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Relapse | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | see comment | 78 (1 study) | Moderate2 | The number of adverse events in this study was small and results were presented narratively. For hydrocortisone, 1 person developed acne and 3 experienced irritation. 2 patients who were assigned to fluocinonide experienced burning. There was no discontinuation in either group. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The assumed risk was the mean risk for the study population 1Downgraded by two levels due to imprecision and high risk of bias (incomplete outcome data). | ||||||
| Acitretin 50 mg daily compared with hydroxychloroquine 400 mg daily for discoid lupus erythematosus | ||||||
| Patients or population: people with discoid lupus erythematosus Settings: specialised lupus clinic Intervention: acitretin Comparison: hydroxychloroquine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| hydroxychloroquine | acitretin | |||||
| Clearing or excellent improvement (after 8 weeks of treatment) | 50 per 100 | 46 per 100 (27 to 80) | 0.93 (0.54 to 1.59) | 58 (1 study) | Low1 | ‐ |
| At least 50% reduction in erythema (after 8 weeks of treatment) | 68 per 100 | 42 per 100 (25 to 72) | 0.61 (0.36 to 1.06) | 49 (1 study) | Low1 | ‐ |
| Quality of life measure | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Relapse | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | see comment | 58 (1 study) | Moderate2 | Information on adverse events was presented narratively. 27 out of 28 participants receiving acitretin had at least one adverse event compared with 17 out of 30 patients treated with hydroxychloroquine. 4 people receiving acitretin discontinued treatment due to dry lips and gastrointestinal symptoms. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The assumed risk was the mean risk for the study population. 1Downgraded by two levels due to imprecision and high risk of bias (non‐comparable treatment arms). | ||||||
| Pimecrolimus 1% compared with betamethasone 17‐valerate 0.1% for discoid lupus erythematosus | |||||
| Patients or population: people with discoid lupus erythematosus Settings: dermatology clinics Intervention: pimecrolimus Comparison: betamethasone | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments |
| Clearing or excellent improvement (after 8 weeks of treatment) | see comment | see comment | 10 (1 study) | see comment | There was a statistically significant reduction in the disease severity score in both treatment groups; however, clearing or improvement was not presented as a percentage and no comparative analyses were performed. |
| At least 50% reduction in erythema | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Quality of life measure | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Relapse | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| R‐salbutamol 0.5% topical cream compared with placebo for discoid lupus erythematosus | |||||
| Patient or population: people with discoid lupus erythematosus Settings: dermatology departments Intervention: R‐salbutamol Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments |
| Clearing or excellent improvement | see comment | see comment | 37 (1 study) | see comment | While data on overall improvement were provided in the study, the number of participants with complete resolution in Jemec 2009 could not be obtained from the trial report. |
| At least 50% reduction in erythema | see comment | see comment | 37 (1 study) | see comment | While data on erythema were provided in the study report, the number of participants with at least 50% reduction in erythema in Jemec 2009 could not be obtained from the trial report. |
| Quality of life measure | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Relapse | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | 37 (1 study) | Moderate1 | Results for adverse events were presented narratively. There were 15 events in the placebo group (experienced by 12 participants) and 24 in the salbutamol group (experienced by 9 participants). None of the adverse events were considered serious. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for imprecision (small sample size). | |||||
| Tacrolimus 0.1% cream compared with placebo for discoid lupus erythematosus | |||||
| Patient or population: people with discoid lupus erythematosus Settings: dermatology departments Intervention: tacrolimus Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks (95% CI) | Illustrative comparative risks (95% CI) | No of Participants | Quality of the evidence | Comments |
| Clearing or excellent improvement | see comment | see comment | see comment | see comment | No participants with DLE in either group in the study by Kuhn 2011 experienced complete clearing. Unable to GRADE due to 0 events in both groups. |
| At least 50% reduction in erythema | see comment | see comment | see comment | see comment | The results for erythema in the study by Kuhn 2011 were not reported separately. |
| Quality of life measure | see comment | see comment | see comment | see comment | This outcome was not measured. |
| Relapse | see comment | see comment | see comment | see comment | This outcome was not measured. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | 14 (1 study) | Moderate1 | Results for adverse events were presented narratively. In the tacrolimus group, 5 participants complained of slight burning and itching, and in 1 person a herpes simplex infection was reactivated. There were no serious adverse events. |
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for imprecision (small sample size). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of skin lesions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of skin lesions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clearing of erythema Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

