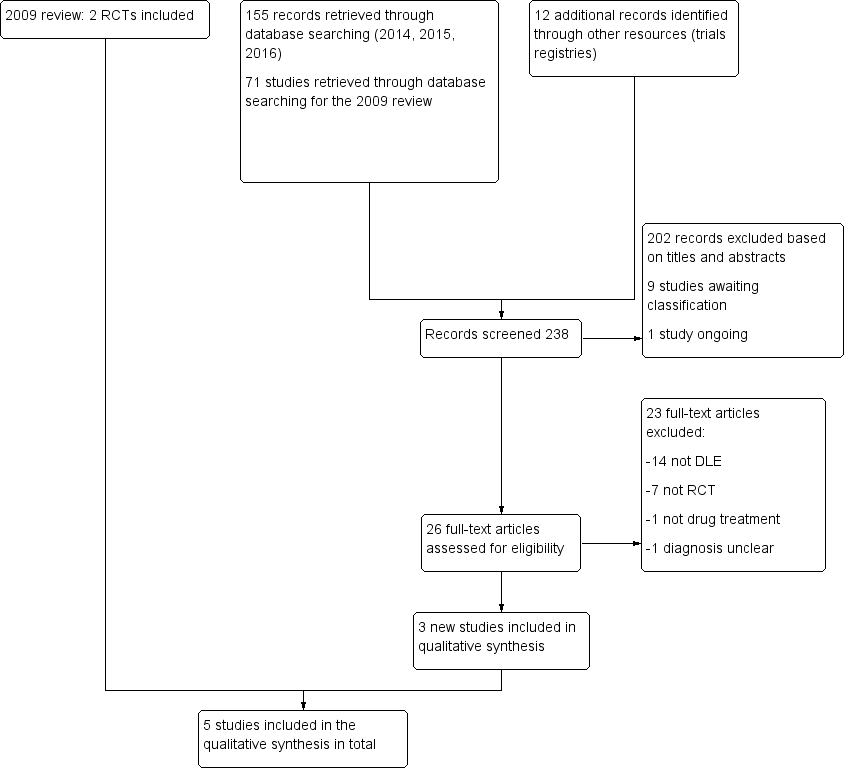
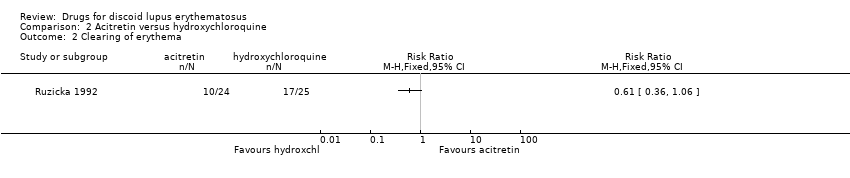
Contenido relacionado
Revisiones y protocolos relacionados
Cora W Hannon, Collette McCourt, Hermenio C Lima, Suephy Chen, Cathy Bennett | 9 marzo 2021
Jade Cury Martins, Ciro Martins, Valeria Aoki, Aecio FT Gois, Henrique A Ishii, Edina MK da Silva | 1 julio 2015
Maulina Sharma, Cathy Bennetta, Stuart N Cohena, Ben Carter | 14 noviembre 2014
Justin Gabriel Schlager, Stefanie Rosumeck, Ricardo Niklas Werner, Anja Jacobs, Jochen Schmitt, Christoph Schlager, Alexander Nast | 26 febrero 2016
Ching‐Chi Chi, Gudula Kirtschig, Maha Baldo, Fabia Brackenbury, Fiona Lewis, Fenella Wojnarowska | 7 diciembre 2011
Ratree Sawangjit, Piyameth Dilokthornsakul, Antonia Lloyd-Lavery, Nai Ming Lai, Robert Dellavalle, Nathorn Chaiyakunapruk | 14 septiembre 2020
Anne R Mason, James Mason, Michael Cork, Gordon Dooley, Helen Hancock | 28 marzo 2013
Helena Kastarinen, Tuija Oksanen, Enembe O Okokon, Vesa V Kiviniemi, Kristiina Airola, Johanna Jyrkkä, Tuomas Oravilahti, Piia K Rannanheimo, Jos H Verbeek | 19 mayo 2014
Miny Samuel, Rebecca Brooke, Sally Hollis, Christopher EM Griffiths | 2 junio 2015
Ausama Atwan, John R Ingram, Rachel Abbott, Mark J Kelson, Timothy Pickles, Andrea Bauer, Vincent Piguet | 10 agosto 2015