Fármacos para el lupus eritematoso discoide
Información
- DOI:
- https://doi.org/10.1002/14651858.CD002954.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 mayo 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Piel
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
SJ was the contact person with the editorial base, SJ co‐ordinated contributions from the co‐authors, and wrote the final draft of the review, with DW.
SJ, DW, PJ screened papers against eligibility criteria.
SJ obtained data on ongoing and unpublished studies.
SJ, DW, PJ appraised the quality of papers.
SJ, DW extracted data for the review and sought additional information about papers.
SJ, MG, DW entered data into RevMan 5.
MG, DW, SJ analysed and interpreted data.
MG, SJ, DW worked on the methods sections.
SJ, DW drafted the clinical sections of the background and responded to the clinical comments of the referees.
MG responded to the methodology and statistics comments of the referees.
Disclaimer
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Sources of support
Internal sources
-
Nottingham University, UK.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Sue Jessop: I receive a small annual amount from royalties relating to the sale of a book on Primary Care Dermatology, which is not related to the subject of the review.
David A Whitelaw: nothing to declare.
Matthew J Grainge: nothing to declare.
Prativa Jayasekera: nothing to declare.
Acknowledgements
This review was performed with the guidance and assistance of members of the Cochrane Skin Group, Nottingham, UK, in particular Hywel Williams, Finola Delamere, Laura Prescott, Helen Scott, Tina Leonard, and Kayode Adetugbo.
The editorial base would like to thank Jennifer Humbles (consumer), for reading the review, and the following people, who were the external referees for this review: Sam Gibbs, Enno Schmidt, Yong Cui, Thomas Chu, Ching‐Chi Chi, Ankur Barua.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 May 05 | Drugs for discoid lupus erythematosus | Review | Sue Jessop, David A Whitelaw, Matthew J Grainge, Prativa Jayasekera | |
| 2009 Oct 07 | Drugs for discoid lupus erythematosus | Review | Sue Jessop, David A Whitelaw, Finola M Delamere | |
| 2009 Jul 08 | Drugs for discoid lupus erythematosus | Review | Sue Jessop, Dave Whitelaw, Francois Jordaan | |
Differences between protocol and review
Differences between the protocol and the current update
For differences between other published versions, please see the 'Differences between protocol and review section' within the original publications.
Note: many of the methods section headings are missing from the protocol and previously published versions. In this update, we have tried to rectify this, in line with the current Cochrane Handbook and RevMan software.
Background: this section has been updated.
Objectives: the sentence 'To identify the need for further study to make rational clinical decisions possible when treating cutaneous lupus' was omitted from this update in line with current Cochrane Handbook for Systematic Reviews of Interventions and RevMan 5 software and the previously published versions of the review as it was considered redundant.
Types of interventions: we planned to include other anti‐malarial quinines other than chloroquine and hydroxychloroquine, but did not find any RCTs for these interventions. Although not planned in the protocol, in this update we did additionally include lenalidomide, biological agents, (including abatacept, adalimumab, belimumab, etanercept, efalizumab, infliximab, rituximab, sifalimumab and sirukumab), topical calcineurin antagonists, (tacrolimus and pimecrolimus) and topical salbutamol as these agents had become available and trials had been performed in connective tissue disorders. We also clarified the types of comparators we would accept, as this detail was not specified in the protocol. We expanded the list of excluded interventions to include phototherapy and photoprotection, which were not originally listed in the protocol, for clarification only. This does not represent a change in methodology.
Types of outcome measures: in a previous version of the review, under Primary outcomes, we added 'percentage of people with' to our 'complete resolution of skin lesions…' and 'clearing of erythema…' outcomes, and these have been retained (Jessop 2009). Note that we accepted the following terms to describe complete resolution: complete clearing, clearing, marked improvement and excellent improvement.
Searching other resource: although not planned in the protocol, we searched Index Medicus by hand for studies relating to treatment of discoid lupus erythematosus for the years 1956 to 1966 because we thought it important, and we recorded all adverse events reported in the included and excluded studies as we recognise the need to report adverse events.
Search methods for identification of studies: the databases and date ranges that we planned to search in the protocol have been extended for this update review, in line with current standard search methods.
Data collection and analysis: In the original protocol, we had not made plans at all regarding how to deal with within‐patient studies, missing data, sensitivity analysis, and 'Summary of findings' tables, but we now have in line with current requirements.
Where results are estimated for individual studies with low numbers of outcomes (< 10 in total) or where the total sample size is less than 30 participants, we decided to report the proportion of outcomes in each treatment group together with a P value from a Fisher’s Exact test. This follows guidance provided by the Skin Group Statistics editors on how to deal with small numbers, made available since the last update of this review.
"We tested statistical heterogeneity using the I² statistic (0% to 40%: may not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: represents considerable heterogeneity) (Higgins 2011).” An assessment of how we would deal with heterogeneity was missing from the original protocol therefore we have amended this at this stage in the event that future updates of this review will contain a pooled analysis of two or more studies.
Assessment of risk of bias in included studies: the text within this section was written in the 'Quality rating of included studies' section of the protocol. The review has been amended to follow the RevMan 5 recommended headings and the new Cochrane Handbook for Systematic Reviews of Interventions guidelines.
Two additional headings have been added to this section: Comparability of the two arms and Intention to treat analysis.
According to the Cochrane Handbook for Systematic Reviews of Interventions, controlled trials that allocate participants by quasi‐randomisation, or that fail to conceal allocation during recruitment, are at risk of selection bias. We have included such trials, but have indicated that there is high risk of selection bias.
Imbalance at baseline has been assessed in this version of the review and added to the risk of bias tables.
Measures of treatment effect: although not planned in the protocol, for any significant outcomes (P < 0.05) we wanted to present the number needed to treat for an additional harmful outcome (NNTH) where the first treatment in the comparison is harmful (risk ratio (RR) > 1 for safety outcomes). We did not do this as no significant harms were reported.
In the protocol, we did not pre‐state the effect measures that we would report. For dichotomous outcomes (primary outcomes 1 and 2 and adverse events), results are presented as RR with 95% confidence intervals (CI). For this update, for continuous outcomes (primary outcome 3), we planned to present results in the form of mean differences (with 95% CIs). Where there were treatment comparisons where studies used different scales for the same outcome, we planned to convert results to standard mean differences (SMD) to allow pooling of results. We could not carry out these plans because the studies were few and heterogeneous.
Unit of analysis issues > Cross‐over trials: in the protocol we did not plan how to analyse cross‐over trials; but having included such a study, we followed the guidance provided in Section 16.4.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where no evidence of a carry‐over effect was present, analyses of paired differences were presented if these data could be obtained from the trial report. Otherwise, only data from the first treatment period were used.
Unit of analysis issues >Within‐patient studies: although not planned in the protocol, internally controlled trials were analysed using techniques for paired designs (e.g. paired t‐test, McNemar's test). Where appropriate, these were included in additional data tables and not pooled with parallel group trials.
Assessment of heterogeneity: in the protocol, we had planned to use statistical tests for homogeneity between studies. However, assessment of heterogeneity was not performed as no findings were based on pooled results from 2 or more trials.
Data synthesis: in the protocol, we planned to draw up a synthesis of included trials; however, this was not possible with the data in the studies we found, due to heterogeneity of study methods and interventions. No two trials investigated the same interventions.
Subgroup analysis and investigation of heterogeneity: in the protocol, we had planned to perform subgroup analysis on the following measures: disseminated DLE versus localised DLE; DLE with systemic lupus versus DLE without systemic lupus; histologically proven versus clinically diagnosed DLE; and effect of ethnic group on outcome. However, we were unable to address these issues, as none of the studies provided data on individual subgroups, (and two of the trials enrolled small numbers of participants).
Trials that included more than one subset of cutaneous lupus were included if most participants had DLE and the outcome data described outcomes for DLE.
Other: we planned where possible to calculate cost effectiveness ratios using quality‐of life‐measures; however, the studies identified did not provide these data.
'Summary of findings' table: although not planned in the protocol, we used GRADEpro 2008 to create a 'Summary of findings' table as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In this, we have summarised the primary outcomes for the most important comparisons. Subgroup analysis was not possible.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acitretin [adverse effects, therapeutic use];
- Albuterol [therapeutic use];
- Calcineurin Inhibitors [therapeutic use];
- Dermatologic Agents [adverse effects, *therapeutic use];
- Fluocinonide [therapeutic use];
- Hydrocortisone [therapeutic use];
- Hydroxychloroquine [therapeutic use];
- Lupus Erythematosus, Discoid [*drug therapy];
- Randomized Controlled Trials as Topic;
- Tacrolimus [analogs & derivatives, therapeutic use];
- Treatment Outcome;
Medical Subject Headings Check Words
Humans;
PICO
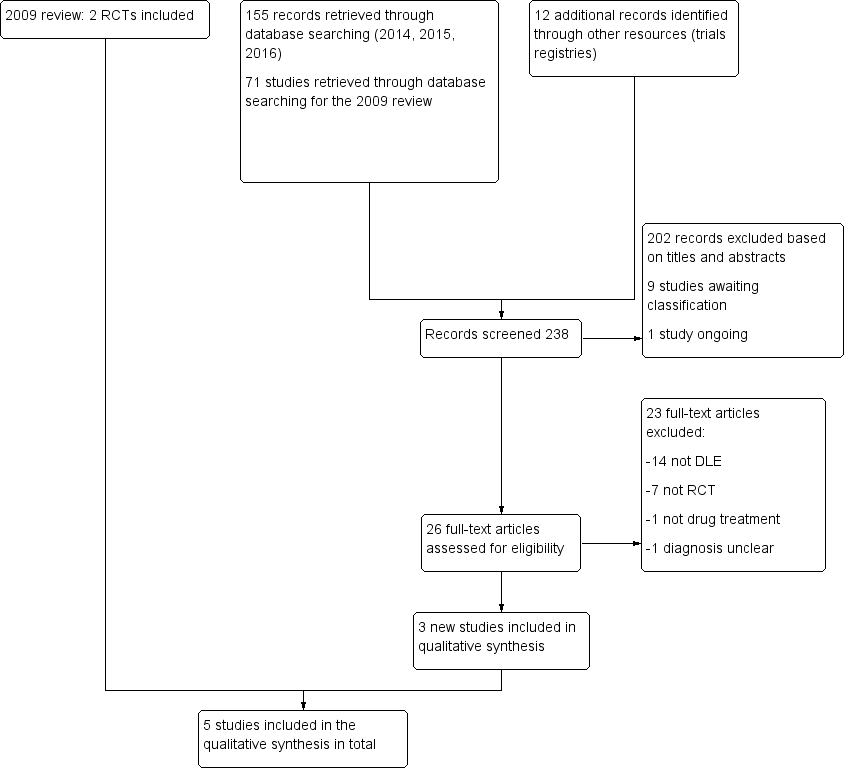
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Fluocinonide versus hydrocortisone cream, Outcome 1 Resolution of skin lesions.

Comparison 2 Acitretin versus hydroxychloroquine, Outcome 1 Resolution of skin lesions.
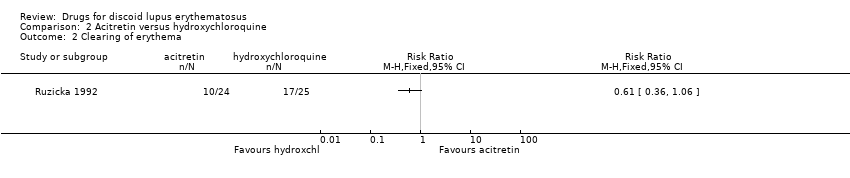
Comparison 2 Acitretin versus hydroxychloroquine, Outcome 2 Clearing of erythema.
| Fluocinonide 0.05% compared with hydrocortisone 1% for discoid lupus erythematosus | ||||||
| Patient or population: people with discoid lupus erythematosus Settings: not stated Intervention: fluocinonide Comparison: hydrocortisone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| hydrocortisone | fluocinonide | |||||
| Clearing or excellent improvement (after 6 weeks of treatment) | 10 per 100 | 27 per 100 (9 to 79) | 2.77 (0.95 to 8.08) | 78 (1 study) | Low1 | ‐ |
| At least 50% reduction in erythema | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Quality of life measure | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Relapse | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | see comment | 78 (1 study) | Moderate2 | The number of adverse events in this study was small and results were presented narratively. For hydrocortisone, 1 person developed acne and 3 experienced irritation. 2 patients who were assigned to fluocinonide experienced burning. There was no discontinuation in either group. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The assumed risk was the mean risk for the study population 1Downgraded by two levels due to imprecision and high risk of bias (incomplete outcome data). | ||||||
| Acitretin 50 mg daily compared with hydroxychloroquine 400 mg daily for discoid lupus erythematosus | ||||||
| Patients or population: people with discoid lupus erythematosus Settings: specialised lupus clinic Intervention: acitretin Comparison: hydroxychloroquine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| hydroxychloroquine | acitretin | |||||
| Clearing or excellent improvement (after 8 weeks of treatment) | 50 per 100 | 46 per 100 (27 to 80) | 0.93 (0.54 to 1.59) | 58 (1 study) | Low1 | ‐ |
| At least 50% reduction in erythema (after 8 weeks of treatment) | 68 per 100 | 42 per 100 (25 to 72) | 0.61 (0.36 to 1.06) | 49 (1 study) | Low1 | ‐ |
| Quality of life measure | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Relapse | see comment | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | see comment | 58 (1 study) | Moderate2 | Information on adverse events was presented narratively. 27 out of 28 participants receiving acitretin had at least one adverse event compared with 17 out of 30 patients treated with hydroxychloroquine. 4 people receiving acitretin discontinued treatment due to dry lips and gastrointestinal symptoms. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The assumed risk was the mean risk for the study population. 1Downgraded by two levels due to imprecision and high risk of bias (non‐comparable treatment arms). | ||||||
| Pimecrolimus 1% compared with betamethasone 17‐valerate 0.1% for discoid lupus erythematosus | |||||
| Patients or population: people with discoid lupus erythematosus Settings: dermatology clinics Intervention: pimecrolimus Comparison: betamethasone | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments |
| Clearing or excellent improvement (after 8 weeks of treatment) | see comment | see comment | 10 (1 study) | see comment | There was a statistically significant reduction in the disease severity score in both treatment groups; however, clearing or improvement was not presented as a percentage and no comparative analyses were performed. |
| At least 50% reduction in erythema | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Quality of life measure | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Relapse | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| R‐salbutamol 0.5% topical cream compared with placebo for discoid lupus erythematosus | |||||
| Patient or population: people with discoid lupus erythematosus Settings: dermatology departments Intervention: R‐salbutamol Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments |
| Clearing or excellent improvement | see comment | see comment | 37 (1 study) | see comment | While data on overall improvement were provided in the study, the number of participants with complete resolution in Jemec 2009 could not be obtained from the trial report. |
| At least 50% reduction in erythema | see comment | see comment | 37 (1 study) | see comment | While data on erythema were provided in the study report, the number of participants with at least 50% reduction in erythema in Jemec 2009 could not be obtained from the trial report. |
| Quality of life measure | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Relapse | see comment | see comment | see comment | see comment | This outcome was not assessed. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | 37 (1 study) | Moderate1 | Results for adverse events were presented narratively. There were 15 events in the placebo group (experienced by 12 participants) and 24 in the salbutamol group (experienced by 9 participants). None of the adverse events were considered serious. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for imprecision (small sample size). | |||||
| Tacrolimus 0.1% cream compared with placebo for discoid lupus erythematosus | |||||
| Patient or population: people with discoid lupus erythematosus Settings: dermatology departments Intervention: tacrolimus Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks (95% CI) | Illustrative comparative risks (95% CI) | No of Participants | Quality of the evidence | Comments |
| Clearing or excellent improvement | see comment | see comment | see comment | see comment | No participants with DLE in either group in the study by Kuhn 2011 experienced complete clearing. Unable to GRADE due to 0 events in both groups. |
| At least 50% reduction in erythema | see comment | see comment | see comment | see comment | The results for erythema in the study by Kuhn 2011 were not reported separately. |
| Quality of life measure | see comment | see comment | see comment | see comment | This outcome was not measured. |
| Relapse | see comment | see comment | see comment | see comment | This outcome was not measured. |
| Adverse events of medication, leading to discontinuation or significant morbidity | see comment | see comment | 14 (1 study) | Moderate1 | Results for adverse events were presented narratively. In the tacrolimus group, 5 participants complained of slight burning and itching, and in 1 person a herpes simplex infection was reactivated. There were no serious adverse events. |
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by one level for imprecision (small sample size). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of skin lesions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of skin lesions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clearing of erythema Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

